Abstract
Fear conditioning studies have led to the view that the amygdala contains neurons that signal threat and in turn elicit defensive behaviors through their brain stem and hypothalamic targets. In agreement with this model, a prior unit-recording study in rats performing a seminaturalistic foraging task revealed that many lateral amygdala (LA) neurons are predator responsive. In contrast, our previous study emphasized that most basolateral (BL) amygdala neurons are inhibited at proximity of the predator. However, the two studies used different methods to analyze unit activity, complicating comparisons between them. By applying the same method to the sample of BL neurons we recorded previously, the present study revealed that most principal cells are inhibited by the predator and only 4.5% are activated. Moreover, two-thirds of these cells were also activated by nonthreatening stimuli. In fact, fitting unit activity with a generalized linear model revealed that the only task variables associated with a prevalent positive modulation of BL activity were expectation of the predator’s presence and whether the prior trial had been a failure or success. At odds with the threat-coding model of the amygdala, actual confrontation with the predator was usually associated with a widespread inhibition of principal BL neurons.
NEW & NOTEWORTHY The basolateral amygdala (BL) is thought to contain neurons that signal threat, in turn eliciting defensive behaviors. In contrast, the present study reports that very few principal BL cells are responsive to threats and that most of them are also activated by nonthreatening stimuli. Yet, expectation of the threat’s presence was associated with a prevalent positive modulation of BL activity; actual confrontation with the threat was associated with a widespread inhibition.
Keywords: amygdala, fear, foraging, predator, threat
INTRODUCTION
A long series of studies have established that the basolateral complex of the amygdala (BLA) plays an essential role in the acquisition and expression of appetitive and aversive conditioned behaviors (Janak and Tye 2015; Maren and Quirk 2004; Pape and Paré 2010). However, most of these studies relied on artificial laboratory paradigms that bear little resemblance to naturalistic conditions: pure tones typically served as conditioned stimuli, and the experimental context usually allowed for only one conditioned behavior (e.g., freezing). As a result, conditioned responses seemed to be triggered reflexively by the conditioned stimuli after training.
Although such paradigms allow investigators to minimize the influence of confounding variables, they are of limited utility to understand how the amygdala contributes to the complex computations needed for survival in the wild. During foraging for instance, multiple factors (current metabolic needs, food palatability, familiarity with the environment, availability of escape routes) influence how animals weigh the need to attain food against the risk of being attacked by a predator (Mobbs et al. 2018). Seminaturalistic foraging tasks (Blanchard and Blanchard 1989; Fanselow et al. 1988) constitute a promising approach to examine the role of the amygdala in natural settings.
In the foraging task of Choi and Kim (2010), rats are attacked by a mechanical predator when they leave their nest to retrieve food pellets in an elongated arena. Whereas rats behave apprehensively in control conditions, following muscimol infusion in the BLA, they ignore the predator and appear fearless (Choi and Kim 2010). Because fear conditioning studies have disclosed the presence of BLA neurons signaling threat and triggering defensive behaviors, this finding suggests that by inhibiting threat-coding neurons, BLA inactivation abolished defensive behaviors. Consistent with this interpretation, ~13% of lateral amygdala (LA) cells increased their firing rates at proximity of, or immediately after being “attacked” by, the predator (Kim et al. 2018).
However, different results were obtained in another BLA nucleus. Recording neurons in the basolateral nucleus (BL) during the foraging task, Amir et al. (2015) identified three types of principal neurons (PNs). Most (type 1, ~70%) displayed a pronounced and persistent reduction in firing rate during foraging, becoming nearly silent near the predator, even as they escaped it. Yet, when rats hesitated at the edge of their nest before initiating foraging, their activity matched the threat-coding model: they fired at higher rates when rats aborted rather than started foraging or in the presence compared with absence of the predator. A second, less numerous class of PNs (type 2, 7%) displayed activity fluctuations that were generally opposite to those of type 1 cells. Finally, a third cell class (type 3, ~13%) lacked persistent changes in firing rates during foraging but exhibited brief alterations in activity, usually on food retrieval. Because of their heterogeneous activity patterns, type 3 cells were not studied further.
The findings of Amir et al. (2015) offer a different interpretation for the effects of muscimol infusions in the BLA: they suggest that BLA inactivation might not decrease defensive behaviors by reducing threat signaling, but by mimicking the state of type 1 cell suppression that occurs when rats forage. However, the two studies relied on different methods to analyze neuronal activity. Whereas Kim et al. (2018) referenced unit activity to when the predator surged forward or when rats retrieved the food pellet, Amir et al. (2015) compared the cells’ average firing rates during foraging or escape to their activity in the nest. Thus the Kim study was more likely to detect cells with transient changes in firing rates, whereas the Amir study was biased toward cells that displayed sustained changes in activity. The present study aims to clarify the role of the BLA during risky foraging decisions and reconcile the two studies by applying the methods used by Kim et al. (2018) to the cells recorded in the Amir et al. (2015) study.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Rutgers University approved the procedures described below, in agreement with the “Guide for the Care and Use of Laboratory Animals.” We used six male Sprague-Dawley rats (310–360 g; Charles River Laboratories, New Field, NJ) that were maintained on a 12:12-h light-dark cycle. Rats were habituated to the animal facility and handling for 1 wk before the surgery. These are the same rats as described in our prior study (Amir et al. 2015). Experiments were carried out during the light cycle.
Surgery
Rats deeply anesthetized with isoflurane were administered atropine sulfate (0.05 mg/kg im) to aid breathing and mounted in a stereotaxic apparatus. In aseptic conditions, the local anesthetic bupivacaine was injected subcutaneously into the scalp, 15 min before the first incision. A craniotomy was then performed above the amygdala, and silicon probes (NeuroNexus, Ann Arbor, MI) were stereotaxically aimed at the BL nucleus. The silicon probes were attached to Buzsáki-style microdrives (Vandecasteele et al. 2012). They consisted of either four (3 rats) or eight (3 rats) shanks (inter-shank distance, 200 µm). All shanks had eight recording leads (deinsulated area, 144 µm2) that were separated by ~20 µm dorsoventrally. A second craniotomy was performed above nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) in three of the rats, and pairs of closely spaced (1–1.7 mm) tungsten electrodes were inserted into these structures. The stereotaxic coordinates (all expressed in mm relative to bregma, and where AP, ML, and DV stand for anteroposterior, mediolateral, and dorsoventral, respectively) we used were as follows: BL, AP −2.2 to −3.6, ML 5 to 5.3, DV 8.8; NAc, AP 1.5, ML 1.35, DV 6.7; mPFC, AP 2.7 to 3.7, ML 0.5, DV 3.6 to 5.2.
Foraging Task
Rats were housed individually with ad libitum access to water after the surgery. After complete recovery from the surgery, daily access to food was restricted in time so that the rats’ bodyweight was maintained at ~90% of age-matched subjects with continuous access to food. This mild food restriction was required to ensure proper motivation in the behavioral tasks.
Foraging apparatus.
The foraging apparatus consisted of a long rectangular alley (length, 245 cm; width, 60 cm) with high walls (60 cm) and no ceiling. A door subdivided the apparatus in two compartments. One compartment (length, 30 cm) was a small and dimly lit “nest” with a water bottle. The second was longer (215 cm) and much brighter (200 lx) and served as a foraging arena.
Mechanical predator.
On a subset of trials, a mechanical predator (Mindstorms; LEGO, Billund, Denmark) on wheels was positioned at the end of the foraging arena, facing the nest. It had the following dimensions: length, 34 cm; width, 17 cm; height, 14 cm. It featured a proximity sensor that triggered a sudden forward movement (80 cm at 60 cm/s) and repetitive jaw openings and closings, followed by return to its original position.
Habituation to nest (days 0 to 1).
In a first phase, we habituated our subjects to the nest for two sessions of 7 h/day with the door shut during which rats could consume up to 6 g of food (sweet cereal in pellet form).
Foraging in the absence of predator (days 2 and 3).
For the following 2 days, rats were trained to retrieve sweetened food pellets (80–100 mg) in the foraging arena. The predator was absent on those days, and there was no food in the nest. Before each trial, one food pellet was placed at variable distances from the nest and the door was then opened. After some hesitation at the door threshold, rats retrieved the pellet in their mouth and went back to the nest to eat it, at which point we closed the door. After three successful trials at a given distance, we progressively lengthened the distance between the nest and food pellet from 25 to 150 cm, in steps of 25 cm, after which the distance randomly varied from trial to trial.
Foraging in the presence of the predator (days 4 and 5).
On the following 2 days, ~60% of trials were conducted with the predator present, alternating trial blocks with (n = 10–20) or without (n = 10–15) the predator. The results described below are based on data acquired during days 4 and 5.
Behavioral Analyses
An overhead video camera (frame rate, 29.97 Hz) recorded the rats’ behavior for off-line analysis. Two approaches were used to analyze behavior. First, an experienced investigator analyzed the video file frame by frame to identify critical task events. These included door opening (when the door to the nest opened), baseline phase (which started at the time the rat was in the nest and ended when the rat started waiting), waiting phase (which started when the rat’s snout extended past the door into the foraging arena and ended when the rat initiated or aborted foraging), foraging phase (which started on the last frame of immobility before the rat moved out of the nest completely and ended when the rat escaped, retrieved the food, or retreated back to the nest), food retrieval (when rats seized the food pellet), predator activation (when the predator started moving), escape phase (when rats, after approaching the pellet, suddenly turned around and ran all the way to the nest), and nest reentry (when the rat’s snout entered back into the nest). The outcome of each trial (failure or success to retrieve the food) was also noted. Second, the rats’ speed and position were detected using a MATLAB script (The MathWorks, Natick, MA) based on the shifting distribution of light intensity across frames.
Unit Recording and Clustering
Except for habituation, we recorded unit activity during all the phases of the foraging task. We used a 64-channel Plexon amplifier (model PBX3/64wb-G50; Dallas, TX) and a National Instruments data acquisition system (PCI-6225; Austin, TX). Rats were implanted with stimulating electrodes in mPFC and NAc, and a silicon probe in BL. Electrical stimuli (300–600 µA, 0.1 ms) were delivered with a stimulator from AM Systems (model 2100; Carlsborg, WA) at 1 Hz to the mPFC and NAc electrodes at the end of most sessions to test whether cells were antidromically responsive. The silicon probe (model 32L or 64L; NeuroNexus, Ann Arbor, MI) was then lowered ≥30 µm using a Buzsáki-style microdrive to ensure mechanical stability during the next recording session on the following day. Two recording sessions were obtained in each of the six rats (days 4 and 5), with an average yield of 38.8 ± 7.7 cells per session. In half of the rats, 32-channel silicon probes were used, whereas in the other half, 64-channel probes were used. In the latter half, the yield was expectedly approximately double (range 34–101 cells per session) that of the former (12–19 principal cells per session). Of note, it is possible that some cells were sampled on different days, but this is difficult to assess because the probes were moved daily, causing changes in spike shapes. In acknowledgment of this uncertainty, we use the terms “unit recordings” or “units” throughout methods and results.
