Abstract
Background and Aims
Fruit heteromorphism is considered to be a bet-hedging strategy to cope with spatially or temporally heterogeneous environments. The different behaviours of the fruit morphs of the same species might also be beneficial during naturalization, once the species has been introduced to a new range. Yet, no study to date has tested the association between fruit heteromorphism and global-scale naturalization success for a large set of plant species.
Methods
We compiled two large datasets on fruit heteromorphism in Asteraceae. One dataset was on native species in Central Europe (n = 321) and the other was on species frequently planted as ornamentals (n = 584). Using phylogenetic linear and logistic regressions, we tested whether heteromorphic species are more likely to naturalize outside their native range, and in more regions of the world than monomorphic species. We also tested whether the effect of heteromorphism is modulated by life history and height of the species.
Key Results
We show that heteromorphic species were more likely to naturalize outside their native range. However, among the naturalized species, heteromorphic and monomorphic species did not differ in the number of world regions where they became naturalized. A short life span and tall stature both promoted naturalization success and, when life history and height were included in the models, the effect of fruit heteromorphism on the ability to naturalize became non-significant. Nevertheless, among tall plants, heteromorphic ornamental species were significantly more likely to become naturalized in general and in more regions than monomorphic species.
Conclusions
Our results provide evidence that in Asteraceae the production of heteromorphic fruits is associated with naturalization success. It appears, however, that not fruit heteromorphism per se, but a successful combination of other biological traits in fruit heteromorphic species, namely short life span and tall stature, contributes to their naturalization success.
Keywords: Alien species, Asteraceae, Compositae, dispersal, fruit heteromorphism, heterocarpy, invasiveness, monocarpy, naturalization, non-native species, seed heteromorphism
INTRODUCTION
In some species, a single plant produces distinctly different types of seeds or fruits, a phenomenon called seed and fruit heteromorphism (also termed heterospermy and heterocarpy, respectively; Hannan, 1980; Venable, 1985; Imbert, 2002). This type of heteromorphism occurs in at least 26 families of angiosperms, and is most common in the Asteraceae and Chenopodiaceae (Lei et al., 2010; Doudová et al., 2017). In the Asteraceae, morphological differentiation mostly occurs between peripheral and central achenes (i.e. fruits) of the capitula (i.e. the flower heads) with differences in size, colour, shape and the presence/absence of dispersal structures (Imbert et al., 1997).
The potential ecological and evolutionary benefits of seed and fruit heteromorphism (hereafter called collectively ‘fruit heteromorphism’) are manifold. First, the fruit morphs usually differ in their dispersal ability. Pappus- or wing-bearing morphs in Asteraceae are usually lighter and can disperse over longer distances (disperser fruits sensuCheptou et al., 2008) compared with morphs without such appendages (non-disperser fruits). The distinct fruit morphs can also be dispersed by different vectors (Imbert, 2002), resulting again in different dispersal distances. Secondly, differences in seed and fruit anatomy such as pericarp thickness and structure, size of the embryo and embryo/pericarp mass ratio lead to variation in germination dynamics, because they influence water absorption and gas exchange of the seeds (Mohamed-Yasseen et al., 1994). Thus, in several heteromorphic Asteraceae species, disperser achenes from the centre of the capitulum germinate immediately when conditions are optimal, while germination of peripheral non-disperser achenes is delayed (Imbert, 1999; de Waal et al., 2016). Thirdly, hardness and chemical components of the fruit wall and seed coat differ between morphs and influence seed viability and dormancy, respectively (Imbert, 2002). Fourthly, due to differences in embryo size between morphs, distinct seedling survival, growth and tolerance spectra have been documented in several heteromorphic species. For example, in Crepis sancta, a European species of hawksbeard, offspring that are derived from peripheral achenes produce more biomass and have a higher competitive ability than offspring derived from central achenes (Imbert et al., 1997). These developmental and life history differences between fruit morphs suggest that heteromorphism has evolved as a bet-hedging strategy to cope with spatially or temporally heterogeneous environments (Imbert, 2002).
The potential ecological benefits of fruit heteromorphism are also likely to favour spread of introduced alien plant species in new regions, and could potentially contribute to the naturalization success of these species. During naturalization, when self-sustaining populations are established (sensuRichardson et al., 2000), heteromorphic species may better tolerate local disturbance and variation in availability of resources (Richardson and Pyšek, 2012) as a result of divergent dormancy and germination requirements of the seed morphs (Venable, 1985; Brändel, 2007; Doudová et al., 2017). During the subsequent spread phase, heteromorphism can contribute to dispersal via the disperser morphs and to establishment in different environments by the non-disperser fruits. Indeed, results of the handful of studies investigating the link between invasion success and fruit heteromorphism support these theoretical benefits (e.g. Rai and Tripathi, 1987; Sendek et al., 2015). However, no study has yet attempted to assess the generality of these findings by testing the association between heteromorphism and global naturalization success for a large set of species.
To assess the relationship between heteromorphism and naturalization success, we compiled two large and comprehensive datasets on heteromorphism in the Asteraceae. The first dataset focuses on Asteraceae species that are native to Central Europe and was used to assess their naturalization success outside of Europe. The other dataset consists of Asteraceae species that are widely used as garden plants outside their native range. For all species, we assessed their naturalization success worldwide (van Kleunen et al., 2015). Moreover, as it is often argued but rarely tested (Küster et al., 2008; Feng et al., 2016) that the role of species traits in invasion success depends on other traits (Kueffer et al., 2013), we also included several other traits in the analyses. We focused here on two traits, life history and plant height, because they were available for most species in our datasets and have been linked to invasion success in previous studies (e.g. Pyšek and Richardson, 2007; Razanajatovo et al., 2016). In addition, we collected for all species data on whether the species produce disperser fruits (i.e. produce achenes with structures that promote anemochory or epizoochory) or not, as this may be confounded with fruit heteromorphism. We asked the following main questions. (1) Are fruit heteromorphic Asteraceae species more likely to become naturalized somewhere in the world than monomorphic species? (2) Among species that are naturalized, are the species with fruit heteromorphism naturalized in more regions than monomorphic species? (3) Do the effects of fruit heteromorphism on naturalization success depend on life history and height of the species?
MATERIALS AND METHODS
Compilation of datasets
We focused on Asteraceae (Compositae) because it is the most speciose plant family, includes in absolute terms the highest number of naturalized species worldwide (Pyšek et al., 2017) and comprises by far the most species with fruit heteromorphism (Imbert, 2002). Moreover, several of the most widely naturalized species in the world are Asteraceae (Pyšek et al., 2017), and the family includes many highly invasive alien species (Weber, 1997; Pyšek, 1998; Lambdon et al., 2008). We collated the two datasets.
