Abstract
A defining characteristic of pulmonary hypertension (PH) is the extensive remodeling of pulmonary arteries (PAs), which results in progressive increases in vascular resistance and stiffness and eventual failure of the right ventricle. There is no cure for PH and identification of novel molecular mechanisms that underlie increased proliferation, reduced apoptosis, and excessive extracellular matrix production in pulmonary artery smooth muscle cells (PASMCs) is a vital objective. Galectin-3 (Gal-3) is a chimeric lectin and potent driver of many aspects of fibrosis, but its role in regulating PASMC behavior in PH remains poorly understood. Herein, we evaluated the importance of increased Gal-3 expression and signaling on PA vascular remodeling and cardiopulmonary function in experimental models of PH. Gal-3 expression was quantified by qRT-PCR, immunoblotting, and immunofluorescence imaging, and its functional role was assessed by specific Gal-3 inhibitors and CRISPR/Cas9-mediated knockout of Gal-3 in the rat. In rat models of PH, we observed increased Gal-3 expression in PASMCs, which stimulated migration and resistance to apoptosis, whereas silencing or genetic deletion reduced cellular migration and PA fibrosis and increased apoptosis. Gal-3 inhibitors attenuated and reversed PA remodeling and fibrosis, as well as hemodynamic indices in monocrotaline (MCT)-treated rats in vivo. These results were supported by genetic deletion of Gal-3 in both MCT and Sugen Hypoxia rat models. In conclusion, our results suggest that elevated Gal-3 levels contribute to inappropriate PA remodeling in PH by enhancing multiple profibrotic mechanisms. Therapeutic strategies targeting Gal-3 may be of benefit in the treatment of PH.
Keywords: cell migration, Galectin-3, fibrosis, knockout rat, pulmonary hypertension, vascular smooth muscle
INTRODUCTION
Pulmonary arterial hypertension (PH) is a progressive disease of the lung vasculature that is characterized by the sustained elevation of pulmonary arterial pressure, increased pulmonary vascular resistance, increased pulmonary artery (PA) stiffness, and eventual failure of the right ventricle (47). PH is more frequent in women than men and is a deadly, incurable disease that without treatment has a survival time of less than 3 yr (50).
Hyperplastic changes in medial PA smooth muscle cells (PASMCs) are a salient feature of PH (28) and progressive muscularization of small distal PAs provides increased resistance to blood flow (57). The present clinical approach to PH centers on drugs that increase pulmonary blood flow by reducing pulmonary artery tone. Although initially this is effective at decreasing resistance and increasing blood flow, none of the therapeutics currently available provide long-term success as they do not adequately address the multiple pathways that promote progressive remodeling of PA (56), which include arterial fibrosis, hyperproliferation, and aberrant cellular apoptosis.
Galectin-3 (Gal-3) is a member of the lectin family of proteins, which recognize specific carbohydrate motifs on glycosylated proteins (14). Gal-3 has a unique chimeric structure that includes an N-terminal oligomerization domain and a C-terminal carbohydrate recognition domain, which together enable the formation of lattices or bridges between molecules and cells and thus the ability to regulate a wide range of cellular processes. Gal-3 binds to numerous substrates, including (but not limited to) signaling molecules (Ras), transcriptional regulators (β-catenin), ribonucleoproteins (RNA splicing), cell surface receptors (integrins, transforming growth factor receptor β), and matrix proteins (fibronectin, collagen) (15, 43) to influence a variety of processes, including proliferation, migration, apoptosis, fibrosis, and inflammation (10, 27, 29, 34, 45). A pathogenic role for Gal-3 has been proposed in cancer (21), inflammatory (49), and fibroproliferative disorders such as pulmonary, cardiac, and hepatic fibrosis (35, 55, 64). Depending on the disease, Gal-3 can be expressed in high levels (15) and in different cell types, including macrophages (44, 55), fibroblasts (42), and cancer cells (69). Although it has been reported that Gal-3 blood levels are elevated in human PH (9, 16, 38), a role for Gal-3 in the pathogenesis of PH has only recently emerged.
In the systemic circulation, an important pathogenic role of Gal-3 in regulating vascular smooth muscle cell function has been identified. Gal-3 is expressed in vascular smooth muscle cell and mediates aldosterone-induced vascular fibrosis in rats through the increased expression of collagen type I (10). Furthermore, in human PH, increased Gal-3 levels are associated with right ventricular systolic dysfunction and cardiac remodeling (1, 16). More recent studies have implicated Gal-3 in the development of PH in the mouse hypoxia model (23) and the rat hypoxia and monocrotaline (MCT) models (25, 36, 67); however, the cell type where Gal-3 is expressed and the functional implications remain key barriers to our understanding of its role in the pathogenesis of PH.
Recently, we reported that increased Gal-3 expression in the media of PA regulates PASMC proliferation in PH in a letter to the editor (5). In this abbreviated study, novel findings include the identification of PASMC as the predominant cell type expressing Gal-3 in hypertensive PA, reversal of PH with a Gal-3 inhibitor, and prevention of Sugen/Hypoxia (Su/H)-induced PH in a Gal-3 knockout (KO) rat along with evidence of a pro-proliferative role of Gal-3 in PASMC. The current study provides not only a much more comprehensive understanding of the role of Gal-3 in PH but identifies novel roles of Gal-3 in mediating PASMC migration, apoptosis, and fibrosis.
METHODS
Cell culture and reagents.
Human PASMCs (HPASMCs) were purchased from Lonza and were grown in smooth muscle growth media with defined growth factors and supplemented with FBS to 5% final concentration (Lonza). HPASMCs stained positive for smooth muscle α-actin and negative for von Willebrand (factor VIII) as indicated by the supplier. Rat PASMCs were isolated from male rats and cultured from PAs by methods previously described (7, 30). Rat PASMCs were authenticated using morphology and positive staining for smooth muscle actin and vimentin. All cells were grown at 37°C in 5% CO2 incubator and used from passage 2–6. All chemicals were purchased from Sigma unless indicated otherwise.
DNA/adenoviral constructs.
The Gal-3 adenovirus was constructed using a plasmid encoding Gal-3 (Origene) via the Invitrogen Virapower system. Gal-3 and control viruses were propagated and titered as described (71). Cells were incubated with adenoviral constructs for 3 h in opti-MEM at the indicated viral titers; the cells were washed with PBS and reconstituted with growth media for siRNA transfection.
Cell proliferation and migration.
HPASMCs were incubated overnight with control red fluorescent protein (RFP) or Gal-3 adenoviruses (MOI 30), media changed to 0.1% FBS, and cell proliferation determined 24 h later using an automated counter (Invitrogen) or the MTT assay. In other experiments, Gal-3 was silenced in HPASMCs using RNAimax (30–100 nM of siRNA, Invitrogen) for 24 h and cell proliferation in complete media determined using the MTT assay or cell count. Alternatively, proliferation of rat PASMCs was determined over time by manual count or the MTT assay as indicated. Recombinant Gal-3 (1–10 µg/ml, Peprotech) was dissolved in PBS and administered to control (SD) or Gal-3 KO PASMC at the indicated concentrations. Cell migration was determined in PASMC exposed to control or Gal-3 adenovirus [multiplicity of infection (MOI) 30] using the ORIS Cell Migration Assay (Platypus Technologies).
Measurement of apoptosis.
HPASMCs were exposed to proapoptotic stimuli (nutrient deprivation, H2O2), and apoptosis was determined using cleaved caspase-3 expression and TUNEL staining as previously described (31).
Rat models of PH.
Two rat models of PH were employed. The MCT model was induced by a single intraperitoneal injection of MCT (60 mg/kg), which produces progressive and severe PH after 4 wk of MCT exposure (58). PH in the Su/H model resulted from injection of the VEGF receptor antagonist SU5416 (20 mg/kg, sc) followed by 3 wk of hypoxia (10% O2) and 3 wk of normoxia (21% O2) as previously described (62). Adult age-matched male Sprague-Dawley (250–300 g) rats were used as controls for both rat models of PH. The Animal Care and Use Committee at Augusta University approved all procedures and protocols, and this study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). All groups of rats were housed under temperature-controlled conditions (21°C–23°C), maintained on standard rat chow, allowed free access to food and water, and exposed to a 12:12-h light/dark cycle.
Human studies.
In the studies with human lungs, patient identifiers, including name, age, sex, and ethnic group were concealed. Waiver of informed consent was approved by the Human Assurance Committee of Augusta University and Institutional Review Board of the University of Colorado and by Pulmonary Hypertension Breakthrough Initiative (PHBI).
