Abstract
Severe asthma develops as a result of heightened, persistent symptoms that generally coincide with pronounced neutrophilic airway inflammation. In individuals with severe asthma, symptoms are poorly controlled by high-dose inhaled glucocorticoids and often lead to elevated morbidity and mortality rates that underscore the necessity for novel drug target identification that overcomes limitations in disease management. Many incidences of severe asthma are mechanistically associated with T helper 17 (TH17) cell-derived cytokines and immune factors that mediate neutrophilic influx to the airways. TH17-secreted interleukin-17A (IL-17A) is an independent risk factor for severe asthma that impacts airway smooth muscle (ASM) remodeling. TH17-derived cytokines and diverse immune mediators further interact with structural cells of the airway to induce pathophysiological processes that impact ASM functionality. Transforming growth factor-β1 (TGF-β1) is a pivotal mediator involved in airway remodeling that correlates with enhanced TH17 activity in individuals with severe asthma and is essential to TH17 differentiation and IL-17A production. IL-17A can also reciprocally enhance activation of TGF-β1 signaling pathways, whereas combined TH1/TH17 or TH2/TH17 immune responses may additively impact asthma severity. This review seeks to provide a comprehensive summary of cytokine-driven T cell fate determination and TH17-mediated airway inflammation. It will further review the evidence demonstrating the extent to which IL-17A interacts with various immune factors, specifically TGF-β1, to contribute to ASM remodeling and altered function in TH17-driven endotypes of severe asthma.
Keywords: airway remodeling, airway smooth muscle, asthma, IL-17A, TGF-β1
INTRODUCTION
Asthma is a chronic inflammatory disease of the lower airways characterized by enhanced structural remodeling, diminished epithelial integrity, and phenotypic switching of airway smooth muscle (ASM) cells. Ancient societies noted respiratory complications as far back as 2600 BC, yet asthma was not described formally in the medical literature until the late fifth century BC when Hippocrates published Corpus Hippocraticum (125, 158, 200). Although epidemiological associations and physical triggers of asthma have been long known, it was not until the early twentieth century that asthma was fully recognized as a heterogenous inflammatory disease with genetic components. Despite treatment advances, asthma remains an exceedingly prevalent noncommunicable health disorder with over 300 million cases worldwide and projected incidence growth of 100 million cases by the year 2025 (22, 160, 168, 187). In the United States and Europe, annual health care costs surpass $82 billion and $22 billion, respectively, and the added burden of an enlarged patient population will further compound treatment expenditures (23, 177). This disconcerting expansion is attributable to intensified industrialization and an upsurge in exposure to occupational pollutants, tobacco smoke, and naturally occurring environmental allergens (241).
When considering approaches to disease management, one must bear in mind that the term “asthma” is a clinical diagnosis encompassing a spectrum of airway obstructive inflammatory diseases. The subclassification of severe asthma constitutes 10% of the asthmatic population yet presents with the gravest of symptoms, has the highest morbidity and mortality rates, and necessitates half of all asthma-related health care costs within the United States and Europe (82, 151, 168, 209, 221). Clinical management of severe asthma is exceedingly burdensome, as patients fail to effectively respond to prevailing treatments of high-dose inhaled and/or oral glucocorticoids in conjunction with other bronchodilator therapies, such as long-acting β2-receptor agonists (31, 98, 140). Although differences in therapeutic efficacy may be informative when assessing comparable disease presentations, they do not account for underlying pathogenic mechanisms surrounding steroid insensitivity. Accounting for these discrepancies, endotyping has emerged as an approach to overcome therapeutic limitations by facilitating specific, therapeutic innovation that links distinguishable phenotypes with unique molecular mechanisms (34, 152). For example, individuals with severe asthma endure persistent airflow obstruction and irreversible airway remodeling associated with a predominantly neutrophilic immune response (2, 236). Heightened neutrophil-induced airway inflammation is linked with infiltration of T helper 17 (TH17) cells and their secreted cytokines. Additionally, many individuals with severe asthma present with elevated levels of transforming growth factor-β1 (TGF-β1), which significantly contributes to airway remodeling and abnormal function that correlates with enhanced TH17 activity (2). Interestingly, TGF-β1 potently suppresses the differentiation of TH1 and TH2 cells but is essential to polarizing naïve T cells toward a TH17 fate (102). Identification of this TH17-driven endotype has increased our understanding of severe asthma pathogenesis, yet the complex interactions linking its unique signaling pathways with those of other T cells and structural airway cells remain marginally understood. This notion, along with increased disease prevalence and severity, underscores the challenge of advancing therapeutic alternatives for individuals with severe asthma (19, 26, 171). This review seeks to provide a comprehensive summary of TH17 fate determination and modulation of airway inflammation through interactions with disparate T cell- and airway-derived immune and regulatory growth factors. We aim to analyze evidence that TH17-secreted interleukin-17A (IL-17A), acting in concordance with known TGF-β1 mechanisms, contributes to enhanced ASM remodeling and altered function in TH17-driven endotypes of severe asthma.
IMMUNITY IN ASTHMA
Role of Innate and Adaptive Immunity in Asthma
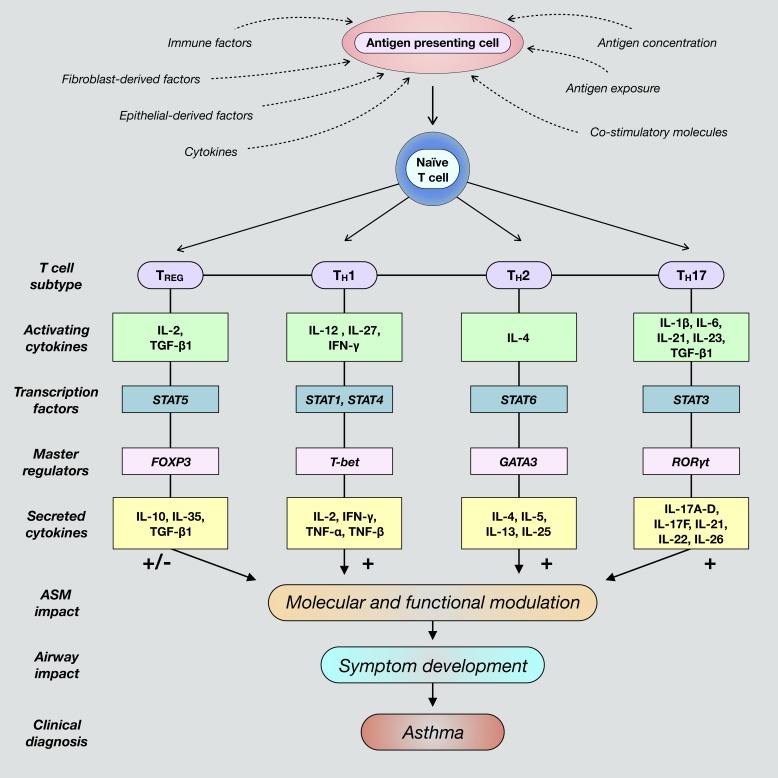
The innate and adaptive branches of the human immune system work in tandem to coordinate host defense, and their role in aberrant immune responses culminates in the development of inflammation-induced disease processes. Innate immunity provides for an immediate, albeit short-lived defense against pathogenic infection that ultimately activates and directs adaptive immunity. Adaptive immunity functions through production of long-lasting immunological memory and a sustained inflammatory response until clearance of foreign pathogens is achieved (153). T helper cells are a vital component of cell-mediated adaptive immunity, and their role in promoting airway inflammation is well established, as they contribute to coordination of immune cells and secretion of proinflammatory factors (Fig. 1; 127, 149). Naïve cluster of differentiation 4-positive (CD4+) T cells express receptors with an affinity for major histocompatibility complex (MHC) class II molecules. Following foreign antigen recognition by specialized antigen-presenting cells (APCs), which constitutively bear MHC class II molecules, APCs bind with cognate MHC receptors on naïve T cells. This distinctive interaction is a crucial initiator of immune signaling cascades, as it induces and directs differentiation of naïve CD4+ T cells into specific effector T cells (Fig. 1). In addition to antigen-specific APC interaction, T cell polarization is determined by the presence of costimulatory molecules and the precise cytokine composition within a given inflammatory microenvironment (153). Mature T cells mediate inflammatory immune responses through activation of cytotoxic CD8+ T cells, maturation of B cells, antibody secretion, and direct/indirect production of cytokines, chemokines, and growth factors (153). Although the regulation of T cell lineage commitment has been extensively studied, mechanisms through which complex downstream signaling networks impact ASM remodeling and functionality, particularly in severe asthma, remain uncertain.
Fig. 1.
Differentiation of naïve cluster of differentiation 4-positive (CD4+) T cells toward polarized, individual effector T cell fates is determined by the presence of unique activating factors and molecules present within a given inflammatory airway microenvironment. These commonly include various factors secreted by epithelial cells and fibroblasts, such as cytokines and chemokines. Additionally, T cell differentiation is promoted when antigen-presenting cells are exposed to foreign antigens and subsequently interact with naïve T cells. Classical T cell subtypes involved in airway immunity include regulatory T (TREG), T helper 1 (TH1), TH2, and TH17 cells. Mature T cells are characterized by the expression and activation of subtype-specific transcription factors and master regulators that ultimately induce secretion of unique cytokines contingent upon differentiation fate. These cytokines activate cognate receptors expressed by structural airway cells that direct immune responses and contribute to dysregulated airway smooth muscle (ASM) function and asthma pathogenesis. FOXP3, forkhead box protein P3; GATA3, trans-acting T cell-specific transcription factor GATA-3; RORγt, retinoic acid receptor (RAR)-related orphan receptor-γ, thymus; T-bet, T cell-specific T-box transcription factor T-bet; TGF-β1, transforming growth factor-β1.
Distinctions Between Nonsevere and Severe Asthma
There are currently two broadly accepted forms of asthma, allergic and nonallergic, with the latter comprising the majority of severe asthma cases (123). The mechanistic distinctions between nonsevere and severe asthma can partially be attributed to pronounced infiltration of TH17 cells within the airways. Allergic asthma is an early-onset disease associated with TH2-mediated eosinophilic airway inflammation, increased immunoglobulin E (IgE) levels, and mild-to-moderate severity that effectively responds to glucocorticoid treatment. As a result of complex genetic and environmental interactions, this extrinsic form of asthma usually develops subsequent to allergen exposure. This culminates in an exaggerated TH2 immune response that induces production of proinflammatory factors that promote airway inflammation (170). TH2-mediated inflammation is normally constrained by TH1-derived immune mediators, which are prominent in the airways of nonasthmatic individuals following allergen exposure. This observation is the foundation of the established paradigm that individuals with allergic asthma acquire a shift from baseline TH1 responses to unrestrained TH2 immune responses. Moreover, single-nucleotide polymorphisms of TH2-associated genes known to promote allergic immune responses can be used to predict inherent disease susceptibility of individuals with allergic asthma (62). T cell contribution to airway inflammation and ASM functionality is neither mutually exclusive nor mechanistically straightforward. This is underscored by the known role of TH1-derived interferon-γ (IFN-γ) in the development of airway hyperresponsiveness (AHR), which is further contingent upon its interaction with numerous regulatory factors (210). Whereas the mechanisms surrounding TH2-mediated allergic asthma are well defined and identify a number of therapeutic targets with varying efficacy, those associated with TH17-driven severe asthma and nonallergic airway immunity remain less understood (226).
Severe asthma is often a late-onset disease that can develop as a result of acquired airway hypersensitivity, tissue damage, and functional complications following bacterial, fungal, and/or viral infections (Fig. 1; 65, 216). Recurrent infections, physical stress, genetic susceptibilities, and exposure to a multitude of nonspecific environmental stimuli induce chronic ASM remodeling and can trigger persistent bronchospasms that result in life-threatening airway obstruction. Heightened serum levels of IL-17A are an independent risk factor for development of severe asthma and neutrophil-linked ASM remodeling and hypercontractility (1, 35). In addition to pleiotropic effects on ASM, TH17-secreted mediators reduce epithelial integrity and induce airway inflammation, fibroblast migration, and mucus cell metaplasia, which can reciprocally impact ASM phenotype (Fig. 5; 41, 42, 48, 52, 135, 176). Moreover, glucocorticoid resistance in severe asthma may partially be explained by a TH17-predominant immune response, as TH17 activity is largely absent or inconsequential in steroid-responsive forms of asthma. However, a dialogue surrounding the explicit role of TH17 cells in severe asthma remains, as evidenced by findings suggesting that IL-17A mediates anti-inflammatory pathways and alone is not sufficient to trigger asthmatic symptoms under certain conditions (63). Interestingly, TH1, TH2, and TH17 immunity may have cooperative roles that additively enhance asthma severity or interactions that suppress the activity of one another (Fig. 2; 15, 56, 121).
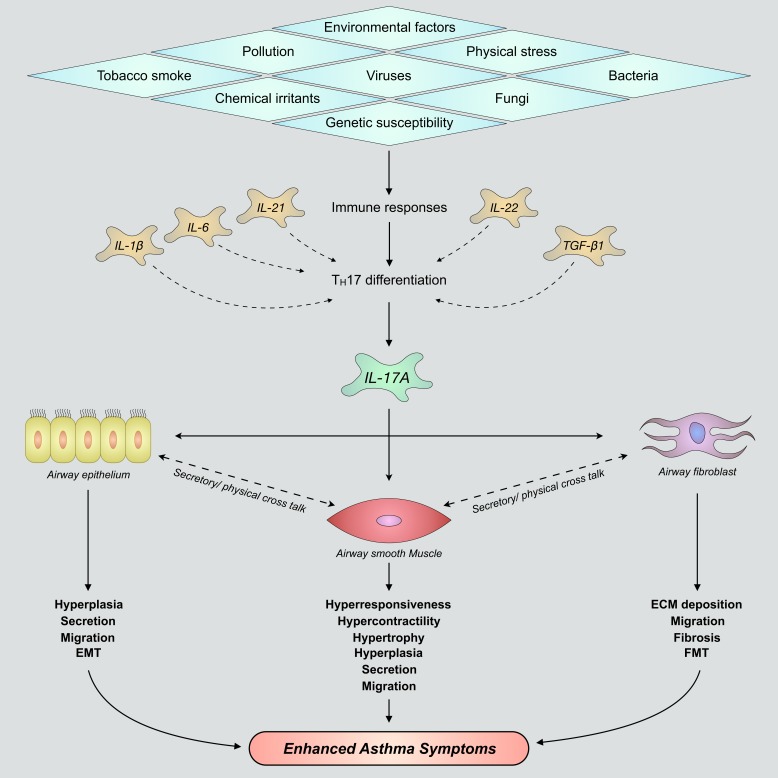
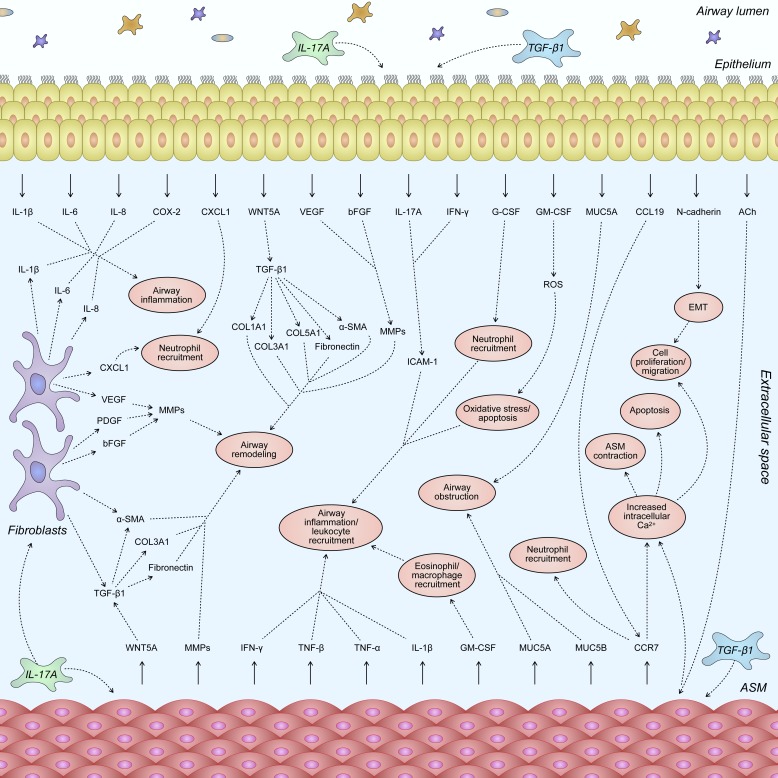
Fig. 5.
Nonspecific inflammatory stimuli induce immune responses that promote T helper 17 (TH17) cell differentiation and production/secretion of IL-17A within immune-infiltrated airways. IL-17A variably acts upon airway epithelium, airway smooth muscle, and lung fibroblast cells to promote elevated airway remodeling and development of severe asthma. Although each of these structural cells imparts a unique effect on airway remodeling following stimulation with IL-17A, there is significant cellular cross talk between tissues. This bears potential to further enhance TH17-mediated effects surrounding airway smooth muscle hyperplasia and hypertrophy, goblet cell mucus secretions, epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) deposition, fibroblast-to-myofibroblast transition (FMT), and airway hyperresponsiveness. TGF-β1, transforming growth factor-β1.
