Abstract
Corticospinal output pathways have typically been considered to be the primary driver for voluntary movements of the hand/forearm; however, more recently, reticulospinal drive has also been implicated in the production of these movements. Although both pathways may play a role, the reticulospinal tract is thought to have stronger connections to flexor muscles than to extensors. Similarly, movements involuntarily triggered via a startling acoustic stimulus (SAS) are believed to receive greater reticular input than voluntary movements. To investigate a differential role of reticulospinal drive depending on movement type or acoustic stimulus, corticospinal drive was transiently interrupted using high-intensity transcranial magnetic stimulation (TMS) applied during the reaction time (RT) interval. This TMS-induced suppression of cortical drive leads to RT delays that can be used to assess cortical contributions to movement. Participants completed targeted flexion and extension movements of the wrist in a simple RT paradigm in response to a control auditory go signal or SAS. Occasionally, suprathreshold TMS was applied over the motor cortical representation for the prime mover. Results revealed that TMS significantly increased RT in all conditions. There was a significantly longer TMS-induced RT delay seen in extension movements than in flexion movements and a greater RT delay in movements initiated in response to control stimuli compared with SAS. These results suggest that the contribution of reticulospinal drive is larger for wrist flexion than for extension. Similarly, movements triggered involuntarily by an SAS appear to involve greater reticulospinal drive, and relatively less corticospinal drive, than those that are voluntarily initiated.
NEW & NOTEWORTHY Through the use of the transcranial magnetic stimulation-induced silent period, we provide novel evidence for a greater contribution of reticulospinal drive, and a relative decrease in corticospinal drive, to movements involuntarily triggered by a startle compared with voluntary movements. These results also provide support for the notion that both cortical and reticular structures are involved in the neural pathway underlying startle-triggered movements. Furthermore, our results indicate greater reticulospinal contribution to wrist flexion than extension movements.
Keywords: cortical drive, reticulospinal tract, startle, transcranial magnetic stimulation
INTRODUCTION
Human movements are carried out via several descending pathways, although it has traditionally been held that motor preparatory and initiation-related neural activity for voluntary actions is predominantly cortically driven. Specifically, the corticospinal tract is believed to be the primary output pathway for most voluntary movements, in particular for distal movements and those requiring fine fractionation of small muscle groups (e.g., hand movements) (Baker 2011; Kuypers 1981). In contrast, the reticulospinal tract has been shown to contribute more strongly to more automatic movements such as locomotion (Matsuyama and Drew 2000) and postural adjustments (Schepens and Drew 2004), as well as playing an important role in voluntary movements of proximal muscles (Kuypers 1981). However, recent evidence suggests that the reticulospinal tract may also contribute to actions involving the muscles of the hand and fingers (Baker 2011; Lemon 2008; Ziemann et al. 1999). Specifically, it has been shown that direct stimulation of the reticulospinal pathway in anesthetized monkeys produces responses in motoneurons projecting to intrinsic hand muscles (Riddle et al. 2009). Similarly, stimulation of the reticulospinal tract results in activation of spinal interneurons involved in the control of the hand and fingers (Riddle and Baker 2010). Together, these results suggest that the reticulospinal tract may play a larger role in the control of voluntary movement than has been previously acknowledged.
Neural recordings from the reticular formation have provided evidence for reticular involvement in both the preparation and movement phases of reaching movements in monkeys (Buford and Davidson 2004). These reticular outputs tend to be bilateral in nature, though reticular involvement may differ between flexor and extensor muscles of the upper limb (Davidson and Buford 2006; Davidson et al. 2007). Specifically, although corticospinal axons innervate both flexor and extensor muscles predominantly contralaterally for all muscle groups, some research suggests that these connections tend to be stronger for the extensors (Cheney et al. 1991; McKiernan et al. 1998). In contrast, research investigating stimulus-triggered averaging of electromyograms (EMG) from forelimb muscles has shown similar cortical contributions to the flexors and extensors (Park et al. 2004). Looking at reticular input, evidence for stronger input to contralateral and ipsilateral flexor muscles has been provided by studies investigating the effects of stimulation of the pontomedullary reticular formation on muscles of the shoulder, elbow, and wrist (Davidson and Buford 2006; Davidson et al. 2007). Although reticular drive to the muscles in control of the fingers has also been studied, no consistent differences were found between the connections to flexor and extensor muscles of the hand (Riddle et al. 2009). These findings can possibly be attributed to the small number of motoneurons that were recorded, as well as the fact that muscles of the wrist and finger were not separated (Baker 2011). Conversely, it is well known that projections from cortex to the reticular formation are bilateral (Fisher et al. 2012; Fregosi et al. 2017); thus the lack of differences may be due to the fact that cortex projects to both flexors and extensors via a cortico-reticulospinal pathway. Because of the discrepancy in results seen across studies, the influence of reticular projections to the wrists on differing movement types remains unclear.
One technique that may be used to further elucidate differential contributions of cortical and reticular drive to flexion versus extension movements is high-intensity transcranial magnetic stimulation (TMS), which can be used to elicit a “cortical silent period.” This silent period can be induced via the application of high-intensity suprathreshold TMS over the primary motor cortex (M1), which results in suppression of ongoing muscle activity for 100–200 ms. Suppression beyond the first 50 ms has been attributed to the interruption of voluntary cortical drive, due to suppression of corticomotoneuronal excitability (Chen et al. 1999; Fuhr et al. 1991; Tergau et al. 1999). Notably, when high-intensity TMS is applied during the reaction time (RT) interval in a simple RT paradigm, movement initiation is significantly delayed due to the interruption of voluntary cortical processing and suppression of M1 output by the TMS (Day et al. 1989). Thus, to investigate differential contributions of cortical and reticular centers to flexion versus extension movements, the present study examined if the RT delay resulting from a TMS-induced cortical silent period would be different depending on the anatomical requirements of the task.
Preprogrammed movements that can be triggered early by a startling acoustic stimulus (SAS), such as wrist extension, have also been argued to receive strong inputs from the reticulospinal system. When an SAS replaces the go signal in simple RT tasks, it not only elicits a stereotypical startle reflex but also results in significant reductions in RT, a phenomenon termed the “StartReact” effect (Carlsen et al. 2012; Valls-Solé et al. 1999). Because the startle reflex is mediated by the pontomedullary reticular formation (Yeomans and Frankland 1995), several studies have argued that these RT reductions are attributable to involuntary response initiation through the engagement of reticulospinal circuits, allowing for the bypassing of cortical response initiation processing (Carlsen et al. 2004; Nonnekes et al. 2015; Valls-Solé et al. 1999, 2008). However, this explanation has been challenged by studies examining the effects of the TMS-induced silent period on the response latency of startle-triggered movements. For example, Alibiglou and MacKinnon (2012) applied suprathreshold TMS in a simple RT paradigm where participants were required to perform a wrist extension movement in response to either a control go signal or an SAS. When TMS was applied during the RT interval, it was found that TMS led to significantly longer RT in both the control and startle trials. Furthermore, no differences were reported in the RT delay induced by the TMS between the control and SAS conditions (see also Stevenson et al. 2014). Although this result points to similar cortical involvement for both voluntary and startle-triggered movements, this previous TMS + SAS study employed very high TMS intensities (>180% of threshold) to induce a silent period, which may have overwhelmed any potential between-condition differences. Thus, by using a rigorously controlled and more sensitive TMS intensity, this study aimed to investigate any potential differences in TMS-induced RT delays between control and startle-inducing stimulus conditions as an indication of cortical and reticular contribution differences.
The purpose of the present study was to determine if the RT delay resulting from a TMS-induced cortical silent period is modulated by the anatomical requirements of the task or the acoustic stimulus condition. In a first experiment, participants were required to perform targeted wrist flexion or extension movements in a simple RT paradigm where an SAS was randomly presented on some trials, and suprathreshold TMS was applied at a fixed intensity and a fixed time within the RT interval. It was hypothesized that application of TMS would result in delayed RT in all conditions; however, this RT delay would be reduced for flexion movements due to increased involvement of reticulospinal outputs. Furthermore, it was hypothesized that RT delays would be smaller for both movements in the SAS condition compared with the control condition due to increased reticular contributions to SAS-triggered responses. A second experiment was conducted to control for possible differences seen in the first experiment due to the length of the silent period induced in the wrist flexor and extensor muscles, and for individual differences in RT across conditions. Similar to experiment 1, it was hypothesized that TMS would result in greater RT delays for extension compared with flexion movements and that, across movements, greater RT delays would be seen in control trials than in SAS trials.
METHODS
Experiment 1
Participants.
Eight right-handed or ambidextrous adults with normal or corrected-to-normal vision, no obvious upper body abnormalities, and no contraindications to TMS (Rossi et al. 2011) participated in this experiment. The experiment was completed in a single session approximately 2 h in length. Two participants did not exhibit consistent sternocleidomastoid (SCM) activation in response to an SAS, which was used as an indicator of a startle reflex (Carlsen et al. 2011); as a result, their data were not included in the final data analysis. Therefore, data from six participants (1 man, 5 women; mean age = 21 yr, SD 1.2) was analyzed. All participants provided written informed consent before participating, and the experiment was approved by and conducted in accordance with all ethical guidelines of the Health Sciences and Science Research Ethics Board at the University of Ottawa and conformed to the seventh revision (2013) of the Declaration of Helsinki.
