Abstract
Skeletal muscle contractures represent the permanent shortening of a muscle-tendon unit, resulting in loss of elasticity and, in extreme cases, joint deformation. They may result from cerebral palsy, spinal cord injury, stroke, muscular dystrophy, and other neuromuscular disorders. Contractures are the prototypic and most severe clinical presentation of increased passive mechanical muscle force in humans, often requiring surgical correction. Intraoperative experiments demonstrate that high muscle passive force is associated with sarcomeres that are abnormally stretched, although otherwise normal, with fewer sarcomeres in series. Furthermore, changes in the amount and arrangement of collagen in the extracellular matrix also increase muscle stiffness. Structural light and electron microscopy studies demonstrate that large bundles of collagen, referred to as perimysial cables, may be responsible for this increased stiffness and are regulated by interaction of a number of cell types within the extracellular matrix. Loss of muscle satellite cells may be related to changes in both sarcomeres and extracellular matrix. Future studies are required to determine the underlying mechanism for changes in muscle satellite cells and their relationship (if any) to contracture. A more complete understanding of this mechanism may lead to effective nonsurgical treatments to relieve and even prevent muscle contractures.
Keywords: biomechanics, cerebral palsy, extracellular matrix, sarcomere length, skeletal muscle mechanics
INTRODUCTION
Skeletal muscle contractures represent the permanent shortening of a muscle-tendon unit that occurs when soft tissue loses elasticity and cannot be stretched, either passively or by antagonistic muscles. A contracture is probably the most clinically significant consequence of altered passive mechanical properties in muscle, which is the topic of this special issue. Contractures may occur because of an upper motor neuron lesion, such as stroke, head injury, or cerebral palsy (CP), or muscle disease, such as spinal muscular atrophy or muscular dystrophy. In such conditions, immobilization, muscle weakness, or paralysis with or without spasticity are key factors that lead to contracture development. The resulting structural alterations, described in more detail below, result in tissue that is stiffer than normal muscle and may eventually restrict joint mobility, causing deformity. When deformity begins to affect quality of life, and conservative treatments such as range of motion exercises, splinting, and neurotoxin injection fail, surgery is indicated. A number of surgical procedures are used to alleviate muscle contracture and deformity: muscle-tendon lengthening, muscle release, fascial and aponeurotical transection, and aponeurectomy with or without skin widening (Table 1). These procedures lengthen the tendon in series with the muscle to allow joint movement, release the tendon from its insertion, or allow the aponeurosis to lengthen, spreading the insertion area of the muscle. As the contracture is released during surgery, the muscle tissue retracts, creating a surgical gap (Fig. 1A), the magnitude of which varies with the particular surgery. The gap can be allowed to remain or sutured to surrounding connective tissue at a new length, resulting in a permanent change in the length of the muscle-tendon unit. Thus, all surgeries allow muscles to shorten while series connective tissue lengthens. Surgeons choose the particular approach depending on the patient’s functional needs and the surgeon’s prior experience. Over the past 20 years, such surgeries have allowed us the unique opportunity to study human muscle tissue adaptation to contracture. In this review, we summarize some of our major findings. The reader is also referred to other presentations of neural aspects related to muscle stiffness (51, 62).
Table 1.
Contracture conditions and surgical treatment options
| Deformity | Muscle Affected | Procedure |
|---|---|---|
| Adducted and internally rotated shoulder | Pectoralis Subscapularis |
Tendon lengthening Tendon lengthening |
| Flexed elbow | Biceps brachialis | Tendon lengthening Muscle-fascia transection (see Fig. 1) |
| Pronated forearm | Pronator teres | Muscle-tendon release |
| Flexed wrist | Flexor carpi radialis | Tendon lengthening |
| Flexed fingers | Flexor digitorum profundus Flexor digitorum superficialis Interossei and lumbricals |
Tendon lengthening Tendon lengthening Proximal phalanx aponeurectomy |
| Adducted and flexed thumb | Flexor pollicis brevis/longus Adductor pollicis |
Tendon lengthening Muscle-tendon release Widening 1st web space |
| Equinus contracture | Gastrocnemius and soleus | Achilles tendon lengthening Gastrocnemius recession |
| Knee flexion contracture | Gracilis and semitendinosus | Gracilis and semitendinosus tendon lengthening |
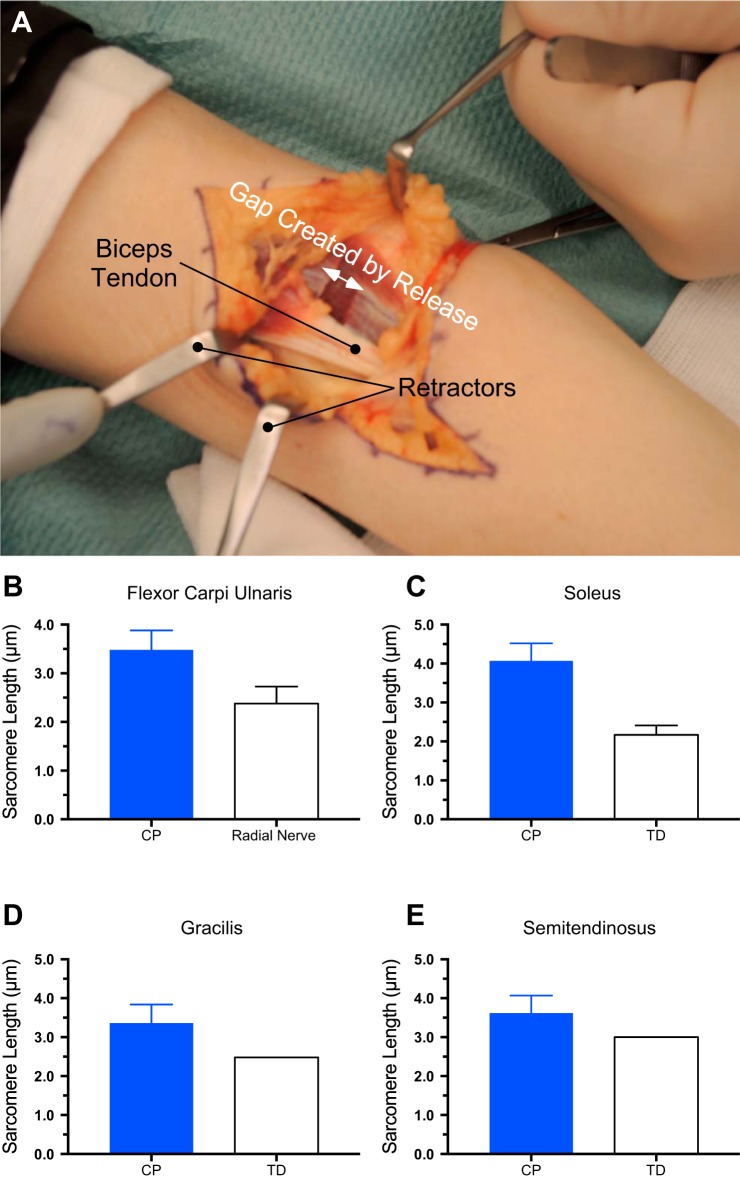
Fig. 1.
