Abstract
The management and treatment of tissue infection, especially chronic infection, represents a significant challenge. Application of autologous platelet-rich plasma (PRP) has emerged as a promising adjunct therapy for facilitating the healing of surgical wounds and tissue injuries. PRP is extracted from whole blood using a sequential centrifugation technique and when activated, can release a vast array of antimicrobial proteins, cytokines, and growth factors. These bioactive molecules are responsible for the ability of PRP to kill pathogens, resolve necrotic tissue, and promote wound healing. PRP is emerging as a useful supplement to prevent postoperative infection and treat chronic wound or bone infections. PRP displays a synergistic effect with antibiotics, which provides unique advantages when treating antibiotic-resistant bacteria. This review will describe the method for PRP preparation and its antibacterial properties, as well as discuss both preclinical in vivo results and evidence from clinical practice of PRP use for the treatment of wound and bone infections.
Impact Statement
The clinical application of platelet-rich plasma (PRP) has been widely studied for its effects on trauma or injury repair/regeneration, however the antibacterial property of PRP has been overlooked. Increasing evidence suggests PRP as a good antibacterial agent and that it could help prevent/treat tissue infection. This review emphasizes the importance of PRP's antibacterial property and summarizes the preclinical and clinical findings regarding the application of PRP in the prevention and treatment of wound and bone infection. The use of biocompatible PRP may be advantageous for tissue infection treatment due to its inherent antibacterial and healing promoting properties.
Keywords: antibacterial, wound infection, bone infection, osteomyelitis, PRP
Introduction
Platelets are well known for their essential hemostasis function.1 Another important characteristic of platelets is to modulate inflammation and wound healing.2 Platelets contain alpha granules, which harbor numerous proteins, cytokines, and growth factors. Some examples include epidermal growth factor (EGF), platelet-derived angiogenesis factor, insulin-like growth factor, platelet factor (PF) 4, transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF).3 Once activated at sites of injury, platelets degranulate, releasing these bioactive substances that can facilitate chemotaxis, cytoprotection, cell proliferation and differentiation, angiogenesis, and extracellular matrix (ECM) remodeling.4,5 Activated platelets also contain serotonin and histamine which enhance local capillary permeability, facilitating leukocyte extravasation into sites of inflammation.6
Platelet-rich plasma (PRP) is a blood derivative, which contains concentrated platelets and other blood components. Similar to platelets, PRP can release high concentrations of various bioactive molecules stored in alpha granules, including growth factors, chemokines, and cytokines,7 which promote angiogenesis, modulate ECM, and accelerate cellular recruitment, proliferation, and differentiation.8–11 There are numerous advantages to harnessing the protective effects of PRP. First, PRP can be easily prepared from peripheral blood samples, which is a relatively noninvasive technique. Second, PRP can be used as an autologous biologic, which minimizes the risk of immune reactions and pathogen transmission compared with nonautologous material.12
Numerous reports have described the use of PRP to promote tissue regeneration in several settings, such as stomatology,13 maxillofacial surgery,14 bone healing,15 sports medicine,16 ophthalmology,17 and peripheral nerve repair.18 To date, few adverse effects from PRP have been reported.19 However, the clinical efficacy of PRP is controversial20 and can differ among patients.21 One possible explanation to account for these discrepancies is the variability in protocols used to prepare PRP in clinical practice.22 This lack of consensus likely contributes to the controversy regarding the clinical efficacy of PRP and hinders its further application.23,24
Currently, the most widely studied application of PRP is its effects on trauma or injury repair/regeneration. PRP has been shown to exert beneficial effects by relieving postsurgical discomfort and preventing infection,25 suggesting that PRP possesses anti-inflammatory and antimicrobial properties. In recent years, there has been an increased interest in exploring the utility of PRP for the treatment of tissue infection.
Although much progress has been made in clinical practice, the treatment of chronic wound and bone infections remains challenging.26 Conventional therapies for treating chronic bone infections typically include surgical debridement of infected wounds, utilization of antibiotics, and restoring soft tissue coverage of bone with healthy tissue if the infected bone is exposed.27 Often times these treatments are ineffective in producing a timely resolution of the infection, which can lead to significant morbidity and economic burden for patients. Recent findings have suggested that PRP possesses antimicrobial properties that may make it a promising adjunct biological therapy, particularly when combined with conventional therapy.28
The purpose of this review is to describe advances in PRP preparation and to propose novel viewpoints toward the establishment of a standardized protocol for PRP preparation. This review will also discuss the antibacterial activity of PRP, as well as its utility for the treatment of chronic soft tissue and bone infections in preclinical animal models and clinical practice.
PRP Preparation
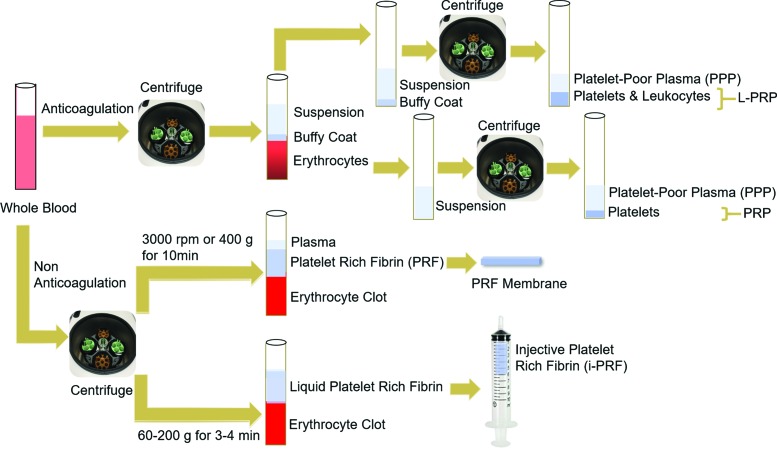
The classic method of PRP preparation involves two sequential centrifugation steps (Fig. 1); namely, separation and concentration spins. The first spin (separation spin) pellets plasma, leukocytes (neutrophils, lymphocytes, and monocytes), and platelets, from erythrocytes. The second spin (concentration spin) collects concentrated platelets in a small volume of plasma (designated as PRP). PRP can be activated by various compounds, such as autologous thrombin or calcium chloride, which induces the formation of a PRP gel.29 The activation process not only forms a fibrin gel for platelet attachment and adhesion, but also initiates degranulation, resulting in the release of bioactive substances.
FIG. 1.
Flowchart of platelet-derivative preparations. Whole blood is collected with an anticoagulant (top). Two sequential centrifugation steps occur under different force, speed, and duration conditions. The first step separates erythrocytes from the buffy coat layer and the upper suspension, containing plasma and platelets. After a second spin, the buffy coat and upper suspension are separated into PPP and L-PRP. A second centrifugation step of the upper suspension layer produces PPP and PRP. To generate PRF, whole blood is processed by a single centrifugation steep without an anticoagulant. By using different force, speed, and duration conditions, a PRF membrane and platelet-rich fibrin (i-PRF) are generated separately. PPP, platelet-poor plasma; PRP, platelet-rich plasma; L-PRP, leukocyte-rich PRP; PRF, platelet-rich fibrin; i-PRF, injective platelet-rich fibrin. Color images are available online.
