Abstract
Cellular senescence results in cell cycle arrest with secretion of cytokines, chemokines, growth factors, and remodeling proteins (senescence-associated secretory phenotype; SASP) that have autocrine and paracrine effects on the tissue microenvironment. SASP can promote remodeling, inflammation, infectious susceptibility, angiogenesis, and proliferation, while hindering tissue repair and regeneration. While the role of senescence and the contributions of senescent cells are increasingly recognized in the context of aging and a variety of disease states, relatively less is known regarding the portfolio and influences of senescent cells in normal lung growth and aging per se or in the induction or progression of lung diseases across the age spectrum such as bronchopulmonary dysplasia, asthma, chronic obstructive pulmonary disease, or pulmonary fibrosis. In this review, we introduce concepts of cellular senescence, the mechanisms involved in the induction of senescence, and the SASP portfolio that are relevant to lung cells, presenting the potential contribution of senescent cells and SASP to inflammation, hypercontractility, and remodeling/fibrosis: aspects critical to a range of lung diseases. The potential to blunt lung disease by targeting senescent cells using a novel class of drugs (senolytics) is discussed. Potential areas for future research on cellular senescence in the lung are identified.
Keywords: aging, airway, alveoli, bronchi, senolytic
INTRODUCTION
Despite significant advancements in our understanding of the cellular processes that drive normal lung development, growth, and aging, as well as pathophysiological processes involved in diseases such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis (PF), and asthma, lung diseases across the age spectrum continue to be a significant health and financial burden (122, 138, 143). A major limitation in this regard is the complexity of normal growth and aging processes per se and their further complex interactions with different factors throughout life, including normal events (e.g., puberty, pregnancy), comorbid conditions that influence the lung (e.g., inflammation and inflammatory disease, cardiovascular diseases), environmental exposures (e.g., diesel or tobacco smoke, household air pollution, allergens), and iatrogenic exposures (e.g., oxygen explores following premature birth, radiation and chemotherapy in cancer). Despite these complexities, certain mechanisms regulating normal versus abnormal cellular structure and function could broadly contribute to lung growth and aging, as well as to disease pathogenesis. In this regard, emerging studies highlight the importance of cellular senescence in the lung and the contribution of senescent cells in aging and diseases, including COPD, PF, and asthma.
The concept of cellular senescence is most commonly associated with biological aging (31, 32, 33, 97, 168), and much exciting research has focused on understanding senescence pathways with a goal of blunting their effects and improving the healthspan of the elderly (95, 96, 136) (i.e., lifespan free from pain, disability, and dependence). Senescence occurs in response to a variety of insults across a range of cell types (31, 38, 39, 40, 173, 184). Accumulation of senescent cells has been observed in vivo in relation to chronological aging (48, 86), chemotherapy and radiotherapy (152), supplemental oxygen (101), and cigarette smoke (CS) (3) to name a few. However, what is less clear in any organ system is the extent to which phenotypic changes associated with normal chronological aging or in disease states are mechanistically attributable to cellular senescence or effects of senescent cells per se, i.e., how important are senescent cells in pathophysiology? Thus, it is recognized that there is a need to understand the contributions of senescence to aging and disease and if significant, the processes that lead to enhanced senescent cell burden. Such a task will then inform the need to determine whether current or future therapies targeting disease states should be evaluated in the context of their ability to reduce senescent cell burden [i.e., senolytic drug therapies (39, 97, 99)] or the influence of senescent cells per se.
Senescent cells have been reported in both human and animal models of pulmonary diseases (2, 5, 6, 41, 71, 107, 158, 187). Indeed, given that chronologic age is a major risk factor for most chronic diseases, including COPD, asthma, and PF, there is relevance to identifying senescence as a fundamental aging process that, if targeted appropriately, could impact on disease alleviation and overall health in the elderly population. However, the mechanistic contributions of senescence in healthy lung aging and in lung disease pathophysiology are still under exploration. In this review, we briefly highlight the processes involved in cellular senescence, the extent of senescent processes in the lung across the age spectrum, the potential mechanisms by which senescent cells contribute to lung structure and function in health and disease, and finally emerging therapeutics that could and perhaps should target senescent cells in the lung. Within these contexts, we highlight current approaches and potential limitations for identification and characterization of senescent cells. Finally, we identify key unanswered questions relevant to the lung that drive future research in an important and timely area.
BRIEF BACKGROUND ON SENESCENCE
Cellular senescence was first observed 60 years ago by Hayflick and Moorehead (75) who reported that primary human lung cells in culture undergo a limited number of divisions before entering a state of replicative arrest, leading to the hypothesis that aging may be a result of cells entering a senescent state. Furthermore, they found that primary human cells isolated from fetal tissue could be cultured longer and through more passages without undergoing replicative senescence compared with adult cells (75). These initial observations highlighted the ability of fetal tissues to resist senescence: a feature that likely wanes with environmental exposures and/or chronological aging. However, what also became clear with subsequent studies is that senescent processes play an important role in normal and pathological embryogenesis (22, 58, 167) beyond their contribution to aging-associated processes.
Senescence essentially involves the exit of a cell from the cycle of cellular replication. A cell’s exit from the cell cycle may serve a teleological role as an integral part of the developmental machinery in embryogenesis or a way to repress replication of cells destined to become oncogenic, resulting in pathology when/if inappropriately triggered (35, 38, 47, 131, 154, 167). At its core, senescence refers to an irreversible arrest of a cell in the cell cycle, resulting in long-lasting survival. While this hypothesis has been confirmed in vitro in multiple models, the in vivo means to accurately detect such an exit has remained elusive (38). Previously, such cells were considered to be inert. However, the discovery of a senescence-associated secretory phenotype (SASP) with cellular secretions consisting of inflammatory mediators, including cytokines, proteins involved in matrix remodeling, and other factors, has challenged this perspective and highlighted roles of senescent cells in autocrine/paracrine effects within cellular microenvironments (2, 140) (discussed further below).
MECHANISMS OF SENESCENCE
The variety of processes leading to cellular senescence encapsulates a large spectrum of mechanisms that are both clinically relevant and significant for cellular function (38). The classic trigger described by Hayflick and Moorehead (75, 77) is progressive telomere shortening leading to DNA damage and activation of tumor suppressors by phosphorylation of p53 and increased production of p21 via an ATM/ARF/CHK1/CHK2 pathway. However, a subset of cells were found to undergo activation of p16 resulting in a mosaic culture, indicating that inducers of senescence may activate several pathways (77). The downstream pathways coalesce on inhibition of cyclin-dependent kinase 2, 4, and 6 or direct interaction between p53 and retinoblastoma, with resultant constitutive activation of retinoblastoma, preventing progression into the S phase (52, 77). The exact mechanism by which p16 is triggered remains unknown; however, transcription factors such as Tbx2/3, JunB, and Ets are thought to play a role (15). The sequence of activation of pathways leading to senescence may be serial, parallel, or there may be crossover between pathways (15).
There are a number of known inducers of senescence relevant to the lung: reactive oxygen species (ROS) (113), endoplasmic reticulum (ER) stress (113), epigenetic modifications (163), oncogene activation (17), mitochondrial dysfunction (1), radiation-induced DNA damage (42), and less well-defined pathways (38) (Fig. 1). Certainly, any or all of these stressors have been demonstrated in COPD, asthma, cystic fibrosis, or pulmonary fibrosis; however, mechanistic links between these stressors, senescence, and disease pathophysiology have not been established definitively.
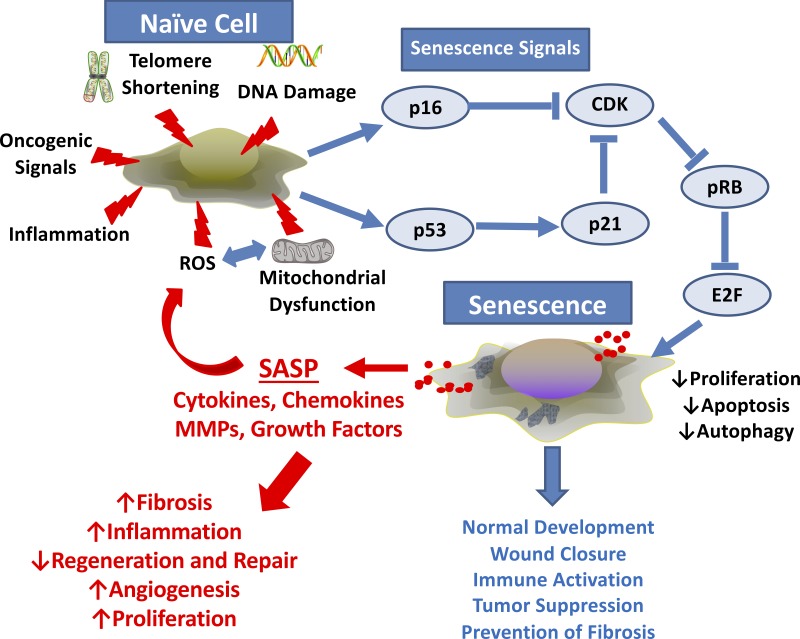
Fig. 1.
Cellular senescence and the senescence-associated secretory phenotype (SASP): naive cells can be exposed to a number endogenous or exogenous stimuli that initiate an intracellular signaling cascade that induces senescence. Factors such as DNA damage from radiation, chemotherapy etc., aging-associated telomere shortening, oncogenic signals, and the influence of inflammation, reactive oxygen species (ROS), and/or mitochondrial dysfunction can all serve as triggers. Here, it is possible, indeed likely, that interplay between these factors is temporally involved. Senescent cells do not proliferate, but they are also resistant to apoptosis and autophagy and are thus long-living. Senescent cells secrete cytokines, chemokines, matrix-modulating factors (e.g., matrix metalloproteinases, MMPs), and growth factors that can have paracrine influences on surrounding naive cells to promote inflammation, mitochondrial dysfunction, and other disease-promoting pathways.
A key feature of senescent cells is their longevity and resistance to apoptosis. While the induction of cell cycle arrest is a key step in senescence, it is important to consider why these cells are resistant to apoptosis (38). Activation of p53 typically results in progression to apoptosis. Using RNA Seq in a radiation-induced model of senescence in IMR-90 human fetal fibroblast cells, Baar et al. (10) noted elevated levels of proapoptotic p53-upregulated modulatory of apoptosis (PUMA) and BIM, a proapoptotic Bcl-family member, with lower levels of antiapoptotic BCL-2 family of genes. They further noted that inhibition of FOXO4, acting in its role as a transcriptional regulator, resulted in release of cytochrome c and cleavage of caspase-3. As such, a central role for FOXO4 in induction of senescence as opposed to apoptosis and the potential utility of FOXO4 inhibitors to reverse senescent cell effects has been demonstrated (10). In addition to p53, p21 may prevent apoptosis by inhibiting cyclin-dependent kinases and therefore blocking formation of the caspase complex or by activation of retinoblastoma, leading to inhibition of E2F1-mediated apoptosis (92). In an effort to identify prosurvival pathways that could be targeted, studies have noted elevated expression of the Ephrins, phosphatidylinositol 3-kinase (PI3K)/Akt, p21, BXL-xl, SERPINE1, hypoxia-inducible factor 1α (HIF1α) αnd BCL-2/XL genes. They also noted differential activation of these pathways in different cell types (198). Modulating these pathways may thus be critically important when considering therapeutics within the realm of senescence (discussed further below).
