Abstract
Short-term muscle disuse is characterized by skeletal muscle insulin resistance, although this response is divergent across subjects. The mechanisms regulating inactivity-induced insulin resistance between populations that are more or less susceptible to disuse-induced insulin resistance are not known. RNA sequencing was conducted on vastus lateralis muscle biopsies from subjects before and after bed rest (n = 26) to describe the transcriptome of inactivity-induced insulin resistance. Subjects were separated into Low (n = 14) or High (n = 12) Susceptibility Groups based on the magnitude of change in insulin sensitivity after 5 days of bed rest. Both groups became insulin-resistant after bed rest, and there were no differences between groups in nonmetabolic characteristics (body mass, body mass index, fat mass, and lean mass). The High Susceptibility Group had more genes altered >1.5-fold (426 high versus 391 low) and more than twofold (73 high versus 55 low). Twenty-four genes were altered more than twofold in the High Susceptibility Group that did not change in the Low Susceptibility Group. 95 gene changes correlated with the changes in insulin sensitivity; 6 of these genes changed more than twofold in the High Susceptibility Group. Participants in the High Susceptibility Group were uniquely characterized with muscle gene responses described by a decrease in pathways responsible for lipid uptake and oxidation, decreased capacity for triglyceride export (APOB), increased lipogenesis (i.e., PFKFB3, FASN), and increased amino acid export (SLC43A1). These transcriptomic data provide a comprehensive examination of pathways and genes that may be useful biomarkers, or novel targets to offset muscle disuse-induced insulin resistance.
NEW & NOTEWORTHY Short-term muscle disuse results in skeletal muscle insulin resistance through mechanisms that are not fully understood. Following a 5-day bed rest intervention, subjects were divided into High and Low Susceptibility Groups to inactivity-induced insulin resistance. This was followed by a genome-wide transcriptional analysis on muscle biopsy samples to gain insight on divergent insulin sensitivity responses. Our primary finding was that the skeletal muscle of subjects who experienced the most inactivity-induced insulin resistance (high susceptibility) was characterized by a decreased preference for lipid oxidation, increased lipogenesis, and increased amino acid export.
Keywords: bed rest, DAVID gene ontology, insulin resistance, RNAseq
INTRODUCTION
Physical activity is a potent mediator of insulin sensitivity for maintaining optimal glucose homeostasis (50, 51). Chronic physical inactivity increases risk for obesity and Type 2 diabetes mellitus (58), conditions that are typically characterized by insulin resistance. Inactivity-induced insulin resistance occurs primarily in muscle (12, 47), making this tissue a key site for molecular investigation for future targeted therapies to offset inactivity-induced insulin resistance during periods of muscle disuse (e.g., recovery from surgery).
As a model to investigate the molecular mechanisms underpinning muscle dysfunction associated with physical inactivity, our laboratory has conducted several studies using models of disuse (i.e., bed rest) to investigate various mechanisms that may regulate muscle mass, glucose tolerance, strength, and physical function in young and old subjects (39, 40, 48). We have demonstrated that during this short-term disuse, older subjects lose more lean mass and quadriceps cross-sectional area (CSA) than young subjects, in spite of having similar baseline muscle sizes (39, 48). While on average, both young and old subjects rapidly develop insulin resistance during this time period, no delineation was found between age groups. This suggests that the mechanisms that govern muscle size do not necessarily also regulate muscle insulin sensitivity (39), a theory supported by some (6), but not all, researchers (11).
Attempts to characterize the transcriptome of skeletal muscle insulin resistance have been done in the form of microarray analysis on basal and insulin-stimulated muscle biopsies after 9 days of bed rest (1), before and after 3 wk of unilateral limb suspension (27), and cross sectionally in insulin-sensitive and insulin-resistant obese individuals (55). Some of the pathways identified to be impacted with disuse include those related to oxidative phosphorylation (1, 27), fatty acid metabolism (1, 27) and transport (27), and BCAA degradation (1). Although these studies provide insight into disuse-induced transcriptional changes, no prospective studies have compared the transcriptome response to short-term inactivity-induced insulin resistance between high (i.e., large change in insulin resistance) and low (i.e., small change in resistance) susceptibility in healthy individuals. This may provide meaningful information on individuals who may be more vulnerable to insulin resistance during short-term disuse. Therefore, the purpose of this study was to use skeletal muscle vastus lateralis samples (n = 26) banked from our previous studies (40, 48) to examine the transcriptome response from two unique cohorts with divergent insulin sensitivity responsiveness to 5 days of bed rest. We hypothesize key pathways and molecular regulators involved in skeletal muscle metabolism, such as altered mitochondrial function and substrate metabolism, will respond to a greater extent in the participants most susceptible to insulin resistance during bed rest.
