Abstract
This article comments on:
Jennifer L. Ison, Elizabeth S. L. Tuan, Matthew H. Koski, Jack S. Whalen and Laura F. Galloway. 2019. The role of pollinator preference in the maintenance of pollen colour variation. Annals of Botany 123(6): 951–960.
The diversity of colourful patterns and structures of flowers has attracted the attention of human observers for many centuries. In the 18th century, the German naturalist Christian Konrad Sprengel noted that they might serve to secure visits from insects in order to transfer pollen between plants. Since then, most attempts to classify interactions between insects and flowers have focused on striking and obvious features, such as the form and colour of petals and floral odours. However, floral traits that are less perceptible to humans have also been shown to influence the behaviour of pollinating insects. These include small colourful structures such as pollen on stamens, as reported by Ison and colleagues (2019) in the current issue.
Pollen comes in many colours, and in the majority of angiosperms it is fully or partially exposed and accessible to a range of pollinators. It is paradoxically a costly reward that pollen-collecting and pollen-eating pollinators will seek out at the expense of a plant’s fitness. Some species have evolved flower shapes and mechanisms that protect pollen from unsuitable pollinators, and florivores, whilst others defend their exposed pollen chemically, all of which can help to reduce the loss of gametes. However, in many plants the colour of the exposed pollen adds to the set of cues that comprise a flower’s advertisement to pollinators – a costly but seemingly effective signalling and reward strategy. How the use of these signals by insect pollinators affects the evolution of floral traits continues to be a widely debated question. Ison et al. (2019) in this issue show using field and experimental observations that pollen-collecting bees are capable of distinguishing and developing a preference for one pollen morph over another in otherwise identical flower displays of co-occurring Campanula vulgaris. This could explain how colour variability is maintained in the pollen of a plant and how pollinators exert selection pressures on this trait. An intriguing question the authors raise is how bees do this, given that the visual acuity of their eyes is low.
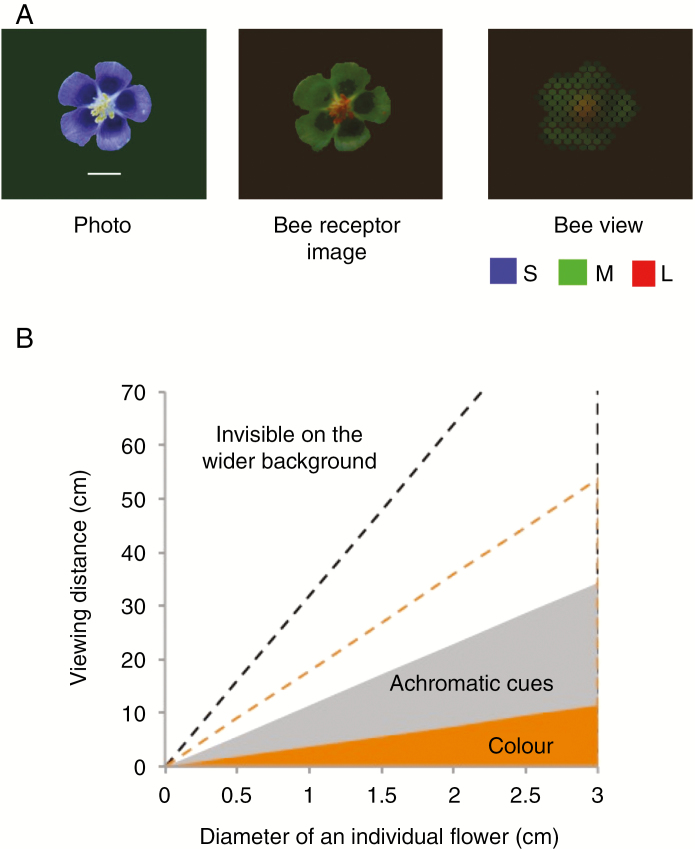
An insect’s visual system is well adapted to its particular lifestyle. Bees, like many other pollinators, have excellent colour and achromatic vision that serves them well for foraging, flight and navigation. However, the spatial resolution is low because their bodies can only accommodate small eyes (Fig. 1). Tiny anthers and pollen sacs can thus only be resolved when the insect come close and lands on the flower. In contrast, larger structures such as coloured petals, bracts or combined displays in inflorescences, trees or patches of co-flowering plants provide positional cues that are effective over longer distances. This can aid the detection of a flower patch or inflorescence and guide the pollinator’s approach towards an individual flower. Although the fine spatial details of flower displays are invisible from any longer distance they become increasingly visible when the insect lands and moves on the flower, an advantage of the optical structure of their compound eyes (Hempel de Ibarra et al., 2014). Small-sized colourful structures can effectively guide the arriving insect towards the reward location on the flower. Stamens that are sufficiently large, merged or protruded may form a convenient structure for landing, and their colourful anthers act as beacons towards which the landing insect moves. As Ison et al. (2019) observed in naturally foraging bees, this can result in selective removal of pollen of a particular colour.
