Abstract
Previous studies have demonstrated an inverse relation between resting muscle sympathetic nerve activity (MSNA) and vasoconstrictor responsiveness (i.e., sympathetic transduction), such that those with high resting MSNA have low vascular responsiveness, and vice versa. The purpose of this investigation was to determine whether biological sex influences the balance between resting MSNA and beat-to-beat sympathetic transduction. We measured blood pressure (BP) and MSNA during supine rest in 54 healthy young adults (27 females: 23 ± 4 yr, 107 ± 8/63 ± 8 mmHg; 27 males: 25 ± 3 yr, 115 ± 11/64 ± 7 mmHg; means ± SD). We quantified beat-to-beat fluctuations in mean arterial pressure (MAP, mmHg) and limb vascular conductance (LVC, %) for 10 cardiac cycles after each MSNA burst using signal averaging, an index of sympathetic vascular transduction. In females, there was no correlation between resting MSNA (burst incidence; burst/100 heartbeats) and peak ΔMAP (r = −0.10, P = 0.62) or peak ΔLVC (r = −0.12, P = 0.63). In males, MSNA was related to peak ΔMAP (r = −0.50, P = 0.01) and peak ΔLVC (r = 0.49, P = 0.03); those with higher resting MSNA had blunted increases in MAP and reductions in LVC in response to a burst of MSNA. In a sub-analysis, we performed a median split between high- versus low-MSNA status on ΔMAP and ΔLVC within each sex and found that only males demonstrated a significant difference in ΔMAP and ΔLVC between high- versus low-MSNA groups. These findings support an inverse relation between resting MSNA and sympathetic vascular transduction in males only and advance our understanding on the influence of biological sex on sympathetic nervous system-mediated alterations in beat-to-beat BP regulation.
Keywords: blood pressure, muscle sympathetic nerve activity, sex differences, vascular physiology, vasoconstriction
INTRODUCTION
The ability of the sympathetic nervous system to modulate vascular tone plays a vital role in the regulation of blood pressure (BP) (27, 28, 29). Sympathetic nerve activity varies widely among healthy normotensive young adults with similar BP, resulting in a lack of correlation between resting sympathetic nerve activity and resting BP (8, 14, 16, 24, 25, 31). Previous findings demonstrate an inverse relation between resting muscle sympathetic nerve activity (MSNA) and vascular adrenergic responsiveness to pharmacological agonists, such that individuals with lower resting sympathetic outflow have greater α-adrenergic responsiveness, and vice versa (5, 17). Further mechanistic evidence comes from rodent studies demonstrating that experimental disruption of sympathetic outflow (via spinal cord transection) enhances sympathetically mediated arterial vasoconstrictor responses to external stimuli (39, 40). Thus there are both rodent and human studies that support an inverse relation between vascular adrenergic responsiveness and sympathetic nerve activity.
Wallin and Nerhed (35) were the first to report an inverse relation between spontaneous bursts of MSNA and beat-to-beat vasoconstrictor responsiveness (i.e., sympathetic vascular transduction) in humans, but these analyses were performed on a heterogeneous group of participants without regard to potential sex differences. Additionally, this study was performed in 1982, predating the technology we have today to routinely assess conduit artery blood flow on a beat-to-beat basis via ultrasonography. Indices of sympathetic vascular transduction have since been characterized using MSNA burst-triggered averaging to define the time course and magnitude of cardiovascular responses [mean arterial pressure (MAP), limb vascular conductance (LVC)] on a beat-to-beat basis (9, 10, 33, 34, 35). A strength of this approach is measuring the conductance in the vasculature that is innervated by the nerve signal that is simultaneously being measured. However, it is unknown if sex influences the relation between resting MSNA and beat-to-beat differences in indices of sympathetic vascular transduction.
In terms of known sex differences, norepinephrine causes less vasoconstriction in females compared with males (20). This may be due to β-adrenergic receptor-mediated vasodilator responses blunting the vasoconstrictor effects of α-adrenergic vasoconstriction in healthy young females (13, 15). Therefore, we assessed the relation between resting MSNA and indices of transduction within male and female participants and hypothesized that the relation between resting MSNA and indices of transduction would be stronger in male compared with female participants. If this hypothesis proved to be correct, the aforementioned inverse relation previously reported between spontaneous bursts of MSNA and beat-to-beat vasoconstrictor responsiveness may be primarily driven by males (35).
The assessment of resting MSNA and indices of sympathetic transduction have implications for BP variability (34), which is prognostic of subclinical target organ damage and future cardiovascular events (2, 22, 36). One previous study reported similar sympathetic transduction (vasoconstrictor and pressor responses to MSNA) between sexes (33), and it has been reported that young males have higher resting MSNA (6, 24, 25). Therefore, we also tested the hypothesis that males would have greater BP variability than females. Further understanding of the balance between resting MSNA and beat-to-beat differences in vasoconstrictor responsiveness, as well as possible sex differences in BP variability, may have important implications for our understanding of BP regulation.
METHODS
To achieve satisfactory statistical power, the data reported here are part of multiple ongoing protocols, including NCT02881515 and NCT03560869. We also included data from a previously published study (n = 19) (3), which did not contain the calculation of indices of sympathetic transduction, as was done herein. All participants provided written and verbal consent. The Institutional Review Board of the University of Delaware approved all study protocols and procedures, and they conform to the provisions of the Declaration of Helsinki.
