Significance
Ser/Thr phosphorylation is critical for T cell receptor signal transduction as well as cell survival/death regulation in developing thymocytes. In this work, we established a Ser/Thr phosphorylation profile during thymocytes selection. Substrate analysis revealed PP2A as the most important enzyme responsible for the dephosphorylation events, which was proved in the PP2A cKO model. Thymic-specific depletion of PP2A caused impaired thymocyte selection. The decrease of DP thymocytes upon PP2A cKO is accompanied by dysregulated dephosphorylation of apoptosis-related proteins and increased cell apoptosis, which could be rescued by Bcl2 transgene or p53 knockout. This studies the role of PP2A in thymocyte development and provides insights to understand T cell development through dephosphorylation regulation of apoptosis-related molecules.
Keywords: thymocyte selection, PP2A, apoptosis
Abstract
The development of thymocytes to mature T cells in the thymus is tightly controlled by cellular selection, in which only a small fraction of thymocytes equipped with proper quality of TCRs progress to maturation. It is pivotal to protect the survival of the few T cells, which pass the selection. However, the signaling events, which safeguard the cell survival in thymus, are not totally understood. In this study, protein Ser/Thr phosphorylation in thymocytes undergoing positive selection is profiled by mass spectrometry. The results revealed large numbers of dephosphorylation changes upon T cell receptor (TCR) activation during positive selection. Subsequent substrate analysis pinpointed protein phosphatase 2A (PP2A) as the enzyme responsible for the dephosphorylation changes in developing thymocytes. PP2A catalytic subunit α (Ppp2ca) deletion in the T cell lineage in Ppp2caflox/flox-Lck-Cre mice (PP2A cKO) displayed dysregulated dephosphorylation of apoptosis-related proteins in double-positive (DP) cells and caused substantially decreased numbers of DP CD4+ CD8+ cells. Increased levels of apoptosis in PP2A cKO DP cells were found to underlie aberrant thymocyte development. Finally, the defective thymocyte development in PP2A cKO mice could be rescued by either Bcl2 transgene expression or by p53 knockout. In summary, our work reveals an essential role of PP2A in promoting thymocyte development through the regulation of cell survival.
T lymphocytes are the major form of adaptive immune cells that target pathogens (1, 2). T cell precursors originate in the bone marrow and then migrate to the thymus to initiate differentiation into mature T cells. In the thymus, CD4–CD8– double-negative (DN) thymocytes (which can be subcategorized as stages DN1–DN4) acquire T cell receptor (TCR) expression through VDJ recombination and develop into CD4+CD8+ double-positive (DP) thymocytes, which subsequently give rise to CD4+ or CD8+ single-positive (SP) T cells. Two pivotal selection processes occur, namely positive selection and negative selection, and both serve as gatekeepers in the progress of DP T cells to the SP stage. Notably, only a small percentage of DP thymocytes survive through the selection process to become mature T cells bearing TCRs with suitable reactivity. Secure survival of the T cells which do pass selection is then of pivotal importance during thymic development.
It has been shown that TCRs on the DP cell surface can bind to self-peptide–major histocompatibility complex (MHC) complexes on thymic epithelial and dendritic cells, which provide signals for thymocyte survival (3–5). Up-regulation of expression of survival-related proteins is one of the known mechanisms to promote thymocyte survival in this context. Well-studied examples include the Bcl-2 family prosurvival proteins Bcl-xL, whose stage-specific enrichment promotes the survival of DP thymocytes (6). In addition to regulations at the level of gene expression, further studies revealed that posttranslational modifications such as Ser/Thr phosphorylation of prosurvival proteins is also vital for thymocyte survival. For example, phosphorylation of different sites in Mcl-1 play opposing roles in thymocyte survival, while changes in Ser/Thr phosphorylation status of the proapoptotic protein Bim have also been implicated in the decision of cell survival or cell death during negative selection (7, 8). ERK activation, which is also marked by Ser/Thr phosphorylation, has been shown to be essential for the positive selection of thymocytes (9). However, despite these findings, the landscape of Ser/Thr phosphorylation during thymocyte selection has not been completely characterized.
In contrast to Ser/Thr phosphorylation, which is governed by a plethora of kinases, dephosphorylation of proteins is regulated by only a handful of phosphatases. Many studies have suggested that phosphatases sensitive to the inhibition by okadaic acid are involved in the regulation of T cell signaling and activation. However, the role of threonine phosphatase PP2A, one of the most important targets of okadaic acid, in thymocyte selection remains unclear. PP2A consists of three subunits: A (scaffold subunit), B (regulatory subunit), and C (catalytic subunit). PP2A C subunit isoforms, α (Ppp2ca) and β (Ppp2cb), share a 97% identity in their amino acid sequence. However, Ppp2ca is 10 times more abundant than Ppp2cb and has been demonstrated to play a dominant role in mouse cells (10). PP2A is able to dephosphorylate a range of proteins such as Akt, p53, and c-Myc in different T cell types and plays a fundamental role in cell survival, signal transduction, and proliferation (11).
In this study, we established a profile of Ser/Thr phosphorylation in developing thymocytes. Among the rates of protein dephosphorylation we identified, two of the top five are known substrates of phosphatase PP2A (12, 13), suggesting a central role of PP2A in this process. In T cell-specific PP2A-deficient mice, there is a dramatic decrease in levels of CD4+CD8+ DP T cells, owing to an increased susceptibility to apoptosis. Furthermore, we found changes in phosphorylation of apoptosis-related proteins underlies the phenotype of PP2Ac-deficient thymocytes. Thus, our study reveals a critical role of PP2Ac in thymocyte development by ensuring cell survival.
