Abstract
OBJECTIVE
To determine if the vascular damage in bladders of prostate cancer (PCa) survivors with radiation cystitis can be detected through altered angiogenic growth factors in urine.
METHODS
Urine samples from PCa survivors with a history of external beam radiation therapy were tested for a panel of angiogenic growth factors by Luminex assay. Urine creatinine levels were measured through high performance liquid chromatography. Through a patient survey, data on patient demographics, radiation history, and urinary symptoms were collected.
RESULTS
Hepatocyte growth factor (HGF), placental growth factor (PlGF), and vascular endothelial growth factor (VEGF) were altered in urine of PCa survivors with a history of radiation therapy. HGF and PlGF were elevated in response to irradiation, while VEGF had a decreasing trend. Within the irradiated population, HGF was also increased in patients diagnosed with radiation cystitis and patients with hematuria. PlGF and VEGF were only increased in the first year postirradiation, and VEGF was elevated in patients with hematuria. Finally, creatinine levels were increased in PCa survivors with a history of radiation therapy.
CONCLUSION
Radiation cystitis is a debilitating bladder condition that cancer survivors are at risk of developing after pelvic radiation. In this study, we identified 3 pro-angiogenic factors that may be urine biomarkers and, if validated in future studies, could indicate new strategy approaches to treat radiation cystitis.
Prostate cancer (PCa) is the most common cancer in males. Over 164,000 men will be diagnosed with PCa in the United States in 2018.1 Due to advances in early detection and cancer therapy, PCa 5 year survival rate has reached 99.3%. Approximately 3.3 million PCa survivors currently live in the United States.2 With a growing number of survivors, long-term side effects from cancer therapy are a growing concern. Radiation therapy (RT) for pelvic cancers can result in radiation cystitis (RC), a radiation-induced chronic inflammatory condition of the bladder. This is especially true for PCa given its intimate proximity with the bladder.
RC is a severely debilitating bladder condition that can develop months to decades after radiation exposure. Urologic adverse events caused by RC include frequency, dysuria, urgency, nocturia, suprapubic pain, bladder infection, fatigue, and both microscopic and gross hematuria.3 The severity of RC is classified from grade 1-5 based on the patient's symptoms according to the common terminology criteria for adverse events version 4.0 grading system. RC symptom range from microscopic hematuria with minimal increase in frequency, urgency, dysuria or nocturia, and new onset of incontinence (grade 1) to gross hematuria, resulting in the need for blood transfusions and/or hospitalization (grade 3-4), and in the most severe cases, death (grade 5).
Despite technical advances in radiation oncology to minimize radiation scatter to normal tissue without jeopardizing cancer treatment, PCa survivors remain at risk for developing bladder complications. In a recent study, the incidence of acute and chronic RC in PCa survivors who received a combination of intensity modulated radiation therapy (IMRT) followed by high dose brachytherapy was 36% and 30% respectively.4 In a large population-based study, Sheets and colleagues compared the incidence of urinary adverse effects in PCa patients of IMRT vs conformal RT, and of IMRT vs proton therapy. Neither IMRT nor proton therapy were able to improve radiation-induced urinary incontinence incidence in comparison to conformal RT or IMRT respectively.5 IMRT is the most commonly used strategy to target PCa; the benefit of proton therapy in PCa patients remains controversial. Due to high costs, the American association for radiation oncology only recommends proton therapy for insurance coverage for patients treated under the coverage with evidence development paradigm, for example IRB approved clinical trials. Thus, bladder related complications from RT in PCa survivors remain a major concern.
