Abstract
The main objective of these studies was to characterize metabolic, body composition, and cardiovascular responses to a free-choice high-fat, high-sucrose diet in female cycling and pregnant rats. In the nonpregnant state, female Sprague-Dawley rats offered a 3-wk free-choice high-fat, high-sucrose diet had greater energy intake, adiposity, serum leptin, and triglyceride concentrations compared with rats fed with standard chow and developed glucose intolerance. In addition, choice-diet-fed rats had larger cardiac ventricular weights, smaller kidney and pancreas weights, and higher blood pressure than chow-fed rats, but they did not exhibit resistance artery endothelial dysfunction. When the free-choice diet continued throughout pregnancy, rats remained hyperphagic, hyperleptinemic, and obese. Choice pregnant rats exhibited uterine artery endothelial dysfunction and had smaller fetuses compared with chow pregnant rats. Pregnancy normalized mean arterial blood pressure and pancreas weights in choice rats. These studies are the first to provide a comprehensive evaluation of free-choice high-fat, high-sucrose diet on metabolic and cardiovascular functions in female rats, extending the previous studies in males to female cycling and pregnant rodents. Free-choice diet may provide a new model of preconceptual maternal obesity to study the role of increased energy intake, individual food components, and preexisting maternal obesity on maternal and offspring physiological responses during pregnancy and after birth.
Keywords: diet-induced obesity, energy intake, female, uterine artery, vascular function
INTRODUCTION
Women with obesity are at risk of developing gestational diabetes and preeclampsia during pregnancy and have a high incidence of labor complications, emergency cesarean sections, and miscarriages (34). In addition to adverse maternal outcomes, obesity affects fetal development and growth, leading to macrosomia (7) or intrauterine growth restriction (13, 42). Given the increasing prevalence of overweight and obesity among women of reproductive age (3, 30, 42), there is an urgent need to understand the mechanisms underlying adverse effects of obesity on the pregnant mother and her offspring, and to develop therapeutic strategies to prevent and manage these effects.
High-fat or high-energy diets are often used to induce an obesogenic phenotype in experimental animals. Diets with a choice element result in overconsumption and hyperphagia, reflecting a more typical Western-style diet (25, 44, 45). Indeed, rats offered a free-choice high-fat, high-sucrose diet exhibited hyperphagic behavior, rapid onset of obesity, and impaired glucose tolerance (1, 18, 24). Importantly, increases in adiposity, metabolic dysfunction, and leptin resistance were observed as early as 3 wk in male rats offered a free-choice diet (18).
The majority of investigations using choice dietary interventions have been conducted in male rodents (1, 2, 18, 24, 25). Yang et al. (59) recently showed that in rodents, diet choice patterns were mediated by various factors, including sex. Others have demonstrated that feeding a high-fat diet or a high-fat high-sugar diet interfered with normal estrous cycle and altered reproductive hormone concentrations in female cycling rats (20, 55). These findings suggest that male responses to a dietary intervention may not reflect responses of female rats at various reproductive states. In addition to limited information with regard to the effects of choice diet in female cycling rats, studies in pregnant rats have mostly focused on fetal and offspring outcomes (9, 27, 28, 29, 47) and minimally on maternal responses. Thus the main objective of this study was to determine the effects of a free-choice high-fat, high-sucrose diet on both female cycling and pregnant rats. First, we sought to characterize the effects of a choice diet on energy intake, body composition, and cardiovascular responses in female cycling rats. Then, we utilized this diet-induced obesity model to determine the effects of prenatal maternal obesity on maternal food consumption and preference and maternal metabolic and cardiovascular functions during pregnancy. We hypothesized that free access to chow, sucrose solution, and lard would cause maternal hyperphagia and metabolic dysfunction and alterations in maternal adipose tissue morphology and cardiovascular function.
METHODS
Animals
All protocols were approved by the Institutional Animal Care and Use Committees of Augusta University and University of North Texas Health Science Center in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
A total of 60 Sprague-Dawley (Harlan, Indianapolis, IN) female rats were used for the following three studies. In study 1, 16 virgin female rats (age: 10 wk, body weight: 188–210 g) were individually housed in hanging wire mesh cages. These rats were used to characterize the effects of free-choice diet on daily energy intake, body composition, and serum metabolic profile in cycling female rats. Wire mesh cages provided the opportunity for accurate evaluation of daily food intakes correcting for spillage. In study 2, 16 virgin female rats (age: 11 wk, body weight: 205−217 g) were individually housed in rat cages with standard rodent bedding (corncob). The objective of this second study was to replicate study 1 in optimal conditions for mating and pregnancy studies. In addition to energy intake determination, we examined the effects of free-choice diet on adipose tissue morphology and cardiovascular function. In study 3, 28 virgin female rats (age: 12–13 wk, body weight: 203–235 g) were individually housed and followed from the preconceptual period to late pregnancy. Here we utilized the optimized protocol developed in study 2 to assess the effects of preconceptual free-choice diet on maternal energy intake, adipose tissue morphology, and cardiovascular function during pregnancy.
All animals were housed in a room maintained at 20–23°C with lights on/off for 12 h/day and were allowed to acclimate for 1 wk before the initiation of any experimentation. Rats had free access to water throughout all studies. Body weights as well as sucrose, lard, and chow intakes were recorded daily between 7:00 and 8:30 AM throughout all studies. Food intakes were corrected for spillage only in study 1. All cycling rats had normal duration of estrous cycle (4–5 days), and this did not change with diet.
Experimental Design and Methodology
Study 1: Assessing effects of free-choice high-fat, high-sucrose diet on energy intake, body composition, and serum metabolic profile in female cycling rats.
Baseline measurements of food intakes and body weights were recorded for 5 days following acclimatization. All rats were given free access (ad libitum) to pelleted chow (24% minimum protein, 4% minimum fat, 0.14 MJ/g; Harlan Teklad Rodent Diet 8604) at acclimatization and baseline. After baseline, rats were assigned by minimization into two groups (chow vs. choice) using body weight as stratifying factor; thus the groups were weight matched. The chow rats maintained free access to pelleted chow for the entire experiment. The choice group retained free access to pelleted chow but also were provided free access to a 30% sucrose solution (wt/vol; liquid sucrose, Kroger Sugar, Hood Packing, Hamlet, NC) and lard (Armour, ConAgra Foods, Omaha, NE) (1, 2, 18, 24). The choice rats were provided free access to pelleted chow, liquid sucrose, and lard for the duration of the experiment. The liquid sucrose and lard were separate components and were not integrated into a powdered or pelleted diet. Thus the choice was among pelleted chow, liquid sucrose, and lard in the choice group. The liquid sucrose was prepared daily in distilled water. Also, all rats were provided the sucrose solution along with a water bottle. The chow was not a purified diet and thus exact diet composition was not available. However, it was 24% minimum protein, 4% minimum fat, and 0.14 MJ/g. Sucrose is a sugar composed of glucose and fructose, and a 30% sucrose solution would be equivalent to 75 g sucrose dissolved into 250 ml water. The lard was lard (from pork), thus 100% fat and solid at room temperature. It was composed of 0% trans fat, 38% monounsaturated fat, and ~45% saturated fat.
A glucose tolerance test (GTT) was performed on day 19 (of the diet) starting at 1:00 PM after food was removed from rat cages for 6 h. A bolus of 1 g glucose/kg body wt was delivered intraperitoneally, and blood glucose was measured in tail blood samples (EasyGluco blood glucose test strips, US Diagnostics) at 0, 15, 30, 45, 60, 90, and 120 min after glucose injection. Tail blood samples were collected for subsequent measurements of serum insulin (Rat Insulin RIA kit, Millipore). Food was returned to cages immediately after the test was completed.
All rats were euthanized after 3 wk on a chow or choice diet. On the day of euthanasia, food was removed from rat cages at 7:00 AM and rats were decapitated at 8:30–9:30 AM. Trunk blood was collected for measurements of serum triglycerides (L-Type Triglyceride H kit, Wako Chemicals) and leptin (rat leptin RIA, Millipore). Organs (heart, kidney, and liver) and fat pads [periuterine, inguinal, mesenteric, retroperitoneal, and intrascapular brown (IBAT)] were excised, weighed (wet weights), and returned to the carcass. One lobe of the liver was flash frozen for liver lipid content measurements. Liver lipid was determined by chloroform-methanol extraction as previously described (16). Composition of the carcass (after the gastrointestinal tract was removed) was analyzed as described previously (17).
Study 2: Examining effects of free-choice high-fat, high-sucrose diet on adipose tissue morphology and cardiovascular function in female cycling rats.
After 5 days of baseline measurements of body weights and food intakes, rats were divided into two weight-matched groups as described in study 1. Sucrose solution was prepared by dissolving sugar in tap water.
A GTT was performed, as described in study 1, in all rats during the last week of the dietary intervention. Rats on choice diet and their weight-matched chow counterparts were tested on the same day. Whole blood glucose was measured in tail blood during the GTT (FreeStyle Lite, Alameda, CA).
At the end of 3 wk, blood pressure was measured in anesthetized rats. Rats were anesthetized with isoflurane (initially with 5% and then maintained at 2% in 100% oxygen), and blood pressure was measured via a P-10 catheter inserted into the right femoral artery and attached to a pressure transducer, as previously described (15). After 10 min of stable arterial pressure recordings, rats were euthanized by the removal of their hearts. In study 2, rats were euthanized between 6:00 AM and 12:00 PM. Organs (heart, kidneys, liver, and pancreas) and fat pads (periuterine, inguinal, mesenteric, retroperitoneal, and IBAT) were excised and weighed. Uterine and mesenteric arteries were also excised and immediately placed in cold physiological salt solution (PSS) of the following composition (in mM): 130 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 dextrose, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 1.6 CaCl2, and 0.026 EDTA.
VASCULAR REACTIVITY EXPERIMENTS.