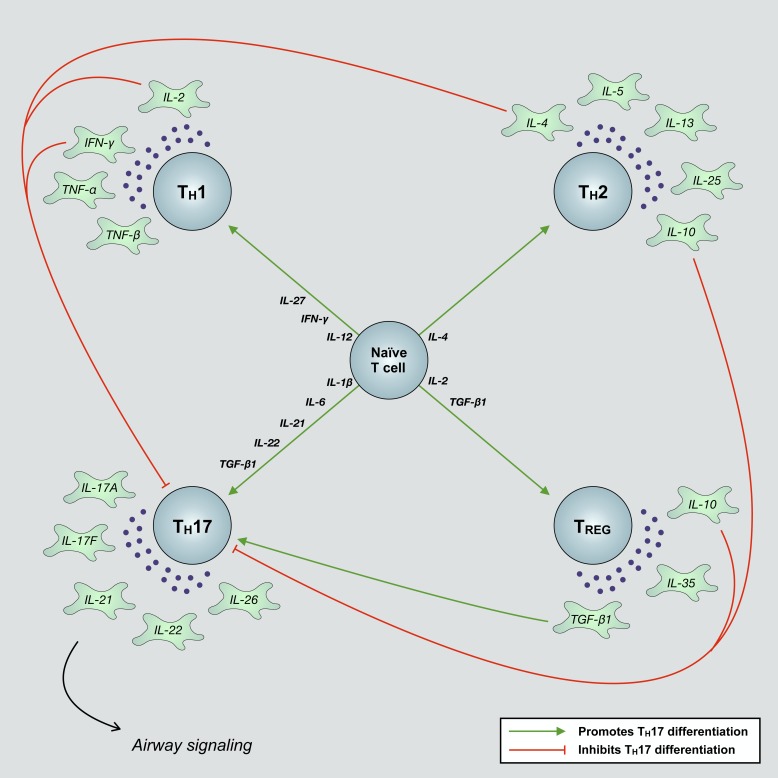
Fig. 2.
Various immune and structural airway cells secrete inflammatory factors that promote the differentiation of naïve T cells into mature effector subtypes. These T cells further secrete subtype-dependent cytokines that bear the ability to promote and/or suppress the differentiation of disparate T cell subtypes. T helper 1 (TH1)- and TH2-derived IL-2, IL-4, IL-10, and IFN-γ inhibit the differentiation of naïve T cells into mature TH17 effector cells. Regulatory T (TREG) cells, depending on the concentration of their secreted cytokines, transforming growth factor-β1 (TGF-β1) and IL-10, can either promote or inhibit polarization toward a TH17 fate. Observations of such mechanisms, which ultimately alter airway function, underscore the complex immune signaling networks that regulate T cell differentiation and may in part explain the acquisition of mixed T cell endotypes in distinct individuals with asthma.
The complexity of TH17-driven severe asthma pathogenesis is further compounded by its interactions with TGF-β1. TGF-β1 is required for the differentiation of mature TH17 cells from naïve T cells, whereas IL-17A is known to enhance TGF-β1 signaling via cognate receptor upregulation (73, 234). Enhanced TGF-β1 expression is positively correlated with disease development, as its levels are elevated beyond those typically seen in mild-to-moderate asthma (2). Moreover, TGF-β1 is a well-known contributor to airway remodeling and ASM contraction and is a potential therapeutic target for reducing asthma morbidity and mortality (180, 212). This makes a compelling argument that IL-17A and TGF-β1 may cooperatively act to promote ASM-mediated pathophysiology. To appreciate how this relationship is impacted by opposing actions of diverse T cell populations, one must understand how the precise composition of the inflammatory milieu differentially regulates TH17-mediated ASM functionality. Further investigation into TH17-mediated ASM remodeling may aid in identifying novel therapeutic targets that overcome ineffective and/or detrimental effects of present treatments (4, 241, 242).
STRUCTURE AND SIGNALING OF IL-17 CYTOKINES
Discovery and Significance of TH17 Cells and IL-17A
In 1993, the gene encoding IL-17A was discovered by subtractive screening of a hybridoma cDNA library as mouse cytotoxic T lymphocyte-associated antigen-8 (CTLA8; 201). TH17 cells were distinguished as the primary cellular source of IL-17A following their identification a few years later (80, 97, 249). TH17 cells secrete four additional members of the IL-17 cytokine family known as IL-17B, IL-17C, IL-17D, and IL-17F, whereas IL-17E is secreted by TH2 cells (Fig. 3). IL-17E, hereinafter referred to as IL-25, has low sequence homology to IL-17A and activates signaling pathways with unique functional outcomes compared with other IL-17 cytokines (Fig. 3). TH17 cells further secrete proinflammatory IL-21, IL-22, and IL-26, which induce structural airway cells to secrete, among others, IL-6, TGF-β1, tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and C-X-C motif chemokine ligand 8 (CXCL8/IL-8; 57). Jointly, these cytokines promote autocrine/paracrine feedback loops of TH17 differentiation through enhanced activation of mitogen-activated protein kinase (MAPK), nuclear factor-κ-light chain enhancer of activated B cells (NF-κB), and retinoic acid receptor (RAR)-related orphan receptor-γ, thymus (RORγt), pathways. TH17 enhancement of TGF-β1 signaling (Fig. 8) underscores the reciprocal relationship of these pathways in maintaining TH17 activity and promoting IL-17A-dependent ASM remodeling. Although TH17-induced production of G-CSF enhances levels of various anti-inflammatory factors, it maintains the capacity to promote proliferation, survival, and chemotaxis of neutrophils, much like GM-CSF. TH17-induced neutrophil influx to the airways and aberrant IL-17A secretion also enhance cyclooxygenase-2 (COX-2) expression and activity (Fig. 7; 202). By enhancing prostacyclin (PGI2) and prostaglandin F2α (PGF2α) autocrine signaling, COX-2 enhances maintenance of IL-17A signaling, airway inflammation, and AHR (146). Apart from severe asthma, dysregulated TH17 immunity is implicated in multiple human autoimmune and inflammatory diseases. Enhanced TH17 activity is correlated with the development of rheumatoid arthritis, systemic lupus, and multiple sclerosis, whereas acquisition of hyper-IgE syndrome, staphylococcal abscesses, and mucocutaneous candidiasis has been observed in TH17-deficient individuals (119, 165, 176, 185). These observations highlight the significance of TH17 homeostasis surrounding autoimmunity and maintenance of mucosal barriers, as even slight alterations promote the acquisition of inflammatory diseases. The composition of cytokines within the inflammatory milieu selectively alters differentiation of naïve T cells (159). Counter to pathogenic TH17 cells, regulatory T (TREG) cells are protective cells that maintain immune homeostasis and restrain proinflammatory responses. Although TREG cells require TGF-β1 for their differentiation, much like TH17 cells, the precise concentration of TGF-β1 influences activation of opposing transcription factors (Fig. 4). Thus, understanding the regulation of TH17 and TREG differentiation in conjunction with various cytokines and growth factors is pivotal to gaining an understanding of mechanistic discrepancies within TH17-mediated severe asthma.
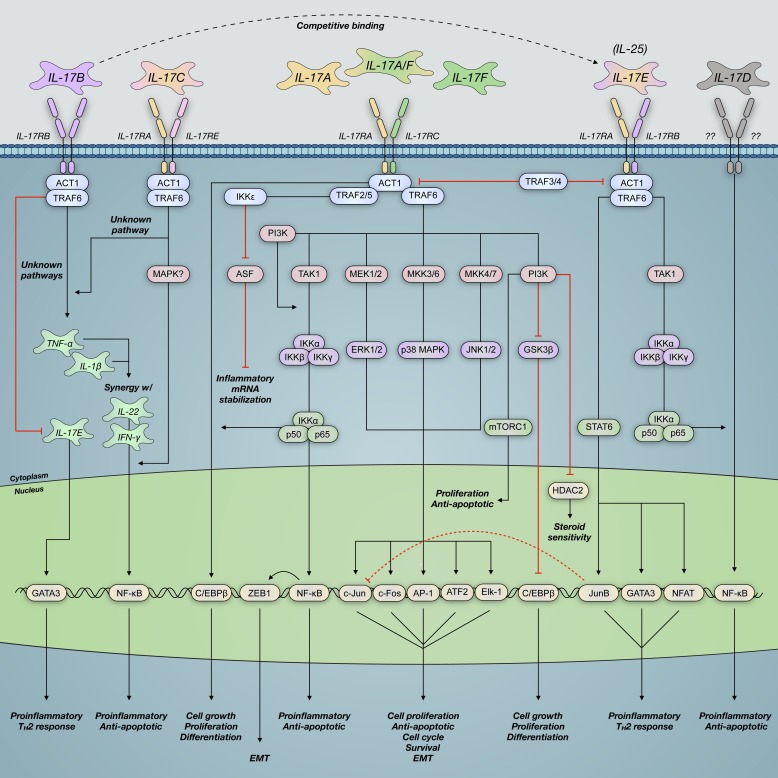
Fig. 3.
IL-17 family cytokines signal through heterodimeric IL-17 surface receptor subunits. This results in NF-κB activator (ACT)/TNF receptor-associated factor (TRAF) recruitment and activation of downstream effectors that impact gene transcription airway functionality. IL-17A binds its cognate IL-17 receptor A and C (IL-17RA/C) complex and activates mitogen-associated protein kinase (MAPK), phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K), and nuclear factor-κ-light chain enhancer of activated B cells (NF-κB) signaling pathways. These pathways contribute to altered airway smooth muscle function, airway remodeling, and development of symptoms consistent with severe asthma. Although other IL-17 cytokines signal via similar pathways, their mechanisms and functions within the asthmatic airway remain poorly characterized compared with those of IL-17A. AP-1, transcription factor AP-1; ASF, arginine- and serine-rich splicing factor; ATF2, cyclic AMP-dependent transcription factor ATF-2; C/EBPβ, CCAAT/enhancer-binding protein-β; Elk-1, E26 transformation-specific (ETS) domain-containing protein Elk-1; EMT, epithelial-mesenchymal transition; GATA3, trans-acting T cell-specific transcription factor GATA-3; HDAC2, histone deacetylase 2; mTORC1, mammalian target of rapamycin complex 1; NFAT, nuclear factor of activated T cells; TAK1, transforming growth factor-β (TGF-β)-activated kinase 1; TH2, T helper 2; w/, with; ZEB1, zinc finger E-box-binding homeobox 1.
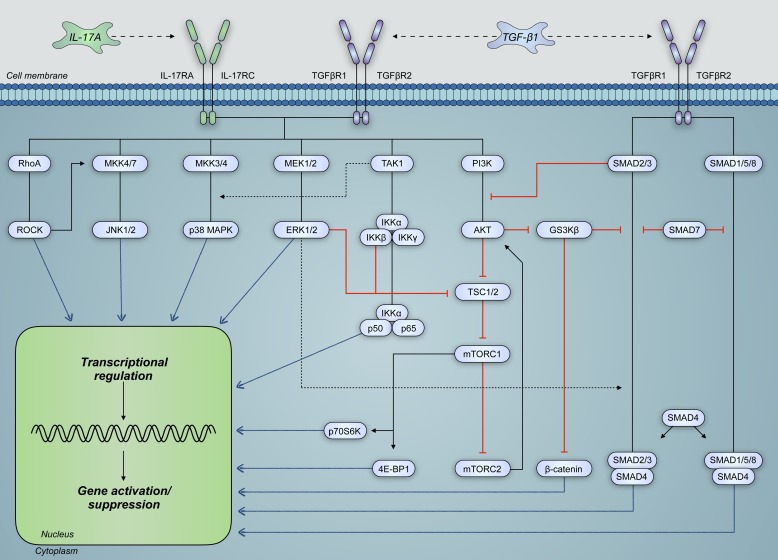
Fig. 8.
IL-17A and transforming growth factor-β1 (TGF-β1) share significant cross talk in the regulation and activation of signaling pathways pertinent to structural airway cell function. These signaling pathways, which are intricately connected, can modulate the activity of one another such that overall transcriptional regulation is altered. Moreover, TGF-β1 signaling activates signal transducer mothers against decapentaplegic homolog (SMAD) pathways, which can interact with IL-17A-activated pathways to modulate gene expression. Such pathways include those of Ras homolog gene family, member A (RhoA), mitogen-activate protein kinases (MAPKs), and phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K). 4E-BP1, eukaryotic translation initiation factor 4E-binding protein-1; GS3Kβ, glycogen synthase kinase-3β; IL-17RA and IL-17RC, IL-17 receptors A and C, respectively; mTORC, mammalian target of rapamycin complex; p70S6K, ribosomal protein S6 kinase β-1; ROCK, Rho-associated protein kinase; TAK1, TGF-β-activated kinase 1; TGFBR, TGF-β receptor; TSC, tuberous sclerosis protein.
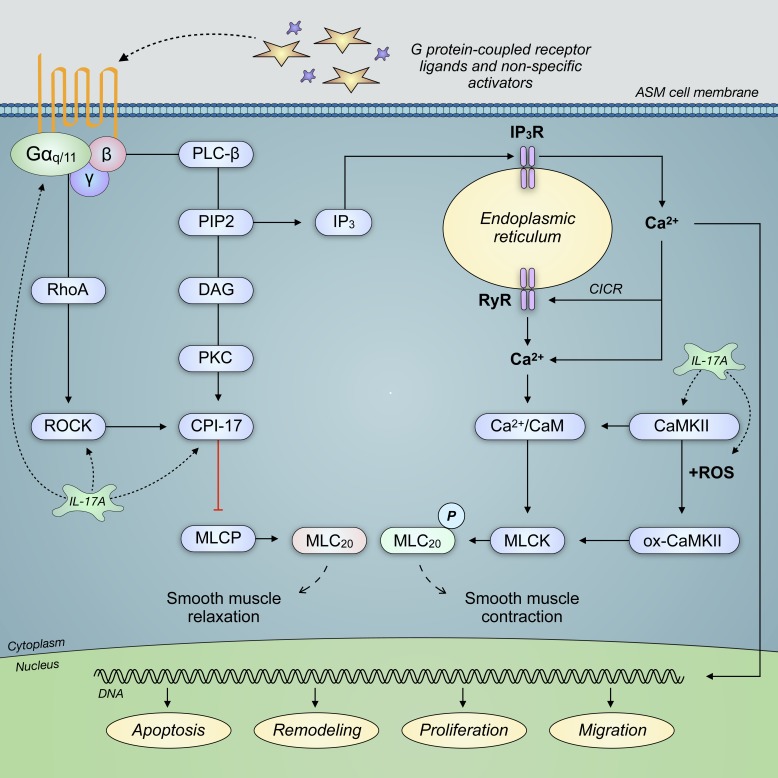
Fig. 7.
IL-17A signaling is correlated with enhanced expression and activation of Ras homolog gene family, member A (RhoA), Rho-associated protein kinase (ROCK), C-kinase-activated protein phosphatase-1 inhibitor (CPI-17), and Ca2+/calmodulin-dependent protein kinase II (CaMKII). These G protein-coupled receptor effectors regulate the phosphorylation (P) of myosin light chain, which modulates smooth muscle contraction. Enhanced contractile force of airway smooth muscle (ASM) cells is known to lead to airway obstruction through a reduced airway lumen diameter. Moreover, oxidized CaMKII (ox-CaMKII) is a product of CaMKII interaction with reactive oxygen species (ROS), which may promote airway reactivity. CICR, calcium-induced calcium release; DAG, diacylglycerol; IP3, inositol (1,4,5)-trisphosphate; IP3R, IP3 receptor; MLC20, myosin regulatory light chain 20 kDa; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; PIP2, phosphatidylinositol 4,5-bisphosphate; RyR, ryanodine receptor.
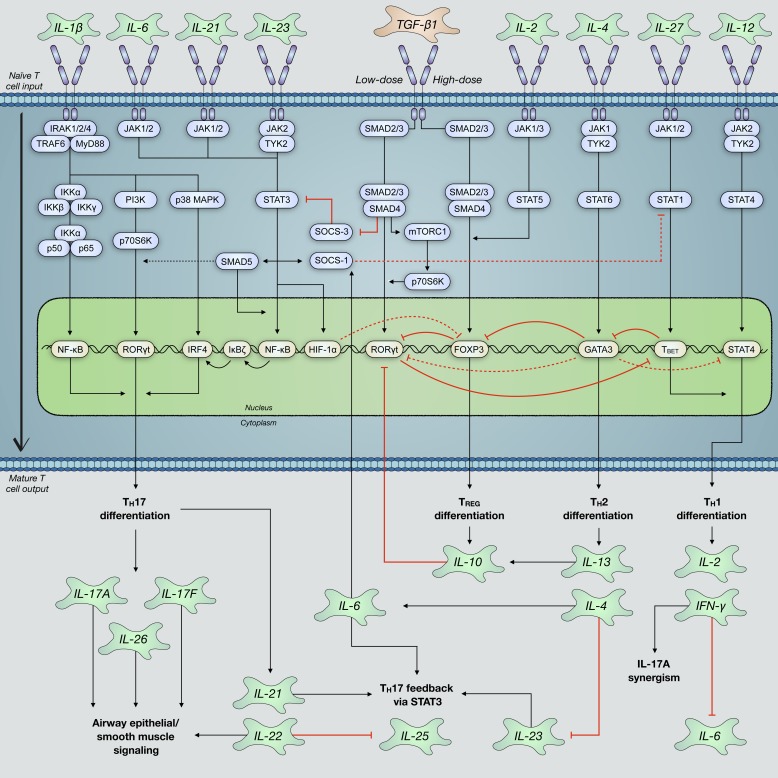
Fig. 4.