Intraoperative measurement of dramatic sarcomere length increases in muscle contractures. A: intraoperative view of the anterior elbow of a person with an elbow contracture secondary to brain damage from prenatal cytomegalovirus infection. This image shows the gap created by surgical release (transection of the anterior fascia of the brachialis muscle and intramuscular tendon bundles and the biceps brachii distal tendon) before stair step lengthening. These releases allow passive elbow extension and, thus, basic activity of daily living functions such as self-care, feeding, and wheelchair driving. Intraoperative sarcomere length was measured by laser diffraction (17, 38) in upper extremity muscles from children with cerebral palsy (CP) or typically developing (TD) children or radial nerve injury patients: flexor carpi ulnaris muscle (B), soleus muscle (C), gracilis muscle (D), and semitendinosus muscle (E). (Data replotted from Refs. 35, 46, and 66.) Data represent means ± SE, n = 6–10 subjects/group. No error bars are provided for TD sarcomere lengths in D or E since these were single sarcomere length estimates based on a mathematical model.
SARCOMERE LENGTH IN MUSCLE CONTRACTURE
Clinical exam and intraoperative inspection clearly reveal that muscle contractures represent a muscle-tendon unit that is under extremely high passive mechanical tension. This is true in patients with CP, patients after stroke, and traumatic brain injury (TBI) patients. The first and most paradoxical observation made during intraoperative measurement of contractured muscles was that, although significantly shortened, the muscles were composed of sarcomeres that were highly stretched (34), sometimes even beyond their normal in vivo operating range. We first observed stretched sarcomeres in contractured wrist flexor muscles in patients with CP and TBI (Fig. 1B, see Ref. 34) and have since made similar observations for the soleus, (Fig. 1C, see Ref. 46), gracilis (Fig. 1D, see Ref. 66), and semitendinosus (Fig. 1E, see Ref. 66) muscles in children with CP.
Functionally, extremely long sarcomeres mean that the muscle will generate very low active tension in an abnormal part of its range of motion (40). Thus, it is no wonder that contractured muscles are very weak. Sarcomere lengths in the 3.5–4.0 µm range (Fig. 1, B–D) would, on average, only result in generation of ~20% of normal maximum tetanic tension, given the dimensions of myosin and actin filaments in humans (35, 68).
Using a combination of ultrasound and intraoperative measurements, we subsequently showed that, while gross muscle fascicle length was relatively normal in plantarflexor contractures of children with CP, serial sarcomere number was much lower (see Fig. 5 of Ref. 45). Many other laboratories have measured fascicle length in children with CP and have reported fascicles that are longer (54), shorter (52), or the same (61) as typically developing (TD) children (reviewed in Ref. 3). From an anatomical point of view, all of these results may be consistent with our findings because sarcomere length was not measured in any other ultrasound study referenced. This suggests that ultrasound measurements may provide limited information with regard to muscle adaptation after upper motor neuron lesion since, in our study, the fascicles were actually highly stretched (long sarcomere length) even though fascicle length was normal. Normal muscles have a stereotypical number of sarcomeres in series, which gives rise to their standard architecture (33) and sarcomere length operating range (5). We suggested that this design is “programmed” in the development of various muscle-tendon units (32, 40). Serial and parallel sarcomere numbers and arrangement are highly consistent among humans and, indeed, among species. The extremely long sarcomeres and high passive tension in contractures indicate impairment of the “sensing” mechanisms that normally regulate length and tension. The dramatic changes in muscle architecture observed in contractures signal a significant loss of structural or developmental homeostasis. Cellular, tissue, and molecular signals are likely all involved in creating the disorganized muscle architecture in contracture.
PASSIVE BIOMECHANICAL PROPERTIES OF CONTRACTURES
Intraoperative access permitted direct measurement of the composite properties of contractured and normal muscle and led to an improved understanding of both muscle fiber and extracellular matrix (ECM) properties. Our early study of contractured wrist flexors suggested that muscle fibers had increased stiffness relative to permeabilized muscle fibers from patients with TD but that this modest increase could not account for the large differences in stiffness between normal and contractured muscle (18, 37) determined by measuring the stiffness of muscle fascicles and whole muscles (see Fig. 2 of Ref. 36). Interestingly, the morphology of the wrist flexors was highly disrupted in children with fixed wrist flexion contractures, resulting in very large areas of inflamed and distorted tissue [compare the morphology of Fig. 2 (a bundle from a child with CP) with Fig. 3 (a bundle from a TD child) in Ref. 37]. Thus, we suspect our ECM area fractions in these muscles were artificially inflated, perhaps leading to the conclusion that the ECM of these spastic muscle was qualitatively inferior to muscles we measured in the lower extremity (summarized in Fig. 2A). In lower extremity, the morphology was much more normal (see Fig. 7 of Ref. 66), and thus ECM areas could be more accurately quantified. In these cases, the results were mixed. For hamstring muscles, gracilis and semitendinosus, muscle fiber bundles from children with CP were significantly stiffer than those from TD children (Fig. 2B, see Ref. 66); however, for the plantarflexors, soleus and gastrocnemius, there was no significant difference in fiber bundle stiffness between CP and TD muscle (Fig. 2A, see Ref. 44). Interestingly, CP soleus muscle fibers were significantly stiffer than normal muscle fibers, suggesting that both intracellular and extracellular adaptations occurred in this particular muscle. The structural basis for this adaptation is not yet known.