Study participants.
All participants provided a complete medical history during screening. Screening consisted of height and weight for calculation of body mass index (BMI; kg/m2) and oscillometric assessment of seated BP, taken in triplicate, after at least 5 min of quiet sitting. Exclusion criteria included hypertension diagnosis, cardiovascular disease, malignancy, diabetes mellitus, renal impairment, pregnancy, obesity (BMI >30 kg/m2), tobacco usage, and postmenopausal status.
Study visit.
Participants reported to the laboratory after a ≥4-h fast. All participants were either provided 2,300 mg sodium/day diets (controlled feeding study, n = 13) or instructed to consume a 2,300 mg sodium/day diet for 3 days preceding the study visit (n = 41). A research dietitian analyzed participant’s 3-day diet records using Nutrient Data System for Research (NDSR), and 24-h urinary sodium excretion was calculated to ensure dietary composition was similar across protocols. A spot urine sample was obtained at the beginning of each study visit, and urine specific gravity was measured to confirm normal hydration status. The reported data were collected under resting conditions before any sympathoexcitatory maneuvers. While menstrual status has been shown to influence MSNA and baroreflex sensitivity (23), menstrual status was not controlled for in female participants taking part in one of our ongoing protocols (controlled feeding study, n = 9). However, menstrual status did not alter the relation between resting MSNA and indices of sympathetic transduction in female participants (see results); thus data from all female participants tested are included.
Body composition and weight were determined at the commencement of each visit (TNF-300A, Tanita). We instrumented participants for single-lead (lead II) electrocardiogram (ECG), and oscillometric BP was assessed with an upper arm cuff placed on the dominant arm (Dash 2000, GE Medical Systems). Beat-to-beat BP, using a cuff placed on the middle finger of the participant’s nondominant arm, was assessed via servo-controlled photoplethysmography (Finometer, Finapres Medical Systems).
Muscle sympathetic nerve activity.
Multiunit, postganglionic MSNA was recorded with an active tungsten microelectrode inserted into the peroneal nerve using standard microneurography techniques (15, 32, 37). The raw signal was amplified (80–90,000×), band-pass filtered (0.7–2.0 kHz), rectified, and integrated (time constant, 0.1 s) using a nerve traffic analyzer (model 662c-4, Nerve Traffic Analyzer, Univ. of Iowa Bioengineering) similar to our previous publications (3, 12). The presence of MSNA was confirmed by a pulse-synchronous signal that responded to an end-expiratory breath hold and stimulation of muscle (tendon tapping), but not skin afferents (gentle skin stroke and/or startle stimulus). Experienced microneurographers (W. B. Farquhar, A. T. Robinson, and M. M. Wenner) performed all microneurography.
Leg blood flow.
Continuous measures of common femoral artery (CFA) diameter and velocity were obtained via duplex Doppler ultrasound (GE Logiq P5) as previously described (10, 34). Briefly, a 10- or 12-MHz linear array transducer was selected for optimal image quality and positioned at the CFA via a custom-designed clamp, ~2–3 cm proximal to the bifurcation of the superficial and deep femoral arteries. Blood velocity was simultaneously obtained with diameter in pulsed-wave mode at an insonation angle of ≤60° and operating at a linear frequency of 5 MHz. The sample volume encompassed the entire vessel lumen without extending beyond the walls.
Hemodynamic data analysis.
CFA diameter and blood velocity were analyzed and synchronized with beat-to-beat ECG, BP, and MSNA signals using custom LabVIEW programs. Femoral blood flow was determined via the continuous recordings of CFA diameter and blood velocity and calculated as: π × (diameter/2)2 × mean blood velocity × 60. LVC was determined by dividing femoral blood flow by MAP, which was calculated as the integral of the arterial BP waveform. Because of technical difficulties, LVC was obtained in 43 of the participants. Of the 43 participants, data on 3 male participants and 1 female participant were excluded from the resting MSNA versus peak changes in MAP/LVC correlation analyses following outlier screening (final n = 39, see criteria below in Statistics).
The sympathetic neurogram was analyzed on a beat-to-beat basis to determine the presence/absence of MSNA bursts using custom LabView software and visually inspected by laboratory members trained in MSNA data analysis and processing (M. C. Babcock, J. C. Watso, and M. S. Brian). Regarding burst detection among different laboratory members, we have a laboratory intraclass correlation coefficient of 0.98. Bursts were identified in accordance with recent guidelines (15, 30, 37) via the following criteria: 1) >3:1 signal-to-noise ratio, 2) burst morphology consistent with MSNA bursts, and 3) a pulse-synchronous signal. As an index of resting sympathetic outflow, MSNA was quantified as burst frequency (bursts/min) and burst incidence (bursts/100 cardiac cycles).
To quantify indices of sympathetic transduction (i.e., the functional effect of individual bursts of MSNA on BP and blood flow), a spike-triggered averaging methodology was used that has been previously described (9, 10, 33, 34). Briefly, each cardiac cycle containing a burst of MSNA was identified and set as cardiac cycle 0 whether or not the burst was a singlet (bursts directly bordered by >1 heartbeat lacking MSNA) or part of a cluster [bursts adjacent to other burst(s) of MSNA]. Both MAP and LVC of cardiac cycle 0 were determined and followed for 10 subsequent cardiac cycles. The absolute change in MAP and percent change in LVC were determined in each of these 10 cardiac cycles. We also performed this technique for cardiac cycles not containing a burst of MSNA (i.e., nonbursts). These methods reliably quantify beat-to-beat changes in BP and blood flow induced by bursts of MSNA (9, 10, 33, 34). An illustration depicting original ECG, microneurographic, and BP tracings, along with calculated LVC, is presented as Fig. 1.