T helper 17 (TH17) cell differentiation relies on the coordination of several well-characterized cytokines and their activation of cognate receptors on the surface of naïve T cells. IL-6 and transforming growth factor-β1 (TGF-β1) are the most prominent drivers of TH17 polarization via canonical signal transducer mothers against decapentaplegic homolog (SMAD) and signal transducer and activator of transcription 3 (STAT3) pathways. This enhances activation of TH17-necessary transcription factor retinoic acid receptor (RAR)-related orphan receptor-γ, thymus (RORγt). Other cytokines, including IL-1β, IL-21, and IL-23, can further drive TH17 differentiation and, moreover, promote their sustained maintenance. Regulatory T (TREG)-, TH1-, and TH2-associated cytokines typically activate signaling pathways that repress RORγt activation and inhibit TH17 differentiation. However, depending on the precise composition of cytokines exposed to naïve T cell surface receptors and interactions between intracellular signaling pathways, there is evidence of increased TH17 differentiation by these cytokines. FOXP3, forkhead box protein P3; HIF-1α, hypoxia-inducible factor 1α; IRAK, IL-1 receptor-associated kinase; IRF-4, interferon regulatory factor 4; mTORC1, mammalian target of rapamycin complex 1; MyD88; myeloid differentiation primary response protein MyD88; p70S6K, ribosomal protein S6 kinase β-1; PI3K, phosphatidylinositol 4,5-bisphosphate 3-kinase; SOCS, suppressor of cytokine signaling; T-bet, T cell-specific T-box transcription factor T-bet; TRAF6, TNF receptor-associated factor 6; TYK2, nonreceptor tyrosine-protein kinase TYK2.
IL-17A and IL-17F
IL-17A and IL-17F are the best characterized IL-17 cytokine family members. Their encoding genes, Il17a and Il17f, share the highest homology (~56%) and have adjacently located transcripts, suggesting evolutionary gene duplication and shared regulatory elements (87, 114, 253). IL-17A and IL-17F can be secreted as disulfide-linked homodimers or as a heterodimer, which has implications in their overlapping functions (Fig. 3). These molecules signal via a heterodimeric receptor complex composed of the IL-17 receptor A (IL-17RA) and IL-17 receptor C (IL-17RC) subunits yet have differing binding affinities. IL-17A has >100-fold the affinity for IL-17RA than IL-17F has but binds with equal affinity to IL-17RC (244). The IL-17A/F heterodimer possesses an intermediate binding affinity, such that biological activity positively correlates with an affinity gradient of IL-17A > IL-17A/F > IL-17F (81, 244). Following ligand activation of the IL-17RA/C complex, binding of adapter protein CIKS [NF-κB activator 1 (ACT1)] promotes recruitment of TNF receptor-associated factor 6 (TRAF6) to the IL-17RC subunit for ubiquitination (38, 40, 81). This activates downstream TGF-β-activated kinase 1 (TAK1), leading to NF-κB activation and induction of stress-induced cellular responses, including enhanced IL-6 production (Fig. 3; 172).
Although IL-17A is a fairly weak activator of NF-κB, its pathogenic potential lies within its ability to synergize with various cytokines to augment proinflammatory responses (87). NF-κB-dependent luciferase activity confirms that low-dose TH17 secretion of TNF-α and IL-17A activates NF-κB more than IL-17A alone, leading to expression of unstable proinflammatory mRNAs such as IL-8, CXCL1, and COX-2 (74, 99, 104, 105). This contributes to efficient translation of mRNAs, as evidenced by IL-17A alone inducing the expression of 248 proinflammatory genes and inducing 9,803 when combined with TNF-α (110). Moreover, through activation of an IκB kinase-ε (IKKε)/TRAF2/TRAF5-dependent pathway and inhibition of arginine- and serine-rich splicing factor (ASF)-dependent 3′-untranslated region cleavage, IL-17A enhances the stability of normally unstable proinflammatory mRNAs such as CXCL1 (Fig. 3; 28, 222). IL-17A can synergize with IL-1β and IFN-γ to augment proinflammatory pathways and further induce IL-1β expression (110, 182). This mechanism enhances expression of IL-8 mRNA and protein translation through MAPK-modulated promotor activity and cooperation with NF-κB cis-acting elements and transcription factor AP-1 (AP-1; 69). Increased secretion of IL-6 and IL-8, along with weak induction of intercellular adhesion molecule 1 (ICAM-1), is a direct result of IL-17A and IFN-γ synergism (231). As an integrin-binding protein, ICAM-1 is involved in the migration and recruitment of proinflammatory immune lymphocytes to airway epithelial and smooth muscle cells (86, 231). ICAM-1 is significantly increased in human subjects with severe asthma and is a marker of persistent airway inflammation (83). Interestingly, TH17 differentiation is negatively regulated by IFN-γ; thus, further elucidation of the mechanisms regulating TH17 differentiation and modulation is necessary. In addition to airway inflammation, IL-17A and IL-17F induce numerous antimicrobial factors, matrix metalloproteinases (MMPs), and granulopoetic factors that can contribute to airway remodeling (10, 79, 118, 124).
Although IL-17A and IL-17F share significant biological activity, their production and inhibition are partially regulated by distinct mechanisms. The phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway and prostaglandin EP receptor agonists, such as prostaglandin E2 (PGE2), enhance the production and secretion of IL-17A (163, 233). However, IL-17F production is inhibited by PGE2 and increased via NF-κB activation through Toll-like receptor 2 (TLR2) and TLR5 pathways (163). These observations contradict evidence of PGE2 acting as an anti-inflammatory mediator in asthma and other lung disorders; however, TH17-independent mechanisms may explain this discrepancy (17, 206). PI3K is an intriguing therapeutic target, as studies highlight its role in ASM remodeling, migration, contraction, and cytokine secretion. Although PI3K activation can also be triggered by IL-1β signaling, IL-17A can impart regulation through its ability to enhance IL-1β production. Induction of PI3K signaling is also sufficient for enhanced DNA synthesis and cellular growth, which may directly impact ASM cell proliferation and remodeling (250). Moreover, findings suggest that IL-17A contributes to glucocorticoid insensitivity in airway epithelial cells via PI3K activation and ensuing reduction in histone deacetylase 2 (HDAC2; Fig. 3; 260). Glucocorticoid treatment can significantly reduce IL-8 serum levels in patients with allergic asthma yet is largely ineffective in steroid-resistant individuals, who often bear signatures of nonallergic asthma (252). Considering that IL-17A promotes activation of PI3K and IL-8 secretion, poor glucocorticoid responses in individuals with severe asthma could potentially be explained through this mechanism (148). Reciprocal IL-17A-induced PI3K activation and PI3K regulation of IL-17A identify a targetable signaling pathway that could hold therapeutic promise against neutrophilic and steroid-resistant severe asthma. As IL-17A-induced PI3K activation contributes to the glucocorticoid insensitivity, this argument could become increasingly compelling following mechanistic elucidation (260). These observations warrant further investigation into the modulation of IL-17A and PI3K, along with their compounding effects on ASM-mediated airway obstruction in severe asthma.
Negative feedback loops can counteract aberrant TH17 activity and inflammatory responses. IL-17A-induced recruitment of TRAF4 to the IL-17RA/C complex competes with TRAF6 for ACT1 binding (251). Along with TRAF3, which binds the complex directly, TRAF4 is a negative regulator of IL-17A-induced NF-κB activation (Fig. 3; 258). Loss of negative regulation is rapidly detrimental, as IL-17A-dependent NF-κB activation leads to downregulation of micro-RNA (miR)-23b. Under normal conditions, miR-23b functions to suppress NF-κB via gene silencing of IKKα and TAK1-binding protein-2 (TAB2; 259). Downregulation of miR-23b creates a feedback loop of sustained NF-κB activation and proinflammatory cytokine expression. Interestingly, miR-23b also functions as an inhibitor of TGF-β1-induced asthmatic ASM remodeling and proliferation via silencing of mothers against decapentaplegic homolog 3 (SMAD3). This demonstrates another example of how IL-17A and TGF-β1 signaling pathways can function to enhance the activity of one another in a manner that promotes airway complications in severe asthma. Together, observations suggest that numerous mechanisms surrounding IL-17A signaling exist that regulate or enhance ASM remodeling and identify potential targets for miR/effector-based therapeutics in IL-17A-induced severe asthma (44).
IL-17B, IL-17C, IL-17D, and IL-25
The remaining TH17-derived members of the IL-17 cytokine family have considerably less sequence identity with IL-17A but continue to share a highly conserved COOH terminus and several cysteine residues (183). The functions of IL-17B, IL-17C, and IL-17D, which are encoded by the Il17b, Il17c, and IL17d genes, respectively, remain relatively obscure compared with those of IL-17A and IL-17F. Biochemical analysis indicates that IL-17B secretes as a non-disulfide-linked homodimer and binds specifically to IL-17 receptor B (IL-17RB; Fig. 3; 214). IL-17B-enhanced production of IL-1β and TNF-α positively correlated with inflammatory pathogenesis in a murine model of collagen-induced arthritis, which corresponds to similar regulation in airway cells by IL-17A (147, 245). However, whereas IL-1β reciprocally elevated IL-17B levels in bovine cartilage explants subjected to mechanical injury, IL-17B failed to induce secretion of proinflammatory mediators in colonic epithelial cells and fibroblasts (147, 199, 219). Considering that IL-17A promotes IL-1β secretion that may in turn enhance IL-17B production and IL-17B-induced production of IL-1β and TNF-α bears the potential to promote synergism with IL-17A, these observations identify a potential feedback mechanism of IL-17-driven inflammation that could exist within TH17-infiltrated airways. IL-17B binding to the heterodimeric IL-17RA/B complex, which is also necessary for IL-25 signaling, inhibits IL-25-mediated IL-6 production via competitive binding and may in part function as a protective cytokine by suppressing TH2 immune responses and reducing TH17 differentiation (199).
IL-17C signals through a heterodimeric IL-17A/E receptor complex and enhances ACT1-dependent activation of NF-κB and MAPK pathways (Fig. 3). IL-17C similarly enhanced production of IL-1β and TNF-α in immature fibroblasts, known as fibrocytes, and synergized with IL-22 to increase production of antimicrobial peptides in a murine model of Citrobacter rodentium challenge (147, 217, 245). Reciprocally, IL-1β and TNF-α induced IL-17C secretion from mucosal epithelial cells, and in an in vivo model of dextran sulfate sodium-induced colitis, IL-17C induced pathogenic activity (194). Loss of IL-17C signaling resulted in initial reduction in epithelial defense, whereas intranasal administration of adenovirus expressing IL-17C in mice resulted in increased bronchoalveolar lavage fluid neutrophilia and induction of proinflammatory genes (113). Primary human bronchial epithelial cells had significantly increased IL-17C mRNA and protein expression following treatment with polyinosinic-polycytidylic acid, which activates TLR3 signaling (139). This correlated with increased secretion of antimicrobial peptides and proinflammatory cytokines, including human β-defensin 2 (hBD2) and colony-stimulating factor 3 (CSF3; 139). These observations suggest that IL-17C may enhance TH17-mediated neutrophilic infiltration and inflammation within airways, although further investigation is necessary.
IL-17D is the least understood IL-17 cytokine, as its cognate signaling receptor and mechanistic function in the human airways remain controversial. Although there is no observed synergy between IL-17D and other proinflammatory cytokines, treatment of human endothelial cells with IL-17D upregulated IL-6, IL-8, and GM-CSF production (218). Although the full mechanism is not known, IL-8 promoter-reporter assays indicate that IL-17D increases IL-8 in an NF-κB-dependent manner (218).
IL-25 shares the least homology (~16%) with IL-17A and is secreted by activated TH2 cells (112). Signaling occurs via the IL-17RA/B heterodimeric complex, the subunits of which are expressed by various cell types and are also required for IL-17A-, IL-17B-, IL-17C-, and IL-17F-mediated signaling (Fig. 3; 142). Much like TH17-derived cytokines, IL-25 activates NF-κB and enhances secretion of proinflammatory cytokines, such as IL-8, yet primarily functions to enhance TH2 immune responses. IL-25 may additionally signal through TRAF6-independent mechanisms that activate extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 MAPK pathways (39, 223). Considering that IL-25 requires recruitment of ACT1 to the receptor complex, there may exist downstream variations surrounding ACT1 signaling via TRAF-independent factors. IL-25 signaling promotes IL-4-, IL-5-, IL-10-, and IL-13-mediated eosinophilic allergic responses that sharply contrast and suppress TH17-potentiated neutrophilic recruitment (77, 87, 142). Although IL-25 is produced by lung epithelial and endothelial cells following allergen stimulation, the signaling mechanisms that regulate expression are poorly known (7, 117). However, epithelial MMP7 enhanced allergic lung inflammation in mice through promotion of IL-25-induced eosinophilia and extracellular matrix (ECM) remodeling (84). Further mechanistic clarification surrounding enhanced IL-25 activity could provide insight into the development of dual-positive TH2/TH17 endotypes and how distinct responses simultaneously exist within individual patients with asthma and/or how they silence one another under specific inflammatory conditions.
TH17 DIFFERENTIATION
Regulation by TGF-β1 and IL-6
TH17 differentiation is a multifactorial process involving transcription factors dissimilar to those of TH1 and TH2 lineages, including RORγt and signal transducer and activator of transcription 3 (STAT3; Fig. 1; 122). TH17 polarization requires a far higher antigen concentration compared with TH1 and TH2 cells, which ultimately enhances necessary CD40 ligand (CD40L) expression on naïve T cells (116). CD40 mediates dendritic production of IL-6, which synergizes with cellular sources of TGF-β1 to modulate TH17 differentiation in a dose-dependent manner. TGF-β1 is a crucial immunomodulatory and remodeling mediator secreted by airway-infiltrating immune, smooth muscle, epithelial, endothelial, and fibroblast cells (96). However, TGF-β1 function surrounding TH17 differentiation remains controversial, as it plays divergent roles contingent upon its endogenous concentration.
Low-dose TGF-β1 synergizes with IL-6 to promote a positive feedback loop of TH17 differentiation through increased STAT3 signaling, activation of RORγt, and suppression of IL-2 signaling and T cell-specific T-box transcription factor T-bet (T-bet; Fig. 4; 157, 228). Activation of RORγt promotes Il17a gene expression via binding of the Il17a regulatory region and enhances its secretion along with that of IL-17B, IL-17C, IL-17D, and IL-17F (247). Reciprocally, IL-17A promotes secretion of IL-6 via induction of STAT3/JNK2 signaling in an AKT-dependent manner, supporting a positive feedback loop of TH17 differentiation (Fig. 4; 88). In contrast, high-dose TGF-β1 induces the master regulator transcription factor of TREG cells, forkhead box protein P3 (FOXP3), and suppresses TH17 differentiation by inhibiting transcriptional activation of RORγt (Fig. 4; 157, 257). However, STAT3 activation inhibits FOXP3 expression via hypoxia-inducible factor 1α (HIF-1α) and restrains TREG development (213). By increasing IL-10 signaling, high-dose TGF-β1 potentiates autoimmunity by restraining TH17 development and overriding preceding lineage commitment (161). Together, low-dose TGF-β1 and IL-6 enhance STAT3-mediated RORγt transcriptional activation and promote feedback loops of TH17 maintenance, whereas high-dose TGF-β1 opposes these actions to maintain immune homeostasis. This is further evidenced by low-dose TGF-β1-induced inhibition of suppressor of cytokine signaling 3 (SOCS-3), which functions as a negative regulator of STAT3 and TH17 differentiation (190).
Regardless of concentration, TGF-β1 regulates T cell polarization and TH17-driven asthma through canonical pathways involving signal transducer SMAD effectors (Fig. 4). TGF-β1 binds the TGF-β receptor I and II (TGFBRI/II) heteromeric complex to induce phosphorylation of SMAD2/3, which leads to SMAD4 association and nuclear translocation (75). SMAD2 is suggested to be essential to TH17 differentiation, whereas SMAD3 plays a redundant role. In naïve T cells, treatment with TGFBRI kinase inhibitors and/or overexpression of SMAD7, which inhibits SMAD2/3 activity (Fig. 8), diminished SMAD2/3 phosphorylation and subsequent TH17 differentiation (100). However, T cells with TGFBRII deficiency completely failed to differentiate, suggesting a more prominent role of TGFBRII in activation of SMAD2/3 signaling and subsequent TH17 differentiation than of TGFBRI. TH17-polarized T cells with increased expression and activity of several SMAD-independent signaling molecules within noncanonical TGF-β1 pathways have been identified, including p38 MAPK, serine/threonine-protein kinases AKT1/AKT2, and mTOR. As a proliferation and survival regulator of structural airway cells, mTOR is positively correlated with loss of TH17/TREG homeostasis (Fig. 4; 255). PI3K- and AKT-dependent mTOR complex 1 (mTORC1) and mTORC2 expression positively regulates TH17 differentiation, as is demonstrated by its inhibition following rapamycin-dependent mTOR inhibition. Interestingly, a highly pathogenic subpopulation of TH17 cells was observed with a molecular signature of increased SMAD1/5 expression and activity, which is further regulated by TGF-β1 signaling (122). SMAD1/5 enhances activation of NF-κB and mTOR/ribosomal protein S6 kinase β-1 (p70S6K) signaling pathways, promoting TH17 differentiation. Despite associations with clinical induction of severe asthma and airway remodeling, SMAD-specific DNA-binding sites have yet to be identified within TH17-associated genes (96, 133). Nevertheless, evidence indicates that TGF-β1- and IL-6-driven TH17 differentiation is a dynamic process involving both SMAD-dependent and SMAD-independent pathways.
