Significance
Cognitive function decreases during aging, but the mechanisms involved remain unclear. Revolutionary recent studies revealed that introduction of blood from young mice into old mice increased cognitive abilities and synaptic connectivity, suggesting that young blood contains specific factors supporting cognitive function. Here, we asked whether young blood is enriched in factors that act directly on neurons to promote synapse formation. We show that serum from young but not old mice indeed directly boosts synapse formation in cultured neurons, and identify two factors, thrombospondin-4 and SPARCL1, that are enriched in young blood and mediate these effects. Thus, our experiments show that young blood is enriched in multiple factors that directly promote synaptic connectivity between neurons.
Keywords: aging, synaptogenesis, NMDA receptors, synaptic transmission, synapse
Abstract
Aging drives a progressive decline in cognition and decreases synapse numbers and synaptic function in the brain, thereby increasing the risk for neurodegenerative disease. Pioneering studies showed that introduction of blood from young mice into aged mice reversed age-associated cognitive impairments and increased synaptic connectivity in brain, suggesting that young blood contains specific factors that remediate age-associated decreases in brain function. However, whether such factors in blood from young animals act directly on neurons to enhance synaptic connectivity, or whether they act by an indirect mechanism remains unknown. Moreover, which factors in young blood mediate cognitive improvements in old mice is incompletely understood. Here, we show that serum extracted from the blood of young but not old mice, when applied to neurons transdifferentiated from human embryonic stem cells, directly increased dendritic arborization, augmented synapse numbers, doubled dendritic spine-like structures, and elevated synaptic N-methyl-d-aspartate (NMDA) receptors, thereby increasing synaptic connectivity. Mass spectrometry revealed that thrombospondin-4 (THBS4) and SPARC-like protein 1 (SPARCL1) were enriched in serum from young mice. Strikingly, recombinant THBS4 and SPARCL1 both increased dendritic arborization and doubled synapse numbers in cultured neurons. In addition, SPARCL1 but not THBS4 tripled NMDA receptor-mediated synaptic responses. Thus, at least two proteins enriched in young blood, THBS4 and SPARCL1, directly act on neurons as synaptogenic factors. These proteins may represent rejuvenation factors that enhance synaptic connectivity by increasing dendritic arborization, synapse formation, and synaptic transmission.
Normal aging drives a progressive decline in cognitive function and predisposes healthy individuals to neurodegenerative disorders. Once believed to arise substantially from neuronal cell death (1), age-induced cognitive decline is now generally thought to be due to a decrease in neuronal function (2, 3). Numerous studies in rodents and primates have shown that aging decreases the number of synapses and dendritic spines in cortex and reduces the length and arborization of dendrites (4–10). Moreover, in nonhuman primates, aging reduces the frequency of spontaneous excitatory postsynaptic events (11), impairs synaptic plasticity (2), and lowers expression of AMPA receptors (AMPARs) and N-methyl-d-aspartate receptors (NMDARs) (12, 13). Together, these findings suggest that a general loss of synaptic connectivity without concomitant changes in neuronal survival may underlie aging-induced memory impairments and cognitive decline.
The molecular mechanisms that decrease synaptic connectivity and promote neurodegeneration during aging remain unclear. Several hypotheses have been advanced. One major hypothesis suggests that aging is driven by excessive neuroinflammation. This hypothesis posits that neuroinflammation during aging is not a physiological defense reaction to a pathological process, but becomes pathogenic in itself (14–17). Another widely shared idea is that age-related cognitive impairments result from decreased adult neurogenesis, although only a small percentage of neurons are actually generated by adult neurogenesis (18–20). A third hypothesis posits that aging is an intrinsic process in neurons, which as nonrenewable cells become incrementally less functional over the lifetime of an individual, thereby leading to a corresponding decrease in brain function (21). Assuming that age-related cognitive decline arises from a loss of synaptic connectivity, the first hypothesis thus suggests that neuroinflammation mediates aberrant destruction of synapses. The second hypothesis conversely implies that the adult generation of a small number of neurons influences the brain’s overall synaptic connectivity; and the third hypothesis predicts that aging impairs the ability of neurons to maintain, restructure and/or regenerate synapses, with neuroinflammation as a secondary response. As a result, antiaging therapies should aim to inhibit neuroinflammation according to the first hypothesis, boost adult neurogenesis according to the second hypothesis, and directly enhance synaptic function according to the third hypothesis.
Although these three hypotheses are well established, recent heterochronic parabiosis experiments provided major surprising insights into brain aging, and suggested a fourth hypothesis, namely, that aging is not an intrinsic process of the brain at all, but instead is driven by changes in systemic factors circulating in the blood (22–25). Specifically, introduction of blood from young into aged mice reversed impairments in learning and memory and increased dendritic spine numbers (23). Moreover, injection of aged mice with human umbilical cord plasma, but not with plasma from young or old persons, reversed cognitive impairments, enhanced immediate early-gene expression in response to environmental stimuli, and restored hippocampal long-term potentiation (26). Strikingly, the rejuvenating effects observed in these studies involved no changes in neuroinflammatory parameters or neurogenesis, suggesting that the driver of aging in the brain may be a decrease in specific factors in the blood. What remained unclear from these experiments, however, is whether blood from young animals acts directly on the brain, or indirectly by activating additional pathways.
The new systemic hypothesis of brain aging fueled efforts to find and characterize blood-borne factors that rejuvenate brain function when administered systemically. A survey of proteins enriched in human umbilical cord plasma identified tissue inhibitor of metalloproteinase 2 (TIMP2) as a candidate (26). Injection of aged mice with recombinant TIMP2 reversed cognitive impairments similar to injections of umbilical cord plasma, and depletion of TIMP2 from umbilical cord plasma abolished its rejuvenating effect (26). Moreover, TIMP2 knockout mice exhibited phenotypes that resembled accelerated cognitive aging. These results suggested that TIMP2 is a key driver for maintaining the structural plasticity of the brain. However, no cellular effects were examined, and the mechanism of action of TIMP2 remained unclear. Moreover, it was unknown why only umbilical cord plasma mediated rejuvenation but not plasma from young individuals, or why other TIMP isoforms did not substitute for TIMP2.
In addition to TIMP2, other candidate rejuvenation factors were described using systemic applications. However, these other factors are also surrounded by uncertainty. For example, gonadotropin-releasing hormone (GnRH) that acts on the hypothalamus was shown to promote brain rejuvenation but the mechanism remained unknown (27). As another example, growth and differentiation factor 11 (GDF11) was demonstrated to increase peripheral muscle strength and brain function (24, 25), but the results were disputed (28, 29).
In the present study, we took an approach that diametrically differs from the standard in vivo strategies for studying systemic factors. Instead of using systemic administration of candidate factors as an approach, we examined the effect of serum extracted from young and old mice (henceforth termed “young serum” and “old serum”) directly on cultured neurons. For this purpose, we used neurons differentiated from human embryonic stem (ES) cells (Fig. 1A). These neurons form abundant synapses that exhibit short-term plasticity and integrate into neuronal networks when transplanted into mouse brain, but largely lack NMDARs (30). We observed that young serum strongly promoted synapse formation, enhanced neurotransmitter release, and generated robust NMDAR-mediated synaptic responses. We then identified circulating factors enriched in young serum, including thrombospondin-4 (THBS4) and secreted protein acidic and rich in cysteine-like protein 1 (SPARCL1). Both THBS4 and SPARCL1 are secreted multidomain proteins that play diverse roles in growth factor signaling, cytoskeletal reorganization, and cell–matrix interactions throughout the body (31). Treatment of human neurons with either recombinant THBS4 or SPARCL1 dramatically enhanced synapse formation and activity, even in neurons cultured previously with old serum. In addition, SPARCL1 but not THBS4 greatly increased NMDAR-mediated synaptic responses. Our results suggest that THBS4 and SPARCL1, enriched in young serum, may be systemic factors that directly activate synapse formation and function in neurons. Ultimately, these factors could serve as tools for generating more mature and robust excitatory neurons from human ES cells and may provide avenues to neurodegenerative disease therapies.