Main uterine arteries and second-order mesenteric arteries were cleaned of connective and adipose tissues, cut in 2-mm-long segments, and mounted onto 40-μm stainless steel wires on a wire myograph (Danish Myo Technology, Aarhus, Denmark). Vascular reactivity experiments were performed as described previously (15). Briefly, arterial segments were equilibrated for 45 min at a resting tension determined via a length-tension curve in myograph chambers filled with 5 ml PSS (95% O2-5% CO2, 37°C). Vascular viability was determined by contracting the arteries with potassium chloride (KCl, 120 mM), and endothelial integrity was examined by assessing responses to acetylcholine (ACh: uterine, 2 × 10−6 M; mesenteric, 3 × 10−6 M) after constriction with phenylephrine (PE: uterine, 2 × 10−6 M; mesenteric, 3 × 10−6 M). To assess vascular endothelial function, we precontracted arteries with PE (uterine, 2 × 10−6 M; mesenteric, 3 × 10−6 M) and performed concentration-response curves to ACh (muscarinic receptor agonist). To examine the nitric oxide (NO) contribution to ACh-induced relaxation, concentration-response curves to ACh were performed in the presence of NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M, 30-min incubation). Concentration-response curves to PE (α1-adrenergic receptor agonist) and U46619 (thromboxane A2 mimetic) were also performed to assess vascular contractile responses.
PE, ACh, l-NAME, and all components of PSS and KCl solution were obtained from Sigma (Sigma Aldrich, St. Louis, MO). U46619 was purchased from Cayman (Cayman Chemical, Ann Arbor, MI). Stock solutions were prepared in distilled water for PE, ACh, and l-NAME and in dimethyl sulfoxide (DMSO) for U46619.
ADIPOSE TISSUE MORPHOLOGY ANALYSIS.
Periuterine adipose tissue was weighed and fixed in 4% paraformaldehyde at 4°C for 24 h. Then, it was transferred to 70% ethanol and stored at 4°C until further processing. Samples were embedded in paraffin, sliced into 5-μm sections, and stained with hematoxylin and eosin (Augusta University Histology Core, Augusta, GA). Adipose tissue histological sections were digitally captured using Nikon microscope and digital camera (Nikon Instruments, Tokyo, Japan) at ×10 magnification and analyzed using NIS Elements software (Nikon Instruments). Two sections for each sample and five different measurement frames (439 × 402 μm) for each section were taken. Linear contrast was applied to enhance the visibility of cell membranes and distinguish them from the cell interior where lipid droplets are present. To determine adipose tissue hypertrophy, we measured cross-sectional area/cell using NIS Elements software. Two independent investigators performed evaluation of blinded histological sections, and intraindividual coefficient of variation (%CV) was calculated.
Study 3: Assessing effects of preconceptual free-choice high-fat, high-sucrose diet on energy intake, food preference, adipose tissue morphology, and cardiovascular function in late pregnant rats.
Rats were single-housed in cages with standard bedding (corncob) and divided into two weight-matched groups to consume either standard chow or a free-choice diet as described in study 2 before (preconceptual period, 3 wk) and throughout pregnancy. In all rats, estrous cycle was determined daily, and mating procedures were performed as previously described (38). The morning on which spermatozoa were found in the vaginal lavage was considered day 1 of gestation (term = 22–23 days). All rats were successfully mated. In one cohort of animals, body weights, chow, sucrose solution (30%), and lard intakes were recorded daily before pregnancy to assess reproducibility of study 2 and during pregnancy to examine the effects of free-choice diet on maternal daily energy intake and food preference. In another cohort of animals, we assessed the effects of free-choice diet on blood pressure and vascular reactivity (as described in study 2). All rats were euthanized in late pregnancy (gestational days 19–21) following procedures described in study 2. Before euthanasia, whole blood glucose was measured in tail blood in conscious dams (FreeStyle Lite, Alameda, CA). Then, with the rat under anesthesia, blood was collected from the vena cava for determining the concentrations of circulating insulin and leptin. After euthanasia, periuterine adipose tissue was collected, weighed, and processed for histology experiments as described in study 2. Fat pads, organs, and fetal weights were also measured. Fetuses were euthanized via decapitation. Enzyme-linked immunosorbent assay (ELISA) was used to quantify circulating concentrations of insulin (catalog no. 80-INSRT-E01, ALPCO, Salem, NH) and leptin (catalog no. 22-LEPMS-E01, ALPCO) in maternal serum. For quantifying insulin, no serum dilution was required. Insulin concentration in serum was generated by plate reader based on the recommended five-parameter curve and was quantified in nanograms per milliliter. For leptin, serum samples from lean and obese pregnant rats required 1:10 dilution. Leptin concentration in serum was generated by plate reader using the recommended four-parametric logistic (4-PL) curve fit and was quantified in picograms per milliliter. Final concentrations are reported as nanograms per milliliter.
Data Analysis
Vascular reactivity studies: concentration-response curve analysis.
Curves were analyzed as previously described (38). Briefly, curves were fitted using robust nonlinear regression analysis: equation, log (agonist) vs. response – variable slope; model, y = bottom + (top – bottom)/{1 + 10^[(log EC50 – x)Hill slope]}. Emax was defined as the maximum response to agonist and EC50 (expressed as log EC50) was defined as the concentration of agonist that gives a response halfway between top and bottom (plateaus in sigmoidal curves). Contractile responses are expressed as percentage of maximum response to KCl (120 mM). Concentration-response curve fitting was performed using Prism (version 6.0; GraphPad Software, San Diego, CA).
Statistical analysis.
All statistical analyses were performed using Prism (version 6.0; GraphPad Software), unless indicated otherwise. Normality was assessed using the D’Agostino and Pearson omnibus test. Group differences (chow vs. choice groups) in single end-point measures were determined by Mann-Whitney U test (for not normally distributed data) or Student’s t-test (for normally distributed data). When variances were determined unequal, Welch’s correction was used. Group differences in daily recordings of energy intake and body weight (study 1) and glucose/insulin responses to GTT (studies 1 and 2) were determined by a two-way analysis of variance (ANOVA) with repeated measures followed by Sidak’s post hoc test. Area under the curve (AUC) for glucose was calculated by the linear trapezoid method, and group comparisons were made using a Student’s t-test. In study 2 and study 3, group differences in daily recordings of energy intake were determined using a two-way mixed model ANOVA with repeated measures followed by Tukey’s post hoc test (JMP Pro 12; SAS Institute, Cary, NC).
Weights of fat depot and body organs are presented in absolute values (g) or relative to body weight (per 100 g body wt). Values in all tables and figures are presented as means ± SE. Exact P values are presented for all tests.
RESULTS
Study 1: Effects of Free-Choice High-Fat, High-Sucrose Diet Over 3 Weeks on Energy Intake, Body Composition, and Serum Metabolic Profile in Female Cycling Rats
Body weight increased throughout the 3-wk dietary intervention in both groups (Fig. 1A), but there were no group differences in body weight gain [chow (n = 8) 21 ± 3 g vs. choice (n = 8) 23 ± 4 g; Student’s t-test, P = 0.74] or body weight at the end of the study [chow (n = 8) 221 ± 4 g vs. choice (n = 8) 219 ± 4 g; P = 0.74]. There was a group effect for daily energy intake, with choice-diet-fed rats having greater energy intake compared with chow-fed rats (Fig. 1B). Lard was the major contributor to total energy intake (lard 822 ± 85 kcal/21 days, sucrose 459 ± 84 kcal/21 days, chow 291 ± 23 kcal/21 days; Tukey’s post hoc test: lard vs. chow, P = 0.002).
Fig. 1.
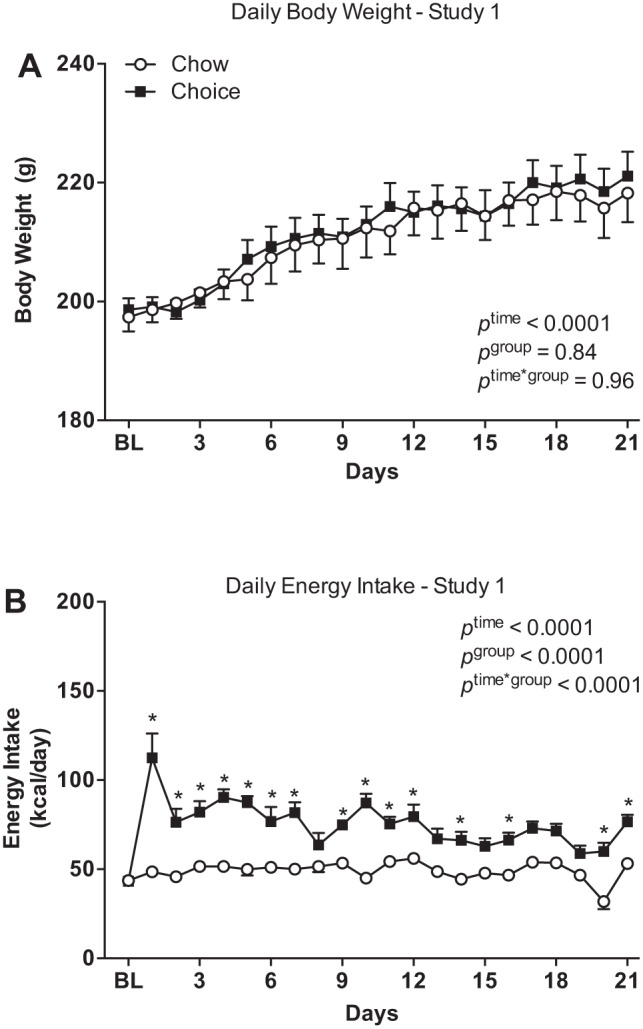
Daily body weights (A) and energy intakes (B) over 3 wk on chow or choice diet in study 1. Rats were individually housed in hanging wire mesh cages and divided into two weight-match groups: one group of rats (chow group) remained on chow and the other group (choice group) had free access to chow, 30% sucrose solution, and lard. Choice-diet-fed rats had greater daily energy intake compared with chow-diet-fed rats, but there were no group differences in daily body weights. BL, baseline. Data are means ± SE (n = 8 rats/group). Two-way ANOVA with repeated measures followed by Sidak’s post hoc test. *P ≤ 0.04.
Choice-diet-fed rats had heavier carcass fat than chow-diet-fed rats, and there were no group differences in carcass weight and lean body mass (water plus protein) (Table 1). In addition, choice rats had greater fat depot weights and left ventricular weights, but smaller kidney weights compared with chow controls (Table 1). These differences remained significant when organ and fat depot weights were as absolute data (data not shown). Liver lipid content as well as serum concentrations of leptin and triglycerides were higher in the choice group compared with the chow group, while there were no group differences in fasting insulin concentrations (Table 1). There was a positive correlation between carcass fat weight and total energy intake (r2 = 0.795, P < 0.0001), but there was no relationship between total energy intake and body weights (r2 = 0.10, P = 0.23).
Table 1.