Regulation by IL-1β, IL-21, and IL-23
Increased levels of the proinflammatory, pleiotropic cytokine IL-1β are observed in the airways of individuals with asthma, whereas murine induction of IL-1β enhances neutrophil and macrophage airway infiltration (141). IL-1β regulates TH17 differentiation through NF-κB, PI3K, and p38 MAPK pathways (Fig. 4) and modulates epithelial goblet cell secretion of mucins, smooth muscle cell hyperplasia, and airway fibrosis in individuals with asthma (Fig. 7; 61, 141). IL-1β-dependent induction of interferon regulatory factor 4 (IRF-4) further modulates IL-21 autocrine function and supports a positive feedback loop of TH17 differentiation via STAT3 (45, 134, 238). IRF-4 deficiency resulted in decreased RORγt expression and complete inhibition of TH17 differentiation in an allergic mouse model (27). In addition to IL-17A, IL-1β promotes the secretion of TH17-derived IL-26, which augments airway inflammation and reduces epithelial integrity by disrupting cellular adhesion molecules (192, 239). This identifies a mechanism by which TH17-derived IL-26 could promote epithelial-mesenchymal transition (EMT) and increased airway wall mass. IL-26 also enhances neutrophil chemotaxis and reciprocally induces the secretion of IL-1β along with IL-8, GM-CSF, and TNF-α via IL-20R1/IL-20R activation and signaling (230). Whereas IL-1β drives TH17 differentiation, IL-21 and IL-23 play central roles in maintaining differentiation feedback loops (Fig. 4; 47, 156). IL-21 and IL-23 induction of STAT3 activates NF-κB and inhibitor of NF-κBζ (IκBζ), resulting in NF-κB/IκBζ/RORγt cooperativity that can drive TH17 differentiation in the absence of IL-6 and TGF-β1 (181). IL-21 cyclically maintains TH17 differentiation through autocrine enhanced activation of STAT3, which increases levels and duration of IL-17A secretion (238). Synergistically, IL-1β and IL-23 were found to enhance differentiation of IL-17A-producing TH17 cells, as IL-17A was not produced in the absence of both cytokines (198). In TH17 cells, activation of IL-23R induces production of TGF-β3, a growth factor implicated in chronic airway remodeling consistent with severe asthma (122, 145). IL-23R signaling further promotes secretion of IL-22, a cytokine with proinflammatory and anti-inflammatory properties, as demonstrated by IL-22-mediated attenuation of allergic inflammation via IL-25 inhibition and enhancement of IL-17A-dependent airway inflammation (14, 225). Although IL-23 is largely secreted by dendritic cells and not solely TH17 dependent, IL-23 promotes TH17 differentiation by reducing T cell sensitivity to IL-12 and reduced STAT4 phosphorylation (215). Considering that IL-12 promotes TH1-derived IL-2, IL-23 functions to enhance TH17 maintenance through suppression of TH1-mediated responses, as TH17 activity and Il17a gene expression are impaired by IL-2-mediated activation of STAT5 (248).
Regulation by IL-2, IL-4, IL-12, and IL-27
Although TH1 immune responses partially function to counteract eosinophil-dominated TH2 inflammation in mild-to-moderate asthma, there is increasing evidence of a significant TH1 presence in severe asthma despite its ability to suppress TH17 differentiation. However, an emerging paradigm suggests that although TH2-mediated responses are predominant in allergic asthma, their capacity to cooperate with or antagonize TH1- and TH17-mediated responses bears significance in severe asthma pathogenesis. This demonstrates the ability of self-promoting T cell subsets to suppress and/or enhance the activity of disparate subsets, complicating dissection of immune signaling within asthmatic airways. An essential observation here is the notion that therapeutics targeting one T cell subset could potentially enhance undesired responses of others because of the vast mechanistic dysregulation within asthma.
Severe asthma is generally associated with TH17 cytokines, but findings indicate higher percentages of TH1-derived IFN-γ+/IL-17A+ CD4+ T cells in bronchoalveolar lavage fluid from some human subjects (195). A murine model of severe asthma was developed by challenging with house dust mite (HDM) allergen with and without the bacterial second messenger cyclic diguanylate, which is a mucosal adjuvant capable of inducing a TH1/TH17-high and TH2-low immune response (72). This resulted in pronounced levels of IFN-γ and IL-17A that correlated with increased AHR and neutrophilic infiltration of the airways, respectively. Treatment with dexamethasone, a therapeutic glucocorticoid effective in TH2-high endotypes, failed to attenuate airway inflammation. However, therapeutic inhibition of IFN-γ reduces disease manifestations in acute asthma, while correlating with the development of TH17-driven severe asthma (136, 229). Undoubtedly, disease discrepancies underscore the pathogenic repercussions of dysregulated T cell polarization and effects on activation, suppression, and maintenance of distinct immunopathological pathways. TH17 function is mediated not only by IFN-γ but also by IL-27-dependent T-bet activation and subsequent inhibition of RORγt (see Fig. 4; 67, 193). Moreover, TH17 activity is impaired by TH1-derived, IL-2-mediated activation of STAT5, which directly reduces Il17a gene expression (248). As TH17 cells can induce secretion of TNF-α, a diagnostic signature for elevated TH1 activity, this observation may have implications in the identification of dual-positive TH1/TH17 endotypes of severe asthma.
Although requisite to TH17 differentiation, STAT3 enhances the interaction of TH2-promoting STAT6 with pertinent gene loci involved in T cell development (220). In addition to IL-17A, TH2-secreted IL-4 promotes the induction and secretion of TH17-inducing IL-6 (Fig. 4). Not only does IL-6 reciprocally amplify naïve T cell production of IL-4 through upregulation of nuclear factor of activated T cells (NFAT) to contribute to a positive feedback loop of TH2 differentiation, but also it is instrumental in TH1 inhibition via upregulation of SOCS-1 (66). As IL-6 is essential to TH17 differentiation, this identifies a potential route to development of dual-positive TH2/TH17 severe asthma. Whereas TH1 inhibition can promote TH17 differentiation, IL-4 limits TH17 differentiation via STAT6/trans-acting T cell-specific transcription factor GATA-3 (GATA3) activation, selective silencing of IL-23R signaling, and inhibition of RORγt (90, 157). IL-13, arguably the most studied TH2 cytokine, contributes to asthma pathogenesis via activation of eosinophils, mucus hypersecretion, and development of AHR and remodeling (226). TH17 differentiation is negatively regulated by TH2- and macrophage-derived IL-10 through an IL-13-dependent mechanism, restraining IL-17A-induced effects on structural airway cells (Fig. 4; 175). IL-10, which is also secreted by TREG cells, is an anti-inflammatory cytokine that restores immune homeostasis through inhibition of various immune cells following pathogen clearance (54, 167). However, the viewpoint surrounding TH17 inhibition remains conflicting, as IL-10 can enhance TH17-associated NF-κB transcriptional activation via promoter binding (190). Together, evidence indicates that an intricate network of endogenous cytokines tightly regulates TH17 differentiation. Considering the ability of TH1-, TH2-, and TREG-secreted cytokines to impact TH17 differentiation and vice versa, it is apparent that investigation into these mechanisms is essential to deciphering severe asthma pathophysiology.
AIRWAY REMODELING AND IL-17A
Role of ASM and IL-17A
Remodeling of the airway wall encompasses structural alterations absent in nonasthmatic individuals that contribute to asthma pathogenesis. ASM remodeling is reversible in many individuals with mild-to-moderate asthma, yet prevailing therapeutics are ineffective against persistently remodeled phenotypes associated with severe asthma. In individuals with severe asthma, remodeling is chronic and can present as a combination of increased ASM mass, EMT, fibroblast-to-myofibroblast transition (FMT), subepithelial fibrosis, ECM deposition, and/or mucus cell metaplasia (Fig. 5). Along with inflammatory mediators, ASM function is in part regulated by cross talk from various tissues such as epithelial cells, which border the airway lumen and form a barrier between ASM and the external environment (Fig. 5; 108). Epithelial exposure to physical stressors and foreign antigens induces secretion of proinflammatory factors that promote development of ASM hyperresponsiveness, hypercontractility, and remodeling. Deposition of ECM, which envelops ASM cells and provides the physical support required for maintenance of ASM function, can be influenced by ASM-secreted collagens and fibronectin, a potent chemoattractant of fibroblasts (64). Fibroblasts, which further contribute to collagen deposition, interact with airway epithelial, smooth muscle, and inflammatory cells to promote airway remodeling (246). Moreover, differentiation of fibroblasts into myofibroblasts contributes to increased ASM mass, ECM deposition, and release of proinflammatory cytokines in the presence of IL-17A (12). TH17-secreted cytokines, including IL-17A, have been shown to mediate ASM remodeling through modulation of phenotypic plasticity. Phenotypic plasticity is a reversible process of cellular maturation between proliferative and contractile states that requires changes in expression of phenotype-specific genes (94). ASM myocytes are not known to secrete IL-17A, but their functionality can be regulated through IL-17A signaling and IL-17RA/C complex activation (186). IL-17A-dependent modulation of ASM cannot be fully understood without also knowing the role of TGF-β1, as these cytokines have tightly interwoven pathways that mediate their activation and maintenance. Highlighted previously, TGF-β1 is elevated in individuals with severe asthma and positively correlates with TH17 activity. Investigation into cooperative TGF-β1 and TH17 mechanisms has the potential to identify mechanisms and distinct targets that overcome the limitations of present therapies that fail to reverse airway remodeling in severe asthma.
IL-17A- and TGF-β1-Induced EMT and Airway Remodeling
Airway EMT is a normal wound-healing process that results in reduced epithelial cell-cell interactions; however, excessive EMT results in diminished mucosal barrier integrity and increased secretion and dedifferentiation of epithelial cells into mesenchymal-like cells (49). Mucins, which are epithelium-secreted glycosylated proteins, positively correlate with chronic inflammation and enhanced asthma severity (58, 79). Mucins MUC5A and MUC5B are prominent markers of airway remodeling and are hypersecreted by epithelial goblet cells via IL-17A-dependent NF-κB and IL-6 feedback loops (Fig. 6; 46, 78, 79). Moreover, airway sputum from individuals with severe and fatal asthma is distinctively identified by abundant levels of MUC5A and MUC5B (240). Decreased epithelial integrity through downregulation of the cell adhesion molecules, such as E-cadherin, contributes to disruption of contact junctions between epithelial cells, increasing airway wall permeability to foreign antigens, secretory molecules, and inflammatory mediators (85). Dedifferentiated mesenchymal cells have highly migratory, stem cell-like properties that allow them to redifferentiate into various cell types depending on their surrounding cellular milieu. TGF-β1-induced reduction in epithelial integrity via cellular apoptosis drives subepithelial remodeling, whereas IL-17A has a correlative association between increased SMAD7 expression and TGF-β1-mediated airway remodeling (60, 92, 96). Although SMAD7 functions to inhibit TGF-β1-induced antiapoptotic effects, it can be counteracted by TGF-β1-induced signaling that promotes apoptosis in airway epithelial cells. Under normal healing conditions, glucocorticoid or Fas death receptor (CD95)-induced airway epithelial apoptosis is abrogated by TGF-β1 (96, 232). However, overexpression of SMAD7 inhibits this protective effect via suppression of SMAD2/3 signaling, and following chronic inflammation or exposure to physical stressors, TGF-β1 enhances p38 MAPK-dependent cellular apoptosis (232). In SMAD7-overexpressing mice, there were significantly reduced levels of IL-1β, IL-6, IL-17A, G-CSF, and GM-CSF, demonstrating the importance of TGF-β1-induced SMAD2/3 surrounding an elevated TH17 immune presence (154). Moreover, SMAD7-deficient T cells secreted higher levels of IL-17A, whereas SMAD7 overexpression resulted in decreased IL-17A, suggesting an inversely proportional relationship that correlates with RORγt activity (132). Interestingly, one study found that IL-17A-dependent EMT increased SMAD3 activation and downregulated SMAD7 expression in vitro (235). Considering that SMAD7 expression is thought to be inversely proportional to human bronchial hyperreactivity and airway remodeling, these observations indicate that the associations of IL-17A and TGF-β1 signaling are unreconciled and warrant further investigation (173).
Fig. 6.
IL-17A and/or transforming growth factor-β1 (TGF-β1) signaling induce secretion of an array of inflammatory factors from airway epithelial, smooth muscle, and fibroblast cells. Moreover, several of these factors promote enhanced airway remodeling and phenotypic switching between structural airway cells. Dynamic cellular cross talk between cells within the airway architecture elevates expression of remodeling proteins beyond that seen within isolated cells following IL-17A and/or TGF-β1 treatment. In individuals with severe asthma, these interactions are suggested to be responsible for increased cellular migration, reduced epithelial integrity, thickened airway smooth muscle (ASM) layers, and a marked degree of airway obstruction and reduced airflow. bFGF, basic fibroblast growth factor; CCL19, C-C motif chemokine 19; CCR7, C-C chemokine receptor 7; COL, collagen; COX-2, cyclooxygenase-2; CXCL1, C-X-C motif chemokine ligand 1; EMT, epithelial-mesenchymal transition; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MMPs, matrix metalloproteinases; MUC, mucin; ROS, reactive oxygen species; α-SMA, α-smooth muscle actin; WNT5A, protein Wnt-5a.
Although the impact of EMT on ASM remodeling remains an evolving topic, present evidence suggests that IL-17A- and TGF-β1-induced EMT increases ASM mass in a manner independent of ASM cell proliferation. In human epithelial lung cells, IL-17A reduced E-cadherin and increased vimentin expression via an NF-κB and zinc finger E-box-binding homeobox 1 (ZEB1) transcription factor pathway (Fig. 3; 89). Vimentin, an intermediate filament, supplements force development and passive tension in ASM cells by providing enhanced cellular structure. Considering the ability of mesenchymal cells to differentiate into ASM cells, IL-17A-induced overexpression of vimentin in transitioning epithelial cells may promote ASM layer thickening in TH17-driven severe asthma. Moreover, the ZEB1 transcription factor negatively regulates IL-2 gene transcription, which functions as a TH1-derived inhibitor of TH17 differentiation. This identifies an EMT-promoting feedback loop of IL-17A-induced TH17 differentiation that can, to an extent, suppress TH1-mediated immune responses. Increased migration of lung epithelial cells was further found to be a result of increased IκBα phosphorylation subsequent to IL-17A stimulation (89). Whereas IL-17A negatively regulates E-cadherin in stimulated human lung cells, it potentiates N-cadherin expression (111). N-cadherin-dependent cell-cell contacts, along with increased TGF-β1 and fibronectin, contribute to reduced epithelial integrity along with increased fibroblast motility and FMT (see Fig. 6; 115, 164, 211). Knockdown of STAT3 reversed IL-17A-induced reduction of E-cadherin expression and restrained increased expression of N-cadherin and vimentin in lung adenocarcinoma tissues (111). This resulted in diminished human EMT that correlated with reduced TH17 differentiation, and in murine lung cells, IL-17A-dependent N-cadherin expression was also identified (111). IL-17A stimulation of human epithelial cells further resulted in α-smooth muscle actin (α-SMA) upregulation and enhanced TGF-β1 secretion, which promoted EMT via SMAD2/3 and ERK1/2 phosphorylation (Fig. 6; 237).
Activation of proteinase-activated receptors (PARs) induces ASM proliferation and may contribute to EMT (11, 36). In human lung fibroblasts, activation of PAR-1 and PAR-2 enhanced the expression of α-SMA and secretion of IL-6 and IL-8, suggesting their role in airway remodeling (9). PAR-2 further enhances IL-17A-induced production of IL-8 and CXCL1, which is involved in airway neutrophil recruitment and activation (207, 225a). Neutrophil cathepsin G is a serine proteinase that induces PARs and is localized in azurophilic granules stored in neutrophil extracellular traps. These extensive networks of extracellular fibers are composed of neutrophilic DNA that binds pathogens and localizes cathepsin G given its high affinity for DNA (25). As TH17 cells promote the recruitment of neutrophils to sites of tissue damage and inflammation, TH17-induced IL-17A expression may correlate with increased PAR activity. PAR-4 is highly expressed in lung epithelial cells, and its activation reduces E-cadherin expression and increases α-SMA in a manner similar to that of TGF-β1 (5). PAR-4 activity was further associated with induction of EMT and increased myofibroblast marker expression. Both observations were abrogated via inhibition of epidermal growth factor receptor kinase and Src kinase, which are phosphorylated by PAR-4 stimulation (5). Whether this mechanism exists within TH17-predominant asthmatic airways and contributes to EMT ASM layer thickening requires further investigation.
IL-17A-Induced ASM Hyperplasia and Hypertrophy
Although their roles remain controversial in vivo, ASM cell hyperplasia and hypertrophy are thought to be significant contributors to airway remodeling by directly increasing ASM layer mass (162). ASM proliferation is largely dependent on activation of ERK1/2 and PI3K signaling pathways, which enhance DNA synthesis and cyclin D1 promoter activity (13). IL-17A and IL-17F can induce ASM proliferation via p38 MAPK and ERK1/2 activation, whereas TH17-derived IL-22 induces ASM proliferation, migration, and inhibition of apoptosis via activation of ERK1/2 and NF-κB signaling pathways (Fig. 3; 41, 42). Knockdown of ERK1/2 and/or NF-κB subunit p65 expression in human ASM cells inhibited proliferation, demonstrating their potential role in airway remodeling (41, 42). Prior to IL-17A treatment, IL-17RA or IL-17RC blockade resulted in inhibition of the proliferative and migratory functions of human ASM cells, which further highlights the necessary role of IL-17A signal activation (41, 42). Although it has been shown that individuals with severe asthma have enhanced epithelial cell proliferation and IL-17A is implicated in the development of epithelial glucocorticoid insensitivity, the direct effects of IL-17A on ASM have yet to be fully examined (54). However, it has been suggested that ASM layer mass is increased via IL-17A synergism with IL-4 and TGF-β1, which promotes EMT and secretion of epithelial-derived immune mediators (235).
