Abstract
Transcription factor 21 (Tcf21) is a basic helix-loop-helix transcription factor required for mesenchymal development in several organs. Others have demonstrated that Tcf21 is expressed in embryonic lung mesenchyme and that loss of Tcf21 results in a pulmonary hypoplasia phenotype. Although recent single-cell transcriptome analysis has described multiple mesenchymal cell types in the lung, few have characterized the Tcf21 expressing population. To explore the Tcf21 mesenchymal lineage, we traced Tcf21-expressing cells during embryogenesis and in the adult. Our results showed that Tcf21 progenitor cells at embryonic day (E)11.5 generated a subpopulation of fibroblasts and lipofibroblasts and a limited number of smooth muscle cells. After E15.5, Tcf21 progenitor cells exclusively become lipofibroblasts and interstitial fibroblasts. Lipid metabolism genes were highly expressed in perinatal and adult Tcf21 lineage cells. Overexpression of Tcf21 in primary neonatal lung fibroblasts led to increases in intracellular neutral lipids, suggesting a regulatory role for Tcf21 in lipofibroblast function. Collectively, our results reveal that Tcf21 expression after E15.5 delineates the lipofibroblast and a population of interstitial fibroblasts. The Tcf21 inducible Cre mouse line provides a novel method for identifying and manipulating the lipofibroblast.
Keywords: fibroblast, lipofibroblast, lung, perilipin 2, transcription factor 21
INTRODUCTION
The lung contains a diversity of cell types that interact with one another during development. Recent studies suggest that mesenchymal cells scattered between the epithelial and endothelial layers may be essential for branching morphogenesis, alveolar formation, and maturation (6, 7, 50, 51). Whereas multiple transcription factors and signaling pathways associated with lung epithelial progenitor cell fate have been identified (21, 31, 39), less is known about the transcription factors controlling mesenchymal lineage commitment.
Mesenchymal cells include pericytes, endothelial cells, smooth muscle cells, and fibroblasts. Fibroblasts have been further divided on the basis of anatomic and physiological features, including myofibroblasts [α-smooth muscle actin (α-SMA)+ cells] (22, 23), interstitial fibroblasts, adventitial fibroblasts, and lipofibroblasts (lipid-laden fibroblasts) (32, 35). Recently, alternative categories have been proposed on the bassis of their involvement in alveolar cell differentiation and their contribution to fibrosis (15, 26, 36, 56, 59, 61). Even when these newer classifications are considered, few of the known lung mesenchymal genes are unique to a single lineage. Tbx4 is broadly expressed by progenitors of pericytes, fibroblasts, and smooth muscle cells (12, 58). Platelet-derived growth factor receptor-α (PDGFRα) expression has been broadly used to identify lung fibroblasts, although its expression may vary greatly between subsets (16, 19, 34) and developmental stages (9, 37). Cells expressing PDGFRα surrounding blood vessels and bronchi identify perivascular and peribronchial smooth muscle (17, 37, 45). Although there is great interest in understanding the classes of lung fibroblasts, relatively little is known about the lung lipofibroblast.
Lipofibroblasts potentially transfer neutral lipids to alveolar type II (ATII) cells to support surfactant and phospholipid synthesis (35). Neutral lipids are detected in the murine lung during the late pseudoglandular stage. The number of lipid droplet-containing cells gradually increases up to the early saccular stage at embryonic day (E)18.5, and triglyceride levels peak during the second postnatal week (3, 54). Aside from the direct detection of lipid droplets by lipophilic stains such as Oil red O and LipidTOX, only a few additional markers have been used to distinguish the lipofibroblast from other lung fibroblast populations. Adipose differentiation-related protein (also known as perilipin 2 [Plin2], ADRP, and ADFP) is involved in lipid droplet formation and is commonly used to discriminate between lipofibroblasts and other fibroblast types. Also, expression of Thy1 (CD90) and zinc finger protein 423 (Zfp423) has been associated with neutral lipid accumulation mediated by peroxisome proliferator-activated receptor-γ (PPARγ) (27, 55). Recent findings have demonstrated that embryonic fibroblast growth factor 10 (FGF10)+ lineages include lipofibroblasts (3, 13) and airway smooth muscle cells (14). Reduced PDGFRα expression has been correlated with lipofibroblast Plin2 expression during early postnatal lung development (16, 34). Although there is great interest in understanding the lipofibroblast identity, a definitive marker for the lipofibroblast has not yet been verified.
Tcf21 (also known as Pod1, capsulin, and epicardin) is a basic helix-loop-helix (bHLH) transcription factor expressed in mesenchymal cells during lung development (1, 29, 43, 44). Tcf21-null embryos have hypoplastic lungs exhibiting defects in branching morphogenesis and epithelial cell differentiation (42), demonstrating that Tcf21 is essential for early lung organogenesis. However, a detailed analysis of fibroblast subtypes derived from Tcf21 progenitors has not been previously reported. Therefore, in this study, we investigated the differentiation potential of Tcf21 lung mesenchymal progenitors by use of genetic cell lineage tracing and found that Tcf21 is expressed preferentially in lipofibroblasts after E15.5, suggesting that Tcf21 expression may help identify the lipofibroblast lineage.
MATERIALS AND METHODS
Mice.
Tcf21LacZ/+ (28), Tcf21mCrem/+ (1), PDGFRαGFP/+ (Jackson Laboratories, no. 007669) (20), Collagen1a1-GFP (60), R26RtdT (Rosa 26 reporter; Jackson Laboratories, no. 007914) (30), and Rpl22tm1.1Psam, henceforth Rpl22HA (ribosomal protein L22; Jackson, no. 011029) (47) mice were maintained on a mixed C57Bl/6 background. Tamoxifen induction of Rpl22HA/+;Tcf21mCrem/+ enables the isolation of ribosomal RNA specifically from Tcf21 lineage cells. All procedures were approved by the University of Hawaii Institutional Animal Care and Use Committees and were conducted in accordance with the National Institutes of Health guidelines for care and use of laboratory animals. Tcf21mCrem/+;R26tdT/tdT mice were back-crossed a minimum of four generations to C57BL/6 and contained the J mutation of the NNT gene. Embryos and adults of both sexes were analyzed.
Tamoxifen administration.
Tamoxifen (MP Biomedicals, no. 0215673891; AdipoGen, no. 50-149-0595, 20 mg/ml stock solution) was dissolved in sunflower seed oil containing 10% ethanol. Tamoxifen (0.1 mg/g body wt) was administrated by a single oral gavage to pregnant dams at embryonic days (E)9.5, E11.5, and E15.5. For postnatal induction, tamoxifen was diluted in sunflower seed oil at a concentration of 5 mg/ml and administered to the mice at postnatal day (P)1 or P2 at a dose of 0.15 mg/g body wt by a single intragastric injection. For adult RiboTag and FACS analysis, tamoxifen (0.3 mg/g body wt) was administrated by oral gavage three times on nonconsecutive days unless otherwise specified. We found that two or three tamoxifen gavages were more efficient than a single induction. Adult mice were between the ages of 2–3 mo. In the absence of tamoxifen administration, no reporter-labeled cells were observed in the lung (data not shown).
Immunoprecipitation of polyribosomes.
Immunoprecipitation and purification of polysome-bound mRNAs was performed from snap-frozen lungs isolated from Tcf21mCrem/+;Rpl22HA/+ mice. Lung tissue was homogenized in (10% wt/vol) ice-cold polysome buffer (50 mM Tris·HCl, pH 7.5, 100 mM KCl, 12 mM MgCl2, 1 mM DTT, 1% IGEPAL (Sigma-Aldrich; no. 18896), 200 U/ml RNasin Plus RNase Inhibitor (Promega, PAN2615), 1 mg/ml heparin, and 0.1 mg/ml cycloheximide), and homogenates were centrifuged at 10,000 g for 10 min to create a postmitochondrial supernatant; 1% of supernatant was reserved as input before immunoprecipitation. The remaining supernatant was immunopurified using anti-HA-tag mAb (MBL, M180-11) at 4°C for 2–4 h. Beads were washed with high-salt buffer consisting of 50 mM Tris·HCl (pH 7.5), 300 mM KCl, 12 mM MgCl2, 1 mM DTT, and 1% IGEPAL, and RNA was extracted with a Quick-RNA MicroPrep kit (Zymo Research, R1050) or RNeasy Plus Micro Kit (Qiagen, 74034) according to the manufacturer’s instructions.
