Abstract
Background and Aims
Pollinators often drive the evolution of floral traits, but their capacity to influence the evolution of pollen colour remains unclear. Pollen colour in Campanula americana is variable and displays a longitudinal cline from prevalence of deep purple in western populations to white and light-purple pollen in eastern populations. While selection for thermal tolerance probably underlies darker pollen in the west, factors contributing to the predominance of light pollen in eastern populations and the maintenance of colour variation within populations throughout the range are unknown. Here we examine whether pollinators contribute to the maintenance of pollen colour variation in C. americana.
Methods
In a flight cage experiment, we assessed whether Bombus impatiens foragers can use pollen colour as a reward cue. We then established floral arrays that varied in the frequency of white- and purple-pollen plants in two naturally occurring eastern populations. We observed foraging patterns of wild bees, totalling >1100 individual visits.
Key Results
We successfully trained B. impatiens to prefer one pollen colour morph. In natural populations, the specialist pollinator, Megachile campanulae, displayed a strong and consistent preference for purple-pollen plants regardless of morph frequency. Megachile also exhibited a bias toward pollen-bearing male-phase flowers, and this bias was more pronounced for purple pollen. The other main pollinators, Bombus spp. and small bees, did not display pollen colour preference.
Conclusions
Previous research found that Megachile removes twice as much pollen per visit as other bees and can deplete pollen from natural populations. Taken together, these results suggest that Megachile could reduce the reproductive success of plants with purple pollen, resulting in the prevalence of light-coloured pollen in eastern populations of C. americana. Our research demonstrates that pollinator preferences may play a role in the maintenance of pollen colour variation in natural populations.
Keywords: Bombus, Campanula americana, Campanulastrum americanum, floral traits, geographic cline, Megachile, plant–pollinator interaction, pollen colour, pollen depletion
INTRODUCTION
Natural selection and genetic drift can decrease phenotypic variation in populations, especially for traits related to fitness or when populations are small (e.g. Wright, 1943; Schemske and Bradshaw, 1999). However, in plants, petal colour variation has been reported in a number of populations, even when one colour has an apparent selective advantage (i.e. higher pollinator visitation; Stanton, 1987; Campbell et al., 2010). While the maintenance of variation in petal colour has been well studied (e.g. Rebelo and Siegfried, 1985; Gigord et al., 2001; Jones and Reithel, 2001; Eckhart et al., 2006; Thairu and Brunet, 2015; Twyford et al., 2018), until recently variation in pollen colour has received less attention (Jorgensen et al., 2006; Koski and Galloway, 2018; Austen et al., 2018; Wang et al., 2018). In addition, we still lack knowledge of the role of pollinator-mediated selection on the maintenance of pollen colour variation.
In many species, pollen colour is determined by flavonoid and/or carotenoid compounds that accumulate in the pollen grains (Wiermann and Vieth, 1983; Mo et al., 1992; Okinaka et al., 2003; Tanaka et al., 2008). The presence and amount of flavonoid compounds has been correlated with variation in pollen germination and tube growth rates (Mo et al., 1992; Ylstra et al., 1992). In species polymorphic for pollen colour, variation in pollen viability between colour morphs has important evolutionary implications. For example, in polymorphic Epimedium pubescens, green pollen has higher germination rates than yellow pollen, but mixed pollen loads have lower siring success than either type alone (Wang et al., 2018). These results suggest that there is likely to be selection against polymorphic populations in E. pubescens. Flavanoids are also suggested to confer protection against environmental stressors (Winkel-Shirley, 2002), and a growing body of work has found that patterns of pollen colour variation are correlated with the abiotic conditions of a population (Jorgensen and Andersson, 2005; Jorgensen et al., 2006; Koski and Galloway, 2018).
Pollen colour is also likely to be under pollinator-mediated selection. Insect pollinators are selective when foraging, using floral cues such as flower size, corolla length, nectar reward, polarization patterns and petal or pollen colour (Lunau, 1991; Foster et al., 2014; Nicholls et al., 2017). Therefore, pollinators can exert selective pressure on specific floral characteristics (Brown and Clegg, 1984; Schemske and Horvitz, 1984; Castellanos et al., 2003), including pollen colour. For example, solitary bee pollinators showed a site-specific pollen colour preference in a dramatic red/yellow pollen colour polymorphism in Erythonium americanum (Austen et al., 2018). In addition, pollinator preferences for floral traits fluctuate depending on trait frequencies. For example, bee pollinators displayed a frequency-dependent preference for petal spot morphs in Clarkia xantiana. Hesperapis regularis (Melittidae) preferentially visited arrays that mimicked the natural morph frequency, while other pollinators preferentially visited arrays that contained a greater frequency of morphs that were the minority in the natural population (Eckhart et al., 2006).
Visual systems and learning processes play key roles in the behaviours of foraging insects and can aid in the development of pollinator preferences (Gumbert, 2000). While pollen preferences exist in honeybees, the preference has been linked to odour (Pernal and Currie, 2002), and it is unclear how much of a role vision plays in discriminating pollen-based rewards. Most bee species have trichromatic colour vision, with photoreceptors sensitive to green, blue and ultraviolet wavelengths (Briscoe and Chittka, 2001). Floral colour cues can help bees distinguish potential resources from the background (Jones and Buchmann, 1974). Even if pollinators can discern the colour differences, they may not have the visual acuity to distinguish smaller structures, such as pollen, as a floral cue. Researchers have used artificial flowers and coloured discs to demonstrate that pollinators can associate colour with pollen reward quality (Nicholls and Hempel de Ibarra, 2014). However, more research is needed to determine if insect pollinators can or do develop a pollen colour preference in plant species with variable pollen colour.
The American bellflower (Campanula americana) is a herbaceous plant commonly found throughout eastern North America (Barnard-Kubow et al., 2015). It is insect pollinated by members of several bee families: Apidae, Megachilidae and Halictidae (Lau and Galloway, 2004; Koski et al., 2018a). In C. americana, pollen colour is variable (ranging from white to deep purple) and heritable (Koski and Galloway, 2018). Pollen colour variation displays a longitudinal cline where westerly populations have a prevalence of purple pollen, probably due to abiotic selection for heat stress resistance, and plants in eastern populations have mostly light-purple or white pollen (Koski and Galloway, 2018). Factors contributing to the predominance of white and light-purple pollen in the eastern populations and the overall maintenance of colour variation in populations throughout the range remain unclear. We examined pollinator-mediated mechanisms for the pollen colour variation by asking the following questions. (1) Are bees able to use pollen colour as a visual cue in C. americana? (2) Do natural bee pollinators exhibit a preference for pollen colour? (3) If so, does the preference vary based on pollen colour frequencies?