Results
Ser/Thr Phospho-Peptide Profiling of Thymocytes and Generation of PP2Ac Conditional Knockout Mice.
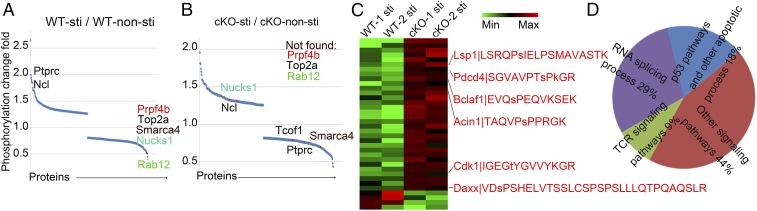
We used anti-CD3 to stimulate thymocytes imitating TCR stimulation during DP development and then analyzed the Ser/Thr phosphorylation by using mass spectrometry-based iTRAQ phosphor-peptide analysis (14, 15). This analysis revealed more than 4,000 phosphorylated sites with different phosphorylation levels between the nonstimulated and stimulated thymocytes. To our surprise, and despite that TCR stimulation is known to activate a Ser/Thr signaling cascade, our experiment did not reveal an obvious increase in phosphorylation over dephosphorylation. Upon TCR stimulation, the proportion of sites with increased phosphorylation levels (52.1%) was similar to those with decreased phosphorylation (47.9%) (Fig. 1A). Among these, 27 proteins showed phosphorylation level changes that were greater than 1.5-fold, while 30 proteins showed dephosphorylation to a similar extent. This result suggests that dephosphorylation might play an equally important role as phosphorylation during this process. Notably, two of the top five proteins (TOP2a and Smarca4) with the most substantial dephosphorylation changes (Fig. 1A) have been previously reported as direct phosphorylation targets of protein phosphatase PP2A, suggesting the involvement of PP2A in DP cell development. To verify our conjecture, we crossed the Ppp2caflox/flox mice with proximal lymphocyte-specific protein tyrosine kinase (Lck) promoter-driven Cre (Lck-Cre) transgenic mice to generate the PP2Ac conditional knockout (cKO) mice with T cell-specific deletion (16) to avoid early embryonic lethality at embryonic day (E)6.5 upon complete loss of PP2Ac. Genotyping (SI Appendix, Fig. S1A), RT-PCR (SI Appendix, Fig. S1C), and Western blot (SI Appendix, Fig. S1B) analysis all demonstrated the specific deletion of PP2Ac in CD4+CD8+ DP thymocytes but not in CD4−CD8− DN thymocytes. Given that the absence of PP2A would directly affect the dephosphorylation of its target proteins, we profiled Ser/Thr phosphorylation sites in PP2A cKO thymocytes using iTRAQ phosphor-peptide analysis. The loss of PP2A caused significant phosphorylation status changes in 370 proteins in TCR-stimulated thymocytes (Fig. 1B). As expected, TOP2A, which is a potential PP2A target protein observed in T cells, no longer altered its phosphorylation level upon TCR stimulation, while dephosphorylation of another target protein Smarca4 was also less apparent. Among the top 35 proteins with increased phosphorylation levels of more than 1.75-fold in PP2A cKO cells, six of them are related to cell survival (Fig. 1C, marked with red font, and Fig. 1D). Some of them have been reported to be regulated by phosphorylation. For example, phosphorylation is important for Daxx in sensitizing stress-induced apoptosis (17). Similarly, phosphorylation of LSP1 S243 is critical for triggering B lymphocyte apoptosis (18). Interestingly, when we checked the expression levels of proapoptosis (Bim, Bax, Bad) and antiapoptosis (Bcl2, Bcl-xl) proteins (19), no obvious differences were observed between WT (Lck-Cre+) and cKO (Lck-Cre+ Ppp2caflox/flox) thymocytes, in either unstimulated cells or cells stimulated with anti-CD3 (SI Appendix, Fig. S1E). This result strongly suggested that PP2A might ensure cell survival via dephosphorylation-mediated regulation of proteins in the apoptosis pathways.
Fig. 1.
Ser/Thr phospho-peptide profiling of thymocytes and generation of PP2Ac conditional knockout mice. (A) Ser/Thr phosphorylation measured by MS-based iTRAQ phosphor-peptide analysis in C57BL/6 resting and activated thymocytes. About 350 proteins changed their phosphorylation (log2). Proteins in which phosphorylation was notably changed are annotated such as Rab12 (green font) and Prpf4b (red font). (B) Ser/Thr phosphorylation measured by MS-based iTRAQ phosphor-peptide analysis in PP2Ac cKO resting and activated thymocytes. Proteins which cannot be found but which notably changed in wild-type cells (A) are annotated, for example Rab12 Prpf4b. The dephosphorylation of some proteins was reduced such as Smarca4. (C) Heatmap showing the 35 proteins showing the greatest change in phosphorylation as measured by mass spectrometry (mean change 1.75-fold). Six of them are related to cell survival (marked with red font). (D) Pathway analysis of the 35 proteins showed the greatest change in phosphorylation in CD3-stimulated PP2A-deficient thymocytes.
PP2A Ablation at the Early Development Stage Resulted in Aberrant Thymocyte Development.