IMPLICATIONS OF SENESCENCE AND THE SENESCENCE-ASSOCIATED SECRETORY PHENOTYPE
Certainly, inert cells that are no longer replicating are not helpful in repair of tissues within a microenvironment following injury. This raises the question as to what role senescent cells play if not repair and regeneration. Here, it is now clear that far from being inert, senescent cells remain metabolically active, producing a variety of inflammatory cytokines, remodeling proteins and growth factors, providing a more viable explanation for the widespread effects of a small population of cells (44, 103). Such a “secretome” is termed senescence-associated secretory phenotype (SASP). Here, it is important to emphasize that although the term SASP is used broadly to define the secretions from senescent cells, SASP profiles are increasingly recognized to be cell- and context-specific, increasing the complexity of understanding effects of senescent cells and furthermore developing broadly applicable senolytic therapies. For example, Coppé et al. (44) noted the production of a variety of factors, including IL-1, IL-6, IL-8, MCP-2, MIP-3α, GRO, and IGFBPs with differential expression depending on the cell type affected. Further work in this realm has demonstrated that the upstream stressor leading to senescence may also affect the components of the SASP and that it may be intimately linked to activation of NF-κB activation via a p38-dependent mechanism (116, 155). SASP components such as plasminogen activator inhibitor-1 (PAI-1), IL-8, VEGF, and others may themselves promote senescence in adjacent naive cells in a paracrine fashion, whereas others such as IL-1α may reinforce senescence resulting in an exponential effect over time (87, 135). These findings demonstrate the importance of SASP in the context of senescent cell effects and the difficulty in combating each specific SASP factor if senolytics are to be ultimately successful yet highlight the need for eliminating senescent cells in the context of disease states mediated by senescence. However, to reach that step, it is first important to address a number of questions: 1) what is the pattern of senescence across the lifespan?, 2) what is the role of senescence in normal development and aging?, and 3) how does senescence change in disease? And certainly, in the lung, this will need to be addressed in the many different cell types that contribute to normal lung structure and function such as epithelium, endothelium, airway and vascular smooth muscle, and fibroblasts, while conversely in the context of disease where inflammatory cells may hugely complicate interpretation, particularly when many of the SASP factors are cytokines (Fig. 1).
DEFINING AND IDENTIFYING A SENESCENT CELL
Regardless of organ or cell system, the relative proportion of senescent cells within a population is quite small, while conversely pathways that lead to cellular senescence are ubiquitous and important for normal cell function. This raises a critical issue in the field of senescence biology as to what defines a senescent cell and how to identify or characterize it in vitro, in situ, and importantly, in vivo.
In vitro studies find that senescent cells exit the cell cycle, undergo irreversible arrest, are relatively resistant to apoptosis, and are metabolically active; however, this has not been definitively established in vivo (32, 75, 104). Further, owing to a lack of a current gold standard, the identification of senescent cells relies on a variety of layers of evidence. Arguably the most frequently used biomarker is senescence-associated β-galactosidase (SA-β-Gal) staining; this process takes advantage of galactosidase activity in the lysosome at a pH of 6.0 to identify senescent cells that metabolize a substrate to stain tissue and cells blue (48). Additionally, expression of p16 and/or p21 either at the RNA or protein level indicates activity of effector pathways; although the reliability of p16 antibodies in mouse models has been limited. Other markers include loss of LaminB1 expression (62), high-mobility group protein B1 (HMBGB1) translocation (18, 185), γH2a.x expression (104), and production of SASP actors such as PAI-1 (18, 104).
In vivo approaches to study senescence have utilized a variety of strategies, including a combination of flow cytometry with green fluorescent protein-tagged reporter genes for p21, p16, or p53 (12, 18), luciferase-tagged promoters (27), with further generation of evidence using SASP factors and SA-β-Gal staining. Another unique approach has been to identify heterochromatic nuclei using in situ hybridization (57, 134, 171) and DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS) (150). The utility of telomere length (which should be shortened) to identify senescence is controversial, as several escape mechanisms have been identified that can lengthen telomeres, although attrition of telomere length does increase the risk for a DNA damage response and senescence (38, 104).
What is clear from these studies is that several complementary approaches are necessary to unambiguously identify and further characterize senescent cells. There remain challenges in the identification of in vivo senescence as it can be difficult to concurrently use multiple lines of evidence (104). An important limitation with any of these approaches is that accepted markers of senescence tend to be intracellular in nature and are thus currently applied to fixed or otherwise nonliving cells. There is urgent need for surface markers that will unambiguously identify senescent cells and could thus be used in a sensitive and specific fashion to sort living cells and thus obtain relatively pure populations of these cells and further explore their properties in health and disease.
SENESCENCE AS A NORMAL PART OF DEVELOPMENT
The area of research that most challenges the concept that senescence is an indicator of aging or of disease is in the field of developmental biology. While the contribution of senescence in the development of the lung has not been well investigated, two key studies by Munoz and Storer (131, 167) suggest its potential importance. Using senescence-associated β-galactosidase staining (a standard in the field) on whole mounts of mouse and chick embryo they were able to identify senescent cells in a variety of different locations, including neural tube, hind limb, pharyngeal arches, and gut endoderm (a known precursor for the trachea) and the midline fusing sternum. Whether the embryonic lung parenchyma shows senescent cells is not known. However, the importance of appropriate regulation of the senescence machinery in lung differentiation is suggested by findings that the loss of Sin3a [which forms a corepressor complex with histone deacetylases (HDACs)] results in abnormal pulmonary branching, and induction of senescence of pulmonary epithelium with abrogated signaling between pulmonary endoderm and mesoderm (190). Data from the LungMAP project also indicate elevations in cyclin-dependent kinase inhibitor 1A (CDKN1a)/p21 mRNA in mouse pulmonary mesenchyme, endothelium, and epithelium from E16.5 to E18.5 (7). Interestingly, neither Munoz nor Storer identified p16-positive cells within their studies, and thus it is possible that p21 is more relevant during lung development. While none of these studies provide much detail regarding the role of senescence, these findings have two potential implications; first, that senescence can be a normal biological process with advantageous consequences for lung development. Second, immune cells (macrophages) are able to recognize senescent cells and induce apoptosis: processes that could be leveraged in the context of targeting senescent cells. Certainly, various pathways that are important in lung development have been noted to be important in senescence as well (43, 112), but much more work in this area is required to understand how senescence can regulate the complex spatiotemporal aspects of lung development and whether senescence pathways are relevant to lung pathologies that manifest in the prenatal period.
PLACENTAL AND ENVIRONMENTAL FACTORS
Maternal influences may negatively impact pulmonary structure and function of the neonate regardless of gestation at delivery (25). Prenatally, aberrant acute senescence in the syncytiotrophoblasts of the placenta driven by telomere shortening or epigenetic DNA methylation contributes to early-onset preeclampsia and intrauterine growth restriction (21, 120), both diseases known to be associated with long-term pulmonary and cardiovascular disease in the newborn. Upregulation of mammalian target of rapamycin (mTOR), p21, p53, p38MAPK, and proinflammatory SASP in the maternal decidual membranes also correlates with preterm birth (45). Placental membranes also express senescent markers in association with parturition and may generate SASP in conjunction with decidual membrane senescence to induce preterm or term labor. Perinatal factors such as obesity and diabetes can influence fetal development and in one investigation of maternal obesity in humans and rats there was an increased incidence of cellular senescence and a resultant programing switch toward decreased glucose metabolism and insulin resistance (36). Children of diabetic mothers have decreased alveolar type II cell differentiation, with pulmonary tissues exhibiting increased oxidative stress and extracellular matrix remodeling (25). To put this data into context, emerging data suggest the role of SASP factors such as CXCL1, IL-8, and VEGF in mediating sustained senescence of neighboring tissue in a paracrine fashion (2). While these data show indirect effects of senescence in nonpulmonary organs on lung development, investigation of the role of senescence in linking prenatal factors to postnatal lung outcomes represents a novel approach to understanding maternal-fetal medicine in the context of the lung.
Exposure to environmental toxins such PM2.5, Bisphenol A (BPA), and cigarette smoke pre- and postnatally may influence pulmonary function in newborns and children. Previous work has demonstrated that children of nonsmoking mothers with high exposure to PM2.5 have a higher risk of wheezing; in addition, PM2.5 has been implicated in inducing senescence of human dermal fibroblasts (85). A study of 730 mother-baby pairs from Belgium demonstrated that increased exposure to PM2.5 correlated with shortened telomeres in placental tissue by 13.2% and umbilical cord blood by 8.8% (119). Shortened telomeres result in a reduced buffer region for coding DNA sequences and could leave cells more sensitive to premature senescence by accelerated activation of DNA damage response upon cellular insults. However, further work is required to demonstrate a definitive link between PM2.5 and pulmonary senescence. Furthermore, as discussed in the copd section, a definitive link between telomere shortening and senescence remains to be established in lung disease.
Other environmental toxins known to cross the placenta and potentially affect pulmonary development include Bisphenol A (13, 147). While the action of BPA on androgen and estrogen receptors has been demonstrated to result in a Th2 inflammatory cascade, more recent data support a role for activation of senescent DSB-ATM-p53 pathways in fetal fibroblasts exposed to BPA in mediating a potential causative role in childhood respiratory diseases (118).
CIGARETTE SMOKE EXPOSURE
Cigarette smoke is known to lead to neonatal and childhood airway dysfunction, and with 6–10% of women smoking during pregnancy (110), special attention must be paid to the impact of CS on the developing fetus and neonate. The impact of maternal smoking on the development of childhood asthma is well established with several studies demonstrating airway hyperactivity, wheezing, asthma, and a host of chronic pulmonary conditions (54, 68, 82, 108, 109, 166, 195). While cigarette smoke has been demonstrated to induce senescence and airway dysfunction in a COPD model (137), studies thus far have failed to demonstrate a definitive role for CS exposure in inducing senescence in the developing lung per se. Certainly, most fibroblast cell lines used for research such as IMR-90 and MRC-5 are fetal in their origin, and as such, models that use these lines to study the role of senescence in adult lung disease may be applicable to fetal life. However, there is currently insufficient evidence that CS directly results in fetal pulmonary senescence. Drummond et al. (53) exposed dams to CS for 2 wk prenatally in a randomized fashion with no postnatal exposure of the pups until day 21; at this point they were randomized to CS or no CS exposure until day 49 of life. Analysis with whole-lung extract at these points did not demonstrate an increase in mRNA for p16 or p21 with an increase in p53 levels only at day 49 in exposed pups. However, there was a trend toward an increase in p16 mRNA levels at D21, which was noted to be statistically different after evaluation of alveolar p16 protein levels using immunohistochemistry. The authors concluded that senescence did not contribute to the airway dysfunction they noted, although further research is probably needed to establish relationships between CS, senescence, and airway dysfunction within the developing lung (53). Interestingly, Mirzakhani et al. (125) demonstrated a trend toward shortening of telomeres in the respiratory epithelium of abortuses exposed to maternal smoking at 93 days after conception but longer telomeres in the same group at an earlier time period less than three months after conception. Further, higher placental concentrations of cotinine were associated with shorter fetal respiratory epithelial telomeres. In a mouse model of CS-induced emphysema, elimination of p16 in the epithelial layer did not attenuate senescence, but there seemed to be downregulation of the inflammatory cascade (170). Overall, it is likely that perinatal environmental insults such as CS (either via the mother or directly in the postnatal period) can induce lung pathophysiology via senescence. However, more research is needed to establish the processes involved and the contribution of senescent cells per se. Here, it is important to note that animal models may or may not be informative, and there is clear need to examine the human condition.
