Abstract
Motor-evoked potentials (MEPs), elicited by transcranial magnetic stimulation (TMS) over the motor cortex, are reduced during the preparatory period in delayed response tasks. In this study we examined how MEP suppression varies as a function of the anatomical organization of the motor cortex. MEPs were recorded from a left index muscle while participants prepared a hand or leg movement in experiment 1 or prepared an eye or mouth movement in experiment 2. In this manner, we assessed if the level of MEP suppression in a hand muscle varied as a function of the anatomical distance between the agonist for the forthcoming movement and the muscle targeted by TMS. MEP suppression was attenuated when the cued effector was anatomically distant from the hand (e.g., leg or facial movement compared with finger movement). A similar effect was observed in experiment 3 in which MEPs were recorded from a muscle in the leg and the forthcoming movement involved the upper limb or face. These results demonstrate an important constraint on preparatory inhibition: it is sufficiently broad to be manifest in a muscle that is not involved in the task, but it is not global, showing a marked attenuation when the agonist muscle belongs to a different segment of the body.
NEW & NOTEWORTHY Using transcranial magnetic stimulation, we examined changes in corticospinal excitability as people prepared to move. Consistent with previous work, we observed a reduction in excitability during the preparatory period, an effect observed in both task-relevant and task-irrelevant muscles. However, this preparatory inhibition is anatomically constrained, attenuated in muscles belonging to a different body segment than the agonist of the forthcoming movement.
Keywords: motor cortex, movement, preparatory inhibition, somatotopy, TMS
INTRODUCTION
Transcranial magnetic stimulation (TMS) has proven to be a powerful tool to assess the dynamics of corticospinal (CS) excitability during response preparation in humans (Bestmann and Duque 2016; Cos et al. 2014; Klein et al. 2012; Leocani et al. 2000). In a delayed response task, a cue is provided to indicate the forthcoming response, with that response initiated after the onset of an imperative signal (e.g., cue left or right index finger movement). TMS studies have shown local increases in cortical excitability in primary motor cortex (M1) during the delay period (Davranche et al. 2007; Duque and Ivry 2009; Tandonnet et al. 2010), as well as broad suppression of CS excitability during the same time window (Greenhouse et al. 2015b; Hannah et al. 2018). Indeed, when single-pulse TMS is delivered over the primary motor cortex during the delay period, motor-evoked potentials (MEPs) elicited from the targeted muscle show profound suppression, regardless of whether that muscle is required to perform the cued movement (i.e., selected) or is not required for the forthcoming response (nonselected) (Duque et al. 2017; Duque and Ivry 2009; Quoilin et al. 2016). After the imperative, the selected muscle shows a rapid increase in excitability, whereas the nonselected muscles remain suppressed (Duque et al. 2014; Duque and Ivry 2009; Klein et al. 2016).
Interestingly, in many studies, the strongest level of MEP suppression during the delay period is observed when the muscle is the agonist for the selected response (Duque and Ivry 2009; Klein et al. 2016). This observation led to the hypothesis that preparatory inhibition is designed to prevent premature movement. In contrast, MEP suppression of nonselected muscles has been considered a useful mechanism for action selection, helping to sharpen the preparation of a selected movement by inhibiting alternative representations (Duque et al. 2005, 2010; Leocani et al. 2000; Tandonnet et al. 2011). This process might be implemented by inhibitory interactions between competing alternatives or might rely on a more generic form of inhibition whereby the choice of one action is accompanied by broad inhibition of the motor system to lower interference from irrelevant motor representations (Duque et al. 2017).
Using a reaction time (RT) task in which the response was fixed for an entire block of trials, Greenhouse et al. (2015b) observed substantial preparatory inhibition in task-irrelevant muscles (e.g., in a left index finger agonist when the right pinky was always used to make the response). Indeed, the magnitude of the MEP suppression was similar in task-irrelevant muscles compared with task-relevant muscles (e.g., in a left index finger agonist when the cued response was either the left index finger or the left pinky). These findings are difficult to reconcile with the hypothesis that preparatory inhibition assists action selection and points to a more generic process.
However, other findings suggest that preparatory inhibition is not generic. In choice RT tasks, the magnitude of MEP suppression in a nonselected muscle varies as a function of the relationship between the members of the response set: a bigger reduction in excitability is found when the response set involves homologous effectors compared with when the response set involves nonhomologous effectors (Duque et al. 2014; Labruna et al. 2014), a result that may reflect functional or anatomical links between homologous representations across the two hemispheres (van den Heuvel and Hulshoff Pol 2010). Similarly, MEP suppression in a nonselected muscle (e.g., left index finger) is greater when the planned movement involves another upper limb effector (e.g., right index finger) compared with when the planned movement involves a lower limb effector (e.g., right leg).
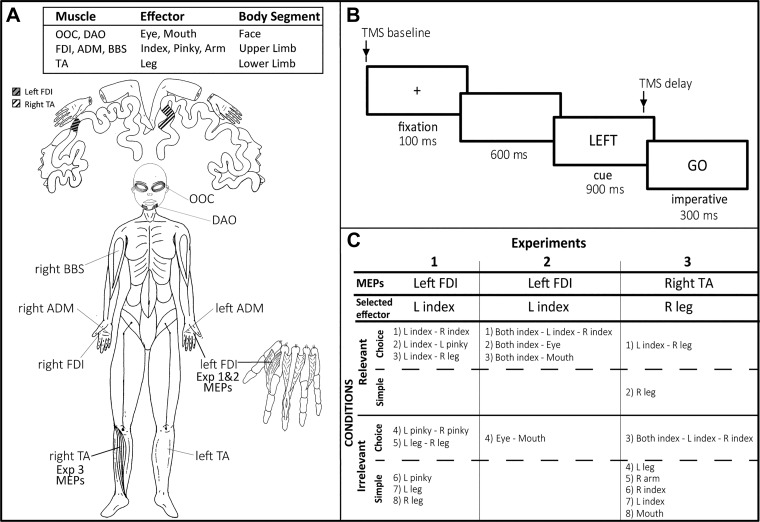
Taken together, these findings suggest that preparatory inhibition is subject to anatomical constraints. To further explore this hypothesis, we systematically manipulated the response set to derive comparisons between conditions in which the probed muscle was close to or distant from the members of the response set in terms of anatomy or function. An overview of the experimental plan is presented in Fig. 1. In experiments 1 and 2, MEPs were always elicited from the left first dorsal interosseous (FDI) muscle, the agonist for left index finger abduction movements. We created conditions in which this muscle was selected or not selected for the forthcoming response or was task irrelevant. Of primary interest, we manipulated the response set to examine whether CS excitability in left FDI varied as a function of the other candidate movements, choosing a range of movements that involved the same or different side of the body or same or different body segment.
Fig. 1.
Overview of the 3 experiments. A: primary agonist muscles used for the responses. A schematic of the cortical homunculus is shown (top), with highlighted regions indicating approximate location of the left first dorsal interosseous (FDI), the muscle targeted for transcranial magnetic stimulation (TMS) in experiments 1 and 2, and the right tibialis anterior (TA), the muscle targeted for TMS in experiment 3. ADM, abductor digiti minimi; BBS, biceps brachii short; DAO, depressor anguli oris; Exp, experiment; OOC, orbicularis oculi. B: sequence of events in the delayed response task. The TMS pulse was either coincident with the onset of the fixation cross (TMS baseline) or occurred 800 ms into the delay period (TMS delay). C: response set for each condition in the experiments. Relevant and irrelevant refer to conditions in which the targeted muscle was either part of or not part of the response set, respectively. In simple conditions, the same movement was cued on each trial, whereas in choice conditions, the cue specified the forthcoming movement. L, left; R, right.
In experiment 1, this involved a comparison of left FDI MEPs between different sets of hand and leg movements. We sought to replicate our earlier findings (Duque et al. 2014; Labruna et al. 2014) showing that reduced excitability is modulated by anatomical similarity, but not by task relevance. In particular, we expected to observe greater MEP suppression in the left FDI when the selected response involved a finger movement not requiring left FDI, compared with when the selected response involved a leg movement. In experiment 2, the focus was on response sets in which index finger movements were paired with either eye or mouth movements. By combining hand and facial movements, we obtained a second test of intersegmental interactions in preparatory inhibition. To ensure that our results are not specific to hand muscles, the TMS probe was targeted at a lower leg muscle, the right tibialis anterior (TA) muscle, in experiment 3. The focus here was to determine if the patterns of intra- and intersegmental interactions observed in a hand muscle would be similar in a leg muscle.