To test whether fruit heteromorphic species are more likely to become naturalized than monomorphic species, we used a ‘source area approach’ (Pyšek et al., 2004, 2015; van Kleunen et al., 2010). We focused on Asteraceae species that are native to three countries in Central Europe – Germany, the Czech Republic and Slovakia – a geographically well-defined region with a good availability of data on fruit heteromorphism. First, we extracted all Asteraceae species for which detailed information on the presence/absence of fruit heteromorphism was available (665 species) from the D3 database (Diaspore Dispersal Database, http://www.seed-dispersal.info, accessed 2 December 2015; Hintze et al., 2013). From this list, we selected species that are native to the three Central European countries based on the BiolFlor database of the German flora (Kühn et al., 2004), and the checklists of vascular plants of the Czech Republic (Danihelka et al., 2012; Pyšek et al., 2012) and Slovakia (Medvecká et al., 2012). The final list consisted of 328 species. Their life history (annual, biennial or perennial) and maximum plant height were extracted from the FloraWeb database, an Internet portal of the German Federal Agency for Nature Conservation (BfN; www.floraweb.de, accessed 2 December 2015) and from the Flora Europaea (Tutin et al., 1976). In total, we had complete trait data for 321 of the 328 species in this dataset.
The approach of focusing on a single source area reduces variation in the chance of dispersal by humans from different source areas (Pyšek et al., 2004, 2015). Nevertheless, some species from the same source area may not have been introduced elsewhere and thus have not had the opportunity to naturalize. Therefore, and because generally most of the naturalized species have been introduced for ornamental horticulture (Reichard and White, 2001; van Kleunen et al., 2018b), we also compiled a dataset of Asteraceae that are frequently planted as ornamental garden plants. For this, we extracted a list of 864 species from the European Garden Flora (Cullen et al., 2011). This encyclopedia includes >20 000 ornamental plant species planted in European gardens. It is, however, likely that most of these species are also grown as ornamentals in other parts of the world due to intensive international trade in ornamental plants (Dehnen-Schmutz et al., 2007; Bradley et al., 2012; van Kleunen et al., 2018b). For data on fruit heteromorphism, life history and height of the species, we screened the D3 and BiolFlor databases, and the scientific literature. Based on this extensive internet search, we found detailed information on achene morphology and height data for 584 of the 864 species. The native ranges of these species are highly heterogeneous, covering most of the continents, but most species are native to North America and Europe.
The fruit monomorphic Asteraceae species are highly diverse regarding their dispersal ability, as some of the species have specialized fruit appendages for long-distance dispersal, while the others have no or only rudimentary appendages. Similarly, there are also some fruit heteromorphic species in which none of the fruit morphs has appendages that enable long-distance dispersal. To be able to account for this potential confounding, we also collated dispersability data of the species. Following the D3 database, we classified species as ‘dispersers’ when they produce fruits that are adapted for anemochory or epizoochory by having specialized appendages (wings, elongated or hooked appendages), and we classified species as ‘non-dispersers’ when they produce fruits without such appendages or if they have only rudimentary structures. We used the D3 database and various scientific literature with detailed morphological descriptions of the fruits to obtain these data.
To characterize the performance of the species as naturalized aliens, we used the Global Naturalized Alien Flora (GloNAF) database, version 1.1 (van Kleunen et al., 2015; Pyšek et al., 2017). This database provides information on the naturalized occurrence of >13 000 vascular plant species in 843 non-overlapping regions, covering approx. 83 % of the terrestrial area of the world (van Kleunen et al., 2015; Pyšek et al., 2017). Following (Razanajatovo et al., 2016), we used two proxies of naturalization success: (1) incidence of naturalization is a presence/absence variable that describes whether a species is naturalized in at least one GloNAF region outside its native range; and (2) extent of naturalization is a continuous variable calculated as the number of GloNAF regions in which the species is reportedly present as naturalized. An alternative measure of the extent of naturalization is the cumulative area of the regions, but, as this is strongly correlated with the number of regions (Pyšek et al., 2017), we used only the number of regions.
Phylogeny
To test whether there is a phylogenetic signal in fruit heteromorphism, and to account for phylogenetic non-independence among the Asteraceae species in the analyses of naturalization success, we constructed phylogenetic trees for our two datasets. As a basis for these trees, we used the largest seed plant phylogeny presently available (the ALLMB tree of Smith and Brown, 2018). To build this dated tree, Smith and Brown (2018) combined genetic data of seed plants from public repositories (GenBank) with phylogenetic data (Open Tree of Life project). We first pruned the ALLMB to contain only those species that also occur in our two datasets, and then the species from our datasets that were missing from the resulting trees were added manually as polytomies at the genus level.
Statistical analysis
To test for a significant phylogenetic signal in fruit heteromorphism (binary variable; heteromorphic: yes or no), we calculated the D statistic, which has been developed for binary data by Fritz and Purvis (2010), by using the phylo.d function in the ‘caper’ package (Orme et al., 2013) of R (R Core Team, 2017). A D-statistic value <1 implies that related taxa resemble each other less than expected (regarding fruit heteromorphism) under the Brownian motion model of evolution, and D = 0 indicates the random expectation (i.e. no phylogenetic signal) (Fritz and Purvis, 2010). Values of D <0 indicate that the binary trait is phylogenetically more conserved than expected under the Brownian model (Orme et al., 2013). The phylo.d function also provided significance tests for departure from a random model (Prand) and from the Brownian motion model (PBM).
We used similar analyses for the native Central European Asteraceae dataset and for the ornamental Asteraceae dataset. To test whether fruit heteromorphism (heteromorphic: yes, no) has an effect on the incidence of naturalization (binary variable, i.e. whether a species has or has not been recorded as naturalized outside its native range), we first carried out a Pearson’s χ2 test of contingency. Then, to account for phylogeny, we used phylogenetic logistic regression as implemented in the phyloglm function of the package ‘phylolm’ (Ho and Ané, 2014) with a binomial error distribution. Finally, to analyse the effects of all three biological traits (fruit heteromorphism, life history and height) on the incidence of naturalization in the same model, we used generalized linear models (GLMs) and phylogenetic logistic regressions. In these models, fruit heteromorphism and life history (annual/biennial or perennial) were entered as fixed factors, and maximum height as a continuous covariate standardized to a mean of 0 and an s.d. of 1. As there were only few biennial species in our list, we merged annual and biennial species into a single category. To test whether the effect of fruit heteromorphism depends on the other traits, we included pairwise interactions between fruit heteromorphism and the other two traits.
To test for the effect of fruit heteromorphism on the extent of naturalization (continuous variable, natural log-transformed number of regions where the species is naturalized) once a species is naturalized in at least one region, we excluded all non-naturalized species. We first ran linear models (LMs) and phylogenetic linear regressions using the phylolm function of the package ‘phylolm’ (Ho and Ané, 2014) in R with fruit heteromorphism as a fixed factor. Then, we built LMs and phylogenetic linear regression models to test for the effect of all three biological traits in the same model, where the same explanatory variables were used as in the phylogenetic logistic regressions described above for the analysis of the incidence of naturalization.
