Abstract
General safety and toxicology assessments supporting in vivo lentiviral vector-based therapeutic development are sparse. We have previously demonstrated the efficacy of a lentiviral vector expressing fumarylacetoacetate hydrolase (LV-FAH) to cure animal models of hereditary tyrosinemia type 1. Therefore, we performed a complete preclinical toxicological evaluation of LV-FAH, in a large cohort (n = 20/group) of wildtype mice and included matched groups of N-nitrosodiethylamine/carbon tetrachloride (DEN/CCl4)–induced liver injury mice to assess specific toxicity in fibrotic liver tissue. Mice receiving LV-FAH alone (109 TU/mouse) or in combination with DEN/CCl4 presented clinically similar to control animals, with only slight reductions in total body weight gains over the study period (3.2- to 3.7-fold vs. 4.2-fold). There were no indications of toxicity attributed to administration of LV-FAH alone over the duration of this study. The known hepatotoxic combination of DEN/CCl4 induced fibrotic liver injury, and co-administration with LV-FAH was associated with exaggeration of some findings such as an increased liver:body weight ratio and progression to focal hepatocyte necrosis in some animals. Hepatocellular degeneration/regeneration was present in DEN/CCl4-dosed animals regardless of LV-FAH as evaluated by Ki-67 immunohistochemistry and circulating alpha fetoprotein levels, but there were no tumors identified in any tissue in any dose group. These data demonstrate the inherent safety of LV-FAH and support broader clinical development of lentiviral vectors for in vivo administration.
Keywords: lentiviral vectors, gene therapy, toxicology, hereditary tyrosinemia type 1, tumorigenicity
Introduction
Hereditary tyrosinemia type 1 (HT1) is an inborn error of liver metabolism resulting from mutation of the gene (Fah) for fumaryl acetoacetate hydrolase, the enzyme that catalyzes the last step of tyrosine metabolism. HT1 is fatal if untreated, and the only current cure is liver transplantation. Patients are clinically managed with daily self-administration of the protective drug 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) and dietary modulation of protein intake.1 However, patients can still proceed to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) while taking NTBC,2 and as many as 10% of patients are unresponsive to NTBC,3 subsequently requiring liver transplantation to cure their disease. Of those patients for whom NTBC is an effective metabolic treatment, cognitive impairment is a major concern. Indeed, duration of continued NTBC administration in humans has been negatively correlated with intelligence quotient.4 Variations in dosing regimen continue to be evaluated to improve metabolite profiles,5 but cognitive impairment is likely due to chronically elevated tyrosine levels in the blood6 that would be largely unaffected by minor dose administration variations. Furthermore, mouse models of HT1 have been shown to develop HCC as early as 18 months despite NTBC administration and protein-restricted diet,7 indicating that NTBC administration is not completely preventative of disease progression. HT1 patients currently await a true cure that does not incur neurotoxicity or have the lifelong implications of daily maintenance therapy, dietary restrictions, or liver transplantation.
Gene therapy provides an ideal cure for monogenic loss-of-function diseases, such as HT1. Furthermore, lentivirus presents an interesting vector to provide long-term correction and durable cures based on genomic integration of the delivered transgene.8 Therefore, we have generated a lentiviral vector expressing fumarylacetoacetate hydrolase, the gene defective in hereditary tyrosinemia type 1 for evaluation as a clinical gene therapy candidate. We have previously demonstrated the efficacy of lentiviral vector (LV)-FAH to stably integrate into mouse and pig hepatocytes, effectively curing small and large animal models of HT1. In both mice and clinically relevant porcine models of HT19 ex vivo gene therapy using this vector on autologous hepatocytes completely cures the test subjects,10 showing durable efficacy.11 However, the genomic integration that supports such duration of effect also causes concern for insertional mutagenesis relative to nonintegrating vectors, such as adenoviral and adeno-associated viral (AAV) vectors. This concern has largely limited previous lentiviral vector development to ex vivo approaches to minimize systemic viral exposure and mitigate genotoxicity of off-target tissues.
However, ex vivo gene therapy is a complicated, invasive, and costly proposition, and an in vivo lentiviral vector approach would offer many advantages, such as ease of access, minimal invasiveness, and improved consistency between patients due to minimization of variability in patient cell isolation, transduction, and autotransplantation. To assess toxicity and specifically address hepatotoxicity, we conducted a complete preclinical toxicology study whereby a therapeutic dose of LV-FAH was administered to wild type mice and mice subjected to a chemical liver injury via DEN induction and chronic CCl4 exposure demonstrated to cause significant liver injury.12 While the wild type mice would generate data to demonstrate any inherent toxicity of the genomic integration of a replication incompetent lentiviral vector in vivo, the chemical injury model would demonstrate any contextual toxicity of lentiviral vector exposure/integration in ongoing profibrotic hepatocyte injury. This latter condition is of interest because there would be a modified gene expression profile in stressed cells, and emphasis would be placed on the consequences of additional integration sites of lentiviral vectors in the genome. Although the relevance of this model to any specific disease state or metabolic insufficiency is important to consider during evaluation, these data provide a unique opportunity to generally evaluate toxicity of in vivo lentiviral vector administration to a healthy animal model. Furthermore, they demonstrate possible additional consequences of lentiviral integration in the context of cell injury, having implications on patient enrollment criteria for potential future clinical evaluations of in vivo administration of lentiviral vectors in multiple liver indications.
