Abstract
Reactive oxygen species (ROS) are ubiquitous signaling molecules involved in diverse physiological processes, including stomatal closure. Photosynthetic electron transport (PET) is the main source of ROS generation in plants, but whether it functions in guard cell signaling remains unclear. Here, we assessed whether PET functions in abscisic acid (ABA) signaling in guard cells. ABA‐elicited ROS were localized to guard cell chloroplasts in Arabidopsis thaliana, Commelina benghalensis, and Vicia faba in the light and abolished by the PET inhibitors 3‐(3, 4‐dichlorophenyl)‐1, 1‐dimethylurea and 2, 5‐dibromo‐3‐methyl‐6‐isopropyl‐p‐benzoquinone. These inhibitors reduced ABA‐induced stomatal closure in all three species, as well as in the NADPH oxidase‐lacking mutant atrboh D/F. However, an NADPH oxidase inhibitor did not fully eliminate ABA‐induced ROS in the chloroplasts, and ABA‐induced ROS were still observed in the guard cell chloroplasts of atrboh D/F. This study demonstrates that ROS generated through PET act as signaling molecules in ABA‐induced stomatal closure and that this occurs in concert with ROS derived through NADPH oxidase.
Keywords: abscisic acid, Arabidopsis thaliana, Commelina benghalensis, guard cell photosynthesis, reactive oxygen species, Vicia faba
1. INTRODUCTION
Reactive oxygen species (ROS) are partially reduced or excited forms of atmospheric oxygen and include superoxide anion (O2 −), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radical (HO•). ROS were long regarded as unwanted and toxic by‐products of aerobic physiological metabolism. However, ROS are now recognized as central players in the complex signaling networks of plant cells as well as animal cells (Mittler, 2017). In plants, they modulate responses to pathogens (Lamb & Dixon, 1997; Mehdy, 1994), growth and morphogenesis (Liu et al., 2016; Mangano, Juárez, & Estevez, 2016), programmed cell death (Breusegem & Dat, 2006; Levine, Tenhaken, Dixon, & Lamb, 1994), and guard cell signaling (McAinsh, Clayton, Mansfield, & Hetherington, 1996; Pei et al., 2000; Song, Miao, & Song, 2014).
Stomata are regulated pores on the surface of aerial plant organs. Stomata sense and rapidly respond to environmental signals, such as light, carbon dioxide, and pathogens, and also respond to hormones including abscisic acid (ABA), auxin, and ethylene (Melotto, Underwood, Koczan, Nomura, & He, 2006; Schroeder, Allen, Hugouvieux, Kwack, & Waner, 2001). ABA, an abiotic stress phytohormone, triggers ROS production, which elicits stomatal closure and inhibits stomatal opening (Pei et al., 2000; Yan, Tsuichihara, Etoh, & Iwai, 2007). NADPH oxidase has been considered to be the main source of ROS for ABA signaling in guard cells. Indeed, the NADPH oxidase inhibitor diphenyleneiodonium (DPI) partially inhibits ABA‐induced stomatal closure (Zhang et al., 2001), and the NADPH oxidase double‐mutant atrboh D/F shows reduced ABA‐induced stomatal closure and ROS generation compared with the wild type (Kwak et al., 2003).
Chloroplasts generate ROS under both normal and stress conditions. Various forms of ROS including the superoxide anion, hydrogen peroxide, and singlet oxygen are generated in chloroplasts, and photosynthetic electron transport (PET) in chloroplast is regarded as the main source of ROS generation in plant cells (Asada, 1999; Foyer & Noctor, 2003). Guard cells contain chloroplasts that are smaller and have fewer grana stacks than mesophyll cell chloroplasts (Lawson, 2009). The guard cell chloroplasts are photosynthetically active, producing sugars by photosynthetic carbon assimilation (Lawson, Oxborough, Morison, & Baker, 2002, 2003; Melis & Zeiger, 1982; Shimazaki, Terada, Tanaka, & Kondo, 1989; Shimazaki & Zeiger, 1985). Many studies indicate that chloroplasts do contribute to stomatal opening (Santelia & Lawson, 2016; Suetsugu et al., 2014; Tominaga, Kinoshita, & Shimazaki, 2001). However, the contradictory results has been reported on its role in ABA‐induced ROS generation leading stomatal closure. For example, norflurazon‐treated fava bean (Vicia faba), which lacks functional photosynthetic activity in guard cells, still responds to ABA (Roelfsema et al., 2006). In addition, Azoulay‐Shemer et al. (2015) showed that ABA induces stomatal closure in an Arabidopsis (Arabidopsis thaliana) mutant lacking chlorophyll in the guard cells. Wang et al. (2016) reported that the photosynthetic electron transport (PET) inhibitor 3‐(3, 4‐dichlorophenyl)‐1, 1‐dimethylurea (DCMU) does not affect ABA‐induced stomatal movement or ABA‐induced ROS production in Arabidopsis leaves. These authors concluded that guard cell photosynthesis is not a direct mediator of ABA‐induced stomatal closure. On the contrary, many studies have demonstrated chloroplastic ROS accumulation when plants or tissues are treated with extracellular Ca2+ (Nomura, Komori, Kobori, Nakahira, & Shiina, 2008; Wang et al., 2012), ozone (Joo, Wang, Chen, Jones, & Fedoroff, 2005; Vahisalu et al., 2010), and ABA (Leshem, Golani, Kaye, & Levine, 2010; Watkins, Chapman, & Muday, 2017; Zhang et al., 2001), leading to stomatal closure. In addition, Xu et al. (2012) found that the light‐harvesting chlorophyll a/b‐binding protein is involved in ABA guard cell signaling by modulating ROS homeostasis. These results suggest that ROS derived from guard cell PET functions in guard cell ABA signaling. Thus, in terms of ABA‐induced stomatal closure, the role of chloroplastic ROS remains an open question.
Here, we examined the possibility that ROS evolved from PET mediate ABA‐induced stomatal closure. We found that ABA‐elicited ROS were localized in guard cell chloroplasts and that their accumulation was abolished by the PET inhibitors DCMU and 2, 5‐dibromo‐3‐methyl‐6‐isopropyl‐p‐benzoquinone (DBMIB). These two inhibitors also reduced ABA‐induced stomatal closure in the NADPH oxidase mutant atrboh D/F and in wild‐type plants treated with DPI. We conclude that ROS generated through PET is crucial for ABA‐induced stomatal closure. Moreover, both PET and NADPH oxidase contribute to ABA‐induced ROS generation in guard cells.
