Abstract
Subdural empyema (SDE), a common neurosurgical emergency in the developing countries, accounts for 15%–20% of localised paediatric intracranial infections. In regions where modern diagnostic tools are scarce and inaccessible, detection of SDE may be delayed with subsequent poor outcome. Percutaneous subdural aspiration in patients with open anterior fontanel may be the only surgical option in resource-poor regions of the world. This review focuses on the management outcome, including neurological outcome of these children. Clinical charts of children with SDE and treated by percutaneous subdural tap between February 2006 and August 2014 were reviewed. Demographic, clinical, radiological, bacteriological parameters and outcome data were analysed. Forty-five children with a mean age of 10.6 ± 6.2 months (range: 2–17 months) and followed up for a median duration of 16.4 months were included. The most frequent clinical features were enlarged head circumference, fever, focal neurologic deficits and altered level of consciousness. Diagnosis of SDE was confirmed using trans-fontanel ultrasound scan in 32 (71.1%) children, computerised tomography in 12 (26.7%) children and magnetic resonance imaging in one (2.2%) child. SDE was unilateral in 73.3% and bilateral in 26.7%. In 23 (51.1%) children with a positive culture, Staphylococcus aureus (n = 10), anaerobes (n = 7), Escherishia coli and Haemophilus influenza (n = 6 each) were the most common organisms. Forty-three children (95.6%) survived, 36 of which had good Glasgow outcome score. Seven children still had moderate deficits at 3 months. Treatment of SDE in young children with patent fontanel using percutaneous subdural tap has good therapeutic and neurological outcome.
Keywords: Infantile subdural empyema, Meningitis, Outcome, Percutaneous aspiration, Surgical treatment, Nigeria
INTRODUCTION
Infantile subdural empyema (SDE) is a common neurosurgical emergency in the developing countries [1]. SDE accounts for 15%–20% of localised intracranial infections in the paediatric population [1–3], and its outcome closely depends on early diagnosis and prompt medical and surgical intervention [4–6]. Morbidity and mortality of SDE is associated with the delayed diagnosis, level of consciousness and presence of focal neurological deficits and seizures [1,4,5,7–9]. Often, SDE is associated with meningitis, and due to its non-specific clinical presentation requires neuro-imaging to confirm the diagnosis. In the developing countries, resources are often limited and radiological examinations are not widely available or too costly. This results in delay or under-diagnosis, consequently leading to poor outcome, including increased mortality. Furthermore, due to cost and remoteness, access to operating facilities is inadequate in the developing countries resulting in fewer children with SDE receiving the preferred surgical intervention of a craniotomy or a burr hole.
A previous study involving a small cohort of children with SDE diagnosed with trans-fontanelle ultrasound reported good short and medium term neurological outcome [10]. In this present report, we present these outcomes in a larger group of children with SDE from multiple centres in an urban area in Lagos, Nigeria.
PATIENTS AND METHODS
This is a retrospective multi-centre review of young children managed in three medical centres in Lagos, Nigeria between February 2006 and August 2014. One of the centres was a public-funded fee-for-service tertiary hospital and the others were private hospitals. We included children younger than 2 years with SDE treated by percutaneous subdural tap via an open anterior fontanel approach.
Our approach in draining SDE via the percutaneous approach has been described previously [10]. We use an 18-guage plastic cannula with metal trocar and achieve pain-free procedure using local anaesthetics. The cannula is inserted at the lateral angle of the anterior fontanel and the metal trocar removed when location is confirmed by the flow of subdural collection. The diagnosis of SDE is confirmed at aspiration by the presence of purulent aspirate. Repeated aspiration is made with cannula in place till evacuation is completed. The use of plastic cannulae made it possible to remove almost all the subdural collection in one procedure using a single tap with multiple syringes. At the end of the procedure, the plastic cannula is immediately removed. Patients with bilateral SDE had the procedure also performed on the contralateral side. Post-drainage imaging was done using trans-fontanel ultrasound scan (TFUSS). Sample of the aspirates was sent for gram stain, culture and sensitivity testing (MCS). Combination broad spectrum antibiotics were administered to all the children and revised when antibiotic sensitivity reports were available. The most common combination antibiotics used was ceftriaxone, amikacin and metronidazole. Following drainage, antibiotics were administered for 6 weeks; four weeks of parenteral administration followed by 2 weeks of oral intake depending on the clinical course.
