Abstract
The ability of bi-layer silk fibroin (BLSF) matrices to mediate functional tissue formation in porcine bladders subjected to acute partial bladder outlet obstruction (pBOO) was investigated. Sixteen female swine were fitted with a transient urinary catheter containing controlled release valves capable of producing either mild (m-pBOO, 35 ± 10 cmH2O, N = 5) or severe (s-pBOO, 70 ± 15 cmH2O, N = 11) urinary outlet resistance for 2 or 4 weeks, respectively. After obstructive insults, augmentation cystoplasty was performed with BLSF grafts and animals were harvested up to 3 months postrepair. Urodynamic evaluations of swine after m-pBOO and s-pBOO injury displayed significant reductions in bladder capacity reflecting 63% ± 19% and 39% ± 13% of noninjured levels, while the s-pBOO cohort demonstrated a 61% decline in bladder compliance from baseline. By 3 months postreconstruction, bladder capacity and compliance in the augmented s-pBOO group were significantly increased over post-pBOO values reflecting 79% ± 19% and 171% ± 75% of baseline quantities, respectively. Neotissues with contractile properties were present at graft sites in both injury groups and displayed SM22α+ smooth muscle, pan-cytokeratin+ epithelia, vessels lined with CD31+ endothelial cells, and neurofilament 200+ nerve trunks. We demonstrate that BLSF scaffolds can support improvements in bladder capacity and compliance and promote the formation of neotissues in diseased bladders.
Impact Statement
The search for an ideal “off-the-shelf” biomaterial for augmentation cystoplasty remains elusive and current scaffold configurations are hampered by mechanical and biocompatibility restrictions. In addition, preclinical evaluations of potential scaffold designs for bladder repair are limited by the lack of tractable large animal models of obstructive bladder disease that can mimic clinical pathology. The results of this study describe a novel, minimally invasive, porcine model of partial bladder outlet obstruction that simulates clinically relevant phenotypes. Utilizing this model, we demonstrate that acellular, bi-layer silk fibroin grafts can support the formation of vascularized, innervated bladder tissues with functional properties.
Keywords: bladder, silk fibroin, regeneration
Introduction
Partial bladder outlet obstruction (pBOO) commonly results from neurogenic bladder and sphincter dysfunction from spinal cord injury or spinal dysraphisms, posterior urethral valves, and acquired (surgical) obstruction in patients with sling or bladder neck reconstruction procedures.1–3 Irrespective of the etiology, pBOO causes bladder overdistension resulting in fibroproliferative remodeling of the detrusor that ultimately leads to diminished bladder capacity, poor compliance, and incomplete emptying.4–6 In cases that are nonresponsive to pharmacotherapy, enterocystoplasty is the primary surgical procedure utilized to increase bladder capacity, reduce urinary storage pressures, preserve renal function, and achieve urinary continence.7,8 While enterocystoplasty is the surgical gold standard, the transposition of gastrointestinal segments into the urinary tract is associated with significant complications, including chronic urinary tract infection (UTI), mucus production, urinary calculi, metabolic abnormalities, bowel dysfunction, bowel obstruction, bladder perforation, and secondary malignancies.9–11 These side effects are too common and adversely affect patients' safety and quality of life. Therefore, there is a profound need to develop alternative strategies for reconstruction of pathological bladders.
Biodegradable scaffolds derived from decellularized bowel and bladder tissues and synthetic polyesters have been previously explored as alternatives to enterocystoplasty either as acellular matrices or grafts seeded with primary autologous cells.12–15 Despite successful demonstrations of bladder tissue regeneration in nondiseased animal models,16–20 none of these technologies have been adopted into widespread clinical practice due to poor urodynamic outcomes, abnormal tissue formation, and serious adverse events encountered in human trials.12–14 Bi-layer silk fibroin (BLSF) matrices derived from Bombyx mori silkworm cocoons represent promising candidates for repair of obstructed bladder tissues due to their low immunogenicity, robust mechanical properties, and tunable biodegradability.21–24 We have previously demonstrated the feasibility of these scaffolds to promote constructive remodeling in healthy rodent and porcine models of augmentation cystoplasty21,22 and improve bladder function in a neurogenic rat model of spinal cord injury.23 However, the ability of BLSF grafts to mediate tissue regeneration and restore normal urodynamic parameters in a large animal model of bladder disease is required before human translation can be considered.
Preclinical validation of biomaterials in animal systems that mimic the pathology of pBOO is necessary to accurately evaluate their potential to facilitate tissue repair and increase bladder capacity and compliance in diseased settings.25 However, several limitations exist in available animal models of pBOO that hamper their translational value for prospective graft screening. Specifically, ligature or ring-based techniques for surgical induction of pBOO in mice, rats, rabbits, and swine produce variable degrees of bladder dysfunction due to deviations in operator technique and animal size that can result in fluctuations in urinary outlet resistance. Therefore, standardization of diseased cohorts for bladder augmentation studies is difficult to establish.26,27
An additional approach for creating pBOO in male swine has utilized penile urethral ligation coupled with suprapubic catheter drainage via a controlled release valve to create defined bladder outlet resistance.25,28 This model is capable of provoking increases in smooth muscle hypertrophy and reductions in bladder compliance; features consistent with clinical pathology. Unfortunately, drawbacks of this system include occurrence of leaks or fistula formation around the penile stump that can compromise the level and duration of the obstructive insult, highly invasive nature involving multiple surgical procedures for model creation, and requirement for meticulous animal husbandry to monitor the integrity of the artificial bladder outlet mechanism.25,28 Given the shortcomings with traditional pBOO models, new methods for creating obstructed bladder phenotypes are desired to allow for adequate preclinical testing of biomaterial candidates.
