Abstract
Faecal disimpaction is very important for successful management of the constipation in children. Lactulose is cheap and widely available medicine compared to other polyethylene glycol (PEG) preparations. From our experience, lactulose is effective and safe medicine for both disimpaction and maintenance therapy in constipated children. The purpose of the present study was to evaluate the safety and efficacy of lactulose in faecal impaction management in children with constipation. We conducted a prospective controlled trial in children with functional constipation, who presented with faecal impaction to Queen Rania Hospital for Children from April 15, 2018 until October 15, 2018. Two randomised matched groups; group A included 33 constipated children treated for disimpaction with higher dose lactulose (10 g/15 ml) 4–6 ml/kg/day (max. 120 ml/day) and group B included 32 children treated for disimpaction with macrogol (PEG 4000) 1–1.5 g/kg (max. 30 g/day). Both groups received treatment until resolution or up to 6 days. Patients were followed over 1 week and success of disimpaction was observed. Moreover, any adverse events were recorded. All the patients in both groups achieved successful disimpaction by seventh day of the therapy, group B showed significant faster response. Both therapies were tolerated and no significant adverse events were reported. Both agents were safe, effective and well tolerated. Lactulose may be a good alternative to PEG in the treatment of faecal impaction in constipated children.
Keywords: Lactulose, Disimpaction, Constipation, Children
INTRODUCTION
Constipation is a common problem in children as well as in adults, and it contributes to about 5% paediatric visits to the primary care physicians and 25% to the paediatric gastroenterology (GI) clinic [1,2]. According to the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition, constipation is defined as a delay or difficulty in defecation, present for two or more weeks and sufficient to cause significant distress to the patient [3]. Paediatric chronic constipation is functional in the vast majority of the cases and good clinical history and examination can exclude most organic causes of constipation, and minority of organic causes could be identified beyond the neonatal period [4].
Faecal impaction is the presence of faecal mass (faecaloma) that can be assessed radiologically or by rectal exam revealing dilated rectum, full of hard stool [4–6]. Early diagnosis and effective optimised management are recommended to relieve associated symptoms and prevent complications [6]. The majority of the cases respond well to management if good education and regular follow up are guaranteed [5,6]. Without disimpaction, no management plan will succeed for chronic constipation and laxatives will cause more cramps, bloating and increase in faecal soiling if present initially [6–8].
Disimpaction can be achieved by oral, rectal or manual routs. Manual and rectal routes are reserved for rare refractory cases [3,4]. The first line recommended and well-studied oral medicine for disimpaction is polyethylene glycol (PEG) with or without electrolytes [9–11]. Lactulose is a frequently mentioned other oral choice for disimpaction but without evidence based recommendations [4–6].
Both lactulose and PEG act as osmotic agents which increase water content of stools, soften stool and promote colonic peristalsis. Unlike PEG, which is not absorbable medicine, only less than 5% of lactulose is absorbed but even absorbed lactulose is not metabolised and excreted in the urine and unabsorbed lactulose is extensively metabolised to organic acids by colonic bacteria. With optimised therapy, both agents are safe and have minimal adverse reactions [7,8].
In our institute, which is a public health institute, the choice of laxatives for constipated children depends mainly on the available medicines in the institute. Lactulose, always available unlike PEG, is inexpensive and available as a solution formula. We are used to using lactulose for disimpaction therapy for many years in higher doses (double the recommended doses for maintenance therapy) with good results, so the aim of this randomised study was to compare the safety, efficacy, tolerability of this therapy compared to a reference PEG formulation in resolving faecal impaction.
MATERIALS AND METHODS
This was a prospective parallel groups randomised clinical trial of a lactulose versus a PEG in the resolution of faecal impaction in constipated children. The study was conducted at Queen Rania Hospital for Children in Amman, Jordan over 6 months’ duration, from April 15, 2018 until October 15, 2018.