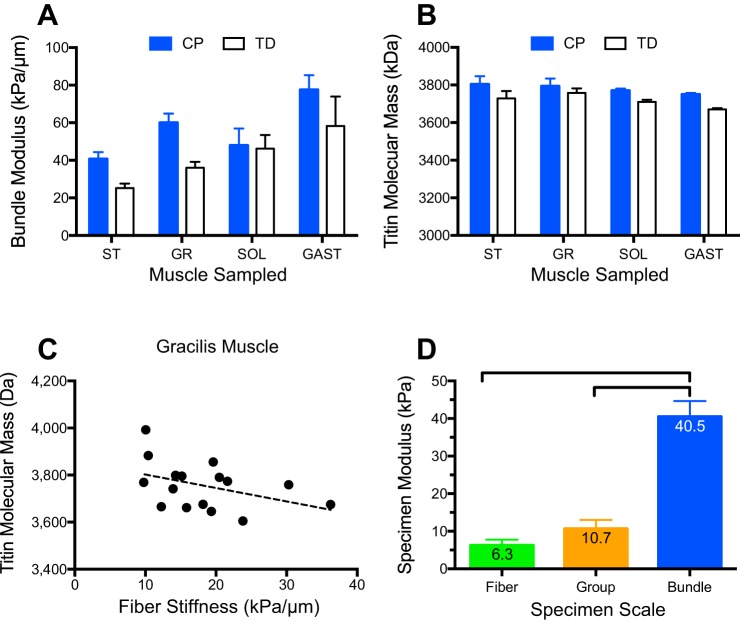
Fig. 2.
Passive biomechanical properties of muscle specimens are altered in contractures because of cerebral palsy (CP) compared with typically developing (TD) muscle. A: bundle modulus measured in vitro from intraoperative muscle biopsies in children with CP is typically stiffer than that of bundles from TD children. B: titin molecular mass measured using specialized electrophoresis (70) from muscle biopsies reveals no meaningful differences between CP and TD muscles (note expanded vertical scale). C: in the gracilis muscle, linear regression reveals a weak correlation between muscle fiber stiffness and titin molecular mass (r2 = 0.3) that is not significant (P > 0.2). This was typical for all muscles tested, suggesting that, in contrast to rabbit muscle (58), titin mass is not a major determinant of fiber stiffness in humans. D: modulus of three different specimen types from the mouse extensor digitorum longus muscle clearly demonstrates that the skeletal muscle extracellular matrix, present in fiber bundles only, bears most of the tensile load (for experimental details, see Ref. 50). SOL, soleus muscle; GR, gracilis muscle; ST, semitendinosus muscle; GAST, gastrocnemius muscle. Modulus data replotted from Refs. 44 and 66. Data represent means ± SE, n = 6–10/group.
The most obvious intracellular cause for altered passive mechanical properties of single muscle fibers is the giant intramuscular protein titin (30). A previous study showed the particular isoform of cardiac titin expressed could change in response to altered pacing (55), so it was conceivable that altered neural activity associated with upper motor neuron lesions could alter titin isoform expression in skeletal muscle. We quantified titin isoform mass in each of the semitendinosus, gracilis, gastrocnemius, and soleus muscles and found either no significant change or a significant increase in titin mass in CP compared with TD muscle (Fig. 2B, see Refs. 44 and 66), which would have indicated a change in isoform (note the low variability of titin gel measurements in Fig. 2B). If anything, increased titin mass would be expected to decrease muscle stiffness, which was never observed. Additionally, in upper extremity muscles we observed a decrease in slack sarcomere length measured from isolated fibers as the length where muscle fiber force is zero (18, 37). Given most of the studies on titin, this would also suggest a decrease in titin size, opposite to what was measured. Future studies are required to sort out the structural basis for altered slack sarcomere length.
It has been demonstrated in mammalian muscle cells that titin bears from 10 to 50% of single fiber tension (58), and, recently, we demonstrated that mammalian muscle fibers bear ~45% of bundle tension (49). Should this be true in general for mammalian muscle, it leads to the conclusion that titin bears, at most, ~23% of fiber bundle tension (50% of 45%). In support of this, our recent report that fiber bundles bear 5–10% of the passive stiffness of whole muscles (T. M. Winters, S. M. O’Connor, R. L. Lieber, and S. R. Ward, unpublished observations) implies that titin would bear only 1–2% of whole muscle tension in mammalian muscle (5–10% of 23%, which is not likely to be functionally important, even if titin stiffness changed by orders of magnitude). Taken together, these experiments contradict the hypothesis that titin is functionally load bearing at the whole muscle level in mammalian skeletal muscle. In further support of this idea, titin molecular mass showed very weak or no significant correlation with muscle fiber stiffness (see example, for gracilis muscle in Fig. 2C). This view that titin does not provide a mechanical function in whole muscle is not universal. For example, titin has been proposed as a tunable spring to regulate muscle stiffness for various types of muscle action (53) and has been correlated to the effects of calcium in myofibrils (27). Thus, this is also an active area of current research.
Recent studies in mouse demonstrate that, in mammalian muscle, the ECM bears approximately five times the load of a muscle fascicle compared with intracellular proteins (Fig. 2D, see Ref. 50). Our experiments clearly demonstrated that the increased load bearing of fiber bundles is not the result of either inhomogeneity between fibers or simple scaling issues (Fig. 2D). We showed this by comparing the sarcomere length-stress relationship among isolated muscle fibers, small muscle fiber bundles, and “groups” of fibers (collections of individually dissected single muscle fibers tied together to form a specimen that was approximately the size of a fiber bundle). The Young’s modulus (a normalized indicator of passive stiffness) was similar for single fibers and groups of fibers (~10 kPa), neither of which has any ECM (control experiments showed no measurable collagen content in fiber groups), while small bundles were more than four times stiffer at over 40 kPa (Fig. 2D). These data clearly indicate that mouse muscle fibers are less stiff, and their ECM much stiffer, than frog muscle (42, 50). Unfortunately, additional comparative biomechanical data on the ECM of different species are lacking and represent an area of future research.