Large numbers of leukocytes may be present in the final PRP preparation, which is referred to as leukocyte-rich PRP (L-PRP) and can potentiate PRP action.30 If an anticoagulant is not added, platelet-rich fibrin (PRF) is formed, which consists of a fibrin matrix, platelet-derived cytokines, growth factors, and entrapped leukocytes devoid of erythrocytes.31 The advantages of using PRF include ease of preparation, no addition of biochemical reagents or anticoagulants, ease of application, and capacity for more sustained release of bioactive factors.32 This review discusses the use of PRP, PRP gel, and PRF for the treatment of tissue infections.
The number of platelets in PRP can exceed 1 × 109/mL.33 There are many variations in PRP formulations due to disparate processing and handling methods, as well as individual variability between patients. Even in the context of autologous transfer, different PRP isolation protocols can create varying platelet and growth factor concentrations.34 In addition, gender differences may also affect PRP composition. For example, PRP from males was reported to contain more inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF), the anti-inflammatory IL-1 receptor antagonist protein, as well as growth factors (basic fibroblast growth factor, PDGF-BB, and TGF-β1), compared with PRP from females.35
PRP is activated by degranulation at target sites and forms fibrin clots in the local ECM. This leads to high concentrations of various bioactive molecules in the microenvironment, whose half-life is extended in fibrin clots compared with unbound forms.36 In addition, multiple cytokines work synergistically and have more efficient effects than individual mediators.37
As mentioned previously, the controversy surrounding the clinical efficacy of PRP can be partially explained by the inconsistent methods used to prepare PRP, which result in variable platelet and cytokine concentrations.38 Most protocols focus on how to harvest concentrated platelets and preserve hemostatic activities; however, we suggest that more attention should be paid to standardizing the PRP preparation to harness its therapeutic potential. For example, PRP is often prepared at 37°C or room temperature, without dilution or supplements.39 It is known that low temperatures enhance platelet activation, improving the release of dense and alpha granules.40 Etulain et al.41 designed an experiment to evaluate how different preparation methods affected the angiogenic and regenerative properties of PRP. They concluded that preincubating PRP at 4°C maximizes the release of platelet-derived proangiogenic molecules.41 In addition, dilution of PRP to 25% and supplementation with a plasma cryoprecipitate synergized with the precooling condition to promote angiogenesis and wound healing.41
Another important factor affecting PRP efficacy is centrifugation conditions, such as force and duration. The double-spin method, which calls for centrifugation at 160 g for 10 min followed by 250 g for 15 min, results in increased platelet, cytokine, and growth factor yields, and accelerates cellular migration and proliferation.42 In canines, a centrifugation protocol of 1000 g for 5 min and 1500 g for 15 min concentrated the number of platelets six fold.43 The centrifuge speed (revolutions per minute) and duration have also been reported to influence platelet quantity, enrichment percentages, and growth factor release.44
Platelet activation is a crucial step before cytokine release, which can be elicited by several factors, including calcium chloride, collagen, thrombin, and mechanical stress.5 PRP activation is more susceptible to autologous thrombin than calcium, and growth factor release (i.e., PDGF, VEGF, and EGF) is independent of thrombin concentrations.45
Another study compared the ability of pulse electric field (PEF), bovine thrombin, and thrombin receptor-activating peptide (TRAP) to promote human PRP activation. PEF was more effective than bovine thrombin or TRAP to yield platelet-derived microparticles, EGF, procoagulant particles, and P-selectin-positive particles.46 In a rabbit model, PRP activated by nanosecond-pulsed electric field-enhanced blood reperfusion in areas of large surgical skin flaps and ischemic hindlimb wounds.47 The utility of PEF to activate PRP may reduce variability and eliminate immune-mediated complications associated with the use of bovine thrombin, and thus has great clinical potential.48
PRP technology has the potential to revolutionize regenerative medicine. Currently, researchers are struggling to maximize the amount of platelets and function of bioactive molecules in a limited volume of plasma. Many attempts to standardize an optimal protocol have been made, with regard to centrifuge force, speed, duration, and PRP activating factors, all of which have their own shortcomings.49 None of the published methods has gained traction as an optimized universal protocol. PRP methodology should be standardized, to screen for optimal protocols to address the variability originating from gender and age differences in PRP preparations.
PRP Antibacterial Properties
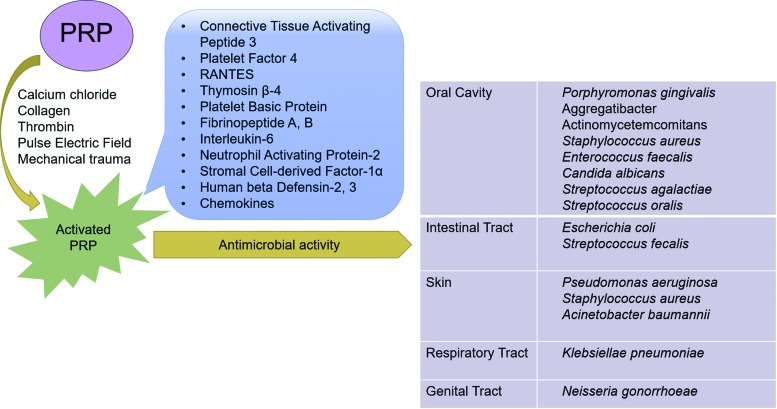
PRP releases several antibacterial proteins upon activation (Fig. 2), including connective tissue activating peptide 3, PF 4, normal T cell expressed and secreted chemokines (RANTES), thymosin β-4, platelet basic protein, fibrinopeptide A, and fibrinopeptide B.50 These molecules interact with the bacterial outer cell membrane and increase membrane permeability, which impedes protein synthesis.51,52 Apart from the cell membrane, these molecules may also target intracellular proteins to affect DNA synthesis or inhibit enzyme activity.53,54 In vitro, these molecules have preferential antimicrobial activity for bacteria compared with fungi, especially in acidic microenvironments.51
FIG. 2.
Antibacterial properties of PRP. Upon activation by numerous stimuli, PRP releases many antibacterial molecules. The organisms listed on the right are susceptible to the bactericidal properties of PRP. Color images are available online.
Bacterial adherence and growth are important initial steps during the process of colonization. In vitro, PRP can also inhibit the adherence and growth of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, which are periodontal pathogens; however, PRF does not kill these organisms.55 PRP can also inhibit the growth of many oral cavity microorganisms in vitro, such as Enterococcus faecalis, Candida albicans, Streptococcus agalactiae, and Streptococcus oralis.56
Importantly, PRP only exerts antibacterial properties upon activation57 and is effective against a broad range of both Gram-positive and Gram-negative bacteria58 (Fig. 2). Studies have shown that Klebsiella pneumoniae (Gram-negative), Staphylococcus aureus (Gram-positive), and Streptococcus faecalis (Gram-positive) are sensitive to molecules contained within PRP, including chemokine (C–C motif) ligand-3 (CCL3), CCL5, and chemokine (C–X–C motif) ligand-1 (CXCL1).59 Both TGF-β1 and PDGF-BB contained within PRP have been reported to exert bacteriostatic effects against Staphylococcus aureus.60 Although several of these molecules are recognized for their chemotactic (CCL3, CCL5, and CXCL1) and immune regulatory activity (TGF-β1), evidence suggests they can also act as antimicrobials upon cleavage.61,62
Staphylococcus aureus is a common pathogen in infected wounds.63 Depending on its acquisition of methicillin resistance, the bacterium is categorized into methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). In vitro, PRP inhibits the growth of both MRSA64 and MSSA65 as well as Group A streptococcus and Neisseria gonorrhoeae, but has little effect on Enterobacter cloacae.66 However, the antimicrobial activity of PRP is only observed within the first few hours of treatment.67
Cetinkaya et al. utilized PRP together with vancomycin in a rat model of MRSA surgical wound infection to determine if synergistic effects were evident.68 Vancomycin is normally used to treat serious infections caused by pathogens that are methicillin resistant (e.g., MRSA). The antibacterial efficacy of different experimental groups was ranked in descending order: vancomycin + PRP, vancomycin, PRP, and vehicle. This suggests that PRP may synergize with antibiotics and can be considered as an adjunct therapy to treat infection. Another in vivo study demonstrated that platelet gel supernatant possessed bactericidal activity against skin bacteria in a rat model.69
As mentioned earlier, PRP preparations also contain leukocytes, which are thought to contribute significantly to the antibacterial activity of PRP.70 For example, Anitua et al. showed that PRP containing a large number of leukocytes (L-PRP) could effectively reduce MSSA and MRSA titers.71 However, Mariani et al. reported that leukocytes did not enhance the microbicidal activity of PRP in vitro, when L-PRP and pure PRP (P-PRP) were compared.72 They concluded that the similar in vitro antibacterial activity of L-PRP and P-PRP may result from antimicrobial peptide release from both formulations, such as neutrophil-activating protein-2.