METHODS
Participants
Thirty-six healthy, right-handed participants (Oldfield 1971) were tested, 12 in each experiment[experiment 1: 20.8 yr old (SD 1.1), 10 men; experiment 2: 22.6 yr old (SD 5.4), 6 men; experiment 3: 21.0 yr old (SD 1.7), 5 men]. Participants were recruited from a website maintained by University of California, Berkeley to assist investigators in identifying individuals willing to participate in scientific research. The participants were naive to the purpose of the study and were financially compensated for their participation. The recruitment process used in the present study excluded professional musicians or individuals with an extensive history of experience in playing a musical instrument. The protocol was approved by the institutional review board of the University of California, Berkeley. As part of the written informed consent, participants completed a TMS safety checklist before the start of the experiment.
Procedure
TMS.
In all experiments, participants sat in front of a computer screen with both hands resting on a pillow, palms down, with the arms relaxed in a semiflexed position. TMS was applied over the M1 during a delayed response task to measure changes in the excitability state of the CS pathway during response preparation. The TMS was positioned to elicit MEPs in a single targeted muscle across all conditions in a given experiment (for a review of general procedures used to measure CS excitability during response preparation, see Bestmann and Duque 2016; Duque et al. 2017).
TMS pulses were delivered with a monophasic Magstim 2002 magnetic stimulator (Magstim, Whitland, UK). In experiments 1 and 2, a 90-mm figure-of-eight coil was positioned over the participant’s scalp above the right M1. The coil was placed tangentially, in the posterior-anterior direction, with the handle oriented toward the back of the head, and laterally at a 45° angle from the midline, an orientation that is approximately perpendicular to the central sulcus. We identified the optimal position to elicit MEPs in the left FDI muscle. In experiment 3, the coil was positioned to optimize MEPs in the TA of the right leg, the agonist for adduction movements of the right foot. Given that the leg region is in the depth of the sulcus, we used a 110-mm double cone coil that produces a higher induced current (Deng et al. 2014). The coil was positioned over the left M1, in a posterior-anterior orientation, 1 cm above and 1 cm to the right of the vertex.
Once identified, the optimal position for eliciting MEPs in the targeted muscle (left FDI or right TA) was marked on the scalp to provide a reference point for the experimental session. The participant’s resting motor threshold (rMT) was identified at the hotspot and defined as the minimum TMS intensity required to evoke MEPs of ~50-μV peak-to-peak amplitude on 5 of 10 consecutive trials (Rossini et al. 1994). Averaged across experiments 1 and 2, the mean rMT for the left FDI corresponded to 45% (SD 7) of maximum stimulator output (MSO). In experiment 3, the mean rMT for the right TA was 78% (SD 13) of MSO. The intensity of TMS was set to 115% of the individual rMT.
Electromyography recording.
Electromyography (EMG) was recorded with surface electrodes placed above selected muscles (see below). The EMG signal was continuously monitored online to ensure that participants maintained a relaxed posture over the course of the experiment. The EMG signals were amplified and bandpass filtered online between 20 and 450 Hz (Delsys). The signals were digitized at 2,000 Hz for off-line analysis.
In experiment 1, six EMG electrodes were used, positioned to record from FDI, abductor digiti minimi (ADM), and TA on both sides. In experiment 2, we used four electrodes. Two were placed on the left and right FDI. The other two were placed on the face, one over the left orbicularis oculi, and the other over the left depressor anguli oris (DAO), to record EMG for eye and mouth muscles, respectively. We only considered activity on one side given that movements with the face effectors, when produced, entailed a relatively symmetric activation in the left and right side muscles (Cattaneo and Pavesi 2014). In experiment 3, six electrodes were used to record activity from FDI and TA bilaterally and from left DAO (mouth muscle) and the short head of right biceps brachii, the agonist for arm flexion.
Delayed response task.
A delayed response task was used to study changes in CS excitability during response preparation (Fig. 1B). Each trial began with the brief presentation (100 ms) of a cross at the center of the computer monitor, followed by a 600-ms blank screen and then the presentation of a preparatory cue for 900 ms. The cue consisted of one or two words, positioned at the screen center, specifying the effector for the forthcoming response (e.g., “LEFT,” see below). At the end of the 900-ms delay period, the word “GO” appeared for 300 ms, providing a signal to the participant to produce the cued response. The participants were instructed to prepare their response during the delay period to respond as quickly as possible once the imperative stimulus appeared.
A single TMS pulse was applied on each trial. The pulse was either coincident with the onset of the fixation cross (TMS baseline) or occurred 100 ms before the imperative, 800 ms into the delay period (TMS delay). The TMS baseline and delay trials were randomized, with the constraint that the two timings occurred equally often for each cue. The variation in MEP amplitudes at TMS delay with respect to TMS baseline provided a probe of changes in CS excitability during movement preparation. Although preparatory time may vary for different movements, the long delay period used here ensured that participants had sufficient time to reach an optimal state of preparation before the imperative. The duration of the intertrial interval was variable and fluctuated between 3,000 and 3,500 ms. We note that, with this design, the participants can anticipate the TMS pulse during the delay period if it did not occur at baseline. However, prior work in our laboratory showed that changes in CS excitability during the delay period are not related to the anticipation of a TMS pulse (Greenhouse et al. 2015b).
In each experiment (summarized in Fig. 1C), trials were grouped in blocks, with each block involving only one condition. Participants were informed of the response set and their associated cues before the start of each block (see below). In choice RT conditions, there were two or three possible responses and their order was randomized within the block. In simple RT conditions, the response set consisted of a single response. There were 60 trials in each choice condition, three of which were catch trials (no imperative). There were 40 trials in each simple condition, two of which were catch trials. We recorded 20 baseline MEPs for each condition and 20 MEPs for each cue condition in the delay period, a sample size recommended to obtain reliable MEP measures (Biabani et al. 2018; Cavaleri et al. 2017; Chang et al. 2016; Goldsworthy et al. 2016). The blocks lasted approximately 8 and 6 minutes for the choice and simple RT conditions, respectively. The order of the blocks was randomized across participants (but see constrains in experiment 1).
The muscle from which the MEPs were recorded (left FDI in experiments 1 and 2, right TA in experiment 3) was always relevant or irrelevant for a given block (as highlighted in Fig. 1C). The former situation occurred in blocks where the targeted muscle was the agonist for an effector that was part of the response set (and either selected or nonselected on each trial). In contrast, the targeted muscle was irrelevant when it was the agonist for an effector that was not part of the response set in the block. We use the terminology task-relevant and task-irrelevant blocks to describe this aspect of the design.
experiment 1.
In experiment 1, we examined CS excitability changes in left FDI as the participants prepared movements with either the left or right hand/leg. There were eight conditions, five of which involved choice RT tasks. For three of these, left FDI was relevant, with left index finger paired with either the right index finger, the left pinky, or the right leg. These three conditions were selected to compare preparatory inhibition in a hand muscle when the alternative response involved a homologous effector, another effector on the same hand, or an effector of another body segment. For the other two choice conditions, the left index finger was irrelevant, with the response set consisting of either left/right pinky movements or left/right leg movements. Here we evaluated preparatory inhibition in left FDI when the left index finger was irrelevant but either at the same body segment (intrasegmental) or at a different body segment (intersegmental) as the effectors included in the response set. Left FDI was also irrelevant in the three simple RT blocks. These conditions allowed us to ask the same question as with the irrelevant choice blocks, but without the choice component given that the response was fixed for a given block (left pinky, right or left leg).
When the response set involved a left and right effector, the cues were “Left” and “Right.” When the response set involved two left hand options, the cues were “Index” and “Pinky.” The word “Left” or “Right” was used as the cue in the three simple RT conditions. Index and pinky responses required an abduction of the specified finger, bringing it away from the center of the hand. For leg responses, the participant produced adduction movements, lifting the foot toward the body midline.
The block order was randomized across participants with the constraint that the left index-right index pairing was always tested last. We did so because we were concerned that some participants might tire over the duration of a 120-min experiment. Given that the left-right index pairing has been used in numerous other studies, we opted to test this one last because the present results could be compared with prior results, providing a crude reliability check.
experiment 2.