RT-qPCR.
Lung tissues and sorted cells were lysed in IBI Isolate (IBI Scientific, no. IB47600) or in TRIzol Reagent (Thermo Fisher Scientific, no. 15596026). Total RNA was prepared according to the manufacturers’ recommendations. RNA quality and concentration were determined by NanoDrop ND-1000 (Thermo Fisher Scientific). For first-strand cDNA synthesis from total lung RNA and sorted cell RNA, M-MLV reverse transcriptase and buffer (Sigma, no. M1302-40KU) and random hexamers (Thermo Fisher Scientific, no. S0142) were used. RNA from input and immuniprecipitation (IP) samples was reverse-transcribed using a SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific, no. 11766050). RT-qPCR reactions were performed with IBI KleenGreen qPCR Master Mix (IBI Scientific, no. IB43143) or PowerUP SYBR Master Mix (Applied Biosystems, no. A25776) on the LightCycler 480 instrument (Roche). Genes were normalized to Gapdh expression. Primer sequences are available upon request.
Primary neonatal lung fibroblast culture.
Lung tissues were dissected from mice at P7, rinsed briefly in Dulbecco's phosphate-buffered saline (DPBS), minced, digested in DPBS with 0.26 Wunsch U/ml Collagenase Liberase Research Grade (Sigma, no. 05401119001) for 30 min with frequent agitation at 37°C. Dissociated cells were then washed twice with DPBS, and placed in tissue culture plates with DMEM-F-12 medium (Corning, no. MT10090CV) with 10% fetal bovine serum (FBS; GIBCO, no. 10437028), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Lonza, no. 17-602E). To enrich for fibroblasts, cells were incubated at 37°C, 5% CO2 for 2 h, and nonadherent cells were washed away. Adenoviral transduction was used to express Tcf21 in primary neonatal lung fibroblasts. Cells (passages 2–4, 2×105 cells/well of a 6-well plate) were transduced with adenoviral (Ad)-Tcf21 at a MOI of 0.2 or 0.4 in Opti-MEM I Reduced-Serum Medium (Thermo Fisher Scientific, no. 31985070). Ad-LacZ or Ad-GFP was used as control. Transduced cells were cultured in fresh DMEM-F-12 medium for 4 days, and neutral lipids were detected by HCS LipidTOX green or LipidTOX red either by flow cytometry or by fluorescence microscopy.
Histology and immunostaining.
Adult lungs were perfused with ice-cold DPBS, followed by 1% paraformaldehyde (PFA)-DPBS and fixed overnight with 1% PFA-DPBS at 4°C. Embryonic and perinatal lungs were fixed overnight with 1% PFA-DPBS at 4°C. Tissues were sequentially incubated with 30% sucrose in DPBS for 4 h, and a 1:1 mixture of 30% sucrose in DPBS and Tissue-Tek O.C.T medium (VWR, no. 102094-106) for 1 h. Tissues were then flash-frozen in Tissue-Tek O.C.T medium with ice-cold 2-methylbutane (Fisher Scientific, no. O3551-4). Ten-micrometer sections were made, permeabilized in 0.1% Triton-X-100 (Sigma-Aldrich, no. T8532-50ml)-DPBS for 10 min, blocked for 1 h (1.5% goat or donkey serum, 1% BSA in DPBS), and incubated with primary antibodies at 4°C overnight (PDGFRα, 1:100 dilution, R&D Systems, no. AF1062; α-SMA-FITC, 1:500 dilution, eBioscience, no. 53-9760-80; cytokeratin, 1:200 dilution, Dako, no. Z0622; proSP-C, 1:200 dilution, Abcam, no. AB3786; CD31-FITC, 1:50 dilution, eBioscience, no. 11-0311-81; DsRed, 1:200 dilution, Clontech Laboratories, no. 632496; anti-Plin2, 1:500 dilution, Abcam, no. ab52356; and Hopx, 1:200 dilution, Santa Cruz Biotechnology, no. sc-30216. For Plin2, Hopx, and CD31 immunostaining, tissue sections were postfixed with cold methanol-acetone (1:1) mixture for 10 min before permeabilization in 0.1% Triton-X-100-DPBS. Secondary antibodies, donkey anti-goat Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 488, and goat anti-rabbit Alexa Fluor 488 (1:500 dilution, Thermofisher) were used. Sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI, Roche, no. 10-236-276-001) for 15 min at room temperature (RT). β-Galactosidase activity was detected as described previously (1). For whole mount lung imaging, tissue clearing was performed before imaging using Visikol HISTO-1 (Visikol) according to manufacturer recommendations after embryonic lungs were fixed with 4% PFA-DPBS overnight at 4°C.
Detection of neutral lipids in cells.
For Oil red O staining, frozen lung tissue sections were stained with 0.6% (wt/vol) Oil red O staining solution (60% isopropanol, 40% water) for 15 min at RT. Tissue sections were washed with running tap water for 20 min and then mounted with 66% glycerol under glass coverslips.
Primary neonatal lung fibroblasts grown on glass coverslips were fixed with 4% PFA for 15 min. Cells were stained with HCS LipidTOX red (1:300 diluted in DPBS) for 30 min at RT. DAPI was used to stain nuclei. For flow cytometry analysis, cells were trypsinized and fixed with 4% PFA in DPBS for 15 min. Fixed cells and frozen lung tissue sections were stained with HCS LipidTOX green (1:300 diluted in DPBS) for 30 min at RT, and samples were immediately analyzed by flow cytometry or fluorescence microscopy.
Flow cytometry and cell sorting.
Adult lungs were minced and digested with collagenase type IV (600 U/ml, Worthington, LS004188) and dispase II (1.2 U/ml, Thermo Fisher, no. 17105041) in DPBS containing 0.9 mM Ca2+ for 45 min at 37°C with frequent agitation. Perinatal lungs were digested as described above. Cell suspensions were sequentially filtered through 40-μm and 30-μm filters and washed in 1% BSA-DPBS. Cells were incubated in primary antibodies anti-CD31 APC (1:50 dilution, BioLegend, no. 102409) and anti-CD45 Pacific blue (1:500 dilution, BioLegend, no. 103125) in 1% BSA-DPBS for 30 min at RT, washed, and fixed with 4% PFA in DPBS for 15 min. Cells were stained with HCS LipidTOX green as described above. Compensation beads (eBioscience, no. 01-2222-42) were used for antibody compensation analysis, and data were acquired on a LSRFortessa (BD Bioscience). Gating analyses were performed using FlowJo (TreeStar).
Lungs from three P7 mice (P2 single tamoxifen induction) were pooled, and single cell dissociation was performed as described above. Red blood cells were lysed with 1× RBS lysis buffer (Thermo Fisher Scientific, no. 00-4300-54) for 5 min. Cell suspensions were filtered through 40-μm filters and incubated with primary antibodies, anti-CD31 APC (1:50 dilution, BioLegend, no. 102409), anti-CD45 APC (1:250 dilution, Tonbo Biosciences, no. 20-0451), and anti-EpCAM (1:200 dilution, Bioss, no. bs-1513R) in 1% BSA-DPBS for 30 min at RT. EpCAM antibody was detected by incubation with secondary antibody (1:500 dilution, goat anti-rabbit antibody, APC, Invitrogen) for 30 min. Cells were washed with 1% BSA-DPBS and stained with HCS LipidTOX green. Prior to cell sorting, cell suspensions were filtered through 30-μm filters. Flow cytometry analyses were performed at the Molecular and Cellular Imaging Core (John A. Burns School of Medicine, Univ. of Hawaii at Manoa). Cells were sorted on a FACS Aria II (BD Biosciences) with the following surface markers: epithelial cell adhesion molecule (EpCAM)/CD31/CD45-(lin−); LipidTOX−, lin−; LipidTOX+, lin+; and LipidTOX+. RNA was extracted from sorted cells as described in RT-qPCR.
Imaging and statistical analysis.
A Zeiss Axiovert 200M was used for fluorescence imaging and a Zeiss Axioskop 2 Plus was used for bright-field images. An Olympus IX81 DSU spinning disk microscope was used for whole mount lung imaging and fluorescence imaging. In the majority of figures, the left lobe was imaged. For quantification, both left and right lobes were included. Fiji software was used for cell counting and area quantification of Oil red O.
