Abstract
The monosynaptic stretch reflex (MSR) plays an important role in feedback control of movement and posture but can also lead to unstable oscillations associated with tremor and clonus, especially when increased with spinal cord injury (SCI). To control the MSR and clonus after SCI, we examined how serotonin regulates the MSR in the sacrocaudal spinal cord of rats with and without a chronic spinal transection. In chronic spinal rats, numerous 5-HT receptor agonists, including zolmitriptan, methylergonovine, and 5-HT, inhibited the MSR with a potency highly correlated to their binding affinity to 5-HT1D receptors and not other 5-HT receptors. Selective 5-HT1D receptor antagonists blocked this agonist-induced inhibition, although antagonists alone had no action, indicating a lack of endogenous or constitutive receptor activity. In normal uninjured rats, the MSR was likewise inhibited by 5-HT, but at much higher doses, indicating a supersensitivity after SCI. This supersensitivity resulted from the loss of the serotonin transporter SERT with spinal transection, because normal and injured rats were equally sensitive to 5-HT after SERT was blocked or to agonists not transported by SERT (zolmitriptan). Immunolabeling revealed that the 5-HT1D receptor was confined to superficial lamina of the dorsal horn, colocalized with CGRP-positive C-fibers, and eliminated by dorsal rhizotomy. 5-HT1D receptor labeling was not found on large proprioceptive afferents or α-motoneurons of the MSR. Thus serotonergic inhibition of the MSR acts indirectly by modulating C-fiber activity, opening up new possibilities for modulating reflex function and clonus via pain-related pathways.
NEW & NOTEWORTHY Brain stem-derived serotonin potently inhibits afferent transmission in the monosynaptic stretch reflex. We show that serotonin produces this inhibition exclusively via 5-HT1D receptors, and yet these receptors are paradoxically mostly confined to C-fibers. This suggests that serotonin acts by gating of C-fiber activity, which in turn modulates afferent transmission to motoneurons. We also show that the classic supersensitivity to 5-HT after spinal cord injury results from a loss of SERT, and not 5-HT1D receptor plasticity.
Keywords: motoneuron, nociceptive, pain, serotonin, spasticity, spinal cord injury
INTRODUCTION
The monosynaptic stretch reflex (MSR) is mediated by activation of proprioceptive group Ia afferents that directly synapse onto motoneurons. This reflex participates in the feedback control of posture and movement, resisting disturbances. However, when the MSR becomes excessive, it can lead to tremor and clonus and generally disturb precision movements (Bennett et al. 1994, 1996; De Serres et al. 2002; Enjin et al. 2012). After spinal cord injury (SCI), there is a widespread loss of normal inhibitory control over afferent transmission (Millan 2002), and the MSR becomes exaggerated (Nielsen et al. 2007). This is thought to participate in the production of clonus and hyperreflexia, although perhaps not in other aspects of spasticity such as muscle spasms (Bennett et al. 1999; Heckman et al. 2005; Li et al. 2004a).
One source of inhibition over the MSR is 5-HT, which is known to inhibit the MSR via 5-HT1 receptors (Honda et al. 2003, 2004, 2006) and modulate general afferent transmission in the spinal cord (García-Ramírez et al. 2014; Iwasaki et al. 2013; Lu and Perl 2007; Millan 2002; Zhao et al. 2016). After SCI, the MSR is likely increased in part because of loss of this 5-HT, because 5-HT normally arises entirely from the brain stem (Murray et al. 2010; Schmidt and Jordan 2000). However, much is still unknown about which specific 5-HT1 receptors (e.g., 5-HT1A, 5-HT1B, 5-HT1D) are involved in regulating the MSR and where these receptors are specifically located in the MSR pathway. It is possible that 5-HT1 receptors are on proprioceptive afferents involved in the MSR, directly producing presynaptic inhibition, similar to the presynaptic action of other similar metabotropic receptors in many neuronal systems (e.g., GABAB, ATP, and ACh receptors) (Curtis and Lacey 1994; Engelman and MacDermott 2004; Li et al. 2004c). We know that 5-HT1D receptors inhibit activity and transmitter release from small nociceptive afferents (Amrutkar et al. 2012; Zhao et al. 2016), but it remains uncertain whether similar presynaptic inhibition occurs in larger proprioceptive afferents. Alternatively, these 5-HT receptors may act indirectly by modulation of GABAergic interneurons (Lu and Perl 2007; Todd 2010), which themselves produce a presynaptic inhibition of afferents. Thus, determining the receptor location is important. We therefore investigated 1) which 5-HT receptors control the MSR in rats, 2) where these receptors are located, 3) whether replacing lost 5-HT with 5-HT1 receptor agonists brings back inhibition of the MSR after SCI, and, finally, 4) whether there is any adaptation in these receptors to compensate for lost 5-HT innervation after SCI (e.g., change in sensitivity or constitutive activity) (Harvey et al. 2006a; Murray et al. 2010).
The 5-HT1 receptors generally produce inhibition by Gi-coupled proteins, decreasing cAMP and ultimately producing neuronal inhibition in both afferent terminals and spinal interneurons (Millan 2002). Our ability to define the functional roles of these receptors has improved with the advent of highly selective drugs that act on the various receptor subtypes: 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F (Amrutkar et al. 2012; Millan 2002). Much of this progress has come from pharmaceutical efforts to develop treatments for migraine headaches, which involve 5-HT1 receptors. The first generation anti-migraine compounds derived from ergots included methysergide and its metabolic derivative, methylergonovine, and likely acted via 5-HT1B and 5-HT1D receptors (Dahlöf and Maassen Van Den Brink 2012). However, these drugs are not selective. Thus the triptans were developed as improved antimigraine drugs, with highly selective action on 5-HT1B and 5-HT1D receptors. Zolmitriptan is one of the most potent 5-HT1D agonists in this class (Glen et al. 1995; Martin et al. 1997). We have therefore used these 5-HT1 agonists, including zolmitriptan, to explore the role of the 5-HT1 receptors in regulation of the monosynaptic reflex. We found that only 5-HT1D receptors, and not other serotonergic receptors, inhibited the MSR in adult rats. Interestingly, when we examined the location of this receptor with a 5-HT1D receptor antibody, we failed to find immunolabeling on the proprioceptive afferents or motoneurons in the MSR, and instead found 5-HT1D receptors mainly on nociceptive C-fibers. Thus serotonin must regulate the MSR indirectly by regulating C fiber activity, which in turn modulates the MSR.
After SCI, muscle spasms and hyperreflexia emerge as a major part of the general spasticity syndrome (Ashby and McCrea 1987; Dietz and Sinkjaer 2007; Gorassini et al. 2004; Heckman et al. 2005; Kuhn and Macht 1949; Li et al. 2004a; Murray et al. 2010, 2011b). Spasms have been shown to be generated by prolonged polysynaptic inputs to the motoneurons, which in turn initiate persistent calcium currents (PICs) in motoneurons that ultimately produce many seconds of motoneuron firing (spasms) (D’Amico et al. 2014; Li et al. 2004a; Murray et al. 2010). Exogenously applied 5-HT modulates spasms by decreasing the polysynaptic excitatory postsynaptic potentials (EPSPs) via 5-HT1B receptors (D’Amico et al. 2013a; Murray et al. 2011b) and increasing motoneurons PICs via 5-HT2 receptors (D’Amico et al. 2013b; Murray et al. 2011a). We have previously found that these spasms (and long-lasting reflexes, LLRs) are not triggered by the monosynaptic EPSPs underlying the MSR, because these EPSPs are too short to trigger PICs (Li et al. 2004a, 2004b, 2004c). Furthermore, in animals that tend to have large spasms, the monosynaptic reflexes are often small, whereas in less spastic animals, the MSR is large, especially if the spinal cord is hemisected (Li et al. 2004b). Thus an additional purpose of the present experiments was to further explore the relationship between the MSR and spasms. Interestingly, selective 5-HT1D receptor agonists, such as zolmitriptan (at doses selective for this receptor), were found to not affect spasms even though they potently inhibit the MSR. Furthermore, mixed 5-HT1D and 5-HT2 receptor agonists, such as methylergonovine, were found to increase spasms at the same time that they decreased the MSR. Thus the 5-HT receptors that control the MSR (and related clonus) and spasms appear different, suggesting that clonus and spasms may be treated independently after SCI.
METHODS
The monosynaptic reflex was recorded from the ventral roots of the sacrocaudal spinal cord in spastic adult rats with chronic SCI and in age-matched uninjured rats (3.5–5 mo old). Adult female rats were transected at the S2 sacral spinal level at 2 mo of age, and recordings were made months later when the affected muscles became spastic (1.5–3 mo after injury), as detailed previously (Bennett et al. 1999; Li et al. 2004a, 2004b; Murray et al. 2010). For recording, the whole sacrocaudal spinal cord was removed and maintained in vitro. All experimental procedures were approved by the University of Alberta Animal Care and Use Committee: Health Sciences.
Spinal transection, afferent labeling and rhizotomy.
The S2 sacral spinal cord was transected in adult rats as follows. Under general anesthetic (pentobarbital sodium 58.5 mg/kg and isoflurane <4%) and sterile conditions, a laminectomy was performed on the L2 vertebrae to expose the S2 spinal cord. The dura was slit transversely, and 0.1–0.3 ml of Xylocaine (1%) was applied topically. Under a surgical microscope, the spinal cord was transected by holding the pia with fine forceps and sucking under the pia with a fine suction tip (made by heating and pulling a 1-ml syringe to a 0.1- to 0.2-mm tip). Extreme caution was needed to avoid damaging the anterior artery or dorsal vein because the sacrocaudal spinal cord dies without this midline vasculature. Also, rats breathed pure oxygen or 95% oxygen with 5% CO2 during this period when the vessels were manipulated to improve the outcome. Usually, a vertical V-shaped 1- to 2-mm section of the cord was removed to provide good visibility and to ensure that the transection was complete. The dura was closed with two 8-0 silk sutures, and the muscle layers and skin were tightly sutured over the cord.
A rhizotomy was performed in some rats. Under the same sterile anesthesia used for spinal transection, the dorsal roots on the left side were cut along the whole sacrocaudal spinal cord. The roots attached to the cauda equina could not be cut without damaging the anterior artery, so these roots were injured by a brief crush. These rats were euthanized 4 wk later, and the tissue was fixed for immunolabeling as detailed below.
A further group of rats had large afferents labeled with neurobiotin, as follows. Under the same sterile anesthesia used for spinal transection, the S4 dorsal root was exposed and a drop of 10% neurobiotin in distilled water was applied to the central cut end of dorsal root in a small grease well around the root to prevent drying. After 4 h, the root was cleaned with sterile saline and the animal was sutured closed in layers. After 2–3 days for neurobiotin transport in afferents, the rats were euthanized and the neurobiotin imaged in fixed spinal cord tissue.
In vitro preparation.
The details of the in vitro experimental procedures have been described in previous publications (Harvey et al. 2006b; Li et al. 2004a; Murray et al. 2010). Briefly, all the rats were anesthetized with urethane (0.18 g/100 g; with a maximum dose of 0.45 g), and the sacrocaudal spinal cord was removed and transferred to a dissection chamber containing modified artificial cerebrospinal fluid (mACSF). Spinal roots were removed, except for the sacral S4 and caudal Ca1 ventral roots and the Ca1 dorsal roots. After 1.5 h in the dissection chamber (at room temperature), the cord was transferred to a recording chamber containing normal ACSF (nACSF) maintained near 23°C and with a flow rate >5 ml/min. A 1-h period in nACSF was given to wash out the residual anesthetic and mACSF before recording, at which time the nACSF was recycled in a closed system with a peristaltic pump.
Ventral root reflex recording and averaging.
Dorsal and ventral roots were mounted on silver-silver chloride wires above the nACSF of the recording chamber and covered with a 3:1 mixture of petroleum jelly and mineral oil for monopolar stimulation and recording (Li et al. 2004a). Ventral roots on both sides of the cord were mounted for recording, including S3 and S4 sacral roots and Ca1 caudal roots. We generally evoked ventral root reflexes in sacral roots with a low-threshold stimulation of the ipsilateral Ca1 caudal dorsal root [single pulse, 0.1 ms, 0.02 mA, corresponding to 2–3 × T, where T is Ia afferent threshold; afferent and reflex threshold are similar (Bennett et al. 2004)] using a constant-current stimulator (ISO-Flex; A.M.P.I., Jerusalem, Israel), because this Ca1 root was most effective in evoking reflexes. The stimulation intensity is compatible with supramaximal activation of low-threshold proprioceptive group Ia afferents of the tail. The stimulation was repeated five times at 10-s intervals for each trial. The ventral root recordings were amplified (2,000 times), high-pass filtered at 100 Hz, low-pass filtered at 3 kHz, and recorded with a data-acquisition system sampling at 6.7 kHz (Axoscope 8; Axon Instruments/Molecular Devices, Burlingame, CA).