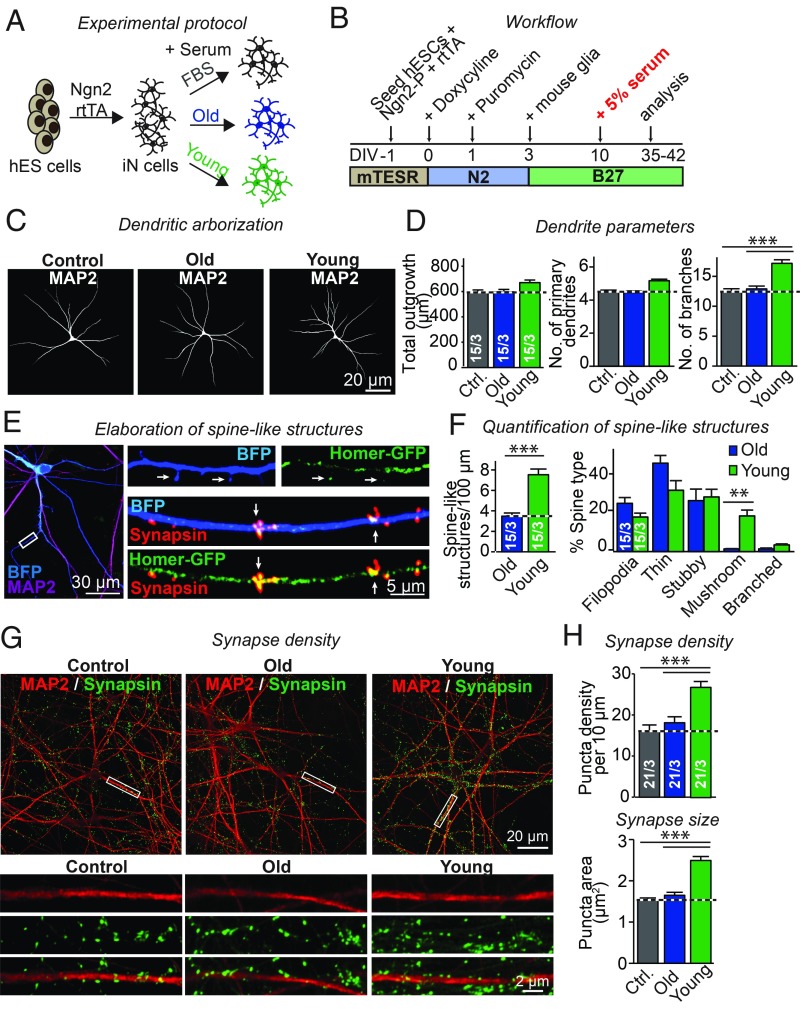
Fig. 1.
Young but not old serum increases formation of dendrites, spine-like protrusions, and synapses in human excitatory neurons derived from ES cells. (A and B) Experimental strategy. The iN cells were generated from H1 embryonic stem cells by forced expression of Ngn2, cocultured with mouse glia from DIV3, and treated with 5% FBS (control) or sera from old and young mice starting at DIV10. (C) Young and old serum do not alter overall neuronal morphology, as illustrated by representative images of sparsely plated human neurons immunostained for MAP2 to visualize dendritic arborization. (D) Quantifications showing that relative to old serum and FBS, young serum does not alter total dendritic outgrowth (Left summary graph) nor the number of primary dendrites (Middle summary graph), but does increase the total number of dendritic branches (Right summary graph). (E) Homer1 and synapsin puncta colocalize along dendrites and within the heads of spine-like structures. Representative images show neurons transfected with eBFP and Homer1-GFP, treated with FBS (control) or young or old serum, and counterstained for MAP2 and synapsin. (F) Quantifications showing that young serum greatly increases the density of spine-like structures in human neurons (Left summary graph), but induces only a minor shift in the shape of spine-like structures from thin to mushroom type (Right summary graph). (G) Representative images showing that young serum increases synapse density as visualized by immunostaining for MAP2 and synapsin. Images are from neurons treated with FBS (control) or young or old serum, with the higher-magnification images at the Bottom taken from the boxed areas in the Top images. (H) Quantifications showing that young but not old serum increases the density (Top summary graph) and size of synapses (Bottom summary graph). All bar graphs display means ± SEM; numbers of cells per independent cultures analyzed are shown within bars. Statistical significance (**P < 0.01; ***P < 0.001) was evaluated by one-way ANOVA with Tukey’s post hoc comparisons (D and H) or Student’s t test (F); nonsignificant relations are not indicated. For additional data, see SI Appendix, Fig. S1.
Results
Serum from Young but Not Old Mice Promotes Dendritic Branching, Development of Spine-Like Protrusions, and Synapse Formation in Human Neurons.
We induced human neurons from H1 ES cells by forced expression of the transcription factor neurogenin 2 (Ngn2). As described in previous studies (30, 32), human neurons produced by this method represent a relatively homogeneous population of excitatory layer 2/3 cortical neurons that exhibit a pyramidal morphology and form functional synaptic networks. After 3 d in vitro (DIV3), we cocultured these neurons with mouse glia to boost neuronal viability and synaptogenesis (refs. 30 and 32 and Fig. 1 A and B). At DIV10, we supplemented the growth medium with serum, using FBS according to our standard protocol to serve as a control, or serum extracted from young mice (postnatal day 15; young serum) or old mice (aged 12–15 mo; old serum). We then analyzed the neurons at DIV35–42.
Overall, neurons treated with FBS or old serum appeared indistinguishable and exhibited no difference in viability (SI Appendix, Fig. S1 A and B). The length and the number of primary dendrites were also identical among neurons treated with FBS or old serum. However, neurons treated with young serum exhibited more dendritic branch points (∼30% increase) and an enhanced dendritic arbor complexity (Fig. 1 C and D).
To examine dendrites in further detail, we transfected human neurons with eBFP and counterstained them for microtubule-associated protein 2 (MAP2) (Fig. 1E and SI Appendix, Fig. S1C). Treatment with old serum yielded smooth dendritic surfaces with occasional thin spine-like extensions that were devoid of MAP2 and thus likely actin based. In contrast, treatment with young serum generated abundant spine-like outgrowths (∼220% increase). These structures exhibited substantial morphological diversity. Some spine-like structures appeared short and stubby, while others possessed distinguishable necks and heads that resembled mushroom spines (SI Appendix, Fig. S1C). To determine if these spine-like extensions contained postsynaptic densities, we expressed Homer-YFP in neurons treated with young serum and counterstained them for synapsin and MAP2 (Fig. 1E). Within the heads of spine-like structures, we observed Homer puncta that colocalized with synapsin, indicating that they represent synapses. Mushroom-type spines comprised the majority of dendritic protrusions induced by young serum (Fig. F).
The observed increase in spine-like protrusions suggests that young serum may promote synapse formation. To directly test this hypothesis, we analyzed human neurons cultured in FBS or in young or old serum by immunocytochemistry for synapsin as a synapse marker and for MAP2 (Fig. 1G and SI Appendix, Fig. S1D). Quantifications of synapses per dendrite length showed that young serum significantly enhanced the density (∼65% increase) and size of synaptic puncta (∼70% increase) compared with FBS or to old serum (Fig. 1H). Collectively, our results show that young serum promotes dendritic branching, generates more spine-like outgrowths, and increases synapse numbers.
Serum from Young Mice Increases Spontaneous Synaptic Activity.
Using whole-cell patch-clamp recordings, we next investigated whether young or old serum differentially altered the electrical properties or functional synaptic connectivity of human neurons. In terms of electrical properties, young serum caused a modest increase in capacitance (∼20%) consistent with the observed increase in dendritic arborization, but produced no changes in input resistance, resting potential, neuronal excitability, or the firing threshold or amplitude of action potentials (SI Appendix, Fig. S2 A–D).
To assess functional synaptic connectivity, we first recorded miniature excitatory postsynaptic currents (mEPSCs) in the presence of tetrodotoxin from human neurons treated with the different sera. Young serum dramatically increased (∼250%) the frequency of spontaneous mEPSCs and significantly elevated the amplitude of mEPSCs (∼20%; Fig. 2A). Conversely, old serum had no effect compared with FBS. These changes are consistent with the selective increase in synapse density and size induced by young but not old serum (Fig. 1 G and H), suggesting that young serum boosts the functional synaptic connectivity of human neurons, even in the presence of cocultured glia, which in itself encourages synapse formation.