ASM hypertrophy is enhanced following exposure to TGF-β1, a well-known inducer of contractile proteins such as α-SMA and smooth muscle myosin heavy chain (13). PI3K and mTOR signaling pathways, which regulate enhanced smooth muscle myosin heavy chain expression and ASM cell elongation, are activated by IL-17A and TGF-β1 (93). Activation of PI3K further induces secretion of MMP-1, which increases deposition of a proproliferative ECM that promotes airway remodeling (174, 179). Hyperproliferative ASM cells can migrate toward the epithelial layer and secrete remodeling mediators IL-1β, TGF-β1, and TNF-α, which in part function to induce autocrine smooth muscle cell hypertrophy (35). In canine ASM cells, SMAD7 increased TGF-β1-induced gene transcription via serum response factor (SRF)-dependent promoter function (33). While SRF is a cofactor for the transcription factor E26 transformation-specific (ETS) domain-containing protein Elk-1 (Elk-1), which promotes vascular smooth muscle cell differentiation and proliferation, SRF interaction with its cofactor myocardin enhances equine ASM cell hypertrophy (50). Expression of protein Wnt-5a (WNT5A), a wingless/integrase 1 family member that potentiates TGF-β1 production that is a known inducer of ASM cell hypertrophy, is increased by IL-17A in airway epithelial and ASM cells (see Fig. 6; 59, 138). Wnt5a gene expression additionally results in increased mRNA and protein expression of β-catenin and NFAT5, which contribute to asthmatic airway remodeling and inflammation (71, 137). Gene knockdown of Wnt5a has been shown to significantly decrease the expression of α-SMA and collagen I, enhance SMAD7 expression, and suppress proliferation in hepatic stellate cells (HSCs; 71). These cells are prominent players in liver fibrosis, but whether similar mechanisms are present within structural airway cells remains to be seen. Hypertrophic ASM cells are known to secrete elevated levels of collagens, which are correlated with ineffective dexamethasone and budesonide treatments that fail to inhibit ECM deposition and ASM hyperplasia (21, 29). These observations indicate that alterations to ASM function and structure have potentially compounding effects that enhance the degree of airway remodeling in individuals with severe asthma with heightened levels of IL-17A and TGF-β1.
IL-17A-Mediated Subepithelial Fibrosis and Airway Remodeling
Fibrocytes, which are precursors to mature fibroblasts, secrete elevated levels of proinflammatory IL-6, IL-8, CXCL1, and TNF-α following IL-17A stimulation (12). IL-17A and IL-17F have been found to stimulate maturation of inactive fibrocytes into α-SMA expression fibroblasts that deposit collagen within the ECM (Fig. 6; 12, 103, 109, 166). Reciprocally, naïve CD4+ T cells cocultured with fibrocytes produced IL-17A via CD40-dependent signaling and mediated IL-6 production and enhanced expression of type I collagen (COL1A) and vascular endothelial growth factor (VEGF) mRNA (103). This suggests a cooperative role between fibrocytes and TH17 cells via CD40-mediated IL-17A and IL-17F signaling, which promotes ECM deposition airway remodeling consistent with asthma (103). VEGF secretion in ASM cells, which can be a result of loss of histone methyltransferase G9A recruitment and trimethylation of histone H3 at lysine 9 (H3K9me3), promotes bronchial remodeling and chronic airway inflammation (53). Interestingly, loss of G9A in naïve T cells removes a homeostatic checkpoint and results in increased sensitivity to TGF-β1 signaling and enhanced TH17 differentiation (8).
In mature fibroblasts, TGF-β1 mediates asthma pathogenesis by inducing morphological changes to fibroblasts that elevate their migratory capacity (68). Fibroblasts also secrete immunomodulatory cytokines and chemokines that regulate immune cell migration, cellular adhesion, and inflammation. IL-1β is capable of enhancing these adaptations and promotes their resistance to glucocorticoid treatment (68). Since IL-1β stimulates TH17 differentiation and IL-17A secretion, resistance could be a result of IL-17A-induced insensitivity via PI3K activation and HDAC2 repression, much as in human airway epithelial cells. Furthermore, IL-17A-stimulated fibroblasts secreted increased levels of potent ASM mitogenic factors that signal via receptor tyrosine kinases, including basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and VEGF (Fig. 6; 128). IL-17A was found to selectively enhance bFGF, PDGF, and VEGF in human endothelial cells and a murine model of allergic asthma, which promoted airway remodeling in an MMP-9-dependent manner (167, 224). Activation of receptor tyrosine kinases induces p38 MAPK, ERK1/2, and PI3K signaling and enhances the hyperproliferative function of ASM cells (128). Moreover, both severe asthma-derived fibroblasts and ASM cells have shown greater resistance to dexamethasone treatment and maintain higher C-C chemokine receptor 7 (CCR7) expression compared with nonsevere asthma cells (150). C-C motif chemokine 19 (CCL19), which is secreted by airway epithelial cells, activates CCR7 and mediates both the recruitment of fibroblasts and migration of ASM cells.
Increased levels of fibroblasts contribute to ECM deposition through synthesis of collagens, glycosaminoglycans, and connective tissue fibers that compose the structural framework of the airway (109). IL-17A antibody blockade in murine airways reduced levels of ECM-associated collagens, actin, and fibronectin (32). This model of chronic allergic airway inflammation further showed decreased levels of TGF-β1 following IL-17A blockade that correlated with reduced FOXP3 and NF-κB activity, suggesting that IL-17A may enhance TGF-β1 production in addition to its signaling. When stimulated with IL-17A, human airway epithelial cells had increased expression and deposition of COL5A, which is negligibly expressed under normal conditions, but intercalates with the primary collagen in human lungs, COLA1 (235). COL5A1 is positively correlated with autophagy-related 5 (ATG5), a protein associated with asthmatic fibrogenic remodeling via autophagy-dependent cellular degradation (189). Moreover, TGF-β1-induced expression of fibronectin is promoted via TGFBR1 signaling, and pharmacological inhibition of this pathway was shown to significantly decrease fibronectin, COL1A, and COL3A mRNA expression that resulted in myofibroblast proliferation (106). Interestingly, expression of αvβ3-integrin on inflammatory TH17 cells induced fibronectin-dependent migration to the airways, as fibronectin is a strong ligand of αvβ3-integrin (70). Integrins, which are transmembrane proteins that mediate cell-cell and cell-ECM interaction, mediate airway remodeling and hyperresponsiveness in individuals with asthma. Furthermore, collagen-binding α2β1-integrin is the TH17-specific receptor for COL1A and COL2A (20). Functional studies have demonstrated α2β1-integrin to be a costimulatory molecule capable of promoting TH17 secretion of IL-17A, IL-17F, and, to an extent, IFN-γ. TH17-derived IL-21 and IL-22, along with IL-23, activate JAK signaling and STAT3 phosphorylation, contributing to the persistency of airway fibroblasts and endothelial cells (144, 196). Together, evidence suggests that the associations between IL-17A and TGF-β1 and profibrogenic factors modulate interactions between subepithelial fibrosis and ASM to promote airway remodeling.
IL-17A-Induced FMT and ASM Remodeling
Although lung fibroblast proliferation, migration, and survival are elements of asthma pathogenesis, FMT is implicated in severe airway remodeling and ASM thickening (150, 197). Following chronic inflammation, fibroblasts may acquire the ability to differentiate into myofibroblasts, which are phenotypic intermediates between fibroblasts and ASM cells (164). Although FMT refers to the process by which subepithelial fibroblasts acquire ASM characteristics, ASM cells may also acquire proliferative, fibrogenic features that lead to generation of myofibroblasts (120). Similar to airway epithelial cells and fibrocytes, myofibroblasts of individuals with severe asthma have significantly decreased sensitivity to dexamethasone (150). Studies investigating the role of IL-17A and TH17-derived cytokines in airway FMT are limited, yet mechanistic observations in disparate tissues could potentially guide future research. In colonic subepithelial myofibroblasts (SEMFs), IL-17A enhances the production of profibrotic IL-6 and IL-8 via activation of NF-κB and MAPK signaling pathways (101). Much as in structural airway cells, IL-6 secretion was reciprocally enhanced via IL-17A synergy with IL-1β or TNF-α. Crohn’s disease is a TGF-β1- and TH17-associated inflammatory bowel disease that results in narrowing of the intestinal lumen via strictures, which are scarlike fibrotic structures with thickened smooth muscle layers. IL-17A was overexpressed in vivo within Crohn’s disease strictures compared with nonstrictured intestinal regions, which further correlated with IL-17RB and IL-17RC expression in isolated Crohn’s disease myofibroblasts (16). In response to in vitro IL-17A stimulation, isolated myofibroblasts increased production and release of MMP-3, MMP-12, several collagens, and tissue inhibitor of metalloproteinases 1 (TIMP-1; 16). Severe asthma is positively correlated with MMP-3 and MMP-12 expression, which are negatively associated with IL-13 (107). Considering that TH17 immunity largely functions to counteract TH2-mediated IL-13 activity, this could identify a potential mechanism of myofibroblast/MMP-dependent airway remodeling specific to severe asthma. A further study investigating IL-22-stimulated SEMFs found that collagen synthesis and proliferation were unaltered but secretion of IL-6, IL-8, and several MMPs was increased via NF-κB and AP-1 activation, transcriptions factors involved in IL-17A-mediated airway remodeling (6). Costimulation of SEMFs with IL-17A and IL-22 additively enhanced IL-6 and IL-8 production, suggesting cooperative promotion of these profibrogenic cytokines. HSCs, which are supportive connective tissue cells [pericytes (PCs)], are involved in liver fibrosis and formation of scar tissue. They can undergo PC-fibroblast transition, undergo PC-myofibroblast transition, and contribute to fibrotic ECM deposition (37). Alone, IL-17A stimulation failed to induce activation of HSCs or their transition (73). However, IL-17A functioned to sensitize HSCs to low-dose exposure of TGF-β1, which enhanced gene and protein expression of α-SMA and COL1A. ECM-depositing airway myofibroblasts, along with contractile ASM cells, have increased expression of α-SMA (91). In an IL-17RA−/− murine model of liver fibrosis, there was a significant reduction in the number of α-SMA+ myofibroblasts along with decreased expression of IL-1β, IL-6, IL-13, TNF-α, TGF-β1, and COL1A compared with wild-type mice (254). A similar mechanism may exist in asthmatic airways, which have increased TGF-β1-dependent α-SMA expression in myofibroblasts and ASM cells, but further studies are needed to investigate such pathways (13).
EFFECTS OF IL-17A ON AHR AND HYPERCONTRACTILITY
ASM-Mediated AHR and Hypercontractility
AHR is a characteristic feature of asthma positively correlated with disease severity, therapeutic reliance, and airflow obstruction and defined by the reduction in forced expiratory volume per 1 s (208). AHR encompasses enhanced sensitivity to contractile agonists that ultimately result in ASM-mediated airway narrowing (30, 178). Inflammation-induced hyperresponsiveness is pronounced in individuals with asthma, yet mechanisms regulating its acquisition remain obscure and vary between individuals. Allergen-induced AHR only partly explains the mechanisms surrounding persistent AHR in individuals with severe asthma, and elucidating the role of structural ASM remodeling could provide insight into inherent disease susceptibilities (30, 178). Enhanced ASM contraction contributes to AHR, and investigation into alterations surrounding contractile signaling will increase our understanding of endotype-specific mechanisms within severe asthma (205). As summarized in Fig. 7, activation of G protein-coupled receptors (GPCRs) and corresponding Gq α-subunits regulates the activity of myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP) pathways. This ultimately modulates phosphorylation of regulatory myosin light chain and subsequent ASM cell shortening/contraction via actin-myosin cross-bridging via Ca2+-dependent and Ca2+-independent signaling. Although levels of smooth muscle MLCK are increased in nearly all individuals with asthma, a unique gene variant is associated with severe asthma within the African American population (76, 143, 155). This highlights a potential link between TH17-driven severe asthma and increased airway contractility via direct alterations to the ASM contractile apparatus. Furthermore, human and murine bronchial smooth muscle cells treated with IL-17A had increased expression of Ras homolog gene family, member A (RhoA), and C-kinase-activated protein phosphatase-1 inhibitor (CPI-17), which potentiate Ca2+ sensitization and modulate ASM contraction in a Ca2+-independent manner (205). Focused studies investigating the role of TH17-derived cytokines, particularly IL-17A, could provide mechanistic clarification and identify potential therapeutic targets surrounding TH17-mediated AHR in severe asthma.
IL-17A-Induced AHR and Hypercontractility
AHR is a characteristic feature of asthma that encompasses increased sensitivity to contractile agonists that results in a greater magnitude of force production by ASM cells. IL-17A has been suggested to enhance human and murine ASM contractility through activation of NF-κB signaling. When stimulated with IL-17A, human bronchial ASM cells had increased levels of RhoA protein that correlated with increased JNK1/2 activity and downregulation of IκBα (52). Contrastingly, one study found decreased JNK1/2 phosphorylation in asthmatic ASM cells compared with nonasthmatic controls (3). However, ASM was collected from donors with mild-to-moderate asthma, and this may have failed to identify mechanistic discrepancies of IL-17A stimulation within individuals with severe asthma. Another study found that intranasal administration of IL-17A to naïve mice resulted in increased RhoA expression and enhanced bronchial hyperresponsiveness (52). RhoA upregulation was a direct result of IL-17A-dependent NF-κB activation, as phosphorylation of the IL-13-dependent inducer of RhoA, STAT6, was unchanged following IL-17A treatment (52). These same mice had decreased levels of miR-133a-3p, which is a negative regulator of RhoA gene expression via mRNA silencing. RhoA induces Ca2+-independent AHR by upregulation of Rho-associated protein kinase 2 (ROCK2) and subsequent inhibition of MLCP via activation of CPI-17 (51). ROCK2-mediated inhibition of MLCP was further enhanced by NF-κB activation, which reciprocally enhanced RhoA and ROCK2 expression in an allergen challenge murine model of airway inflammation (135). In addition to ROCK2, CPI-17 is activated by protein kinase C (PKC) following GPCR activation in a RhoA-independent manner, leading to inhibition of MLCP in ASM cells (169, 204). This is evidenced by studies showing increased contractility in response to methacholine in human asthmatic bronchi compared with nonasthmatic bronchi following IL-17A stimulation (243). Coinciding with these studies, mice with IL-17A and IL-17RA deficiency had reduced AHR and neutrophilic infiltration of their airways (256). Interestingly, IL-17A administration in mice lacking dendritic αvβ8-integrin, which activates latent TGF-β1 and promotes TH17 differentiation, resulted in diminished tracheal ring contractility (135).
Reactive oxygen species (ROS) are molecules that play an important role in cell signaling and adaptation to cellular stresses that can contribute to AHR. Heighted levels of ROS result in significant damage to cells, and individuals with severe asthma have impaired oxidative stress defense mechanisms that are associated with elevated ROS following exposure to cigarette smoke, ozone, and other industrial pollutants (18, 184). Mentioned previously, IL-17A secretion enhances PI3K activity and increases airway inflammation and AHR (130). Through activation of NF-κB signaling, PI3K can further enhance activation of endoplasmic reticulum-associated ROS and regulated inositol-requiring enzyme 1 (RIDD)-retinoic acid-inducible gene I (RIG-1; 129). Activation of these proteins is a direct result of cellular stress and mechanical strain, which has wide-reaching consequences on gene transcription that regulates cellular function. One impact of ROS elevation is the oxidation of Ca2+/calmodulin-dependent protein kinase II (ox-CaMKII), which is linked to the development of asthma (Fig. 7; 191). CaMKII is a regulator of MLCK phosphorylation that itself is phosphorylated at a rate slower than the rate of increased cytosolic Ca2+ concentration and thus does not significantly alter ASM reactivity (227). Although it remains poorly understood how ox-CaMKII contributes to disease pathophysiology, the observation that IL-17A promotes endoplasmic reticulum-associated ROS via PI3K signaling provides incentive for further investigation toward identifying IL-17A-dependent mechanisms of CaMKII functionality and ASM-mediated AHR.
Individuals with severe asthma are highly susceptible to bronchospasms triggered by industrial pollutants, and investigating the correlations between these factors and TH17-driven AHR is necessary. In murine airways, ozone increases expression of IL-1β, IL-17A, and G-CSF and enhances neutrophilic inflammation (43). This study demonstrated that IL-17A production was largely a product of γδ-T cells, whereas neutrophilic inflammation in IL-17A-knockout mice was attenuated. Isolated macrophages from the same mice had increased caspase-1 activation and secretion of IL-1β, which was shown to be requisite for IL-17A production in IL-1R1-knockout mice. As mentioned, IL-1β is required for TH17 polarization and maintains a positive feedback loop of sustained TH17 differentiation and activity. Ozone-exposed mice had enhanced secretion of IL-1β and IL-17A in addition to p38 MAPK activation, all of which were diminished in IL-17R-knockout mice (188). Ozone was also found to induce AHR via ROCK2-mediated inhibition of MLCP in a murine model of asthma, with a ROCK2 inhibitor reducing contractile force in human ASM cells (126). Diesel exhaust particles (DEPs), including polycyclic aromatic hydrocarbons and other ultrafine particles, are common pollutants of automobiles that trigger respiratory immune responses and irritation. DEP exposure alone is not known to induce AHR in mice, but coexposure with HDM allergen increased AHR more than HDM allergen alone (24). This suggests that environmental and industrial pollutants may additively stimulate the development of asthma symptoms but alone may not always be enough to trigger AHR-inducing immune responses. However, in a comparison of 235 children with asthma exposed to low and high levels of DEPs, IL-17A serum levels were nearly 6 times higher in those exposed to high DEP levels (24). This correlated with 32.2% of children exposed to high levels of DEPs having more frequent asthma symptoms over the course of a year compared with 14.2% of the low-DEP group. Combination DEP and HDM allergen treatment was found to induce a mixed TH2/TH17 immune response that was identified by the presence of dual-positive IL-13+/IL-17A+ T cells, whereas IL-17A neutralization inhibited DEP-dependent enhancement of AHR (24). Together, these studies highlight the potential role of IL-17A-induced expression of ROS and how it potentially contributes to AHR in pollutant-sensitive individuals with severe asthma residing within highly industrialized communities.