Ventral root reflexes were quantified using custom-written software (MATLAB, The MathWorks, Natick, MA); that is, data were rectified and the earliest monosynaptic component averaged across the five trials in a window 2–6 ms poststimulation. Following dorsal root stimulation, the afferent volley arrives at the cord 0.8–1 ms later (due to root conduction delay), and then the MSR arises ~1 ms later (in vitro the synaptic delay is ~1 ms at room temperature); thus the overall monosynaptic latency is 2 ms after the stimulation. Polysynaptic reflexes arrive ~4–8 ms after the MSR, corresponding to a 6- to 10-ms latency after the stimulation (Murray et al. 2011b). Thus the 2- to 6-ms window used to average the MSR was not contaminated by polysynaptic reflexes. Average background ventral root activity before stimulation was measured over the 800 ms before the first stimulus.
The recording procedure was repeated at 15-min intervals, and 5-HT receptor agonists were added immediately after each recording, giving them time to fully act by the next recording session (15 min later). Cumulative dose-response relations were computed by increasing agonist doses at these 15-min intervals (0.003, 0.01, 0.03. 0.1, …, 30 µM doses used). Antagonists took longer to act, and responses reached near steady state typically >30 min after application, at which time responses were averaged. The effect of some agonists on the reflexes were reversible on washout of the agonist, but full recovery to baseline only occurred after several hours, likely due to the large size of the whole cord preparation. Thus washout of agonists was not feasible between doses of the agonists used in the construction of dose-response relations.
C-fiber stimulation.
To activate C-fibers in isolation, we took advantage of their resistance to a low dose of TTX (100–200 nM). We and others have found that this TTX dose blocks all afferents except small C-fibers (Lucas-Osma et al. 2018; Russo et al. 2000), effectively isolating them for subsequent electrical stimulation. To use this TTX without affecting the spinal cord, we led a long dorsal root out of the main bath through a grease barrier into a side bath containing the low-dose TTX and then stimulated this root at C-fiber intensity (50×T, 5-ms pulse).
Drugs and solutions.
The mACSF was composed of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. The nACSF was composed of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. Drugs were added to the nACSF as indicated in the text, including: 5-HT, (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI), LP44, and pargyline (obtained from Sigma-Aldrich, St. Louis, MO); 2-methyl-5-HT, 5-carboxyamidotryptamine maleate (5-CT), (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT), α-methyl-5-HT, BW723C86, EMD386088, granisetron, LY344864, methylergonovine, methysergide, MK-212, citalopram, paroxetine, SB216641, SB206553, SB224289, and GR127935 (obtained from Tocris); N,N-diethyllysergamide (LSD) and TTX (TTX-citrate; obtained from Toronto Research Chemicals, Toronto, Canada); and zolmitriptan (kindly donated by AstraZeneca Canada). All drugs were first dissolved as a 10–50 mM stock in water before final dilution in ACSF, with the exception of BW723C86, EMD386088, LP44, methylergonovine, and SB224289, which were dissolved in minimal amounts of DMSO (final concentration in ACSF <0.04%; by itself, DMSO had no effect on the LLR in vehicle controls).
Immunolabeling.
Rats were euthanized with Euthanyl (700 mg/kg; BimedaMTC) and perfused intracardially with 100 ml of saline containing sodium nitrite (1 g/l; Fisher) and heparin (300 IU/l, from 1,000 U/ml stock; Leo Pharma) for 3–4 min, followed by 400 ml of 4% paraformaldehyde (PFA; in phosphate buffer at room temperature), over 15 min. Spinal cords were postfixed in PFA overnight at 4°C, cryoprotected in 30% sucrose in phosphate buffer, frozen, and cut on a cryostat NX70 (Fisher Scientific) in horizontal or transverse 20-µm sections. We mounted spinal cord sections on slides and rinsed with phosphate-buffed saline (PBS; 50 mM) containing 0.3% Triton X-100 (PBS-TX; 3 × 15-min rinses used for all PBS-TX rinses). Sections were incubated overnight at room temperature with the following primary antibodies in PBS-TX with 2% normal goat serum: rabbit anti-5-HT1D (1:50,000; provided by Dr. Andy Ahn), mouse anti-Neurofilament 200 (NF200; 1:2,000; product no. N0142, Sigma-Aldrich), sheep calcitonin gene-related polypeptide α antibody (CGRP; 1:5,000; Abcam, ab22560) and chicken myelin basic protein antibody (MBP) (1:200, Abcam, ab106583. The slides were rinsed again in PBS-TX. To visualize the labeling of 5-HT1D, NF200, CGRP, neurobiotin and MBP, fluorescent secondary antibodies were used, including goat anti-rabbit Alexa Fluor 555 (1:500; catalog no. ab150174, Abcam), goat anti-mouse Alexa Fluor 488 (1:500; catalog no. A11029, Invitrogen), donkey anti-sheep Alexa Fluor 647 (1:500; catalog no. ab150179, Abcam), Cy5-conjugate streptavidin (1:200; code 016-170-084, Jackson ImmunoResearch; for neurobiotin), or goat anti-chicken Alexa Fluor 405 (1:200; catalog no. ab175674, Abcam) in PBS-TX, applied on slides for 2 h at room temperature. After being rinsed with PBS-TX (2 × 15 min) and PBS (2 × 15 min), the slides were coverslipped in Fluoromount-G (product no. 00-4958-02; ThermoFisher Scientific, Waltham, MA).
Alternatively, to view diaminobenzidine (DAB) labeling of 5-HT1D, a biotinylated goat anti-rabbit secondary antibody (1:200; Vector ABC kit) was applied at room temperature for 2 h in PBS-TX, followed by DAB-ABC amplification according to manufacturer guidelines (ABC, Vector PK-6101; DAB, Vector SK-4100). After being rinsed with PBS-TX, the slides were serially dehydrated with alcohol, cleared with xylene, and coverslipped in Permount (Sakura Finetek, Torrance, CA). Image acquisition was performed by both conventional microscopy (for DAB) and epifluorescence (Leica DM 6000 B). Standard controls in which the primary antibody was omitted or quenched with its antigen (Potrebic et al. 2003) were used to confirm the selectivity of the antibody staining.
Data analysis.
Data were analyzed in Clampfit 8.0 (Axon Instruments) and SigmaPlot (Jandel Scientific). Data are shown as means ± SD. A Student’s t-test or ANOVA was used to test for statistical differences before and after drug applications as appropriate, with a significance level of P < 0.05. A Kolmogorov-Smirnov test for normality was applied to each data set, with a P < 0.05 level set for significance. Most data sets were found to be normally distributed, as is required for a t-test. For those that were not normally distributed, a Wilcoxon signed-rank test was instead used with a significance level of P < 0.05.
Standard sigmoidal curves were fit to the relation between agonist dose and reflex responses, with doses expressed in log units, and with a Hill slope of unity. The dose that produced 50% effect (EC50) was measured from the curve, and –log(EC50) was used to quantify the drug potency: pEC50 = −log(EC50). Also, the maximum drug-induced response (efficacy) was computed from the curve (peak of curve). For comparison with our computed potencies (pEC50), the binding affinity of each drug at the rat 5-HT receptors was also reported, with values taken from the literature (Table 1). The binding of an agonist to a receptor is expressed in terms of its Ki value (nM), which corresponds to the dose that produces 50% binding to that receptor (Knight et al. 2004). This is typically measured by the agonist’s ability to displace a standard radiolabeled ligand, such as 5-[3H]HT, from the receptor expressed in isolated cells. Binding affinity is computed as pKi = −log(Ki) (Knight et al. 2004). When possible, binding affinities of different drugs for a given receptor were taken from large studies or summary reviews (Boess and Martin 1994), usually using isolated cloned receptors. Also, high-affinity agonist-preferring binding sites were always used, measured with radioactive agonists (usually 5-[3H]HT), rather than radioactive antagonists that bind to a low-affinity site (Egan et al. 2000; Knight et al. 2004). If rat receptor Ki values were not available, human values were used instead, because these are similar for most receptors (Boess and Martin 1994).
Table 1.
5-HT receptor agonists and their receptor bind affinity
| Receptor | Agonist | Ki, nM | pKi (−log Ki) | Reference |
|---|---|---|---|---|
| 5-HT1A | 5-HT | 1.65 | 8.78 | Hoyer et al. 1985 |
| 8-OH-DPAT | 0.97 | 9.01 | Boess and Martin 1994 | |
| LP44 | 52.7 | 7.28 | Leopoldo et al. 2007 | |
| LSD | 1.00 | 9.00 | Boess and Martin 1994 | |
| Methysergide | 25.12 | 7.60 | Peroutka 1986 | |
| 5-HT1B | 5-HT | 24.49 | 7.61 | Hoyer et al. 1985 |
| α-Methyl-5-HT | 85.11 | 7.07 | Ismaiel et al. 1990 | |
| LSD | 170.0 | 6.77 | Peroutka 1986 | |
| Methylergonovine | 3.22 | 8.50 | Auch-Schwelk et al. 2000 | |
| Methysergide | 1585.0 | 5.80 | Adham et al. 1992 | |
| Zolmitriptan | 5.01 | 8.30 | Martin et al. 1997 | |
| 5-HT1D | 2-Methyl-5-HT | 388.11 | 6.40 | Adham et al. 1992 |
| 5-HT | 2.5 | 8.60 | Boess and Martin 1994 | |
| α-Methyl-5-HT | 151.36 | 6.82 | Ismaiel et al. 1990 | |
| LSD | 12.59 | 7.90 | Boess and Martin 1994 | |
| Methylergonovine | 0.65 | 9.20 | Auch-Schwelk et al. 2000 | |
| Methysergide | 7.76 | 8.11 | Boess and Martin 1994 | |
| Zolmitriptan | 0.63 | 9.20 | Martin et al. 1997 | |
| 5-HT1E | 5-HT | 6.16 | 8.21 | |
| α-Methyl-5-HT | 120.22 | 6.92 | ||
| LSD | 93.00 | 7.03 | Nichols et al. 2002 | |
| Methylergonovine | 89.12 | 7.05 | ||
| Methysergide | 323.59 | 6.49 | ||
| 5-HT1F | 5-HT | 67.60 | 7.17 | |
| α-Methyl-5-HT | 181.97 | 6.74 | ||
| LY344864 | 6.02 | 8.22 | Phebus et al. 1997 | |
| Methylergonovine | 30.90 | 7.51 | ||
| Methysergide | 13.49 | 7.87 | ||
| Zolmitriptan | 63.09 | 7.20 | Martin et al. 1997 | |
| 5-HT2A | 5-HT | 5.75 | 8.24 | Boess and Martin 1994 |
| α-Methyl-5-HT | 127.05 | 6.90 | Engel et al. 1986 | |
| DOI | 0.79 | 9.10 | ||
| LSD | 7.07 | 8.15 | ||
| Methylergonovine | 0.35 | 9.45 | Knight et al. 2004 | |
| Methysergide | 3.31 | 8.48 | Boess and Martin 1994 | |
| 5-HT2B | 2-Methyl-5-HT | 316.23 | 6.50 | |
| 5-HT | 10.23 | 7.99 | ||
| α-Methyl-5-HT | 10.47 | 7.98 | ||
| DOI | 27.54 | 7.56 | ||
| LSD | 30.00 | 7.52 | ||
| Methylergonovine | 0.50 | 9.30 | Knight et al. 2004 | |
| Methysergide | 6.31 | 8.20 | Boess and Martin 1994 | |
| 5-HT2C | 5-HT | 10.99 | 7.96 | Egan et al. 2000 |
| α-Methyl-5-HT | 2.69 | 8.57 | Knight et al. 2004 | |
| DOI | 9.30 | 8.03 | ||
| LSD | 2.80 | 8.49 | ||
| Methylergonovine | 4.57 | 8.34 | Knight et al. 2004 | |
| Methysergide | 1.00 | 9.00 | Egan et al. 2000 | |
| MK-212 | 97.72 | 7.01 | Knight et al. 2004 | |
| 5-HT3 | 2-Methyl-5-HT | 85.11 | 7.07 | Milburn and Peroutka 1989 |
| MK-212 | 29.00 | 7.54 | Glennon et al. 1989 | |
| 5-HT4 | 5-HT | 6.31 | 8.20 | Adham et al. 1996 |
| α-Methyl-5-HT | 263.03 | 6.58 | Adham et al. 1996 | |
| 5-HT5A | 5-HT | 7.94 | 8.10 | |
| 8-OH-DPAT | 50.12 | 7.30 | ||
| LSD | 3.23 | 8.49 | ||
| Methysergide | 194.98 | 6.71 | ||
| 5-HT6 | 2-Methyl-5-HT | 52.48 | 7.28 | Boess et al. 1997 |
| 5-HT | 56.23 | 7.25 | Boess and Martin 1994 | |
| LSD | 6.90 | 8.16 | Nichols et al. 2002 | |
| Methysergide | 338.84 | 6.47 | ||
| EMD386088 | 7.41 | 8.13 | ||
| 5-HT7 | 5-HT | 1.51 | 8.82 | |
| 8-OH-DPAT | 34.67 | 7.46 | ||
| LP44 | 0.22 | 9.66 | Leopoldo et al. 2007 | |
| LSD | 6.60 | 8.18 | Nichols et al. 2002 | |
| Methysergide | 12.59 | 7.90 | Boess and Martin 1994 |
Agonist values for Ki and binding affinity (pKi = –log Ki) were obtained from high-affinity agonist radioligand bindings studies cited. Each agonists is considered to activate a receptor if Ki < 400 nM and is listed with that receptor. The exception is methysergide, which is shown in the 5-HT1B receptor category for comparison with the 5-HT1D receptor, even though its affinity is >400 nM. DOI, (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride; LSD; N,N-diethyllysergamide; 8-OH-DPAT, (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide.