Materials and Methods
Animals and animal care
All animals received humane care in compliance with the regulations of the institutional animal care and use committee at Mayo Clinic, Rochester, MN. Mice for the study were wild type C57/bl6 background bred in house from the laboratory's research colony, with the exception of 3 HT1 mice used to demonstrate efficacy. Daily observations were performed by animal care/laboratory staff, and any clinical concerns were addressed by on-site veterinarians. Body weights were evaluated weekly as part of the toxicological assessment and used as an additional measure of animal health. Animals were dosed intravenously via lateral tail vein (109 TU of LV-FAH or vehicle) or by intraperitoneal injection (1 mg/kg DEN, saline, 0.2 mL/kg CCl4, or olive oil). DEN was obtained from Sigma Aldrich (St Louis, MO). CCl4 was obtained from Acros Organics (Fair Lawn, NJ). Olive oil was obtained from MP Biomedicals (Solon, OH). LV-FAH was formulated in Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Waltham, MA). Animals were sacrificed by CO2 asphyxiation followed by cervical dislocation consistent with institutional policies for rodent euthanasia. Blood samples were collected postmortem via the inferior vena cava at necropsy for evaluation of clinical pathology parameters. Tissues were collected immediately after termination, dabbed to remove excess blood, and weighed for calculation of ratios to terminal body weights.
Lentiviral vector construct
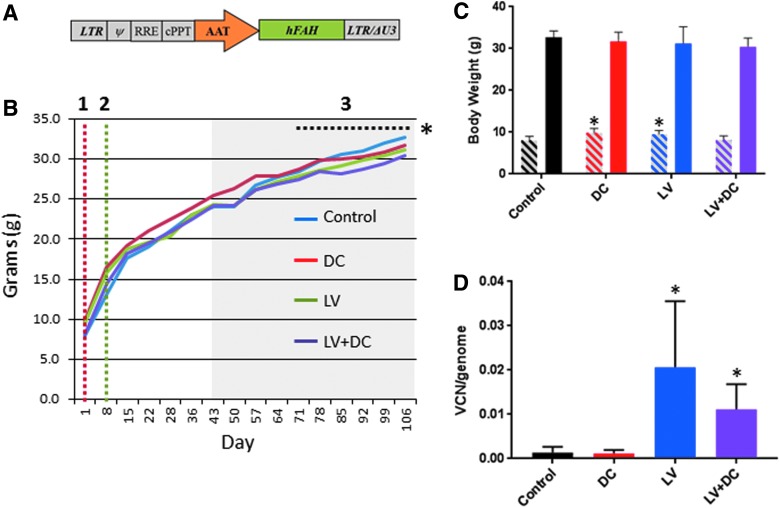
In order to generate the viral vectors, a plasmid containing Fah under the control of the alpha-1 antitrypsin promoter, or green fluorescent protein under control of the cytomegalovirus promoter, was co-transfected with the packaging plasmid p8.91 and the vesicular stomatitis virus glycoprotein G-encoding plasmid pVSV-G into 293T cells using 1 mg/mL polyethylenimine (Polysciences, Warrington, PA). Viral supernatant was harvested 48 h and 72 h after transfection, filtered through a 0.45 μm filter, and concentrated by ultracentrifugation (25,000 rpm, 1.5 h at 4°C). After resuspension in serum-free media (Dulbecco's modified Eagle medium, Thermo Fisher Scientific), lentiviral vectors were aliquoted and stored at −80°C. Total vector particles were determined by p24 enzyme-linked immunosorbent assay and titer was subsequently determined by qPCR using the Lenti-X Provirus Quantitation Kit (Clontech, Mountain View, CA) to detect transduction (as actual integrations into recipient cell genomes). A schematic representation of the lentiviral vector carrying human FAH is provided in Fig. 1A.
Figure 1.
Transduction of lentiviral vector fumarylacetoacetate hydrolase (LV-FAH) into wild type and chemical liver injury mice. (A) Diagram of LV-FAH containing a human FAH transgene regulated by the alpha-1 antitrypsin promoter. The construct also includes LTRs with the 3′ ΔU3 mutation, Ψ element, RRE, and cPPT. (B) Diagram of study design with body weights by group showing the timing of the initiator N-nitrosodiethylamine (DEN) dose on day 1 (red dotted line [1]), administration of the LV-FAH on day 8/9 (green dotted line [2]) and carbon tetrachloride (CCl4) repeat dosing phase of the study from days 43–106 (shaded gray region). Although body weights were similar throughout the study, the lentivirus DEN/CCl4 (LV+DC) group (group 4) was statistically lower than control (group 1) from day 71 through the end of the study (*p < 0.05). (C) Bar graph showing the body weights on day 1 (left bar of each set, angled lines) compared with the end of the study, day 106 (right bar of each set, solid) for each group. Notably, DC and LV groups were statistically heavier than control and LV+DC at the initiation of the study despite age matching (*p < 0.001 compared to control and LV+DC groups). (D) Analysis of genomic DNA demonstrated that in the LV group and LV+DC group 1–2% of cells were integrated with LV-FAH by the end of the study, while control and DC groups had negligible lentiviral vector genomes detected, similar to negative control sample (*p < 0.01 compared with control and DC groups). AAT, alpha-1 antitrypsin promoter; cPPT, central polypurine track; DEN, N-nitrosodiethylamine/chronic carbon tetrachloride; hFAH, human FAH transgene; LTR, long terminal repeat; RRE, rev response element.
Vector copies per genome
Three mice each were randomly selected from groups that did not receive LV-FAH (groups 1 and 2) to establish lack of exposure and reasonable data cut points for lentiviral vector integration positivity, while 7 (of 20) animals were randomly selected from each of the groups that did receive LV-FAH (n = 14 total from groups 3 and 4). Liver tissue was recovered from paraffin blocks using Gentra Puregene Tissue Kit (QIAgen, Hilden, Germany) per the manufacturer's instructions. Genomic DNA was diluted in Tris-EDTA buffer to 300 ng/μL in 50 μL final volumes for quantitative PCR. A standard curve and duplicates of each liver sample were amplified using SYBR green qPCR with included forward and reverse primers (proprietary to Lenti-X Provirus Quantitation Kit, Takara Bio, Mountain View, CA, formerly Clontech). Reactions were performed in the ViiA 7 System (Applied Biosystems, Carlsbad, CA).