METHODS
Subject characteristics.
The subject characteristics from healthy older and younger male and female adults before and after 5 days of bed rest (which include body composition and metabolic end points) were pooled together from two identical previously published studies (40, 48). Subjects were then categorized into High (n = 12, 5 men/7 women) and Low (n = 14, 8 men/6 women) Susceptibility Groups to measure inactivity-induced insulin resistance (described in further detail below). These characteristics can be found in Table 1.
Table 1.
Subject characteristics
| Low Susceptibility | High Susceptibility | ||||
|---|---|---|---|---|---|
| Age, yr | 52 (SD 23) (5/9) | 53 (SD 23) (4/8) | |||
| Sample Size (male/female) | 14 (8/6) | 12 (5/7) | ANOVA P Values | ||
| Height, cm | 172 (SD 8) | 175 (SD 5) | |||
| Pre | Post | Pre | Post | Bed Rest | Low vs. High | Interaction | |
|---|---|---|---|---|---|---|---|
| Body mass, kg† | 74 (SD 14) | 73 (SD 14) | 71 (SD 9) | 69 (SD 8) | <0.001 | 0.435 | 0.263 |
| BMI† | 25.03 (SD 3.8) | 24.78 (SD 3.7) | 23.04 (SD 2.2) | 22.65 (SD 2.1) | <0.001 | 0.103 | 0.337 |
| Fat mass, kg | 23 (SD 7) | 23 (SD 7) | 22 (SD 6) | 22 (SD 5) | 0.238 | 0.569 | 0.803 |
| Whole body lean mass, kg† | 48 (SD 10) | 47 (SD 9) | 46 (SD 7) | 45 (SD 6) | <0.001 | 0.508 | 0.136 |
| Insulin sensitivity (CLIX-IR)† | 5.51 (SD 2.0) | 4.64 (SD 1.7) | 7.02 (SD 2.7) | 3.65 (SD 1.5) | <0.001 | 0.729 | <0.001 |
| HOMA-IR | 1.54 (SD 0.8) | 1.50 (SD 0.8) | 0.97 (SD 0.3) | 1.18 (SD 0.6) | 0.223 | 0.118 | 0.121 |
| Matsuda Index† | 7.78 (SD 4.1) | 7.59 (SD 6.0) | 12.4 (SD 4.5)* | 7.75 (SD 2.9) | 0.002 | 0.166 | 0.003 |
| Fasting glucose, mg/dl† | 92 (SD 6) | 94 (SD 6) | 91 (SD 10) | 96 (SD 14) | 0.020 | 0.895 | 0.309 |
| Fasting insulin, µU/ml‡ | 6.68 (SD 3.2) | 6.35 (SD 3.3) | 3.99 (SD 1.9) | 4.88 (SD 1.7) | 0.435 | 0.046 | 0.091 |
| 120 min glucose, mg/dl† | 116 (SD 23) | 122 (SD 35) | 108 (SD 21) | 134 (SD 55) | 0.048 | 0.839 | 0.176 |
| 120 min insulin, µU/ml† | 58.4 (SD 70) | 63.4 (SD 35) | 21.7 (SD 19) | 32.0 (SD 18) | 0.041 | 0.120 | 0.468 |
| AUC glucose, g·dl−1·min−1† | 15244 (SD 2185) | 16154 (SD 2859) | 14228 (SD 2827) | 17493 (SD 4756) | 0.001 | 0.889 | 0.048 |
| AUC insulin, µU·ml−1·min−1† | 5914 (SD 4749) | 6846 (SD 2859) | 2749 (SD 934.9) | 4896 (SD 1968) | <0.001 | 0.089 | 0.049 |
Values are expressed as means (SD). BMI, body mass index; CLIX-IR, Clamp-Like Index; HOMA-IR, homeostatic model assessment of insulin resistance.
Different from Low Susceptibility with interaction (Sidak’s multiple-comparison test, P < 0.05).
Bed rest effect;
Group difference.
