Abstract
Patients suffering from heart failure with reduced ejection fraction (HFrEF) experience impaired limb blood flow during exercise, which may be due to a disease-related increase in α-adrenergic receptor vasoconstriction. Thus, in eight patients with HFrEF (63 ± 4 yr) and eight well-matched controls (63 ± 2 yr), we examined changes in leg blood flow (Doppler ultrasound) during intra-arterial infusion of phenylephrine (PE; an α1-adrenergic receptor agonist) and phentolamine (Phen; a nonspecific α-adrenergic receptor antagonist) at rest and during dynamic single-leg knee-extensor exercise (0, 5, and 10 W). At rest, the PE-induced reduction in blood flow was significantly attenuated in patients with HFrEF (−15 ± 7%) compared with controls (−36 ± 5%). During exercise, the controls exhibited a blunted reduction in blood flow induced by PE (−12 ± 4, −10 ± 4, and −9 ± 2% at 0, 5, and 10 W, respectively) compared with rest, while the PE-induced change in blood flow was unchanged compared with rest in the HFrEF group (−8 ± 5, −10 ± 3, and −14 ± 3%, respectively). Phen administration increased leg blood flow to a greater extent in the HFrEF group at rest (+178 ± 34% vs. +114 ± 28%, HFrEF vs. control) and during exercise (36 ± 6, 37 ± 7, and 39 ± 6% vs. 13 ± 3, 14 ± 1, and 8 ± 3% at 0, 5, and 10 W, respectively, in HFrEF vs. control). Together, these findings imply that a HFrEF-related increase in α-adrenergic vasoconstriction restrains exercising skeletal muscle blood flow, potentially contributing to diminished exercise capacity in this population.
Keywords: blood flow, exercise, heart failure, neurohumoral, sympathetic
INTRODUCTION
Heart failure (HF) with reduced ejection fraction (HFrEF) is associated with debilitating dyspnea and fatigue during tasks of everyday living, limiting the ability for physical exertion and impairing quality of life (48). Interestingly, while systolic ventricular dysfunction is, by definition, a fundamental characteristic of HFrEF, it does not fully explain the severe exercise intolerance experienced by this patient group (9, 24, 32, 51, 54). Indeed, the importance of disease-related adaptations in the peripheral circulation has become increasingly recognized in HFrEF. Interestingly, there is evidence for (31, 49, 56) and against (21, 56) a disease-related decrement in exercising skeletal muscle blood flow in patients with HFrEF, a discrepancy that is likely the result of differences in medical management, exercise modality, and disease severity. Using small muscle mass exercise that does not provoke significant cardiopulmonary stress, our group recently identified a marked reduction in limb blood flow during both handgrip and knee-extensor (KE) exercise in patients with HFrEF on optimized pharmacotherapy compared with healthy, age-matched controls (8). Considering the lack of evidence for alterations in perfusion pressure during exercise in patients with HFrEF (35, 43, 48), a blunted rise in vascular conductance is likely the primary contributing factor to an attenuated exercise hyperemia in this population.
A likely culprit inhibiting the rise in vascular conductance during exercise in HFrEF is the underlying increase in sympathetic nervous system (SNS) activity and norepinephrine (NE) spillover across the active muscle bed in these patients (14, 20, 29, 53). In an effort to better understand the end-organ expression of this exaggerated sympathoexcitation in HFrEF, a small number of studies have investigated the effective changes in resting vasomotor tone induced by local intra-arterial infusion of α-adrenergic receptor agonist and antagonist drugs. At rest, a qualitatively greater percent increase in forearm blood flow has been documented in response to brachial artery infusion of the nonspecific α-adrenergic receptor antagonist phentolamine (Phen) in patients with HFrEF than in healthy controls (61). Additionally, similar dose-dependent reductions in forearm blood flow were observed in response to subsequent administration of both phenylephrine (PE) and BHT-993 (selective α1- and α2-adrenegeric receptor agonist drugs, respectively) in the presence of significantly elevated plasma NE (25). Together, these studies indicate a heightened contribution of α-adrenergic receptor vasoconstriction to forearm vascular tone in patients with HFrEF, coupled with an apparent lack of desensitization of α-adrenergic receptors of the forearm vasculature, that might be expected in the presence of chronically elevated sympathoexcitation (25).
These findings, at rest, raise the following question: could the exaggerated sympathoexcitation experienced by patients with HFrEF (33, 35, 43, 46), coupled with the preserved α-adrenergic receptor responsiveness (25), generate a degree of vasoconstriction in the exercising skeletal muscle vasculature that could attenuate exercise hyperemia in this cohort? Using intra-arterial infusion of Phen to locally abolish sympathetic restraint, Zelis et al. (61) documented that α-adrenergic receptor-mediated vasoconstriction plays a greater role in restraining blood flow during handgrip exercise in patients with HFrEF than in control subjects, although this response was not evident in subsequent studies using a similar pharmacological approach during maximal upright cycling (55). However, to our knowledge, the degree to which α-adrenergic receptor responsiveness is reduced during exercise to optimize blood flow to exercising skeletal muscle, a phenomenon termed “functional sympatholysis” (37), has yet to be determined in patients with HFrEF.
Thus the present study sought to further determine the role of α1-adrenergic receptor responsiveness and endogenous α-adrenergic tone in the regulation of skeletal muscle blood flow in the leg at rest and to determine if these drug-induced changes in vascular tone and blood flow differ as a consequence of exercise in patients with HFrEF and healthy age-matched controls. We hypothesized that 1) at rest, vasoconstriction in response to intra-arterial infusion of an α1-adrenergic receptor agonist (PE) would reduce leg blood flow to a similar degree in patients with HFrEF and controls; 2) at rest, the increase in leg blood flow in response to infusion of an α-adrenergic antagonist drug (Phen) would be greater in patients with HFrEF than controls; 3) during exercise, patients with HFrEF would exhibit a sustained responsiveness to PE infusion into the exercising limb, indicating attenuated functional sympatholysis in patients with HFrEF compared with controls; and 4) during exercise, patients with HFrEF would experience a greater vasodilation to Phen infusion into the exercising limb, indicating a heightened sympathetic restraint of exercising limb blood flow in patients with HFrEF compared with controls.
METHODS
Subjects
Eight New York Heart Association (NYHA) class II–III HFrEF patients (63 ± 4 yr, n = 7 men and 1 woman) and eight healthy, age- and sex-matched control subjects (63 ± 2 yr, n = 7 men and 1 woman) were recruited either by word of mouth or in the HF clinics at the University of Utah Health Sciences Center and the Salt Lake City Veterans Affairs Medical Center. All age-matched control subjects were nonsmokers, were not taking prescription medication, and were free from overt cardiovascular disease, as indicated by a health history questionnaire. Protocol approval and written informed consent were obtained according to University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Board requirements. All data were collected at the Utah Vascular Research Laboratory located at the Veterans Affairs Salt Lake City Geriatric, Research, Education, and Clinical Center.
Experimental Design
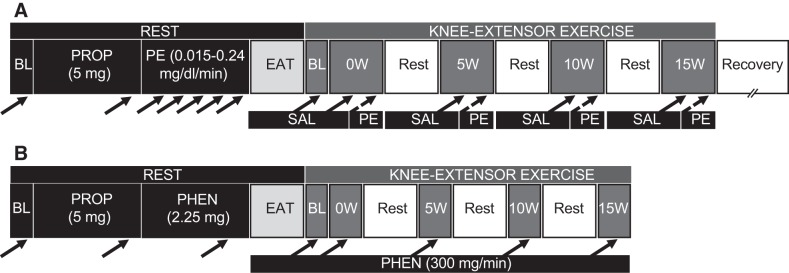
A schematic outline of the overall protocol design is illustrated in Fig. 1. All studies were performed in a thermoneutral environment, with subjects reporting to the laboratory fasted and not having performed any exercise within 24 h of the study. Subjects reported to the laboratory on a preliminary day to complete a health history questionnaire and physical examination, perform a graded single-leg KE exercise test to determine maximal work rate, and to determine thigh volume for the calculation of drug dosing (5, 27). Subjects returned to the Utah Vascular Research Laboratory within 2 wk of this preliminary visit at 0800 for the experimental day. After 20 min of supine rest, two catheters [one in the common femoral artery (CFA) and the other in the common femoral vein (CFV)] were placed using sterile technique, as described previously (2, 6, 7). After catheter placement, subjects rested for ~30 min and then were seated in a KE ergometer in a semirecumbent (60° reclined) position. After baseline measurements, propranolol (Prop) was administered intravenously to achieve systemic β-adrenergic receptor blockade in the control group. Prop was not administered in the patients with HFrEF because of the presence of nonspecific β-adrenergic receptor antagonists in all the patients’ respective regimen of daily medication. After Prop administration, the α1-adrenergic receptor agonist PE was administered. Subjects were then given a small, standardized meal (1/2 cup of corn flakes and 1/2 cup of skim milk) that has been shown to minimally affect leg blood flow at rest and during exercise (7). Subjects then completed four bouts of KE exercise, with PE administered during the final 2 min of each exercise stage. After recovery from exercise, a second Prop bolus was administered intravenously (controls only). Phen was then administered for 10 min followed by a maintenance dose that continued for the remainder of the protocol. Participants consumed a second small meal, after which an additional bout of KE exercise was performed. Because of the long-lasting effects of Phen, this portion of the protocol always occurred after the PE component of the study.
