Abstract
Radiation‐induced cardiovascular disease is well described as a late effect in cancer patients treated with radiation therapy. Advancements in surgery, radiotherapy, and chemotherapy have led to an increasing number of cancer survivors with resultant long‐term side effects related to their cancer treatments. In this review, we describe the short‐ and long‐term cardiovascular consequences of mediastinal radiotherapy and discuss the optimal cardiovascular assessments and diagnostic tools in asymptomatic and symptomatic patients.
Keywords: Cardiovascular disease, Prevention, Radiotherapy
1. INTRODUCTION
More than 50% of patients with cancer undergo radiation therapy (RT). RT is an integral part of achieving long‐term cure in a number of intrathoracic malignancies, especially among patients with breast cancer and Hodgkin lymphoma (HL). It is also frequently employed in patients with lung, esophagus, and thyroid cancer. With improved surgical procedures, radiation regimes, and chemotherapeutic treatments, the number of cancer survivors is increasing—and so is the number of patients with long‐term RT‐induced cardiovascular (CV) complications.1, 2
In 1987, Cuzick et al published an analysis of trials comparing radical mastectomy without RT against simple mastectomy with RT. Overall, no significant difference in survival was seen, but there was a nonsignificant trend for patients given RT to do worse after 15 years of follow‐up.3 A few years later, it was documented that excess mortality was primarily related to cardiac deaths among patients irradiated for left‐sided breast cancer.4 Subsequently, there has been much focus on the risk of RT‐induced heart morbidity and mortality. There seems to be a clear dose‐response relationship,5, 6 although no threshold dose of RT has been documented.5 Meta‐analyses show that RT‐induced heart disease develops slowly and is normally not seen before ≥15 years of follow‐up.4
In a study of 6039 HL patients followed for a median of 9 years after RT, CV complications were seen in 703 patients (11.6%), the most common being coronary heart disease (19%), arrhythmia (16%), heart failure (12%), heart valve disease (11%), and pericardial disease (5%).7
In this review we will focus on the different manifestations of RT‐induced heart and vascular disease (an overview is shown in the Table 1).
Table 1.
Overview of cardiovascular structures being affected after RT
| Tissue Involved | Diagnostic Consideration | Time of Presentation After RT | Cardiac Evaluation |
|---|---|---|---|
| Pericardium | Acute pericarditis | Days to weeks | TTE |
| Chronic effusion | Weeks to months | TTE | |
| Constrictive pericarditis | Years | TTE, MRI, right‐sided catheterization | |
| Vascular tree | Premature CAD | Years | CT, SPECT, catheterization, TTE (stress), risk factors |
| MI | Months to years | Catheterization | |
| Asymptomatic CAD | Years | CT, SPECT, catheterization, TTE (stress), risk factors | |
| Aortic arch calcification | Years | CT, MRI | |
| Carotid stenosis | Years | Perfusion imaging, MRI, risk factors | |
| TIA/stroke | Months to years | Perfusion imaging, MRI, risk factors | |
| PVD | Months to years | Perfusion imaging, CT‐flow | |
| Endocardium | Valvular disease | Years | Echo (stress), TEE |
| Myocardium | Myocarditis | Weeks to months | Echo, MRI, biomarkers |
| Cardiomyopathy | Months to years | TTE | |
| Chronic HF | Years | TTE | |
| Diastolic dysfunction | Years | TTE | |
| Conduction system | Heart block | Months to years | ECG, Holter |
Abbreviations: CAD, coronary artery disease; CT, computed tomography; ECG, electrocardiogram; Echo, echocardiography; HF, heart failure; MI, myocardial infarction; MRI, magnetic resonance imaging; PVD, peripheral vascular disease; RT, radiotherapy; SPECT, single‐photon emission computed tomography; TEE, transesophageal echocardiography; TIA, transient ischemic attack; TTE, transthoracic echocardiography.
2. METHODS
We comprehensively reviewed the Cochrane Library, PubMed, and Embase databases through April 29, 2016, for relevant studies evaluating the association between RT and cardiovascular disease (CVD). A total of 178 clinical and observational studies, meta‐analyses, and review studies were evaluated. Further searches were performed using reference lists, and recent literature reviews from recent papers were added; a total of 40 references were found eligible.