2. METHODS
2.1. Plant materials
Seeds of Arabidopsis (Arabidopsis thaliana) ecotype Col‐0 and of the mutant atrboh D/F (CS 68522) were obtained from the Arabidopsis Biological Research Center (Columbus, OH, USA). Seedlings were grown in soil in a growth chamber for 1–2 months at 20–25°C under an 11‐hr‐light/13‐hr‐dark cycle (50 μmol m−2 s−1). Fava bean (Vicia faba L. cv. Ryousai) was obtained from Nakahara Seed Product Co., Ltd. (Fukuoka, Japan). Commelina (Commelina benghalensis L.) was a line maintained at Kyushu University (Fukuoka, Japan). Fava bean and commelina were grown in a glasshouse under natural light conditions.
2.2. Stomatal aperture assay
The stomatal aperture was assayed as described previously (Joudoi et al., 2013). Briefly, epidermal strips were peeled from the abaxial surface of young but fully expanded leaves. Epidermal strips were kept in opening medium (10 mM MES‐KOH, pH 6.15, 50 mM KCl, 0.1 mM CaCl2) for 3 hr in the light (50 μmol m−2 s−1) and then transferred to opening medium with or without inhibitors (10 μM DCMU, 0.2 μM DBMIB, 10 μM DPI). After incubating for 20 min, 10 μM ABA and/or 100 μM hydrogen peroxide was added to the medium and then incubated for another 2 hr in the light. During treatment, epidermal strips were kept at 23°C. After treatment, epidermal strips were photographed with a charge‐coupled device (CCD) camera (DS‐Fi1; Nikon Corp.) mounted on a microscope (Eclipse E600; Nikon Corp.) with a 10 × /0.30 or 20 × /0.50 numerical aperture air objective. Stomatal apertures (inner diameters of the stomatal pores) were measured using a digital micro analyzer (Japan Polaroid Digital Products). At least four strips, with 25 stomata in each strip, were evaluated for each treatment. ABA and DPI were dissolved in dimethyl sulfoxide. DCMU and DBMIB were dissolved in ethanol. ABA, DCMU, and DBMIB were from Sigma‐Aldrich. Other reagents were from Wako Pure Chemical Industries. Experiments were repeated at least three times, and representative results are shown. The data were statistically analyzed by one‐way analysis of variance (one‐way ANOVA) followed by Tukey's test. In our experimental conditions, the apertures of preopened stomata changed with season and day by day. In Arabidopsis, they were 4–6 μm in spring, summer, and early autumn, but 3–4 μm in late autumn and winter. In commelina, they were 16–18 μm in late spring and summer, but 12–15 μm in autumn. In fava bean, they were 13–15 μm in spring and autumn, but 11–12 μm in winter. The results in Figure 1 were obtained in spring and summer. The results in Figure 6a (Arabidopsis) and Figure 6c (fava bean) were obtained in winter, and those in Figure 6b (commelina) were obtained in autumn.
Figure 1.
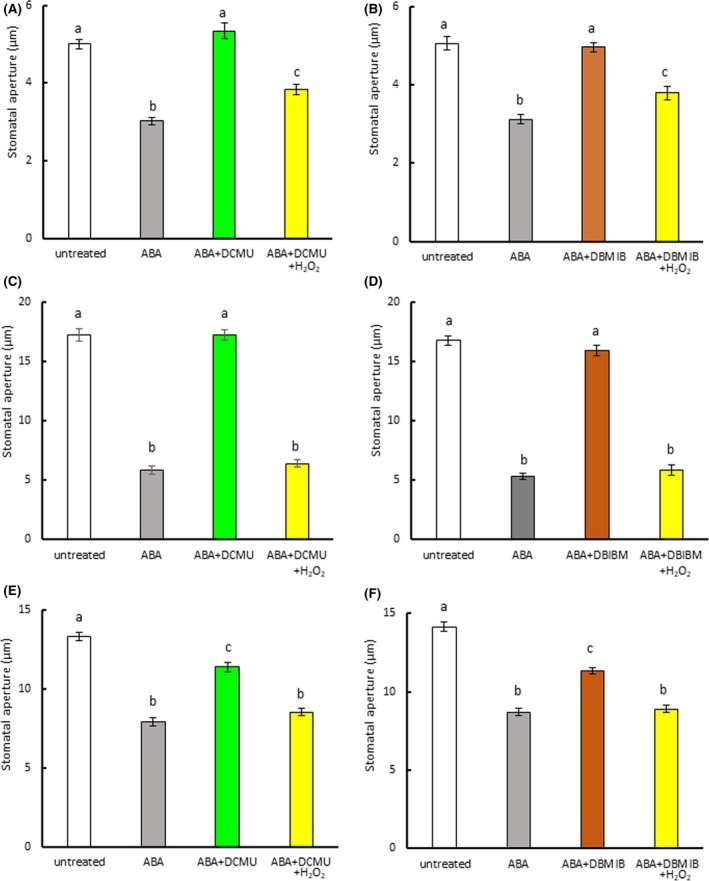
Photosynthetic inhibitors reduce abscisic acid (ABA)‐induced stomatal closure. (a) Effect of DCMU on ABA‐induced stomatal closure in Arabidopsis. (b) Effect of DBMIB on ABA‐induced stomatal closure in Arabidopsis. (c) Effect of DCMU on ABA‐induced stomatal closure in commelina. (d) Effect of DBMIB on ABA‐induced stomatal closure in commelina. (e) Effect of DCMU on ABA‐induced stomatal closure in fava bean. (f) Effect of DBMIB on ABA‐induced stomatal closure in fava bean. Epidermal strips containing preopened guard cells were pretreated with 10 μM DCMU or 0.2 μM DBMIB for 20 min and then incubated with 10 μM ABA and/or H2O2 for 2 hr. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Error bars represent standard error of the mean (n = 100). Different letters indicate significant differences at p < 0.01
2.3. ROS detection in guard cells
Reactive oxygen species were detected as described previously (Joudoi et al., 2013). Briefly, epidermal strips were incubated in opening medium for 3 hr in the light (50 μmol m−2 s−1) and then transferred to opening medium containing 10 μM ABA and incubated for 5 min in the light. During treatment, epidermal strips were kept at 23°C. Epidermal strips treated with 10 μM H2DCFDA for 20 min in the dark were observed with a fluorescence microscope (excitation, 465–495 nm; emission, 515–555 nm; 10 × /0.30 or 20 × /0.50 numerical aperture air objective; Eclipse E600; Nikon Corp., Tokyo, Japan) equipped with a CCD camera (DS‐Fi1; Nikon Corp.). At least 30 guard cells were evaluated. Data are presented as average values from at least three independent experiments. The statistical significance between two groups was determined by Student's t test. For subcellular localization of ROS, fluorescence was observed using a confocal laser scanning microscope (EZ‐C1; Nikon Corp.) with the following settings: 20 × /0.75 or 40 × /0.95 numerical aperture air objective; excitation, 488 nm; emission, 515/30 nm for fluorescence derived from H2DCFDA, and 605/75 nm for chlorophyll autofluorescence.