Body composition, organ weights, and serum metabolic profile in cycling rats fed with pelleted chow (chow group) versus a free-choice high-fat, high-sucrose diet (choice group): study 1
| Chow | Choice | P Value | |
|---|---|---|---|
| Carcass composition, g | |||
| Carcass weight | 182.2 ± 4.1 (8) | 188.6 ± 3.5 (8) | 0.26 |
| Water | 123.4 ± 2.9 (8) | 118.6 ± 1.3 (8) | 0.16 |
| Ash | 7.8 ± 0.9 (8) | 6.9 ± 0.5 (8) | 0.43 |
| Protein | 43.5 ± 0.7 (8) | 46.8 ± 2.8 (8) | 0.28 |
| Fat | 7.5 ± 0.5 (8) | 19.7 ± 2.3 (8) | 0.0002 |
| body mass | 166.9 ± 3.1 (8) | 165.4 ± 3.9 | 0.77 |
| Fat pad weights, g/100 g body wt | |||
| Periuterine | 0.96 ± 0.09 (7) | 2.30 ± 0.10 (6) | 0.001 |
| Inguinal | 0.88 ± 0.05 (8) | 1.35 ± 0.09 (8) | 0.0004 |
| Mesenteric | 0.64 ± 0.06 (8) | 1.27 ± 0.09 (8) | <0.0001 |
| Retroperitoneal | 0.24 ± 0.03 (8) | 0.84 ± 0.05 (8) | <0.0001 |
| IBAT | 0.13 ± 0.02 (8) | 0.28 ± 0.04 (8) | 0.002 |
| Organ weights, g/100 g body wt | |||
| Left ventricle | 0.23 ± 0.00 (8) | 0.25 ± 0.00 (8) | 0.002 |
| Right ventricle | 0.06 ± 0.00 (8) | 0.06 ± 0.00 (8) | 0.14 |
| Left kidney | 0.31 ± 0.01 (8) | 0.29 ± 0.01 (8) | 0.01 |
| Right kidney | 0.32 ± 0.01 (8) | 0.29 ± 0.01 (8) | 0.02 |
| Liver composition | |||
| Liver weight, g/100 g body wt | 3.00 ± 0.09 (8) | 3.17 ± 0.17 (8) | 0.56 |
| Liver lipid, mg/liver | 0.27 ± 0.01 (8) | 0.38 ± 0.04 (8) | 0.01 |
| Serum metabolic parameters | |||
| Leptin, ng/ml | 2.0 ± 0.2 (8) | 4.4 ± 0.7 (8) | 0.01 |
| TG, mg/dl | 31.0 ± 2.5 (6) | 45.2 ± 3.8 (8) | 0.003 |
| Fasting glucose, mg/dl | 110 ± 2.43 (8) | 122 ± 3.92 (8) | 0.01 |
| Fasting insulin, ng/ml | 0.21 ± 0.04 (7) | 0.19 ± 0.04 (8) | 0.52 |
Data are means ± SE; number of rats is given in parentheses. IBAT, intrascapular brown adipose tissue; TG, triglycerides. Student’s t-test for carcass weight, protein, fat, lean body mass, retroperitoneal adipose tissue, right ventricle, left kidney, right kidney, and leptin. Mann-Whitney U test for other variables.
Choice rats had higher blood glucose compared with chow rats (Table 1). A GTT showed no group differences in glucose and insulin responses (Fig. 2, A and B). In addition, there were no differences in AUC for glucose between choice and chow rats [chow (n = 8) 3,001 ± 322 mg·dl−1·min−1 vs. choice (n = 8) 2,678 ± 370 mg·dl−1·min−1; Student’s t-test, P = 0.52].
Fig. 2.
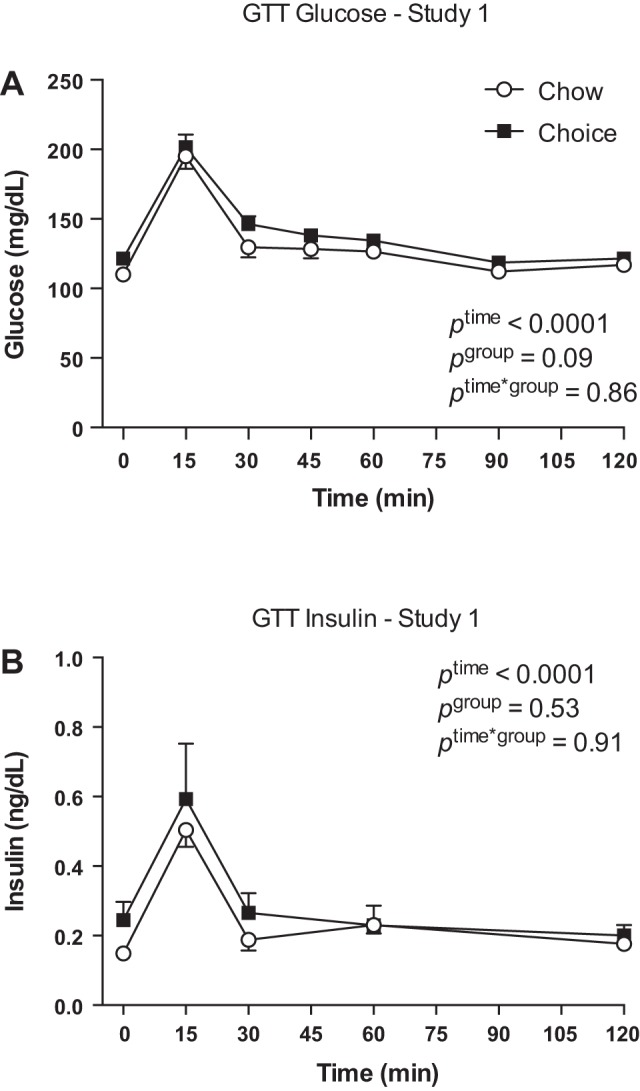
Glucose (A) and insulin (B) responses to a glucose tolerance test (GTT) on day 19 of study 1. There were no group differences in glucose and insulin responses to a GTT. Data are means ± SE (A: n = 8 rats/group; B: n = 4 rats in chow group and n = 7 rats in choice group). Two-way ANOVA with repeated measures.
In summary, a free-choice high-fat, high-sucrose diet increased energy intake, adiposity, serum leptin, and blood glucose over 3 wk in female cycling rats.
Study 2: Effects of Free-Choice High-Fat, High-Sucrose Diet on Adipose Tissue Morphology and Cardiovascular Function in Female Cycling Rats
There was a significant time-by-group interaction for daily body weight, with choice-diet-fed rats exhibiting a greater increase in body weight over 3 wk compared with chow-diet-fed rats (Fig. 3A). Daily energy intake was greater in choice compared with chow group throughout the study (Fig. 3B). The majority of energy was derived from sucrose (lard: 709 ± 71 kcal/20 days, sucrose: 1,500 ± 136 kcal/20 days, chow: 394 ± 15 kcal/20 days; Tukey’s post hoc tests: lard vs. sucrose, P < 0.0001; sucrose vs. chow, P < 0.0001; lard vs. chow, P = 0.05).
Fig. 3.
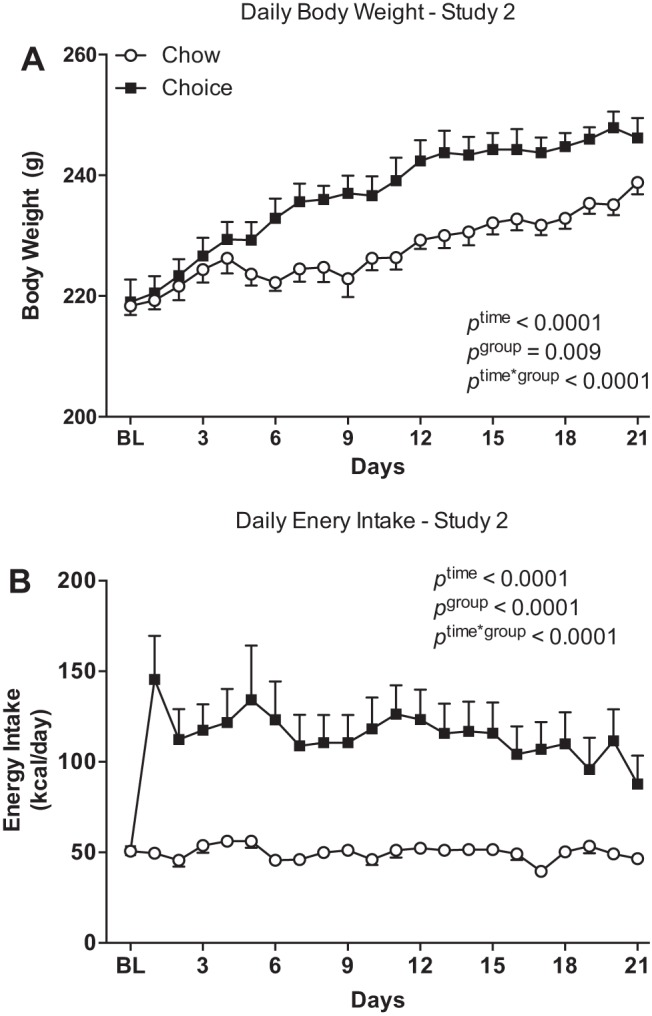
Daily body weights (A) and energy intakes (B) over 3 wk on chow or choice diet in study 2. Rats were individually housed in rat cages with standard rodent bedding and divided into two weight-matched groups: one group of rats (chow group) remained on pelleted chow and the other group (choice group) had free access to pelleted chow, 30% sucrose solution, and lard. Choice-diet-fed rats had greater body weights (after 5 days on a choice diet) and daily energy intakes compared with chow-diet-fed rats. BL, baseline. Data are means ± SE (n = 8 rats/group). Two-way mixed model ANOVA with repeated measures.