Experiment 2 was designed to further investigate anatomical constraints on preparatory inhibition. A key comparison in experiment 1 involved changes in the MEPs of a hand muscle when preparing a leg movement. In experiment 2, we extended this intersegmental test, but now examined changes in the MEPs of a hand muscle when preparing a facial movement. Moreover, by comparing different facial gestures, we can assess if the spread of preparatory inhibition is a function of cortical distance. Based on the classic motor homunculus, we would expect MEPs from left FDI would show more suppression when the selected response involves the eye compared with the mouth, given that the eye representation is anatomically closer to the hand area (Fig. 1A).
Given that facial movements are generally bilateral (Cattaneo and Pavesi 2014), we thought it important to compare these movements with bilateral hand movements. There were four conditions (Fig. 1C), with the order randomized across participants. For three of these, the left FDI was relevant, with bimanual index finger movements combined with either eye or mouth movements, or with unimanual left and right index finger movements. The latter block was used as a control condition to establish a baseline. In the fourth block, the choice was between a mouth and an eye movement, with the left FDI being irrelevant.
Finger movements were cued with the words “Left index,” “Right index,” or “Both index.” Eye and mouth movements were cued with the words “Eyes” or “Mouth,” respectively. Finger responses were as in experiment 1 (index finger abduction). Eye movements consisted of a single volitional squint with both eyes. The mouth movements required the participants to make a volitional smile, with the instruction to show as much of the teeth as possible.
experiment 3.
To ensure that the CS excitability changes observed in experiments 1 and 2 were not specific to MEPs elicited in a hand muscle, we targeted the TA muscle of the right leg in experiment 3. MEPs are more difficult to elicit from leg muscles: not only is the leg region in the depth of the sulcus, but the motor representations of leg muscles may contain fewer or weaker CS projections (Kesar et al. 2018). Given this challenge, the thresholding phase of experiment 3 also served as a screening procedure: we recruited 23 participants to identify 12 individuals for whom we were able to consistently elicit MEPs in the right TA.
There were a total of eight conditions, with the order randomized across these 12 participants. The right TA muscle was relevant in two conditions, one in which the right leg was tested in a simple RT task and one in which the right leg was paired with the left index finger in a choice RT task. Note that we opted to record MEPS from the right TA rather than the left TA given that, by doing so, we have a condition that is identical to one tested in experiment 1 (left index paired with right leg).
The right TA was irrelevant in the other six conditions. Five of these were simple RT tasks, with the responses made (in separate blocks) with either the mouth, right arm, left index finger, right index finger, or left leg. For the remaining choice RT condition, we used the 3-choice manual condition of experiment 2 (left, right, or bimanual index finger movement).
For the simple RT blocks, the words “Left Index,” “Right Index,” “Right Arm,” “Mouth,” or “Left Leg” were used. In the choice RT blocks, the cues were “Left Index,” “Right Index,” “Both Index,” or “Right Leg.” The required movements for each effector were as in experiments 1 and 2.
Data and Statistical Analysis
The EMG data were analyzed offline using customized routines within MATLAB, as well as visual inspection of individual traces to identify artifacts. From the EMG data, we extracted two dependent variables: the peak-to-peak amplitude of the MEP (left FDI in experiments 1 and 2; right TA in experiment 3) and the RT. To prevent contamination of the MEP measurements by fluctuations in background EMG, trials were excluded if the background EMG activity was >0.01 mV in the 200-ms window preceding the TMS pulse (Duque et al. 2014; Quoilin et al. 2016; Wilhelm et al. 2016). We also excluded MEPs that were above or below 3 SD of the mean MEP amplitude for that condition, as well as those in which there was EMG activity associated with a noncued response (selection errors). Overall, 9% of the trials (SD 2%) were excluded from the analysis (~50% of these were due to the outlier exclusion criterion).
The mean MEP values were calculated for the TMS baseline and delay probes, with the latter calculated separately for each cued effector. To assess CS excitability changes during response preparation, we subtracted the mean delay period MEPs from the mean baseline MEPs on an individual basis and normalized these values by dividing the difference by the mean baseline value. The scores were multiplied by 100 to express as percentage scores, with negative values indicative of preparatory inhibition. Given that many studies have confirmed the existence of preparatory inhibition (for reviews see Bestmann and Duque 2016; Duque et al. 2017), one-tailed t-tests were used in within-condition comparisons to evaluate whether the MEPs were inhibited relative to baseline (i.e., comparison of the normalized scores for each condition to the null hypothesis that the scores would be distributed around zero). The Shapiro-Wilk’s test was used to assess if the scores for a given condition met the normality assumption. When this test indicated a violation of the normality assumption, we analyzed the data with the nonparametric Wilcoxon signed-rank test.
For comparisons of the preparatory MEP changes between conditions, we used repeated-measures analyses of variance (ANOVARM), with post hoc tests based on the Bonferroni method, adjusted for multiple comparisons. When the contrast included a condition that violated the assumption of normality, we used the nonparametric Friedman test, with the Wilcoxon signed-rank test for post hoc comparisons. The post hoc tests in experiments 1 and 2 were two-tailed because we did not have strong a priori hypotheses. In experiment 3, a one-tailed test was employed given that the results of the first two experiments led to a test of a specific hypothesis. Effect sizes are reported using partial eta-squared () for the ANOVA and Cohen’s d for the planned contrasts in which the data met the normality assumption. For the nonparametric Wilcoxon signed-rank test, the effect size r was calculated as (Rosenthal 1991). The reported P values for these are adjusted for multiple comparisons.
RT was defined as the time interval between the onset of the imperative signal and the time point at which the EMG activity of the agonist muscle for the cued response exceeded 3 SD of the mean of the rectified signal for the entire trial epoch. ANOVARM were also used to analyze these data.
RESULTS
CS Excitability
The goal of this study was to explore constraints on preparatory inhibition. We assessed whether changes in CS excitability observed during the delay period varied as a function of the effectors involved in the task and their anatomical relationship with the muscle probed with TMS. To assess whether CS excitability was inhibited during the preparatory period, MEPs elicited during the delay period were compared with MEPs elicited at baseline (i.e., trial onset). A summary of these within-condition comparisons for all three experiments is presented in Table 1.
Table 1.
Within-condition test of preparatory inhibition for all three experiments
| Cued Effector | t(11) or Z Value | P Value |
|---|---|---|
| Experiment 1: left FDA MEPs | ||
| Relevant choice | ||
| L index* | 3.06 | 0.001 |
| R index | 8.98 | 0.000 |
| L index | 7.05 | 0.000 |
| L pinky | 6.76 | 0.000 |
| L index | 3.97 | 0.001 |
| R leg | 4.83 | 0.000 |
| Irrelevant choice | ||
| L pinky | 6.20 | 0.000 |
| R pinky | 5.67 | 0.000 |
| L leg | 3.40 | 0.003 |
| R leg | 2.05 | 0.032 |
| Irrelevant simple | ||
| L pinky | 10.01 | 0.000 |
| L leg | 2.82 | 0.008 |
| R leg | 1.81 | 0.048 |
| Experiment 2: left FDI MEPs | ||
| Relevant choice | ||
| Both index | 4.95 | 0.000 |
| L index* | 3.06 | 0.001 |
| R index* | 2.75 | 0.003 |
| Both index | 10.08 | 0.000 |
| Eye* | 2.00 | 0.020 |
| Both index | 6.15 | 0.000 |
| Mouth | 1.60 | 0.069 |
| Irrelevant choice | ||
| Eye* | 1.53 | 0.126 |
| Mouth | 0.47 | 0.468 |
| Experiment 3: right TA MEPs | ||
| Relevant choice | ||
| L index | 1.80 | 0.049 |
| R leg | 3.59 | 0.002 |
| Relevant simple | ||
| R leg | 3.23 | 0.004 |
| Irrelevant choice | ||
| Both index | −0.22 | 0.415 |
| Left index | −0.21 | 0.419 |
| R index* | −0.71 | 0.237 |
| Irrelevant simple | ||
| L leg* | 1.49 | 0.068 |
| R arm | 1.38 | 0.097 |
| R index | 2.19 | 0.026 |
| L index | 1.28 | 0.116 |
| Mouth | −0.81 | 0.217 |
Data are results of a within-condition test of preparatory inhibition for all 3 experiments, operationalized as the normalized change in motor-evoked potential (MEP) during the delay period relative to the baseline period [(MEPbase – MEPdelay)/MEPbase]. The comparisons were conducted with one-tailed t-tests, motivated by prior studies showing an attenuation of MEPs during the delay period. FDI, first dorsal interosseous; L, left; R, right; TA, tibialis anterior.