2.4. Accession numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: atrboh D, AT5G47910; atrboh F, AT1G64060.
3. RESULTS
3.1. PET is involved in ABA‐induced stomatal closure
To assess the possibility that PET is involved in ABA‐induced stomatal closure, we treated strips of Arabidopsis epidermis with the PET inhibitors DCMU and DBMIB. At a concentration of 10 μM, DCMU alone had no effect on the stomatal aperture (Figure S1a). DBMIB was previously reported to trigger stomatal closure at 14 μM (Wang et al., 2016). We examined the effect of lower DBMIB concentrations on stomatal movement and found that although DBMIB‐induced stomatal closure at concentrations above 0.5 μM, it did not at 0.2 μM (Figure S1b). On the basis of these results, we selected 10 μM DCMU and 0.2 μM DBMIB for use in further studies. When DCMU and ABA were simultaneously added to the assay medium, DCMU had no effect on ABA‐induced stomatal closure (Figure S3). However, when the epidermal strips were pretreated with DCMU for 20 min, ABA‐induced stomatal closure in Arabidopsis was eliminated (Figure 1a). When hydrogen peroxide was applied in combination with ABA and DCMU, the stomata closed. DBMIB pretreatment also inhibited ABA‐induced stomatal closure in Arabidopsis, but H2O2 eliminated this inhibition (Figure 1b). Similar results were observed in commelina (Commelina benghalensis; Figure S2a; Figure 1c,d) and fava bean (Figure S2b, Figure 1e,f). These results indicate that PET contributes to ABA‐induced stomatal closure in these three species.
3.2. ABA‐induced ROS generation depends on PET
We used 2, 7‐dichlorofluorescin diacetate (H2DCFDA) to detect ROS generation in guard cells. We previously reported that guard cell ROS concentrations peaked 3–5 min after the initiation of ABA treatment, then gradually decreased (Joudoi et al., 2013). Therefore, in this study, we monitored ROS generation in guard cells treated with ABA for 5 min. We next studied the effect of pinhole size on confocal micrographs (Figure S4). The images show the same guard cell taken with a pinhole of 30, 60, and 100 μm. The other conditions were the same among the three different micrographs. When the pinhole size of the excitation laser beam in the confocal laser scanning microscope was small (30 μm), the results showed that H2O2 localized in the chloroplast (Figure S4a), but the large pinhole size (60 and 100 μm) showed H2O2 in the chloroplasts and cytoplasm (Figure S4b,c). The large pinhole created a low‐resolution micrograph, and we could not distinguish between the fluorescence in the chloroplast and that in the other cellular compartments. The small pinhole gave a high‐resolution micrograph that showed that ROS accumulated in the chloroplasts. On the basis of these results, we used micrographs taken with a 30 μm pinhole. We used epidermal strips to detect ROS generation. The epidermal strips might be injured by peeling and the mechanical injury might cause NADPH oxidase dependent ROS generation in apoplast (Miller et al., 2009). Assuming that mechanical injury elicit ROS, ROS will be observed in apoplast. However, ROS were not observed in the apoplast (Figure 2), indicating that we can neglect ROS induced by mechanical injury in our experimental condition.
Figure 2.
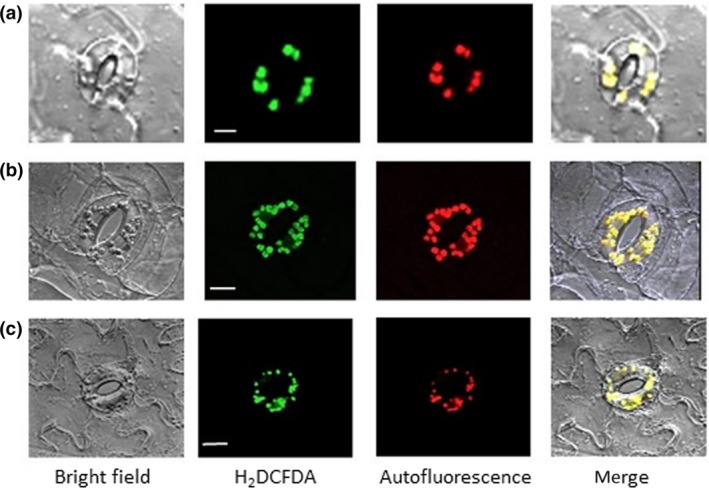
Abscisic acid (ABA)‐induced reactive oxygen species were localized at guard cell chloroplasts. (a) Arabidopsis. (b) commelina. (c) fava bean. Confocal micrographs of guard cells treated with 10 μM ABA for 5 min. Reactive oxygen species and chloroplasts were visualized by H2 DCFDA fluorescence and chlorophyll autofluorescence, respectively. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Bars = 10 μm for a, and 20 μm for b and c
Intense H2DCFDA fluorescence signals were located at chloroplasts in guard cells of Arabidopsis, commelina, and fava bean but were hardly observed in cytoplasm and the plasma membrane (Figure 2a–c; Figure S5). ABA induced a 250 to 280% increase in ROS generation in the light, but it did not increase ROS generation in the dark (Figure 3a), indicating that light is required for ABA‐induced ROS generation in guard cells. To examine the possibility that PET is involved in ROS generation in guard cells, we used the PET inhibitors DCMU and DBMIB, which abolished the increase in ABA‐induced ROS generation (Figure 3a,b). These results show that PET is involved in ABA‐induced ROS generation in guard cells.