PERINATAL LUNG INJURY
While antenatal insults may alter postnatal pulmonary structure and function, there is a much more robust body of literature supporting the negative consequences of postnatal exposures in causing neonatal and childhood pulmonary disease. The fetus grows in a hypoxic environment, and several primary studies have demonstrated dysregulation of normal branching and vascular morphogenesis and proximal to distal differentiation in the setting of normoxia (21% O2) (65, 66, 181). While the majority of the literature in this realm focuses on prematurity as an instigator for iatrogenic pulmonary injury, it is equally important to recognize that many of the same exposures, which result in chronic pulmonary diseases in adults, do so in children and neonates as well. In the neonate, there have been several changes in therapy over the past decade, including a decreased reliance on supplemental oxygen due to an increased recognition of oxidative damage, an increased emphasis on natural transition to postnatal life, and utility of noninvasive positive airway ventilation (159, 183). Vento et al. (183) noted that transitioning to a less invasive form of ventilation with reliance on moderate levels of supplemental oxygen in neonates born before 28 wk has actually led to an increase in dependence on supplemental oxygen at 36 wk and worse spirometry measurements in children at 8 yr of age. Certainly, extremely premature neonates who require intubation and ventilation for prolonged periods of time, developing the “classic” form of bronchopulmonary dysplasia (BPD) still exist, but the “new” form of BPD is an equally important challenge. To contrast the two, the new BPD reflects an arrest in pulmonary vasculature and alveolar development, whereas the pathology of classical BPD demonstrated a higher degree of fibrosis (46). Furthermore, in children with obstructive pulmonary processes such as asthma biopsies have demonstrated large airway remodeling and inflammation (25).
Studying childhood diseases on a cellular and molecular level with in vivo data is difficult; however, there are several observations supporting the concept that senescence may play a role in neonatal and childhood airway dysfunction. First, the use of oxygen in neonatal resuscitation, which may occur at any gestation, can lead to fibroblast senescence. Saretzki (156) noted that MRC-5 fibroblasts exposed to 40% oxygen for 4–6 wk demonstrated gene expression consistent with senescence. A follow-up study using 70% oxygen to induce senescence noted that in their model, the p53/pRb pathways mediated the induction and maintenance of senescence independent of telomere shortening, with complete reversal of this phenotype upon suppressing these pathways (101). Even 21% oxygen represents a relatively hyperoxic environment for fetal lung in the context of prematurity (46). Several primary studies have demonstrated dysregulation of normal branching and vascular morphogenesis and proximal to distal differentiation in the setting of 21% O2 (65, 66, 181). Other studies modulating environmental oxygen between 1% and 20% demonstrated an increased replicative lifespan for IMR-90s under hypoxic conditions, with a rapid induction of senescence when cells were moved back to room air (153). Gebb and Jones (66) alluded to the importance of fetal hypoxia in the developing lung with specific respect to angiogenesis and branching. Our own data using primary fetal pulmonary fibroblasts and airway smooth muscle exposed to 40% oxygen demonstrate activation of senescent pathways and associated SASP along with associated markers in autopsy specimens of peri-viable neonates. Moreover, there were increases in airway smooth muscle contractility, inflammation, and fibrosis noted in our model suggesting a etiopathogenesis for the asthma-like phenotype identified in postneonatal intensive care unit infants and children (141). Further, alveolar epithelial cells from mice with shortened telomeres activated the DNA damage response resulting in activation of the p53-p21 pathway and an inability to support recovery of alveoli after bleomycin-induced damage (116). Londhe et al. (114a) studied the impact of 80% supplemental oxygen on mouse pups in the alveolar stage of lung development and demonstrated decreased epithelial HDAC activity with activation of the p53-p21 pathway, in the setting of impaired alveolarization as seen in BPD. The influence of senescent cells on the surrounding microarchitecture of the lung is likely mediated through the SASP, which depends on the underlying cause of the senescence; hence, the resultant damage to the developing lung may vary (116). To this end, our data in fetal airway smooth muscle demonstrate that the SASP tends to be proinflammatory, profibrotic, and even procontractile and may explain the wheeze and/or asthma experienced by neonates exposed to supplemental oxygen (141). Faksh et al. (55) demonstrated that LPS in combination with 50% O2 in a mouse model resulted in increased thickness of the smooth muscle layer of conducting airways, inflammation and upregulation of matrix remodeling genes collagen-3, transforming growth factor-β (TGF-β), and connective tissue growth factor. Furthermore, in a model of CS- and LPS-induced lung inflammation, knockout of p21 resulted in decreased inflammation and airspace enlargement via NF-κB and ROS pathway (192). Within endothelial progenitors with senescent phenotypes, decreased angiogenic potential has been demonstrated in older patients; however, there is a lack of information in this realm with respect to challenges of neonatal life (93). Further, senescence may lead to permissiveness for infectious pathology as one model demonstrated where exposure to SASP from senescent A549 cells increased ligands for pneumococcal bacteria in naive cells, which may lead to detrimental outcomes after discharge from the neonatal intensive care unit (161). Such effects have been studied in children with cystic fibrosis, where high levels of neutrophil elastase induce p16 and lead to higher levels of senescence in airway epithelium resulting in a compounding of insults (59). Put together, there is reasonable evidence to support a role for senescence in neonatal airway disease with further work needed to establish this link definitively both in the vascular and pulmonary compartments. Further investigation into the role of senescence in both old and new models of BPD and the impact of stretch or ventilation without supplemental oxygen on the development of senescence in pulmonary lineages is the natural next step.
SENESCENCE IN THE ADULT LUNG
An increasing lifespan is accompanied by increase in chronic diseases, including asthma, COPD, and pulmonary fibrosis, raising the question of whether and how senescence pathways play a role not only in normal aging lung but also lung disease that shows greater propensity with aging. Here, the lung may be particularly susceptible given that it has cumulative exposures to environmental pollutants, allergens, smoke, and respiratory infections with cycles of injury and repair resulting in lung aging (4, 94, 100, 193). Little is known about the balance between chronic environmental exposure, repair, and cellular senescence with aging and how this impacts lung function and development of airway diseases, including asthma, COPD, and PF (Fig. 2) (124).
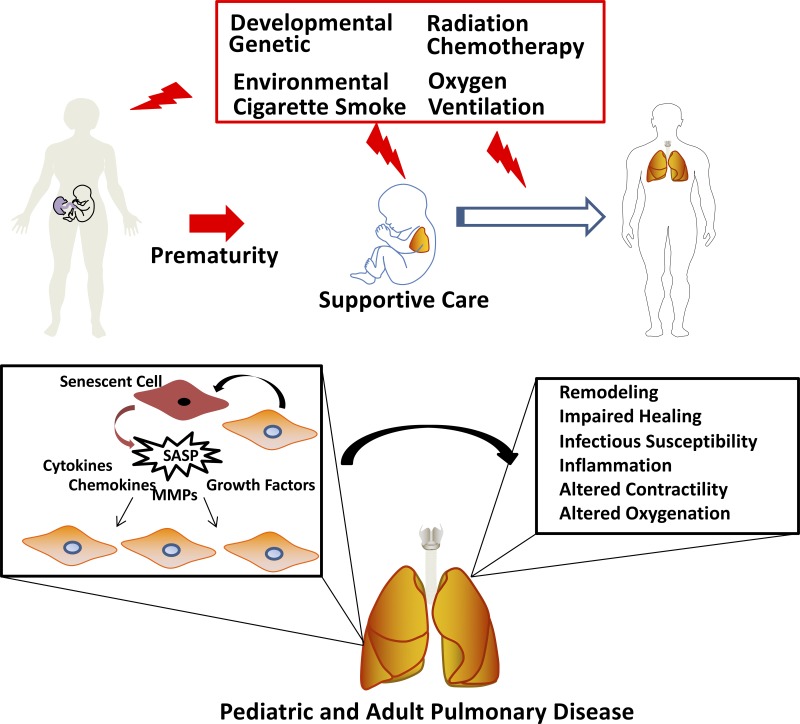
Fig. 2.
Throughout the lifespan, including during embryonic development, endogenous and exogenous factors can lead to structural and functional dysfunction of lung cells, promoting disease. Here, senescent cells and senescence-associated secretory phenotype (SASP) could play a role at each life stage, sometimes as part of normal developmental processes or in response to insults. Senescence/SASP in pregnancy can lead to premature birth, which in itself is a risk factor for diseases such as asthma. Interventions such as supplemental oxygen and mechanical ventilation in the context of prematurity place the infant at further risk for asthma and bronchopulmonary dysplasia. Environmental exposures such as allergens, pollution, and tobacco smoke from birth onwards and certainly throughout life contribute to asthma, chronic obstructive pulmonary disease (COPD), and fibrosis. Here, senescent cells may play variable roles that are not yet entirely clear. Finally, senescent cells could contribute to normal aging-associated changes in the lung, as well as to aging-associated diseases such as COPD and fibrosis (and even asthma). MMPs, matrix metalloproteinases.
AGING LUNG AND SENESCENCE
Aging-related changes in lung morphology begin at age 50, where patients develop enlargement of small airways caused by changes in the collagen fiber network around the alveolar ducts, resulting in homogeneous enlargement of alveolar air spaces (84, 124, 189) decreased alveolar surface tension, and a compliant distensible lung (164, 165), i.e., a senile emphysema that is homogenous with no alveolar wall destruction (164, 182). Clinical studies show that airway contractility remains unchanged (79, 176) or is lowered with normal aging (26).
In the lung, senescent cells increase with age, accompanied by increased SASP that have autocrine/paracrine effects inducing further senescence, stem cell dysfunction, increased inflammation, and cancer progression (1, 90). SASP is known to aid in wound repair by recruiting inflammatory cells (32). Such recruitment of inflammatory cells may also subsequently eliminate senescent cells that were induced by the original stresses; however, the immune clearance mediated by natural killer (NK) cells is imperfect, likely leading to an accumulation of senescent cells over a lifespan (32). With aging, clearance of senescent cells is slowed, due either to failure of inflammatory cells to respond to SASP (immune senescence) or alternatively to an excessive number of senescent cells or SASP effects altering the local environment, resulting in aging or development of disease (105).
Aging in the lung is associated with chronic low-grade inflammation (115, 123, 151, 174, 189). IL-6 and IL-8, the most common cytokines associated with SASP, are in fact elevated in the bronchoalveolar lavage of aging patients (123, 149). IL-6 is a chemoattractant and survival factor for neutrophils and monocytes (63, 91). Therefore, accumulation of senescent cells and release of SASP in the lung may contribute to chronic inflammation observed in the lungs of aging patients. Furthermore, oxidative stress may increase SASP release by senescent cells. Thus, stress induction of senescent cells following inhalation of particles or environmental pollutants such as ozone may contribute to lung aging and propagation of disease (90). Additionally, both proinflammatory and anti-inflammatory factors may play a role in shaping the outcome of the aging process. Cellular defects can cause inflammation, which may exacerbate existing damage. Thus, aging-associated inflammation and structural changes in the lungs may be caused by an accumulation of senescent cells and a failure to eliminate cells damaged by reactive oxygen species (Fig. 2) (83, 100).