3. RESULTS
3.1. RT with dose‐response association
During the last decade, most RT departments changed to computed tomography (CT)‐based dose planning. This provides opportunity for definition of relevant targets and organs at risk, as, for example, the heart.8 The largest groups of patients being treated with RT are those with breast cancer and lymphoma.
In particular, patients with breast cancer and lymphoma are subject to potential cardiotoxic chemotherapy used in the treatment of both types of cancer, especially anthracyclines. In assessing the likelihood that a given heart disease is induced by RT, it is appropriate to examine whether there is overlap between heart damage and previous radiation fields.
In a population‐based study of 5‐year survivors of HL age <41 years at time of treatment (1965–1995), the risk of CVD was 3× to 5× higher than expected, even after a follow‐up time of 25 years.9 The authors found a 30‐year cumulative incidence of any CVD of 34.5%, with an incidence of myocardial infarction (MI) of 12.9% and incidence of valvular disorders of 19.7%.9 CV side effects of RT are directly related to RT dose and volume of the heart being irradiated.10 The mediastinal RT dose of 36, 30, 25, or 20 centigray (cGy), or no RT, showed 25‐year cumulative incidence of CVD of 21% in the 36‐cGy RT dose, decreasing to 10%, 6%, 5%, and 3% (no RT), respectively.10 Similar dose‐response association has been shown in several studies.11
In a study of 14 358 survivors of cancer, of which >50% received chemotherapy and radiation, survivors of cancer were significantly more likely than siblings to report congestive heart failure (CHF; hazard ratio [HR]: 5.9, 95% confidence interval [CI]: 3.4‐9.6, P < 0.001), MI (HR: 5.0, 95% CI: 2.3‐10.4, P < 0.001), pericardial disease (HR: 6.3, 95% CI: 3.3‐11.9, P < 0.001), or valvular abnormalities (HR: 4.8, 95% CI: 3.0‐7.6, P < 0.001). Exposure to ≥250 mg/m2 of anthracyclines increased the relative hazard of CHF, pericardial disease, and valvular abnormalities by 2× to 5× compared with survivors who had not been exposed to anthracyclines. Cardiac radiation exposure of ≥1500 cGy increased the relative hazard of CHF, MI, pericardial disease, and valvular abnormalities by 2‐fold to 6‐fold compared with nonirradiated survivors. The cumulative incidence of adverse cardiac outcomes in cancer survivors continued to increase up to 30 years after diagnosis.11
3.2. Pericardial disease
Acute pericarditis can develop in experimental animals within days to weeks after a single high irradiation dose of 20 to 40 cGy, due to inflammation.12 Acute exudative pericarditis is a rare condition that can occur during RT or within weeks post‐RT and can be revealed by asymptomatic pericardial effusion or symptomatic pericarditis (Figure 1). Spontaneous clearance of an effusion may occur, but some patients may develop large pericardial effusion. Pericardiocentesis is indicated if the effusion is large or causing hemodynamic compromise.13
Figure 1.
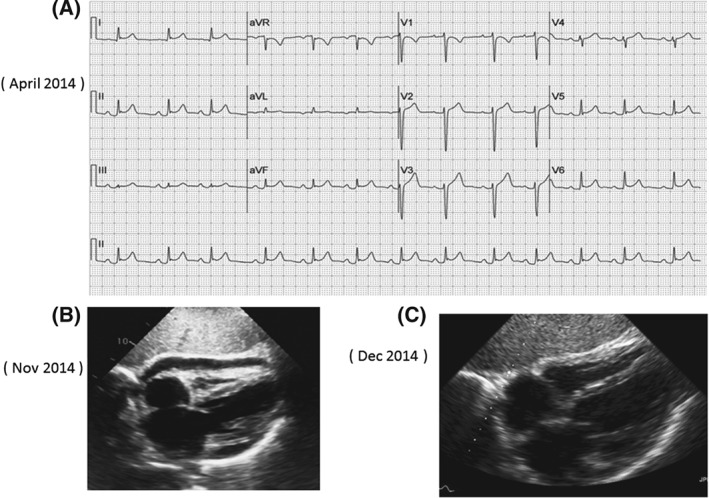
ECG and Echocardiogram showing pericarditis/pericardial effusion related to radiation therapy. (A): ECG shows diffuse ST elevation suggestive of pericarditis. (B): Echocardiogram (subcostal view) showing small pericardial effusion during attack of recurrence. (C): Echocardiogram (subcostal view) showing complete resolution of the pericardial effusion. A 60 year female with left lung cancer, who presented in April 2014, within 2 weeks of radiation therapy with typical pain of pericarditis. She had recurrence of pericarditis in Nov 14, for which she was given NSAID and cochicine with good effect.