Fig. 1.
Study timeline. A: after baseline (BL) measurements, a propranolol (Prop) bolus was administered intravenously (to controls only) followed by a resting infusion of phenylephrine (PE). Participants then consumed a small meal, after which knee-extensor exercise was performed (4 bouts, 5 min per bout, with 5-min rest periods). Saline (Sal) was infused at rest and during the first 3 min of each exercise bout, and flow-adjusted doses of PE were administered during the final 2 min of each exercise bout. Solid black arrows indicate the time point for assessment of femoral artery blood flow, arterial and venous blood pressure, and heart rate; dashed black arrows indicate PE infusion and subsequent assessment of femoral artery blood flow, arterial and venous blood pressure, and heart rate. B: after 60 min of rest following the protocol described in A, baseline measurements were obtained and a second Prop bolus was administered intravenously (to controls only). Phentolamine (Phen) was administered for 10 min followed by a maintenance dose that continued for the remainder of the protocol. Participants then consumed a small meal, after which a second bout of knee-extensor exercise was performed (4 bouts, 3 min per bout, with 5-min rest periods).
Study Drugs
Prop (propranolol hydrochloride; APP Pharmaceuticals, Schaumburg, IL) was administered to the controls as the nonselective β-adrenergic receptor antagonist to minimize confounding effects of β-adrenergic receptor stimulation. Prop was prepared at a concentration of 0.5 mg/ml in 0.9% sterile saline, and 10 ml (5 mg of Prop) were administered intravenously over the course of 30 s before the beginning of the PE and Phen protocols (Fig. 1). This dose has been documented to block β-adrenergic receptors in the peripheral circulation, ablating the forearm vasodilation response to infusion of epinephrine (26). As previously stated, Prop was not administered to patients with HFrEF.
PE (Sigma-Aldrich, St. Louis, MO) was administered as a selective α1-adrenergic receptor agonist. PE was prepared at a concentration of 2.5 μg/ml of 0.9% sterile saline. At rest, PE was infused intra-arterially (0.015, 0.03, 0.06, 0.12, and 0.24 µg·min−1·dl thigh volume−1, 2 min per dose) via a constant-speed infusion pump (Harvard Apparatus, Holliston, MA). During exercise, real-time CFA blood flow of the exercising leg was determined by Doppler ultrasound before PE infusion, and infusion rate was adjusted to blood flow according to these “on-the-fly” blood flow values to a blood flow-adjusted rate of 8.3 ng/ml of leg blood flow (0.5–15 ml/min) to ensure a similar effective concentration of the drug at rest and during exercise (Fig. 1A). This flow-adjusted dose is similar to the 0.12 µg·min−1·dl thigh volume−1 dose of PE administered at rest, which we previously reported as sufficient to elicit maximal PE-induced vasoconstriction while limiting the risk of systemic spillover during the high infusion rates that occur during single-leg KE exercise (57).
Phentolamine mesylate (Phen, Regitine, Bedford Laboratories, Bedford, OH) was administered as the nonselective α-adrenergic receptor antagonist. Phen was prepared at a concentration of 0.3 mg/ml in 0.9% sterile saline and infused intra-arterially for 10 min (total dose 2.25 mg) (Fig. 1B) followed by a maintenance dose (300 µg/min) that continued for the remainder of the protocol. This dose exceeds the dose that has previously been documented to achieve complete α-adrenergic blockade, as confirmed by an α-adrenergic agonist challenge (18).
Knee-Extensor Exercise
The single-leg KE paradigm that was implemented in this study has been described previously (4, 5, 7, 28, 38, 60). Briefly, subjects were seated in a semirecumbent position on an adjustable chair with a cycle ergometer (model 828E, Monark Exercise, Vansbro, Sweden) positioned behind them. Resistance was created by application of friction to the flywheel, which was turned by the subject via a metal bar connecting the crank arm of the ergometer to a metal boot in which the subject’s foot was placed. Subjects exercised for 3 min at 0, 5, and 10 W, maintaining 60 contractions per minute, with measurements and blood samples taken during the 3rd min of each stage. During the PE trial (Fig. 1A), subjects exercised for an additional 2 min per stage while PE was infused, with PE-induced measurements taken during the 2nd min of infusion. Additionally, the preinfusion and PE-infusion portions of each work rate were divided into two separate 3-min stages if the subject could not exercise for the full 5 min. The patients were allowed to recover for 5 min following each stage; the rest period was extended for subjects who required more rest to complete the predetermined stages.
Measurements
Doppler ultrasound assessments.
CFA blood velocity and vessel diameter in the infused leg were measured using a Doppler ultrasound system (Logiq 7, GE Medical Systems, Milwaukee, WI) operating in duplex mode. The Logic 7 was equipped with a linear-array transducer operating at an imaging frequency of 14 MHz. The CFA was insonated 2–3 cm proximal to its bifurcation into the superficial and deep branches. The blood velocity profile was obtained using the same transducer with a Doppler frequency of 5 MHz operated in the high pulsed repetition frequency mode (2–25 kHz). Scale adjustments were used, especially during exercise, to avoid aliasing the blood velocity spectra. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of ≤60° (30). The sample volume was maximized according to vessel size and was centered within the vessel on the basis of real-time ultrasound visualization. At all sample points, arterial diameter (cm) and angle-corrected, time-averaged, and intensity-weighted mean blood velocity (Vmean, cm/s) values were calculated using commercially available software (Logiq 7). Measured arterial diameter and Vmean were used to calculate leg blood flow according to the following equation: leg blood flow (ml/min) = Vmean × π × (arterial diameter/2)2 × 60.
Blood pressure, vascular conductance, and heart rate assessment.
Arterial and venous blood pressure measurements were collected continuously from the indwelling catheters placed in the CFA and CFV, with the pressure transducers placed at the level of the catheters (Transpac IV, Abbott Laboratories). Mean arterial pressure (MAP) was calculated as follows: MAP (mmHg) = diastolic arterial pressure + (arterial pulse pressure × 0.33). Leg perfusion pressure was calculated as follows: leg perfusion pressure (mmHg) = MAP – venous pressure. Leg vascular conductance was calculated as follows: leg vascular conductance (ml·min−1·mmHg−1) = leg blood flow/leg perfusion pressure. Heart rate (HR) was monitored from a standard three-lead ECG recorded in duplicate on the data acquisition device (Biopac, Goleta, CA) and the Logiq 7.
Blood chemistry.
A lipid panel was obtained for all subjects by standard techniques. In the last 30 s of each exercise stage, femoral arterial and venous blood samples (3–4 ml) were collected. A blood-gas analyzer (GEM 4000) and CO-oximeter (Instrumentation Laboratories, Bedford, MA) and 1 ml of anaerobic arterial and venous blood were used to obtain arterial and venous total hemoglobin (tHb), oxyhemoglobin saturation (So2), partial pressure of O2 (Po2), and hematocrit (Hct). Arterial and venous blood O2 content ( and ) was calculated as follows: blood O2 content (ml/dl) = 1.39 (tHb) × (So2/100) + 0.003 × Po2. Leg O2 consumption (V̇o2) was calculated as follows: leg V̇o2 (ml/min) = ( − ) × leg blood flow. Leg O2 delivery was calculated as follows: leg O2 delivery (ml/min) = leg blood flow × .