RESULTS
MSR in chronic spinal rats, recorded in vitro.
When a dorsal root of the sacrocaudal spinal cord was stimulated at an intensity to fully activate all proprioceptive group Ia afferents (2–3 × T), a large brief MSR usually occurred on the ipsilateral ventral roots, as shown in Fig. 1, A–B, and previously described for this preparation (Li et al. 2004b; 2004c). The same stimulation also evoked a polysynaptic reflex that produced many seconds of motoneuron activity (LLR; Fig. 1, B, G, and H), which we have previously shown to underlie muscle spasms in spastic chronic spinal rats (Li et al. 2004a). This polysynaptic reflex begins ~4–8 ms after the MSR, and thus the MSR is readily studied in isolation.
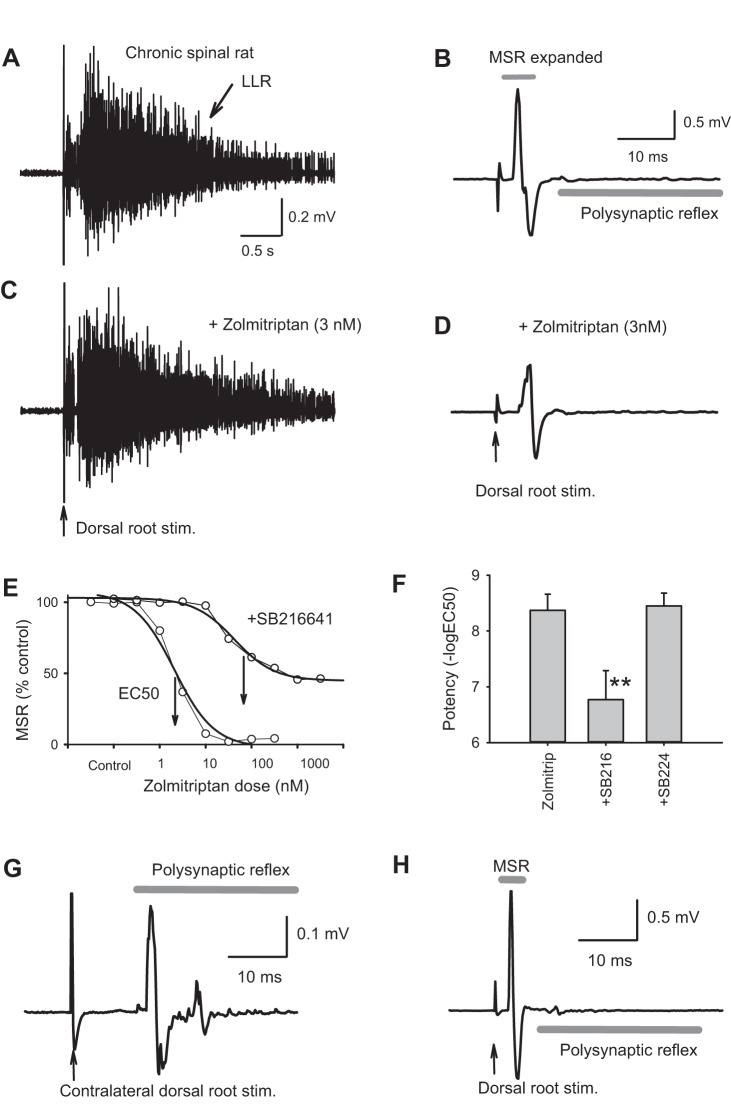
Fig. 1.
5-HT1D receptors selectively inhibit the monosynaptic reflex. A: long-lasting reflex (LLR; spasm) in a chronic spinal rat recorded from S4 sacral ventral root in response to dorsal root stimulation (nearby Ca1 caudal root, 3 × T, where T is Ia afferent threshold). B: expanded timescale of reflex in A, showing monosynaptic component (monosynaptic stretch reflex, MSR). C and D: application of low-dose zolmitriptan (3 nM) to selectively activate 5-HT1D receptors did not inhibit the LLR (C) but did inhibit the MSR (D). E: cumulative dose-response relation for inhibition of MSR by zolmitriptan before (left) and after 5-HT1D receptors were blocked with SB216641 (from a single representative rat with spinal cord injury). The EC50 indicated by arrows is shifted by 2 orders of magnitude with SB216641. Each point in the plot is 15 min apart, recorded just before addition of new dose, with a total time course of ~2 h for the entire experiment. F: group means of potency [−log(EC50)] of zolmitriptan at inhibiting the MSR, which is significantly reduced by prior application of SB216641 (+SB216), but not the 5-HT1B antagonist SB224289 (+SB224). Error bars are SD. **P < 0.05, significant change; n = 26 for each bar. G and H: polysynaptic reflex (G) and monosynaptic reflexes (H) recorded on an S4 ventral root in response to ipsilateral and contralateral Ca1 dorsal root stimulation, to show the typical latency difference between these two reflex components. Stim., stimulation.
5-HT1D receptor agonists inhibit the MSR.
Application of the selective 5-HT1D receptor agonist zolmitriptan (Table 1) significantly and potently inhibited the MSR (Fig. 1D) in chronic spinal rats, with an average inhibition of 63% (efficacy, peak reduction in MSR) and half-maximal inhibition occurring at low nanomolar doses (EC50 = 4 nM), as summarized in Fig. 1E and Table 2, where potency = −log(EC50). This extremely low EC50 is consistent with activation of the 5-HT1D receptors, which selectively and potently bind zolmitriptan (Table 1). Previously, we have shown that zolmitriptan decreases the long-lasting polysynaptic reflexes (LLRs) that underlie spasms after injury, but only with much higher doses (EC50 ≈ 100 nM; Murray et al. 2011b). This inhibitory action on spasms we attributed to nonselective binding of zolmitriptan to 5-HT1B and 5-HT1F receptors, the only other two receptors that bind zolmitriptan appreciably, albeit with a much lower affinity than 5-HT1D receptors. Consistent with this, we found the low nanomolar doses of zolmitriptan that decrease the MSR have no effect on the LLRs (spasms; Fig. 1, A and C; Murray et al. 2011b). Furthermore, application of the antagonist SB216641 that selectively blocks 5-HT1D and 5-HT1B receptors (at 3 µM) inhibited the action of zolmitriptan on the MSR, significantly increasing the EC50 of subsequent applied zolmitriptan by more than two orders of magnitude (Fig. 1E–F and Table 2). In contrast, the selective 5-HT1B antagonist SB224289 (3 µM) did not significantly affect the EC50 of zolmitriptan on the MSR (Fig. 1F; Table 2), whereas we previously showed that this antagonist inhibited the action of 5-HT1B agonists on the LLR and spasms (Murray et al. 2011b). Taken together, these data suggest that 5-HT1D receptors, but not 5-HT1B or 5-HT1F receptors, selectively inhibit the MSR.
Table 2.
Inhibition of the MSR by 5-HT1B agonists
| Receptor Activation | Inhibition of the MSR | Comparison with 5-HT1D Affinity |
|||||
|---|---|---|---|---|---|---|---|
| Agonist | Antagonist present | Receptors that can be activated | Efficacy, %change | pEC50 (−log EC50) | EC50, nM | pKi at 5-HT1D (−log Ki) | Relative potency (pKi − pEC50) |
| Zolmitriptan | None | 5-HT1B,1D,1F | −63.54 ± 20.35* | 8.36 ± 0.31 | 4.40 | 9.20 | −0.86 |
| Zolmitriptan | SB224289 | 5-HT1D,1F | −72.60 ± 31.07* | 8.45 ± 0.25 | 3.53 | 9.20 | −0.75 |
| Methylergonovine | None | 5-HT1B,1D,1E,1F, 5-HT2,7 | −79.24 ± 38.39* | 8.42 ± 0.17 | 3.80 | 9.20 | −0.78 |
| 5-HT | None | All 5-HTRs | −28.88 ± 11.12* | 7.62 ± 0.38 | 23.99 | 8.60 | −0.98 |
| Methysergide | None | 5-HT1D,1F, 5-HT2,5,6,7 | −93.8 ± 2.90* | 7.00 ± 0.30 | 100.00 | 8.11 | −1.11 |
| LSD | None | 5-HT1A,1B,1D,1E, 5-HT2,5,6,7 | −55.11 ± 28.95* | 6.59 ± 0.15 | 252.27 | 7.90 | −1.31 |
| α-Methyl-5-HT | None | 5-HT1B,1D,1E,1F, 5-HT2,4 | −58.75 ± 20.92* | 6.12 ± 0.17 | 765.72 | 6.82 | −0.70 |
| α-Methyl-5-HT | SB206553 | 5-HT1B,1D,1E,1F, 5-HT4 | −62.21 ± 27.20* | 6.10 ± 0.25 | 793.46 | 6.82 | −0.72 |
| 2-Methyl-5-HT | Granisetron | 5-HT1D, 5-HT2B,6 | −53.87 ± 26.89* | 5.28 ± 0.40 | 5263.23 | 6.40 | −1.12 |
| Zolmitriptan | SB216664 | 5-HT1F | −43.72 ± 27.41*† | 6.77. ± 0.51*† | 169.82 | ||
| α-Methyl-5-HT | GR127935 | 5-HT1E,1F, 5-HT2,4 | −3.99 ± 25.67*† | ND | ND | ||
| LY344864 | None | 5-HT1F | 1.55 ± 7.82 | ND | ND | ||
| DOI | None | 5-HT2A,2B,2C | 1.04 ± 3.89 | ND | ND | ||
| MK-212 | None | 5-HT2C,3 | −2.85 ± 34.10 | ND | ND | ||
| LP44 | None | 5-HT1A, 5-HT7 | 0.51 ± 12.64 | ND | ND | ||
| 8-OH-DPAT | None | 5-HT1A, 5-HT5,7 | −8.08 ± 32.42 | ND | ND | ||
| EMD386088 | GR127935 | 5-HT6 | −2.01 ± 12.32 | ND | ND | ||
Agonists with varying selectivity for the different 5-HT receptors were applied to chronic spinal rats, sometimes after prior application of 5-HT receptor antagonists to effectively make the agonist action more selective (pretreatment). The receptors that can be activated by this agonist/antagonist combination are indicated in (Ki < 400 nM; see details in Table 1). The antagonists used in combination with some drugs were SB216641 (5-HT1D/1B receptor antagonist; 3 µM), SB224289 (5-HT1B; 3 µM), GR127935 (5-HT1D/1B; 3 µM), SB206553 (5-HT2; 3 µM), and granisetron (5-HT3; 3 µM). The efficacy of these agonists in inhibiting the MSR is indicated, normalized by the predrug reflex amplitudes [100 × (postdrug − predrug)/predrug]. The potency and EC50 of these drugs at inhibiting the monosynaptic stretch reflex (MSR) are also indicated. ND, no change in efficacy with drug, and so no EC50 or potency could be detected. The relative potency is computed from the affinity and potency, as indicated.
P < 0.05, significant change in reflex with drug (efficacy); n > 8 per condition.
P < 0.05, significant decrease in potency of 5-HT after application of antagonist; n > 8 per condition.
Other less selective 5-HT1D receptor agonists (Table 1) also significantly inhibited the MSR, including 5-HT itself, as well as α-methyl-5-HT, LSD, methysergide, and methylergonovine (Fig. 2 and Table 2). Again, consistent with the involvement of 5-HT1D receptors, the 5-HT1D/1B antagonist GR127935 (3 µM) blocked this inhibition of the MSR by nonselective agonists (α-methyl-5-HT; Fig. 2F and Table 2). Despite the nonselective binding of methysergide to many 5-HT receptors, methysergide is useful because it is a 5-HT1D agonist that does not bind appreciably to 5-HT1B receptors in rats (>100 times less potent; Table 1), unlike zolmitriptan. Thus the potent inhibition of the MSR by methysergide further supports the role of 5-HT1D and not 5-HT1B receptors in inhibiting the MSR. Methysergide is also peculiar because it is an antagonist, rather than an agonist, to many other 5-HT receptors, including the 5-HT2 receptors that are known to facilitate the LLR. Thus methysergide does not by itself inhibit the LLR in chronic spinal rats, because there is no 5-HT present to antagonize after spinal transection (Murray et al. 2010).