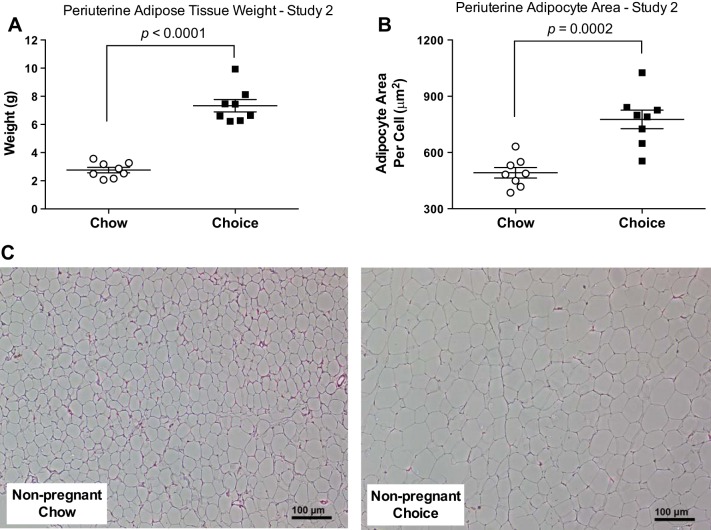
Choice-diet-fed rats had greater concentrations of blood glucose at 15 and 30 min of a GTT (Fig. 4A) and larger AUC for glucose (Fig. 4B) compared with chow-fed rats. Periuterine fat weight and adipocyte area were larger in the choice group compared with the chow group (Fig. 5, A and B). The intraindividual %CV for histology section evaluation was 10.5 ± 0.04%.
Fig. 4.
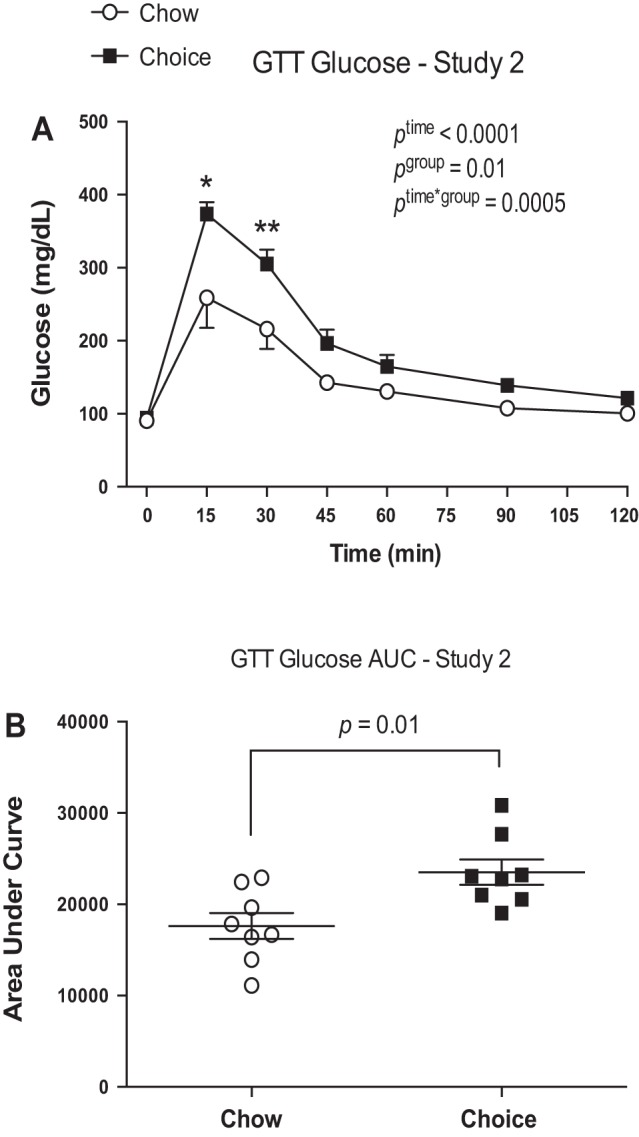
Glucose responses during a glucose tolerance test (GTT) in study 2. Choice-diet-fed rats had higher glucose responses at 15 and 30 min of GTT and greater area under the curve (AUC) compared with chow-diet-fed rats. Data are means ± SE (n = 8 rats/group). Two-way ANOVA with repeated measures followed by Sidak’s post hoc test and Student’s t-test. *P < 0.0001, **P = 0.0023.
Fig. 5.
Periuterine adipose tissue weight (A), adipocyte area/cell (B), and representative images of histological sections of periuterine adipose tissue (C) in study 2. Periuterine adipose tissue weights were heavier and adipocyte area was larger in choice-diet-fed rats compared with chow-diet-fed rats. Data are means ± SE (n = 8 rats/group). Student’s t-test for A and B.
Fat pad weights normalized to body weight (Fig. 6A) were greater in choice-diet-fed rats compared with chow rats. Similar results were found when absolute values were compared (data not shown). Absolute left and right ventricular weights were higher in choice rats compared with the chow group, but when normalized to body weight, this difference remained significant only for the right ventricular weights (Fig. 6B). Kidney weights normalized to body weight (Fig. 6B) were reduced in choice compared with chow rats.
Fig. 6.
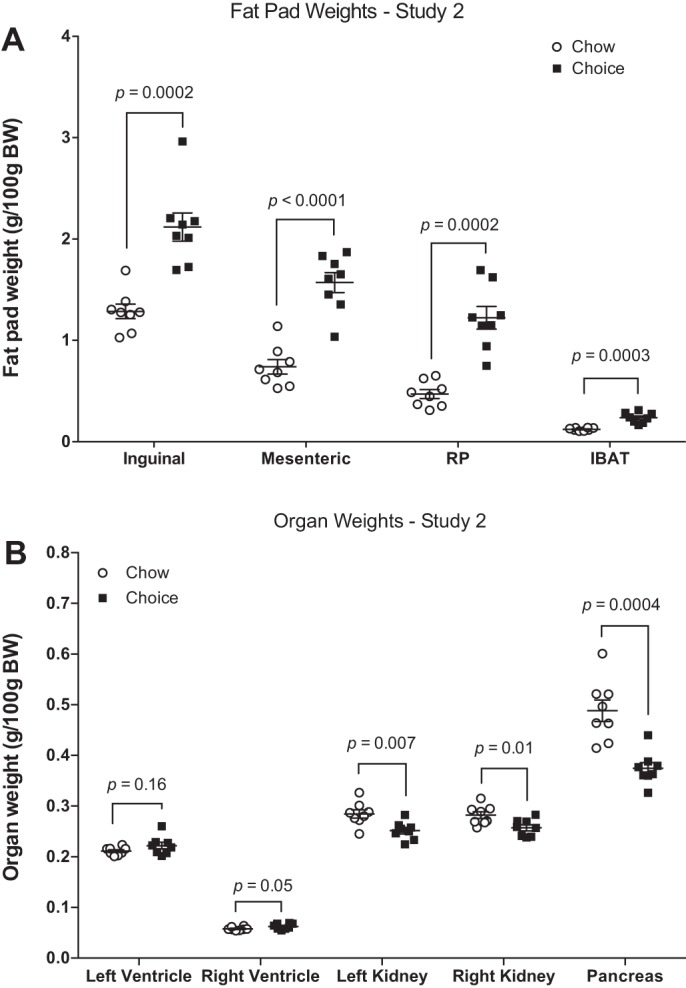
Weights of inguinal, mesenteric, retroperitoneal (RP), and intrascapular brown (IBAT) depots (A) as well as left and right ventricles, left and right kidneys, and pancreas (B) in study 2. Choice-diet-fed rats had heavier fat depots, larger right ventricular weights, and smaller kidney and pancreas weights compared with chow-diet-fed rats. BW, body weight. Data are means ± SE (n = 8 rats/group for all depots and organs). Mann-Whitney U test for inguinal adipose tissue. Student’s t-test for other variables.
Mean arterial pressure was higher in choice-diet-fed rats compared with the chow group (95.8 ± 1.8 vs. 88.5 ± 1.3 mmHg; P = 0.005). There were no group differences in sensitivity to ACh in mesenteric arteries (Table 2), and addition of l-NAME reduced total relaxation to ACh in both groups [AUC, ACh alone vs. ACh + l-NAME: chow (n = 6) 313.9 ± 10.09 vs. 170.0 ± 23.0; Tukey’s post hoc test, P = 0.0009; choice (n = 7) 313.4 ± 9.92 vs. 140.3 ± 32.3; Tukey’s post hoc test, P < 0.0001]. Uterine arteries from choice rats were more sensitive to ACh compared with arteries from chow rats (Table 2). l-NAME abolished total relaxation to ACh in uterine arteries from chow and choice rats [AUC, ACh alone vs. ACh + l-NAME: chow (n = 7) 235.1 ± 26.0 vs. 46.3 ± 16.3; Tukey’s post hoc test, P < 0.0001; choice (n = 7) 227.0 ± 6.07 vs. 54.2 ± 16.03; Tukey’s post hoc test, P < 0.0001]. In addition, there were no group differences in uterine and mesenteric artery responses to PE and U46619 (data not shown).
Table 2.
Relaxation responses to acetylcholine in mesenteric and uterine arteries from cycling and late pregnant rats fed with pelleted chow (chow group) or a free-choice high-fat, high-sucrose diet (choice group) for 3 wk (cycling rats) or for 3 wk before conception and throughout pregnancy (pregnant rats)
| Chow |
Choice |
|||
|---|---|---|---|---|
| ACh | ACh + l-NAME | ACh | ACh + l-NAME | |
| Cycling rats | ||||
| Mesenteric | ||||
| pEC50 | 7.80 ± 0.17 (6) | ND | 7.73 ± 0.09 (7) | ND |
| Emax | 99.8 ± 0.2* (6) | 48.6 ± 19.7 (6) | 97.1 ± 1.2† (7) | 34.8 ± 15.2 (7) |
| Uterine | ||||
| pEC50 | 6.88 ± 0.04 (7) | ND | 7.10 ± 0.05§ (7) | ND |
| Emax | 92.9 ± 3.0* (7) | 25.1 ± 8.9 (7) | 92.0 ± 2.3† (7) | 30.5 ± 11.2 |
| Pregnant rats | ||||
| Mesenteric | ||||
| pEC50 | 7.80 ± 0.17 (6) | ND | 7.73 ± 0.09 (7) | ND |
| Emax | 98.0 ± 1.3 (9) | 80.1 ± 8.2 (9) | 97.2 ± 1.3* (8) | 72.3 ± 9.0‡ (8) |
| Uterine | ||||
| pEC50 | 7.22 ± 0.15 (8) | ND | 7.41 ± 0.09 (7) | ND |
| Emax | 92.3 ± 1.9* (8) | 7.8 ± 6.7 (8) | 89.0 ± 3.8* (7) | 42.7 ± 8.6 (7) |
Data are means ± SE; number of rats per group is given in parentheses. ACh, acetylcholine; l-NAME, NG-nitro-l-arginine methyl ester; pEC50, concentration of agonist that gives a response halfway between top and bottom (plateaus in sigmoidal curves); Emax, maximum response to agonist. One-way ANOVA followed by Tukey’s post hoc test. ND, not determined;
P ≤ 0.04 vs. chow ACh + l-NAME, choice ACh + l-NAME;
P ≤ 0.04 vs. choice ACh + l-NAME, chow ACh + l-NAME;
P ≤ 0.04 vs. chow ACh, choice ACh;
P < 0.007.