Gal-3 KO rat model.
Gal-3 KO rats were developed using CRISPR technology on the Sprague-Dawley background (Sage). CRISPR guides were selected targeting the fifth exon, and gene disruption was confirmed by genomic sequencing and immunoblot analysis for Gal-3 protein expression in lung tissue.
Pharmacological targeting of Gal-3 in models of PH.
Two different Gal-3 inhibitors (Galectin therapeutics, courtesy of Peter Traber) were administered to MCT-treated rats: (GM-CT-01 or GR-MD-02; 60 mg/kg iv), which were delivered twice weekly for 4 wk starting at the time of the single MCT injection in Sprague-Dawley rats. In a “reversal protocol,” the inhibitors were given at post-3-wk MCT treatment, which marked the first time point of a significant (detectable using ultrasound, Vevo 2100) increase in right ventricle (RV) thickness. A Gal-3 inhibitor or vehicle (saline) was then given twice weekly, and cardiopulmonary indices were monitored on a weekly basis using ultrasound (Vevo 2100) for 3 additional wk, which correspond to weeks 4, 5, and 6 of MCT exposure.
Assessment of RV function and hypertrophy.
Rats were anesthetized (pentobarbital, 50 mg/kg, ip) and the trachea intubated. The diaphragm was surgically exposed through the abdomen, and a 25-gage needle connected to a pressure transducer (ADInstruments) was inserted into the RV through the diaphragm, and RV pressure was continuously monitored for 10–15 min. Right ventricular systolic pressure (RVSP) was recorded using a PowerLab data acquisition system (ADInstruments). With this approach, the diaphragm remains intact without opening the chest. It has previously been established that measurements of RVSP using this system are comparable to measurements obtained using the right jugular vein (37). Following measurement of hemodynamics, rats were euthanized by thoracotomy. Blood in the pulmonary vasculature was removed by PBS infusion through the pulmonary artery and the heart and lungs removed en bloc. The free wall of the RV, left ventricle (LV), and septum (S) were carefully dissected free and weighed individually to calculate the RV/LV + S ratio (Fulton index) as an index of RV hypertrophy.
Measurement of systolic blood pressure.
Systolic blood pressure (SBP) was measured by tail cuff in (age-matched) control (SD), MCT-treated, and Gal-3 KO rats. Rats were placed in a medium-sized rodent restrainer to prevent excessive movement. The restrained rat was placed within a temperature-controlled compartment maintained at 32°C (IITC Life Science, Wookland Hills, CA) and housed in a quiet, isolated room. Rat tails were passed through a 7/16-inch cuff with a photoelectric sensor, and the rats were acclimated to the tail-cuff technique by following this procedure for 2 consecutive days before data collection. SBP of each rat was determined by the average of approximately five independent readings with at least a 1-min break between each inflation of the pressure cuff. All readings were taken in the morning. An average SBP was then calculated for each rat.
Noninvasive measurement of cardiopulmonary parameters.
Rats were temporally anesthetized (1%–4% inhaled isoflurane), and RV hypertrophy, cardiac output (CO), and functional parameters of PH and PA remodeling (RV thickness, velocity time integral; VTI) were measured using the VEVO 2100 digital ultrasound micro-imaging system (VisualSonics).
Histology and morphometric analysis.
The right lungs were removed and snap-frozen in liquid nitrogen for preparation of homogenates, and the left lungs were filled with 4% paraformaldehyde solution with 0.5% agarose at 25 cm H2O and fixed in 4% paraformaldehyde for 24 h. The fixed lungs were then sliced midsagittally and embedded in paraffin. The slides (7-μm thickness) were stained with hematoxylin and eosin for morphometric analysis and with trichrome for collagen I expression and were examined with an Olympus BX41 microscope. An Olympus DP72 digital camera and ImageJ software (https://imagej.nih.gov/ij/) were used to analyze slides. A minimum of 10 microscopic fields were examined for each slide. To quantitate pulmonary arterial wall thickness, the lumen area at the level of the basement membrane and total vascular area at the adventitial border in 20 muscular arteries with diameters of 50–100 μm per lung section were outlined, and area sizes were measured using ImageJ. The vascular wall thickness was calculated as follows: wall thickness = (total vascular area − lumen area)/total vascular area.
Confocal microscopy.
To determine the location of specific cellular markers in pulmonary arterial vessels, both normotensive and PH lung sections were stained with α-actin (Abcam; 1:700 dilution) for 30 min before being double-stained with antibodies against Gal-3 (Santa Cruz, Santa Cruz, CA; 1:1,000 dilution) for 30 min. For (negative) control IgG images, vessels were incubated first with nonimmune rabbit IgG and mouse IgG (dilution 1:100) overnight and then with goat anti-rabbit IgG AlexaFluor-488 and goat anti-mouse IgG AlexaFluor-594 for 2 h. All fluorescence-labeled lung sections were examined using a Zeiss LSM 510 laser scanning confocal microscope. Apoptosis was detected in lung sections using ApopTag Plus Fluorescent in situ Apoptosis Detection Kit (Millipore, Temecula, CA) according to the manufacturer’s instructions. Nuclei were stained with DAPI. Slides were evaluated by Zeiss LSM 510 laser scanning confocal microscope using filters for FITC and DAPI (excitation/emission 490/520 nm and 358/461 nm, respectively). Alternatively, the endothelial marker, von Willebrand Factor (vWF) and SMC-actin in rat lung sections were detected using anti-vWF antibody (Dako, Santa Clara, CA) and anti-SMC-actin (Santa Cruz) followed by staining with anti-rabbit AlexaFluor-488 and anti-mouse AlexaFluor-594 secondary antibodies respectively. Nuclei were stained with DAPI. Slides were evaluated by Zeiss LSM 510 laser scanning confocal microscope using filters for FITC, AlexaFluor-594, and DAPI (excitation/emission 490/520 nm, 590/617 nm, and 358/461 nm, respectively).
Analysis of gene expression.
PAs (down to fourth order) were dissected from the surrounding pulmonary parenchyma, snap-frozen in liquid nitrogen, pulverized and RNA extracted using TRIZOL or proteins solubilized in 2× Laemmli buffer. cDNA was synthesized from total RNA using the iScript cDNA Synthesis Kit (Biorad) and used to assess relative PA gene expression using real-time RT-PCR (Bio Rad iQ SYBR Green).
Analysis of protein expression.
Protein levels in lysates of PA or lung sections were determined by immunoblot (IB) as previously described (6, 12), using the following antibodies: Gal-3 (Novus; 1:1,000 dilution), smooth muscle actin (Santa Cruz Biotechnology, 1:700 dilution) (37), vWF (Dako, 1:700 dilution) (59), cell FN1 (SCBT; 1:500 dilution) (68), thrombospondin-4 (Santa Cruz; 1:250 dilution) (66), periostin (Novus; 1:500 dilution) (40), vimentin (V9, Thermo Fisher; 1:1,000 dilution) (26), and collagen I (Novus) (46) with GAPDH (SCBT) (5) and Hsp90 (Cell Signaling Technology) (11) serving as the protein loading controls. Relative protein densitometry was determined using ImageJ software (NIH).
Statistical analysis.
Statistical analysis was performed using IBM SPSS Statistics version 25 for Mac and GraphPad Instat. The mean ± SE was calculated in all experiments. Data sets were assessed for normal distribution, and statistical significance was determined either by the unpaired t-test (for two groups) or two-way ANOVA (for ≥ three groups). In data sets analyzed by ANOVA, the Bonferroni post hoc test was employed. A value of P < 0.05 was considered statistically significant.
RESULTS
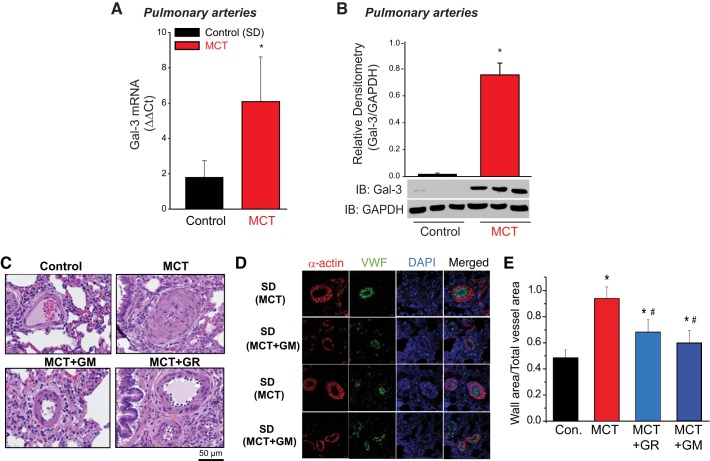
The relative expression of Gal-3 was assessed in the MCT animal model of PH (Fig. 1). Increased mRNA expression of Gal-3 was detected in PA isolated from MCT-exposed rats versus control PA using real-time RT-PCR normalized to GAPDH (Fig. 1A). Analysis of protein expression via IB confirmed increased expression of Gal-3 protein in the MCT rat model of PH (Fig. 1B).