Figure 3.
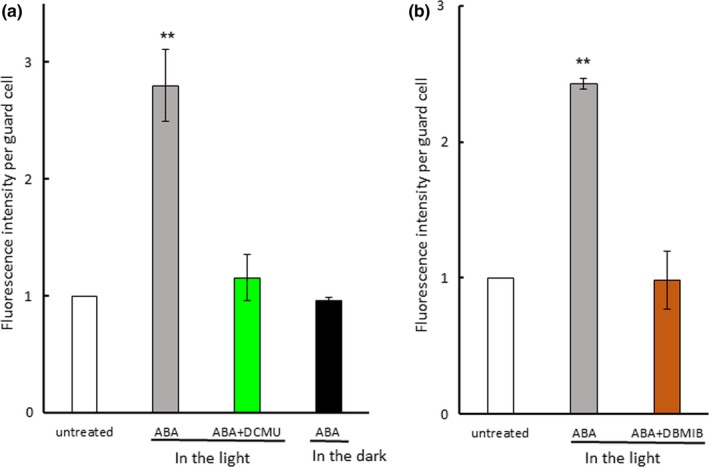
Photosynthetic inhibitors eliminated abscisic acid (ABA)‐induced generation of reactive oxygen species. (a) Effect of DCMU and light on ABA‐induced generation of reactive oxygen species in Arabidopsis guard cells. (b) Effect of DBMIB on ABA‐induced generation of reactive oxygen species in Arabidopsis guard cells. Epidermal strips containing preopened guard cells were pretreated with 10 μM DCMU or 0.2 μM DBMIB for 20 min and then incubated with 10 μM ABA for 5 min. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Fluorescence intensity per guard cell was expressed as the ratio of treated cells to untreated cells. Error bars represent standard error of the mean (n = 3). Double asterisks (**) indicate a significant difference (p < 0.01) compared with values for untreated cells
3.3. PET functions in concert with NADPH oxidase
To examine the role of NADPH oxidase in generating ROS in guard cell chloroplasts, we used the NADPH oxidase inhibitor DPI and the NADPH oxidase‐lacking mutant atrboh D/F. The production of ROS from chloroplasts was not abolished by DPI in Arabidopsis (Figure 4a), commelina (Figure 4b), or fava bean (Figure 4c). In addition, ABA‐induced ROS generation was observed in guard cell chloroplasts of the NADPH oxidase‐lacking mutant (Figure 5).
Figure 4.
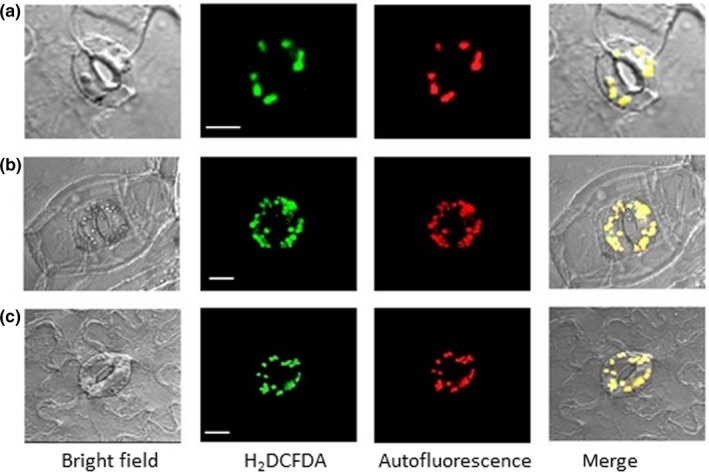
Abscisic acid (ABA)‐induced reactive oxygen species at guard cell chloroplasts was not eliminated by NADPH oxidase inhibitor. (a) Arabidopsis. (b) commelina. (c) fava bean. Confocal micrographs of guard cells treated with 10 μM ABA for 5 min. Reactive oxygen species and chloroplasts were visualized by H2 DCFDA fluorescence and autofluorescence of chlorophyll, respectively. Epidermal strips containing preopened guard cells were pretreated with NADPH oxidase inhibitor (10 μM diphenyleneiodonium) for 20 min and then incubated with 10 μM ABA for 5 min. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Bars = 10 μm for a, and 20 μm for b and c
Figure 5.
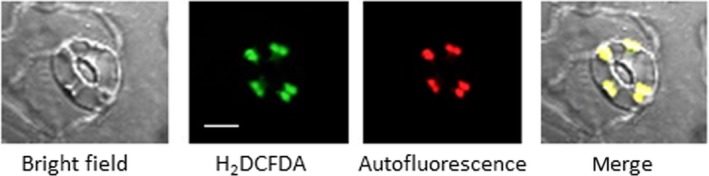
Abscisic acid (ABA)‐induced reactive oxygen species were localized at guard cell chloroplasts of the NADPH oxidase mutant atrboh D/F. Confocal micrographs of guard cell treated with 10 μM ABA for 5 min. Reactive oxygen species and chloroplasts were visualized by H2 DCFDA fluorescence and autofluorescence of chlorophyll, respectively. Bar = 10 μm
In epidermal strips treated with DPI, we observed that DPI partially inhibited ABA‐induced stomatal closure in Arabidopsis and fava bean (Figure 6a,c), consistent with previous results (Kwak et al., 2003; Zhang et al., 2001). In commelina, similar results were observed (Figure 6b). However, when the epidermal strips were treated with both the NADPH oxidase inhibitor DPI and the PET inhibitors DCMU and DBMIB, ABA‐induced stomatal closure was fully abolished in Arabidopsis (Figure 6a,b), commelina (Figure 6c,d), and fava bean (Figure 6e,f). We also examined whether PET inhibitors affected ABA‐induced stomatal closure in the NADPH oxidase mutant atrboh D/F (Figure 7). ABA treatment induced partial closure of stomata in this mutant, consistent with previous results (Kwak et al., 2003), but this closure was fully abolished in the presence of DCMU and DBMIB. These results indicate that both PET and NADPH oxidase contribute to ABA‐induced stomatal closure.
Figure 6.