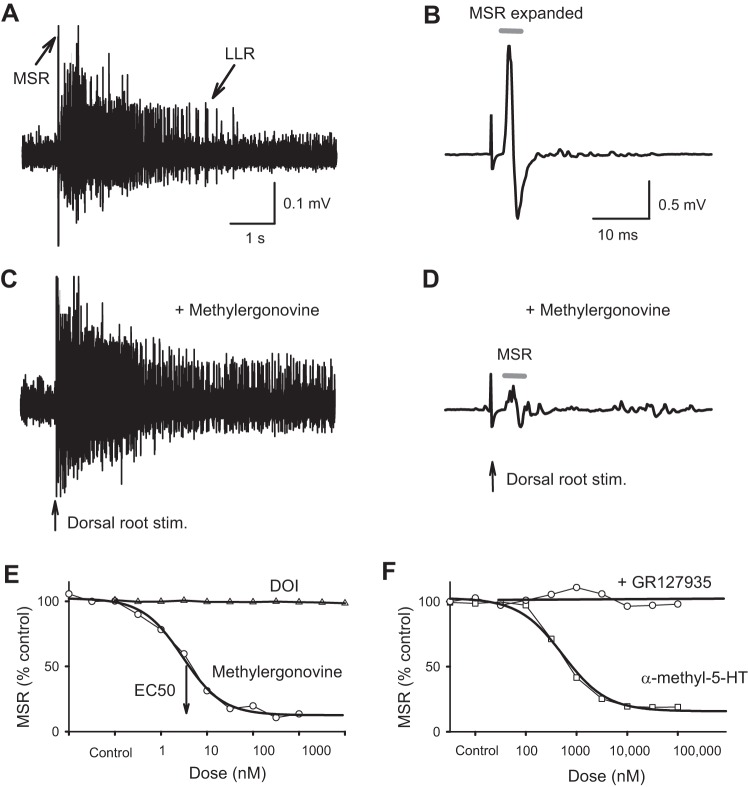
Fig. 2.
Monosynaptic stretch reflex (MSR) and long-lasting reflexes (LLRs; spasms) are independently modulated by 5-HT1D and 5-HT2 receptors, respectively. A and B: LLR underlying muscle spasms and MSR evoked by dorsal root stimulation in chronic spinal rat (B shows an expanded timescale of reflex in A; details as in Fig. 1). C and D: the mixed 5-HT1D and 5-HT2 agonist methylergonovine (3 nM) increases the LLR and decreases the MSR. 5-HT2 receptors are known to selectively inhibit the LLR. E: the selective 5-HT2 agonist (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) does not affect the MSR, whereas methylergonovine inhibits the MSR, with a nanomolar E50 consistent with its very high affinity to 5-HT1D receptors (cumulative dose-response from representative rat as in Fig. 1E; see group means in Table 1). F: another mixed 5-HT1D and 5-HT2 agonist, α-methyl-5-HT, acts similarly, simultaneously increasing the LLR (Murray et al. 2011a) and decreasing the MSR. The 5-HT1D antagonist GR127935 blocks the inhibition of the MSR by α-methyl-5-HT (cumulative dose-response relations for representative rat; see group means in Table 1). Stim., stimulation.
In contrast, application of methylergonovine, a potent agonist of both 5-HT1D and 5-HT2 receptors (Table 1; also called methylergometrine) had dramatically opposing actions on the MSR and LLR. That is, low nanomolar doses of methylergonovine significantly inhibited the MSR (Fig. 2, D and E) and at the same time increased the LLR (spasms; Fig. 2C; see details of LLR action in Murray et al. 2011a). Similar results occurred for other mixed 5HT2 and 5-HT1 agonists such as 5-HT, α-methyl-5-HT, and LSD (Fig. 2F and Tables 1 and 2; Murray et al. 2011a). Along with the increased LLR, there was more overall motoneuron excitability with these mixed agonists, as previously detailed (Murray et al. 2010, 2011a); thus the opposing action of mixed 5-HT2 and 5-HT1D agonists suggests that the inhibition of the MSR by 5-HT1D receptors does not depend on postsynaptic motoneuron changes, but rather presynaptic action on afferents. Furthermore, 5-HT2 receptor antagonists (SB206553; Table 2) did not block the inhibition of the MSR by α-methyl-5-HT, even though they inhibited the 5-HT2 receptor-mediated facilitation of the LLR by α-methyl-5-HT (Murray et al. 2011b).
Agonists to other 5-HT receptors do not inhibit the MSR.
Application of other 5-HT receptor agonists that do not appreciably activate 5-HT1D receptors (Table 1) did not significantly reduce the MSR (Table 2 and DOI in Fig. 2E), further ruling out the involvement of these receptors. These drugs lacking action included LY344864 (5-HT1F agonist), MK-212 (5-HT2C, 5-HT3), DOI (5-HT2A/B/C), LP44 (5-HT1A, 5-HT7), BW723C86 (5-HT2B), EMD386088 (5-HT6), and 8-OH-DPAT (5-HT1A, 5-HT5, 5-HT7). This suggests that none of these associated receptors modulate the MSR. We did not test agonists selective to 5-HT1E and 5-HT4 receptors over the 5-HT1D receptor, and instead used other methods to rule out action of these receptors. In the presence of the 5-HT1D/B antagonist GR127935, α-methyl-5-HT did not significantly decrease the MSR (Fig. 2F and Table1), even though this agonist then acted selectively at just 5-HT1E, 5-HT1F, 5-HT2, and 5-HT4 receptors, thus ruling out these receptors, as well.
Agonist potency on MSR is correlated to agonist binding affinity to 5-HT1D receptors.
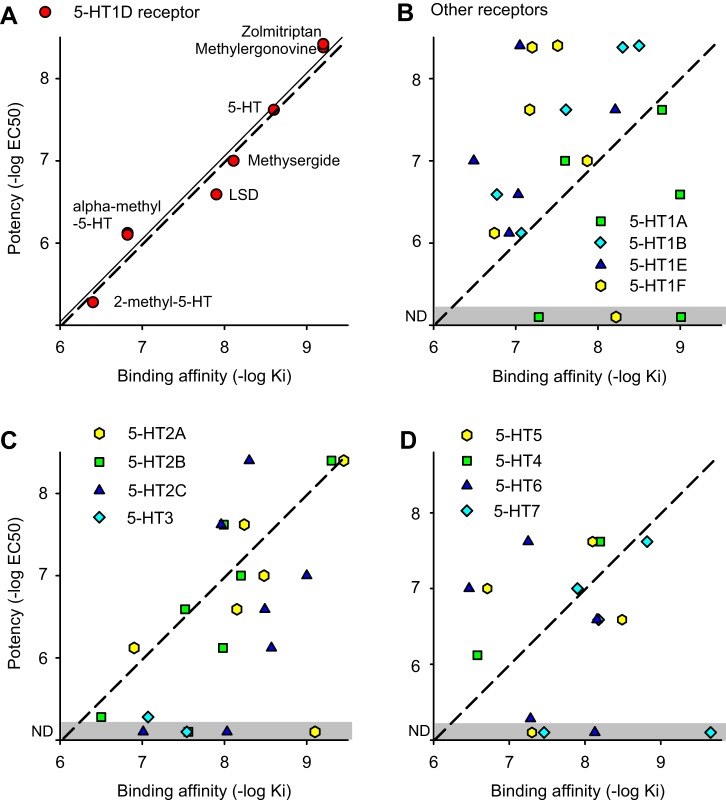
Although the observed doses to inhibit the MSR (EC50 values) varied by orders of magnitude from drug to drug (Table 2), this variation was largely accounted for by the known binding affinities of these drugs to the 5-HT1D receptor (Table 1). That is, a significant linear relation was found between the binding affinities of each drug to the 5-HT1D receptor [pKi = −log(Ki)] and the potency of the drug at inhibiting the MSR [Fig. 3A; where potency = pEC50 = −log(EC50); for example, pEC50 = 9.0 if EC50 = 1 nM]. Overall, the affinity and potency were highly correlated (correlation coefficient r = 0.91; thin line in Fig. 3A; P < 0.05, n = 7, significant). The form of the linear relationship was approximately pEC50 = pKi − 1.0 (dashed line in Fig. 3A), as observed for other receptors in our whole cord in vitro preparation (Murray et al. 2011a, 2011b). In contrast, the potency of 5-HT receptor agonists in regulating the MSR was not significantly correlated to the binding affinity of these agonists to other 5-HT receptors, including the closely related 5-HT1 receptors and 5-HT2, 5-HT5, 5-HT6, and 5-HT7 receptors (r < 0.50; P > 0.05; Fig. 3, B–D). The correlation could not be assessed for 5-HT3 and 5-HT4 receptors because only two agonists to these receptors were tested. However, the potency obtained with these agonists was far from the potency predicted by the binding affinities for 5-HT3 and 5-HT4 receptors (dashed lines in Fig. 3; see relative potency discussion below), and one such 5-HT3 agonist had no detectable effect on the MSR at all (MK-212).
Fig. 3.
Potency of the agonists at inhibiting the monosynaptic stretch reflex (MSR) is correlated to their binding affinity [−log(Ki)] to 5-HT1D receptors. A: mean potency of each agonist from Table 2 plotted against the affinity of these agonists to 5-HT1D receptors. Thin line shows linear regression (significant correlation coefficient r = 0.91, P < 0.05, n = 7). Dashed line shows unity slope. Each drug is indicated next to the red circle representing its response. Data are for chronic spinal rats. B–D: agonists to other 5-HT receptors (from Table 1) plotted in the same way as in A, showing no relation between agonist potency at inhibiting the MSR (no significant correlation, P > 0.05; Table 2) and agonist binding affinity to each receptor (drug names associated with each affinity are indicated in Table 1). ND, no detected response (gray zone).
Relative potency can be used to confirm involvement of 5-HT1D receptors.
To directly compensate for the variable receptor binding affinity of different agonists, we computed the potency of each agonist in regulating the MSR relative to its binding affinity at each receptor, which we term the relative potency: pEC50 − pKi (Murray et al. 2011a, 2011b). This essentially gives a measure of the effectiveness of each drug at a receptor, after the known differences in drug dose to bind to a receptor are taken into account, and thus the relative potency should be similar for all drugs that bind to a common functional receptor. Indeed, for the 5-HT1D receptor we found that the relative potency of all 5-HT1D agonists was invariant, and on average −0.94 ± 0.22 (thin line in Fig. 3A, pEC50 − pKi = −0.94), consistent with each drug acting at a common receptor (5-HT1D). Furthermore, each 5-HT1D agonist had a relative potency within 2 SD from −1.0 (the confidence interval; Table 2). Remarkably, this relative potency value of about −1.0 was observed for three other functional 5-HT receptors in our preparation (Murray et al. 2011a, 2011b), though different for adrenergic receptors (Rank et al. 2011). Thus, in this preparation, if a 5-HT receptor is functional, then the relative potency pEC50 − pKi = −1.0, which indicates that the EC50 is an order of magnitude greater than the Ki dose. This is likely because of common diffusion barriers, such as the pia and dense white matter, impeding the drugs from reaching the receptor, or drug metabolism (Murray et al. 2011a, 2011b). In contrast, the agonist binding affinity (pKi) and associated Ki values are typically computed by applying agonists to cloned receptors in cultured cells, where there are no diffusion barriers (see methods), and lower Ki doses are measured, compared with our EC50 values.
In contrast to the 5-HT1D receptor, other 5-HT receptors had highly variable relative potency values for inhibiting the MSR (difference in potency from Table 2 and affinity in Table 1), with at least one agonist for each receptor showing relative potencies more than 2 SD from −1.0 (the confidence interval). This is consistent with the lack of correlation of affinity and potency for these other receptors and provides additional support for the lack of involvement of these receptors in cases when there are only a few agonists tested at a given receptor. For example, the computed relative potency of zolmitriptan for the 5-HT1F receptor was too high for this receptor to be involved (0.34 greater than −1.0; outside confidence interval), since this drug has a much lower affinity for 5-HT1F than for 5-HT1D receptors. Likewise, the action of methysergide on the MSR was not potent enough to be accounted for by the 5-HT1B receptor, because the relative potency was 0.2, again significantly greater than −1.0 (>2 SD), whereas methysergide has a relative potency for 5-HT1D receptors very close to −1.0 (−0.88, well within the 2 SD confidence interval). Furthermore, 5-HT and α-methyl-5-HT both had relative potencies computed for the 5-HT4 receptor of about −0.3, which is too high to be accounted for by this 5-HT4 receptor (>2 SD from −1.0).
5-HT1D receptors are not endogenously active in chronic spinal rats.
To explore possible endogenous activity in 5-HT1D receptors, we next examined the effects of 5-HT1D receptor antagonists and inverse agonists alone in chronic spinal rats (Brys et al. 2000). The antagonists GR127935 and SB216641 did not increase the MSR in all rats tested (Fig. 4A; n = 12/12 for each drug applied at 3–5 µM), even though they antagonized the inhibition of the MSR by zolmitriptan (Fig. 4B), as detailed above (Table 2). These two drugs are known to only competitively block 5-HT1D receptors that have been activated by 5-HT or other ligands (as in Fig. 1; also partial agonists) and not block constitutive receptor activity (Price et al. 1997; Watson et al. 1996). Thus the lack of increase in the MSR with these drugs indicates that there is no endogenous 5-HT producing tonic inhibition of the MSR by activating the 5-HT1D receptor, consistent with the complete loss of functional 5-HT after spinal transection injury (Murray et al. 2010). Other drugs that are known to block constitutive activity in the 5-HT1D receptor (inverse agonists), including high doses of SB224289 (30 µM) and methiothepin (3 µM) (Audinot et al. 2001; Millán et al. 2001), also did not increase the MSR in any of the roots tested (Fig. 4C; n = 12/12 each). This suggests that there was no constitutive activity in the 5-HT1D receptors after injury. Whereas SB224289 is selective to 5-HT1B receptors at low doses (3 µM has no action in Fig. 1), it also binds the 5-HT1D receptors at this 10-fold higher dose and acts as an inverse agonist (Audinot et al. 2001; Millán et al. 2001; Selkirk et al. 1998).
Fig. 4.