RC patients generally present with hematuria are diagnosed by cystoscopy and ruling out other causes of lower urinary tract symptoms (LUTS), including hematuria. Hematuria is a result from radiation-induced damage to the bladder vasculature and indicates an advance stage of RC. Hematuria may also be indicative of bladder cancer, and urine cystology and cystoscopy may be indicated in patients who are at risk of developing bladder cancer. Upon diagnosis, arresting bleeding is the primary focus. Treatments are dependent on the severity of hematuria and include clot evacuation, continuous irrigation, fulguration of bleeding sites, hyperbaric oxygen therapy, instillation of astringent agents, and, as a last resort, formalin instillation or cystectomy.3 Accumulated damage from RC may be irreversible and hence early diagnosis is essential in treating all RC symptoms and improving quality of life. Thus, there is a need to identify biomarkers for early detection of RC. In addition, biomarkers could aid in identifying novel and individualized treatment methods for PCa survivors with radiation damage to the bladder as current treatment modalities only offer temporary relief, and are time consuming and ineffective. Urine is an ideal bio sample for biomarker evaluation as it is in direct contact with the bladder and can be collected easily and non-invasively. As vascular damage is a prominent feature of RC, we sought to identify changes in vascular growth factors in the urine in response to RT.
MATERIALS AND METHODS
Urine and Data Collection
This study had Beaumont IRB approval (IRB 2015-302). All subjects provided written, informed consent with guarantees of confidentiality. PCa survivors with pelvic RT history filled out a survey and provided a urine sample. Urine samples were randomly collected, for example not first void of the day.6 De identified patient information including demographics, symptom severity, bladder health history, smoking status, and radiation history, were collected at Hospital Clínico San Borja Arriarán, Santiago de Chile, Chile with approval of the Chilean IEC, CEC-SSMC N° 96/16. On the questionnaire, patients ranked the incidence of urgency, hematuria (with or without blood clots), nocturia, incontinence, stress incontinence, and urogenital spasm on a scale from 0 (never) to 5 (multiple times a day). Exclusion criteria were history of chemotherapy, interstitial cystitis, recurrent urinary tract infection, kidney and/or bladder stones, and prostatitis. In this patient cohort, patients were diagnosed with RC based on the presence of pale bladder mucosa and telangiectasia with or without ulcers and a decrease in bladder capacity as determined by the radiation therapy oncology group classification. Midstream urine was collected in sterile urine cups and mixed with a urine preservative (Norgen Biotek) to stabilize proteins at room temperature. Urine was subsequently transferred to a low-binding tube, and shipped to Beaumont Research Institute, Royal Oak, MI. The presence of hematuria and inflammation, as defined by the presence of nitrites or leukocytes, was verified using urine test strips (LW Scientific).
Luminex Assay
Urine samples were spun down for 5 minutes at 3000 rpm in a tabletop centrifuge to remove any debris. The supernatant was then vortexed before use. Levels of angiogenic growth factors were assessed in undiluted urine using milliplex human angiogenesis and/or growth factor magnetic bead panel (EMD Millipore) according to the manufacturer's instructions. All samples were run in duplicate on a Bio-Plex 200 system (BioRad). RC and control samples were run on the same plates. Standard curve and quality control samples were included on every plate.
Creatinine Levels
Creatinine determination was performed with a Shimadzu Prominence high performance liquid chromatography system including a dual pump system, delivering a mobile phase consisting of 25 mM citric acid and 3% acetonitrile (pH 6.0) at a flow rate of 0.5 ml/min. After diluting urine 1:50 in mobile phase, separation was done on a kinetix 2.6μ XB-C18 100A (100 × 4.60 mm) column (Phenomenex) with UV absorbance detection at 250 nm. Quantification was based on peak height as compared to creatinine standards (Acros Organics).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0. Statistical significance was determined with an unpaired student t- test using the Holm-Sidak method. Computations assume all samples are from populations with the same scatter. Results are expressed as mean ± standard error mean (SEM).
Data Statement
Data will be made available upon request.