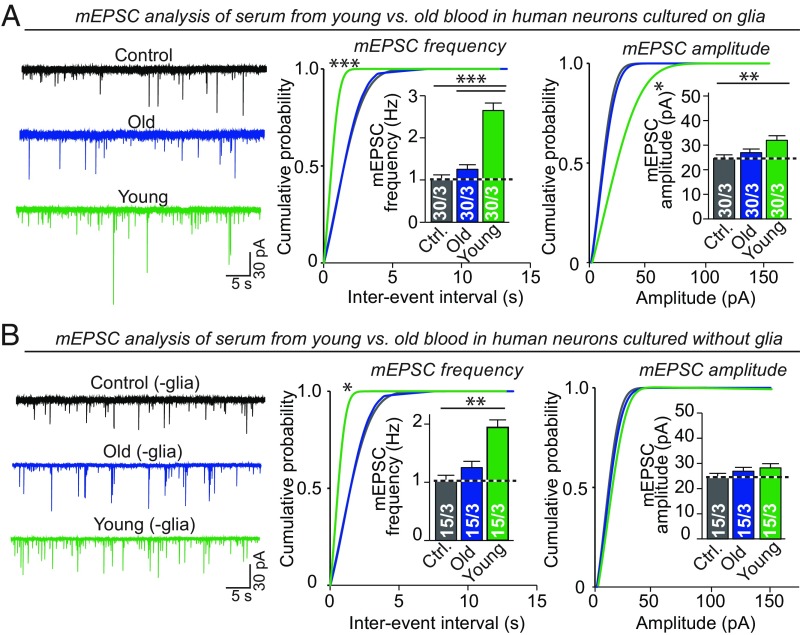
Fig. 2.
Young but not old serum acts in a dominant fashion to increase spontaneous synaptic activity in human neurons independent of glial cocultures. (A) Young but not old serum elevates spontaneous synaptic activity as monitored by the mEPSC frequency and amplitude in human neurons cocultured with glia (Left, representative traces; Right, cumulative probability plots of the mEPSC interevent intervals and of the mEPSC amplitudes. Insets: summary graphs of the mEPSC frequency and mEPSC amplitude, respectively). Sera were added at DIV10, and neurons were analyzed at DIV35. (B) Same as A, but for neurons cultured in the absence of glia. All bar graphs are means ± SEM; numbers of cells per independent cultures analyzed are shown in bars. Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was evaluated by one-way ANOVA with Tukey’s post hoc comparisons (bar graphs) or Kolmogorov–Smirnov test (cumulative probability plots); nonsignificant relations are not indicated. For measurements of passive and active electrical properties of neurons, see SI Appendix, Fig. S2.
It is puzzling that, relative to old mouse serum, FBS derived from fetal cows was not as effective as young mouse serum in boosting synaptic connectivity. At least two hypotheses could plausibly account for this observation: the commercial production of FBS may inactivate some of its components, or mouse serum may exert a species-specific effect on cocultured mouse glia. To examine whether young serum operates directly on neurons or indirectly through mouse glia, we tested human neurons cultured alone on Matrigel. Even in the absence of glia, young serum massively increased mEPSC frequency (∼200%), demonstrating that it acts directly on the neurons (Fig. 2B). We did not observe a corresponding increase in mEPSC amplitude, possibly because the neurons were not as robust when cultured on Matrigel alone versus when cultured on glia (32). Thus, it is likely that young serum acts directly on human neurons to increase synapse numbers and synaptic activity.
The synaptogenic activity of young serum compared with old serum could be mediated by specific young serum factors that promote synapse formation, or old serum factors that inhibit synaptic formation. To distinguish between these two possibilities, we investigated whether addition of young serum to neurons cultured previously in old serum still enhanced synaptic activity, or conversely whether addition of old serum to neurons cultured previously in young serum suppressed synaptic activity. In this experiment, we maintained human neurons in young or old serum from DIV10 to DIV35, and replaced half of their medium with fresh medium containing serum of the opposite type at DIV35. Finally, we recorded mEPSCs from these neurons at DIV42 (SI Appendix, Fig. S2G). Addition of young serum to human neurons cultured previously in old serum raised the mEPSC frequency and amplitude, similar to neurons treated only with young serum. Thus, the synaptogenic effect of young serum overrides the effects of old serum (SI Appendix, Fig. S2G). Conversely, addition of old serum to neurons cultured previously in young serum modestly decreased the mEPSC frequency and amplitude compared with young serum alone (SI Appendix, Fig. S2G). These results suggest that young serum contains factors that activate synapse formation even in the presence of old serum.
Young Serum Enhances Both AMPAR- and NMDAR-Mediated Synaptic Responses.
To characterize action potential-dependent synaptic transmission in neurons treated with young or old serum, we measured evoked EPSCs mediated by AMPARs and NMDARs (Fig. 3A and SI Appendix, Figs. S2E and S3F). Consistent with the enhancement of synapse density and the elevaton of the mEPSC frequency (Figs. 1 and 2), young serum dramatically increased (∼250%) the amplitude of AMPAR EPSCs relative to neurons treated with FBS or with old serum. Unexpectedly, young serum produced an even more dramatic increase (>300%) in the amplitude of NMDAR-mediated EPSCs (Fig. 3A). We validated the distinct measurements of AMPAR- and NMDAR-mediated EPSCs by pharmacologically isolating each component (SI Appendix, Fig. S2). The increase in NMDAR-mediated responses was surprising because NMDAR-mediated responses, as opposed to AMPAR-mediated responses, are barely detectable in human neurons produced by Ngn2 transduction of ES or iPS cells (30) and are also generally lacking in human neurons produced by other protocols. Consistent with this observation, young serum greatly increased (>250%) the NMDAR/AMPAR ratio of synaptic responses (Fig. 3A). Collectively, these data show that young serum not only potently promotes synapse formation, but also changes the receptor composition of synapses by increasing their NMDAR responses.
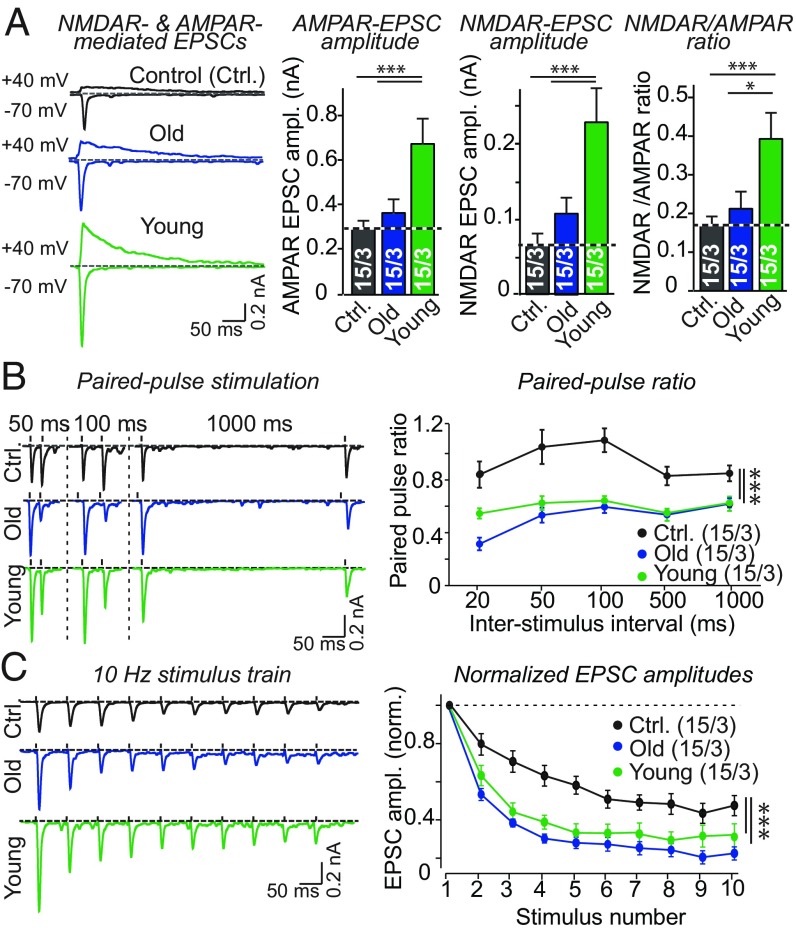
Fig. 3.
