Significance
Activity in the lateral habenula (LHb), a brain region implicated in depression, provides a reward prediction error (RPE): rewards reduce, while punishment or expected-reward omission increase LHb activity. These signals are important in shaping future choices. Here, we image the activity of individual LHb neurons in behaving mice. We find that stress, a major risk factor for depression, acutely transforms LHb signals in a remarkable manner: under stress, rewards produce punishment-like LHb output, and reward omission increases its punishment-like output. That is, under stress, LHb signals interpret “good as bad and bad as worse.” This effect is rapid and coincides with reduced reward responsivity. These results potentially explain why an individual under stress is more prone to depression.
Keywords: habenula, stress, reward, anhedonia, prediction error
Abstract
Neuronal activity in the lateral habenula (LHb), a brain region implicated in depression [C. D. Proulx, O. Hikosaka, R. Malinow, Nat. Neurosci. 17, 1146–1152 (2014)], decreases during reward and increases during punishment or reward omission [M. Matsumoto, O. Hikosaka, Nature 447, 1111–1115 (2007)]. While stress is a major risk factor for depression and strongly impacts the LHb, its effect on LHb reward signals is unknown. Here we image LHb neuronal activity in behaving mice and find that acute stress transforms LHb reward responses into punishment-like neural signals; punishment-like responses to reward omission also increase. These neural changes matched the onset of anhedonic behavior and were specific to LHb neurons that distinguished reward and its omission. Thus, stress distorts LHb responsivity to positive and negative feedback, which could bias individuals toward negative expectations, a key aspect of the proposed pathogenesis of depression [A. T. Beck, Depression: Clinical, Experimental, and Theoretical Aspects, sixth Ed (1967)].
Lateral habenula (LHb) neurons encode numerous stimuli including rewards, their omission, and punishment (1–7). In particular, the LHb provides reward prediction error (RPE) (2) signals—the difference between expected and actual reward value—a computation thought to be essential for an animal to learn from its environment (8–10). In this way, LHb activity, which is aversive (1, 11–13), can provide “teaching” signals to an animal: increased LHb activity (i.e., if actual reward value is less than expected) discourages repeating a behavior in the future (11, 12, 14), while decreased LHb activity is thought to reinforce a behavior.
Human and nonhuman animal studies indicate that stress-induced changes in LHb activity may contribute to depression by suppressing reward-based behavior (1, 15–18). While stress decreases reward sensitivity (19, 20), is a major risk factor for depression (21, 22), forms the basis for most animal models of depression (23–25), and causes plasticity in the LHb (26–31), its effects on LHb reward and RPE signals are not known. Here, we use calcium-imaging techniques to monitor RPE from individual LHb neurons in awake, behaving mice in the absence and presence of intermittent tail shock stress. Surprisingly, we find that stress causes the LHb to respond to rewards as if they were punishment. This switch is tightly linked temporally with onset of anhedonic behavior, suggesting that this aberrant LHb responsivity contributes to anhedonia (29–35). These changes were also accompanied by a larger (i.e., “more negative”) LHb signal to reward omission. Our results indicate that stress causes a negative shift in LHb signaling of reward and its omission. While potentially adaptive in some conditions (e.g., suppressing reward-seeking behavior during threat), repeated occurrence of such effects could contribute to the pathogenesis of depression.
Results
RPE Encoding in a Subpopulation of LHb Neurons.
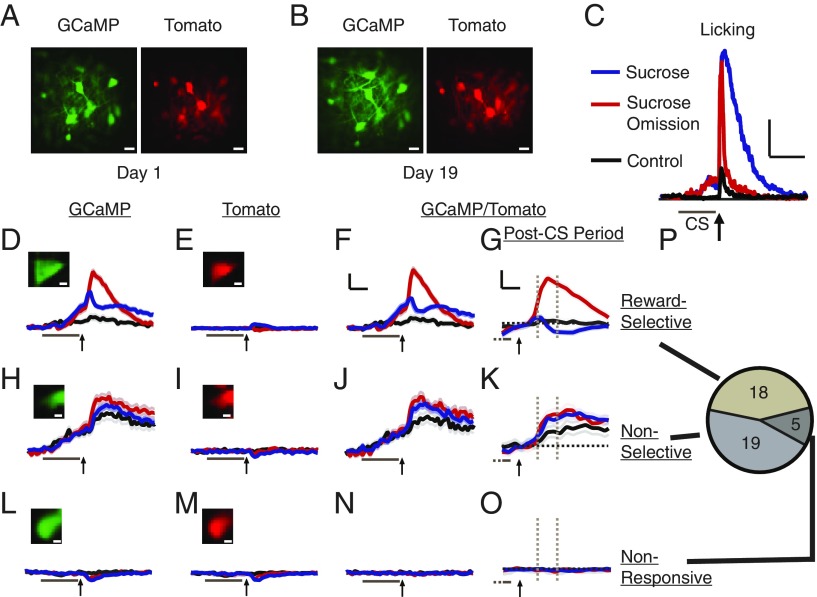
We monitored the activity of multiple independent LHb neurons in awake, behaving mice using calcium-imaging techniques (see Materials and Methods for details). We injected into the LHb viruses expressing a genetically encoded calcium indicator, GCaMP6S, and a cytoplasmic marker, tdTomato. We gained chronic optical access to the LHb, which is several millimeters deep in the mouse brain, by permanently implanting a gradient refractive index (GRIN) lens (SI Appendix, Fig. S1). Three-to-four weeks after injection and implantation, individual LHb neurons expressing GCaMP and tdTomato were observed in awake, head-fixed mice with 2-photon laser-scanning microscopy. After several sessions of acclimatization, neuronal activity was monitored during trials consisting of a 10-s auditory stimulus (CS+) followed 0.5 s after CS+ termination with a drop of sucrose presented from a spout close to their mouth. Behavioral (licking) responses were measured as the percent of time during which a light beam positioned between the animal’s mouth and the spout was crossed. Such sucrose reward trials were interleaved with control trials wherein a different auditory stimulus (CS−) was terminated without a reward. After several days of such training trials, mice licked more during the CS+ than CS− (compared with licking before CS, ∆CS; ∆CS+ = 13 ± 4% and ∆CS− = −2 ± 1%; P < 0.02, n = 4 mice, paired t test; each mouse, P < 0.02, n = 32–36 trials, bootstrap; see Materials and Methods). Mice were then intermittently exposed to trials wherein sucrose was omitted after the CS+ (sucrose omission trials). Such omission trials confirmed that mice learned to expect sucrose after the CS+ and not the CS−, as evidenced by significantly more licking during the sucrose omission period after the CS+ than during a similar period after the CS− (P < 0.05, n = 4 mice, paired t test; each mouse, P < 0.001, n = 33–108 trials, bootstrap; Fig. 1C).