In summary, a free-choice high-fat, high-sucrose diet increased energy intake, adiposity, and mean arterial pressure and augmented uterine artery sensitivity to ACh over 3 wk in female cycling rats.
Study 3: Effects of Preconceptual Free-Choice High-Fat, High-Sucrose Diet on Energy Intake, Food Preference, Adipose Tissue Morphology, and Cardiovascular Function in Late Pregnant Rats
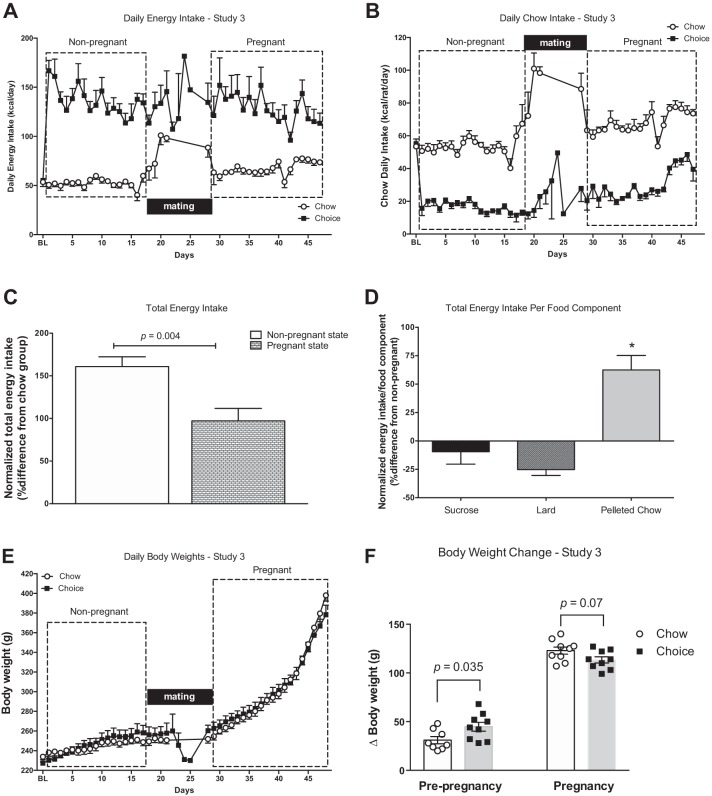
Figure 7A illustrates daily energy intakes for chow-fed and choice-diet-fed rats in the nonpregnant and pregnant states. Overall, choice rats had greater energy intakes compared with chow rats (main effect of diet, P < 0.0001). There was a significant diet-by-state interaction (P = 0.02: diet, chow vs. choice; state, nonpregnant vs. pregnant) but no significant main effect for state alone (P = 0.11). Chow rats increased their energy intake during pregnancy (compared with nonpregnant state; Tukey’s post hoc test, P = 0.02), while choice rats did not (Tukey’s post hoc test, P = 0.94; Fig. 7A). Chow intake was greater in pregnant compared with nonpregnant rats in both chow and choice groups (Fig. 7B). In the nonpregnant state, and in agreement with study 2, the choice group derived the majority of energy from sucrose (n = 8; lard 683 ± 78 kcal/18 days, sucrose 1,371 ± 140 kcal/18 days, chow 285 ± 14 kcal/18 days; Tukey’s post hoc tests: lard vs. sucrose, P = 0.0001; sucrose vs. chow, P < 0.0001; lard vs. chow, P = 0.02). During pregnancy, choice rats continued deriving most energy from sucrose (n = 7; lard 511 ± 35 kcal/18 days, sucrose 1,241 ± 150 kcal/18 days, chow 464 ± 36 kcal/18 days; Tukey’s post hoc tests: lard vs. sucrose, P < 0.0001; sucrose vs. chow, P < 0.0001; lard vs. chow, P = 0.93). There was a difference in relative total energy intake between nonpregnant and pregnant states, such that choice rats increased their energy intake to a greater extent at preconception as compared with pregnancy (Fig. 7C). In addition, choice rats consumed less sucrose and lard and more pelleted chow during pregnancy as compared with the nonpregnant state (Fig. 7D).
Fig. 7.
Daily energy intakes (A), daily chow intakes (B), relative total energy intake (C), relative total energy intake per food component (D), daily body weights (E), and body weight change (F) in the nonpregnant and pregnant states in chow and choice rats. Choice-diet-fed rats had greater daily energy intakes compared with chow-diet-fed rats and increased intake of pelleted chow during pregnancy as compared with the prenatal period. Although energy intakes remained higher in choice group during pregnancy, choice-diet-fed rats gained less weight during pregnancy compared with chow-diet-fed rats (calculated in a subset of animals that were euthanized on gestational day 20). BL, baseline. Data are means ± SE (n = 8 rats/group). Two-way mixed model ANOVA with repeated measures followed by Tukey’s post hoc test (A, B, and E), Student’s t-test (C), one-way ANOVA followed by Tukey’s post hoc test (D), and two-way ANOVA with repeated measures followed by Sidak’s post hoc test (F). *P < 0.0002 vs. lard, vs. sucrose
There was a significant main effect of pregnancy (P < 0.0001) but no main effect of diet (P = 0.81) or diet-by-state interaction (P = 0.75) for body weight (Fig. 7E). Preconception body weights were greater in choice compared with chow rats [choice (n = 9) 262.4 ± 5.4 g vs. chow (n = 9) 246.3 ± 5.3 g; Student’s t-test, P = 0.04]. In the nonpregnant state, choice rats gained more body weight compared with chow rats, but during pregnancy, choice-fed rats had a smaller weight gain compared with gestational age-matched (gestational day 20) chow rats (Fig. 7F).
There were no group differences in blood glucose [choice (n = 12) 79.2 ± 4.4 mg/dl vs. chow (n = 13) 86.6 ± 4.6 mg/dl; Mann-Whitney U test, P = 0.19] or serum insulin [choice (n = 7) 2.11 ± 1.7 ng/ml vs. chow (n = 5) 1.05 ± 0.1 ng/ml; Mann-Whitney U test, P = 0.53]. Serum leptin concentrations were higher in choice-diet-fed compared with chow-fed pregnant rats [choice (n = 6) 1.65 ± 0.41 ng/ml vs. chow (n = 6) 0.29 ± 0.05 ng/ml; Mann-Whitney U test, P = 0.002].
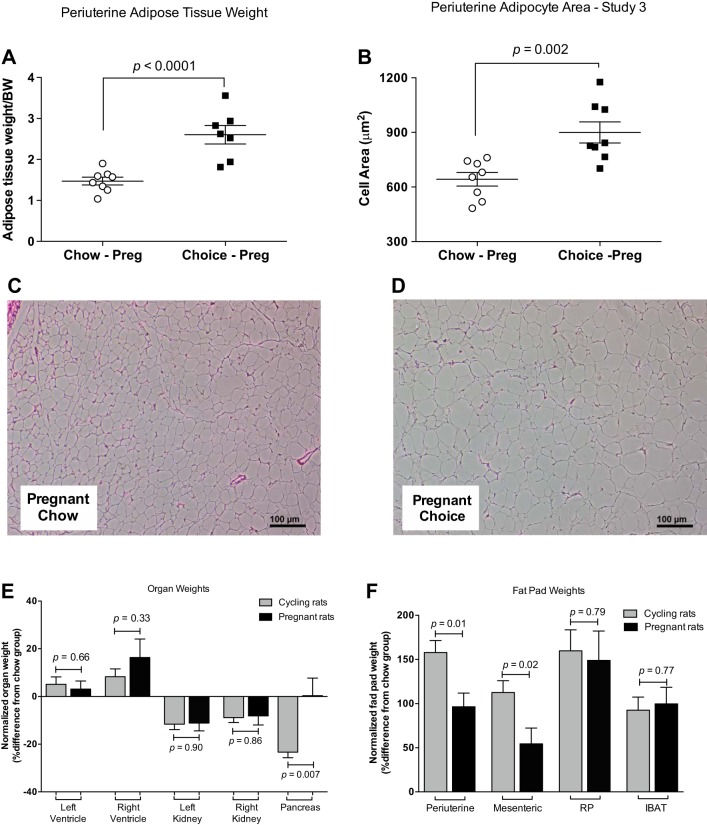
Absolute and normalized (to body weight) periuterine fat pad weights were heavier (Fig. 8A), and adipocyte area was larger in choice compared with chow pregnant rats (Fig. 8, B–D). Mesenteric, retroperitoneal, and intrascapular brown fat pads (absolute and normalized values) were heavier in choice compared with chow pregnant rats (Table 3). When normalized to body weight, right ventricular weights were larger and left kidney weights were smaller in choice compared with chow pregnant rats (Table 3). In contrast to the effects of choice diet on pancreas weight in the nonpregnant state (see study 2), there were no differences in pancreas weight between choice and chow pregnant rats (Table 3). This outcome did not change when absolute values were compared (data not shown). To compare the effects of choice diet on fat pad and organ weights between cycling (study 2) and pregnant rats (study 3), we expressed weights of choice rats relative to weights of chow (control) rats. Although periuterine and mesenteric adipose depot weights increased in response to choice diet in both cycling and pregnant rats, this increase was smaller during pregnancy (Fig. 8E). Furthermore, pancreas weight was reduced in response to choice diet only in the cycling rats (Fig. 8F).
Fig. 8.
Periuterine fat pad weight (A), adipocyte area/cell (B), representative images of histological sections of periuterine adipose tissue (C, D) in chow and choice pregnant rats, organ weights (E), and fat pad weights (F) of choice rats relative to those of chow rats within cycling (study 2) and pregnant rats (study 3). Periuterine adipose tissue weights were heavier and adipocyte area was larger in pregnant choice-diet-fed rats compared with pregnant chow-diet-fed rats. RP, retroperitoneal; IBAT, intrascapular brown adipose tissue. Data are means ± SE (n = 8 rats/group). Student’s t-test (A and B).
Table 3.