Most of the heteromorphic species produce at least one disperser morph, whereas the monomorphic species produce either disperser or non-disperser morphs. This implies that the effect of fruit heteromorphism could be confounded with whether or not the species produces any disperser fruits. Therefore, we reran all the analyses using only the disperser species of the monomorphic and heteromorphic groups, and present the results in Supplementary Data Tables S1 and S2.
To evaluate potential problems arising from collinearity of explanatory variables, we calculated variance-inflation factors (VIFs) for each variable in each model using the vif() function from the package ‘car’ in R. VIFs for all models were <3, far less than the threshold of 5 above which collinearity may adversely affect regression results (Rogerson, 2001). To measure the variation explained by our phylogenetic models, the likelihood ratio R2lr was calculated as implemented in the ‘rr2’ package in R (Ives, 2017). We also calculated the relative importance of each variable (R2par) as the difference in deviance between the full model and a model without the variable of interest. All analyses were performed using the R statistical environment, version 3.4.2 (R Core Team, 2017).
RESULTS
Fruit heteromorphism and naturalization success of Central European Asteraceae
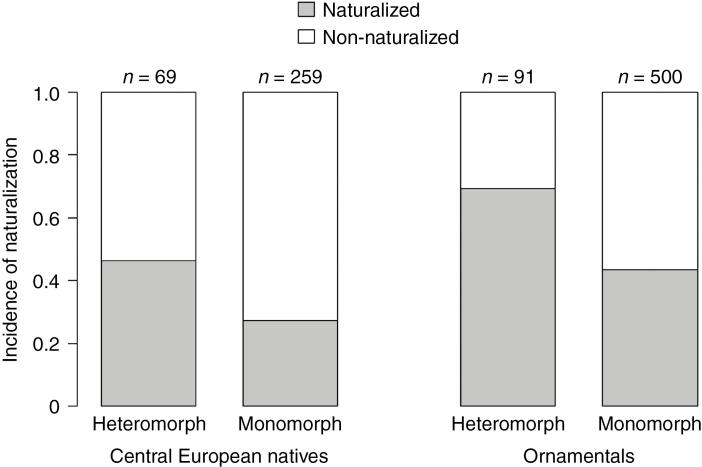
Among the 328 species in our dataset of Asteraceae native to Central Europe, 69 species (i.e. 21 %) are fruit heteromorphic. The percentage of heteromorphic species that have become naturalized outside of Europe (46 %; 32 out of 69) was significantly higher than for monomorphic species (27 %; 71 out of 259; Table 1; Fig. 1).
Table 1.
Effects of fruit heteromorphism vs. monomorphism on the incidence of naturalization (expressed as being naturalized outside of Europe or not) and extent of naturalization (natural log-transformed number of regions where the species is naturalized) of native Central European Asteraceae species (Models 1), and effects of heteromorphism vs. monomorphism, annual/biennial vs. perennial life history, plant height and pair-wise interactions between heteromorphism and the other two traits on the same response variables (Models 2)
| Response variables | Incidence of naturalization | Extent of naturalization (log) | ||||
|---|---|---|---|---|---|---|
| Explanatory variables | χ 2 test/GLM | Phylo GLM | R 2 par | LM | Phylo LM | R 2 par |
| Models 1 | n = 328, R2lr = 0.051 | n = 103, R2lr = 0.029 | ||||
| Fruit heteromorphism (FHM) | 8.236** | 2.580** | 0.045 | 1.175 | 1.162 | 0.013 |
| Models 2 | n = 321, R2lr = 0.195 | n = 102, R2lr = 0.079 | ||||
| FHM | –0.838 | –0.263 | 0.001 | –0.450 | –0.248 | 0.005 |
| Life history | –4.386*** | –4.376*** | 0.091 | –1.867 | –1.940* | 0.031 |
| Plant height | 2.648** | 2.564* | 0.047 | 0.122 | –0.297 | 0.000 |
| FHM × life history | 1.490 | 1.765 | 0.012 | 0.767 | 0.880 | 0.007 |
| FHM × plant height | 0.186 | –0.393 | 0.000 | 0.715 | 0.957 | 0.008 |
Pearson’s χ2 test of contingency in univariate models and generalized linear models (GLMs) in multivariate models, followed by phylogenetic logistic regressions, were used in the case of incidence of naturalization (z statistics are shown), and linear models (LMs) followed by phylogenetic linear regressions in the case of extent of naturalization (t statistics are shown) in both models.
Asterisks denote significance at *P < 0.05, **P < 0.01, ***P < 0.001.
Total explained variance of the models (R2lr), and the partial variances explained by each variable (R2par) are also shown.
Sample size refers to the total number of species in the analysed datasets.
Fig. 1.
Frequency of species naturalized somewhere according to the GloNAF database (i.e. incidence of naturalization) among fruit heteromorphic vs. fruit monomorphic species of Central European native Asteraceae species and ornamental Asteraceae species. Sample sizes (n) refer to the number of species in different species groups.
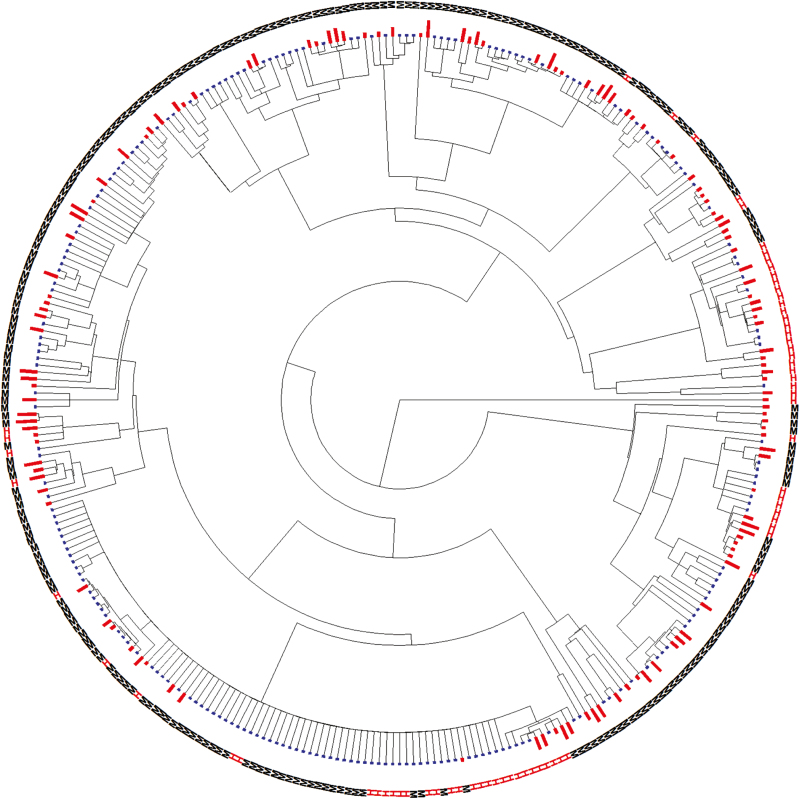
Fruit heteromorphism was significantly phylogenetically clustered (D = –0.038, Prand < 0.001; PBM = 0.571; Fig. 2), but the difference in naturalization incidence between heteromorphic and monomorphic Central European Asteraceae remained significant in a phylogenetic logistic regression (Table 1). However, for the sub-set of 103 species that have become naturalized outside Europe, heteromorphic species were not naturalized in more regions than monomorphic species (Table 1).