For quantification and analysis of percentage of Tcf21 lineage labeled cells, immunofluorescence images were taken at ×400 magnification using a Zeiss Axiovert 200M inverted microscope. The percentage of lineage-traced smooth muscle, PDGFRα, and lipofibroblasts was calculated using merged tdTomato, DAPI, and the respective cellular marker. The number of double-positive tdTomato and α-SMA, PDGFRα, or Plin2 was divided by the total number of tdTomato+ cells. DAPI staining was used to identify individual cells. Between six and ten fields of view (FOV) per lung were analyzed.
All statistical calculations were performed using Prism 7 (GraphPad). Data were analyzed using two-sided t-tests or one-way ANOVA, as noted in the figure legends. Statistical variability is expressed as mean ± SD.
RESULTS
Tcf21 expression in late- gestation lung.
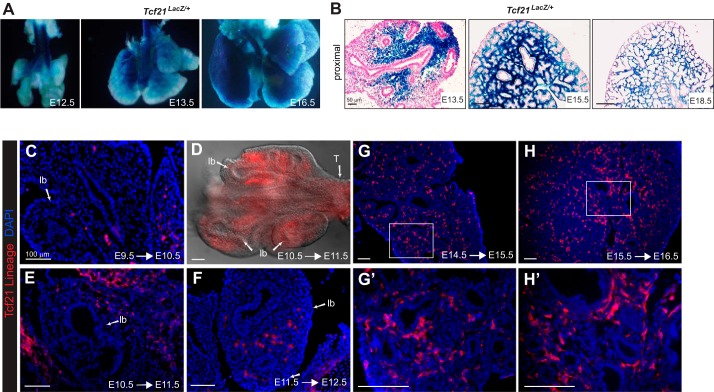
Tcf21-null animals die at birth, in part due to hypoplastic lungs (28, 42). Although Tcf21 expression in lung mesenchyme has been previously described by in situ hybridization and LacZ reporters (28, 42, 44), whole mount views of the Tcf21LacZ reporter allele (29) suggested a temporal and spatial expression of Tcf21 that had not been previously appreciated. To understand the dynamic expression of Tcf21 during lung embryogenesis, we examined Tcf21LacZ lungs at E12.5 and E13.5. β-Galactosidase activity was observed surrounding the proximal conducting airway but was absent from the distal lung mesenchyme (Fig. 1, A and B). After E15.5, β-galactosidase activity was detected throughout the lung mesenchyme but was not present in all mesenchymal cells (Fig. 1B).
Fig. 1.
Transcription factor 21 (Tcf21) expression in embryonic lung. A: whole mount images of Tcf21LacZ/+ reporter embryonic lungs. B: histologic sections of Tcf21LacZ/+ reporter lungs counterstained with nuclear fast red. Representative images are top left lobe at embryonic day (E)13.5, E15.5, and E18.5; n = 3–5. Scale bars, 50 μm. C–H: Cre-mediated tdTomato expression in embryonic lungs after 1-day labeling. tdTomato expression was visualized using DsRed antibody. Boxed regions in G and H are magnified in G' and H'. D: whole mount fluorescence image of E11.5 lung; lb, lung bud. lb, lung bud; T, trachea. Scale bars, 100 μm.
Because the β-galactosidase protein is relatively stable and can remain in cells that have lost Tcf21 expression, we performed 24-h lineage tracing using a tamoxifen-inducible Cre allele of Tcf21 (Tcf21mCrem) (1). Tamoxifen treatment at E9.5 resulted in Tcf21 lineage-tagged cells associated with lung and trachea primordia (Fig. 1C), whereas E10.5 and E11.5 labeled cells were concentrated in mesenchyme adjacent to the proximal airways (Fig. 1, D–F). Single day traces of Tcf21-expressing cells labeled at E14.5 and E15.5 revealed Tcf21-expressing cells throughout the lung mesenchyme (Fig. 1, G, G', H, and H').
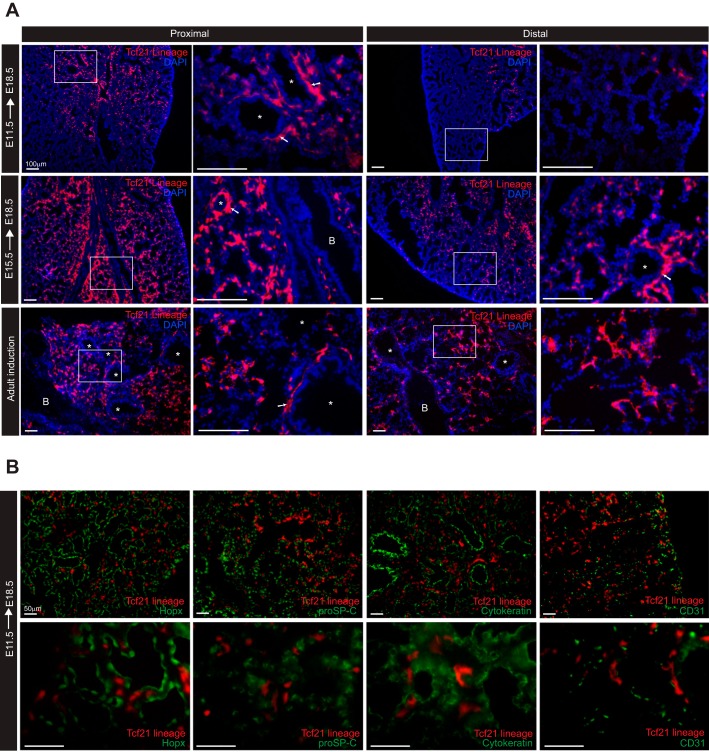
To determine the location and fate of the Tcf21+ progenitor cells, we induced at embryonic time points and examined lungs at E18.5. Progeny of Tcf21-expressing cells labeled at E11.5 were present near the proximal bronchioles but were rare in distal regions. When labeled at E15.5, descendants of Tcf21-expressing cells were found throughout the lung mesenchyme but were more prevalent near the proximal bronchi (Fig. 2A). Adult Tcf21 lineage tracing demonstrated labeled cells in the interstitium as well as in close proximity to bronchioles and blood vessels (Fig. 2A). No embryonic or adult Tcf21 lineage-tagged cells were positive for cytokeratin, Hopx, prosurfactant protein C (proSP-C), or CD31 expression, indicating that Tcf21-expressing progenitors do not give rise to epithelial cells, ATI, ATII, or endothelial cells, respectively (Fig. 2B).
Fig. 2.
Cre-mediated transcription factor 21 (Tcf21) expression pattern in embryonic and adult lung. A: representative images of Tcf21 lineage-tagged, tdTomato cells in proximal and distal embryonic and adult lungs from Tcf21mCrem/+;R26RtdT/tdT mice (R26R, Rosa 26 reporter). Thin white boxed regions indicate regions magnified on the right. Lungs were isolated at embryonic day (E)18.5 after a single dose of tamoxifen administration at E11.5 and E15.5. Adult lungs were isolated 3 mo after a single dose of tamoxifen administration. White arrows point to tdTomato+ cells found in regions surrounding vessel; n = 5–6. B, bronchiole; *, pulmonary vessels. Scale bars, 100 μm. B: representative images of E18.5 lungs stained for Hopx [HOP homeobox; alveolar type 1 (ATI) cells], proSP-C [prosurfactant protein C; (ATII) cells], cytokeratin (epithelial cells), and CD31 (endothelial cells). Tamoxifen induction at E11.5; n = 3. Scale bars, 50 μm.
Tcf21 expression identifies a multipotent mesenchymal progenitor at E11.5.