Biochemical analysis
For clinical chemistry analysis, serum was analyzed with the Piccolo Xpress chemistry analyzer (Abaxis, Union City, CA) according to the manufacturer's instructions. For hematology analysis, whole blood was analyzed with the VetScan HM5 analyzer (Abaxis) according to the manufacturer's instructions. Alpha-fetoprotein (AFP) was analyzed in serum with the Beckman Coulter Access AFP immunoenzymatic assay on the Beckman Coulter UniCel DXI 800 (Beckman Coulter Inc., Fullerton, CA). Tyrosine values were determined using tandem mass spectrometry and chromatography via Mayo Clinic's internal biochemical phenylketonuria test.
Histology analysis
For histological analysis, tissue samples were fixed in 10% neutral buffered formalin (Protocol, Fisher Scientific, Pittsburgh, PA) and processed for paraffin embedding and sectioning. For hematoxylin and eosin staining, slides were prepared with standard protocols and evaluated by a board-certified veterinary pathologist for variations. Ki-67 immunohistochemistry was performed using a monoclonal anti-Ki67 primary antibody (MIB-1; Dako/Agilent, Santa Clara, CA) as performed with a Bond III automatic stainer (Leica, Buffalo Grove, IL) with a 20-min antigen retrieval step using Bond Epitope Retrieval Solution 2 (Leica), and stained with diaminobenzidine (Leica). FAH immunochemistry was performed as previously described.10 Slides used to evaluate fibrosis were stained with Masson's trichrome stain using standard protocols. Ki-67 quantification was performed by selecting up to three random cross sections per slide manually verified to avoid staining artifacts. Areas were analyzed and quantified using an Aperio ImageScope algorithm that quantifies nuclear staining. Results are reported as percentage of nuclear positivity among cells analyzed.
Statistical analysis
Numerical data are expressed as mean (± standard deviation). Calculations and statistical analysis were performed using Microsoft Excel 2010, and additional statistical analyses were performed with GraphPad Prism software version 7.03 (San Diego, CA). All numerical data were analyzed by 2-tailed Student's t-test, and differences were considered significant at p < 0.05.
Results
A total of 86 mice were randomized into one of four groups (Table 1) to receive either vehicle (groups 1 and 2) or LV-FAH (groups 3 and 4), with (groups 2 and 4) or without (groups 1 and 3) induction of chemical liver injury. A maximum feasible dose of lentivirus was evaluated to maximize potential to characterize toxicity, which was determined to be 1 × 109 TU based on volume (6.3–6.9 × 1010 TU/kg), which is sufficient for transduction of hepatocytes and sufficient to cure the HT1 mouse model with eventual complete repopulation (Supplementary Fig. S1). Mice were vehicle treated or induced with DEN on day 1, administered LV-FAH (Fig. 1A) on day 8/9, and received twice weekly intraperitoneal injections of CCl4 or olive oil beginning on day 43 (Fig. 1B), and body weights were evaluated weekly (starting and ending group mean body weight presented in Fig. 1C). Mice were observed for 106 days based on previously published accounts of the chemical injury model,12 at which time all mice were necropsied for post mortem evaluations of toxicity. There were 9 early procedural deaths on the study, primarily resulting from frequent injections of CCl4 or olive oil vehicle, with no increased incidence from LV-FAH or CCl4 administration (5, 2, 1, and 1 early deaths in groups 1–4, respectively). There was no measurable exposure to LV-FAH for mice in groups 1 and 2, while animals in groups 3 and 4 were positive for 0.021 and 0.011 lentiviral vector copies per mouse genome, respectively, consistent with 1–2% transduced hepatocytes by the end of the observation period (Fig. 1D).
Table 1.
Experimental design
| Group | n (males) | DEN in PBS (mg/kg) | Volume (mL/kg) | CCl4 in Olive Oil (mL/kg) | Volume (mL/kg) | LV-FAH (TU)a | TU/kgb | n Necropsy day 106 |
|---|---|---|---|---|---|---|---|---|
| 1 | 21 | 0 | 15 | 0 | 15 | – | 0 | 16 |
| 2 | 22 | 1.0 | 15 | 0.2 | 15 | – | 0 | 20 |
| 3 | 21 | 0 | 15 | 0 | 15 | 109 | 6.3 × 1010 | 20 |
| 4 | 22 | 1.0 | 15 | 0.2 | 15 | 109 | 6.9 × 1010 | 21 |
“–” Indicates treatment not administered to this group.
Based on mean group starting body weights of 15.8 g (group 3) and 14.4 g (group 4).
FAH, human fumarylacetoacetate hydrolase; LV, lentiviral vector; TU, transducing units.
There were no effects of DEN/CCl4, LV-FAH, or their combination on observational data (not shown). Changes in clinical observations were limited to background findings with similar frequency and severity between groups, including control animals. Although animals in all groups gained weight over the course of the study, there was a slight attenuation of total body weight gains in all treated groups relative to the control cohort, whereby weight in control animals increased 4.2-fold over their starting weight of 8.0 ± 1.0 g compared with 3.2-, 3.3-, and 3.7-fold over starting weights of 9.8 ± 1.1 g, 9.5 ± 0.9 g, and 8.2 ± 0.9 g for DEN/CCl4 (DC), LV, and LV+DC groups, respectively (Fig. 1C). This resulted in a slight net decrease in final body weights for the treated groups compared to control animals that was statistically significant in the LV+DC combination-treated group only (group 4). Although the final difference for all groups was minimal, the DC group and the LV group (groups 2 and 3) had statistically higher body weights than the control animals at day 1.