In this study, we describe a novel model of pBOO in female swine whereby voiding occurs through a transient urinary catheter fitted with controlled release valves to produce a precise degree of urinary outlet resistance. By manipulating the magnitude and duration of pBOO with system parameters, we demonstrate that tissue level damage and urodynamic outcomes can be modulated to achieve various states of obstructive bladder disease. In addition, we show that acutely obstructed bladders reconstructed with acellular BLSF grafts display de novo tissue formation and improved bladder function. These findings may have important implications for urologic tissue engineering applications.
Materials and Methods
Biomaterials
BLSF scaffolds were fabricated from aqueous silk fibroin solutions derived from B. mori silkworm cocoons using a solvent-casting/salt-leaching protocol in combination with SF film casting as previously described.21 The tensile and structural properties of the scaffold have been reported in published reports.21 Biomaterials were autoclaved sterilized before surgical manipulations.
Surgical procedures
All animal procedures and husbandry followed guidelines prescribed by the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals and were reviewed and approved by the Boston Children's Hospital Animal Care and Use Committee before experimentation and executed under protocol 17-07-3515R.
pBOO was performed in 16 adult female, Yucatan mini-swine (∼30–33 kg; Sinclair BioResources, Windham, ME). Male swine were excluded from the study since their urethra cannot be reliably catheterized due to excessive tortuosity. Under general anesthesia using 1–4% isoflurane after induction with intramuscular injection of 0.4 mg/kg atropine, 2.2 mg/kg xylazine, and 4.4 mg/kg tiletamine+zolazepam, a three-way, 22 French Foley catheter was inserted through the urethra into the urinary bladder, inflated, and fixed into place via two peri-urethral purse sutures (2-0 polypropylene) to generate a fluid tight seal around the urethral meatus (Fig. 1A, B). The catheter was secured to the animal's flank with interrupted 2-0 polypropylene sutures to prevent dislodgement. Swine were then divided into two groups and subjected to either “mild” (m-pBOO, N = 5) or “severe” (N = 11, s-pBOO) obstructive conditions for a period of 2 or 4 weeks, respectively. For m-pBOO and s-pBOO settings, Foley catheter outlets were fitted in line with controlled release valves (Lee Company, Westbrook, CT) containing respective cracking pressures of 35 ± 10 cmH2O or 70 ± 15 cmH2O (Fig. 1C). Catheters were exchanged in all swine every 2 weeks during the pBOO phase to minimize the risk of UTIs. Four weeks after commencement of pBOO, all swine were evaluated by urodynamic and cystographic analyses and then either euthanized for end-point analyses (N = 2, s-pBOO) or subjected to bladder augmentation.
FIG. 1.
Porcine pBOO and augmentation cystoplasty models. (A) Creation of pBOO in female swine whereby voiding occurs through a transient urinary catheter fitted with controlled release valves to produce a precise degree of urinary outlet resistance. (B) Peri-urethral purse string placement to ensure fluid tight seal around urethral meatus and urethral catheter. (C) Three-way, 22 French Foley catheter outlets fitted with controlled release valves (insert) to produce pBOO. (D) Gross image of BLSF graft before implantation. (E) Cystotomy of diseased bladder dome before scaffold placement. (F) Implantation of BLSF grafts into pathological bladders. BLSF, bi-layer silk fibroin; pBOO, partial bladder outlet obstruction.
Augmentation cystoplasty with BLSF grafts (Fig. 1D, area = 28 cm2) was performed in swine after acute pBOO (m-pBOO, N = 5; s-pBOO, N = 9) using an open technique (Fig. 1E, F) previously described.22 Before scaffold implantation, bladder biopsies were collected and evaluated with the outcome analyses described below to ascertain pathological effects of pBOO. Nonabsorbable sutures (2-0, 4-0 polypropylene) were placed around the periphery of the scaffold to mark the anastomotic boundaries. Urethral catheterization was performed without controlled release valves for a period of 2 weeks post-op after scaffold integration and swine were maintained on prophylactic antibiotics in parallel (20 mg/kg/day). After this period, catheters were removed, and swine were allowed to voluntary void. Animals were euthanized at 1 or 3 months postscaffold implantation and isolated bladder specimens were harvested from the anterior, posterior, and central regions of the original graft site and nonaugmented control (NAC) areas. These samples were assessed for the outcome analyses described below. Nonsurgical control (NSC) tissues harvested from normal swine were analyzed similarly in parallel.