One factor that should be considered when evaluating the efficacy of L-PRP is the length of time from preparation to analysis. For example, fresh L-PRP will contain more viable leukocytes than preparations that are not evaluated immediately. Therefore, additional studies are needed to systematically compare the antibacterial activities of L-PRP and P-PRP, especially in vivo, before any conclusions regarding efficacy are drawn.
To summarize these findings, PRP has definitive antibacterial properties, which are supported by several in vitro and in vivo studies. In general, the antibacterial properties attributed to PRP to date are against common pathogens (Fig. 2). Because the exact mechanism of action is not known, further research on the bactericidal properties of PRP should be conducted.
The antibacterial effects of PRP are evident immediately following activation; however, they are somewhat weak and short lived when PRP is used alone. PRP can act synergistically with antibiotics and can be considered as an adjunct treatment for infections once the pathogen has been identified,73 especially in cases involving antibiotic-resistant bacteria. The consequences of leukocytes contained in PRP on its antibacterial properties is still controversial. There is no doubt that leukocytes play an important role in host defense, suggesting that the presence of leukocytes should augment the antibacterial properties of PRP. Further optimization of leukocyte abundance and composition in PRP, identifying the mechanism of action, and in vivo studies should be performed to better understand the role of leukocytes on the antibacterial properties in L-PRP.
PRP Application for the Treatment of Wound Infection
Both acute and chronic wound infections can present a difficult therapeutic issue. Many diseases can increase susceptibility to chronic wound infections, such as diabetes,74 arteriosclerotic obliterans, chronic venous insufficiency,75 malnutrition, and soft tissue defects due to trauma. In some circumstances, physical pressure, poor blood supply, or aging may also be contributing factors.76 PRP and its derivatives have been examined for their utility in promoting wound healing and curtailing infection, as described below.
Treatment for chronic ulcers
Ulcers are chronic wounds that involve a full-thickness layer of skin or even subcutaneous tissue and muscle, which are usually complicated by bacterial infection. For example, chronic ulcers can develop in sedentary patients secondary to pressure, and rarely heal spontaneously. Over time, bacterial contamination, chronic inflammation, diminished blood supply, and growth factor imbalance exert negative effects on the wound, delaying the healing process. Conventional therapies for treating ulcers include surgical debridement, infection management, and ulcer bed revascularization.77
Topical applications of autologous PRP have rendered unique advantages, especially for refractory wounds.78 The advantages of PRP may originate mainly from the release of highly concentrated cytokines, growth factors, and antibacterial proteins.79 Cytokines and growth factors are helpful for cellular proliferation, migration, and the differentiation of fibroblasts, endothelial cells, and keratinocytes.80,81 Growth factors released from PRP are encapsulated in exosomes, which exert their effect through the activation of Yes-associated protein.82
As described above, antibacterial proteins can kill or inhibit local pathogens, which may facilitate wound healing. Collectively, these secreted molecules can synergize to establish a microenvironment that is beneficial for vascularization, granulation tissue growth, and epithelialization, effectively accelerating ulcer resolution. Additionally, after PRP is activated and applied onto ulcer surfaces, the remaining plasma fibronectin can also influence ECM remodeling and tissue regeneration by serving as a scaffold.83
The ability of PRP to facilitate ulcer healing has been evaluated in several clinical studies. One report examined 10 patients with chronic lower limb venous stasis ulcers that failed traditional treatment modalities. All patients in this study showed complete resolution of these ulcers over 4–10 weeks after receiving adjunctive PRP therapy.84 Another retrospective study examined 39 patients with chronic lower extremity wounds to evaluate the effectiveness of standard protocols combined with PRP gel.85 This study demonstrated that the addition of PRP gel promoted wound healing after an average of 145 days in 83% of the wounds and decreased the amputation rate.
In another prospective autocontrolled clinical study, 21 patients with severe lower extremity ulcers, who had failed to improve after at least 4 months of conventional compression therapy, were recruited to evaluate the efficacy of PRF treatment.86 After a 16-week follow-up, 66.7% of venous ulcers and 44% of nonvenous ulcers achieved complete healing with PRF therapy, without any treatment-associated complications or adverse reactions.
In another prospective, randomized, and controlled clinical study, the ability of PRP gel to treat refractory diabetic foot ulcers was compared with a saline gel dressing. After a strict follow-up protocol, 16 cases of PRP gel and 19 cases of saline gel dressing were included in the study cohort. Interestingly, 81.3% of the wounds were resolved in the PRP gel group compared with only 42.1% in the saline gel group. Again, PRP gel did not exhibit any adverse effects.87 In another study, PRP gel was found to be superior to antiseptic ointment dressing.88 Similar positive effects on accelerating the healing of infected wounds has also been demonstrated in clinical trials,89,90 even showing efficacy in patients with severe arterial disease.91
Treatment for hidradenitis suppurativa
Hidradenitis suppurativa is a chronic, recurrent, suppurative skin condition.92 Several options exist to treat hidradenitis suppurativa, including antibiotics, topical bactericidal lotions, corticosteroids, retinoids, and surgery for severely infected cases.93 When combined with Hyalomatrix, PRP gel displayed efficacy in treating resistant nuchal lesions after surgical excision with excellent safety and no disease recurrences after a 1-year follow-up.94 In another case report of four patients, PRP improved wound healing in autologous split-thickness skin grafting after wide excisions of scalp and gluteal skin.95
Treatment for other skin ulcers and infections
Pressure ulcers are injuries to skin and underlying tissue resulting from prolonged pressure, which commonly occur on prominences of the hip, feet, and buttocks of chronically sedentary patients. These wounds are commonly infected by fecal and urinary pathogens. PRP has been shown to decrease Staphylococcus aureus colonization of pressure ulcers, which has been attributed to its antibacterial properties.96
AIDS is a serious complication of HIV-induced immunosuppression, and some opportunistic pathogens may be fatal to HIV-positive patients. After L-PRP was adopted to treat lower limb ulcers among five AIDS patients, Staphylococcus aureus and Pseudomonas aeruginosa levels were reduced concomitant with increased VEGF, FLK, and CD34, suggesting that the beneficial effects of L-PRP may be mediated, in part, by stimulating angiogenesis within the ulcer bed.97
Thalassemia is a type of congenital hemoglobinopathy, which may increase the risk of skin ischemia and ulceration. After 100 chronic thalassemic leg wounds were treated successfully with PRP gel, its uses were expanded further.98
When considering the potential benefits of PRP as an adjunctive therapy, the aggressive debridement used to treat wound infections should be considered.99 Basic research and clinical studies have demonstrated that topical application of PRP is a useful auxiliary therapy for wound infections. Although there are many studies supporting that PRP accelerates wound healing by promoting tissue growth and inhibiting bacteria colonization, large-scale, randomized, and controlled clinical studies are needed to further standardize the applications for PRP, as well as its antimicrobial specificity.