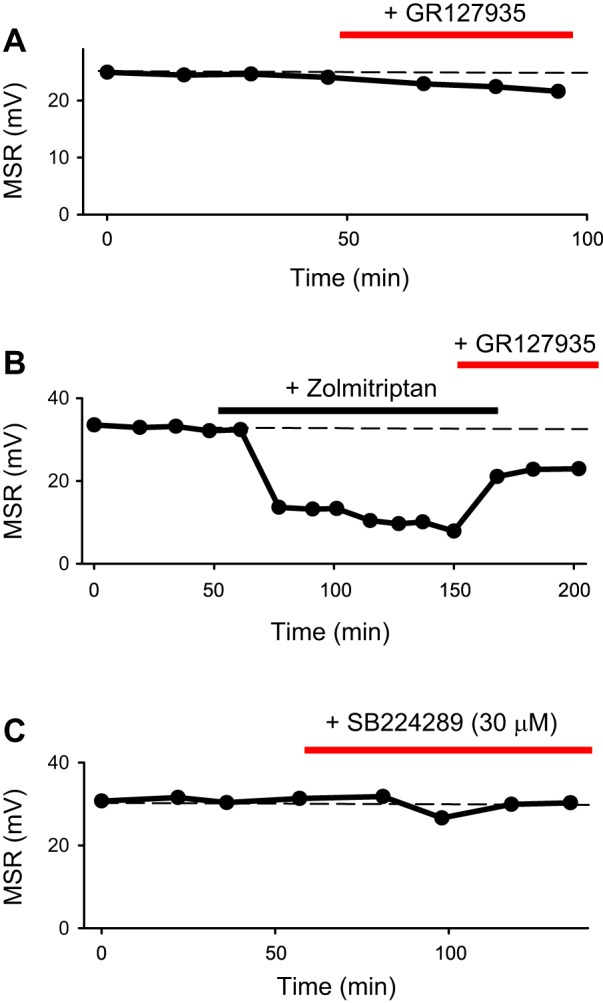
5-HT1D receptors are not endogenously active after spinal cord transection. A: mean monosynaptic stretch reflex (MSR) in S4 ventral roots in response to dorsal root stimulation (as in Fig. 1), plotted over time in a chronic spinal rat. The 5-HT1D antagonist GR127935 (5 µM) by itself does not increase the MSR, indicating no endogenous 5-HT activating this receptor. The slight decrease in MSR is likely due to the weak partial agonist action of GR127935. B: in contrast, GR127935 reduced the inhibition of the MSR by zolmitriptan (3 nM). C: at high doses, SB224289 (30 µM) is an inverse agonist at the 5-HT1D receptor but does not increase the MSR, indicating that there is no constitutive 5-HT1D receptor activity after spinal cord injury. n = 12 per condition, with similar results for each (12/12).
5-HT1D receptors also inhibit the MSR in normal rats, with similar potency to that in chronic spinal rats.
The sacrocaudal spinal cords from normal rats also exhibited an MSR (Fig. 5A), although these MSRs were significantly smaller than in chronic spinal rats, by half (14.12 ± 13.25 compared with 24.90 ± 12.58 mV; n = 12, P < 0.05). Nevertheless, these MSRs were significantly inhibited by the selective 5-HT1D receptor agonist zolmitriptan, just as in chronic spinal rats, with an inhibition of 82.21 ± 26.38% (efficacy; n = 12), potency of 8.48 ± 0.20 [−log(EC50)], and corresponding EC50 of 3.32 nM (Fig. 5, A and B). Interestingly, compared with chronic spinal rats, the MSR in normal rats was just as sensitive to this 5-HT1D receptor agonist (potency 8.48 ± 0.20; not significantly different, P > 0.05, n = 12; Table 2). This is in contrast to the marked supersensitivity of chronic spinal rats to 5-HT2 receptor agonists compared with the sensitivity of normal rats to 5-HT2 receptor agonists (PIC and LLR responses; Harvey et al. 2006a).
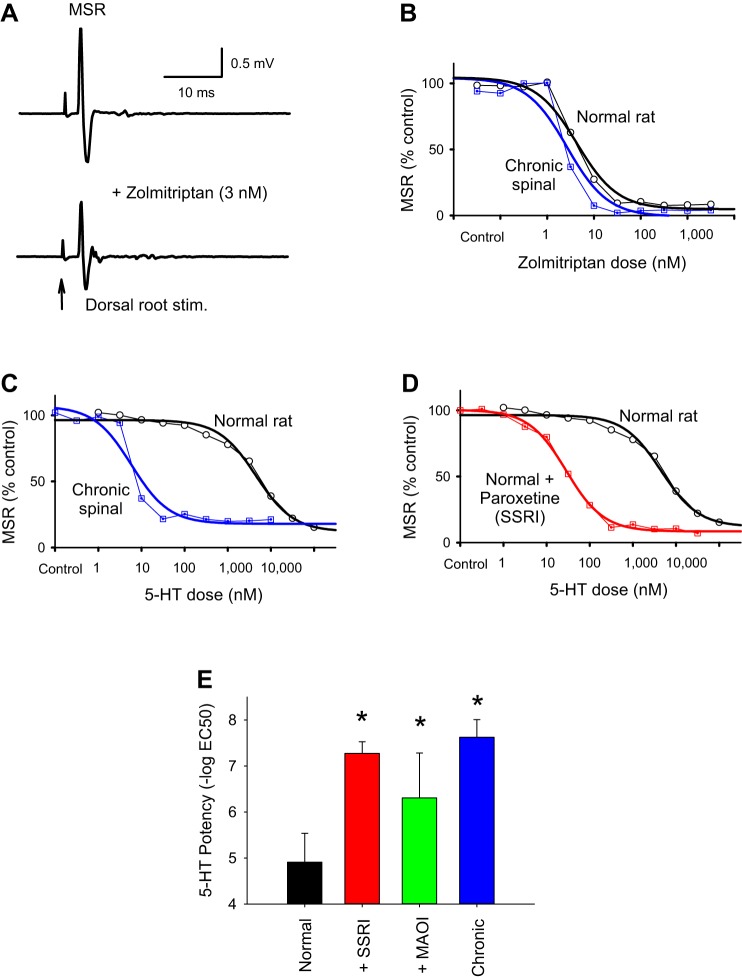
Fig. 5.
Supersensitivity of monosynaptic stretch reflex (MSR) to 5-HT is due to a loss of the serotonin transporter SERT and monoamine oxidase (MAO) after spinal cord injury (SCI). A: MSR in a normal rat, before and after low-dose zolmitriptan (3 nM; same parameters as in Fig. 1A). B: the cumulative dose-response relation for zolmitriptan inhibiting the MSR (as in Fig. 1E) is nearly identical in normal rats and chronic spinal rats (blue), suggesting that the 5-HT1D receptor is not itself supersensitive after SCI. Data are from single representative normal and injured rats. C: in contrast, the cumulative dose-response relation for 5-HT is shifted by over 2 orders of magnitude after SCI, with much less 5-HT needed to inhibit the MSR in chronic spinal rats. D: blocking SERT with paroxetine (5 µM; a selective serotonin reuptake inhibitor, SSRI; red) in normal rat mimics the chronic spinal rat, with much less 5-HT needed to inhibit the MSR. Because 5-HT is transported by SERT and zolmitriptan is not, these results indicate that the supersensitivity to 5-HT is due to a loss of SERT after SCI. E: mean potency of 5-HT at inhibiting the MSR in normal rats (n = 12; black) and an increase in potency with SERT block (+SSRI: paroxetine or citalopram, 10 µM; n = 16 normal rats; red) or MAO block (MAO inhibitor: pargyline, 20 µM; n = 12 normal rats; green) to levels not different from that in chronic spinal rats (n = 12; blue; without drugs). *P < 0.05, significant change. Error bars are SD.
SERT changes the sensitivity of the MSR to 5-HT.
In contrast to zolmitriptan, 5-HT itself inhibited the MSR much less potently in normal uninjured rats compared with in chronic spinal rats, with up to 100–1,000 times more 5-HT needed to inhibit the MSR in normal rats (Fig. 5, C and E). Thus the MSR is supersensitive to 5-HT, but not zolmitriptan, after SCI. The lack of supersensitivity to zolmitriptan rules out a simple upregulation of 5-HT1D receptors producing the supersensitivity after SCI. A key difference between triptans (zolmitriptan) and 5-HT is that triptans are not taken up by the major high-affinity serotonin transporter SERT, unlike 5-HT and 5-HT2 receptor agonists such as α-methyl-5-HT (Henry et al. 2006; Honda et al. 2006). We thus turned to evaluating the role of SERT, because spinal transection eliminates SERT together with the loss of brain stem-derived 5-HT fibers (Hayashi et al. 2010; Murray et al. 2010). We reasoned that a loss of SERT with spinal transection would allow our exogenously applied 5-HT to more easily reach the receptors, unimpeded by this high-affinity transporter (Blakely et al. 1994). To mimic spinal transection, we applied the SERT blocker paroxetine (a selective serotonin reuptake inhibitor, SSRI) in normal rats and found that the MSR was then significantly more sensitive to 5-HT, and not significantly different from the high sensitivity in chronic spinal rats (Fig. 5, D and E). Likewise, monoamine oxidase (MAO) inhibitors (MAOI) increased the sensitivity of the MSR to 5-HT in normal rats (Fig. 5E). In contrast, SERT or MAO inhibitors had no effect on the MSR in chronic spinal rats (not shown; n = 12/12 each), consistent with a lack of SERT and MAO with spinal transection. Taken together, these results suggest that applied 5-HT is normally rapidly taken up into monoamine fibers by SERT and continuously metabolized by MAO in these fibers (Li et al. 2014), and this function is lost after SCI.
Oddities in the action of 5-HT1D receptors.
Interestingly, the amount of inhibition of the MSR by zolmitriptan (efficacy) was significantly greater in normal compared with chronic spinal rats (82% compared with 63% inhibition; Table 2). Furthermore, a small fraction of ventral root MSRs in chronic spinal rats did not respond at all to zolmitriptan (6/32). These nonresponders were not included in the group averages in Table 2 (because an EC50 value could not be determined from them), whereas including them further decreased the efficacy of zolmitriptan on the MSR to 51% inhibition. This is striking considering that in normal rats, the MSR was always affected by zolmitriptan and on most ventral roots largely eliminated the MSR (Fig. 5A). Possibly the nonresponding ventral roots in chronic spinal rats had reflexes that were saturated, and indeed these were always the roots that had the largest reflexes to start with (overall, chronic spinal rats had larger reflexes, as mentioned above).
Another oddity we found with zolmitriptan was that it irreversibly inhibited the MSR, with long periods of washing out the drug not reverting the reflex back to control levels in normal and injured rats. Although drugs are in general difficult to wash from the large whole sacrocaudal spinal cord preparation, zolmitriptan was completely resistant to the wash.
5-HT1D receptors are restricted to small primary sensory afferents and are not on spinal neurons.
Considering that 5-HT1D receptors potently inhibit the MSR while having no direct effect on motoneurons (Murray et al. 2011b), we wondered whether this receptor was on the large proprioceptive afferents involved in the MSR. To examine this, we employed a highly selective 5-HT1D receptor antibody to explore its distribution in the spinal cord (Ahn and Basbaum 2006; Potrebic et al. 2003). In spinal cords from both normal and chronic spinal rats, intense 5-HT1D-positive immunolabeling was seen in the dorsal root entry zone and superficial dorsal horn (Fig. 6, A and B), consistent with previous reports in normal rats (Ahn and Basbaum 2006; Potrebic et al. 2003). Interestingly, this 5-HT1D labeling was exclusively on fine beaded fibers confined to the superficial dorsal horn laminae I–III, with a distribution and morphology that suggests they may be axons of C-fibers (Fig. 6, C and D, arrows). Indeed, many of these 5-HT1D-positive fibers appeared to be connected to the dorsal roots, sometimes even expressing 5-HT1D within the dorsal root (Fig. 6D, arrowhead). In contrast, 5-HT1D labeling was absent from large-diameter axons. In particular, no labeling was seen in large axons in the ventral horn, ruling out 5-HT1D receptors being on the proprioceptive afferents that project to motoneurons (Vincent et al. 2017), as detailed further below. We occasionally saw weak 5-HT1D labeling on small γ-motoneurons in the ventral horn, although these neurons are not involved in the MSR and so we did not pursue this further (Enjin et al. 2012). With the exception of these γ-motoneurons, 5-HT1D immunolabeling was not seen anywhere outside the superficial dorsal horn and was absent from cell bodies or major axon tracts in the spinal cord, including being absent from large α-motoneurons (Fig. 6, A and B). Also, the 5-HT1D immunolabeling intensity and dorsal distribution were not different in chronic spinal rats (Fig. 6B) compared with uninjured rats (Fig. 6A), indicating that descending axons (degenerated in latter rats) do not appreciably contain 5-HT1D receptors.
Fig. 6.