EXTRACELLULAR MATRIX BIOCHEMICAL CHANGES IN CONTRACTURE
The structure, function, and composition of skeletal muscle ECM are much more poorly understood than those of connective tissues, such as skin, tendon, and ligament. These tissues have a much lower cell density compared with muscle and orders of magnitude more ECM, making their ECM much easier to study structurally and functionally (6, 7). To further understand increased muscle stiffness in contractures, we applied assays used in other connective tissues to quantify collagen content (quantified as mg collagen/muscle tissue mass; Fig. 3) and histological appearance of contractured muscle. Histologically, increased ECM appears as a larger space between muscle fibers in sections stained for almost any extracellular matrix protein, such as collagen or laminin (Fig. 3, A and B). However, while the “area fraction” of ECM can be quantified, it is difficult to relate this to any structural or mechanical measurements, since extracellular space can change because of increased ECM, decreased muscle fiber size, or a combination of the two. Thus, a more precise biochemical quantification of ECM was performed in a number of different muscles. The values obtained depended on the particular muscle studied, but we observed collagen content ranged from <5% by mass to >10% by mass in whole contractured muscle samples (44, 66) (Fig. 3C). Interestingly, the change in collagen content in CP relative to TD also depended on the particular muscle studied, with hamstring muscles showing increases in collagen content while plantarflexor muscles actually showed decreases relative to TD (Fig. 3C). Overall, the general increase in collagen content in contractured hamstrings and the dramatic sarcomere length alterations led us to conclude that this condition represents a type of muscle fibrosis (39). We wanted to determine whether the increased collagen content observed in hamstring muscles could explain increased muscle stiffness, so, as with the titin studies described above, we measured biochemical composition and biomechanical properties in the same specimens (66). Unfortunately, although collagen content increased as did mechanical tensile stiffness, the correlation was very weak (r2 = 0.3, P > 0.2; Fig. 3D). In other words, increased collagen content is characteristic of contracture but does not entirely account for the increased mechanical tension in a quantitative fashion. Interestingly, triceps surae muscles demonstrated a decreased collagen content (Fig. 3D) that also did not correlate with mechanical stiffness. At this point, it is not clear whether the structural alteration is the difference between upper and lower extremities or differences between specific muscle groups. Such questions are the topic of current studies.
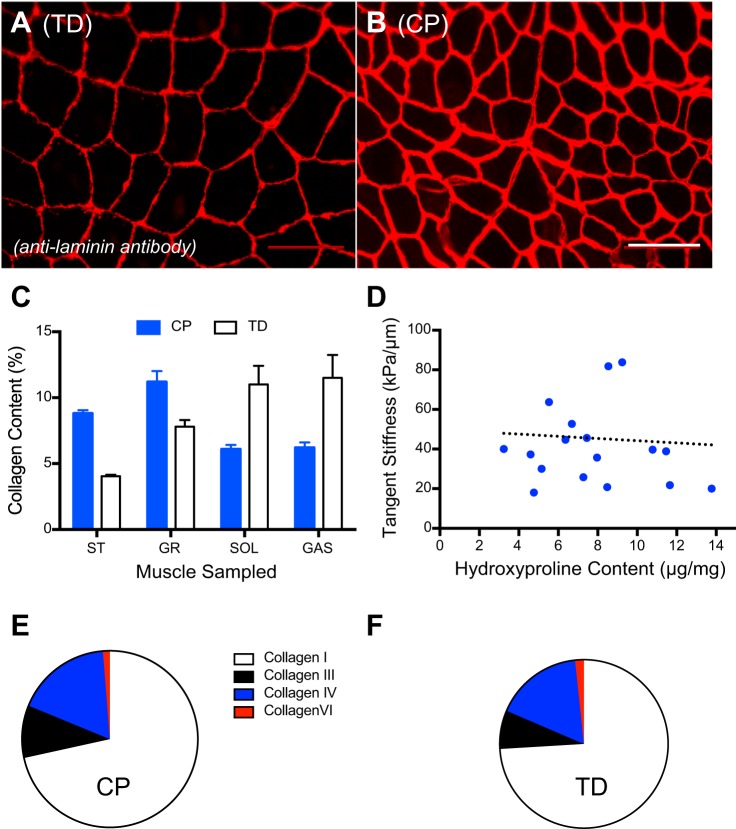
Fig. 3.
Extracellular matrix proteins change in contractures because of cerebral palsy (CP) compared with typically developing (TD) muscles. Cross sections of muscle from a TD child (A) and child with CP (B) immunolabeled with antilaminin antibody and imaged at the same magnification with the same laser intensity show increased thickness of laminin stain in CP. Calibration bar = 100 µm. C: collagen content (%collagen mass/muscle mass) measured by hydroxyproline assay of four different intraoperative muscle biopsies (SOL, soleus muscle; GR, gracilis muscle; ST, semitendinosus muscle; GAST, gastrocnemius muscle) varies by muscle type. D: relationship between collagen concentration (measured as hydroxyproline content) and tangent stiffness for CP muscle. Note that, in spite of the generally higher collagen content in CP muscle compared with TD, there is a clear lack of correlation between collagen content and stiffness in this human ST muscle (r2 = 0.01, P > 0.8). Similar results were obtained for GR, SOL, and GAS. Ratios of collagen isoforms in CP muscle (E) and TD muscle (F) indicate no difference between CP and TD conditions. Data replotted from Refs. 44 and 66. Data represent means ± SE, n = 6–24/group.
Biochemical changes in ECM that could modify muscle stiffness, other than the amount of collagen, include the type of collagen expressed or the posttranslational processing of collagen fibers. Collagen exists in multiple isoforms, and changes in isoform composition often correlate with different biomechanical properties (16). This observation, based primarily on a comparison between tendons and ligaments, reveals a correlation between increased compliance in tendons and an increase in the type 3-to-type 1 collagen ratio (29). Tendons designed for very stiff biomechanical function, such as human digital flexors (69), have lower ratios than those that are compliant enough to stretch and store energy, such as the Achilles tendon (1, 2, 29). We thus quantified collagen types I, III, IV, and VI in contractured muscle. While the overall collagen concentration increased by almost threefold for each of these collagen isoforms, compared with TD muscle, the ratio among isoforms remained remarkably constant (Fig. 3, E and F) (57). We thus conclude that, in contractures, there is no selective synthesis or expression of a single collagen isoform.
Posttranslational processing of collagen fibrils can also affect their stiffness (43). Collagen fibrils are heavily cross-linked to one another, and it is known that the degree of cross-linking can correlate with mechanical stiffness (41). Thus, we measured the degree of collagen cross-linking in normal and contractured human muscle but found no difference between the two. However, because of the high degree of variability in these human samples, we repeated the analysis in a more highly controlled study of mouse muscle fibrosis (10) and found that lysyl-pyridinoline, hydro-xylysyl-pyridinoline, and pentosidine collagen cross-links were also not causally related to tissue stiffness (9). Thus, while increased collagen cross-links can affect tissue stiffness, increased tissue stiffness does not necessary require increased collagen cross-links.