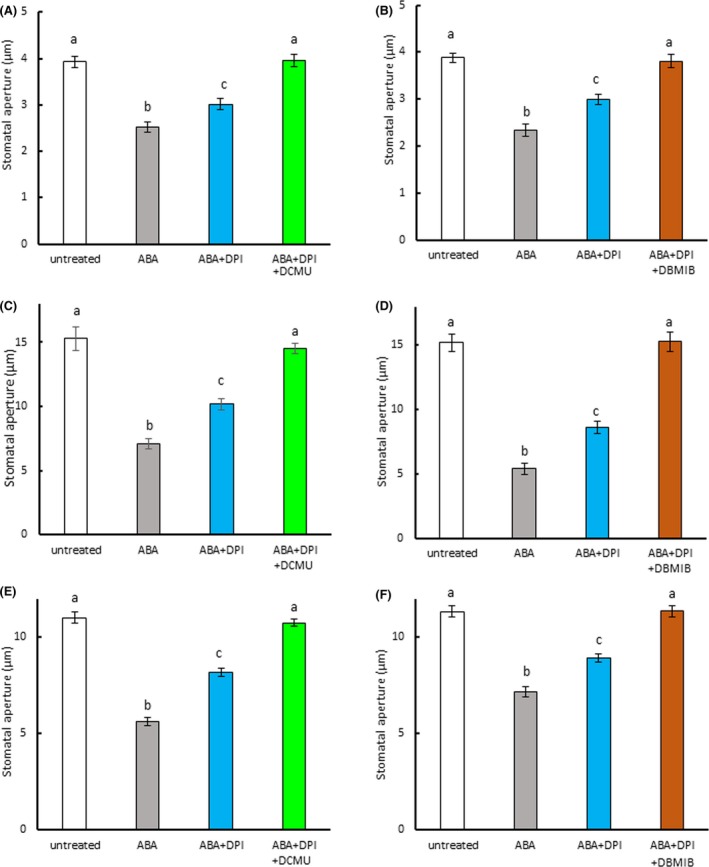
Photosynthetic inhibitors further reduced abscisic acid (ABA)‐induced stomatal closure in epidermal strips treated with NADPH oxidase inhibitor. (a) Effect of DCMU on ABA‐induced stomatal closure in NADPH oxidase inhibitor (diphenyleneiodonium, DPI)‐treated strips of Arabidopsis epidermis. (b) Effect of DBMIB on ABA‐induced stomatal closure in DPI‐treated strips of Arabidopsis epidermis. (c) Effect of DCMU on ABA‐induced stomatal closure in DPI‐treated strips of commelina epidermis. (d) Effect of DBMIB on ABA‐induced stomatal closure in DPI‐treated strips of commelina epidermis. (e) Effect of DCMU on ABA‐induced stomatal closure in DPI‐treated strips of fava bean epidermis. (f) Effect of DBMIB on ABA‐induced stomatal closure in DPI‐treated strips of fava bean epidermis. Epidermal strips containing preopened guard cells were pretreated with 10 μM DCMU, 0.2 μM DBMIB and/or 10 μM DPI for 20 min and then incubated with 10 μM ABA for 2 hr. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Error bars represent standard error of the mean (n = 100). Different letters indicate significant differences at p < 0.01
Figure 7.
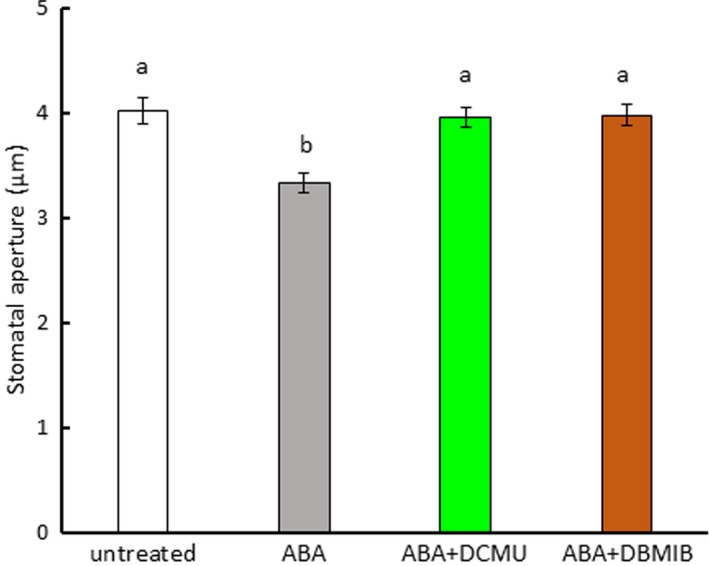
Photosynthetic inhibitors eliminated abscisic acid (ABA)‐induced stomatal closure in the NADPH oxidase mutant atrboh D/F. Epidermal strips containing preopened guard cells were pretreated with 10 μM DCMU or 0.2 μM DBMIB for 20 min and then incubated with 10 μM ABA for 2 hr. Experiments were conducted at 23°C in the light (50 μmol m−2 s−1). Error bars represent standard error of the mean (n = 100). Different letters indicate significant differences at p < 0.01
4. DISCUSSION
The role of guard cell photosynthesis in ABA signaling remains widely debated, mainly because of contradictory evidence from different experiments in different laboratories using different species (Santelia & Lawson, 2016). Some laboratories have shown that guard cell photosynthesis is important in ABA‐induced ROS generation and stomatal closure (Leshem et al., 2010; Watkins et al., 2017; Zhang et al., 2001). However, others have concluded that it has no function in ABA‐induced ROS generation and stomatal closure (Azoulay‐Shemer et al., 2015; Roelfsema et al., 2006; Wang et al., 2016). In this report, we provide evidence that guard cell PET mediates ABA‐induced stomatal closure in three phylogenetically distant species: Arabidopsis (Cruciferae, dicot), commelina (Commelinaceae, monocot), and fava bean (Leguminosae, dicot). The PET inhibitors DCMU and DBMIB suppressed ABA‐induced stomatal closure in Arabidopsis (Figure 1a,b), commelina (Figure 1c,d), and fava bean (Figure 1e,f), indicating that guard cell PET mediates ABA‐induced stomatal closure.