Fig. 1.
(A) A view of Aquilegia vulgaris, a relatively large flower with protruding anthers and coloured pollen (from Hempel de Ibarra et al., 2014). The left image shows a photo, the middle a UV-VIS multispectral image in RGB colour values obtained by modelling the spectral measurements and photoreceptor sensitivity for the S, M and L receptors of the honeybee. The right image shows the flower as seen through the honeybee (Apis mellifera) ommatidia from a distance of 10 cm, at which a honeybee can perceive the colours of this individual flower. (B) The limits of spatial resolution of honeybee vision as determined in behavioural experiments (see also Hempel de Ibarra et al., 2014). Shown are the distance ranges over which single-coloured individual displays of differently-sized flowers can be detected by an approaching bee through achromatic and colour cues. The dashed lines show the corresponding limits for average-sized bumblebee workers, Bombus terrestris. Foraging bumblebee workers tend to be larger in body size and have therefore larger-sized and more sensitive eyes, an advantage for flying and nesting in a wide range of diverse habitats and grounds with dense vegetation.
Whilst visual signals are indispensable for guiding movement and enabling foraging decisions, the most important aspect of a flower from a pollinator’s perspective is nevertheless the reward it expects to obtain. The evaluation of reward is a cognitive process that shapes a pollinator’s foraging preferences. Thus, an insect may be attracted to colourful structures of a flower and decide to execute an approach followed by a landing and extraction of the reward. Alternatively, it can decide to end an approach flight at any distance, or even to ignore a flower altogether. The decision exercised depends on its sensory biases, frequency and previous experience of rewards as well as the cost of searching and choosing between co-flowering plants. The preferences that result from such foraging decisions will depend on the combination of colour cues in flower displays that guide an insect towards a sufficiently rich and reliable reward. Thus, pollen-foraging bumblebees in the Ison et al. (2019) study ignored the variations of colour under field conditions, but learned to use them when sucrose solution was manipulated in an experiment to be available in just one pollen morph.
Pollinators can discover and attend to minute floral details, such as pollen colours, patterns or nectar guides. Some of these structures emanate distinctive odour bouquets (Dobson and Bergström, 2000), which may facilitate their discovery. Importantly, they provide effective visual cues when contrasted against petals or a foliage background, depending on the viewing distance and direction. The presence of visually-contrasting features is essential for guiding the insect’s elaborate movements during a flower visit. The seemingly effortless execution of highly coordinated sequences of motor responses during approach, landing and reward extraction is a considerably demanding task for the insect brain and nervous system. It is costly in terms of energy expended, more so when flowers move due to wind or due to the force exerted by the pollinator itself on the flower. Insects therefore readily associate the presence of salient structures and cues with a smooth landing and a swift extraction of reward, forming memories that are presumably multimodal and that influence subsequent decisions during and between foraging bouts.
Pollen offers both visual and chemical cues. We are only beginning to uncover how pollinators detect and learn them, with few experimental and field studies so far conducted predominantly on few species of bees (for a review see Nicholls and Hempel de Ibarra, 2017). Only recently it has been shown that pollen-foraging bumblebees easily learn the colour of both pollen and associated petals, under experimental conditions that control for previous experience and sensory biases. Learning, thus, underpins the adaptive development of preferences for a particular type of pollen in individual foragers. Foraging preferences in individuals may change with learning, when bees continuously gain experience, and as a result bees specialize on different types of pollen over a period of time.
This flexibility is based on the continuous assessment of reward quality, associative learning processes and experience of handling flowers for pollen removal. Nevertheless, it is still often argued that innate preferences dominate foraging preferences and thus determine pollinator-mediated selection of floral traits. We refer to innate preferences as unlearned, spontaneous preferences because they appear to be unstable and variable. Experimental studies with bees and butterflies show that their expression is context-dependent and transformed after just a single or very few exposures to a rewarding colour (for example, Blackiston et al., 2011; Balamurali et al., 2018). Fast colour and odour learning are the main mechanisms that determine foraging choices and the development of preferences in pollinating insects. Adaptive limitations to such flexibility might exist and vary across pollinator taxa (for instance hoverflies, Lunau et al., 2018). However, so far the evidence is scarce and the underlying neural mechanisms are unknown.