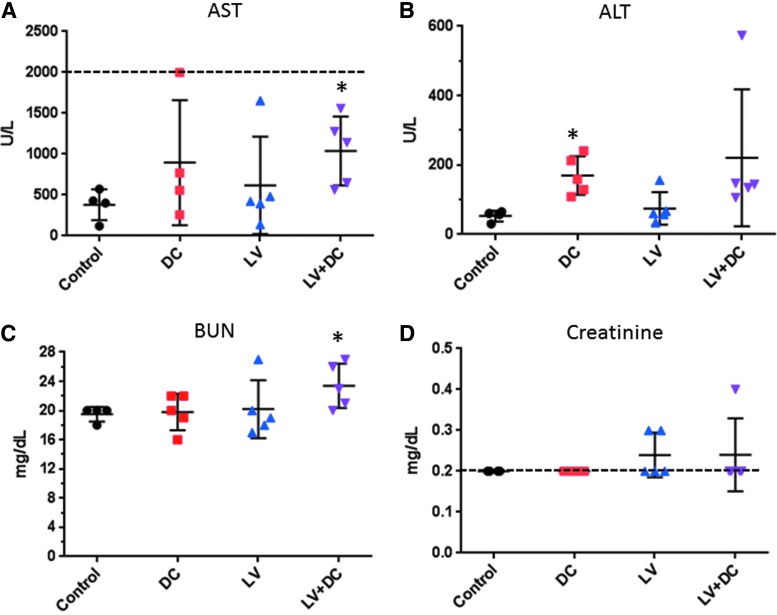
LV-FAH administration alone was not associated with changes in clinical pathology parameters by the end of the study (group 3; Tables 2 and 3). The DEN/ CCl4 chemical induction of liver injury (group 2) was associated with a 2.4 × increase in AST and a 3.2 × increase in ALT compared with control values (Fig. 2A and 2B, respectively), and co-administration with LV-FAH trended toward further increases (2.7 × and 4.2 × , respectively). There was a slight elevation in blood urea nitrogen (BUN) in the combined treatment group only (Fig. 2C) which was present in most animals, and slight elevations in creatinine above the lower limit of detection in a few animals in both LV-FAH-treated groups (Fig. 2D); however, the kidneys were morphologically normal based on histopathology, therefore the slight variations in BUN and creatinine levels were considered not to be toxicologically meaningful. Complete group mean serum chemistry data from Day 106 are presented in Table 2. There were no noteworthy changes in any dose group in hematology parameters tested on Day 106 (Table 3), although LV-treated groups trended toward slightly lower red blood cell parameters, hematocrit was similar in all groups (HCT 31-37%) and within expected variation.
Table 2.
Serum chemistry parameters
| Group | Na | K | CO2 | Cl | Glu | Ca | BUN | Cre | ALP | ALT | AST | Tbil | Alb | TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mean | 148.5 | 7.1 | 20.3 | 109.5 | 201.3 | 9.8 | 19.5 | 0.20 | 47.5 | 53.0 | 380.0 | 0.2 | 1.7 | 5.1 |
| SD | 1.7 | 0.5 | 2.8 | 1.7 | 11.3 | 0.3 | 1.0 | 0.00 | 4.5 | 15.7 | 189.2 | 0.1 | 0.1 | 0.1 | |
| 2 | Mean | 151.0 | 8.1 | 20.2 | 107.6 | 179.6 | 9.8 | 19.8 | 0.20 | 49.2 | 169.8 | 896.3 | 0.2 | 1.9 | 5.4 |
| SD | 1.9 | 0.5 | 2.3 | 2.3 | 32.8 | 0.8 | 2.5 | 0.00 | 4.5 | 55.6 | 765.1 | 0.0 | 0.1 | 0.3 | |
| 3 | Mean | 150.4 | 7.6 | 18.0 | 110.4 | 192.8 | 9.7 | 20.2 | 0.24 | 43.8 | 74.8 | 618.4 | 0.2 | 1.7 | 5.1 |
| SD | 2.6 | 0.3 | 3.4 | 2.8 | 12.6 | 0.4 | 4.0 | 0.05 | 8.6 | 47.0 | 594.3 | 0.0 | 0.1 | 0.2 | |
| 4 | Mean | 150.6 | 8.1 | 21.0 | 106.8 | 142.8 | 9.7 | 23.4 | 0.24 | 48.2 | 221.0 | 1037.6 | 0.4 | 1.9 | 5.2 |
| SD | 1.5 | 0.4 | 2.3 | 2.3 | 19.5 | 0.4 | 3.0 | 0.09 | 12.2 | 197.5 | 422.7 | 0.4 | 0.1 | 0.2 | |
Alb, albumin (g/dL); ALP, alkaline phosphatase (U/L); ALT, alanine aminotransferase (U/L); AST, aspartate aminotransferase (U/L); BUN, blood urea nitrogen (mg/dL); Ca, Calcium (mg/dL); Cl, chloride (mmol/L); CO2, carbon dioxide (mmol/L); Cre, creatinine (mg/dL); Glu, glucose (mg/dL); K, potassium (mmol/L); Na, sodium (mmol/L); SD, standard deviation; Tbil, total bilirubin (mg/dL); TP, total protein (g/dL).
Table 3.