Urodynamics and cystography
Bladder compliance and capacity were determined under general anesthesia at baseline before pBOO, 4 weeks after initiation of pBOO, and at 1 or 3 months postgraft implantation utilizing a Triton® Urodynamic System (Laborie Medical, Williston, VT). Briefly, a three-way 22 French Foley catheter was inserted into the bladder and outlets were connected to either a bladder infusion channel or a pressure transducer. A 10–12 French red rubber rectal catheter was inserted in the anus to monitor intra-abdominal pressure. The pressure transducer was positioned at the level of the urinary bladder and sterile saline mixed with contrast reagent (Optiray, Raleigh, NC) was continuously infused into the bladder at 37°C. Intravesical and intra-abdominal pressure, infusion rate, and infused volume were monitored and recorded via system software. Bladder capacity was determined at an intravesical pressure of ∼20 cmH2O to avoid organ rupture or vesicoureteral reflux and cystogram images were acquired using an AMX-4 portable X-ray machine (General Electric, CT). Bladder compliance was determined by calculating the Δvolume/Δpressure between start and end-point pressures achieved during cystometry.
Histological, immunohistochemical, and histomorphometric analyses
Bladder tissue specimens from NSC, 4 weeks after obstructive insults, and 3 months after augmentation cystoplasty from both the original implant site (anterior, center, and posterior areas) and NAC regions were excised for standard histological processing. Samples were fixed in 10% neutral-buffered formalin for 24 h, dehydrated in graded alcohols, and paraffin embedded. Tissue sections (10 μm) were cut, stained with Masson's trichrome (MTS), and collagen content was determined using previously published methods.29 Briefly, MTS-stained samples were digitally imaged using a PathScan Enabler IV and color segmented using a subprogram within MATLAB (R2014b; MathWorks, Natick, MA) that separates and quantifies color elements, allowing for quantitation of blue-stained areas corresponding to extracellular collagen deposition. Collagen content was determined in at least three independent fields per experimental replicate and displayed as the percentage of blue-stained area per total area examined.
For immunohistochemical (IHC) assessments, parallel sections were deparaffinized, rehydrated in graded alcohols, and subjected to antigen retrieval in 10 mM sodium citrate buffer, pH 6.0. Specimens were then stained with primary antibodies overnight at 4°C to select antigens including pan-cytokeratin (CK, 1:150 dilution; Dako, Carpinteria, CA); uroplakin (UP) 3A (goat IgG, 1:200 dilution, Cat. No. sc-15186; Santa Cruz Biotechnology, Santa Cruz, CA); SM22α (1:200 dilution; Abcam, Cambridge, MA); neurofilament 200 (NF200, 1:250 dilution; Sigma-Aldrich); and CD31 (Cat. No. ab228364, 1:100 dilution; Abcam). Sections were incubated with species-matched Cy3 conjugated secondary antibodies (Millipore) and nuclear counterstain was performed with DAPI (4′,6-diamidino-2-phenylindole). An Axioplan-2 microscope (Carl Zeiss MicroImaging, Thornwood, NY) was utilized for sample visualization and representative fields were obtained with Axiovision software (version 4.8). Histomorphometric evaluations (N = 3–4 animals/group) were performed on four to nine independent microscopic fields (5 × or 10 × magnification) to quantify stained elements in experimental cohorts using published methods.30
Ex vivo contractility/relaxation responses
Bladder tissue segments harvested from NSCs, 4 weeks after obstructive insults, and 3 months after bladder augmentation from both the original graft site and NAC were subjected to ex vivo isometric tension analysis as previously described.22 Briefly, longitudinal strips of bladder tissue with either intact or denuded mucosa were suspended in organ baths containing Krebs's solution maintained at 37°C and bubbled with carbogen (95% O2 and 5% CO2). Tissues were attached to a force transducer (Grass Instruments), stretched to a resting tension of 2 g, and equilibrated for at least 1 h. Bladder smooth muscle contractions in response α,β-methyleneATP (αβmeATP, purinergic agonist, 10 μM) and KCl (120 mM) were measured. Dose- and frequency-response curves were generated for the cholinergic agonist carbachol (CCh, 1 nM–10 μM), and electrical field stimulation (EFS, 1–64 Hz, 16 V, 0.5 ms pulse width, 10 s duration). The relaxation response to a β-adrenergic agonist isoproterenol (ISO, 10 μM) was measured after precontracting the tissue with carbachol (10 μM). Conditioned signals from force transducers were continuously acquired at 30 Hz by a 16-channel analog-to-digital converter (DataQ, DI-720) and recorded to disk using Windaq data acquisition software. Data were expressed as force (mN) normalized by tissue cross-sectional area.
Statistical analyses
Multi-group comparisons of parametric data derived from urodynamic and histomorphometric analyses were performed using a one-way ANOVA (analysis of variance) with a two-tailed Student's t-test utilized for two group comparisons. Multi-group comparisons of nonparametric data acquired from ex vivo contractility/relaxation assessments were evaluated with Kruskal–Wallis test while Dunn's method was executed for pairwise assessments. Statistical evaluations were performed with Microsoft Excel or Sigmaplot softwares. Statistically significant values were defined as p < 0.05. Data were displayed as means ± standard deviation unless otherwise noted.
Results
All animals survived primary obstructive damage and subsequent bladder augmentation and were capable of voluntary voiding after 2 weeks of catheterization immediately after scaffold implantation. Approximately 2 months after bladder augmentation, three swine in the s-pBOO cohort were subjected to early euthanasia owing to adverse complications including an infected abscess at the abdominal incision site (N = 1); rectal/vaginal prolapse (N = 1); and an idiopathic bowel obstruction (N = 1). One animal in the m-pBOO was also removed from the study at 2 months postrepair due to severe hematuria associated with unilateral hydrouretronephrosis detected during necropsy. No other adverse effects or events were noted in the remaining animals over the course of the experimental period.