Fig. 2.
Phylogenetic tree of 328 Central European native Asteraceae species. Fruit heteromorphic species are denoted with a red ‘H’ and monomorphic species with a black ‘M’. The red bars beside the letters are for species that are naturalized somewhere outside of Europe (i.e. naturalization incidence >0); blue bars are for species not known to be naturalized outside Europe. The sizes of the red bars are proportional to the log10(+2) of the number of GloNAF regions (i.e. extent of naturalization). A high-resolution version of the same tree that also shows the names of all species can be seen as Supplementary Data Fig. S1.
Fruit heteromorphism was significantly more frequent among annual/biennial species than among perennial species, and was also significantly associated with tall species (Supplementary Data Figs S3 and S4). Consequently, when including both life history and plant height in the models, the significant effect of fruit heteromorphism on the incidence of naturalization disappeared (Table 1). Life history significantly influenced the incidence and extent of naturalization achieved by the species. Among the annual/biennial species, 70 % have become naturalized outside of Europe, compared with only 25 % of the perennial species (Table 1). Similarly, among the 102 species that have become naturalized, the extent of naturalization was significantly higher for annual/biennial species compared with perennials (Table 1). Plant height had a significantly positive effect on the incidence of naturalization of Central European Asteraceae species outside of Europe, but not on the extent of naturalization (Table 1). When we restricted the dataset to those 289 species with long-distance dispersal ability, we found highly similar results (Supplementary Data Table S1).
Fruit heteromorphism and naturalization success of ornamental Asteraceae
Among the 591 ornamental Asteraceae listed in the European Garden Flora, 91 species (i.e. 15 %) are heteromorphic. The percentage of heteromorphic ornamental Asteraceae that have become naturalized somewhere in the world (69 %; 63 out of 91) was significantly higher than for monomorphic ornamental Asteraceae (44 %; 218 out of 500; Table 2; Fig. 1).
Table 2.
Effects of fruit heteromorphism vs. monomorphism on the incidence of naturalization (expressed as being naturalized somewhere in the world or not) and extent of naturalization (natural log-transformed number of regions where the species is naturalized) of ornamental Asteraceae species (Models 1), and effect of heteromorphism vs. monomorphism, annual/biennial vs. perennial life history, plant height and pair-wise interactions between heteromorphism and the other two traits on the same response variables (Models 2).
| Response variables | Incidence of naturalization | Extent of naturalization (log) | ||||
|---|---|---|---|---|---|---|
| Explanatory variables | χ 2/GLM | Phylo GLM | R 2 par | LM | Phylo LM | R 2 par |
| Models 1 | n = 591, R2lr =0.123 | n = 281, R2lr = 0.020 | ||||
| Fruit heteromorphism (FHM) | 19.26*** | 4.100*** | 0.085 | 0.931 | 0.668 | 0.001 |
| Models 2 | n = 584, R2lr = 0.179 | n = 279, R2lr = 0.100 | ||||
| FHM | 2.208* | 2.824** | 0.003 | 1.134 | 0.922 | 0.000 |
| Life history | –4.983*** | –3.323*** | 0.038 | –3.409*** | –3.317** | 0.052 |
| Plant height | 3.532*** | 4.007*** | 0.107 | –0.075 | 0.099 | 0.001 |
| FHM × life history | 0.506 | 0.342 | 0.000 | –0.742 | –0.564 | 0.001 |
| FHM × plant height | 2.223* | 2.976** | 0.029 | 2.516* | 2.879** | 0.032 |
Pearson’s χ2 test of contingency in univariate models and generalized linear models (GLMs) in multivariate models, followed by phylogenetic logistic regressions, were used in the case of incidence of naturalization (z statistics are shown), and linear models (LMs) followed by phylogenetic linear regressions in the case of extent of naturalization (t statistics are shown) in both models.
Asterisks denote significance at *P < 0.05, **P < 0.01, ***P < 0.001.
Total explained variance of the models (R2lr), and the partial variances explained by each variable (R2par) are also shown.
Sample size refers to the total number of species in the analysed datasets.
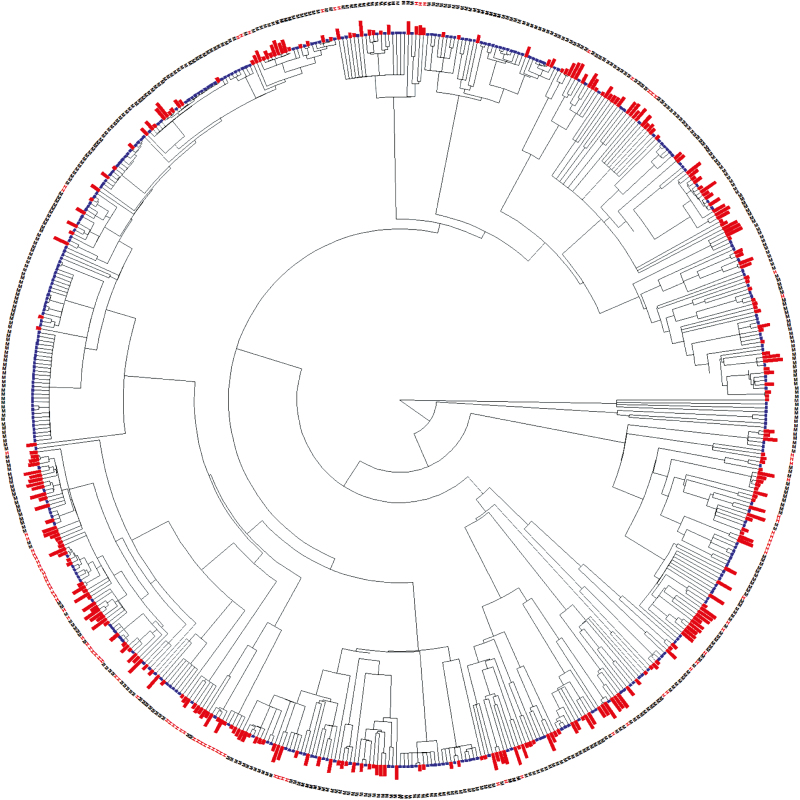
As for the Central European Asteraceae, fruit heteromorphism among ornamental Asteraceae showed a significant phylogenetic clustering (D = 0.164; Prand < 0.001; PBM = 0.149; Fig. 3), but the difference in the incidence of naturalization between heteromorphic and monomorphic ornamental Asteraceae remained significant in a phylogenetic logistic regression (Table 2). However, for the sub-set of 281 ornamental Asteraceae that have become naturalized somewhere in the world, fruit heteromorphism had no significant effect on their extent of naturalization (Table 2).