Chronic pericarditis appears months to years after RT. Among asymptomatic patients with previous mediastinal radiation, pericardial thickening was present in 21% of patients and small pericardial effusion was noted in 3% of the patients.14 The prevalence of pericardial thickening increased with longer latency period, with a prevalence of 33% for the >20 years latency group.14 Most patients are relatively asymptomatic, even with large pericardial effusions. The condition may be detected as a coincidental finding on CT or X‐ray of the chest.
Treatment of acute pericarditis comprises conventional therapy, including nonsteroidal anti‐inflammatory drugs and colchicine.15 Steroids are used as second‐line agent. In resistant cases, an interleukin‐1β receptor antagonist (anakinra) may be useful.16
Constrictive pericarditis is the most severe form of RT‐induced pericardial disease.16 Symptoms are those of intractable CHF. Diuretics are used for symptomatic relief, but the definitive treatment is surgical pericardiectomy.17
Pericardiectomy is associated with a perioperative mortality of 6%.17 Long‐term outcome depends upon the etiology, left ventricular systolic function, pulmonary artery pressure, and age. Prior RT is associated with a poor outcome.17
3.3. Symptomatic CAD
In animal studies, the mechanism of coronary artery disease (CAD) is believed to be related to vascular endothelium damage leading to arteritis, significant fibrosis of the adventitia, media destruction, and secondary lipid deposition.18
A population‐based case‐control study of major coronary events was conducted in women in Sweden and Denmark who received RT for breast cancer between 1958 and 2001. Out of 35 000 RT‐treated women, 963 women suffered a major coronary event (MI, coronary revascularization, or death from ischemic heart disease [IHD]) and were included for study, as well as 1205 controls. Forty‐four percent suffered the coronary event within 10 years, 33% occurred within 10 to 19 years, and 23% occurred ≥20 years afterward.5, 19 Women being irradiated for cancer of the left breast had higher rates of major coronary events than women being irradiated to the right.5 There was an increase in risk of a major coronary event by 7.4% per 1‐cGy increase in mean heart RT dose. The risk was 6.7× as high in women with prior IHD as compared with women with no such history. The risk of major coronary events was also elevated among women with other circulatory diseases, diabetes mellitus (DM), chronic obstructive pulmonary disease, smoking, and high body mass index.5
A study of HL patients also identified a dose‐response relationship of the same magnitude and also confirms the long‐term risk of CAD, with a median time interval from diagnosis of HL and CAD of 19 years.6
An increased risk of fatal MI of 2.2‐fold to 7.7‐fold (as compared with the general population) have been documented in several studies of HL survivors who had prior treatments with supradiaphragmatic RT.6 More than 1 classical CAD risk factor further increased the risk of suffering symptomatic MI or angina.20 The risk of developing CAD increases with time. However, one randomized study assessing morbidity and mortality from IHD in patients treated with post‐mastectomy RT or not, did not show an increased risk of IHD in the RT group compared with the non‐RT group within 12 years of treatment.21