Fat pad and organ weights of late pregnant rats fed with pelleted chow (chow group) versus a free-choice high-fat, high-sucrose diet (choice group) 3 wk before conception and throughout pregnancy
| Chow | Choice | P Value | |
|---|---|---|---|
| Fat pad weights, g/100 g body wt | |||
| Mesenteric | 0.79 ± 0.05 (8) | 1.22 ± 0.14 (7) | 0.02 |
| Retroperitoneal | 0.52 ± 0.02 (8) | 1.28 ± 0.17 (7) | 0.004 |
| IBAT | 0.11 ± 0.01 (8) | 0.23 ± 0.02 (7) | 0.0002 |
| Organ weights, g/100 g body wt | |||
| Left ventricle | 0.16 ± 0.01 (9) | 0.17 ± 0.01 (8) | 0.22 |
| Right ventricle | 0.04 ± 0.00 (9) | 0.05 ± 0.00 (8) | 0.04 |
| Left kidney | 0.23 ± 0.01 (8) | 0.20 ± 0.01 (7) | 0.046 |
| Right kidney | 0.23 ± 0.01 (8) | 0.21 ± 0.02 (7) | 0.17 |
| Pancreas | 0.34 ± 0.03 (8) | 0.34 ± 0.02 (7) | 0.97 |
Data are means ± SE; number of rats is in parentheses. Student’s t-test.
In pregnancies terminated on gestational day 20, fetal weights were 25% lower in choice-diet-fed rats compared with chow-fed rats [choice (n = 5) 3.03 ± 0.36 g vs. chow (n = 5) 2.26 ± 0.08 g; Student’s t-test, P = 0.07]. There were no group differences in litter size [choice (n = 5) 14 vs. chow (n = 5) 13; Mann-Whitney U test, P = 0.28] and placental weights [choice (n = 5) 0.471 ± 0.021 g vs. chow (n = 5) 0.506 ± 0.016 g; Student’s t-test, P = 0.23].
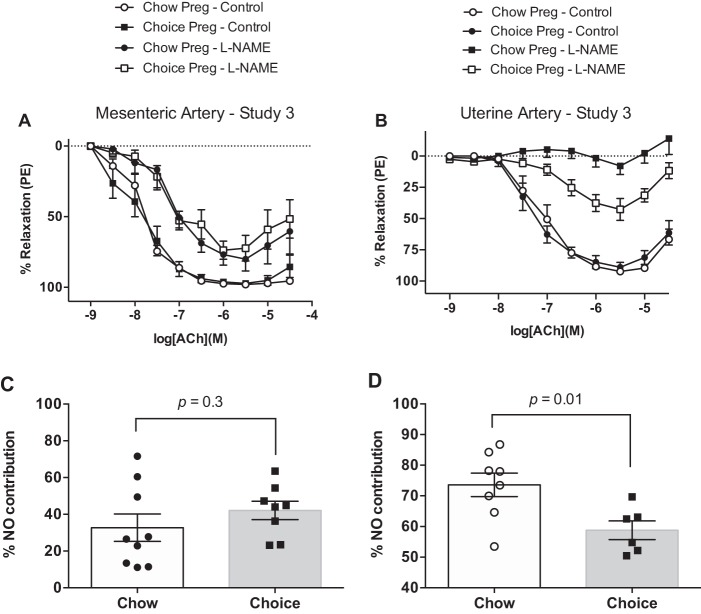
Mean arterial pressure did not differ between choice-diet-fed dams and chow-fed dams [choice (n = 6) 85.0 ± 3.1 mmHg vs. chow (n = 7) 81.1 ± 2.0 mmHg; Student’s t-test, P = 0.31]. There were no group differences in relaxation responses to ACh either in mesenteric or in uterine artery (Table 3, Fig. 9, A and B). In mesenteric arteries, incubation with l-NAME reduced total relaxation to ACh in both groups [AUC, ACh alone vs. ACh + l-NAME: chow (n = 9) 319.5 ± 8.9 vs. 216.3 ± 25.9; Tukey’s post hoc test, P < 0.004; choice (n = 8) 322.5 ± 20.7 vs. 186.8 ± 21.0; Tukey’s post hoc test, P < 0.0004]. l-NAME reduced ACh-induced relaxation (AUC) in uterine arteries from chow and choice rats as well [AUC, ACh alone vs. ACh + l-NAME: chow (n = 8) 230.8 ± 11.7 vs. 58.7 ± 6.5; Tukey’s post hoc test, P < 0.0001; choice (n = 7) 231.1 ± 17.0 vs. 84.7 ± 13.4; Tukey’s post hoc test, P < 0.0001]. The extent of this inhibition was smaller in chow-diet-fed rats compared with choice-diet-fed rats, indicating reduced NO contribution to ACh-induced relaxation in uterine arteries of choice-diet-fed compared with chow pregnant rats (Fig. 9, C and D).
Fig. 9.
Concentration-response curves to acetylcholine (ACh) in the presence and absence of NG-nitro-l-arginine methyl ester [l-NAME; nitric oxide (NO) synthase inhibitor] in mesenteric (A) and uterine (B) arteries from pregnant rats in study 2. Cumulative effect of ACh in the presence and absence of l-NAME was quantified as area under the curve (AUC) for mesenteric (C) and uterine (D) arteries. NO contribution was defined as the difference between ACh AUC and ACh + l-NAME AUC. There were no group differences in mesenteric and uterine artery responses to ACh, but choice diet reduced NO contribution to ACh-induced relaxation in uterine arteries. Data are means ± SE (A and C: n = 8 rats/group; B and D: n = 8 chow rats and n = 6 choice rats). Student’s t-test.
In summary, free-choice high-fat, high-sucrose diet before and throughout rat pregnancy increased maternal energy intake, serum leptin concentrations, and adiposity and reduced fetal weights. In addition, access to free-choice diet altered regulatory mechanisms of endothelium-dependent dilation in uterine arteries of pregnant rats.
DISCUSSION
Our main findings were 1) in the nonpregnant state, a free-choice high-fat, high-sucrose diet induced hyperphagia and features of the metabolic syndrome, including adipose tissue expansion, hypertriglyceridemia, high blood pressure, and glucose intolerance in female rats; and 2) rats exposed to a free-choice diet before and throughout pregnancy remained hyperphagic and hyperleptinemic and developed uterine artery endothelial dysfunction but did not have high blood pressure in late pregnancy. To the best of our knowledge, this is the first study to provide a comprehensive characterization of physiological responses to a free-choice high-fat, high-sucrose diet in female rats at various reproductive states, including pregnancy.
Initial exposure to choice diet (first 3–4 days) induced a spike in energy intake, which was attributed to the novelty of the diet. Although energy intake was reduced after the initial spike, it remained higher in the choice group compared with chow group until the end of the study, suggesting that high-fat, high-sucrose diet induced a sustained hyperphagic response in female cycling and pregnant rats. It has been demonstrated that the hyperphagic and metabolic responses of choice diet are primarily due to the form of sucrose (i.e., liquid vs. solid) and the element of choice (1, 18, 24, 53).
In choice cycling and pregnant rats, the majority of total calories were derived from sucrose solution. The metabolic responses to a choice diet and the preference for sucrose were similar to those reported previously in adult male rats offered a similar choice diet (1, 2, 18, 24, 25). Interestingly, lard was the preferred food in study 1, in which sucrose solution was created by mixing sugar in distilled water. This may be attributed to lower palatability of distilled water compared with tap water. Differences in housing (wire mesh vs. standard bedding) may also account for group differences in food preferences. The results of study 1 informed modifications of experimental design in study 2 and study 3 to increase food palatability and caloric intake. It is noteworthy that adipose tissue expansion, hyperphagia, and changes in kidney/cardiac weights were common outcomes of both studies. This indicates that an increase in caloric intake was adequate to induce adipose tissue and cardiovascular organ remodeling. Remarkably, increase in intake also induced changes in eating behavior of female cycling rats, regardless of whether sucrose or lard was the major caloric contributor. On the other hand, glucose intolerance required a higher caloric intake and possibly overconsumption of sucrose (instead of lard) to manifest (study 1 vs. study 2).
All rats increased their energy intake during pregnancy compared with the nonpregnant state, but this increase was smaller in choice compared with chow rats. Furthermore, choice rats increased intake of pelleted chow during pregnancy, while slightly reducing lard and sucrose intakes. Given that pelleted chow was the only source of protein and micronutrients for the choice group, this change in eating behavior and food preference may be explained by an increase in need for protein and micronutrients during pregnancy. These findings support previous studies demonstrating that animals eat for their protein needs (14, 22, 23, 46). Adult nonpregnant rats require ~7% of energy to derive from natural (nonpurified) diet to maintain their weight (4), while these requirements increase during pregnancy to ~18–22% (26). The pelleted chow provided in this study had 24% minimum protein. Thus we estimated (1) that chow rats consumed ~4.1 g/day protein in the nonpregnant state and ~5.1 g/day during pregnancy, while choice rats consumed ~1.3 g/day protein in the nonpregnant state and ~2.1 g/day during pregnancy. Hence, in the nonpregnant state, choice rats consumed ~23% more protein, whereas during pregnancy they consumed ~11% more protein than was needed for weight maintenance and gestation, respectively. This evidence suggests that choice rats were not protein deficient either in the nonpregnant or pregnant state. The carcass analysis in cycling rats provided further support that choice rats were not protein deficient. There were no differences in protein or lean body mass (Table 1). While not significant, the choice cycling rats actually had more total body protein. Even if choice rats were not protein deficient, we cannot exclude that the reduction in protein intake in combination with increased caloric intake might have an adverse effect on end points measured in this study (e.g., fetal weights).
Free-choice high-fat, high-sucrose diet induced adipocyte hypertrophy in periuterine adipose tissue in both cycling and pregnant rats. The increase in adipocyte size is in line with previous studies examining visceral fat depots in other animal models of obesity (57) and reflects adipose tissue expansion and possibly inflammation (5, 48, 56). Expansion of all adipose depots, concomitant with an increase in serum leptin concentrations, suggests morphological but also functional changes of adipose tissue in response to free-choice diet. In pregnant women, inflammatory and immune pathways are activated in white adipose tissue and are associated with normal metabolic adaptations that facilitate successful pregnancy outcomes (33, 43). In obese pregnancies, however, excess adipose tissue inflammation, immune cell infiltration, and enhanced adipose tissue hypertrophy are associated with maternal metabolic dysfunction and poor pregnancy outcomes (11, 52). Choice-diet-fed cycling and pregnant rats overconsumed throughout the dietary intervention, suggesting that they might have also developed leptin resistance. Indeed, Apolzan and Harris (2) have demonstrated that free access to sucrose, lard, and chow for 3 wk increased circulating leptin and caused leptin resistance in male rats.