Cooperativity of IL-17A and IL-13 in AHR
Mixed T cell populations can additively enhance detrimental effects that would otherwise be less severe in individual subtype-driven diseases. Much like IL-17A and TGF-β1 cooperativity in severe asthma remodeling (Fig. 8), IL-17A and IL-13 cooperativity has implications in the development and maintenance of AHR. Compared with intratracheal administration of IL-13 alone, coadministration with IL-17A has been shown to enhance murine inflammation, AHR, and induction of IL-13-associated gene expression (95). In vitro expression of IL-13-induced genes was increased following IL-17A treatment, whereas IL-13/IL-17A coexposure inhibited IL-17A gene expression both in vivo and in vitro (95). This suggests that IL-17A-driven enhancement of IL-13-induced gene expression involves IL-13-driven STAT6 activation. Considering that STAT6 induces GATA3 expression and differentiation of TH2 cells, this may identify a feedback loop of constitutive STAT6 activation. The rationale for this lies in the ability of TH2-derived IL-4 and IL-13 to suppress reactivation of differentiated TH17 cells via STAT3 inhibition and Il17a promoter binding despite exposure to IL-6, TGF-β1, and other TH17-promoting cytokines (55). However, this has been found to apply only to early-stage TH17 cells, as TH17 cells that have undergone prolonged inflammatory stimulation retain their functionality (55). An additional study coadministered IL-13 with either low- or high-dose IL-17A (131). This resulted in reduced infiltration of IL-17+ γδ-T cells and increased eosinophilia in low-dose IL-17A groups, whereas high-dose IL-17A treatment contrastingly reduced IL-13-induced inflammatory responses. Moreover, both IL-13 and IL-13/IL-17A treatment significantly increased AHR, whereas IL-17A alone failed to induce significant changes in AHR compared with nontreated individuals. Together, these data indicate that IL-17A has a dose-dependent effect of IL-13-associated AHR that is synergistically enhanced in the presence of various immune factors within murine models of asthma. However, studies investigating the dynamics between the relationship of IL-17A with IL-13 and TGF-β1 along with the contributions of those relationships to AHR, specifically ASM-mediated AHR, are limited. Further research is needed in both murine and human airways if the intricacies surrounding TH17-induced, ASM-mediated AHR are to be fully deciphered.
CONCLUSIONS
In summary, emerging evidence indicates that TH17-derived cytokines, specifically IL-17A, contribute to the pathogenesis of asthma. In response to industrial pollutants, infectious agents, tobacco smoke, and other nonspecific stimuli, injured airway epithelial cells release a multitude of factors that initiate innate and adaptive immunity. Secreted mediators specifically direct immune responses that drive T cell differentiation and promote persistent inflammation and structural remodeling of the airways in individuals with severe asthma. Epithelial-secreted TGF-β1 independently enhances airway remodeling via cognate signaling pathways and inhibits TH1 and TH2 differentiation. Although TGF-β1 is essential to TH17 differentiation and the production of IL-17A, its own activity can be reciprocally enhanced by IL-17A signaling. This highlights the cooperative nature of these immune mediators and how they contribute to the development of enhanced and persistent airway remodeling in TH17-high endotypes of severe asthma. Environmental induction of immune mediators can be further compounded by unique genetic susceptibilities that drive TH17-dependent pathophysiology. Thus, intrinsic gene-environment interplay is pivotal not only to the development of severe asthma but also to the identification of unique endotypes of disease. TH17-driven severe asthma is unquestionably correlated with increased production and secretion of IL-17A, which plays a significant role in neutrophilic airway infiltrations, EMT, FMT, ASM remodeling, and AHR. Although this is achieved as a direct result of IL-17A signaling, it can be further modulated by the capacity of ASM and other structural airway cells to respond to IL-17A-induced secretion of various immune mediators within the airway architecture. The mechanisms by which IL-17A influences ASM phenotype and function are largely distinct from those of TH1 and TH2 immunity, making identification of novel drug targets within these pathways appealing. Although IL-17A undoubtedly plays a significant role in severe asthma pathogenesis, the full extent of its inherent regulation and signaling impacts on ASM in varied inflammatory microenvironments requires further investigation. As the mechanisms underlying severe asthma pathogenesis, including those of ASM, are neither linear nor straightforward, identification of distinct severe asthma endotypes will provide a much-needed foundation for the mechanistic studies required for development of therapeutic innovations. This need is underscored by an ever-increasing worldwide rate of asthma incidence that imparts heavy individual burden and detrimental socioeconomic consequences. As further studies search for therapeutic targets within TH17-mediated pathways, there should be a heightened attentiveness surrounding how alterations to already dysregulated pathways could impact regulatory cross talk with disparate cellular mechanisms pertinent to severe asthma pathogenesis and the acquisition of life-threatening symptoms.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL127192-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.E. prepared figures; J.M.E. drafted manuscript; J.M.E. and C.A.S. edited and revised manuscript; J.M.E. and C.A.S. approved final version of manuscript.
REFERENCES
- 1.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med 104: 1131–1137, 2010. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor β and severe asthma: a perfect storm. Respir Med 108: 1409–1423, 2014. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Alrashdan YA, Alkhouri H, Chen E, Lalor DJ, Poniris M, Henness S, Brightling CE, Burgess JK, Armour CL, Ammit AJ, Hughes JM. Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement. Am J Physiol Lung Cell Mol Physiol 302: L1118–L1127, 2012. doi: 10.1152/ajplung.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372: 1107–1119, 2008. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 5.Ando S, Otani H, Yagi Y, Kawai K, Araki H, Fukuhara S, Inagaki C. Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res 8: 31, 2007. doi: 10.1186/1465-9921-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984, 2005. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 7.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 204: 1509–1517, 2007. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antignano F, Burrows K, Hughes MR, Han JM, Kron KJ, Penrod NM, Oudhoff MJ, Wang SK, Min PH, Gold MJ, Chenery AL, Braam MJ, Fung TC, Rossi FM, McNagny KM, Arrowsmith CH, Lupien M, Levings MK, Zaph C. Methyltransferase G9A regulates T cell differentiation during murine intestinal inflammation. J Clin Invest 124: 1945–1955, 2014. doi: 10.1172/JCI69592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asokananthan N, Lan RS, Graham PT, Bakker AJ, Tokanović A, Stewart GA. Activation of protease-activated receptors (PARs)-1 and -2 promotes alpha-smooth muscle actin expression and release of cytokines from human lung fibroblasts. Physiol Rep 3: e12295, 2015. doi: 10.14814/phy2.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baines KJ, Wright TK, Simpson JL, McDonald VM, Wood LG, Parsons KS, Wark PA, Gibson PG. Airway β-defensin-1 protein is elevated in COPD and severe asthma. Mediators Inflamm 2015: 407271, 2015. doi: 10.1155/2015/407271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, Thumerel M, Ousova O, Kolbeck R, Coyle AJ, Woods J, Tunon de Lara JM, Marthan R, Berger P. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med 185: 715–722, 2012. doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 12.Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol 5: 140–149, 2012. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 13.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 5: 89–96, 2008. doi: 10.1513/pats.200705-063VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F, Warszawska K, Wolk K, Quesniaux V, Ryffel B, Togbe D. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med 183: 1153–1163, 2011. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 15.Bhakta NR, Erle DJ. IL-17 and “TH2-high” asthma: adding fuel to the fire? J Allergy Clin Immunol 134: 1187–1188, 2014. doi: 10.1016/j.jaci.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Biancheri P, Pender SLF, Ammoscato F, Giuffrida P, Sampietro G, Ardizzone S, Ghanbari A, Curciarello R, Pasini A, Monteleone G, Corazza GR, Macdonald TT, Di Sabatino A. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair 6: 13, 2013. doi: 10.1186/1755-1536-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, Belvisi MG. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax 70: 740–747, 2015. doi: 10.1136/thoraxjnl-2014-206592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishopp A, Sathyamurthy R, Manney S, Webbster C, Krishna MT, Mansur AH. Biomarkers of oxidative stress and antioxidants in severe asthma: a prospective case-control study. Ann Allergy Asthma Immunol 118: 445–451, 2017. doi: 10.1016/j.anai.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Blakey JD, Wardlaw AJ. What is severe asthma? Clin Exp Allergy 42: 617–624, 2012. doi: 10.1111/j.1365-2222.2012.03962.x. [DOI] [PubMed] [Google Scholar]
- 20.Boisvert M, Chetoui N, Gendron S, Aoudjit F. Alpha2beta1 integrin is the major collagen-binding integrin expressed on human Th17 cells. Eur J Immunol 40: 2710–2719, 2010. doi: 10.1002/eji.201040307. [DOI] [PubMed] [Google Scholar]
- 21.Bonacci JV, Schuliga M, Harris T, Stewart AG. Collagen impairs glucocorticoid actions in airway smooth muscle through integrin signalling. Br J Pharmacol 149: 365–373, 2006. doi: 10.1038/sj.bjp.0706881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, Casale TB, Chan-Yeung M, Chen R, Chowdhury B, Chung KF, Dahl R, Drazen JM, Fabbri LM, Holgate ST, Kauffmann F, Haahtela T, Khaltaev N, Kiley JP, Masjedi MR, Mohammad Y, O’Byrne P, Partridge MR, Rabe KF, Togias A, van Weel C, Wenzel S, Zhong N, Zuberbier T. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol 126: 926–938, 2010. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Braman SS. The global burden of asthma. Chest 130, Suppl: 4S–12S, 2006. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 24.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol 132: 1194–1204.e2, 2013. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 35: 513–530, 2013. doi: 10.1007/s00281-013-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruijnzeel PL, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease-relevant neutrophil phenotype? J Leukoc Biol 98: 549–556, 2015. doi: 10.1189/jlb.3VMR1214-600RR. [DOI] [PubMed] [Google Scholar]
- 27.Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat Immunol 8: 958–966, 2007. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 28.Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, Lauer M, Hascall V, Misra S, Chance M, Aronica M, Hamilton T, Li X. The inducible kinase IKKi is required for interleukin 17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol 12: 844–852, 2011. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess JK, Oliver BGG, Poniris MH, Ge Q, Boustany S, Cox N, Moir LM, Johnson PRA, Black JL. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. J Allergy Clin Immunol 118: 649–657, 2006. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation. Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138, Suppl: 4S–10S, 2010. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol 106: 1033–1042, 2000. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- 32.Camargo LD, Righetti RF, Aristóteles LR, Dos Santos TM, de Souza FC, Fukuzaki S, Cruz MM, Alonso-Vale MI, Saraiva-Romanholo BM, Prado CM, Martins MA, Leick EA, Tibério IF. Effects of anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol 8: 1835, 2018. doi: 10.3389/fimmu.2017.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camoretti-Mercado B, Fernandes DJ, Dewundara S, Churchill J, Ma L, Kogut PC, McConville JF, Parmacek MS, Solway J. Inhibition of transforming growth factor β-enhanced serum response factor-dependent transcription by SMAD7. J Biol Chem 281: 20383–20392, 2006. doi: 10.1074/jbc.M602748200. [DOI] [PubMed] [Google Scholar]
- 34.Campo P, Rodríguez F, Sánchez-García S, Barranco P, Quirce S, Pérez-Francés C, Gómez-Torrijos E, Cárdenas R, Olaguibel JM, Delgado J; Severe Asthma Workgroup; SEAIC Asthma Committee . Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol 23: 76–88, 2013. [PubMed] [Google Scholar]
- 35.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111: 1293–1298, 2003. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 36.Chambers LS, Black JL, Poronnik P, Johnson PR. Functional effects of protease-activated receptor-2 stimulation on human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 281: L1369–L1378, 2001. doi: 10.1152/ajplung.2001.281.6.L1369. [DOI] [PubMed] [Google Scholar]
- 37.Chang FC, Chou YH, Chen YT, Lin SL. Novel insights into pericyte-myofibroblast transition and therapeutic targets in renal fibrosis. J Formos Med Assoc 111: 589–598, 2012. doi: 10.1016/j.jfma.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res 17: 435–440, 2007. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 39.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 23: 1069–1075, 2011. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281: 35603–35607, 2006. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 41.Chang Y, Al-Alwan L, Risse P-A, Roussel L, Rousseau S, Halayko AJ, Martin JG, Hamid Q, Eidelman DH. TH17 cytokines induce human airway smooth muscle cell migration. J Allergy Clin Immunol 127: 1046–1053, 2011. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 42.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J 26: 5152–5160, 2012. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 43.Che L, Jin Y, Zhang C, Lai T, Zhou H, Xia L, Tian B, Zhao Y, Liu J, Wu Y, Wu Y, Du J, Li W, Ying S, Chen Z, Shen H. Ozone-induced IL-17A and neutrophilic airway inflammation is orchestrated by the caspase-1-IL-1 cascade. Sci Rep 6: 18680, 2016. doi: 10.1038/srep18680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M, Shi J, Zhang W, Huang L, Lin X, Lv Z, Zhang W, Liang R, Jiang S. MiR-23b controls TGF-β1 induced airway smooth muscle cell proliferation via direct targeting of Smad3. Pulm Pharmacol Ther 42: 33–42, 2017. doi: 10.1016/j.pupt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity 29: 899–911, 2008. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 278: 17036–17043, 2003. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum 56: 2936–2946, 2007. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chesné J, Braza F, Chadeuf G, Mahay G, Cheminant MA, Loy J, Brouard S, Sauzeau V, Loirand G, Magnan A. Prime role of IL-17A in neutrophilia and airway smooth muscle contraction in a house dust mite-induced allergic asthma model. J Allergy Clin Immunol 135: 1643–1643.e3, 2015. doi: 10.1016/j.jaci.2014.12.1872. [DOI] [PubMed] [Google Scholar]
- 49.Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med 190: 1094–1101, 2014. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 50.Chevigny M, Guérin-Montpetit K, Vargas A, Lefebvre-Lavoie J, Lavoie JP. Contribution of SRF, Elk-1, and myocardin to airway smooth muscle remodeling in heaves, an asthma-like disease of horses. Am J Physiol Lung Cell Mol Physiol 309: L37–L45, 2015. doi: 10.1152/ajplung.00050.2015. [DOI] [PubMed] [Google Scholar]
- 51.Chiba Y, Misawa M. The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J Smooth Muscle Res 40: 155–167, 2004. doi: 10.1540/jsmr.40.155. [DOI] [PubMed] [Google Scholar]
- 52.Chiba Y, Tanoue G, Suto R, Suto W, Hanazaki M, Katayama H, Sakai H. Interleukin-17A directly acts on bronchial smooth muscle cells and augments the contractility. Pharmacol Rep 69: 377–385, 2017. doi: 10.1016/j.pharep.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Clifford RL, John AE, Brightling CE, Knox AJ. Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma. J Immunol 189: 819–831, 2012. doi: 10.4049/jimmunol.1103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M; NHLBI Severe Asthma Research Program (SARP) . Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med 176: 138–145, 2007. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol 187: 4440–4450, 2011. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, Liotta F, Parronchi P, Maggi E, Romagnani S, Annunziato F. Identification of a novel subset of human circulating memory CD4+ T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol 125: 222–230.e4, 2010. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Cosmi L, Santarlasci V, Maggi L, Liotta F, Annunziato F. Th17 plasticity: pathophysiology and treatment of chronic inflammatory disorders. Curr Opin Pharmacol 17: 12–16, 2014. doi: 10.1016/j.coph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Crespo-Lessmann A, Mateus E, Torrejón M, Belda A, Giner J, Vidal S, Sibila O, Plaza V. Asthma with bronchial hypersecretion: expression of mucins and toll-like receptors in sputum and blood. J Asthma Allergy 10: 269–276, 2017. doi: 10.2147/JAA.S142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daud T, Parmar A, Sutcliffe A, Choy D, Arron J, Amrani Y, Bradding P, Siddiqui S. The role of WNT5a in Th17 asthma. Eur Respir J 48: PA3416, 2016. doi: 10.1183/13993003.congress-2016.PA3416. [DOI] [Google Scholar]
- 60.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc 6: 678–682, 2009. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De S, Zelazny ET, Souhrada JF, Souhrada M. IL-1β and IL-6 induce hyperplasia and hypertrophy of cultured guinea pig airway smooth muscle cells. J Appl Physiol (1985) 78: 1555–1563, 1995. doi: 10.1152/jappl.1995.78.4.1555. [DOI] [PubMed] [Google Scholar]
- 62.Dean K, Niven R. Asthma phenotypes and endotypes: implications for personalised therapy. BioDrugs 31: 393–408, 2017. doi: 10.1007/s40259-017-0242-5. [DOI] [PubMed] [Google Scholar]