MATERIALS AND METHODS
Study system
The American bellflower (Campanula americana L., Campanulaceae) is a herbaceous annual or biennial plant found at forest edges throughout the eastern USA (Fig. 1). Campanula americana is protandrous and capable of self-fertilization. Flowers open in the male phase, where pollen is presented on pollen-collecting hairs along the style. Flowers transition to the female phase after pollen is removed and the stigmatic lobes open (Koski et al., 2018b). The reflectance of all pollen colours peaks at 460 nm, and white/light-purple pollen has higher reflectance than purple pollen (Koski and Galloway, 2018). Petal colour is also variable but does not co-vary with pollen colour (see the Results). Petals have peak reflectance in the violet range at 439 nm with an average of 30 % reflectance (± 5.13 s.d.).
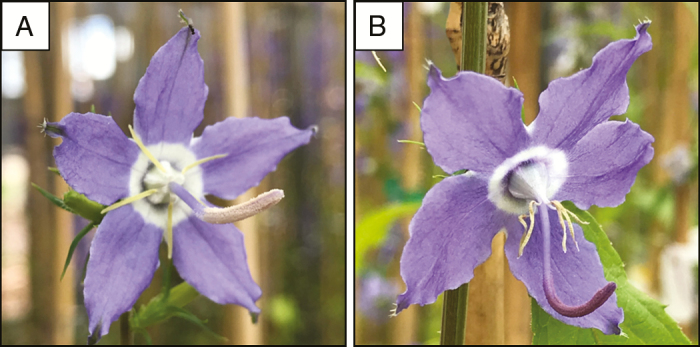
Fig. 1.
Male-phase Campanula americana flowers with pollen present on an unreceptive style. (A) Flower with white pollen (colour score =1; see the Materials and Methods). (B) Flower with deep-purple pollen (colour score = 6; see the Materials and Methods). Photo credit: M. H. Koski.
Campanula americana is insect pollinated and is visited by a variety of pollinators including Bombus spp. (Apidae), Megachile campanulae (Megachilidae) and small ground-nesting bees (including Augochlorella spp. and Lasioglossum spp. in the Halictidae, and Ceratina spp. in the Apidae; Lau and Galloway, 2004; Koski et al., 2018a). All insect pollinators forage for nectar, but M. campanulae and the small bees also forage for pollen. Per visit, Bombus are significantly more effective pollinators than M. campanulae and small bees. Megachile campanulae removes more pollen per visit than Bombus spp. and small bees, and small bees deposit less pollen per visit than the other pollinator taxa (Koski et al., 2018a).
Pollen colour as a visual cue
To determine whether pollinators have the ability to distinguish differences in pollen colour in a natural system, we trained Bombus impatiens, a natural pollinator of C. americana, to use pollen colour as a reward cue. We used two B. impatiens colonies (BioBest® and Natupol®) consisting of female workers and a queen. One colony was kept in an agricultural landscape at the College of Wooster’s field station, Fern Valley. The other colony was kept in a residential area in Wooster, OH. Outside of experimental trials, the bees were free to forage in the surrounding area and we provided them with sugar water and pollen. However, we withheld food and prevented natural foraging for 24 h prior to training and testing days to ensure foraging. To identify bees, we caught individuals in the flight cage and labelled them by applying small dots of acrylic paint on the thorax between their wings.
We used C. americana plants from six populations in Alabama, Indiana, Kansas, Minnesota, Pennsylvania and Wisconsin (Koski et al., 2017). Plants were grown from seed at the University of Virginia and transported to Wooster, OH where they were kept in a greenhouse at Ohio State University Agricultural Technical Institute. We recorded the pollen colour and petal colour for all plants used in this study. We scored pollen and petal colour from 1 to 7 (hereafter colour score) using Sherwin Williams’ Interior Color Answers paint sample #119, ranging from white to deep purple.
We set up two displays of C. americana in a flight cage (Coleman™ Instant Screenhouse; 3 × 3m mesh tent) – one display had four plants with deep purple pollen (colour scores 5–7; Fig. 1) and the other, presented at the same time, had four plants with white pollen (colour scores 1 or 2; Fig. 1). We tested if petal and pollen colour co-varied by comparing the petal colour score of white-pollen plants and purple-pollen plants with an independent sample t-test. All female-phase flowers were removed and the location of each display within the tent was randomized daily to ensure that the bees were not learning to forage by location. Light conditions varied slightly due to cloud coverage (sunny to slightly overcast), although the trials were not conducted on rainy or cold days.
We trained the foragers using pollen as a reward cue by arbitrarily making purple-pollen flowers rewarding and white-pollen flowers non-rewarding. To do this, we removed the nectar from each flower and filled the nectaries with 20 μL of water (white-pollen flowers) or 20 μL of a 1:3 sucrose:water mixture (purple-pollen flowers). The sucrose solution was within the range of C. americana’s nectar sugar concentration in the greenhouse, but less concentrated than the greenhouse mean (57.7 %; M. H. Koski and L. F. Galloway, unpubl. data). During the training session, we allowed the bees to forage on the C. americana displays inside the flight cage. We tracked individual bees as they foraged, and recorded pollen colour and bee ID. A full training session for a bee consisted of at least six visits. Each bee was conditioned for a minimum of four training sessions before the testing session.
In the testing session, the floral displays were the same as in the training session; however, all flowers were non-rewarding and filled with 20 μL of water. We then recorded the foraging visits of previously trained bees. Bees with incomplete training were not permitted to forage. To assess whether B. impatiens learned to use pollen colour as a reward cue, we conducted G-tests for Goodness of Fit (DescTools package, R v.1.0.143). We compared the number of observed first visits to each pollen colour morph with the expected number of visits (50 %) for training session one and the testing session. A lack of preference for pollen colour in the first training session, but a preference for purple pollen in the testing session, indicates that B. impatiens can learn to associate purple pollen with a nectar reward.