In addition to adipose depots, organ weights also differed between choice and chow cycling and pregnant rats, suggesting potential metabolic and cardiovascular functional changes. Pregnancy per se is associated with extensive remodeling of the cardiovascular system, and thus it is unknown whether changes in kidney and heart weights were a consequence of an interaction between pregnancy and diet. Interestingly, pancreatic weights were smaller in choice compared with chow cycling rats but did not differ between diet groups at the pregnant state. A reduction in pancreas size has been previously associated with type 1 and type 2 diabetes (12, 31, 58). In our study, choice-diet-fed cycling rats were glucose intolerant with normal basal insulin concentrations. With regard to pregnancy, previous studies have shown that pregnancy restores insulin secretion from pancreatic islets in cafeteria-diet-induced obese rats, possibly because of increased metabolic activity associated with restored calcium handling mechanisms (54). Although we did not assess pancreatic islet function in the present study, the lack of changes in pancreas weights in response to choice diet during pregnancy concomitant with normal insulin levels suggests that pregnancy might have reversed choice-diet-induced pancreatic atrophy and dysfunction in these animals.
Choice-diet-fed rats had higher blood pressure compared with chow rats at the end of 3 wk on the choice diet at preconception. A reduction in kidney weights and an increase in cardiac ventricular weights suggest renal and cardiac changes that might contribute to a hypertensive phenotype in the choice group. An alternative interpretation is that choice-diet-induced hypertension in cycling rats was not the consequence but instead was the cause of renal and cardiac weights. The differences between chow and choice rats in blood pressure were abolished during pregnancy. It has been reported that exposure to high-fat diet from prepubertal age until young adulthood before mating and throughout pregnancy increased maternal blood pressure (19). Similar findings were reported in female mice exposed to high-fat diet for 4 wk before and throughout pregnancy (32). Others, however, demonstrated no changes in maternal mean arterial pressure in response to a high-fat diet in rats (40). These discrepancies may be attributed to different timing of diet (i.e., pubertal stage), diet duration, percentage of fat consumed, and different methods used to assess blood pressure (i.e., tail cuff vs. indwelling catheters). In the present study, blood pressure was measured in anesthetized rats. It is unknown whether anesthesia differentially affected chow compared with choice rats and if measurements in conscious rats would reveal a different outcome.
Uterine but not resistance mesenteric arteries from choice cycling rats were more sensitive to ACh compared with arteries from chow rats, while during pregnancy there were no differences in endothelium-dependent relaxation of uterine arteries between chow and choice rats. In addition, the contribution of NO to endothelium-dependent relaxation responses was significantly reduced in uterine arteries from choice-diet-fed pregnant rats compared with chow dams. A reduction in NO contribution concomitant with no change in cumulative vascular relaxation responses suggests that activation of other regulatory mechanisms (e.g., prostacyclin, non-NO endothelium-dependent hyperpolarization) compensated for the deficiency in NO-dependent vasodilatory pathways. Similar results have been reported in uterine arteries from diabetic mice (49). On the other hand, no changes in endothelium-dependent relaxation were found in uterine arteries from pregnant rats fed with a diet supplemented with 20% wt/wt lard (50). These authors, however, did not assess the contribution of NO to uterine artery dilatory responses (50).
Rats consuming the free-choice high-fat, high-sucrose diet during pregnancy had smaller fetal weights compared with controls. Both fetal macrosomia (7) and intrauterine growth restriction (13, 42) have been reported in human obese pregnancies. In our study, the reduction in fetal weights could be a result of the detrimental effects of lard and sucrose or reduced protein intake or a secondary effect of systemic and adipose tissue inflammation. Maternal hyperleptinemia may have also affected fetal growth. Human studies have provided mixed results, with some reporting a positive association between hyperleptinemia and macrosomia or elevated adiposity at birth (21), while others showing lower maternal leptin concentrations in women with small-for-gestational-age infants (8) or no association between maternal leptin and fetal growth (6). Studies in rodents reported an association between hyperleptinemia and low birth weight in models of intrauterine growth restriction induced by maternal glucocorticoid treatment. Furthermore, treatment with exogenous leptin during pregnancy results in smaller fetuses in rats (39).
Perspectives and Significance
Panchal and Brown (41) suggested that male rats fed high-fat, high-carbohydrate diets represent an ideal experimental model to study the mechanisms and consequences of human metabolic syndrome. Given that maternal obesity is one of the largest obstetric risk factors for maternal health, we wanted to extend these previous findings to female cycling and pregnant rats. Others have previously studied the effect of high-fat, high-sucrose diets on pregnancy outcomes (9, 27–29, 37, 47), but their focus was geared toward understanding how these diets affect fetal development and offspring health. Our studies are the first to provide a comprehensive evaluation of a free-choice high-fat, high-sucrose diet on maternal metabolic and cardiovascular functions in female rats, extending the previous studies in males to female cycling and pregnant rodents. Another novel aspect of our studies is that we demonstrated that although 3 wk on a free-choice diet were not adequate to induce endothelial dysfunction in resistance arteries, it was sufficient to induce hypertension, potentially via renal and cardiac mechanisms. This evidence provides an experimental window to examine diet-induced maternal hypertension in the absence of resistance artery endothelial dysfunction at preconception. We reported a reduction in fetal weights in choice-fed rats. Given that maternal obesity is a risk factor for fetal health and offspring cardiometabolic disease, further investigation of the effects of choice diet on fetal development and developmental programming is warranted. Such studies should consider species differences in the timing of development relative to birth between rats and humans to maximize the translational implications of their findings, especially if therapeutic interventions are tested (10, 35, 36, 51). We conclude that a free-choice high-fat, high-sucrose diet may provide a new and alternative rodent model of preconceptual maternal obesity to study the role of increased energy intake, individual food components, and preexisting maternal obesity on maternal and offspring physiological responses during pregnancy and after birth.
GRANTS
This research was supported in part by National Institutes of Health Grants T32-HL066993-09 (to S. Goulopoulou) and U54 GM10490 (to J. W. Apolzan), the Heart and Stroke Foundation Canada (to J. L. Hannan), a University of North Texas Health Science Center Intramural Pilot Grant and the Department of Physiology and Anatomy in the University of North Texas Health Science Center (to S. Goulopoulou).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.H., J.W.A., and S.G. conceived and designed research; H.A., J.L.H., J.W.A., O.O., S.C.C., and S.G. performed experiments; H.A., J.L.H., J.W.A., O.O., S.C.C., S.A.R., and S.G. analyzed data; H.A., J.L.H., J.W.A., S.A.R., and S.G. interpreted results of experiments; H.A. and S.G. prepared figures; H.A. and S.G. drafted manuscript; H.A., J.L.H., J.W.A., O.O., S.C.C., S.A.R., and S.G. edited and revised manuscript; H.A., J.L.H., J.W.A., O.O., S.C.C., S.A.R., and S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Ruth Harris (Dept. of Physiology, Augusta University) for assisting with body carcass processing and liver lipid measurements. In addition, we thank Dr. R. Clinton Webb (Dept. of Physiology, Augusta University) for providing laboratory space and resources and guidance through study design.
REFERENCES
- 1.Apolzan JW, Harris RBS. Differential effects of chow and purified diet on the consumption of sucrose solution and lard and the development of obesity. Physiol Behav 105: 325–331, 2012. doi: 10.1016/j.physbeh.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apolzan JW, Harris RBS. Rapid onset and reversal of peripheral and central leptin resistance in rats offered chow, sucrose solution, and lard. Appetite 60: 65–73, 2013. doi: 10.1016/j.appet.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet 115, Suppl 1: S6–S10, 2011. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 4.Bricker ML, Mitchell HH. The protein requirements of the adult rat in terms of the protein contained in egg, milk and soy flour. J Nutr 34: 491–505, 1947. doi: 10.1093/jn/34.5.491. [DOI] [PubMed] [Google Scholar]
- 5.Brune JE, Kern M, Kunath A, Flehmig G, Schön MR, Lohmann T, Dressler M, Dietrich A, Fasshauer M, Kovacs P, Stumvoll M, Blüher M, Klöting N. Fat depot-specific expression of HOXC9 and HOXC10 may contribute to adverse fat distribution and related metabolic traits. Obesity (Silver Spring) 24: 51–59, 2016. doi: 10.1002/oby.21317. [DOI] [PubMed] [Google Scholar]
- 6.Castro NP, Euclydes VV, Simões FA, Vaz-de-Lima LR, De Brito CA, Luzia LA, Devakumar D, Rondó PH. The relationship between maternal plasma leptin and adiponectin concentrations and newborn adiposity. Nutrients 9: 182, 2017. doi: 10.3390/nu9030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DR, Persson B, Hod M, Oats JJ; HAPO Study Cooperative Research Group . The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35: 780–786, 2012. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, Roberts JM. Maternal leptin across pregnancy in women with small-for-gestational-age infants. Am J Obstet Gynecol 196: 558.e1–558.e8, 2007. doi: 10.1016/j.ajog.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Crew RC, Waddell BJ, Mark PJ. Maternal obesity induced by a “cafeteria” diet in the rat does not increase inflammation in maternal, placental or fetal tissues in late gestation. Placenta 39: 33–40, 2016. doi: 10.1016/j.placenta.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson H, Moss TJ, Gatford KL, Moritz KM, Akison L, Fullston T, Hryciw DH, Maloney CA, Morris MJ, Wooldridge AL, Schjenken JE, Robertson SA, Waddell BJ, Mark PJ, Wyrwoll CS, Ellery SJ, Thornburg KL, Muhlhausler BS, Morrison JL. A review of fundamental principles for animal models of DOHaD research: an Australian perspective. J Dev Orig Health Dis 7: 449–472, 2016. doi: 10.1017/S2040174416000477. [DOI] [PubMed] [Google Scholar]