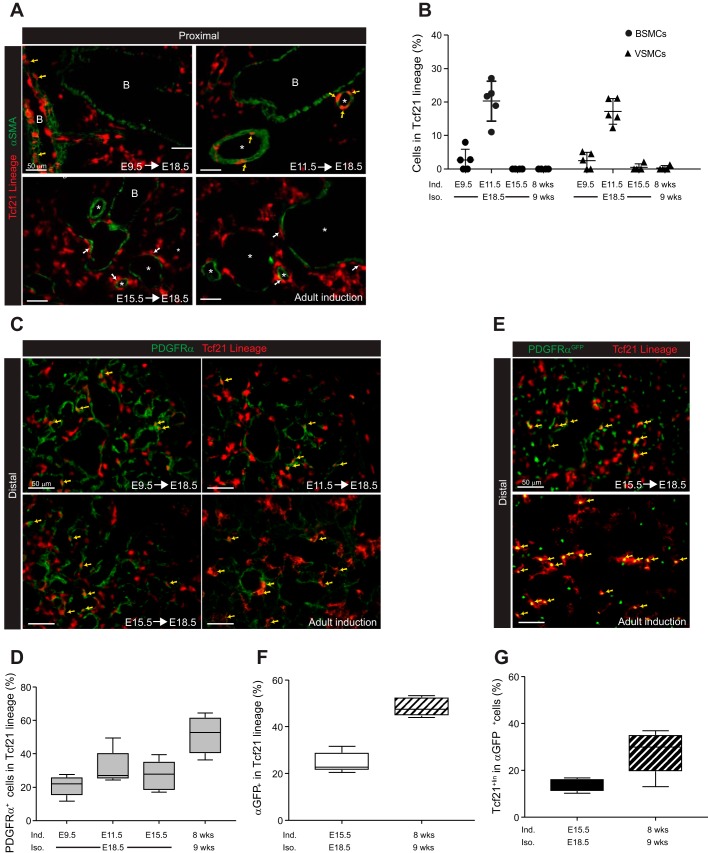
Recent lineage tracing and single-cell transcriptome analyses of mesenchymal cells in the lung have suggested that several subsets of mesenchymal cells exist (26, 59, 61). To identify the mesenchymal populations derived from Tcf21-expressing progenitors, we costained Tcf21 lineage-traced lungs with mesenchymal cell markers. First, we stained lineage-traced lungs with α-SMA to identify smooth muscle cells and myofibroblasts. When Tcf21-expressing cells were labeled at E9.5 and lungs examined at E18.5, lineage-tagged cells surrounded bronchioles and vessels in the proximal embryonic lung (Fig. 3A), but only a few bronchial smooth muscle cells (BSMCs) and vascular smooth muscle cells (VSMCs) were lineage tagged (Fig. 3, A and B). When lineage-tagged at E11.5, a small but definitive population of α-SMA+ smooth muscle cells were Tcf21lin+ in E18.5 lungs (BMSCs: 20.3 ± 6.0%; VSMCs: 17.2 ± 3.8%). However, lineage tagging at E14.5 and later resulted in less than 1% of the Tcf21 lineage-tagged cells being α-SMA+ (Fig. 3, A and B, and data not shown). These results indicated that, at the early pseudoglandular stage, a fraction of Tcf21-expressing progenitors become smooth muscle cells, but by E14.5, Tcf21 was no longer expressed in this population.
Fig. 3.
Identification of smooth muscle cells and platelet-derived growth factor receptor-α (PDGFRα)-expressing cells derived from transcription factor 21 (Tcf21) progenitors. A: representative images of α-smooth muscle actin (α-SMA) staining in embryonic day (E)18.5 and adult lungs of Tcf21mCrem/+;R26RtdT/tdT mice treated with tamoxifen at indicated time points. Adult lungs were isolated 1 wk after a single tamoxifen induction. Yellow arrows indicate cells with coexpressed tdTomato and α-SMA; white arrows indicate tdTomato+ cells surrounding a bronchiole or vessel; n = 4–5. Scale bars, 50 μm. B: quantification of α-SMA+ cells in Tcf21 lineage; n = 4–5 with 6–10 fields of view (FOV). BSMCs, bronchial smooth muscle cells; VSMCs, vascular smooth muscle cells. Data are shown as scatter dot plot with means ± SD. C: representative images of PDGFRα immunohistochemistry. Yellow arrows indicate cells with overlapping tdTomato and PDGFRα; n = 4–5. Scale bars, 50 μm. D: quantification of PDGFRα+ cells in Tcf21 lineage; n = 4–5, 10 FOV were analyzed. E: representative images of distal E18.5 and adult lungs from Tcf21mCrem/+;R26RtdT/tdT;PDGFRαGFP/+ mice. Yellow arrows indicate cells with overlapping fluorescence. Note that PDGFRαGFP cells contain a nuclear localized green fluorescent protein (GFP); n = 5. Scale bars, 50 μm. F and G: quantification of fluorescence overlap shown in E; n = 5, 10 FOV were analyzed. Data are shown as box and whisker plot with whiskers indicating max/min values.
Since PDGFRα-expressing cells represent the myofibroblast and lung fibroblast population (37), we examined the overlap between PDGFRα expression and Tcf21 lineage-tagged cells. Depending on the time of labeling, 20–30% of the embryonic Tcf21 lineage cells also expressed PDGFRα protein. This overlap was consistent between proximal and distal regions (Fig. 3, C and D, and data not shown). These data were confirmed by using lineage-tagged PDGFRαGFP mice (20), where ~25% of lineage-tagged cells labeled at E15.5 were GFP+ in E18.5 lungs (Fig. 3, E and F). Conversely, less than 30% of the GFP-expressing cells were Tcf21 lineage tagged (Fig. 3, E and G). However, in the adult lung, a higher percentage of Tcf21 lineage-tagged cells expressed GFP (Fig. 3, E and F). Therefore, some Tcf21-expressing cells become a mesenchymal population distinct from smooth muscle cells and PDGFRα-expressing fibroblasts.
Interstitial Tcf21-expressing cells are lipofibroblasts.
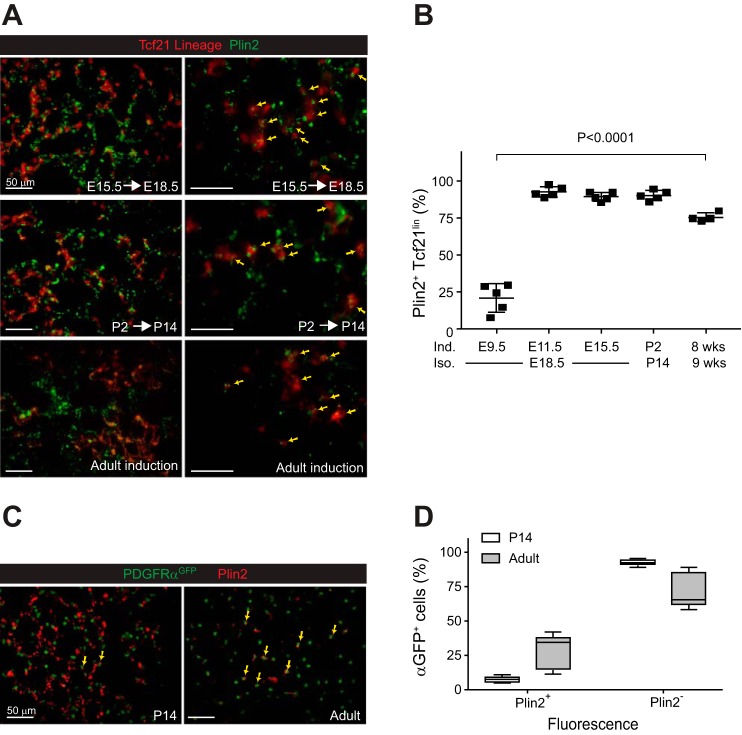
Given the limited number of Tcf21 lineage-tagged cells that overlapped with PDGFRα+ fibroblasts or smooth muscle cells, we next determined whether the Tcf21 lineage becomes the specialized lipofibroblast. Plin2 (ADRP and ADFP) is a protein that associates with intracellular lipid droplets in lipofibroblasts and other lung cells (3, 40, 41). A modest percentage (21.1 ± 9.5%) of Tcf21-expressing cells labeled at E9.5 were Plin2+ in lungs isolated at E18.5. However, Tcf21-expressing progenitors labeled at either E11.5 or E15.5 were predominantly Plin2+ cells in both proximal and distal areas of E18.5 harvested lungs (92.6 ± 3.5% and 89.4 ± 3.0%, respectively; Fig. 4, A and B). To determine whether Tcf21-expressing cells contribute to postnatal lipofibroblasts, we induced Tcf21mCrem/+;R26RtdT/tdT mice either at P2 or in the adult (8 wk old). A majority of the Tcf21 lineage also expressed Plin2 (Fig. 4, A and B). By contrast, only a subpopulation of nuclear PDGFRαGFP+ cells were Plin2+ at P14 (Fig. 4, C and D). These results indicated that Tcf21-expressing cells during the pseudoglandular stage and beyond develop primarily into lipofibroblasts. It has been suggested that PDGFRαGFPdim cells represent the P7 lipofibroblast population (16, 34). Therefore, we assessed PDGFRαGFP expression at P7 in the postnatal Tcf21 lineage. We found that ~50% of the Tcf21 lineage were PDGFRαGFPdim, whereas 23% of the Tcf21 lineage were PDGFRαGFPhi (data not shown).