Whether sensory cues, such as the colour or taste, are linked to nutritional quality of pollen, and whether or not pollinators preferentially select flowers based on the nutritional value of their pollen continues to be controversial discussed. It is commonly assumed that foragers should maximize the intake of nutrients by evaluating pollen quality during collection. However, as the vast majority of bee species are generalist pollen collectors, they could also maximize their foraging efficiency by increasing the mass of collected pollen through learning of floral displays and higher efficiency in handling a particular flower type. Thus, pollen-collecting pollinators may not necessarily rely on the taste or nutritional quality of pollen when deciding which flowers to visit (Nicholls and Hempel de Ibarra, 2017). Indeed, foragers of social bee species, such as honeybees and bumblebees, do not eat pollen, yet develop individual pollen preferences for different plants. As a result, a diverse mix of pollen is stored in a colony providing a range of nutrients.
Solitary female bees also form mixed-species pollen stores, but in addition regularly feed on pollen (Cane et al., 2017). Bees mix pollen with regurgitated nectar, which improves its digestibility and thus nutritional value (Nicolson et al., 2018). It is still to be demonstrated whether active ingestion also occurs on flowers, but it might be disadvantageous to any foraging bee. Digesting raw pollen might release high concentrations of psychoactive or toxic secondary metabolites that can disrupt the insect’s motor and cognitive performance. Nevertheless, it is conceivable that the consumption of pollen in the nest may influence the formation of pollen preferences in solitary bees through taste cues or post-ingestive mechanisms. Interestingly, Ison et al. (2019) observed a strong preference in the solitary bees collecting dark-coloured pollen in Campanula vulgaris, whilst bumblebees collected indiscriminately from both pollen morphs ignoring the stark differences in pollen colouration. It could well be that the observed preferences in solitary bees were reinforced by nutritional or taste cues, and thus it would be of interest to explore how pollen morphs differ in their chemical composition or other non-colour features.
The perception of small floral structures, such as colourful pollen-bearing anthers and pollen sacs, will vary to some extent across pollinators, and most likely the same is true for the perception of rewards. The resulting selection pressures from pollinators will thus change across plant and pollinator communities, with different trends in converging and diverging floral traits both within and between plant species. From the plant’s perspective, it remains to be explored whether the colouration of pollen reflects a trade-off between attracting suitable pollinators and avoiding antagonists such as florivores, which can depress plant fitness. How the variation in pollen colour and reinforcement of insect preferences affect plants is a fascinating question that should be explored in further phylogenetic studies and behavioural observations of different pollinator species. Last but not least, such work can provide substantial insights that will be important for developing and testing predictive plant-pollinator network models.
Literature Cited
- Balamurali G, Nicholls E, Somanathan H, Hempel de Ibarra N. 2018. A comparative analysis of colour preferences in temperate and tropical social bees. The Science of Nature 105: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Briscoe AD, Weiss MR. 2011. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). Journal of Experimental Biology 214: 509–520. [DOI] [PubMed] [Google Scholar]
- Cane JH, Dobson HE, Boyer B. 2017. Timing and size of daily pollen meals eaten by adult females of a solitary bee (Nomia melanderi) (Apiformes: Halictidae). Apidologie 48: 17–30. [Google Scholar]
- Dobson HE, Bergström G. 2000. The ecology and evolution of pollen odors. Plant Systematics and Evolution 222: 63–87. [Google Scholar]
- Hempel de Ibarra N, Vorobyev M, Menzel R. 2014. Mechanisms, functions and ecology of colour vision in the honeybee. Journal of Comparative Physiology A 200: 411–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JL, Tuan ES, Koski MH, Whalen JS, Galloway LF. 2019. The role of pollinator preference in the maintenance of pollen colour variation. Annals of Botany 123: 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunau K, An L, Donda M, Hohmann M, Sermon L, Stegmanns V. 2018. Limitations of learning in the proboscis reflex of the flower visiting syrphid fly, Eristalis tenax. PLoS One 13: e0194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls E, Hempel de Ibarra N. 2017. Assessment of pollen rewards by foraging bees. Functional Ecology 31: 76–87. [Google Scholar]
- Nicolson SW, Neves SDSD, Human H, Pirk CW. 2018. Digestibility and nutritional value of fresh and stored pollen for honey bees (Apis mellifera scutellata). Journal of Insect Physiology 107: 302–308. [DOI] [PubMed] [Google Scholar]