Weekly TFUSS was repeated while the patient was hospitalised and receiving parenteral antibiotics. Clinical outcome and treatment response was assessed using clinical symptoms and signs and weekly TFUSS. Outpatient follow-up visits were scheduled every 2 months. During follow-up, the resolving neurologic deficits were assessed and addressed as needed. The Henk W. Mauser grading scale [2] for morbidity of survivors of SDE was used to assess the subjects in this study.
For this study, the demographic, clinical, radiological, bacteriological parameters and outcome data were collected and analysed. Statistical Analysis was performed using Excel 2013, (Microsoft, USA).
RESULTS
Demographics
Forty-five infants were included in this review. Twenty-six (57.8%) were males and the mean age was 10.6 ± 6.2 months (range: 2–17 months). Median follow-up period was 16.4 months (range: 12–36 months).
Clinical Presentation
The most common clinical signs and symptoms were rapidly increasing occipito-frontal head circumference (OFC) (45; 100%), fever (39; 86.7%), new focal neurologic deficits (40; 88.9%) and altered level of consciousness (31; 68.9%). Bulging fontanel was present in all of the children (Table 1). Table 2 shows the level of consciousness based on the Bannister and William [11] grading of level of consciousness. Over 70% of the patients presented with grades I and II level of consciousness. The mean duration of symptoms prior to surgical intervention was 29 days with a range of 7–94 days. The longest period was recorded in a child who was referred from a rural area after the treatment of bacterial meningitis failed.
Table 1.
Common Clinical presentations.
| Clinical presentations | Frequency (N =45) |
|---|---|
| Increasing head circumference (bulging fontanel) | 45 (100%) |
| Meningeal irritation | 41 (88.9%) |
| Focal deficit/hemiparesis | 40 (86.7%) |
| Fever | 39 (86.7%) |
| Altered consciousness | 31 (68.9%) |
| Seizures | 17 (37.8%) |
Table 2.
Bannister and William [11] grading for level of consciousness in intracranial SDE.
| Grade | Frequency (N = 45) | Description |
|---|---|---|
| I | 14 (31.1%) | Awake and alert |
| II | 20 (44.4%) | Drowsy and disoriented |
| III | 8 (17.8%) | Responsive to stimuli |
| IV | 3 (6.7%) | Unresponsive |
Employed Imaging Modalities
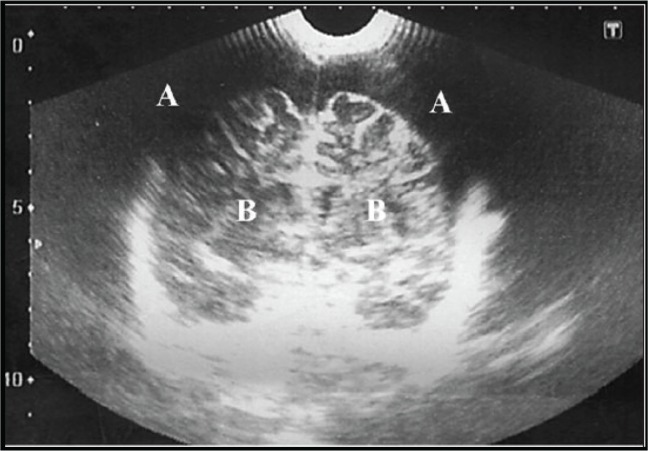
SDE was diagnosed using trans-fontanel ultrasound scan (TFUSS), cranial computerised tomographic (CT) scan or magnetic resonance imaging (MRI), in 32 (71.1%), 12 (26.7%) and one (2.2%) children, respectively. Thirty-three (73.3%) children had unilateral SDE and 12 (26.7%) patients had bilateral SDE with involvement of the parafalcine region in one child. Figure 1 shows a typical TFUSS in a child with bilateral SDE.
Figure 1.
A typical trans-fontanel ultrasound scan (TFUSS) in a child with bilateral SDE. (A) Subdural collection and (B) Brain parenchyma.
Bacteriology
Subdural aspirates from 22 (48.9%) patients did not yield any bacterial growth. In 23 children with positive culture, the following organisms were isolated: Staphylococcus aureus (n = 10; 43.5%), anaerobes (n = 7; 30.4%) and 6 (26.1%) children each of Escherishia coli and Haemophilus influenza. Multiple bacterial organisms were isolated in six children.