Fig. 4.
Transcription factor 21 (Tcf21) lineage cells become lipofibroblasts. A: representative images of perilipin 2 (Plin2)-stained lungs from Tcf21mCrem/+;R26RtdT/tdT mice treated with tamoxifen at indicated time points. Yellow arrows indicate cells with overlapping fluorescence; n = 4–5. Scale bars, 50 μm. Adult lungs were isolated 1 wk after a single tamoxifen induction. B: quantification of Plin2+ cells in Tcf21 lineage; n = 4–5, 6–10 fields of view (FOV), one-way ANOVA. Adult lungs were isolated 1 wk after a single tamoxifen induction. E, embryonic day; P, postnatal day; Ind, tamoxifen induction; Iso, lung isolation. C: representative images of Plin2-stained lungs from P14 and adult platelet-derived growth factor receptor-α (PDGFRα)GFP/+ mice. GFP, green fluorescent protein. Yellow arrows indicate cells with overlapping red and green fluorescence; n = 5. Scale bars, 50 μm. D: quantification of Plin2+ cells in PDGFRαGFP+ cells; n = 5, 10 FOV. Data are shown as box and whisker plot with whiskers indicating max/min values.
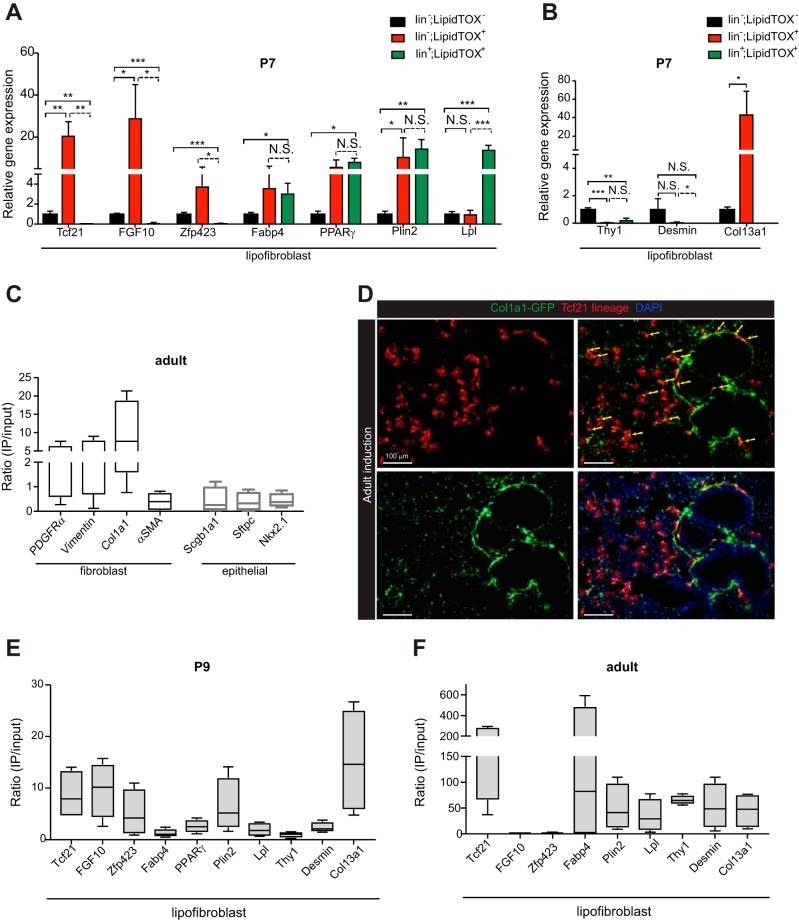
To compare gene expression between lipofibroblasts and other lipid droplet-containing cells in the lung, we sorted cells from freshly isolated lungs at P7. Using LipidTOX, a neutral lipid dye to identify the lipid droplet-containing cells, we sorted three populations of cells. The lipid droplet-containing endothelial, hematopoietic, and epithelial cells were sorted in one channel using CD31, CD45, and CD326 (EpCAM). This population was termed lin+;LipidTOX+. The second population, which represented the lipofibroblast, was lin−;LipidTOX+. The third population was lin−;LipidTOX−. We performed qPCR for genes previously reported to be expressed in lipofibroblasts and found that fatty acid binding protein-4 (Fabp4), peroxisome proliferator-activated receptor-γ (PPARγ), and Plin2 were expressed in lipofibroblasts and other lipid droplet-containing cells. Lipoprotein lipase (Lpl), Tcf21, fibroblast growth factor 10 (FGF10), and zinc finger protein (Zfp)423 were differentially expressed between the two lipid-containing populations at P7 (Fig. 5A). Because recent single cell transcriptome analysis suggested that Tcf21 expression was strongly associated with a collagen (Col)13a1 fibroblast subtype in the adult lungs (59), we determined Col13a1 expression in the sorted cells and found that Col13a1 expression was enriched in lin−;LipidTOX+ cells (Fig. 5B). Other fibroblast markers, Thy1 and desmin, were not highly expressed in lin−;LipidTOX+ cells compared with the lin−;LipidTOX−population.
Fig. 5.
Gene expression in perinatal and adult lipofibroblasts. A and B: expression of putative lipofibroblast genes in lipofibroblasts and other lipid droplet-containing cells in the lung. Postnatal day (P)7 lungs from 3 tamoxifen-treated mice were pooled and sorted. Lineage− (lin−) with antibodies for CD45, CD31, and CD326 (epithelial cell adhesion molecule, EpCAM) that were positive (lin−;LipidTOX+) or negative (lin−;LipidTOX−) for LipidTOX and lin+ that were positive for LipidTOX (lin+LipidTOX+); n = 3 replicates of pooled samples. FGF10, fibroblast growth factor 10; Zfp423, zinc finger protein-423; Fabp4, fatty acid-binding protein-4; PPARγ, peroxisome proliferator-activated receptor-γ; Plin2, perilipin 2; Lpl, lipoprotein lipase; Thy1, thymus cell antigen 1; Col13a1, collagen type XIIIα1. C: expression of lung fibroblast and epithelial genes in adult transcription factor 21 (Tcf21) lineage. PDGFRα, platelet-derived growth factor receptor-α; Col1a1, collagen type 1α1; α-SMA, α-smooth muscle actin; Sftpc, surfactant protein C; Nkx2.1, NK2 homeobox 1. D: representative images of Col1a1 reporter in adult lungs from Tcf21mCrem/+;R26RtdT/tdT;Col1a1-GFP mice. GFP, green fluorescent protein. Adult lungs were isolated 3 mo after 3 doses of tamoxifen induction. Yellow arrows indicate fluorescence overlap; n = 4. Scale bars, 100 μm. E and F: expression of putative lipofibroblast genes in P2 induced P9 isolated Tcf21 Ribotag lungs (E) and adult induced Tcf21 Ribotag lungs (F). Expression levels were normalized to that of GADPH and represented as a ratio of gene expression in the Tcf21 lineage (IP) to total lung RNA (input). Lungs were isolated after a single tamoxifen induction for P9 mice or 2–3 doses of tamoxifen induction for adult mice. Data are shown as box and whisker plot with whiskers indicating max/min values (n = 4). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
To examine the gene expression of Tcf21 lineage cells, we isolated ribosomal-associated transcripts from the Tcf21 lineage by using a Cre-inducible ribosomal tag, Rpl22HA (RiboTag) (47). We found that fibroblast genes, including PDGFRα, vimentin, and collagen 1a1 (Col1a1) were enriched in the adult Tcf21 lineage, whereas α-SMA and epithelial genes were not (Fig. 5C). Consistent with these results, we observed that a population of postnatal Tcf21 lineage cells express an active type I collagen promoter (60) (Fig. 5D). Using RiboTag to isolate ribosomal associated transcripts, we also determined the expression of putative lipofibroblast genes in perinatal and adult Tcf21 lineage cells (Fig. 5, E and F). Both Plin2 and Col13a1 expressions were enriched in perinatal and adult Tcf21 lineage cells compared with total lung RNA. FGF10 and Zfp423 were enriched in perinatal Tcf21 lineage cells but not in the adult Tcf21 lineage, while additional putative lipofibroblasts genes Thy1 and desmin were enriched only in the adult Tcf21 lineage. Taken together, these data indicated that the Tcf21 lineage had gene expression consistent with that of fibroblasts and lipofibroblasts.
Tcf21 lineage cells contain lipid droplets.