Conditions in which the sample distribution deviated from normality (Shapiro-Wilk test). For these conditions, the Z statistic and corresponding P value from the nonparametric Wilcoxon signed-rank test are presented.
Experiment 1.
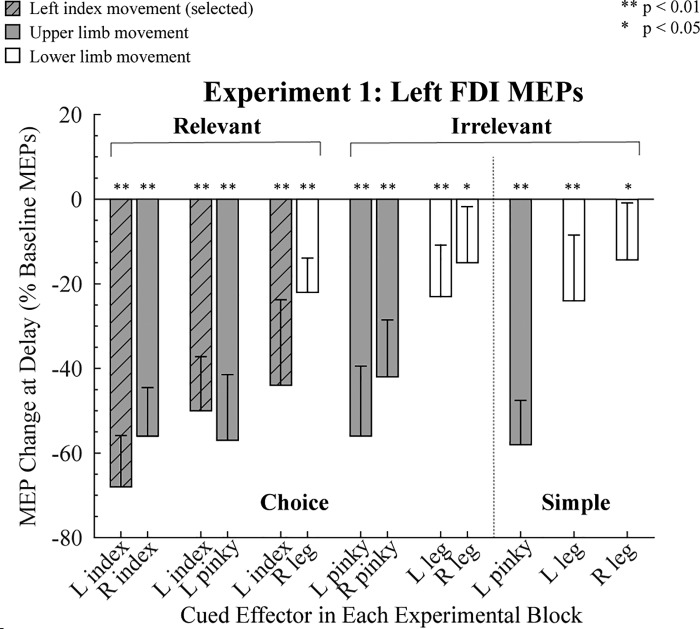
Baseline MEPs for the left FDI averaged 1.02 mV (SD 1.02). Relative to this baseline level, MEPs elicited in the delay period were attenuated in all conditions in which the cue indicated that the participant should prepare a finger movement (all P < 0.01; Fig. 2). A similar pattern was present when the cue indicated a leg movement (all P < 0.05). Thus we observed broad suppression of cortical excitability during response preparation (Greenhouse et al. 2015b), evident when the targeted muscle was part of the task set (choice relevant), in most of the conditions in which the muscle was not part of the task set (choice irrelevant), and even when there was no choice (simple irrelevant RT conditions).
Fig. 2.
Modulation of motor-evoked potentials (MEPs) in experiment 1. MEPs recorded from left first dorsal interosseous (FDI) during the delay period are expressed as a percentage of baseline (0%). Shaded bars indicate trials in which an upper limb movement was cued, and open bars indicate trials in which a lower limb movement was cued. Hatched gray bars indicate when left FDI was the agonist for the forthcoming response. Error bars indicate 95% confidence intervals, depicting if the MEP change during the delay period, relative to baseline, was significantly different from zero (1-tailed test). L, left; R, right.
To compare the strength of preparatory inhibition between the different experimental conditions, we used a 3 (task: choice relevant, choice irrelevant, and simple irrelevant) × 2 (effector: left pinky and right leg) ANOVARM. We focused on these two effectors because they were included in each of the three types of tasks; the left leg and index fingers were not included in the relevant and irrelevant conditions and thus could not be used to test the effect of relevance. The effect of effector was significant [F(1,11) = 42.53, P < 0.01, = 0.79], but there was no effect of task [F(2,22) = 3.43, P = 0.71, = 0.03], nor was there an interaction between these factors [F(2,22) = 0.40, P = 0.67, = 0.03]. The degree of MEP suppression in left FDI was greater when the cued action required a left pinky movement compared with when it required a right leg movement [mean difference = −39.8% (4.4), P < 0.01, Cohen’s d = 6.87]. These results indicate that the demands on response selection (choice vs. simple) and task relevance do not influence the level of preparatory inhibition. However, the magnitude of MEP suppression varied as a function of the movements forming the response set. We recognize that including the left pinky finger and right leg in the first analysis confounds body segment (upper limb vs. lower limb) and body side (left vs. right). Given this confound, we performed separate analyses ANOVARM for each of the tasks (relevant choice, irrelevant choice, irrelevant simple), including in each ANOVA all of the conditions for the task under consideration (see Fig. 1).
For the relevant choice conditions (Fig. 2, left) we first focused on the three conditions in which the left index finger was cued (selected). The degree of MEP suppression in left FDI varied as a function of the other, nonselected member of the response set (χ2 = 8.21, P = 0.02). In terms of the post hoc comparisons, the only reliable difference was that there was stronger suppression of the left FDI when it was paired with the homologous right index compared with when it was paired with the left pinky (Z = −2.51, P = 0.03, r = −0.51). Thus MEP suppression was greatest in the selected muscle when the choice involved homologous muscles. Second, we examined MEP suppression of left FDI when the left index was not cued (nonselected) in the choice conditions. Here suppression of left FDI MEPs was weaker when the cued movement was the right leg compared with when the cued movement was either the right index finger (P < 0.01, Cohen’s d = 1.81) or left pinky (P < 0.01, Cohen’s d = 1.49). Hence, the amount of left FDI suppression when the left index finger was not selected was stronger when the selected effector was a hand muscle compared with when it was a leg muscle (intrasegmental vs. intersegmental).
Additional comparisons of anatomy can be made with the data from the irrelevant conditions in which the left index finger is not part of the response set. For the choice irrelevant conditions (Fig. 2, middle), we conducted a 2 × 2 ANOVARM with the factors body side (left, right) and effector (pinky, foot). There was a main effect for effector [F(1,11) = 14.88, P < 0.01, = 0.57], with greater MEP suppression of left FDI when the choice was between two finger movements compared with two leg movements [mean difference = −30% (SD 8)]. The effect of body side was marginally significant [F(1,11) = 4.82, P = 0.05, = 0.30], with MEP suppression greater when the forthcoming response was on the left side compared with the right side. The interaction was not significant [F(1,11) = 0.87, P = 0.37, = 0.07]. In the simple irrelevant conditions (Fig. 2, right), a 1-way ANOVARM with the factor competing effector (left pinky, left leg, right leg) was significant [F(2,22) = 11.44, P < 0.01, = 0.51]. Post hoc tests showed that left FDI MEP suppression was stronger when participants prepared a left pinky movement compared with a left (P < 0.01, Cohen’s d = 1.35) or right (P < 0.01, Cohen’s d = 3.01) leg movement. For the two leg movement conditions, there was no effect of body side (P = 0.35, Cohen’s d = 1.34).
In summary, the results of experiment 1 indicate that preparatory inhibition of left FDI is greatest when this muscle is the agonist for the selected response compared with when it is nonselected, replicating earlier results (e.g., Duque and Ivry 2009; Labruna et al. 2014). In terms of our primary question concerning the spread of preparatory inhibition, the magnitude of left FDI MEP suppression was greater when the response set was restricted to finger movements compared with when the response set included a leg muscle. MEP suppression also tended to be greater when the cued response was on the left side of the body compared with when it was on the right side of the body, although this effect was not systematic.
Experiment 2.
The observations made in experiment 1 are consistent with the hypothesis that the reduced excitability is related to anatomical similarity: MEPs in a hand muscle showed greater suppression when the cued response involved a hand movement compared with when the cued response involved a leg movement. In experiment 2, we further explored anatomical constraints on preparatory inhibition, measuring MEPs in left FDI while people prepared finger movements or facial gestures.