Bed rest.
Subjects were recruited within the Salt Lake City (Utah) area, and bed rest (5 days; Monday–Friday) took place at the University of Utah Center for Clinical and Translational Science using protocol and safety guidelines thoroughly described in our previous studies (40, 48). All subjects read and signed the informed consent form. The current study was approved by the University of Utah Institutional Review Board (no. 50933, 72083) and conformed to the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, “Protection of Human Subjects”. This study was registered at the clinical trials registry at ClinicalTrials.org (NCT01669590, NCT02566590). During bed rest, caloric intake (determined using the Harris–Benedict equation adjusted for no physical activity) for each subject was evenly distributed across meals and days predetermined by a research dietician. Bathroom and hygiene activities were performed in a wheelchair, while the remainder of time was spent in a bed. Nursing staff was available 24 h/day for care during the 5 days of bed rest.
Body composition and insulin sensitivity.
Whole body lean and fat mass was determined using dual-energy X-ray absorptiometry. Administration of an oral glucose tolerance test (OGTT) after a 10-h overnight fast occurred before bed rest and on the 4th day of bed rest. Measurements of insulin sensitivity included the HOMA-IR (33), the Matsuda Index (32), and Clamp-Like Index (CLIX-IR) (3), which analyze serum at each time point of an OGTT to categorize insulin sensitivity. CLIX-IR was determined using the following equation: Serum creatinine (0.85 male, 1.0 female) / mean AUC of glucose × mean AUC of C-peptide) × 6600, where CLIX-IR <5.0 is the threshold for insulin resistance and AUC is area under the curve (3). Using serum collected at each time point during the OGTT, glucose (Yellow Springs Instrument, Yellow Springs, OH), serum creatinine (no. 80189; Crystal Chem, Elk Grove Village, IL), and C-peptide (no. E6020-K; EMD Millipore, Burlington, MA) levels were determined according to manufacturers’ instructions. Because of the distinct association and tight correspondence between the gold standard insulin sensitivity measurement method, the hyperinsulinemic-euglycemic clamp, and the clamp-derived CLIX-IR measurement, we chose CLIX-IR values to determine two distinct cohorts of High and Low Susceptibility for this study (3). A 40% or greater decrease in CLIX-IR was the cut-off criteria for High Susceptibility (average: 49 ± 8.2%), with less than a 40% drop in CLIX-IR for Low Susceptibility (average: 15 ± 23%), as this allowed for near-equal sample sizes and with an equal distribution of young and old and men and women subjects in each susceptibility group to mitigate potential confounders of age and sex.
Muscle biopsies and RNA extraction.
Muscle biopsies were obtained from the vastus lateralis after a 12-h fast using the Bergström needle approach, with manual suction after anesthetizing with 1% lidocaine. Biopsies were obtained before bed rest (Pre: day 1), after bed rest (Post: day 5), and a standardized and similar meal was provided to the participant the night before each muscle biopsy. Total RNA was isolated as described previously (48). Briefly, 10–15 mg of tissue was placed in Tri reagent LS (Molecular Research Center, Cincinnati, OH) and disrupted via a handheld homogenizer (Bio-Gen PRO200; Pro Scientific, Oxford, CT). Chloroform was added to separate the RNA into an aqueous phase that was then precipitated using isopropanol. Extracted RNA was then washed with ethanol and suspended in nuclease-free water with EDTA. RNA purity and quantity were determined using a spectrophotometer (EPOCH; Take3; BioTek, Winooski, VT). RNA integrity has been reported on some of these samples previously (~8.8) (48), while the remaining samples were recorded to have a RIN of ~8.7.
RNA sequencing and library construction.
RNA sequencing and library construction were performed by the University of Utah High-Throughput Genomics Core. Total RNA samples (100–500 ng) were hybridized with Ribo-Zero Gold to substantially deplete cytoplasmic and mitochondrial rRNA from the samples. Sequencing libraries (25 pM) were chemically denatured and applied to an Illumina HiSeq v4 single-read flow cell using an Illumina cBot. Hybridized molecules were clonally amplified and annealed to sequencing primers with reagents from an Illumina HiSeq SR Cluster Kit v4-cBot (GD-401-4001). Following transfer of the flow cell to an Illumina HiSeq 2500 instrument (HCSv2.2.38 and RTA v1.18.61), a 50-cycle single-read sequence run was performed using HiSeq SBS kit v4 sequencing reagents (FC-401-4002).