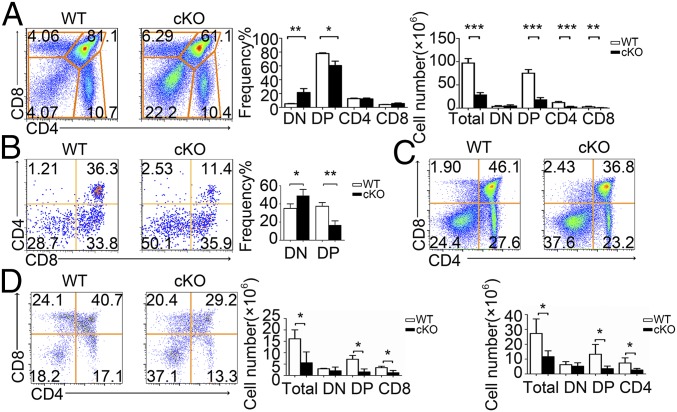
We next analyzed the thymocyte development in PP2A cKO mice. Significant reductions in both thymus size (SI Appendix, Fig. S2A) and changes in cell levels were observed in PP2A cKO mice compared with the WT littermate controls (Fig. 2A). The cKO mice showed an increase in the percentage of DN cells and a substantial decrease in DP T cells. In terms of absolute cell numbers, similar numbers of DN cells and substantially fewer DP and SP thymocytes were found in cKO mice (Fig. 2A). CD4−CD8− DN thymocytes can be further subdivided into four developmental stages in the following order: CD25− CD44+ (DN1), C25+ CD44+ (DN2), CD25+ CD44− (DN3), and CD25− CD44− (DN4) (20). The proportions and numbers of DN1–DN4 cells were not affected in cKO mice as expected (SI Appendix, Fig. S2B), suggesting the loss of thymocytes occurs before, or upon development to the DP stage along with the elevated Cre expression during this stage.
Fig. 2.
Defective T cell development in PP2Ac cKO mice. (A) Cell surface staining of CD4 and CD8 (Left) on WT and PP2Ac cKO thymocytes. Numbers in or adjacent to outlined areas (or in quadrants) indicate percentage. Bar charts indicate the frequency and cell numbers for total thymocytes DN, DP, CD4+, or CD8+ SP thymocyte subpopulations. (B) Representative flow cytometry analysis of CD4 versus CD8 in total thymocyte, and from thymocytes cocultured with OP9-DL1 cells at day 5. Percentages are indicated in the quadrants. Bar chart indicates the frequency of DN and DP. (C and D) Flow cytometry of thymocytes from WT or cKO mice expressing a transgene encoding the MHC class II-restricted OT-II (C) and OT-I (D) TCR. Bar charts indicate the cell number of different subpopulations. *P < 0.05; **P < 0.01; ***P < 0.001.
When we isolated DN3 precursor cells from both WT and cKO mice and cocultured them in vitro with stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1) (21), cKO cells gave rise to fewer DP cells than WT cells (Fig. 2B), despite the fact that a similar number of DN4 cells were produced (SI Appendix, Fig. S2C). As such, this result reproduced the defects observed in vivo and support a cell-autonomous role for PP2Ac in thymocyte development.
Since developmental defects associated with thymocyte selection can be masked by compensatory changes in the TCR repertoire (20), we thus introduced αβ TCR transgenes into PP2Ac cKO mice to mitigate such compensation. We analyzed the effect of PP2Ac deficiency in both OT-II and OT-I TCR-transgenic mice (20). PP2Ac cKO led to defective thymocyte development in either OT-II or OT-I mice, as evidenced by fewer DP or CD4+ or CD8+ SP cells (Fig. 2 C and D). PP2Ac deficiency did not affect immature single CD8+ positive (ISP) cell numbers (22) (SI Appendix, Fig. S2D), but did cause slightly decreased numbers of thymic regulatory T cells (tTregs) and natural killer T cells (NKT) (SI Appendix, Fig. S2 E and F).
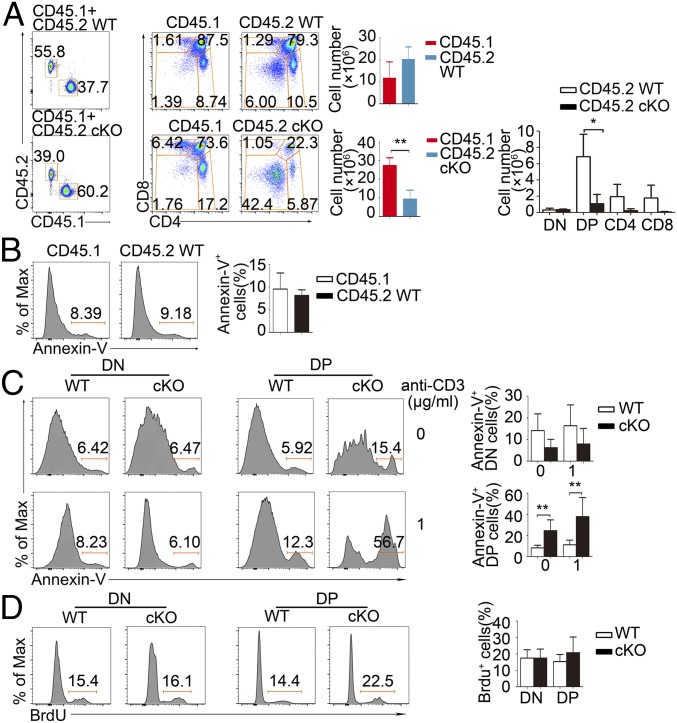
It has been previously reported that the Lck promoter-driven Cre expression exerted obvious cytotoxicity during T cell development (23, 24). We thus compared total thymocyte numbers of Lck-Cre+ mice and Lck-Cre− mice but found no obvious number reduction (SI Appendix, Fig. S1D). To further rule out the possible cytotoxic influence, we have also crossed Ppp2caflox/flox mice to the hCD2-Cre mice to minimize cytotoxicity (23, 24). Consistently, decreased thymocyte numbers since the DP stage was observed (SI Appendix, Fig. S2G). When we directly compared the apoptosis rate of Lck-Cre+ (CD45.2+) and Lck-Cre− (CD45.1+) thymocytes in Rag1−/− host in the mixed bone marrow chimera experiment, they are almost same (see Fig. 5B). Thus, the defective thymocyte development in PP2A cKO mice is not the result from the cytotoxic effect from the Lck-Cre transgene.