EXTRACELLULAR MATRIX STRUCTURAL CHANGES IN CONTRACTURE
Lack of correlation between collagen content, collagen isoform, or collagen cross-linking and mechanical stiffness was somewhat surprising, based on studies of other connective tissues in which one or more of these parameters typically correlates well with tensile stiffness. However, other tissues are more structurally homogeneous. Skeletal muscle represents a true composite tissue with relatively compliant muscle fibers embedded in a relatively stiff connective tissue matrix. The structure of this matrix, which is largely unknown, may also affect mechanical properties. Few studies have examined muscle ECM structure, with the exception of the few excellent studies by Purslow and Trotter (59) that focused on the endomysial connective tissue surrounding the muscle fibers. The endomysium is composed primarily of type IV collagen and forms a classic “mesh” that both rotates and deforms with muscle length change (59).
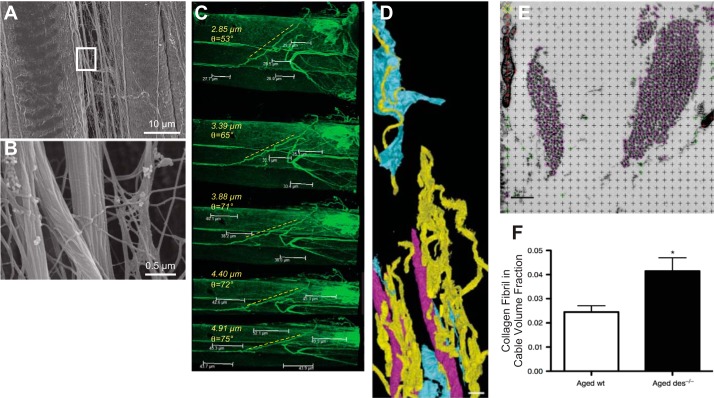
We performed confocal microscopy and scanning electron microscopy (SEM) of small mouse muscle fiber bundles and found a large number of previously unobserved collagen cables dispersed throughout the perimysial space (Fig. 4). When mouse tibialis anterior muscles were stretched and fixed for observation by SEM (21), we observed longitudinal cables that ran parallel to muscle fibers, ideal for bearing tensile load (Fig. 4A). High-magnification views of these cables revealed the typical collagen fibrillar structure (Fig. 4B); thus, we concluded that they were composed of types I and III fibrillar collagen. These cables were seen to undulate through the extracellular space often, as shown by SEM, extending hundreds of microns and changing morphology depending on muscle sarcomere length (21). However, these images were obtained from specimens that were dried and coated in preparation for SE and thus perhaps distorted (15), and only a single static snapshot could be obtained. We therefore immunolabeled small muscle fiber bundles with a type I collagen antibody and were able to observe the deformation of the cables during muscle stretching using confocal microscopy (23); the cables both stretched longitudinally and rotated with increasing sarcomere length (Fig. 4C). Cables were observed in close proximity to muscle fibers, but we were again concerned that preparatory methods for microscopy might distort or disrupt the cables relative to the rest of the ECM and thus obscure their true location. We therefore adapted a block-face serial sectioning method, initially used to quantify cerebellar structures (13), whereby perfusion-fixed muscle tissue was serially sectioned within the vacuum of the SEM chamber and the remaining block face imaged by electrons reflected from the block (22). The beauty of this method is that the embedded tissue can be perfusion fixed and embedded in plastic with minimal distortion. Also, the serial sections need not be collected and organized, since the block face is imaged, and each successive section is discarded. These images, which are acquired by SEM but actually look more like transmission electron micrographs, can be reconstructed and quantified in three dimensions to reveal the native structure of the cables (Fig. 4D). While studies of the detailed structure and function of these cables are still in progress, it is clear that the number and size of these cables can vary. We reported this finding for fibrotic mouse muscle in which passive stiffness increased ~10 times compared with normal muscle, and the major structural change observed was in the organization of the collagen cables. Detailed dimensions of the collagen cables and number of fibrils were measured by stereology of high-magnification micrographs of the ECM (Fig. 4E), and we found that the total number of cables did not change significantly, but their size changed as did the number of free collagen fibrils in the ECM (Fig. 4F) (23). Our interpretation of these data is that there is an equilibrium between free isolated fibrils and those assembled in cables. If true, this represents an underlying biological process that is almost certainly mechanically sensitive. The cellular processes responsible for the creation, maintenance, and adaptation of these cables are currently unknown. It is fascinating to speculate that this process is regulated in skeletal muscle, based on mechanical, neural, or molecular environmental cues. In support of the idea of altered ECM homeostasis, we have also measured dramatic transcriptional changes in contractured muscle ECM from children with CP, with hamstring (65) or wrist flexor (67) contractures. In both cases, expression of numerous genes for collagen, cross-linking enzymes, proteases, and protease inhibitors is dramatically altered in CP. This observation paves the way for an exciting area of future research.
Fig. 4.
Images of perimysial collagen cables. A: low-magnification scanning-electron micrograph of perimysial cables running along the side of stretched mouse muscle fibers. B: higher-magnification scanning-electron micrograph of boxed area in A; this close-up of perimysial cables reveals the collagen fibrillar structure. C: sequential confocal images of a perimysial cable being stretched and rotating during imaging. Specimen was labeled with an anti-type I collagen antibody. Sarcomere length is shown in microns; perimysial cable angle is shown in degrees. D: 3-dimensional reconstruction of serial block-face images taken from mouse skeletal muscle color coded for capillaries (pink), fibroblasts (blue), or perimysial collagen cables (yellow). Scale bar = 5 µm. E: high-magnification (×11,000) transmission electron micrograph with stereology grid overlaid. Each point (at crosshair) was classified as a single collagen fibril (green circle), collagen fibril in cable (pink circle), or interstitial space (black). Points lying on objects that were not part of the extracellular space were not classified (red) or included in quantification. Scale bar = 250 nm. F: volume fraction of collagen fibrils located within perimysial cables determined by stereological analysis of transmission electron micrographs from transgenic mice lacking the desmin gene, resulting in fibrosis. (Images taken from studies published in Refs. 21–23.)