Fig. 1.
Monitoring LHb neuronal activity and behavior during reward and reward omission. (A) Dual-channel, 2-photon laser-scanning image of GCaMP- and Tomato-expressing LHb neurons through GRIN lens. (B) Image of same region as A, 18 d later. (C) Mean licking for indicated trial type (n = 4 mice). (Scale bars, 10 s, 20% of trials licking.) Arrow, sucrose or sucrose omission; bar, CS+ (sucrose-conditioned) or CS− (control) stimulus. (D–P) Classification of LHb neurons. Example images and mean responses (dark lines; 84–216 trials per trial type) ± SEM (light lines for all figures) for indicated cell class, trial type, and channel. Arrow and gray bar, as in C. [Scale bars, 5 s (2 s for post-CS period responses), 20% ΔF/F.] Post-CS period responses measured as mean response between dashed gray lines relative to dashed black lines, which indicate zero (value just before start of sucrose delivery/omission; 100-ms time bin; shown in G, K, and O). GCaMP/Tomato responses displayed in subsequent figures; see Materials and Methods for GCaMP/Tomato calculation. Pie chart displays number of neurons in each class. (Scale bars, A, B: 10 μm; D, E, H, I, L, M: 2 μm).
Two-photon imaging using both GCaMP and tdTomato channels and normalizing the GCaMP signal by the tdTomato signal (∆G/T; see Materials and Methods) permitted correction of the neural signal for movement artifacts that could not be corrected by single-channel movement-correction software (e.g., transient movement of tissue out of the imaging plane; Fig. 1 D–O). Activity of the same LHb neurons could be imaged daily for several weeks (Fig. 1 A and B). Neurons were classified according to their activity patterns. Consistent with primate studies (2, 36, 37), many neurons were “reward-selective” (18 of 42; 43%), displaying significantly different activity during sucrose consumption and sucrose omission (Fig. 1 D–G and P). However, there was also a large population of “nonselective” neurons (19 of 42; 45%) displaying activity that did not differ significantly between sucrose consumption and sucrose omission (Fig. 1 H–K and P); and a small number of neurons displayed no response (“nonresponsive” neurons; 5 of 42; 12%; Fig. 1 L–O and P). Reward-selective neurons typically had higher activity during sucrose omission than sucrose consumption (89%); 72% displayed responses that were reduced by reward and enhanced by reward omission, as RPE signaling is defined in primate LHb (2), and also observed in mouse neurons projecting to the LHb (38); ventral tegmental area dopamine neurons, which are functionally inhibited by the LHb (2, 39), display an RPE inverted with respect to what we observed, as expected (10, 40).