Young but not old serum enhances evoked synaptic transmission and increases the NMDAR/AMPAR ratio, but young serum does not affect short-term synaptic plasticity. (A) Young but not old serum significantly increases the amplitudes of evoked AMPAR- and NMDAR-EPSCs and enhances the NMDAR/AMPAR ratio in human neurons (Left, representative traces of evoked AMPAR- and NMDAR-EPSCs monitored in control neurons or in old or young serum-treated neurons; Right, summary graphs of AMPAR- and NMDAR-EPSC amplitudes and of the NMDAR/AMPAR ratio calculated separately for each neuron). Sera were added at DIV10 and neurons were analyzed by DIV42 (for validation of the AMPAR- and NMDAR-EPSC measurements, see SI Appendix, Fig. S2 E and F). (B) Neurons treated with young or old serum exhibit a similar degree of paired-pulse depression of evoked AMPAR-EPSCs that is significantly lower than the paired-pulse depression observed in control neurons treated with FBS (Left, representative traces; Right, summary plot of paired-pulse ratios as a function of the interstimulus interval). (C) Neurons treated with young or old serum exhibit similar synaptic depression of evoked AMPAR-EPSCs during a 10-Hz stimulus train. Again, synaptic depression is significantly higher in FBS-treated control neurons than in neurons treated with mouse sera (Left, representative traces; Right, summary plot of the EPSC amplitudes normalized to the first response as a function of time during the stimulus train). All bar and line graphs show means ± SEM; numbers of cells per independent cultures analyzed are shown within the bars. Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was evaluated by one- or two-way ANOVAs and Tukey’s post hoc comparisons. Nonsignificant relations are not indicated.
To examine whether young serum additionally impacts the release probability at synapses, we analyzed short-term synaptic plasticity. Relative to old serum, young serum did not significantly alter the paired-pulse ratio of two closely spaced EPSCs (Fig. 3B) or the use-dependent, short-term synaptic depression induced by high-frequency stimulus trains (Fig. 3C). Interestingly, in both tests control neurons cultured in FBS exhibited significantly less depression than neurons cultured in young or old mouse serum. This finding suggests that compared with mouse sera, FBS inhibits neurotransmitter release, possibly owing to the presence of inhibitory contaminants and/or degradation of permissive constituents in commercial serum (Fig. 3 B and C). Viewed together, these results indicate that young serum promotes synapse formation and synaptic NMDAR recruitment without dramatically reorganizing the presynaptic release machinery.
Identification of Protein Factors Enriched in Old and Young Serum by Tandem Mass Spectrometry.
In search of specific factors in young or old serum that might increase or suppress synapse numbers, we fractionated proteins in young and old sera. We first filtered the sera through a cellulose membrane that retains proteins larger than 3 kDa (“concentrated fraction”) as confirmed by protein assays (SI Appendix, Fig. S3A). We then passed the retained serum proteins through columns packed with an albumin-specific affinity resin and an immobilized Protein-A resin. The resultant eluates were depleted of most albumin and immunoglobulins (“serum factors”; see SI Appendix, Fig. S3B). Next, we treated human neurons with each fraction and recorded mEPSCs to test their synaptogenic activity. We focused specifically on fractions obtained from young serum because it has such a dramatic and selective effect on mEPSC frequency in human neurons (Fig. 2A). The concentrated serum and the serum factors that were depleted of albumin and immunoglobulins (IgG) caused the same dramatic increase in mEPSC frequency (∼300%) as total young serum (Fig. 4 A–C). In contrast, the serum filtrate and the flow through containing albumin and IgG were relatively inactive.
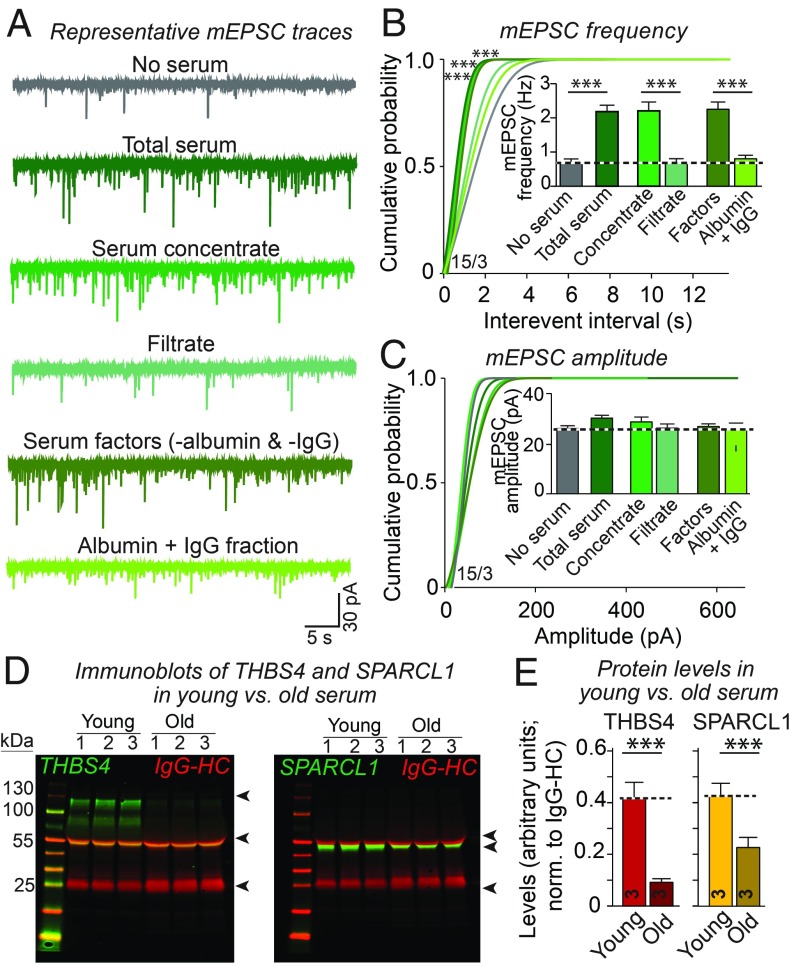
Fig. 4.
Partial purification of synaptogenic proteins from young serum and identification of protein factors enriched in young or old serum by tandem mass spectrometry. (A–C) mEPSC recordings show that protein concentrates from young serum that were depleted of IgGs and albumin potently activate synaptic responses in human neurons [A, representative traces; B and C, cumulative probability plots of mEPSC interevent intervals (Inset: summary graph of the mEPSC frequency) and of mEPSC amplitudes (Inset: summary graph of mEPSC amplitudes)]. For further data on the fractionation of serum proteins, see SI Appendix, Fig. S3. (D and E) THBS4 and SPARCL1 are enriched in young blood serum as quantified by immunoblotting (see SI Appendix, Fig. S4 for additional data). Bar graphs are means ± SEM; numbers of cells per independent cultures analyzed are shown within the bars. Statistical significance (***P < 0.001) was evaluated by one-way ANOVA and Tukey’s post hoc test (bar graphs) or Kolmogorov–Smirnov test (cumulative probability plots). Nonsignificant comparisons are not indicated.
We next analyzed the serum factors fraction from old and young mice by tandem mass spectrometry (SI Appendix, Fig. S3C). To accurately determine proteins enriched in young or old serum, we differentially labeled the peptides in each sample with TMT isobaric tags containing variable mass reporter regions. This enabled us to determine the ratiometric abundance of proteins in each serum sample in reference to the pooled control. Spectral analyses from two independent experiments identified ∼450–600 proteins in each sample. To select candidate proteins for further study in an unbiased manner, we consolidated similar proteins into groups, ranked the groups according to coverage, and calculated the reciprocal fold-elevation ratios from the abundance of a protein in each sample. In this manner, we identified a series of proteins enriched in young or old blood (SI Appendix, Fig. S3C).
Among the proteins that we identified as enriched in young serum were THBS4 and SPARC-like protein 1 (SPARCL1). Because we found in subsequent experiments that these two proteins recapitulate the enhancement of synapse formation mediated by young serum (see below), we aimed to independently confirm their enrichment in young serum by quantitative immunoblotting. These measurements showed that THBS4 levels were ∼400% higher in young than in old serum, whereas SPARCL1 levels were ∼200% higher (Fig. 4E and SI Appendix, Fig. S4). Thus, it is likely that the proteins identified by mass spectrometry truly differ in abundance between young and old sera.
THBS4 and SPARCL1 Increase Spontaneous Synaptic Activity, Synapse Density, and Dendritic Branching.