Fig. 1.
Gal-3 expression is increased in hypertensive pulmonary arteries and contributes to pulmonary vascular remodeling. Real-time qRT-PCR (ΔΔCt) of Gal-3 mRNA normalized to GADPH in PA isolated from control (SDR), and 4 wk MCT-treated male rats (A). Immunoblot (IB) of Gal-3 expression in control, MCT male rat PA. *Different from control, P < 0.05 [n = 4 (RNA), n = 3 (protein), unpaired t-test] (B). H&E staining of lung segments from male rats treated with vehicle or MCT (60 mg/kg, ip) for 4 wk in presence/absence of Gal-3 inhibitor GM or GR. Magnification ×40 (C). Confocal images of lung sections from male rats treated with vehicle or MCT (60 mg/kg, ip) for 4 wk in the presence/absence of the Gal-3 inhibitors, GM or GR and stained with smooth muscle actin (red) or von Willebrand Factor (vWF) in green (D). Quantitative morphometry of PA vascular remodeling (E). *Different from control, #different from MCT P < 0.05 analyzed by two-way ANOVA followed by a Bonferroni post hoc test (n = 5 for each group). Gal-3, Galectin-3; GM, GM-CT-01; GR, GR-MD-02; H&E, hematoxylin-eosin; MCT, monocrotaline; PA, pulmonary artery; SDR, Sprague-Dawley rat.
To determine the significance of Gal-3 in PA vascular remodeling in PH, we used two structurally distinct inhibitors of Gal-3, GM-CT-01 (GM) and GR-MD-02 (GR), which bind directly to Gal-3 and function as competitive inhibitors (24, 65). Following MCT administration, animals were administered either vehicle (saline), GM, or GR (60 mg/kg iv twice a week) for 4 wk using a dose range and frequency established previously in rats (64). Histological analysis and immunofluorescent staining of lung sections from MCT-exposed rats revealed significant PA remodeling that was abrogated by treatment with the Gal-3 inhibitors (Fig. 1, C–D). Specifically, both GM and GR attenuated intimal/medial hypertrophy of PA (~100 μm) as determined by quantitative morphometry of hematoxylin-eosin staining (Fig. 1E).
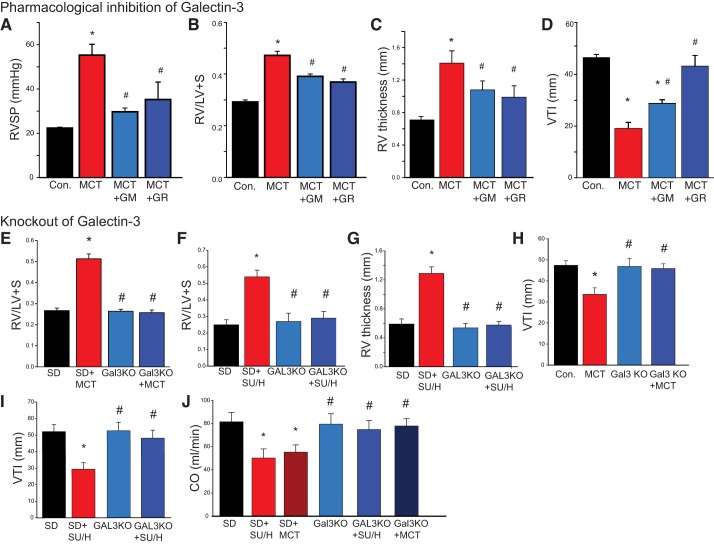
The effects of Gal-3 inhibition on cardiac remodeling and indices of cardiopulmonary function in the MCT rat model of PH are shown in Fig. 2. MCT significantly increased RVSP (Fig. 2A) and RV hypertrophy as shown by the Fulton index (RV/LV+S, Fig. 2B) and increased RV wall thickness (Fig. 2C) and decreased PA blood flow as determined by analysis of hemodynamics using the VTI as measured by noninvasive digital ultrasound [Vevo 2100 (VTI; Fig. 2D)]. In MCT-exposed rats, both RVSP and RV hypertrophy were significantly reduced by Gal-3 inhibition with GM and GR (Fig. 2, A–C), and VTI was significantly increased by GM and GR (Fig. 2D). Multiple inhibitors with different structures were used to ensure pharmacological rigor. Given the ability of Gal-3 inhibitors to prevent MCT-induced PH when administered before the development of PH, we next determined whether inhibition of Gal-3 can reverse or arrest established PH, which is more relevant to clinical therapy. In a reversal protocol, GM or GR was administered (60 mg/kg, twice weekly iv) to rats after 3 wk of MCT exposure. This time period was selected because it is the first significant change in noninvasive measures of PH (RV thickness) (6). We found that both Gal-3 inhibitors (GM and GR) significantly decreased RV hypertrophy as compared with MCT-exposed animals alone (Supplemental Fig. S1A; Supplemental Material for this article is available online at https://doi.org/10.6084/m9.figshare.7637663.v1).
Fig. 2.
Gal-3 inhibition and genetic ablation reduces RV remodeling and improves hemodynamics and reverses PH in MCT-treated male rats. In the MCT model, Gal-3 inhibitors reduce increases in RVSP (A), Fulton index (RV/LV+S) (B), and RV thickness (C) and decreases in PA blood flow (VTI) (D) in 4 wk MCT and MCT + GM/GR inhibitor-treated male rats. *P < 0.05 versus control (vehicle), #P < 0.05 versus MCT, analyzed by two-way ANOVA followed by a Bonferroni post hoc test (n = 5 for each group). RV remodeling as determined by the Fulton index (RV/LV + S) in control and Gal-3 KO rats with and without MCT (E) or SU/H (F) and ultrasound measurements of RV thickness in SU/H rats (G). PA hemodynamics (VTI) in control and Gal-3 KO rats with and without MCT (H) or SU/H (I). Cardiac output (CO) in control and Gal-3 KO rats with and without MCT or SU/H (J). *Versus control (SD), #P < 0.05 versus MCT, P < 0.05, analyzed by two-way ANOVA followed by a Bonferroni post hoc test (n = 5 for each group). Gal-3, Galectin-3; GM, GM-CT-01; GR, GR-MD-02; KO, knockout; LV, left ventricle; MCT, monocrotaline; PA, pulmonary artery; PH, pulmonary hypertension RV, right ventricle; RVSP, RV systolic pressure; S, spetum; SU/H, Sugen/Hypoxia; VTI, velocity time integral.
To provide a complementary molecular approach to our pharmacological studies, we used genome engineering to generate a Sprague-Dawley rat lacking Gal-3 protein expression, as shown previously (5). Briefly, the rat Gal-3 gene was disrupted within exon5 using a CRISPR/Cas9 based strategy, and the loss of protein expression was determined by IB and CRISPR-induced changes in the genomic DNA revealed by sequencing (Supplemental Fig. S2A, https://doi.org/10.6084/m9.figshare.7637672.v1). Cardiopulmonary function was then measured in control (Sprague-Dawley) and Gal-3 KO rats administered MCT (Fig. 2, E, H, and J) and in SU/H-exposed rats (Fig. 2, F, G, I, and J). Compared with wild-type Sprague-Dawley rats, Gal-3 KO rats were resistant to both MCT- and SU/H-induced changes in RV hypertrophy (Fulton index and RV wall thickness) (Fig. 2, E–G) and PA remodeling (VTI; Fig. 2, H–I). Expression of Gal-3 in the RV of MCT-treated rats was apparent at 3 wk and not further increased at 4 wk. In addition, although MCT-exposed Sprague-Dawley rats exhibited advanced signs of PH by 4 wk as evidenced by progressive RV hypertrophy, Gal-3 KO rats administered MCT were able to survive at least 8 wk post-MCT with cardiopulmonary function and RV thickness in the normal range. (Supplemental Fig. S2B). Ultrasound measurements of CO were significantly decreased in both the MCT and SU/H-exposed Sprague-Dawley rats but not different in Gal-3 KO rats (Fig. 2J). The MCT rat model of PH exhibited significantly increased RVSP (Supplemental Fig. S2C), which was lower in Gal-3 KO rats and not different from Sprague-Dawley control rats. Vascular remodeling in small PAs was assessed in lung sections from Sprague-Dawley and Gal-3 KO rats that were administered MCT (Supplemental Fig. S2, D–E). MCT evoked significant medial hypertrophy in PA from Sprague-Dawley rats, and these changes were abrogated in rats lacking Gal-3. Systolic blood pressure was also measured by tail cuff revealing no significant difference in SBP between the Sprague-Dawley (control) rats and MCT-exposed Sprague-Dawley rats. However, SBP in the Gal-3 KO rats was slightly lower than that in Sprague-Dawley rats (Supplemental Fig. S2F).