Reduced Peripheral T Cell Numbers in PP2Ac cKO Mice.
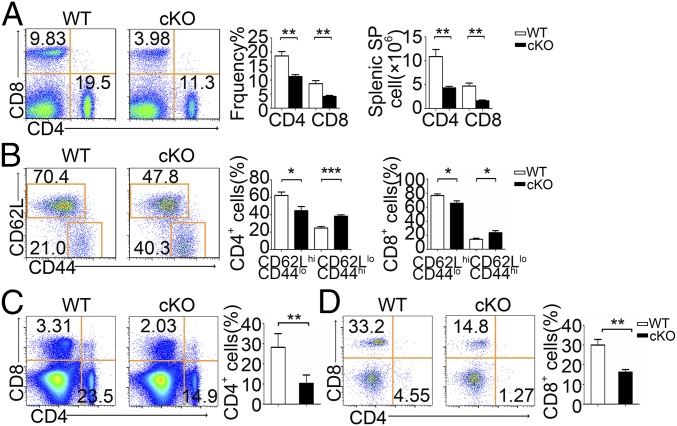
PP2Ac deficiency also resulted in fewer peripheral CD4+ and CD8+ T cells in the spleen (Fig. 3A) and lymph node (LN) (SI Appendix, Fig. S3A). We also observed decreased splenic Foxp3+ regulatory T cells (pTreg) in cKO mice (SI Appendix, Fig. S3C). As expected, the B cell compartment in spleen was not affected (SI Appendix, Fig. S3D). Both splenic (Fig. 3B) and LN (SI Appendix, Fig. S3B) CD4+ or CD8+ T cell populations from PP2Ac cKO mice had higher frequencies of effector/memory (CD44hi CD62Llo) cells than littermate control mice, as is frequently observed in lymphopenic mice and which reflects homeostatic expansion. PP2Ac-deficient OT-II or OT-I mice also had fewer mature peripheral CD4+ or CD8+ T cells (Fig. 3 C and D). Taken together, these experiments show that defective thymocyte development in PP2Ac cKO mice leads to lymphopenia in the periphery.
Fig. 3.
Phenotype of peripheral splenic T cells in PP2Ac cKO mice. (A) Cell surface staining of CD4 and CD8 on WT and PP2Ac cKO splenocytes, Bar charts indicate frequency and cell number of splenocyte subpopulations. (B) Expression of CD62L and CD44 on CD4+ splenocytes from WT and cKO mice; bar charts show frequency of CD4+ cells in the CD62LhiCD44lo and CD62LloCD44hi subpopulations. Data are representative of five experiments. (C and D) Flow cytometry of splenocytes from WT or cKO mice expressing a transgene encoding the MHC class II-restricted OT-II (C) and OT-I (D) TCR. *P < 0.05; **P < 0.01; ***P < 0.001.
Intact TCR Signaling and Unaltered Cell Proliferation Capability in PP2Ac cKO Thymocytes.
To determine whether the defect in thymocyte development in PP2A cKO mice is due to defects in TCR signaling, we analyzed downstream TCR signaling events in both WT and cKO cells. To our surprise, TCR-mediated calcium flux was comparable in WT and cKO cells in all subsets including DP or CD4+ or CD8+ SP thymocytes (SI Appendix, Fig. S4A). WT and cKO cells also showed no substantial differences in the phosphorylation of PLC-γ1, AKT, Erk1/2, and Jnk upon TCR stimulation (SI Appendix, Fig. S4B). Equivalent up-regulation of the adhesion molecule CD5 on the cell surface suggested intact TCR signaling in PP2Ac cKO cells (25) (SI Appendix, Fig. S4C). Developing thymocytes depend on TCR signaling for survival through direct induction of the interleukin 7 receptor (IL-7R) and the prosurvival factor Bcl-2 (26, 27). Thus, we next analyzed the expression of these two proteins in WT and cKO cells. We found that surface IL-7R was not substantially altered in cKO thymocytes (SI Appendix, Fig. S4C). In an in vitro stimulation assay, recombinant IL-7 was able to up-regulate Bcl-2 expression to a similar level in DN and DP cells from both WT and cKO mice (SI Appendix, Fig. S4D), indicating intact IL-7R responses.
The reduced numbers of DP thymocytes in the absence of PP2Ac could result from either reduced proliferation or increased cell death. To measure the proliferation capacity of WT and cKO thymocytes, 1 mg of BrdU was injected into both WT and cKO mice and BrdU incorporation in thymocytes was assessed 18 h later (20). We found that both DN and DP thymocytes from the WT and cKO mice showed equivalent BrdU incorporation (SI Appendix, Fig. S4F). At the same time, cell cycle analysis revealed no difference between WT and PP2Ac cKO DP thymocytes (SI Appendix, Fig. S4E). Consistent with the observations above, PP2Ac cKO thymocytes also showed no metabolic alterations in either oxygen consumption rate (OCR) or extracellular acidification rate (ECAR) (28) (SI Appendix, Fig. S4G). Taken together, the defective thymocyte development in PP2A cKO mice does not result from alterations in TCR signaling or from changes in cell proliferation capacity.