AGING AND ASTHMA
Asthma affects 10% of the elderly population and is associated with increased morbidity and mortality (9, 13, 24, 67, 72, 88, 174). Diagnosis of asthma in elderly patients is difficult due to abnormal asthma triggers and normal-aging related changes in lung structure and function that lead to shortness of breath, airflow limitation, and inflammation (9, 24, 29, 51, 67, 88, 182). Comorbidities including heart failure can mimic asthmatic symptoms making diagnosis difficult. Additionally, elderly patients with asthma have an increased frequency of airway hyperresponsiveness (13, 24, 127). However, the treatment of asthma in the elderly is difficult since these patients tend to be resistant to standard therapies, including long acting β-agonists and corticosteroids and are often excluded from clinical trials (9, 23).
While the mechanisms that drive asthma development and exacerbation have been studied extensively in the general population, the contribution of senescent cells in the asthmatic lung, particularly in the development and progression of asthma is not well studied. Epithelium from asthmatic airways show increased protein expression of senescent markers p16 and p21 when compared with normal nonasthmatic airways (142, 187). Furthermore, increased senescent marker expression corresponds to an increase in βgal-staining (187). In human epithelial cells, thymic stromal lymphopoetin (TSLP), a cytokine that is known to increase in the lungs of persons with asthma, elevates p21 and p16 in a dose-dependent manner (187). Silencing of both p21 and p16 with siRNA prevents TSLP-induced senescence and remodeling (187), highlighting a role for senescent epithelial cells in airway remodeling (187). Elevated p21 in asthmatic epithelium is not decreased with corticosteroid treatment (142). These limited data suggest that increased epithelial senescence occurs with asthma and may impact epithelial repair independent of inflammation (142). Additionally, senescent fibroblasts may be involved, as suggested by one study where fibroblasts isolated from asthmatic lungs and cultured with normal airway epithelial cells blunt epithelial proliferation and increase expression of p21 (160). What factors released from asthmatic fibroblasts contribute in this regard remains to be determined. These limited in vitro data are supported by explorations in an ovalbumin (OVA) mouse model that shows increased cellular senescence in the lung (187). Blocking TSLP signaling with a STAT3 inhibitor prevents airway hyperreactivity, epithelial senescence, and blunts remodeling (187).
Chronic low-grade inflammation increases disease burden in elderly asthmatics (29, 61, 174, 189). Senescence is commonly associated with an increase in the proinflammatory IL-6 (149). In asthmatic lungs, IL-6 is elevated in bronchoalveolar lavage, macrophages, airway epithelium, endothelium, and airway smooth muscle (74, 144). Furthermore, a single-nucleotide polymorphism in the IL-6 receptor was identified as a marker for asthma susceptibility and severity in individuals of European descent (74). Elderly asthmatics have higher sputum IL-6 than young asthmatics (29). Elderly patients with asthma have increased neutrophils (as with aging) and eosinophils in airway tissue and sputum than both elderly patients without asthma and young patients with asthma (29, 30). In addition to being associated with senescence, IL-6 is a potent activator of neutrophil chemo-attraction toward IL-8 (186). Thus, elevated IL-6 in asthmatic lungs could contribute to an increase in the number of senescent cells, while simultaneously promoting disease progression and airway obstruction through the recruitment of eosinophils and neutrophils to the lungs.
Senescent cells may also be part of a feed-forward loop that drives asthma progression in the face of immunosenescence that blunts the efficacy of anti-inflammatory therapies. SASP release may increase inflammation, impair cellular function, and prevent repair in elderly patients with asthma (90). Furthermore, aging may cause glucocorticoid receptor resistance so that common steroidal treatments are rendered ineffective due to aging-related changes in glucocorticoid receptor function (37, 69, 187). While the above concepts are appealing and there are some disparate data to support the role of senescent cells in asthma with aging, further research is needed to establish definitive, mechanistic links between aging biology, senescent cell induction and effects, and asthma pathophysiology.
COPD
Unlike the homogenous nondestructive changes in the lung parenchyma with aging, COPD is characterized by emphysema due to destruction of the lung parenchyma, an increase in mucus producing cells, and inflammation of both central and peripheral airways (83). However, since reduction in lung function [measured by decrease in forced expiratory volume in 1 s (FEV1)], progresses slowly over decades, the majority of COPD patients are middle aged or elderly (49). Furthermore, the major risk factor for development of COPD is long-term inhalation of small particles most commonly from cigarette smoke, fossil fuels, and noxious gases (49, 83), all of which lead to chronic inflammation of small airways and parenchyma (83). This suggests that repeated episodes of damage and repair alter the lung in some way that accelerates aging. Why then, in some individuals, do the same insults not lead to the development of an accelerated lung disease later in life? Recent data have shown that several childhood risk factors in addition to adult smoking may lead to the development of COPD (28), including maternal or paternal asthma, maternal prenatal smoking, childhood asthma, respiratory infections, and antenatal exposure to smoke and pesticides (28). Whether senescence-associated processes are involved (including genetic components) remains to be determined.
While COPD is a disease that develops later in life, the increase in life expectancy has resulted in a growing population of elderly patients with the potential to develop COPD. Furthermore, in contrast to normal aging, the rate at which lung function declines in a patient with COPD is faster than normal aging (60, 61). Additionally, the inflammation observed in the lungs of patients with COPD (61) is similar to the chronic low-grade inflammation observed in normal aging. The question remains how do senescent cells contribute to the development and progression of COPD? Patients with COPD show a greater number of senescent lung fibroblasts, Clara cells, and epithelial cells when compared with normal elderly patients (130, 178, 196). Mouse models of COPD show cigarette exposure activates the p21-poly(ADP-ribose) polymerase (PARP)-1 pathway, DNA damage, and increases cellular senescence (191). Additionally, exposing cultured human lung epithelial cells to cigarette smoke extract induces senescence with upregulation of p21 (179). In patients with COPD, induction of epithelial senescence may prevent epithelial repair following environmental damage leading to alveolar destruction and the chronic inflammation associated with COPD (179). Interestingly, Sundar et al. (170) reported that silencing of p16 in the epithelial layer did not protect against cellular senescence present in a mouse model of COPD but did attenuate SASP, raising questions regarding the mechanistic importance of p16 (beyond being a marker of senescent cells) and the perhaps greater importance of SASP components.
Given the observed inflammation in COPD lungs (16, 50) it makes sense to investigate the interplay between inflammation and senescence/SASP in this disease. Lung tissue from patients with COPD contain a higher percentage of senescent type II alveolar epithelial cells expressing p16 and pNFκB. These senescent cells produced greater amounts of the SASP cytokines IL-6, IL-8, IL-13, IL-1, and TNF-α than lung tissue from asymptomatic smokers or nonsmokers (105, 178). During COPD exacerbations, both IL-6 and IL-8 levels are further potentiated (16, 50). Elevated sputum neutrophils over time (50) and increased neutrophils and eosinophils during exacerbations (50) are also associated with COPD. Thus, increased senescence cells in the lung may lead to increased release of SASP that causes additional inflammatory cell influx, increased survival of neutrophils and eosinophils, and thus exacerbation of disease.
Oxidative stress causes inactivation of antiproteinases, airspace epithelial injury, and mucus hypersecretion (117). Oxidative stress also plays a role in increased airway inflammation observed in the lungs of patients with COPD via activation of redox-sensitive transcription factors NF-κB and AP-1 (117). Markers of oxidative stress are elevated in the blood of patients with COPD, and these markers are further potentiated during periods of disease exacerbation (145). Patients with COPD have been shown to have mitochondrial dysfunction resulting in overproduction of ROS and oxidative damage to mitochondrial DNA (56). While each of these aspects can contribute to increased senescence per se, and conversely, senescent cells or SASP can induce oxidative stress and mitochondrial dysfunction, mechanistic links between these elements in the context of COPD are still not established to truly implicate the role of senescence in COPD. Nonetheless, links between senescence/SASP, oxidative stress, and mitochondrial biology do represent an important area for future research, especially if suppression of senescent cell induction or their downstream effects is considered a significant therapeutic approach in lung disease.
One interesting aspect of senescence in COPD is the role of telomeres. The rate of telomere attrition is a result of replication history that can be impacted by several factors, including chronic inflammation and oxidative stress (56). Patients with COPD have shorter telomeres when compared with control subjects (56). Additionally, there is a correlation to the length of the telomeres and the number of years smoked (56, 128, 180). However, the role of telomere shortening per se versus telomere dysfunction is poorly understood. Studies have found accelerated telomere shortening in leukocytes from patients with COPD (81, 129), and leukocyte telomere length negatively correlates with plasma IL-6 levels in COPD (157). There is also a dose-dependent relationship between smoking history and telomere length of circulating lymphocytes (128), and short telomere length is associated with decreased lung function and increased risk of COPD (148), although this correlation is not found in other studies (81, 157). However, of note, none of the studies necessarily show a mechanistic link between telomere shortening and COPD pathophysiology particularly in the context of senescence.
Telomere dysfunction is strongly implicated in induction and maintenance of senescence (20). One recent study found that in the aging mouse lung and following CS exposure and in patients with COPD, telomere-associated DNA damage foci increase in small airway epithelial cells but without significant telomere shortening (19). However, it should be noted that mice have long telomeres, and thus shortening may not be as important. It is possible that telomere shortening is more important in humans, but the relevance in lung aging and/or COPD remains to be determined. Nonetheless, increases in telomere-associated foci in small airway epithelial cells with aging are accelerated by CS exposure (19), and such foci predict age-dependent emphysema as also suggested by enhanced emphysema in transgenic mice that harbor dysfunctional telomeres. In this model, CS-induced telomere dysfunction involves reactive oxygen species and is associated with ATM-dependent secretion of the SASP factors IL-6 and IL-8 (19). Overall, it is likely that both telomere shortening and length-independent telomere damage per se occur in COPD since telomeres are susceptible to oxidative modifications and show impaired repair (78, 146).
PULMONARY FIBROSIS
Wound healing and fibrosis are interrelated processes, driven by injury, inflammation, fibroblast migration and proliferation, extracellular matrix deposition, and remodeling (158, 188). Effective wound healing decreases with age (76, 158). Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease that has garnered much attention recently in the context of senescence, given that senescent cells contribute to wound healing and aging (32, 47). In patients with IPF, both alveolar and bronchial epithelial cells express senescent markers p21 and p53 (106). In fibrotic foci there is an increase in epithelial p16, TGF-β, and Ki67 (114). The presence of an intact epithelial layer controls proliferation of the surrounding fibroblasts. This increase in epithelial senescence is accompanied by an increase in fibroblast proliferation (114). Thus, in IPF where the epithelium expresses high levels of the senescence markers p21 and p53, there may be a progression fibrosis driven by senescent epithelium that may induce fibroblast proliferation and extracellular matrix deposition. Additionally, SASP from damaged senescent epithelium may also promote fibroblast proliferation, leading to a cyclic problem where senescent epithelium fails to undergo repair and simultaneously induces fibroblast proliferation and extracellular matrix deposition (106).
Epithelial senescence is thought to contribute to overgrowth of pulmonary fibroblasts in patients with IPF (106). In the bleomycin mouse model of pulmonary fibrosis, DNA and oxidative damage and have been noted to induce senescence in multiple cell types, including endothelium, fibroblasts, and epithelium (6, 158). In a mouse model of IPF, the number of epithelial cells to undergo senescence following exposure to bleomycin was decreased in caveolin-1 knockout mice (162). Inhibition of epithelial cell division allows increased proliferation of airway fibroblasts and progression of airway fibrosis (6).