We also modelled pollinator perception of petal and pollen colour to assess the degree to which pollen contrasts from petals of C. americana. To estimate the average petal colour of plants used in flight cage and field array experiments, we measured spectral reflectance from 71 flowers across the six source populations from which arrays were constructed (n = 7–14 per population) using an Ocean Optics Spectrophotometer with a UV-VIS Deuterium light source (Ocean Optics, Dunedin, FL, USA). The average petal reflectance was calculated using the ‘aggspec’ function in R (pavo package). We measured spectral reflectance of pollen for 2–5 plants with five colour categories (described in Koski and Galloway, 2018). We modelled the perceived distance between petal and pollen colour using two separate insect visual systems – B. impatiens and Osmia rufa. The colour photoreceptors of B. impatiens have peak sensitivity at 347, 424 and 539nm (Skorupski and Chittka, 2010). While the photoreceptor sensitivity for M. campanulae is unknown, the Megachilidae species, O. rufa, also has trichromatic vision with peak sensitivities at 344, 432 and 560 nm (Peitsch et al., 1992).
We measured contrast between each pollen colour category and the average petal for B. impatiens and O. rufa. For each pollinator type, we measured photons of light captured by each of the three photoreceptors (quantum catch) using spectral inputs (average petal and pollen of each colour morph) with Standard Illuminant D65, and a green background with the ‘vismodel’ function using the pavo package in R (Maia et al., 2013). We visualized the relative locations of petals and pollen in hexagonal insect colour perceptual space using the ‘colspace’ function (Chittka and Menzel, 1992). Finally, we measured Euclidean distances between mean petal colour and each pollen colour class in hexagonal space (chromatic contrast), as well as long-wavelength photoreceptor distance (achromatic contrast) with the ‘coldist’ function.
Pollen colour preferences in natural populations
To determine if wild pollinators have a pollen colour preference, we selected two naturally occurring populations of C. americana in north-east Ohio. The first site, along the Chuckery Trail in the Cascade Valley Metro Park (Akron, OH; 41°06’50.5’’N, 81°31’12.6’’W), had a large and widespread C. americana population. The second site, located along a natural trail (40°42’32.3’’N, 81°58’54.2’’W) within the Killbuck Marsh Wildlife Area in Shreve, OH, had occasional clumps of C. americana. We scored pollen colour in the populations using the same method as for the experimental plants (see ‘Pollen colour as a visual cue’). Pollen colour in both populations ranged from white to purple, with a mean pollen colour score of 2.63 (Chuckery Trail, n = 286) and 2.83 (Killbuck, n = 36; Supplementary Data Fig. S1).
In each population, we established arrays of 12 potted C. americana plants to evaluate pollen colour preference of insect visitors. Each array was 60 × 90 cm, and individual plants were placed 30 cm from each other. To assess the influence of pollen colour morph frequency on colour preference, we set up 6P:6W arrays with an equal number of purple- (np = 6) and white- (nw = 6) pollen morphs. We also set up purple-skewed arrays (8P:4W; np = 8; nw = 4) and white-skewed arrays (4P:8W; np = 4; nw = 8). For the white-pollen morphs, we used plants with a colour score of 1 or 2 (rarely 3), and the purple-pollen morphs had a colour score of 5–7 (rarely 4; Fig. 1; Supplementary Data Table S1). We positioned each array adjacent to the natural populations to ensure that local pollinators were accustomed to foraging on the plant. Arrays were initiated by mid to late morning so that data collection occurred before or during peak pollinator activity (Evanhoe and Galloway, 2002). Each array type was repeated on 5 d, i.e. 3 d at Chuckery Trail and 2 d at Killbuck (n = 15 arrays; Supplementary Data Table S1). We used a randomized block design to determine the order of arrays. We used the same stock of plants for these arrays as we used in the flight cage study.
For each plant in the arrays, we reduced floral display size to two male-phase flowers and two female-phase flowers. Males were identified by the presence of pollen on the style and females by the reflexed three-lobed stigma and no pollen remaining on the style. When a plant had more than two flowers in the male or female phase, we excluded the extra flowers by covering them with a split drinking straw. We observed pollen levels throughout the day. If a male flower was depleted of pollen, we would uncover a new male flower if available. However, if 30 % of male-phase flowers were stripped of pollen, we ended data collection for that array. We observed pollinators, defined as floral visitors making contact with the style. For each insect, we recorded the plant position and flower sex phase for all visits in an array. We also collected foraging data as bees transitioned between plants and flowers within the array; however, pollinators were shooed away after ten consecutive transitions between flowers. We replicated arrays until each array type received at least 30 visits from each of three pollinator groups: Bombus spp. (hereafter Bombus), Megachile campanulae (hereafter Megachile) and small bees (including Augochlorella spp., Lasioglossum spp. and Ceratina spp.). Data were collected between July and August 2017, the natural flowering time of plants in north-eastern Ohio.
To determine whether naturally occurring pollinators displayed a preference for different pollen colour morphs and whether the preference depended on morph frequency, we used a generalized mixed linear model with a Poisson distribution (SAS v. 9.4, PROC GLIMMIX). In each array replicate, we totalled the number of first visits made by a pollinator to each colour morph and floral sex phase. First visits represent the initial choice made by a pollinator upon entering an array. We modelled the number of first visits as a function of array type (i.e. ‘morph frequency’; white biased, mixed and purple biased), pollinator type (Bombus, Megachile and small bee), pollen colour morph (purple and white) and floral sex phase (male and female). All two-way and three-way interactions were included. Four-way interactions were not significant and were removed from the model. Array replicate nested within array type was modelled as a random effect. We did not have the replication to test for site-specific effects. There was a significant pollinator type by pollen colour morph interaction, so we assessed which pollinator type(s) displayed a colour preference using a SLICE statement in SAS. We generated least-squares means from models and back-transformed them to visualize the data. We also conducted this analysis using the first male-phase flower (pollen-bearing) each pollinator visited. The results were very similar between the two models.
We used a similar model to test whether colour morphs experience differential pollinator visitation taking into account entire pollinator foraging bouts. In this model, the response was the number of total visits to each floral colour morph and flower sex phase by each pollinator type in each array. We removed all visits from the data set that resulted from movement of a pollinator between flowers on the same plant. Visits resulting from movement between flowers on the same plant were removed because these were unlikely to reflect a choice made by a pollinator based on floral traits. Again, we assessed differences between groups within significant interactions terms using a SLICE statement in SAS and visualized the data as noted above.