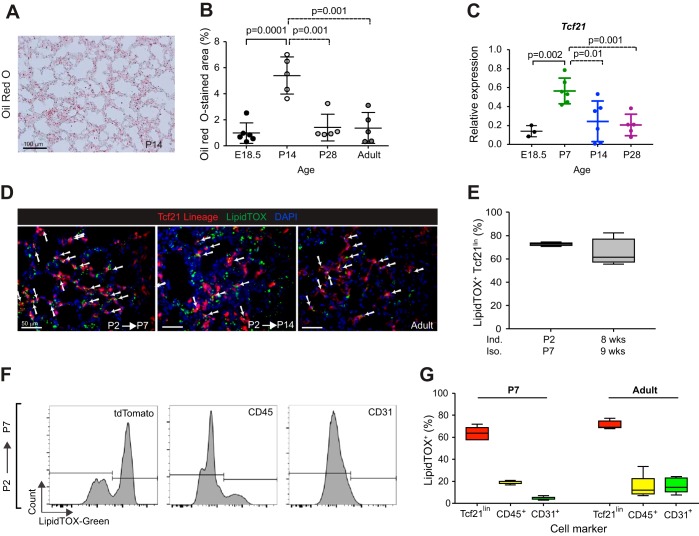
Consistent with reports documenting peak lipid content in the lung during alveolar septation (4, 32), we observed increased lipid content at P14, which decreased by P28 (Fig. 6, A and B). Relative Tcf21 expression peaked at P7 and then decreased (Fig. 6C). Since the distinguishing feature of lipofibroblasts is the presence of neutral lipid droplets within the cells, we determined by histology whether Tcf21 lineage-tagged cells contain lipid droplets. At P7 and in the adult lung, more than 60% of Tcf21 lineage-tagged cells were LipidTOX+ in distal lungs (Fig. 6, D and E).
Fig. 6.
Neutral lipid-containing cells in the lung. A: representative image of Oil red O-stained postnatal day (P)14 lung section; n = 5. B: quantification of Oil red O-stained area in lungs at stages indicated. E, embryonic day. Data are shown as scatter dot plot with means ± SD. Ten fields of view (FOV) from lungs were analyzed, unpaired t-test. C: expression of transcription factor 21 (Tcf21) in lungs at stages indicated. Data were normalized to expression of GADPH and are shown as scatter dot plot with means ± SD, unpaired t-test. D: representative images of LipidTOX green-stained postnatal and adult lungs from Tcf21mCrem/+;R26RtdT/tdT mice. White arrows indicate LipidTOX+ Tcf21 lineage. Adult lungs were isolated 1 wk after a single tamoxifen induction; n = 4. Scale bars, 50 μm. E: quantification of LipidTOX+ cells in Tcf21 lineage at indicated time points. Data are shown as box and whisker plot with whiskers indicating max/min values; n = 4. Seven to ten FOV from lungs were analyzed. Ind, induced; Iso, isolated. F: representative flow cytometry histograms of LipidTOX fluorescence intensity within tdTomato, CD45, or CD31 gates at P7 lungs. Tamoxifen induction at P2; n = 5. G: quantification of percent LipidTOX+ cells in P7 and adult lungs, gated on tdTomato+, CD45+, or CD31+. Adult lungs were isolated 2 wk after 3 doses of tamoxifen induction. Data are shown as box and whisker plot with whiskers indicating max/min values (n = 4–5).
Because others have suggested that macrophages and endothelial cells also accumulate neutral lipid droplets (25, 46), we determined by flow cytometry what proportion of each cell type contained lipid droplets. At P7 and in the adult, a majority of Tcf21 lineage cells were LipidTOX positive (60–70%), whereas relatively fewer CD45+ leukocytes and CD31+ endothelial cells contained lipid droplets (Fig. 6, F and G). Whereas the proportion of LipidTOX+;CD45+, and LipidTOX+;Tcf21lin remained relatively similar, the proportion of LipidTOX+ endothelial cells increased in the adult lung (Fig. 6G). These results reinforce data from others demonstrating that there are several lipid-containing cell types in the lung and demonstrate that the postnatal Tcf21 lineage represents a portion of the lipid-containing cells.
Lipid droplet accumulation with Tcf21 overexpression.
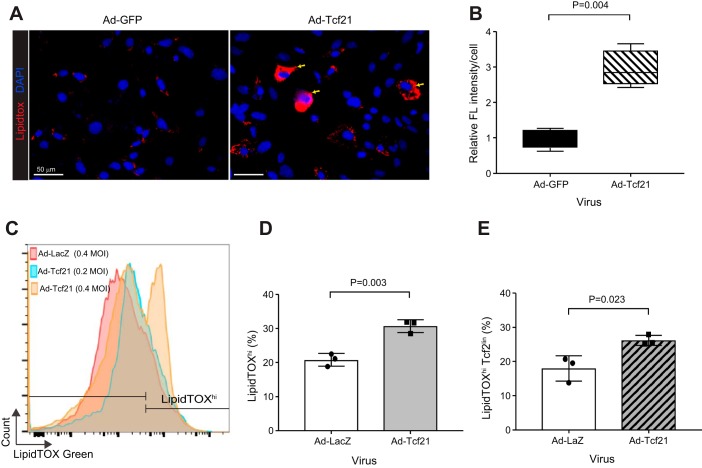
The data above suggested that Tcf21 lineage tracing overlaps with the lipofibroblast population. To investigate if Tcf21 has a role in neutral lipid accumulation, we overexpressed Tcf21 in postnatal primary lung fibroblasts. Adenoviral-driven Tcf21 (Ad-Tcf21) resulted in an ~200-fold increase in Tcf21 transcript levels 4 days after transduction (data not shown). We observed no difference in the total number of neutral lipid-containing cells with Tcf21 overexpression (data not shown), but LipidTOX accumulation increased on a per-cell basis compared with Ad-GFP-transduced fibroblasts (Fig. 7, A and B). To further document the neutral lipid accumulation, we labeled Tcf21lin cells at P2 and isolated primary lung fibroblasts at P7 and transduced with adenovirus. Using flow cytometry to detect neutral lipid accumulation, we observed that Tcf21 overexpression resulted in increased fluorescence intensity in a dose-dependent manner (Fig. 7C). Indeed, Tcf21 overexpression resulted in an overall increase in the percentage of LipidTOXhi fibroblasts (Fig. 7D). When we focused specifically on the Tcf21 lineage cells, which presumably already express Tcf21, the increase in lipid content was not as dramatic (Fig. 7E). These results suggested that Tcf21 expression leads to lipid accumulation.
Fig. 7.
Increased neutral lipid content in lung fibroblasts after transcription factor 21 (Tcf21) overexpression. A: representative images of lipid accumulation in wild-type postnatal day (P)7 primary lung fibroblasts after adenoviral GFP [Ad-GFP, 0.4 multiplicity of infection (MOI)] or adenoviral Tcf21 (Ad-Tcf21, 0.4 MOI) transduction. Yellow arrows indicate cells with increased neutral lipids; n = 4. B: quantification of fluorescent intensity per cell. Images and data analysis were performed 4 days after adenoviral transduction of wild-type P7 primary lung fibroblasts; n = 4, paired t-test. Data are shown as box and whisker plot with whiskers indicating max/min values. C: flow cytometry histograms of neutral lipid staining in P7 primary lung fibroblasts after transduction with adenoviral Tcf21 at MOI of 0.2 and 0.4 or adenoviral LacZ at a MOI of 0.4 as control. D and E: quantification of LipidTOXhi cells in P7 lung fibroblasts after Tcf21 overexpression. D: quantification of percent LipidTOXhi cells in P7 fibroblast after Ad-βgal (0.4 MOI) or Tcf21 (0.4 MOI) transduction. E: quantification of percent LipidTOXhi cells in P7 Tcf21 lineage. Tcf21 lineage cells were labeled at P1; n = 3, unpaired t-test.
DISCUSSION
Based on their proximity to ATII cells, lipid-laden fibroblasts in the lung have the potential to shuttle lipids for surfactant production (54), but this function has only been demonstrated in vitro (53). Others have demonstrated that lipid-laden fibroblasts can support epithelial cell expansion and differentiation (3, 5). Because lipid content in lipofibroblasts is maximal for only a few weeks during postnatal development and other cell populations also possess lipid droplets, unambiguous identification of this cell population has been problematic. In addition, there has been little consensus confirming that lipofibroblasts are a distinct lineage. In vivo data have not determined whether lipofibroblasts and lung interstitial fibroblasts derive from a common progenitor. Although Ntokou et al. (37), demonstrated that a subpopulation of PDGFRα lineage cells labeled immediately after birth become Plin2+, they also demonstrated that by P5 PDGFRα lineage cells lose their ability to differentiate into lipofibroblasts and are predominantly interstitial fibroblasts.