Stress Decreases Licking for Reward.
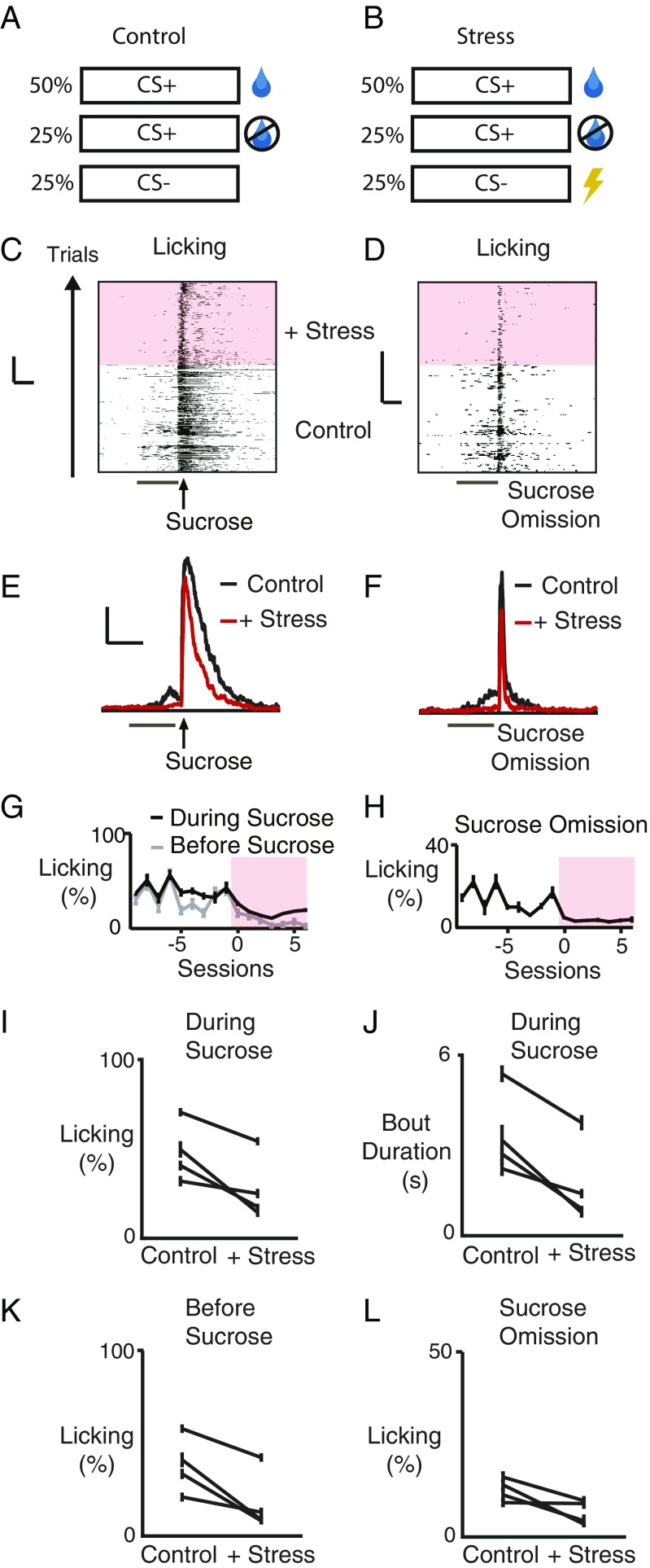
To study the effect of stress on reward processing, we introduced intermittent trials with tail shocks (0.3-s duration), presented after the CS− to minimize contextual fear conditioning (41) (which might interfere with reward consumption; Fig. 2 A and B). After initiating such trials, a number of changes were observed in the animals’ behavior. The overall duration of licking during sucrose presentation was reduced (48 ± 10% to 28 ± 10%; P < 0.05, n = 4 mice, paired t test; each mouse, P < 0.02, n = 33–216 trials, bootstrap; Fig. 2 C, E, G, and I). Further analysis of the licking microstructure showed this decrease was due to a reduction in the duration of licking bouts (3.4 ± 0.8–1.7 ± 0.8 s; P < 0.02, n = 4 mice, paired t test; each mouse, P < 0.001, n = 33–216 trials, bootstrap; Fig. 2J; see Materials and Methods) consistent with reduced reward value (42–47), with no effect on the interlick interval within a licking burst, excluding a motor impairment (interlick interval: 12.7 ± 0.8–12.2 ± 1.2 ms, P > 0.05, n = 4 mice, paired t test). There was also reduced licking before sucrose delivery (39 ± 9% to 18 ± 10%; P < 0.05, n = 4 mice, paired t test; each mouse, P < 0.002, n = 66–324 trials, bootstrap; Fig. 2 G and K) as well as during sucrose omission (13 ± 2% to 7 ± 2%; P = 0.07, n = 4 mice, paired t test; 3/4 mice P < 0.05, n = 33–108 trials, bootstrap; Fig. 2 D, F, H, and L), consistent with reduced motivation to receive a reward.
Fig. 2.
Stress decreases licking for reward. (A) Trial types before stress. (Top) Sucrose delivered 0.5 s after end of 10-s auditory CS+. (Middle) Sucrose omission. (Bottom) 10-s auditory CS− control. (B) Trial types during stress. Same as A except 0.3-ms tail shock delivered 0.5 s after end of CS−. (C and D) Raster plots of licking data from one mouse before and during stress (pink shading, all figures) for indicated trial type. Ticks denote licking. (Scale bars, 5 s, 50 trials.) (E and F) Mean licking before and during stress for indicated trial type (n = 4 mice). (Scale bars, 10 s, 20% of trials.) Bars and arrows as in Fig. 1. (G and H) Mean (percentage of trials, ± SEM) licking of one mouse for indicated trial type. Before sucrose indicates measurements during 0.5-s period after CS and before sucrose. (I–L), Licking for indicated conditions for each mouse. (J) Duration of licking bouts during sucrose delivery for each mouse (see Materials and Methods).
Stress Distorts Reward Signaling in LHb Neurons.