Fig. 1.
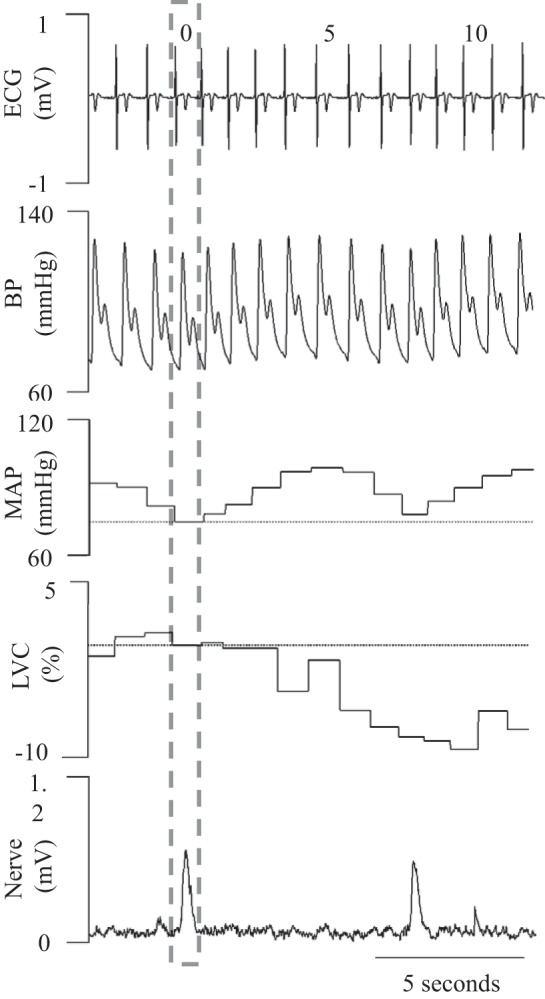
Illustration depicting original electrocardiogram (ECG), microneurographic, and blood pressure (BP) traces, along with a calculated vascular conductance curve in a male participant. MAP, mean arterial pressure; LVC, limb vascular conductance.
Blood pressure variability.
Blood pressure variability was calculated using the average real variability (ARV) index. The ARV index calculates the average of the absolute differences between consecutive BP measurements and is thought to provide further prognostic value compared with traditional indices of BP variability (i.e., standard deviation of BP) (2, 22). Blood pressure ARV was assessed over 10 min of quiet rest using the beat-to-beat BP signal derived from the Finometer (described in Hemodynamic data analysis). Additionally, ambulatory BP was assessed in a subset of participants (14 female participants, 13 male participants) during the 24-h period preceding the study visit to derive ambulatory BP ARV. Participants wore an ambulatory BP monitor (Oscar 2 with Sphygmacor, SunTech Medical) on their nondominant arm. The monitor measured BP every 20 min from 0601 to 2200 h and every 30 min from 2201 to 0600 h. In the subset of participants who performed 24-h ambulatory BP collection, participants were instructed to abstain from caffeine and exercise for the 24 h before and during 24-h ambulatory BP collection. All other participants were instructed to abstain from caffeine and exercise for the 24 h before their study visit.
Statistics.
The statistical approaches reported here were informed by recent guidelines in statistical reporting of cardiovascular research (21). The primary outcome measure was the relation between baseline resting MSNA and peak changes in MAP and LVC. Therefore, we performed a power analysis for Pearson's correlation. Power was calculated using G*Power 3.0.10 for Windows (11). Statistical power was set at 0.8, α at 0.05, and the anticipated correlation (r = −0.57) was determined from a prior study that performed this analysis without regard to sex (35). We determined 22 participants per group were needed to satisfy these criteria. Pearson correlations were performed to determine the relation between indices of resting MSNA and peak ΔMAP, and between resting MSNA and peak ΔLVC. The peak values of MAP and LVC were taken from each individual irrespective of cardiac cycle. These peak values usually occurred in cardiac cycles 6–8 following a burst of MSNA, consistent with reporting for prior studies (9, 10, 33). Sex differences in participant characteristics and ARV were compared using unpaired, two-tailed t-tests. A Bonferroni correction for multiple comparisons (two measures) was applied for ARV comparisons since two indices (ambulatory and beat-to-beat) were performed, and adjusted P values are reported here. Sex differences in sympathetic transduction were compared using two-way ANOVA (sex × cardiac cycle following a MSNA burst). For a separate analysis using two-way ANOVA, we performed a median split on resting MSNA data from both male and female participants to derive upper and lower halves of resting MSNA data. An unpaired, two-tailed, t-test was used to confirm the subgroups were statistically different. For ANOVAs, where there was a significant omnibus F, Sidak's multiple comparisons test was used for post hoc analysis. Outliers were screened using the criteria of removing values outside of the mean ± 1.5 (interquartile range) (38). Statistical outliers were removed from the burst incidence vs. peak change in MAP/LVC correlation analysis if either burst incidence, peak ΔMAP, or peak ΔLVC was determined to be an outlier. Levene’s test was used to assess the equality of variances and the Shapiro-Wilk test to assess normality of data. When data were not normally distributed, the independent samples Mann-Whitney U test was used in place of the paired sample t-test. Statistical significance was set at P < 0.05, and results are reported as means ± SD. Statistics were analyzed using IBM SPSS version 24.0 and GraphPad Prism version 7.0.