RESULTS
Description of Patient Population
One hundred twelve urine samples were collected between March 2016 and September 2017. Demographic data and LUTS are summarized in Table 1. Seventy five patients were PCa survivors with a history of RT (irradiation (IRR) group), of which 10 (13.3%) had officially been diagnosed with RC at time of urine collection and 65 patients (86.7%) had not been diagnosed with RC (non-RC group). Patients with RC diagnosis did not undergo any therapy to treat RC-related symptoms at time of urine collection. All patients received external beam RT with a median of 3 years (range 1-10 years) for RC group and 5 years (range 0-29 years) for non-RC group post treatment at time of urine collection. The majority of patients (86.7%, n = 65/75) received 36-40 radiation treatments. Three patients (4.5%) received a higher number of treatments (41-45), while 9.3% (n=7/75) of participants received 16-20 or 31-35 treatments. The mean age was 70.5 (SEM= 2.4) and 72.8 (SEM= 0.85) years for the RC and non-RC groups respectively. Mean body mass, marital status, smoking status, and education level were equally distributed among both groups. Diabetes was more prevalent in the non-RC group. Higher incidence of frequency, urge and genitourinary spasm were reported in the RC group vs the non-RC group. Nocturia was a common complaint among RC and non-RC patients (100% and 73.8%; n = 10/10 and n = 48/65 respectively). Of all patients with nocturia, 81% (n = 47/58) had multiple voiding episodes per night. Only non-RC patients reported urinary incontinence (6.3%; n= 4/64). Four (40%) RC patients reported hematuria, of which 3 experienced gross hematuria. One non-RC patient reported hematuria. The control population consisted of 37 participants: 28 healthy men with no history of PCa and RT, and 9 PCa survivors that did not receive RT. None of the control men reported other urologic complications.
Table 1.
Demographic data of study participants
| Control | RC diagnosis | ||
|---|---|---|---|
| Total | (n = 37) | Yes (n = 10) | No (n = 65) |
| Age (y) | 69.0 (1.45) | 70.5 (2.4) | 72.8 (0.85) |
| BMI (kg/m2) | 26.4 (1.45) | 27.0 (3.1) | 27.2 (3.2) |
| Marital Status | n = 18 | n = 9 | n = 65 |
| Married and/or living together | 15 | 7 | 49 |
| Widow | 1 | 0 | 3 |
| Divorced and/or Separated | 1 | 1 | 8 |
| Single | 1 | 1 | 5 |
| Smoking Status | n = 9 | n = 9 | n = 65 |
| Never | 6 | 5 | 28 |
| Past | 3 | 3 | 25 |
| Yes | 0 | 1 | 12 |
| Education | n = 18 | n = 9 | n = 65 |
| < High School | 3 | 4 | 28 |
| High School | 7 | 3 | 24 |
| ≥ College | 8 | 2 | 13 |
| Diabetes | Unknown | 10% (n = 1) | 24.6% (n = 16) |
| Years Post IRR (median) | n.a. | 3 (range:1-10) | 5 (range: 0-29) |
| Radiation dose | n.a. | n = 10 | n = 65 |
| 16-20 | 0% | 6.2% (n = 4) | |
| 31-35 | 10% (n = 1) | 3.1% (n = 2) | |
| 36-40 | 90% (n = 9) | 86.2% (n = 56) | |
| 41-45 | 0% | 4.6% (n = 3) | |
| Lower urinary tract Symptoms | |||
| Frequency (>8 voids) | Unknown | 56% (n = 5/9) | 27.9% (n = 17/65) |
| 9-11 voids | 60% (n = 3) | 76.5% (n = 13) | |
| 12-14 voids | 0% | 23.5% (n = 4) | |
| 14-16 voids | 40% (n = 2) | 0% | |
| Urge | Unknown | 60% (n = 6/10) | 28.8% (n = 19/65) |
| Hematuria | Unknown | 40% (n = 4/10) | 1.5% (n = 1/65) |
| Gross hematuria | 75% (n = 3) | 0% (n = 0) | |
| Nocturia | Unknown | 100% (n = 10/10) | 72.7% (n = 48/65) |
| Once and/or night | 20% (n = 2) | 18.75% (n = 9) | |
| Multiple times and/or night | 80% (n = 8) | 81.25% (n = 39) | |
| Urinary incontinence | Unknown | 0% (n = 0/10) | 6.1% (n = 4/65) |
BMI, body mass index; RC, radiation cystitis.