Fig. 3.
Phylogenetic tree of 591 ornamental Asteraceae species. Fruit heteromorphic species are denoted with a red ‘H’ and monomorphic species with a black ‘M’. The red bars beside the letters are for species that are naturalized somewhere outside of Europe (i.e. naturalization incidence >0); blue bars are for species not known to be naturalized somewhere. The sizes of the red bars are proportional to the log10(+2) of the number of GloNAF regions (i.e. extent of naturalization). A high-resolution version of the same tree that also shows the names of all species can be seen as Supplementary Data Fig. S2.
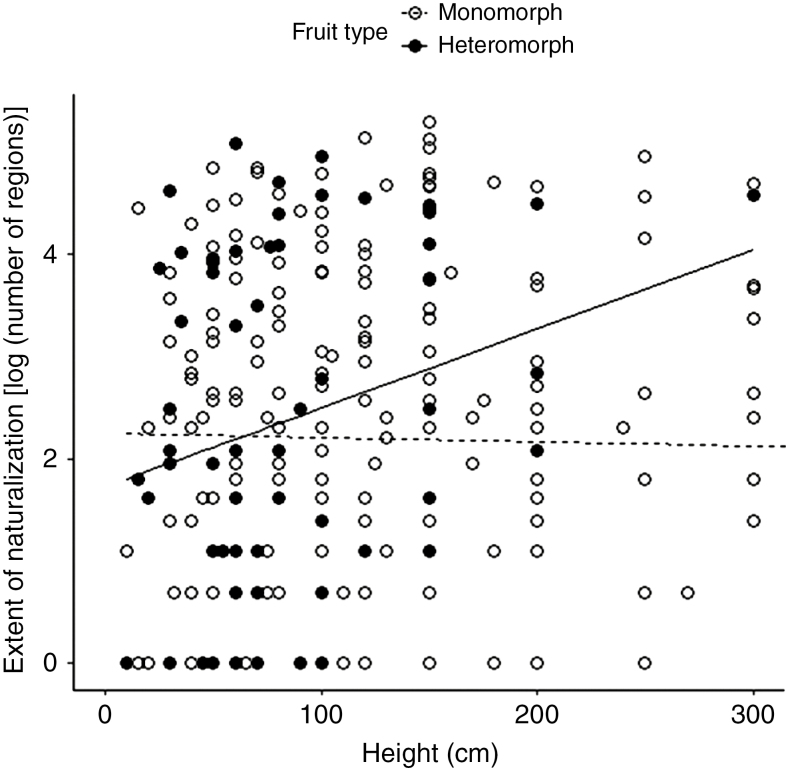
For the ornamental Asteraceae, fruit heteromorphism was also significantly more frequent among annual/biennial species than among perennial species (Supplementary Data Fig. S3). However, in contrast to the Central European Asteraceae, ornamental species with fruit heteromorphism were not taller than monomorphic species (Supplementary Data Fig. S4). In this dataset, the positive effect of fruit heteromorphism on the incidence of naturalization of ornamental Asteraceae remained significant (Table 2) when we included both life history and height in the models, but it explained only a small amount of variation (0.3 % out of 17 % of the full model). Plant height had a significant positive effect on the incidence of naturalization, but not on its extent (Table 2). The effect of fruit heteromorphism on the incidence of naturalization became stronger with increasing plant height (Table 2). Similarly, although there was no significant main effect of fruit heteromorphism on the extent of naturalization, the effect of heteromorphism became stronger with increasing plant height (Table 2; Fig. 4). Among the annual/biennial species, 82 % are naturalized somewhere in the world, while among the perennial species, the corresponding figure is only 42 % (Table 2). Among the 279 species that have become naturalized, the extent of naturalization was significantly higher for annual/biennial species compared with perennials (Table 2). When we restricted the ornamental Asteraceae dataset to those 441 species with long-distance dispersal ability, the results remained largely the same (Supplementary Data Table S2). However, the positive effect of fruit heteromorphism on the incidence of naturalization disappeared in the model with both life history and height (Supplementary Data Table S2).
Fig. 4.
Extent of naturalization (natural log-transformed number of regions where the species is naturalized) of ornamental Asteraceae species plotted against plant height for fruit heteromorphic and monomorphic species. Each dot in the scatter plot represents a species.
DISCUSSION
In this study, we provide the first multispecies test of whether fruit heteromorphism promotes global naturalization success of plant species. We show that within the two Asteraceae datasets analysed, fruit heteromorphism is phylogenetically conserved, and associated with the incidence of naturalization, but not with its extent. In the dataset of Central European native Asteraceae, the effect of fruit heteromorphism on the incidence of naturalization disappeared when we included life history and plant height in the model, suggesting a possible confounding effect between heteromorphism and the other traits. However, in the dataset of ornamental Asteraceae, the effect of fruit heteromorphism on the incidence of naturalization remained significant after including the other species traits, although it explained far less variation in the incidence of naturalization than life history and plant height. Interestingly, among ornamental species with high stature, the heteromorphic species are significantly more likely to become naturalized and in more regions than monomorphic species, whereas this was not the case among short species. This indicates that fruit heteromorphism per se has only a moderate effect on the naturalization success of Asteraceae species, but that for tall plants it can significantly enhance naturalization success.
Fruit heteromorphism: another small piece to the naturalization success puzzle
Many studies have been conducted to identify traits associated with invasion success, but few universal patterns have emerged due to the inherent idiosyncrasies of invasions (van Kleunen and Richardson, 2007; Pyšek and Richardson, 2007; Kueffer et al., 2013; Pyšek et al., 2015; Maurel et al., 2016; van Kleunen et al., 2018a). The use of a relatively homogeneous set of species regarding their phylogeny, life history and origin or introduction history might allow detection of traits that promote global invasion success and might contribute to a more mechanistic understanding of the influences of traits in alien plant naturalization (Richardson et al., 2011; Canavan et al., 2018). It is also important to discern traits that contribute to invasion success in different phases such as introduction, establishment (naturalization) and spread. Richardson and Pyšek (2012) suggested that the determinants of naturalization success are more robust than those of invasiveness, as invasive alien species also require effective dispersal, which introduces an additional set of complex biological traits and processes. In addition, the definition of naturalization is much more consistent across studies compared with that of invasiveness, which made it possible to build a relatively comprehensive global database of naturalized species (van Kleunen et al., 2015; Pyšek et al., 2017).