Signals were sampled at 25 kHz, stored on hard drives, high-pass filtered using a median filter (window size of 1.1 ms), and then thresholded to extract spikes. After principal components analysis (PCA) was run on the spikes and the first three components were clustered using KlustaKwik (http://klustakwik.sourceforge.net/), spike clusters were refined manually using Klusters (Hazan et al. 2006). We verified the reliability of cluster separation by inspecting auto- and cross-correlograms. We required that autocorrelograms display a refractory period of at least 2 ms and that cross-correlograms not show evidence of a refractory period, because this would indicate overlap between clusters. We excluded units with unstable spike shapes.
Identification of PNs and interneurons.
Antidromic action potentials were identified as such when they had a fixed latency (≤0.1 ms jitter) and collided with spontaneously occurring spikes. Units with spike peak-to-trough times >0.6 ms and firing rates <6 Hz were classified as PNs; units with peak-to-trough spike times ≤0.6 ms and firing rates ≥6 Hz were classified as interneurons (ITNs). Peak-to-trough times, the intervals between spike troughs and peaks (Barthó et al. 2004), were determined by using the channel where the spikes of a given unit had the highest peak-to-trough amplitude. Two lines of evidence support the validity of this classification scheme. First, units antidromically responsive to electrical stimulation of NAc or mPFC were only found among presumed PNs (see Fig. 2 of Amir et al. 2015). Second, few misclassifications (~1% of PNs and ~4% of ITNs) were disclosed by cross-correlation analyses where we sought instances of monosynaptic inhibition from putative PNs to other units (when an ITN was misclassified as a PN) or of excitatory connections from putative ITNs to other units (when a PN was misclassified as an ITN; Amir et al. 2018).
Fig. 2.
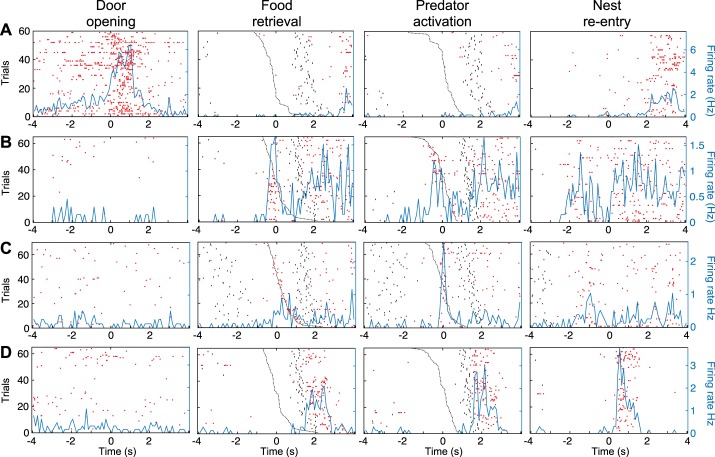
Examples of units specifically activated by only one task event. A–D: data for 4 different principal neurons (PNs), respectively, are spike rasters (red dots) and superimposed perievent time histograms (PETHs) of neuronal discharges (blue lines) referenced to the event listed at top of each column. In second column, raster trials were rank ordered by latency of predator activation (black dashed line). In third column, raster trials were rank ordered by latency of food retrieval (black dashed line). Black dots before or after time 0 in the PETHs mark when rats started foraging (left of origin) or reentered the nest (right of origin).
Histology
On completion of the experiments, rats were deeply anesthetized with isoflurane. One of the recording sites on each shank was marked with an electrolytic lesion (10 µA between a channel and the animal’s tail for 10 s). Rats were perfused-fixed, their brains removed and sliced with a vibrating microtome, and the sections counterstained with cresyl violet. All the units considered below were recorded in BL.
Analyses and Statistics
Except for clustering and the generalized linear model (GLM), all analyses were performed using custom scripts in MATLAB. In the Kim et al. (2018) study, each testing day consisted of three sequential blocks of 5–10 trials: “pre-predator,” “predator,” and “post-predator.” Thus, for all recording sessions, there were trials with and without the predator. Additionally, because the food pellet was relatively close to the predator in the Kim et al. study, rats usually failed to retrieve it when the predator was present (~97% of trials) and always succeeded when the predator was absent. Therefore, in the Kim et al. study, predator activation and food retrieval rarely occurred in the same trials. Below, such trials are termed “simple trials.”
In the present study, on days 4 and 5, rats also alternated between trial blocks with or without the predator. However, because the nest to food distance varied in our study and our arena was wider than in the Kim et al. (2018) study, rats often (~80%) retrieved the food pellet when the predator was present, complicating analyses in two ways. First, in some recording sessions, the number of simple trials was insufficient for meaningful statistical analyses. Second, on mixed trials, food retrieval, predator activation, and nest reentry were temporally contiguous. That is, food retrieval and predator activation were usually separated by <1 s, and it often took rats <1.5 s to retreat into the nest.
When 10 or more simple trials of each type were available (with only food retrieval or predator activation), we used the approach of Kim et al. (2018). That is, unit data were binned (0.5 s) and each trial was referenced to when the predator was activated or when rats reached the food pellet. Also as in the Kim et al. study, activity from −5 to −1.5 s before the event of interest (baseline period) was used to z-score data from −1.5 to 1 s surrounding the reference time (test window). However, when the food was placed at a short distance from the nest, the baseline period was shortened (−3 to −1.5 s) so that it overlapped entirely with the foraging phase. As in the Kim et al. study, predator- or food-responsive units were identified as such when one or more bins in the test window exceeded a z score of 3. The same approach was used to identify units that increased their firing rates when the door to the nest was opened. It should be noted that although the data were segmented in 0.5-s bins for statistical testing, a shorter bin (0.1 s) was used for data display.
In complex trials, three task events occurred in close temporal contiguity: food retrieval, predator activation, and reentry into the nest. Thus a different approach was needed to determine whether the units’ firing rates increased in relation to one or more of these events, as described below. However, it should be noted that for recording sessions where a sufficient number of simple and complex trials were available, the agreement between the two methods was 85%. Using binned unit data (0.5 s), we constructed three perievent time histograms (PETHs), each referenced to one of the three events. Each PETH consisted of a baseline period and a test window, as above, and the baseline period was used to z-score the data. Next, we determined whether one or more bins in the test window exceeded a z score of 3. If the criterion (z > 3) was exceeded in one or more of the PETHs, then we compared the timing of the spikes with respect to the three events using the following approach. For each trial with at least one spike in the test and baseline windows, we measured the interval between the reference event and the nearest spike. Next, we used a Kruskal-Wallis ANOVA to determine if the interval between the three reference times and the nearest spikes differed significantly (P < 0.05). If so, we next sought to identify the reference event or events with the shortest spike latency using Dunn’s test (P < 0.05). If one reference event had significantly shorter spike latencies than the other two, the unit was classified as responsive to that event. If there was no difference in spike latencies between two of the events but both were significantly shorter than the third, then the unit was classified as being responsive to the first two events. Finally, if there was no significant difference between the spike latencies of the three references, then the unit was classified as responsive to the three events.
For a unit to be classified as being activated in response to nest reentry, an additional requirement was added because we previously noticed that type 1 units had higher firing rates when in the nest compare with during foraging and escape. As a result, we needed to distinguish units whose firing rates increased in relation to nest reentry from units whose firing rates simply returned to baseline after foraging and escaping. To distinguish these two situations, we added the requirement that the increase in firing rate detected on nest reentry be transient [the peak firing rate reached on nest reentry (during the test window) had to be ≥200% that seen later in the nest].
For a unit to be classified as activated in response to door opening, an additional requirement was added because many units increased their firing rates when rats started waiting. To distinguish units whose firing rates increased in relation to door opening vs. waiting, we added the requirement that the increase in firing rate detected on door opening had to be transient [the peak firing rate reached on door opening (in the test window) had to be ≥200% that seen during the waiting phase].
Alternative procedure for units with low firing rates.
For units with baseline firing rates lower than 0.1 Hz, instead of the z-score approach described above, we used a Kruskal-Wallis ANOVA followed by Dunn’s test for firing rate to determine if the units’ activity in the test windows was higher than in the baseline windows. Although Kim et al. (2018) used a different approach to deal with units with very low firing rates, all the units that were deemed significant using their method were also deemed significant using our approach. However, our approach detected additional units that had visually obvious task correlates, which the approach used in the Kim et al. study classified as nonsignificant. For complex trials, if a significant difference was detected using Dunn’s test, then the latency analysis described above was applied. For simple trials, if significant differences were detected using Dunn’s test, then the unit was considered to be significant for the given event. Although the Dunn’s test also detected significantly inhibited units, we only considered units with significant increases in firing rates, as in Kim et al. (2018).
Generalized Linear Model
A regularized regression, group lasso (least absolute shrinkage and selection operator) with Poisson distribution (grpreg version 3.2-0 R package; Breheny and Huang 2015) and sevenfold cross-validation was used to fit the spiking of individual units. Spiking was binned (~66.7-ms bins, 2 video frames) across all trials. Each trial consisted of four task phases: baseline when rats were in the nest, waiting, foraging and escape.
The variables considered for the GLM included baseline, waiting, foraging, escape, door opening, food retrieval, predator activation, nest reentry, speed, distance from nest, distance from food, trial type (without or with predator), and prior trial outcome (failure or success). Each variable was either dummy coded or convolved with a set of basis functions.
Trial type (dummy coded): trial type was set to 1 in trials when the robot was present (this included the entire trial duration) and set to 0 in trials where the robot was absent (entire trial duration).
Prior trial (dummy coded): prior trial was set to 1 for the whole trial duration if on the previous trial the rat failed to retrieve the food pellet; otherwise, it was set to 0.
Waiting (dummy coded): waiting was set to 1 during the waiting phase of the task; otherwise, it was set to 0.
Foraging (dummy coded): foraging was set to 1 during the foraging phase of the task; otherwise, it was set to 0.
Escape (dummy coded): escape was set to 1 during the escape phase of the task; otherwise, it was set to 0.
Nest re-entry (basis functions): the nest re-entry variable was set to 1 at the moment the rat entered the nest; otherwise, it was set to 0. Nest reentry events were convolved with 14 basis functions (Fig. 1A).
Food retrieval (basis functions): the food retrieval variable was set to 1 at the moment the rat grabbed the food; otherwise, it was set to 0. Food retrieval events were convolved with 14 basis functions (Fig. 1A).
Door opening (basis functions): the door opening variable was set to 1 at the moment the door was opened, otherwise it was set to 0. Door opening events were convolved with 14 basis functions (Fig. 1A).