- 63.De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, Serone AP, Wright TJ, Jenkins MM, Panesar IS, Thiagarajah SS, Wenzel SE. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol 133: 989–996, 2014. doi: 10.1016/j.jaci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 65.Denning DW, Pashley C, Hartl D, Wardlaw A, Godet C, Del Giacco S, Delhaes L, Sergejeva S. Fungal allergy in asthma: state of the art and research needs. Clin Transl Allergy 4: 14, 2014. doi: 10.1186/2045-7022-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincón M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13: 805–815, 2000. doi: 10.1016/S1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 67.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 182: 5748–5756, 2009. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 68.Doerner AM, Zuraw BL. TGF-β1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respir Res 10: 100, 2009. doi: 10.1186/1465-9921-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1β-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1023–L1029, 2007. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 70.Du F, Garg AV, Kosar K, Majumder S, Kugler DG, Mir GH, Maggio M, Henkel M, Lacy-Hulbert A, McGeachy MJ. Inflammatory Th17 cells express integrin αvβ3 for pathogenic function. Cell Rep 16: 1339–1351, 2016. doi: 10.1016/j.celrep.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du J, Niu X, Wang Y, Kong L, Wang R, Zhang Y, Zhao S, Nan Y. MiR-146a-5p suppresses activation and proliferation of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis through directly targeting Wnt1 and Wnt5a. Sci Rep 5: 16163, 2015. doi: 10.1038/srep16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebensen T, Schulze K, Riese P, Link C, Morr M, Guzmán CA. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 25: 1464–1469, 2007. doi: 10.1016/j.vaccine.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 73.Fabre T, Kared H, Friedman SL, Shoukry NH. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol 193: 3925–3933, 2014. doi: 10.4049/jimmunol.1400861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3′-untranslated region of COX-2 mRNA. J Biol Chem 278: 26897–26907, 2003. doi: 10.1074/jbc.M212790200. [DOI] [PubMed] [Google Scholar]
- 75.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 76.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol 31: 296–305, 2007. doi: 10.1002/gepi.20210. [DOI] [PubMed] [Google Scholar]
- 77.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985–995, 2001. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 78.Fujisawa T, Chang MM, Velichko S, Thai P, Hung LY, Huang F, Phuong N, Chen Y, Wu R. NF-κB mediates IL-1β- and IL-17A-induced MUC5B expression in airway epithelial cells. Am J Respir Cell Mol Biol 45: 246–252, 2011. doi: 10.1165/rcmb.2009-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1β and IL-17A; the NF-κB paradigm. J Immunol 183: 6236–6243, 2009. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol 23: 613–619, 2011. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goepfert A, Lehmann S, Wirth E, Rondeau JM. The human IL-17A/F heterodimer: a two-faced cytokine with unique receptor recognition properties. Sci Rep 7: 8906, 2017. doi: 10.1038/s41598-017-08360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez-Bastero A, Soto-Campos JG, Campo P, Moreira A, DáVila I. Economic costs of severe asthma in omalizumab-treated patients. FENOMA Study (Abstract). Eur Respir J 50, Suppl 61: PA636, 2017. doi: 10.1183/1393003.congress-2017.PA636. [DOI] [Google Scholar]
- 83.Gorska-Ciebiada M, Ciebiada M, Gorski P, Grzelewska-Rzymowska I. ICAM-1 and TNF-α in patients with persistent asthma: relationship with the disease severity. J Allergy Clin Immunol 117, Suppl: S258, 2006. doi: 10.1016/j.jaci.2005.12.1022. [DOI] [PubMed] [Google Scholar]
- 84.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song L, Redding D, Singh B, Sur S, Woodruff P, Dong C, Corry DB, Kheradmand F. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol 10: 496–503, 2009. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goto Y, Uchida Y, Nomura A, Sakamoto T, Ishii Y, Morishima Y, Masuyama K, Sekizawa K. Dislocation of E-cadherin in the airway epithelium during an antigen-induced asthmatic response. Am J Respir Cell Mol Biol 23: 712–718, 2000. doi: 10.1165/ajrcmb.23.6.4031. [DOI] [PubMed] [Google Scholar]
- 86.Grunstein MM, Hakonarson H, Maskeri N, Kim C, Chuang S. Intrinsic ICAM-1/LFA-1 activation mediates altered responsiveness of atopic asthmatic airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 278: L1154–L1163, 2000. doi: 10.1152/ajplung.2000.278.6.L1154. [DOI] [PubMed] [Google Scholar]
- 87.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine 64: 477–485, 2013. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 10: 150, 2011. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S, Yu Y. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer Res 5: 1169–1179, 2015. [PMC free article] [PubMed] [Google Scholar]
- 90.Guenova E, Skabytska Y, Hoetzenecker W, Weindl G, Sauer K, Tham M, Kim KW, Park JH, Seo JH, Ignatova D, Cozzio A, Levesque MP, Volz T, Köberle M, Kaesler S, Thomas P, Mailhammer R, Ghoreschi K, Schäkel K, Amarov B, Eichner M, Schaller M, Clark RA, Röcken M, Biedermann T. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci USA 112: 2163–2168, 2015. doi: 10.1073/pnas.1416922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Günther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev 21: 152–160, 2012. doi: 10.1183/09059180.00001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-β1. Am J Respir Crit Care Med 180: 122–133, 2009. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 93.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol 31: 266–275, 2004. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 94.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc 5: 80–88, 2008. doi: 10.1513/pats.200705-057VS. [DOI] [PubMed] [Google Scholar]
- 95.Hall SL, Baker T, Lajoie S, Richgels PK, Yang Y, McAlees JW, van Lier A, Wills-Karp M, Sivaprasad U, Acciani TH, LeCras TD, Myers JB, Kovacic MB, Lewkowich IP. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol 139: 462–471.e14, 2017. doi: 10.1016/j.jaci.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol 44: 127–133, 2011. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 97.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132, 2005. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 98.Hartley R, Berair R, Brightling CE. Severe asthma: novel advances in the pathogenesis and therapy. Pol Arch Med Wewn 124: 247–254, 2014. doi: 10.20452/pamw.2253. [DOI] [PubMed] [Google Scholar]
- 99.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol 179: 4135–4141, 2007. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 100.Hasan M, Neumann B, Haupeltshofer S, Stahlke S, Fantini MC, Angstwurm K, Bogdahn U, Kleiter I. Activation of TGF-β-induced non-Smad signaling pathways during Th17 differentiation. Immunol Cell Biol 93: 662–672, 2015. doi: 10.1038/icb.2015.21. [DOI] [PubMed] [Google Scholar]
- 101.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-κB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 282: G1035–G1044, 2002. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 102.Hatton RD. TGF-β in Th17 cell development: the truth is out there. Immunity 34: 288–290, 2011. doi: 10.1016/j.immuni.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayashi H, Kawakita A, Okazaki S, Yasutomi M, Murai H, Ohshima Y. IL-17A/F modulates fibrocyte functions in cooperation with CD40-mediated signaling. Inflammation 36: 830–838, 2013. doi: 10.1007/s10753-013-9609-z. [DOI] [PubMed] [Google Scholar]
- 104.Henness S, Johnson CK, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A augments TNF-α-induced IL-6 expression in airway smooth muscle by enhancing mRNA stability. J Allergy Clin Immunol 114: 958–964, 2004. doi: 10.1016/j.jaci.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 105.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-α-induced IL-8 mRNA in human ASM. Am J Physiol Lung Cell Mol Physiol 290: L1283–L1290, 2006. doi: 10.1152/ajplung.00367.2005. [DOI] [PubMed] [Google Scholar]
- 106.Higashiyama H, Yoshimoto D, Kaise T, Matsubara S, Fujiwara M, Kikkawa H, Asano S, Kinoshita M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp Mol Pathol 83: 39–46, 2007. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanović R, Kurukulaaratchy RJ, Chauhan A, Howarth PH. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol 138: 61–75, 2016. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int 57: 1–10, 2008. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 109.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 38: 872–897, 2008. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 110.Hot A, Lenief V, Miossec P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann Rheum Dis 71: 768–776, 2012. doi: 10.1136/annrheumdis-2011-200468. [DOI] [PubMed] [Google Scholar]
- 111.Huang Q, Han J, Fan J, Duan L, Guo M, Lv Z, Hu G, Chen L, Wu F, Tao X, Xu J, Jin Y. IL-17 induces EMT via Stat3 in lung adenocarcinoma. Am J Cancer Res 6: 440–451, 2016. [PMC free article] [PubMed] [Google Scholar]
- 112.Huang XD, Zhang H, He MX. Comparative and evolutionary analysis of the interleukin 17 gene family in invertebrates. PLoS One 10: e0132802, 2015. doi: 10.1371/journal.pone.0132802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169: 443–453, 2002. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 114.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J 20: 5332–5341, 2001. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ierodiakonou D, Postma DS, Koppelman GH, Boezen HM, Gerritsen J, Ten Hacken N, Timens W, Vonk JM. E-cadherin gene polymorphisms in asthma patients using inhaled corticosteroids. Eur Respir J 38: 1044–1052, 2011. doi: 10.1183/09031936.00194710. [DOI] [PubMed] [Google Scholar]
- 116.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci USA 106: 876–881, 2009. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 34: 149–162, 2011. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 118.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226: 57–79, 2008. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 119.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol 74: 1–13, 2011. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 120.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 185: 1058–1064, 2012. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 121.Ji X, Li J, Xu L, Wang W, Luo M, Luo S, Ma L, Li K, Gong S, He L, Zhang Z, Yang P, Zhou Z, Xiang X, Wang CY. IL4 and IL-17A provide a Th2/Th17-polarized inflammatory milieu in favor of TGF-β1 to induce bronchial epithelial-mesenchymal transition (EMT). Int J Clin Exp Pathol 6: 1481–1492, 2013. [PMC free article] [PubMed] [Google Scholar]
- 122.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect 2: e60, 2013. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 113: 832–836, 2004. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 124.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol 173: 3482–3491, 2004. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 125.Karamanou M, Androutsos G. Aretaeus of Cappadocia and the first clinical description of asthma. Am J Respir Crit Care Med 184: 1420–1421, 2011. doi: 10.1164/ajrccm.184.12.1420b. [DOI] [PubMed] [Google Scholar]
- 126.Kasahara DI, Mathews JA, Park CY, Cho Y, Hunt G, Wurmbrand AP, Liao JK, Shore SA. ROCK insufficiency attenuates ozone-induced airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol 309: L736–L746, 2015. doi: 10.1152/ajplung.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy 91: 59–75, 2006. doi: 10.1159/000090230. [DOI] [PubMed] [Google Scholar]
- 128.Khan MA. Inflammation signals airway smooth muscle cell proliferation in asthma pathogenesis. Multidiscip Respir Med 8: 11, 2013. doi: 10.1186/2049-6958-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim HK, Lee GH, Bhattarai KR, Junjappa RP, Lee HY, Handigund M, Marahatta A, Bhandary B, Baek IH, Pyo JS, Kim HK, Chai OH, Kim HR, Lee YC, Chae HJ. PI3Kδ contributes to ER stress-associated asthma through ER-redox disturbances: the involvement of the RIDD-RIG-I-NF-κB axis. Exp Mol Med 50: e444, 2018. doi: 10.1038/emm.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, Lee YR, Kim JS, Hong SJ, Lee YC. PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease. J Immunol 179: 6820–6829, 2007. doi: 10.4049/jimmunol.179.10.6820. [DOI] [PubMed] [Google Scholar]
- 131.Kinyanjui MW, Shan J, Nakada EM, Qureshi ST, Fixman ED. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J Immunol 190: 3859–3868, 2013. doi: 10.4049/jimmunol.1200506. [DOI] [PubMed] [Google Scholar]
- 132.Kleiter I, Song J, Lukas D, Hasan M, Neumann B, Croxford AL, Pedré X, Hövelmeyer N, Yogev N, Mildner A, Prinz M, Wiese E, Reifenberg K, Bittner S, Wiendl H, Steinman L, Becker C, Bogdahn U, Neurath MF, Steinbrecher A, Waisman A. Smad7 in T cells drives T helper 1 responses in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 133: 1067–1081, 2010. doi: 10.1093/brain/awq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koćwin M, Jonakowski M, Przemęcka M, Zioło J, Panek M, Kuna P. The role of the TGF-SMAD signalling pathway in the etiopathogenesis of severe asthma. Pneumonol Alergol Pol 84: 290–301, 2016. doi: 10.5603/PiAP.2016.0037. [DOI] [PubMed] [Google Scholar]
- 134.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448: 484–487, 2007. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-γ as a possible target in chronic asthma. Inflamm Allergy Drug Targets 5: 253–256, 2006. doi: 10.2174/187152806779010909. [DOI] [PubMed] [Google Scholar]
- 137.Kumawat K, Koopmans T, Gosens R. β-Catenin as a regulator and therapeutic target for asthmatic airway remodeling. Expert Opin Ther Targets 18: 1023–1034, 2014. doi: 10.1517/14728222.2014.934813. [DOI] [PubMed] [Google Scholar]
- 138.Kumawat K, Menzen MH, Bos IS, Baarsma HA, Borger P, Roth M, Tamm M, Halayko AJ, Simoons M, Prins A, Postma DS, Schmidt M, Gosens R. Noncanonical WNT-5A signaling regulates TGF-β-induced extracellular matrix production by airway smooth muscle cells. FASEB J 27: 1631–1643, 2013. doi: 10.1096/fj.12-217539. [DOI] [PubMed] [Google Scholar]
- 139.Kusagaya H, Fujisawa T, Yamanaka K, Mori K, Hashimoto D, Enomoto N, Inui N, Nakamura Y, Wu R, Maekawa M, Suda T, Chida K. Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respir Cell Mol Biol 50: 30–39, 2014. doi: 10.1165/rcmb.2013-0130OC. [DOI] [PubMed] [Google Scholar]
- 140.Lang DM. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc 36: 418–424, 2015. doi: 10.2500/aap.2015.36.3908. [DOI] [PubMed] [Google Scholar]
- 141.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 32: 311–318, 2005. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 142.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem 276: 1660–1664, 2001. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 143.Léguillette R, Laviolette M, Bergeron C, Zitouni N, Kogut P, Solway J, Kachmar L, Hamid Q, Lauzon AM. Myosin, transgelin, and myosin light chain kinase: expression and function in asthma. Am J Respir Crit Care Med 179: 194–204, 2009. doi: 10.1164/rccm.200609-1367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277: 33676–33682, 2002. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 145.Li G, Fox J III, Liu Z, Liu J, Gao GF, Jin Y, Gao H, Wu M. Lyn mitigates mouse airway remodeling by downregulating the TGF-β3 isoform in house dust mite models. J Immunol 191: 5359–5370, 2013. doi: 10.4049/jimmunol.1301596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, Wang PM, Bortner CD, Maruoka S, Lih FB, Cook DN, Tomer KB, Jetten AM, Zeldin DC. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med 184: 37–49, 2011. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA 97: 773–778, 2000. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu G, Zhu R, Li B. TNF-α and IL-8 of the patients with allergic asthma. J Huazhong Univ Sci Technolog Med Sci 25: 274–275, 2005. doi: 10.1007/BF02828140. [DOI] [PubMed] [Google Scholar]
- 149.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity 31: 438–449, 2009. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lo CY, Michaeloudes C, Bhavsar PK, Huang CD, Wang CH, Kuo HP, Chung KF. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. J Allergy Clin Immunol 135: 1186–1195.e1-6, 2015. doi: 10.1016/j.jaci.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 151.Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int 111: 847–855, 2014. doi: 10.3238/arztebl.2014.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 127: 355–360, 2011. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 153.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol 2012: 925135, 2012. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo X, Ding Q, Wang M, Li Z, Mao K, Sun B, Pan Y, Wang Z, Zang YQ, Chen Y. In vivo disruption of TGF-β signaling by Smad7 in airway epithelium alleviates allergic asthma but aggravates lung carcinogenesis in mouse. PLoS One 5: e10149, 2010. doi: 10.1371/journal.pone.0010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol 283: L1181–L1189, 2002. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 156.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol 9: 641–649, 2008. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manni ML, Robinson KM, Alcorn JF. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med 8: 25–42, 2014. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marketos SG, Ballas CN. Bronchial asthma in the medical literature of Greek antiquity. J Asthma 19: 263–269, 1982. doi: 10.3109/02770908209104771. [DOI] [PubMed] [Google Scholar]
- 159.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol 3: 129, 2012. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Masoli M, Fabian D, Holt S, Beasley R; Global Initiative for Asthma (GINA) Program . The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478, 2004. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 161.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol 8: 1390–1397, 2007. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 162.McParland BE, Macklem PT, Paré PD. Airway wall remodeling: friend or foe? J Appl Physiol (1985) 95: 426–434, 2003. doi: 10.1152/japplphysiol.00159.2003. [DOI] [PubMed] [Google Scholar]