CELLULAR CHANGES IN MUSCLE CONTRACTURE
Changes in the passive mechanical properties of contractured muscle are thus thought to result from a combination of changes in ECM structure and function as well as an aberrant muscle regulatory system that allows long sarcomere lengths. It is not an overstatement to say that the dramatic sarcomere length changes seen in contractures are unprecedented in any species, much less in mammals. While it has been known since the 1970s that serial sarcomere number can change in response to prolonged muscle length change, such as occurs during immobilization, previous animal studies demonstrate that such adaptations always return to a resting sarcomere length that is near optimal. Indeed, in their classic studies, Williams and Goldspink demonstrated that a chronically stretched muscle (i.e., long sarcomere length) will synthesize and add serial sarcomeres to muscle fibers to shorten sarcomeres to their optimal length (71, 72). Conversely, a chronically shortened muscle (i.e., short sarcomere length) will subtract serial sarcomeres from muscle fibers to return remaining sarcomeres to their optimal length. It has never been demonstrated that a normal muscle will change serial sarcomere number to achieve the highly stretched sarcomere length observed in human muscle contractures. What is the mechanism for this change? Absent an animal model, since no animal model recapitulates these dramatic sarcomere length changes, we initiated further biological studies of human contractured tissue.
In addition to structural proteins described above, skeletal muscle ECM contains a variety of cell types, any or all of which could be involved in adaptation to contracture (8, 21, 63). One of the most interesting cell types are skeletal muscle satellite cells (SC), named as such because they are located at the periphery of the muscle cell, sandwiched between the sarcolemma and the basal lamina (47). SCs are located extracellularly, even though at low magnification they resemble an intracellular myonucleus. These cells remain in a quiescent state until activated by any of a number of different mechanical and chemical stimuli (11, 25). Activated SCs are a type of muscle stem cell that can rebuild muscle tissue. Sophisticated experiments using transgenic mouse models have conclusively demonstrated that SCs are required for muscle regeneration from a toxin-induced degeneration injury (31). In the absence of SCs, other cells such as fibroblasts and fibroadipogenic progenitors proliferate unchecked to create stiff, fibrotic, and fat-infiltrated tissue (26, 56). SCs may also be required for muscle fiber hypertrophy although there is also evidence to the contrary, reflecting the technical difficulty of performing such experiments (19, 20, 48). Maintenance of quiescence and self-replication after activation of SCs is a highly regulated critical process; if the muscle SC pool is depleted (as occurs in Duchenne muscular dystrophy because of chronic injury and regeneration of tissue), no SCs will be available for muscle regeneration, repair, or growth (4, 60).
Muscle fibers from contractures in children with CP are small in diameter and have highly stretched sarcomeres. We thus suspected that contractured muscles might have impaired growth, which could implicate SC dysfunction. The idea that muscles from children with CP are “unable” to grow has been in the literature for decades based primarily on muscle volumes measured by ultrasound (3, 24). This difference is also observed at the muscle fiber level (see comparative regression lines for CP and TD in Fig. 4 of Ref. 12). To begin to understand SCs in CP, we performed direct measurement of SC number in muscle biopsies obtained from children with CP and TD children. SCs were quantified in homogenized tissue by antibody labeling and fluorescence-activated cell sorting analysis; SC numbers were expressed as cells per milligram tissue. Surprisingly, we found a dramatic ~75% decrease in SC concentration in CP muscle, which represents a tremendous maladaptation of the muscle tissue (64). We were very excited by this initial finding, but a potential serious limitation of this study was that the abnormality of the ECM in CP tissues that were described above may have made it more difficult to isolate SCs from CP muscle than from TD muscle. Thus, we repeated the study using the much more laborious method of counting antibody-labeled SCs in serial sections of frozen tissue (12). In this method, SCs were visualized by high-powered fluorescence microscopy (×40 objective) and counted only if all three of the following criteria were met: 1) presence of Pax7, a known SC transcription factor, 2) localization in the subbasal laminal region, and 3) colocalization of Pax7 with a nucleus (see Fig. 1 of Ref. 12). This immunohistochemistry study likewise demonstrated that numbers of SC cells per muscle fiber were decreased by ~70% in CP muscle compared with TD muscle. [Because the units of SC concentration are different between the two methods (SC/mg tissue for fluorescence-activated cell sorting and SC/fiber in immunohistochemistry), a direct quantitative comparison cannot be made between the studies.]
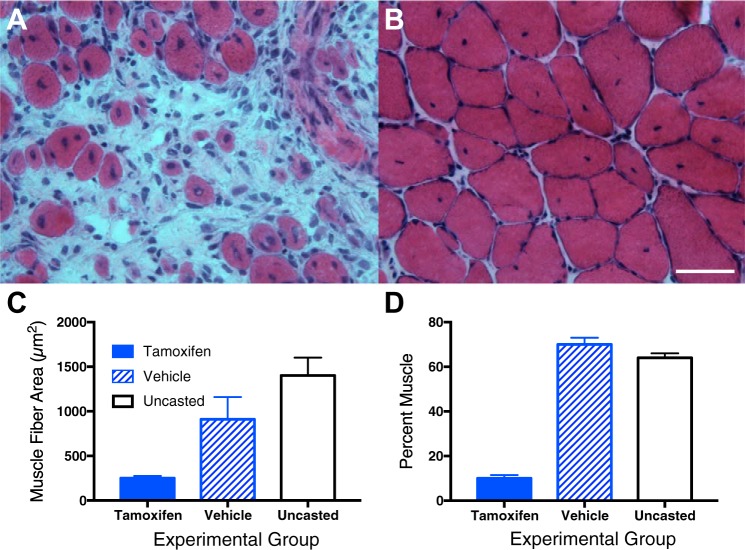
The decrease in SC numbers in CP tissue leads to an obvious working hypothesis for the mechanism of contracture formation: an upper motor neuron lesion results in a reduction in SC numbers that makes it impossible for muscles to “grow” in length and girth as bone length increases with development. This hypothesis is consistent with the clinical observation that contractures often appear or become worse in children during the growth spurts that occur near puberty, usually between 8 and 13 yr of age in girls and 10–15 yr in boys. Also in support of this hypothesis is our recent study that demonstrated the inability of mouse muscles with decreased SC content to add a normal complement of sarcomeres to muscles that are stretched by cast immobilization (28). These studies used a transgenic mouse strain in which SC number was decreased by administration of tamoxifen over 5 days, resulting in depletion of SC numbers by ~70% (similar to that seen in CP). After a 5-day washout period, mouse ankles were immobilized in a fully dorsiflexed position, thus stretching the soleus complex and shortening the tibialis anterior and extensor digitorum longus muscles. These immobilization conditions mimic the classic studies of Williams and Goldspink and others who showed that, under these conditions, muscles add sarcomeres to stretched muscles to restore sarcomere length to normal. We found that the muscles stretched after tamoxifen treatment had a highly abnormal morphological appearance (Fig. 5A) with an excess of hypercellular ECM compared with vehicle control mice (Fig. 5B), as well as decreased numbers of SCs. While the serial sarcomere number in those soleus muscle fibers that were formed was essentially normal, only ~25% fibers were created compared with untreated muscles, and the entire ECM was hypertrophic (Fig. 5A). This was easily quantified as a decreased area fraction of muscle (Fig. 5D) with dramatically decreased muscle fiber size (Fig. 5C). Thus, these data provide support for the concept that a reduction in SC number may be mechanistically related to changes in muscle fiber adaptation to stretch. While the mechanism of these effects is unknown, further studies are underway (14).