Patients with prior RT might have involvement of the proximal coronary arteries22, 23 and also myocardial perfusion defects, in nonanatomical territories, suggesting small‐vessel damage with RT.24 Development of CAD is related to the age of the patient, proximity of the tumor to the heart, radiation dose, and presence of coexisting risk factors.5 Treatment of radiation‐induced CAD is similar to general population,2 although the risk of restenosis in these patients is higher than usual. In patients with prior RT treatment, the risk after surgical procedures, such as coronary artery bypass grafting, is higher than in patients without prior RT treatment.25 Evaluation of potential porcelain aorta must be performed before deciding on surgery.25
3.4. Asymptomatic CAD
In a study of 294 asymptomatic HL survivors who had no known CAD, 21.4% had abnormal ventricular images at rest, suggesting prior myocardial injury.26 In this study, 49% were male, mean age was 42 years (SD = 9), with only 9% having a history of hypertension (HTN), 27% smokers, and 1% with DM. During stress testing, 14% developed perfusion defects. A coronary angiography (performed in 90% of patients with perfusion defects) showed stenosis >50% in 55% of the patients.26 Of 294 patients, screening led to bypass graft surgery in 2.4%, and 7.8% developed coronary events during a median 6.5 years of follow‐up.26
In a prospective study of postoperative RT in left‐sided breast cancer, 12 patients were examined before and 1 year after RT.24 Six of the 12 patients had new fixed scintigraphic defects after RT. The average size of the defect on the bull's‐eye view was 63.3 pixels (range, 36–80 pixels) and average depth was 247.8 pixels (range, 143–333 pixels), which on an average was 3.9× SD. The localization of the defects corresponded well with the irradiated volume of the left ventricle.24 Neither electrocardiographic (ECG) changes nor left ventricular segmental wall‐motion abnormalities could be detected by echocardiography.24
Computed tomography angiography is a useful evaluation tool in asymptomatic patients. In a study of HL survivors age 17 to 28 years, coronary abnormalities was detected 6.8× as often in patients who received RT compared with patients who did not receive RT.27
In another study of HL survivors, the prior radiation doses to stenotic segments were compared with those received by normal segments (from cases and controls). In 11 cases out of 12, the highest of the coronary dose distribution was on a damaged segment.20 The coronary ostia and proximal segments were most often involved.23
The use of single‐photon emission computed tomography gated myocardial perfusion imaging is also useful in early detection of perfusion defects, which is seen in 44% to 47% of asymptomatic patients as early as 6 months after RT.14, 28 In a prospective study of 114 patients with left‐sided breast cancer, the incidence of new perfusion defects at 6, 12, 18, and 24 months after RT was 27%, 29%, 38%, and 42%, respectively.29
Identification of classical CAD risk factors such as family history of CAD, smoking, dyslipidemia, DM, HTN, adiposities, and inactivity are important factors to treat and modify. A thorough clinical evaluation of potential CV symptoms is of importance. In high‐risk patients, noninvasive testing should be considered, preferably with either computed tomography angiography, single‐photon emission computed tomography, or stress echocardiography.1, 2, 30 Figure 2 illustrates the case of asymptomatic CAD.
Figure 2.
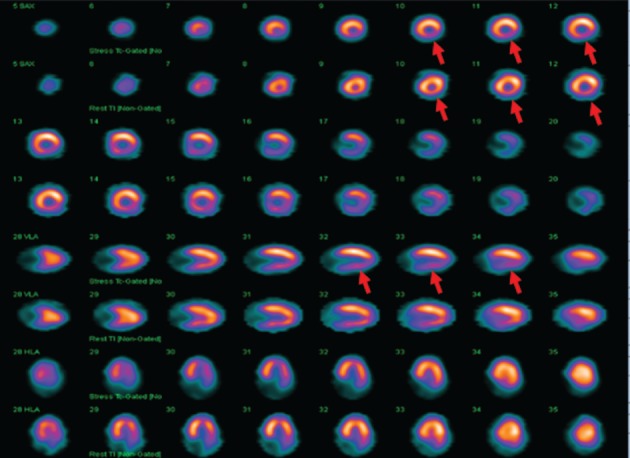
Myocardial perfusion study showing ischemia in the inferior wall (region of radiation theraphy). A 57 year old asymptomatic man, who had received a total of 50 Gy radiation 2 years previously for cancer esophagus. Myocardial perfusion study shows ischemia in the inferior wall.