Children were eligible if they were aged between 1 and 14 years and had diagnosis of functional constipation according to Rome III criteria [7] with evidence of faecal impaction. Exclusion criteria included children with suspected organic causes of constipation such as Hirschsprung’s disease, spina bifida, hypothyroidism, children with growth failure, children who received recent laxatives or children with suspected bowel obstruction.
We estimated lactulose response rate to be 70% and PEG response rate of 90% as reported [9–11]. So, a sample size of 60 children, 30 in each side, is needed to elicit the difference with 80% power and a 95% confidence level. We estimated dropout rate of 10% so a sample size of 70 children was planned to achieve 60 evaluable children.
To ensure a balanced allocation of treatment among different ages, separate computer generated randomisation lists were used for the three age groups (1–5, 6–11 and 11–14 years), because of obvious differences in the treatment appearance and taste, blinding of the participants and part of study personnel was not possible.
According to the randomisation process, the patients were divided into two groups: group A (lactulose group) treated with lactulose 4–6 ml/kg/day (double the usual dose for maintenance therapy) divided in two doses (maximum 120 ml/day) until resolution or for 6 days and group B (PEG group) received PEG (macrogol 4000) 1.5 g/kg/day divided in two doses (maximum 30 g/day) until resolution or for 6 days.
Written instructions were given to the care givers about the medicine, usage, doses and possible side effects, and we followed up patients over 1 week of outpatient treatment. Data collected by the investigators included age, gender, growth parameters (height and weight), day of disimpaction, possible adverse events, parents and child satisfaction, compliance and acceptability.
The statistical analyses were performed using statistical package for the social science (SPSS) software version 22 for windows. A cut-off p-value of 0.05 for statistical significance was presumed.
RESULTS
A total of 72 children were enrolled, among them, seven children dropped out as they missed their follow up; therefore, analysis was completed for 65 patients (33 for lactulose group and 32 for PEG group).
Comparing the two groups before starting of therapy, they were matched and no relevant clinical or demographic differences at baseline were observed (Table 1).
Table 1.
Baseline demographics and clinical characteristics.
| Lactulose group | PEG group | p-value | |
|---|---|---|---|
| Patients no. | 33 | 32 | |
| Age range: | 1–13 | 1–14 | |
| 1–5 years | 19 | 17 | 0.718 |
| 6–10 years | 11 | 10 | 0.858 |
| 11–14 years | 3 | 5 | 0.423 |
| Mean ± SD | 5.2 ± 2.6 | 5.4 ± 3.1 | 0.779 |
| Gender: | |||
| Male | 18 | 18 | 0.890 |
| Female | 15 | 14 | 0.890 |
| Weight (kg) Mean ± SD |
18.2 ± 6.6 | 19.1 ± 8.2 | 0.627 |
| Height (cm) Mean ± SD |
102 ± 19 | 103 ± 21 | 0.841 |
SD, standard deviation.
Successful disimpaction was achieved in all the 65 patients (100%) in both groups by day seven of therapy. In the lactulose group, faecal disimpaction was observed in nine children on day 2, 12 children on day 3, four children on day 4, four children on day 5 and four children on day 6, while in the PEG group, disimpaction was achieved in 19 children on day 2, nine children on day 3, three children on day 5 and one child on day 6. Comparing both groups, p-values were 0.001, 0.122, 0.266, 0.197 and 1.000 on days 2, 3, 4, 5, and 6, respectively (Table 2; Figure 1). In comparison, the only significant difference is that PEG group showed faster response (p-value = 0.001) by day 2 of the treatment (Table 2).
Table 2.