PRP Application in Treatment of Bone Infections
Bone infection or osteomyelitis, is typically caused by hematogenous spread or contamination of open wounds with bacteria that penetrate the bone. Organisms most typically associated with osteomyelitis include Staphylococcus aureus, Group B Streptococcus, Pseudomonas aeruginosa, and Escherichia coli.100,101 Acute osteomyelitis is managed with antibiotics and aggressive debridement is often necessary for chronic infections,102 especially with abscess and subsequent sequestrum formation.
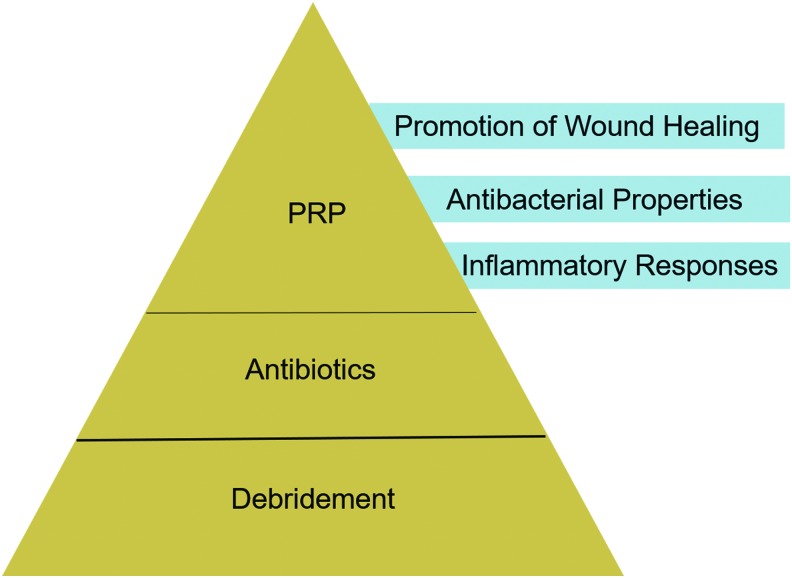
New strategies are needed for treating osteomyelitis due to the increased prevalence of antibiotic-resistant bacteria. It would be interesting to combine PRP with conventional techniques used for the treatment of infections (e.g., antibiotics, debridement), to achieve a possible synergistic effect (Fig. 3). In this review, we will focus on the utilization of PRP as a topical treatment for chronic osteomyelitis.
FIG. 3.
Application of PRP for osteomyelitis. Debridement is a fundamental step for the treatment of chronic osteomyelitis. The antibacterial strength of PRP is short acting and appears weaker than antibiotics, but PRP displays a synergistic effect with antibiotics. Color images are available online.
Possible mechanisms of PRP treatment for osteomyelitis
Sites of chronic osteomyelitis are usually typified by poor blood supply, necrotic bone or soft tissue, and insufficient growth factors.103 Many properties of PRP have the ability to overcome these challenges.104 First, macrophages may be activated by several PRP-derived proteins, including α-1-microglobulin, α-2-macroglobulin, and vitamin D-binding protein, leading to increased proinflammatory cytokine production (i.e., TNFα, IL-1β, and IL-6)105 to augment the antibacterial response. This may be magnified if there are leukocytes in the PRP (L-PRP), which induce NF-κB signaling in fibroblasts and osteoblasts to further amplify the proinflammatory environment.106
Ultimately, these inflammatory responses help to resolve necrotic bone and soft tissue and promote pathogen uptake and killing, which is facilitated by the antimicrobial proteins in PRP. In addition, the concentrated growth factors in PRP increase revascularization, promote mesenchymal stem cell and osteoblast development into osteocytes, and encourage granulation tissue formation to fill the defect.107,108
PRP treatment for osteomyelitis in preclinical animal models
A rabbit model of MRSA tibial bone infection has been used to determine the efficacy of L-PRP gel implanted into the infected bone. Six weeks after debridement, antibacterial efficacy and new bone formation were measured. The results showed that L-PRP gel acted synergistically with vancomycin to combat infection and restore bone defects compared with vancomycin or L-PRP gel alone. It is possible that the ability of L-PRP to increase local capillary networks promotes antibiotic delivery and bactericidal effects.109 In another animal model of spinal implant-associated infection, PRP significantly decreased bacterial burden and promoted new bone formation.66
Clinical applications for PRP treatment of osteomyelitis
Several clinical studies have evaluated the use of PRP in combination with antibiotics and debridement for osteomyelitis.110 Scafati et al.111 utilized PRP with autologous bone grafts to treat sinus obliteration subsequent to chronic frontal sinusitis. After a 12-month follow-up, a gradual reduction in disease symptoms was observed concomitant with improvement in sinus patency, as determined by computerized tomography scans.111 Patients with sternal bone infections also achieved better outcomes with a combination of negative pressure drainage and PRP therapy.112
Another serious condition is gunshot-related injuries, especially when fractures are involved. Chronic tissue necrosis and secondary infections caused by antibiotic-resistant bacteria can be associated with significant morbidity and even mortality. PRP may prove beneficial for salvaging extremities with these types of injuries.113
PRP application in osteomyelitis prophylaxis
The effect of PRP as a prophylactic agent combined with preoperative IV antibiotics to prevent localized osteitis in mandibular bone was evaluated after third molar extraction in 200 patients. The incidence of localized osteitis was 1% when PRP was used in the extraction site, which was lower than 9.5% without topical PRP application.114
A deep sternal wound infection may threaten the lives of patients undergoing cardiac surgery. The effect of PRP on reducing the occurrence of deep sternal wound infections was examined in 1093 patients. Subjects receiving PRP had an infection occurrence of 1 in 422 (0.2%), which was significantly lower than 10 in 671 (1.5%) of patients without PRP application.115 According to another large-scale clinical study of 2000 patients during a 7-year period, PRP was found to prevent sternal wound infections and to reduce cost and readmission rates following cardiac surgery. Therefore, the use of PRP is recommended for patients undergoing a sternotomy in cardiac surgery.116 The implementation of platelet derivatives in the treatment of wound and bone infections in both preclinical animal models and clinical research has been summarized in Table 1.
Table 1.