3.5. Vascular disease
RT of the head and neck can induce early endothelial damage and extensive fibrosis with lower flow in the carotid artery being irradiated.31 An increased risk of transient ischemic attack or stroke has been documented in 5‐year survivors of HL.32 Aortic‐arch calcification with porcelain aorta is seen >10 years after RT and is of importance when heart surgery is considered.25 The risk of dying after surgery is higher in RT‐treated patients, and alternative strategies should be considered.25
Involvement of the femoral arteries and claudication can occur after abdominal RT. Peripheral arterial disease has been documented as early as 5 years after RT.33
Medical therapy with aspirin and statins should be considered. Due to fibrosis in the neck, symptomatic severe carotid disease may be more amenable to percutaneous intervention. Patients who undergo neck radiation are good candidates for carotid stenting and can undergo this procedure with good results.34
3.6. Valvular heart disease
Valvular heart disease is frequently seen in patients with RT‐induced heart disease. In one postmortem series, the incidence was high, with 81% of patients showing evidence of mild valvular damage.22 The aortic and mitral valves are more commonly involved than the tricuspid and pulmonary valves. Asymptomatic HL survivors after 20 years (as compared with survivors after 10 years) have a higher incidence of aortic regurgitation (60% vs 4%) and aortic stenosis (16% vs 0%), and also a higher incidence of mitral regurgitation (52% vs 26%, respectively).14 Echocardiography shows unusually marked calcification of the intervalvular fibrosa of the aortic valve (Figure 3) and the anterior mitral leaflet.14
Figure 3.
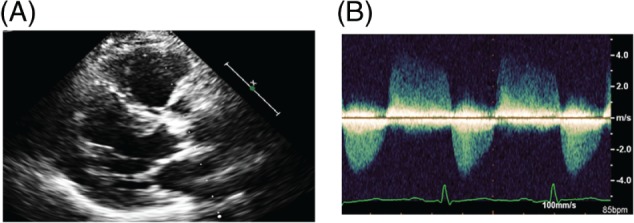
Echocardiogram showing moderate arotic stenosis mild regurgitation. A 57 year old man with history of Hodgkin's lymphoma treated with mediastinal radiation in 1982. In February 2012 he was evaluated because of symptoms of fatigue. Echocardiogram showed mild mitral annular calcification and moderate calcific aortic stenosis. A cardiac catherization also showed CAD, with signficant RCA disease for which he underwent stenting. (A): Echocardiogram (parasternal view) showing mild mitral annular calcification and severe aortic valve calcification. (B): Doppler shows moderate aortic stenosis with mild regurgitation.
In a nested case‐control study from a cohort of 1852 five‐year HL survivors (diagnosed at ages 15–41 years), 89 patients had valvular heart disease of at least moderate severity as their first CV diagnosis following HL treatment. Of 89 patients with moderate valve disease, the aortic valve was affected in 71% and the mitral valve in 47%. Risks increased more than linearly with radiation dose.35 With lower doses and newer RT techniques in recent decades, the risk of valvular heart disease is reduced, and the authors stated that in patients treated in recent years with RT of 20 to 30 cGy, the 30‐year risk of valvular heart disease will be increased by only about 1.4% compared with the general population.35
Patients with murmurs and echocardiographic findings suggestive of valve disease should be evaluated with echocardiography at regular intervals, and the patients should be educated in signs of progression. In patients with severe valve disease, surgery should be considered. A high preoperative EuroSCORE and an increased thickness of the junction between the base of the anterior mitral leaflet and the aortic root is a predictor of poor outcome.25, 36 In appropriate cases, other treatment strategies such as transcatheter aortic valve replacement and percutaneous mitral valve clips may be considered.
3.7. Myocarditis, cardiomyopathy
Myocardial injury after irradiation is known to be associated with damage to the microvasculature, which is demonstrated by decreased microvessel density, focal loss of endothelial alkaline phosphatase, and increased expression of von Willebrand factor.37 Acute myocarditis related to RT‐induced inflammation with transient ST‐ and T‐wave ECG abnormalities and myocardial dysfunction may occur. Measurement of cardiac biomarkers, echocardiography with global longitudinal strain (GLS), and magnetic resonance imaging of the heart should be considered.