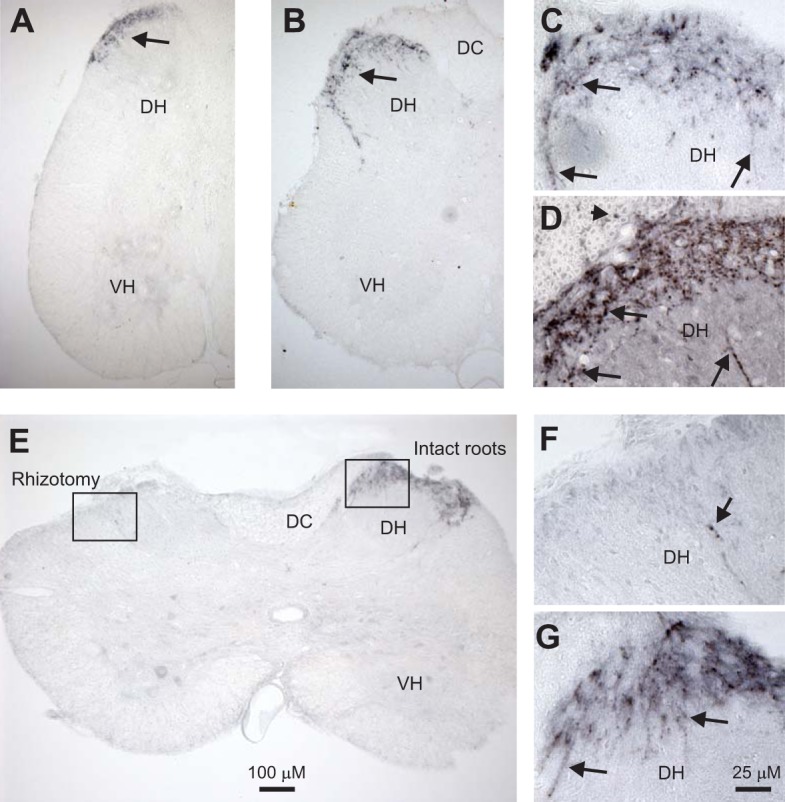
5-HT1D receptors are mostly expressed on small sensory afferents in the superficial dorsal horn. A: 5-HT1D receptor immunolabeling (black, diaminobenzidine) in sacral spinal cord of a normal rat. B: similar immunolabeling in a chronic spinal rat, caudal to injury. Note immunolabeling is only in the superficial dorsal horn (DH; at arrows). Also, labeling is absent from the dorsal columns (DC), where large propriospinal axons travel, and mostly absent from the ventral horn (VH), where propriospinal afferents synapse onto motoneurons in the monosynaptic stretch reflex (MSR) pathway. Very weak labeling of small γ-motoneurons was sometimes seen in the VH (see also E). C and D: magnified immunolabeling in the DH of normal and injured rats, respectively, showing labeling on fine beaded fibers confined mostly to the superficial laminae (arrows). Labeling was also seen on fine fibers in the dorsal root (arrowhead in D), consistent with the 5-HT1D labeling arising from fine sensory C-fibers. E: cutting the dorsal roots on the left side (rhizotomy) 4 wk before tissue fixation eliminated most labeling, indicating that the 5-HT1D receptors were mostly on fine sensory afferents. F and G: magnified images of the DH on the left and right sides (boxes) in E, showing only one weakly labeled fiber after rhizotomy. Scales for low- and high-magnification images are shown in E and G, respectively; n = 5 animals per condition, all showing the same results.
To determine the extent to which the 5-HT1D expression arose from afferents, rather than from dorsally located interneurons, we next removed the dorsal roots from the left side of chronic spinal rats (rhizotomy). When these animals were examined 4 wk later after the degeneration of sensory afferents, we observed a near complete loss of 5-HT1D-positive fibers on the side of the dorsal rhizotomy (Fig. 6E), with only a few fibers remaining in the entire spinal cord (Fig. 6F, arrow). These few fibers likely arose from extremely small dorsal root fragments that attach to the cauda equina and are thus not easy to detect or transect (see methods). In contrast, on the side with intact dorsal roots, the usual intense pattern of 5-HT1D-positive fibers at the dorsal root entry zone was observed (Fig. 6E, right, and expanded in Fig. 6G) and not different from normal or chronic spinal rats. Similar results of rhizotomy on 5-HT1D expression were seen in lumbar (not shown) and sacral cords (Figs. 6 and 7). These data indicate that the majority of the 5-HT1D receptors in the spinal cord are restricted to small sensory afferents, likely C-fibers (or Aδ).
Fig. 7.
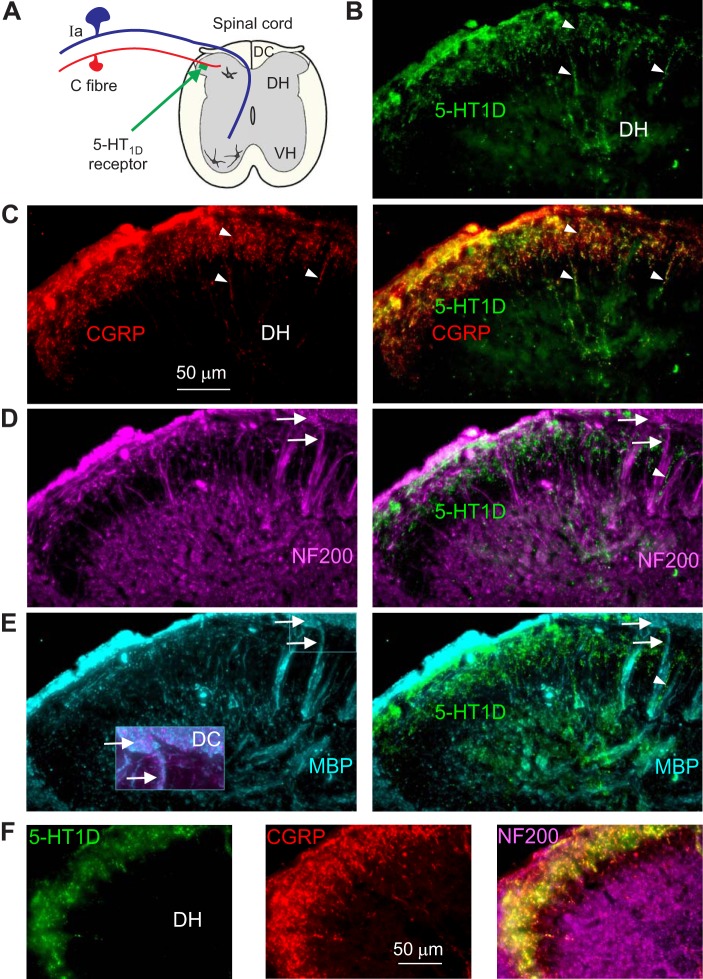
5-HT1D receptors colocalize with small CGRP-positive C-fibers and are absent from large myelinated sensory afferents. A: schematic of spinal cord showing 5-HT1D receptors on C-fibers. B: immunolabeling for 5-HT1D receptors (green fluorescent microscopy) in the dorsal horn (DH), with examples of fine labeled fibers marked with arrows. Image shows normal uninjured tissue from sacral spinal cord. C: immunolabeling for CGRP (red) and extensive colocalization with 5-HT1D receptors (yellow and orange). D: immunolabeling for neurofilament (NF200; purple) to show large axons and neurons in the spinal cord. Large proprioceptive afferents project from the dorsal columns (top right corner) in bundles (arrows) that descend to the motoneurons. The 5-HT1D labeling (green; from B) is not colocalized with NF200, including being absent from large proprioceptive afferents, even though some fine 5-HT1D-labeled fibers (arrowhead) traveled parallel to NF200-labeled axons (arrows). E: immunolabeling for myelin basic protein (MBP; blue) colocalizes with large myelinated NF200-positive afferents (arrow) arising from the dorsal columns (DC; inset), but these again lack 5-HT1D receptor labeling. F: immunolabeling of DH in chronic spinal rat (caudal to injury), again showing colocalization of 5-HT1D and CGRP (yellow) and lack of colocalization with NF200 (purple). n = 5 rats per condition, all showing similar results, including 5-HT1D expression.
5-HT1D receptors are confined to C-fibers and absent from large afferents.
To confirm that the 5-HT1D immunolabeling was exclusively on C-fibers, we immunolabeled C-fibers with the neuropeptide CGRP (Fig. 7C). As expected, we found extensive colocalization of the 5-HT1D receptor and CGRP immunolabeling, with CGRP and 5-HT1D labeling seen on very fine beaded fibers confined to the superficial dorsal horn (Fig. 7, B and C, arrowheads), as seen with 5-HT1D staining alone (Fig. 6). In contrast, labeling large myelinated axons with a neurofilament antibody (NF200) and a myelin basic protein (MBP) antibody revealed no colocalization with 5-HT1D immunolabeling with large axons (Fig. 7, D and E). Small myelinated axons (including Aδ-fibers) also lacked 5-HT1D immunolabeling. The large NF200-positive axons colocalized MBP but mostly avoided the superficial dorsal horn regions innervated by CGRP-positive C-fibers. These NF200-positive axons included large proprioceptive afferents in the dorsal roots that projected to the dorsal columns (Fig. 7E, inset expanded) and then dove ventrally in bundles to the ventral horn (Fig. 7, D and E, arrows), consistent with the projection patterns of proprioceptive afferents (Burke and Glenn 1996; Lucas-Osma et al. 2018; Nicol and Walmsley 1991; Vincent et al. 2017). These NF200-positive axons and their terminal boutons lacked 5-HT1D immunolabeling. When we filled large afferents in the dorsal root with neurobiotin, once again these axons and their terminal arbors lacked 5-HT1D immunolabeling in the spinal cord (not shown; n = 10/10). 5-HT1D was similarly colocalized with CGRP-positive C-fibers in uninjured and chronic spinal rats (Fig. 7, C and F, respectively). Taken together, these data indicate that 5-HT1D receptors are selectively expressed on C-fibers in the spinal cord. Importantly, 5-HT1D receptors are not expressed on any large or myelinated afferents and, in particular, are absent from large proprioceptive afferents involved in the monosynaptic stretch reflex.
C-fiber activation facilitates the MSR, and 5-HT1D receptors block this facilitation.
Considering that 5-HT1D receptors are mainly on C-fibers and somehow inhibit the MSR, we next examined the effect of C-fiber activation on the MSR. C-fibers were selectively activated 300 ms before MSR testing, by electrically stimulating a long dorsal root (50 × T; S4) led out of the main bath through a grease barrier into a side bath containing low-dose TTX (100–200 nM) to block all afferents except TTX-resistant C-fibers (see methods) (Lucas-Osma et al. 2018; Russo et al. 2000). Following this selective C-fiber stimulation, the MSR was significantly increased (to 214 ± 151% of control; average of 10 trials at 10-s intervals; n = 20, P < 0.05), without changes in background ventral root activity at the time of MSR testing, implying that the C-fiber activity somehow presynaptically facilitates the MSR. Application of zolmitriptan decreased the MSR, as already described, but the residual MSR was no longer facilitated by the same C-fiber stimulation (90 ± 105% of control, P > 0.05), indicating that zolmitriptan does indeed act via inhibiting C-fibers.
DISCUSSION
5-HT1D receptors exclusively inhibit the MSR.
Our results demonstrate that 5-HT1D receptors strongly inhibit the MSR in the sacrocaudal spinal cord of adult normal and chronic spinal rats, whereas no other 5-HT receptor influences the MSR. In particular, all 5-HT1D receptor agonists tested inhibit the MSR with a potency highly correlated to the binding affinity of these agonists to the 5-HT1D receptor, and this inhibition is only reduced by selective 5-HT1D antagonists. Furthermore, selectively activating other 5-HT receptors, including 5-HT1B and 5-HT1F receptors, does not inhibit the MSR. Thus the MSR is controlled by a different 5-HT receptor than the receptor that inhibits the polysynaptic reflexes that trigger muscle spasms (5-HT1B) and the receptors that control motoneuron excitability (5-HT2B/C) in this rat preparation (Murray et al. 2010, 2011b) and also in humans (D’Amico et al. 2013a, 2013b). These results further support the idea that separate pathways control the MSR and muscle spasms, suggesting that clinically we may be able to design drugs that selectively inhibit clonus (MSR), muscle spasms, or excessive motoneuron excitability.
The conclusions drawn from our in vitro recordings are consistent with the recordings from in vivo chronic spinal rats, where endogenously released 5-HT is also shown to inhibit the MSR via the 5-HT1D receptor (Honda et al. 2004). However, we additionally show that no other 5-HT receptor inhibits the MSR, which is in contrast to the in vivo finding that exogenously applied 5-hydroxytryptophan (5-HTP) inhibits the MSR by actions on 5-HT1B receptors in chronic spinal rats (Honda et al. 2003). Honda et al. (2004) suggested that the difference in the 5-HT receptors involved in modulating the MSR with exogenous 5-HTP vs. endogenous 5-HT results from differences in the locations of the receptors involved, with perhaps 5-HT1D receptors located in synapses and 5-HT1B receptors located extrasynaptically. However, we feel that the in vivo action of 5-HT1B receptors is likely indirect via changes in blood flow, because this receptor is on capillary pericytes that modulate blood flow and associated spinal motor function after SCI (Li et al. 2014; Li et al. 2017; Millan 2002). The exogenous 5-HTP that Honda et al. (2003) applied is a precursor that must be converted in the spinal cord to 5-HT by the enzyme AADC, and after spinal transection, AADC is for the most part only expressed on capillaries (Li et al. 2014, 2017). We recently showed that this capillary-derived 5-HT (or other amines) has potent effects on blood flow via local 5-HT1B receptors on pericytes, which indirectly influences motoneuron output (Li et al. 2017), whereas this capillary-derived 5-HT has weaker effects on more distant 5-HT receptors on neurons (Li et al. 2014).