Hematology parameters
| Group | WBC | Lym | Mon | Neu | RBC | Hgb | Hct | MCV | MCH | MCHC | RDW | Plt | MPV | PDWc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mean | 3.43 | 2.77 | 0.29 | 0.38 | 7.72 | 13.3 | 35.2 | 46 | 17.7 | 38.9 | 16.3 | 16.4 | 7.1 | 33.2 |
| SD | 2.65 | 2.01 | 0.33 | 0.33 | 1.66 | 1.0 | 7.5 | 0 | 2.4 | 5.3 | 0.4 | 10.4 | 0.9 | 1.5 | |
| 2 | Mean | 3.68 | 2.94 | 0.31 | 0.43 | 8.22 | 12.1 | 37.0 | 45 | 14.8 | 32.9 | 17.4 | 6.0 | 6.9 | 32.0 |
| SD | 1.37 | 1.21 | 0.13 | 0.13 | 0.64 | 0.8 | 3.1 | 0 | 0.8 | 1.8 | 0.3 | 12.3 | 1.0 | 2.1 | |
| 3 | Mean | 2.72 | 1.90 | 0.06 | 0.76 | 6.71 | 9.5 | 30.4 | 45 | 14.3 | 31.6 | 16.7 | 18.8 | 6.8 | 32.7 |
| SD | 3.10 | 1.93 | 0.04 | 1.18 | 1.75 | 2.8 | 7.7 | 1 | 3.2 | 7.1 | 0.7 | 15.7 | 0.6 | 1.9 | |
| 4 | Mean | 5.22 | 3.51 | 0.13 | 1.57 | 6.83 | 9.2 | 31.1 | 46 | 13.4 | 29.4 | 17.6 | 15.8 | 6.5 | 32.2 |
| SD | 5.3 | 3.0 | 0.1 | 2.3 | 1.8 | 4.2 | 8.1 | 0.9 | 4.8 | 10.7 | 0.9 | 18.4 | 0.5 | 2.2 | |
Hct, hematocrit (%); Hgb, hemoglobin (g/dL); Lym, lymphocytes (109/L); MCH, mean corpuscular hemoglobin (pg); MCHC, mean corpuscular hemoglobin concentration (g/dL); MCV, mean corpsuscular volume (fL); Mon, monocytes (109/L); MPV, mean platelet volume (fL); Neu, neutrophils (109/L); PDWc, platelet distribution width (%); Plt, platelets (109/L); RBC, red blood cells (1012/L); RDW, red cell distribution width (%); WBC, white blood cells (109/L).
Figure 2.
Serum chemistry parameters of liver and kidney effects of chemical liver injury and LV-FAH. Results of serum chemistry analysis at the end of the study (day 106) from mice by groups for (A) aspartate aminotransferase, (B) alanine aminotransferase, (C) blood urea nitrogen, and (D) creatinine. Results indicate increased liver enzymes in the DC-treated groups, regardless of LV and slight elevations in kidney parameters in some animals in LV-treated groups. Where applicable, upper and lower assay limits are indicated for aspartate aminotransferase and creatinine by dashed lines, respectively. *p < 0.05 compared to control. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
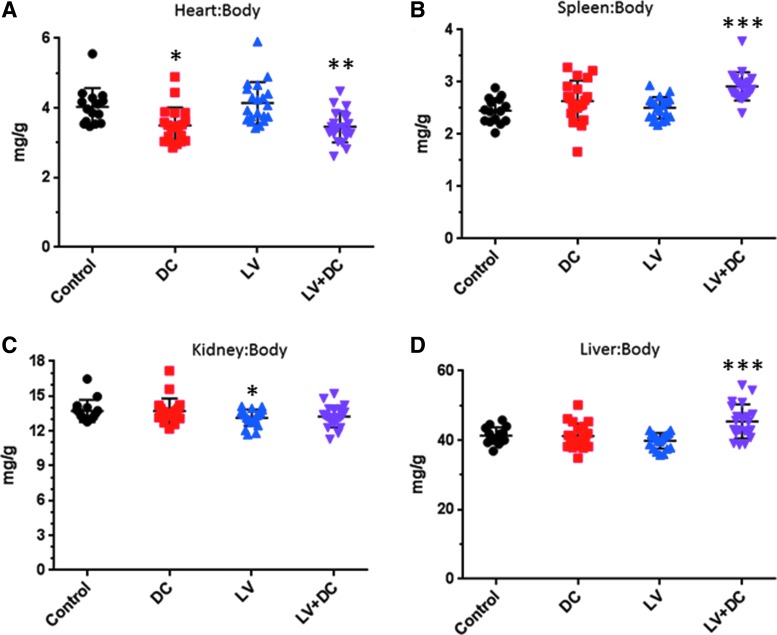
Administration of DEN/ CCl4 was associated with slight decreases in heart weight:body weight ratios (Fig. 3A) and increases in spleen weight:body weight ratios (Fig. 3B), regardless of LV-FAH co-administration, although the LV-FAH group trended toward greater increases in the spleen than DEN/ CCl4 alone. Administration of LV-FAH alone was associated with a slight decrease in kidney weight ratios relative to the control group (0.93 × ; p < 0.05) which was consistent with the decrease present in the combined treatment group (0.91 × ; nonsignificant), indicating that this slight variation may be associated with LV-FAH administration independent of DEN/ CCl4 (Fig. 3C). These decreases were not considered adverse based on the marginal effect in the associated clinical pathology parameters (BUN and Cre) and the absence of gross or microscopic lesions in the kidneys. Interestingly, DEN/ CCl4–induced liver injury was not associated with organ weight changes except in combination with LV-FAH, where liver:body weight ratio was 45 mg/g compared to 41 mg/g in control animals (Fig. 3D; p < 0.05), contributed to by both a marginal increase in raw liver weights and a significant decrease in body weights. The reduced bodyweight in this group did not appreciably affect ratios of other organs.