Fig. 5.
Muscle morphology after serial sarcomere addition as a result of casting in normal and satellite cell-depleted muscle (see text for details). Hematoxylin- and eosin-stained sections of muscle with depleted satellite cell number (tamoxifen treated) (A) and vehicle control (B) after immobilization and stretch show smaller fibers and hypercellular extracellular matrix after satellite cell depletion. C: muscle fiber area in cross sections from tamoxifen-treated mice, vehicle control-treated mice, and uncasted mice. D: %muscle tissue from histological cross sections in tamoxifen-treated mice, vehicle control-treated mice, and uncasted mice. Data represent means ± SE, n = 4–5/group. (Data replotted from Ref. 28.)
SUMMARY
Human muscle contracture represents the prototypic most severe clinical case of increased passive mechanical muscle force, which often requires surgical release. The high passive force is associated with abnormally stretched sarcomeres, which otherwise are fairly normal, and a lower serial sarcomere number. Furthermore, changes in the amount and arrangement of collagen in the ECM also affect stiffness. Observational studies suggest that perimysial collagen cables, which are regulated through the interaction of a number of cells within the ECM, may be largely responsible for increased stiffness. Reduced SCs may be related to both of these phenomena; future studies are required to determine the underlying mechanism for changes in muscle SCs and their relationship (if any) to contracture. A more complete understanding of this mechanism may lead to nonsurgical treatments to relieve or even prevent muscle contractures.
GRANTS
This work was generously supported by NIH Grants R01-AR-057393, R24-HD-050837, and P30-AR-061303. We also acknowledge the support of the Department of Veterans Affairs. We also acknowledge the outstanding graduate work described herein by former Ph.D. students Drs. Allison Gillies, Margie Mathewson, Gretchen Meyer, and Lucas Smith.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L.L. and J.F. conceived and designed research; R.L.L. and J.F. prepared figures; R.L.L. and J.F. drafted manuscript; R.L.L. and J.F. edited and revised manuscript; R.L.L. and J.F. approved final version of manuscript.
REFERENCES
- 1.Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature 265: 114–117, 1977. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RM. Elastic Mechanisms in Animal Movement. Cambridge, MA: Cambridge University Press, 1988. [Google Scholar]
- 3.Barrett RS, Lichtwark GA. Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev Med Child Neurol 52: 794–804, 2010. doi: 10.1111/j.1469-8749.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 4.Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 80: 4856–4860, 1983. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204: 1529–1536, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of Ligaments and Tendons. San Francisco, CA: The Franklin Institute Press, 1978, p. 125–182. [PubMed] [Google Scholar]
- 7.Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res 26: 1–9, 2008. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 8.Chapman MA, Mukund K, Subramaniam S, Brenner D, Lieber RL. Three distinct cell populations express extracellular matrix proteins and increase in number during skeletal muscle fibrosis. Am J Physiol Cell Physiol 312: C131–C143, 2017. doi: 10.1152/ajpcell.00226.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman MA, Pichika R, Lieber RL. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech 48: 375–378, 2015. doi: 10.1016/j.jbiomech.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman MA, Zhang J, Banerjee I, Guo LT, Zhang Z, Shelton GD, Ouyang K, Lieber RL, Chen J. Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum Mol Genet 23: 5879–5892, 2014. doi: 10.1093/hmg/ddu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14: 329–340, 2013. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dayanidhi S, Dykstra PB, Lyubasyuk V, McKay BR, Chambers HG, Lieber RL. Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J Orthop Res 33: 1039–1045, 2015. doi: 10.1002/jor.22860. [DOI] [PubMed] [Google Scholar]
- 13.Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2: e329, 2004. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domenighetti AA, Mathewson MA, Pichika R, Sibley LA, Zhao L, Chambers HG, Lieber RL. Loss of myogenic potential and fusion capacity of muscle stem cells isolated from contractured muscle in children with cerebral palsy. Am J Physiol Cell Physiol 315: C247–C257, 2018. doi: 10.1152/ajpcell.00351.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg BR, Kuda AM. Retrieval of cryostat section for comparison of histochemistry and quantitative electron microscopy in a muscle fiber. J Histochem Cytochem 25: 1169–1177, 1977. doi: 10.1177/25.10.72099. [DOI] [PubMed] [Google Scholar]
- 16.Fang F, Lake SP. Experimental evaluation of multiscale tendon mechanics. J Orthop Res 35: 1353–1365, 2017. doi: 10.1002/jor.23488. [DOI] [PubMed] [Google Scholar]
- 17.Fridén J, Lieber RL. Physiologic consequences of surgical lengthening of extensor carpi radialis brevis muscle-tendon junction for tennis elbow. J Hand Surg Am 19: 269–274, 1994. doi: 10.1016/0363-5023(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 18.Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve 27: 157–164, 2003. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 19.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21: 76–80, 2015. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillies AR, Bushong EA, Deerinck TJ, Ellisman MH, Lieber RL. Three-dimensional reconstruction of skeletal muscle extracellular matrix ultrastructure. Microsc Microanal 20: 1835–1840, 2014. doi: 10.1017/S1431927614013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillies AR, Chapman MA, Bushong EA, Deerinck TJ, Ellisman MH, Lieber RL. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J Physiol, 595: 1159–1171, 2017. doi: 10.1113/JP273376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gough M, Shortland AP. Could muscle deformity in children with spastic cerebral palsy be related to an impairment of muscle growth and altered adaptation? Dev Med Child Neurol 54: 495–499, 2012. doi: 10.1111/j.1469-8749.2012.04229.x. [DOI] [PubMed] [Google Scholar]