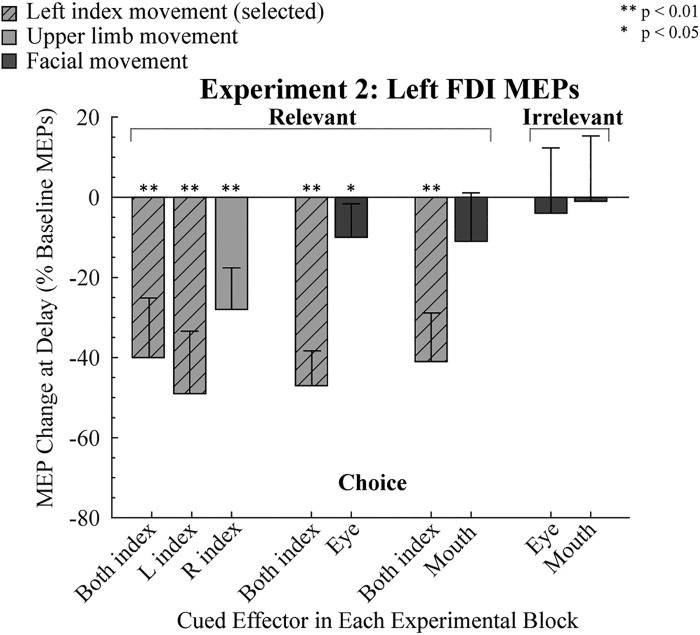
Baseline MEPs for the left FDI averaged 0.82 mV (SD 0.51). As in experiment 1, MEPs elicited in the delay period were attenuated in all conditions in which the cue indicated that the participant should prepare a finger movement (all P < 0.01; Fig. 3). In contrast, when the participants prepared a facial movement, preparatory inhibition in left FDI was only significant in the condition in which the eye movement was prepared in the choice context (relevant task, P = 0.02; see Table 1).
Fig. 3.
Modulation of motor-evoked potentials (MEPs) in experiment 2. MEPs recorded from left first dorsal interosseous (FDI) during the delay period are expressed as a percentage of baseline (0%). Light shaded bars indicate trials in which an upper limb movement was cued, and dark shaded bars indicate trials in which a facial movement was cued. Hatched light shaded bars indicate when left FDI was the agonist for the forthcoming response. Error bars indicate 95% confidence intervals, depicting if the MEP change during the delay period, relative to baseline, was significantly different from zero (1-tailed test). L, left; R, right.
To compare preparatory inhibition between conditions, we first focused on the condition in which the response set was limited to finger movements (Fig. 3, left). Given that the MEP values in a number of conditions violated the normality assumption (see Table 1), the nonparametric Friedman test was used to compare MEP suppression in left FDI when the cued response was for a left index, right index, or bimanual index finger response. There were no significant difference between the three conditions (χ2 = 5.17, P = 0.08), and planned comparisons showed that the magnitude of MEP suppression in the bimanual condition did not differ from either unimanual condition (left: Z = 1.69, P = 0.38, r = 0.34; right: Z = 1.77, P = 0.16, r = 0.36). The main result to be taken from these analyses is that preparatory inhibition is similar in the bimanual condition compared with the unimanual conditions. We saw this as a prerequisite for the analysis of the facial movement conditions given that the facial gestures are produced bilaterally.
We next compared the three conditions in which participants were cued to prepare a bimanual response (e.g., selected). MEP suppression of left FDI was similar across the conditions (χ2 = 0.129, P > 0.94), indicating that the strength of preparatory inhibition was similar when the competing response required a hand or facial movement. However, when the left index finger was not selected, MEP suppression differed across the three conditions (χ2 = 6.25, P > 0.04), with the post hoc comparisons indicating that left FDI was more inhibited when the cue indicated a right index finger movement compared with when the cue indicated an eye movement (Z = 2.51, P = 0.03, r = −0.51). A similar pattern was observed when the cue indicated a mouth movement, but this comparison did not approach significance (Z = 1.84, P = 0.18, r = 0.38). There was no difference between the mouth and eye movement conditions (Z = 0.27, P = 2.37, r = 0.06). Thus the results suggest that the suppression of left FDI is reduced when the participants prepared a facial movement. This conclusion is further supported when the results from the irrelevant conditions are considered (Fig. 3, right). As noted above in the within-condition results, left FDI MEPs in the delay period were not significantly reduced, relative to baseline, when participants had to choose between a mouth or eye movement, and there was no difference between these conditions (Z = −0.55, P = 0.58, r = 0.11; Fig. 3, right).
In summary, the results of experiment 2 provide further evidence that the degree of preparatory inhibition varies as a function of the members of the response set. MEP suppression of a hand muscle was greater when the cued response was for a finger movement compared with when the cued response was for a facial movement. In a comparison of the two types of facial responses, we did not observe greater MEP suppression when the participants prepared an eye movement, a strong test of the cortical distance hypothesis. We recognize that the distance from the hand area to the face area may be greater than the extent of preparatory inhibition, an issue we return to in the discussion. Nonetheless, with this caveat in mind, the results of the first two experiments indicate that the spread of preparatory inhibition is strong within a body segment and weak or absent between segments.
Experiment 3.
The results of experiments 1 and 2 showed that preparatory inhibition in a finger muscle is much larger when the cued response entails an upper limb movement compared with when the cued response entails a different body segment (lower limb or facial). To ensure that these effects are not specific to upper limb movements, we reversed the situation in experiment 3, measuring MEPs in a leg muscle while participants prepared movements of a leg, finger, or mouth. We opted to stimulate over the left hemisphere, targeting the TA muscle in the right leg. This allowed us to include exact replications of conditions from experiment 1 (choice: left index/right leg; simple: left leg), but now with preparatory inhibition probed in a lower limb. As noted above, we only included participants in the main experiment for whom we were able to reliably elicit MEPs in right TA. For these participants, the mean MEPs during baseline were 0.22 mV (SD 0.09), a value that is considerably lower than that for baseline MEPs elicited in FDI in experiments 1 and 2. Nonetheless, we did observe MEPs of at least 0.05 mV on 90% of the trials in the baseline period.
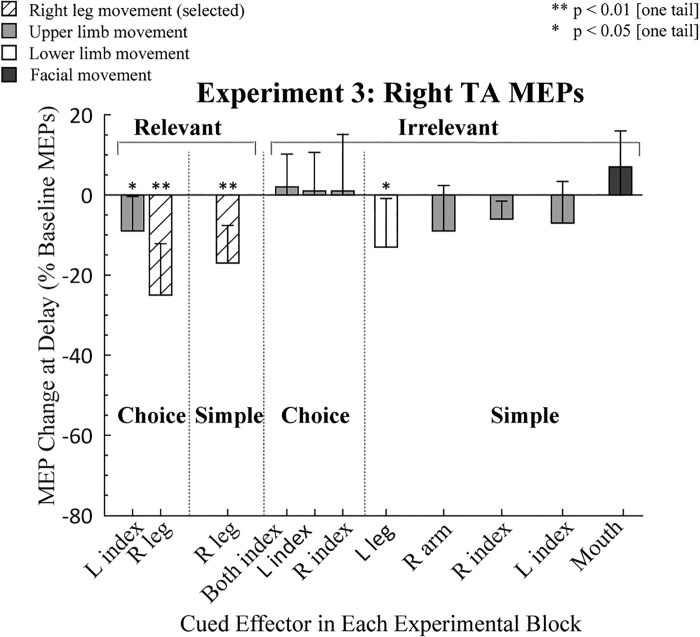
As in the first two experiments, we first conducted within-condition t-tests to assess preparatory inhibition for each condition (Table 1). MEPs elicited in right TA during the delay period were significantly reduced in the two choice conditions in which the participants prepared a lower limb movement (all P < 0.05; Fig. 4). A similar trend was observed in the simple irrelevant RT condition (P = 0.07). In contrast, MEP suppression during the delay period was only observed in two of the seven conditions when an upper limb movement was prepared (left index relevant choice and right index irrelevant simple, both P < 0.05) and was not significant when a mouth response was prepared. Thus preparatory inhibition in right TA was robust when participants prepared a leg movement (right or left leg) but inconsistent or absent when an upper limb movement or facial gesture was prepared.
Fig. 4.
Modulation of motor-evoked potentials (MEPs) in experiment 3. MEPs recorded from right tibialis anterior (TA) during the delay period are expressed as a percentage of baseline (0%). Light shaded, open, and dark shaded bars indicate trials in which the cued response required an upper limb, lower limb, or facial movement, respectively. Hatched open bars indicate when right TA was the agonist for the forthcoming response. Error bars indicate 95% confidence intervals, depicting if the MEP change during the delay period, relative to baseline, was significantly different from zero (1-tailed test). L, left; R, right.
Turning to the between-condition comparisons, preparatory inhibition in right TA was greater when that muscle was selected for the forthcoming response compared with when it was not selected [choice relevant: t(12) = 3.14, P = 0.01, Cohen’s d = 1.19]. No differences were found when the right leg was selected as part of either a choice or a simple task [t(12) = −1.04, P = 0.32, Cohen’s d = 0.35], consistent with the results of the first experiment, indicating that preparatory inhibition is independent of the task context.