Anthropometrics.
Thigh length (l), circumferences (O1, O2, and O3), and skinfold thickness (S) were measured, and thigh volume (L) was calculated as follows: L = l·(12π)−1·(O12 + O22 + O32) – (S − 0.4)·2−1·l·(O1 + O2 + O3)·2−1.
Data Analysis
Drug-induced changes were calculated as follows: drug-induced change (Δ) = drug trial value – preinfusion value, or drug-induced percent change (%Δ) = [drug-induced change (Δ)/preinfusion value] × 100. When exercise was compared with resting drug-induced changes for the PE trial, the response to the fourth dose of the PE dose response was always used, as this dose was similar to the concentration of drug infused during exercise. The control condition (i.e., preinfusion), with which the exercise portion of Phen trial was compared, was the preinfusion portion of the PE trial (Fig. 1). Statistics were performed using commercially available software (SigmaStat 3.10, Systat Software, Point Richmond, CA). Repeated-measures ANOVA (α < 0.05) and Student’s t-test were used to identify significant changes in measured variables within and between drug groups and across exercise intensities. The Holm-Sidak method was used for α adjustment and post hoc analysis. All group data are expressed as means ± SE. Significance was established at P < 0.05.
RESULTS
Subject Characteristics
Baseline characteristics of the control subjects and patients with HFrEF are displayed in Table 1. Disease-specific characteristics and medications of patients with HFrEF are presented in Table 2.
Table 1.
Subject characteristics
| Control (n = 8) | HFrEF (n = 8) | |
|---|---|---|
| Age, yr | 63 ± 2 | 63 ± 4 |
| Height, cm | 174 ± 4 | 174 ± 3 |
| Weight, kg | 76 ± 7 | 82 ± 4 |
| Body mass index, kg/m2 | 26 ± 1 | 27 ± 1 |
| Thigh volume, liters | 7.7 ± 0.5 | 5.9 ± 0.1* |
| Quadriceps muscle mass, kg | 2.7 ± 0.1 | 2.2 ± 0.0* |
| Systolic blood pressure, mmHg | 120 ± 3 | 120 ± 6 |
| Diastolic blood pressure, mmHg | 79 ± 2 | 77 ± 3 |
| MAP, mmHg | 93 ± 3 | 91 ± 4 |
| KEmax, W | 33 ± 6 | 18 ± 2* |
| Glucose, mg/dl | 86 ± 4 | 99 ± 8 |
| Total cholesterol, mg/dl | 195 ± 14 | 139 ± 19* |
| Triglycerides, mg/dl | 117 ± 24 | 103 ± 10 |
| HDL, mg/dl | 47 ± 4 | 37 ± 3 |
| LDL, mg/dl | 131 ± 13 | 86 ± 15* |
Values are means ± SE; n = number of subjects. HFrEF, heart failure with reduced ejection fraction; MAP, mean arterial pressure; KEmax, maximum knee-extensor exercise; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 vs. control.
Table 2.
Disease-specific characteristics and medications
| HFrEF (n = 8) | |
|---|---|
| Disease-specific characteristics | |
| Left ventricular ejection fraction, % (mean ± SE) | 30 ± 3 |
| Diagnosis | |
| Ischemic | 6/8 |
| Nonischemic | 2/8 |
| NYHA class | |
| II | 4/8 |
| III | 4/8 |
| Diabetic | 4/8 |
| Medications | |
| β-Blocker | 8/8 |
| ACE inhibitor | 6/8 |
| Angiotensin receptor inhibitor | 2/8 |
| Statin | 7/8 |
| Diuretic | 8/8 |
| Aldosterone inhibitor | 2/8 |
| Digoxin | 1/8 |
| Anticoagulant | 7/8 |
n = number of subjects. HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme.
β-Adrenergic receptor antagonism.
β-Adrenergic receptor blockade by intravenous infusion of Prop induced a significant reduction in HR, leg blood flow, and leg vascular conductance, with no marked change in leg perfusion pressure or CFA diameter (Table 3).
Table 3.
Hemodynamic responses to α1-adrenergic agonist (phenylephrine) administration at rest
| Phenylephrine Concentration, μg·min−1·dl thigh volume−1 |
|||||||
|---|---|---|---|---|---|---|---|
| BL | Propranolol (5 mg) | 0.015 | 0.03 | 0.06 | 0.12 | 0.24 | |
| Control | |||||||
| Leg blood flow, ml/min | 253 ± 33 | 227 ± 33# | 212 ± 33 | 185 ± 33# | 174 ± 33# | 142 ± 24# | 141 ± 19# |
| Leg vascular conductance, ml·min−1·mmHg−1 | 3.0 ± 0.4 | 2.7 ± 0.4# | 2.5 ± 0.4 | 2.2 ± 0.4# | 2.1 ± 0.4# | 1.6 ± 0.3# | 1.6 ± 0.2# |
| Leg perfusion pressure, mmHg | 85 ± 3 | 85 ± 3 | 83 ± 3 | 84 ± 3 | 85 ± 3 | 86 ± 3 | 87 ± 3 |
| CFA diameter | |||||||
| cm | 1.02 ± 0.06 | 1.02 ± 0.06 | 1.01 ± 0.06 | 0.99 ± 0.06# | 0.97 ± 0.07# | 0.97 ± 0.07# | 0.93 ± 0.7# |
| %Δ | 0.0 ± 0.1 | −0.6 ± 0.4 | −3.0 ± 1.7 | −4.8 ± 2.5# | −6.8 ± 2.9# | −9.6 ± 3.5# | |
| HR, beats/min | 60 ± 4 | 54 ± 3# | 54 ± 2 | 53 ± 3 | 53 ± 2 | 51 ± 2 | 52 ± 2 |
| HFrEF | |||||||
| Leg blood flow, ml/min | 218 ± 26 | 215 ± 20 | 202 ± 22 | 189 ± 19 | 179 ± 16# | 174 ± 18# | |
| Leg vascular conductance, ml·min−1·mmHg−1 | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.5 ± 0.3 | 2.3 ± 0.2# | 2.2 ± 0.3# | 2.1 ± 0.2# | |
| Leg perfusion pressure, mmHg | 80 ± 5 | 80 ± 5 | 82 ± 5 | 82 ± 5 | 83 ± 4 | 82 ± 3 | |
| CFA diameter | |||||||
| cm | 0.88 ± 0.05 | 0.87 ± 0.05 | 0.86 ± 0.05 | 0.84 ± 0.05# | 0.82 ± 0.05# | 0.80 ± 0.06# | |
| %Δ | −1.2 ± 0.3 | −2.6 ± 0.6 | −4.3 ± 0.8# | −6.2 ± 1.0# | −8.8 ± 2# | ||
| HR, beats/min | 67 ± 2 | 65 ± 2* | 66 ± 3* | 67 ± 2* | 68 ± 3* | 72 ± 6* | |
Values are means ± SE. In controls, propranolol was administered immediately before the phenylephrine dose response (0.015–0.24 μg·min−1·dl thigh volume−1, 2 min per dose) to minimize β-adrenergic effects. BL, baseline; HR, heart rate; CFA, common femoral artery; HFrEF, heart failure with reduced ejection fraction.
P < 0.05 vs. control;
P < 0.05 vs. preinfusion.
α1-Adrenergic receptor responsiveness at rest.