Altered Levels of HGF, VEGF, and PlGF in Urine of Patients With Radiation Treatment History
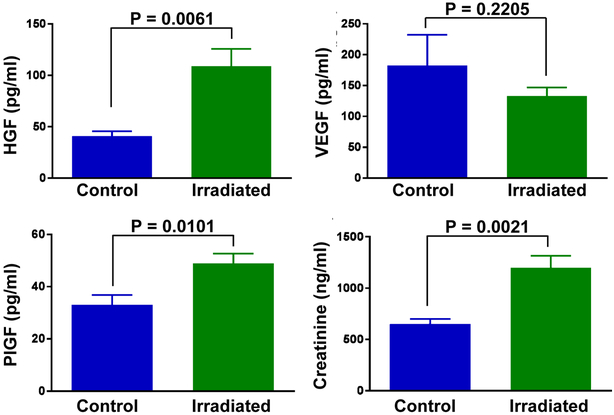
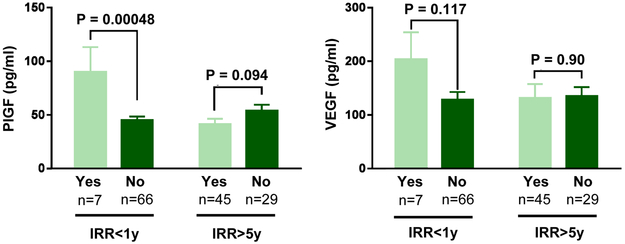
Urine samples were tested for detection of ten angiogenic growth factors. Hepatocyte growth factor (HGF), placental growth factor (PlGF), and vascular endothelial growth factor (VEGF) levels were altered in urine samples of cancer survivors with a history of RT. Both HGF and PlGF were significantly elevated in the IRR group in comparison to control (Fig. 1). HGF was also significantly increased in RC patient samples and in urine samples from patients with hematuria in comparison to non-RC and nonhematuria samples respectively (Table 2). Patients with high symptom scores had significantly higher levels of HGF, though growth factor levels were not altered in patients with frequency, as defined by 9 or more voids per day (Table 2). VEGF was significantly elevated in samples with hematuria (Table 2), but showed a decreasing trend in control vs IRR groups (Fig. 1). This trend was reversed when comparing RC and non-RC samples. In addition, urinary PlGF and VEGF were elevated within the first 12 months postirradiation, though this increase was only significant for PlGF (Fig. 2). Of the remaining proteins analyzed, Angiopoetin-2, BMP-9, endoglin, follistatin, and VEGF-D were undetectable in urine. No difference was found between control and IRR groups for epidermal growth factor, heparin binding epidermal growth factor like growth factor, and leptin (data not shown).
Figure 1.
Altered angiogenic growth factors and creatinine levels in urine of PCa survivors with a history of RT. Urine samples of control and/or nonirradiated (n = 37) and irradiated (n = 75) participants were tested for the presence of HGF, PlGF, VEGF, and creatinine. Data is shown as mean ± SEM. Statistical significance was determined using unpaired t test. (Color version available online.)
Table 2.
Urine protein values in RC versus non-RC PCa survivors. Data is shown as mean (± SEM). Statistical significance was determined using unpaired t test
| Yes | No | P Value | |
|---|---|---|---|
| RC Diagnosis | n = 10 | n = 65 | |
| HGF (pg/ml) | 208.6 (108.4) | 93.6 (10) | .0197 |
| PlGF (pg/ml) | 46.7 (7.5) | 49.2 (4.3) | .828 |
| VEGF (pg/ml) | 146.7 (50.8) | 131.0 (14.2) | .703 |
| Creatinine (ng/ml) | 929.1 (127.4) | 1251.3 (134.2) | .357 |
| Hematuria | n = 5 | n = 70 | |
| HGF (pg/ml) | 380.5 (204) | 89.5 (8.6) | < .0001 |
| PlGF (pg/ml) | 55.2 (12.5) | 48.4 (4)) | .659 |
| VEGF (pg/ml) | 244.7 (84.9) | 125.1 (13.4) | .0309 |
| Creatinine (ng/ml) | 1718.1 (735.2) | 1171.9 (116) | .251 |
| Frequency (>8 voids) | n = 22 | n = 47 | |
| HGF (pg/ml) | 80.4 (12.0) | 115.5 (25.3) | .36 |
| PlGF (pg/ml) | 46.6 (8.9) | 51.2 (4.3) | .6 |
| VEGF (pg/ml) | 96.6 (15.4) | 144.2 (19.5) | .122 |
| Creatinine (ng/ml) | 1018 (145.3) | 1271.1 (158) | .139 |
| Symptom Score >9 | n = 20 | n = 55 | |
| HGF (pg/ml) | 188.7 (56.2) | 79.9 (8.5) | .00374 |
| PlGF (pg/ml) | 49.5 (6.4) | 48.6 (4.7) | .925 |
| VEGF (pg/ml) | 173.6 (39.7) | 118.4 (12) | .0789 |
| Creatinine (ng/ml) | 1280 (227.5) | 1182.3 (139.1) | .717 |
HGF, hepatocytegrowthfactor; PlGF, placentalgrowthfactor; SEM, standarderrormean; VEGF, vascular endothelial growth factor.