RESULTS
Participant characteristics are reported in Table 1. Male participants had a significantly greater height, mass, and age but similar BMI compared with female participants. Although the values were within normal, healthy values, male participants also had a lower heart rate and higher systolic BP, whereas diastolic BP was similar between sexes. Male participants also demonstrated greater resting MSNA as indicated by significantly higher burst frequency (bursts/minute) and burst incidence (bursts/100 heartbeats). We report the relation between resting MSNA and peak changes in transduction (ΔMAP and ΔLVC) with MSNA presented as burst incidence in the figures due to the sex difference in heart rate. There were similar trends in the relation between resting MSNA expressed as burst frequency (bursts/min) and peak changes in transduction (ΔMAP and ΔLVC) (see below).
Table 1.
Participant characteristics
| Participant Characteristics | Females (n = 27) | Males (n = 27) | P Value |
|---|---|---|---|
| Age, yr | 23 ± 4 | 25 ± 3 | 0.03 |
| Height, cm | 163 ± 9 | 179 ± 8 | <0.01 |
| Mass, kg | 65 ± 12 | 78 ± 9 | <0.01 |
| BMI, kg/m2 | 24 ± 4 | 24 ± 3 | 0.44 |
| Resting HR, beats/min | 60 ± 9 | 54 ± 7 | 0.03 |
| Systolic BP, mmHg | 107 ± 8 | 115 ± 11 | <0.01 |
| Diastolic BP, mmHg | 63 ± 8 | 64 ± 7 | 0.65 |
| Burst frequency, bursts/min | 10 ± 5 | 14 ± 6 | 0.02 |
| Burst incidence, bursts/100 beats | 17 ± 10 | 25 ± 12 | 0.02 |
Data are means ± SD. BMI, body mass index; BP, blood pressure; HR, heart rate.
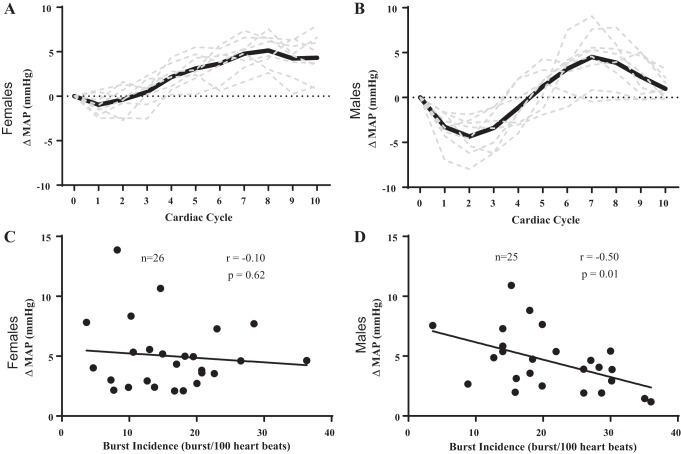
We present the relation between burst incidence and peak change in MAP in Fig. 2 using within-sex analyses. Examples of the signal averaging for a female participant (Fig. 2A) and a male participant (Fig. 2B) illustrate the changes in MAP following spontaneous bursts of MSNA. There was no relation between burst incidence and peak change in MAP (Fig. 2C) in female participants. With regard to burst frequency, there was no relation between burst frequency and peak change in MAP in female participants (r = −0.30, P = 0.13). There was still no relation between burst incidence (r = −0.07, P = 0.78) or burst frequency (r = −0.26, P = 0.31) and peak change in MAP in female participants when we excluded female participants who were not controlled for menstrual status. However, there was a significant negative correlation between burst incidence and peak change in MAP (Fig. 2D) in male participants. There was also a relation between burst frequency and peak change in MAP in male participants (r = −0.50, P = 0.01).
Fig. 2.
Influence of resting sympathetic outflow on peak changes in beat-to-beat mean arterial pressure (MAP) in females vs. males. Examples of the signal averaging for a female participant (A) and a male participant (B) illustrate the changes in MAP following spontaneous bursts of muscle sympathetic nerve activity. There was no relation between peak change in MAP and burst incidence in female participants (C). However, there was a significant negative correlation between peak change in MAP and burst incidence in male participants (D). Sample size (n) is provided within each graph.