Outcome
Forty-three children (95.6%) survived. Two patients (4.4%) died from sepsis by 7th and 19th day of the treatment. Of the survivors, 36 (83.7%) had a Glasgow Outcome Score (GOS) of 4 or above at 3 months. Seven of the 43 children (16.3%) had moderate deficits (GOS of 3) at 3 months.
Table 3 summarises the morbidity of survivors based on the Mauser grading system [2] at 1 year of follow-up.
Table 3.
Mauser and Tulleken [2] grading system for morbidity of survivors of intracranial SDE.
| Grade | Frequency (%) n = 45 |
Description |
|---|---|---|
| A | 36 (80) | Survival without or with a minor, not disabling focal deficit |
| B | 7 (15.6) | Survival with no disabling seizures and with or without a minor focal |
| C | Nil (0) | Survival with severe disability |
| D | 2 (4.4) | Death |
Residual deficits (Table 4) still present by the third month post aspiration were seizures (8), hemiparesis (4), hydrocephalus (7), speech deficit (3) and recurrence of SDE (4). Patients with recurrent SDE were treated by the same procedures and they have remained stable.
Table 4.
Residual deficits.
| Deficit | Frequency (at 3 months) | Intervention | Remarks (at 1 year) |
|---|---|---|---|
| Seizures | 8 | Anticonvulsant | Resolved |
| Hydrocephalus | 7 | Shunt/ETV | Stable |
| Hemiparesis | 4 | Physiotherapy | Resolved |
| Speech | 3 | Speech therapy | On-going therapy |
| Recurrence | 4 | Re-drained | Resolved |
ETV = Endoscopic third ventriculostomy.
DISCUSSION
SDE is a common neurosurgical emergency in the developing countries [1,12]. Infants with meningitis are highly predisposed to the development of SDE. Subdural collections in general, as a complication of meningitis, occur in 39%–60% of infants with pyogenic meningitis [13–15]. It has been suggested that SDE often occurs when a subdural effusion related to meningitis becomes infected [9].
There is a male preponderance (57.8%) in the present study, which has been previously published [8,15–17]. The most common clinical features in our series were bulging fontanel with rapidly increasing head circumference, meningism, focal neurological signs and fever. These features are similar to those observed in other studies [2,5,6,18–20]. The average duration of symptoms before diagnosis and intervention was approximately 1 month (7–94 days), much longer than the 2 weeks reported by Liu et al. [9] in Taiwan. In contrast, Yilmaz et al. [16] in Turkey recorded a longer duration of symptoms averaging 2 months. A delay in diagnosis is a common problem in the developing world [10].
Thirty-two patients (71%) in our series were investigated using TFUSS. Though CT and MRI are the preferred imaging modalities for diagnosis [5,18–20], TFUSS has been proven to be a reliable imaging modality in infants with the ability to distinguish between reactive subdural effusion and SDE [9,10,21]. Other benefits include the ease of deployment, no risk of irradiation and cheaper cost. These features recommend it as a screening tool for SDE in young children with bacterial meningitis [22]. We used the other diagnostic modalities when affordable, but TFUSS often saved us significant amount of time of diagnosis and intervention in a cohort presenting late with severe disease.
The treatment options for SDE, though controversial, include burr-holes and craniotomy, and the choice is often based on the surgeon’s preference and experience [1,9,15,16]. The role of percutaneous subdural tap in the management of SDE in young children has been documented and is still useful in the developing world for patients where operating time is a factor and cost of surgical intervention is hardly affordable. In this series, we demonstrated that percutaneous subdural tap remains effective in the treatment of SDE in children with patent fontanels. Eighty percent of our patients had favourable outcome (GOS of 4 and 5). This compares with outcomes in studies by Liu et al. [9] (73%) and Banerjee et al. [8] (87%). Two children (4.4%) died in our series and both had Bannister and Williams grade IV level of consciousness. Whereas Liu et al. [9] recorded a mortality of 3%, Banerjee et al. [8] and Smith and Hendrick [15] recorded slightly higher mortality rates of 10.8% and 13%, respectively, in their series using burr holes and craniotomy as modalities of treatment. In addition, most residual deficits had resolved at 1-year post treatment and parents confirmed significant improvement of functions and return to activity.
Furthermore, the outcome in this series may have been better than presently observed if the children presented early. Only in four children (8%) did the SDE recur and were subsequently drained with satisfactory outcome. Liu et al. [9] showed no difference in recurrence rate, number of days until afebrile, number of days of postoperative antibiotic treatment and GOS score between the bur hole and craniotomy groups. Time of presentation appears to be the most significant prognostic factor.