Impaired Cell Survival of PP2Ac cKO Thymocytes.
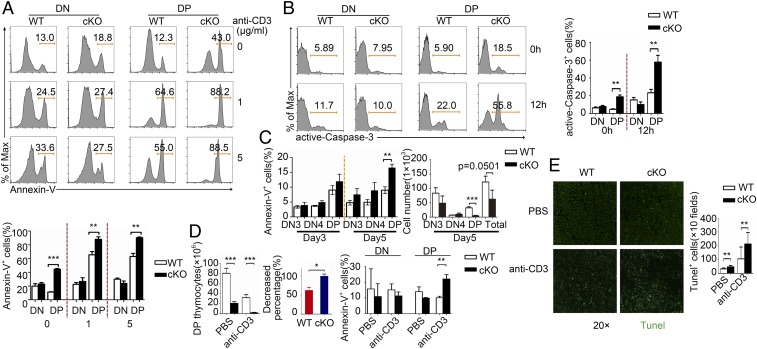
We next wanted to assess the survival of PP2Ac cKO thymocytes. Thymocytes isolated from WT and cKO mice cells were either left unstimulated or stimulated with different doses of anti-CD3 antibody overnight before annexin V staining. We found that PP2Ac deletion led to a marked increase in apoptosis in DP thymocytes (Fig. 4A), along with enhanced caspase-3 activity (29) (Fig. 4B). Increased apoptosis and reduced cell numbers could also be observed in PP2Ac cKO DP cells in the in vitro OP9-DL1–mediated differentiation assay (Fig. 4C).
Fig. 4.
Impaired cell survival of PP2Ac cKO thymocytes. (A) Cells were gated on a single cell by the exclusion of dead cells for analysis: Annexin V staining of DN and DP cells left unstimulated or stimulated for 12 h in vitro with different doses of anti-CD3; bar chart shows quantification of the results (Lower). Data are representative of five experiments. (B) Active Caspase-3 staining of DN and DP cells left unstimulated or stimulated for 12 h in vitro with anti-CD3 bar chart shows quantification of the results. (C) Percentages and cell numbers of annexin V staining of DN3, DN4, DN, and DP cells after coculture with OP9-DL1 at day 5. (D) Number of DP cells (Left) and percentage of WT and cKO DP cells (Center) after i.p. injected with PBS or anti-CD3 for 12 h. Percentages and quantification of annexin V+ of DN and DP cells (Right). (E) Staining of total thymus with TUNEL (Green) in frozen sections from WT and cKO mice i.p. injected with PBS or anti-CD3 for 12 h and quantification of total TUNEL+ thymocytes. *P < 0.05; **P < 0.01; ***P < 0.001.
To analyze cell death in vivo, we examined thymocyte apoptosis after anti-CD3 antibody administration, which is known to induce cell death of immature thymocytes (20, 30). We observed a more substantial decrease in DP cell number (Fig. 4 D, Left and Center) and increased annexin V labeling in the PP2Ac cKO mouse thymus (Fig. 4 D, Right). Furthermore, by using the TUNEL assay, we found an increase in TUNEL-positive cells in the PP2Ac cKO thymus irrespective of anti-CD3 antibody treatment intraperitoneally (i.p.) (Fig. 4E). Increased apoptosis of DP thymocytes was also observed in PP2Ac cKO cells after TNF-α or Fas-L stimulation (Fig. 5 A and B and SI Appendix). Thus, PP2Ac is essential for the survival of DP thymocytes.
Fig. 5.
Bone marrow-chimera experiments. (A) Flow cytometry of thymus cells from bone marrow chimeras deficient in recombination-activating gene 1 (Rag1−/−) 8 wk after transfer of bone marrow cells from WT C57BL/6 (CD45.1+) mice and WT or PP2A cKO(CD45.2+) mice, assessed for expression of CD4 and CD8 after gating on CD45.1 or CD45.2. Data are representative of three experiments. (B) Apoptosis of C57BL/6 (CD45.1+) and WT (CD45.2+) total thymocytes in reconstituted Rag1−/− mice. (C) The apoptosis of DN and DP cells by annexin V staining before and after anti-CD3 stimulation in WT or PP2A cKO(CD45.2+) derived cells. (D) BrdU incorporation in WT or PP2A cKO(CD45.2+) mice. Data are representative of three experiments. *P < 0.05 and **P < 0.01.
To determine whether the developmental defect in PP2A cKO mice was T cell autonomous, we generated bone marrow chimeric mice by injecting a mixture of Lck-Cre+ WT or PP2A cKO (CD45.2+) bone marrow cells along with C57BL/6 (CD45.1+) bone marrow cells, into irradiated Rag1−/− mice. The thymus of the reconstituted mice had a lower proportion of CD45.2+ cells derived from PP2A cKO cells than those derived from Lck-Cre+ WT cells (Fig. 5A). In accordance, there are significantly reduced DP, CD4, and CD8 SP thymocytes among the CD45.2+ cells derived from PP2A cKO mice (Fig. 5A), which indicated an intrinsic defect in PP2A cKO thymocyte development. Simultaneously, increased annexin V+ DP cells were observed in PP2A cKO (CD45.2+) reconstituted mice, nevertheless with anti-CD3 stimulation (Fig. 5C). Comparable BrdU+ DP cells between these two groups again indicate that PP2A inactivation does not affect DP thymocytes proliferation (Fig. 5D). These results demonstrate that PP2A has an intrinsic role during T cell development.