The Antibacterial Efficacy of Platelet Derivatives and Treatment for Tissue Infection in Preclinical and Clinical Research
| Study design | Types of platelet derivatives | Subjects and sites | Main findings | Reference | |
|---|---|---|---|---|---|
| Wound infection | |||||
| Preclinical | Prospective, randomized, and controlled study | PRP gel | Male rats, subcutaneous wounds | Antibacterial efficacy against MRSA was ranked in descending order: Vancomycin + PRP, Vancomycin, PRP, and no treatment group | 59 |
| Prospective, randomized, and controlled study | Platelet gel supernatant | Male rats, skin inoculated | inactivating Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus | 60 | |
| Prospective, randomized, and controlled study | PRP | 20 Rabbits, skin defects in ears | 19 of 20 defects healed in PRP group and 7 of 20 defects healed in conservative group | 79 | |
| Clinical | Prospective autocontrolled study | PRP | 10 Patients, lower limb ulcers | Ulcers healed completely | 74 |
| Prospective autocontrolled study | PRP gel | 39 Patients, lower extremity wounds | 83% Wounds healed | 75 | |
| Prospective autocontrolled study | PRF | 21 Patients, lower extremity ulcers | 66.7% Venous ulcers and 44% nonvenous ulcers healed | 76 | |
| Prospective, randomized, and controlled study | PRP gel | 35 Patients, diabetic foot ulcers | 81.3% and 42.1% ulcers healed in PRP gel group and saline group, separately | 77 | |
| Prospective and controlled study | PRP gel | 56 Patients, diabetic foot ulcers | 86% Ulcers healed in PRP gel group and 68% healed in group of antiseptic ointment dressing | 78 | |
| Prospective autocontrolled study | PRP | 26 Patients with chronic ulcers: lower limb (15), trunk (9), upper limb (1), and head (1) | 10 Patients healed, 16 patients improved healing | 79 | |
| Case report | PRP | 1 Patient, diabetic foot infection | Wound healed | 80 | |
| Retrospective study | PRP | 72 Patients with peripheral arterial disease, diabetic foot ulcers | Ulcer reduction >90% in 52/72 patients | 81 | |
| Case report | PRP gel | 1 Patient with hidradenitis suppurativa, nuchae lesion | Wound healed, no recurrence during 1 year postoperatively | 84 | |
| Case report | PRP | 4 Patients with hidradenitis suppurativa: gluteal lesion (3) and scalp (1) | Wounds healed, no complications | 85 | |
| Prospective study | L-PRP | 5 Patients with AIDS and lower vessel dysfunction, crural ulcers | Wounds healed in 60% cases | 87 | |
| Prospective autocontrolled study | PRP gel | 100 Patients with thalassemia, leg ulcers | Wounds healed | 88 | |
| Osteomyelitis | |||||
| Preclinical | Prospective, randomized, and controlled study | L-PRP gel | Rabbit model, tibia bone infected by MRSA | The group of vancomycin combined with L-PRP gel was best with antibacterial efficacy and new bone formation | 99 |
| Prospective, randomized, and controlled study | PRP | Rabbit model, implant-associated spinal infection | PRP treatment reduced bacterial colonies and increased mineralized tissues | 57 | |
| Clinical | Case report | PRP | 7 Patients, chronic frontal sinusitis | PRP combined with autologous calvarial bone graft succeeded to treat chronic frontal sinusitis | 101 |
| Retrospective and controlled study | PRP gel | 62 Patients, sternal osteomyelitis and sinus tract after thoracotomy | PRP combined with negative-pressure wound therapy reduced healing time and odds of secondary repair surgery | 102 | |
| Retrospective and controlled study | PRF | 200 Patients, extraction sites of both mandibular third molars | incidence of localized osteitis was 1% in PRF group, lower than the 9.5% of the control group | 104 | |
| Retrospective and controlled study | PRP | 1093 Patients with cardiac surgery through median sternotomy | Incidence of deep sternal wound infection in PRP group was 1 out of 422 (0.2%), significantly lower than 10 out of 671 (1.5%) of control group | 105 | |
| Retrospective and controlled study | PRP | 2000 Patients with cardiac surgery through sternotomy | PRP application reduced the incidence of deep sternal wound infection from 2.0% to 0.6%, superficial sternal wound infections from 8.0% to 2.0%, and the readmission rate from 4.0% to 0.8%. | 106 | |
PRP, platelet-rich plasma; L-PRP, leukocyte-rich PRP; PRF, platelet-rich fibrin; MRSA, methicillin-resistant Staphylococcus aureus.
Conclusions and Future Perspectives
PRP is an extract prepared from whole blood following centrifugation. Activated PRP can release various antimicrobial proteins, inflammatory cytokines, and growth factors; however, there is currently no standardized protocol to prepare PRP to allow efficacy comparisons across studies. PRP-derived bioactive molecules are responsible for its antimicrobial properties, resolving necrotic tissue, and promoting wound healing. PRP has been shown to have a beneficial rule in treating chronic wound infections and osteomyelitis.
One of the limitations of PRP is that its antibacterial strength is short acting and is weaker than antibiotics; however, PRP displays a synergistic effect with antibiotics, suggesting they should be used together for the treatment of bacterial infections. In addition, the effectiveness of PRP treatment has some reported variability due to inherent differences between individuals and disease models.
Currently, autologous PRP has been most widely used. Alternatively, allogeneic PRP from well-characterized donors represents an off-the-shelf solution.117 Allogeneic PRP may generate more consistent and reliable therapeutic effects and could be more suitable for patients requiring long-term treatment.118,119 However, no studies to date have systematically compared the safety and efficacy of allogeneic PRP over autologous PRP. Therefore, more clinical and translational research should be conducted to verify the feasibility of using allogeneic PRP to replace its autologous counterpart.
The bioactive molecules secreted from PRP facilitate the ability of leukocytes to clear pathogens and necrotic tissue, which promotes wound healing. Leukocytes in L-PRP may potentiate inflammatory cytokine production and phagocytic activity; however, this should be investigated in more depth. More convincing and robust clinical evidences is needed to support the application of PRP for the treatment of chronic wound infections and osteomyelitis. Therefore, future large-scale, prospective, randomized, and controlled clinical studies should be conducted to standardize the indication, contradiction, and detailed procedure of PRP use in the clinical setting.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke R01 NS107369 (to T.K.) and National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR073225 (To B.D.).
Disclosure Statement
No competing financial interests exist.