Predator activation (basis functions): the predator activation variable was set to 1 when the predator started moving; otherwise, it was set to 0. This variable was convolved with 14 basis functions (Fig. 1A).
Speed (basis functions): speed was defined as the absolute rat velocity in pixels per frame based on the rat’s position (x, y coordinates). Speed was convolved with 14 basis functions (Fig. 1A).
Distance to nest (basis functions): distance to nest was the absolute difference between the rat’s position and the position of the nest in pixels. Distance 0 represented when the rat was in the nest. Distance increased as the rat moved toward the food/predator and decreased as the rat moved back toward the nest. Both the x- and y-axes were used to calculate distance to nest. Distance to nest was convolved with 14 basis functions (Fig. 1A).
Distance to food (basis functions): distance to food was defined as the difference between the position of the food and that of the rat. When rats were in the nest, the distance was at its highest; when rats started moving toward the food, it decreased; and when rats grabbed the food, it was zero. Both the x- and y-axes were used to calculate distance to food. Distance to food was convolved with 14 basis functions (Fig. 1A).
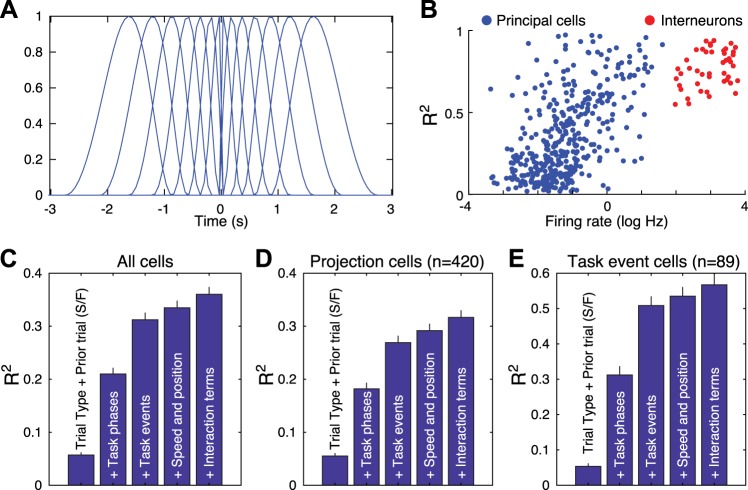
Fig. 1.
A: basis functions. B: correlation between R2 and firing rates in all principal neurons (PNs; blue, n = 420) and presumed interneurons (red; n = 45). C–E: impact of sequentially adding different classes of variables on R2 in all cells (C; n = 465), only PNs (D: n = 420), or only PNs responsive to task events (E; n = 89). Prior trial (S/F) indicates prior trial success and failure. The increases in R2 values caused by adding the various classes of variables were all significant. Friedman one-way ANOVAs revealed χ2 ranging between 1,110 and 1,637, with P values <3.4e10−239. Dunn’s tests revealed that all differences were significant at P < 0.01 for the 3 sets of cells.
Task events were convolved with 14 basis functions defined by log-timescaled raised cosine bumps. Three reasons motivated our decision to use this basis function family. First, spiking activity does not necessarily occur in lockstep with behavioral or task events; sometimes it can lag or lead. To account for this, convolution kernels that were offset in time from 0 are needed. Second, although one does have a great deal of flexibility in the choice of basis functions for the kernels, it is best to choose a set that captures the properties of the time series one wants to model. In doing so, the number of kernels needed can be minimized, which allows for more parsimonious models. For instance, we could have chosen time-lagged Dirac delta functions (a value of 1 at a single time bin, 0 everywhere else, a different kernel for each time lag considered), but this approach requires more kernels than necessary and ignores the fact that spiking activity is often correlated between adjacent time bins. With finite data, this can impair the fitting process. Third, the raised-cosine bump basis function family satisfies the first two requirements and has proven especially successful in modeling spiking activity across a variety of systems, such as retinal ganglion cells (Pillow et al. 2005) and neurons in lateral intraparietal cortex (Park et al. 2014).
The properties of this particular family are appealing because they embody the temporal dynamics of event-related spiking. Raised cosine bumps reflect the fact that neuronal responses, whether they be excitatory or inhibitory, tend to ramp up faster than they fall off. They also capture the fact that spiking activity driven near an event tends to be more temporally precise (e.g., an onset response to a tone) than that occurring sometime later (e.g., a ramp of activity reflecting a stimulus-induced decision). Last, we have found that they are capable of capturing the variety of event-related modulations observed in our study, such as onset responses, offset responses, tonic shifts in firing rate, ramped activity, and evoked responses at long latencies.
The basis function we chose has the following form:
where the subscript j denotes a particular basis and a is a time stretch factor (a = 10 in our study). Both b, which is the time the basis peaked, and t, a specified time lag, are log transformed, divided by the time the basis peaks, and multiplied by π. Thus our N bases tile the time range from −1.6 to 1.6 s. Visualization of our basis functions can be found in Fig. 1A.
The values chosen were based on previous work we published on the basolateral amygdala and striatum Kyriazi et al. 2018). In that work, we found that our choice of time stretch factor and range of time lags produced models that adequately fit our spiking data. For the present study, the parameters were slightly adjusted to better fit the time course of the foraging task. The basis functions were symmetric around the event onset with seven bases before onset and seven bases after. Each event kernel was represented as a linear combination of basis functions spanning 3 s before and 3 s after event onset. Basis functions included time before event onset to capture spiking activity that anticipated and followed events of interest. Indeed, Kim et al. (2018) observed units that fired in anticipation of predator activation and food retrieval. In addition, we noticed that many fast-spiking units increase their activity before these two events. Finally, we used basis functions that span ±3 s from event onset to match Kim et al. (2018).
The model was fit by minimizing the error Q over β in
where y represents the spike train across time, X is the design matrix with the basis functions, β values are the least-squares regression coefficients, λ is the regularization penalty for an L1 norm, and Kj normalizes across groups of different sizes. The lasso penalty parameter (λ) to the L1 norm is based on the lowest cross-validation error and is applied to each group, creating sparsity and variable selection at the group level (Breheny and Huang 2015; Tibshirani 1996; Yuan and Lin 2006). Cross-validation sets were assigned by dividing the recording session into seven equal segments. These approaches allowed us to be confident that our β coefficients were significantly different from zero.
The simplified formula that describes the full GLM model is as follows:
| FR ~ Waiting + Foraging + Escape + Door_Opening + Food_Retrieval + Predator_Activation + Nest_reentry + Speed + DistanceToNest + DistanceToFood + TrialType + PriorTrial, |
where FR is firing rate.
Kernel calculation.
After completion of model fitting, the basis functions were scaled by the model’s β values and summed within time bins to create a single kernel for each event. Most importantly, if a unit did not encode a parameter, the group lasso GLM gave β values of 0 for the corresponding basis functions. Thus only parameters that best fit observed spiking of a given unit are selected by the model.
Modulation of firing rate.
For each unit and variable, we normalized the GLM estimated spiking using the following formula: KernNorm = [Kern(t) − baseline]/[Kern(t) + baseline], where t ranged from −3 to 3 s and Kern(t) represents the GLM estimated spiking at time t, using the kernel fit of the corresponding variable. Baseline is the value of Kern at time −3 s. The advantage of transforming Kern(t) to KernNorm(t) is that in KernNorm(t), inhibition (bound by −1) and excitation (bound by +1) are scaled similarly and can be compared directly.
After KernNorm was calculated, we used the absolute value of KernNorm to find the largest peak from −1 to 3 s. The peak was then adjusted by the sign (positive or negative of KernNorm). Finally, the modulation of firing rate for each unit and event was calculated by averaging the activity around the peak (KernNorm peak) from −0.25 to +0.25 s, to match the 0.5-s bins we used for the PETHs.
Firing rate modulation values >0 represent increases in firing rate. Specifically, positive modulation values of trial type indicate increased firing rate when the predator is present compared with when the predator is absent. Positive modulation values of prior trial indicate increased firing rate on trials following a failed attempt compared with a successful attempt of picking up the food pellet. Positive modulation values of behavior phases (waiting, foraging, escape) indicate increased firing rate during the corresponding task phase compared with the baseline phase of the task. Positive modulation values of the task events food retrieval, predator activation, nest reentry, and door opening indicate increases in firing rate during the event compared with 3 s before the event.
For speed, distance to nest, and distance to food, the modulation was calculated by aligning the GLM-estimated firing to the onset of foraging. Positive modulation for speed indicates increased firing rate with increasing speed. Positive modulation for distance to nest indicates increased firing rate as the rat moved away from the nest (toward the food). Positive modulation for distance from food means that firing rate increases as the rat gets closer to the food.
Importantly, we also applied the GLM when all the variables including task phases (baseline, waiting, foraging, and escape) were treated using the same basis functions as for the other variables. Qualitatively identical results were obtained with this approach, including the observation that task phases had a more important influence over firing rates than task events in PNs and the opposite in ITNs.
Assessing the independence of GLM variables.
When variables included in a GLM are correlated, the variance of regression parameters is increased, causing incorrect predictor selection. To address this problem, penalized regression methods such as ridge and lasso regression are recommended (Dormann et al. 2013), allowing variable selection through cross-validation, as was done in this study. This method can handle correlated variables (Breheny and Huang 2015; Tibshirani 1996). To identify collinearity among predictors, the correlation between them is assessed: it should not exceed a threshold r of 0.7 (Dormann et al. 2013). We verified whether this was the case in our data set. Specifically, we computed all pairwise correlations between the variables used in our regression. Across all sessions, the mean (±SE) absolute pairwise correlation was very low: 0.11 ± 0.03. Of 66 pairwise correlations, only 1–3 (depending on the session) had a value that exceeded 0.7 (maximal value of 0.9), and the correlated variables only included speed and distance to nest, foraging and speed, or foraging and distance to nest.
Model with interaction terms.
To assess the potential influence of interactions between the variables considered in our GLM, we also ran another model that included interaction variables. The interaction model consisted of the 12 original single variables (see above) and 42 interaction variables. Each interaction variable was created by computing the product of two of the individual variables. For example, TrialType_PriorTrial was constructed by multiplying the TrialType variable by the PriorTrial variable. If both single variables in an interaction term were dummy coded in the original model, then their interaction variable was also dummy coded. If one or both of the single variables were convolved with the basis functions, then the interaction variable was also convolved with the basis functions.
Note that even though there are a total of 66 possible combinations for interactions, 24 of them were not used for the following reasons. First, in some cases the variables did not overlap in time, creating a zero vector. Second, in some cases multiplying the two variables resulted in a vector that was zero because one variable canceled another out. For example, Nest reentry and Distance to Nest resulted in a zero vector because when Nest reentry was 1, Distance to Nest was 0. Finally, in some cases the multiplication of the two variables resulted in a vector that was identical to one of the single vectors. For example, TrialType and Predator Activation resulted in the same vector as Predator Activation when multiplied together.