In contrast to these results, Wang et al. (2016) reported that DCMU did not affect ABA‐induced stomatal closure in Arabidopsis. While we used epidermal strips, they used leaves and added DCMU and ABA to the assay medium simultaneously. When we used leaves and added DCMU and ABA at the same time, we obtained similar results to theirs: DCMU failed to inhibit ABA‐induced stomatal closure (Figure S6). Intact leaves usually require pretreatment with DCMU for several hours to inhibit photosynthesis, perhaps because DCMU does not penetrate to the chloroplasts of intact leaves during a short period (e.g., Basra, Dhawan, & Goyal, 2002; Tóth, Schansker, & Strasser, 2005). To avoid the possibility of DCMU not reaching the guard cell chloroplasts, we used epidermal strips pretreated with DCMU. We found that when epidermal strips were treated with DCMU and ABA simultaneously, DCMU did not affect ABA‐induced stomatal closure (Figure S3). However, when epidermal tissue was pretreated with DCMU for 20 min, DCMU inhibited ABA‐induced stomatal closure (Figure 1). The effect of DBMIB on ABA‐induced stomatal closure has remained unknown due to technical restrictions: DBMIB (14 μM) alone was reported to directly induce stomatal closure in isolated epidermis, which prevented it from being used to explore the effects on ABA‐induced stomatal closure (Wang et al., 2016). Here, we also found that DBMIB alone induced stomatal closure at concentrations higher than 0.5 μM (Figure S1b). To overcome this technical restriction, we used low concentrations of DBMIB. At 0.2 μM, DBMIB alone did not affect stomatal aperture under normal conditions (Figure S1b) but did inhibit ABA‐induced stomatal closure (Figure 1). Thus, the differences between the effects of PET inhibitors may be attributed to the experimental conditions employed by the two groups.
We further showed that short‐term ABA treatment induced ROS production in the guard cell chloroplasts of Arabidopsis (Figure 2a), commelina (Figure 2b), and fava bean (Figure 2c; Figure S5). Furthermore, both DCMU and DBMIB abolished ABA‐induced ROS generation in guard cells (Figure 3), indicating that ABA‐induced ROS generation was mediated by guard cell PET. Three major sites of ROS generation in PET have been reported: (a) the triplet P680 of PSII that reacts with ground state oxygen to form singlet oxygen (Roh, Shingles, Cleveland, & Mccarty, 1998; Triantaphylides et al., 2008); (b) the reduced plastoquinone (PQ) pool that reduces molecular oxygen to superoxide anions, which are in turn converted to H2O2 by a disproportionation reaction (Ivanov, Mubarakshina, & Khorobrykh, 2007; Mubarakshina, Khorobrykh, & Ivanov, 2006); and (c) the electron acceptors of PSI that direct electrons to molecular oxygen and generate superoxide anions and H2O2 (Asada, 1999). It was reported that Ca2+ affects the redox state of the PQ pool in guard cell, resulting in ROS generation, whereas ABA does not affect the redox state of the PQ pool (Wang et al., 2016). Apparently, ABA‐induced ROS are generated from a site other than the reduced PQ pool. DCMU blocks electron transfer from QA to PQ, resulting in the oxidation of the PQ pool, whereas DBMIB blocks PET at the PQ oxidation site in the cytochrome b 6 f complex, resulting in reduction in the PQ pool. Using these inhibitors, we can distinguish between the sites of ROS generation in ABA‐treated guard cell chloroplasts: (a) when both DCMU and DBMIB promote ROS generation, the site is PSII; (b) when DCMU abolishes ROS generation and DBMIB elicits it, the site is the reduced PQ pool; and (c) when both DCMU and DBMIB suppress ROS generation, the site is PSI. Our results show that both 10 μM DCMU and 0.2 μM DBMIB abolished ABA‐induced ROS generation in guard cells (Figure 3a.b), indicating that ABA‐induced ROS are derived from PSI.
We conclude that PET mediates ABA‐induced stomatal closure in three phylogenetically distant species: Arabidopsis (Cruciferae, dicot), commelina (Commelinaceae, monocot), and fava bean (Leguminosae, dicot).
4.1. PET functions in parallel with NADPH oxidase in ABA‐induced stomatal closure
NADPH oxidase is believed to be the main ROS generator in ABA guard cell signaling. Plant NADPH oxidase has six transmembrane domains that oxidize cytoplasmic NADPH, translocate electrons across the plasma membrane, and reduce extracellular ambient oxygen to yield superoxide anion in the apoplast (Suzuki et al., 2011). The superoxide anion is rapidly converted to H2O2 that is transported into the cytoplasm via aquaporin (Bienert, Schjoerring, & Jahn, 2006). Assuming that membrane‐bound NADPH oxidase is the main ROS producer, ROS will be observed first in the apoplast and subsequently in cytoplasm. However, we observed the ROS in the chloroplast but not in the apoplast or cytoplasm in three different species. These observations provide information that the chloroplast is the main site producing ROS, indicating that NADPH oxidase was not main ROS generator in ABA guard cell signaling.
Nevertheless, these results do not ruled out the possibility that NADPH oxidase also play a role. This raises the question as to how PET and NADPH oxidase function together in ABA‐induced stomatal closure. The NADPH oxidase inhibitor DPI partly inhibited ABA‐induced stomatal closure in Arabidopsis, commelina, and fava bean (Figure 6). ABA‐induced stomatal closure was still observed in the NADPH oxidase‐lacking mutant atrboh D/F (Figure 7), consistent with the report by Kwak et al. (2003). The PET inhibitors DCMU and DBMIB further inhibited ABA‐induced stomatal closure in atrboh D/F (Figure 7). These two inhibitors also inhibited ABA‐induced stomatal closure in epidermal strips treated with DPI in wild‐type Arabidopsis, commelina, and fava bean (Figure 6). Our results thus indicate the existence of two separate branches, involving PET and NADPH oxidase pathways, which both contribute to stomatal closure. The next question is, what is the role of PET and NADPH oxidase in ABA‐induced ROS generation? Zhang et al. (2001) showed that ABA induces ROS accumulation in guard cell chloroplasts significantly earlier than in other regions of guard cells in fava bean. The NADPH oxidase inhibitor DPI partly abolishes ABA‐induced ROS production but does not inhibit the production of ROS in chloroplasts of fava bean. We showed that DPI did not eliminate ROS generation in the guard cell chloroplasts in Arabidopsis, commelina, or fava bean (Figure 4). In the NADPH oxidase‐lacking Arabidopsis mutant atrboh D/F, ROS generation was still observed in guard cell chloroplasts (Figure 5), indicating that NADPH oxidase do not function upstream of PET in ABA‐induced ROS generation. We conclude that both PET and NADPH oxidase contribute to ABA‐induced ROS generation, which leads to stomatal closure (Figure S7). The mechanism by which ABA elicits photosynthetic ROS generation in guard cells and the interaction between PET and NADPH oxidase require further study.