As lung injury and fibrotic foci spontaneously resolve at 3–4 wk after treatment (158), improvement in lung function is accompanied by a decrease in p16 mRNA. Additionally, these mice also show lower expression of SASP proteins, including Col1a1, Mmp10, and Pai1 (158). This suggests that reduction or elimination of senescent cells and associated SASP signaling may be important for the spontaneous resolution observed in animal models of IPF. Furthermore, reduction of epithelial senescence via reduction in caveolin-1 expression may prevent epithelial senescence, fibroblast proliferation, and matrix deposition in patients with IPF.
Fibroblast senescence is recognized as a pathogenic mechanism of IPF. Senescent fibroblasts isolated from the lungs of patients with IPF have reduced expression of caveolin-1 (80). Additionally, senescent fibroblasts from patients with IPF failed to undergo Fas Ligand (FasL)- or TRAIL-induced apoptosis (80). Conversely, senescent fibroblasts from the lungs of normal healthy patients underwent FasL-induced apoptosis (80). When senescent fibroblasts isolated from patients with IPF were treated with the senolytic quercetin, caveolin-1 was upregulated, and sensitivity to TRAIL and FasL were restored (80). Similarly, in a mouse model of IPF, senolytic treatment with quercetin reversed bleomycin-induced injury and reduced expression of the senescence marker p21 and SASP secretion in aged mice (80). This suggests caveolin-1 contributes to the sensitivity of senescent fibroblasts in the lungs of patients with IPF, and upregulating caveolin-1 expression may restore sensitivity to apoptotic signals and decrease fibroblast proliferation and extracellular matrix deposition.
Patients with IPF have increased markers of oxidative stress both systemically and in the lungs (56). Fibroblasts from patients with IPF show an imbalance in cellular redox homeostasis caused by increased expression ROS generating enzyme (NADPH oxidase-4) and an inability to induce antioxidant response [nuclear factor erythroid 2-related factor 2 (nrf2)] (76). This increase in oxidative stress can lead to cellular senescence via DNA damage and telomere shortening. Additionally, as the severity of the fibrotic lesion increases, so do the number of double-stranded DNA breaks found in bronchial and alveolar epithelium of patients with IPF (106). Patients with IPF also show mutations in telomerase (8, 169, 177) and telomere-lengthening component (8, 56). These defects result in shortened telomeres in both circulating lymphocytes (8, 169) and lung cells (121). Thus, defects in telomere stability and increased oxidative stress may predispose patients for the development of epithelial senescence and the development of fibrotic lesions.
Additional work in a mouse model of IPF found that deletion of the telomere shelterin protein TRF1 from alveolar type II cells leads to pulmonary fibrosis in mice (133). Loss of TFR1 resulted in collagen deposition, increased in β-galactosidase-positive epithelial cells, and increased number of alpha smooth muscle actin-positive mesenchymal cells (133). In contrast, deletion of TRF1 in collagen-expressing cells caused pulmonary edema but not fibrosis (133). Thus, defects in telomere stability and induction of senescence in alveolar type II cells may drive progression of fibrosis in patients with IPF.
SENOLYTICS TO TARGET SENESCENT CELLS
Even the limited data in the lungs and certainly in other tissues highlight that senescence has both positive and negative physiologic consequences. Senescence pathways have evolved as a tumor suppressor pathway, and that fact should be taken into consideration when designing therapeutic approaches for diseases where senescent cells may have detrimental contributions (5). This makes careful selection of therapeutic strategies targeting the negative aspects of senescent cells critical yet complex. For example, it would be undesirable to simply interrupt a prosenescent pathway to allow for uncontrolled proliferation of a cell with damaged DNA where the goal should be to ensure senescent cells undergo apoptosis while minimizing effects on nonsenescent tissues. Additionally, removing/targeting senescent cells may be more beneficial to preventing the detrimental effects than trying to prevent senescence in the first place (5).
The drive to target senescent cells was originally based on findings in different mouse models that senescent cells accumulate with age and that senescent cell burden is associated with lifespan (98). For example, caloric restriction to increase lifespan was found to be associated with decreased expression of a senescence marker, p16Ink4a (102), while progeroid models such as the BubR1H/H mice show increased senescent cells (11, 173). In the context of senescent cell removal, genetically targeting senescent cells in INKATTAC;BubR1H/H progeroid mice expressing a drug-activated suicide gene specifically in senescent cells was found to enhance healthspan (12). Here, clearance of the entire burden of senescence may be ideal but unnecessary. For example, Baker et al. (12) demonstrated that even clearance of 30% of senescent cells can improve age-related complications. These initial findings have driven development of small molecule senolytic agents and other approaches to decrease senescent cell burden, and current approaches include peptides, RNA interference, and vaccines (98). The lung represents an attractive target for development of such therapies as airways permit direct drug delivery in a fashion that is impossible in most other organ systems. As such, the minimum dose required to achieve a given therapeutic level while reducing the risk of off-target effects is much lower (34). Conversely, given the many different cell types in the lung and the complex roles they play in normal lung structure/function as well as disease, directed delivery may be only one hurdle to overcome.
There are various challenges with development of therapeutics in the realm of senolytics, with specific reference to the variety of pathways that can be activated and lead to a senescent phenotype. Kirkland et al. demonstrated that prosurvival networks were activated in both human umbilical vein endothelial cells and preadipocytes that were senescent, with specific reference to EFNB1, PI3KCD, p21, PAI-2, and BXL-xl. However, they noted that not all lineages activate the same pathways to the same extent, and as such therapeutics to interrupt these pathways should be carefully selected based on gene activation profiles (198). Further, ensuring the elimination of senescent cells in vivo remains a challenge as there is no gold standard to adequately prove the relative burden of senescent cells and their location(s). Several transgenic models using luciferase- or fluorescent-tagged promoters have been developed (12, 18, 27) to evaluate the efficacy of potential therapeutics, taking advantage of the activation of p16, p21, or p53 pathways in senescence. Although none of these pathways/targets are ideal in themselves (p16 deletion can be oncogenic, p21-insufficient, etc.), utilization of these tools has led to the nascent field of senolytic drug discovery. The best-described approach involves the nonspecific tyrosine kinase inhibitor, dasatinib and the flavonoid quercetin (139, 158, 198). Quercetin targets a variety of pathways, including BCL-2/XL, PI3K/AKT, and p53/p21/PAI1, whereas dasatinib inhibits tyrosine kinases, as expected (97). The utility of a combination of dasatinib and quercetin in targeting idiopathic pulmonary fibrosis has been investigated in several studies (64, 126, 158). In fact, inhalational quercetin has been demonstrated in a guinea pig model to improve asthmatic responses in ovalbumin-sensitized guinea pigs, although the mechanism is likely through inhibition of PDE4, providing proof of concept with regards to aerosolized delivery (126, 175). Drug discovery efforts have revealed other potential drugs such as Navitoclax, fisetin, A1331852, and A1155463 with varying degrees of efficacy in different cell types (158, 194). Navitoclax, which is an inhibitor of antiapoptotic pathways Bcl-2, Bcl-xl, and Bcl-w resulted in reduced viability of senescent HUVEC and IMR-90 cells; however, it is was not efficacious in preadipocytes (197). Combinations of drugs may target different prosurvival and proapoptotic pathways and result in synergistic efficacy such as the activity of piperlongumine combined with Navitoclax in WI-38 fibroblasts (172). However, dasatinib, a well-known nonspecific tyrosine kinase inhibitor, has several off-target effects (pulmonary vasculature, fetal, hematologic) that reduce its viability as a potential therapeutic agent (14, 70, 111).
Most recently, selective elimination of senescent cells has been demonstrated using a galactose-bound delivery system, which preferentially delivers senolytics to senescent cells, known to have increased activity of lysosomal β-galactosidase. This system improved pulmonary function in a bleomycin model of IPF, with a simultaneous reduction in toxicity (132). The discovery of additional small molecules and delivery systems that may be efficacious in reducing the burden of senescence without off-target effects will be critically important in advancing the utility of these therapies in clinical settings and trials.
Certainly in addition to demonstrating a reduction in senescent cells using gold standards such as β-galactosidase, it is imperative to demonstrate reversal of the SASP and improvement of relevant functional parameters. Several approaches have been attempted to date in animal models of disease. For example, Schafer et al. (158) noted improved exercise capacity in a bleomycin model of IPF treated with combination gavage of dasatinib and quercetin. This was confirmed, with a further mechanistic understanding of quercetin’s action in this setting by Hohmann et al. (80). Furthermore, in an aging model of pulmonary dysfunction, elimination of p19ARF-expressing cells improved pulmonary compliance at baseline and with exposure to diphtheria toxin along with markers of SASP (73). Most recently, Justice et al. (89) completed an open-label, single-arm clinical study to evaluate the potential utility of dasatinib and quercetin in improving functional and molecular markers of senescence in a cohort of 14 patients with IPF. Their findings indicate an improvement in physical function (6-min walk test, 4-meter gait speed, and chair stand times) among these patients; however, there was no improvement in spirometric lung measurements. Interestingly, while SASP markers of senescence did not decrease, there was a decrease in proinflammatory and profibrotic SASP markers. We would be interested in a 6-mo and 1-yr follow-up of this group to determine the slope of change of their spirometric outcomes and functional outcomes compared with a normative population to determine if there are ongoing benefits from this intervention. This trial represents the culmination of a wealth of data from in vitro and in vivo models supporting a role of senescence in IPF and will likely be followed by additional randomized control trials in the future and emphasizes the importance of continued translational research within the field of adult and neonatal pulmonary medicine.
RELATIVE IMPORTANCE OF SENESCENCE
The above discussion highlights the potential for cellular senescence to occur at different life stages and for senescent cells to contribute to lung diseases such as asthma, COPD, and PF. Certainly, each of these diseases is well recognized as involving a multitude of inflammatory and resident lung cells, along with a number of intracellular and intercellular mechanisms. It is of course unlikely that senescence represents a root cause of lung disease, yet it is possible that the many other mechanisms involved lie either upstream or downstream to cellular senescence and senescent cells. Identifying where senescent cells lie in the spatiotemporal matrix of disease mechanisms and how important they are compared with other pathways remains to be determined. Indeed, without robust ways to identify, sort, and track senescent cells especially in the lung, which involves >40 different cell types; given the heterogeneity of senescence mechanisms and SASP profiles, this may be a Sisyphean task especially as our understanding of cell roles and disease processes keeps expanding. This is only made more complicated as the limited data in the lungs and other tissues show both beneficial and detrimental roles for senescent cells. Nonetheless, studies to date underline the potential for senescent cells and SASP to play detrimental roles in the lung particularly with aging. Thus, beyond gaining understanding of such roles, there is at least the need to determine the impact of removing or interfering with senescent cells in disease alleviation.
With the above framework, a number of questions for future research should be considered:
1) What are the key senescence pathways, and what is the heterogeneity of SASP profiles within each lung cell type (including inflammatory cells)? How do these pathways and profiles change with age, sex, and, importantly, time? Indeed, which cells within the lung undergo senescence and when?
2) How distinct are senescent cells from the native cells (e.g., are senescent fibroblasts just a committed long-lasting subpopulation of fibroblasts in the lung?) Importantly, how do they differ in terms of transcriptome and proteome?