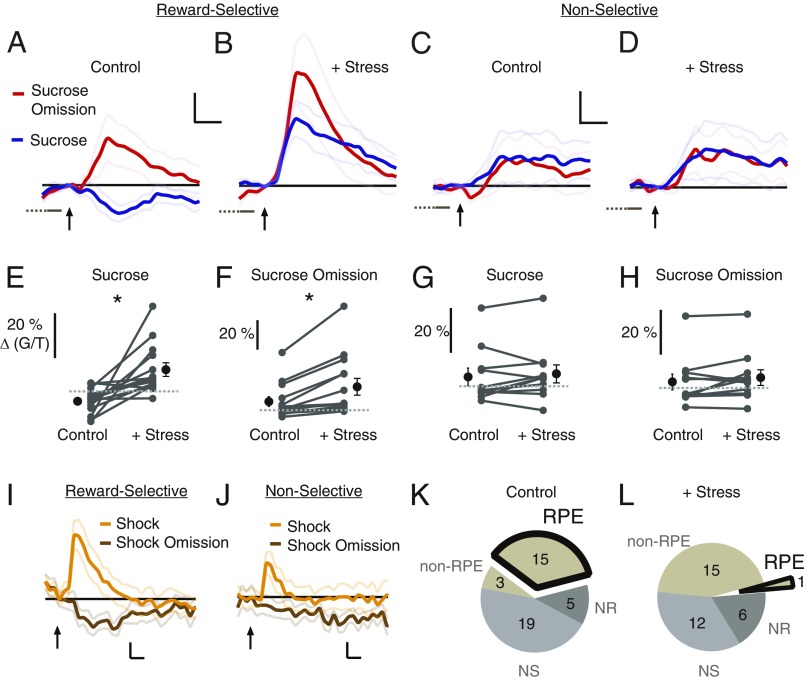
To determine the neural basis by which such a stress protocol could produce these behavioral effects, we examined reward-processing signals in the LHb. As expected, stressful stimuli (i.e., the intermittent shock trials) produced an increased neural response, consistent with LHb responses to punishment seen in primates (4) and rodents (3) (Fig. 3 I and J, shock traces; SI Appendix, Fig. S2). Also as expected, before the stress protocol, reward-selective neurons typically decreased their activity during sucrose consumption and increased their activity during sucrose omission (Fig. 3A), displaying RPE encoding as defined in primate LHb (2). During the CS+, unlike other studies (2, 3), there was a delayed increase in activity as the mice waited for reward (SI Appendix, Figs. S3 and S4), perhaps because of the relatively long CS–sucrose time interval in our study. Remarkably, after initiating a stress protocol, the activity of these neurons during sucrose consumption flipped, displaying an increased rather than decreased response (before stress: −3.8 ± 2% ΔG/T; with stress: 10.1 ± 3% ΔG/T; P < 0.005, n = 14 neurons, Wilcoxon signed-rank test; Fig. 3 B and E and SI Appendix, Figs. S3 and S5; data in ref. 48), suggesting that stress caused the LHb to “interpret” rewards as punishment. Notably, the activity of these neurons also increased during sucrose omission (before stress: 6.7 ± 3% ΔG/T; with stress: 17.5 ± 6% ΔG/T; P < 0.005, n = 14 neurons, Wilcoxon signed-rank test; Fig. 3 B and F and SI Appendix, Figs. S3 and S5). Importantly, these stress-induced changes in neural activity during the post-CS+ period were not due to generalization following any CS, as this increase in activity was not seen following the CS not predicting a reward (Fig. 3 I and J, shock omission traces; SI Appendix, Fig. S6), and similar stress-induced changes were observed if either the shock or sucrose was unsignaled (SI Appendix, Fig. S7) or if we only included trials with anticipatory licking in the analysis (SI Appendix, Fig. S8). The stress-induced change in responses to sucrose effectively erased the typical LHb RPE signal: before stress, 36% of LHb neurons (84% of reward-selective neurons) displayed RPE coding (Fig. 3K; see Materials and Methods); after stress, only 3% of LHb neurons (6% of reward-selective neurons) displayed RPE coding (Fig. 3L; χ2 = 12.1, P < 0.001, n = 42, 34 neurons). In contrast to the effect on reward-selective neurons, there was no significant effect of stress on the responses of nonselective neurons to sucrose consumption (before stress: 4.0 ± 4% ΔG/T; with stress: 5.3 ± 4% ΔG/T; P > 0.05, n = 11 neurons, Wilcoxon signed-rank test; Fig. 3 C, D, and G) nor sucrose omission (before stress: 3.1 ± 3% ΔG/T; with stress: 5.0 ± 3% ΔG/T; P > 0.05, n = 11 neurons, Wilcoxon signed-rank test; Fig. 3 C, D, and H).
Fig. 3.
Stress transforms reward coding in LHb neurons. (A–D) Mean post-CS neural responses (for 3 or 6 d before or after stress introduction, respectively) of all responses for indicated cell type and condition (reward-selective, RS, n = 14 neurons; Nonselective, NS, N =11 neurons; four mice). (Scale bars, 2 s, 5% ΔG/T.) Arrows as in Fig. 1. Black lines indicate zero. (E–H) Mean response of indicated neuron type (as in A–D) and trial type, for individual neurons (connected with line) before and during stress. Dashed lines indicate zero. (I and J) Mean responses of indicated neuron type during shock and shock omission trials (RS, n = 8 neurons; NS, n = 10 neurons; three mice). (Scale bars, 2 s, 5% ΔG/T.) Black lines indicate zero. (K and L) Pie chart indicating number of neurons behaving as indicated class before (n = 42 neurons) and during stress (n = 34 neurons). Non-RPE indicates neurons with significantly different activity during sucrose and its omission (reward-selective), but with the same polarity (see Materials and Methods). NR, nonresponsive. Trials with no licking in the first second after sucrose delivery or omission were excluded in A–H (see Materials and Methods). *P < 0.01.
Correlation Between LHb Reward Signals and Reward-Related Behavior.
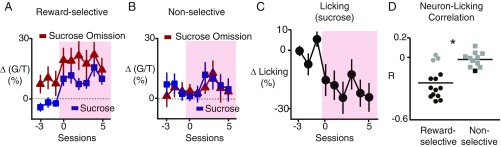
To gain insight into the relation between the stress-induced changes in neural signals and behavior, we compared their time courses. Changes in both behavior and neural signals from reward-selective neurons occurred soon after shock trials were introduced and persisted for the duration of sessions with such stress (Fig. 4 A and C). This was the case for both the inversion of the neural response to sucrose and the enhanced neural response to sucrose omission (Fig. 4A). No significant changes were observed in nonselective neurons (Fig. 4B). In addition to similar time courses, the increased sucrose consumption responses of reward-selective neurons were correlated with reduced licking on a trial-by-trial basis. For reward-selective neurons, the mean Pearson’s correlation coefficient (R) for all neurons was −0.24 ± 0.04 (P < 0.0001, n = 14 neurons, one-sample t test); for 11 of 14 reward-selective neurons the R value was statistically significant. In contrast, nonselective neurons showed no significant relationship; the mean R was −0.01 ± 0.02 (P > 0.05, n = 11 neurons, one-sample t test); only 1 of 11 neurons displayed a significant R value (Fig. 4D). These data provide an additional link between the stress-induced changes in LHb neural responses and anhedonic behavior.