Diffuse myocardial fibrosis can occur, often after a >25‐to‐30‐cGy RT dose, with resultant systolic and diastolic dysfunction, conduction disturbance, and autonomic dysfunction. Restrictive cardiomyopathy represents an advanced stage of myocardial damage due to fibrosis with severe diastolic dysfunction and signs and symptoms of heart failure.14 The risk of developing symptoms of CHF after RT is 5.9× as high as in siblings not being treated with RT.11
In one study, 1820 young cancer survivors exposed to anthracycline chemotherapy (n = 1050), RT (n = 306), or both (n = 464) were evaluated with echocardiographic assessment, including 3‐dimensional left ventricular ejection fraction (LVEF), GLS and circumferential myocardial strain, and diastolic function (median age, 31 years, with a median time from diagnosis of 23 years).38 Only 5.8% of survivors had abnormal 3D LVEFs (<50%); however, 32.1% of patients with normal 3D LVEFs had evidence of cardiac dysfunction by GLS (28%) or diastolic assessment (8.7%), or both.38 Abnormal GLS was associated with RT at 1 to 19.9 cGy (relative risk [RR]: 1.38), 20 to 29.9 cGy (RR: 1.65), and >30 cGy (RR: 2.39).38 Also, anthracycline dose >300 mg/m2 was associated with abnormal GLS (RR: 1.72). Survivors with metabolic syndrome were twice as likely to have abnormal GLS.38
In recent consensus statements, it was recommended to use echocardiography as first choice of LVEF evaluation with use of GLS.30, 39 Treatment with angiotensin‐converting enzyme inhibitors and β‐blockers should be initiated if LVEF is <53% or if LVEF or GLS decrease by >10 percentage points.30, 39
3.8. Conduction system
The conduction system is rarely involved after RT. Changes in the ECG after RT may range from nonspecific ST‐T changes to low voltage and right bundle branch block.22, 40 Right bundle branch block is commonly associated with mediastinal RT because the right bundle lies close to the endocardium on the right side. Injury to bundle may occur either directly from RT or indirectly from associated myocardial fibrosis and ischemia. A serious manifestation is complete atrioventricular block. Extensive heart disease with CAD, valve disease, and constriction is concomitantly present in a large fraction of patients who developed complete heart block.23
3.9. Cardiac biomarkers (Tn and BNP)
In one prospective study, serial cardiac biomarkers (troponin I [TnI] and brain natriuretic peptide [BNP]) were measured in 25 patients treated with RT with high‐dose cardiac exposure.40 After the first RT fraction, no changes were noted in ECG or median TnI or BNP levels; at the end of RT, 2 patients had elevated TnI and BNP, but neither difference was statistically significant. At first follow‐up, TnI had returned to normal, but the median BNP remained elevated. BNP did not increase over time in the 18 patients who received only RT. Twelve patients experienced acute ECG changes during RT, which resolved in 7 patients by the next measurement. No patients experienced clinically significant RT‐related events.40 Based on current data, routine measurement of cardiac biomarkers is not recommended routinely after RT.
3.10. Surveillance and prevention after RT in cancer survivors
The incidence of RT‐induced heart disease in general is low,19 but in a small fraction of cancer survivors, especially those with known heart disease or with prior RT doses >30 cGy, >1 CV risk factor, or prior treatment with anthracycline, lifelong follow‐up and surveillance is recommended.2, 30 Modifiable risk factors such as HTN, lipids, DM, smoking habits, adiposities, and inactivity should be treated as per current guidelines wherever applicable (Figure 4).2, 30
Figure 4.