3) In the context of identification, what are the key surface markers that differentiate senescent cells? How stable are such markers over time and with exposures? How distinct are they between cell types?
4) What are the interactions between established or emerging lung disease mechanisms such as inflammation, ROS or mitochondria, and senescence pathways? How important are such interactions in mediating or modulating disease?
5) Is cellular senescence a part of normal embryonic lung growth, and if so, do/how do senescent cells contribute to spatiotemporal patterning that leads to a functional lung at birth? If not, what are the mechanisms by which senescent cells are cleared in utero, from which we may glean approaches for removal of senescent cells in the context of disease?
6) In the context of perinatal insults such as placental pathologies, premature birth, and iatrogenic process such as oxygen and mechanical ventilation, is there enhanced senescent cell burden, and what roles do senescent cells play in lung diseases of prematurity? Here, do different cell types, including inflammatory cells, show differential susceptibility to induction of senescence and/or in their contribution to disease?
7) There are currently no data on the time course of senescent cell induction during childhood, which raises the question, do chronic environmental, allergic, or infectious exposures result in a progressive (or accelerated) increase of senescent cell burden through to adulthood?
8) Do senescent cells substantially contribute to the peripubertal changes in incidence of asthma in women or in the greater susceptibility for bronchitis and COPD in women who smoke, i.e., are there sex differences in lung senescence biology?
9) Certainly, many of the SASP factors are cytokines highly relevant to inflammatory airway disease but compared with COPD or PF, less is known regarding senescent cell burden or the role of such cells or SASP in asthma. Thus, the question becomes where senescence lies in the induction or progression of asthma and which cells are involved: inflammatory cells versus resident airway cells, including epithelium and smooth muscle.
10) If senescence is part of aging biology, what are the key features of senescent cells that drive the “good” versus the “bad” in contributing to lung diseases of aging? This will help drive specificity for senolytic therapies that have goals of alleviating disease and enhancing healthspan in the elderly.
GRANTS
This work was supported by the Mayo Clinic Department of Obstetrics and Gynecology (P. Parikh), NIH Grants T32 HL105355 (S. Wicher), R00 HL131682 (R. Britt), R01 HL138402 (C. Pabelick), and R01 HL056470 (Y. S. Prakash), an American Heart Association Postdoctoral Fellowship Award (S. Wicher), and by a pilot grant from the Center for Biomedical Discovery and the Mayo Clinic Center for Clinical and Translational Science (R. Britt and C. Pabelick) NCATS UL1 TR002377.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.P., S.W., and Y.S.P. prepared figures; P.P., S.W., and K.K. drafted manuscript; P.P., S.W., K.K., C.M.P., R.D.B., and Y.S.P. edited and revised manuscript; P.P., S.W., K.K., C.M.P., R.D.B., and Y.S.P. approved final version of manuscript.
REFERENCES
- 1.Abbas M, Jesel L, Auger C, Amoura L, Messas N, Manin G, Rumig C, León-González AJ, Ribeiro TP, Silva GC, Abou-Merhi R, Hamade E, Hecker M, Georg Y, Chakfe N, Ohlmann P, Schini-Kerth VB, Toti F, Morel O. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity: role of the Ang II/AT1 receptor/NADPH Oxidase-mediated activation of MAPKs and PI3-kinase pathways. Circulation 135: 280–296, 2017. doi: 10.1161/CIRCULATIONAHA.116.017513. [DOI] [PubMed] [Google Scholar]
- 2.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990, 2013. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29: 2912–2929, 2015. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexeeff SE, Litonjua AA, Wright RO, Baccarelli A, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure, antioxidant genes, and lung function in an elderly cohort: VA normative aging study. Occup Environ Med 65: 736–742, 2008. doi: 10.1136/oem.2007.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antony VB, Thannickal VJ. Cellular senescence in chronic obstructive pulmonary disease: multifaceted and multifunctional. Am J Respir Cell Mol Biol 59: 135–136, 2018. doi: 10.1165/rcmb.2018-0061ED. [DOI] [PubMed] [Google Scholar]
- 6.Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 22: 436–443, 2003. doi: 10.1183/09031936.03.00011903. [DOI] [PubMed] [Google Scholar]
- 7.Ardini-Poleske ME, Clark RF, Ansong C, Carson JP, Corley RA, Deutsch GH, Hagood JS, Kaminski N, Mariani TJ, Potter SS, Pryhuber GS, Warburton D, Whitsett JA, Palmer SM, Ambalavanan N; LungMAP Consortium . LungMAP: the molecular atlas of lung development program. Am J Physiol Lung Cell Mol Physiol 313: L733–L740, 2017. doi: 10.1152/ajplung.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA III, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 9.Athanazio R, Cukier A. Phenotyping asthma: more complex than just age. Respirology 22: 1485–1486, 2017. doi: 10.1111/resp.13120. [DOI] [PubMed] [Google Scholar]
- 10.Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169: 132–147.e16, 2017. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederländer NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol 10: 825–836, 2008. [Erratum in: Nat Cell Biol Cell 14: 649, 2012.] doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol 202: 393.e1–393.e7, 2010. doi: 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Barkoulas T, Hall PD. Experience with dasatinib and nilotinib use in pregnancy. J Oncol Pharm Pract 24: 121–128, 2018. doi: 10.1177/1078155217692399. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 37: 961–976, 2005. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 55: 114–120, 2000. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, Edwards MG, Rathinam MK, Zhou T, Tauler J, Borowicz S, Lussier YA, Parr BA, Cool CD, Winn RA. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene 34: 5317–5328, 2015. [Erratum in: Oncogene 34: 5406, 2015.] doi: 10.1038/onc.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell 16: 661–671, 2017. doi: 10.1111/acel.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, Green NJ, Moisey E, Birrell MA, Belvisi MG, Black F, Taylor JJ, Fisher AJ, De Soyza A, Passos JF. DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 309: L1124–L1137, 2015. doi: 10.1152/ajplung.00293.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birch J, Barnes PJ, Passos JF. Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharmacol Ther 183: 34–49, 2018. doi: 10.1016/j.pharmthera.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 202: 381.e1–381.e7, 2010. doi: 10.1016/j.ajog.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Bonney EA, Krebs K, Saade G, Kechichian T, Trivedi J, Huaizhi Y, Menon R. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta 43: 26–34, 2016. doi: 10.1016/j.placenta.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulet LP. Asthma in the elderly patient. Asthma Res Pract 2: 3, 2016. doi: 10.1186/s40733-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braman SS. Asthma in the elderly. Clin Geriatr Med 33: 523–537, 2017. doi: 10.1016/j.cger.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Britt RD JR, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med 7: 515–531, 2013. doi: 10.1586/17476348.2013.838020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton J, Pavord I, Richards K, Wisniewski A, Knox A, Lewis S, Tattersfield A, Weiss S. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet 344: 357–362, 1994. doi: 10.1016/S0140-6736(94)91399-4. [DOI] [PubMed] [Google Scholar]
- 27.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152: 340–351, 2013. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush A. Lung development and aging. Ann Am Thorac Soc 13, Suppl 5: S438–S446, 2016. doi: 10.1513/AnnalsATS.201602-112AW. [DOI] [PubMed] [Google Scholar]
- 29.Busse PJ, Birmingham JM, Calatroni A, Manzi J, Goryachokovsky A, Fontela G, Federman AD, Wisnivesky JP. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol 139: 1808–1818.e6, 2017. doi: 10.1016/j.jaci.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, Srivastava K, Li XM. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol 104: 236–246.e2, 2010. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705, 2013. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campisi J. Cellular senescence and lung function during aging. Yin and yang. Ann Am Thorac Soc 13, Suppl 5: S402–S406, 2016. doi: 10.1513/AnnalsATS.201609-703AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campisi J, Robert L. Cell senescence: role in aging and age-related diseases. Interdiscip Top Gerontol 39: 45–61, 2014. doi: 10.1159/000358899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cazzola M, Matera MG, Rogliani P, Calzetta L. Senolytic drugs in respiratory medicine: is it an appropriate therapeutic approach? Expert Opin Investig Drugs 27: 573–581, 2018. doi: 10.1080/13543784.2018.1492548. [DOI] [PubMed] [Google Scholar]
- 35.Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol 17: 42–48, 2010. doi: 10.1097/PAP.0b013e3181c66f4e. [DOI] [PubMed] [Google Scholar]
- 36.Chen JR, Lazarenko OP, Blackburn ML, Rose S, Frye RE, Badger TM, Andres A, Shankar K. Maternal obesity programs senescence signaling and glucose metabolism in osteo-progenitors from rat and human. Endocrinology 157: 4172–4183, 2016. doi: 10.1210/en.2016-1408. [DOI] [PubMed] [Google Scholar]
- 37.Chen KC, Blalock EM, Curran-Rauhut MA, Kadish I, Blalock SJ, Brewer L, Porter NM, Landfield PW. Glucocorticoid-dependent hippocampal transcriptome in male rats: pathway-specific alterations with aging. Endocrinology 154: 2807–2820, 2013. doi: 10.1210/en.2013-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21: 1424–1435, 2015. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16: 718–735, 2017. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest 128: 1217–1228, 2018. doi: 10.1172/JCI95146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chilosi M, Facchetti F, Caliò A, Zamò A, Brunelli M, Martignoni G, Rossi A, Montagna L, Piccoli P, Dubini A, Tironi A, Tomassetti S, Poletti V, Doglioni C. Oncogene-induced senescence distinguishes indolent from aggressive forms of pulmonary and non-pulmonary Langerhans cell histiocytosis. Leuk Lymphoma 55: 2620–2626, 2014. doi: 10.3109/10428194.2014.887713. [DOI] [PubMed] [Google Scholar]
- 42.Citrin DE, Shankavaram U, Horton JA, Shield W III, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst 105: 1474–1484, 2013. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coppé JP, Kauser K, Campisi J, Beauséjour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem 281: 29568–29574, 2006. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 44.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868, 2008. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox LS, Redman C. The role of cellular senescence in ageing of the placenta. Placenta 52: 139–145, 2017. doi: 10.1016/j.placenta.2017.01.116. [DOI] [PubMed] [Google Scholar]
- 46.Day CL, Ryan RM. Bronchopulmonary dysplasia: new becomes old again! Pediatr Res 81: 210–213, 2017. doi: 10.1038/pr.2016.201. [DOI] [PubMed] [Google Scholar]
- 47.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733, 2014. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Divo MJ, Celli BR, Poblador-Plou B, Calderón-Larrañaga A, de-Torres JP, Gimeno-Feliu LA, Bertó J, Zulueta JJ, Casanova C, Pinto-Plata VM, Cabrera-Lopez C, Polverino F, Carmona Píréz J, Prados-Torres A, Marin JM; EpiChron–BODE Collaborative Group . Chronic obstructive pulmonary disease (COPD) as a disease of early aging: evidence from the EpiChron Cohort. PLoS One 13: e0193143, 2018. doi: 10.1371/journal.pone.0193143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, Maccallum PK, Wedzicha JA. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 128: 1995–2004, 2005. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dow L. The diagnosis of asthma in older people. Clin Exp Allergy 24: 156–159, 1994. doi: 10.1111/j.1365-2222.1994.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 52.Drayton S, Peters G. Immortalisation and transformation revisited. Curr Opin Genet Dev 12: 98–104, 2002. doi: 10.1016/S0959-437X(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 53.Drummond D, Baravalle-Einaudi M, Lezmi G, Vibhushan S, Franco-Montoya ML, Hadchouel A, Boczkowski J, Delacourt C. Combined effects of in utero and adolescent tobacco smoke exposure on lung function in C57Bl/6J mice. Environ Health Perspect 125: 392–399, 2017. doi: 10.1289/EHP54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duijts L, Jaddoe VWV, van der Valk RJP, Henderson JA, Hofman A, Raat H, Steegers EAP, Moll HA, de Jongste JC. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children: the Generation R Study. Chest 141: 876–885, 2012. doi: 10.1378/chest.11-0112. [DOI] [PubMed] [Google Scholar]