RESULTS
Pollen colour as a visual cue
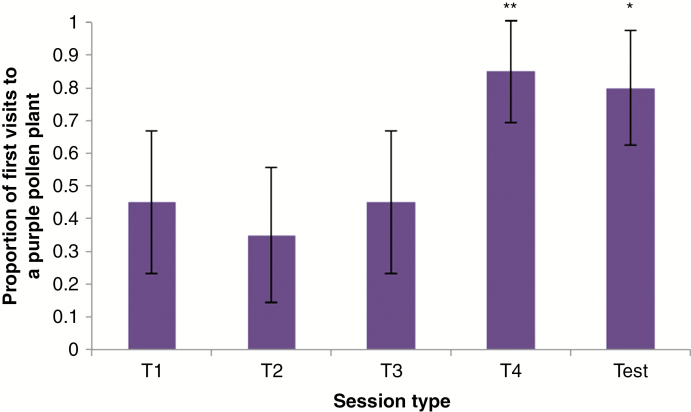
Pollen and petal colour for the plants used in this study did not covary (mean petal colour for white-pollen plants = 5.15, s.d. = 0.24; mean petal colour for purple-pollen plants = 5.13, s.d. = 0.36; t = 0.18, d.f. = 28, P = 0.86). In the flight cage study, we completed four training sessions and one testing session for 20 different B. impatiens foragers. The bees displayed no initial preference for pollen colour during training session one (T1, Fig. 2; G = 0.20, d.f. = 1, P = 0.65). However, by the final training session, T4, 17 of 20 foragers visited the rewarding purple-pollen morph first. During the testing session, when all plants were unrewarding, 16 of the 20 foragers (80 %) visited a purple-pollen plant first (Fig. 2; G = 7.71, d.f. = 1, P = 0.005).
Fig. 2.
Proportion of purple-pollen plants Bombus impatiens foragers visited first during the training sessions (T1–T4) and the testing session (Test). In training sessions, plants with purple pollen were rewarding and those with white pollen were not. In the test session, both colour morphs were non-rewarding. Asterisks indicate significant overvisiting of purple-pollen plants. Error bars represent the binomial 95 % confidence interval. *P < 0.05. **P < 0.01.
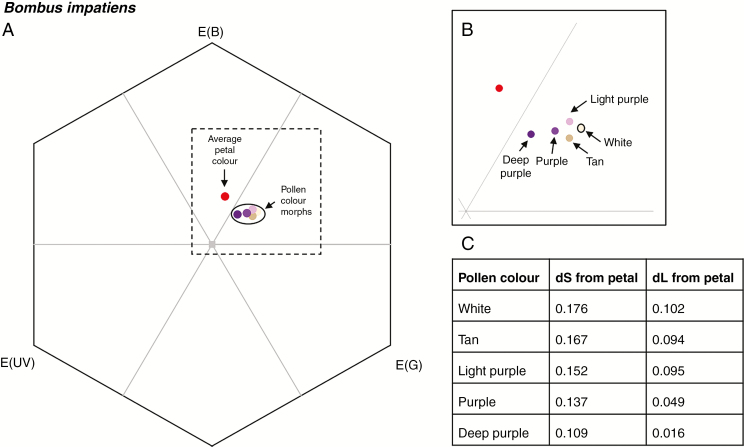
Results from the colour vision model demonstrated that the perception of pollen and petal colour was largely the same for Bombus and Osmia (Fig. 3; Supplementary Data Fig. S2). Petals of C. americana fall within the ‘blue’ area of hexagonal colour space for both pollinator types, indicating that quantum catch of the mid-wavelength photoreceptor is higher than the shorter and long-wavelength receptors. All pollen colour morphs are in the ‘blue-green’ range of hexagonal colour space so pollen excites the long-wavelength green receptor more than the petal does. White pollen displays the highest chromatic and achromatic contrast from the petal, and both chromatic and achromatic contrasts from the petal decline with increasing darkness of pollen (Fig. 3; Supplementary Data Fig. S2).
Fig. 3.
(A and B) The average Campanula americana petal and pollen colour of five colour morphs placed in hexagonal colour space for Bombus impatiens. (C) Chromatic (dS) and achromatic (dL) distance between each pollen colour morph and the average petal colour.
Pollen colour preferences in natural populations
We recorded 1108 pollinator visits by Bombus (98), Megachile (428) and small bees (582) to the floral arrays (Supplementary Data Fig. S3). All array types had a similar number of visits (array effect in all models P > 0.6; Table 1; Supplementary Data Table S2). However, there were more Megachile and small bee visits compared with Bombus, with only two Bombus visits recorded at the Killbuck population (pollinator type effect in all models P < 0.001; Table 1; Supplementary Data Tables S2 and S3).
Table 1.
Analysis of the number of flowers visited in a foraging bout by three pollinator types to C. americana arrays that differed in the frequency of flowers with purple and white pollen
| Effect | Num. d.f. | Den. d.f. | F-value | P-value |
|---|---|---|---|---|
| Array type | 2 | 12 | 0.41 | 0.671 |
| Pollinator | 2 | 136 | 122.08 | <0.001 |
| Pollen colour | 1 | 136 | 10.55 | 0.015 |
| Sex phase | 1 | 136 | 14.81 | 0.002 |
| Array type × pollinator | 4 | 136 | 2.66 | 0.035 |
| Array type × pollen colour | 2 | 136 | 56.71 | <0.001 |
| Array type × sex phase | 2 | 136 | 8.51 | 0.003 |
| Pollinator × pollen colour | 2 | 136 | 8.76 | 0.003 |
| Pollinator × sex phase | 2 | 136 | 2.85 | 0.024 |
| Sex phase × pollen colour | 1 | 136 | 4.99 | 0.027 |
| Array type × pollinator × pollen colour | 4 | 136 | 0.54 | 0.707 |
| Array type × pollinator × sex phase | 4 | 136 | 1.51 | 0.202 |
| Array type × sex phase × pollen colour | 2 | 136 | 5.77 | 0.004 |
| Pollinator × sex phase × pollen colour | 2 | 136 | 0.70 | 0.500 |
Fixed effects of the generalized mixed linear model where the response is the number of visits in a foraging bout (see Figs 4B and 5; Supplementary Data Table S4).