Fig. 4.
Relationship between LHb neuronal activity and licking. (A and B) Mean responses of indicated neuron type during indicated trial type over sessions before (−3 to −1) and during stress (0–5). (C) Mean change in licking during sucrose presentation, normalized to sessions before stress (n = 4 mice). Sessions as in A and B. (D) Pearson’s correlation coefficients for trial-by-trial correlations between neuronal response and licking percentage during sucrose consumption for indicated neuron type. Black, correlation P < 0.05; gray, not significant. *P < 0.005. Trials with no licking in the first second after sucrose delivery or omission were excluded in A, B, and D (see Materials and Methods).
Discussion
Studies with healthy humans indicate that acute stress can reduce reward responsiveness (20, 49–51). Such effects may play a role in the development of pathologies including eating disorders (52, 53), posttraumatic stress disorder (54), and depression-linked anhedonia (20, 55). In concordance with these human data, we find that acute stress through introduction of intermittent shock trials reduces licking for a sucrose reward, consistent with anhedonic behavior (42–47).
Previous studies in rodents found that stress increases metabolic and neuronal activity in the LHb (17, 26–29), indicating that stress causes depression-relevant behaviors by increasing baseline tonic or bursting LHb activity in vivo (1, 13, 18, 29, 56). Our results suggest an unexpected, additional mechanism for how stress decreases reward-motivated behavior: negatively distorted RPE signaling in LHb neurons. Notably, stress changed the polarity of neuronal responses to rewards, making them appear as punishment signals. This neural modification could explain the observed reduced hedonic responses to sucrose rewards; that is, receiving a punishment neural signal during a behavior (licking) would be expected to reduce that behavior.
In addition, we observed an increase of punishment-like signals during reward omission. This is surprising, and runs counter to learning theories: an omission signal should decrease, not increase, if the reward is less valued. This result thus challenges the view that LHb signals simply reflect the difference between actual and expected value. Alternatively, during stressful conditions, while the actual value of a reward to the animal may be reduced (as evidenced by reduced licking), signals for reward omission could pathologically increase, thus generating a very large punishment-like omission signal.
Such distorted signals could have significant impact on an individual’s behavior, as the result of any trial would be a punishment signal from the LHb. This might be expected to reduce the motivation to pursue subsequent rewards. In this way, aberrant LHb signaling during repeated exposure to stress could produce system-wide modifications of the reward system (8–10, 57–61), leading to persistent anhedonic (59), negative-bias (62, 63), and motivational impairments (64) seen in depression.
Materials and Methods
Experimental Design.
The objective of this study was to characterize neuronal responses of mouse LHb neurons to reward and reward omission and determine the effects of stress on these responses. The relatively small size of the mouse brain facilitated implantation of short (<7 mm) GRIN lenses into and above the LHb. We used GCaMP6s to maximize signal/noise of intracellular calcium concentration fluctuations and tdTomato to correct for small movements of the brain outside of the imaging plane that could not be corrected with software. The use of 2-photon microscopy minimized out-of-plane fluorescence that could confound single-neuron fluorescence measurements. Change in fluorescence, rather than absolute fluorescence, was calculated as a measure of (change in) activity because absolute fluorescence is determined in large part by the amount of fluorophore present in the cell (especially in neurons with high baseline firing rates, like lateral habenula neurons, where individual spiking events cannot be resolved). Change in fluorescence during sucrose consumption and omission was determined relative to the time bin just before sucrose delivery (or omission) because the recent change in activity of neurons is thought to signal reinforcement (rather than relative to some distant time point). We used a 2-s time bin starting 2 s after the start of reward delivery/omission for measurement of sucrose and omission neuronal responses because this was where the greatest neuronal activity difference between sucrose consumption and omission occurred. We measured licking during the 10-s time window after sucrose delivery/omission to ensure that all consumption or anticipation-related licking was used in our analyses [licking appeared to continue after all sucrose was consumed, and this, rather than lick frequency, is thought to relate to the palatability of the solution during sucrose consumption (44)]. We determined sample size based on previous experience (S.J.S.) with neuronal recording in behaving animals. Our within-subject design reduced the number of neurons and mice needed for comparisons of neuronal activity and behavior before and during stress. Data were processed in large batches, with different conditions (before or during stress) grouped together and software analysis run in an automated way for the whole dataset. Thus, there were no between-groups comparisons that required blinding.
Animals.
Male C57/Bl6 mice (50–90 d old at time of surgery) were singly housed during the experiment. Four mice passed all of the elements (see below for details of each element) required for the study: viral injection, GRIN lens implantation surgery, sufficient number of neurons in viewing field, survival past the ∼40 imaging sessions (at most 1/d) needed for conditioning and testing, before and after stress introduction. All procedures involving mice were approved by the Institute Animal Care and Use Committee of the University of California, San Diego.
Surgery.