Apparatus and task.
Participants were seated in a padded, height-adjustable chair ~1.5 m in front of a 24-in. LCD computer monitor (Asus VH242H). The right arm was abducted ~30° at the shoulder and flexed ~90° at the elbow, with the forearm resting parallel to the floor in a custom-made manipulandum such that the palm faced inward with the axis of rotation of the wrist aligned with the axis of rotation of the manipulandum. To restrict movement to only the wrist, the forearm was fastened in place by Velcro straps placed just distal and proximal to the elbow and wrist joints, respectively. Participants were required to perform 20° targeted wrist extension or flexion movements in response to an auditory go signal starting from a neutral position (wrist neither flexed nor extended). Participants were instructed to react to the go signal as quickly as possible while also performing the movement as accurately as possible. Feedback, which consisted of RT and movement accuracy, was provided on the computer monitor following each trial. To encourage fast RTs, a points system rewarded participants for fast RTs (<150 ms) and penalized them for slow RTs (>250 ms); however, points were used only as an incentive and were not analyzed.
Recording equipment.
Surface EMG was collected from the superficial muscle bellies of the right extensor carpi radialis (ECR; agonist for extensor movement), right flexor carpi radialis longus (FCR; agonist for flexor movement), and left sternocleidomastoid (SCM; startle reflex indicator) muscles using bipolar preamplified (gain = 10) surface electrodes (Delsys Bagnoli DE-2.1), which were connected to an external amplifier (Delsys Bagnoli-8) via shielded cabling. Electrodes were prepped with electrode gel (Spectra 360) to improve electrical conductance and were then placed parallel to the muscle fibers and attached to the skin with double-sided adhesive tape. A reference electrode (Dermatrode HE-R) was placed on the right lateral epicondyle. To minimize electrical impedance, all sites of electrode attachment were cleaned with abrasive skin prepping gel (NuPrep) and alcohol wipes before attachment. Wrist angular position was provided by a potentiometer attached to the axis of rotation of the wrist manipulandum. Raw bandpassed (20–450 Hz) EMG and potentiometer data were digitally sampled at 4,000 Hz (National Instruments PCIe-6321) using a customized LabVIEW program, and data were stored for later offline analysis. On each trial, data collection was initiated by the computer 1,000 ms before presentation of the imperative stimulus and continued for 3,000 ms.
Transcranial magnetic stimulation.
TMS was delivered with the use of a Magstim 2002 stimulator with a figure-of-eight coil (D70mm; Magstim, Whitland, UK) with a maximum stimulator output of 2.2 T. TMS was applied over the M1 representation of the right ECR muscle. This area was first estimated by finding the midpoint between the nasion and inion, and the midpoint between the left and right preauricular notches, followed by measuring ~4.5 cm laterally leftward and ~1 cm anteriorly. This location was marked with a red grease pencil, and TMS pulses were delivered to locations near this mark on the scalp in ~0.5-cm steps to determine the stimulation location that elicited the largest motor evoked potential (MEP) within the right ECR. For this process, TMS was initially applied at ~50% of maximum stimulator output and then adjusted on an individual basis to allow identification of the site that resulted in the largest and most consistent MEPs, which was saved using neuronavigation hardware and software (visor2; ANT Neuro, Madison, WI). The resting motor threshold (rMT) of the right ECR was then defined to the nearest 1% of stimulator output by determining the minimum intensity required to elicit a peak-to-peak MEP of 100 µV in 5 of 10 trials (Rossini et al. 1994). This process was repeated to determine the rMT of the right FCR. Because of the substantial overlap of the ECR and FCR representations within M1 (Suzuki et al. 2014), the same location on the scalp was used to determine the rMT within FCR. During the experiment, TMS was delivered at 140% of participants’ individual rMT for each given muscle. This TMS intensity was chosen because it has been shown to excite all high-threshold, fast-conducting corticospinal motoneurons (Rossini et al. 2015) and was expected to be high enough to cause marked differences in RT while still allowing smaller differences between conditions to be seen. All TMS pulses were delivered with the coil placed tangentially on the scalp and perpendicular to the central sulcus to elicit a posterior-anterior (PA) current direction.
Experimental procedure.
Before beginning testing, participants completed a block of 20 practice trials, 10 each of the flexion and extension movements, to allow them to become comfortable with the task. Trials in the practice block were identical to the experimental trials, with the exception that there were no SAS or TMS trials. This was followed by the experimental trials, which consisted of eight blocks of 24 trials, for a total of 192 trials. In four of the eight blocks, participants performed wrist extension movements, and in the other four blocks they performed wrist flexion movements. Each block consisted of 12 control trials, 4 control trials with TMS, 4 SAS trials, and 4 SAS trials with TMS. All four blocks of one task were performed before the other task, with task order counterbalanced across participants.
All trials began with the words “Get Ready!” being displayed on the computer screen for 1,000 ms, followed immediately by an auditory warning signal (100 ms, 200 Hz, 80 dB). After presentation of the warning signal, the computer screen went blank, and following a variable foreperiod of 1,500–2,500 ms, the auditory imperative stimulus (82 dB, 25 ms, 1,000-Hz sine wave) was presented (Fig. 1A). The intensities of all acoustic stimuli were measured using a high-precision, low-latency sound level meter (Cirrus Research model CR:162C) at a distance of 30 cm from the loudspeaker. Both the warning signal and imperative stimulus were generated with digital-to-analog hardware (National Instruments PCIe-6321) and were amplified and presented by a loudspeaker (MG Electronics M58-H, frequency response 300 Hz to 11 Hz, rise time <1 ms) located 30 cm behind the participant, as measured individually from the opening of the participant’s auditory canal. After completion of the movement, trial feedback was displayed on the computer monitor for 3,500 ms, consisting of displacement RT, a visual representation of movement accuracy with respect to the target, and the points earned/lost (based on displacement RT). A customized LabVIEW (National Instruments) program controlled the timeline for each trial, as well as the display of information to the participant.
Fig. 1.
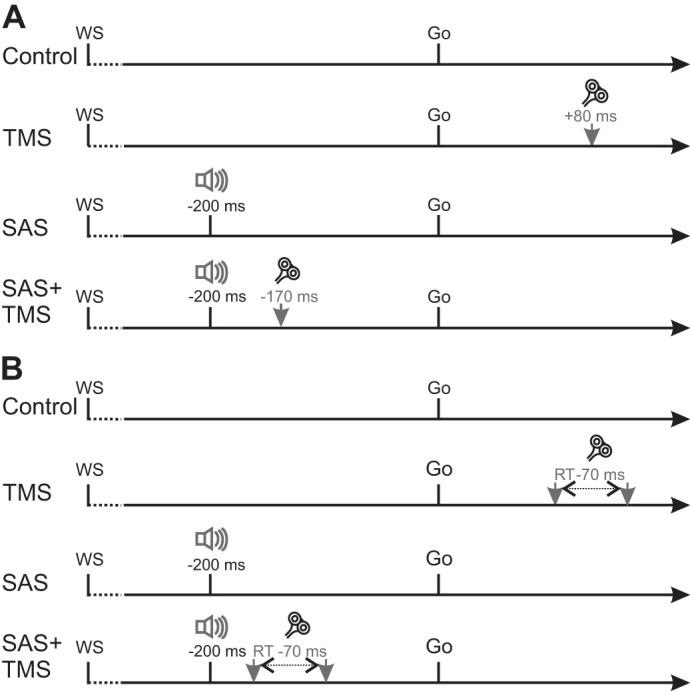
Schematic timeline of experimental trials for experiment 1 (A) and experiment 2 (B). In all trials, an auditory warning signal (WS) was presented, followed by a 1.5- to 2.5-s foreperiod and the auditory go signal (82 dB). On a subset of trials, a 120-dB startling acoustic stimulus (SAS) was presented 200 ms before the go signal. A: in experiment 1, transcranial magnetic stimulation (TMS) was applied on a subset of trials 80 ms following the go signal in TMS trials and 170 ms before the go signal in SAS+TMS trials. B: in experiment 2, TMS was applied 70 ms before participants’ baseline RT values for both the TMS and SAS+TMS trials. RT, reaction time.
In control trials with TMS, the TMS pulse was applied 80 ms following the go signal. This timing of TMS application was chosen because silent period suppression beyond the first 50 ms has been primarily attributed to interruptions in cortical drive (Fuhr et al. 1991). As such, TMS applied more than 50 ms before agonist EMG burst onset in a RT paradigm can be used to investigate the involvement of voluntary cortical drive to movement preparation and initiation. With RTs of ~130 ms commonly seen for targeted wrist movements in response to an auditory go signal (Maslovat et al. 2014; Smith and Carlsen 2018), TMS applied 80 ms following the go signal falls within the expected time interval for cortical suppression.