Figure 3.
Organ to body weight ratios for chemical liver injury and LV-FAH treated mice. Organ weights were collected at necropsy and normalized to terminal body weights for (A) heart, (B) spleen, (C) kidney, and (D) liver. Heart to body weight ratio was slightly decreased in liver injury mice regardless of LV-FAH administration. Spleen and liver weight ratios were greatest in the combination (LV+DC) group. *p < 0.05 compared with control; **p < 0.05 compared with control and LV; ***p < 0.05 compared with control, DC, and LV.
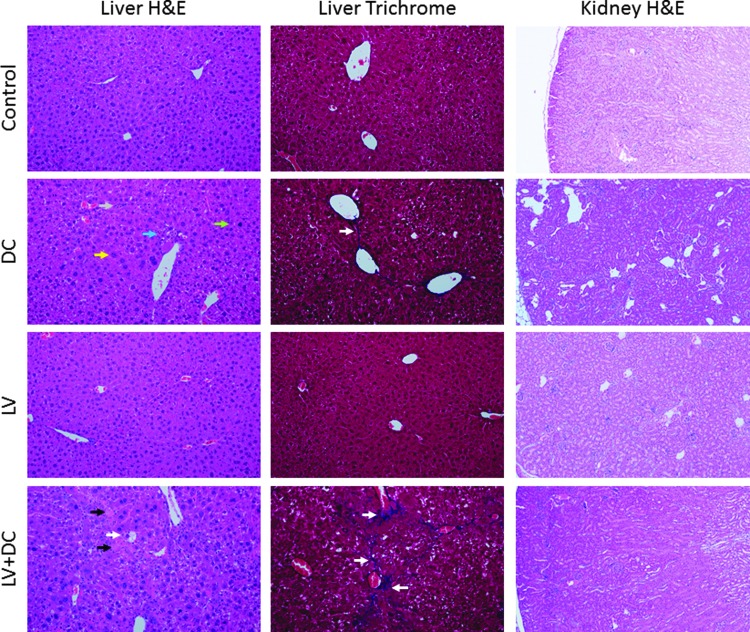
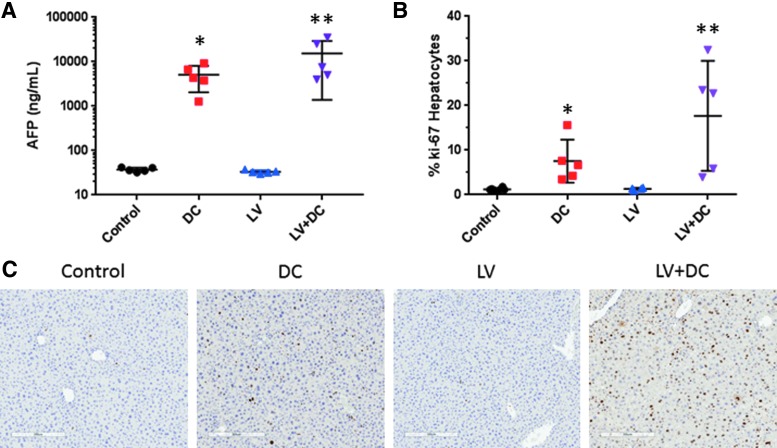
Administration of DEN/CCl4 induced diffuse hepatocellular hypertrophy and hyperplasia in the livers of the mice, with karyocytomegaly, biliary stasis and bridging fibrosis, and fibroplasia (Fig. 4, left and central panels; see arrows). When combined with LV-FAH, the DEN/CCl4-induced changes advanced to include hepatocyte degeneration and necrosis. Neither the chemical liver injury nor LV-FAH was associated with histological changes in kidney (Fig. 4, right panels). Considering the liver is the target organ for the pseudotype of the LV-FAH (VSV), and the significant effects of DEN/CCl4 present on liver weight and pathology, we evaluated circulating levels of AFP as a biomarker for tumorigenic potential and Ki-67 staining as an indicator of hepatocyte proliferation. Indeed, DEN/CCl4 was associated with over 100-fold increase in AFP compared with control or LV-FAH groups (Fig. 5A; p < 0.05 vs. control). Although the difference between the combination treatment and DC alone was an additional three-fold increase, this trend was the result of exaggerated responses in only 2 of the 5 animals tested and was not significant between the DEN/ CCl4 groups. LV-FAH alone had no effect on AFP levels, which would not portend tumor formation as a result of transduction and integration at this level in hepatocytes. LV-FAH alone did not have an effect on baseline Ki-67 positivity compared to control animals (Fig. 5B and C). However, DEN/ CCl4 treatment was associated with increased Ki-67 positivity of hepatocytes, with a trend for greater positivity in the context of LV-FAH co-administration.
Figure 4.
Liver and kidney histopathology for chemical liver injury and LV-FAH treated mice. Routine hematoxylin and eosin (H&E) and Masson's trichrome staining were performed on sections of liver and kidney from mice in all groups. Lentivirus alone was not associated with histological changes in liver or kidney. DEN/CCl4 was associated with histological changes in liver including hepatocellular cytomegaly (H&E panels, yellow arrow), increased mitoses (H&E panels, green arrow), inflammatory cell infiltrates (H&E panels, gray arrow), pigment-laden macrophages (H&E panels, blue arrow), and fibrosis (trichrome panels, white arrows), with exacerbation to bridging fibrosis, cell necrosis (H&E panels, black arrow), and vacuolar degeneration (H&E panels, white arrow) only when in combination with lentivirus. No histological changes were present in kidneys in any dose groups (right-hand panels).