Urodynamic evaluations (Fig. 2A, B) of swine post-pBOO, but before augmentation cystoplasty demonstrated that both m-pBOO and s-pBOO cohorts displayed significant reductions in bladder capacity reflecting 63% ± 19% and 39% ± 13% of noninjured baseline values, respectively. By comparison, only swine subjected to s-pBOO displayed a significant attenuation of compliance exhibiting 39% ± 14% of baseline levels. By 3 months postrepair, swine exposed to m-pBOO had achieved a similar degree of bladder capacity compared to baseline. Moreover, bladder capacity and compliance were significantly increased in the s-pBOO cohort over post-pBOO values reflecting 79% ± 19% and 171% ± 75% of baseline quantities, respectively. Cystographic evaluations (Fig. 2C) qualitatively confirmed expansion and contraction trends in bladder size detected by urodynamic analyses. In addition, no evidence of contrast extravasation in reconstructed organs was observed. These data demonstrate that the decline in bladder capacity and compliance depend on the severity of obstructive bladder injury and that diseased bladders reconstructed with BLSF grafts display increased bladder capacity and compliance.
FIG. 2.
BLSF grafts improve urodynamic features of pathological bladders subjected to acute pBOO. Longitudinal urodynamic evaluations of bladder capacity (A) and compliance (B) in swine before injury (baseline), after acute pBOO for up to 4 weeks (post-pBOO) at urinary outlet resistance pressures of 35 ± 10 cmH2O (m-pBOO) or 70 ± 15 cmH2O (s-pBOO), and 1 or 3 months after repair (AUG) with BLSF grafts. N = 3–10 animals per group were assessed per data point. All urodynamic data are displayed relative to respective baseline group. *p < 0.05 in comparison to noninjured baseline. †p < 0.05 in comparison to post-pBOO. §p > 0.05 in comparison to noninjured baseline. Data were analyzed with one-way ANOVA and post hoc Student's two-tailed t-test. Means ± SD (C) Cystographic evaluations of bladder morphology after filling bladders to 20 cmH2O. ANOVA, analysis of variance; m-pBOO, mild partial bladder outlet obstruction; SD, standard deviation; s-pBOO, severe partial bladder outlet obstruction.
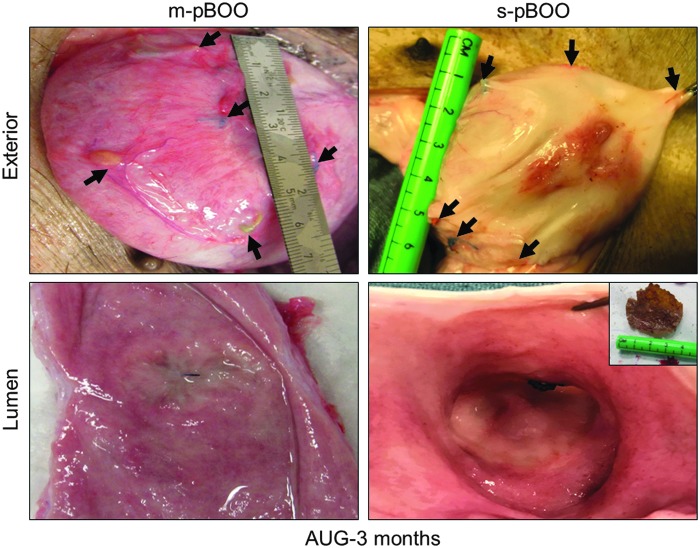
At scheduled euthanasia, necropsy observations of swine at 1 (s-pBOO, N = 3) or 3 (m-pBOO, N = 4; s-pBOO, N = 3) months after bladder augmentation demonstrated that BLSF scaffolds supported extensive de novo tissue formation throughout the original implantation site with minimal graft contracture between marking sutures (Fig. 3). In the s-pBOO cohort, intraluminal scaffold fragments were apparent in 100% of swine at 1 month, while only 33% of animals exhibited matrix remnants at 3 months. Residual BLSF pieces were also detected in 25% of the m-pBOO group at 3 months postrepair. Mild to moderate bowel adhesions were detected at all graft sites at both timepoints. Upper urinary tract anomalies at 3 months post-op were limited to one animal in the m-pBOO group that displayed a unilateral, communicating renal cyst.
FIG. 3.
BLSF scaffolds promote de novo tissue formation with minimal graft site contracture in pathological bladders. Gross morphology of regenerated tissues at 3 months postrepair in diseased porcine bladders subjected to acute m-pBOO (35 ± 10 cmH2O) and s-pBOO (70 ± 15 cmH2O) conditions. Arrows denote original marking sutures around implant periphery. Inset: intraluminal scaffold fragment.
Histological assessments (MTS) of regenerated tissues in both m-pBOO and s-pBOO groups 3 months after bladder reconstruction displayed cross-sectional organization similar to NSC architecture with distinct compartments consisting of a luminal epithelium, an extra-cellular matrix-rich lamina propria, and an outer layer of smooth muscle bundles (Fig. 4A–C). However, in comparison to NSC, significant fourfold to fivefold increases in collagen content were detected in both m-pBOO and s-pBOO biopsies after the obstruction period and in corresponding regenerated and NAC tissues 3 months after augmentation (Fig. 4D). Subepithelial mononuclear inflammatory cells were also detected throughout obstructed tissues and neotissues and NAC. These results show that fibrosis caused by obstructive insults of varying degrees persist throughout the bladder repair process and is prevalent in neotissues.