- 55.Faksh A, Britt RD JR, Vogel ER, Kuipers I, Thompson MA, Sieck GC, Pabelick CM, Martin RJ, Prakash YS. Effects of antenatal lipopolysaccharide and postnatal hyperoxia on airway reactivity and remodeling in a neonatal mouse model. Pediatr Res 79: 391–400, 2016. doi: 10.1038/pr.2015.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186: 306–313, 2012. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 57.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23: 1072–1079, 2017. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: a sign of premature senescence? J Matern Fetal Neonatal Med 29: 1283–1288, 2016. doi: 10.3109/14767058.2015.1046045. [DOI] [PubMed] [Google Scholar]
- 59.Fischer BM, Wong JK, Degan S, Kummarapurugu AB, Zheng S, Haridass P, Voynow JA. Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol 304: L394–L400, 2013. doi: 10.1152/ajplung.00091.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1: 1645–1648, 1977. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92–105, 2007. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23: 2066–2075, 2012. doi: 10.1091/mbc.e11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 8, Suppl 2: S3, 2006. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, Curtis JL, Martinez FJ, Zick S, Hershenson MB, Sajjan US. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res 11: 131, 2010. doi: 10.1186/1465-9921-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebb SA, Fox K, Vaughn J, McKean D, Jones PL. Fetal oxygen tension promotes tenascin-C-dependent lung branching morphogenesis. Dev Dyn 234: 1–10, 2005. doi: 10.1002/dvdy.20500. [DOI] [PubMed] [Google Scholar]
- 66.Gebb SA, Jones PL. Hypoxia and lung branching morphogenesis. Adv Exp Med Biol 543: 117–125, 2003. doi: 10.1007/978-1-4419-8997-0_8. [DOI] [PubMed] [Google Scholar]
- 67.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet 376: 803–813, 2010. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 68.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 163: 429–436, 2001. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 69.Grasso G, Lodi L, Lupo C, Muscettola M. Glucocorticoid receptors in human peripheral blood mononuclear cells in relation to age and to sport activity. Life Sci 61: 301–308, 1997. doi: 10.1016/S0024-3205(97)00386-X. [DOI] [PubMed] [Google Scholar]
- 70.Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Manéglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 126: 3207–3218, 2016. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hadj Salem I, Dubé J, Boulet LP, Chakir J. Telomere shortening correlates with accelerated replicative senescence of bronchial fibroblasts in asthma. Clin Exp Allergy 45: 1713–1715, 2015. doi: 10.1111/cea.12611. [DOI] [PubMed] [Google Scholar]
- 72.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK; Asthma in Elderly workshop participants . Asthma in the elderly: current understanding and future research needs—a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol 128, Suppl: S4–S24, 2011. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, Sugimoto K, Sato T, Maruyama M, Sugimoto M. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight 1: e87732, 2016. doi: 10.1172/jci.insight.87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, Howard TD, Busse WW, Erzurum SC, Wenzel SE, Peters SP, Meyers DA, Bleecker ER; National Heart, Lung, and Blood Institute-sponsored Severe Asthma Research Program (SARP) . The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol 130: 510–515.e1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621, 1961. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 76.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14: 501–513, 2004. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 78.Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3: 708, 2012. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higgins MW, Enright PL, Kronmal RA, Schenker MB, Anton-Culver H, Lyles M. Smoking and lung function in elderly men and women. The Cardiovascular Health Study. JAMA 269: 2741–2748, 1993. doi: 10.1001/jama.1993.03500210041029. [DOI] [PubMed] [Google Scholar]
- 80.Hohmann MS, Habiel DM, Coelho AL, Verri WA JR, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent ipf fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol 60: 28–40, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houben JM, Mercken EM, Ketelslegers HB, Bast A, Wouters EF, Hageman GJ, Schols AM. Telomere shortening in chronic obstructive pulmonary disease. Respir Med 103: 230–236, 2009. doi: 10.1016/j.rmed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Hylkema MN, Blacquière MJ. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 660–662, 2009. doi: 10.1513/pats.200907-065DP. [DOI] [PubMed] [Google Scholar]
- 83.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 135: 173–180, 2009. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 84.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 14: 1454–1457, 1999. doi: 10.1183/09031936.99.14614549. [DOI] [PubMed] [Google Scholar]
- 85.Jedrychowski WA, Perera FP, Maugeri U, Mroz E, Klimaszewska-Rembiasz M, Flak E, Edwards S, Spengler JD. Effect of prenatal exposure to fine particulate matter on ventilatory lung function of preschool children of non-smoking mothers. Paediatr Perinat Epidemiol 24: 492–501, 2010. doi: 10.1111/j.1365-3016.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128: 36–44, 2007. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang C, Liu G, Luckhardt T, Antony V, Zhou Y, Carter AB, Thannickal VJ, Liu RM. Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease. Aging Cell 16: 1114–1124, 2017. doi: 10.1111/acel.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SC, Iverson D, Burns P, Evers U, Caputi P, Morgan S. Asthma and ageing: an end user’s perspective–the perception and problems with the management of asthma in the elderly. Clin Exp Allergy 41: 471–481, 2011. doi: 10.1111/j.1365-2222.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- 89.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40: 554–563, 2019. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kadota T, Fujita Y, Yoshioka Y, Araya J, Kuwano K, Ochiya T. Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol Aspects Med 60: 92–103, 2018. doi: 10.1016/j.mam.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24: 25–29, 2003. doi: 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 92.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst) 42: 63–71, 2016. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Kaur I, Rawal P, Rohilla S, Bhat MH, Sharma P, Siddiqui H, Kaur S. Endothelial progenitor cells from aged subjects display decreased expression of sirtuin 1, angiogenic functions, and increased senescence. Cell Biol Int 42: 1212–1220, 2018. doi: 10.1002/cbin.10999. [DOI] [PubMed] [Google Scholar]
- 94.Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 52: 820–827, 1997. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol 48: 1–5, 2013. doi: 10.1016/j.exger.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci 64A: 209–212, 2009. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine 21: 21–28, 2017. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 68: 19–25, 2015. doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc 65: 2297–2301, 2017. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirkwood TB. Understanding the odd science of aging. Cell 120: 437–447, 2005. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 101.Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP, Schumacker PT, Budinger GR, Chandel NS. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J 23: 783–794, 2009. doi: 10.1096/fj.08-114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98: 12072–12077, 2001. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 24: 2463–2479, 2010. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 51: 323–333, 2014. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 106.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 154: 477–483, 1996. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 107.Kuźnar-Kamińska B, Mikula-Pietrasik J, Ksiazek K, Tykarski A, Batura-Gabryel H. Lung cancer in chronic obstructive pulmonary disease patients: importance of cellular senescence. Pol Arch Intern Med 128: 462–468, 2018. [DOI] [PubMed] [Google Scholar]
- 108.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20: 115–126, 1996. doi: 10.1016/S0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 109.Landau LI. Tobacco smoke exposure and tracking of lung function into adult life. Paediatr Respir Rev 9: 39–44, 2008. doi: 10.1016/j.prrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health 6: e769–e776, 2018. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- 111.Latagliata R, Stagno F, Annunziata M, Abruzzese E, Iurlo A, Guarini A, Fava C, Gozzini A, Bonifacio M, Sorà F, Leonetti Crescenzi S, Bocchia M, Crugnola M, Castagnetti F, Capodanno I, Galimberti S, Feo C, Porrini R, Pregno P, Rizzo M, Antolino A, Mauro E, Sgherza N, Luciano L, Tiribelli M, Russo Rossi A, Trawinska M, Vigneri P, Breccia M, Rosti G, Alimena G. Frontline dasatinib treatment in a “real-life” cohort of patients older than 65 years with chronic myeloid leukemia. Neoplasia 18: 536–540, 2016. doi: 10.1016/j.neo.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol 270: 214–231, 2004. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Yang JR, Chen XM, Cai GY, Lin LR, He YN. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am J Physiol Cell Physiol 308: C621–C630, 2015. doi: 10.1152/ajpcell.00096.2014. [DOI] [PubMed] [Google Scholar]
- 114.Lomas NJ, Watts KL, Akram KM, Forsyth NR, Spiteri MA. Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int J Clin Exp Pathol 5: 58–71, 2012. [PMC free article] [PubMed] [Google Scholar]
- 114a.Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH, Rahman I. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and upregulation of p21 in neonatal mouse lung. Pediatr Res 69: 371–377, 2011. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging 8: 1489–1496, 2013. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maciel-Barón LA, Morales-Rosales SL, Aquino-Cruz AA, Triana-Martínez F, Galván-Arzate S, Luna-López A, González-Puertos VY, López-Díazguerrero NE, Torres C, Königsberg M. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr) 38: 26, 2016. doi: 10.1007/s11357-016-9886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacNee W. Oxidants/antioxidants and chronic obstructive pulmonary disease: pathogenesis to therapy. Novartis Found Symp 234: 169–185, 2001. [PubMed] [Google Scholar]
- 118.Mahemuti L, Chen Q, Coughlan MC, Qiao C, Chepelev NL, Florian M, Dong D, Woodworth RG, Yan J, Cao XL, Scoggan KA, Jin X, Willmore WG. Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch Toxicol 92: 1453–1469, 2018. doi: 10.1007/s00204-017-2150-3. [DOI] [PubMed] [Google Scholar]
- 119.Martens DS, Cox B, Janssen BG, Clemente DBP, Gasparrini A, Vanpoucke C, Lefebvre W, Roels HA, Plusquin M, Nawrot TS. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr 171: 1160–1167, 2017. doi: 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayne BT, Leemaqz SY, Smith AK, Breen J, Roberts CT, Bianco-Miotto T. Accelerated placental aging in early onset preeclampsia pregnancies identified by DNA methylation. Epigenomics 9: 279–289, 2017. doi: 10.2217/epi-2016-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McDonough JE, Martens DS, Tanabe N, Ahangari F, Verleden SE, Maes K, Verleden GM, Kaminski N, Hogg JC, Nawrot TS, Wuyts WA, Vanaudenaerde BM. A role for telomere length and chromosomal damage in idiopathic pulmonary fibrosis. Respir Res 19: 132, 2018. doi: 10.1186/s12931-018-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer KC. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med 11: 343–359, 2017. doi: 10.1080/17476348.2017.1312346. [DOI] [PubMed] [Google Scholar]
- 123.Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med 153: 1072–1079, 1996. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- 124.Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med 31: 521–527, 2010. doi: 10.1055/s-0030-1265893. [DOI] [PubMed] [Google Scholar]
- 125.Mirzakhani H, De Vivo I, Leeder JS, Gaedigk R, Vyhlidal CA, Weiss ST, Tantisira K. Early pregnancy intrauterine fetal exposure to maternal smoking and impact on fetal telomere length. Eur J Obstet Gynecol Reprod Biol 218: 27–32, 2017. doi: 10.1016/j.ejogrb.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 126.Moon H, Choi HH, Lee JY, Moon HJ, Sim SS, Kim CJ. Quercetin inhalation inhibits the asthmatic responses by exposure to aerosolized-ovalbumin in conscious guinea-pigs. Arch Pharm Res 31: 771–778, 2008. doi: 10.1007/s12272-001-1225-2. [DOI] [PubMed] [Google Scholar]