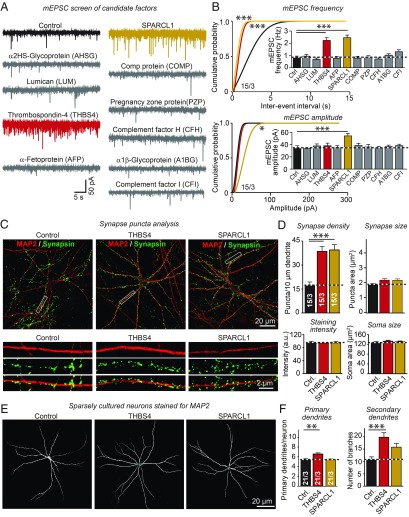
We selected for further analysis six and four proteins, respectively, that were enriched more than 1.5-fold in young vs. old serum. These proteins were picked based on their plausibility as signaling factors (SI Appendix, Fig. S3C). We expressed these proteins in recombinant form and recovered them from the supernatants of transfected HEK293 cells, which were cultured in the absence of serum (SI Appendix, Fig. S5 A and B; ref. 32). We then analyzed their effects on human neurons using supernatant from HEK293 cells transfected with mClover as a negative control. We added the factors to the serum-free growth medium of human neurons at DIV35 and recorded mEPSCs from these neurons at DIV40–42 (Fig. 5A). Strikingly, two factors enriched in young serum—THBS4 and SPARC-like protein 1 (SPARCL1)—substantially raised the mEPSC frequency (∼250%; Fig. 5B). In addition, SPARCL1 significantly elevated the mEPSC amplitude (∼30%; Fig. 5B). All other factors tested, including factors enriched in old blood, had no significant effect on spontaneous synaptic activity.
Fig. 5.
THBS4 and SPARC-like protein 1 (SPARCL1) increase spontaneous synaptic responses, synapse density, and dendritic branching. (A and B) Screen of recombinant candidate proteins enriched in young or old serum identifies THBS4 and SPARCL1 as potent stimulators of synapse formation. Proteins secreted into the medium of transfected HEK293T cells expressing candidate factors were concentrated by ultrafiltration and added at DIV35 to the neuronal growth medium (final concentration, 1.43%) in the absence of serum. Neurons were analyzed by mEPSC recordings at DIV40–42 (A, representative traces; B, cumulative probability plots of mEPSC interevent intervals. Inset: summary graph of the mEPSC frequency; and of mEPSC amplitudes, Inset: summary graph of mEPSC amplitudes). (C and D) Recombinant THBS4 and SPARCL1 increase the synapse density of human neurons (C, representative images of control and treated neurons immunostained for MAP2 and synapsin; B, quantification of synapsin puncta density, area and intensity on proximal dendrites of control, and factor-treated human neurons). (E and F) Recombinant THBS4 and SPARCL1 increase dendritic branching in human neurons (E, representative images of sparsely plated human neurons immunostained with MAP2 to visualize dendritic arborization; F, quantification of primary dendrites per neuron and number of dendritic branches). All bar graphs are means ± SEM; numbers of cells per independent cultures analyzed are shown within the bars. Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was evaluated with one-way ANOVA followed by Dunnett’s post hoc test (B) or Tukey’s post hoc test (D and F), or by Kolmogorov–Smirnov test (cumulative probability plots in B). For the production of recombinant THBS4 and SPARCL1 and sample images, see SI Appendix, Fig. S5.
We next measured the effect of THBS4 and SPARCL1 on synapse density by immunocytochemistry (Fig. 5C and SI Appendix, Fig. S5C). Consistent with the higher mEPSC frequencies, THBS4 and SPARCL1 both greatly increased the density of synaptic puncta (∼250%) without changing the size and staining intensity of synaptic puncta nor the size of the neuronal soma (Fig. 5D). We additionally measured the length and arborization of dendrites as a function of THBS4 or SPARCL1 treatment (Fig. 5E). Although neither THBS4 nor SPARCL1 altered the number of primary dendrites, both increased dendritic branching and thus enhanced the complexity of dendritic arbors (Fig. 5F). Viewed together, these results show that THBS4 and SPARCL1 recapitulate the synaptogenic phenotype of young blood.
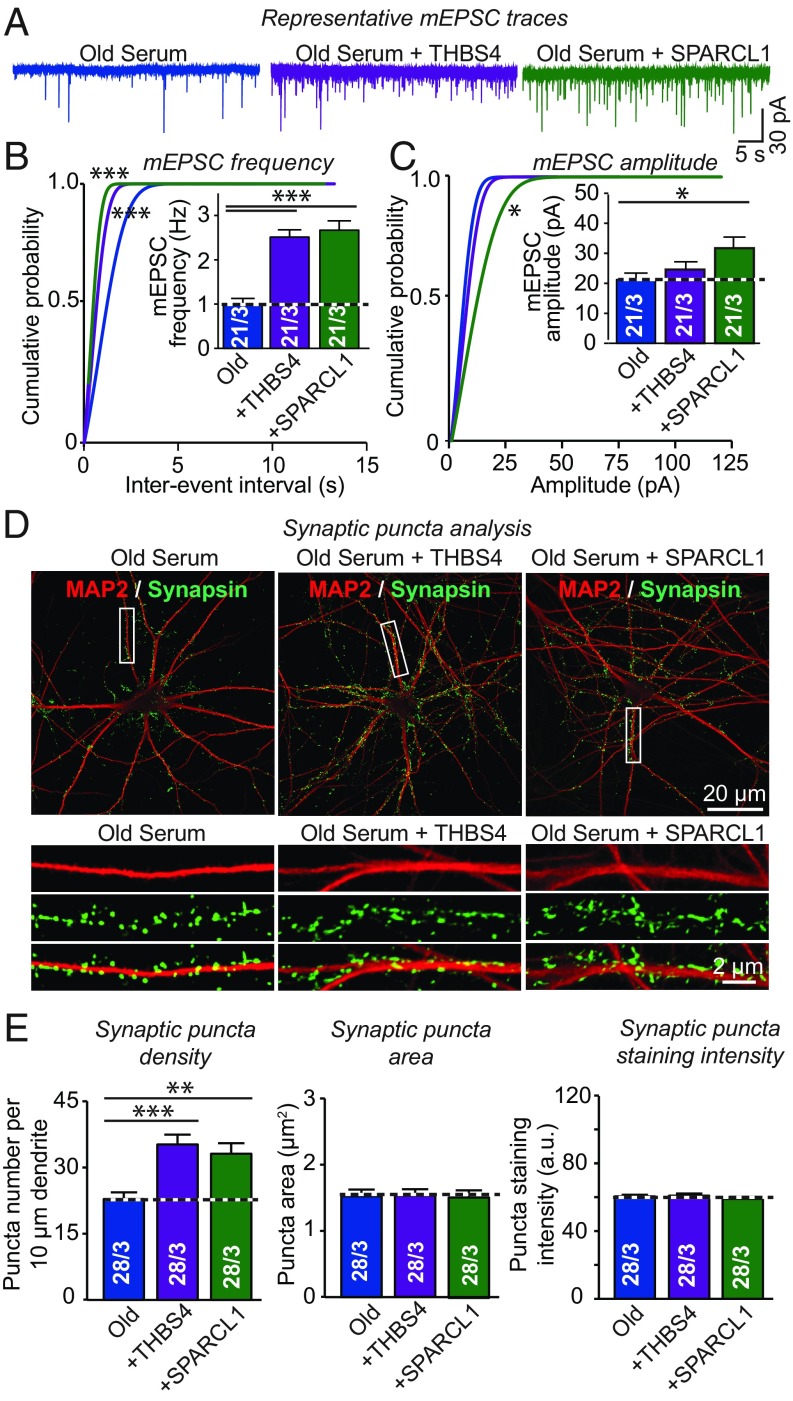
In the experiments above, we examined THBS4 and SPARCL1 using neurons cultured in the absence of serum. Although it is plausible that young serum is more active than old serum because it contains higher levels of THBS4 and SPARCL1, it is also possible that the much lower levels of THBS4 and SPARCL1 in old serum would be sufficient to rejuvenate neurons but are counteracted by deleterious factors in old serum. To test this possibility, we examined the effect of THBS4 and SPARCL1 on human neurons cultured in old serum (Fig. 6). We found that both THBS4 and SPARCL1 greatly increased the mEPSC frequency (∼250%) and significantly elevated the synapse density (∼80%) in human neurons cultured in old serum (Fig. 6). Thus, the relative lack of THBS4 and SPARCL1 in old serum accounts for at least part of its inability to promote synaptic connectivity in human neurons.
Fig. 6.