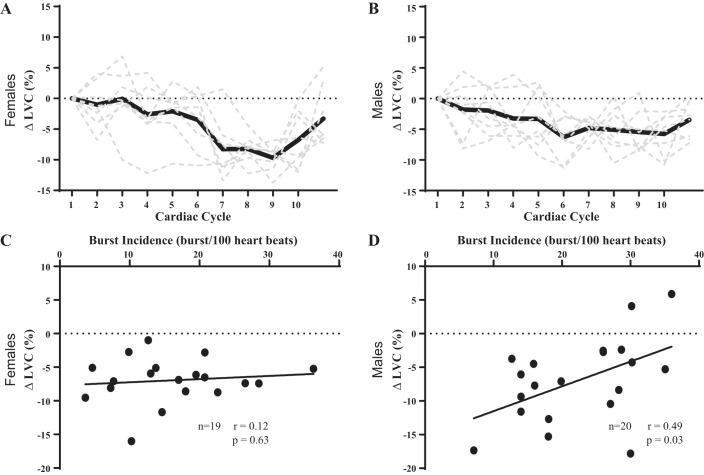
The relation between burst incidence and peak change in LVC is presented in Fig. 3 using within-sex analyses. Examples of the signal averaging for a female participant (Fig. 3A) and a male participant (Fig. 3B) illustrate the changes in LVC following spontaneous bursts of MSNA. There was no relation between burst incidence and peak change in LVC (Fig. 3C) in female participants. There was no relation between burst frequency and peak change in LVC in female participants (r = 0.12, P = 0.63). There was no relation between burst incidence (r = 0.09, P = 0.78) or burst frequency (r = 0.18, P = 0.55) and peak change in LVC in female participants when we excluded female participants who were not controlled for menstrual status. However, there was a significant positive relation between burst incidence and peak change in LVC (Fig. 3D) in male participants. There was a relation between burst frequency and peak change in LVC in male participants (r = 0.49, P = 0.03).
Fig. 3.
Influence of resting sympathetic outflow on peak changes in beat-to-beat limb vascular conductance (LVC) in females vs. males. Examples of the signal averaging for a female participant (A) and a male participant (B) illustrate the changes in LVC following spontaneous bursts of muscle sympathetic nerve activity. There was no relation between peak change in LVC and burst incidence in female participants (C). There was a significant positive correlation between peak change in LVC and burst incidence in male participants (D). Sample size (n) is provided within each graph.
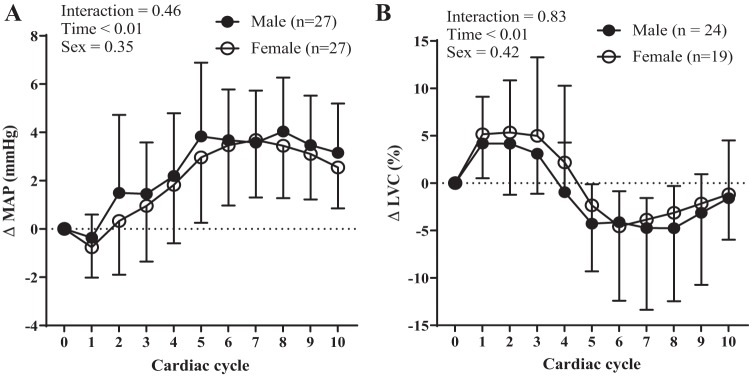
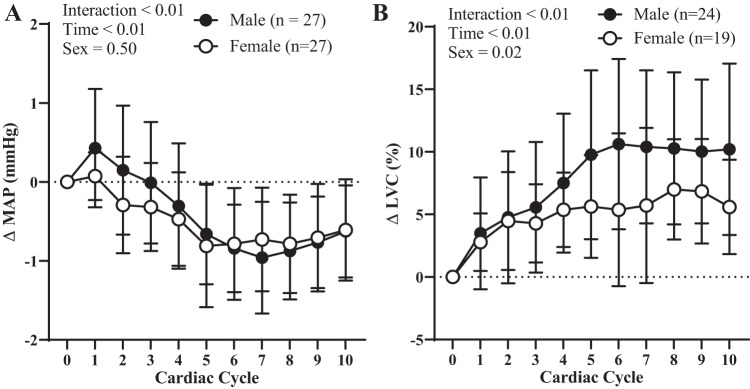
We depict the influence of sex on indices of transduction over 10 cardiac cycles in Fig. 4 using a between-sex comparison. These data represent the changes in MAP and LVC following bursts of MSNA over 10 cardiac cycles rather than the peak changes in MAP or LVC as reported in Figs. 1 and 2. There was no effect of sex on transduction when quantified as either MAP (Fig. 4A) or ΔLVC (Fig. 4B).
Fig. 4.
Resting sympathetic vascular transduction in female vs. male participants. There was no effect of sex on transduction when quantified as either mean arterial pressure (MAP) (A) or limb vascular conductance (LVC) (B). Sample size (n) is provided within each graph. Data are means ± SD.