- 127.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE; National Heart, Lung, Blood Institute’s Severe Asthma Research Program . Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morlá M, Busquets X, Pons J, Sauleda J, MacNee W, Agustí AG. Telomere shortening in smokers with and without COPD. Eur Respir J 27: 525–528, 2006. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- 129.Mui TS, Man JM, McElhaney JE, Sandford AJ, Coxson HO, Birmingham CL, Li Y, Man SF, Sin DD. Telomere length and chronic obstructive pulmonary disease: evidence of accelerated aging. J Am Geriatr Soc 57: 2372–2374, 2009. doi: 10.1111/j.1532-5415.2009.02589.x. [DOI] [PubMed] [Google Scholar]
- 130.Müller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, Krug N, Nakashima M, Branscheid D, Magnussen H, Jörres RA, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 7: 32, 2006. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118, 2013. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 132.Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M, Llanos S, Chaib S, Muñoz-Martín M, Ucero AC, Garaulet G, Mulero F, Dann SG, VanArsdale T, Shields DJ, Bernardos A, Murguía JR, Martínez-Máñez R, Serrano M. A versatile drug delivery system targeting senescent cells. EMBO Mol Med 10: e9355, 2018. doi: 10.15252/emmm.201809355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, Wolters PJ. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: e86704, 2016. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716, 2003. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 135.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11: 345–349, 2012. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niedernhofer LJ, Kirkland JL, Ladiges W. Molecular pathology endpoints useful for aging studies. Ageing Res Rev 35: 241–249, 2017. doi: 10.1016/j.arr.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 35: 681–688, 2006. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Conor R, Martynenko M, Gagnon M, Hauser D, Young E, Lurio J, Wisnivesky JP, Wolf MS, Federman AD; Supporting Asthma Self-Management Behaviors Among Aging Adults (SAMBA) investigators . A qualitative investigation of the impact of asthma and self-management strategies among older adults. J Asthma 54: 39–45, 2017. doi: 10.1080/02770903.2016.1193602. [DOI] [PubMed] [Google Scholar]
- 139.Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8: 15691, 2017. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Özcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, Galderisi U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 8: 1316–1329, 2016. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parikh P, Britt RD JR, Manlove LJ, Wicher SA, Roesler A, Ravix J, Teske J, Thompson MA, Sieck GC, Kirkland JL, LeBrasseur N, Tschumperlin DJ, Pabelick CM, Prakash YS. Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Am J Respir Cell Mol Biol rcmb.2018-0176OC, 2018. doi: 10.1165/rcmb.2018-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Puddicombe SM, Torres-Lozano C, Richter A, Bucchieri F, Lordan JL, Howarth PH, Vrugt B, Albers R, Djukanovic R, Holgate ST, Wilson SJ, Davies DE. Increased expression of p21(waf) cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol 28: 61–68, 2003. doi: 10.1165/rcmb.4715. [DOI] [PubMed] [Google Scholar]
- 143.Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom 3: e4, 2018. doi: 10.1017/gheg.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quante T, Ng YC, Ramsay EE, Henness S, Allen JC, Parmentier J, Ge Q, Ammit AJ. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. Am J Respir Cell Mol Biol 39: 208–217, 2008. doi: 10.1165/rcmb.2007-0014OC. [DOI] [PubMed] [Google Scholar]
- 145.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 146.Richter T, Saretzki G, Nelson G, Melcher M, Olijslagers S, von Zglinicki T. TRF2 overexpression diminishes repair of telomeric single-strand breaks and accelerates telomere shortening in human fibroblasts. Mech Ageing Dev 128: 340–345, 2007. doi: 10.1016/j.mad.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 147.Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep 2: 379–387, 2015. doi: 10.1007/s40572-015-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rode L, Bojesen SE, Weischer M, Vestbo J, Nordestgaard BG. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax 68: 429–435, 2013. doi: 10.1136/thoraxjnl-2012-202544. [DOI] [PubMed] [Google Scholar]
- 149.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979, 2009. [Erratum in: Nat Cell Biol 11: 1272, 2009.] doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124: 68–81, 2011. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rubini A. Interleukin-6 and lung inflammation: evidence for a causative role in inducing respiratory system resistance increments. Inflamm Allergy Drug Targets 12: 315–321, 2013. doi: 10.2174/1871528111312050003. [DOI] [PubMed] [Google Scholar]
- 152.Sabin RJ, Anderson RM. Cellular senescence - its role in cancer and the response to ionizing radiation. Genome Integr 2: 7, 2011. doi: 10.1186/2041-9414-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saito H, Hammond AT, Moses RE. The effect of low oxygen tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp Cell Res 217: 272–279, 1995. doi: 10.1006/excr.1995.1087. [DOI] [PubMed] [Google Scholar]
- 154.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev 28: 99–114, 2014. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24: 835–845, 2012. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 156.Saretzki G, Feng J, von Zglinicki T, Villeponteau B. Similar gene expression pattern in senescent and hyperoxic-treated fibroblasts. J Gerontol A Biol Sci Med Sci 53A: B438–B442, 1998. doi: 10.1093/gerona/53A.6.B438. [DOI] [PubMed] [Google Scholar]
- 157.Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, Le Corvoisier P, Rideau D, Boczkowski J, Dubois-Randé JL, Chouaid C, Adnot S. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179: 566–571, 2009. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schreiber MD, Marks JD. Noninvasive ventilation in the premature newborn - is less always more? N Engl J Med 377: 386–388, 2017. doi: 10.1056/NEJMe1707439. [DOI] [PubMed] [Google Scholar]
- 160.Semlali A, Jacques E, Rouabhia M, Milot J, Laviolette M, Chakir J. Regulation of epithelial cell proliferation by bronchial fibroblasts obtained from mild asthmatic subjects. Allergy 65: 1438–1445, 2010. doi: 10.1111/j.1398-9995.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 161.Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 10: 798–806, 2011. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shivshankar P, Brampton C, Miyasato S, Kasper M, Thannickal VJ, Le Saux CJ. Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 47: 28–36, 2012. doi: 10.1165/rcmb.2011-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sidler C, Kovalchuk O, Kovalchuk I. Epigenetic regulation of cellular senescence and aging. Front Genet 8: 138, 2017. doi: 10.3389/fgene.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skloot GS. The effects of aging on lung structure and function. Clin Geriatr Med 33: 447–457, 2017. doi: 10.1016/j.cger.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 165.Skloot GS, Busse PJ, Braman SS, Kovacs EJ, Dixon AE, Vaz Fragoso CA, Scichilone N, Prakash YS, Pabelick CM, Mathur SK, Hanania NA, Moore WC, Gibson PG, Zieman S, Ragless BB; ATS ad hoc Committee on Asthma in the Elderly . An official American Thoracic Society workshop report: evaluation and management of asthma in the elderly. Ann Am Thorac Soc 13: 2064–2077, 2016. doi: 10.1513/AnnalsATS.201608-658ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology 8: 266–285, 2003. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 167.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130, 2013. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 168.Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 32: 9–19, 2017. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, Torres F, Kaza V, Girod CE, Jones KD, Elicker BM, Ma SF, Vij R, Collard HR, Wolters PJ, Garcia CK. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med 2: 557–565, 2014. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sundar IK, Rashid K, Gerloff J, Li D, Rahman I. Genetic ablation of p16INK4a does not protect against cellular senescence in mouse models of chronic obstructive pulmonary disease/emphysema. Am J Respir Cell Mol Biol 59: 189–199, 2018. doi: 10.1165/rcmb.2017-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol 203: 929–942, 2013. doi: 10.1083/jcb.201306073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tai H, Wang Z, Gong H, Han X, Zhou J, Wang X, Wei X, Ding Y, Huang N, Qin J, Zhang J, Wang S, Gao F, Chrzanowska-Lightowlers ZM, Xiang R, Xiao H. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy 13: 99–113, 2017. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123: 966–972, 2013. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, Busse PJ, Tuder RM, Antony VB, Sznajder JI, Budinger GR. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med 191: 261–269, 2015. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Townsend EA, Emala CW SR. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am J Physiol Lung Cell Mol Physiol 305: L396–L403, 2013. doi: 10.1152/ajplung.00125.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Trigg CJ, Bennett JB, Tooley M, Sibbald B, D’Souza MF, Davies RJ. A general practice based survey of bronchial hyperresponsiveness and its relation to symptoms, sex, age, atopy, and smoking. Thorax 45: 866–872, 1990. doi: 10.1136/thx.45.11.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA 104: 7552–7557, 2007. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 80: 59–70, 2010. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- 179.Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol 31: 643–649, 2004. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- 180.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet 366: 662–664, 2005. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 181.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 288: L167–L178, 2005. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- 182.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci 67A: 264–275, 2012. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vento M, Asensi M, Sastre J, García-Sala F, Pallardó FV, Viña J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647, 2001. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 184.Wiley CD, Campisi J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23: 1013–1021, 2016. doi: 10.1016/j.cmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S, Campisi J. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16: 1043–1050, 2017. doi: 10.1111/acel.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wright HL, Cross AL, Edwards SW, Moots RJ. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford) 53: 1321–1331, 2014. doi: 10.1093/rheumatology/keu035. [DOI] [PubMed] [Google Scholar]
- 187.Wu J, Dong F, Wang RA, Wang J, Zhao J, Yang M, Gong W, Cui R, Dong L. Central role of cellular senescence in TSLP-induced airway remodeling in asthma. PLoS One 8: e77795, 2013. doi: 10.1371/journal.pone.0077795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med 208: 1339–1350, 2011. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, Peters S, Niimi A, Ledford DK, Katial R, Fabbri LM, Celedón JC, Canonica GW, Busse P, Boulet LP, Baena-Cagnani CE, Hamid Q, Bachert C, Pawankar R, Holgate ST. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J 7: 8, 2014. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yao C, Carraro G, Konda B, Guan X, Mizuno T, Chiba N, Kostelny M, Kurkciyan A, David G, McQualter JL, Stripp BR. Sin3a regulates epithelial progenitor cell fate during lung development. Development 144: 2618–2628, 2017. doi: 10.1242/dev.149708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yao H, Sundar IK, Gorbunova V, Rahman I. P21-PARP-1 pathway is involved in cigarette smoke-induced lung DNA damage and cellular senescence. PLoS One 8: e80007, 2013. doi: 10.1371/journal.pone.0080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O’Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol 39: 7–18, 2008. doi: 10.1165/rcmb.2007-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yoo JK, Kim TS, Hufford MM, Braciale TJ. Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol 132: 1263–1276, 2013. doi: 10.1016/j.jaci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD, Niedernhofer LJ. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36: 18–28, 2018. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res 2: 00042-2016, 2016. doi: 10.1183/23120541.00042-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou F, Onizawa S, Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res 12: 78, 2011. doi: 10.1186/1465-9921-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15: 428–435, 2016. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658, 2015. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]