In startle trials, the SAS (120 dB, 25 ms, white noise waveform) was presented 200 ms before the imperative go signal. This time of SAS presentation was chosen to allow comparison with previous experiments investigating the differences in TMS-induced RT delay between movement conditions (Alibiglou and MacKinnon 2012). This SAS timing also ensures that rapid RTs seen in response to a SAS can be attributed to involuntary response triggering, rather than simply to fast voluntary responses caused by stimulus intensity effects. The presentation of trials was controlled by a computer and pseudorandomized such that an SAS was never presented on the first two trials of a block, and an SAS was never presented on two consecutive trials. In SAS trials with TMS, TMS was applied 30 ms following the SAS (170 ms before the imperative go signal). RTs of ~80–90 ms are often seen in response to an SAS for targeted wrist extension movements (Maslovat et al. 2014; Smith and Carlsen 2018; Stevenson et al. 2014); thus this timing of TMS application occurred ~50 ms before the expected agonist EMG burst onset, allowing investigation of cortical involvement in StartReact responses.
Data reduction and analysis.
SAS trials where no discernible EMG activation was seen in the SCM within 50–120 ms following SAS presentation were discarded, because this is considered a robust and reliable indicator of a startle response sufficient to elicit a StartReact effect (Carlsen 2015; Carlsen et al. 2007, 2011). Two participants failed to exhibit startle reflex-related SCM activity in all SAS trials for a given movement type; thus their data were excluded from the final analysis. In the remaining participants, 54 trials were excluded due to lack of SCM activation. Trials where participants reacted too quickly (RT < 50 ms, anticipation; 30 trials) or too slowly (RT > 300 ms, not paying sufficient attention; 50 trials), as well as trials with movement errors (e.g., performing the wrong movement; 1 trial), were also discarded. These errors were fairly evenly distributed across participants (mean = 17.5; range = 6–36) and resulted in an overall inclusion rate of 88% (1,017/1,152 trials).
The primary dependent measure was premotor RT, measured as the time interval between presentation of the imperative stimulus and the time of EMG onset of the right ECR or right FCR for extension and flexion movements, respectively. EMG onset for all muscles (including SCM) was defined as the point where rectified and filtered EMG activity reached 2 SD above baseline (measured as the mean EMG in a 100-ms interval following the warning signal) and remained elevated for at least 20 ms. EMG traces were displayed on a computer monitor along with EMG onset markers computed using a custom LabVIEW algorithm and then manually adjusted to correct for any possible errors due to the strictness of the algorithm (Hodges and Bui 1996), such as marking a small muscle twitch before movement as EMG burst onset. The integrated area of the raw rectified agonist EMG was calculated for 30 ms beginning from EMG onset (iEMG30). Thirty milliseconds was chosen because this time is often used to quantify the intensity of motor neuron excitation and represents the initial slope of the rise in EMG activity (Corcos et al. 1989; Gottlieb et al. 1989; Khan et al. 1999).
Statistical analysis.
To ensure all data met the assumptions for analysis of variance (ANOVA), dependent measures were analyzed using Shapiro-Wilk’s tests of normality. Data used in linear mixed-effects (LME) models were examined for homoscedasticity and approximate normal distribution of residuals, as well as being scanned for influential cases. The proportion of SAS trials where a burst of EMG was observed in SCM was analyzed using a 2 (movement: extension, flexion) × 2 (TMS condition: none, TMS) repeated-measures ANOVA to determine if there was a difference in incidence of startle reflexes elicited across SAS conditions. Premotor RT was analyzed using LME analysis with movement type (extension, flexion), acoustic stimulus (control, SAS), and TMS condition (none, TMS) as interacting fixed effects, with trial number as a noninteracting fixed effect to account for any learning effects. Intercepts for participants were specified as a random effect, and random slopes were specified for the effects of TMS condition and acoustic stimulus by subject, respectively. A similar model was used to analyze iEMG30, but due to the different nature of the muscles and actions, movement type was not included as a fixed factor. Similarly, to determine if TMS presentation or movement condition had an effect on the onset of the startle response, onset of SCM activity was analyzed with the use of a similar model to that used for premotor RT, but because this analysis was only performed on SAS trials, acoustic stimulus type was not used in the model. Thus an LME analysis included movement type (extension, flexion) and TMS condition (none, TMS) as interacting fixed effects, with trial number as a noninteracting fixed effect to account for any learning effects. Intercepts for participants were specified as a random effect, and random slopes were specified for the effects of TMS condition by subject. Participants’ rMT and the TMS intensity used throughout the experimental trials were analyzed using paired-samples t-tests to determine if there was any difference between the extension and flexion conditions. LME analyses were performed with R statistical software (R Core Team 2018) using the lme4 package (Bates et al. 2015) along with the lmerTest package (Kuznetsova et al. 2017) for providing P values. ANOVAs and t-tests were performed using SPSS software for windows (IBM, Armonk, NY). The significance value for all statistical tests was set at P < 0.05, and r values are reported to provide an estimate of effect size for t-tests where appropriate.
Experiment 2
Rationale.
In experiment 1, the TMS intensity employed during both the extension and flexion tasks was 140% of rMT for the respective target muscles. However, the rMT was found to differ between muscles (see results, Experiment 1), resulting in a significantly higher intensity being used throughout the experiment for the flexor movements. Thus a second experiment was conducted to control for any differences in the length of the silent period evoked between wrist flexor and extensor muscles, as well as to normalize TMS delivery time with respect to individualized RTs.
Participants.
Twenty-seven right-handed or ambidextrous adults with normal or corrected-to-normal vision and no contraindications to TMS participated in this experiment (2 of the participants from experiment 1 also completed experiment 2). The experiment was completed in a single session, which was ~2 h in length. The data of three participants were excluded from analysis, because they did not exhibit a startle reflex in response to the SAS for all trials of a movement type (see Experiment 1, Data reduction and analysis, for details). This resulted in a final sample size of 24 participants (8 men, 18 women; mean age = 25.3 yr, SD 8.4) being included in the final analysis. All participants provided written informed consent before participating, and the experiment was approved by and conducted in accordance with the ethical guidelines set by the Health Sciences and Science Research Ethics Board at the University of Ottawa and conformed to the seventh revision (2013) of the Declaration of Helsinki.
Experimental procedure.
The experimental procedure was similar to that of experiment 1, with the following modifications. First, whereas the TMS intensity used to induce a cortical silent period in the wrist extensor muscle was 140% of rMT (as in experiment 1), the intensity used for flexion movements was that required to induce a silent period of a similar length as that seen in the wrist extensor. This was done to ensure that the silent period duration was matched between effectors. To achieve this, participants underwent a silent period matching procedure before beginning testing. Participants were seated in front of a computer monitor with their right arm abducted ~30° at the shoulder and ~90° at the elbow, with the forearm resting semipronated and parallel to the floor on the armrest portion of a custom-made wrist force measurement device. The device restricted movement solely to isometric flexion/extension of the wrist with the palmar and dorsal surfaces of the hand secured against a brace made of molded plastic that was attached to force transducers (Nano25; ATI Industrial Automation). The force transducers were mounted on a solid surface and contacted the molded brace approximately at the third metacarpophalangeal joint. Each participant’s maximum voluntary contraction (MVC) force was then determined by having them perform maximum isometric contractions of both the wrist extensors and flexors. Participants performed three trials of each movement in alternation, with each trial requiring participants to exert the greatest amount of force possible through contraction of solely the muscles of the wrist joint for 3 s. The MVC was then calculated as the mean peak force across the three trials for each movement. Participants were then required to isometrically contract their wrist extensors at 10% of their extensor MVC, and 10 TMS pulses were applied at an intensity of 140% rMT over the M1 representation of the right ECR. A customized LabVIEW program was used to calculate the silent period length for the wrist extensor. In brief, the program created an ensemble average of the rectified EMG activity time locked to the TMS pulse, which was displayed on a computer monitor in front of the researcher, along with a cursor indicating the point at which EMG activity spontaneously resumed following the suppression. This point was determined as the first point where the ensemble averaged EMG increased and remained above 2 SD of the mean activity observed in a 40-ms window following the TMS pulse. If needed, this cursor was adjusted by the researcher, and the time between the TMS pulse and the cursor was used as the silent period duration for the wrist extensor. Participants were then instructed to isometrically contract their wrist flexors at 10% of their flexor MVC. During this, TMS pulses were applied over the FCR representation of M1 starting at 140% rMT. TMS intensity was then adjusted until a silent period of approximately the same duration as that seen in the wrist extensor was achieved. Ten TMS pulses were then applied at this intensity, and EMG was recorded to confirm the duration of the induced silent period in the wrist flexor, measured as the time between onset of the MEP and resumption of EMG activity.
The second modification in experiment 2 was the timing of TMS application in TMS trials. TMS was applied 70 ms before each participant’s individual median premotor RT for each movement (flexion and extension) and acoustic stimulus (control and startle) condition. This was done to control for individual differences in RT, ensuring that the time of TMS application resulted in the cortical silent period occurring when each participant was expected to initiate their movements. Following the silent period procedure, participants completed one block of 10 practice trials to become comfortable with the wrist extension movement, which was identical to that used in experiment 1 (20° targeted wrist extension). To determine each participant’s individual baseline RT values, participants then completed one block of 25 RT trials. Each block consisted of 20 control auditory imperative stimulus trials and 5 SAS trials. Following each trial, a customized LabVIEW program displayed the agonist EMG activity along with a marker indicating EMG onset to the researcher, who adjusted the marker as necessary (see Experiment 1, Data reduction and analysis). The median EMG onset times were then calculated for each acoustic stimulus condition to give each participant’s baseline values. This process was repeated for the wrist flexion movement.