FIG. 4.
Neotissues in reconstructed pathological bladders display mucosal and smooth muscle compartments with elevated collagen content. (A–C) Gross and magnified photomicrographs of porcine bladder cross-sections stained with MTS from NSCs, acutely injured groups (post-pBOO) subjected to urinary outlet resistance pressures of 35 ± 10 cmH2O (m-pBOO) or 70 ± 15 cmH2O (s-pBOO), and regenerated (RT) and NAC tissues 3 months after bladder reconstruction with BLSF grafts. For (A–C), scale bars for first columns are 5 mm, second columns are 2 mm, and third to fourth columns are 200 μm. (D) Quantitation of collagen content in MTS-stained specimens described in (A–C). For all panels, N = 3–10 animals per group were assessed per data point. *p < 0.05 in comparison to NSC. †p > 0.05 in comparison to RT. Data were analyzed with one-way ANOVA and post hoc Student's two-tailed t-test. Means ± SD. MTS, Masson's trichrome; NAC, nonaugmented control; NSCs, nonsurgical controls.
Regenerated and control tissues were evaluated by IHC (Fig. 5A) and histomorphometric (Fig. 5B) analyses to assess the extent of obstructive damage and determine the degree of maturation achieved at implant sites. In comparison to NSC and m-pBOO cohorts, bladder tissues exposed to s-pBOO injury displayed a significantly reduced level of SM22α+ smooth muscle, elevated density of CD31+ vessels, and urothelial hyperplasia characterized by significantly increased pan-CK immunoreactivity. Mild urothelial dysplasia was also noted in the s-pBOO group illustrated by focal loss of apical UP3A expression. These results contrasted with m-pBOO specimens that displayed a urothelial phenotype resembling NSC. Both mild and severe obstructive injury led to an ∼30% reduction in NF200+ nerve trunks relative to NSC. Overall, these observations demonstrate that the severity of pBOO leads to differential bladder injury responses. After 3 months postrepair, neotissues in both augmented groups contained ∼65% of the level of SM22α+ smooth muscle observed in NSC. In addition, smooth muscle content was similar in corresponding NAC regions. Urothelial hyperplasia persisted in all de novo tissues, however, urothelial dysplasia was evident in regenerated areas and NAC regions present in the s-pBOO cohort alone. Vascular density at graft sites was increased in both experimental groups in respect to NSC, while the extent of innervation mirrored the degree exhibited after obstructive insults. Taken together, BLSF scaffolds can promote innervated, vascularized epithelial and smooth muscle tissues after reconstruction of obstructed bladders. However, significant alterations in urothelial maturation and vasculature persist in regenerated tissues formed in bladders exposed to severe, obstructive insult.
FIG. 5.
Injury and regenerative responses in acutely obstructed bladder tissues. IHC assessments of porcine bladder cross sections from NSCs, acutely injured groups (post-pBOO) subjected to urinary outlet resistance pressures of 35 ± 10 cmH2O (m-pBOO) or 70 ± 15 cmH2O (s-pBOO), and regenerated (RT) and NAC tissues 3 months after bladder repair with BLSF grafts. (A) Photomicrographs of SM22α, pan-CK, UP 3A, CD31, and NF200 in control and experimental groups. For all panels, respective marker expression is displayed in red (Cy3 labeling) and blue denotes DAPI nuclear counterstain. Scale bars in all panels = 200 μm. (B) Histomorphometric analysis of stained elements displayed in (A). N = 3–4 animals per group and four to eight sections analyzed per animal for each data point. *p < 0.05 in comparison to NSC. †p < 0.05 in comparison to m-pBOO. ‡p > 0.05 in comparison to NSC. §p > 0.05 in comparison to RT. For NF200 analysis, p > 0.05 for all groups. Data were analyzed with one-way ANOVA and post hoc Student's two-tailed t-test. Means ± SD. CK, cytokeratin; DAPI, 4′,6-diamidino-2-phenylindole; IHC, immunohistochemical; NF200, neurofilament 200; UP, uroplakin.
Ex vivo tissue bath studies on bladder tissue with denuded or intact mucosa were performed to assess contractile responses of regenerated and control specimens to CCh, αβmeATP, KCl, and EFS (Fig. 6A–D). Contractile responses to stimulation were increased in the m-pBOO cohort and attenuated in the s-pBOO group compared to NSC, consistent with compensated and decompensated function respectively. These findings indicate that this animal model appropriately reflects the stages of bladder dysfunction observed clinically in response to outlet obstruction. We observed that s-pBOO injury markedly attenuated all contractile responses tested in comparison to tissues exposed to m-pBOO. At 3 months postrepair, evaluations of neotissues and NAC in both injury groups revealed contractile responses to direct smooth muscle activation, and nerve stimulation; however, in general, levels of force generation were substantially lower than those achieved in NSC. The relaxation response to ISO in precontracted tissues was maintained after bladder augmentation in regenerated and native samples from both groups (Fig. 6E).
FIG. 6.