References
- 1. Caviglia H., Landro M., Daffunchio C., et al. Platelet rich plasma for chronic synovitis treatment in patients with haemophilia. Haemophilia 23, 613, 2017 [DOI] [PubMed] [Google Scholar]
- 2. Cervelli V., Lucarini L., Spallone D., et al. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv Skin Wound Care 24, 176, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Davis V.L., Abukabda A.B., Radio N.M., et al. Platelet-rich preparations to improve healing. Part I: workable options for every size practice. J Oral Implantol 40, 500, 2014 [DOI] [PubMed] [Google Scholar]
- 4. De Pascale M.R., Sommese L., Casamassimi A., and Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev 29, 52, 2015 [DOI] [PubMed] [Google Scholar]
- 5. Davis V.L., Abukabda A.B., Radio N.M., et al. Platelet-rich preparations to improve healing. Part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol 40, 511, 2014 [DOI] [PubMed] [Google Scholar]
- 6. Yun S.-H., Sim E.-H., Goh R.-Y., Park J.-I., and Han J.-Y. Platelet activation: the mechanisms and potential biomarkers. BioMed Res Int 2016, [Epub ahead of print]; DOI: 10.1155/2016/9060143, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marques L.F., Stessuk T., Camargo I.C., Sabeh Junior N., dos Santos L., and Ribeiro-Paes J.T. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets 26, 101, 2015 [DOI] [PubMed] [Google Scholar]
- 8. Burnouf T., Strunk D., Koh M.B., and Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 76, 371, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Qian Y., Han Q., Chen W., et al. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem 5, 89, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kakudo N., Morimoto N., Kushida S., Ogawa T., and Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 47, 83, 2014 [DOI] [PubMed] [Google Scholar]
- 11. Holmes H.L., Wilson B., Goerger J.P., et al. Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS One 13, e0194567, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones I.A., Togashi R.C., and Vangsness C.T. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med 11, 558, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaigler D., Avila G., Wisner-Lynch L., et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther 11, 375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simonpieri A., Del Corso M., Vervelle A., et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharmaceut Biotechnol 13, 1231, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Intini G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials 30, 4956, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kreuz P.C., Krüger J.P., Metzlaff S., et al. Platelet-rich plasma preparation types show impact on chondrogenic differentiation, migration, and proliferation of human subchondral mesenchymal progenitor cells. Arthroscopy 31, 1951, 2015 [DOI] [PubMed] [Google Scholar]
- 17. L Alio J., Arnalich-Montiel F., and E Rodriguez A. The role of “eye platelet rich plasma”(E-PRP) for wound healing in ophthalmology. Curr Pharmaceut Biotechnol 13, 1257, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Sanchez M., Garate A., Delgado D., and Padilla S. Platelet-rich plasma, an adjuvant biological therapy to assist peripheral nerve repair. Neural Regen Res 12, 47, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Picard F., Hersant B., Niddam J., and Meningaud J.-P. Injections of platelet-rich plasma for androgenic alopecia: a systematic review. J Stomatol Oral Maxillofac Surg 118, 291, 2017 [DOI] [PubMed] [Google Scholar]
- 20. Calori G., Tagliabue L., Gala L., d'Imporzano M., Peretti G., and Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury 39, 1391, 2008 [DOI] [PubMed] [Google Scholar]
- 21. SanGiovanni T.P., and Kiebzak G.M. Prospective randomized evaluation of intraoperative application of autologous platelet-rich plasma on surgical site infection or delayed wound healing. Foot Ankle Int 37, 470, 2016 [DOI] [PubMed] [Google Scholar]
- 22. Russell R.P., Apostolakos J., Hirose T., Cote M.P., and Mazzocca A.D. Variability of platelet-rich plasma preparations. Sports Med Arthrosc Rev 21, 186, 2013 [DOI] [PubMed] [Google Scholar]
- 23. Dhurat R., and Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg 7, 189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chahla J., Cinque M.E., Piuzzi N.S., et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am 99, 1769, 2017 [DOI] [PubMed] [Google Scholar]
- 25. Knezevic N.N., Candido K.D., Desai R., and Kaye A.D. Is platelet-rich plasma a future therapy in pain management? Med Clin 100, 199, 2016 [DOI] [PubMed] [Google Scholar]
- 26. Jia W.T., Zhang C.Q., Wang J.Q., Feng Y., and Ai Z.S. The prophylactic effects of platelet-leucocyte gel in osteomyelitis: an experimental study in a rabbit model. J Bone Joint Surg Br 92, 304, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Yuan T., Zhang C., and Zeng B. Treatment of chronic femoral osteomyelitis with platelet-rich plasma (PRP): a case report. Transfus Apher Sci 38, 167, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Andia I., and Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 8, 645, 2013 [DOI] [PubMed] [Google Scholar]
- 29. Velier M., Magalon J., Daumas A., et al. Production of platelet-rich plasma gel from elderly patients under antithrombotic drugs: perspectives in chronic wounds care. Platelets 29, 496, 2018 [DOI] [PubMed] [Google Scholar]
- 30. Ehrenfest D.M.D., Andia I., Zumstein M.A., Zhang C.-Q., Pinto N.R., and Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 4, 3, 2014 [PMC free article] [PubMed] [Google Scholar]
- 31. Naik B., Karunakar P., Jayadev M., and Marshal V.R. Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent 16, 284, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khiste S.V., and Naik Tari R. Platelet-rich fibrin as a biofuel for tissue regeneration. ISRN Biomater 2013, Article ID 627367, 2013 [Google Scholar]
- 33. Amable P.R., Carias R.B., Teixeira M.V., et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 4, 67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magalon J., Bausset O., Serratrice N., et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy 30, 629, 2014 [DOI] [PubMed] [Google Scholar]
- 35. Xiong G., Lingampalli N., Koltsov J.C., et al. Men and women differ in the biochemical composition of platelet-rich plasma. Am J Sports Med 46, 409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng L., Chang W., Tian B., Zeng W., and Zhou D. Bone regeneration combining platelet rich plasma with engineered bone tissue. J Biomater Tissue Eng 7, 841, 2017 [Google Scholar]
- 37. Castro A.B., Meschi N., Temmerman A., et al. Regenerative potential of leucocyte-and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 44, 67, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohn C.S., and Lockhart E. Autologous platelet-rich plasma: evidence for clinical use. Curr Opin Hematol 22, 527, 2015 [DOI] [PubMed] [Google Scholar]
- 39. Magalon J., Chateau A., Bertrand B., et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med 2, e000060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Egidi M.G., D'Alessandro A., Mandarello G., and Zolla L. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus 8, s73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Etulain J., Mena H.A., Meiss R.P., et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep 8, 1513, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin W., Xu H., Sheng J., et al. Optimization of pure platelet-rich plasma preparation: a comparative study of pure platelet-rich plasma obtained using different centrifugal conditions in a single-donor model. Exp Therapeut Med 14, 2060, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shin H.-S., Woo H.-M., and Kang B.-J. Optimisation of a double-centrifugation method for preparation of canine platelet-rich plasma. BMC Vet Res 13, 198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. SabaRiSh R., LaVu V., and Rao S.R. A comparison of platelet count and enrichment percentages in the platelet rich plasma (prp) obtained following preparation by three different methods. J Clin Diagn Res 9, ZC10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huber S.C., Júnior J.L.R.C., Montalvão S., et al. In vitro study of the role of thrombin in platelet rich plasma (PRP) preparation: utility for gel formation and impact in growth factors release. J Stem Cells Regen Med 12, 2, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frelinger A.L., 3rd Torres, A., Caiafa A., et al. Platelet-rich plasma stimulated by pulse electric fields: platelet activation, procoagulant markers, growth factor release and cell proliferation. Platelets 27, 128, 2016 [DOI] [PubMed] [Google Scholar]