Array replicate nested within array type was modelled as a random effect. Floral pollen morph frequency (6P:6W, 8P:4W and 4P:8W) = array type; pollinator group (Bombus spp., Megachile campanulae and small bees = pollinator type; model degrees of freedom = num. d.f.; and denominator degrees of freedom = Den. D.f.
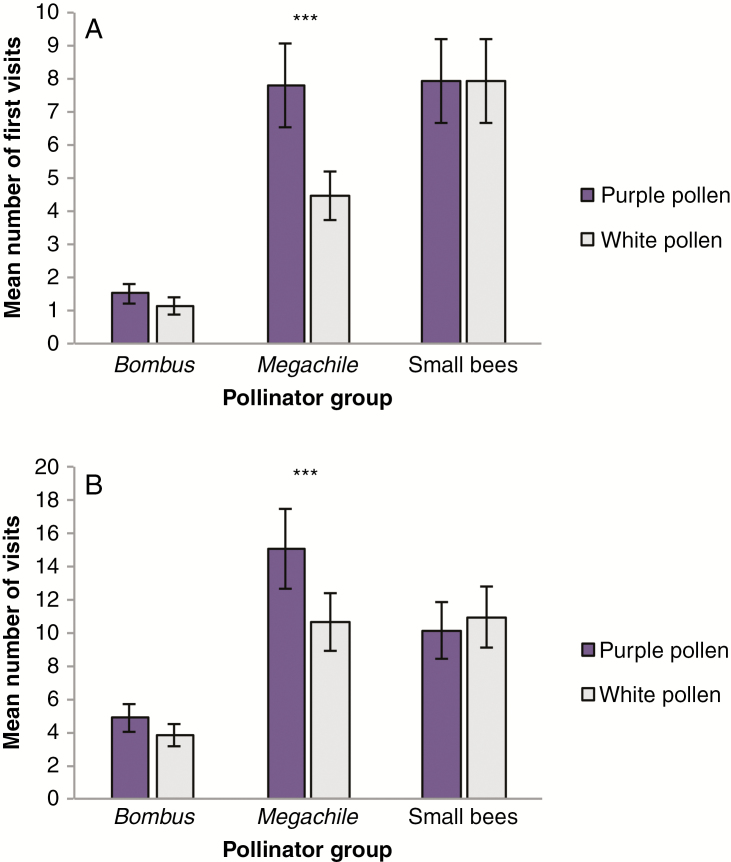
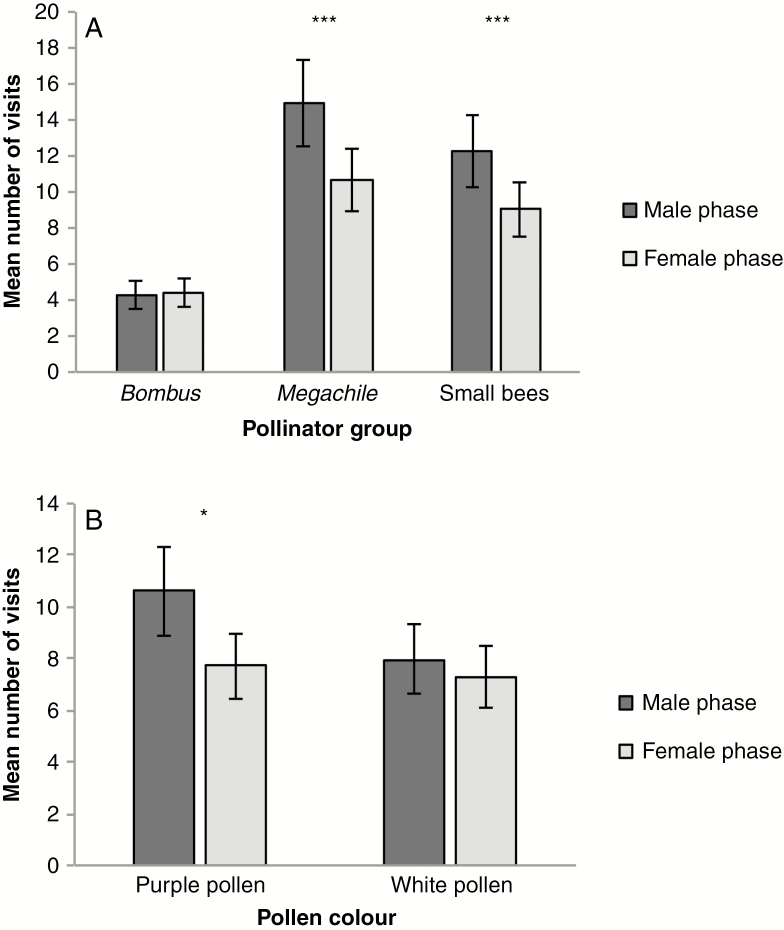
For all visitation metrics, pollen colour preferences varied by pollinator group, with Megachile displaying a strong and consistent preference for plants with purple pollen (pollinator type × pollen colour; all models P < 0.01; Fig. 4; Supplementary Data Fig.S4; Table 1; Supplementary Data Tables S2 and S3). Megachile demonstrated a preference for purple-pollen plants on their first visit to the array (Fig. 4A; Supplementary Data Table S2), their first visit to a male-phase flower (Supplementary Data Fig. S4, Table S3) and across their foraging bout (Fig. 4B; Table 1). In contrast, Bombus and small bees showed no pollen colour preference in their first visit, their first male-phase visit or within a foraging bout (Fig. 4; Supplementary Data Fig. S4; Table 1; Supplementary Data Tables S2 and S3). In all models, there was no difference in frequency-dependent pollen morph preference among pollinators (pollinator type × pollen colour × array type; all models P > 0.6; Table 1; Supplementary Data Tables S2 and S3). Both Megachile and small bees had a significant preference for male-phase flowers both within a foraging bout and for their first visit (Fig. 5A; Supplementary Data Tables S2 and S4). In contrast, Bombus showed no sex phase preference (Fig. 5A). Interestingly, the bias for male-phase flowers was stronger for purple pollen compared with white pollen (Fig. 5B; sex phase × pollen colour P < 0.05; Table 1; Supplementary Data Table S4).