The interaction terms used in the model were: 1) TrialType_PriorTrial, 2) TrialType_Waiting, 3) TrialType_Foraging, 4) TrialType_Escape, 5) TrialType_Nest reentry, 6) TrialType_Food Retrieval, 7) Trial type_Door Opening, 8) TrialType_Speed, 9) TrialType_Distance to Nest, 10) TrialType_Distance to Food, 11) PriorTrial_Waiting, 12) PriorTrial_Foraging, 13) PriorTrial_Escape, 14) PriorTrial_Nest reentry, 15) PriorTrial_Food Retrieval, 16) PriorTrial_Door Opening, 17) PriorTrial_Predator Activation, 18) PriorTrial_Speed, 19) PriorTrial_Distance to Nest, 20) PriorTrial_Distance to Food, 21) Waiting_Door Opening, 22) Waiting_Distance to Food, 23) Foraging_Nest reentry, 24) Foraging_Predator Activation, 25) Foraging_Speed, 26) Foraging_Distance to Nest, 27) Foraging_Distance to Food, 28) Escape_Nest reentry, 29) Escape_Predator Activation, 30) Escape_Speed, 31) Escape_Distance to Nest, 32) Escape_Distance to Food, 33) Nest reentry_Distance to Food, 34) Food Retrieval_Predator Activation, 35) Food Retrieval_Distance to Nest, 36) Door Opening_Distance to Food, 37) Predator Activation_Speed, 38) Predator Activation_Distance to Nest, 39) Predator Activation_Distance to Food, 40) Speed_Distance to Nest, 41) Speed_Distance to Food, and 42) Distance to Nest_Distance to Food.
Coefficient of determination.
To compare the model fit to observed spiking, we used the coefficient of determination (R2). For each cell, we tested five different models. For each model, new terms were added to the previous model to calculate the contribution of the additional variables to the model fit. The five models are:
model1 = TrialType + PriorTrial
model2 = model1 + task phases of Waiting, Foraging, and Escape
model3 = model2 + task events of Door Opening, Food Retrieval, Predator Activation, and Nest reentry
model4 = model3 + Speed, Distance to Nest, and Distance to Food
model5 = model4 + 42 interaction terms
For each model and cell, we compared the estimated firing rate of that model using Kernels fit with all parameters with the observed firing rate. We constructed nine PETHs for comparison, with each PETH starting 10 s before and ending 10 s after the following events: door opening, foraging onset, predator activation, food retrieval, and nest reentry. For each event (except predator activation), we constructed two sets of PETHs: one set included trials with the predator present, and the other set included trials without the predator. This was performed to account for possible interactions between TrialType and the five events, making a fair comparison for model5 where interaction terms are included. Similarly, observed PETHs for the above events were also constructed. These model-estimated kernels and observed PETHs were used to calculate the coefficient of determination as follows:
where yi represents the observed PETH and fi is the model-estimated kernel at different time points i, and is the mean of the observed PETH.
We examined the impact of adding the different classes of variables one at a time on R2 (Fig. 1). Qualitatively identical results were obtained for all cells (Fig. 1C), only PNs (Fig. 1D), or only PNs responsive to task events (Fig. 1E). See Fig. 1 legend for statistics. Only considering the trial type and the prior trial outcomes yielded low values of R2 (~0.05). Also considering task phases increased R2 to ~0.2–0.3, and then adding task events also markedly increased R2. In contrast, then sequentially adding the rats’ speed/position and interaction terms caused marginal increases in R2. This analysis also revealed that R2 values were higher in PNs responsive to task events, presumably because these cells had higher firing rates and this variable correlated positively with R2 (Fig. 1B). Because consideration of interaction terms only caused a marginal improvement in the model’s performance, they are not included in results.
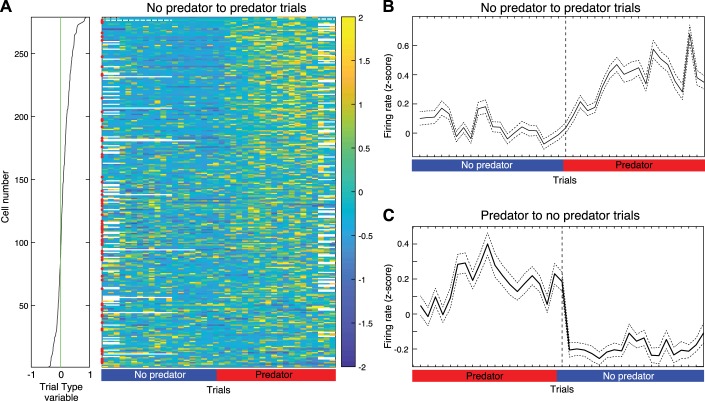
Transition from no-predator to predator trials and from predator to no-predator trials.
For each cell and its corresponding session, the transitions when trials switched from the predator being present to the predator being absent (and vice versa) were identified. The average firing rate for 20 trials before and 20 trials after the transition was then calculated. For each trial, the average firing rate was derived by counting the number of spikes during the baseline and waiting phases divided by the duration of the baseline and waiting phases. This resulted in a total of 20 trials of averaged activity around the transition trial. For sessions with multiple transitions, this process was repeated for each one and then averaged across all. Last, for each cell, we z-scored the average firing rate around the transition points. If a transition had fewer than 20 trials around it, then the missing trials were ignored in the calculations.
RESULTS
Procedural Differences Between the Studies of Kim et al. (2018) and Amir et al. (2015)
We first highlight procedural differences between the Kim et al. (2018) and Amir et al. (2015) studies (Table 1), because they will impact the unit analyses and their interpretation. Particularly significant is the fact that because the distance between the nest and food was fixed at 125 cm (near the predator) in the Kim et al. study, rats usually failed to retrieve the food on predator trials. Thus there was a near perfect dissociation between predator activation and food retrieval in the Kim et al. study, because food retrieval only occurred on no-predator trials. In contrast, because the distance between the nest and food varied in the Amir et al. study, there were trials with food retrieval but no predator movement, trials with food retrieval and predator movement, and trials with only predator movement.
Table 1.
Procedural differences between the Kim et al. (2018) and Amir et al. (2015) studies
| Kim et al. (2018) | Amir et al. (2015) | |
|---|---|---|
| Recording site | LA | BL |
| Trial types within recording sessions | All recording sessions featured trials with or without the predator | Two recording sessions included only no-predator trials and two subsequent sessions featured trials with or without the predator |
| Forward movement of predator | Variable (23, 60, or 140 cm) | Fixed (80 cm) |
| Location of food pellet | Fixed (125 cm) | Variable (25–150 cm) |
| Successful predator trials | 3% | 80% excluding aborted trials;69% including aborted trials |
| Arena width | 21 cm | 60 cm |
BL, basolateral amygdala; LA, lateral amygdala.
Database and Neuronal Classification
We recorded a total of 705 units histologically determined to be located in BL. Figure 3 of Amir et al. (2015) shows where they were recorded. A subset of these units (n = 233) were recorded while rats were trained to retrieve food pellets in the absence of the predator (days 2 and 3; see methods). The other units (n = 472) were recorded during trial blocks without or with the predator (days 4 and 5). The present report focuses on these 472 units.
Recorded units were classified as presumed PNs or ITNs based on their activity in the nest with the door closed. By using cutoffs of 0.6 ms for the spike peak-to-trough interval and 6 Hz for firing rate, units were classified as PNs (n = 420) when they fired at low rates and generated spikes of long duration, and conversely for presumed fast-spiking ITNs (n = 45). Units that met only one of the two criteria (n = 7) were not considered further. See methods for additional evidence that this classification scheme is valid.
Overview of Methods Used to Analyze Activity Related to Predator Activation and Food Retrieval
To examine BL activity in relation to task events of interest, we adhered to the approach used in the Kim et al. (2018) study as closely as possible. However, some modifications were required due to procedural differences between the two studies (Table 1). Moreover, to assess coding specificity, we analyzed the relation between unit activity and two additional variables, not considered in the Kim et al. study: door opening and reentry into the nest. The latter was included because when rats escaped from the predator, reentry into the nest often occurred in the test window used to identify predator-responsive units in our study. The following summarizes our approach to analyze unit data; a detailed description can be found in methods.
As in the Kim et al. (2018) study, binned (0.5 s) unit data were referenced to the events of interest (door opening, food retrieval, predator activation, nest reentry), and activity from −5 to −1.5 s of the reference time (baseline period) was used to normalize (z score) the data from −1.5 to 1 s of the reference time (test window). Responsive units were identified as such when one or more bins in the test window exceeded a z score of 3, as in the Kim et al. study. In contrast with the Kim et al. study, where there was almost no overlap between trials with predator activation or food retrieval, the two events often occurred in the same trials in our experiments. Thus we added an additional requirement for applying the above approach to a given unit, namely, that there be at least 10 trials with only one of the two events (10 with predator activation; 10 with food retrieval). For units with fewer than 10 trials of each type, we considered trials where both events occurred (termed mixed trials) and constructed separate PETHs around each event. We then used a two-step process to assess whether the unit’s activity was related to the events of interest: first, the z-score approach described above, and second, a comparison between the interval from the event of interest to the nearest spike using a Kruskal-Wallis ANOVA followed by Dunn’s test. See methods for details.
PNs Activated by Specific Task Events
With four task events (door opening, predator activation, food retrieval, nest reentry), there were 16 theoretically possible combinations of responses. Fourteen of these possibilities were observed (Table 2). Of the 420 PNs, ~21% increased their firing rates in relation to one or more of the four task events. In decreasing order of incidence were units activated in relation to nest reentry (10.7%), door opening (10%), predator activation (4.5%), and food retrieval (3.6%). We first consider examples of units that increased their firing rate in relation to only one task event, hereafter termed “simple units” (n = 61), and then consider more complex units (n = 28).
Table 2.
Incidence of PNs with significant firing rate increases in relation to one or more task events
| Task Event |
|||||
|---|---|---|---|---|---|
| Predator activation | Food retrieval | Nest reentry | Door opening | No. of PNs | Incidence, % |
| 0 | 0 | 0 | 1 | 24 | 5.71 |
| 0 | 0 | 1 | 0 | 27 | 6.43 |
| 0 | 0 | 1 | 1 | 9 | 2.14 |
| 0 | 1 | 0 | 0 | 5 | 1.19 |
| 0 | 1 | 0 | 1 | 1 | 0.24 |
| 0 | 1 | 1 | 0 | 4 | 0.95 |
| 1 | 0 | 0 | 0 | 5 | 1.19 |
| 1 | 0 | 0 | 1 | 5 | 1.19 |
| 1 | 0 | 1 | 0 | 3 | 0.71 |
| 1 | 0 | 1 | 1 | 1 | 0.24 |
| 1 | 1 | 0 | 0 | 3 | 0.71 |
| 1 | 1 | 0 | 1 | 1 | 0.24 |
| 1 | 1 | 1 | 1 | 1 | 0.24 |
| 4.52% | 3.57% | 10.71% | 10% | 89 | 21.19% |
Values 1 and 0 indicate whether units were responsive to a given task event or not, respectively. With 4 task events, there were 16 theoretically possible combinations of responsiveness; 14 of these possibilities were observed. The number and incidence of responsive principal neurons (PNs) are indicated for each possible combination. The case of units unresponsive to all task events is not listed. Totals indicate the percentage or number of PNs (n = 420) that increased their firing rates in relation to 1 or more of the 4 task events.