Finally, in a subset of participants (n = 6), EMG was collected from the right orbicularis oculi (OOc) muscle, which was used as a secondary startle indicator. Activation in SCM is generally regarded as a robust and reliable indicator of a startle response (Carlsen 2015; Carlsen et al. 2007); however, during testing it was observed that a larger than normal proportion of participants in experiment 2 did not exhibit SCM activation in response to a SAS. This may have been due to the TMS coil being held on participants’ heads throughout the experiment, resulting in participants bracing their necks against the coil and interfering with a typical startle response being elicited in SCM. As such, EMG activity from both the right SCM and OOc were used as startle indicators in the final six participants tested in experiment 2. In these participants, startle trials were discarded if a lack of activation was seen in either SCM or OOc.
Participants then completed the experimental trials, which were completed as eight blocks of 18 trials, for a total of 144 trials. In four of the eight blocks, participants were required to perform wrist extension movements, and in the other four blocks they were required to perform wrist flexion movements. Each block consisted of 10 control trials, 4 control trials with TMS, 2 SAS trials, and 2 SAS trials with TMS. All four blocks of one task were performed before the other task, with task order counterbalanced across participants. The timeline of the experimental trials was identical to that of experiment 1, with the exception that on TMS trials, TMS was applied 70 ms before each participant’s baseline RT in both control and SAS trials for each task (Fig. 1B). The remainder of the experimental protocols were identical to those of experiment 1. As stated in Experiment 2, Participants, the data reduction protocol resulted in the exclusion of three participants, because they did not exhibit SCM activation following SAS presentation in any SAS trials for a given movement type. In the remaining 24 participants, 301 SAS trials were discarded due to lack of SCM activation and 6 SAS trials were discarded due to lack of activation in both SCM and OOc. Furthermore, 61 trials were discarded for RTs that were too fast (<50 ms), 61 trials were discarded for RTs that were too slow (>300 ms), and 16 trials were excluded due to movement errors. These errors were fairly evenly distributed across participants (mean = 19.6; range = 1–38) and resulted in an overall inclusion rate of 87% (3,011/3,456).
The statistical analyses performed for experiment 2 were similar to those used in experiment 1, with the addition of analysis of MEP amplitude on TMS trials and descriptive statistics regarding the distribution of RT with respect to the value used to normalize TMS delivery to each individual. MEP amplitude was defined as the greatest peak-to-peak amplitude recorded in the 30-ms window beginning 15 ms following the time of TMS presentation. MEPs were rejected if 1) root mean square EMG activity in the 100 ms before the TMS pulse exceeded 100 µV and 2) if root mean square EMG activity in the 100 ms before the TMS pulse exceeded two times the resting root mean square value for that trial (determined from a mean of the first 500 ms of EMG in the trial). In addition, MEPs were rejected if the peak-to-peak amplitude was less than 0.05 mV. This resulted in the exclusion of 121 MEPs and an overall MEP inclusion rate of 89% (1,031/1,152). To determine if there was a difference in the size of MEPs elicited in the control and startle conditions, MEP peak-to-peak amplitude was analyzed using LME analysis with movement type (extension, flexion) and acoustic stimulus (control, SAS) as interacting fixed effects. Intercepts for participants were specified as a random effect, and random slopes were specified for the effect of acoustic stimulus by subject. In addition, mean duration of the silent period elicited during baseline testing was analyzed using a paired samples t-test to compare mean silent period duration between the extensor and flexor muscles. This analysis was added to ensure there were no differences between the size of MEPs elicited in the control imperative stimulus and SAS conditions, allowing for comparison of the TMS-induced RT delay between conditions. As mentioned previously, time of TMS delivery was normalized to each individual’s median baseline RT in experiment 2. To ensure that median RT was a useful timing parameter, subjects’ RT distributions were quantified with respect to their median value from baseline testing. To do this, the proportion of test trials where RT fell within a particular range (±30 and ±40 ms) of the median value was obtained for each participant and each no-TMS condition. The no-TMS conditions were used as a surrogate for what was expected to have occurred during TMS trials, because it was expected that TMS would influence RT. The remainder of the statistical analyses were identical to those in experiment 1.
RESULTS
Experiment 1
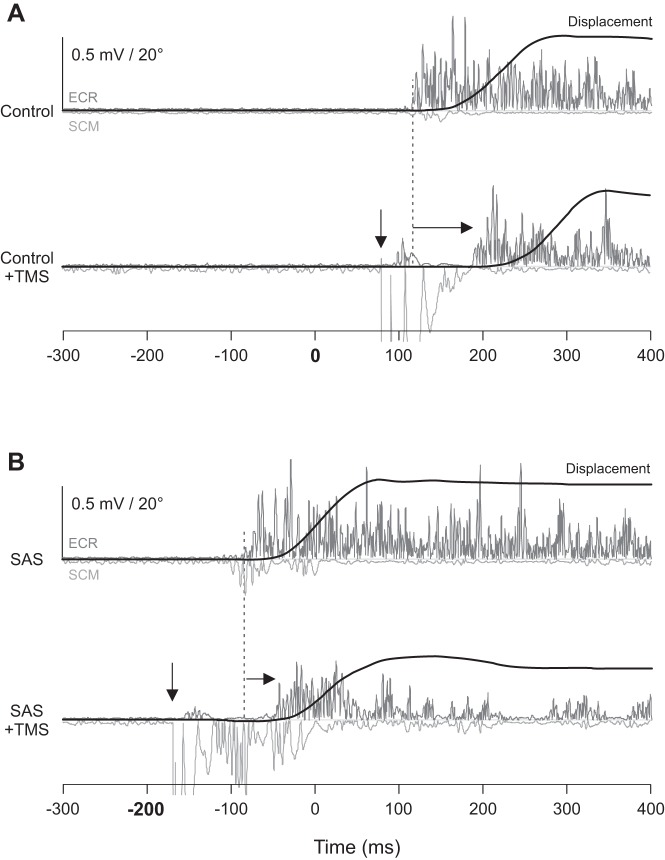
Figure 2 shows individual behavioral data from no-TMS and TMS trials during extension movements in the control acoustic stimulus conditions (A) and in SAS conditions (B).
Fig. 2.
Experiment 1 example individual trial displacement data (black) and electromyogram (EMG) from the extensor carpi radialis (ECR; dark gray) and sternocleidomastoid (SCM; light gray) muscles from a representative participant in the extension movement conditions. A: control acoustic stimulus occurred at 0 ms. Top traces show EMG from a no-transcranial magnetic stimulation (TMS) control trial; bottom traces show EMG from a trial where TMS was delivered 80 ms following the acoustic stimulus (down arrow; large artifact visible in SCM EMG trace followed ~20 ms later by a motor evoked potential in ECR and silent period). Note that TMS leads to delayed premotor reaction time (RT) compared with no TMS (right arrow from dashed vertical line). B: startling acoustic stimulus (SAS) occurred at −200 ms. Top traces show EMG from a no-TMS SAS trial; bottom traces show EMG from a trial where TMS was delivered 30 ms after the SAS. Note that in SAS trials, TMS leads to a smaller delay in premotor RT compared with control trials. Only the extension movements are shown, because similar effects were observed between flexion and extension conditions.
Transcranial magnetic stimulation.
Analysis revealed a significant difference between rMT values and, by extension, the TMS intensity throughout the experimental trials [t(5) = −3.115, P = 0.026, r = 0.81]. Mean rMT was found to be significantly greater in the flexor muscle (45.2%, SD 8.2) than in the extensor muscle (39.7%, SD 6.0), with an average difference of 4.7% (SD 4.5) between extensors and flexors. Because the intensity used was 140% of rMT for each muscle (rounded to the nearest percentage), a similar significant difference was also found for the TMS intensity used throughout the experiment for the extensor and flexor muscles [t(5) = −3.068, P = 0.028, r = 0.81]; the intensity applied in experimental trials was significantly greater in the flexor muscle (63.3%, SD 11.7) than in the extensor muscle (55.3%, SD 8.4).
Premotor RT.
Premotor RT for all eight experimental conditions is shown in Fig. 3A. Estimates of fixed effects are provided in Table 1. Examination of the effects reveals that compared with the extension movement, movement type (flexion), TMS, and presentation of a SAS all had significant effects on RT. In general, flexion movements were slightly faster, and whereas the application of TMS led to RT slowing, presentation of an SAS led to RT speeding. However, the presence of a significant interaction effect between TMS and SAS indicates that the RT delay induced by TMS was shortened in the presence of a SAS. Estimates of marginal means suggests that whereas the delay induced by TMS (collapsed across movement types) in control (no SAS) trials was 81.4 ms (SE 7.8), it was only 50.4 ms (SE 9.4) in the presence of an SAS (Fig. 3B). Furthermore, a significant interaction effect between TMS and flexion indicates that the RT delay induced by a TMS was longer during flexion movements, with estimates of marginal means suggesting that whereas the delay induced by TMS (collapsed across acoustic stimulus types) was 55.5 ms (SE 8.7) for extension movements, it was 76.3 ms (SE 8.5) for flexion movements (Fig. 3C).