Library construction was performed using the Illumina TruSeq stranded total RNA sample preparation kit with Ribo-Zero human/mouse/rat (cat. nos. RS-122-2201 and RS-122-2202). Ribosomal RNA was removed from total RNA samples (100 ng to 4 µg) using biotinylated Ribo-Zero oligos attached to magnetic beads that are complementary to cytoplasmic rRNA. Following purification, the rRNA-depleted sample was fragmented with divalent cations under elevated temperatures and primed with random hexamers in preparation for cDNA synthesis. First-strand reverse transcription was accomplished using Superscript II reverse transcriptase (cat. no. 18064-014; Invitrogen, Carlsbad, CA). Second-strand cDNA synthesis was accomplished using DNA polymerase I and RNase H under conditions in which dUTP is substituted for dTTP, yielding blunt-ended cDNA fragments in which the second strand contains dUTP. An A-base was added to the blunt ends as a means to prepare the cDNA fragments for adapter ligation and block concatamer formation during the ligation step. Adapters containing a T-base overhang were ligated to the A-tailed DNA fragments. Ligated fragments were PCR-amplified (12–15 cycles) under conditions in which the PCR reaction enables amplification of the first-strand cDNA product, whereas attempted amplification of the second-strand product stalls at dUTP bases, and, therefore, was not represented in the amplified library.
The PCR-amplified library was purified using Agencourt AMPure XP beads (cat. no. A63881; Beckman Coulter Genomics, Danvers, MA). Following amplification, the library was purified by bead-based methodologies. The concentration of the amplified library was measured with a NanoDrop spectrophotometer, and an aliquot of the library was resolved on an Agilent 2200 Tape Station using a D1K (cat. nos. 5067-5361 and 5067-5362) or a high-sensitivity D1K (cat. nos. 5067-5363 and 5067-5364) assay to define the size distribution of the sequencing library. Libraries were adjusted to a concentration of ~10 nM, and quantitative PCR was performed using the Kapa Biosystems Kapa Library quantification kit (cat. no. KK4824) to calculate the molarity of an adapter-ligated library molecules. The concentration was further adjusted following quantitative PCR to prepare the library for Illumina sequence analysis. These data have been submitted to the National Center for Biotechnology Information’s Gene Expression Omnibus (accession number: GSE113165).
Bioinformatics.
To analyze RNA sequencing data, human GRCh38 FASTA and GTF files were downloaded from Ensembl release 87, and the reference database was created using STAR version 2.5.2b with splice junctions optimized for 50-base pair reads (14, 57). Reads were aligned to the reference database using STAR in two-pass mode to output a BAM file sorted by coordinates. Mapped reads were assigned to annotated genes in the GTF file using featureCounts (v1.5.1) (30). The output files from FastQC, STAR, and featureCounts were summarized using MultiQC to check for any sample outliers (15).
Differentially expressed genes were identified using a 5% false discovery rate with DESeq2 version 1.16.0 (31). When assessing differences in gene expression with respect to CLIX-IR category, since each subject had two observations (pre- and post-bed rest) and can only be in one group (High or Low), the design formula was constructed by following the section on group-specific condition effects, individuals nested within groups in the DESeq2 vignette. The design included CLIX-IR category + CLIX-IR:nested + CLIX-IR:time to test for differences in bed rest in High Susceptibility and Low Susceptibility Groups, and their interaction, in this case, if bed rest effects are different between the two CLIX-IR categories (where CLIX-IR is High or Low, nested is subject number nested by CLIX-IR, and time is pre- or post-bed rest). Fold changes were determined using maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered.
Volcano plot and DAVID gene ontology.
All results P value (−log10 adjusted P value) and fold changes (maximum-likelihood estimates of the log2 fold change) were loaded into Prism (v. 7.00 for Windows; GraphPad Software, La Jolla CA) for generation of the volcano plot. The significant results were processed using DAVID bioinformatics enrichment tools to determine the gene expression pathways altered by bed rest within each IR susceptibility group (22, 23). Gene ontology (GO) databases for biological process were presented as the top 10-enriched pathways organized and presented by P value (−log10).
OxPhos Western blot analysis.