Fig. 4.
Pollen colour visitation of the primary pollinator groups, Bombus, Megachile and small bees. Displayed are the least square means (± 1 s.e.) for (A) the first pollen colour morph a pollinator visited upon entering the array and (B) across a pollinator’s foraging bout. Asterisks represent a preference for purple pollen for Megachile. ***P < 0.001.
Fig. 5.
Flower sex phase visitation of the primary pollinator groups, Bombus, Megachile and small bees. Displayed are the least square means (± 1 s.e.) for visitation across a foraging bout (A) by each pollinator type and (B) by pollen colour. Asterisks represent a preference for male-phase flowers. *P < 0.05. ***P < 0.001.
DISCUSSION
Our study examined the role of pollinator preference in the prevalence of light pollen in eastern populations of C. americana. Using a flight cage experiment, we found that Bombus have the ability to perceive differences in pollen colour and use pollen colour as a visual cue while foraging (Fig. 2). Data from our field study demonstrated that the specialist Megachile bee had a strong and consistent preference for purple pollen (Fig. 4). The purple preference of Megachile was not dependent on the frequency of pollen colours in the arrays and was observed in both sites (Fig. 4; Supplementary Data Fig. S4; Table 1; Supplementary Data Tables S2–S4). In contrast, Bombus and small bees did not show a pollen colour preference in any of the arrays regardless of pollen morph frequencies. Similar to previous studies, both Megachile and small bees showed a bias toward male-phase flowers, but Megachile’s bias for male-phase flowers was stronger in purple-pollen plants than in white-pollen plants (Fig. 5; Table 1; Supplementary Data Table S4). A concurrent study in the same populations found that Megachile removed nearly twice as many pollen grains per visit to male-phase flowers than small bees or Bombus (approx. 10 500 grains compared with around approx. 5700 grains for small bees and approx. 5000 for Bombus), but deposited significantly fewer pollen grains than Bombus (Koski et al., 2018a). Because of Megachile’s strong biases for male-phase flowers and purple pollen, it could reduce the reproductive success of plants with purple pollen, resulting in light pollen across the range of C. americana, and the potential to shape geographic variation in pollen colour.
Visual abilities of bees to distinguish pollen colour variation
Visual acuity in insect pollinators is generally considered to be low (e.g. Bombus visual acuity is estimated at 0.36 cycles per degree, Jander and Jander, 2002) and, prior to our study, it was not known whether insect pollinators are able to distinguish and respond to subtle pollen colour variation in a natural system. Our flight cage results demonstrated that Bombus were able to use pollen colour as a reward cue in C. americana. Individual bees initially displayed no preference in pollen colour (Fig. 2), but by the fourth training session most foragers exhibited a notable preference for purple pollen. This preference continued into the testing session when both pollen colour morphs were unrewarding (Fig. 2). Ideally, we would have trained Bombus workers to prefer the white-pollen phenotype too. Logistically, however, we could not train some Bombus workers on purple pollen as the rewarding phenotype and others on white pollen, because each worker experienced four complete training sessions and we could not control which worker foraged at any given time. Yet, it is likely that Bombus workers could have been trained to prefer the white-pollen phenotype for two reasons (1) Bombus workers showed no initial preference for pollen colour in both the flight cage study and the natural populations (Figs 2 and 4) and (2) based on the vision modelling, the white-pollen phenotype is more distinct from the petal colour background than the purple-pollen phenotype (Fig. 3). Our results demonstrating that the colour of pollen can be learned by Bombus workers is an important first step for understanding whether pollinators can exert selection on this trait in a natural system.
Colour vision models demonstrated that in relation to average petal colour, white pollen is more distinct than purple pollen (Fig. 3; Supplementary Data Fig. S2) when viewed by both B. impatiens and O. rufa (Megachilidae). Since B. impatiens learned to associate purple pollen with a reward, it is likely to be able also to utilize the even more obvious white pollen in foraging decisions. These results, in combination with our field study results, show that pollinating bees can perceive pollen colour variation in C. americana and associate it with a reward. To the best of our knowledge, ours is one of the first studies to demonstrate that bees can distinguish and learn to prefer a given pollen colour morph using naturally occurring pollen colour variants.
Implications of pollen colour foraging preferences in natural populations
Pollinator-mediated selection on floral traits is often due to pollinators that are both efficient and abundant (Fenster et al., 2004). Yet, in many populations, the most abundant pollinator is not always the most efficient pollinator. In fact, when in low abundance, efficient pollinators probably do not exert significant selective pressures and therefore do not influence floral trait evolution. For example, in Heterotheca subaxillaris, some of the most efficient pollinators are generally rare and, as a result, of low importance to seed production, whereas the most important pollinators are less effective but more abundant (Olsen, 1996). Similarly, the influence of both pollinator foraging behaviour and abundance could drive the prevalence of white and light-coloured pollen in eastern populations of C. americana.
Specifically, we hypothesize that the abundant specialist pollinator, Megachile, is exerting selection against purple pollen. While Bombus is the most efficient pollinator per visit (Koski et al., 2018a), we observed fewer Bombus visits than either Megachile or small bees, with Bombus visits nearly non-existent at the Killbuck population (Supplementary Data Fig. S3). In contrast, Megachile and small bees were common at both sites in all array types. Megachile always preferred purple-pollen plants and had a stronger male-phase flower bias when a plant had purple pollen compared with white pollen (Fig. 4B). Since Megachile removes nearly twice as much pollen per visit as either Bombus or a small bee (Koski et al., 2018a), we hypothesize that Megachile’s preference for purple pollen is reducing the male fitness of purple-pollen plants. In Claytonia virginica, specialist Andrena erigeniae also removes significantly more pollen from flowers than any other pollinator, and populations with high A. erigeniae visitation produce fewer seeds (Parker et al., 2016). Megachile’s strong preference towards purple-pollen plants and its male-phase bias may similarly deplete purple pollen from C. americana populations.