Lack of constitutive receptor activity.
We also found that 5-HT1D receptors were not endogenously active after SCI, unlike the constitutively active 5-HT2 receptors that control motoneurons after injury (Di Narzo et al. 2015; Murray et al. 2010). This lack of plasticity in the 5-HT1D receptor action helps explain the heightened MSR after spinal cord injury. 5-HT innervation of the spinal cord is nearly entirely derived from the brain stem (Murray et al. 2010; Schmidt and Jordan 2000), and before injury, this brain stem 5-HT tends to endogenously inhibit the MSR via the 5-HT1D receptor pathway that we and others have described (Honda et al. 2004). After spinal transection, this 5-HT innervation is lost (Murray et al. 2010) and thus endogenous brain stem-derived inhibition of the MSR is removed, leaving the MSR larger and thus contributing to ongoing muscle clonus and spasticity (Heckman et al. 2005). The lack of plasticity seen in 5-HT1D receptors leaves the MSR disinhibited chronically after SCI. In contrast, 5-HT2 receptors become constitutively active to compensate for lost 5-HT, ultimately aiding the recovery of motoneuron excitability after SCI (D’Amico et al. 2013b; Murray et al. 2010, 2011a). Also, 5-HT1B receptors on capillaries become endogenously activated (via trace amines) to compensate for lost 5-HT, helping to restore capillary tone after SCI (Li et al. 2017). Interestingly, knocking out the 5-HT1D receptor at birth indirectly leads to an increased MSR, so this receptor must somehow be involved in the development and maturation of the circuits that regulate the MSR (Enjin et al. 2012). Thus it may well be that the loss of 5-HT and associated 5-HT1D receptor activity with spinal transection could also contribute indirectly to an increase in the MSR, although the mechanism for such plasticity remains to be determined.
Guarding effect of SERT, and loss of SERT causing supersensitivity to 5-HT.
Our results additionally demonstrate that the 5-HT1D receptor itself does not become supersensitive to agonists after SCI, because the agonist zolmitriptan is equally potent at inhibiting the MSR in normal and injured rats. Instead, we demonstrate that the supersensitivity of the MSR to 5-HT after SCI results from a loss of SERT and MAO after SCI, as previously suggested from indirect evidence (Honda et al. 2006). In particular, we show that 5-HT itself is much more potent at inhibiting the MSR in chronic spinal rats than in normal rats, and this lower potency in normal rats can be entirely accounted for by SERT. That is, the potent inhibition of the MSR by 5-HT in chronic spinal rats can be mimicked in normal rats by blocking SERT or MAO before applying 5-HT. Bearing in mind that SERT is present in 5-HT fibers in the spinal cord of normal but not transected or severely contused rats (Blakely et al. 1994; Hayashi et al. 2010), these results indicate that in normal rats applied 5-HT is taken up by SERT and continuously metabolized by MAO, reducing the amount of 5-HT that reaches the receptors. Essentially, SERT near receptors acts to “guard” the receptors from extrasynaptic 5-HT, and so this 5-HT is much less likely to reach receptors. In contrast, SERT is lost with SCI (Hayashi et al. 2010), and thus 5-HT reaches the receptors much more readily, making applied 5-HT or related drugs more effective. Consistent with this interpretation, the drug zolmitriptan (and related sumatriptan) is not transported by SERT (Honda et al. 2006) and accordingly affects 5-HT1D receptors equally in chronic spinal rats, normal rats, and normal rats treated with SERT blockers. Importantly, these results indicate that previous conclusions of receptor supersensitivity after SCI are likely contaminated by changes in transporters such as SERT after SCI, including our previous work (Harvey et al. 2006a). Many 5-HT agonists, such as α-methyl-5-HT, are transported by SERT (Henry et al. 2006), changing our previous interpretation of the role of supersensitivity of spasms to this drug after SCI. More generally, it is clear that to determine the actual sensitivity of 5-HT receptors to particular drugs in normal central nervous system tissue, it is necessary to block SERT (and related transporters such as the norepinephrine transporter NET) or eliminate SERT entirely (chronic spinal rat), depending on the affinity these drugs have for SERT.
5-HT1D expression on C-fibers.
Interestingly, we find that 5-HT1D receptors are exclusively expressed on small C-fibers, and not on other larger afferents or interneurons in the sacrocaudal spinal cord. Although this result complicates our interpretation of the how 5-HT1D receptors inhibit the MSR, it is broadly consistent with previous studies showing 5-HT1D receptors on small primary afferents in the lumbar cord of rats (Ahn and Basbaum 2006; Potrebic et al. 2003) and mice (Enjin et al. 2012). Like our results, Potrebic et al. (2003) found that the bulk of expression is on peptidergic nociceptive C-fibers, although they also found a small amount of expression on larger cutaneous afferents (also see review Millan 2002). Previous reports have demonstrated 5-HT1D expression on small gamma motoneurons in the spinal cord, although not on the α-motoneurons involved in the MSR (γ-motoneurons do not themselves receive a monosynaptic input) (Enjin et al. 2012). We also saw some 5-HT1D expression on small putative γ-motoneurons, although immunolabeling was weak in comparison to the expression on C fibers. Regardless of these other weak central sources of this receptor, we find that removal of all dorsal roots from one side of the cord eliminates most 5-HT1D receptor expression, demonstrating that small afferents are the main location of these receptors.
5-HT1D/B receptors are often found as autoreceptors on 5-HT terminals in the brain, inhibiting 5-HT release (Millan 2002). Thus it is interesting that we did not see 5-HT1D receptors throughout the uninjured spinal cord following the extensive innervation pattern of fine 5-HT fibers in normal rats, which includes dense ventral and intermediate lamina projections (Murray et al. 2010; Schmidt and Jordan 2000). This suggests that in many places in the spinal cord, 5-HT1D receptors do not function as autoreceptors on descending 5-HT fibers, as Millan (2002) concluded. Morphologically, 5-HT fibers appear as fine beaded fibers, like C-fibers. Thus it is possible that some of the fine 5-HT1D-labeled fibers that we see in the superficial dorsal horn of uninjured rats are 5-HT fibers. However, we did not see obvious changes in 5-HT1D expression with spinal transection, likely ruling out these 5-HT fibers containing 5-HT1D receptors in uninjured rats. Furthermore, we found that dorsal root rhizotomy eliminates all 5-HT1D, and indeed, most of the 5-HT1D expression is on CGRP-positive C-fibers (Millan 2002; Potrebic et al. 2003). Also, any minor contribution from autoreceptors on dorsal 5-HT fibers is eliminated by spinal transection. Overall, these findings indicate that autoreceptors on 5-HT fibers cannot affect the modulation of the MSR in injured rats.
Reconciling 5-HT1D inhibition of the MSR with 5-HT1D expression on C-fibers.
The finding that 5-HT1D receptors selectively inhibit the MSR somehow has to be reconciled with our finding that these receptors are not expressed on the MSR pathway and are instead exclusively expressed on C-fibers in the spinal cord (Potrebic et al. 2003). These 5-HT1D receptors must act indirectly to alter the MSR by modulating C-fiber actions, and indeed, we find that direct C-fiber activation facilitates the MSR and that 5-HT1D receptors inhibit this action. C-fiber activity and transmission to spinal neurons are more generally known to be modulated by 5-HT1 receptors, and indeed, this is partly the basis for brain-derived serotonergic control over pain transmission (Iwasaki et al. 2013; Lu and Perl 2007; Millan 2002; Todd 2010). Specifically, we know that 5-HT1D receptors inhibit transmitter release from central terminals of C-fibers (CGRP containing), although we do not know whether this is via direct 5-HT1D action on C-fibers or via indirect circuit actions (Amrutkar et al. 2012). Nociceptive C-fibers in turn are known to facilitate the MSR (Hopkins and Stencil 2002; Schomburg et al. 2007), and thus it makes sense that 5-HT1D receptors can indirectly modulate the MSR via actions on C-fibers.
In general, most C-fibers are directly inhibited by 5-HT1 receptors on the fibers themselves (Amrutkar et al. 2012; Millan 2002; Zhao et al. 2016), although the mechanisms remain unclear. A small subgroup of C-fibers that sense cool temperatures (TRPM8) are instead facilitated by 5-HT1 receptors (coupling to TRPM8) (Vinuela-Fernandez et al. 2014). However, these particular TRPM8 C-fibers indirectly inhibit effects of most other C-fibers (via GABAergic circuits) (Zheng et al. 2010), so even in this case, the net effect is an antinociceptive action of 5-HT1 receptors, essentially enhancing the analgesic effect of cooling (Vinuela-Fernandez et al. 2014). Thus overall 5-HT1 receptor action is inhibitory to C-fibers. Taken together with our findings, this suggests the following mechanism for 5-HT1D receptor action: C-fibers somehow exert an endogenous tonic facilitation over the MSR, and 5-HT1D receptors inhibit this C-fiber action, thereby inhibiting the MSR. This explanation requires C-fibers to exhibit a low level of activity or at least spontaneous transmitter release, which is actually compatible with in vivo recordings of C-fiber activity (Hulse et al. 2010). It is also plausible that the many TRP channels on the central terminals of C-fibers contribute to transmitter release in the absence of peripheral excitation, due to variations in temperature, oxygen level, and pH (even in vitro) (Szallasi and Sheta 2012; Takahashi et al. 2011; Takashima et al. 2007). This conclusion is supported by our study and other studies showing that nociceptive (C-fiber) activity increases the MSR (Hopkins and Stencil 2002; Schomburg et al. 2007), and 5-HT1D receptors block this C-fiber action. Thus, overall, our results indicate that 5-HT1D receptors function to reduce the MSR indirectly by inhibiting endogenous C-fiber-mediated facilitation of the MSR.
However, the mechanism for a modulation of the MSR with C-fiber activity and 5-HT1D receptors remains unclear. Possibly, C-fiber-activated circuits act presynaptically on proprioceptive afferents of the MSR, or alternatively, they might act postsynaptically to facilitate motoneurons. 5-HT1D receptors do not affect α-motoneuron excitability via C-fibers, because zolmitriptan does not directly affect motoneuron properties (no change in input resistance, membrane potential, spike properties, or PICs), despite dramatically inhibiting sensory-evoked polysynaptic EPSPs on motoneurons (Murray et al. 2011b). Thus 5-HT1D receptor-mediated changes in C-fiber activity and the MSR are unlikely to be mediated postsynaptically on α-motoneurons.
Presynaptic inhibition of the MSR is thought to be largely mediated by GABAA receptors on proprioceptive terminals contacting motoneurons, although other transmitters and receptors may in principle presynaptically inhibit afferent transmission, including opiate and GABAB receptors (Engelman and MacDermott 2004; Millan 2002). Thus a plausible explanation for the inhibition of the MSR by 5-HT1D receptors is an increase in GABAergic presynaptic inhibition (albeit via indirect C-fiber actions). Although this remains to be investigated, we suspect that the solution is not that simple, because C-fibers have been shown to induce a tonic facilitation of GABAA receptor-mediated primary afferent depolarization (PAD) (Lucas-Osma et al. 2018; Russo et al. 2000), and an increase in PAD is classically associated with presynaptic inhibition and a decreased, not increased, MSR (Rudomin and Schmidt 1999). Furthermore, 5-HT decreases rather than increases PAD (García-Ramírez et al. 2014), consistent with the general finding that 5-HT1D receptors decrease C-fiber activity (and thus PAD). However, our recent finding that C-fiber activity evokes a GABAA receptor-induced tonic PAD that facilitates spikes in proprioceptive afferents might help explain the increased MSR with C-fiber activity and the inhibitory effect of 5-HT1D receptor activity (Lucas-Osma et al. 2018). Thus we suggest 5-HT1D receptors decrease C-fiber activity, which in turn reduces tonic GABAergic input onto proprioceptive afferents and decreases the MSR. Further studies are in progress to confirm these ideas.
Summary and clinical implications.
We have demonstrated that 5-HT1D receptors potently inhibit the MSR, but do so indirectly via an action on C-fibers, where they are mostly expressed. Although the mechanism of this action is unclear, these findings open up new possibilities for treating hyperreflexia and clonus after SCI. Drugs such as zolmitriptan can serve to reduce the MSR and spasms (D’Amico et al. 2013a), and drugs that more generally modulate pain pathways (gabapentin, opioids) (Ahn and Basbaum 2006) likely also play a role in reducing spasticity, consistent with clinical experience. Furthermore, our finding that SERT plays a major role in modulating the sensitivity of the MSR to 5-HT suggests that with incomplete SCI, drugs that modulate SERT (SSRIs) may also regulate clonus and muscle spasms. Previous studies have shown that unique 5-HT receptors separately modulate locomotion, motoneuron excitability, and polysynaptic EPSPs that activate spasms (D’Amico et al. 2013a, 2013b, 2014; Fouad et al. 2010; Schmidt and Jordan 2000). Taken together with our current findings, these results indicate that the many aspects of motor function and recovery after SCI can be selectively regulated by pharmaceutical treatments selective to particular 5-HT receptors and transporters.