- 47. Hargrave B., and Li F. Nanosecond pulse electric field activated-platelet rich plasma enhances the return of blood flow to large and ischemic wounds in a rabbit model. Physiol Rep 3, pii: , 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frelinger A.L., III, Gerrits A.J., Neculaes V.B., et al. Tunable activation of therapeutic platelet-rich plasma by pulse electric field: differential effects on clot formation, growth factor release, and platelet morphology. PLoS One 13, e0203557, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lang S., Loibl M., and Herrmann M. Platelet-rich plasma in tissue engineering: hype and hope. Eur Surg Res 59, 265, 2018 [DOI] [PubMed] [Google Scholar]
- 50. Mussano F., Genova T., Munaron L., Petrillo S., Erovigni F., and Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 27, 467, 2016 [DOI] [PubMed] [Google Scholar]
- 51. Tang Y.-Q., Yeaman M.R., and Selsted M.E. Antimicrobial peptides from human platelets. Infect Immun 70, 6524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeaman M.R., and Bayer A.S. Antimicrobial peptides from platelets. Drug Resist Updates 2, 116, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Bechinger B., and Gorr S.-U. Antimicrobial peptides: mechanisms of action and resistance. J Dent Res 96, 254, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ho Y.-H., Shah P., Chen Y.-W., and Chen C.-S. Systematic analysis of intracellular-targeting antimicrobial peptides, bactenecin 7, hybrid of pleurocidin and dermaseptin, proline-arginine-rich peptide, and lactoferricin B, by using Escherichia coli proteome microarrays. Mol Cell Proteomics 15, 1837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Badade P.S., Mahale S.A., Panjwani A.A., Vaidya P.D., and Warang A.D. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res 27, 300, 2016 [DOI] [PubMed] [Google Scholar]
- 56. Drago L., Bortolin M., Vassena C., Taschieri S., and Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol 13, 47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drago L., Bortolin M., Vassena C., Romanò C.L., Taschieri S., and Del Fabbro M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One 9, e107813, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tohidnezhad M., Varoga D., Podschun R., et al. Thrombocytes are effectors of the innate immune system releasing human beta defensin-3. Injury 42, 682, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Mariani E., Filardo G., Canella V., et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy 16, 1294, 2014 [DOI] [PubMed] [Google Scholar]
- 60. Lopez C., Carmona J., Giraldo C., and Alvarez M. Bacteriostatic effect of equine pure plateletrich plasma and other blood products against methicillin-sensitive Staphylococcus aureus. Vet Comp Orthopaed Traumatol 27, 372, 2014 [DOI] [PubMed] [Google Scholar]
- 61. Valdivia-Silva J., Medina-Tamayo J., and Garcia-Zepeda E.A. Chemokine-derived peptides: novel antimicrobial and antineoplasic agents. Int J Mol Sci 16, 12958, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen L.T., and Vogel H. Structural perspectives on antimicrobial chemokines. Front Immunol 3, 384, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morris J., Kelly N., Elliott L., et al. Evaluation of bacteriophage anti-biofilm activity for potential control of orthopedic implant-related infections caused by Staphylococcus aureus. Surg Infect (Larchmt) 20, 16, 2018 [DOI] [PubMed] [Google Scholar]
- 64. Çetinkaya R.A., Yenilmez E., Petrone P., et al.. Platelet-rich plasma as an additional therapeutic option for infected wounds with multi-drug resistant bacteria: in vitro antibacterial activity study. Eur J Trauma Emerg Surg [Epub ahead of print]; DOI: 10.1007/s00068-018-0957-0, 2018 [DOI] [PubMed] [Google Scholar]
- 65. Intravia J., Allen D.A., Durant T.J., et al. In vitro evaluation of the anti-bacterial effect of two preparations of platelet rich plasma compared with cefazolin and whole blood. Muscles Ligaments Tendons J 4, 79, 2014 [PMC free article] [PubMed] [Google Scholar]
- 66. Li H., Hamza T., Tidwell J.E., Clovis N., and Li B. Unique antimicrobial effects of platelet-rich plasma and its efficacy as a prophylaxis to prevent implant-associated spinal infection. Adv Healthc Mater 2, 1277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fabbro M.D., Bortolin M., Taschieri S., Ceci C., and Weinstein R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 27, 276, 2016 [DOI] [PubMed] [Google Scholar]
- 68. Cetinkaya R., Yilmaz S., Ünlü A., et al. The efficacy of platelet-rich plasma gel in MRSA-related surgical wound infection treatment: an experimental study in an animal model. Eur J Trauma Emerg Surg 44, 859, 2018 [DOI] [PubMed] [Google Scholar]
- 69. Edelblute C.M., Donate A.L., Hargrave B.Y., and Heller L.C. Human platelet gel supernatant inactivates opportunistic wound pathogens on skin. Platelets 26, 13, 2015 [DOI] [PubMed] [Google Scholar]
- 70. D'asta F., Halstead F., Harrison P., Zecchi Orlandini S., Moiemen N., and Lord J. The contribution of leucocytes to the antimicrobial activity of platelet-rich plasma preparations: a systematic review. Platelets 29, 9, 2018 [DOI] [PubMed] [Google Scholar]
- 71. Anitua E., Alonso R., Girbau C., Aguirre J., Muruzabal F., and Orive G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol Exp Dermatol 37, 652, 2012 [DOI] [PubMed] [Google Scholar]
- 72. Mariani E., Canella V., Berlingeri A., et al. Leukocyte presence does not increase microbicidal activity of Platelet-rich plasma in vitro. BMC Microbiology 15, 149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Różalski M.I., Micota B., Sadowska B., Paszkiewicz M., Więckowska-Szakiel M., and Różalska B. Antimicrobial/anti-biofilm activity of expired blood platelets and their released products. Postepy Hig Med Dosw (Online) 67, 321, 2013 [DOI] [PubMed] [Google Scholar]
- 74. Boulton A.J., Vileikyte L., Ragnarson-Tennvall G., and Apelqvist J. The global burden of diabetic foot disease. Lancet 366, 1719, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Woźniak W., Tarnas M., Miłek T., Mlosek R.K., and Ciostek P. The effect of local platelet rich plasma therapy on the composition of bacterial flora in chronic venous leg ulcer. Pol J Microbiol 65, 353, 2016 [DOI] [PubMed] [Google Scholar]
- 76. Han G., and Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 34, 599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Waniczek D., Kozowicz A., Muc-Wierzgoń M., Kokot T., Świętochowska E., and Nowakowska-Zajdel E. Adjunct methods of the standard diabetic foot ulceration therapy. Evid Based Complement Alternat Med 2013, 243568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yotsu R.R., Hagiwara S., Okochi H., and Tamaki T. Case series of patients with chronic foot ulcers treated with autologous platelet-rich plasma. J Dermatol 42, 288, 2015 [DOI] [PubMed] [Google Scholar]
- 79. Cieslik-Bielecka A., Bielecki T., Gazdzik T.S., Arendt J., Król W., and Szczepanski T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus Apheres Sci 41, 9, 2009 [DOI] [PubMed] [Google Scholar]
- 80. Kanemaru H., Kajihara I., Yamanaka K., et al. Platelet-rich plasma therapy is effective for the treatment of refractory skin ulcers in patients with systemic sclerosis. Mod Rheumatol 25, 660, 2015 [DOI] [PubMed] [Google Scholar]
- 81. Greene-Roos J.A., and Laughlin M. Umbilical cord derived monocytes and platelet rich plasma for diabetic wound healing. J Immunol 198, 81.27, 2017 [Google Scholar]