Fig. 3.
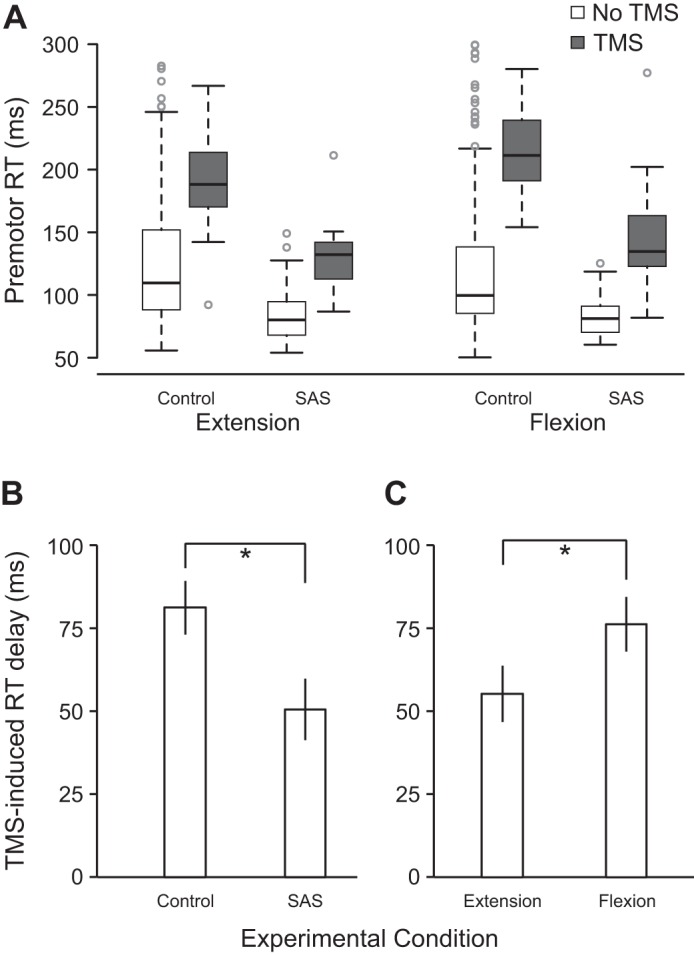
Premotor reaction time (RT) as a function of movement, acoustic stimulus, and transcranial magnetic stimulation (TMS) conditions in experiment 1 (n = 6). A: boxplots of RT in all 8 conditions. Box ends (fences) represent upper and lower quartiles, the line dividing the box represents the median, the whiskers represent the highest and lowest data points within 1.5 times the interquartile range from the fences, and circles represent outlier data points. B: RT delay induced by TMS (means ± SE) collapsed across movement type for control and startling acoustic stimuli (SAS). *P < 0.05, significant difference between variables. C: RT delay induced by TMS (means ± SE) collapsed across acoustic stimulus type for extension and flexion movements. Linear mixed-effects analysis statistical values are shown in Table 1. *P < 0.05, significant difference between variables.
Table 1.
Statistical values for LME analysis of premotor RT in experiment 1
| Fixed Effect | Estimate, ms | df | t Value | P Value |
|---|---|---|---|---|
| Intercept (extension) | 126.3 (8.8) | 4.9 | 14.33 | <0.001 |
| Flexion | −6.9 (2.8) | 997.1 | −2.47 | 0.014 |
| TMS | 67.9 (9.0) | 11.0 | 7.55 | <0.001 |
| SAS | −38.5 (8.6) | 17.3 | −4.48 | <0.001 |
| Trial | −0.2 (0.2) | 997.4 | −0.94 | 0.348 |
| TMS × SAS | −25.0 (11.8) | 1,002.8 | −2.11 | 0.035 |
| TMS × flexion | 27.0 (8.7) | 997.2 | 3.10 | 0.002 |
| SAS × flexion | 6.8 (10.2) | 997.3 | 0.66 | 0.507 |
| TMS × SAS × flexion | −12.3 (16.3) | 999.0 | −0.75 | 0.451 |
Values are mean (SE) fixed-effect estimates, degrees of freedom (df), and statistical values from linear mixed-effects (LME) analysis of premotor reaction time (RT) in experiment 2. SAS, startling acoustic stimulation; TMS, transcranial magnetic stimulation.
Agonist EMG activity.
LME analysis revealed a significant effect of acoustic stimulus on iEMG30 for the agonist burst, with estimates of marginal means indicating that the initial agonist burst was significantly greater (P = 0.012) in SAS trials (5.4 µV·ms, SE 1.3, 95% CI [2.0–8.8], where CI is confidence interval) than in control trials (3.5 µV·ms, SE 0.6, 95% CI [1.9–5.1]). There were no significant differences attributable to TMS condition, as well as no interaction between the factors (all P values >0.05).
Startle reflex activity.
No significant differences between conditions and no significant interactions were found in the proportion of trials where a startle reflex was observed (all P values >0.05). In extension conditions where a SAS was presented, a startle reflex was elicited in 71% (SD 20) of SAS trials without TMS and in 63% (SD 37) of SAS trials with TMS. In flexion conditions, a startle response was elicited in 67% (SD 29) of SAS trials without TMS and in 83% (SD 17) of SAS trials with TMS. In SAS trials where SCM activity was elicited, LME analysis revealed no significant difference in SCM onset latency between movement (P = 0.995) or TMS (P = 0.480) conditions, as well as no interaction between the factors (P = 0.105). In SAS trials where a startle reflex was elicited, onset latency was 78 ms (SE 6) in extension trials without TMS and 73 ms (SE 6) in extension trials with TMS. In flexion conditions, SCM onset latency was 78 ms (SE 6) in trials without TMS and 82 ms (SE 5) in trials with TMS.
Experiment 2
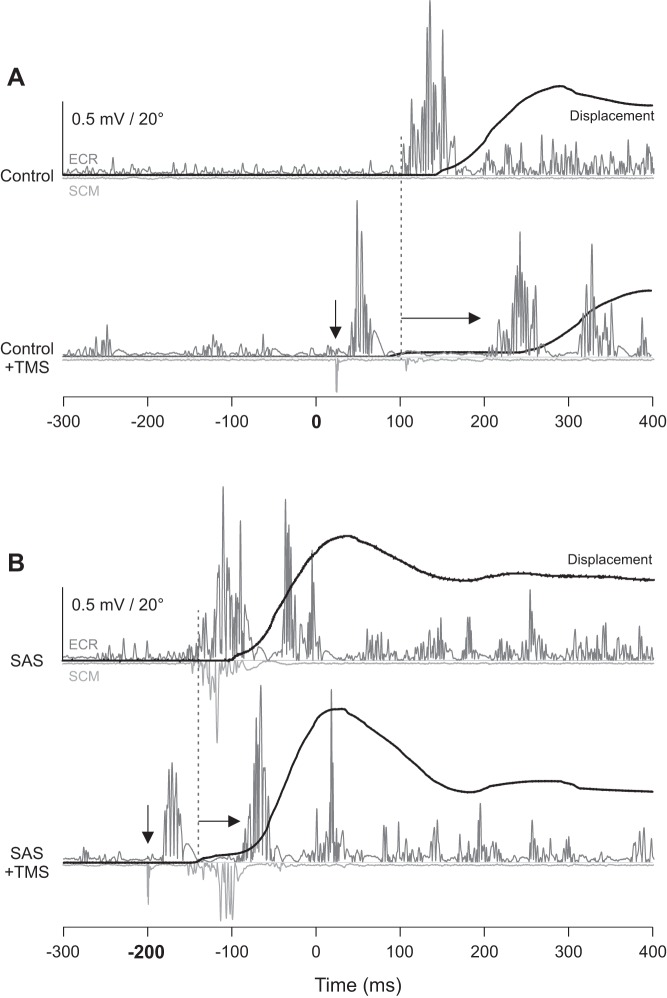
Figure 4 shows individual behavioral data from no-TMS and TMS trials during extension movements in the control acoustic stimulus conditions (A) and in SAS conditions (B).
Fig. 4.
Experiment 2 example individual trial displacement data (black) and electromyogram (EMG) from the extensor carpi radialis (ECR; dark gray) and sternocleidomastoid (SCM; light gray) muscles from a representative participant in the extension movement conditions. A: control acoustic stimulus occurred at 0 ms. Top traces show EMG from a no-transcranial magnetic stimulation (TMS) control trial; bottom traces show EMG from a trial where TMS was delivered 70 ms before expected EMG onset (down arrow, artifact visible in SCM EMG trace followed ~20 ms later by a motor evoked potential in ECR and silent period). Note that TMS leads to delayed premotor reaction time (RT) compared with no-TMS (right arrow from dashed vertical line). B: startling acoustic stimulus (SAS) occurred at −200 ms. Top traces show EMG from a no-TMS SAS trial; bottom traces show EMG from a trial where TMS was delivered 70 ms before expected EMG onset. Note that in SAS trials, TMS leads to a smaller delay in premotor RT compared with control trials. Only the extension movements are shown, because similar effects were observed between flexion and extension conditions.
Transcranial magnetic stimulation.