Because we observed that mitochondrial GO:biological process pathways were altered by bed rest, an OxPhos blot was used to determine mitochondrial complex protein abundance in subject samples with remaining lysate (Low Susceptibility: n = 10; High Susceptibility: n = 6). As previously described (40, 48), tissue samples were homogenized 1:10 (wt/vol) using a prechilled glass tube and mechanical glass pestle in an ice-cold buffer (50 mm Tris (pH 7.5), 250 mm mannitol, 40 mm NaF, 5 mm pyrophosphate, 1 mm EDTA, 1 mm EGTA, and 1% Triton X-100, with a protease inhibitor cocktail), and centrifuged for 10 min at 4°C. A Bradford protein assay was conducted, and protein abundance was measured by a spectrophotometer (EPOCH; BioTek). Equal amounts of protein were separated on a 12% gel and transferred onto a polyvinylidene difluoride membrane. Total OxPhos WB antibody cocktail (no. 110413; Abcam, Cambridge, MA) was incubated 1:1,000 overnight, and HRP conjugated mouse 2° (no. 7076; Cell Signaling Technology, Danvers, MA) was incubated 1:5,000 for 1 h. Chemiluminescence reagent (ECL Prime; Fisher Scientific, Hampton, NH) was applied to each blot for 5 min before imaging (ChemiDoc XRS; Bio-Rad, Hercules, CA). Blots were quantified with Image Lab software (Bio-Rad).
Statistical analysis.
We used a two-way ANOVA examining the interaction between time (Pre versus Post) and CLIX-IR (High versus Low) for body mass, body mass index (BMI), fat mass, lean mass, HOMA-IR, Matsuda Index, CLIX-IR, glucose from the OGTT (fasting, 120 min, AUC), and insulin from the OGTT (fasting, 120 min, AUC). Post hoc analysis (Sidak’s multiple-comparison test) was performed when a significant interaction was detected. A χ2 test was used to determine differences in sex and age between susceptibility groups. To identify specific gene targets, which may describe development of insulin resistance during bed rest, the change in CLIX-IR (log2 post - log2 pre) was correlated with the change in gene expression using the difference in regularized log-transformed values (rlog post − rlog pre). The rlog values in DESeq2 are similar to log2-normalized counts, except the variance in low-count genes is reduced. All statistical analysis and creation of figures were performed using GraphPad Prism (v. 7.00 for Mac, GraphPad Software).
RESULTS
Characteristics.
Subject characteristics in the Low and High Susceptibility Groups before (Pre) and after (Post) bed rest are presented in Table 1. Most all characteristics (body mass, BMI, lean mass, CLIX-IR, Matsuda Index, fasting glucose, 120 min glucose and insulin, AUC glucose, and insulin) with the exception of fat mass, HOMA-IR, and fasting insulin, were altered by bed rest in both Low and High Susceptibility Groups. Both Low and High Susceptibility Groups became insulin-resistant after bed rest (CLIX-IR <5). Low and High Susceptibility Groups exhibited differential responses to bed rest for the metabolic end points of the Matsuda Index (Interaction: P = 0.003), CLIX-IR (Interaction: P < 0.001), AUC glucose (Interaction: P = 0.048), and AUC insulin (Interaction: P = 0.049). In the high response group, CLIX-IR (insulin sensitivity) was lower by 49% (SD 8.2) [Pre:7.02 (SD 2.7), Post: 3.65 (SD 1.5)], while the low response group demonstrated a 15% (SD 23) [Pre: 5.51 (SD 2.0), Post: 4.64 (SD 1.7)] decrease after bed rest. Matsuda Index was higher in the High Susceptibility Group [low: 7.78 (SD 4.1) vs. high: 12.4 (SD 4.5)] via post hoc (P < 0.05), accompanied by lower fasting insulin values both before and after bed rest [Low: Pre, 6.68 µU/ml (SD 3.2); Post, 6.35 µU/ml (SD 3.3); vs. High: Pre 3.99 µU/ml (SD 1.9), Post 4.88 µU/ml (SD 1.7); group difference, P = 0.046]. No differences between High and Low Susceptibility or interactions were detected for any of the nonmetabolic characteristics (body mass, BMI, fat mass, and lean mass). Neither the sex (P = 0.695) nor the age (P > 0.999) of the participants was different between susceptibility groups. Activity level (step count) before bed rest did not explain the change in insulin resistance induced by bed rest (R2 = 0.0166, P = 0.5299), or before bed rest (R2 = 0.0596, P = 0. 2294).