To determine the level of Gal-3 expression in specific cell types we performed immunofluorescence staining on lung sections from control and MCT-exposed rats as well as in human lungs from a control and a patient with PH followed by confocal imaging. Using a different antibody to that used previously (5), we found that Gal-3 expression is robustly expressed in the thickened media of PA from MCT-exposed rats and human with PH, which colocalized with smooth muscle actin (Fig. 3A). Given that Gal-3 can be found in the cytoplasm, nucleus, or secreted into the extracellular milieu, we next investigated whether the subcellular localization of Gal-3 and its secretion is altered in a rat model of PH. In PASMC from control and MCT-exposed rats, we found that the majority of Gal-3 expression was cytosolic with smaller amounts present in the nucleus (Fig. 3B). Higher amounts of Gal-3 were found in PASMCs from MCT-exposed rats, but there was no bulk change in its subcellular distribution with proportional increases detected in the cytosol and nucleus (Fig. 3, B–C). To assess the extracellular release of Gal-3 we constructed a novel adenoviral expression vector comprised of the small luciferase, gaussia (gluc), fused in frame to the C-terminus of Gal-3 (pAD-Gal-3-gluc). PASMCs were transduced with pAD-Gal-3-gluc, and the amount of Gal-3 released into the extracellular medium was measured via luciferase activity. Gal-3 secretion into the extracellular medium was not different in PASMCs from control or MCT-administered rats relative to total cellular luciferase (Fig. 3D).
Fig. 3.
Gal-3 is expressed in the media of rat PAs and within the cytosol of PASMCs. Confocal images of lung sections from control and experimental rat PH (4 wk MCT) (A). Subcellular fractionation of Gal-3 in PASMCs from control and MCT-treated rats. Nuclear and cytoplasmic fractions were isolated and expression of Gal-3 and respective markers of nuclear and cytoplasmic fractions determined by immunoblot (IB) (B). Densitometric analysis of the subcellular fractionation in B, Data analyzed by unpaired t-test [n = 4 (MCT), n = 5 (control)] (C). Extracellular release of Gal-3 (D). Gaussia luciferase (gLUC) lacking the signal peptide was fused in frame to the C-terminus of human Gal-3 and cloned into the pDEST adenoviral expression vector. PASMCs were isolated from control and MCT-treated rats and transduced with 10 MOI of Gal-3-gLUC adenovirus. To control for possible differences in viral transduction, the extracellular gLUC signal was normalized to the intracellular signal. Data were analyzed by unpaired t-test (n = 4). Gal-3, Galectin-3; MCT, monocrotaline; MOI, multiplicity of infection; PA, pulmonary artery; PASMC, PA smooth muscle cell; PH, pulmonary hypertension.
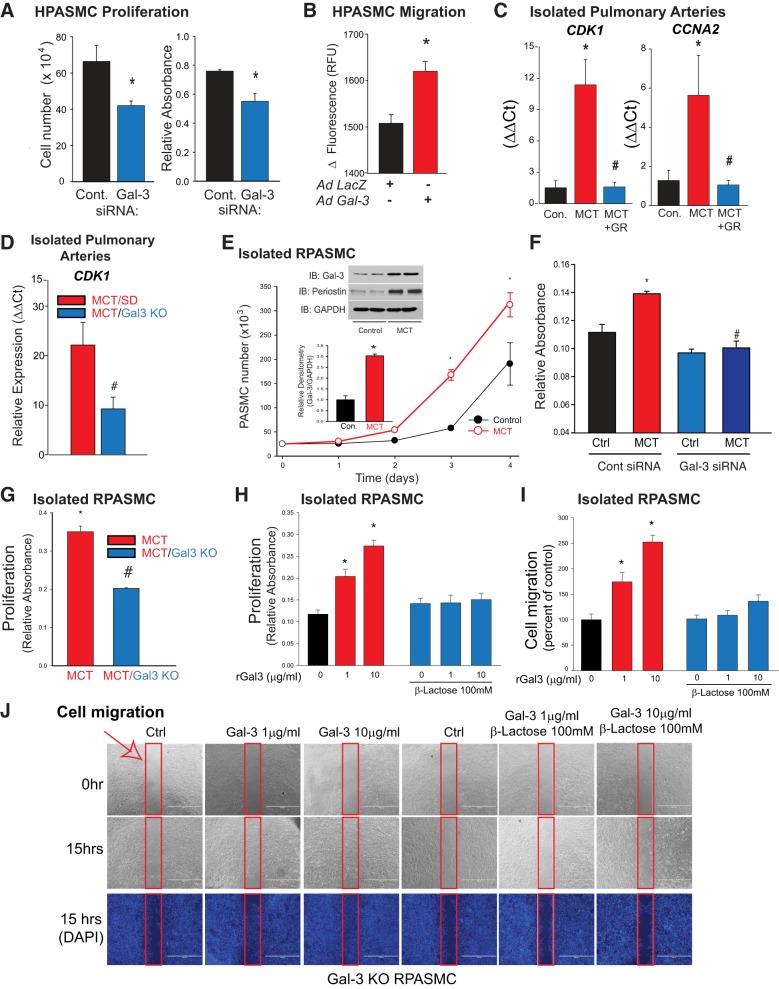
We next assessed the effect of Gal-3 on PASMC behavior. Silencing Gal-3 reduced HPASMC proliferation as measured by both MTT absorbance and cell number (Fig. 4A). In contrast, increased expression of Gal-3 via adenovirus stimulated the migration of HPASMCs (Fig. 4B). In isolated PAs, the expression of markers of cell proliferation (cyclin-dependent kinase 1, cyclin-A2) were increased in MCT-exposed rats compared with control and reduced in MCT-exposed rats administered Gal-3 inhibitors and in knockout rats lacking Gal-3 (Fig. 4, C–D). To assess changes within hypertensive PASMCs, cells were isolated from the PAs of control or 4 wk MCT-exposed rats and grown in culture. We observed increased rates of proliferation in PASMCs from MCT rats as compared with control cells (Fig. 4E), and increased proliferative capacity was associated with significantly increased expression of Gal-3 (inset). Silencing Gal-3 and KO of Gal-3 significantly reduced proliferation in PASMCs from MCT-exposed rats as compared with control cells (Fig. 4, F–G). In Gal-3 KO rat PASMCs, the addition of recombinant Gal-3 dose-dependently stimulated cell proliferation, and these effects were completely inhibited in the presence of an inhibitor of the lectin-domain of Gal-3, lactose (Fig. 4H). Recombinant Gal-3 also dose-dependently increased the ability of Gal-3 KO rat PASMCs to migrate, effects that were also sensitive to inhibition of the carbohydrate recognition domain with lactose (Fig. 4, I–J).
Fig. 4.