GRANTS
Research was supported by Canadian Institutes of Health Research Grant MOP 14697 and National Institutes of Health Grant R01 NS47567.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L., C.J.H., K.F., and D.J.B. conceived and designed research; A.M.L.-O., Y.L., K.C.M., S.L., A.H.A., C.J.H., K.K.F., K.F., and D.J.B. performed experiments; A.M.L.-O., Y.L., K.C.M., M.J.S., A.H.A., K.F., and D.J.B. analyzed data; Y.L., C.J.H., K.K.F., K.F., and D.J.B. interpreted results of experiments; A.M.L.-O., Y.L., A.H.A., K.F., and D.J.B. prepared figures; K.F. and D.J.B. drafted manuscript; A.M.L.-O., Y.L., S.L., S.B., M.J.S., A.H.A., C.J.H., K.K.F., K.F., and D.J.B. edited and revised manuscript; A.M.L.-O., Y.L., K.C.M., S.L., S.B., M.J.S., A.H.A., C.J.H., K.K.F., K.F., and D.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Leo Sanelli for technical assistance and Tia Bennett for editing the document.
REFERENCES
- Adham N, Gerald C, Schechter L, Vaysse P, Weinshank R, Branchek T. [3H]5-hydroxytryptamine labels the agonist high affinity state of the cloned rat 5-HT4 receptor. Eur J Pharmacol 304: 231–235, 1996. doi: 10.1016/0014-2999(96)00122-7. [DOI] [PubMed] [Google Scholar]
- Adham N, Romanienko P, Hartig P, Weinshank RL, Branchek T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol 41: 1–7, 1992. [PubMed] [Google Scholar]
- Ahn AH, Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain. J Neurosci 26: 8332–8338, 2006. doi: 10.1523/JNEUROSCI.1989-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 153: 830–838, 2012. doi: 10.1016/j.pain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Ashby P, McCrea DA. Neurophysiology of spinal spasticity. In: Handbook of the Spinal Cord, edited by Davidoff RA. New York: Dekker, 1987, p. 119–143. [Google Scholar]
- Auch-Schwelk W, Paetsch I, Krackhardt F, Gräfe M, Hetzer R, Fleck E. Modulation of contractions to ergonovine and methylergonovine by nitric oxide and thromboxane A2 in the human coronary artery. J Cardiovasc Pharmacol 36: 631–639, 2000. doi: 10.1097/00005344-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Audinot V, Newman-Tancredi A, Millan MJ. Constitutive activity at serotonin 5-HT1D receptors: detection by homologous GTPγS versus [35S]-GTPγS binding isotherms. Neuropharmacology 40: 57–64, 2001. doi: 10.1016/S0028-3908(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol 496: 837–850, 1996. doi: 10.1113/jphysiol.1996.sp021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16: 69–84, 1999. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Prochazka A. Catching a ball: contributions of intrinsic muscle stiffness, reflexes, and higher order responses. Can J Physiol Pharmacol 72: 525–534, 1994. doi: 10.1139/y94-076. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 196: 263–281, 1994. [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology 33: 275–317, 1994. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Boess FG, Monsma FJ Jr, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology 36: 713–720, 1997. doi: 10.1016/S0028-3908(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Brys R, Josson K, Castelli MP, Jurzak M, Lijnen P, Gommeren W, Leysen JE. Reconstitution of the human 5-HT1D receptor-G-protein coupling: evidence for constitutive activity and multiple receptor conformations. Mol Pharmacol 57: 1132–1141, 2000. [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol 372: 465–485, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lacey G. GABA-B receptor-mediated spinal inhibition. Neuroreport 5: 540–542, 1994. doi: 10.1097/00001756-199401000-00002. [DOI] [PubMed] [Google Scholar]
- D’Amico JM, Condliffe EG, Martins KJ, Bennett DJ, Gorassini MA. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 8: 36, 2014. doi: 10.3389/fnint.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Li Y, Bennett DJ, Gorassini MA. Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans. J Neurophysiol 109: 1485–1493, 2013a. doi: 10.1152/jn.00822.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Murray KC, Li Y, Chan KM, Finlay MG, Bennett DJ, Gorassini MA. Constitutively active 5-HT2/α1 receptors facilitate muscle spasms after human spinal cord injury. J Neurophysiol 109: 1473–1484, 2013b. doi: 10.1152/jn.00821.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlöf C, Maassen Van Den Brink A. Dihydroergotamine, ergotamine, methysergide and sumatriptan – basic science in relation to migraine treatment. Headache 52: 707–714, 2012. doi: 10.1111/j.1526-4610.2012.02124.x. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Bennett DJ, Stein RB. Stretch reflex gain in cat triceps surae muscles with compliant loads. J Physiol 545: 1027–1040, 2002. doi: 10.1113/jphysiol.2002.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Narzo AF, Kozlenkov A, Ge Y, Zhang B, Sanelli L, May Z, Li Y, Fouad K, Cardozo C, Koonin EV, Bennett DJ, Dracheva S. Decrease of mRNA editing after spinal cord injury is caused by down-regulation of ADAR2 that is triggered by inflammatory response. Sci Rep 5: 12615, 2015. doi: 10.1038/srep12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6: 725–733, 2007. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse 35: 144–150, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol 332: 1–7, 1986. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci 5: 135–145, 2004. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Enjin A, Leão KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. Sensorimotor function is modulated by the serotonin receptor 1d, a novel marker for gamma motor neurons. Mol Cell Neurosci 49: 322–332, 2012. doi: 10.1016/j.mcn.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Rank MM, Vavrek R, Murray KC, Sanelli L, Bennett DJ. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J Neurophysiol 104: 2975–2984, 2010. doi: 10.1152/jn.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ramírez DL, Calvo JR, Hochman S, Quevedo JN. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9: e89999, 2014. doi: 10.1371/journal.pone.0089999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen RC, Martin GR, Hill AP, Hyde RM, Woollard PM, Salmon JA, Buckingham J, Robertson AD. Computer-aided design and synthesis of 5-substituted tryptamines and their pharmacology at the 5-HT1D receptor: discovery of compounds with potential anti-migraine properties. J Med Chem 38: 3566–3580, 1995. doi: 10.1021/jm00018a016. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Ismaiel AE, McCarthy BG, Peroutka SJ. Binding of arylpiperazines to 5-HT3 serotonin receptors: results of a structure-affinity study. Eur J Pharmacol 168: 387–392, 1989. doi: 10.1016/0014-2999(89)90802-9. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006b. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Jacob-Vadakot S, Dugan EA, McBride S, Olexa R, Simansky K, Murray M, Shumsky JS. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp Neurol 221: 68–78, 2010. doi: 10.1016/j.expneurol.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem 281: 2012–2023, 2006. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- Honda M, Imaida K, Tanabe M, Ono H. Endogenously released 5-hydroxytryptamine depresses the spinal monosynaptic reflex via 5-HT1D receptors. Eur J Pharmacol 503: 55–61, 2004. doi: 10.1016/j.ejphar.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Honda M, Tanabe M, Ono H. Serotonergic depression of spinal monosynaptic transmission is mediated by 5-HT1B receptors. Eur J Pharmacol 482: 155–161, 2003. doi: 10.1016/j.ejphar.2003.09.070. [DOI] [PubMed] [Google Scholar]
- Honda M, Tanabe M, Ono H. Spinal cord injury-specific depression of monosynaptic spinal reflex transmission by l-5-hydroxytryptophan results from loss of the 5-HT uptake system and not 5-HT receptor supersensitivity. Exp Neurol 202: 258–261, 2006. doi: 10.1016/j.expneurol.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Stencil R. Ankle cryotherapy facilitates soleus function. J Orthop Sports Phys Ther 32: 622–627, 2002. doi: 10.2519/jospt.2002.32.12.622. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (−)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol 118: 13–23, 1985. doi: 10.1016/0014-2999(85)90658-2. [DOI] [PubMed] [Google Scholar]
- Hulse R, Wynick D, Donaldson LF. Intact cutaneous C fibre afferent properties in mechanical and cold neuropathic allodynia. Eur J Pain 14: 565.e1–565.e10, 2010. doi: 10.1016/j.ejpain.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents α-methylserotonin and 2-methylserotonin. J Med Chem 33: 755–758, 1990. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Otsuguro K, Kobayashi T, Ohta T, Ito S. Endogenously released 5-HT inhibits A and C fiber-evoked synaptic transmission in the rat spinal cord by the facilitation of GABA/glycine and 5-HT release via 5-HT2A and 5-HT3 receptors. Eur J Pharmacol 702: 149–157, 2013. doi: 10.1016/j.ejphar.2013.01.058. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 370: 114–123, 2004. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp 84: 43–75, 1949. [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R. Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. 2. J Med Chem 50: 4214–4221, 2007. doi: 10.1021/jm070487n. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91: 2236–2246, 2004b. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Li L, Stephens MJ, Zenner D, Murray KC, Winship IR, Vavrek R, Baker GB, Fouad K, Bennett DJ. Synthesis, transport, and metabolism of serotonin formed from exogenously applied 5-HTP after spinal cord injury in rats. J Neurophysiol 111: 145–163, 2014. doi: 10.1152/jn.00508.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004c. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 23: 733–741, 2017. doi: 10.1038/nm.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol 582: 127–136, 2007. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Osma AM, Li Y, Lin S, Black S, Singla R, Fouad K, Fenrich KK, Bennett DJ. Extrasynaptic α5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord. J Neurophysiol 120: 2953–2974, 2018. doi: 10.1152/jn.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Robertson AD, MacLennan SJ, Prentice DJ, Barrett VJ, Buckingham J, Honey AC, Giles H, Moncada S. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5-HT1B/1D receptor partial agonist, 311C90 (zolmitriptan). Br J Pharmacol 121: 157–164, 1997. doi: 10.1038/sj.bjp.0701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn CM, Peroutka SJ. Characterization of [3H]quipazine binding to 5-hydroxytryptamine3 receptors in rat brain membranes. J Neurochem 52: 1787–1792, 1989. doi: 10.1111/j.1471-4159.1989.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Millán J, Qaidi M, Alonso MA. Segregation of co-stimulatory components into specific T cell surface lipid rafts. Eur J Immunol 31: 467–473, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol 66: 355–474, 2002. doi: 10.1016/S0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105: 731–748, 2011a. doi: 10.1152/jn.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106: 925–943, 2011b. doi: 10.1152/jn.01011.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J Med Chem 45: 4344–4349, 2002. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. A serial section electron microscope study of an identified Ia afferent collateral in the cat spinal cord. J Comp Neurol 314: 257–277, 1991. doi: 10.1002/cne.903140205. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem 47: 529–540, 1986. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer AD Jr, Branchek TA, Flaugh ME. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61: 2117–2126, 1997. doi: 10.1016/S0024-3205(97)00885-0. [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci 23: 10988–10997, 2003. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. SB-216641 and BRL-15572 – compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol 356: 312–320, 1997. doi: 10.1007/PL00005056. [DOI] [PubMed] [Google Scholar]
- Rank MM, Murray KC, Stephens MJ, D’Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol 105: 410–422, 2011. doi: 10.1152/jn.00775.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Russo RE, Delgado-Lezama R, Hounsgaard J. Dorsal root potential produced by a TTX-insensitive micro-circuitry in the turtle spinal cord. J Physiol 528: 115–122, 2000. doi: 10.1111/j.1469-7793.2000.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. doi: 10.1016/S0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H, Maznychenko AV, Pilyavskii AI, Hellström F, Kostyukov AI, Maisky VA. Acute muscle inflammation enhances the monosynaptic reflexes and c-fos expression in the feline spinal cord. Eur J Pain 11: 579–586, 2007. doi: 10.1016/j.ejpain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, Price GW. SB-224289 – a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol 125: 202–208, 1998. doi: 10.1038/sj.bjp.0702059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Sheta M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expert Opin Investig Drugs 21: 1351–1369, 2012. doi: 10.1517/13543784.2012.704021. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7: 701–711, 2011. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157, 2007. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11: 823–836, 2010. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T, Cope TC. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol 118: 2687–2701, 2017. doi: 10.1152/jn.00497.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuela-Fernandez I, Sun L, Jerina H, Curtis J, Allchorne A, Gooding H, Rosie R, Holland P, Tas B, Mitchell R, Fleetwood-Walker S. The TRPM8 channel forms a complex with the 5-HT1B receptor and phospholipase D that amplifies its reversal of pain hypersensitivity. Neuropharmacology 79: 136–151, 2014. doi: 10.1016/j.neuropharm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Watson JM, Burton MJ, Price GW, Jones BJ, Middlemiss DN. GR127935 acts as a partial agonist at recombinant human 5-HT1Dα and 5-HT1Dβ receptors. Eur J Pharmacol 314: 365–372, 1996. doi: 10.1016/S0014-2999(96)00579-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Qin W, Cao J, Zhang Y, Ni J, Sun Y, Jiang X, Tao J. Serotonin type-1D receptor stimulation of A-type K+ channel decreases membrane excitability through the protein kinase A- and B-Raf-dependent p38 MAPK pathways in mouse trigeminal ganglion neurons. Cell Signal 28: 979–988, 2016. doi: 10.1016/j.cellsig.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol 588: 2065–2075, 2010. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]