Bed rest-induced changes in gene expression.
The number of genes changed within each group, as well as changed 1.5+ fold and 2+ fold, are presented in Fig. 1A. The Low Susceptibility Group had 1,235 (610 up, 625 down) gene changes with bed rest, while the High Susceptibility Group had 954 (390 up, 564 down) gene changes, with 409 (202 up, 207 down) uniquely altered genes in the High Susceptibility, but not in the Low Susceptibility Group. While High Susceptibility subjects demonstrate less total significant changes when compared with Low Susceptibility subjects, they had more gene changes that were >1.5-fold (426 High vs. 391 Low) and 33% more gene changes that were >2-fold (73 High vs. 55 Low).
Fig. 1.
Global gene response to 5 days of bed rest. Total numbers of significantly altered genes tabulated delineate all significant changes, changes >1.5-fold and >2-fold (A), and a volcano plot highlighting the top 10 significant gene changes in the high susceptibility group (B) contrast the global gene change differences between high and low susceptibility. Statistical design included CLIX-IR category + CLIX-IR:nested + CLIX-IR:time. Fold changes were determined using maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. Low Susceptibility Group: n = 14; High Susceptibility Group: n = 12.
Highly altered genes unique to high susceptibility.
Graphical representation of altered genes are presented as a volcano plot (Fig. 1B), with delineation of differences (P ≤ 0.05), and a twofold change. The top 10 significantly increased High Susceptibility genes (by fold change: PFKFB3, RP11-369K17.1, CXCL2, CTD-2207A17.1, BBOX1-AS1, HSD52, MIR133B, USP6, THRSP, GREM2) are labeled within the volcano plot. The top 10 significantly altered genes (by fold change) upregulated and downregulated for both High and Low Susceptibility Groups are presented in Table 2.
Table 2.
Top 10 genes upregulated and downregulated by 5 days of bed rest
| Low |
High |
||||
|---|---|---|---|---|---|
| Gene Name | Fold Change | Adjusted P Value | Gene Name | Fold Change | Adjusted P Value |
| Upregulated | |||||
| CTD-2207A17.1 | 3.01 | 1.52E−03 | PFKFB3 | 4.72 | 1.30E−12 |
| WNT4 | 2.20 | 1.18E−03 | RP11–369K17.1 | 3.46 | 1.43E−04 |
| PDE11A | 2.17 | 1.41E−05 | CXCL2 | 3.38 | 1.36E−03 |
| CHRNA1 | 2.12 | 1.66E−03 | CTD-2207A17.1 | 2.88 | 7.06E−03 |
| CHRND | 2.09 | 6.57E−07 | BBOX1-AS1 | 2.74 | 4.19E−04 |
| PKD1L2 | 2.09 | 3.35E−03 | HSD52 | 2.65 | 1.31E−10 |
| NME9 | 2.07 | 1.34E−02 | MIR133B | 2.43 | 1.21E−02 |
| MSTN | 2.06 | 3.47E−10 | USP6 | 2.30 | 2.29E−09 |
| PFKFB3 | 2.04 | 2.36E−03 | THRSP | 2.30 | 1.41E−04 |
| CHAD | 2.02 | 5.27E−03 | GREM2 | 2.28 | 4.12E−02 |
| Downregulated | |||||
| RP11–145A3.1 | 0.11 | 3.39E−11 | RP11–145A3.1 | 0.11 | 9.34E−10 |
| SLC26A9 | 0.15 | 9.60E−06 | SLC26A9 | 0.19 | 3.01E−04 |
| BDNF | 0.21 | 2.46E−04 | THY1 | 0.23 | 3.71E−14 |
| LBP | 0.22 | 2.78E−04 | LBP | 0.23 | 8.76E−04 |
| TNC | 0.26 | 4.27E−04 | ABCC12 | 0.25 | 2.43E−07 |
| CNN1 | 0.26 | 1.51E−03 | SERPINE1 | 0.28 | 4.58E−04 |
| HMGCS2 | 0.28 | 7.74E−04 | IGFN1 | 0.32 | 1.88E−02 |
| APLN | 0.29 | 3.35E−08 | TPPP3 | 0.32 | 2.42E−16 |
| ANGPTL4 | 0.30 | 1.34E−02 | NOV | 0.33 | 3.94E−07 |
| TNFRSF12A | 0.31 | 2.12E−04 | MYL12A | 0.34 | 3.63E−13 |
The values are organized by fold change.