Mice were anesthetized with ketamine (70 mg/kg) and dexmedetomidine (1 mg/kg) and unilaterally injected in the lateral habenula with AAV2/1-synapsin-GCaMP6s (UPenn Viral Core) and AAV2/8-synapsin-tdTomato (made in Malinow laboratory; ∼0.3 μL total virus; A-P: -1.8–2.0, M-L: 0.6, D-V: 2.8–3.3 mm from bregma) with a glass pipette and picospritzer over 8–15 min. After viral injection, a 0.5-mm diameter GRIN lens (Inscopix) was implanted in or just above the LHb (A-P: −1.8–2.0, M-L: 0.6, D-V: 2.8 mm) by slowly lowering it over 20 min. An ∼20 × 3 mm metal headbar was implanted anterior to bregma. The lens and headbar were secured to the skull with Metabond dental cement. A well was built around the lens with dental cement for the water needed for imaging.
Behavior.
Three-to-five weeks after surgery, mice were habituated to head restraint under the microscope while scanned for fluorescent cells through the GRIN lens. If greater than three dual-labeled cells were detected, mice continued restraint habituation for 3–4 d (15–45 min each day) before conditioning began. If three or fewer dual-labeled cells were detected, the mice were removed from the study. Mice were restricted to 15 min of water access per day until their weight stabilized (∼10–15% body weight loss). On the first day of conditioning, the lickometer spout was positioned in front of the mouth, and drops of 10% sucrose were delivered until the mouse licked the spout. Tone and white noise stimuli (10 s; ∼75–80 dB) were randomly assigned to be CS+ and CS−. Each conditioning session consisted of an equal number of CS+ and CS− trials (16−18 per session) delivered in random order. Mice were imaged for 70 s per trial, with 25 or 35 s (interleaved randomly) of imaging preceding the start of the CSs. One drop of sucrose (0.3-s duration) was delivered 0.5 s after the end of the CS+ by opening of a valve (NResearch, part 161K011) that was located outside of the imaging chamber in a styrofoam box. If mice learned to lick selectively for the CS+ (13–25 sessions), they advanced to sessions with occasional sucrose omission (1/3 of all CS+ trials) in addition to CS+/sucrose and CS− trials. During these omission and subsequent stress sessions, one mouse was given an extra drop of sucrose 5 s after the first drop on 1/3 of CS+ trials (these trials were excluded from the analysis). After 3–9 sucrose omission sessions, stress sessions began. Stress sessions (6–9 total) were identical to sucrose omission sessions, except a 0.3-s, 0.6-mA tail shock was delivered inaudibly 0.5 s after the end of the CS− via a tail cuff placed ∼1 cm from the end of the tail. Three animals were tested for responses to shock omission (1/3 of trials) in separate sessions after the stress sessions (Fig. 3 I and J and SI Appendix, Fig. S6). One animal was tested with sessions consisting of unsignaled tail shocks (no CS− preceding tail shocks) and other sessions with unsignaled, longer-duration sucrose delivery (2 s; no CS+ preceding sucrose delivery; SI Appendix, Fig. S7).
Data Collection and Analysis.
Images were collected at 30 Hz with a 20× water-immersion Olympus XLUMPlanF 0.95 NA objective and Bruker 2-photon laser resonant scanning microscope. Motion correction was performed offline with custom code written in MATLAB, and images were saved as averages of 20 frames. Licking data (100-Hz sampling rate) were synchronized with images in Prairie View (Bruker) software and exported separately for analysis. Licking data were transformed into binary yes or no values for every 100-ms bin (except for licking microstructure analysis, Fig. 2J). Regions of interest were drawn over fluorescent neurons in average motion-corrected images in ImageJ. Data from regions of interest (ROIs) were extracted in ImageJ and saved in Excel files, which were analyzed in MATLAB.
Green/red (GCaMP/Tomato) values of dual-labeled neurons were calculated for each average frame by first subtracting background green and red fluorescence (in a region distant from ROIs) from green and red fluorescence in each ROI, then dividing green by red. For Figs. 3 and 4 A, B, and D and SI Appendix, Figs. S5 and S7, only sucrose and sucrose omission trials in which the mouse licked (within 1 s after the start of sucrose delivery or omission) were included to ensure we only included neuronal responses to sucrose consumption and unexpected sucrose omission. For SI Appendix, Fig. S8, only trials with licking in the 0.5-s interval between the end of the sucrose CS+ and sucrose presentation (or omission) were included. For Fig. 1 D–F, H–J, and L–N, activity was normalized to the mean green/red during the 5-s baseline before the CS. Normalization is calculated: ([(green/red in 100-ms time bin) – (green/red during baseline of same trial)]/[green/red during baseline]) × 100; where background green and red is first subtracted from each ROI’s green and red value. For Fig. 1 G, K, and O, activity was normalized to the time bin (100 ms) just before sucrose delivery or omission. For Fig. 3 I and J, neuronal activity was normalized to the time bin (100 ms) just before shock delivery or omission. Neuronal responses to sucrose consumption and sucrose omission were calculated as percent change in GCaMP/Tomato (∆G/T) during a 2-s window 2.5 s after the end of the CS+, relative to the time bin (100 ms) just before sucrose delivery or omission. Correlation between neuronal responses to sucrose consumption and licking during sucrose consumption were calculated using all trials in which the mouse licked (as above for Figs. 3 and 4) during all sucrose omission and stress sessions.
Reward-selective neurons were defined as neurons that had statistically significant differences (P < 0.05) in activity during sucrose presentation and sucrose omission trials in the time windows indicated above. Nonselective neurons were defined as neurons that had significant differences (P < 0.05) in activity during sucrose presentation compared with a 5-s baseline period before the CS+, but no significant difference (P > 0.05) between activity during sucrose presentation and sucrose omission. Nonresponsive neurons were defined as neurons which had no significant change in activity during sucrose presentation compared with the pre-CS baseline period. RPE neurons were defined as reward-selective neurons with opposite direction changes in activity during sucrose consumption and sucrose omission. Non-RPE neurons were defined as reward-selective neurons with the same direction changes in activity during sucrose consumption and sucrose omission. Neurons were included in the analyses for each session if they were fluorescent in both the green and red channels above background fluorescence (measured in a region with no cells toward edge of the image). Over time, although most neurons could be imaged for weeks, there was often a slight shift in the field of view that prevented chronic imaging of some neurons.