Success of disimpaction related to days of therapy.
| Group | Lactulose | Total | PEG | Total | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 1–5 | 6–10 | 11–14 | 33 | 1–5 | 6–10 | 11–14 | 32 | |
| Patients no. | 19 | 11 | 3 | 17 | 10 | 5 | |||
| Day 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Day 2 | 5 | 3 | 1 | 9 (9/33) |
11 | 5 | 3 | 19 (19/32) |
0.001* |
| Day 3 | 6 | 5 | 1 | 12 (21/33) |
5 | 3 | 1 | 9 (28/32) |
0.122 |
| Day 4 | 2 | 2 | 0 | 4 (25/33) |
0 | 0 | 0 | 0 (28/32) |
0.266 |
| Day 5 | 1 | 2 | 1 | 4 (29/33) |
1 | 2 | 0 | 3 (31/32) |
0.197 |
| Day 6 | 1 | 3 | 0 | 4 (33/33) |
0 | 0 | 1 | 1 (32/32) |
1.000 |
Figure 1.
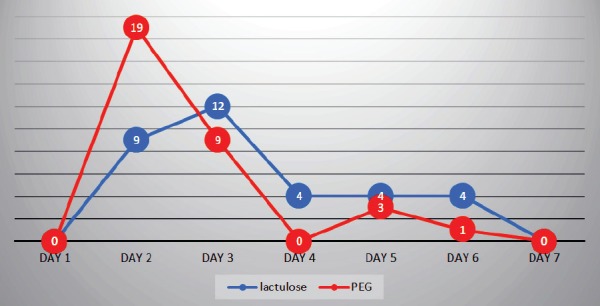
Disimpaction success using lactulose or PEG.
Only three children from those who completed the study period had adverse reactions presumed to be treatment related and were mild. These were diarrhoea and vomiting in one child in the lactulose group for which the treatment was suspended for 2 days and he did well thereafter; abdominal pain (one in each group) and treatment were interrupted for 1 day and then has been tolerated.
DISCUSSION
The importance of this study is that it constitutes the first trial of using lactulose in higher doses for faecal disimpaction, so we carried out a randomised comparison between a lactulose and a PEG formulation for resolution of faecal impaction in children with functional constipation.
All published studies and review articles showed the efficacy and safety of both PEG and lactulose in the maintenance (long-term) management of chronic constipation in children [11–15], with probable superiority of PEG in Cochrane database systematic review research [11] and cost effectiveness of PEG over lactulose in one review article in UK in adults [15]. Studies that have proven the first line therapy with PEG for faecal disimpaction in children are scarce [9,10], but we could not find in the literature any study for the use of lactulose laxative for disimpaction therapy in children or in adults.
Our study showed that the lactulose was effective and well tolerated as PEG in achieving disimpaction by sixth day of therapy but there was significant difference; PEG group had faster disimpaction response by day 2 (p-value < 0.001) and no significant difference thereafter (p-values were 0.122, 0.266, 0.197 and 1.000 by days 3, 4, 5 and 6, respectively).
Both lactulose and PEG were reported to be safe and well-tolerated osmotic laxatives with no serious adverse events in a Cochrane systematic review [11]. This was in accordance with our results during disimpaction period. Only three events were reported in both groups (two in lactulose group and one in PEG group) as being related to treatment. They were considered as mild events and not requiring stopping disimpaction therapy. This indicates no significant safety issues in using lactulose, which is in keeping with our long time experience.
There are some notable limitations in the present trial. It is an open-label trial, since blinding was not feasible as both used treatments are different in appearance, taste, dose volume and dosage instruction. More studies should be designed to reach the optimum dose and duration of lactulose with minimum adverse events for disimpaction therapy as we have used double the recommended doses. Moreover, our study reported no issues regarding compliance and tolerability in both the groups during the short disimpaction period, so long-term (namely, maintenance therapy) compliance and tolerability could not be assessed properly. Our survey showed predilection to lactulose over PEG as 82% of parents and caregivers preferred liquid formulae over powder formulae and 83% preferred small dose volumes as in lactulose.
CONCLUSION
Lactulose is effective and may be a good alternative to PEG in the treatment of faecal impaction in constipated children, and proved to be safe and well-tolerated medicine. We recommend more studies to provide further evidence of this practice.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and their families, the administrative and clinical staff of Queen Rania Hospital for Children for their cooperation and support.
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
There are no conflicts of interest.