- 25.Haller S, Kapuria S, Riley RR, O'Leary MN, Schreiber KH, Andersen JK, Melov S, Que J, Rando TA, Rock J, Kennedy BK, Rodgers JT, Jasper H. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell 21: 806–818.e5, 2017. doi: 10.1016/j.stem.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joumaa V, Herzog W. Calcium sensitivity of residual force enhancement in rabbit skinned fibers. Am J Physiol Cell Physiol 307: C395–C401, 2014. doi: 10.1152/ajpcell.00052.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinney MC, Dayanidhi S, Dykstra PB, McCarthy JJ, Peterson CA, Lieber RL. Reduced skeletal muscle satellite cell number alters muscle morphology after chronic stretch but allows limited serial sarcomere addition. Muscle Nerve 55: 384–392, 2017. doi: 10.1002/mus.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 30.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270: 293–296, 1995. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 31.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber RL. Skeletal Muscle Structure and Function and Plasticity. Baltimore, MD: Williams & Wilkins, 2010, p. 393. [Google Scholar]
- 33.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23: 1647–1666, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Lieber RL, Fridén J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 25: 265–270, 2002. doi: 10.1002/mus.10036. [DOI] [PubMed] [Google Scholar]
- 35.Lieber RL, Loren GJ, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol 71: 874–881, 1994. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- 36.Lieber RL, Murray WM, Clark DL, Hentz VR, Fridén J. Biomechanical properties of the brachioradialis muscle: Implications for surgical tendon transfer. J Hand Surg Am 30: 273–282, 2005. doi: 10.1016/j.jhsa.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28: 464–471, 2003. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 38.Lieber RL, Schmitz MC, Mishra DK, Fridén J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol (1985) 77: 1926–1934, 1994. doi: 10.1152/jappl.1994.77.4.1926. [DOI] [PubMed] [Google Scholar]
- 39.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol 305: C241–C252, 2013. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos Trans R Soc Lond B Biol Sci 366: 1466–1476, 2011. doi: 10.1098/rstb.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López B, Querejeta R, González A, Larman M, Díez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension 60: 677–683, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196113. [DOI] [PubMed] [Google Scholar]
- 42.Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science 230: 1280–1282, 1985. doi: 10.1126/science.4071053. [DOI] [PubMed] [Google Scholar]
- 43.Magnusson SP, Heinemeier KM, Kjaer M. Collagen homeostasis and metabolism. Adv Exp Med Biol 920: 11–25, 2016. doi: 10.1007/978-3-319-33943-6_2. [DOI] [PubMed] [Google Scholar]
- 44.Mathewson MA, Chambers HG, Girard PJ, Tenenhaus M, Schwartz AK, Lieber RL. Stiff muscle fibers in calf muscles of patients with cerebral palsy lead to high passive muscle stiffness. J Orthop Res 32: 1667–1674, 2014. doi: 10.1002/jor.22719. [DOI] [PubMed] [Google Scholar]
- 45.Mathewson MA, Chapman MA, Hentzen ER, Fridén J, Lieber RL. Anatomical, architectural, and biochemical diversity of the murine forelimb muscles. J Anat 221: 443–451, 2012. doi: 10.1111/j.1469-7580.2012.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathewson MA, Ward SR, Chambers HG, Lieber RL. High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J Orthop Res 33: 33–39, 2015. doi: 10.1002/jor.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer G, Lieber RL. Frog muscle fibers bear a larger fraction of passive muscle tension than mouse fibers. J Exp Biol, 2018. doi: 10.1242/jeb.182089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 141: 446–459, 2001. doi: 10.1007/s00221-001-0901-z. [DOI] [PubMed] [Google Scholar]
- 52.Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon) 22: 718–724, 2007. doi: 10.1016/j.clinbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Monroy JA, Powers KL, Gilmore LA, Uyeno TA, Lindstedt SL, Nishikawa KC. What is the role of titin in active muscle? Exerc Sport Sci Rev 40: 73–78, 2012. doi: 10.1097/JES.0b013e31824580c6. [DOI] [PubMed] [Google Scholar]
- 54.Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol 51: 800–806, 2009. doi: 10.1111/j.1469-8749.2009.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation 106: 1333–1341, 2002. doi: 10.1161/01.CIR.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 56.Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood: the skeletal muscle niche. Trends Mol Med 18: 599–606, 2012. doi: 10.1016/j.molmed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Pichika R, Meza R, Smith L, Ward SR, Lieber RL. Collagen, proteoglycan and SLRP contribute to stiffness in human skeletal muscle contractures (Abstract) Orthopaedic Research Society (ORS) 2015 Annual Meeting. Las Vegas, NV, 28–31 March 2015, p. 451. [Google Scholar]
- 58.Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126: 461–480, 2005. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil 15: 299–308, 1994. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 60.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143: 1059–1071, 2010. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol 44: 158–163, 2002. doi: 10.1017/S0012162201001864. [DOI] [PubMed] [Google Scholar]
- 62.Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain 117: 355–363, 1994. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- 63.Smith LR, Barton ER. Regulation of fibrosis in muscular dystrophy. Matrix Biol 68-69: 602–615, 2018. doi: 10.1016/j.matbio.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol 55: 264–270, 2013. doi: 10.1111/dmcn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith LR, Chambers HG, Subramaniam S, Lieber RL. Transcriptional abnormalities of hamstring muscle contractures in children with cerebral palsy. PLoS One 7: e40686, 2012. doi: 10.1371/journal.pone.0040686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith LR, Pontén E, Hedström Y, Ward SR, Chambers HG, Subramaniam S, Lieber RL. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics 2: 44–56, 2009. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec 178: 63–81, 1974. doi: 10.1002/ar.1091780107. [DOI] [PubMed] [Google Scholar]
- 69.Ward SR, Loren GJ, Lundberg S, Lieber RL. High stiffness of human digital flexor tendons is suited for precise finger positional control. J Neurophysiol 96: 2815–2818, 2006. doi: 10.1152/jn.00284.2006. [DOI] [PubMed] [Google Scholar]
- 70.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24: 1695–1702, 2003. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 71.Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat 127: 459–468, 1978. [PMC free article] [PubMed] [Google Scholar]
- 72.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat 116: 45–55, 1973. [PMC free article] [PubMed] [Google Scholar]