TMS was presented at 70 ms before each participant’s median RT from baseline. During testing, it was found that in non-SAS trials, RT was within ±30 ms of each participant’s median baseline RT in 67.3% (SD 19.4) of extension trials and in 66.5% (SD 14.2) of flexion trials. This percentage grew to 75.6% (SD 16.6) of extension trials and 78.4% (SD 10.4) of flexion trials if a range of ±40 ms was used. Similarly, for SAS trials, RT was within ±30 ms of each participant’s median SAS trial baseline RT in 82.0% (SD 3 5.9) of extension trials and in 83.8% (SD 32.1) of flexion trials, and this percentage grew to 97.1% (SD 1.6) and 87.6% (SD 7.0), respectively, if a range of ±40 ms was used. These results suggest that use of this parameter was effective in estimating timing for TMS delivery.
As in experiment 1, analysis of rMT revealed a significant difference between the extensor and flexor muscles [t(23) = −6.073, P < 0.001, r = 0.78], indicating that mean rMT was significantly greater for the flexor muscle (47.6%, SD 8.3) than for the extensor muscle (41.4%, SD 7.5), with an average difference of 6.3% (SD 5.0) between extensors and flexors. In contrast, analysis revealed no significant difference between the extensor and flexor muscles with respect to the TMS intensity used throughout the experimental trials [t(23) = −0.564, P = 0.578, r = 0.12]. The TMS intensity used throughout the experiment was 58.0% (SD 10.5) for the extensor muscle and 58.5% (SD 10.0) for the flexor muscle. Analysis of the duration of the silent period confirmed that during baseline testing, there was no difference in the silent period duration between the extensors (142 ms, SD 3) and the flexors (142 ms, SD 2) [t(23) = 0.134, P = 0.894, r = 0.03]. Example silent period data from a representative participant are provided in Fig. 5.
Fig. 5.
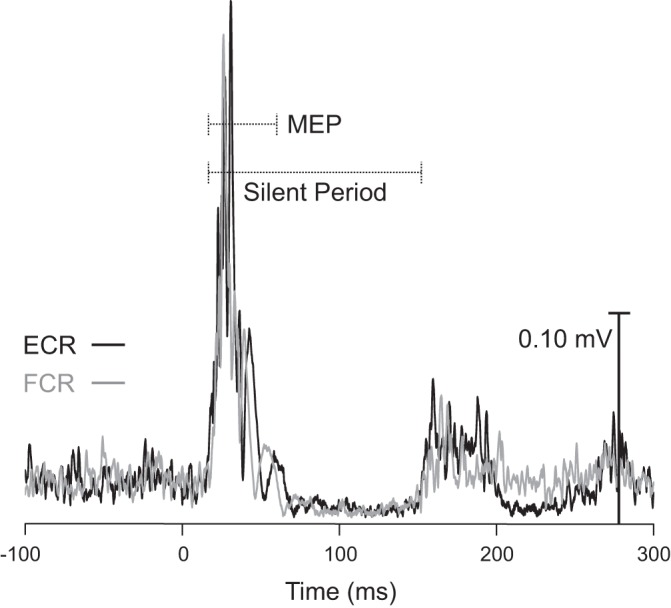
Ensemble average of rectified electromyography (EMG) during silent period determination trials (see methods). Data are from a single participant and are time-locked to transcranial magnetic stimulation (TMS) delivery time (0 ms) in both the extensor carpi radialis (ECR; black) and flexor carpi radialis (FCR; gray) muscles. Note that the ongoing EMG activity is interrupted by a TMS-induced motor evoked potential (MEP; short dashed line) and an ensuing EMG silent period (long dashed line), with EMG activity returning ~150 ms after the onset of the TMS pulse in both muscles.
Premotor RT.
Premotor RT values across all eight conditions are shown in Fig. 6A. Estimates of fixed effects are provided in Table 2. Examination of the effects reveals that compared with the extension movement, movement type (flexion), TMS, and presentation of a SAS all had significant effects on RT. In general, flexion movements were slightly slower, and the application of TMS led to slower RTs, whereas presentation of an SAS led to faster RTs. Although trial number also had a significant effect on RT, the effect was extremely small. However, similar to experiment 1, the presence of a significant interaction effect between TMS and SAS indicates that the RT delay induced by TMS was shortened in the presence of an SAS. Estimates of marginal means (collapsed across movement types) suggests that whereas the delay induced by TMS in control (no SAS) trials was 44.6 ms (SE 6.0), it was only 27.3 ms (SE 6.6) in the presence of an SAS (Fig. 6B). Furthermore, a significant interaction effect between TMS and flexion indicates that the RT delay induced by a TMS was shorter during flexion movements, with estimates of marginal means (collapsed across acoustic stimulus types) suggesting that whereas the delay induced by TMS was 40.0 ms (SE 6.3) for extension movements, it was only 32.0 ms (SE 6.3) for flexion movements (Fig. 6C).
Fig. 6.
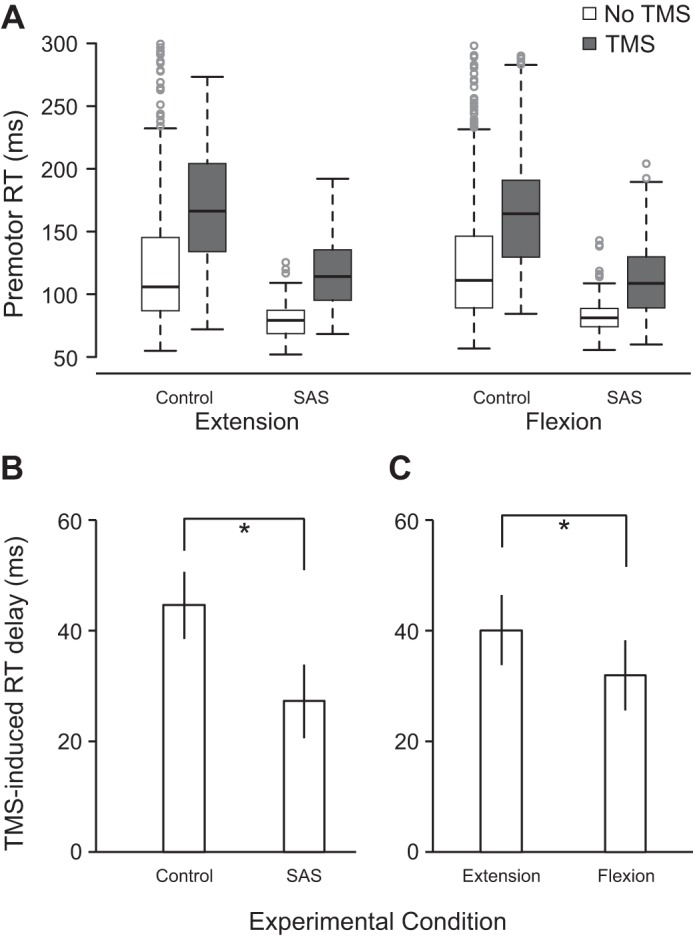
Premotor reaction time (RT) as a function of movement, acoustic stimulus, and transcranial magnetic stimulation (TMS) conditions in experiment 2 (n = 24). A: boxplots of RT in all 8 conditions. Box ends (fences) represent upper and lower quartiles, the line dividing the box represents the median, the whiskers represent the highest and lowest data points within 1.5 times the interquartile range from the fences, and circles represent outlier data points. B: RT delay induced by TMS (means ± SE) collapsed across movement type for control and startling acoustic stimuli (SAS). *P < 0.05, significant difference between variables. C: RT delay induced by TMS (means ± SE) collapsed across acoustic stimulus type for extension and flexion movements. Linear mixed-effects analysis statistical values are shown in Table 2. *P < 0.05, significant difference between variables.
Table 2.
Statistical values for LME analysis of premotor RT in experiment 2
| Fixed Effect | Estimate, ms | df | t Value | P Value |
|---|---|---|---|---|
| Intercept (extension) | 122.2 (5.3) | 25.7 | 22.89 | <0.001 |
| Flexion | 3.6 (1.5) | 2,938.5 | 2.39 | 0.017 |
| TMS | 48.6 (6.2) | 25.9 | 7.88 | <0.001 |
| SAS | −36.5 (5.1) | 50.3 | −7.21 | <0.001 |
| Trial | −0.1 (0.0) | 2,944.4 | −3.16 | 0.002 |
| TMS × SAS | −17.3 (4.7) | 2,856.4 | −3.65 | <0.001 |
| TMS × flexion | −7.9 (2.8) | 2,938.7 | −2.86 | 0.004 |
| SAS × flexion | 1.1 (4.6) | 2,932.9 | 0.247 | 0.805 |
| TMS × SAS × flexion | 0.1 (6.7) | 2,942.7 | 0.013 | 0.990 |
Values are mean (SE) fixed-effect estimates, degrees of freedom (df), and statistical values from linear mixed-effects (LME) analysis of premotor reaction time (RT) in experiment 2. SAS, startling acoustic stimulation; TMS, transcranial magnetic stimulation.
Agonist EMG activity.