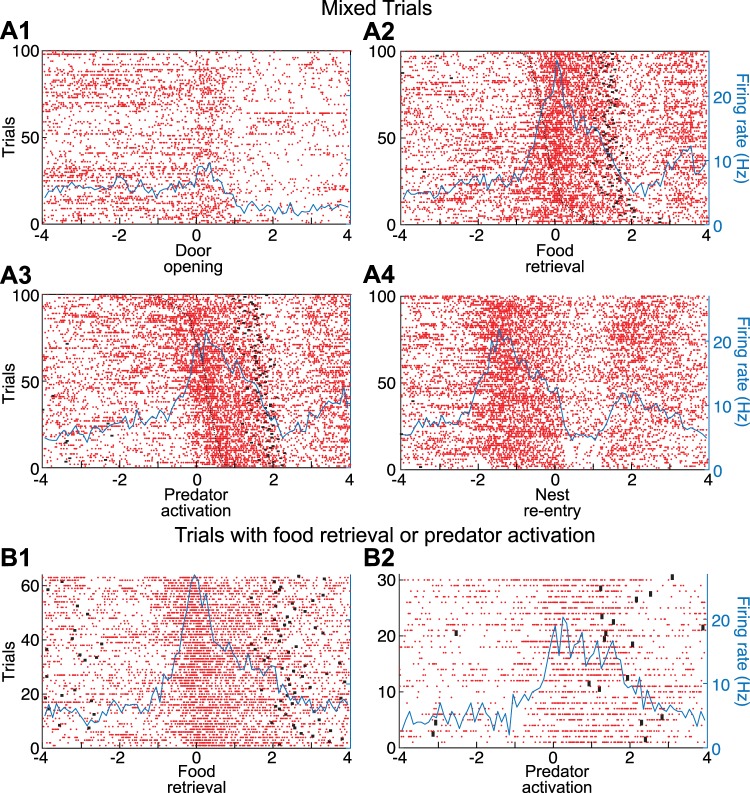
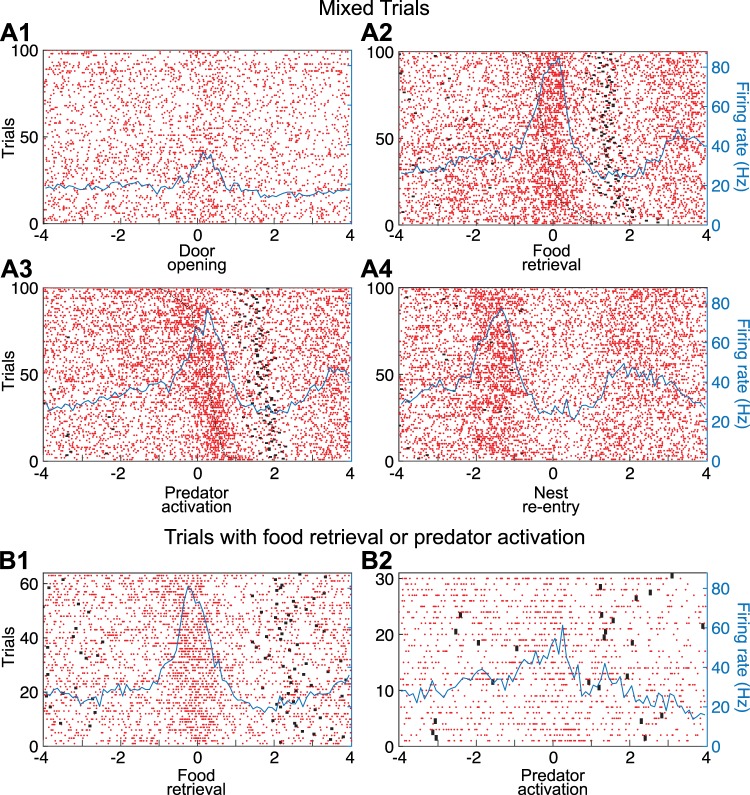
Figure 2 illustrates four examples of simple units. In all cases, we provide four PETHs of neuronal discharges, each referenced to one of the four task events (left to right): door opening, food retrieval, predator activation, and nest reentry. The same order, but from top to bottom, was used to order the four units by responsiveness; thus Fig. 2A shows a unit activated upon door opening, Fig. 2B shows a different unit activated upon food retrieval, Fig. 2C when the predator surged forward, and Fig. 2D when the rat reentered the nest.
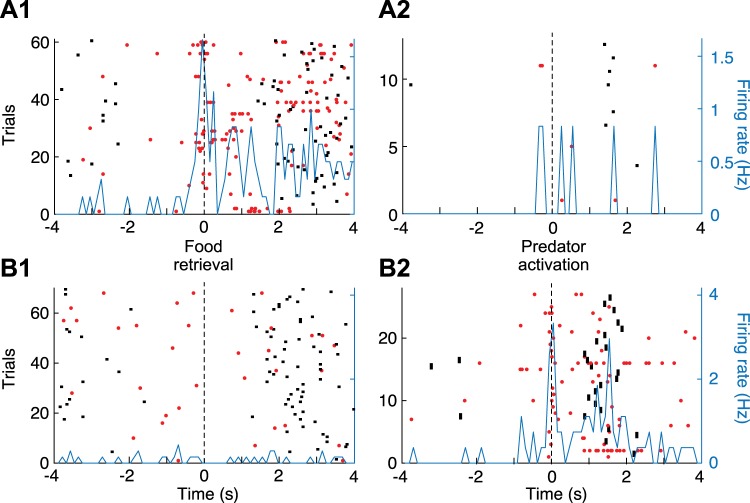
To facilitate determination of whether the units shown in Fig. 2, B and C, were activated in relation to food retrieval or predator activation, raster trials in PETHs referenced to food retrieval were rank ordered by latency of predator activation, whereas raster trials in PETHs referenced to predator activation were rank ordered by latency of food retrieval. This revealed that the firing rate increase displayed by the units in Fig. 2, B and C, were time-locked to food retrieval or predator activation, respectively. This was formally tested by comparing the time interval between the reference events and the nearest spike (see methods), revealing that for the unit shown in Fig. 2B, it was shorter for food retrieval than for predator activation and nest reentry (Kruskal-Wallis ANOVA, df = 2, χ2 = 8.36, P = 0.0153, Dunn’s test, P < 0.05), whereas for the unit shown in Fig. 2C, the interval was shorter for predator activation than for food retrieval and nest reentry (Kruskal-Wallis for latencies df = 2, χ2 = 24.76, P = 4.21 × 10−6, Dunn’s test, P < 0.05). For the unit shown in Fig. 2B, additional evidence that it fired in relation to food retrieval but not predator activation was obtained by comparing two additional subsets of trials where only one of these two events occurred (Fig. 3A). Although no such trials were available for the unit shown in Fig. 2C, we illustrate a different unit that fired in relation to predator activation (Fig. 3B).
Fig. 3.
Principal neurons (PNs) activated at the time of food retrieval or predator activation. A: PN activated at the time of food retrieval but not predator activation (same unit as in Fig. 1B). B: different PN activated at the time of predator activation but not food retrieval. Data are spike rasters (red dots) and superimposed perievent time histograms (PETHs) of neuronal discharges (blue lines). A1 and B1: trials with food retrieval but no predator activation. PETH is referenced to the time of food retrieval. A2 and B2: trials with predator activation but no food retrieval. PETH is referenced to the time of predator activation. Black dots before or after time 0 in the PETHs mark when rats started foraging (left of origin) or reentered the nest (right of origin).
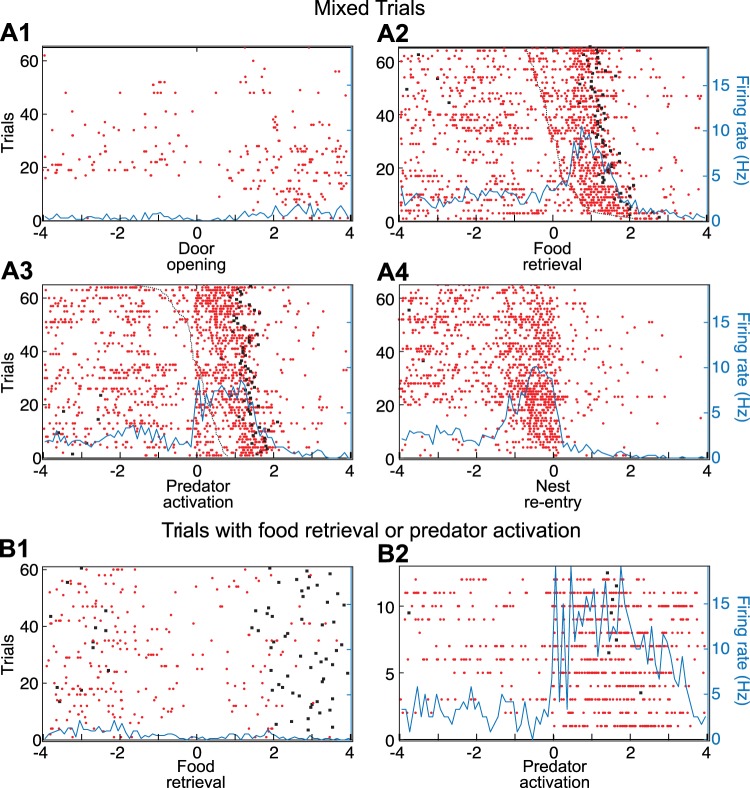
Complex units (Figs. 4 and 5) accounted for roughly one-third of responsive units (Table 2). They tended to have higher baseline firing rates (1.54 ± 0.33 Hz, n = 28) than simple units (0.61 ± 0.12 Hz, n = 61; rank-sum test, P = 8.5 × 10−4). Here too, the task of determining what variables were related to the increased activity was complicated by the fact that many task events occur in rapid succession. However, rank ordering raster trials by the latency of events preceding or following the one used to reference the PETHs and comparing the time intervals between the reference events and the nearest spikes allowed us to circumvent this difficulty. For instance, in the PETH referenced to food retrieval (Fig. 4A2), it is clear that the rise in firing rate parallels the time when the predator is activated. Consistent with this, the time interval between the reference events and the nearest spike was shorter for predator activation and nest reentry than for food retrieval (Kruskal-Wallis ANOVA, df = 2, χ2 = 29.11, P = 4.76 × 10−7, Dunn’s test, P < 0.05). This predator-locked event is then followed by a second and more persistent rise in firing rate, which is time-locked to reentry into the nest (Fig. 4A4). Additional evidence that the critical variable was not food retrieval but predator activation was obtained by comparing two additional subsets of trials where only one of these two events occurred (Fig. 4B).