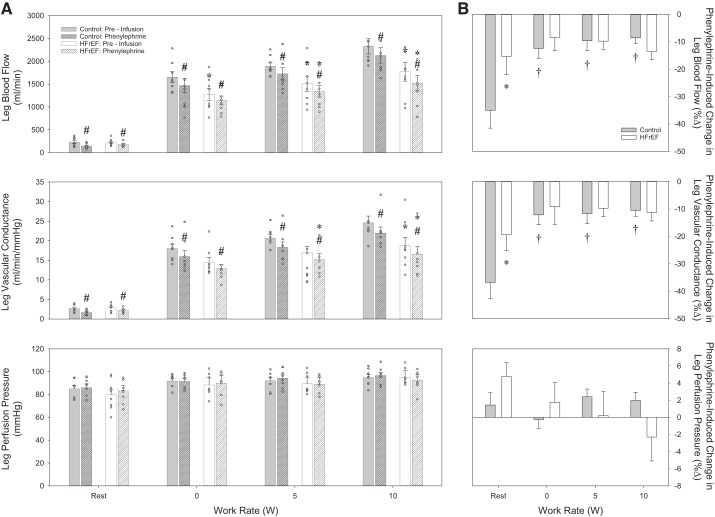
In the control and HFrEF groups, HR or perfusion pressure was not significantly changed by administration of the α1-adrenergic receptor agonist PE in increasing concentrations (Table 3). PE did provoke significant, dose-dependent reductions in leg blood flow, leg vascular conductance, and CFA diameter in either group (Figs. 2 and 4, and Table 3). However, compared with the control group, the HFrEF group exhibited a significantly smaller reduction in leg blood flow and leg vascular conductance (Fig. 2) induced by PE infusion.
Fig. 2.
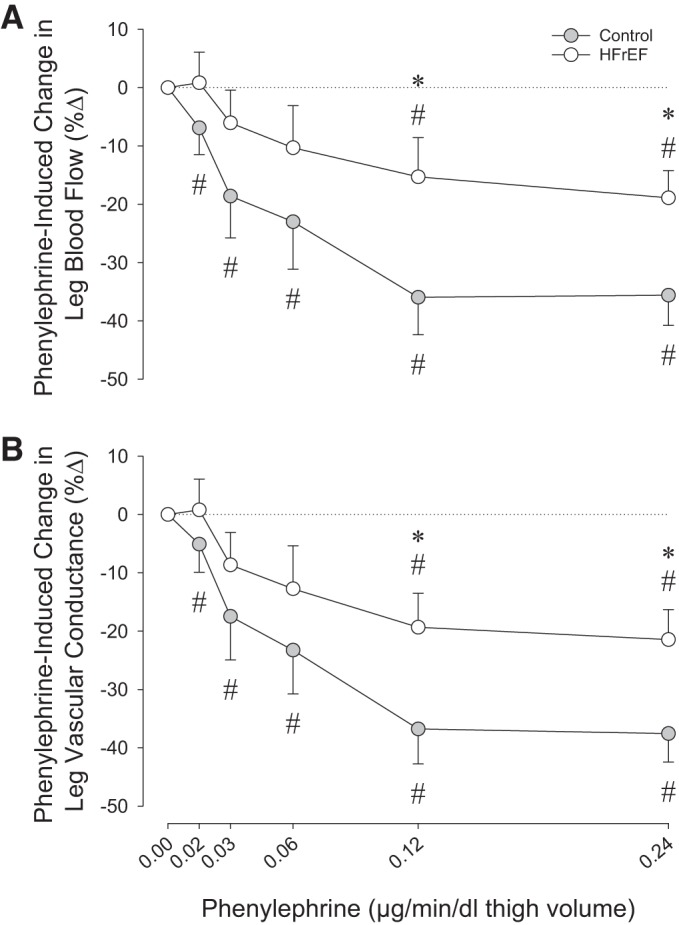
α1-Adrenergic agonist dose response. A and B: changes (%Δ) in resting leg blood flow and leg vascular conductance in response to local, intra-arterial infusion of phenylephrine in control subjects and heart failure patients with reduced ejection fraction (HFrEF). *P < 0.05 vs. control; #P < 0.05 vs. preinfusion.
Fig. 4.
α1-Adrenergic agonist drug infusion responses. A: absolute values for leg blood flow (top), leg vascular conductance (middle), and leg perfusion pressure (bottom) in control subjects and heart failure patients with reduced ejection fraction (HFrEF) at rest and during knee-extensor exercise in control conditions (preinfusion) and during phenylephrine infusion. B: percent changes (%Δ) in leg blood flow (top), leg vascular conductance (middle), and leg perfusion pressure (bottom) in response to phenylephrine in control subjects and patients with HFrEF at rest and during knee-extensor exercise. *P < 0.05 vs. control; #P < 0.05 vs. preinfusion; †P < 0.05 vs. rest.
Nonselective α-adrenergic receptor antagonism at rest.
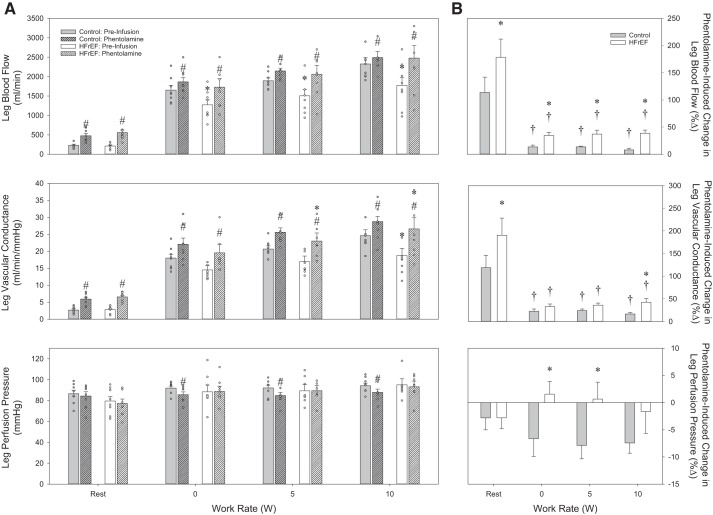
After 10 min of continuous infusion of Phen, no significant changes in HR or leg perfusion pressure were observed in either group (Table 4). Both groups demonstrated a marked increase in leg blood flow and leg vascular conductance in response to Phen (Figs. 3 and 5). However, the increase in these variables was significantly greater in the HFrEF group. Additionally, the increase in CFA diameter was significantly greater in patients with HFrEF than in control subjects (Table 4).
Table 4.
Hemodynamic responses to α-adrenergic antagonist (phentolamine) administration at rest
| BL | Prop (5 mg) | Phentolamine (2.25 mg) | |
|---|---|---|---|
| Control | |||
| Leg blood flow, ml/min | 234 ± 26 | 230 ± 27 | 477 ± 60# |
| Leg vascular conductance, ml·min−1·mmHg−1 | 2.7 ± 0.3 | 2.7 ± 0.3 | 5.7 ± 0.6# |
| Leg perfusion pressure, mmHg | 87 ± 3 | 86 ± 4 | 84 ± 4 |
| CFA diameter | |||
| cm | 1.02 ± 0.06 | 1.03 ± 0.05 | 1.05 ± 0.05 |
| %Δ | 1.1 ± 1.1 | 1.7 ± 1.2 | |
| HR, beats/min | 56 ± 3 | 54 ± 2 | 58 ± 3 |
| HFrEF | |||
| Leg blood flow, ml/min | 212 ± 24 | 557 ± 66# | |
| Leg vascular conductance, ml·min−1·mmHg−1 | 2.8 ± 0.3 | 7.4 ± 1.0*# | |
| Leg perfusion pressure, mmHg | 79 ± 4 | 77 ± 4 | |
| CFA diameter | |||
| cm | 0.88 ± 0.05 | 0.91 ± 0.06# | |
| %Δ | 4.1 ± 0.9*# | ||
| HR, beats/min | 67 ± 3* | 67 ± 3* |
Values are means ± SE. In controls, propranolol was administered immediately before phentolamine infusion (0.225 ml/min for 10 min) to minimize β-adrenergic effects. CFA, common femoral artery; HR, heart rate; HFrEF, heart failure with reduced ejection fraction.
P < 0.05 vs. control;
P < 0.05 vs. preinfusion.
Fig. 3.