Addition of THBS4 or SPARCL1 enhances synaptic responses and synapse numbers even when neurons are cultured in old serum, demonstrating that both proteins override the effect of putative old serum factors. Old serum was added to human neurons cocultured with glia at DIV10; THBS4 or SPARCL1 were subsequently added at DIV35; and neurons were analyzed at DIV42. (A–C) Addition of THBS4 or SPARCL1 dramatically increases synaptic activity in neurons cultured in old serum, as evidenced by analyses of mEPSCs (A, representative traces; B, cumulative distribution of the interevent interval of mEPSCs with the mean mEPSC frequency shown in the bar diagram Inset; C, cumulative distribution of the mEPSC amplitude with the mean amplitude shown in the bar diagram Inset). (D and E) Addition of THBS4 or SPARCL1 dramatically increases synapse numbers in human neurons cultured in the presence of old serum (D, representative images of neurons stained for MAP2 and synapsin; E, summary graphs of the synapse density, size, and staining intensity). All bar graphs are means ± SEM; numbers of cells per independent cultures analyzed are shown in bars. Statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001) was evaluated by one-way ANOVA followed by Tukey’s post hoc tests (bar graphs) or Kolmogorov–Smirnov test (cumulative probability plots). Nonsignificant comparisons are not indicated.
THBS4 and SPARCL1 Enhance Evoked Neurotransmission Without Altering Short-Term Synaptic Plasticity.
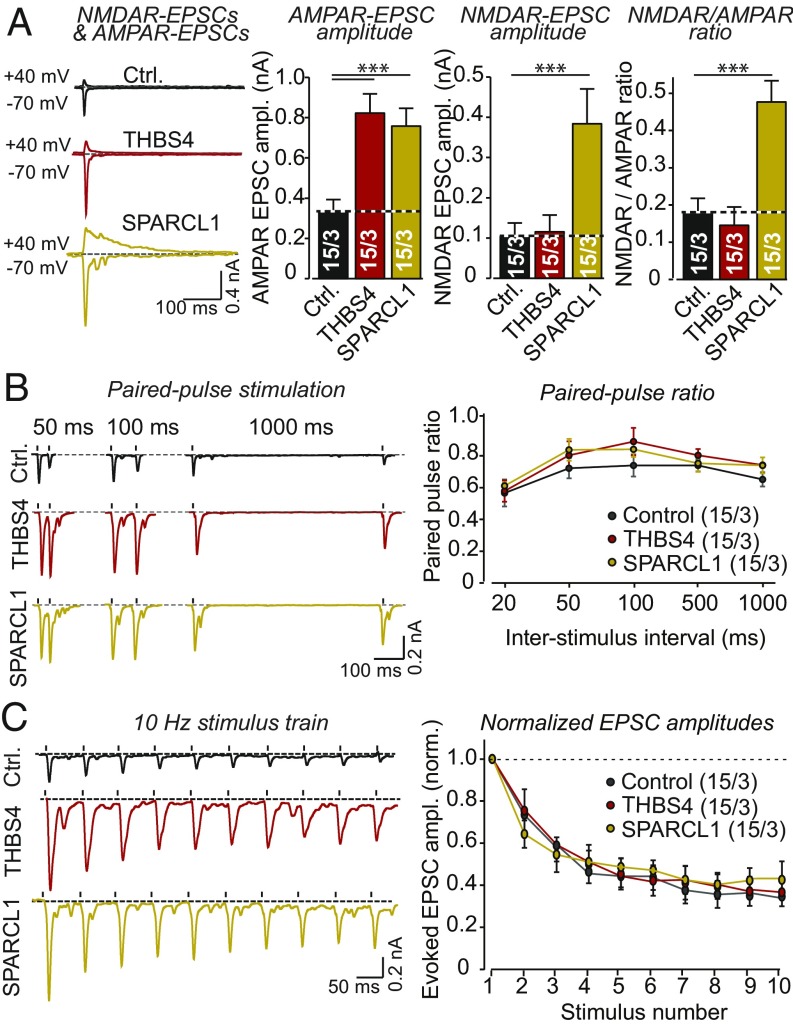
To characterize action potential-dependent synaptic transmission in neurons treated with THBS4 and SPARCL1, we measured evoked AMPAR- and NMDAR-mediated EPSCs (Fig. 7A). Both THBS4 and SPARCL1 more than doubled the amplitude of AMPAR-EPSCs (∼250% increase; Fig. 7A). Additionally, SPARCL1 but not THBS4 massively enhanced the amplitude of NMDAR-EPSCs (∼400%). Because SPARCL1 increased NMDAR-EPSCs more than AMPAR-EPSCs, it also greatly enhanced the NMDAR/AMPAR ratio (∼250%; Fig. 7A).
Fig. 7.
THBS4 and SPARCL1 increase AMPAR-EPSCs, and SPARCL1 additionally enhances NMDAR-EPSCs, without altering short-term synaptic plasticity. (A) THBS4 and SPARCL1 significantly increase the amplitudes of evoked AMPAR-EPSCs, while only SPARCL1 enhances the amplitude of evoked NMDAR-EPSCs. Recombinant factors (or control medium) were added at DIV35 to neurons cultured in the absence of serum, and neurons were analyzed at DIV42 by recording of EPSCs evoked by extracellular stimulation (Left, representative traces of EPSCs monitored at −70 mV and +40 mV holding potentials; Right, summary graphs of AMPAR- and NMDAR-EPSC amplitudes and of the NMDAR/AMPAR ratios). (B) THBS4 and SPARCL1 do not significantly alter paired-pulse depression in human neurons treated as described in A (Left, representative traces of synaptic responses to two closely spaced stimuli; Right, summary plots of the paired-pulse ratio as a function of the interstimulus interval). (C) THBS4 and SPARCL1 do not significantly alter synaptic depression induced by a 10-Hz stimulus train in neurons treated as described in A (Left, representative EPSC traces; Right, summary plots of the EPSC amplitudes normalized to the first response). All bar and line graphs represent means ± SEM; numbers of cells per independent cultures analyzed are shown within the bars. Statistical significance (***P < 0.001) was evaluated by one- or two-way ANOVAs with Tukey’s post hoc comparisons; nonsignificant comparisons are not indicated.
Thus, THBS4 and SPARCL1 separately mimic the effect of young serum by enhancing synaptic connectivity. To extend this comparison, we asked whether THBS4 and SPARCL1 modulate the probability of neurotransmitter release and analyzed short-term synaptic plasticity in control and THBS4- and SPARCL1-treated human neurons. Paired-pulse recordings uncovered no difference in paired-pulse depression between the three conditions (Fig. 7B). Moreover, high-frequency stimulus trains produced similar degrees of depression in control, THBS4-treated, and SPARCL1-treated neurons (Fig. 7C). Consistent with the phenotype of neurons treated with young serum (Figs. 1–3), THBS4 and SPARCL1 both increase synaptic connectivity, but both factors act by distinct mechanisms: SPARCL1 but not THBS4 enhances NMDAR synaptic responses.
Discussion
Aging is thought to drive a progressive decline in synaptic connectivity in brain, resulting in cognitive impairments and predisposition to neurodegenerative diseases (2, 3, 14, 17). Recent heterochronic parabiosis experiments revealed that aging is, at least in part, mediated by changes in systemic factors circulating in blood (23–25). Introduction of blood from young mice into old mice caused rejuvenation of multiple organ systems, especially the brain. Strikingly, this rejuvenation reversed cognitive impairments and increased dendritic spines in cortical neurons (23). Moreover, injection of plasma from human cord blood or injection of TIMP2, a protein that is enriched in human cord blood plasma, similarly rejuvenated the brain of aged mice (26). These studies tantalizingly suggested that the levels of a single protein, TIMP2 that is enriched in blood of young animals, may determine brain age. However, these studies also raised puzzling questions. Are other rejuvenation factors present in young blood, or are the rejuvenating effects of young blood entirely due to TIMP2? Do these rejuvenating factors act directly on neurons to counteract the loss of synaptic connectivity observed during aging, or do they improve brain function via an indirect mechanism?
In the present study, we have addressed these questions using an approach that differs from the commonly employed approach involving systemic application of blood or serum factors: We directly tested the effects of serum and candidate factors on cultured neurons. By examining the effects of serum and its constituent factors on synapse formation and synaptic function in human neurons, we could test whether serum from young mice is enriched in specific synaptogenic factors that act on human neurons, or conversely whether serum from old mice contains inhibitory factors that override the effects of synaptogenic factors. Our experiments show that young but not old serum dramatically increased synapse numbers, synaptic responses, and NMDAR content in human neurons (Figs. 1–3). These changes were accompanied by increases in dendritic arborization and spine-like structures. Fractionations revealed that proteins in young serum mediate these effects (Fig. 4), and mass spectrometry, immunoblotting, and treatment of human neurons with recombinant proteins identified THBS4 and SPARCL1 as key factors that increased dendritic arborization, dendritic spines, and synapse numbers (Figs. 4–7). In addition, SPARCL1 but not THBS4 dramatically increased synaptic NMDAR-mediated responses. Human neurons transdifferentiated from ES cells notoriously lack NMDARs (30), and SPARCL1 is the only factor to our knowledge that induces synaptic NMDAR responses in neurons derived from ES cells. Finally, both THBS4 and SPARCL1 acted when added to neurons cultured in old serum (Fig. 6), suggesting that old serum does not contain inhibitory factors. Viewed together, our results indicate that multiple circulating factors regulate dendrite and synapse formation in neurons, and that a gradual loss of such factors may contribute to the decline in brain function during aging.