Fig. 4.
Principal neuron (PN) with complex responsiveness. Data are spike rasters (red dots) and superimposed perievent time histograms (PETHs) of neuronal discharges (blue lines). A: PETHs of neuronal discharges referenced to the time of door opening (A1), food retrieval (A2), predator activation (A3), or reentry into the nest (A4). B1: trials with food retrieval but no predator activation. PETH is referenced to the time of food retrieval. B2: trials with predator activation but no food retrieval. PETH is referenced to the time of predator activation. In A2, raster trials were rank ordered by latency of predator activation (black dashed line). In A3, raster trials were rank ordered by latency of food retrieval (black dashed line). Black dots before or after time 0 in the PETHs mark when rats started foraging (left of origin) or reentered the nest (right of origin).
Fig. 5.
PN responsive to 3 of the 4 task events. Data are spike rasters (red dots) and superimposed perievent time histograms (PETHs) of neuronal discharges (blue lines). A: PETHs of neuronal discharges referenced to the time of door opening (A1), food retrieval (A2), predator activation (A3), or reentry into the nest (A4). B1: trials with food retrieval but no predator activation. PETH is referenced to the time of food retrieval. B2: trials with predator activation but no food retrieval. PETH is referenced to the time of predator activation. In A2, raster trials were rank ordered by latency of predator activation (black dashed line). In A3, raster trials were rank ordered by latency of food retrieval (black dashed line). Black dots before or after time 0 in the PETHs mark when rats started foraging (left of origin) or reentered the nest (right of origin).
Figure 5 illustrates a PN with an even more complex responsiveness. This unit showed increased levels of activity in relation to three of the four task events: door opening (Fig. 5A1), food retrieval (Fig. 5, A2 and B1), and predator activation (Fig. 5, A3 and B2). In this case, disambiguation of the activity related to food retrieval or predator activation could not be achieved by considering variations in the latency of these responses, but only by comparing the activity of the units on trials where only one of these two events occurred (Fig. 5B).
ITNs Activated by Specific Task Events
As detailed in Table 3, the proportion of presumed ITNs responsive to one or more of the four task events (87%) was four times higher than in PNs (21.2%; Fisher exact test, P = 2.3 × 10−18). Moreover, their pattern of responsiveness was strikingly different (Fig. 6). Indeed, whereas food recovery was the event least likely to drive PNs (3.6%), a majority of ITNs (75.5%) increased their firing rates in relation to this event (Fisher exact test, P = 2.24 × 10−30). In fact, ITNs that only activated in relation to this event accounted for nearly half of the responsive local circuit cells (18 of 39; Table 2 and Fig. 6). Inversely, whereas reentry into the nest was the event most likely to drive PNs (10.71%), very few ITNs (2.2%) increased their firing rates in relation to this event (Fisher exact test, P = 0.11; Table 2 and Fig. 6). Last, the proportion of ITNs with a complex responsiveness was much higher than in PNs. For instance, 26.7% of them were responsive to three or four of the task events, compared with 0.71% of PNs (Fisher exact test, P = 5.7 × 10−11). Figure 7 shows a representative example of a unit with such a complex responsiveness.
Table 3.
Incidence of ITNs with significant firing rate increases in relation to one or more task events
| Task Event |
|||||
|---|---|---|---|---|---|
| Predator activation | Food retrieval | Nest reentry | Door opening | No. of ITNs | Incidence, % |
| 0 | 0 | 0 | 1 | 3 | 6.7 |
| 0 | 1 | 0 | 0 | 18 | 40 |
| 1 | 0 | 0 | 1 | 2 | 4.4 |
| 1 | 1 | 0 | 0 | 4 | 8.9 |
| 1 | 1 | 0 | 1 | 11 | 24.4 |
| 1 | 1 | 1 | 1 | 1 | 2.2 |
| 40% | 75.5% | 2.2% | 37.8% | 39 | 86.7 |
Values 1 and 0 indicate whether units were responsive to a given task event or not, respectively. With 4 task events, there were 16 theoretically possible combinations of responsiveness; 7 of these possibilities were observed. The number and incidence of responsive interneurons (ITNs) are indicated for each possible combination. The case of units unresponsive to all task events is not listed. Totals indicate the percentage or number of ITNs (n = 45) that increased their firing rates in relation to 1 or more of the 4 task events.
Fig. 6.
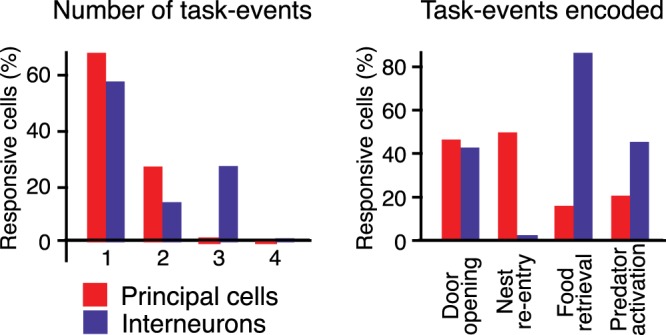
Contrasting responsiveness of principal neurons (PNs) and interneurons (ITNs). Left: proportion of responsive units (y-axis) that increased their activity in relation to 1, 2, 3, or 4 task events. Red, PNs; blue, ITNs. Right: proportion of units (y-axis) responding to the task events listed along the x-axis.
Fig. 7.
Interneuron (ITN) with complex responsiveness. Data are spike rasters (red dots) and superimposed perievent time histograms (PETHs) of neuronal discharges (blue lines). A: PETHs of neuronal discharges referenced to the time of door opening (A1), food retrieval (A2), predator activation (A3), or reentry into the nest (A4). B1: trials with food retrieval but no predator activation. PETH is referenced to the time of food retrieval. B2: trials with predator activation but no food retrieval. PETH is referenced to the time of predator activation. In A2, raster trials were rank ordered by latency of predator activation (black dashed line). In A3, raster trials were rank ordered by latency of food retrieval (black dashed line). Black dots before or after time 0 in the PETHs mark when rats started foraging (left of origin) or reentered the nest (right of origin). Note that for clarity, we depict only 1 of 5 spikes. Illustrated spikes were selected randomly.
Relative Importance of Coding for Task Phases vs. Task-Events in PNs and Local Circuit Cells
The results above indicate that small subsets of PNs show activity variations in relation to the task events we considered. However, the temporal contiguity of these events and the presence of potentially confounding variables such as the rats’ speed hinder identification of the variables BL neurons encode. To overcome these obstacles, we fit the activity of individual BL units using a group lasso GLM. This approach takes advantage of variability in the timing and duration of relevant variables to determine which one(s) neurons encode. It also allows one to consider other variables such as the rats’ speed and position, their distance from the food pellet, the influence of trial types (with or without predator), and task phases (waiting at the door, foraging, escape). Critically, this type of GLM permits dimensionality reduction of correlated data and favors sparsity in the identification of the variables related to neuronal activity (Breheny and Huang 2015; Tibshirani 1996; Yuan and Lin 2006).
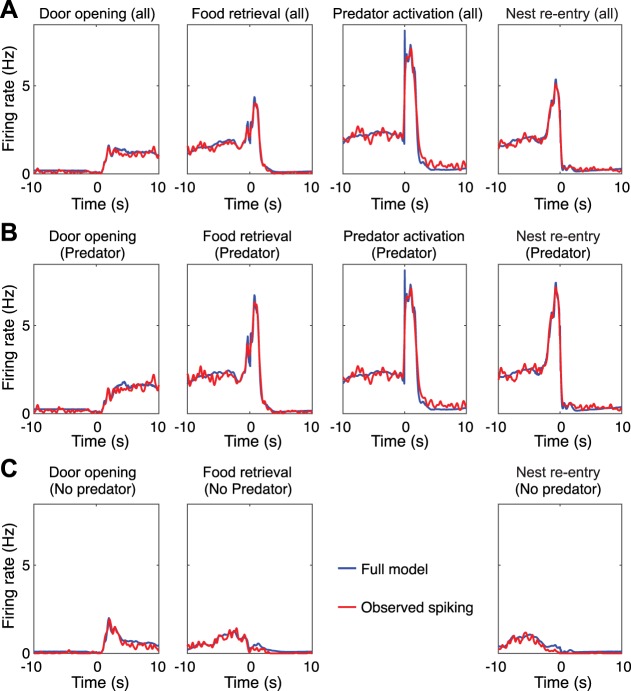
To test the validity of this approach, we first examined whether GLM coding estimates matched the results obtained with PETHs constructed around different task events. Overall, for the four task events considered above, there was generally agreement between PETH analyses and the GLM’s output. For instance, in the example unit shown in Fig. 8, the model-estimated spiking combined across all task-related variables closely matched observed spiking (Fig. 8B).
Fig. 8.
Generalized linear model (GLM)-estimated coding of a principal neuron (PN). A–C: observed (red lines) and predicted (blue lines) firing of a PN during all available trials (A), only predator trials (B), or only no-predator trials (C). In A–C, the plots from left to right show observed and estimated activity around door opening, food retrieval, predator activation, and nest reentry.
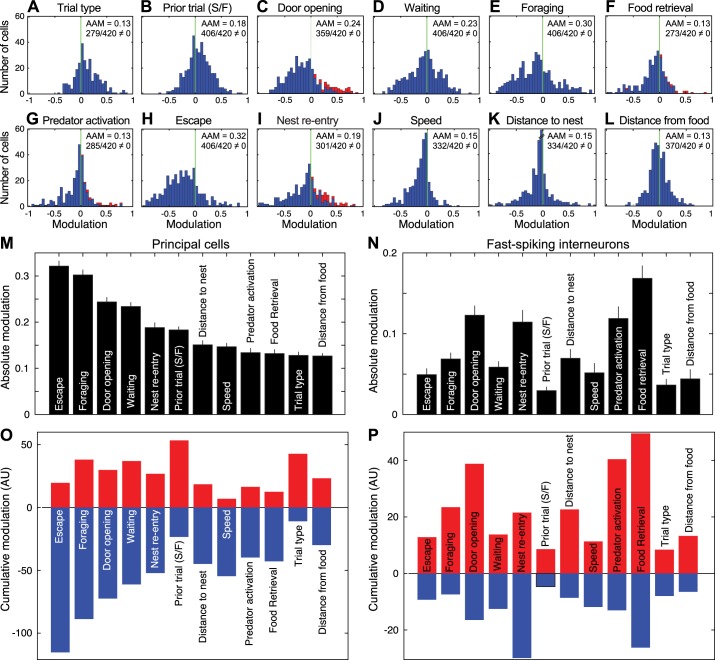
Figure 9, A–L, shows frequency distributions of the firing rate modulation by 12 different variables in PNs (n = 420). In each case, the average absolute modulation (AAM) is indicated, along with the proportion of units with modulations significantly different from zero. Inhibitory and excitatory modulations are plotted to the left and right of the origin, respectively. Units that were deemed to show significant excitatory modulations using PETHs are indicated. Note that they also tended to have high positive modulations based on the GLM analyses.