The role of pollinators in the maintenance of intraspecific pollen colour variation
Our research, along with previous research in this system, can start to elucidate why populations with variable pollen colour are found throughout the range of C. americana as well as the prevalence a light-coloured pollen in the east of the range. In western populations, more abundant deep-purple pollen is favoured by selection due to its resistance to heat stress, whereas the germination of white pollen is reduced under high temperatures (Koski and Galloway, 2018). Greater thermal tolerance of purple pollen may be conferred by elevated flavonol content since some flavonols are crucial for pollen germination (Mo et al., 1992); this has yet to be tested in C. americana. Therefore, abiotic selection is predicted to drive C. americana populations to purple pollen. However, we demonstrate that pollinators have the ability to discern intraspecific pollen colour variation and that an abundant wild pollinator prefers one pollen colour over another. Previous research has found that opposing selective pressures maintain petal colour variation in Claytonia virginica populations (Frey and Williams, 2004). Similarly, our results suggest that opposing selection between abiotic factors and pollinator preferences help to maintain pollen colour variation in C. americana.
While we found no frequency-dependent preference (i.e. Megachile always prefers purple-pollen plants), the evolutionary implications of pollen colour preference could still be context dependent. For instance, in populations with a high frequency of purple pollen, Megachile’s purple preference may have little impact since they may not deplete all the purple pollen from the population. It is also important to note that we only measured preference in two eastern populations even though all three pollinator groups are common throughout C. americana’s range (Koski et al., 2017). While pollen colour preference did not vary in Ohio populations based on morph frequencies, preference may vary across the range. In a Virginia population of C. americana, light pollen is preferred by small bees when only male-phase flowers are available (Lau and Galloway, 2004) and site-specific pollen colour preferences have been observed in other systems (Austen et al., 2018).
Our results do not rule out the role of neutral genetic processes and dispersal limitation in the observed pollen colour cline and population-level variation. However, given the strong and consistent preference of the specialist Megachile pollinator for purple pollen, it seems likely that Megachile visits are imposing a selective pressure on pollen colour that is in opposition to abiotic selection. Previous research in other C. americana populations supports our interpretation that pollen colour variation is not solely driven by neutral genetic structure. For example, nearly all populations have pollen colour variation, even small populations (Koski and Galloway, 2018). In addition, C. americana’s northward pattern of post-glacial migration would be expected to cause a latitudinal not longitudinal cline due to population genetic structure associated with migration (Barnard-Kubow et al., 2015).
In conclusion, our study suggests that opposing selection may maintain floral trait variation and contribute to observed geographic patterns in floral traits. Megachile are relatively inefficient pollinators of C. americana, preferentially visiting male-phase flowers and removing twice as much pollen as the other pollinators while depositing less than Bombus. Since Megachile have a strong and consistent preference for purple pollen, they are probably depleting purple pollen from natural populations. This preference may result in selection against plants with purple pollen. However, selection against purple pollen is opposed by abiotic selection favouring purple pollen since it is more heat resistant. These opposing selective forces may help to maintain pollen colour variation throughout C. americana’s range, with a prevalence of white and light-colour pollen in the eastern part of the range where abiotic selection is probably relaxed.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: sample sizes and phenotype information for the floral arrays. Table S2: fixed effects and least square means of the generalized mixed linear model for first flower a pollinator visited upon entering the array. Table S3: fixed effects and least square means of the generalized mixed linear model for first male-phase flower a pollinator visited upon entering the array. Table S4: least square means from the generalized mixed linear model for visitation across a foraging bout. Figure S1: pollen colour frequencies in the two natural populations, Chuckery Trail and Killbuck in OH, USA. Figure S2: the average Campanula americana petal and pollen colour of five colour morphs placed in hexagonal colour space for Osmia rufa (Megachilidae). Figure S3: the number of each pollinator group that visited arrays at Chuckery Trail and Killbuck populations. Figure S4: the number of first visits to a male-phase flower upon entering an array for the primary pollinator groups.
ACKNOWLEDGEMENTS
We thank S. Garcia and A. Padilla for field assistance; W. Crannage for plant care; R. J. Mitchell for assistance locating populations, and J. R. K. Forrest for insight on maintaining the bumble-bee colonies. This work was supported by NSF DEB 1457037 to L.F.G. and a supplemental ROA award to L.F.G., M.H.K. and J.L.I. Additional funding was provided through The College of Wooster’s Wilson and Copeland Awards.
LITERATURE CITED
- Austen EJ, Lin S-Y, Forrest JRK. 2018. On the ecological significance of pollen color: a case study in American trout lily (Erythronium americanum). Ecology 99: 926–937. [DOI] [PubMed] [Google Scholar]
- Barnard-Kubow KB, Debban CL, Galloway LF. 2015. Multiple glacial refugia lead to genetic structuring and the potential for reproductive isolation in a herbaceous plant. American Journal of Botany 102: 1842–1853. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annual Review of Entomology 46: 471–510. [DOI] [PubMed] [Google Scholar]
- Brown BA, Clegg MT. 1984. Influence of flower color polymorphism on genetic transmission in a natural population of the common morning glory, Ipomoea purpurea. Evolution 38: 796–803. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Bischoff M, Lord JM, Robertson AW. 2010. Flower color influences insect visitation in alpine New Zealand. Ecology 91: 2638–2649. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. 2003. Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon. Evolution 57: 2742–2752. [DOI] [PubMed] [Google Scholar]
- Chittka L, Menzel R. 1992. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. Journal of Comparative Physiology A 171: 171–181. [Google Scholar]
- Eckhart VM, Rushing NS, Hart GM, Hansen JD. 2006. Frequency-dependent pollinator foraging in polymorphic Clarkia xantiana ssp. xantiana populations: implications for flower colour evolution and pollinator interactions. Oikos 112: 412–421. [Google Scholar]