Gal-3 stimulates PASMC proliferation and migration. Silencing Gal-3 in HPASMCs reduces proliferation [MTT (n = 8) and cell number (n = 3)] in serum replete media (A). Increased Gal-3 expression increases HPASMC migration. PASMC cells were exposed to Gal-3 adenovirus (30 MOI) and migration determined by fluorescent staining using the ORIS assay, n = 4 (B). qRT-PCR of markers of proliferation, cyclin-dependent kinase 1 (CDK1) and Cyclin-A2 (CCNA2) in isolated PA from 4 wk MCT male SD rats and 4 wk MCT-treated male rats with the Gal-3 inhibitor GR (n = 4) (C). qRT-PCR of CDK1 in isolated PA from 4 wk MCT male SD rats and 4 wk MCT male Gal-3 KO rats (n = 4) (D). Cultured SMCs from the PAs of male SD rats treated with MCT exhibit increased proliferation rates and increased Gal-3 expression compared with cells from control male rats. SMCs were isolated from control (vehicle) and MCT-hypertensive PAs and cultured in DMEM. Growth of SMCs was determined by cell number. Inset: relative expression of Gal-3 in control and MCT-PASMC, (n = 3) (E). Gal-3 silencing reduces proliferation in rat PASMC from MCT rats. PASMC were transfected with control siRNA or Gal-3 siRNA and cell proliferation determined by MTT assay (F). PASMC proliferation as determined by the MTT assay in cells isolated from PAs of control, 4 wk male MCT SD, and 4 wk MCT male Gal-3 KO rats (n = 4 for each group) (G). Cell proliferation (H) and migration (I) were monitored over 24 h in serum-free conditions in response to increasing amounts of recombinant human Gal-3 (1 and 10 µg/ml) in the presence and absence of lactose, a competitive inhibitor of Gal-3 carbohydrate binding. Representative images of cell migration (J). Red arrow and border indicates area analyzed for cell migration (n = 4). *P < 0.05 versus control, #P < 0.05 versus MCT. Data were analyzed by either an unpaired t-test or one-way ANOVA followed by a Bonferroni post hoc test. Gal-3, Galectin-3; GR, GR-MD-02; HPASMC, human PASMC; KO, knockout; MCT, monocrotaline; MOI, multiplicity of infection; PA, pulmonary artery; PASMC, PA smooth muscle cell; rGal-3, recombinant Gal-3; RPASMC, rat PASMC; SD, Sprague-Dawley.
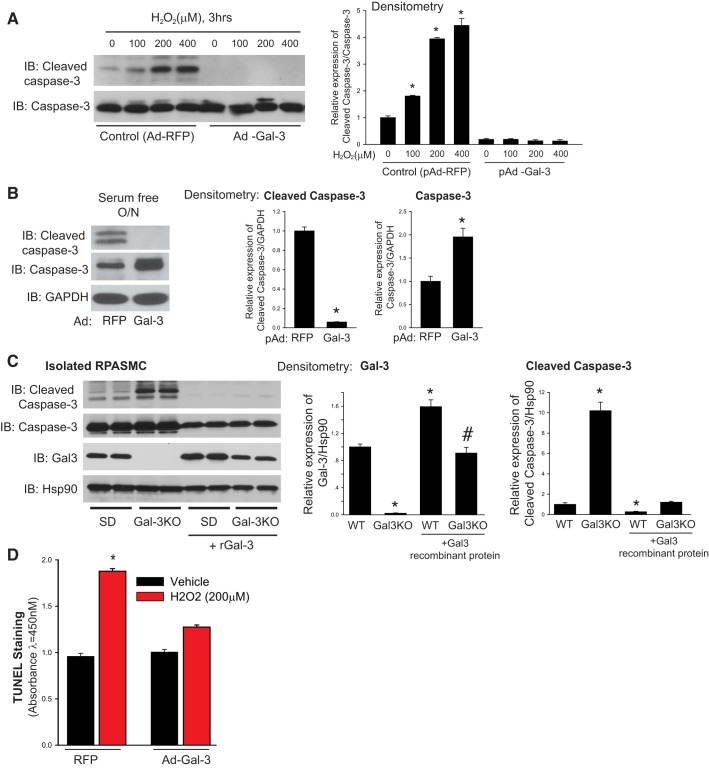
Increased numbers of medial SMCs may also reflect reduced apoptosis, and to determine whether Gal-3 regulates apoptosis in PASMC, HPASMC were transduced with adenoviruses encoding a control gene (RFP) or Gal-3 and exposed to proapoptotic stimuli (H2O2 or removal of growth factors) and apoptosis determined by the expression of cleaved caspase-3 and TUNEL staining. As shown in Fig. 5, HPASMCs treated with the Gal-3 adenovirus had no observable cleaved caspase-3 when exposed to increasing doses of H2O2 (Fig. 5A) or serum deprivation (Fig. 5B) when compared with control conditions. PASMC were isolated from SD or Gal-3 KO rats and assessed for levels of cleaved caspase-3 in serum-free conditions in the presence or absence of recombinant Gal-3 (10 µg/ml). PASMCs from Gal-3 KO rats exhibited increased levels of cleaved caspase-3 compared with PASMCs from Sprague-Dawley rats and the presence of recombinant Gal-3 dramatically decreased cleaved caspase-3 levels in both wild-type and knockout cells (Fig. 5C). Increased TUNEL staining was also observed in HPASMCs exposed to H2O2 and decreased in cells transduced with pAd-Gal-3 (Fig. 5D).
Fig. 5.
Gal-3 potently suppresses apoptosis in PASMC. HPASMC were treated with control (RFP) or Gal-3 adenoviruses (Ad; 30 MOI) and exposed to increasing concentrations of H2O2 for 3 h (A) or serum-free media overnight (O/N) and the expression of cleaved caspase-3, caspase-3 and GAPDH were determined by immunoblot (IB). Densitometric analysis of changes in expression is shown in the panels on the right (B). Expression of cleaved caspase-3, caspase-3, Gal-3, and Hsp90 (loading control) in SMCs isolated from the PAs of male control (SD) and male Gal-3 KO rats and then treated with or without recombinant Gal-3 (10 µg/ml). Densitometric analysis is shown (right) (C). HPASMC were treated with Ad-RFP or pAd-Gal-3 and exposed to 200 µM H2O2 for 9 h and TUNEL determined by TiterTACs (D). [n = 3 (A–C) and n = 5 (D)], *P < 0.05 versus control, #P < 0.05 versus MCT. Data were analyzed by an unpaired t-test or a one-way or two-way ANOVA followed by a Bonferroni post hoc test. Gal-3, Galectin-3; HPASMC, human PASMC; KO, knockout; MCT, monocrotaline; MOI, multiplicity of infection; PA, pulmonary artery; PASMC, PA smooth muscle cell; RFP, red fluorescent protein; WT, wild-type.
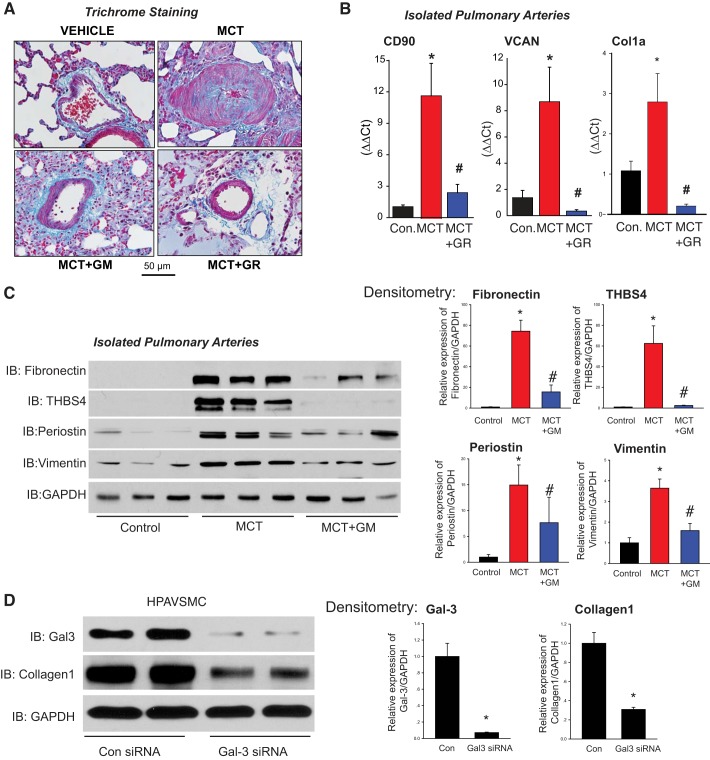
Gal-3 is an established and potent regulator of fibrosis. To assess whether Gal-3 regulates fibrosis in PH, lung sections were stained with trichrome revealing increased expression of collagen in the media of small PAs from MCT-exposed rats compared with controls (Fig. 6A). Furthermore, in MCT rats treated with the Gal-3 inhibitors (GM and GR), there were lower levels of collagen staining in the media (Fig. 6A, bottom). More quantitative assessment of other markers of fibrosis using qRT-PCR and IB revealed higher levels of mRNA expression of CD90, VCAN, and Col1A as well as protein expression of cellular fibronectin, thrombspondin-4, periostin, and vimentin in isolated PAs from MCT-exposed rats (Fig. 6, B–C), which were decreased in the presence of the Gal-3 inhibitor GM or GR. In isolated HPASMCs, Gal-3 silencing resulted in a significant decrease in collagen 1 expression (Fig. 6D).