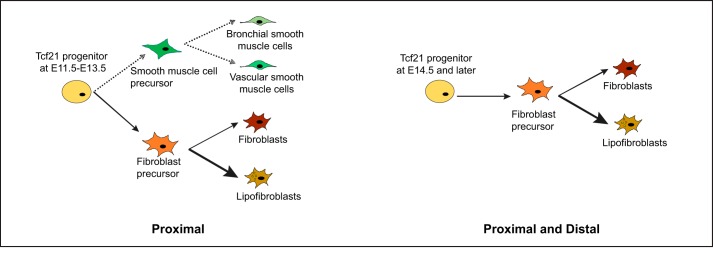
Here, we show that Tcf21-expressing embryonic mesenchymal progenitors become lipofibroblasts and a subpopulation of fibroblasts. Within a brief embryonic time frame, E11.5 to E13.5, Tcf21 mesenchymal progenitors can become either fibroblasts or smooth muscle cells, but around E15.5 Tcf21 progenitors develop predominantly into interstitial fibroblasts and lipofibroblasts (Fig. 8). This differentiation potential is reminiscent of the differentiation of mesenchymal progenitors in the heart, where Tcf21 expression marks a bipotent mesenchymal progenitor, but after E13.5, expression is restricted to the cardiac fibroblast lineage (2). A similar pattern of mesenchymal cell fate was proposed by a recent single-cell map of lung development (24).
Fig. 8.
Summary of fibroblast subtypes derived from transcription factor 21 (Tcf21) progenitors. Ad-GFP, adenoviral green fluorescent protein. Tcf21 lineage tracing results of the present study demonstrate that Tcf21 progenitors at embryonic day (E)11.5 to E13.5 generate smooth muscle cells, interstitial fibroblasts, and lipofibroblasts. After E14.5, Tcf21 progenitors give rise exclusively to lipofibroblasts and a subpopulation of fibroblasts.
In contrast to the heart, where there is synchrony between the Tcf21 lineage and PDGFRα expression (2), in the lung, only a subset of the Tcf21 lineage coexpresses PDGFRα. It is possible that Tcf21 is expressed in the same FGF10 progenitor that can differentiate into fibroblasts, lipofibroblasts, and smooth muscle cells (13), but later in embryonic development, Tcf21 expression is restricted to interstitial fibroblasts and lipofibroblasts. Unlike FGF10, which does not label adult lipofibroblasts (20), Tcf21 continues to be expressed in the lipofibroblast and some interstitial fibroblasts. One limitation to our studies is that we have assumed that Tcf21 expression is synonymous with Tcf21mCrem recombination, as we showed in the heart (2). There is the possibility that Cre recombination occurs only in cells with a threshold of Tcf21 promoter activity. Therefore, we may not be detecting cells with lower levels of Tcf21 expression.
Consistent with lineage tracing results of embryonic lung, the Tcf21 lineage constitutes a population of interstitial fibroblasts and lipofibroblasts in neonatal and adult lungs. Interestingly, recent single-cell data from adult mouse lungs found that Tcf21 was expressed in a Col13a1-expressing cell population that was labeled matrix fibroblasts (59), and Col13a1 was also identified as one of the genes differentially expressed in the PDGFRαGFPdim population (19). Our data suggest that Col13a1 expression not only occurs in Tcf21 lineage cells but can also be found in lipid-laden, CD31−, CD45−, EpCAM− cells, suggesting that Col13a1 may be enriched in lipofibroblasts. Unlike FGF10 and Zfp423 expression, which are relatively reduced in adult lipofibroblasts, Col13a1 and Tcf21 expressions remain enriched in this population. As might be expected from the predicted role of lipofibroblasts in lung development (33), our data suggest that lipofibroblast gene expression changes over the course of lung maturation and that some of the suggested lipofibroblast genes are differentially expressed. Although another group has reported Tcf21 expression in the perinatal lipofibroblast (34), additional single-cell reports from adult lungs have not identified a unique Tcf21 lipofibroblast subset (26, 59, 61), possibly because Tcf21 is a transcription factor and its expression is below detection limits of adult lung single-cell analyses.
The lung lipofibroblast remains a relative mystery. Identification has relied on expression of proteins related to lipid droplets and neutral lipid detection. Over the past few decades, numerous lung lipofibroblast genes have been suggested, including those associated with lipid droplet regulation: Plin2 (3, 55), Lpl (8), Zfp423 (27), and Fapb1, -4, -5 (27, 59). As lipid handling is an essential process not only for lipofibroblasts but also for other cell types in the lung, the unambiguous labeling of a lipofibroblast by this gene profile is not possible. In fact, several of the lipid metabolism genes used to define the lung lipofibroblast population (Pparγ, Lpl, and Plin2) are also expressed in alveolar macrophages (10, 18, 24, 38, 49). While some of these genes are clearly involved in regulating lipofibroblast development and function, most are also broadly expressed in other cell types in the lung.
FGF receptor signaling was one of the first pathways shown to affect lipofibroblast development, where disruption of signaling impaired lipofibroblast development (3). Other receptors that have been implicated in lipofibroblast development include transforming growth factor-β type I receptor Alk5 and PDGFRα. Loss of either of these receptors leads to reduced myofibroblasts and increased lipofibroblasts (27). Those authors suggested that Alk5 signaling is involved in a cell fate decision between myofibroblast and lipofibroblast, but our lineage tracing does not identify a lipofibroblast progenitor that can also become an alveolar myofibroblast. Perhaps the phenotype observed with Alk5 loss was not cell autonomous. Further studies are required to determine whether Tcf21 is required for lipofibroblast development and function.
Overexpression of Tcf21 resulted in increased lipid vesicle density, suggesting that Tcf21 may transcriptionally regulate lipid vesicle processing. Others have noted Tcf21 expression in cells with lipid-storing capacity, including white preadipocytes (52), hepatic stellate cells (unpublished observation, M. J. Ivey and M. D. Tallquist), and steroidogenic cells of the adrenal gland (57) and testes (11). Chromatin immunoprecipitation sequencing combined with gene expression in human VSMCs found that Tcf21 associates with loci of genes in the category of lipoprotein particle clearance and cholesterol import (48). Our data suggest that Tcf21 expression not only identifies lipofibroblasts, but also may be involved in the regulation of lipid storage.
In conclusion, in this study, we present data delineating the distribution and identification of Tc21 progenitor cells during lung development. Here, we have shown that the Tcf21 lineage develops exclusively into lipofibroblasts and interstitial fibroblasts. These data suggest that Tcf21 can be used to identify cells with lipofibroblast potential even in the absence of lipid droplets. This finding represents an essential step toward the development of new therapeutic strategies by modulating distinct progenitor cell types in a disease context. Further experiments will be required to understand how Tcf21 transcriptional targets determine lipofibroblast development and differentiation.
GRANTS
Flow cytometry cell sorting was performed by the Molecular and Cellular Immunology Core, which is supported, in part, by Grant P30 GM-114737 from the Centers of Biomedical Research Excellence (COBRE) program of the National Institute of General Medical Sciences, a component of the National Institutes of Health. This work was supported by National Heart, Lung, and Blood Institute Grants HL-074257, HL-144067, and HL-100401 (M. D. Tallquist), F31 HL-126512 (M. J, Ivey), F31 HL-128048 (J. M. Swonger), and T32 HL-115505 (M. J. Ivey and J. M. Swonger), University of Hawaii Undergraduate Research Opportunities Program funding (G. Y. Park), and Hawaii Community Foundation Grant 18ADVC-90699 (J. Park).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P. and M.D.T. conceived and designed research; J.P., M.J.I., Y.D., K.R., E.S., V.S., C.S., T.H., G.Y.P., J.M.S., and T.R. performed experiments; J.P., M.J.I., Y.D., K.R., E.S., C.S., T.H., G.Y.P., T.R., and M.D.T. analyzed data; J.P., M.J.I., E.S., and M.D.T. interpreted results of experiments; J.P., M.J.I., and Y.D. prepared figures; J.P. and M.D.T. drafted manuscript; J.P., M.J.I., Y.D., K.R., E.S., V.S., J.M.S., T.R., R.E.M., K.A., and M.D.T. edited and revised manuscript; J.P. and M.D.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Greg Gojanovich for providing the Tcf21mCrem/+;Rpl22HA/+ lung tissues and Sharmaine Sebastian for technical assistance.