Ex vivo contractile responses in injured and regenerated tissues. Contractile responses from denuded (A–E) bladder strips from NSCs, acutely injured groups (post-pBOO) subjected to urinary outlet resistance pressures of 35 ± 10 cmH2O (m-pBOO) or 70 ± 15 cmH2O (s-pBOO), and regenerated (RT) and NAC tissues 3 months after bladder repair with BLSF grafts. Contractile responses to (A) α,β-methyleneATP (10 μM) (B) extracellular KCl (120 mM). *p < 0.05 in comparison to post m-pBOO. (C) Dose–response curves for CCh (10−9 to 10−5 M). (D) Frequency response curves for EFS (1–64 Hz). *p < 0.05 in comparison to post m-pBOO and all doses and frequencies. (E) Relaxation responses to ISO (10 μM) in CCh precontracted bladder strips. *p < 0.05 in comparison to post m-pBOO and NAC m-pBOO. (F) The effect of the mucosa on the contractile response to CCh (1 μM). Responses from intact tissue were expressed as a percent of responses in denuded tissue. *p < 0.05 in comparison to NSC and RT for m-pBOO group. N = 3–4 animals per group. Data were analyzed with Kruskal–Wallis test with post hoc Dunn's method. Means ± standard error. CCh, carbachol; EFS, electrical field stimulation; ISO, isoproterenol.
Previous studies have shown that contractile responses to muscarinic stimulation are attenuated by the presence of the urothelium in the pig bladder due to the release of a urothelium-derived inhibitory factor.31 We found a similar reduction in the response to carbachol in tissues with an intact mucosa relative to the response in denuded bladder tissue. The inhibitory effect of the mucosa remained evident in tissues after obstruction and postaugmentation in both groups. However, in regenerated tissue from s-pBOO animals, the inhibitory effect of the mucosa was augmented compared with NSC and m-pBOO animals (Fig. 6F), possibly a consequence of the more severe histomorphometric urothelial changes observed in s-pBOO.
Our observations provide evidence for the ability of BLSF grafts to promote functional tissue formation in diseased porcine bladders after acute pBOO. These data also demonstrate that the severity of obstructive bladder injury impacts the degree of tissue remodeling and functional phenotype.
Discussion
The search for a viable graft replacement for enterocystoplasty has been the focus of urologic research for decades. A crucial step in identifying alternative implant configurations for obstructive bladder repair is to assess their efficacy to promote tissue regeneration and improve urodynamic parameters in an appropriate large animal system that mimics patient pathology. Conventional large animal models of bladder disease previously utilized for potential graft screening have employed modes of injury such as partial or trigone-sparing cystectomy,19,32 bladder marsupialization,33 or urinary tract obstruction via urethral ligation coupled with suprapubic outlet resistance.25,28 Unfortunately, these systems do not produce anatomical or functional urinary tract obstruction or are technically challenging to perform. In this study, we have developed and characterized a novel minimally invasive, porcine model of pBOO and evaluated the ability of BLSF scaffolds to serve as “off-the-shelf” grafts for pathological bladder reconstruction.
Adult swine were used as a model species because they display comparable size to humans, have similar abdominal anatomy, and changes in bladder capacity and compliance from obstructive insults or scaffold implantation can be easily assessed because of the absence of growth-related alterations in organ size.34 Urinary outlet pressures of 35 ± 10 cmH2O or 70 ± 15 cmH2O were selected as mild and severe obstructive insults since a detrusor leak point pressure >40 cmH2O has been clinically associated with an increased risk of upper urinary tract damage.35,36 Before augmentation cystoplasty, our findings show that both acute m-pBOO and s-pBOO led to histopathological alterations in bladder tissues such as elevated fibrosis as well as perturbations in urodynamic outcomes including reductions in bladder capacity. However, the nature of the injury response was also correlated to the severity of obstruction. In contrast to m-pBOO, s-pBOO caused a significant decline in bladder compliance, reduction in smooth muscle content, urothelial dysplasia, increased vascularity, and diminished agonist-induced contractility relative to NSC. These differential phenotypes resemble the spectrum of pBOO symptoms encountered in patients4,37 and are likely a result of variations in bladder overdistension produced during mild and severe obstructive settings. Indeed, previous studies of rabbits with pBOO have shown that the degree of bladder overdistension is associated with specific molecular and functional alterations in bladder tissue.38,39 The ability of our model to generate a range of pathological insults in a controllable fashion may serve as a valuable tool for studying subtypes of obstructive bladder disease that encompass either mild or severe obstruction.
Bladders repaired with BLSF grafts after acute, severe pBOO demonstrated partial recovery of bladder capacity and restoration of bladder compliance to levels statistically similar with noninjured baseline values by 3 months post-op. These trends occurred despite initial decline in urodynamic parameters 1 month after scaffold implantation and may have resulted from higher stiffness and diminished elasticity of neotissues during early stages of graft remodeling.40 Augmentation cystoplasty was performed after removal of acute obstruction to allow for bladder cycling to occur during the wound healing process since previous studies have reported that early exposure to site-appropriate mechanical loading plays a significant role in constructive remodeling of bladder defects.41 Our approach mirrors clinical scenarios encountered in certain patients afflicted with posterior urethral valves wherein bladder pathology persists after surgical correction of pBOO, thus necessitating bladder augmentation.42,43 Functional and histomorphometric analyses of NAC regions provided evidence for the lack of spontaneous recovery in our system after acute obstructive damage wherein pathological features such as elevated collagen content and reductions in contractile properties were maintained 3 months after insult. Nevertheless, parallel longitudinal comparisons between acutely obstructed bladders with and without augmentation will be pursued in future experiments to verify that gains in bladder capacity and compliance are solely due to BLSF scaffold implantation and not from partial reversal of disease phenotype.