Thrombospondins and SPARCL1 were previously identified as possible dendritogenic and synaptogenic factors (33–37). As such, thrombospondin-1 and -2 as well as SPARCL1 were suggested to act as astrocytic factors that support synapse formation of cocultured neurons (35, 37). In our analyses, however, recombinant THBS4 and SPARCL1 robustly increased synaptogenesis even when cocultured with astrocytes, implying that both factors are not supplied in significant amounts by the astrocytes. Moreover, it was suggested thrombospondin-1 promotes synapse formation without causing recruitment of postsynaptic receptors, thereby generating “silent” synapses (35). However, we find that thrombospondin-4 caused a massive increase in AMPAR-mediated synaptic responses and thus induced functional synapses—in fact, the increase in synaptic transmission was proportionally higher than the increase in synapse numbers. Thrombospondin-1 and -2 were proposed to act by binding to the α2δ-1 subunit of Ca2+ channels because gabapentin inhibited the effect of thrombospondin-2 on synapse formation (38), but uncertainty about this mechanism has been reported (39). In another study, thrombospondin-1 was shown to act in hippocampal synaptogenesis by binding to neuroligin 1 (40), but neither sparse nor constitutive deletion of neuroligin 1 causes loss of synapses or of AMPAR-mediated responses in the hippocampus (41, 42), arguing against this idea. Other hypotheses suggest that THBS4 may facilitate laminin clustering (33, 36) and thereby increase dendritic branching, that it may bind to integrins (43) to stabilize synapses, or that it may modulate Notch signaling (44) to influence synaptic plasticity. It is possible that some of the differences and even discrepancies between these results are due to differences between experimental conditions. Thus, at present, it remains unclear how precisely THBS4 functions as a synaptogenic factor and whether other thrombospondins have similar functions.
Similar to thrombospondins, SPARCL1 (also named “Hevin”) was proposed as a factor that is secreted by astrocytes to promote synapse formation. SPARCL1 was reported to enhance synapse formation by binding to presynaptic neurexin-1α and postsynaptic neuroligin 1 (45). In at least one study, however, binding of SPARCL1 to neuroligin 1 could not be detected (46). Moreover, deletion of only neuroligin 1 or of all neuroligins from mouse brain does not significantly alter the numbers of most synapses (41, 42), nor does deletion of all neurexins (47, 48).
Thus, the mechanisms of action of THBS4 (and possibly other thrombospondins) and of SPARCL1 in brain are unclear and remain a fertile field for further studies. Since thrombospondins and SPARCL1 perform general functions in shaping the extracellular matrix in many tissues throughout the body (49–51), the most plausible hypothesis at present is that they also perform this role in brain. Given the emerging contribution of the extracellular matrix to synapse function (52), these proteins may regulate dendritogenesis and synapse formation in a manner similar to their action in nonneuronal cells, but by a mechanism that is distinct from that of synaptic cell-adhesion molecules such as neurexins, neuroligins, and latrophilins (53). However, such an extracellular matrix-based mechanism does not account for the potent ability of SPARCL1 to promote NMDAR-mediated synaptic responses, suggesting that this mechanism at best explains part of the activity of SPARCL1.
Why did our experiments not identify TIMP2, previously implicated as the central rejuvenating factor in human cord blood (26), as a synaptogenic factor for human neurons? This discrepancy may be attributable to differences between protein detection methods and/or experimental systems. We did not detect TIMP2 in old and young mouse sera by mass spectrometry, possibly owing to its low abundance relative to other synaptogenic proteins. Cellular targets of TIMP2 have not been investigated, and it remains unknown whether TIMP2 acts directly on neurons to promote synapse formation and regulate structural plasticity. In an in vivo setting, it is possible that TIMP2 promotes synapse formation by an indirect mechanism. For example, TIMP2 may function broadly as a neuroprotective factor by inhibiting metalloprotease activity and preventing excessive degradation of the extracellular matrix. It may also stimulate other synaptogenic factors or facilitate transport of such factors across the blood–brain barrier. Alternatively, young blood may contain multiple factors that exhibit different relative concentrations in human and mouse blood, although in this case it is puzzling to understand why depletion of TIMP2 from human cord blood would have blocked the effects of other factors (16).
Our experiments demonstrate that with THBS4 and SPARCL1, young blood is enriched in two factors that directly act on neurons to stimulate dendritogenesis and synapse formation. The direct dendritogenic and synaptogenic activity of these factors in cultured neurons, however, does not prove that they actually function as rejuvenation factors in young blood in a living animal. It is not clear whether THBS4 and SPARCL1 can cross the blood–brain barrier and be transported from blood into the brain parenchyma. Some proteins appear to cross the blood–brain barrier by receptor-mediated transcytosis (54), which is mediated by several receptors in the endothelial cell membrane, including the low-density lipoprotein receptor-related protein 1 (LRP1; 55). LRP1 binds with high affinity to the N-terminal heparin-binding domain of THBS1, which is shared by all THBS isoforms (56). Therefore, it may be possible for THBS4 to cross the blood–brain barrier by transcytosis. Analogously, as a matricellular protein, SPARCL1 could bind to a presently unidentified scavenger receptor for uptake at the blood–brain barrier. Future experiments will have to explore these questions. Even if systemic administration of THBS4 and SPARCL1 was active in the classical systemic administration assay and if they entered the brain, this still would not prove such activity since these factors are also expressed by cells in brain, in particular glia. Testing this hypothesis will require genetic manipulations of these factors, ideally of multiple factors simultaneously, that selectively deletes them in blood but not in the brain. This is a challenging experiment that may be required to test the hypothesis of whether various candidate rejuvenation factors actually physiologically act as such, possibly in a patchwork of multiple overlapping activities.
Experimental Procedures
For detailed methods, see SI Appendix.
Animal Procedures and Serum Collections.
Male and female CD1 wild-type mice Crl:CD1 (ICR; Charles River Laboratories) were used in all experiments. Serum was collected from 15-d or 12- to 15-mo-old mice immediately after killing by CO2 inhalation. All animal procedures were approved by the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University and are consistent with the policies of the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the Panel on Euthanasia of the American Veterinary Medical Association.
Generation of Human Neurons from ES Cells.
Induced human neurons (iN cells) were generated from H1 ES cells basically as described (30, 57), and treated as described in Fig. 1B. Neurons were assayed after at least DIV35.
Serum Protein Isolation.
Proteins in serum samples from young and aged mice were isolated by centrifugal ultrafiltration at 14,000 × g for 30 min through an Amicon regenerated cellulose membrane with a nominal molecular weight limit of 3 kDa (Millipore). Total protein concentrations were measured using a colorimetric BCA protein assay according to the manufacturer’s protocol (Thermo Fisher Scientific). Serum samples were depleted of albumin and Ig (IgG) using ProteoExtract columns according to the manufacturer’s protocol (Calbiochem).
TMT Isobaric Mass Tagging and Tandem Mass Spectrometry.
Serum peptides were prepared and labeled with TMT isobaric mass tags according to the manufacturer’s protocol (Thermo Fisher Scientific). Digested peptides from each serum sample (100 µg per reaction) were differentially labeled with TMT Label Reagents and subsequently combined in equal amounts. Quantitation of labeled peptides was performed using a tandem mass spectrometer capable of MS/MS fragmentation and spectra were analyzed by the Stanford University Mass Spectrometry Facility (SUMS). Similar proteins were consolidated into groups, and ratiometric abundances were determined in reference to the pooled control.
Recombinant Expression of Candidate Serum Factors.
Mouse full-length cDNA clones were obtained from the NIH Mammalian Gene Collection (Dharmacon, GE Healthcare), and recloned into pEB-Multi-Neo (Wako) using In-Fusion cloning (Clontech). Recombinant proteins were produced in transfected HEK293T cells. The medium from HEK293T cells expressing candidate synaptogenic factors was concentrated by centrifugal ultrafiltration, and concentrates were diluted 1:100 in serum-free neuronal growth medium before adding them to human neuron cultures at 35 DIV. Neurons were assayed within 5–7 d after treatment.