For the irrelevant conditions, we conducted three analyses to compare preparatory inhibition in the right TA when the cued response a different lower limb effector to conditions in which the cued response was from another body segment. For the former, we used left leg movements; for the latter, the cued response either involved upper limb effectors or the mouth. First, we compared the left leg condition to the upper limb condition, taking the average of the three upper limb effectors in the choice condition. This contrast was significant (Z = −2.20, P = 0.03, r = 0.45,), with greater MEP suppression in right TA when the selected limb was from the same body segment. The second contrast was between the left leg and the average of the three upper limb effectors in the simple conditions. Here the difference was not significant (Z = −0.39, P = 0.7, r = 0.08). The third contrast, between the left leg and mouth, approached significance (Z = −1.82, P = 0.07, r = 0.37).
Overall, the results of experiment 3 are consistent with the idea that anatomical constraints on preparatory inhibition are not specific to upper limb muscles but also hold for lower limb muscles. This prediction was supported by two of the contrasts of different body segments; it was not supported by the third (lower vs. upper segment, simple conditions). We note that our sensitivity in this experiment is reduced given the relatively low MEPs elicited from right TA.
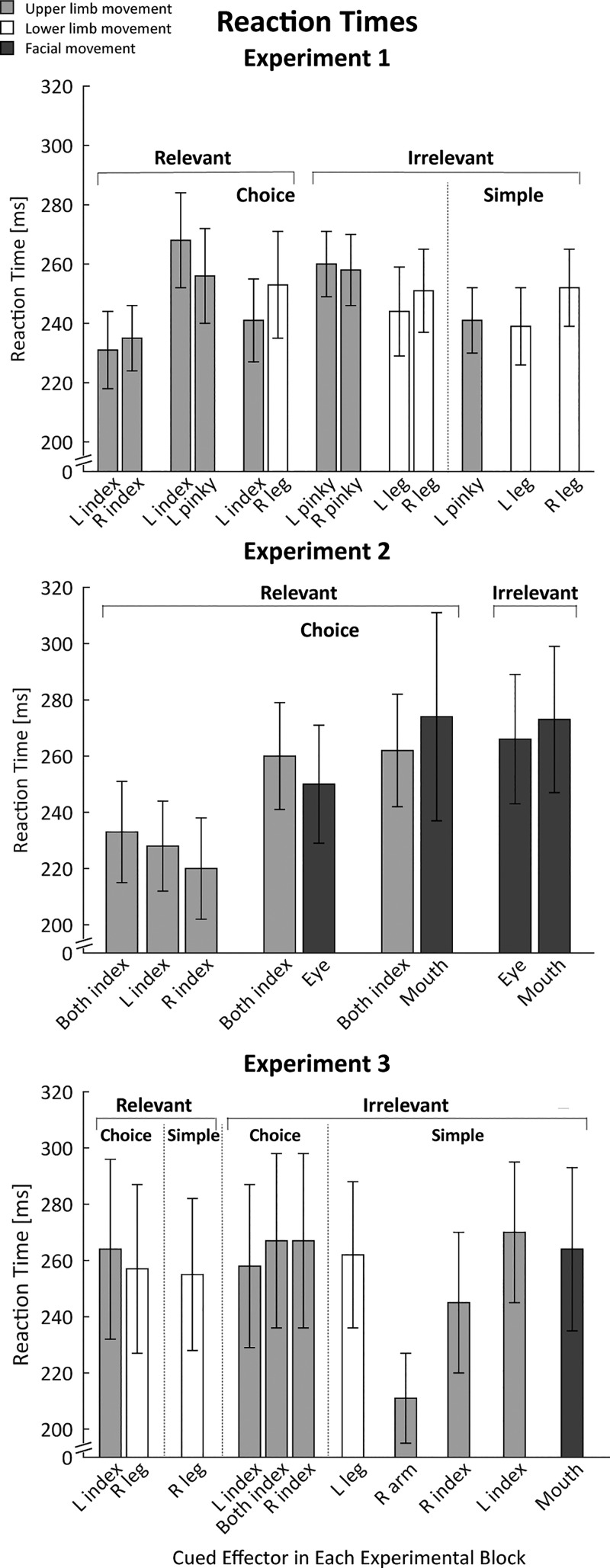
Reaction Times
RTs were relatively fast (around 250 ms), indicating that the participants had used the cues to prepare the forthcoming response during the delay period (Fig. 5). This is most clearly evident in the comparison of choice and simple RTs for each effector in experiments 1 and 3: Mean RTs in the choice RT conditions were similar to those observed in the simple RT conditions. The difference scores ranged from 0 to 22 ms, and even the largest difference (experiment 3, right index finger) was not significant (P = 0.35). RTs were also similar on trials in which the TMS pulse was applied just before the start of the trial (baseline) or when applied during the delay period in all three experiments (P > 0.10), with data collapsed across conditions.
Fig. 5.
Reaction times for experiments 1–3, with data combined from trials in which transcranial magnetic stimulation was applied at baseline and during the delay period. Light shaded, open, and dark shaded bars indicate trials in which the cued response required an upper limb, lower limb, or facial movement, respectively. Values are means; error bars are SE. L, left; R, right.
There were some effector-specific effects on RT. For example, we can compare left- and right-side RTs for the index finger, pinky, and leg in three choice conditions in experiment 1 (Fig. 5, top). Mean RTs were fastest for index finger movements [233 ms (SD 12)], followed by leg movements [247 ms (SD 15)], and slowest for pinky movements [259 ms (SD 10)]. However, a 3 (effector) × 2 (side) ANOVARM showed that these differences were not significant (all P > 0.14).
In the choice RT conditions, the RT for a given effector was modulated by the other member of the response set. For example, a 1-way ANOVARM on the RTs for the left index finger in the three choice conditions showed a main effect [F(2,22) = 7.60, P < 0.01, = 0.58], with slower RTs when the left index finger movement was paired with the pinky of the same hand compared with when it was paired with the right index finger (P = 0.01, Cohen’s d = 2.53) or with the right leg (P = 0.03, Cohen’s d = 1.82). This pattern suggests that the participants adopted, to some degree, a task set in which the speed of movement initiation for a given condition was relatively constant for each choice, adjusted to the rate of the slower member of the response pair.
A similar pattern was evident in experiment 2 (Fig. 5, middle). RTs were slower for the facial gestures compared with the finger responses. When analysis focused on the 3-choice condition that involved bimanual responses (averaging RTs over left and right fingers because the responses were tightly coupled), finger RTs were slower in blocks in which these responses were paired with facial responses than with a unimanual finger response (mean difference with eye 27 ms (SD 49), and with mouth 29 ms (SD 48), although the ANOVARM showed only a marginal effect (P = 0.07, = 0.22).
In experiment 3 (Fig. 5, bottom), finger RTs in the choice conditions were relatively invariant, with no advantage in conditions in which all responses were with the fingers compared with when a finger and leg response were paired. At first glance, RTs in experiment 3 were slower than in the first two experiments. In a post hoc analysis, we compared RTs for the left index finger across experiments, focusing on this finger because it was the only effector paired in all three experiments with another upper limb effector. The outcome of this 1-way ANOVA was not significant (P = 0.52, = 0.38).
RT was not related to the magnitude of the MEPs (see Duque et al. 2017 for a discussion on this issue), similar to what has been observed in previous studies (but see Hannah et al. 2018). This can be seen in a comparison between conditions: for instance, in experiment 2, bimanual RTs tended to be slower when paired with facial responses than when paired with unimanual finger responses, but MEPs elicited from the left FDI were relatively invariant across conditions. Even more compelling, it was not observed in a trial-by-trial analysis performed on an individual basis. When data were pooled across conditions involving the left index finger, there was no consistent pattern of correlation between RT and MEP for the left index finger.
DISCUSSION
Preparing to move entails the recruitment of inhibitory mechanisms. This preparatory inhibition is evidenced by the attenuation of MEPs elicited during a delay period when participants prepare to initiate a cued response. Several studies have identified constraints on the magnitude of this phenomenon; for example, the degree of MEP suppression is modulated by task difficulty (Beck and Hallett 2010; Greenhouse et al. 2015a; Klein et al. 2014). These findings indicate that preparatory inhibition is not generic. In the current study, we extend this work, systematically examining anatomical constraints on preparatory inhibition.