Fig. 6.
Gal-3 contributes to increased fibrosis of hypertensive PA. Collagen staining in lung sections from male control, 4 wk MCT-treated, and 4 wk MCT + Gal-3 inhibitors GM or GR rats (A). qRT-PCR of CD90 (fibroblasts), VCAN (matrix), and collagen1A (matrix) in PA isolated from male control, 4 wk MCT rats, and 4 wk MCT rats treated with the Gal-3 inhibitor, GR (B). IB of genes regulating fibrosis (cellular fibronectin, thrombospondin-4, periostin, vimentin) in PA isolated from male control, MCT and MCT + GM rats (C). Densitometry (right). Silencing of Gal-3 in HPASMCs reduces expression of collagen 1 (D). n = 3 for B–D. *P < 0.05 versus control, #P < 0.05 versus MCT. Data were analyzed by one-way ANOVA followed by a Bonferroni post hoc test. Col1a, Collagen1a; Gal-3, Galectin-3; GM, GM-CT-01; GR, GR-MD-02; HPASMC, human PA smooth muscle cell; KO, knockout; LV, left ventricle; MCT, monocrotaline; PA, pulmonary artery; IB, immunoblot.
DISCUSSION
In this study we show that Gal-3 is a gene that is upregulated in PA from animals with PH. Gal-3 is robustly expressed in PASMCs, and increased expression confers a pro-proliferative, promigratory, and profibrotic phenotype that is resistant to apoptosis. Collectively, these altered phenotypic changes in PASMC contribute to remodeling of the pulmonary vasculature and elevated PA pressures as evidenced by the ability of pharmacological inhibitors of Gal-3 as well as genetic ablation of Gal-3 to prevent and reverse PH in multiple rat models.
Previous studies have linked PH with increased Gal-3 levels. Fenster et al. (16) reported that serum Gal-3 levels were significantly increased in patients with PH, as well as in patients with systemic sclerosis and elevated RVSP (61). In patients with PH, a significant correlation exists between Gal-3 levels and measurements of RV systolic function, diastolic function, hypertrophy, as well as PA pressure (13, 16). In addition, evidence suggests that high Gal-3 levels are associated with a greater risk for the onset of heart failure and RV dysfunction in cases of chronic obstructive pulmonary disease-related PH (1). This concept is supported by data in models of left heart failure that are associated with increased Gal-3, and elevated Gal-3 alone, via direct infusion of recombinant Gal-3 into the pericardium, is sufficient to stimulate ventricular dysfunction and fibrosis (55). We also found that Gal-3 expression in the RV was increased in MCT-exposed rats compared with control RV, and changes in expression coincided with the onset of increased RV hypertrophy. As RV failure is the ultimate cause of death for patients with PH, the ability of Gal-3 inhibition to improve RV function by preventing remodeling and fibrosis would likely be very beneficial in preserving or improving cardiopulmonary function. In support of this, a recent study showed that Gal-3 levels are increased in the RV of mice with PA banding and contribute to fibrosis; however, an interesting finding was that fibrosis did not correlate with RV function (13). Recently we reported a time-dependent increase in Gal-3 expression in PA from the MCT rat that mirrored changes in pulmonary hemodynamics and increased expression in PA from other rat models, including the SU/H model (5). These data are consistent with the current study and also with others that have found increased Gal-3 expression in human, mouse, and rat models of PH (23, 25, 36, 38, 67). The time-dependent increase in Gal-3 expression in PA more closely paralleled changes in pulmonary hemodynamics (expression and hemodynamic changes evident at 2 wk) (5) as compared with the RV (seen at 3 wk) and suggests that PA remodeling may be the initial and more important target of excess Gal-3.
The functional significance of Gal-3 in PH was assessed using two structurally distinct Gal-3 inhibitors, GM and GR, in the MCT model. The rationale for using two compounds was to improve pharmacological rigor in that inhibitors with similar targets but distinct structures are unlikely to share the same nonspecific actions. We found that both inhibitors had similar efficacy and did not observe any toxicity/adverse effects at the doses employed (such as weight loss, lack of appetite, etc.). The MCT model of PH was selected based on the expeditious time course of PH (3–4 wk of exposure), which is more conducive to pharmacological intervention. In prevention strategies, we found that both Gal-3 inhibitors were effective at ameliorating PH as determined by RVSP, indices of right ventricular remodeling, and noninvasive in vivo measurements of RV thickness, CO, and remodeling of PA (VTI). A further distinction of our study is that we also employed Gal-3 inhibitors (GM or GR) in an experimental protocol to assess the ability of these drugs to reverse established PH as measured by the Fulton index and RVSP as shown previously (5) in MCT-exposed rats. This experimental approach is more clinically relevant as the early stages of PH are generally silent, difficult to detect, and rarely treated. Although we found that both GM and GR were effective at slowing the progression of PH, we did not observe the ability of either inhibitor to reverse fully the disease to levels below that measured at the 3-wk time point after MCT administration. The reasons for this are not yet known but may be related to the dose of inhibitor used, which may have been submaximal (i.e., did not completely inhibit Gal-3) or simply that this pathway may be more important in the developing stages of vascular remodeling. Our data support the effectiveness of the pharmacological approach as Gal-3 inhibitors robustly suppressed specific markers of proliferation and fibrosis in PA. To provide an alternative genetic approach to the pharmacological studies, we generated a novel rat model using CRISPR/Cas9 technology that introduced a frame shift insertion into exon5 of the Gal-3 gene, which disrupted protein expression. In Gal-3 knockout rats, we found that PA and RV remodeling were significantly reduced in two different hypertensive models as compared with Sprague-Dawley controls. Moreover, a time course after MCT exposure revealed that Gal-3 KO rats can survive for greater than 8 wk without appreciable changes in RV hypertrophy and PA blood flow as shown elsewhere (5). We did observe a slight reduction in SBP in Gal-3 KO rats administered MCT, but the significance of this is yet to be determined.
To identify how Gal-3 underlies the development of PH, we assessed the cellular distribution in lung sections. Gal-3 expression was concentrated in the medial layer of PA, overlapping with smooth muscle actin, and little expression was seen in the intima or adventitia. The observation of relatively weak Gal-3 staining in the intima and particularly in the adventitia was surprising as it has been shown to be expressed in endothelial cells (63, 70) and fibroblasts (32), which we confirmed. Furthermore, Gal-3 has been shown to be highly expressed in macrophages (33, 55), and macrophage-specific markers are primarily detected in PA adventitia (6, 18). Others have reported that Gal-3 is expressed in the adventitia of PAs (67); however, this was not directly demonstrated. Collectively, our data suggest that the bulk of Gal-3 expression is found within PASMCs. Given the ability of SMCs to undergo phenotypic switching and acquire inflammatory/macrophage markers (3, 39, 51, 54), it remains to be determined whether Gal-3-positive cells in the medial layer remain phenotypically consistent with contractile SMCs. In addition to changes in pulmonary vascular remodeling, Gal-3 may contribute to sustained vasoconstriction via the activation of RhoA (8, 53); however, additional studies using ROCK inhibitors are needed to explore this possibility. The factors responsible for elevated Gal-3 expression in hypertensive PASMCs are not yet known, but other investigators have shown that PDGF (22), hypoxia (23), and TGFβ (67) can stimulate increased Gal-3 expression. The persistence of increased Gal-3 expression in PASMCs in culture suggests that other, more enduring mechanisms play a significant role.