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with the work described in this manuscript.
AUTHOR CONTRIBUTIONS
S.I. performed most of the experiments and contributed to the writing of the article with Ken.S. and Kin.S.; S.O. performed the stomatal aperture assay; N.Y. performed the laser scanning confocal microscopy analyses; M.I. grew the plant materials; S.I. and Ken.S. designed the research and analyzed data; All authors discussed the results and commented on the manuscript. S.I. agrees to serve as the author responsible for contact and ensures communication.
Supporting information
ACKNOWLEDGMENTS
We thank the members of the Vegetable Science Laboratory at Kagoshima University, Daiki Shinboku (Kyusyu University) and Eiji Gotoh (Kyusyu University) for help and discussion. We also thank the Center for Advanced Instrumental and Educational Supports, Faculty of Agriculture, Kyushu University for help with Leica TCS SP8 confocal system analysis.
Iwai S, Ogata S, Yamada N, Onjo M, Sonoike K, Shimazaki K‐I. Guard cell photosynthesis is crucial in abscisic acid‐induced stomatal closure. Plant Direct. 2019;3:1–10. 10.1002/pld3.137
Funding information
This work was supported by Grants‐in‐Aid for Scientific Research (grant number 18K06292 to Ken.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- Asada, K. (1999). The water‐water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 601–639. 10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- Azoulay‐Shemer, T. , Palomares, A. , Bagheri, A. , Israelsson‐Nordstrom, M. , Engineer, C. B. , Bargmann, B. O. , … Schroeder, J. I. (2015). Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2 ‐ and ABA‐induced stomatal closing. The Plant Journal, 83, 567–581. 10.1111/tpj.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra, A. S. , Dhawan, A. K. , & Goyal, S. S. (2002). DCMU inhibits in vivo nitrate reduction in illuminated barley (C (3)) leaves but not in maize (C (4)): A new mechanism for the role of light? Planta, 215, 855–861. 10.1007/s00425-002-0802-9 [DOI] [PubMed] [Google Scholar]
- Bienert, G. P. , Schjoerring, J. K. , & Jahn, T. P. (2006). Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta, 1758, 994–1003. 10.1016/j.bbamem.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Breusegem, F. V. , & Dat, J. F. (2006). Reactive oxygen species in plant cell death. Plant Physiology, 141, 384–390. 10.1104/pp.106.078295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer, C. H. , & Noctor, G. (2003). Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum, 119, 355–364. 10.1034/j.1399-3054.2003.00223.x [DOI] [Google Scholar]
- Ivanov, B. , Mubarakshina, M. , & Khorobrykh, S. (2007). Kinetics of the plastoquinone pool oxidation following illumination oxygen incorporation into photosynthetic electron transport chain. FEBS Letters, 581, 1342–1346. 10.1016/j.febslet.2007.02.044 [DOI] [PubMed] [Google Scholar]
- Joo, J. H. , Wang, S. , Chen, J. G. , Jones, A. M. , & Fedoroff, N. V. (2005). Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. The Plant Cell, 17, 957–970. 10.1105/tpc.104.029603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudoi, T. , Shichiri, Y. , Kamizono, N. , Akaike, T. , Sawa, T. , Yoshitake, J. , … Iwai, S. (2013). Nitrated cyclic GMP modulates guard cell signaling in Arabidopsis . The Plant Cell, 25, 558–571. 10.1105/tpc.112.105049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, J. M. , Mori, I. C. , Pei, Z.‐M. , Leonhardt, N. , Torres, M. A. , Dangl, J. L. , … Schroede, J. I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS‐dependent ABA signaling in Arabidopsis . EMBO Journal, 22, 2623–2633. 10.1093/emboj/cdg277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, C. , & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 251–275. 10.1146/annurev.arplant.48.1.251 [DOI] [PubMed] [Google Scholar]
- Lawson, T. (2009). Guard cell photosynthesis and stomatal function. New Phytologist, 181, 13–34. 10.1111/j.1469-8137.2008.02685.x [DOI] [PubMed] [Google Scholar]
- Lawson, T. , Oxborough, K. , Morison, J. I. L. , & Baker, N. R. (2002). Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiology, 128, 52–62. 10.1104/pp.010317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, T. , Oxborough, K. , Morison, J. I. L. , & Baker, N. R. (2003). The response of guard cell photosynthesis to CO2, O2, light and water stress in a range of species are similar. Journal of Experimental Botany, 54, 1743–1752. 10.1093/jxb/erg186 [DOI] [PubMed] [Google Scholar]
- Leshem, Y. , Golani, Y. , Kaye, Y. , & Levine, A. (2010). Reduced expression of the v‐SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid‐dependent stomatal closure. Journal of Experimental Botany, 61, 2615–2622. 10.1093/jxb/erq099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A. , Tenhaken, R. , Dixon, R. , & Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79, 583–593. 10.1016/0092-8674(94)90544-4 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Wang, R. , Zhang, P. , Chen, Q. , Luo, I. , Zhu, Y. , & Xu, J. (2016). The nitrification inhibitor methyl 3‐(4‐hydroxyphenyl) propionate modulates root development by interfering with auxin signaling via the NO/ROS pathway. Plant Physiology, 171, 1686–1703. 10.1104/pp.16.00670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano, S. , Juárez, S. P. , & Estevez, J. M. (2016). ROS regulation of polar growth in plant cells. Plant Physiology, 171, 1593–1605. 10.1104/pp.16.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M. R. , Clayton, H. , Mansfield, T. A. , & Hetherington, A. M. (1996). Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology, 111, 1031–1042. 10.1104/pp.111.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy, M. C. (1994). Active oxygen species in plant defense against pathogens. Plant Physiology, 105, 467–472. 10.1104/pp.105.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis, A. , & Zeiger, E. (1982). Chlorophyll a fluorescence transients in mesophyll and guard cells. Modulation of guard cell photophosphorylation by CO2 . Plant Physiology, 69, 642–647. 10.1104/pp.69.3.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto, M. , Underwood, W. , Koczan, J. , Nomura, K. , & He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell, 126, 969–980. 10.1016/j.cell.2006.06.054 [DOI] [PubMed] [Google Scholar]