Anatomical Constraints on Preparatory Inhibition
Consistent with previous findings, preparatory inhibition was generally greatest when the targeted muscle was the agonist for the forthcoming movement. Moreover, the magnitude of MEP suppression for the selected conditions was independent of the other member of the response set. This was most evident in experiment 2, where MEP suppression in the left FDI was similar across choice conditions in which the left index finger was paired with the right index finger or paired with an eye or mouth movement.
A different pattern was observed when the cue indicated a response other than the left index finger. Preparatory inhibition in left FDI was pronounced if that effector was from the same body segment (e.g., another manual response) but much weaker if the cued effector was from a different body segment. In experiment 1, the mean level of MEP suppression, relative to baseline, was −42% when the cued response involved another finger movement and only −20% when the cued response involved a leg movement. Similarly, in experiment 2, MEPs were reduced by −28% when the cue indicated a right index finger movement and only reduced by −8% when the cue indicated a facial movement. Indeed, in the latter experiment, mean MEP amplitudes were not significantly different from baseline in three of the conditions involving facial responses.
This pattern was similar for conditions in which the left index finger was relevant or irrelevant. Moreover, the magnitude of preparatory inhibition did not depend on whether the cue required a decision between alternative responses (choice conditions) or always specified the same response (simple conditions). For example, on trials in experiment 1 in which the planned response was with the left pinky finger, MEP suppression of left FDI was similar when the left index finger was part of the response set or not part of the response set. Consistent with the results reported in Greenhouse (2015b), the magnitude of preparatory inhibition does not appear to depend on task relevance or choice behavior.
Taken together, the results of experiments 1 and 2 indicate that the magnitude of preparatory inhibition targeted at nonresponding effectors is greater when the planned response is from the same body segment (e.g., hand) compared with when it entails a different body segment (leg or face). To test the generality of this hypothesis, the TMS probe was directed at right TA, the agonist for adduction movements of the lower leg, in experiment 3. Here we also included conditions in which the response set either included or did not include the right leg. The pattern was similar to that observed in experiments 1 and 2. MEPs from right TA were significantly suppressed during the delay period when the cue called for the preparation of either a right or left leg movement. In contrast, MEP suppression of right TA was reduced or absent when the cue indicated a hand, arm, or facial movement.
Qualitatively the magnitude of preparatory inhibition appears to be lower for right TA compared with left FDI. We are hesitant to draw any inferences concerning this pattern. First, this between-experiment comparison confounds side and segment, given our decision to focus on right TA. Second, although we normalize our measure of preparatory inhibition by expressing the change in the delay period relative to baseline, it is important to keep in mind that MEPs are much more difficult to obtain from leg muscles, and when obtained, are weaker than those elicited from FDI (Kesar et al. 2018). Most important, the claims about intra- vs. intersegment differences are evident in the within-experiment comparisons where the TMS probes are always restricted to the same muscle.
Anatomy vs. Function
We interpret the current results to indicate that the extent of preparatory inhibition is constrained by anatomy, dropping in strength when the distance between the selected effector and the muscle targeted by TMS is increased. One variant of this distance hypothesis is that the extent of preparatory inhibition may be related to the motor homunculus. Experiment 2 was designed to test this hypothesis, building on the fact that the hand area is closer to the cortical representation of the eyes compared with the cortical representation of the mouth. The results of experiment 2 failed to support this strong version of the cortical distance hypothesis: when either a squint or smile was planned, there was minimal change in left FDI MEPs, and numerically, the small effects were comparable for the two types of facial gestures.
However, there are a number of caveats to keep in mind when considering the cortical distance hypothesis. First is the general concern with all null results. Second, although the eye representation is closer to the hand area, the distance is still relatively large, at least in comparison to the distance between finger representations (Meier et al. 2008; Weiss et al. 2013). It may be that the spread of excitability changes does follow a cortical gradient, but that it is negligible beyond some maximal distance. A finer-grained analysis would be required to test the cortical distance hypothesis; for example, compare the magnitude of preparatory inhibition in left FDI in conditions in which the cue specifies a finger, wrist, lower arm, or upper arm movement.
The current results do reveal a consistent difference between conditions in which the planned movement is from the same body segment (lower, upper, face) or a different body segment, with the former producing greater reduced excitability in the probed muscle. Rather than these effects being attributed to the cortical distance of motor representations, the difference may reflect the synergistic recruitment of intrasegmental representations. The motor homunculus visualized across the motor cortex is recognized as a simplification given that there is considerable overlap between motor representations. Indeed, it has been proposed that a clear spatial separation is limited to representations of different body segments (Schieber 2001; Zeharia et al. 2012). By this view, the interactions within a segment in terms of preparatory inhibition could arise from the fact that the fingers of one hand, or even fingers between two hands, are frequently coactivated for a given movement. Preparatory inhibition might extend to effectors within the same body segment as the cued one to reduce activation of muscles that are close in cortical space to the agonist for the forthcoming movement.
There are well-defined movements that do involve intersegmental coordination. For example, when one reaches for objects, the eyes and hands move in a coordinated manner, and some of the ethological gestures described by Graziano and colleagues (Desmurget et al. 2014; Fernandino and Iacoboni 2010; Graziano 2016) involve coordinated movements between the upper limbs and face (e.g., eating). The fact that our results failed to reveal consistent MEP suppression in FDI when facial gestures were being prepared argues against these function-based hypotheses. Similarly, in a preliminary study (Labruna et al. 2016), we tested experienced drummers to see if they showed greater preparatory inhibition in an upper limb when preparing a leg movement given that drumming requires extensive intersegmental coordination. The data from this group were similar to those reported currently, with minimal MEP suppression of FDI when the drummers prepared a leg movement. In summary, the present picture suggests that the spread of preparatory inhibition is best defined in terms of a segmental criterion, rather than one based on functional considerations.
Implications for Models of Preparatory Inhibition
A recent review by Duque at al. (2017) summarizes three functional models of preparatory inhibition. The first of these models suggests that inhibition is restricted to task-relevant muscles, reflecting a competition between candidate effectors (Duque et al. 2005, 2010). The second model suggests preparatory inhibition arises from the operation of two processes, one producing a global or broad inhibitory effect and the other focused at only the selected response representation. The third model emphasizes a single process that operates in the form of a “spotlight” centered over the selected response representation with the width of the aperture constrained by the task context, such as whether or not selection entails a choice (Greenhouse et al. 2015b). According to the spotlight model, inhibition, or reduced excitation, facilitates the selection and initiation of motor responses by reducing background noise and, thus, increasing the gain within the motor system.
We observed preparatory inhibition, independent of whether the probed muscle was part of the response set or was task irrelevant. Moreover, we also observed robust MEP suppression when the probed muscle was the sole member of the response set. These findings are at odds with the competition model, because competition is absent in the task-irrelevant conditions and simple conditions. In contrast, the two-process and spotlight models are consistent with the current findings, although we suggest an additional anatomical constraint on preparatory inhibition. A spotlight might operate at the level of body segments, with the strongest influence over the body segment that includes the selected response representation and negligible effect on representations from other body segments. With respect to the two-process model, the current results would indicate that the process producing a broad reduction of excitability is not generic. Rather, its extent appears to be categorical and mostly limited to muscles within the same body segment as the agonist effector. The notion of a categorical constraint based on body segment, however, should be qualified given that we may lack the sensitivity to detect effects in the tail of a gradient, one that spans large cortical distances.
In terms of function, the current data do not differentiate hypotheses that focus on how preparatory inhibition might prevent premature responses or facilitate gain modulation during response planning. Future work may be able to capitalize on the spatial constraints identified here to better address functional questions.
Relationship of Anatomical Constraints in Preparatory and Reactive Inhibition
TMS has been used to characterize the dynamics of cortical excitability in tasks involving reactive inhibition, such as the stop-signal task in which a planned response is aborted. One prominent idea is that when the stop signal requires the termination of all volitional movement (where the planned response involves one or more effectors), the inhibitory signal is broadcast in a global manner, manifest in both task-relevant and task-irrelevant muscles (Badry et al. 2009; Coxon et al. 2006; Greenhouse et al. 2012; Leocani et al. 2000; Majid et al. 2012). Most relevant to the present discussion, reactive inhibition is seen in both intra- and intersegmental muscles.