Figure 2.
PlGF and VEGF levels in urine is upregulated in the first year post irradiation. Data is shown as mean ± SEM. Statistical significance was determined using unpaired t test. (Color version available online.)
Urine samples were also analyzed for creatinine. RT significantly increased urinary creatinine levels (Fig. 1). However, no statistically significant differences were observed within the IRR subgroups (Table 2).
COMMENT
Radiation-induced vascular damage to the bladder is a major hallmark of RC. While the underlying mechanism is not well-understood, it is thought that radiation damages the vasculature which, when repaired, results in fragile vessels. Due to repetitive expansion and contraction of the bladder as it stores then voids urine, brittle vessels are more prone to tearing and repetitive injury than vasculature in other irradiated organ systems. We hypothesize that vascular damage in the bladder in PCa survivors with a history of RT can be detected prior to the onset of hematuria through altered urinary angiogenic growth factor levels. We tested ten angiogenic growth factors in urine of men with and without a history of pelvic RT. HGF, VEGF, and PlGF demonstrated altered levels in comparison to our control population.
HGF was significantly elevated in patients with radiation history. Also, within the irradiated population, HGF was increased in patients with RC diagnosis and in patients with hematuria. HGF is implicated in angiogenesis by promoting endothelial cell proliferation, migration and survival. Nothing is known about HGF in the context of bladder inflammation or fibrosis, however the role of HGF in pulmonary fibrosis is well-established. In the lung, HGF is produced by macrophages and myofibroblasts, and subsequently binds its receptor (c-Met) on epithelial and endothelial cells to promote migration, proliferation, survival and morphogenesis, hereby enhancing normal tissue regeneration.7 HGF inhibits pulmonary fibrosis and prevents collagen deposition as demonstrated in various animal models of pulmonary fibrosis.7 High levels of HGF observed in urine of PCa survivors with a history of RT likely indicates ongoing tissue repair.
In addition to HGF, PlGF is elevated in urine of PCa survivors with a history of RT (Fig. 1). PlGF is a member of the VEGF family and is mainly implicated in pathologic angiogenesis, such as wound healing, colitis, atherosclerosis, liver cirrhosis, obesity and cancer. For example, PlGF promotes wound healing by stimulating vascularization, macrophage recruitment, migration of keratinocytes, and formation of granular tissue.8 PlGF has been proposed as a treatment target, either through PlGF suppression or stimulation, in numerous conditions. At a molecular level, PlGF acts by binding VEGFR1. This binding has a number of downstream effects: (1) PlGF stimulates VEGF activity as VEGF now is forced to bind to VEGFR2, which has stronger tyrosine kinase activity, (2) the activated PlGF:VEGFR1 enhances VEGF:VEGFR2 activity, and (3) PlGF:VEGFR1 increases the expression of various angiogenic growth factors (e.g. VEGF). Through these mechanisms, PlGF acts upon numerous cell types to stimulate vessel growth and maturation.8 Therefore, the observed elevated levels of PlGF in the urine of PCa survivors after RT are indicative of active vascular repair in the bladder tissue. Remarkably, the strongest increase in PlGF expression was observed in patients that had undergone RT within the last 12 months (Fig. 2), suggesting a role for PlGF in repairing initial radiation-induced damage to the bladder vasculature, possibly in a similar fashion as during wound healing. However, no change in PlGF levels was observed in patients who had been diagnosed with RC or who reported hematuria (Table 2). This suggests that PlGF would not be a good biomarker for early detection of RC. However, due to the role of PlGF in vascular repair and vessel maturation, upregulation of PlGF could prove beneficial in strengthening the small fragile blood vessels (telangiectasia) in RC patients. However, PlGF also has pro-inflammatory properties and can recruit macrophages and leukocytes, which needs to be taken into account when considering PlGF as a treatment strategy.9