Certain biological traits have already been linked to naturalization success, such as selfing ability (Razanajatovo et al., 2016), high fecundity (Moravcová et al., 2010, 2015), rapid and profuse seedling emergence (van Kleunen and Johnson, 2007; Gioria and Pyšek, 2017), ability to build a persistent seed bank (Gioria et al., 2012; Gioria and Pyšek, 2015; Pyšek et al., 2015), clonal growth (Milbau and Stout, 2008), cold hardiness (Maurel et al., 2016), long flowering time (Pyšek et al., 2009, 2015) and large specific leaf area (Lavoie et al., 2016). Many of these traits are related to reproduction, but the role of fruit (or seed) heteromorphism in naturalization success had never been explicitly tested before, despite straightforward theoretical arguments supporting its potential role. We found that fruit heteromorphism was significantly associated with naturalization success of Asteraceae species. The explanatory power of fruit heteromorphism on the incidence of naturalization, however, was only 3.7 % for Central European native and 5.7 % for ornamental Asteraceae species in univariate models. These low values are similar to those of other significant invasiveness traits in single-trait studies (Küster et al., 2008; Klonner et al., 2016) or in other ecological and evolutionary studies (Møller and Jennions, 2002). The relatively small contribution of single traits is not surprising given that many other traits, as well as ecological, evolutionary and historical factors (e.g. propagule pressure and time since introduction), play a role in naturalization success (van Kleunen et al., 2018a).
Based on our two Asteraceae datasets, we cannot tell whether fruit heteromorphism plays a role in progression of species along the naturalization–invasion continuum (sensuRichardson et al., 2000; Blackburn et al., 2011). The number of regions where a species has been naturalized, i.e. the extent of naturalization, might indicate the spreading ability of a species, although it is not equivalent to established definitions of invasiveness (e.g. having high potential to spread over a considerable area sensuRichardson et al., 2000). Therefore, the fact that the extent of naturalization was not affected by fruit heteromorphism does not rule out the possibility that fruit heteromorphism might contribute to invasiveness. We assume that the mixed strategy of heteromorphic species might simultaneously contribute to increasing population sizes by local dispersal and also to long-distance dispersal during the invasion phase. The high variation in seed characteristics (mass, dispersal, germination and dormancy) can at least partially explain the successful colonization of heterogeneous environments by certain invasive alien species (Willis and Hulme, 2004; Fumanal et al., 2007). Together with our findings, this suggests that fruit heteromorphism predisposes species to become naturalized and to spread successfully under a wide range of environmental conditions.
Fruit heteromorphism, life history and plant height
Traits often do not evolve independently from each other. Consequently, we also considered life history and plant height as two other traits that might be correlated with fruit heteromorphism or mediate its effects on naturalization success. Including more life history and functional traits would be desirable as it would improve the predictive power of our models. However, as data on most traits are only available for a few species, including more traits would have considerably reduced the number of species involved in our analyses. In line with previous studies (Plitmann, 1986; Imbert, 2002), we found that the frequency of short-lived (i.e. annual and biennial) species among fruit heteromorphic species is significantly higher than among monomorphic species (Supplementary Data Fig. S3). In addition, our results show a greater naturalization success for annuals and biennials than for perennials in both Asteraceae datasets, which is also in line with earlier studies (e.g. Razanajatovo et al., 2016). Short-lived plants often flourish in early successional, highly disturbed habitats (Pianka, 1970; Prach et al., 2017), resulting in a stronger predominance of the ruderal adaptive strategy among naturalized than among non-naturalized plants (Guo et al., 2018). Disturbed habitats have increased in the last centuries due to conversion of natural habitats into arable fields and ruderal sites, types of habitat that have contributed many species to the global naturalized flora (Kalusová et al., 2017).
Regarding plant height, our results are also in line with previous studies analysing the effect of different traits on invasion success of plants: the taller a plant species is, the higher the chance that it becomes a successful alien species (Pyšek and Richardson, 2007). As fruit heteromorphic species were significantly taller than monomorphic species in the dataset of Central European natives, this confounding factor might explain why the main effect of fruit heteromorphism was not significant when we included plant height in the analysis. In summary, our results suggest that the tall stature and high frequency of annual/biennial life history among heteromorphic species rather than fruit heteromorphism per se may have contributed to their naturalization success.
In contrast, the confounding effect of height and heteromorphism was less strong in the dataset of ornamental Asteraceae, as in that dataset there were no significant height differences between heteromorphic and monomorphic species. Nevertheless, fruit heteromorphism was shown to have considerable explanatory power only in interaction with plant height. Heteromorphic species with a high stature had greater naturalization success than monomorphic species. The synergistic effect of height and the presence of fruit heteromorphism in ornamental Asteraceae species might be explained by the fact that one of the morphs is usually wind dispersed, and that wind dispersal distance is strongly correlated with plant height (Tackenberg, 2003; Thomson et al., 2011). Indeed, when we took into account only the disperser species, the significant effect of heteromorphism (both as a main effect and in interaction with plant height) disappeared, as both groups require high stature for efficient dispersal. Efficient dispersal might be beneficial in the naturalization phase as it could reduce kin competition and potential inbreeding (de Waal et al., 2016), and contributes to the establishment of new populations in more suitable environments.
Conclusions
Our results provide evidence that the production of heteromorphic fruits is associated with naturalization success in Asteraceae, the largest of all plant families with fruit heteromorphism. Although the number of families with fruit heteromorphism is relatively small (reported in 26 families), future studies should test whether fruit or seed heteromorphic species from other families have similarly high chances of becoming naturalized to those in the Asteraceae. Moreover, as cryptic heteromorphism (seed or fruits of a single plant with distinct ecological differences without obvious morphological differences) is likely to be an underestimated phenomenon, more research is needed to examine the role of intraindividual variation in seed characteristics in invasion biology, and in ecology in general.
Although we found a correlation between fruit heteromorphism and naturalization success, it appears that it is a particularly successful combination of other biological traits in fruit heteromorphic species that contributes to naturalization success. These results do not diminish the importance of fruit heteromorphism as a trait associated with naturalization success. Instead, our results indicate that plant traits evolve synergistically, and that fruit heteromorphic species possess a particular combination of traits that makes them successful alien species. Therefore, our findings highlight the importance of compiling and analysing multivariate datasets when testing for characteristics that promote plant invasion to reveal how the contribution of one plant trait depends on other traits.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: the effect of fruit heteromorphism, life history and plant height on naturalization success in Central European native Asteraceae with fruits capable of long-distance dispersal. Table S2: the effect of fruit heteromorphism, life history and plant height on naturalization success in ornamental Asteraceae with fruits capable of long-distance dispersal. Figure S1: phylogenetic tree of 328 Central European native Asteraceae species showing the incidence and extent of naturalization of fruit heteromorphic and monomorphic species. Figure S2: phylogenetic tree of 591 ornamental Asteraceae species showing the incidence and extent of naturalization fruit of heteromorphic and monomorphic species. Figure S3: comparing the frequency of different life histories in fruit heteromorphic and monomorphic species. Figure S4: maximum height of fruit heteromorphic vs. monomorphic species in Central European native and ornamental Asteraceae species.