Licking was monitored with an infrared beam placed between the spout and the mouth of the mouse (sampling frequency of 100 Hz). For licking microstructure analysis (Fig. 2J), licking during the first 10 s after sucrose delivery was partitioned into clusters (bouts) separated by > 500 ms with no licking and bursts separated by > 200 ms with no licking, as previously described (44). Bout duration was used as a measure of hedonia, as previously described (44, 46, 47). For other analyses of licking (Fig. 2 C–I, K, and L), each 100-ms bin was examined for licking to calculate the percentage of time mice were licking in a given interval. Change in licking during the CSs was calculated by subtracting the average licking values during a 10-s pre-CS period from the average licking values during the 10-s CSs (reported in text). Licking during sucrose delivery/omission was calculated using the first 10 s after the start of sucrose delivery/omission (Figs. 2 G–J and L and 4 C and D). Licking percentage was used for all figures except Fig. 2J. Licking “before sucrose” (Fig. 2 G and K) was calculated using the 0.5-s interval after the end of the CS+ but before sucrose delivery/omission.
Histology.
Mice were deeply anesthetized and transcardially perfused with 0.9% saline followed by 4% formaldehyde. The 50–100-μm brain slices were imaged with an epifluorescence dissection microscope and confocal microscope. GRIN lens location was determined by inspection of lens tract in the brain. Mice with >20% of the tip of the lens outside the LHb were excluded from the study.
Statistical Analysis.
We used nonparametric bootstrap with 100,000 simulations for comparing means between unpaired groups. For paired groups, we used two-tailed paired t tests (for groups with n < 5), Wilcoxon signed-rank tests (for nonnormal, paired data), and one-sample t tests for comparing data to zero. We used χ2 tests for comparing proportions. For neuron type classification, we used unpaired t tests. Pearson’s R values with significance tests were computed in MATLAB.
Supplementary Material
Acknowledgments
We thank A. Mitani, H. Makino, and T. Komiyama for motion correction MATLAB code and assistance with the behavioral setup. We thank T. Sejnowski and members of the Malinow laboratory for feedback on the manuscript. Funding was provided by NIH Grant RO1MH091119. R.M. is supported by the Shiley-Marcos endowment.
Footnotes
The authors declare no conflict of interest.
Data deposition: Imaging raw data are available on Zenodo (https://zenodo.org/record/3226985#.XOgT-hZKhhE).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903334116/-/DCSupplemental.
References
- 1.Proulx C. D., Hikosaka O., Malinow R., Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 17, 1146–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto M., Hikosaka O., Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Wang D., et al. , Learning shapes the aversion and reward responses of lateral habenula neurons. eLife 6, e23045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto M., Hikosaka O., Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 12, 77–84 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker P. M., et al. , The lateral habenula circuitry: Reward processing and cognitive control. J. Neurosci. 36, 11482–11488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikosaka O., Sesack S. R., Lecourtier L., Shepard P. D., Habenula: Crossroad between the basal ganglia and the limbic system. J. Neurosci. 28, 11825–11829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromberg-Martin E. S., Matsumoto M., Nakahara H., Hikosaka O., Multiple timescales of memory in lateral habenula and dopamine neurons. Neuron 67, 499–510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai H. C., et al. , Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg E. E., et al. , A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz W., Stauffer W. R., Lak A., The phasic dopamine signal maturing: From reward via behavioural activation to formal economic utility. Curr. Opin. Neurobiol. 43, 139–148 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto M., Hikosaka O., Electrical stimulation of the primate lateral habenula suppresses saccadic eye movement through a learning mechanism. PLoS One 6, e26701 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatakis A. M., Stuber G. D., Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 15, 1105–1107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proulx C. D., et al. , A neural pathway controlling motivation to exert effort. Proc. Natl. Acad. Sci. U.S.A. 115, 5792–5797 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stopper C. M., Tse M. T. L., Montes D. R., Wiedman C. R., Floresco S. B., Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84, 177–189 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Sartorius A., et al. , Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry 67, e9–e11 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Yang Y., Wang H., Hu J., Hu H., Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 48, 90–96 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Caldecott-Hazard S., Mazziotta J., Phelps M., Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J. Neurosci. 8, 1951–1961 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuno-Perez A., Tchenio A., Mameli M., Lecca S., Lateral habenula gone awry in depression: Bridging cellular adaptations with therapeutics. Front. Neurosci. 12, 485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willner P., Towell A., Sampson D., Sophokleous S., Muscat R., Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl.) 93, 358–364 (1987). [DOI] [PubMed] [Google Scholar]
- 20.Bogdan R., Pizzagalli D. A., Acute stress reduces reward responsiveness: Implications for depression. Biol. Psychiatry 60, 1147–1154 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton G. C., Coffey C., Posterino M., Carlin J. B., Bowes G., Life events and early onset depression: Cause or consequence? Psychol. Med. 33, 1203–1210 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Daley S. E., Hammen C., Rao U., Predictors of first onset and recurrence of major depression in young women during the 5 years following high school graduation. J. Abnorm. Psychol. 109, 525–533 (2000). [PubMed] [Google Scholar]
- 23.Willner P., Muscat R., Papp M., Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 16, 525–534 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Berton O., et al. , Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Seligman M. E., Learned helplessness. Annu. Rev. Med. 23, 407–412 (1972). [DOI] [PubMed] [Google Scholar]