Immunocytochemistry.
All immunocytochemistry experiments were performed as described (58), using antibodies to synapsin to mark synapses, MAP2 to mark dendrites, and NeuN to mark neuronal nuclei. Images of neurons with pyramidal morphology were acquired as Z stacks using a Nikon A1RSi confocal microscope with constant laser gain and offset settings, scanning speed, and pinhole size. Synaptic puncta were counted along well-isolated primary dendrites (5 × 100-µm dendritic segments per cell) using the “Count Nuclei” application in Metamorph (Molecular Devices). To measure dendritic length and branching, field images of low-density neuronal cultures stained with MAP2 and NeuN were analyzed using the “Neurite Outgrowth” application in Metamorph. Constant threshold settings to exclude background signals were maintained for all experimental conditions.
Electrophysiology.
Whole-cell patch-clamp recordings were performed as described (59, 60). The resistance of pipettes filled with intracellular solution varied between 3.0 and 4.0 MΩ. Intrinsic firing properties were recorded in current-clamp mode. To induce action potentials, increasing currents were injected for 1 s in an incremental manner. Spontaneous miniature synaptic responses (monitored in the presence of 1 µM tetrodotoxin) and evoked synaptic responses were recorded in voltage-clamp mode. Evoked synaptic responses were triggered by 0.5-ms current (100 µA) injection through a local extracellular electrode (FHC concentric bipolar electrode) placed 100–150 µm from the soma of neurons recorded. Data were digitized at 10 kHz with a 2-kHz low-pass filter using a Multiclamp 700B Amplifier (Molecular Devices). Data were analyzed using Clampfit 10.1 software. All experiments were performed at room temperature.
Statistical Analyses.
All experiments were performed on at least 15 cells from three independent cultures. All data shown are means ± SEMs. All statistical analyses were performed using one- or two-way ANOVAs with Tukey’s post hoc tests, comparing control and treated conditions within the same experiments.
Supplementary Material
Acknowledgments
We thank the SUMS for performing the TMT isobaric mass tagging and tandem mass spectrometry. K.J.G. is funded by a postdoctoral research fellowship from the Canadian Institutes of Health Research (MFE-141209). This research was funded by grants from the National Institute of Mental Health (MH052804 and MH092931 to T.C.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902672116/-/DCSupplemental.
References
- 1.Brody H., Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J. Comp. Neurol. 102, 511–516 (1955). [DOI] [PubMed] [Google Scholar]
- 2.Burke S. N., Barnes C. A., Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Dickstein D. L., et al. , Changes in the structural complexity of the aged brain. Aging Cell 6, 275–284 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada A., et al. , Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: A model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 32, 1–14 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Peters A., Sethares C., Moss M. B., The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb. Cortex 8, 671–684 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Peters A., Sethares C., Luebke J. I., Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152, 970–981 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Brabander J. M., Kramers R. J., Uylings H. B., Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur. J. Neurosci. 10, 1261–1269 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S., Akiguchi I., Kameyama M., Mizuno N., Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: A quantitative Golgi study. Acta Neuropathol. 65, 281–284 (1985). [DOI] [PubMed] [Google Scholar]
- 9.Jacobs B., Driscoll L., Schall M., Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J. Comp. Neurol. 386, 661–680 (1997). [PubMed] [Google Scholar]
- 10.Jacobs B., et al. , Regional dendritic and spine variation in human cerebral cortex: A quantitative golgi study. Cereb. Cortex 11, 558–571 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Luebke J. I., Chang Y. M., Moore T. L., Rosene D. L., Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 125, 277–288 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Gazzaley A. H., Siegel S. J., Kordower J. H., Mufson E. J., Morrison J. H., Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc. Natl. Acad. Sci. U.S.A. 93, 3121–3125 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hof P. R., et al. , Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 928, 175–186 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Fan X., Wheatley E. G., Villeda S. A., Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci. 40, 251–272 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J., Microglia in neurodegeneration. Nat. Neurosci. 21, 1359–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labzin L. I., Heneka M. T., Latz E., Innate immunity and neurodegeneration. Annu. Rev. Med. 69, 437–449 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Mattson M. P., Magnus T., Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 7, 278–294 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drapeau E., et al. , Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 100, 14385–14390 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Praag H., Shubert T., Zhao C., Gage F. H., Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith L. K., White C. W. 3rd, Villeda S. A., The systemic environment: At the interface of aging and adult neurogenesis. Cell Tissue Res. 371, 105–113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyss-Coray T., Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villeda S. A., et al. , The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villeda S. A., et al. , Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsimpardi L., et al. , Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha M., et al. , Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellano J. M., et al. , Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G., et al. , Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker R. G., et al. , Biochemistry and biology of GDF11 and myostatin: Similarities, differences, and questions for future investigation. Circ. Res. 118, 1125–1141; discussion 1142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer M. J., et al. , Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 23, 1207–1215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., et al. , Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy-Ullrich J. E., Sage E. H., Revisiting the matricellular concept. Matrix Biol. 37, 1–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y. A., Zhou B., Wernig M., Südhof T. C., ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168, 427–441.e21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arber S., Caroni P., Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J. Cell Biol. 131, 1083–1094 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Au E., et al. , SPARC from olfactory ensheathing cells stimulates Schwann cells to promote neurite outgrowth and enhances spinal cord repair. J. Neurosci. 27, 7208–7221 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christopherson K. S., et al. , Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Dunkle E. T., Zaucke F., Clegg D. O., Thrombospondin-4 and matrix three-dimensionality in axon outgrowth and adhesion in the developing retina. Exp. Eye Res. 84, 707–717 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Kucukdereli H., et al. , Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U.S.A. 108, E440–E449 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eroglu C., et al. , Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lana B., et al. , Thrombospondin-4 reduces binding affinity of [(3)H]-gabapentin to calcium-channel α2δ-1-subunit but does not interact with α2δ-1 on the cell-surface when co-expressed. Sci. Rep. 6, 24531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J., Xiao N., Xia J., Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 13, 22–24 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Varoqueaux F., et al. , Neuroligins determine synapse maturation and function. Neuron 51, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Jiang M., et al. , Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol. Psychiatry 22, 375–383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawler J., Hynes R. O., An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 74, 2022–2027 (1989). [PubMed] [Google Scholar]
- 44.Benner E. J., et al. , Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 497, 369–373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh S. K., et al. , Astrocytes assemble thalamocortical synapses by bridging NRX1α and NL1 via hevin. Cell 164, 183–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elegheert J., et al. , Structural mechanism for modulation of synaptic neuroligin-neurexin signaling by MDGA proteins. Neuron 95, 896–913.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Missler M., et al. , α-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423, 939–948 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Chen L. Y., Jiang M., Zhang B., Gokce O., Südhof T. C., Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron 94, 611–625.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Mosher D. F., Adams J. C., Adhesion-modulating/matricellular ECM protein families: A structural, functional and evolutionary appraisal. Matrix Biol. 31, 155–161 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Sullivan M. M., et al. , Matricellular hevin regulates decorin production and collagen assembly. J. Biol. Chem. 281, 27621–27632 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Sullivan M. M., Puolakkainen P. A., Barker T. H., Funk S. E., Sage E. H., Altered tissue repair in hevin-null mice: Inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 16, 310–319 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Ferrer-Ferrer M., Dityatev A., Shaping synapses by the neural extracellular matrix. Front. Neuroanat. 12, 40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Südhof T. C., Towards an understanding of synapse formation. Neuron 100, 276–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuma P., Hubbard A. L., Transcytosis: Crossing cellular barriers. Physiol. Rev. 83, 871–932 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Tian X., et al. , LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci. Rep. 5, 11990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikhailenko I., et al. , Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J. Biol. Chem. 272, 6784–6791 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Patzke C., Acuna C., Giam L. R., Wernig M., Südhof T. C., Conditional deletion of L1CAM in human neurons impairs both axonal and dendritic arborization and action potential generation. J. Exp. Med. 213, 499–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwinter D. M., Lo K., Mafi P., Silverman M. A., Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience 162, 1001–1010 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Maximov A., Pang Z. P., Tervo D. G., Südhof T. C., Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J. Neurosci. Methods 161, 75–87 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Pak C., et al. , Human neuropsychiatric disease modeling using conditional deletion reveals synaptic transmission defects caused by heterozygous mutations in NRXN1. Cell Stem Cell 17, 316–328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.