FUNDING
This work was supported by the Autoritatea Natională pentru Cercetare Stiintifică | Unitatea Executiva pentru Finantarea Invatamantului Superior, a Cercetarii, Dezvoltarii si Inovarii, CNCS – UEFISCDI [grant no. PN-III-P1-1.1-PD-2016-0731, within PNCDI III to A.F.], Academy of Sciences of the Czech Republic [grant no. 14-36079G to P.P. and J.P. and Praemium Academiae award to P.P.], Centre of Excellence PLADIAS (Czech Science Foundation) [grant no. RVO 67985939 to P.P. and J.P.], the Austrian Science Fund FWF [grant no. I2086-B16 to F.E.] and the Deutsche Forschungsgemeinschaft [grant no. 264740629 to M.v.K.; iDiv via FZT 118 to M.W.].
ACKNOWLEDGEMENTS
We thank Jiří Danihelka (Průhonice) for consultation on the origin and status of the Central European species, and two anonymous reviewers for their helpful comments and suggestions.
LITERATURE CITED
- Blackburn TM, Pyšek P, Bacher S, et al. 2011. A proposed unified framework for biological invasions. Trends in Ecology and Evolution 26: 333–339. [DOI] [PubMed] [Google Scholar]
- Bradley BA, Blumenthal DM, Grosholz ED, Lawler JJ. 2012. Global change, global trade, and the next wave of plant invasions. Frontiers in Ecology and the Environment 10: 20–28. [Google Scholar]
- Brändel M. 2007. Ecology of achene dimorphism in Leontodon saxatilis. Annals of Botany 100: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan S, Meyerson LA, Packer JG, et al. 2018. Tall-statured grasses: a useful functional group for invasion science. Biological Invasions. doi: 10.1007/s10530-018-1815-z. [DOI] [Google Scholar]
- Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences, USA 105: 3796–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen J, Knees SG, Cubey HS (eds). 2011. The European garden flora. Flowering plants: a manual for the identification of plants cultivated in Europe, both out-of-doors and under glass. Cambridge/New York: Cambridge University Press. [Google Scholar]
- Danihelka J, Chrtek J, Kaplan Z. 2012. Checklist of vascular plants of the Czech Republic. Preslia 84: 647–811. [Google Scholar]
- Dehnen-Schmutz K, Touza J, Perrings C, Williamson M. 2007. The horticultural trade and ornamental plant invasions in Britain. Conservation Biology 21: 224–231. [DOI] [PubMed] [Google Scholar]
- Doudová J, Douda J, Mandák B. 2017. The complexity underlying invasiveness precludes the identification of invasive traits: a comparative study of invasive and non-invasive heterocarpic Atriplex congeners. PLoS One 12: e0176455. doi: 10.1371/journal.pone.0176455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Maurel N, Wang Z, Ning L, Yu FH, van Kleunen M. 2016. Introduction history, climatic suitability, native range size, species traits and their interactions explain establishment of Chinese woody species in Europe. Global Ecology and Biogeography 25: 1356–1366. [Google Scholar]
- Fritz SA, Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conservation Biology 24: 1042–1051. [DOI] [PubMed] [Google Scholar]
- Fumanal B, Chauvel B, Sabatier A, Bretagnolle F. 2007. Variability and cryptic heteromorphism of Ambrosia artemisiifolia seeds: what consequences for its invasion in France? Annals of Botany 100: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioria M, Pyšek P. 2015. The legacy of plant invasions: changes in the soil seed bank of invaded plant communities. BioScience 66: 40–53. [Google Scholar]
- Gioria M, Pyšek P. 2017. Early bird catches the worm: germination as a critical step in plant invasion. Biological Invasions 19: 1055–1080. [Google Scholar]
- Gioria M, Pyšek P, Moravcova L. 2012. Soil seed banks in plant invasions: promoting species invasiveness and long-term impact on plant community dynamics. Preslia 84: 327–350. [Google Scholar]
- Guo WY, van Kleunen M, Winter M, et al. 2018. The role of adaptive strategies in plant naturalization. Ecology Letters 21: 1380–1389. [DOI] [PubMed] [Google Scholar]
- Hannan GL. 1980. Heteromericarpy and dual seed germination modes in Platystemon californicus (Papaveraceae). Madroño 27: 164–170. [Google Scholar]
- Hintze C, Heydel F, Hoppe C, Cunze S, König A, Tackenberg O. 2013. D3: The Dispersal and Diaspore Database – baseline data and statistics on seed dispersal. Perspectives in Plant Ecology, Evolution and Systematics 15: 180–192. [Google Scholar]
- Ho LST, Ané C. 2014. A linear-time algorithm for gaussian and non-gaussian trait evolution models. Systematic Biology 63: 397–408. [DOI] [PubMed] [Google Scholar]
- Imbert E. 1999. The effects of achene dimorphism on the dispersal in time and space in Crepis sancta (Asteraceae). Canadian Journal of Botany 77: 508–513. [Google Scholar]
- Imbert E. 2002. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology Evolution and Systematics 5: 13–36. [Google Scholar]
- Imbert E, Escarre J, Lepart J. 1997. Seed heteromorphism in Crepis sancta (Asteraceae): performance of two morphs in different environments. Oikos 79: 325–332. [Google Scholar]
- Ives A. 2017. R2s for correlated data: phylogenetic models, LMMs, and GLMMs. bioRxiv144170. [DOI] [PubMed] [Google Scholar]
- Kalusová V, Chytrý M, van Kleunen M, et al. 2017. Naturalization of European plants on other continents: the role of donor habitats. Proceedings of the National Academy of Sciences, USA 114: 201705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleunen M, Johnson SD. 2007. South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. Journal of Ecology 95: 674–681. [Google Scholar]
- van Kleunen M, Richardson DM. 2007. Invasion biology and conservation biology: time to join forces to explore the links between species traits and extinction risk and invasiveness. Progress in Physical Geography 31: 447–450. [Google Scholar]
- van Kleunen M, Dawson W, Schlaepfer D, Jeschke JM, Fischer M. 2010. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecology Letters 13: 947–958. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Dawson W, Essl F, et al. 2015. Global exchange and accumulation of non-native plants. Nature 525: 100–103. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Bossdorf O, Dawson W. 2018a. The ecology and evolution of alien plants. Annual Review of Ecology, Evolution and Systematics 49: 25–47. [Google Scholar]
- van Kleunen M, Essl F, Perg J, et al. 2018b. The changing role of ornamental horticulture in alien plant invasions. Biological Reviews 93: 1421–1437. [DOI] [PubMed] [Google Scholar]
- Klonner G, Fischer S, Essl F, Dullinger S. 2016. A source area approach demonstrates moderate predictive ability but pronounced variability of invasive species traits. PLoS One 11: e0155547. doi: 10.1371/journal.pone.0155547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueffer C, Pyšek P, Richardson DM. 2013. Integrative invasion science: model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytologist 200: 615–633. [DOI] [PubMed] [Google Scholar]