The most highly altered gene in the High Susceptibility Group was PFKFB3 (4.72-fold increase), greater than twofold higher than the Low Susceptibility Group. A total of 24 genes (12 up, 12 down) were altered >2-fold in the High Susceptibility Group, whereas the Low Susceptibility Group showed no significant changes (Table 3). Increased genes represent many biotypes (protein coding, lincRNA, antisense, miRNA, pseudogenes) while decreased genes were either protein-coding or lincRNA. The top 5 increased protein-coding genes (CXCL2, GREM2, FASN, GADD45A, and RRAD) were all >2-fold with the exception of RRAD (1.96-fold), while the top 5 decreased protein-coding genes (SERPINE1, IGFN1, APOB, FGF6, and CRABP2) were all decreased >2-fold (Table 4).
Table 3.
Genes altered twofold with high susceptibility, yet unresponsive to bed rest with low susceptibility
| Gene Name | Biotype | Fold Change | Adjusted P Value |
|---|---|---|---|
| Upregulated | |||
| RP11-369K17.1 | lincRNA | 3.46 | 1.43E−04 |
| CXCL2 | protein_coding | 3.38 | 1.36E−03 |
| BBOX1-AS1 | antisense | 2.74 | 4.19E−04 |
| CTD-2116N24.1 | lincRNA | 2.52 | 5.44E−02 |
| MIR133B | miRNA | 2.43 | 1.21E−02 |
| GREM2 | protein_coding | 2.28 | 4.12E−02 |
| FASN | protein_coding | 2.25 | 4.10E−02 |
| FAM181A-AS1 | antisense | 2.23 | 1.54E−02 |
| RPS4XP5 | transcribed_processed_ pseudogene | 2.04 | 2.86E−03 |
| GADD45A | protein_coding | 2.04 | 3.46E−02 |
| CTB-52I2.4 | transcribed_processed_ pseudogene | 2.01 | 1.42E−02 |
| KB-1471A8.1 | lincRNA | 2.01 | 3.13E−02 |
| Downregulated | |||
| SERPINE1 | protein_coding | 0.28 | 4.58E−04 |
| IGFN1 | protein_coding | 0.32 | 1.88E−02 |
| APOB | protein_coding | 0.36 | 1.19E−03 |
| FGF6 | protein_coding | 0.40 | 2.71E−05 |
| C20orf197 | lincRNA | 0.41 | 1.68E−03 |
| CRABP2 | protein_coding | 0.42 | 4.95E−03 |
| TCEAL7 | protein_coding | 0.43 | 5.93E−04 |
| PENK | protein_coding | 0.43 | 1.34E−02 |
| HK2 | protein_coding | 0.46 | 1.32E−02 |
| HOGA1 | protein_coding | 0.49 | 2.81E−02 |
| RP11-15I11.3 | lincRNA | 0.49 | 3.79E−02 |
| ABRA | protein_coding | 0.50 | 5.76E−03 |
The values are organized by fold change.
Table 4.
Top 5 protein-coding genes uniquely altered in highly susceptible subjects by 5 days of bed rest
| Gene Name | Fold Change | Adjusted P Value |
|---|---|---|
| Upregulated | ||
| CXCL2 | 3.38 | 1.36E−03 |
| GREM2 | 2.28 | 4.12E−02 |
| FASN | 2.25 | 4.10E−02 |
| GADD45A | 2.04 | 3.46E−02 |
| RRAD | 1.96 | 2.43E−02 |
| Downregulated | ||
| SERPINE1 | 0.28 | 4.58E−04 |
| IGFN1 | 0.32 | 1.88E−02 |
| APOB | 0.36 | 1.19E−03 |
| FGF6 | 0.40 | 2.71E−05 |
| CRABP2 | 0.42 | 4.95E−03 |
The values are organized by fold change.
Pathway analysis and mitochondrial complex expression.