Fig. 9.
Generalized linear model (GLM)-estimated coding of 12 variables by principal neurons (PNs). A–L: frequency distributions of firing modulation (x-axis) in relation to 12 different variables. Red bars represent units that showed statistically significant increases in firing rates, as determined using standard analyses: red bars in C correspond to 42 units responsive to door opening, in F to 15 units responsive to food retrieval, in G to 19 units responsive to predator activation, and in I to 45 units responsive to nest reentry. In B, S/F indicates prior trial success and failure. For each variable, the average absolute modulation (AAM) and the number of units with modulation different from 0 are listed at top right. Units with modulation values of 0 are not included in the histograms. M and N: GLM variables rank ordered by absolute firing rate modulation magnitude in PNs (M; n = 420) and interneurons (ITNs; N; n = 45), both ordered based on the rank of PNs. Values are averages ± SE. O and P: sum of positive (red) and negative (blue) modulations in PNs (O) and ITNs (P). AU, arbitrary units.
In PNs and ITNs, there were significant differences in the magnitude of the firing rate modulations associated with the different variables (PNs: repeated-measures Friedman ANOVA χ2 = 853, df = 11, P = 4.9 × 10−175; ITNs: repeated-measures Friedman ANOVA χ2 = 216, df = 11, P = 1.29 × 10−39). In PNs, task phases escape and foraging had a significantly higher AAM value than all other variables with the exception of door opening (Dunn’s test, P < 0.05), and the task phase waiting had the fourth highest value (Fig. 9M). An opposite pattern was seen in ITNs (Fig. 9N). In these units, task events (food retrieval, door opening, predator activation, and nest reentry) had the four highest AAM values, significantly higher than for all task phases (Dunn’s test, P < 0.05).
In PNs, distributions of firing rate modulations (Fig. 9, A–L) were generally skewed to the left, indicating that for most variables, inhibitory modulations prevailed. This can be more easily seen in Fig. 9O, where inhibitory and excitatory modulations are summed separately. There were only two exceptions to this trend: trial type and prior trial (Fig. 9, A, B, and O). For these variables, the distributions were clearly skewed to the right, reflecting the fact that PNs typically fired at higher rates on trials with than without the predator, or after failed compared with successful trials. Of note, the predator activation and food retrieval variables had some of the lowest firing rate modulations (they ranked 9th and 10th). Here again, an opposite pattern was seen in ITNs. In these units, positive firing rate modulations were generally higher than negative ones (Fig. 9P).
Two of the results listed above appear to contradict each other. On the one hand, predator activation generally caused little change or an inhibition in PNs. On the other hand, trial type, which indicates trials with the predator present vs. absent, was generally associated with higher firing rates when the predator was present. How can these results be reconciled? Here, it is natural to expect that the sight of the immobile predator or of its forward surge should elicit similar changes in BL activity. However, can rats detect the immobile predator from the nest? To address this question, we compared the time from the start of foraging to food retrieval in four different conditions: the last trial of predator blocks (last predator trial), the first trial of no-predator blocks (first no-predator trial), the last trial of no-predator blocks (last no-predator trial), and the first trial of predator blocks (first predator trial). A Kruskal-Wallis ANOVA revealed significant differences between these conditions (χ2 = 26.24, df = 3, P = 4.65 × 10−6). Post hoc comparisons (Dunn’s test, with threshold P of 0.05) revealed that the time to food retrieval did not differ significantly between the last predator trial (16.1 ± 3.3 s) and first no-predator trial (11.8 ± 2.1 s) and that both were significantly higher than the last no-predator trial (5.3 ± 1.3 s) and first predator trial (3.45 ± 0.6 s).
These results suggest that rats did not detect the predator from the nest or that visual information was not a major determinant of their foraging behavior. To determine whether the same applied to the firing rate of BL cells, all available units were rank ordered based on their modulation by trial type (Fig. 10A). Their average z-scored firing rate during baseline and waiting was then plotted at the transition between trial blocks, from no-predator to predator trials (Fig. 10B) as well as from predator to no-predator trials (Fig. 10C). Consistent with the behavioral observations, these new analyses revealed that the change in unit activity did not occur on the first trial of a new trial block but after one or more trials of the new trial block. In other words, only after the first trial of a predator block did rats express in their behavior or could we detect in the activity of BL cells that they “knew” or expected, based on their recent experience, that the predator would be present on subsequent trials. This is in contrast with predator activation, which is akin to a sensory stimulus (since its makes a noise when activated), a suggestion supported by the fact that nearly half of the predator-responsive units were also activated when the door was opened.
Fig. 10.
Change in unit activity at transitions between trial blocks. Units were rank ordered (A) on the basis of their modulation by the trial type variable (see Fig. 9A). Their average firing rate during the baseline and waiting phases was then plotted trial by trial (x-axis) around transitions between trial blocks from no-predator to predator trials (A and B) and from predator to no-predator trials (C). In A, firing rate is z-scored and color coded (bar at right). Red dots on the left of the central panel mark event cells. In B and C, solid lines are average firing rate of all available units; dashed lines are SE.
DISCUSSION
The present study was undertaken to shed light on the influence of the amygdala on risky foraging decisions. A prior study, using a seminaturalistic foraging task, reported that BLA inactivation abolishes the normally apprehensive behavior of rats confronted with a predator (Choi and Kim 2010). In keeping with this observation, another report disclosed the presence of predator-responsive neurons in LA (Kim et al. 2018), suggesting that BLA inactivation rendered rats fearless because it inhibited threat-coding neurons. Contrasting with LA, however, most BL PNs showed markedly reduced firing rates during foraging (Amir et al. 2015), instead suggesting that BLA inactivation rendered rats fearless because it mimicked the suppression of BL activity normally associated with foraging. However, the two studies used different methods to analyze unit activity, raising the possibility that Amir et al. (2015) underestimated the incidence of predator-coding neurons. By applying the methods used by Kim et al. (2018) to BL neurons, the present study revealed that very few PNs (4.5%) are activated by the predator. In addition, we found that coding for task phases was much stronger than for task events. Below, we discuss the significance of these findings for the contribution of the amygdala in risky foraging decisions.
A Minute Proportion of BL PNs are Responsive to the Predator
In a prior study (Amir et al. 2015) on the same sample of BL neurons, we found that most PNs (88%) display significant firing rate changes in the foraging task. Opposite to the threat-coding model emerging from fear conditioning studies, most PNs (type 1 cells, 69% of significant cells) displayed a reduction in firing rate during foraging, were nearly silent near the predator, and during escape. Yet, their activity during the waiting phase was consistent with the threat-coding model. That is, they fired at higher rates during waiting periods followed by retreat into the nest than at initiation of foraging. Similarly, they fired at higher rates during the waiting period of predator than no-predator trials. Furthermore, whereas their firing rates correlated negatively with the speed of forward movements (toward the food), when rats hesitated during foraging, as indicated by alternations between forward and backward movements, their firing rate correlated positively with the speed of backward movements (toward the nest). A second, less numerous (7%) class of PNs (type 2) showed an increase in firing rate during foraging. However, this heightened activity was not threat related because it was also observed on no-predator trials as well as in a control food-seeking shuttle task devoid of explicit threats. Last were type 3 cells (13%), a heterogeneous group of PNs that showed transient alterations in firing rates, typically around the time of food capture.
In contrast with Kim et al. (2018), our prior study did not examine neural responses to predator activation for two reasons. First, the most conspicuous changes in BL activity seemed related to task phases (waiting, foraging, escape), not punctual task events like the surging predator. Second, predator activation coincides with a major change in rat behavior (from foraging to escape) and is contiguous with other task events (food retrieval and nest reentry). As a result, it seemed a priori difficult to determine what variable(s) BL neurons encode during this complex confluence of events. Nevertheless, the present study attempted to address this question in two ways: first, by applying the PETH-based analysis method of Kim et al., and second, by fitting the activity of individual BL cells using GLM.
Using the PETH-based approach, we found that the majority of predator-responsive BL PNs were type 3 cells. Their incidence (4.5%) was even lower than in LA (13%). Moreover, two-thirds of these cells were also responsive to nonthreatening stimuli, and ~25% of them also increased their firing rates when rats retrieved the food, in the absence of predator. The incidence of PNs activated on reentry into the nest was actually higher than that of predator-responsive cells.
GLM Analysis Suggests That BL PNs Encode Task Phases More Strongly Than Valenced Stimuli
A serious problem with the PETH method is that it ignores the potential influence of the switch in rat behavior (foraging to escape) and change in speed that occur around the time of predator activation. In our prior study, we did not analyze this complex period for this exact reason. Fitting BL activity with a GLM allowed us to consider additional variables we had neglected before and to examine the influence of each while factoring out that of the others. This approach revealed that task phases (e.g., escape, foraging, waiting) had a significantly higher influence on the activity of PNs than task events such as predator activation or food retrieval. Moreover, for most variables, inhibitory modulations prevailed. In fact, the predator activation and food retrieval variables had some of the lowest positive firing rate modulations. Two exceptions to this general rule were trial type and the outcome of the prior trial for which excitatory modulations prevailed; even after factoring out the influence of speed, BL PNs tended to fire at higher rates when the predator was present than when absent or when rats had failed rather than succeeded to retrieve the food on the prior trial.
Finally, one might consider the possibility BL neurons encode threats through inhibition. According to this view, the inhibition of BL neurons would be transformed into an excitation of fear effectors via networks of GABAergic neurons in downstream structures. However, this possibility seems unlikely given that nearly all the variables we considered, most of which were not threat related, were associated with a prevalent inhibition of principal BL cells.
ITNs and PNs Show an Opposite Pattern of Responsiveness
Whereas Kim et al. (2018) implied that PNs and ITNs had a similar responsiveness in LA, we found that it differed markedly in BL. They were preferentially responsive to different events (ITNs, food retrieval; PNs, nest reentry). Moreover, whereas most PNs showed transient responses time-locked to task events, ITN responses typically lasted longer and tended to anticipate these events. Also, using the GLM, we found that the activity of ITNs was preferentially modulated by task events (not task phases, as in PNs), and that these modulations were prevalently positive (not negative, as in PNs). Together, the reciprocal pattern of results obtained in PNs and ITNs is consistent with the possibility that extrinsic afferents drive ITNs, which in turn inhibit PNs, dampening their responses to task events.