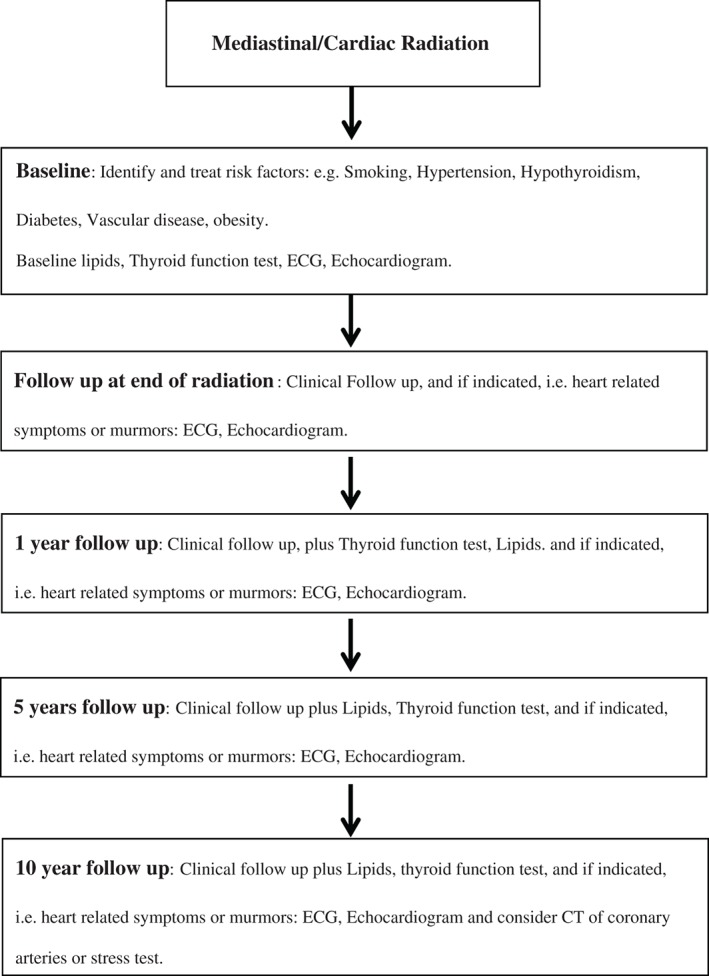
Suggested algorithm for cardiovascular screening after mediastinal radiation therapy. Abbreviations: CT, computed tomography; ECG, electrocardiogram.
4. CONCLUSION
Cancer survivors initially treated with RT are at increased risk of developing CVD with multiple manifestations. Efforts to prevent RT‐induced CVD are of paramount importance. Although modern RT, with heart‐protective techniques, is likely to reduce the prevalence and severity of CV complications, more efforts to diagnose and prevent these side effects are still needed.
Conflicts of interest
The authors declare no potential conflicts of interest.
Nielsen KM, Offersen BV, Nielsen HM, Vaage‐Nilsen M and Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. ClinCardiol, 2017. doi: 10.1002/clc.22634
REFERENCES
- 1. Yusuf SW, Howell RM, Gomez D, et al. Radiation‐related heart and vascular disease. Future Oncol. 2015;11:2067–2076. [DOI] [PubMed] [Google Scholar]
- 2. Iliescu CA, Grines CL, Herrmann J, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio‐oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv. 2016;87:E202–E223. [DOI] [PubMed] [Google Scholar]
- 3. Cuzick J, Stewart H, Peto R, et al. Overview of randomized trials comparing radical mastectomy without radiotherapy against simple mastectomy with radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:7–14. [PubMed] [Google Scholar]
- 4. Roychoudhuri R, Robinson D, Putcha V, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population‐based study. BMC Cancer. 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 6. van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose‐response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. [DOI] [PubMed] [Google Scholar]
- 7. Maraldo MV, Giusti F, Vogelius IR, et al; European Organisation for Research and Treatment of Cancer (EORTC) Lymphoma Group. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC‐LYSA trials. Lancet Haematol . 2015;2:3492–e502. [DOI] [PubMed] [Google Scholar]
- 8. Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol . 2016;118:205–208. [DOI] [PubMed] [Google Scholar]
- 9. Aleman BM, van den Belt‐Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 10. Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow‐up project of the German‐Austrian DAL‐HD studies. Pediatr Blood Cancer. 2010;55:1145–1152. [DOI] [PubMed] [Google Scholar]
- 11. Mulrooney DA, Kawashima TF, Mitby PF, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fajardo LF, Stewart JR. Experimental radiation‐induced heart disease. I. Light microscopic studies. Am J Pathol . 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 13. El Haddad D, Iliescu C, Yusuf SW, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion [published correction appears in J Am Coll Cardiol 2015;66:2269]. J Am Coll Cardiol . 2015;66:1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. [DOI] [PubMed] [Google Scholar]
- 15. Imazio M, Brucato A, Cemin R, et al; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med . 2013;369:1522–1528. [DOI] [PubMed] [Google Scholar]
- 16. Yusuf SW, Hassan SA, Mouhayar E, et al. Pericardial disease: a clinical review. Expert Rev Cardiovasc Ther. 2016;14:525–539. [DOI] [PubMed] [Google Scholar]
- 17. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause‐specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445–1452. [DOI] [PubMed] [Google Scholar]
- 18. Virmani R, Farb A, Carter AJ, et al. Pathology of radiation‐induced coronary artery disease in human and pig. Cardiovasc Radiat Med. 1999;1:98–101. [DOI] [PubMed] [Google Scholar]
- 19. McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35 000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. [DOI] [PubMed] [Google Scholar]