- Miller, G. , Schlauch, K. , Tam, R. , Cortes, D. , Torres, M. A. , Shulaev, V. , … Mittler, R. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling, 2, ra45. [DOI] [PubMed] [Google Scholar]
- Mittler, R. (2017). ROS are good. Trends in Plant Science, 22, 11–19. 10.1016/j.tplants.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Mubarakshina, M. , Khorobrykh, S. , & Ivanov, B. (2006). Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane. Biochimica et Biophysica Acta, 1757, 1496–1503. 10.1016/j.bbabio.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Nomura, H. , Komori, T. , Kobori, M. , Nakahira, Y. , & Shiina, T. (2008). Evidence for chloroplast control of external Ca2+‐induced cytosolic Ca2+ transients and stomatal closure. The Plant Journal, 53, 988–998. [DOI] [PubMed] [Google Scholar]
- Pei, Z. M. , Murata, Y. , Benning, G. , Thomine, S. , Klüsener, B. , Allen, G. J. , … Schroeder, J. I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature, 406, 731–734. 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- Roelfsema, M. R. , Konrad, K. R. , Marten, H. , Psaras, G. K. , Hartung, W. , & Hedrich, R. (2006). Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant, Cell and Environment, 29, 1595–1605. 10.1111/j.1365-3040.2006.01536.x [DOI] [PubMed] [Google Scholar]
- Roh, M. H. , Shingles, R. , Cleveland, M. J. , & Mccarty, R. E. (1998). Direct measurement of calcium transport across chloroplast inner‐envelope vesicles. Plant Physiology, 118, 1447–1454. 10.1104/pp.118.4.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia, D. , & Lawson, T. (2016). Rethinking guard cell metabolism. Plant Physiology, 172, 1371–1392. 10.1104/pp.16.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J. I. , Allen, G. J. , Hugouvieux, V. , Kwack, J. M. , & Waner, D. (2001). Guard cell signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology, 52, 627–658. 10.1146/annurev.arplant.52.1.627 [DOI] [PubMed] [Google Scholar]
- Shimazaki, K. , Terada, J. , Tanaka, K. , & Kondo, N. (1989). Calvin‐Benson cycle enzymes in guard‐cell protoplasts from Vicia faba L. Plant Physiology, 90, 1057–1064. 10.1104/pp.90.3.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki, K. , & Zeiger, E. (1985). Cyclic and noncyclic phosphorylation in isolated guard cell protoplasts from Vicia faba L. Plant Physiology, 78, 211–214. 10.1104/pp.78.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Miao, Y. , & Song, C. P. (2014). Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytologist, 201, 1121–1140. 10.1111/nph.12565 [DOI] [PubMed] [Google Scholar]
- Suetsugu, N. , Takami, T. , Ebisu, Y. , Watanabe, H. , Iiboshi, C. , Doi, M. , & Shimazaki, K. (2014). Guard cell chloroplasts are essential for blue light‐dependent stomatal opening in Arabidopsis. PLoS ONE, 9, e108374 10.1371/journal.pone.0108374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, N. , Miller, G. , Morales, J. , Shulaev, V. , Torres, M. A. , & Mittler, R. (2011). Respiratory burst oxidases: The engines of ROS signaling. Current Opinion in Plant Biology, 14, 691–699. 10.1016/j.pbi.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Tominaga, M. , Kinoshita, T. , & Shimazaki, K. (2001). Guard‐cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant and Cell Physiology, 42, 795–802. 10.1093/pcp/pce101 [DOI] [PubMed] [Google Scholar]
- Tóth, S. Z. , Schansker, G. , & Strasser, R. J. (2005). In intact leaves, the maximum fluorescence level (F M) is independent of the redox state of the plastoquinone pool: A DCMU‐inhibition study. Biochimica et Biophysica Acta, 1708, 275–282. 10.1016/j.bbabio.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Triantaphylides, C. , Krischke, M. , Hoeberichts, F. A. , Ksas, B. , Gresser, G. , Havaux, M. , … Mueller, M. J. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiology, 148, 960–968. 10.1104/pp.108.125690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu, T. , Puzõrjova, I. , Brosché, M. , Valk, E. , Lepiku, M. , Moldau, H. , … Kollist, H. (2010). Ozone‐triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal, 62, 442–453. 10.1111/j.1365-313X.2010.04159.x [DOI] [PubMed] [Google Scholar]
- Wang, W. H. , He, E. M. , Chen, J. , Guo, Y. , Chen, J. , Liu, X. , & Zheng, H. L. (2016). The reduced state of the plastoquinone pool is required for chloroplast‐mediated stomatal closure in response to calcium stimulation. The Plant Journal, 86, 132–144. 10.1111/tpj.13154 [DOI] [PubMed] [Google Scholar]
- Wang, W. H. , Yi, X. Q. , Han, A. D. , Liu, T. W. , Chen, J. , Wu, F. H. , … Zheng, H. L. (2012). Calcium‐sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis . Journal of Experimental Botany, 63, 177–190. 10.1093/jxb/err259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, J. M. , Chapman, J. M. , & Muday, G. K. (2017). Abscisic acid‐induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiology, 175, 1807–1825. 10.1104/pp.17.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. H. , Liu, R. , Yan, L. , Liu, Z. Q. , Jiang, S. C. , Shen, Y. Y. , … Zhang, D. P. (2012). Light‐harvesting chlorophyll a/b‐binding proteins are required for stomatal response to abscisic acid in Arabidopsis . Journal of Experimental Botany, 63, 1095–1106. 10.1093/jxb/err315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. , Tsuichihara, N. , Etoh, T. , & Iwai, S. (2007). Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant, Cell and Environment, 30, 1320–1325. 10.1111/j.1365-3040.2007.01711.x [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, L. , Dong, F. , Gao, J. , Galbraith, D. W. , & Song, C. P. (2001). Hydrogen peroxide is involved in abscisic acid‐induced stomatal closure in Vicia faba . Plant Physiology, 126, 1438–1448. 10.1104/pp.126.4.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