Bcl2 Transgene Expression or p53 Knockout Rescues the Thymocyte Development Defect in PP2A cKO Mice.
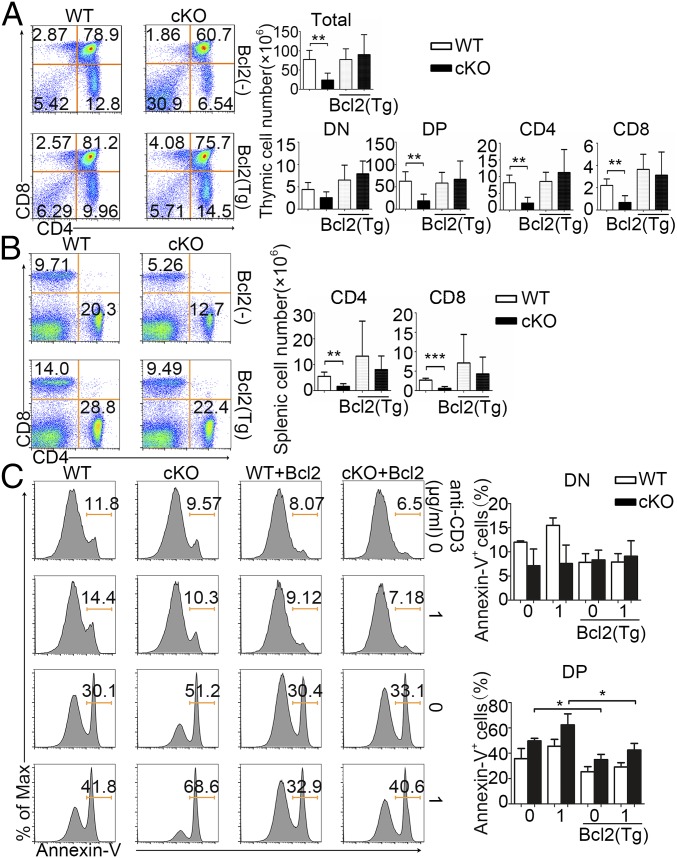
We next examined whether the impaired thymocyte development seen in PP2A-deficient mice could be rescued by enhanced cell survival. We crossed the PP2Ac conditional knockout mice with hBCL2 transgenic mice and p53 knockout (KO) mice, respectively. We found that the reduced cell levels in the thymus form PP2Ac-deficient mice could be largely rescued by the ectopic expression of the antiapoptotic protein BCL2 or through knockout of the proapoptotic p53 gene (Fig. 6A and SI Appendix, Fig. S6A). Both Bcl2 overexpression and p53 knockout resulted in recovery of peripheral T cell numbers in the spleen (Fig. 6B and SI Appendix, Fig. S6B). Annexin V staining also revealed restored survival of the DP thymocytes in BCL2 transgenic PP2A cKO mice (Fig. 6C). These data strongly support the conclusion that PP2A supports thymocyte development through promoting cell survival of thymocytes.
Fig. 6.
Bcl2 transgenic mice can rescue cell apoptosis. (A) Cell surface staining of CD4 and CD8 on WT and cKO on Bcl2− or Bcl2+ background thymocytes. Numbers in or adjacent to outlined areas (or in quadrants) indicate percent. Bar charts show the frequency and cell number of total, DN, DP, CD4+, or CD8+ SP thymocyte subpopulations. (B) Cell surface staining of CD4 and CD8 on WT and cKO on Bcl2− or Bcl2+ background splenocytes, and cell number of splenocyte subpopulations. (C) Cell staining of annexin V of WT, cKO, WT Bcl2+, and cKO Bcl2+ DN and DP thymocytes with or without anti-CD3 stimulation, and quantification of annexin V cell percentage. Data are representative of five experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In this study, by using Lck-proximal Cre-mediated deletion of PP2A in thymocytes, we observed that the absence of PP2A led to a specific increase in DP thymocyte apoptosis and notably decreased thymic cell numbers. Thymocytes must pass both positive and negative selection to become mature T cells. Positive selection ensures the survival and maturation of thymocytes whose TCRs display adequate affinity to self peptide–MHC complexes, while negative selection purges thymocytes whose TCRs exhibit high affinity to self-peptide–MHC complexes to avoid autoimmune diseases. In both cases, ensuring cell survival or prevention of apoptosis is the key cellular event for successful selection. This is achieved by both transcriptional and posttranscriptional regulation of prosurvival and proapoptosis molecules. Both the phenotypic characteristics of PP2A cKO mice and the phospho-profile changes in apoptosis-related proteins in PP2A cKO thymocytes reveal a central role for PP2A in regulating the selection process.
It is worth noting that increased apoptosis of DP thymocytes has been observed not only after anti-CD3 antibody stimulation, but also after Fas-L or TNF-α stimulation (31–33), suggesting a general role for PP2A in regulating cell survival as opposed to a specific downstream regulator of TCR signaling. This concept is also supported by the unaffected TCR downstream signaling seen in PP2A cKO thymocytes.
PP2A is a highly conserved multifunctional Ser/Thr phosphatase playing essential roles in many cellular events. Although its catalytic subunit is broadly expressed to a high level in almost all cell types, its function could be regulated in temporal and spatial manner through the usage of different regulator B subunits. B subunits are usually tissue specific or expressed differently at different stages of cell development (34, 35). Among all of the known B subunits, PPP2R5C (36), PP2A Bβ (37), and B55 (38) have all been shown to be enriched in T cells. Whether any of them is involved in thymocyte apoptosis regulation we describe here still needs to be clarified. Ser/Thr phosphorylation profiling in developing thymocytes revealed the phosphorylation changes of several cell survival-related proteins including Rab12 and Tcof1. Although their roles in regulating cell survival in other cell types have been previously reported, their functions in T cells have not yet been studied in detail. Further research is necessary to clarify the functions and mechanisms of these proteins in T cell development.