Superficially, it may appear that preparatory and reactive inhibition arise from different processes given that we find, at best, modest preparatory inhibition between body segments, whereas reactive inhibition tasks point to a global process. However, it remains unclear if the TMS data provide strong evidence of a difference between preparatory and reactive inhibition. Similar to the effects observed here, the magnitude of reactive inhibition in task-irrelevant muscles is much larger for intrasegmental muscles compared with intersegmental muscles. For example, Badry et al. (2009) used TMS to elicit MEPs in either the thumb or leg after a stop signal had indicated that the participants should abort an index finger response. Relative to baseline, thumb MEPs were reduced by close to 50%, whereas leg MEPs were only reduced by 15% (see also Greenhouse et al. 2012; Majid et al. 2012). Similarly, stopping speech resulted in only a 15% reduction in hand MEPs (Cai et al. 2012).
In sum, the stop-signal literature also points to a gradient in the extent of reactive inhibition, similar to that observed in the current study with preparatory inhibition, with only weak changes in CS excitability when the probed muscle is at a different segmental level from the task-relevant effector. This observation by itself offers only weak evidence for a common mechanism underlying preparatory and reactive stopping. Future studies can be designed to provide more direct tests. Whereas studies using a range of methods have detailed a cortico-basal ganglia circuit recruited for reactive stopping, similar work is needed to understand the networks that result in preparatory inhibition.
Conclusions
The three experiments reported in this article provide converging evidence that preparatory inhibition is constrained by anatomy. A marked reduction in corticospinal excitability was observed when the response involved a muscle from the same body segment, and was reduced or even absent when the response involved a muscle from a different body segment. These results are consistent with models in which an inhibitory process is targeted at specific motor representations, with a spatial extent limited to motor representations within the same body segment.
GRANTS
This work was supported by Belgian National Funds for Scientific Research Grant MIS F.4512.14, the Fondation Médicale Reine Elisabeth, National Institute of Neurological Disorders and Stroke Grants NS092079 and NS097480, and the France-Berkeley fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.L. and R.B.I. conceived and designed research; C.T. and C.C. performed experiments; L.L., C.T., and C.C. analyzed data; L.L., I.G., J.D., F.L., and R.B.I. interpreted results of experiments; C.T. and L.L. prepared figures; L.L. drafted manuscript; I.G., J.D., F.L., and R.B.I. edited and revised manuscript; L.L. and R.B.I. approved final version of manuscript.
ACKNOWLEDGEMENTS
We thank Simone Ewell-Szabo for drawing Fig. 1A.
REFERENCES
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin Neurophysiol 120: 1717–1723, 2009. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol 121: 98–103, 2010. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Duque J. Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist 22: 392–405, 2016. doi: 10.1177/1073858415592594. [DOI] [PubMed] [Google Scholar]
- Biabani M, Farrell M, Zoghi M, Egan G, Jaberzadeh S. The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci Lett 674: 94–100, 2018. doi: 10.1016/j.neulet.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, Aron AR. Stopping speech suppresses the task-irrelevant hand. Brain Lang 120: 412–415, 2012. doi: 10.1016/j.bandl.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Pavesi G. The facial motor system. Neurosci Biobehav Rev 38: 135–159, 2014. doi: 10.1016/j.neubiorev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Cavaleri R, Schabrun SM, Chipchase LS. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis. Syst Rev 6: 48, 2017. doi: 10.1186/s13643-017-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim Y-H, Pascual-Leone A. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol 127: 2892–2897, 2016. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos I, Duque J, Cisek P. Rapid prediction of biomechanical costs during action decisions. J Neurophysiol 112: 1256–1266, 2014. doi: 10.1152/jn.00147.2014. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95: 3371–3383, 2006. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci 25: 3766–3774, 2007. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol 125: 1202–1212, 2014. doi: 10.1016/j.clinph.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Richard N, Harquel S, Baraduc P, Szathmari A, Mottolese C, Sirigu A. Neural representations of ethologically relevant hand/mouth synergies in the human precentral gyrus. Proc Natl Acad Sci USA 111: 5718–5722, 2014. doi: 10.1073/pnas.1321909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Greenhouse I, Labruna L, Ivry RB. Physiological markers of motor inhibition during human behavior. Trends Neurosci 40: 219–236, 2017. doi: 10.1016/j.tins.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex 19: 2013–2024, 2009. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Cazares C, Ivry RB. Dissociating the influence of response selection and task anticipation on corticospinal suppression during response preparation. Neuropsychologia 65: 287–296, 2014. doi: 10.1016/j.neuropsychologia.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci 30: 3793–3802, 2010. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex 15: 588–593, 2005. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Fernandino L, Iacoboni M. Are cortical motor maps based on body parts or coordinated actions? Implications for embodied semantics. Brain Lang 112: 44–53, 2010. doi: 10.1016/j.bandl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience 320: 205–209, 2016. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Graziano MS. Ethological action maps: a paradigm shift for the motor cortex. Trends Cogn Sci 20: 121–132, 2016. doi: 10.1016/j.tics.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J Neurophysiol 107: 384–392, 2012. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Saks D, Hoang T, Ivry RB. Inhibition during response preparation is sensitive to response complexity. J Neurophysiol 113: 2792–2800, 2015a. doi: 10.1152/jn.00999.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Sias A, Labruna L, Ivry RB. nonspecific inhibition of the motor system during response preparation. J Neurosci 35: 10675–10684, 2015b. doi: 10.1523/JNEUROSCI.1436-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Cavanagh SE, Tremblay S, Simeoni S, Rothwell JC. Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J Neurosci 38: 1264–1276, 2018. doi: 10.1523/JNEUROSCI.2869-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Stinear JW, Wolf SL. The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature: challenges and opportunities. Restor Neurol Neurosci 36: 333–348, 2018. doi: 10.3233/RNN-170801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Duque J, Labruna L, Ivry RB. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. Neuroimage 125: 220–232, 2016. doi: 10.1016/j.neuroimage.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Olivier E, Duque J. Influence of reward on corticospinal excitability during movement preparation. J Neurosci 32: 18124–18136, 2012. doi: 10.1523/JNEUROSCI.1701-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Petitjean C, Olivier E, Duque J. Top-down suppression of incompatible motor activations during response selection under conflict. Neuroimage 86: 138–149, 2014. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci 26: 269–278, 2014. doi: 10.1162/jocn_a_00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Tischler C, Levitin DJ, Dabit M, Greenhouse I, Lebon F, Ivry RB. The gradient level of inhibition in response preparation (Abstract). Program 436.06 Society for Neuroscience Annual Meeting, San Diego, CA, November 12–16, 2016. [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123: 1161–1173, 2000. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex 22: 363–371, 2012. doi: 10.1093/cercor/bhr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol 100: 1800–1812, 2008. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Lambert J, Jacob B, Klein P-A, Duque J. Comparison of motor inhibition in variants of the instructed-delay choice reaction time task. PLoS One 11: e0161964, 2016. doi: 10.1371/journal.pone.0161964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. Washington, DC: Sage, 1991. [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107, 2015. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol 86: 2125–2143, 2001. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Garry MI, Summers JJ. Cortical activation during temporal preparation assessed by transcranial magnetic stimulation. Biol Psychol 85: 481–486, 2010. doi: 10.1016/j.biopsycho.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Garry MI, Summers JJ. Selective suppression of the incorrect response implementation in choice behavior assessed by transcranial magnetic stimulation. Psychophysiology 48: 462–469, 2011. doi: 10.1111/j.1469-8986.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Specific somatotopic organization of functional connections of the primary motor network during resting state. Hum Brain Mapp 31: 631–644, 2010. doi: 10.1002/hbm.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Nettekoven C, Rehme AK, Neuschmelting V, Eisenbeis A, Goldbrunner R, Grefkes C. Mapping the hand, foot and face representations in the primary motor cortex - retest reliability of neuronavigated TMS versus functional MRI. Neuroimage 66: 531–542, 2013. doi: 10.1016/j.neuroimage.2012.10.046. [DOI] [PubMed] [Google Scholar]
- Wilhelm E, Quoilin C, Petitjean C, Duque J. A double-coil TMS method to assess corticospinal excitability changes at a near-simultaneous time in the two hands during movement preparation. Front Hum Neurosci 10: 88, 2016. doi: 10.3389/fnhum.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeharia N, Hertz U, Flash T, Amedi A. Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc Natl Acad Sci USA 109: 18565–18570, 2012. doi: 10.1073/pnas.1119125109. [DOI] [PMC free article] [PubMed] [Google Scholar]