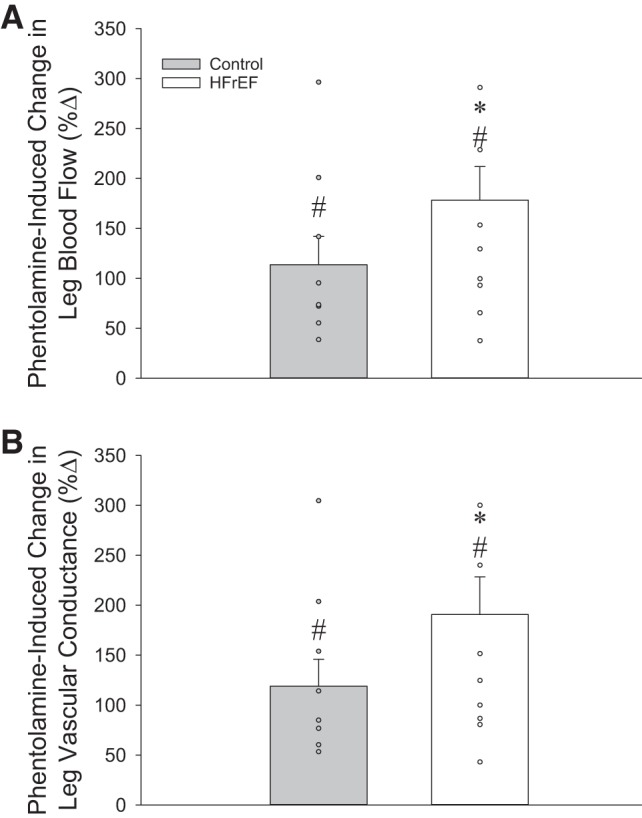
A and B: changes (%Δ) in resting leg blood flow and leg vascular conductance in response to local, intra-arterial infusion of phentolamine in control subjects and heart failure patients with reduced ejection fraction (HFrEF). *P < 0.05 vs. control; #P < 0.05 vs. preinfusion.
Fig. 5.
α-Adrenergic antagonist drug infusion responses. A: absolute values for leg blood flow (top), leg vascular conductance (middle), and leg perfusion pressure (bottom) in control subjects and heart failure patients with reduced ejection fraction (HFrEF) at rest and during knee-extensor exercise in control conditions (preinfusion) and during phentolamine infusion. B: percent changes (%Δ) in leg blood flow (top), leg vascular conductance (middle), and leg perfusion pressure (bottom) in response to phentolamine in control subjects and patients with HFrEF at rest and during knee-extensor exercise. *P < 0.05 vs. control; #P < 0.05 vs. preinfusion; †P < 0.05 vs. rest.
Cardiovascular responses to exercise.
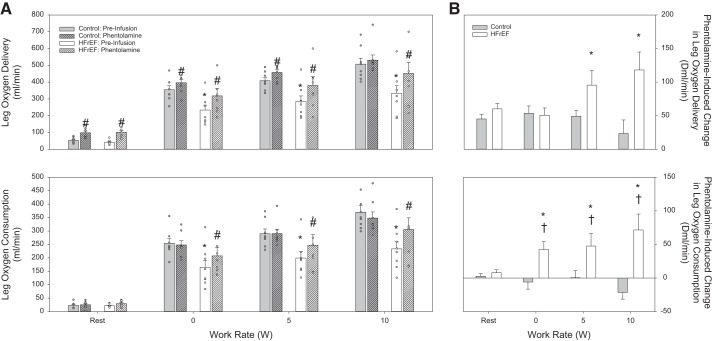
Intensity-dependent increases in leg blood flow, leg vascular conductance, and leg perfusion pressure were observed in both groups (Figs. 4A and 5A). However, the hyperemic and vasodilatory responses were attenuated in patients with HFrEF compared with control subjects (Figs. 3A and 4A, top and middle). This attenuated hyperemic response across work rates in patients with HFrEF contributed to a significantly lower leg O2 delivery (Fig. 6A, top), which, in combination with an attenuated leg arterial-venous O2 difference (Table 5), resulted in an attenuated leg V̇o2 (Fig. 6A, bottom) in the HFrEF group.
Fig. 6.
Leg O2 delivery and leg O2 consumption responses. A: absolute values for leg O2 delivery (top) and leg O2 consumption (bottom) in control subjects and heart failure patients with reduced ejection fraction (HFrEF) at rest and during knee-extensor exercise in control conditions (preinfusion) and during phentolamine infusion. B: phentolamine-induced changes (Δ) in leg O2 delivery (top) and leg O2 consumption (bottom) in control subjects and patients with HFrEF at rest and during knee-extensor exercise. *P < 0.05 vs. control; #P < 0.05 vs. preinfusion; †P < 0.05 vs. rest.
Table 5.
Cardiovascular responses to intra-arterial infusions of phenylephrine and phentolamine at rest and during exercise
| Exercise |
||||
|---|---|---|---|---|
| Rest | 0 W | 5 W | 10 W | |
| Preinfusion | ||||
| Control | ||||
| HR, beats/min | 55 ± 2 | 66 ± 3† | 70 ± 5† | 72 ± 5† |
| MAP, mmHg | 114 ± 3 | 123 ± 3 | 123 ± 3 | 126 ± 4 |
| Leg arterial-venous O2 difference, ml/dl | 9.3 ± 1.3 | 15.4 ± 0.6 | 15.4 ± 0.8 | 16.0 ± 0.6 |
| CFA diameter, cm | 1.02 ± 0.05 | 1.02 ± 0.05 | 1.02 ± 0.05 | 1.02 ± 0.05 |
| HFrEF | ||||
| HR, beats/min | 65 ± 3* | 84 ± 5*† | 86 ± 2*† | 92 ± 3*† |
| MAP, mmHg | 104 ± 5 | 119 ± 7† | 119 ± 6† | 125 ± 6† |
| Leg arterial-venous O2 difference, ml/dl | 10.1 ± 0.6 | 12.7 ± 1.1 | 13.4 ± 0.8 | 13.4 ± 0.8 |
| CFA diameter, cm | 0.88 ± 0.05 | 0.88 ± 0.05 | 0.88 ± 0.05 | 0.88 ± 0.05 |
| Phenylephrine | ||||
| Control | ||||
| HR, beats/min | 52 ± 2 | 65 ± 4† | 68 ± 6† | 71 ± 5† |
| MAP, mmHg | 114 ± 3 | 126 ± 3† | 125 ± 3† | 128 ± 3† |
| CFA diameter | ||||
| cm | 0.95 ± 0.07# | 1.00 ± 0.06† | 1.00 ± 0.05† | 1.01 ± 0.05† |
| %Δ | −6.8 ± 2.9 | −2.2 ± 0.7† | −2.2 ± 0.6† | −1.1 ± 0.5† |
| HFrEF | ||||
| HR, beats/min | 70 ± 6* | 81 ± 5* | 82 ± 4*† | 89 ± 2*† |
| MAP, mmHg | 107 ± 3 | 120 ± 7† | 120 ± 6† | 126 ± 4† |
| CFA diameter | ||||
| cm | 0.82 ± 0.05 | 0.83 ± 0.05* | 0.83 ± 0.05* | 0.83 ± 0.05 |
| %Δ | −6.2 ± 1.0# | −5.3 ± 1.2# | −4.1 ± 1.3# | −2.5 ± 1.0 |
| Phentolamine | ||||
| Control | ||||
| HR, beats/min | 58 ± 3 | 68 ± 5 | 74 ± 7† | 75 ± 6† |
| MAP, mmHg | 109 ± 3 | 114 ± 3#† | 116 ± 4#† | 117 ± 3# † |
| Leg arterial-venous O2 difference, ml/dl | 6.4 ± 1.6# | 13.5 ± 0.8# | 13.6 ± 0.8# | 14.1 ± 0.7# |
| CFA diameter, cm | 1.05 ± 0.05 | 1.05 ± 0.05 | 1.05 ± 0.05 | 1.05 ± 0.05 |
| HFrEF | ||||
| HR, beats/min | 67 ± 3* | 83 ± 6† | 88 ± 5† | 97 ± 6*† |
| MAP, mmHg | 102 ± 4 | 117 ± 5† | 120 ± 6† | 126 ± 6† |
| Leg arterial-venous O2 difference, ml/dl | 5.6 ± 0.6 | 12.0 ± 0.7 | 11.9 ± 0.9 | 12.3 ± 0.6 |
| CFA diameter, cm | 0.91 ± 0.06# | 0.91 ± 0.06# | 0.91 ± 0.06# | 0.91 ± 0.06# |
Values are means ± SE. HFrEF, heart failure with reduced ejection fraction; MAP mean arterial pressure, HR, heart rate; CFA, common femoral artery.