BLSF grafts supported innervated, vascularized bladder tissue formation in swine exposed to both mild and severe, acute pBOO. In regenerated tissues, a number of relevant, though compromised, motor features of intact tissue were identified by evoked responses, including a functioning smooth muscle contractile apparatus, intact neurotransmission, effects of urothelial-derived inhibitory factors, and receptor-mediated contractile/relaxation events. However, aberrant tissue properties acquired from obstructive injury such as fibrosis, urothelial dysplasia, and reduced contractile function were apparent in regenerated tissues and NAC at 3 months post-op. Abnormal urothelial differentiation has the potential to increase bladder permeability to noxious urinary components44 while prolonged fibrotic tissue remodeling can minimize gains in bladder compliance and capacity due to increased organ stiffness.25 It is unknown how these alterations in normal bladder tissue phenotype may affect the long-term ability of BLSF scaffolds to improve urodynamic outcomes and preserve renal function, which is the primary goal of augmentation cystoplasty. Nonetheless, future investigations will explore modifications in the original BLSF scaffold prototype including targeted biomaterial release of antifibrotic agents such as halofuginone45 and pro-differentiation stimuli such as retinoids for urothelial maturation46 by harnessing the drug delivery potential of SF.47
In conclusion, we have generated a new large animal model of pBOO that is minimally invasive, relatively simple to perform, and allows for specific modulation of mild and severe bladder injury responses that are consistent with clinically relevant, disease phenotypes such as those encountered in patients with posterior urethral valves. Utilizing this model, we have shown improvements in pathological bladder capacity and compliance occur after BLSF scaffold implantation and that this matrix technology supports the formation of de novo tissues with functional properties at implant sites after acute obstructive insults. Overall, these results suggest that BLSF grafts offer a promising platform for bladder tissue engineering and may have utility in overcoming the challenges associated with conventional enterocystoplasty.
Acknowledgments
This research was supported by NIH/NIBIB 5R21EB020860 (J.R.M and C.R.E.) and DVA BX001790 (M.P.S.). The authors thank Dr. Arthur Nedder, DVM, Debra Franck, MSc, and Alyssa Savarino, MSc for technical assistance and acknowledge the Tufts University, Tissue Engineering Resource Center, NIH/NIBIB P41 EB002520 (Dr. David Kaplan, PI), for procurement of silkworm cocoons. We also acknowledge Dr. Rosalyn Adam, PhD for helpful discussions.
Authors' Contributions
J.R.M., C.R.E., S.A., and F.M.S. conceived and designed the experiments. S.A., F.-M.S., K.A., X.Y., K.C., G.G., B.S., and C.S. performed the experiments. V.C., M.P.S., M.G.-K., J.A.M., X.Y., K.C., G.G., F.M.S., and C.S. executed study outcome analyses, processed and evaluated data. V.C. and J.R.M. performed statistical analyses. J.R.M. wrote the artcle. All authors edited the article for conceptual and technical content. J.R.M. coordinated and supervised all aspects of the study.
Disclosure Statement
No competing financial interests exist.
References
- 1. Ginsberg D. The epidemiology and pathophysiology of neurogenic bladder. Am J Manag Care 19, s191, 2013 [PubMed] [Google Scholar]
- 2. Clayton D.B., and Brock J.W., III. Lower urinary tract obstruction in the fetus and neonate. Clin Perinatol 41, 643, 2014 [DOI] [PubMed] [Google Scholar]
- 3. Komninos C., and Mitsogiannis I. Obstruction-induced alterations within the urinary bladder and their role in the pathophysiology of lower urinary tract symptomatology. Can Urol Assoc J 8, E524, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mirone V., Imbimbo C., Longo N., and Fusco F. The detrusor muscle: an innocent victim of bladder outlet obstruction. Eur Urol 51, 57, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Guys J.M., Hery G., Haddad M., and Borrionne C. Neurogenic bladder in children: basic principles, new therapeutic trends. Scand J Surg 100, 256, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Mirshemirani A., Khaleghnejad A., Rouzrokh M., Sadeghi A., Mohajerzadeh L., and Sharifian M. Posterior urethral valves; a single center experience. Iran J Pediatr 23, 531, 2013 [PMC free article] [PubMed] [Google Scholar]
- 7. Scales C.D., Jr., and Wiener J.S. Evaluating outcomes of enterocystoplasty in patients with spina bifida: a review of the literature. J Urol 180, 2323, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Baka-Ostrowska M. Bladder augmentation and continent urinary diversion in boys with posterior urethral valves. Cent Eur J Urol 64, 237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flood H.D., Malhotra S.J., O'Connell H.E., Ritchey M.J., Bloom D.A., and McGuire E.J. Long-term results and complications using augmentation cystoplasty in reconstructive urology. Neurourol Urodyn 14, 297, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Husmann D.A., and Rathbun S.R. Long-term follow up of enteric bladder augmentations: the risk for malignancy. J Pediatr Urol 4, 381, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Veeratterapillay R., Thorpe A.C., and Harding C. Augmentation cystoplasty: contemporary indications, techniques and complications. Indian J Urol 29, 322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atala A., Bauer S.B., Soker S., Yoo J.J., and Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Joseph D.B., Borer J.G., De Filippo R.E., Hodges S.J., and McLorie G.A. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol 191, 1389, 2014 [DOI] [PubMed] [Google Scholar]
- 14. Schaefer M., Kaiser A., Stehr M., and Beyer H.J. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol 9, 878, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Horst M., Madduri S., Gobet R., et al. Engineering functional bladder tissues. J Tissue Eng Regen Med 7, 515, 2013 [DOI] [PubMed] [Google Scholar]