Similarly to experiment 1, LME analysis of iEMG30 for the agonist burst revealed a significant effect of acoustic stimulus on iEMG30 for the agonist burst [t(93.7) = 3.72, P < 0.001], with estimates of marginal means indicating that the initial agonist burst was significantly greater in SAS trials (3.9 µV·ms, SE 0.7, 95% CI [2.4–5.3]) than in control trials (3.4 µV·ms, SE 0.6, 95% CI [2.1–4.6]). There was also a significant effect of TMS on the agonist burst [t(23.8) = 2.37, P = 0.026], indicating that the initial agonist burst was significantly larger following TMS (3.9 µV·ms, SE 0.7, 95% CI [2.4–5.4]) than in control trials (3.4 µV·ms, SE 0.6, 95% CI [2.1–4.7]). The interaction between the factors was not significant (P = 0.116).
Startle reflex activity.
No significant differences between movement or TMS conditions were found in the proportion of trials where a startle reflex was observed (all P values >0.05). For the extension movement, a startle response in SCM was elicited in 58% (SD 38) of trials without TMS and in 65% (SD 35) of trials with TMS. For the flexion movement, a startle response was elicited in 57% (SD 39) of SAS trials without TMS and in 58% (SD 38) of trials with TMS. In SAS trials where an SCM response was evoked, LME analysis showed no differences in SCM onset latency between TMS conditions (P = 0.164), but there was a significant effect of movement [t(23.8) = 2.37, P = 0.026], with estimates of marginal means indicating that the onset of SCM EMG activity occurred significantly earlier when the required movement was flexion (75.1 ms, SE 3.7, 95% CI [67.5–82.7]) compared with extension (78.0 ms, SE 3.7, 95% CI [70.4–85.6]). There was no interaction between the factors (P = 0.148).
MEP amplitude.
LME analysis of MEP peak-to-peak amplitude revealed a significant effect of movement type [t(1004.3) = 13.68, P < 0.001], with estimates of marginal means (collapsed across acoustic stimulus types) indicating that MEP amplitude was significantly larger during extension trials (0.80 mV, SE 0.05, 95% CI [0.69–0.92]) compared with flexion trials (0.43 mV, SE 0.05, 95% CI [0.32–0.54]). There was no significant effect of acoustic stimulus condition on MEP amplitude [t(818.9) = −1.41, P = 0.16]. MEP amplitude during control (no SAS) trials was 0.64 mV (SE 0.05, 95% CI [0.53–0.76]), and that during SAS trials was 0.59 mV (SE 0.05, 95% CI [0.48–0.71]). The interaction between the factors was not significant (P = 0.899).
DISCUSSION
It has long been generally accepted that voluntary movements, particularly those of the hands and fingers, are primarily carried out by the corticospinal tract, yet recent findings have provided evidence for the existence of projections from the reticulospinal tract to the motor neurons and interneurons of these distal effectors (Riddle and Baker 2010; Riddle et al. 2009). In particular, it has been shown that the reticulospinal tract may also play a complementary role to that of the corticospinal tract in the control of voluntary movements involving the hand and forearm (Baker 2011). Previous research investigating the effects of the reticular pathway on voluntary movement suggests that there may also be many important differences in the roles of these pathways (Baker 2011; Davidson and Buford 2006; Davidson et al. 2007), such as the corticospinal tract exhibiting stronger connections to extensor muscles than flexor muscles (Cheney et al. 1991; McKiernan et al. 1998). However, this is not a universal finding, with some research suggesting that the contributions of corticospinal and reticulospinal input are similar to both flexion and extension movements (Fregosi et al. 2017; Park et al. 2004). A similar debate exists regarding the neural inputs underlying the involuntary triggering of a response through the use of an SAS, which has been suggested to increase the contribution of reticulospinal drive to movement initiation (Carlsen et al. 2004; Nonnekes et al. 2014; Valls-Solé et al. 1999) and thus may involve a relative decrease in corticospinal contribution. As such, the purpose of the present experiments was to determine if the RT delay resulting from a TMS-induced cortical silent period is modulated by the anatomical task requirements (wrist flexion vs. extension) or the acoustic stimulus condition (control vs. SAS).
It was reasoned that the length of the RT-delay induced by high-intensity TMS could be used as an indicator of the relative cortical and reticular contributions to various movements, with greater delays indicating a greater cortical bias and shorter delays indicating a greater reticular bias. Results showed that TMS resulted in significant increases in RT in all conditions, and these increases were significantly different between flexion and extension movements. In experiment 1, there was a larger increase in RT for flexion movements, which was contrary to the hypothesis; however, in experiment 2, where the TMS silent period duration across movements was explicitly controlled, the RT delay was larger in the extension movement compared with flexion (Fig. 6). When silent period duration is appropriately controlled for, this result suggests that there is indeed a greater degree of corticospinal drive for extension than flexion movements of the wrist. Furthermore, the type of acoustic stimulus presented also appeared to affect the relative contribution of neural drive from corticospinal and reticulospinal centers; the RT delay caused by TMS was found to be significantly shorter for SAS compared with control trials (Fig. 6B). Although a significant RT delay was still present in SAS trials, indicating that corticospinal drive is involved in startle-evoked movements (albeit reduced), the smaller delay is likely due to greater reticular drive, and thus relatively less cortical drive, when movements are initiated in response to an SAS compared with a control imperative stimulus.
As previously mentioned, research investigating the contributions of reticulospinal and corticospinal drive to the flexors and extensors of the wrist has provided mixed results. Some studies have suggested that corticospinal input is greater for the extensors and reticulospinal input is greater for flexors (Baker 2011; Cheney et al. 1991), whereas other studies have found no differences between the inputs of these pathways (Park et al. 2004). The significant difference in the TMS-induced RT delay between the flexion and extension movements in experiment 2 provides evidence that the contribution of corticospinal drive to wrist extension movements is greater than that to wrist flexion movements. Although the opposite result was seen in experiment 1 (Fig. 3C), the TMS intensity used for flexion movements was significantly higher, which likely explains this unexpected finding. Because experiment 2 controlled for silent period duration across movements and had a larger sample size, these results appear to support previous findings that there is relatively smaller corticospinal contribution to wrist flexion vs. extension movements. It could be argued that the withdrawal of voluntary drive seen in the later part of the TMS-induced silent period (Fuhr et al. 1991) is not due simply to the withdrawal of corticospinal drive, but rather to the activation of cortical inhibitory circuits. This would reduce both corticospinal and reticulospinal drive to the muscles; however, the results of the present experiment indicates that this was not the case, suggesting that not all reticular drive to the muscles involves initial drive directly from cortex. Results also showed that in SAS trials, SCM onset latency occurred significantly earlier in flexion than in extension movements, providing further evidence that reticular drive may be greater for flexion movements.
In addition to the significant differences in TMS-induced RT delay between the flexion and extension movements, in both experiments TMS resulted in significantly smaller RT delays in SAS trials than in controls trials (Figs. 3B and 6B). The precise neural mechanism underlying movements involuntarily elicited by a startle remains unclear, with debate regarding the role of the cortex and reticular formation in movement initiation. Some researchers argue that SAS-triggered movements are mediated by a subcortical pathway (i.e., reticular formation) without involvement of the cortex (Nonnekes et al. 2015), whereas others argue the opposite, that it is primarily a cortically mediated process that is no different from that underlying initiation of voluntary movements (Marinovic and Tresilian 2016). In the present study, the shorter RT delay resulting from TMS during SAS trials supports the hypothesis that there is a greater amount of reticular drive (and perhaps a relative decrease in cortical drive) for movements involuntarily elicited by an SAS. In combination with previous research indicating involvement of the reticular formation in the StartReact effect (Carlsen et al. 2007; Honeycutt and Perreault 2012; Nonnekes et al. 2014; Valls-Solé et al. 1999), these results provide evidence for the notion that SAS-elicited movements involve a neural pathway that includes both motor-cortical and subcortical structures (Carlsen et al. 2012). Specifically, a shorter TMS-induced delay in SAS trials indicates the presence of increased noncortical (presumably reticular) drive; nevertheless, the very presence of a TMS-induced RT delay indicates that some cortical drive contributes to RTs in the StartReact effect. It could be argued that the smaller RT delay seen in startle trials was due to a shorter silent period length in this condition, given that loud sounds have been shown to lead to suppression of cortical excitability (Fisher et al. 2004; Ilic et al. 2011). However, previous research employing a similar paradigm provided evidence that an SAS presented in a simple RT paradigm does not lead to suppression within M1 (Marinovic et al. 2014). Analysis of the MEP peak-to-peak amplitude in the present experiment revealed no significant differences between the control and startle conditions. Because previous research has shown that MEP amplitude is highly correlated with silent period duration (Tazoe and Perez 2017), the lack of difference in MEP amplitude between the startle and control conditions suggests that the SAS had little effect on the duration of the silent period.
It is also possible that the shorter delays seen in startle trials were due to an incomplete, or “partial,” response being triggered by the SAS; however, analysis of EMG data revealed agonist muscle bursts (iEMG30) were in fact larger in SAS trials. Small to moderate increases in the integrated EMG of the prime mover have been previously reported in studies employing an SAS (Carlsen et al. 2013; Siegmund et al. 2001; Smith and Carlsen 2018), with the interpretation that this could be the result of startle-related drive summing with the voluntary activation. Indeed, the startle reflex typically exhibits as a flexor-dominant response (Landis et al. 1939), so increased startle-related activation may be expected. However, these data also support the interpretation that the results of the present experiment are due to increased reticular drive related to the movement in startle-triggered trials. Although the current data cannot distinguish between these two possibilities, further studies may be better able to elucidate any differences in contribution from the two systems.