ETHICS
Ethical approval was sought from the Research Ethics Committee and Institute Review Board in the Jordanian Royal Medical Services via letter number 14/4-2018. Written informed consent had been obtained from the children parents or care givers.
REFERENCES
- 1. Van Den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–9. doi: 10.1111/j.1572-0241.2006.00771.x. https://doi.org/10.1111/j.1572-0241.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 2.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders. Child/adolescent. Gastroenterol. 2006;130(5):1527–37. doi: 10.1053/j.gastro.2005.08.063. https://doi.org/10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabbers MM, Di Lorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr. Gastroenterol Nutr. 2014;58(2):258–74. doi: 10.1097/MPG.0000000000000266. https://doi.org/10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 4.Kuizenga W, Benninga MA. Functional constipation and encopresis. In: Wyllie R, editor. Pediatric gastroenterology and liver disease. Philadelphia, PA: Elsevier; 2016. pp. 124–36. [Google Scholar]
- 5.Xinias I, Mavroudi A. Constipation in childhood. An update on evaluation and management. Hippokratia. 2015;19(1):11–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43(3):1–13. doi: 10.1097/01.mpg.0000233159.97667.c3. https://doi.org/10.1097/01.mpg.0000233159.97667.c3. [DOI] [PubMed] [Google Scholar]
- 7.Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Pediatr Drugs. 2015;17:349–60. doi: 10.1007/s40272-015-0142-4. https://doi.org/10.1007/s40272-015-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soo HC, Kie YP, Sung KK, Ki SK, So YN, Hye RY, et al. Prevalence, clinical characteristics, and management of functional constipation at pediatric gastroenterology clinics. Korean Med Sci. 2013;28:1356–61. doi: 10.3346/jkms.2013.28.9.1356. https://doi.org/10.3346/jkms.2013.28.9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youssef NN, Peters JM, Henderson W, Shultz-Peters S, Lockhart DK, Di Lorenzo C. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr. 2002;141:410–4. doi: 10.1067/mpd.2002.126603. https://doi.org/10.1067/mpd.2002.126603. [DOI] [PubMed] [Google Scholar]
- 10.Francesco S, Serena V, Maiullari E, Giovanni D, Salvatore O, Salvatore C, et al. Efficacy and tolerability of peg-only laxative on fecal impaction and chronic constipation in children. A controlled double blind randomized study vs a standard peg-electrolyte laxative. BMC Pediatrics. 2012;12:178. doi: 10.1186/1471-2431-12-178. https://doi.org/10.1186/1471-2431-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee-Robichaud H, Thomas K, Morgan J, Nelson RL. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev. 2010;7:CD007570. doi: 10.1002/14651858.CD007570.pub2. https://doi.org/10.1002/14651858.CD007570.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Thomson MA, Jenkins HR, Bisset WM, Heuschkel R, Kalra DS, Green MR, et al. Polyethylene glycol 3350 plus electrolytes for chronic constipation in children: a double blind, placebo controlled, crossover study. Arch Dis Child. 2007;92:996–1000. doi: 10.1136/adc.2006.115493. https://doi.org/10.1136/adc.2006.115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut. 2011;60(2):209–18. doi: 10.1136/gut.2010.227132. https://doi.org/10.1136/gut.2010.227132. [DOI] [PubMed] [Google Scholar]
- 14.Dupont C, Leluyer B, Maamri N, Morali A, Joye JP, Fiorini JM, et al. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr. 2005;41:625–33. doi: 10.1097/01.mpg.0000181188.01887.78. https://doi.org/10.1097/01.mpg.0000181188.01887.78. [DOI] [PubMed] [Google Scholar]
- 15.Guest JF, Clegg JP, Helter MT. Cost-effectiveness of macrogol 4000 compared to lactulose in the treatment of chronic functional constipation in the UK. Curr Med Res Opin. 2008;24:1841–52. doi: 10.1185/03007990802102349. https://doi.org/10.1185/03007990802102349. [DOI] [PubMed] [Google Scholar]