- Kühn I, Durka W, Klotz S. 2004. BiolFlor – a new plant-trait database as a tool for plant invasion ecology. Diversity and Distributions 10: 363–365. [Google Scholar]
- Küster EC, Kühn I, Bruelheide H, Klotz S. 2008. Trait interactions help explain plant invasion success in the German flora. Journal of Ecology 96: 860–868. [Google Scholar]
- Lambdon PW, Pyšek P, Basnou C, et al. 2008. Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs. Preslia 80: 101–149. [Google Scholar]
- Lavoie C, Joly S, Bergeron A, Guay G, Groeneveld E. 2016. Explaining naturalization and invasiveness: new insights from historical ornamental plant catalogs. Ecology and Evolution 6: 7188–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Ming D, ZhenYing H. 2010. Review of research on seed heteromorphism and its ecological significance. Journal of Plant Ecology 34: 578–590 [in Chinese]. [Google Scholar]
- Maurel N, Hanspach J, Kühn I, Pyšek P, van Kleunen M. 2016. Introduction bias affects relationships between the characteristics of ornamental alien plants and their naturalization success. Global Ecology and Biogeography 25: 1500–1509. [Google Scholar]
- Medvecká J, Kliment J, Májeková J, et al. 2012. Inventory of the alien flora of Slovakia. Preslia 84: 257–309. [Google Scholar]
- Milbau A, Stout JC. 2008. Factors associated with alien plants transitioning from casual, to naturalized, to invasive. Conservation Biology 22: 308–317. [DOI] [PubMed] [Google Scholar]
- Mohamed-Yasseen Y, Barringer SA, Splittstoesser WE, Costanza S. 1994. The role of seed coats in seed viability. The Botanical Review 60: 426–439. [Google Scholar]
- Møller A, Jennions MD. 2002. How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132: 492–500. [DOI] [PubMed] [Google Scholar]
- Moravcová L, Pyšek P, Jarošík V, Havlíčková V, Zákravský P. 2010. Reproductive characteristics of neophytes in the Czech Republic: traits of invasive and non-invasive species. Preslia 82: 365–390. [Google Scholar]
- Moravcová L, Pyšek P, Jarošík V, Pergl J. 2015. Getting the right traits: reproductive and dispersal characteristics predict the invasiveness of herbaceous plant species. PLoS One 10: e0123634. doi: 10.1371/journal.pone.0123634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2013. caper: comparative analyses of phylogenetics and evolution in R. [Google Scholar]
- Pianka ER. 1970. On r- and K-selection. The American Naturalist 104: 592–597. [Google Scholar]
- Plitmann U. 1986. Alternative modes in dispersal strategies, with an emphasis on herbaceous plants of the Middle East. Proceedings of the Royal Society of Edinburgh 89: 193–202. [Google Scholar]
- Prach K, Tichý L, Vítovcová K, Řehounková K. 2017. Participation of the Czech flora in succession at disturbed sites: quantifying species’ colonization ability. Preslia 89: 87–100. [Google Scholar]
- Pyšek P. 1998. Is there a taxonomic pattern to plant invasions? Oikos 82: 282–294. [Google Scholar]
- Pyšek P, Richardson DM. 2007. Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W, ed. Biological invasions. Berlin, Heidelberg: Springer, 97–125. [Google Scholar]
- Pyšek P, Richardson DM, Williamson M. 2004. Predicting and explaining plant invasions through analysis of source areas floras: some critical considerations. Diversity and Distribution 10: 179–187. [Google Scholar]
- Pyšek P, Jarošík V, Pergl J, et al. 2009. The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Diversity and Distributions 15: 891–903. [Google Scholar]
- Pyšek P, Danihelka J, Sádlo J, et al. 2012. Catalogue of alien plants of the Czech Republic (2nd edition): checklist update, taxonomic diversity and invasion patterns. Preslia 84: 155–255. [Google Scholar]
- Pyšek P, Manceur AM, Alba C, et al. 2015. Naturalization of central European plants in North America: species traits, habitats, propagule pressure, residence time. Ecology 96: 762–774. [DOI] [PubMed] [Google Scholar]
- Pyšek P, Pergl J, Essl F, et al. 2017. Naturalized alien flora of the world: species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 89: 203–274. [Google Scholar]
- Rai JPN, Tripathi RS. 1987. Germination and plant survival and growth of Galinsoga parviflora Cav. as related to food and energy content of its ray- and disc-achenes. Acta Oecologica, Oecologia Plantarum 8: 155–165. [Google Scholar]
- Razanajatovo M, Maurel N, Dawson W, et al. 2016. Plants capable of selfing are more likely to become naturalized. Nature Communications 7: 13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reichard SH, White P. 2001. Horticulture as pathways of plant introductions in the United States. BioScience 51: 103–113. [Google Scholar]
- Richardson DM, Pyšek P. 2012. Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytologist 196: 383–396. [DOI] [PubMed] [Google Scholar]
- Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ. 2000. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 6: 93–107. [Google Scholar]
- Richardson DM, Carruthers J, Hui C, et al. 2011. Human-mediated introductions of Australian acacias – a global experiment in biogeography. Diversity and Distributions 17: 771–787. [Google Scholar]
- Rogerson PA. 2001. Statistical methods for geography. London: Sage Publications Ltd/Cromwell Press Ltd. [Google Scholar]
- Sendek A, Herz K, Auge H, Hensen I, Klotz S. 2015. Performance and responses to competition in two congeneric annual species: does seed heteromorphism matter? Plant Biology 17: 1203–1209. [DOI] [PubMed] [Google Scholar]
- Smith SA, Brown JW. 2018. Constructing a broadly inclusive seed plant phylogeny. American Journal of Botany 105: 302–314. [DOI] [PubMed] [Google Scholar]
- Tackenberg O. 2003. Modeling long-distance dispersal of plant diaspores by wind. Ecological Monographs 73: 173–189. [Google Scholar]
- Thomson FJ, Moles AT, Auld TD, Kingsford RT. 2011. Seed dispersal distance is more strongly correlated with plant height than with seed mass. Journal of Ecology 99: 1299–1307. [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al., eds. 1976. Flora Europaea, Vol. 4. Plantaginaceae to Compositae (and Rubiaceae). Cambridge: Cambridge University Press. [Google Scholar]
- Venable DL. 1985. The evolutionary ecology of seed heteromorphism. The American Naturalist 126: 577–595. [Google Scholar]
- de Waal C, Anderson B, Ellis AG. 2016. Dispersal, dormancy and life-history tradeoffs at the individual, population and species levels in southern African Asteraceae. New Phytologist 210: 356–365. [DOI] [PubMed] [Google Scholar]
- Weber EF. 1997. The alien flora of Europe: a taxonomic and biogeographic review. Journal of Vegetation Science 8: 565–572. [Google Scholar]
- Willis SG, Hulme PE. 2004. Environmental severity and variation in the reproductive traits of Impatiens glandulifera. Functional Ecology 18: 887–898. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.