P < 0.05 vs. control;
P < 0.05 vs. preinfusion;
P < 0.05 vs. rest.
α1-Adrenergic responsiveness during exercise.
No significant changes in HR or leg perfusion pressure were observed after PE infusion (Table 5). PE significantly reduced leg blood flow and leg vascular conductance in both groups across increasing exercise intensities (Fig. 4A, top and middle). Similar PE-induced changes in leg blood flow and leg vascular conductance were also observed in both groups (Fig. 4B, top and middle). However, PE-induced changes were attenuated compared with rest only in the control group (Fig. 4B, top and middle). Interestingly, PE significantly reduced CFA diameter only in the HFrEF group during exercise (Table 5), and the degree of CFA vasoconstriction in the HFrEF group across exercise intensities was not different from that at rest (Table 5).
Nonselective α-adrenergic antagonism during exercise.
No significant changes in HR were observed before vs. after Phen infusion in either group (Table 5). Phen infusion contributed to a reduction in leg perfusion pressure in the control group (Fig. 5, bottom), while no significant changes in leg perfusion pressure were observed in the HFrEF group, during exercise (Fig. 5, bottom). Phen significantly increased leg blood flow and vascular conductance in both groups (Fig. 5A, top and middle), with the changes in leg blood flow and leg vascular conductance being significantly greater in patients with HFrEF than control subjects (Fig. 5B, top and middle). Despite the significant increases in leg blood flow and vascular conductance, these changes were significantly less than the response at rest in both groups (Fig. 5B, top and middle). In the control group, the Phen-induced increase in leg blood flow, coupled with a reduction in leg arterial-venous O2 difference (Table 5), resulted in similar leg V̇o2 values in the Phen trial and before infusion (Fig. 6, bottom). While Phen also induced a reduction in leg arterial-venous O2 difference in the patients with HFrEF (Table 5), the Phen-induced increase in leg blood flow was substantial enough to significantly increase leg V̇o2 in this group (Fig. 6, bottom).
DISCUSSION
This study sought to comprehensively examine the importance of α-adrenergic receptor-mediated vasoconstriction in the skeletal muscle vasculature of patients with HFrEF compared with age-matched control subjects at rest and during exercise. At rest, vasoconstriction in response to intra-arterial infusion of PE was significantly reduced in patients with HFrEF, suggesting a disease-related reduction in α1-adrenergic receptor responsiveness. α-Adrenergic receptor antagonism induced by intra-arterial infusion of Phen elicited a greater vasodilation in patients with HFrEF than in controls, supporting the concept of exaggerated sympathetic restraint of resting leg blood flow in this patient group. PE-induced vasoconstriction was reduced during exercise compared with at rest in the control group, confirming exercise-induced functional sympatholysis. In contrast, vasoconstriction to PE was unchanged compared with rest in patients with HFrEF, suggesting that the sympatholytic effect of exercise is impaired in this population. Additionally, Phen infusion during exercise increased leg blood flow to a greater extent in the HFrEF than the control group, indicating a greater sympathetic restraint in the leg vasculature during exercise. The Phen-induced increase in leg blood flow and the accompanying rise in O2 delivery increased leg V̇o2 in the HFrEF group, a response not evident in the control group. Together, these findings imply that disease-related changes in the α-adrenergic receptor pathway represent an important maladaptive process that restrains skeletal muscle blood flow and O2 delivery and, therefore, might contribute to diminished exercise capacity and exaggerated exercise intolerance in this patient group.
α-Adrenergic Receptor Regulation at Rest
Although patients with HFrEF present with a host of symptoms related to cardiac dysfunction, perhaps one of the most detrimental consequences of the disease is an elevation in SNS activity (29), which has implications in both the progression of HFrEF and the mortality of these patients (12). One of the most deleterious effects of heightened sympathoexcitation is the end-organ (i.e., α-adrenergic receptor) expression in the peripheral circulation, resulting in a substantial restraint of skeletal muscle blood flow (13). Thus one of the primary goals of the present study was to determine the role of the α-adrenergic receptors in regulation of skeletal muscle blood flow at rest in patients with HFrEF.
In the resting leg, administration of PE resulted in significant vasoconstriction in both groups. However, the magnitude of the PE-induced reduction in leg blood flow (Fig. 2, top) and the magnitude of leg vascular conductance (Fig. 2, bottom) were significantly blunted in patients with HFrEF compared with control subjects, suggesting a disease-related reduction in α1-adrenergic receptor responsiveness in patients with HFrEF. These findings are in contrast to one of the only previous human studies to examine α1-adrenergic receptor-mediated vasoconstriction in patients with HFrEF. Using multiple doses of PE infused into the brachial artery, Indolfi et al. (25) identified similar reductions in forearm blood flow in NYHA Class II–III HFrEF patients and young, healthy control subjects, despite significantly higher concentrations of plasma NE in patients with HFrEF. While the reasons for this discrepancy between the former and current studies are not immediately apparent, significant differences in methodology, drug dosing, and subject characteristics may explain the divergent findings. It is also noteworthy that the current study was performed in the leg, an important distinction, considering the evidence for nonuniform distribution of α-adrenergic receptors between limbs (36, 42). Thus, using a wide range of drug doses that produced a clear “plateau” in vasoconstriction at the highest doses (Fig. 2), we have identified, for the first time, a reduction in α1-adrenergic receptor-mediated responsiveness at rest in patients with HFrEF.
This reduction in α1-adrenergic receptor responsiveness is congruent with studies in other populations with elevated SNS activity, where a downregulation and/or desensitization of α-adrenergic receptors is observed in response to chronic sympathoexcitation. For example, in the elderly, a population in which SNS activity is also increased, our group (59) and others (15, 44) observed a blunted α-adrenergic receptor vasoconstriction following intra-arterial sympathomimetic drug infusions. This likely represents a protective response to the 200–300% increase in resting SNS activity often reported with advancing age (16, 34, 50). It is tempting to speculate that attenuation of the responsiveness of α1-adrenergic receptors in patients with HFrEF may be the consequence of a disease-associated increase in SNS activity (22, 29). However, an actual determination of a relationship between this diminution in α1-adrenergic receptor responsiveness and a decline in receptor sensitivity or density or changes in receptor distribution is beyond the scope of the present study.
To further explore disease-related changes in the α-adrenergic receptor pathway, we also administered a nonselective α-adrenergic antagonist (Phen) to pharmacologically ablate endogenous sympathetic tone in the resting leg. In support of our hypothesis, Phen infusion induced a greater increase in leg blood flow (Fig. 3, top) and leg vascular conductance (Fig. 3, bottom) at rest in patients with HFrEF than controls. These responses are in agreement with work in a pacing-induced animal model of HF, where intra-arterial infusion of Phen increased resting hindlimb vascular conductance by 50% in healthy control animals and by 226% in animals with HF (47). Similar observations have been documented in humans. Specifically, Zelis et al. (61) reported a greater percent increase in forearm blood flow in response to brachial artery infusion of Phen in patients with HFrEF than in healthy control subjects (61). More recently, Alves et al. (1) performed a similar experiment and reported a significantly greater forearm vasodilation and a corresponding increase in forearm blood flow in patients with HFrEF compared with healthy age-matched control subjects. Thus the current findings of an exaggerated vasodilation in response to “pharmacological sympathectomy” in the skeletal muscle vasculature of the leg confirm and extend previous work in patients with HFrEF, supporting the concept that endogenous α-adrenergic tone is elevated in this population.
α-Adrenergic Receptor Regulation During Exercise
During dynamic exercise, SNS activity increases in an effort to redistribute blood flow and optimize perfusion of the exercising skeletal muscle (41). Considering that patients with HFrEF experience an exaggerated increase in SNS activity during exercise (19), the α-adrenergic receptor pathway provides a logical starting point to probe the attenuated exercise-induced hyperemia observed in this patient group. Indeed, inhibition of sympathetic vasoconstriction via the metabolic by-products produced by exercising skeletal muscle, a phenomenon known as functional sympatholysis (37), is well described in both animals (37) and humans (39, 57). However, until now, no study has examined the sympatholytic effect of exercise in HFrEF.