In numerous diseases, Gal-3 is a common variable that links increased cell proliferation, migration, inflammation, and fibrosis (29, 34, 41). In our study we found that Gal-3 expression in PASMCs promoted smooth muscle proliferation migration, resistance to apoptosis, and increased production of matrix and matricellular proteins. These changes are consistent with the altered phenotype of SMCs in PH and were collectively mitigated in rats administered with Gal-3 inhibitors, Gal-3 gene silencing, or in Gal-3 KO rats. The mechanisms by which Gal-3 impacts these diverse signaling pathways is incompletely understood but is thought to relate to the presence of specific patterns of carbohydrate modifications on its substrates (52) as well as its localization, which can be cytoplasmic, nuclear, or extracellular. In our study, we found that extracellular delivery of recombinant Gal-3 could effectively rescue deficits in migration and apoptosis in Gal-3 KO rat PASMCs. The ability of lactose to block these effects indicates that they are mediated by the lectin-like domain of Gal-3. In rat PASMCs, Gal-3 was found primarily in the cytoplasm and to a lesser extent in the nucleus, and although MCT increased the overall amount of Gal-3 in PASMCs, it did not alter its subcellular distribution. Intracellular Gal-3 may regulate apoptosis, and it is the only member of the Galectin gene family to have sequence similarity to Bcl-2. Mutation of this region in Gal-3 negates its ability to protect against apoptosis (2). We found that supplementation with extracellular recombinant Gal-3 rescued the increased apoptosis seen in Gal-3 KO PASMCs. The precise mechanisms involved and whether Gal-3 uptake in PASMC is required for these effects, remains to be determined. Gal-3 that is released into the extracellular space can also influence fibrosis and inflammation via paracrine actions on other cell types (17). We found that the extracellular secretion of Gal-3 was not different in PASMCs from MCT-exposed rats versus control cells, suggesting that increased expression of Gal-3 in PASMC is the primary driving force behind increased levels in the extracellular milieu. Given its ability to bind to galactose residues on proteins, Gal-3 has been shown to bind and regulate numerous targets, including Lgals3BP (Gal-3 binding protein), integrins, CD147, VEGFR2, and VCAM-1 among others (19, 48, 60). Gal-3 also interacts with the tyrosine kinase cAbl and the phosphorylation of Gal-3 preserves it from degradation (4). The functional significance of nuclear Gal-3 is not known and may include changes in RNA processing, selective expression of mitotic genes, β-catenin signaling, and differentiation. The phosphorylation of Gal-3 is important in regulating nuclear/cytoplasmic traffic (20). We observed a significant increase in Gal-3 expression in the nucleus of PASMCs from MCT-exposed rats and whether this results from changes in Gal-3 phosphorylation remains to be determined. Thus, Gal-3 binds to a wide range of substrates in multiple compartments in and outside the cell and although that makes it a very useful target in complex diseases such as PH, it complicates the identification of specific pathways by which it promotes changes in cellular function.
In summary, our study reveals the effectiveness of genetic and pharmacological strategies targeting Gal-3 in halting the progression of PA and RV remodeling that occurs in experimental PH. We have also found that Gal-3 is robustly expressed in the medial layer of PA and is a key regulator of smooth muscle cell expansion and fibrosis in hypertensive arteries. These data suggest that Gal-3 may be an attractive target for the treatment of PH and other related pulmonary vascular diseases. The ability of Gal-3 inhibitors to impact multiple pathways may be advantageous in treating complex diseases like PH and may also have utility in combinatorial approaches that have significantly greater potential to delay the progression of disease (56).
GRANTS
This work was supported by NIH Grants R01-HL-125926-01A1 (to D. Fulton and S. Barman) and 1R01-HL-124773-01A1 (to D. Stepp and D. Fulton). The Galectin-3 inhibitors were supplied by Galectin Therapeutics, Inc., Norcross, GA (http://galectintherapeutics.com/).
DISCLOSURES
P. Traber was employed by Galectin Therapeutics, Inc. and is now employed by Morphic Therapeutics, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.A.B., X.L., Z.B., P.T., J.S., G.R.C., J.T., Y.S., F.C., and D.J.R.F. conceived and designed research; X.L., S.H., D.K., K.M., Z.B., D.W.S., Y.W., W.S., D.J., J.S., G.R.C., J.T.B., J.T., Y.S., F.C., and D.J.R.F. performed experiments; S.A.B., X.L., S.H., D.K., K.M., Z.B., D.W.S., J.Z., Y.W., D.S.W., W.S., D.J., J.C.S., G.R.C., J.T., Y.S., F.C., and D.J.F. analyzed data; S.A.B., X.L., S.H., D.K., K.M., Z.B., D.W.S., J.Z., Y.W., D.S.W., P.T., W.S., J.C.S., G.R.C., J.T., Y.S., F.C., and D.J.R.F. interpreted results of experiments; S.A.B., Y.S., F.C., and D.J.R.F. prepared figures; S.A.B., F.C., and D.J.R.F. drafted manuscript; S.A.B., D.S.W., F.C., and D.J.R.F. edited and revised manuscript; S.A.B., X.L., S.H., D.K., K.M., Z.B., D.W.S., J.Z., D.S.W., P.T., W.S., D.J., J.S., G.R.C., J.T., Y.S., F.C., and D.J.R.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Correspondence may also be addressed to Feng Chen (fchen@njmu.edu.cn).
REFERENCES
- 1.Agoston-Coldea L, Lupu S, Petrovai D, Mocan T, Mousseaux E. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Med Ultrason 17: 487–495, 2015. doi: 10.11152/mu.2013.2066.174.ech. [DOI] [PubMed] [Google Scholar]
- 2.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res 57: 5272–5276, 1997. [PubMed] [Google Scholar]
- 3.Arar C, Gaudin JC, Capron L, Legrand A. Galectin-3 gene (LGALS3) expression in experimental atherosclerosis and cultured smooth muscle cells. FEBS Lett 430: 307–311, 1998. doi: 10.1016/S0014-5793(98)00683-8. [DOI] [PubMed] [Google Scholar]
- 4.Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem 287: 5192–5198, 2012. doi: 10.1074/jbc.C111.331686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barman SA, Chen F, Li X, Haigh S, Stepp DW, Kondrikov D, Mahboubi K, Bordan Z, Traber P, Su Y, Fulton DJR. Galectin-3 promotes vascular remodeling and contributes to pulmonary hypertension. Am J Respir Crit Care Med 197: 1488–1492, 2018. doi: 10.1164/rccm.201711-2308LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barman SA, Marrero MB. Mechanism of endothelin-1 activation of MAP kinases in neonatal pulmonary vascular smooth muscle. Lung 183: 425–439, 2005. doi: 10.1007/s00408-005-2554-3. [DOI] [PubMed] [Google Scholar]
- 8.Barman SA, Zhu S, White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vasc Health Risk Manag 5: 663–671, 2009. doi: 10.2147/VHRM.S4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvier L, Legchenko E, Grimm L, Sallmon H, Hatch A, Plouffe BD, Schroeder C, Bauersachs J, Murthy SK, Hansmann G. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 102: 390–396, 2016. doi: 10.1136/heartjnl-2015-308365. [DOI] [PubMed] [Google Scholar]
- 10.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, López-Andrés N. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 33: 67–75, 2013. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal 14: 2107–2119, 2011. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, Patel V, Chadli A, Rudic RD, Stepp DW, Catravas JD, Fulton DJ. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler Thromb Vasc Biol 32: 2989–2999, 2012. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crnkovic S, Egemnazarov B, Damico R, Marsh LM, Nagy BM, Douschan P, Atsina K, Kolb TM, Mathai SC, Hooper JE, Ghanim B, Klepetko W, Fruhwald F, Lassner D, Olschewski A, Olschewski H, Hassoun PM, Kwapiszewska G. Disconnect between fibrotic response and right ventricular dysfunction. Am J Respir Crit Care Med rccm.201809-1737OC, 2018. doi: 10.1164/rccm.201809-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50: 7842–7857, 2011. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta 1760: 616–635, 2006. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Fenster BE, Lasalvia L, Schroeder JD, Smyser J, Silveira LJ, Buckner JK, Brown KK. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels 31: 939–946, 2016. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 17.Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavão MS. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol 4: 138, 2014. doi: 10.3389/fonc.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funasaka T, Raz A, Nangia-Makker P. Galectin-3 in angiogenesis and metastasis. Glycobiology 24: 886–891, 2014. doi: 10.1093/glycob/cwu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funasaka T, Raz A, Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol 27: 30–38, 2014. doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guha P, Kaptan E, Bandyopadhyaya G, Kaczanowska S, Davila E, Thompson K, Martin SS, Kalvakolanu DV, Vasta GR, Ahmed H. Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. Proc Natl Acad Sci USA 110: 5052–5057, 2013. doi: 10.1073/pnas.1202653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Feng Z. Galectin-3 mediates the effect of PDGF on pulmonary arterial hypertension. Int J Clin Exp Med 8: 15302–15307, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23.Hao M, Li M, Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep 15: 160–168, 2017. doi: 10.3892/mmr.2016.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, Irish W, Miles MV, Xanthakos SA, Lawitz E, Noureddin M, Schiano TD, Siddiqui M, Sanyal A, Neuschwander-Tetri BA, Traber PG. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther 44: 1183–1198, 2016. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 25.He J, Li X, Luo H, Li T, Zhao L, Qi Q, Liu Y, Yu Z. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens 11: 275–289.e2, 2017. doi: 10.1016/j.jash.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 26.He S, Zhao Z, Yang Y, O’Connell D, Zhang X, Oh S, Ma B, Lee JH, Zhang T, Varghese B, Yip J, Dolatshahi Pirooz S, Li M, Zhang Y, Li GM, Ellen Martin S, Machida K, Liang C. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat Commun 6: 7839, 2015. doi: 10.1038/ncomms8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103: 5060–5065, 2006. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot CM, Dubois-Randé JL, Amsellem V, Adnot S. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol 48: 568–577, 2013. doi: 10.1165/rcmb.2012-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res 245: 294–302, 1998. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JA, Barman SA. Protein kinase C modulation of cyclic GMP in rat neonatal pulmonary vascular smooth muscle. Lung 182: 79–89, 2004. doi: 10.1007/s00408-003-1046-6. [DOI] [PubMed] [Google Scholar]