- 82. Guo S.-C., Tao S.-C., Yin W.-J., Qi X., Yuan T., and Zhang C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 7, 81, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moroz A., and Deffune E. Platelet-rich plasma and chronic wounds: remaining fibronectin may influence matrix remodeling and regeneration success. Cytotherapy 15, 1436, 2013 [DOI] [PubMed] [Google Scholar]
- 84. Waniczek D., Mikusek W., Kamiński T., Wesecki M., Lorenc Z., and Cieślik-Bielecka A. The “biological chamber” method–use of autologous platelet-rich plasma (PRP) in the treatment of poorly healing lower-leg ulcers of venous origin. Pol J Surg 87, 283, 2015 [DOI] [PubMed] [Google Scholar]
- 85. Sakata J., Sasaki S., Handa K., et al. A retrospective, longitudinal study to evaluate healing lower extremity wounds in patients with diabetes mellitus and ischemia using standard protocols of care and platelet-rich plasma gel in a Japanese wound care program. Ostomy Wound Manage 58, 36, 2012 [PubMed] [Google Scholar]
- 86. O'Connell S.M., Impeduglia T., Hessler K., Wang X.J., Carroll R.J., and Dardik H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen 16, 749, 2008 [DOI] [PubMed] [Google Scholar]
- 87. Driver V.R., Hanft J., Fylling C.P., Beriou J.M., and Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 52, 68, 2006 [PubMed] [Google Scholar]
- 88. Ahmed M., Reffat S.A., Hassan A., and Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg 38, 206, 2017 [DOI] [PubMed] [Google Scholar]
- 89. Dionyssiou D., Demiri E., Foroglou P., et al. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: an experimental and clinical study. Int Wound J 10, 397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Deng W., Boey J., Chen B., et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care 25, 393, 2016 [DOI] [PubMed] [Google Scholar]
- 91. Kontopodis N., Tavlas E., Papadopoulos G., et al. Effectiveness of platelet-rich plasma to enhance healing of diabetic foot ulcers in patients with concomitant peripheral arterial disease and critical limb ischemia. Int J Low Extrem Wounds 15, 45, 2016 [DOI] [PubMed] [Google Scholar]
- 92. Mehdizadeh A., Alavi A., Alhusayen R., et al. Proceeding report of the Symposium on Hidradenitis Suppurativa Advances (SHSA). Exp Dermatol 27, 104, 2018 [DOI] [PubMed] [Google Scholar]
- 93. Dini V., Oranges T., Rotella L., and Romanelli M. Hidradenitis suppurativa and wound management. Int J Low Extrem Wounds 14, 236, 2015 [DOI] [PubMed] [Google Scholar]
- 94. Nicoli F., Balzani A., Lazzeri D., et al. Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and hyalomatrix. Int Wound J 12, 338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vossen A.R., van der Zee H.H., and Prens E.P. Accelerated wound healing after wide excisions in hidradenitis suppurativa using autologous split-thickness skin grafting and platelet-rich plasma. Int Wound J 14, 583, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Singh R., Dhayal R.K., Sehgal P.K., and Rohilla R.K. To evaluate antimicrobial properties of platelet rich plasma and source of colonization in pressure ulcers in spinal injury patients. Ulcers 2015, Article ID 749585, 2015 [Google Scholar]
- 97. Cieslik-Bielecka A., Skowroński R., Jędrusik-Pawłowska M., and Pierchała M. The application of L-PRP in AIDS patients with crural chronic ulcers: a pilot study. Adv Med Sci 63, 140, 2018 [DOI] [PubMed] [Google Scholar]
- 98. Afradi H., Saghaei Y., Kachoei Z.A., Babaei V., and Teimourian S. Treatment of 100 chronic thalassemic leg wounds by plasma-rich platelets. Int J Dermatol 56, 171, 2017 [DOI] [PubMed] [Google Scholar]
- 99. Akingboye A.A., Giddins S., Gamston P., Tucker A., Navsaria H., and Kyriakides C. Application of autologous derived-platelet rich plasma gel in the treatment of chronic wound ulcer: diabetic foot ulcer. J Extra Corpor Technol 42, 20, 2010 [PMC free article] [PubMed] [Google Scholar]
- 100. Tande A.J., Osmon D.R., Greenwood-Quaintance K.E., Mabry T.M., Hanssen A.D., and Patel R. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. MBio 5, e01910, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kavanagh N., O'Brien F., and Kerrigan S. The molecular mechanics of inflammatory bone and joint disease caused by microbial infection. In: Kon K., and Rai M., eds. The Microbiology of Skin, Soft Tissue, Bone and Joint Infections. Cambridge, MA: Elsevier, 2017, pp. 125–140 [Google Scholar]
- 102. Wang H.F., Gao Y.S., Yuan T., Yu X.W., and Zhang C.Q. Chronic calcaneal osteomyelitis associated with soft-tissue defect could be successfully treated with platelet-rich plasma: a case report. Int Wound J 10, 105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang J., Zhao K., Liu H., Zhao H., Yang J., and Sun X. Infected bone inactivation combined with transplantation of autologous platelet-rich plasma and bone marrow for treatment of chronic osteomyelitis. Eur Rev Med Pharmacol Sci 19, 4488, 2015 [PubMed] [Google Scholar]
- 104. Zhang J., Middleton K.K., Fu F.H., Im H.-J., and Wang J.H. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS One 8, e67303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andia I., and Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 9, 721, 2013 [DOI] [PubMed] [Google Scholar]
- 106. Anitua E., Zalduendo M., Troya M., Padilla S., and Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One 10, e0121713, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Qi Y., Niu L., Zhao T., et al. Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration. Stem Cell Res Ther 6, 256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Koh Y.-G., Kwon O.-R., Kim Y.-S., and Choi Y.-J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30, 1453, 2014 [DOI] [PubMed] [Google Scholar]
- 109. Li G.Y., Yin J.M., Ding H., Jia W.T., and Zhang C.Q. Efficacy of leukocyte- and platelet-rich plasma gel (L-PRP gel) in treating osteomyelitis in a rabbit model. J Orthop Res 31, 949, 2013 [DOI] [PubMed] [Google Scholar]
- 110. Schuckert K.-H., Jopp S., and Teoh S.-H. Mandibular defect reconstruction using three-dimensional polycaprolactone scaffold in combination with platelet-rich plasma and recombinant human bone morphogenetic protein-2: de novo synthesis of bone in a single case. Tissue Eng Part A 15, 493, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Scafati C.T., Scafati S.T., Aveta A., Cassese M., and Vitale C. Chronic frontal sinus disease: combined use of platelet-rich plasma and calvarial bone grafts for sinus obliteration in aggressive and secondary cases. Rev Stomatol Chir Maxillofac 111, 216, 2010 [DOI] [PubMed] [Google Scholar]
- 112. Hao D., Feng G., Li T., et al.. [Curative effects of platelet-rich plasma combined with negative-pressure wound therapy on sternal osteomyelitis and sinus tract after thoracotomy]. Zhonghua Shao Shang Za Zhi 32, 331, 2016. (Article in Chinese) [DOI] [PubMed] [Google Scholar]
- 113. Franke A., Bieler D., Wilms A., Hentsch S., Johann M., and Kollig E. Treatment of gunshot fractures of the lower extremity: part 2: procedures for secondary reconstruction and treatment results. Der Unfallchirurg 117, 985, 2014 [DOI] [PubMed] [Google Scholar]
- 114. Hoaglin D.R., and Lines G.K. Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. Int J Dent 2013, 875380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Serraino G.F., Dominijanni A., Jiritano F., et al. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J 12, 260, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Patel A.N., Selzman C.H., Kumpati G.S., McKellar S.H., and Bull D.A. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg 11, 62, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Anitua E., Prado R., and Orive G. Allogeneic platelet-rich plasma: at the dawn of an off-the-shelf therapy? Trends Biotechnol 35, 91, 2017 [DOI] [PubMed] [Google Scholar]
- 118. Zhang Z.-Y., Huang A.-W., Fan J.J., et al. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: immunogenicity and defect healing efficacy. Cell Transplant 22, 175, 2013 [DOI] [PubMed] [Google Scholar]
- 119. Asadi M., Alamdari D.H., Rahimi H.R., et al. Treatment of life-threatening wounds with a combination of allogenic platelet-rich plasma, fibrin glue and collagen matrix, and a literature review. Exp Therapeut Med 8, 423, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]