- 16. Kropp B.P., Rippy M.K., Badylak S.F., et al. Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol 155, 2098, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Kropp B.P. Small-intestinal submucosa for bladder augmentation: a review of preclinical studies. World J Urol 16, 262, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kropp B.P., Cheng E.Y., Lin H.K., and Zhang Y. Reliable and reproducible bladder regeneration using unseeded distal small intestinal submucosa. J Urol 172, 1710, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Oberpenning F., Meng J., Yoo J.J., and Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17, 149, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Song L., Murphy S.V., Yang B., Xu Y., Zhang Y., and Atala A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng Part B Rev 20, 163, 2014 [DOI] [PubMed] [Google Scholar]
- 21. Seth A., Chung Y.G., Gil E.S., et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 34, 4758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tu D.D., Chung Y.G., Gil E.S., et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials 34, 8681, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung Y.G., Algarrahi K., Franck D., et al. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials 35, 7452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sack B.S., Mauney J.R., and Estrada C.R. Jr. Silk fibroin scaffolds for urologic tissue engineering. Curr Urol Rep 17, 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akbal C., Lee S.D., Packer S.C., Davis M.M., Rink R.C., and Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol 176, 1706, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Fry C.H., Daneshgari F., Thor K., et al. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn 29, 603, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Kitta T., Kanno Y., Chiba H., et al. Benefits and limitations of animal models in partial bladder outlet obstruction for translational research. Int J Urol 25, 36, 2018 [DOI] [PubMed] [Google Scholar]
- 28. Shaw M.B., Herndon C.D., Cain M.P., Rink R.C., and Kaefer M. A porcine model of bladder outlet obstruction incorporating radio-telemetered cystometry. BJU Int 100, 170, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Ma J., Gharaee-Kermani M., Kunju L., et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol 188, 1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Algarrahi K., Franck D., Cristofaro V., et al. Bi-layer silk fibroin grafts support functional tissue regeneration in a porcine model of onlay esophagoplasty. J Tissue Eng Regen Med 12, e894, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawthorn M.H., Chapple C.R., Cock M., and Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129, 416, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth C.C., Mondalek F.G., Kibar Y., et al. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int 108, 148, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Roelofs L.A., Kortmann B.B., Oosterwijk E., et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int 114, 447, 2014 [PubMed] [Google Scholar]
- 34. Sloff M., Simaioforidis V., de Vries R., Oosterwijk E., and Feitz W. Tissue engineering of the bladder—reality or myth? A systematic review. J Urol 192, 1035, 2014 [DOI] [PubMed] [Google Scholar]
- 35. McGuire E.J., Woodside J.R., Borden T.A., and Weiss R.M. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 126, 205, 1981 [DOI] [PubMed] [Google Scholar]
- 36. Snodgrass W.T., and Gargollo P.C. Urologic care of the neurogenic bladder in children. Urol Clin North Am 37, 207, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Chan Y.Y., Sandlin S.K., and Kurzrock E.A. Urological outcomes of myelomeningocele and lipomeningocele. Curr Urol Rep 18, 35, 2017 [DOI] [PubMed] [Google Scholar]
- 38. Kato K., Wein A.J., Kitada S., Haugaard N., and Levin R.M. The functional effect of mild outlet obstruction on the rabbit urinary bladder. J Urol 140, 880, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Zhang E.Y., Stein R., Chang S., et al. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of non-muscle caldesmon. Am J Pathol 164, 601, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hinz B., Phan S.H., Thannickal V.J., et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180, 1340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boruch A.V., Nieponice A., Qureshi I.R., Gilbert T.W., and Badylak S.F. Constructive remodeling of biologic scaffolds is dependent on early exposure to physiologic bladder filling in a canine partial cystectomy model. J Surg Res 161, 217, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Smith G.H., Canning D.A., Schulman S.L., Snyder H.M., III, and Duckett J.W. The long-term outcome of posterior urethral valves treated with primary valve ablation and observation. J Urol 155, 1730, 1996 [PubMed] [Google Scholar]
- 43. Wolffenbuttel K.P., de Jong B.W., Scheepe J.R., and Kok D.J. Potential for recovery in bladder function after removing a urethral obstruction. Neurourol Urodyn 27, 782, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Slobodov G., Feloney M., Gran C., Kyker K.D., Hurst R.E., and Culkin D.J. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol 171, 1554, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Jaidane M., Ali-El-Dein B., Ounaies A., Hafez A.T., Mohsen T., and Bazeed M. The use of halofuginone in limiting urethral stricture formation and recurrence: an experimental study in rabbits. J Urol 170, 2049, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Gandhi D., Molotkov A., Batourina E., et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26, 469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pritchard E.M., and Kaplan D.L. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv 8, 797, 2011 [DOI] [PubMed] [Google Scholar]