VEGF, a master regulator of angiogenesis and vascular health, plays an important role in vascular development, maintenance and pathology, and is widely studied as a therapeutic target in various diseases such as cancer and diabetic retinopathy.10, 11 VEGF is produced and secreted by various cell types in response to hypoxia, though usually not by endothelial cells. However, PlGF can induce VEGF expression by endothelial cells, giving endothelial cells the capacity to activate themselves. Unlike HGF and PlGF, VEGF levels were not significantly altered in control vs irradiated population. In fact, VEGF levels showed a decreasing trend when comparing irradiated vs nonirradiated samples. Although not statistically significant, VEGF levels were higher in the first 12 months postirradiation, similar to PlGF. This small increase in VEGF can still have a large impact, especially in combination with increased expression of PlGF. VEGF has a higher binding affinity for VEGFR1 than VEGFR2. However, in the presence of PlGF, PlGF will directly compete with VEGF for binding to VEGFR1, forcing VEGF to bind to VEGFR2 instead. VEGFR2 has stronger pro-angiogenic activity. Finally, PlGF:VEGFR1 can stimulate VEGF:VEGFR2 downstream signaling. Thus, in the presence of elevated PlGF, VEGF can become more active, even without increased VEGF expression. In the first 12 months, postirradiation, PlGF, and VEGF likely work together to repair radiation-induced vascular damage. This elevation in PlGF and VEGF can be detected in urine samples. VEGF is also elevated in urine samples from patients with hematuria. Blood is likely the direct source of VEGF, especially since no difference is seen in patients with an RC diagnosis. The low levels of VEGF after irradiation except for the first 12 months post irradiation, is suggestive that VEGF therapy could enhance blood vessel repair. This is supported by a pre-clinical study where irradiated rat bladders treated with a single dose of 50ng recombinant VEGF resulted in significantly decreased fibrotic tissue and enhanced tissue vascularization. 12
Finally, creatinine levels were markedly higher in irradiated patients. Creatinine is a breakdown product of muscle tissue that is filtered and secreted through the kidneys to help maintain stable creatinine levels in the blood. For research purposes, creatinine is typically used to normalize urinary proteins to correct for dilute vs concentrated urine. Altered levels of creatinine can be diet-induced, or can indicate impaired kidney function. Elevated urine creatinine levels in irradiated PCa survivors can have multiple etiologies. It is feasible that creatinine leaks out of damaged blood vessels in the bladder or that high creatinine levels are a direct consequence of detrusor muscle breakdown. Lifestyle differences such as physical inactivity, diet, smoking status, or other co morbidities, can also influence urine creatinine levels as neither IRR nor control groups consisted of healthy men. Thus significance of elevated creatinine levels in the urine in patients with a history of RT will need further exploration.
Due to the lack of reliable symptoms or biomarkers for RC, patients often go years without diagnosis. There is an overwhelming need for early detection methods for RC. Radiation-induced damage to the bladder is currently irreversible, thus early intervention is necessary to prevent further damage and a decline in quality of life. In addition, an early RC diagnosis may improve the effectiveness of current therapies. Currently, RC is diagnosed by ruling out other causes of LUTS. LUTS are common among older individuals as noted in our patient survey and, although more prevalent in RC, they are not indicative of RC. For example, 2 PCa survivors in our cohort without any urinary symptoms other than nocturia, did develop RC. One major hallmark of RC is hematuria. However, in our RC group only 4 patients reported blood in the urine at the time of survey, of which 3 reported hematuria with blood clots. Hematuria is often microscopic and diagnosed with dipstick analysis. In addition, hematuria is not detectable every void, thus it is feasible that more patients in the RC-group have microscopic hematuria without their knowledge. One non-RC patient reported hematuria at time of urine collection. However, this patient was eventually diagnosed with RC through cystoscopy 18 months later. An additional patient was diagnosed with RC 14 months after urine collection. These patients were included in the non-RC group as they were diagnosed after urine collection. These 2 cases indicate the rapid progression of RC.