During exercise, we observed a significant reduction in leg blood flow (Fig. 4A, top) and leg vascular conductance (Fig. 4A, middle) in response to PE administration in both groups across all work rates. However, compared with the respective responses observed at rest, the control group exhibited a “lysing” of α1-adrenergic receptor vasoconstriction (Fig. 4B, top and middle), whereas the HFrEF group demonstrated a preserved α1-adrenergic receptor vasoconstrictor response (Fig. 4B, top and middle). This new finding is in agreement with one of the only other studies to examine functional sympatholysis in HF. Specifically, using a coronary ligation model of HF in rats, Thomas et al. (52) examined changes in femoral artery vascular conductance in response to sympathetic stimulation during both a sham trial and involuntary contraction of the hindlimbs induced by electrical stimulation. The unequivocal finding from this study was that sympathetic vasoconstriction was preserved in these animals during hindlimb contraction compared with the sham trial, indicating an impaired sympatholysis in this animal model of HF. Thus, in the present study, we extend these preclinical data to patients with HFrEF and report, for the first time, a sustained α-adrenergic receptor vasoconstriction during exercise in this population.
To further investigate the sympathetic restraint of leg blood flow in patients with HFrEF during exercise, α-adrenergic receptor antagonism was implemented through intra-arterial infusion of Phen. Compared with the preinfusion condition, Phen administration increased leg blood flow (Fig. 5A, top) and leg vascular conductance (Fig. 5A, middle) in both groups across all work rates. However, somewhat contrary to our expectation, leg perfusion pressure was reduced in the control group during the Phen trial but remained unchanged in the HFrEF group (Fig. 5, bottom). Thus, in the control group, this reduction in perfusion pressure during exercise may have contributed to the attenuated increase in leg blood flow, somewhat overestimating the between-group differences in leg blood flow in response to Phen (Fig. 5, top). However, even when this pressure change was taken into account by calculation of leg vascular conductance (Fig. 5, middle), Phen-induced changes during exercise remain significantly different between groups (main effect for group), particularly at the higher exercise intensities. Together, these data indicate a greater role and capacity of the α-adrenergic receptors in restraining blood flow and, ultimately, O2 delivery in this patient group.
These changes in response to α-adrenergic inhibition are in agreement with animal studies utilizing a similar pharmacological approach in a pacing-induced HF model. Indeed, Stickland et al. (47) observed a substantially greater increase in hindlimb blood flow following Phen administration during treadmill running in HF than control animals, indicating an augmented α-adrenergic receptor restraint of hindlimb blood flow. Compared with this observation in animals, the results in human HF are equivocal. For example, Zelis et al. (61) reported that α-adrenergic receptor-mediated vasoconstriction plays a greater role in restraining blood flow during one moderate level of handgrip exercise in patients with HFrEF than in healthy controls, as documented by a larger increase in exercising forearm blood flow in response to intra-arterial infusion of Phen. In contrast, Wilson et al. (55) documented no change in leg blood flow when Phen was administered before maximal upright cycling exercise in patients with HFrEF. The present data build upon this earlier work through the inclusion of a carefully matched control group, continuous drug infusion, and use of a the single-leg KE exercise modality at multiple work rates, identifying, for the first time, a significant sympathetic restraint of leg blood flow during exercise in patients with HFrEF compared with controls.
α-Adrenergic Receptor Regulation of Leg V̇o2 During Exercise
While the increases in leg blood flow and leg vascular conductance following Phen administration clearly identify the importance of excess sympathetic vasoconstriction in terms of exercise hyperemia in HFrEF, further insight into the functional significance of this blood flow restriction may be gained from evaluation of leg O2 delivery and V̇o2 during exercise. Interestingly, after α-adrenergic receptor blockade, patients with HFrEF demonstrated an increase in leg V̇o2, a response not present in the control group (Fig. 6, bottom). This unique combination of elevated leg V̇o2 in response to increased O2 delivery, after α-adrenergic inhibition, suggests a decrease in intramuscular efficiency, defined as the ratio of work performed to metabolic cost, in this case calculated from V̇o2 (11, 45). At face value, a reduction in intramuscular efficiency might appear detrimental, especially in a patient group where greater efficiency should be considered beneficial; however, a recent study from our group suggests otherwise. Indeed, using the novel technique of lumbar intrathecal injection of fentanyl to inhibit group III and IV afferent fibers in patients with HFrEF during single-leg KE exercise, we observed an increase in leg O2 delivery and V̇o2 that resulted in a substantial increase in fatigue resistance (3). Thus it is tempting to speculate that what is sacrificed in efficiency when leg blood flow is increased acutely via α-adrenergic receptor blockade (Figs. 5 and 6) may be outweighed by reduced fatigue and subsequent improvements in exercise tolerance in patients with HFrEF, although additional studies are needed to directly examine this relationship.
Functional Sympatholysis in HFrEF: A Tale of Two Pharmacological Strategies
Conventionally, the concept of functional sympatholysis during exercise is studied using a “stimulatory approach” via lumbar sympathetic stimulation in animal models (52) or the pharmacological approach of local α-adrenergic receptor agonist drugs (10, 17, 58), with both approaches comparing the resting vasoconstrictor response with the response induced during muscular contraction or exercise (Fig. 4). A complementary approach is the use of α-adrenergic receptor antagonism to compare the change in blood flow at rest with that during exercise (Fig. 5). While both of these pharmacological methods probe the role of the α-adrenergic receptors in the regulation of vascular tone and blood flow to the exercising skeletal muscle, they should be considered mutually exclusive, as both methods tell very different stories. Specifically, the stimulatory approach is informative regarding the level of vasoconstrictor reserve or “active vasoconstriction” (40) provided by the α-adrenergic system. Thus, if sympathetic vasoconstriction during exercise is lysed by metabolic inhibition or α-adrenergic receptor occupancy by NE, a stimulatory approach will indicate the extent to which the α-adrenergic receptors can further induce vasoconstriction and reduce blood flow. In contrast, α-adrenergic antagonism provides insight concerning the level of passive vasodilation (40), or the level to which α-adrenergic vasoconstriction contributes to the endogenous level of vascular tone and blood flow, at rest and during exercise. As these two approaches reveal very different aspects of vascular control, the current study has comprehensively established the role and vasoconstrictor potential of the α-adrenergic receptor pathway at rest and during exercise in this patient group.
Experimental Considerations
We acknowledge that the reduction in leg blood flow at rest and during exercise in patients with HFrEF may be partially explained by lower quadriceps muscle mass in this group (Table 1). However, this concern is somewhat mitigated by recent work that failed to identify a significant relationship between quadriceps muscle mass and leg blood flow during KE exercise in young, healthy adults (23). Given the differences in exercise capacity between groups, we also recognize that the use of absolute work rates, rather than work rates relative to maximum KE, may have resulted in a greater metabolic perturbation in the exercising muscle of patients with HFrEF. As the degree of functional sympatholysis is known to depend on exercise intensity (57), this may have created the potential for a greater sympatholytic effect in the HFrEF group. By design, the present study focused on the role of the α1-adrenergic receptor to explore disease-related changes in sympatholysis, while a nonselective α1/α2-receptor antagonist was utilized to evaluate sympathetic restraint. Therefore, we cannot exclude the role that α2-adrenergic receptors might play in the regulation of peripheral hemodynamics in HFrEF, and we recognize that the use of selective α1- and α2-receptor agonists and antagonists in additional studies may be necessary to fully characterize α-adrenergic vasoconstriction during exercise in this patient group. It is noteworthy that the attenuated sympatholysis in patients with HFrEF is predicated on comparisons with a maximal dose of PE at rest. While use of a resting drug dose that elicited a maximal reduction (i.e., plateau) in leg blood flow and vascular conductance increases the likelihood of achieving similar receptor occupancy between groups, interpretation of the sympatholytic effect of exercise may differ when responses to lower drug doses are compared. Finally, we acknowledge that patients with HFrEF were maintained on optimized pharmacotherapy, with no medications withheld on experimental days. Therefore, we cannot exclude the possibility that existing drug therapy might have affected our measurements, particularly cardiac responses, although it is noteworthy that this represents the “real world” in which patients live.