- 11.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ Jr, Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE. Feto-placental adaptations to maternal obesity in the baboon. Placenta 30: 752–760, 2009. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia TS, Rech TH, Leitão CB. Pancreatic size and fat content in diabetes: a systematic review and meta-analysis of imaging studies. PLoS One 12: e0180911, 2017. doi: 10.1371/journal.pone.0180911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population-based percentiles. Am J Obstet Gynecol 201: 28.e1–28.e8, 2009. doi: 10.1016/j.ajog.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Obes Rev 15: 183–191, 2014. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- 15.Goulopoulou S, Hannan JL, Matsumoto T, Ogbi S, Ergul A, Webb RC. Reduced vascular responses to soluble guanylyl cyclase but increased sensitivity to sildenafil in female rats with type 2 diabetes. Am J Physiol Heart Circ Physiol 309: H297–H304, 2015. doi: 10.1152/ajpheart.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RB. Factors influencing energy intake of rats fed either a high-fat or a fat mimetic diet. Int J Obes Relat Metab Disord 18: 632–640, 1994. [PubMed] [Google Scholar]
- 17.Harris RB. Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. J Nutr 121: 1075–1080, 1991. doi: 10.1093/jn/121.7.1075. [DOI] [PubMed] [Google Scholar]
- 18.Harris RB, Apolzan JW. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol 302: R1327–R1339, 2012. doi: 10.1152/ajpregu.00477.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez-Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC, Raha S. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One 7: e33370, 2012. doi: 10.1371/journal.pone.0033370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain MA, Abogresha NM, Hassan R, Tamany DA, Lotfy M. Effect of feeding a high-fat diet independently of caloric intake on reproductive function in diet-induced obese female rats. Arch Med Sci 12: 906–914, 2016. doi: 10.5114/aoms.2016.59790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josefson JL, Zeiss DM, Rademaker AW, Metzger BE. Maternal leptin predicts adiposity of the neonate. Horm Res Paediatr 81: 13–19, 2014. doi: 10.1159/000355387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriazakis I, Emmans GC, Whittemore CT. The ability of pigs to control their protein intake when fed in three different ways. Physiol Behav 50: 1197–1203, 1991. doi: 10.1016/0031-9384(91)90582-9. [DOI] [PubMed] [Google Scholar]
- 23.Kyriazakis I, Oldham JD. Diet selection in sheep: the ability of growing lambs to select a diet that meets their crude protein (nitrogen × 6.25) requirements. Br J Nutr 69: 617–629, 1993. doi: 10.1079/BJN19930064. [DOI] [PubMed] [Google Scholar]
- 24.La Fleur SE, Luijendijk MC, van Rozen AJ, Kalsbeek A, Adan RA. A free-choice high-fat high-sugar diet induces glucose intolerance and insulin unresponsiveness to a glucose load not explained by obesity. Int J Obes 35: 595–604, 2011. doi: 10.1038/ijo.2010.164. [DOI] [PubMed] [Google Scholar]
- 25.La Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes 31: 1286–1294, 2007. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SM, Barnard DE, Knapka JJ. Nutrition. Burlington, MA: Elsevier Academic, 2006. [Google Scholar]
- 27.Loche E, Blackmore HL, Carpenter AA, Beeson JH, Pinnock A, Ashmore TJ, Aiken CE, de Almeida-Faria J, Schoonejans JM, Giussani DA, Fernandez-Twinn DS, Ozanne SE. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc Res 114: 1372–1384, 2018. doi: 10.1093/cvr/cvy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lock M, McGillick EV, Orgeig S, McMillen IC, Morrison JL. Regulation of fetal lung development in response to maternal overnutrition. Clin Exp Pharmacol Physiol 40: 803–816, 2013. doi: 10.1111/1440-1681.12166. [DOI] [PubMed] [Google Scholar]
- 29.Lock MC, McGillick EV, Orgeig S, McMillen IC, Mühlhäusler BS, Zhang S, Morrison JL. Differential effects of late gestation maternal overnutrition on the regulation of surfactant maturation in fetal and postnatal life. J Physiol 595: 6635–6652, 2017. doi: 10.1113/JP274528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovejoy JC, Sainsbury A; Stock Conference 2008 Working Group . Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 10: 154–167, 2009. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 31.Macauley M, Percival K, Thelwall PE, Hollingsworth KG, Taylor R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One 10: e0126825, 2015. doi: 10.1371/journal.pone.0126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuyama H, Hiramatsu Y. Treatment with a constitutive androstane receptor ligand ameliorates the signs of preeclampsia in high-fat diet-induced obese pregnant mice. Mol Cell Endocrinol 348: 120–127, 2012. doi: 10.1016/j.mce.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 33.Mazaki-Tovi S, Vaisbuch E, Tarca AL, Kusanovic JP, Than NG, Chaiworapongsa T, Dong Z, Hassan SS, Romero R. Characterization of visceral and subcutaneous adipose tissue transcriptome and biological pathways in pregnant and non-pregnant women: evidence for pregnancy-related regional-specific differences in adipose tissue. PLoS One 10: e0143779, 2015. doi: 10.1371/journal.pone.0143779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mission JF, Marshall NE, Caughey AB. Pregnancy risks associated with obesity. Obstet Gynecol Clin North Am 42: 335–353, 2015. doi: 10.1016/j.ogc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, Giussani DA, Gray CL, Harding R, Herrera EA, Kemp MW, Lock MC, McMillen IC, Moss TJ, Musk GC, Oliver MH, Regnault TRH, Roberts CT, Soo JY, Tellam RL. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol 315: R1123–R1153, 2018. doi: 10.1152/ajpregu.00391.2017. [DOI] [PubMed] [Google Scholar]
- 36.Morrison JL, Botting KJ, Darby JRT, David AL, Dyson RM, Gatford KL, Gray C, Herrera EA, Hirst JJ, Kim B, Kind KL, Krause BJ, Matthews SG, Palliser HK, Regnault TRH, Richardson BS, Sasaki A, Thompson LP, Berry MJ. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol 596: 5535–5569, 2018. doi: 10.1113/JP274948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong ZY, Wanasuria AF, Lin MZ, Hiscock J, Muhlhausler BS. Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite 65: 189–199, 2013. doi: 10.1016/j.appet.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Osikoya O, Jaini PA, Nguyen A, Valdes M, Goulopoulou S. Effects of low-dose aspirin on maternal blood pressure and vascular function in an experimental model of gestational hypertension. Pharmacol Res 120: 267–278, 2017. doi: 10.1016/j.phrs.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Palei AC, Spradley FT, Granger JP. Chronic hyperleptinemia results in the development of hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 308: R855–R861, 2015. doi: 10.1152/ajpregu.00286.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palei AC, Spradley FT, Granger JP. Role of nitric oxide synthase on blood pressure regulation and vascular function in pregnant rats on a high-fat diet. Am J Hypertens 30: 240–248, 2017. doi: 10.1093/ajh/hpw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol 2011: 351982, 2011. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, Gillman MW. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 4: 1025–1036, 2016. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 43.Resi V, Basu S, Haghiac M, Presley L, Minium J, Kaufman B, Bernard S, Catalano P, Hauguel-de Mouzon S. Molecular inflammation and adipose tissue matrix remodeling precede physiological adaptations to pregnancy. Am J Physiol Endocrinol Metab 303: E832–E840, 2012. doi: 10.1152/ajpendo.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolls BJ, Van Duijvenvoorde PM, Rowe EA. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol Behav 31: 21–27, 1983. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- 45.Sclafani A, Springer D, Kluge L. Effects of quinine adulterated diets on the food intake and body weight of obese and non-obese hypothalamic hyperphagic rats. Physiol Behav 16: 631–640, 1976. doi: 10.1016/0031-9384(76)90225-0. [DOI] [PubMed] [Google Scholar]
- 46.Shariatmadari F, Forbes JM. Growth and food intake responses to diets of different protein contents and a choice between diets containing two concentrations of protein in broiler and layer strains of chicken. Br Poult Sci 34: 959–970, 1993. doi: 10.1080/00071669308417656. [DOI] [PubMed] [Google Scholar]
- 47.Sinclair KJ, Friesen-Waldner LJ, McCurdy CM, Wiens CN, Wade TP, de Vrijer B, Regnault TRH, McKenzie CA. Quantification of fetal organ volume and fat deposition following in utero exposure to maternal Western Diet using MRI. PLoS One 13: e0192900, 2018. doi: 10.1371/journal.pone.0192900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92: 1023–1033, 2007. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 49.Stanley JL, Ashton N, Taggart MJ, Davidge ST, Baker PN. Uterine artery function in a mouse model of pregnancy complicated by diabetes. Vascul Pharmacol 50: 8–13, 2009. doi: 10.1016/j.vph.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Taylor PD, Khan IY, Lakasing L, Dekou V, O’Brien-Coker I, Mallet AI, Hanson MA, Poston L. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol 88: 389–398, 2003. doi: 10.1113/eph8802495. [DOI] [PubMed] [Google Scholar]
- 51.Taylor PD, Matthews PA, Khan IY, Rees D, Itani N, Poston L. Generation of maternal obesity models in studies of developmental programming in rodents. Methods Mol Biol 1735: 167–199, 2018. doi: 10.1007/978-1-4939-7614-0_9. [DOI] [PubMed] [Google Scholar]
- 52.Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci 1205: 76–81, 2010. doi: 10.1111/j.1749-6632.2010.05667.x. [DOI] [PubMed] [Google Scholar]
- 53.Van de Giessen E, la Fleur SE, de Bruin K, van den Brink W, Booij J. Free-choice and no-choice high-fat diets affect striatal dopamine D2/3 receptor availability, caloric intake, and adiposity. Obesity (Silver Spring) 20: 1738–1740, 2012. doi: 10.1038/oby.2012.17. [DOI] [PubMed] [Google Scholar]
- 54.Vanzela EC, Ribeiro RA, de Oliveira CA, Rodrigues FB, Bonfleur ML, Carneiro EM, Souza KL, Boschero AC. Pregnancy restores insulin secretion from pancreatic islets in cafeteria diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 298: R320–R328, 2010. doi: 10.1152/ajpregu.00256.2009. [DOI] [PubMed] [Google Scholar]
- 55.Volk KM, Pogrebna VV, Roberts JA, Zachry JE, Blythe SN, Toporikova N. High-fat, high-sugar diet disrupts the preovulatory hormone surge and induces cystic ovaries in cycling female rats. J Endocr Soc 1: 1488–1505, 2017. doi: 10.1210/js.2017-00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Yu C, Feng J, Chen J, Jiang Q, Kuang S, Wang Y. Depot-specific differences in fat mass expansion in WT and ob/ob mice. Oncotarget 8: 46326–46336, 2017. doi: 10.18632/oncotarget.17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yagihashi S. Diabetes and pancreas size, does it matter? J Diabetes Investig 8: 413–415, 2017. doi: 10.1111/jdi.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang T, Xu WJ, York H, Liang NC. Diet choice patterns in rodents depend on novelty of the diet, exercise, species, and sex. Physiol Behav 176: 149–158, 2017. doi: 10.1016/j.physbeh.2017.02.045. [DOI] [PubMed] [Google Scholar]