- 20. Moignier A, Broggio D, Derreumaux S, et al. Coronary stenosis risk analysis following Hodgkin lymphoma radiotherapy: a study based on patient specific artery segments dose calculation. Radiother Oncol. 2015;117:467–472. [DOI] [PubMed] [Google Scholar]
- 21. Højris I, Overgaard MF, Christensen JJ, et al; Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Morbidity and mortality of ischaemic heart disease in high‐risk breast‐cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Lancet . 1999;354:1425–1430. [DOI] [PubMed] [Google Scholar]
- 22. Brosius FC 3rd, Waller BF, Roberts WC. Radiation heart disease: analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3500 rads to the heart. Am J Med. 1981;70:519–530. [DOI] [PubMed] [Google Scholar]
- 23. Orzan F, Brusca A, Gaita F, et al. Associated cardiac lesions in patients with radiation‐induced complete heart block. Int J Cardiol. 1993;39:151–156. [DOI] [PubMed] [Google Scholar]
- 24. Gyenes G, Fornander T, Carlens P, et al. Detection of radiation‐induced myocardial damage by technetium‐99m sestamibi scintigraphy. Eur J Nucl Med. 1997;24:286–292. [DOI] [PubMed] [Google Scholar]
- 25. Wu W, Masri A, Popovic ZB, et al. Long‐term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1485. [DOI] [PubMed] [Google Scholar]
- 26. Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease [published correction appears in J Clin Oncol 2007;25:1635]. J Clin Oncol . 2007;25:43–49. [DOI] [PubMed] [Google Scholar]
- 27. Küpeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma [published correction appears in J Clin Oncol 2010;28:1808]. J Clin Oncol . 2010;28:1025–1030. [DOI] [PubMed] [Google Scholar]
- 28. Zhang P, Hu X, Yue J, et al. Early detection of radiation‐induced heart disease using (99m)Tc‐MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiother Oncol. 2015;115:171–178. [DOI] [PubMed] [Google Scholar]
- 29. Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT‐associated cardiac perfusion defects. Int J Radiat Oncol Biol Physics. 2005;63:214–223. [DOI] [PubMed] [Google Scholar]
- 30. Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi‐modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. [DOI] [PubMed] [Google Scholar]
- 31. Cheng SW, Ting AC, Lam LK, et al. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:517–521. [DOI] [PubMed] [Google Scholar]
- 32. De Bruin ML, Dorresteijn LD, van't Meer MB, et al. Increased risk of stroke and transient ischemic attack in 5‐year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. [DOI] [PubMed] [Google Scholar]
- 33. Pherwani AD, Reid JA, Keane PF, et al. Synergism between radiotherapy and vascular risk factors in the accelerated development of atherosclerosis: a report of three cases. Ann Vasc Surg. 2002;16:671–675. [DOI] [PubMed] [Google Scholar]
- 34. Gurm HS, Fayad P, Katzen BT, et al; SAPPHIRE Investigators. Long‐term results of carotid stenting versus endarterectomy in high‐risk patients. N Engl J Med . 2008;358:1572–1579. [DOI] [PubMed] [Google Scholar]
- 35. Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desai MY, Wu W, Masri A, et al. Increased aorto‐mitral curtain thickness independently predicts mortality in patients with radiation‐associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg. 2014;97:1348–1355. [DOI] [PubMed] [Google Scholar]
- 37. Seemann I, Gabriels K, Visser NL et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143–150. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment‐related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol . 2015;65:2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomez DR, Yusuf SW, Munsell MF, et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high‐dose heart exposure. J Thorac Oncol. 2014;9:1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]