- 31.Kondrikov D, Fulton D, Dong Z, Su Y. Heat shock protein 70 prevents hyperoxia-induced disruption of lung endothelial barrier via caspase-dependent and AIF-dependent pathways. PLoS One 10: e0129343, 2015. doi: 10.1371/journal.pone.0129343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther 351: 336–343, 2014. doi: 10.1124/jpet.114.218370. [DOI] [PubMed] [Google Scholar]
- 33.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR JR. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 147: 1016–1028, 1995. [PMC free article] [PubMed] [Google Scholar]
- 34.Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci 1183: 158–182, 2010. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 35.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99: 323–328, 2010. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo H, Liu B, Zhao L, He J, Li T, Zha L, Li X, Qi Q, Liu Y, Yu Z. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J Am Soc Hypertens 11: 673–683.e3, 2017. doi: 10.1016/j.jash.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest 121: 4548–4566, 2011. doi: 10.1172/JCI57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazurek JA, Horne BD, Saeed W, Sardar MR, Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ 26: 1208–1215, 2017. doi: 10.1016/j.hlc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Nachtigal M, Ghaffar A, Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol 172: 247–255, 2008. doi: 10.2353/ajpath.2008.070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakazeki F, Nishiga M, Horie T, Nishi H, Nakashima Y, Baba O, Kuwabara Y, Nishino T, Nakao T, Ide Y, Koyama S, Kimura M, Tsuji S, Sowa N, Yoshida S, Conway SJ, Yanagita M, Kimura T, Ono K. Loss of periostin ameliorates adipose tissue inflammation and fibrosis in vivo. Sci Rep 8: 8553, 2018. doi: 10.1038/s41598-018-27009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr 39: 79–84, 2007. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neidhart M, Zaucke F, von Knoch R, Jüngel A, Michel BA, Gay RE, Gay S. Galectin-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric matrix protein. Ann Rheum Dis 64: 419–424, 2005. doi: 10.1136/ard.2004.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J 19: 527–535, 2002. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 44.Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, Greaves DR. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol 28: 433–440, 2008. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 45.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl) 76: 402–412, 1998. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 46.Pietrovito L, Leo A, Gori V, Lulli M, Parri M, Becherucci V, Piccini L, Bambi F, Taddei ML, Chiarugi P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol Oncol 12: 659–676, 2018. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prewitt AR, Ghose S, Frump AL, Datta A, Austin ED, Kenworthy AK, de Caestecker MP. Heterozygous null bone morphogenetic protein receptor type 2 mutations promote SRC kinase-dependent caveolar trafficking defects and endothelial dysfunction in pulmonary arterial hypertension. J Biol Chem 290: 960–971, 2015. doi: 10.1074/jbc.M114.591057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priglinger CS, Szober CM, Priglinger SG, Merl J, Euler KN, Kernt M, Gondi G, Behler J, Geerlof A, Kampik A, Ueffing M, Hauck SM. Galectin-3 induces clustering of CD147 and integrin-β1 transmembrane glycoprotein receptors on the RPE cell surface. PLoS One 8: e70011, 2013. doi: 10.1371/journal.pone.0070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol 23: 313–320, 2002. doi: 10.1016/S1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 50.Rich S. The current treatment of pulmonary arterial hypertension: time to redefine success. Chest 130: 1198–1202, 2006. doi: 10.1378/chest.130.4.1198. [DOI] [PubMed] [Google Scholar]
- 51.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA 100: 13531–13536, 2003. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saraboji K, Håkansson M, Genheden S, Diehl C, Qvist J, Weininger U, Nilsson UJ, Leffler H, Ryde U, Akke M, Logan DT. The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: ultra-high-resolution structures and water dynamics. Biochemistry 51: 296–306, 2012. doi: 10.1021/bi201459p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serizawa N, Tian J, Fukada H, Baghy K, Scott F, Chen X, Kiss Z, Olson K, Hsu D, Liu FT, Török NJ, Zhao B, Jiang JX. Galectin 3 regulates HCC cell invasion by RhoA and MLCK activation. Lab Invest 95: 1145–1156, 2015. doi: 10.1038/labinvest.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21: 628–637, 2015. [Erratum in Nat Med 22: 217, 2016]. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121–3128, 2004. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 56.Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev 25: 408–417, 2016. doi: 10.1183/16000617.0085-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 58.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 59.Swystun LL, Lai JD, Notley C, Georgescu I, Paine AS, Mewburn J, Nesbitt K, Schledzewski K, Géraud C, Kzhyshkowska J, Goerdt S, Hopman W, Montgomery RR, James PD, Lillicrap D. The endothelial cell receptor stabilin-2 regulates VWF-FVIII complex half-life and immunogenicity. J Clin Invest 128: 4057–4073, 2018. doi: 10.1172/JCI96400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tadokoro T, Ikekita M, Toda T, Ito H, Sato T, Nakatani R, Hamaguchi Y, Furukawa K. Involvement of galectin-3 with vascular cell adhesion molecule-1 in growth regulation of mouse BALB/3T3 cells. J Biol Chem 284: 35556–35563, 2009. doi: 10.1074/jbc.M109.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi T, Asano Y, Akamata K, Noda S, Masui Y, Yamada D, Takahashi T, Ichimura Y, Toyama T, Tamaki Z, Tada Y, Sugaya M, Kadono T, Sato S. Serum levels of galectin-3: possible association with fibrosis, aberrant angiogenesis, and immune activation in patients with systemic sclerosis. J Rheumatol 39: 539–544, 2012. doi: 10.3899/jrheum.110755. [DOI] [PubMed] [Google Scholar]
- 62.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 63.Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: altered expression and localization in activated and tumor endothelial cells. Am J Pathol 172: 545–553, 2008. doi: 10.2353/ajpath.2008.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, Friedman SL. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 8: e75361, 2013. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 8: e83481, 2013. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanhoutte D, Schips TG, Kwong JQ, Davis J, Tjondrokoesoemo A, Brody MJ, Sargent MA, Kanisicak O, Yi H, Gao QQ, Rabinowitz JE, Volk T, McNally EM, Molkentin JD. Thrombospondin expression in myofibers stabilizes muscle membranes. eLife 5: e17589, 2016. doi: 10.7554/eLife.17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Wang Y, Zhang J, Guan X, Chen M, Li Y, Zhang L. Galectin-3 contributes to vascular fibrosis in monocrotaline-induced pulmonary arterial hypertension rat model. J Biochem Mol Toxicol 31: e21879, 2017. doi: 10.1002/jbt.21879. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Chen X, Liu X, Huang S, He C, Chen B, Liu Y. Autophagy regulates TGF-β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Mol Med Rep 17: 3607–3614, 2018. doi: 10.3892/mmr.2017.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu LG. Circulating galectin-3 in the bloodstream: an emerging promoter of cancer metastasis. World J Gastrointest Oncol 2: 177–180, 2010. doi: 10.4251/wjgo.v2.i4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Li YM, Zeng XX, Wang XY, Chen SK, Gui LX, Lin MJ. Galectin-3- mediated transdifferentiation of pulmonary artery endothelial cells contributes to hypoxic pulmonary vascular remodeling. Cell Physiol Biochem 51: 763–777, 2018. doi: 10.1159/000495331. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 28: 1627–1633, 2008. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]