PP2A has been previously reported to be involved in autoimmune diseases in both human patients and mouse models. This could arise from dysregulated T cell differentiation as previously described (37, 39). Our findings here provide another possible mechanism elevated PP2A expression might facilitate for the survival of autoreactive T cells during selection, thus increase the likelihood of autoimmune disease arising.
In summary, our work uncovers a PP2A-mediated regulatory mechanism of thymocyte survival during thymocyte selection. This work not only reveals the importance of phosphorylation/dephosphorylation in regulating thymocyte survival but also provides us an insight to T cell development is regulated through posttranslational modification of proteins.
Materials and Methods
Mice.
Ppp2ca floxed mice were provided by X. Gao, Model Animal Research Center of Nanjing University. Mice with Cre recombinase driven by the proximal promoter of of Lck gene were purchased from The Jackson Laboratory (Jax-006889). Mice harboring the hCD2-iCre transgene have the human CD2 promoter and locus control region (LCR) directing expression of an optimized variant of Cre recombinase (iCre) to T cells and B cells and were purchased from The Jackson Laboratory (JAX: 008520). Mice with transgenic expression of the OT-I, OT-II, and Bcl2 mice were from The Jackson Laboratory. Recombination-activating gene 1 (Rag1−/−) mice were provided by Model Animal Research Center of Nanjing University. All mice were housed in the University Laboratory Animal Center. Animal experiment protocols were approved by the review committee from Zhejiang University School of Medicine and were in compliance with institutional guidelines.
OP9-DL1.
OP9 bone marrow stromal cells expressing the Notch ligand DL-1 (OP9-DL1), provided kindly by J. C. Zúñiga-Pflücker (University of Toronto, Toronto, QC, Canada), were cultured and maintained in α-MEM (Gibco) medium with 10% FBS (Gibco). For DN3 cell cocultures, sorted DN3 cells were plated onto confluent OP9-DL1 monolayers (70–80% confluent) and cultured with 5 ng/mL recombinant murine interleukin-7 (Peprotech) and 5 ng/mL Flt3L (Peprotech).
Apoptosis in Vitro.
Annexin V.
Two million cells were cultured overnight in 96-well plates with 1 µg/mL anti-CD3. Cells were stained with surface marker (CD4, CD8) at 4 °C for 30 min, washed with cold PBS, and stained with 3 μL of annexin V (BioLegend, 640914) in 300 µL of 1× binding buffer at room temperature for 30 min before being collected for FACS analysis (BD Fortessa). The FACS data analysis gated on single cell by exclusion of dead cells. Caspase-3 staining were performed according to the manufacturer's instructions (BioLegend, 640945).
Apoptosis in Vivo.
i.p. injection with anti-CD3 antibody (eBioscience, 1.0 mg/mL) was performed (10 µL per mouse). Twelve hours later, mice were killed to collect thymocytes. Cells for apoptosis markers was performed as apoptosis in vitro.
TUNEL Assay.
i.p. injection with anti-CD3 antibody (eBioscience catalog: 16-0031-86, clone: 145–2C11) was performed (10 µL per mouse). Twelve hours later, mice were killed and snap freeze tissue by optimal cutting temperature (OCT) compound to embed specimen at a temperature range of −18 to −22 °C was performed. Frozen sections were labeled with TUNEL. Staining was performed according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, Fluorescein, 11684795910 from Roche).
Phosphorylation Mass Spectrometry and Data Analysis.
We killed five mice and mixed their thymus for each sample. Each sample had two duplicate measurements. Thymocytes were left unstimulated or stimulated with anti-CD3 (0.3 µg/mL, 4 h), the cells were harvested centrifugation at 400 x g for 10 min, and the supernatant was discarded, then washed twice with ice-cold PBS. These cells were then lysed in SDT [4% wt/vol SDS, 100 mM Tris/HCl, 1 mM 1,4-dithiothreitol (DTT), pH 7.6] at a cell concentration of 5 × 107/mL before treatment at 100 °C for 10 min, before flash freezing in liquid nitrogen by APTBIO, Shanghai, China.
Generation of Bone Marrow Chimeras.
Bone marrow cells collected from congenic C57BL/6 (CD45.1+) mice were mixed at a ratio of 1:2 with bone marrow cells from Lck-Cre+ WT or PP2A cKO (CD45.2+) mice, and the mixture was injected into irradiated Rag1−/− mice (650 Rads). Mice were analyzed 8 wk after bone marrow transfer.
Statistics.
Statistical differences were calculated with Student’s two-tailed t test with assumption of different variances and a confidence level of 95%. GraphPad Prism software was used for calculations. Statistical significance was defined as P < 0.05.
Detailed materials and methods are presented fully in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Richard Sloan for helpful discussions and assistance with manuscript editing. This work was supported by National Natural Science Foundation of China Grants 31770954 and 31530019 (to L.L.), 81570013 (to S.W.), 31500708 (to M.Z.), and 31700766 (to D.S.) and China Postdoctoral Science Foundation Grants 2015M580516 and 2016T90546 (to M.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821116116/-/DCSupplemental.