- Evanhoe L, Galloway LF. 2002. Floral longevity in Campanula americana (Campanulaceae): a comparison of morphological and functional gender phases. American Journal of Botany 89: 587–591. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. [Google Scholar]
- Foster JJ, Sharkey CR, Gaworska AVA, Roberts NW, Whitney HM, Partridge JC. 2014. Bumblebees learn polarization patterns. Current Biology 24: 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey FM, Williams CF. 2004. Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae). Evolution 58: 2426–2437. [DOI] [PubMed] [Google Scholar]
- Gigord LDB, Macnair MR, Smithson A. 2001. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proceedings of the National Academy of Sciences, USA 98: 6253–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology 48: 36–43. [Google Scholar]
- Jander U, Jander R. 2002. Allometry and resolution of bee eyes (Apoidea). Arthropod Structure & Development 30: 179–193. [DOI] [PubMed] [Google Scholar]
- Jones EC, Buchmann SL. 1974. Ultraviolet floral patterns as functional orientation cues in hymenopterous pollination systems. Animal Behaviour 22: 481–485. [Google Scholar]
- Jones KN, Reithel JS. 2001. Pollinator-mediated selection on a flower color polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). American Journal of Botany 88: 447–454. [PubMed] [Google Scholar]
- Jorgensen TH, Andersson S. 2005. Evolution and maintenance of pollen-colour dimorphisms in Nigella degenii: habitat-correlated variation and morph-by-environment interactions. New Phytologist 168: 487–498. [DOI] [PubMed] [Google Scholar]
- Jorgensen TH, Petanidou T, Andersson S. 2006. The potential for selection on pollen colour dimorphisms in Nigella degenii: morph-specific differences in pollinator visitation, fertilisation success and siring ability. Evolutionary Ecology 20: 291–306. [Google Scholar]
- Koski MH, Galloway LF. 2018. Geographic variation in pollen color is associated with temperature stress. New Phytologist 218: 370–379. [DOI] [PubMed] [Google Scholar]
- Koski MH, Grossenbacher DL, Busch JW, Galloway LF. 2017. A geographic cline in the ability to self-fertilize is unrelated to the pollination environment. Ecology 98: 2930–2939. [DOI] [PubMed] [Google Scholar]
- Koski MH, Ison JL, Padila A, Pham AQ, Galloway LF. 2018a Linking pollinator efficiency to patterns of pollen limitation: small bees exploit the plant–pollinator mutualism. Proceedings of the Royal Society B: Biological Sciences 285: 20180635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski MH, Kuo L, Niedermaier KM, Galloway LF. 2018b Timing is everything: dichogamy and pollen germinability underlie variation in autonomous selfing among populations. American Journal of Botany 105: 241–248. [DOI] [PubMed] [Google Scholar]
- Lau JA, Galloway LF. 2004. Effects of low-efficiency pollinators on plant fitness and floral trait evolution in Campanula americana (Campanulaceae). Oecologia 141: 577–583. [DOI] [PubMed] [Google Scholar]
- Lunau K. 1991. Innate flower recognition in bumblebees (Bombus terrestris, B. lucorum; Apidae): optical signals from stamens as landing reaction releasers. Ethology 88: 203–214. [Google Scholar]
- Maia R, Eliason CM, Bitton P-P, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Methods in Ecology and Evolution 4: 609–613. [Google Scholar]
- Mo Y, Nagel C, Taylor LP. 1992. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proceedings of the National Academy of Sciences, USA 89: 7213–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls E, Hempel de Ibarra N. 2014. Bees associate colour cues with differences in pollen rewards. Journal of Experimental Biology 217: 2783–2788. [DOI] [PubMed] [Google Scholar]
- Nicholls E, Hempel de Ibarra N, Nicolson Sue. 2017. Assessment of pollen rewards by foraging bees. Functional Ecology 31: 76–87. [Google Scholar]
- Okinaka Y, Shimada Y, Nakano-Shimada R, Ohbayashi M, Kiyokawa S, Kikuchi Y. 2003. Selective accumulation of delphinidin derivatives in tobacco using a putative flavonoid 3′,5′-hydroxylase cDNA from Campanula medium. Bioscience, Biotechnology, and Biochemistry 67: 161–165. [DOI] [PubMed] [Google Scholar]
- Olsen KM. 1996. Pollination effectiveness and pollinator importance in a population of Heterotheca subaxillaris (Asteraceae). Oecologia 109: 114–121. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Williams NM, Thomson JD. 2016. Specialist pollinators deplete pollen in the spring ephemeral wildflower Claytonia virginica. Ecology and Evolution 6: 5169–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, Souza J de, Ventura DF, Menzel R. 1992. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology A 170: 23–40. [DOI] [PubMed] [Google Scholar]
- Pernal SF, Currie RW. 2002. Discrimination and preferences for pollen-based cues by foraging honeybees, Apis mellifera L. Animal Behaviour 63: 369–390. [Google Scholar]
- Rebelo AG, Siegfried WR. 1985. Colour and size of flowers in relation to pollination of Erica species. Oecologia 65: 584–590. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. 1999. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proceedings of the National Academy of Sciences, USA 96: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemske DW, Horvitz CC. 1984. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225: 519–521. [DOI] [PubMed] [Google Scholar]
- Skorupski P, Chittka L. 2010. Photoreceptor spectral sensitivity in the bumblebee, Bombus impatiens (Hymenoptera: Apidae). PLoS One 5: e12049. doi: 10.1371/journal.pone.0012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ML. 1987. Reproductive biology of petal color variants in wild populations of Raphanus sativus: I. Pollinator response to color morphs. American Journal of Botany 74: 178–187. [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A. 2008. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. The Plant Journal 54: 733–749. [DOI] [PubMed] [Google Scholar]
- Thairu MW, Brunet J. 2015. The role of pollinators in maintaining variation in flower colour in the Rocky Mountain columbine, Aquilegia coerulea. Annals of Botany 115: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyford AD, Caola AM, Choudhary P, Raina R, Friedman J. 2018. Loss of color pigmentation is maintained at high frequency in a monkey flower population. American Naturalist 191: 135–145. [DOI] [PubMed] [Google Scholar]
- Wang X-Y, Quan Q-M, Wang B, Li Y-X, Huang S-Q. 2018. Pollen competition between morphs in a pollen-color dimorphic herb and the loss of phenotypic polymorphism within populations. Evolution 72: 785–797. [DOI] [PubMed] [Google Scholar]
- Wiermann R, Vieth K. 1983. Outer pollen wall, an important accumulation site for flavonoids. Protoplasma 118: 230–233. [Google Scholar]
- Winkel-Shirley B. 2002. Biosynthesis of flavonoids and effects of stress. Current Opinion in Plant Biology 5: 218–223. [DOI] [PubMed] [Google Scholar]
- Wright S. 1943. An analysis of local variability of flower color in Linanthus parryae. Genetics 28: 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylstra B, Touraev A, Moreno RMB, et al. 1992. Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiology 100: 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.