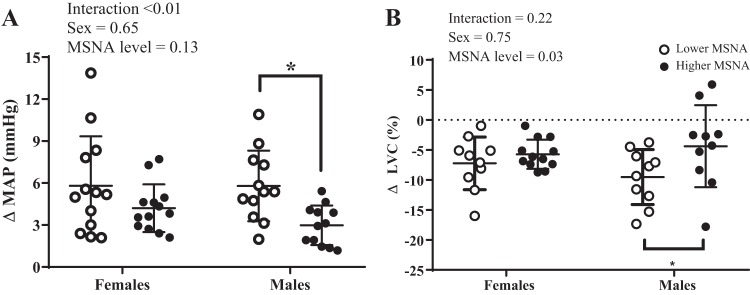
The sex-specific effects of high and low resting MSNA on peak changes in MAP and LVC are reported in Fig. 5. High and low MSNA subgroups for both female and male participants were derived by performing a median split. There was a significant difference in resting burst incidence (low = 10.1 ± 3.7 vs. high = 20.9 ± 3.7 bursts/100 heartbeats, P < 0.001) and burst frequency (low = 6.2 ± 2.4 vs. high = 13.5 ± 5.1 bursts/min, P < 0.001) between the high and low subgroups in female participants. There was also a significant difference in resting burst incidence (low = 16.5 ± 2.7 vs. high = 29.8 ± 3.4 bursts/100 heartbeats, P < 0.001) and burst frequency (low = 8.8 ± 1.7 vs. high = 19.6 ± 4.2 bursts/min, P < 0.001) between the high and low subgroups in male participants. There was a significant interaction effect (sex × MSNA level) on peak changes in MAP (Fig. 5A). Post hoc analysis indicated male participants with lower MSNA demonstrated greater peak changes in MAP compared with male participants with higher MSNA, but this was not the case in female participants. There was also a significant main effect of MSNA level on peak changes in LVC (Fig. 5B). Post hoc analysis indicated male participants with lower resting MSNA demonstrated greater peak changes in LVC compared with male participants with higher resting MSNA, but this was not the case in female participants.
Fig. 5.
The sex-specific effects of high vs. low muscle sympathetic nerve activity (MSNA) on peak changes in mean arterial pressure (MAP) and limb vascular conductance (LVC). There was a significant interaction effect (sex × MSNA level) on ΔMAP (A). Post hoc analyses indicated male participants with lower resting MSNA demonstrated greater changes in ΔMAP compared with male participants with higher resting MSNA (n values for MAP: low MSNA females = 12, high MSNA females = 13, low MSNA males = 12, high MSNA males = 12). There was a significant main effect of resting MSNA level on ΔLVC (B). Post hoc analyses indicated male participants with lower MSNA demonstrated greater changes in ΔLVC compared with male participants with higher resting MSNA, but this was not the case in female participants (n values for LVC: low MSNA females = 10, high MSNA females = 9, low MSNA males = 10, high MSNA males = 10). Data are means ± SD. We performed a median split to determine high vs. low MSNA. *P < 0.05, high vs. low MSNA.
We depict the influence of sex on MAP and LVC over 10 cardiac cycles after nonbursts in Fig. 6 using a between-sex comparison. These data represent the changes in MAP and LVC following bursts over 10 cardiac cycles that were not preceded by a burst of MSNA. There was no effect of sex on changes in MAP (Fig. 6A), but there was a sex difference for ΔLVC (Fig. 6B), such that male participants had a higher increase in ΔLVC following non-bursts.
Fig. 6.
Vascular hemodynamics following non-bursts in female vs. male participants. There was no effect of sex on changes in hemodynamics following nonbursts when quantified as mean arterial pressure (MAP) (A). However, female participants demonstrated smaller changes in limb vascular conductance (LVC) (B). Sample size (n) is provided within each graph. Data are means ± SD.
Measures of BP variability are reported in Table 2. There were no sex differences in measures of resting beat-to-beat systolic BP ARV or resting beat-to-beat diastolic BP ARV. Additionally, in the subset of 28 participants who underwent 24-h ambulatory BP monitoring, there were no sex differences in measures of awake systolic BP ARV or awake diastolic BP ARV.
Table 2.
Measures of resting beat-to-beat blood pressure variability and awake ambulatory blood pressure variability in healthy young adults
| BP Variability Measure | Females | Males | P Value |
|---|---|---|---|
| Resting systolic BP ARV (n = 27) | 1.85 ± 0.48 | 1.85 ± 0.40 | 0.74 |
| Resting diastolic BP ARV (n = 27) | 1.57 ± 0.47 | 1.30 ± 0.48 | 0.14 |
| Awake systolic BP ARV (n = 14) | 11.32 ± 2.36 | 9.79 ± 2.23 | 0.18 |
| Awake diastolic BP ARV (n = 14) | 8.38 ± 1.56 | 8.17 ± 1.89 | 0.99 |
Data are means ± SD. ARV, average real variability; BP, blood pressure.
DISCUSSION
The primary novel finding of this study was a strong inverse relation between resting MSNA and indices of sympathetic vascular transduction in male participants, such that those with higher resting MSNA had smaller peak increases in MAP and peak declines in LVC, and vice versa. However, there was no such relation in female participants. To our knowledge, this is the first study to investigate sex differences in the regulation of transduction using beat-to-beat changes in LVC. The sex difference reported here builds on previous literature suggesting a negative relation between resting MSNA and successive vasoconstrictor responses (35). However, to our knowledge, this is the first study to report a sex difference in the relation between resting MSNA and indices of sympathetic vascular transduction.
Previous studies have demonstrated an important role of sex in the integrative balance between resting MSNA and BP. For example, while there is inter-individual variability, Joyner and colleagues (4, 5, 16) have reported a negative relation between cardiac output (CO) and MSNA, and a positive relation between total peripheral resistance (TPR) and MSNA within a group of healthy normotensive young males. In contrast, these studies have demonstrated no relation between CO or TPR and MSNA within a group of young healthy normotensive females (13, 16). Other studies have also demonstrated reduced autonomic support of BP in young females despite demonstrating similar systemic α1-adrenergic vascular responsiveness (i.e., BP responses to phenylephrine) to their male counterparts (6). Studies have also demonstrated that β-adrenergic receptors, which elicit norepinephrine-dependent vasodilatation, may blunt the vasoconstrictor effect of resting MSNA in young females, but not older females, suggesting an important role of female reproductive hormones (1, 14, 19). It is important to note these prior studies used time-averaged data in contrast to beat-to-beat measures; however, taken together, these findings supported our thinking that β-adrenergic-mediated dilation may buffer changes in α1-adrenergic vascular responsiveness across a range of MSNA in young females. An alternative explanation could be that males with higher levels of MSNA exhibit greater α1-adrenergic receptor desensitization than females with higher levels of MSNA, but the underlying mechanism for this potential explanation remains unclear.