Contribution of Predator-Responsive Cells to the Cautious Behavior of Rats in the Foraging Task
Rats displayed many signs of apprehension in the foraging task. When a trial began, they hesitated at the door threshold before venturing in the foraging arena, sometimes retreating back into the nest instead of initiating foraging. Also, the time to food recovery and the incidence of aborted trials were significantly higher in the presence than in the absence of predator. Yet, our analyses revealed that rats could not detect the predator from the nest or that visual information was not a major determinant of their foraging behavior. Moreover, only 36.8% (7 of 19) of the predator-responsive PNs showed increased baseline activity on predator trials, whereas as many as 50.8% of type 1 PNs (or 156 of 307) did. These results, combined with the fact that foraging initiation coincides with reduced firing rates in type 1 cells, strongly suggest that the behavioral effects of BLA inactivation is not entirely dependent on the inhibition of predator-responsive neurons but also, if not mainly, on reproducing the inhibition of type 1 cells, as seen normally when rats initiate foraging.
Reconciliation of LA and BL Results
In the Kim et al. (2018) study, 8% and 13% of PNs increased their firing rate in relation to food retrieval and predator activation, respectively. In addition, Kim et al. implied that LA ITNs and PNs showed similar response to these stimuli. By contrast, in BL, the proportion of PNs with increased discharge rates at the time of food retrieval (3.5%) or predator activation (4.5%) was lower than in LA. Moreover, in contrast to LA, most BL ITNs were excited before food retrieval and/or predator activation. Many factors may explain the differing response profiles of LA and BL neurons. For example, LA and BL receive inputs from different cortical and subcortical structures (McDonald 1998; Turner and Herkenham 1991), including neuromodulatory inputs (Aitta-Aho et al. 2018; Unal et al. 2015; Zhang et al. 2013). Furthermore, the density of parvalbumin-expressing ITNs is not homogenous across the BLA (McDonald and Betette 2001). It is thus possible that the excitation of fast-spiking ITNs when rats are near the food causes a stronger inhibition of PNs in BL than in LA, resulting in a lower proportion of responsive PNs in BL.
Although the above findings emphasize the possibility that the higher proportion of predator-responsive neurons in LA and BL is due to differences in the connectivity and cellular composition of these nuclei, the possibility remains that predator-responsive neurons are not actually encoding threat. Indeed, Kim et al. (2018) did not examine the responsiveness of LA cells to door opening or nest reentry. In contrast, we found that two-thirds of predator-responsive BL PNs were also activated by these events. These observations raise the possibility that predator-responsive neurons do not encode threat per se, but simply respond to sensory inputs associated with predator activation, such as the noise or visual stimulation associated with its forward surge. Additional analysis in LA will be needed to settle this question.
Paths to the Expression of Defensive Behaviors
Depending on the nature and sensory modality of threatening stimuli, different circuits support innate and conditioned defensive behaviors (Gross and Canteras 2012). For instance, whereas lesions of the central amygdala abolish conditioned responses to discrete conditioned stimuli (Hitchcock and Davis 1987; LeDoux et al. 1988), they do not affect innate and contextual fear responses to predators or their odor (Li et al. 2004; Martinez et al. 2011). Instead, the latter depend on a medial hypothalamic circuit involving the ventromedial hypothalamic nucleus (VMH) and its downstream targets (reviewed in Canteras et al. 2012).
Depending on the sensory modality, different amygdala nuclei relay sensory information about predators to the VMH. The transmission of predator odors is mainly dependent on projections from the accessory olfactory bulb to the medial amygdala and from there to the VMH (Dielenberg and McGregor 2001; Martinez et al. 2011). In contrast, nonolfactory cues about predators are relayed primarily to the VMH by the basomedial nucleus (BM) of the amygdala. Because LA contributes very strong projections to BM (Krettek and Price 1978; Pitkänen et al. 1997; Smith and Paré 1994) and is a major recipient of sensory information of thalamic and cortical origin (McDonald 1998; Turner and Herkenham 1991), it is an ideal position to contribute critically to this circuit. Consistent with this, predator exposure increases Fos expression in LA, and LA lesions reduce the expression of defensive behaviors elicited by predators (Martinez et al. 2011).
Likely because BL contributes limited projections to BM (Paré et al. 1995; Pitkänen et al. 1997) and does not project to the VMH, BL usually does not figure prominently in models of innate defensive behaviors (Canteras et al. 2012). However, numerous studies have implicated BL projections to the striatum and nucleus accumbens in the genesis of active avoidance response to threatening cues (Boeke et al. 2017; Delgado et al. 2009; Ramirez et al. 2015) and in the consolidation of aversive memories (McGaugh 2004). Moreover, a recent study reported that cytotoxic BL lesions disrupt innate and conditioned fear of predators (Bindi et al. 2018). In light of our results, these findings suggest that BL does not promote defensive behaviors by only signaling specific threatening stimuli but by inhibiting behavioral engagement when threats are anticipated, as discussed at length elsewhere (Paré and Quirk 2017).
GRANTS
This material is based on work supported by National Institute of Mental Health Grants R01 MH107239 and R01 MH112505 (to D. Paré).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. and D.P. conceived and designed research; A.A. and S.-C.L. performed experiments; A.A., P.K., D.B.H., and D.P. analyzed data; A.A. and D.P. interpreted results of experiments; A.A. and D.P. prepared figures; D.P. drafted manuscript; A.A., P.K., S.-C.L., and D.B.H. edited and revised manuscript; A.A., P.K., S.-C.L., D.B.H., and D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for S.-C. Lee: Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 20215.
REFERENCES
- Aitta-Aho T, Hay YA, Phillips BU, Saksida LM, Bussey TJ, Paulsen O, Apergis-Schoute J. Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr Biol 28: 2557–2569.e4, 2018. doi: 10.1016/j.cub.2018.06.064. [DOI] [PubMed] [Google Scholar]
- Amir A, Headley DB, Lee SC, Haufler D, Paré D. Vigilance-associated gamma oscillations coordinate the ensemble activity of basolateral amygdala neurons. Neuron 97: 656–669.e7, 2018. doi: 10.1016/j.neuron.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Lee SC, Headley DB, Herzallah MM, Pare D. Amygdala signaling during foraging in a hazardous environment. J Neurosci 35: 12994–13005, 2015. doi: 10.1523/JNEUROSCI.0407-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Bindi RP, Baldo MV, Canteras NS. Roles of the anterior basolateral amygdalar nucleus during exposure to a live predator and to a predator-associated context. Behav Brain Res 342: 51–56, 2018. doi: 10.1016/j.bbr.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103: 70–82, 1989. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Boeke EA, Moscarello JM, LeDoux JE, Phelps EA, Hartley CA. Active avoidance: neural mechanisms and attenuation of Pavlovian conditioned responding. J Neurosci 37: 4808–4818, 2017. doi: 10.1523/JNEUROSCI.3261-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breheny P, Huang J. Group descent algorithms for nonconvex penalized linear and logistic regression models with grouped predictors. Stat Comput 25: 173–187, 2015. doi: 10.1007/s11222-013-9424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Mota-Ortiz SR, Motta SC. What ethologically based models have taught us about the neural systems underlying fear and anxiety. Braz J Med Biol Res 45: 321–327, 2012. doi: 10.1590/S0100-879X2012007500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci USA 107: 21773–21777, 2010. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 3: 33, 2009. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev 25: 597–609, 2001. doi: 10.1016/S0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JR, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46, 2013. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- Fanselow MS, Lester LS, Helmstetter FJ. Changes in feeding and foraging patterns as an antipredator defensive strategy: a laboratory simulation using aversive stimulation in a closed economy. J Exp Anal Behav 50: 361–374, 1988. doi: 10.1901/jeab.1988.50-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci 13: 651–658, 2012. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods 155: 207–216, 2006. doi: 10.1016/j.jneumeth.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav 39: 403–408, 1987. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 517: 284–292, 2015. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kong MS, Park SG, Mizumori SJ, Cho J, Kim JJ. Dynamic coding of predatory information between the prelimbic cortex and lateral amygdala in foraging rats. Sci Adv 4: eaar7328, 2018. doi: 10.1126/sciadv.aar7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–279, 1978. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Kyriazi P, Headley DB, Paré D. Multi-dimensional coding by basolateral amygdala neurons. Neuron 99: 1315–1328.e5, 2018. doi: 10.1016/j.neuron.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529, 1988. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci 118: 324–332, 2004. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852, 2004. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Carvalho-Netto EF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience 172: 314–328, 2011. doi: 10.1016/j.neuroscience.2010.10.033. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332, 1998. doi: 10.1016/S0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D28k. Neuroscience 102: 413–425, 2001. doi: 10.1016/S0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28, 2004. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Trimmer PC, Blumstein DT, Dayan P. Foraging for foundations in decision neuroscience: insights from ethology. Nat Rev Neurosci 19: 419–427, 2018. doi: 10.1038/s41583-018-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010. [Erratum in Physiol Rev 90: 1269, 2010.] doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ. When scientific paradigms lead to tunnel vision: lessons from the study of fear. NPJ Sci Learn 2: 6, 2017. doi: 10.1038/s41539-017-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y, Paré JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 69: 567–583, 1995. doi: 10.1016/0306-4522(95)00272-K. [DOI] [PubMed] [Google Scholar]
- Park IM, Meister ML, Huk AC, Pillow JW. Encoding and decoding in parietal cortex during sensorimotor decision-making. Nat Neurosci 17: 1395–1403, 2014. doi: 10.1038/nn.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillow JW, Paninski L, Uzzell VJ, Simoncelli EP, Chichilnisky EJ. Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. J Neurosci 25: 11003–11013, 2005. doi: 10.1523/JNEUROSCI.3305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523, 1997. doi: 10.1016/S0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Moscarello JM, LeDoux JE, Sears RM. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J Neurosci 35: 3470–3477, 2015. doi: 10.1523/JNEUROSCI.1331-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Paré D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248, 1994. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B 58: 267–288, 1996. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol 313: 295–325, 1991. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci 35: 853–863, 2015. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecasteele M, M S, Royer S, Belluscio M, Berényi A, Diba K, Fujisawa S, Grosmark A, Mao D, Mizuseki K, Patel J, Stark E, Sullivan D, Watson B, Buzsáki G. Large-scale recording of neurons by movable silicon probes in behaving rodents. J Vis Exp 4: e3568, 2012. doi: 10.3791/3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Lin Y. Model selection and estimation in regression with grouped variables. J R Stat Soc B 68: 49–67, 2006. doi: 10.1111/j.1467-9868.2005.00532.x. [DOI] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ. Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience 228: 395–408, 2013. doi: 10.1016/j.neuroscience.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]