- 163.Melton AC, Melrose J, Alajoki L, Privat S, Cho H, Brown N, Plavec AM, Nguyen D, Johnston ED, Yang J, Polokoff MA, Plavec I, Berg EL, O’Mahony A. Regulation of IL-17A production is distinct from IL-17F in a primary human cell co-culture model of T cell-mediated B cell activation. PLoS One 8: e58966, 2013. doi: 10.1371/journal.pone.0058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Michalik M, Pierzchalska M, Włodarczyk A, Wójcik KA, Czyż J, Sanak M, Madeja Z. Transition of asthmatic bronchial fibroblasts to myofibroblasts is inhibited by cell-cell contacts. Respir Med 105: 1467–1475, 2011. doi: 10.1016/j.rmed.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 165.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452: 773–776, 2008. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108: 430–438, 2001. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 167.Moon IJ, Kim DY, Rhee C-S, Lee CH, Min YG. Role of angiogenic factors in airway remodeling in an allergic rhinitis murine model. Allergy Asthma Immunol Res 4: 37–45, 2012. doi: 10.4168/aair.2012.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program . Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morin C, Sirois M, Echave V, Rousseau E. CPI-17 silencing-reduced responsiveness in control and TNF-α-treated human bronchi. Am J Respir Cell Mol Biol 39: 638–643, 2008. doi: 10.1165/rcmb.2008-0177RC. [DOI] [PubMed] [Google Scholar]
- 170.Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem 286: 32883–32889, 2011. doi: 10.1074/jbc.R110.197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol 158, Suppl 1: 96–102, 2012. doi: 10.1159/000337801. [DOI] [PubMed] [Google Scholar]
- 172.Nakajima M, Kawaguchi M, Ota K, Fujita J, Matsukura S, Huang SK, Morishima Y, Ishii Y, Satoh H, Sakamoto T, Hizawa N. IL-17F induces IL-6 via TAK1-NFκB pathway in airway smooth muscle cells. Immun Inflamm Dis 5: 124–131, 2017. doi: 10.1002/iid3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nakao A, Sagara H, Setoguchi Y, Okada T, Okumura K, Ogawa H, Fukuda T. Expression of Smad7 in bronchial epithelial cells is inversely correlated to basement membrane thickness and airway hyperresponsiveness in patients with asthma. J Allergy Clin Immunol 110: 873–878, 2002. doi: 10.1067/mai.2002.129236. [DOI] [PubMed] [Google Scholar]
- 174.Naveed SU, Clements D, Jackson DJ, Philp C, Billington CK, Soomro I, Reynolds C, Harrison TW, Johnston SL, Shaw DE, Johnson SR. Matrix metalloproteinase-1 activation contributes to airway smooth muscle growth and asthma severity. Am J Respir Crit Care Med 195: 1000–1009, 2017. doi: 10.1164/rccm.201604-0822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, Peebles RS Jr. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol 188: 1027–1035, 2012. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Newcomb DC, Peebles RS Jr. Th17-mediated inflammation in asthma. Curr Opin Immunol 25: 755–760, 2013. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc 15: 348–356, 2018. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 178.O’Byrne PM, Inman MD. Airway hyperresponsiveness. Chest 123, Suppl: 411S–416S, 2003. doi: 10.1378/chest.123.3_suppl.411S. [DOI] [PubMed] [Google Scholar]
- 179.Ohta Y, Hayashi M, Kanemaru T, Abe K, Ito Y, Oike M. Dual modulation of airway smooth muscle contraction by Th2 cytokines via matrix metalloproteinase-1 production. J Immunol 180: 4191–4199, 2008. doi: 10.4049/jimmunol.180.6.4191. [DOI] [PubMed] [Google Scholar]
- 180.Ojiaku CA, Yoo EJ, Panettieri RA Jr. Transforming growth factor β1 function in airway remodeling and hyperresponsiveness. The missing link? Am J Respir Cell Mol Biol 56: 432–442, 2017. doi: 10.1165/rcmb.2016-0307TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IκBζ regulates TH17 development by cooperating with ROR nuclear receptors. Nature 464: 1381–1385, 2010. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 182.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129: 311–321, 2010. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pappu R, Ramirez-Carrozzi V, Ota N, Ouyang W, Hu Y. The IL-17 family cytokines in immunity and disease. J Clin Immunol 30: 185–195, 2010. doi: 10.1007/s10875-010-9369-6. [DOI] [PubMed] [Google Scholar]
- 184.Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res 121: 71–78, 2013. doi: 10.1016/j.envres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, Molès JP, Danger Y, Ravon E, Lesaux S, Yssel H, Gascan H. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 180: 7423–7430, 2008. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 186.Peters M, Köhler-Bachmann S, Lenz-Habijan T, Bufe A. Influence of an allergen-specific Th17 response on remodeling of the airways. Am J Respir Cell Mol Biol 54: 350–358, 2016. doi: 10.1165/rcmb.2014-0429OC. [DOI] [PubMed] [Google Scholar]
- 187.Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med 100: 1139–1151, 2006. doi: 10.1016/j.rmed.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 188.Pinart M, Zhang M, Li F, Hussain F, Zhu J, Wiegman C, Ryffel B, Chung KF. IL-17A modulates oxidant stress-induced airway hyperresponsiveness but not emphysema. PLoS One 8: e58452, 2013. doi: 10.1371/journal.pone.0058452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Poon AH, Choy DF, Chouiali F, Ramakrishnan RK, Mahboub B, Audusseau S, Mogas A, Harris JM, Arron JR, Laprise C, Hamid Q. Increased autophagy-related 5 gene expression is associated with collagen expression in the airways of refractory asthmatics. Front Immunol 8: 355, 2017. doi: 10.3389/fimmu.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, Benveniste EN, Cong Y. TGF-β promotes Th17 cell development through inhibition of SOCS3. J Immunol 183: 97–105, 2009. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Qu J, Do DC, Zhou Y, Luczak E, Mitzner W, Anderson ME, Gao P. Oxidized CaMKII promotes asthma through the activation of mast cells. JCI Insight 2: e90139, 2017. doi: 10.1172/jci.insight.90139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramezanpour M, Moraitis S, Smith JL, Wormald PJ, Vreugde S. Th17 cytokines disrupt the airway mucosal barrier in chronic rhinosinusitis. Mediators Inflamm 2016: 9798206, 2016. doi: 10.1155/2016/9798206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-β inhibits human Th17 cell differentiation. J Immunol 183: 5418–5427, 2009. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 194.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de Voss J, Balazs M, Gonzalez L Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12: 1159–1166, 2011. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 195.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest 125: 3037–3050, 2015. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rébé C, Végran F, Berger H, Ghiringhelli F. STAT3 activation: a key factor in tumor immunoescape. JAKSTAT 2: e23010, 2013. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reeves SR, Kolstad T, Lien TY, Herrington-Shaner S, Debley JS. Fibroblast-myofibroblast transition is differentially regulated by bronchial epithelial cells from asthmatic children. Respir Res 16: 21, 2015. doi: 10.1186/s12931-015-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, Poholek AC, McGeachy MJ. IL-23 and IL-1β drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep 22: 2642–2653, 2018. doi: 10.1016/j.celrep.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, Flaherty S, Chang SH, Watarai H, Dong C. Interleukin-17B antagonizes interleukin-25-mediated mucosal inflammation. Immunity 42: 692–703, 2015. doi: 10.1016/j.immuni.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ring J. History of allergy in antiquity. Chem Immunol Allergy 100: 2–14, 2014. doi: 10.1159/000358422. [DOI] [PubMed] [Google Scholar]
- 201.Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 150: 5445–5456, 1993. [PubMed] [Google Scholar]
- 202.Rumzhum NN, Patel BS, Prabhala P, Gelissen IC, Oliver BG, Ammit AJ. IL-17A increases TNF-α-induced COX-2 protein stability and augments PGE2 secretion from airway smooth muscle cells: impact on β2-adrenergic receptor desensitization. Allergy 71: 387–396, 2016. doi: 10.1111/all.12810. [DOI] [PubMed] [Google Scholar]
- 204.Sakai H, Chiba Y, Misawa M. Augmentation of endothelin-1-induced phosphorylation of CPI-17 and myosin light chain in bronchial smooth muscle from airway hyperresponsive rats. Biol Pharm Bull 29: 1897–1899, 2006. doi: 10.1248/bpb.29.1897. [DOI] [PubMed] [Google Scholar]
- 205.Sakai H, Suto W, Kai Y, Chiba Y. Mechanisms underlying the pathogenesis of hyper-contractility of bronchial smooth muscle in allergic asthma. J Smooth Muscle Res 53: 37–47, 2017. doi: 10.1540/jsmr.53.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sastre B, del Pozo V. Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm 2012: 645383, 2012. doi: 10.1155/2012/645383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sawant KV, Xu R, Cox R, Hawkins H, Sbrana E, Kolli D, Garofalo RP, Rajarathnam K. Chemokine CXCL1-mediated neutrophil trafficking in the lung: role of CXCR2 activation. J Innate Immun 7: 647–658, 2015. doi: 10.1159/000430914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sawyer G, Miles J, Lewis S, Fitzharris P, Pearce N, Beasley R. Classification of asthma severity: should the international guidelines be changed? Clin Exp Allergy 28: 1565–1570, 1998. doi: 10.1046/j.1365-2222.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- 209.Sears MR. Trends in the prevalence of asthma. Chest 145: 219–225, 2014. doi: 10.1378/chest.13-2059. [DOI] [PubMed] [Google Scholar]
- 210.Sel S, Wegmann M, Sel S, Bauer S, Garn H, Alber G, Renz H. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J Immunol 178: 7805–7813, 2007. doi: 10.4049/jimmunol.178.12.7805. [DOI] [PubMed] [Google Scholar]
- 211.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol 142: 873–881, 1998. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shaifta Y, MacKay CE, Irechukwu N, O’Brien KA, Wright DB, Ward JP, Knock GA. Transforming growth factor-β enhances Rho-kinase activity and contraction in airway smooth muscle via the nucleotide exchange factor ARHGEF1. J Physiol 596: 47–66, 2018. doi: 10.1113/JP275033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376, 2011. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, Olsen H, Ruben SM, Knyazev I, Cho YH, Kao V, Wilkinson KA, Carrell JA, Ebner R. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem 275: 19167–19176, 2000. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 215.Sieve AN, Meeks KD, Lee S, Berg RE. A novel immunoregulatory function for IL-23: inhibition of IL-12-dependent IFN-γ production. Eur J Immunol 40: 2236–2247, 2010. doi: 10.1002/eji.200939759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smits HH, Hiemstra PS, Prazeres da Costa C, Ege M, Edwards M, Garn H, Howarth PH, Jartti T, de Jong EC, Maizels RM, Marsland BJ, McSorley HJ, Müller A, Pfefferle PI, Savelkoul H, Schwarze J, Unger WW, von Mutius E, Yazdanbakhsh M, Taube C. Microbes and asthma: opportunities for intervention. J Allergy Clin Immunol 137: 690–697, 2016. doi: 10.1016/j.jaci.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 217.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 12: 1151–1158, 2011. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 218.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol 169: 642–646, 2002. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 219.Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics 8: 1475–1489, 2009. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 34: 39–49, 2011. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE; TENOR Study Group . Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy 62: 126–133, 2007. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 222.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing regulatory factor SF2 (ASF). Nat Immunol 12: 853–860, 2011. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol 182: 1631–1640, 2009. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett 98: 189–193, 2005. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 225.Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, Tokoyoda K, Renauld JC, Iwamoto I, Nakayama T, Nakajima H. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol 128: 1067–1076.e1-6, 2011. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 225a.Takei-Taniguchi R, Imai Y, Ishikawa C, Sakaguchi Y, Nakagawa N, Tsuda T, Hollenberg MD, Yamanishi K. Interleukin-17- and protease-activated receptor 2-mediated production of CXCL1 and CXCL8 modulated by cyclosporine A, vitamin D3 and glucocorticoids in human keratinocytes. J Dermatol 39: 625–631, 2012. doi: 10.1111/j.1346-8138.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 226.Takejima P, Agondi RC, Rodrigues H, Aun MV, Kalil J, Giavina-Bianchi P. Allergic and nonallergic asthma have distinct phenotypic and genotypic features. Int Arch Allergy Immunol 172: 150–160, 2017. doi: 10.1159/000458151. [DOI] [PubMed] [Google Scholar]
- 227.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem 269: 9912–9920, 1994. [PubMed] [Google Scholar]
- 228.Tantisira KG, Hwang ES, Raby BA, Silverman ES, Lake SL, Richter BG, Peng SL, Drazen JM, Glimcher LH, Weiss ST. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci USA 101: 18099–18104, 2004. doi: 10.1073/pnas.0408532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Teixeira LK, Fonseca BP, Barboza BA, Viola JP. The role of interferon-γ on immune and allergic responses. Mem Inst Oswaldo Cruz 100, Suppl 1: 137–144, 2005. doi: 10.1590/S0074-02762005000900024. [DOI] [PubMed] [Google Scholar]
- 230.Tengvall S, Che KF, Lindén A. Interleukin-26: an emerging player in host defense and inflammation. J Innate Immun 8: 15–22, 2016. doi: 10.1159/000434646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Teunissen MBM, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 111: 645–649, 1998. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 232.Undevia NS, Dorscheid DR, Marroquin BA, Gugliotta WL, Tse R, White SR. Smad and p38-MAPK signaling mediates apoptotic effects of transforming growth factor-β1 in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L515–L524, 2004. doi: 10.1152/ajplung.00044.2004. [DOI] [PubMed] [Google Scholar]
- 233.Varshney P, Saini N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim Biophys Acta Mol Basis Dis 1864, 5 Part A: 1795–1803, 2018. doi: 10.1016/j.bbadis.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 234.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189, 2006. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 235.Vittal R, Fan L, Greenspan DS, Mickler EA, Gopalakrishnan B, Gu H, Benson HL, Zhang C, Burlingham W, Cummings OW, Wilkes DS. IL-17 induces type V collagen overexpression and EMT via TGF-β-dependent pathways in obliterative bronchiolitis. Am J Physiol Lung Cell Mol Physiol 304: L401–L414, 2013. doi: 10.1152/ajplung.00080.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F, Gibson PG. Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J 38: 567–574, 2011. doi: 10.1183/09031936.00170110. [DOI] [PubMed] [Google Scholar]
- 237.Wang T, Liu Y, Zou JF, Cheng ZS. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-β1 mediated Smad2/3 and ERK1/2 activation. PLoS One 12: e0183972, 2017. doi: 10.1371/journal.pone.0183972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 282: 34605–34610, 2007. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Weiss DI, Dang AT, Montoya D, Wu A, Gilliet M, Pellegrini M, Modlin RL. Innate activation of Th17 cells triggers IL-26 release. J Immunol 198, Suppl: 61–64, 2017.27852745 [Google Scholar]
- 240.Welsh KG, Rousseau K, Fisher G, Bonser LR, Bradding P, Brightling CE, Thornton DJ, Gaillard EA. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest 152: 771–779, 2017. doi: 10.1016/j.chest.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy 42: 650–658, 2012. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 242.Wenzel SE. Severe asthma in adults. Exp Lung Res 31, Suppl 1: 22, 2005. [PubMed] [Google Scholar]
- 243.Willis CR, Siegel L, Leith A, Mohn D, Escobar S, Wannberg S, Misura K, Rickel E, Rottman JB, Comeau MR, Sullivan JK, Metz DP, Tocker J, Budelsky AL. IL-17RA signaling in airway inflammation and bronchial hyperreactivity in allergic asthma. Am J Respir Cell Mol Biol 53: 810–821, 2015. doi: 10.1165/rcmb.2015-0038OC. [DOI] [PubMed] [Google Scholar]
- 244.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 181: 2799–2805, 2008. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 245.Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, Yamamoto K. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. J Immunol 179: 7128–7136, 2007. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 246.Yamauchi E, Shoji S, Nishihara M, Shimoda T, Nishima S. Contribution of lung fibroblast migration in the fibrotic process of airway remodeling in asthma. Allergol Int 57: 73–78, 2008. doi: 10.2332/allergolint.O-06-481. [DOI] [PubMed] [Google Scholar]
- 247.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28: 29–39, 2008. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O’Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol 12: 247–254, 2011. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol 155: 5483–5486, 1995. [PubMed] [Google Scholar]
- 250.Yoo EJ, Ojiaku CA, Sunder K, Panettieri RA Jr. Phosphoinositide 3-kinase in asthma: novel roles and therapeutic approaches. Am J Respir Cell Mol Biol 56: 700–707, 2017. doi: 10.1165/rcmb.2016-0308TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zepp JA, Liu C, Qian W, Wu L, Gulen MF, Kang Z, Li X. Cutting edge: TNF receptor associated factor 4 restricts IL-17-mediated pathology and signaling processes. J Immunol 189: 33–37, 2012. doi: 10.4049/jimmunol.1200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang J, Bai C. Elevated serum interleukin-8 level as a preferable biomarker for identifying uncontrolled asthma and glucocorticosteroid responsiveness. Tanaffos 16: 260–269, 2017. [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang X, Angkasekwinai P, Dong C, Tang H. Structure and function of interleukin-17 family cytokines. Protein Cell 2: 26–40, 2011. doi: 10.1007/s13238-011-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang Y, Huang D, Gao W, Yan J, Zhou W, Hou X, Liu M, Ren C, Wang S, Shen J. Lack of IL-17 signaling decreases liver fibrosis in murine schistosomiasis japonica. Int Immunol 27: 317–325, 2015. doi: 10.1093/intimm/dxv017. [DOI] [PubMed] [Google Scholar]
- 255.Zhang Y, Jing Y, Qiao J, Luan B, Wang X, Wang L, Song Z. Activation of the mTOR signaling pathway is required for asthma onset. Sci Rep 7: 4532, 2017. doi: 10.1038/s41598-017-04826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol 6: 335–346, 2013. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453: 236–240, 2008. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, Liu Y, Zhao L, Li X, Shi Y, Qian Y. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med 207: 2647–2662, 2010. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med 18: 1077–1086, 2012. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 260.Zijlstra GJ, Ten Hacken NH, Hoffmann RF, van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur Respir J 39: 439–445, 2012. doi: 10.1183/09031936.00017911. [DOI] [PubMed] [Google Scholar]