A study limitation was the small sample number which is expected given that RC is an orphan disease. We only obtained 10 samples from patients with RC diagnosis, and 5 samples from patients with hematuria. Additionally, patients only provided a single urine sample after RT without long-term follow-up. The control group included 7 PCa survivors without history of RT and 2 PCa patients awaiting RT to account for any possible PCa specific changes. In a future longitudinal study we intend to collect urine before and after RT, hereby generating an internal control and allowing for monitoring changes in growth factor levels within an individual. Finally, our study is based on urine samples and survey data only. Bladder tissue samples would have allowed us to verify growth factor changes in the bladder.
Although future studies are needed, we envision 2 clinical implications for our findings. First, the pro-angiogenic factors identified in this study could function as noninvasive urinary biomarkers with different roles: (1) a diagnostic marker to diagnose patients with RC, or (2) a monitoring biomarker to determine the effectiveness of RC therapy on vascular health in the bladder or to monitor the progression of RC. These biomarkers could be tested for in PCa survivors as part of their routine checkups by their primary care physician, oncologist or urologist. Second, these vascular growth factors could also be used as therapeutic targets, either by enhancing or blocking their function.
CONCLUSION
RC is a debilitating bladder condition that PCa survivors are at risk of developing after RT. In this study we identified 3 pro-angiogenic factors, VEGF, HGF, and PlGF, that may be early indicators of underlying tissue damage and ongoing vascular and tissue repair in the bladder.
Acknowledgment.
We would like to acknowledge Joe Janicki for analytical support and the Aikens Center for Neurology Research.
Funding Support: This research was supported by the Aikens Center for Neurology Research, the U Can-Cer-Vive Foundation, the Urology Care Foundation Research Scholars Program (BMMZ), and the NIDDK K01 career development award (K01 DK114334;BMMZ).
Footnotes
Financial Disclosure: All authors declare that they have no relevant financial interests.
References
- 1.American Cancer Society: Cancer facts & figures 2018. 2018. [Google Scholar]
- 2.American Cancer Society: Cancer treatment & survivorship facts & figures 2016-2017. 2016. [Google Scholar]
- 3.Zwaans BM, Nicolai HG, Chancellor MB, et al. Challenges and opportunities in radiation-induced hemorrhagic cystitis. Rev Urol. 2016;18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng IWS, Tey JCS, Soon YY, et al. Outcomes of Asian patients with localized prostate cancer treated with combined intensity modulated radiation therapy (IMRT) and high dose rate (HDR) brachytherapy: a single institution experience. Asia Pac J Clin Oncol 2017;1–6. [DOI] [PubMed] [Google Scholar]
- 5.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb LE, Janicki JJ, Bartolone SN, et al. Development of an interstitial cystitis risk score for bladder permeability. PLoS One. 2017;12:e0185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panganiban RA, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmacol Sin. 2011;32:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2:a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skoda M, Stangret A, Szukiewicz D. Fractalkine and placental growth factor: a duet of inflammation and angiogenesis in cardiovascular disorders. Cytokine Growth Factor Rev. 2017;39:116–123. [DOI] [PubMed] [Google Scholar]
- 10.Comunanza V, Bussolino F. Therapy for cancer: strategy of combining anti-angiogenic and target therapies. Front Cell Dev Biol. 2017;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahrami B, Hong T, Gilles MC, et al. Anti-VEGF therapy for diabetic eye diseases. Asia Pac J Ophthalmol (Phila). 2017; 6:535. [DOI] [PubMed] [Google Scholar]
- 12.Soler R, Vianello A, Fullhase C, et al. Vascular therapy for radiation cystitis. Neurourol Urodyn. 2011;30:428. [DOI] [PubMed] [Google Scholar]