Some reports suggest that SDE could be managed with antibiotics only but resolution takes a longer course [3,23,24]. The removal of the empyema affords us a sample for microbial analysis since blood cultures often yield no bacterial growth [18]. Microscopy, culture and sensitivity pattern obviously help to tailor the initial antibiotic therapy, hence reducing drug pressure and antibiotics resistance. We believe that the early treatment of meningitis with appropriate antibiotics will help to reduce the incidence of SDE and other intracranial complications.
CONCLUSIONS
Young children with SDE present late with features indistinguishable and co-existing with those of bacterial meningitis. Trans-fontanel ultrasound scan remains a useful diagnostic tool in young children with open fontanel presenting with SDE. The outcome of children treated with percutaneous subdural tap is satisfactory in an environment with high burden of such emergencies and limited facilities. It is hoped that our findings may help guide the choice of percutaneous subdural tap where access to theatre for open drainage is limited or unavailable.
ACKNOWLEDGEMENTS
The authors would like to thank Andrew A. Kanner, MD for his mentoring, thorough review of the manuscript and useful suggestions. Olubunmi Ojelade, MD provided technical support for this study.
FUNDING
None.
CONFLICT OF INTEREST
None.
ETHICS
This study was approved by the Health Research Ethics Committee of Lagos University Teaching Hospital; ADM/DCST/HREC/APP/564 on 29/10/2015.
REFERENCES
- 1.Nathoo N, Nadvi SS, Van Dellen JR, Gouws E. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529–35. doi: 10.1097/00006123-199903000-00055. discussion 35–6. https://doi.org/10.1097/00006123-199903000-00055. [DOI] [PubMed] [Google Scholar]
- 2.Mauser HW, Tulleken CA. Subdural empyema. A review of 48 patients. Clin Neurol Neurosurg. 1984;86:255–63. doi: 10.1016/0303-8467(84)90286-5. https://doi.org/10.1016/0303-8467(84)90286-5. [DOI] [PubMed] [Google Scholar]
- 3.Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20:372–86. doi: 10.1093/clinids/20.2.372. https://doi.org/10.1093/clinids/20.2.372. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini GL. Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep. 2004;4:448–56. doi: 10.1007/s11910-004-0067-8. https://doi.org/10.1007/s11910-004-0067-8. [DOI] [PubMed] [Google Scholar]
- 5.De Bonis P, Anile C, Pompucci A, Labonia M, Lucantoni C, Mangiola A. Cranial and spinal subdural empyema. Br J Neurosurg. 2009;23:335–40. doi: 10.1080/02688690902939902. https://doi.org/10.1080/02688690902939902. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesh MS, Pandey P, Devi BI, Khanapure K, Satish S, Sampath S, et al. Pediatric infratentorial subdural empyema: analysis of 14 cases. J Neurosurg. 2006;105:370–7. doi: 10.3171/ped.2006.105.5.370. https://doi.org/10.3171/ped.2006.105.5.370. [DOI] [PubMed] [Google Scholar]
- 7.Le Roux PC, Wood M, Campbell RA. Subdural empyema caused by an unusual organism following intracranial haematoma. Childs Nerv Syst. 2007;23:825–7. doi: 10.1007/s00381-007-0313-x. https://doi.org/10.1007/s00381-007-0313-x. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee AD, Pandey P, Devi BI, Sampath S, Chandramouli BA. Pediatric supratentorial subdural empyemas: a retrospective analysis of 65 cases. Pediatr Neurosurg. 2009;45:11–8. doi: 10.1159/000202619. https://doi.org/10.1159/000202619. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZH, Chen NY, Tu PH, Lee ST, Wu CT. The treatment and outcome of postmeningitic subdural empyema in infants. J Neurosurg Pediatr. 2010;6:38–42. doi: 10.3171/2010.4.PEDS09433. https://doi.org/10.3171/2010.4.PEDS09433. [DOI] [PubMed] [Google Scholar]