Figure 5.
Markers of tumorigenicity and regeneration from chemical liver injury and LV-FAH treated mice. (A) Alpha fetoprotein was increased in chemical liver injury, regardless of administration of LV (note logarithmic y-axis). (B) Percent of Ki-67 positive cells in random sections of liver from mice for each group. (C) Representative panels of Ki-67 immunohistochemistry from mice from each group. Ki-67 staining was markedly increased in response to chemical liver injury, regardless of LV co-administration. *p < 0.05 compared with control; **p < 0.05 compared with control and LV.
Discussion
Gene delivery via lentiviral vectors is ideal for gene therapy of many diseases to provide long-term correction and durable cures based on genomic integration of the delivered transgene. However, genomic integration also causes increased concern for insertional mutagenesis relative to non-integrating vectors, such as adenoviral and AAV vectors, which has largely limited lentiviral vector development to ex vivo approaches that minimize systemic exposure. We have generated a lentiviral vector expressing fumarylacetoacetate hydrolase, the gene defective in hereditary tyrosinemia type 1, intended for in vivo application to cure the human disease. As part of development toward human clinical application, a thorough preclinical assessment must be made to evaluate safety and toxicity of systemic lentiviral vector administration.
Preclinical assessments of AAVs have been reported, and a review of this subject with emphasis on genotoxicity was prepared by Chandler et. al. in 2017.13 Administration of different oncolytic adenovirus has been associated with only transient and minor changes systemically or locally in brain14 or transient lymphopenia and transaminitis following intravenous administration without 15local (intraprostatic) administration.15 In a separate published preclinical assessment, another AAV caused no adverse effects in mouse or beagles through multiple toxicity endpoints and was not associated with immune responses in guinea pigs.16 More specific to liver, AAVs expressing LacZ or VEGF were only associated with mild to moderate regenerative changes in hepatocytes at day 14 when administered with an associated chemotherapy regimen.17 Adenovirus and AAV are generally considered safe due to the persistence as an episome, and the previous demonstration that adenoviral integration into a specific genomic locus was associated with HCC could be mitigated by dose, design, and timing of administration.18
Conversely, there are not many preclinical accounts of toxicity from in vivo lentiviral vector administration, especially in wild type animals to describe baseline toxicity of these vectors, primarily from integration and not transgene expression. An interesting finding from our study is that the total integration from a single lentiviral dose without subsequent positive selection for corrected cells was consistent with transduction of 1–2% of the cells by the end of the study. Although this initial population of corrected cells would eventually completely replace all diseased hepatocytes in HT1, findings from the current unexpanded population are applicable to the majority of metabolic diseases where there is no such selection/expansion. This low level of integration was of no toxicological consequence in the wild type mice; however, some aspects of the chemical injury model appeared exacerbated by previous exposure to lentiviral vector. Consistent with the findings of Tuppurainen et al.,17 we do not believe this was related to the specific presence or expression of the Fah transgene, but likely inherent to integration of the lentiviral vector, the latter being the focus of this safety study in wild type mice where actual transgene expression was not assayable due to endogenous FAH positivity. Lentiviral vector administration has previously been described not to be genotoxic in mice based on integration site analysis,19 but it is possible that the context of degeneration/regeneration in the fibrotic liver enhanced effects of integrations that would be inconsequential in healthier tissue. The slight trend for less lentiviral vector detection in the DEN/ CCl4 group by the end of the study might be a product of random variation or sampling accuracy, but it also could be a product of mild selection against the transduced hepatocytes in the regenerative context. Initial transduction rates were not evaluated in this study, but it is possible they were similar, and the chemical injury model resulted in a higher proportion of transduced cells dying off, perhaps due to integration in genes that were important for regenerative capacity.
Lentiviral vectors have shown favorable safety profiles in preclinical and limited clinical studies. Therefore, preclinical studies designed with clinical consideration in vector design, production, and administration are translatable to indicate that minimal genotoxicity would be expected in human patients.20 Indeed, ex vivo lentiviral vectors administered to hematopoietic stem cells did not affect tumorigenesis, even in a tumor-prone mouse model.21 In one instance where increased tumorigencity was observed in an ex vivo gene therapy of a lentivector expressing alpha-iduronidase, there was actually no increase over procedural control groups. Therefore, it was attributed to an irradiation protocol employed to prepare the mice, rather than to the vector itself.22
Lentiviral vector integration is influenced by activity of the targeted cell and cell division but seems to be random within transcriptional units of active genes in both dividing and non-dividing cells.23 Genotoxicity of the current vector construct would therefore be most likely due to inactivation of tumor suppressor genes,24 which would require a highly improbable bi-allelic disruption for tangible risk. There was no evidence of tumorigenicity in any group in this study, which is consistent with our previous ex vivo work with this vector in mouse and pig models of HT1,10,11 and the benign integration profile described by others.25 In one long-term pig from our ex vivo study, no tumorigenicity was present in the liver or elsewhere after 3 years post-transplant.26 Finally, the use of a liver-specific promoter further focusses any potential for genotoxicity from transactivation to hepatocytes, where screening for tumorigenesis is already part of the clinical management of human disease via frequent imaging.
In combination with other studies performed, the current preclinical data set addresses the requirements of the European Medicines Agency guideline for Nonclinical gene therapy studies in 2008,27 and the construct has been optimized to mitigate risk of insertional mutagenesis as described in the European Medicines Agency reflection paper in 201328 such as vector design, insertion profile analysis, dose, transgene product, and target cells. With demonstration of safety in wild type animals, and previous demonstration of safety and efficacy in disease models, in vivo administration of lentiviral vectors such as LV-FAH is ready for clinical consideration.