Although previous studies have also shown that TMS led to RT delays during startle trials, these studies found no significant differences between the RT delays seen in control and startle trials (Alibiglou and MacKinnon 2012; Stevenson et al. 2014), a finding that is in contrast to the results of the current two experiments. One possible reason for this discrepancy in results could be differences in the intensity of TMS used. In the present experiment, TMS was applied at ~120–140% of rMT, whereas past experiments employed TMS intensities of ~150–180% of rMT (Alibiglou and MacKinnon 2012; Stevenson et al. 2014). At these higher intensities, the silent period and RT delay induced by TMS may be too large for a difference between conditions to be seen; however, further research is warranted to substantiate this claim.
When considering these results, it is important to acknowledge the effect that suprathreshold TMS can have on descending tracts other than the corticospinal tract. Specifically, previous research has shown that TMS over M1 leads to responses in the reticular formation, likely due to the activation of a corticoreticular pathway (Fisher et al. 2012). The effects of TMS are considered to primarily affect the corticospinal tract, an assertion supported by the lack of TMS-induced slowing of the brain stem-mediated startle reflex, seen both in the present experiments as well as in previous experiments that applied high-intensity TMS within a startle paradigm (Alibiglou and MacKinnon 2012; Stevenson et al. 2014). Nevertheless, it is possible that at the TMS intensities used in the present experiments, TMS would also affect the reticulospinal tract, as well as other descending motor tracts. Despite this, the significant difference in the TMS-induced RT delay between the control and startle conditions indicates a differential contribution of the cortical and reticular pathways for StartReact and voluntary responses, because the effects of TMS on all areas would be expected to be similar across experimental conditions. Because it has been shown that the latter part of a TMS-induced silent period is largely due to suppression of voluntary cortical drive (Fuhr et al. 1991), this supports the notion that the smaller RT delay seen in startle trials is indicative of greater reticular drive, and relatively less cortical drive, to these movements.
In conclusion, the results of the present study suggest that corticospinal tract contributions to extension movements of the wrist are greater than those to flexion movements. Similarly, the differences seen between the control and startle conditions provide evidence that responses that are triggered early by an SAS involve greater drive from the reticulospinal tract than voluntary movements made in response to a nonstartling go stimulus. This provides strong and novel information regarding the debate about the neural pathway underlying the StartReact effect, indicating an increase in reticular drive likely contributes to the involuntary initiation of movements made in response to an SAS. This difference in TMS-induced RT delay between control and SAS conditions had not been previously observed or reported, and this may have been due to the lower relative TMS employed in the present experiment than in past experiments (Alibiglou and MacKinnon 2012; Stevenson et al. 2014). This reduced TMS intensity also allowed a difference in TMS-induced RT-delay to be seen between flexion and extension movements, which provides evidence that reticular projections to the wrist have differing effects on these movement types. Thus it appears that TMS intensity can affect the sensitivity of the silent-period protocol to detect differences between conditions. Taken together, these results also suggest that the TMS-induced silent period is a possible noninvasive method for elucidating the relative contributions of the corticospinal and reticulospinal pathways to various movement types.
GRANTS
This research was supported by Natural Sciences and Engineering Research Council of Canada Grant RGPIN 418361-2012 and Ontario Ministry of Research, Innovation and Science Grant ER14-10-133 (both to A. N. Carlsen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M. and A.N.C. conceived and designed research; V.S., N.M.D., J.H., and A.L. performed experiments; V.S., N.M.D., J.H., and A.N.C. analyzed data; V.S., D.M., N.M.D., and A.N.C. interpreted results of experiments; V.S. and A.N.C. prepared figures; V.S. and A.N.C. drafted manuscript; V.S., D.M., N.M.D., J.H., and A.L. edited and revised manuscript; V.S., D.M., N.M.D., J.H., A.L., and A.N.C. approved final version of manuscript.
REFERENCES
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol 590: 919–936, 2012. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612, 2011. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Carlsen AN. A broadband acoustic stimulus is more likely than a pure tone to elicit a startle reflex and prepared movements. Physiol Rep 3: e12509, 2015. doi: 10.14814/phy2.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Almeida QJ, Franks IM. Using a startling acoustic stimulus to investigate underlying mechanisms of bradykinesia in Parkinson’s disease. Neuropsychologia 51: 392–399, 2013. doi: 10.1016/j.neuropsychologia.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res 159: 301–309, 2004. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res 176: 199–205, 2007. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol 123: 21–33, 2012. doi: 10.1016/j.clinph.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35: 366–376, 2011. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128: 539–542, 1999. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res 87: 213–252, 1991. doi: 10.1016/S0079-6123(08)63054-X. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single-joint movements. II. A speed-sensitive strategy. J Neurophysiol 62: 358–368, 1989. doi: 10.1152/jn.1989.62.2.358. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain 112: 649–663, 1989. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol 590: 4045–4060, 2012. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Sharott A, Kühn AA, Brown P. Effects of combined cortical and acoustic stimuli on muscle activity. Exp Brain Res 157: 1–9, 2004. doi: 10.1007/s00221-003-1809-6. [DOI] [PubMed] [Google Scholar]
- Fregosi M, Contestabile A, Hamadjida A, Rouiller EM. Corticobulbar projections from distinct motor cortical areas to the reticular formation in macaque monkeys. Eur J Neurosci 45: 1379–1395, 2017. doi: 10.1111/ejn.13576. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81: 257–262, 1991. doi: 10.1016/0168-5597(91)90011-L. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I. A speed-insensitive strategy. J Neurophysiol 62: 342–357, 1989. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101: 511–519, 1996. [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Planning of ballistic movement following stroke: insights from the startle reflex. PLoS One 7: e43097, 2012. doi: 10.1371/journal.pone.0043097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Pötter-Nerger M, Holler I, Siebner HR, Ilic NV, Deuschl G, Volkmann J. Startle stimuli exert opposite effects on human cortical and spinal motor system excitability in leg muscles. Physiol Res 60, Suppl 1: S101–S106, 2011. [DOI] [PubMed] [Google Scholar]
- Khan MA, Garry MI, Franks IM. The effect of target size and inertial load on the control of rapid aiming movements. A test of speed-sensitive and speed-insensitive strategies. Exp Brain Res 124: 151–158, 1999. doi: 10.1007/s002210050609. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Brookheart JM, Mountcastle VB. Bethesda, MD: American Physiological Society, 1981, p. 597–666. doi: 10.1002/cphy.cp010213. [DOI] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Softw 82: 1–26, 2017. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Landis C, Hunt WA, Strauss H. The Startle Pattern. New York: Farrar & Rinehart, 1939, p. 166. [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR. Triggering prepared actions by sudden sounds: reassessing the evidence for a single mechanism. Acta Physiol (Oxf) 217: 13–32, 2016. doi: 10.1111/apha.12627. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR, de Rugy A, Sidhu S, Riek S. Corticospinal modulation induced by sounds depends on action preparedness. J Physiol 592: 153–169, 2014. doi: 10.1113/jphysiol.2013.254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslovat D, Carter MJ, Kennefick M, Carlsen AN. Startle neural activity is additive with normal cortical initiation-related activation. Neurosci Lett 558: 164–168, 2014. doi: 10.1016/j.neulet.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol 84: 2237–2256, 2000. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998. doi: 10.1152/jn.1998.80.4.1961. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neurosci Biobehav Rev 53: 131–138, 2015. doi: 10.1016/j.neubiorev.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, Bloem BR, Weerdesteyn V, Geurts AC. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci 34: 275–281, 2014. doi: 10.1523/JNEUROSCI.2948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol 92: 2968–2984, 2004. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2018. https://www.R-project.org/. [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol 103: 2821–2832, 2010. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol 122: 1686, 2011. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NM, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107, 2015. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535: 289–300, 2001. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V, Carlsen AN. Sub-threshold transcranial magnetic stimulation applied after the go-signal facilitates reaction time under control but not startle conditions. Eur J Neurosci 47: 333–345, 2018. doi: 10.1111/ejn.13827. [DOI] [PubMed] [Google Scholar]
- Stevenson AJ, Chiu C, Maslovat D, Chua R, Gick B, Blouin JS, Franks IM. Cortical involvement in the StartReact effect. Neuroscience 269: 21–34, 2014. doi: 10.1016/j.neuroscience.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kirimoto H, Sugawara K, Oyama M, Yamada S, Yamamoto J, Matsunaga A, Fukuda M, Onishi H. Motor cortex-evoked activity in reciprocal muscles is modulated by reward probability. PLoS One 9: e90773, 2014. doi: 10.1371/journal.pone.0090773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Cortical and reticular contributions to human precision and power grip. J Physiol 595: 2715–2730, 2017. doi: 10.1113/JP273679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res 124: 447–454, 1999. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res 187: 497–507, 2008. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516: 931–938, 1999. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 21: 301–314, 1995. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]