- 10.Kanu OO, Nnoli C, Olowoyeye O, Ojo O, Esezobor C, Adeyomoye A, et al. Infantile subdural empyema: the role of brain sonography and percutaneous subdural tapping in a resource-challenged region. J Neurosci Rural Pract. 2014;5:355–9. doi: 10.4103/0976-3147.139978. https://doi.org/10.4103/0976-3147.139978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister G, Williams B, Smith S. Treatment of subdural empyema. J Neurosurg. 1981;55:82–8. doi: 10.3171/jns.1981.55.1.0082. https://doi.org/10.3171/jns.1981.55.1.0082. [DOI] [PubMed] [Google Scholar]
- 12.Pathak A, Sharma BS, Mathuriya SN, Khosla VK, Khandelwal N, Kak VK. Controversies in the management of subdural empyema. A study of 41 cases with review of literature. Acta Neurochir (Wien) 1990;102:25–32. doi: 10.1007/BF01402182. https://doi.org/10.1007/BF01402182. [DOI] [PubMed] [Google Scholar]
- 13.Snedeker JD, Kaplan SL, Dodge PR, Holmes SJ, Feigin RD. Subdural effusion and its relationship with neurologic sequelae of bacterial meningitis in infancy: a prospective study. Pediatrics. 1990;86:163–70. [PubMed] [Google Scholar]
- 14.Syrogiannopoulos GA, Nelson JD, McCracken GH., Jr Subdural collections of fluid in acute bacterial meningitis: a review of 136 cases. Pediatr Infect Dis. 1986;5:343–52. doi: 10.1097/00006454-198605000-00014. https://doi.org/10.1097/00006454-198605000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Smith HP, Hendrick EB. Subdural empyema and epidural abscess in children. J Neurosurg. 1983;58:392–7. doi: 10.3171/jns.1983.58.3.0392. https://doi.org/10.3171/jns.1983.58.3.0392. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz N, Kiymaz N, Yilmaz C, Bay A, Yuca SA, Mumcu C, et al. Surgical treatment outcome of subdural empyema: a clinical study. Pediatr Neurosurg. 2006;42:293–8. doi: 10.1159/000094065. https://doi.org/10.1159/000094065. [DOI] [PubMed] [Google Scholar]
- 17.Pattisapu JV, Parent AD. Subdural empyemas in children. Pediatr Neurosci. 1987;13:251–4. doi: 10.1159/000120338. https://doi.org/10.1159/000120338. [DOI] [PubMed] [Google Scholar]
- 18.Madhugiri VS, Sastri BV, Bhagavatula ID, Sampath S, Chandramouli BA, Pandey P. Posterior fossa subdural empyema in children-management and outcome. Childs Nerv Syst. 2011;27:137–44. doi: 10.1007/s00381-010-1169-z. https://doi.org/10.1007/s00381-010-1169-z. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Lim M, Harsh GR. Management of epidural abscesses and subdural empyemas. Operat Techniques Neurosurg. 2004;7:182–7. https://doi.org/10.1053/j.otns.2005.06.003. [Google Scholar]
- 20.Salunke PS, Malik V, Kovai P, Mukherjee KK. Falcotentorial subdural empyema: analysis of 10 cases. Acta Neurochir (Wien) 2011;153:164–70. doi: 10.1007/s00701-010-0695-5. https://doi.org/10.1007/s00701-010-0695-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen CY, Huang CC, Chang YC, Chow NH, Chio CC, Zimmerman RA. Subdural empyema in 10 infants: US characteristics and clinical correlates. Radiology. 1998;207:609–17. doi: 10.1148/radiology.207.3.9609881. https://doi.org/10.1148/radiology.207.3.9609881. [DOI] [PubMed] [Google Scholar]
- 22.Chang YC, Huang CC, Wang ST, Chio CC. Risk factor of complications requiring neurosurgical intervention in infants with bacterial meningitis. Pediatr Neurol. 1997;17:144–9. doi: 10.1016/s0887-8994(97)00078-7. https://doi.org/10.1016/S0887-8994(97)00078-7. [DOI] [PubMed] [Google Scholar]
- 23.Heran NS, Steinbok P, Cochrane DD. Conservative neurosurgical management of intracranial epidural abscesses in children. Neurosurgery. 2003;53:893–7. doi: 10.1227/01.neu.0000084163.51521.58. discussion 97–8. https://doi.org/10.1227/01.NEU.0000084163.51521.58. [DOI] [PubMed] [Google Scholar]
- 24.Leys D, Destee A, Petit H, Warot P. Management of subdural intracranial empyemas should not always require surgery. J Neurol Neurosurg Psychiatry. 1986;49:635–9. doi: 10.1136/jnnp.49.6.635. https://doi.org/10.1136/jnnp.49.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]