REFERENCES
- 1.Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis 49: 870–877, 2011. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139: 2139–2149, 2012. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Alam D, El Agha E, Sakurai R, Kheirollahi V, Moiseenko A, Danopoulos S, Shrestha A, Schmoldt C, Quantius J, Herold S, Chao CM, Tiozzo C, De Langhe S, Plikus MV, Thornton M, Grubbs B, Minoo P, Rehan VK, Bellusci S. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 142: 4139–4150, 2015. doi: 10.1242/dev.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Developmental shift in the relative percentages of lung fibroblast subsets: role of apoptosis postseptation. Am J Physiol 277: L848–L859, 1999. doi: 10.1152/ajplung.1999.277.4.L848. [DOI] [PubMed] [Google Scholar]
- 5.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996. doi: 10.1016/S0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Jackson S, Doro M, McGowan S. Perinatal expression of genes that may participate in lipid metabolism by lipid-laden lung fibroblasts. J Lipid Res 39: 2483–2492, 1998. [PubMed] [Google Scholar]
- 9.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47: 517–527, 2012. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Giladi A, Gorki AD, Solodkin DG, Zada M, Hladik A, Miklosi A, Salame TM, Halpern KB, David E, Itzkovitz S, Harkany T, Knapp S, Amit I. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175: 1031–1044.e18, 2018. doi: 10.1016/j.cell.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131: 4095–4105, 2004. doi: 10.1242/dev.01266. [DOI] [PubMed] [Google Scholar]
- 12.del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol 293: 77–89, 2006. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 13.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, Bellusci S. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141: 296–306, 2014. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Agha E, Kheirollahi V, Moiseenko A, Seeger W, Bellusci S. Ex vivo analysis of the contribution of FGF10+ cells to airway smooth muscle cell formation during early lung development. Dev Dyn 246: 531–538, 2017. doi: 10.1002/dvdy.24504. [DOI] [PubMed] [Google Scholar]
- 15.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Szibor M, Kosanovic D, Schwind F, Schermuly RT, Henneke I, MacKenzie B, Quantius J, Herold S, Ntokou A, Ahlbrecht K, Braun T, Morty RE, Günther A, Seeger W, Bellusci S. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20: 571, 2017. [Erratum in: Cell Stem Cell 20: 261 273.e3. 10.1016/j.stem.2016.10.004. 27867035.] 10.1016/j.stem.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch M, Xu Y, Perl AK. Dataset on transcriptional profiles and the developmental characteristics of PDGFRα expressing lung fibroblasts. Data Brief 13: 415–431, 2017. doi: 10.1016/j.dib.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch MT, Xu Y, Perl AK. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol 425: 161–175, 2017. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green J, Endale M, Auer H, Perl AK. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. Am J Respir Cell Mol Biol 54: 532–545, 2016. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development 141: 502–513, 2014. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jostarndt-Fögen K, Djonov V, Draeger A. Expression of smooth muscle markers in the developing murine lung: potential contractile properties and lineal descent. Histochem Cell Biol 110: 273–284, 1998. doi: 10.1007/s004180050289. [DOI] [PubMed] [Google Scholar]
- 23.Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G. “Contractile interstitial cells” in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol 60: 375–392, 1974. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16: 36–44, 2015. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 25.Kuo A, Lee MY, Sessa WC. Lipid droplet biogenesis and function in the endothelium. Circ Res 120: 1289–1297, 2017. doi: 10.1161/CIRCRESAHA.116.310498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170: 1149–1163.e12, 2017. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z, Bellusci S, Li C, Minoo P. Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. BMC Biol 14: 19, 2016. doi: 10.1186/s12915-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Chang P, Richardson JA, Gan L, Weiler H, Olson EN. The basic helix-loop-helix transcription factor capsulin controls spleen organogenesis. Proc Natl Acad Sci USA 97: 9525–9530, 2000. doi: 10.1073/pnas.97.17.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev 73: 23–32, 1998. doi: 10.1016/S0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 30.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev 87: 219–244, 2007. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 32.Maksvytis HJ, Vaccaro C, Brody JS. Isolation and characterization of the lipid-containing interstitial cell from the developing rat lung. Lab Invest 45: 248–259, 1981. [PubMed] [Google Scholar]
- 33.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev 32: 98–105, 2015. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGowan SE, McCoy DM. Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 307: L618–L631, 2014. doi: 10.1152/ajplung.00144.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59: 43–62, 1997. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 36.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359: 1118–1123, 2018. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, Szibor M, Braun T, Morty RE, Seeger W, Voswinckel R, Ahlbrecht K. Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol 309: L942–L958, 2015. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 38.Ntokou A, Szibor M, Rodríguez-Castillo JA, Quantius J, Herold S, El Agha E, Bellusci S, Salwig I, Braun T, Voswinckel R, Seeger W, Morty RE, Ahlbrecht K. A novel mouse Cre-driver line targeting Perilipin 2-expressing cells in the neonatal lung. Genesis 55: e23080, 2017. doi: 10.1002/dvg.23080. [DOI] [PubMed] [Google Scholar]
- 39.Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol 4: a00318, 2012. doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul A, Chan L, Bickel PE. The PAT family of lipid droplet proteins in heart and vascular cells. Curr Hypertens Rep 10: 461–466, 2008. doi: 10.1007/s11906-008-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul A, Chang BH, Li L, Yechoor VK, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res 102: 1492–1501, 2008. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126: 5771–5783, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Quaggin SE, Vanden Heuvel GB, Igarashi P. Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev 71: 37–48, 1998. doi: 10.1016/S0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- 44.Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, Jenkins NA, Harvey RP. epicardin: a novel basic helix-loop-helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Dev Dyn 213: 105–113, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 45.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 108: E1475–E1483, 2011. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero F, Shah D, Duong M, Penn RB, Fessler MB, Madenspacher J, Stafstrom W, Kavuru M, Lu B, Kallen CB, Walsh K, Summer R. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am J Respir Cell Mol Biol 53: 74–86, 2015. doi: 10.1165/rcmb.2014-0343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci USA 106: 13939–13944, 2009. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sazonova O, Zhao Y, Nürnberg S, Miller C, Pjanic M, Castano VG, Kim JB, Salfati EL, Kundaje AB, Bejerano G, Assimes T, Yang X, Quertermous T. Characterization of TCF21 downstream target regions identifies a transcriptional network linking multiple independent coronary artery disease loci. PLoS Genet 11: e1005202, 2015. doi: 10.1371/journal.pgen.1005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 15: 1026–1037, 2014. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 50.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet 21: 138–141, 1999. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 51.Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol 166: 600–614, 1994. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- 52.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 104: 4401–4406, 2007. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torday J, Hua J, Slavin R. Metabolism and fate of neutral lipids of fetal lung fibroblast origin. Biochim Biophys Acta 1254: 198–206, 1995. doi: 10.1016/0005-2760(94)00184-Z. [DOI] [PubMed] [Google Scholar]
- 54.Tordet C, Marin L, Dameron F. Pulmonary di-and-triacylglycerols during the perinatal development of the rat. Experientia 37: 333–334, 1981. doi: 10.1007/BF01959845. [DOI] [PubMed] [Google Scholar]
- 55.Varisco BM, Ambalavanan N, Whitsett JA, Hagood JS. Thy-1 signals through PPARγ to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol 46: 765–772, 2012. doi: 10.1165/rcmb.2011-0316OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyalov SL, Gabbiani G, Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol 143: 1754–1765, 1993. [PMC free article] [PubMed] [Google Scholar]
- 57.Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, Hammer GD. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development 140: 4522–4532, 2013. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, Kumar M, Xiao R, D’Armiento J, Metzger D, Chambon P, Papaioannou VE, Stripp BR, Jiang D, Noble PW. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest 126: 3063–3079, 2016. doi: 10.1172/JCI85328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, Liu N, Kulur V, Yao C, Chen P, Liu Z, Stripp B, Tang J, Liang J, Noble PW, Jiang D. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Reports 22: 3625–3640, 2018. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37: 267–276, 2003. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 61.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170: 1134–1148.e10, 2017. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]