Perspectives and Significance
This study has revealed a significant role of the α-adrenergic receptor pathway in restraining blood flow and O2 delivery to the exercising skeletal muscle in patients with HFrEF, a physiological maladaptation that likely contributes to exercise intolerance and a limited capacity to perform tasks of everyday life evident in this patient population.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grant HL-118313 and US Department of Veterans Affairs Grants RX001311, RX001418, and RX000182.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.B.-O., R.S.R., and D.W.W. conceived and designed research; Z.B.-O., J.F.L., S.J.I., J.D.T., M.A.W., M.J.R., H.J.G., J.R.S., D.E.M., A.D.N., J.S., R.S.R., and D.W.W. performed experiments; Z.B.-O. and D.W.W. analyzed data; Z.B.-O., R.S.R., and D.W.W. interpreted results of experiments; Z.B.-O. and D.W.W. prepared figures; Z.B.-O. and D.W.W. drafted manuscript; Z.B.-O., J.F.L., S.J.I., J.D.T., M.A.W., M.J.R., H.J.G., J.R.S., D.E.M., A.D.N., J.S., R.S.R., and D.W.W. edited and revised manuscript; Z.B.-O., J.F.L., S.J.I., J.D.T., M.A.W., M.J.R., H.J.G., J.R.S., D.E.M., A.D.N., J.S., R.S.R., and D.W.W. approved final version of manuscript.
REFERENCES
- 1.Alves MJ, Rondon MU, Santos AC, Dias RG, Barretto AC, Krieger EM, Middlekauff HR, Negrão CE. Sympathetic nerve activity restrains reflex vasodilatation in heart failure. Clin Auton Res 17: 364–369, 2007. doi: 10.1007/s10286-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and V̇o2max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol 292: H2491–H2497, 2007. doi: 10.1152/ajpheart.01396.2006. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. doi: 10.1152/ajpheart.00603.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol 307: H1512–H1520, 2014. doi: 10.1152/ajpheart.00527.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation 61: 955–959, 1980. doi: 10.1161/01.CIR.61.5.955. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol (1985) 90: 172–178, 2001. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh PR, Kram R. The efficiency of human movement—a statement of the problem. Med Sci Sports Exerc 17: 304–308, 1985. doi: 10.1249/00005768-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 13.Dargie H. Sympathetic activity and regional blood flow in heart failure. Eur Heart J 11, Suppl A: 39–43, 1990. doi: 10.1093/eurheartj/11.suppl_A.39. [DOI] [PubMed] [Google Scholar]
- 14.Davis D, Baily R, Zelis R. Abnormalities in systemic norepinephrine kinetics in human congestive heart failure. Am J Physiol Endocrinol Metab 254: E760–E766, 1988. doi: 10.1152/ajpendo.1988.254.6.E760. [DOI] [PubMed] [Google Scholar]
- 15.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. doi: 10.1161/01.CIR.0000028819.64790.BE. [DOI] [PubMed] [Google Scholar]
- 16.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- 17.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol 262: 39–50, 1976. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55: 1945–1954, 2010. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito F, Mathieu-Costello O, Wagner PD, Richardson RS. Acute and chronic exercise in patients with heart failure with reduced ejection fraction: evidence of structural and functional plasticity and intact angiogenic signalling in skeletal muscle. J Physiol 596: 5149–5161, 2018. doi: 10.1113/JP276678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito F, Wagner PD, Richardson RS. Incremental large and small muscle mass exercise in patients with heart failure: evidence of preserved peripheral haemodynamics and metabolism. Acta Physiol (Oxf) 213: 688–699, 2015. doi: 10.1111/apha.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson DW, Berg WJ, Sanders JS, Kempf JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16: 1125–1134, 1990. doi: 10.1016/0735-1097(90)90544-Y. [DOI] [PubMed] [Google Scholar]
- 23.Garten RS, Groot HJ, Rossman MJ, Gifford JR, Richardson RS. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol (1985) 116: 1204–1209, 2014. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol 51: 52–60, 1983. doi: 10.1016/S0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 25.Indolfi C, Maione A, Volpe M, Rapacciuolo A, Esposito G, Ceravolo R, Rendina V, Condorelli M, Chiariello M. Forearm vascular responsiveness to α1- and α2-adrenoceptor stimulation in patients with congestive heart failure. Circulation 90: 17–22, 1994. doi: 10.1161/01.CIR.90.1.17. [DOI] [PubMed] [Google Scholar]
- 26.Johnsson G, Nyberg G, Sölvell L. Influence of metoprolol and propranolol on hemodynamic effects induced by physical work and isoprenaline. Acta Pharmacol Toxicol (Copenh) 36, Suppl 5: 69–75, 1975. doi: 10.1111/j.1600-0773.1975.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 28.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 29.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 30.Logason K, Bärlin T, Jonsson ML, Boström A, Hårdemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50–69% and 70–99% carotid artery stenosis. Eur J Vasc Endovasc Surg 21: 311–313, 2001. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson G, Kaijser L, Sylvén C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res 33: 297–306, 1997. doi: 10.1016/S0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- 32.Metra M, Raddino R, Dei Cas L, Visioli O. Assessment of peak oxygen consumption, lactate and ventilatory thresholds and correlation with resting and exercise hemodynamic data in chronic congestive heart failure. Am J Cardiol 65: 1127–1133, 1990. doi: 10.1016/0002-9149(90)90326-V. [DOI] [PubMed] [Google Scholar]
- 33.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. doi: 10.1161/01.HYP.21.4.498. [DOI] [PubMed] [Google Scholar]
- 35.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 36.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol (1985) 92: 2105–2113, 2002. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 37.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.RES.11.3.370. [DOI] [PubMed] [Google Scholar]
- 38.Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30: 28–33, 1998. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547: 971–976, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford University Press, 1986. [Google Scholar]
- 41.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876-2003): cycles of revision and new vision. J Appl Physiol (1985) 97: 384–392, 2004. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- 42.Sielatycki JA, Shamimi-Noori S, Pfeiffer MP, Monahan KD. Adrenergic mechanisms do not contribute to age-related decreases in calf venous compliance. J Appl Physiol (1985) 110: 29–34, 2011. doi: 10.1152/japplphysiol.00930.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol (1985) 84: 1551–1559, 1998. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 44.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional α-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stainbsy WN, Gladden LB, Barclay JK, Wilson BA. Exercise efficiency: validity of base-line subtractions. J Appl Physiol Respir Environ Exerc Physiol 48: 518–522, 1980. doi: 10.1152/jappl.1980.48.3.518. [DOI] [PubMed] [Google Scholar]
- 46.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991. doi: 10.1161/01.CIR.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 47.Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res 100: 1371–1378, 2007. doi: 10.1161/01.RES.0000266974.84590.d2. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis 38: 1–22, 1995. doi: 10.1016/S0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 80: 769–781, 1989. doi: 10.1161/01.CIR.80.4.769. [DOI] [PubMed] [Google Scholar]
- 50.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szlachcic J, Masse BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 55: 1037–1042, 1985. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- 52.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- 53.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol 41: 233–243, 1978. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 54.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 65: 1213–1223, 1982. doi: 10.1161/01.CIR.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 55.Wilson JR, Ferraro N, Wiener DH. Effect of the sympathetic nervous system on limb circulation and metabolism during exercise in patients with heart failure. Circulation 72: 72–81, 1985. doi: 10.1161/01.CIR.72.1.72. [DOI] [PubMed] [Google Scholar]
- 56.Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation 69: 1079–1087, 1984. doi: 10.1161/01.CIR.69.6.1079. [DOI] [PubMed] [Google Scholar]
- 57.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of α-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007. doi: 10.1152/ajpheart.00867.2007. [DOI] [PubMed] [Google Scholar]
- 59.Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009. doi: 10.1152/ajpheart.01016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol (1985) 99: 81–86, 2005. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 61.Zelis R, Longhurst J, Capone RJ, Mason DT, Kleckner R. A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in normal subjects and patients with congestive heart failure. Circulation 50: 137–143, 1974. doi: 10.1161/01.CIR.50.1.137. [DOI] [PubMed] [Google Scholar]