In contrast to our secondary hypothesis, there were no sex differences in BP ARV. We speculated that there would be sex differences in BP ARV due to previous studies that have reported similar sympathetic transduction between sexes (33) as well as previous studies that reported that young males have greater levels of resting MSNA (6, 24, 25). Our rationale was that females, who have lower burst incidence, would experience fewer BP excursions over a given number of cardiac cycles. This seems plausible given that in healthy young adults cardiac cycles not preceded by MSNA exhibit very minor fluctuations in BP (ΔMAP = ≤1 mmHg) compared with cycles preceded by MSNA (ΔMAP = 3–4 mmHg) (33). Thus we speculated much of the variability in BP could be attributable to excursions in BP following bursts of MSNA. Indeed, we found similar sympathetic transduction between sexes and differences in burst incidence between males and females. Furthermore, we examined correlations between peak changes in MAP and resting systolic BP ARV and diastolic BP ARV (P < 0.05 for both). Although the relations were significant, the coefficients of determination were only 0.16 and 0.32, respectively (data not displayed in graphs), and BP variability is recognized as a complex phenomenon regulated by several inputs (26). For example, Hart et al. (18) showed changes in BP via the arterial baroreflex are associated with resting MSNA in young males but not in young females. Therefore, several factors not measured here likely contribute to BP variability and may explain why we did not observe sex differences in ARV.
Others have quantified sympathetic vascular transduction by using MSNA burst-triggered averaging to define the time course and magnitude of cardiovascular responses (MAP and LVC) to multiunit postganglionic nerve bursts on a beat-to-beat basis (9, 10, 34). A difference in the analysis used here was that each cardiac cycle containing a burst of MSNA was identified and set as cardiac cycle 0 whether or not the burst was a singlet (bursts directly bordered by >1 heartbeat lacking MSNA) or part of a cluster (bursts adjacent to other bursts of MSNA). Previous analyses have treated clusters of bursts as a single event, thus calculating changes in MAP and LVC for only the first burst of the cluster (not the average change following each burst of the cluster) (10). Although our focus was the relation of baseline MSNA and indices of sympathetic transduction within a group of males and females (i.e., within-group comparisons), we also examined between-sex differences in transduction (between group, males vs. females, Fig. 4). In agreement with Vianna et al. (33), we observed no differences in indices of transduction between the male and female participants tested. While initially counterintuitive, it is reasonable that across a spectrum of resting MSNA levels in both males and females, indices of transduction could center on a similar mean and have a different relation between resting MSNA and peak changes in MAP and LVC. In support of this, female participants with low and high MSNA presented peak changes in MAP and LVC that were not different. However, we show that compared with male participants with higher resting MSNA, male participants with low resting MSNA demonstrate greater peak changes in MAP and LVC. Taken together, these findings may explain why despite a sex difference in the relation between resting MSNA and peak changes in MAP and LVC, the male and female participants present similar sympathetic vascular transduction over 10 cardiac cycles. We report that MAP slightly decreases and LVC increases following non-bursts. These findings are in agreement with previous findings that MAP decreases and total vascular conductance increases following nonbursts (33). We also found that male participants demonstrated greater increases in LVC following nonbursts, but more studies are needed to confirm this observation.
The findings reported here are in agreement with several prior studies demonstrating that MSNA regulates vascular tone on a beat-to-beat time scale (9, 10). It is important to note differences in the temporal resolution of data analyses between previous studies investigating sex-specific interactions between MSNA and BP and this investigation. We measured beat-to-beat changes in MAP and LVC, whereas previous studies examined time-averaged responses from longer term steady-state periods (≥30 s epochs) (13, 17). Given this difference in time resolution, the inverse relation between resting MSNA and indices of sympathetic vascular transduction here and the positive relation between resting MSNA and TPR in previous studies are not in opposition as time-averaged methods would not be adequate to assess beat-to-beat relations. Additionally, the feedback mechanisms over these different time spans likely differ (e.g., adrenergic receptor sensitivity vs. baroreflex mechanisms).
This investigation advances our knowledge on sympathetic transduction by elucidating a sex difference in the relation between resting MSNA and corresponding changes in MAP and vascular conductance following nerve bursts in healthy young adults. However, limitations of our study include the following: our conclusions are restricted to healthy young adults, and there is reason to think the sex difference found here would be altered in older populations. Postmenopausal females lose the protective effects of estrogen, and sympathetic nerve activity increases with age, with resting MSNA becoming more tightly coupled to BP (13, 16, 17, 24). Aging studies also suggest that increases in resting MSNA (8, 31) may lead to desensitization of α-adrenergic vasoconstriction (7, 33). An alternative explanation is that differences in α-adrenergic responsiveness (due to age, genetics, and/or environmental inputs) may make some individuals’ blood vessels less responsive to α-adrenergic agonists leading to a compensatory higher resting MSNA, although more research is needed to elucidate mechanisms that contribute to vasoconstrictor responsiveness. Additional pharmacological studies are needed to determine if β-adrenergic blockade alters the relation between resting MSNA and indices of sympathetic transduction in females, resulting in a pattern similar to young males. Nonetheless, a strength of our study was quantifying endogenous sympathetic-mediated vascular control on a beat-to-beat basis.