- 26.Li B., et al. , Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchenio A., Lecca S., Valentinova K., Mameli M., Limiting habenular hyperactivity ameliorates maternal separation-driven depressive-like symptoms. Nat. Commun. 8, 1135–1142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecca S., et al. , Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 22, 254–261 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., et al. , Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Cui Y., et al. , Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Li K., et al. , βCaMKII in lateral habenula mediates core symptoms of depression. Science 341, 1016–1020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo J. S., Zhong P., Liu A., Yan Z., Greengard P., Elevation of p11 in lateral habenula mediates depression-like behavior. Mol. Psychiatry 23, 1113–1119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han L. N., et al. , Serotonin7 receptors in the lateral habenular nucleus regulate depressive-like behaviors in the hemiparkinsonian rats. Brain Res. 1644, 79–87 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Han L. N., et al. , Activation of serotonin(2C) receptors in the lateral habenular nucleus increases the expression of depression-related behaviors in the hemiparkinsonian rat. Neuropharmacology 93, 68–79 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., et al. , Dorsal raphe projection inhibits the excitatory inputs on lateral habenula and alleviates depressive behaviors in rats. Brain Struct. Funct. 223, 2243–2258 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Hong S., Hikosaka O., The globus pallidus sends reward-related signals to the lateral habenula. Neuron 60, 720–729 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberg-Martin E. S., Matsumoto M., Hong S., Hikosaka O., A pallidus-habenula-dopamine pathway signals inferred stimulus values. J. Neurophysiol. 104, 1068–1076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephenson-Jones M., et al. , A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji H., Shepard P. D., Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J. Neurosci. 27, 6923–6930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J. Y., Haesler S., Vong L., Lowell B. B., Uchida N., Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavlov I. P., Conditioned Reflexes (Oxford University Press, 1927). [Google Scholar]
- 42.Mitra A., Guèvremont G., Timofeeva E., Stress and sucrose intake modulate neuronal activity in the anterior hypothalamic area in rats. PLoS One 11, e0156563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dwyer D. M., Boakes R. A., Hayward A. J., Reduced palatability in lithium- and activity-based, but not in amphetamine-based, taste aversion learning. Behav. Neurosci. 122, 1051–1060 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Dwyer D. M., EPS prize lecture. Licking and liking: The assessment of hedonic responses in rodents. Q J Exp Psychol (Hove) 65, 371–394 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Clarkson J. M., Dwyer D. M., Flecknell P. A., Leach M. C., Rowe C., Handling method alters the hedonic value of reward in laboratory mice. Sci. Rep. 8, 2448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis J. D., Smith G. P., Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav. Neurosci. 106, 217–228 (1992). [PubMed] [Google Scholar]
- 47.Travers J. B., Norgren R., Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav. Neurosci. 100, 544–555 (1986). [DOI] [PubMed] [Google Scholar]
- 48.Shabel S., Wang C., Monk B., Aronson S., Malinow R., Data from “Stress transforms lateral habenula reward responses into punishment signals.” Zenodo. https://zenodo.org/record/3226985#.XOgT-hZKhhE. Deposited 23 May 2019. [DOI] [PMC free article] [PubMed]
- 49.Bogdan R., Perlis R. H., Fagerness J., Pizzagalli D. A., The impact of mineralocorticoid receptor ISO/VAL genotype (rs5522) and stress on reward learning. Genes Brain Behav. 9, 658–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogdan R., Santesso D. L., Fagerness J., Perlis R. H., Pizzagalli D. A., Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J. Neurosci. 31, 13246–13254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berenbaum H., Connelly J., The effect of stress on hedonic capacity. J. Abnorm. Psychol. 102, 474–481 (1993). [DOI] [PubMed] [Google Scholar]
- 52.Fryer S., Waller G., Kroese B. S., Stress, coping, and disturbed eating attitudes in teenage girls. Int. J. Eat. Disord. 22, 427–436 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Boehm I., et al. , The trajectory of anhedonic and depressive symptoms in anorexia nervosa: A longitudinal and cross-sectional approach. Eur. Eat. Disord. Rev. 26, 69–74 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Kashdan T. B., Elhai J. D., Frueh B. C., Anhedonia and emotional numbing in combat veterans with PTSD. Behav. Res. Ther. 44, 457–467 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Whitton A. E., Treadway M. T., Pizzagalli D. A., Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 28, 7–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui Y., Yang Y., Dong Y., Hu H., Decoding depression: Insights from glial and ketamine regulation of neuronal burst firing in lateral habenula. Cold Spring Harb. Symp. Quant. Biol. 83, 036871 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Zhong W., Li Y., Feng Q., Luo M., Learning and stress shape the reward response patterns of serotonin neurons. J. Neurosci. 37, 8863–8875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saunders B. T., Richard J. M., Margolis E. B., Janak P. H., Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 21, 1072–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pizzagalli D. A., Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10, 393–423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Treadway M. T., et al. , Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol. Psychiatry 82, 570–577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berridge K. C., Robinson T. E., What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28, 309–369 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Eshel N., Roiser J. P., Reward and punishment processing in depression. Biol. Psychiatry 68, 118–124 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Herzallah M. M., et al. , Learning from negative feedback in patients with major depressive disorder is attenuated by SSRI antidepressants. Front. Integr. Nuerosci. 7, 67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X. H., et al. , Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 220, 874–882 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.