References
- 1.Doherty D. G., O’Farrelly C., Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174, 5–20 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Schmid D., Dengjel J., Schoor O., Stevanovic S., Münz C., Autophagy in innate and adaptive immunity against intracellular pathogens. J. Mol. Med. (Berl.) 84, 194–202 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Fu G., et al. , Fine-tuning T cell receptor signaling to control T cell development. Trends Immunol. 35, 311–318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr T. K., Jameson S. C., Hogquist K. A., Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Zúñiga-Pflücker J. C., T-cell development made simple. Nat. Rev. Immunol. 4, 67–72 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Ligons D. L., et al. , RORγt limits the amount of the cytokine receptor γc through the prosurvival factor Bcl-xL in developing thymocytes. Sci. Signal. 11, eaam8939 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy D., et al. , HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 8, e3026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissler A., et al. , Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 20, 1317–1329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer A. M., Katayama C. D., Pagès G., Pouysségur J., Hedrick S. M., The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23, 431–443 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Zolnierowicz S., et al. , Diversity in the regulatory B-subunits of protein phosphatase 2A: Identification of a novel isoform highly expressed in brain. Biochemistry 33, 11858–11867 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Seshacharyulu P., Pandey P., Datta K., Batra S. K., Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 335, 9–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escargueil A. E., Larsen A. K., Mitosis-specific MPM-2 phosphorylation of DNA topoisomerase IIalpha is regulated directly by protein phosphatase 2A. Biochem. J. 403, 235–242 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sif S., Stukenberg P. T., Kirschner M. W., Kingston R. E., Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev. 12, 2842–2851 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kweon H. K., Andrews P. C., Quantitative analysis of global phosphorylation changes with high-resolution tandem mass spectrometry and stable isotopic labeling. Methods 61, 251–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitkopf S. B., Asara J. M., (2012) Determining in vivo phosphorylation sites using mass spectrometry. Curr Protoc Mol Biol 98, Unit18.19.1–Unit18.19.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu P., Qi X., Zhou Y., Wang Y., Gao X., Generation of Ppp2Ca and Ppp2Cb conditional null alleles in mouse. Genesis 50, 429–436 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Chang C. C., et al. , Structural and functional roles of Daxx SIM phosphorylation in SUMO paralog-selective binding and apoptosis modulation. Mol. Cell 42, 62–74 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Wu J. L., et al. , Temporal regulation of Lsp1 O-GlcNAcylation and phosphorylation during apoptosis of activated B cells. Nat. Commun. 7, 12526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cory S., Adams J. M., The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2, 647–656 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Wang D., et al. , Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat. Immunol. 13, 560–568 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Schmitt T. M., Zúñiga-Pflücker J. C., Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Xiong J., Armato M. A., Yankee T. M., Immature single-positive CD8+ thymocytes represent the transition from Notch-dependent to Notch-independent T-cell development. Int. Immunol. 23, 55–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carow B., Gao Y., Coquet J., Reilly M., Rottenberg M. E., lck-driven Cre expression alters T cell development in the thymus and the frequencies and functions of peripheral T cell subsets. J. Immunol. 197, 2261–2268 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Shi J., Petrie H. T., Activation kinetics and off-target effects of thymus-initiated cre transgenes. PLoS One 7, e46590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling K. W., et al. , GATA3 controls the expression of CD5 and the T cell receptor during CD4 T cell lineage development. Eur. J. Immunol. 37, 1043–1052 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Boudil A., et al. , IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte β-selection. Nat. Immunol. 16, 397–405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van de Wiele C. J., et al. , Impaired thymopoiesis in interleukin-7 receptor transgenic mice is not corrected by Bcl-2. Cell. Immunol. 250, 31–39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbist K. C., et al. , Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 532, 389–393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimura M., et al. , Room temperature-induced apoptosis of Jurkat cells sensitive to both caspase-1 and caspase-3 inhibitors. Cancer Lett. 132, 7–16 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Shi Y. F., et al. , In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J. Immunol. 146, 3340–3346 (1991). [PubMed] [Google Scholar]
- 31.Volpe E., Sambucci M., Battistini L., Borsellino G., Fas-Fas ligand: Checkpoint of T cell functions in multiple sclerosis. Front. Immunol. 7, 382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmailzadeh S., Huang Y., Su M. W., Zhou Y., Jiang X., BIN1 tumor suppressor regulates Fas/Fas ligand-mediated apoptosis through c-FLIP in cutaneous T-cell lymphoma. Leukemia 29, 1402–1413 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Chen G., et al. , RIP1-dependent Bid cleavage mediates TNFα-induced but Caspase-3-independent cell death in L929 fibroblastoma cells. Apoptosis 20, 92–109 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Santarlasci V., et al. , Musculin inhibits human T-helper 17 cell response to interleukin 2 by controlling STAT5B activity. Eur. J. Immunol. 47, 1427–1442 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Baskaran R., Velmurugan B. K., Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 210, 40–46 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Zheng H., et al. , Expression and distribution of PPP2R5C gene in leukemia. J. Hematol. Oncol. 4, 21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crispín J. C., Apostolidis S. A., Finnell M. I., Tsokos G. C., Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc. Natl. Acad. Sci. U.S.A. 108, 12443–12448 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo C. Y., Brautigan D. L., Larner J. M., ATM-dependent dissociation of B55 regulatory subunit from nuclear PP2A in response to ionizing radiation. J. Biol. Chem. 277, 4839–4844 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Sharabi A., Kasper I. R., Tsokos G. C., The serine/threonine protein phosphatase 2A controls autoimmunity. Clin. Immunol. 186, 38–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.