Supplementary Material
Acknowledgments
This work was supported by the Foundation of Children's Hospital of Minnesota and additional research support was provided by Regenerative Medicine of Minnesota. R.D.H. was funded through a U.S. National Institutes of Health K01 (DK106056) award and a Mayo Clinic Center for Regenerative Medicine Career Development Award.
Author Disclosure
No competing financial interests exist.
Supplementary Material
References
- 1. Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: Long-term outcome in French patients. J Inherit Metab Dis 2008;31:81–87 [DOI] [PubMed] [Google Scholar]
- 2. van Spronsen FJ, Bijleveld CM, van Maldegem BT, Wijburg FA. Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2-(2 nitro-4-3 trifluoro- methylbenzoyl)-1, 3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 2005;40:90–93 [DOI] [PubMed] [Google Scholar]
- 3. Holme E, Lindstedt S. Nontransplant treatment of tyrosinemia. Clin Liver Dis 2000;4:805–814 [DOI] [PubMed] [Google Scholar]
- 4. van Ginkel WG, Jahja R, Huijbregts SC, et al. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J Rare Dis 2016;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kienstra NS, van Reemst HE, van Ginkel WG, et al. Daily variation of NTBC and its relation to succinylacetone in tyrosinemia type 1 patients comparing a single dose to two doses a day. J Inherit Metab Dis 2018;41:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thimm E, Richter-Werkle R, Kamp G, et al. Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J Inherit Metab Dis 2012;35:263–268 [DOI] [PubMed] [Google Scholar]
- 7. Al-Dhalimy M, Overturf K, Finegold M, Grompe M. Long-term therapy with NTBC and tyrosine-restricted diet in a murine model of hereditary tyrosinemia type I. Mol Genet Metab 2002;75:38–45 [DOI] [PubMed] [Google Scholar]
- 8. Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science 2018;359:6372. [DOI] [PubMed] [Google Scholar]
- 9. Hickey RD, Mao SA, Glorioso J, et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res 2014;13:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hickey RD, Mao SA, Glorioso J, et al. Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci Transl Med 2016;8:349ra399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elgilani F, Mao SA, Glorioso JM, et al. Chronic phenotype characterization of a large-animal model of hereditary tyrosinemia type 1. Am J Pathol 2017;187:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uehara T, Pogribny IP, Rusyn I. The DEN and CCl4 -induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Curr Protoc Pharmacol 2014;66:14.30.1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chandler RJ, Sands MS, Venditti CP. Recombinant adeno-associated viral integration and genotoxicity: Insights from animal models. Hum Gene Ther 2017;28:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JW, Auffinger B, Spencer DA, et al. Single dose GLP toxicity and biodistribution study of a conditionally replicative adenovirus vector, CRAd-S-pk7, administered by intracerebral injection to Syrian hamsters. J Transl Med 2016;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freytag SO, Zhang Y, Siddiqui F. Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for prostate cancer. Mol Ther Oncolytics 2015;2 [Epub ahead of print]; DOI: 10.1038/mto.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi Y, Guo H, Hu N, et al. Preclinical pharmacology and toxicology study of Ad-hTERT-E1a-Apoptin, a novel dual cancer-specific oncolytic adenovirus. Toxicol Appl Pharmacol 2014;280:362–369 [DOI] [PubMed] [Google Scholar]
- 17. Tuppurainen L, Sallinen H, Kokki E, et al. Preclinical safety, toxicology, and biodistribution study of adenoviral gene therapy with sVEGFR-2 and sVEGFR-3 combined with chemotherapy for ovarian cancer. Hum Gene Ther Clin Dev 2013;24:29–37 [DOI] [PubMed] [Google Scholar]
- 18. Chandler RJ, LaFave MC, Varshney GK, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 2015;125:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantore A, Ranzani M, Bartholomae CC, et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci Transl Med 2015;7:277ra228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dropulic B. Lentiviral vectors: Their molecular design, safety, and use in laboratory and preclinical research. Hum Gene Ther 2011;22:649–657 [DOI] [PubMed] [Google Scholar]
- 21. Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006;24:687–696 [DOI] [PubMed] [Google Scholar]
- 22. Visigalli I, Delai S, Ferro F, et al. Preclinical testing of the safety and tolerability of lentiviral vector-mediated above-normal alpha-L-iduronidase expression in murine and human hematopoietic cells using toxicology and biodistribution good laboratory practice studies. Hum Gene Ther 2016;27:813–829 [DOI] [PubMed] [Google Scholar]
- 23. David RM, Doherty AT. Viral vectors: The road to reducing genotoxicity. Toxicol Sci 2017;155:315–325 [DOI] [PubMed] [Google Scholar]
- 24. Cesana D, Ranzani M, Volpin M, et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol Ther 2014;22:774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biffi A, Bartolomae CC, Cesana D, et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood 2011;117:5332–5339 [DOI] [PubMed] [Google Scholar]
- 26. Hickey RD, Nicolas CT, Allen KL, et al. Autologous gene and cell therapy provides safe and long-term curative therapy in a large pig model of hereditary tyrosinemia type 1. Cell Transplant 2019;28:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. European Medicines Agency (EMA) Committee for the medicinal products for human use, Guideline on the non-clinical studies required before first clinical use of gene therapy medicinal products, 2008. www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-studies-required-first-clinical-use-gene-therapy-medicinal-products_en.pdf (accessed April4, 2019)
- 28. European Medicines Agency (EMA) Committee for Advanced Therapies, Reflection paper on management of clinical risks deriving from insertional mutagenesis, 2013. www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-management-clinical-riskss-deriving-insertional-mutagenesis_en.pdf (accessed April4, 2019) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.