Perspectives and Significance
The primary finding of this study was that biological sex influences the relation between resting MSNA and indices of sympathetic vascular transduction. There was a strong inverse relation between resting MSNA and peak changes in MAP and vascular conductance in healthy young male but not female adults. We also determined the influence of high versus low resting MSNA status on peak changes in MAP and vascular conductance. Only male participants demonstrated a significant difference in ΔMAP and ΔLVC when comparing high vs. low resting MSNA groups. Understanding sex-specific differences in BP regulation is imperative given that BP dysregulation is a critical public health issue. We conclude that there is a sex-specific relation between resting MSNA and beat-to-beat neurovascular transduction.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 HL128388 (to W. B. Farquhar), NIH Grant P20 GM113125 and American Heart Association Grant 18POST34060020 (to A. T. Robinson), and a University of Delaware Doctoral Fellowship (to J. C. Watso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.R., M.C.B., J.C.W., and W.B.F. conceived and designed research; A.T.R., M.C.B., J.C.W., M.S.B., K.U.M., M.M.W., and W.B.F. performed experiments; A.T.R., M.C.B., J.C.W., and M.S.B. analyzed data; A.T.R., M.C.B., J.C.W., M.S.B., K.U.M., M.M.W., and W.B.F. interpreted results of experiments; A.T.R. prepared figures; A.T.R. and W.B.F. drafted manuscript; A.T.R., M.C.B., J.C.W., M.S.B., K.U.M., M.M.W., and W.B.F. edited and revised manuscript; A.T.R., M.C.B., J.C.W., M.S.B., K.U.M., M.M.W., and W.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank nurses Wendy Nichols and Carolyn Haines for technical assistance; research dietitian Sofia Sanchez for diet planning, preparation, and analysis; laboratory coordinator Liza Walker for scheduling/recruitment assistance; and all of the study participants for their time and commitment to the study.
REFERENCES
- 1.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boggia J, Asayama K, Li Y, Hansen TW, Mena L, Schutte R. Cardiovascular risk stratification and blood pressure variability on ambulatory and home blood pressure measurement. Curr Hypertens Rep 16: 470, 2014. doi: 10.1007/s11906-014-0470-8. [DOI] [PubMed] [Google Scholar]
- 3.Brian MS, Matthews EL, Watso JC, Babcock MC, Wenner MM, Rose WC, Stocker SD, Farquhar WB. The influence of acute elevations in plasma osmolality and serum sodium on sympathetic outflow and blood pressure responses to exercise. J Neurophysiol 119: 1257–1265, 2018. doi: 10.1152/jn.00559.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568: 315–321, 2005. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498, 2005. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. doi: 10.1161/01.CIR.0000028819.64790.BE. [DOI] [PubMed] [Google Scholar]
- 8.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3: 201–205, 1993. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 9.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC II, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. doi: 10.1113/jphysiol.2013.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 12.Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp Physiol 98: 1422–1431, 2013. doi: 10.1113/expphysiol.2013.073189. [DOI] [PubMed] [Google Scholar]
- 13.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 29: 8–15, 2014. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- 14.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension 54: 127–133, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589: 3395–3404, 2011. doi: 10.1113/jphysiol.2011.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, Joyner MJ. Forearm vasodilator responses to a β-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol Rep 2: e12032, 2014. doi: 10.14814/phy2.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. doi: 10.1016/S0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 21.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J Am Heart Assoc 6: e006895, 2017. doi: 10.1161/JAHA.117.006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.CIR.101.8.862. [DOI] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 25.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. doi: 10.1161/01.HYP.21.4.498. [DOI] [PubMed] [Google Scholar]
- 26.Schillaci G, Parati G. Determinants of blood pressure variability in youth: at the roots of hypertension. J Hypertens 28: 660–664, 2010. doi: 10.1097/HJH.0b013e3283391950. [DOI] [PubMed] [Google Scholar]
- 27.Seals DR. Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension 17: 36–43, 1991. doi: 10.1161/01.HYP.17.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol (1985) 66: 2472–2478, 1989. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 30.Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol 119: 1731–1744, 2018. doi: 10.1152/jn.00841.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272: 383–397, 1977. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293–302, 1982. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 36.Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic significance of blood pressure variability on beat-to-beat monitoring after transient ischemic attack and stroke. Stroke 49: 62–67, 2018. doi: 10.1161/STROKEAHA.117.019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitley E, Ball J. Statistics review 1: presenting and summarising data. Crit Care 6: 66–71, 2002. doi: 10.1186/cc1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004. doi: 10.1113/jphysiol.2004.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeoh M, McLachlan EM, Brock JA. Tail arteries from chronically spinalized rats have potentiated responses to nerve stimulation in vitro. J Physiol 556: 545–555, 2004. doi: 10.1113/jphysiol.2003.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]