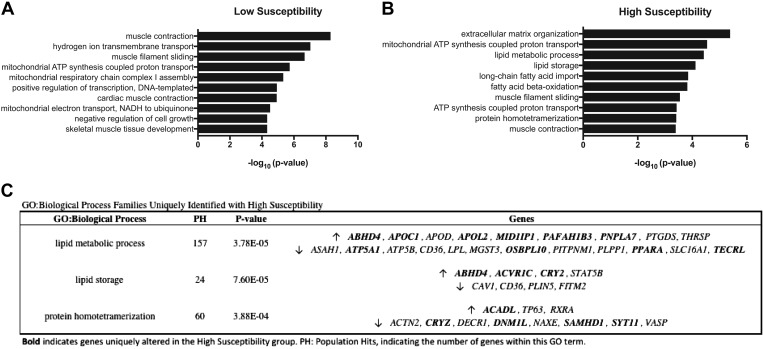
Using DAVID bioinformatics enrichment tools, the top 10 GO:biological process pathways are presented in Fig. 2 (Low: 2A, High: 2B). Many differences were found between High and Low Susceptibility, with only three families in common (muscle contraction, muscle filament sliding, mitochondrial ATP synthesis, and coupled proton transport). Biological process gene families that were among the top 10 altered with high susceptibility (but not altered with low susceptibility) are lipid metabolic processes, lipid storage, and protein homotetramerization. Genes categorized within these pathways are presented in Fig. 2C, with genes uniquely altered with high susceptibility indicated in bold. Only three of the genes identified (APOC1, PAFAH1B3, and ACVR1C) with this method were altered above 1.5-fold, all of which were increased. PAFAH1B3 was also correlated with the change in insulin sensitivity (R2 =0.278, P = 0.006) (Fig. 4B). In agreement with DAVID:GO mitochondrial pathway changes, PPARGC1A gene expression was decreased in both low (18%, P = 0.023) and high susceptibility (30%, P < 0.001). However, differences were not found between groups or for the impact of bed rest on the protein expression of mitochondrial Complexes I–V; however, a trend (P = 0.059) was observed for a decrease in Complex V expression in response to bed rest (Fig. 3).
Fig. 2.
Top 10 DAVID gene ontology (GO):biological process pathways. The top 10 significantly altered GO:biological processes for low susceptibility (A) and high susceptibility (B) are presented. The top three GO:families uniquely altered with high susceptibility, along with the genes within that family are presented (C). Genes uniquely altered with high susceptibility indicated in bold. Statistical design included CLIX-IR category + CLIX-IR:nested + CLIX-IR:time. Fold changes were determined using maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. Low Susceptibility Group: n = 14; High Susceptibility Group; n = 12.
Fig. 4.
Top 15 genes correlated with the change in insulin sensitivity. Venn diagram showing gene changes in High and Low Susceptibility Groups correlated with the change in insulin sensitivity (A) and list of the top 15 (determined by R2) tightest correlated (B) are presented. Statistical design included CLIX-IR category + CLIX-IR:nested + CLIX-IR:time. Fold changes were determined using maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. Low Susceptibility Group: n = 14; High Susceptibility Group: n = 12.
Fig. 3.
Muscle mitochondrial complex expression. Representative Western blot for mitochondrial complex proteins I-V (left), as well as combined expression of all complexes (right). Bottom: individual mitochondrial complexes are shown relative to to expression of Low Susceptibility Pre Bed Rest. ANOVA (insulin sensitivity × time), repeated measures by time (pre and post bed rest). Low Susceptibility Group: n = 10; High Susceptibility Group: n = 6. Low Pre = Low Susceptibility Pre Bed Rest.
Bed rest-induced genes and relationship to insulin sensitivity.
Next, we correlated bed rest-induced gene changes with the bed rest-induced change in insulin sensitivity. This resulted in identification of 95 genes (Fig. 4A). The 15 most tightly correlated genes are presented in Fig. 4B. The genes within these top 15 that changed with high susceptibility after bed rest were (PAN2, SMPD1, SRSF5, SELENOS, SLC25A15, SSFA2, DLG2, PAFAH1B3, SLC43A1, RBM20, and TUBG1). Out of these, only 11 (PAN2, SMPD1, SRSF5, SELENOS, SLC25A15, SSFA2, DLG2, PAFAH1B3, SLC43A1, RBM20, and TUBG1) were altered in the High Susceptibility Group. To capture other important genes describing high susceptibility to inactivity-induced insulin resistance, we identified six genes that changed >2-fold in the High Susceptibility Group and correlated these with the change in insulin sensitivity, four of which are protein coding (Fig. 5). The following genes were increased in the High Susceptibility Group: BBOX1-AS1 (R2 = 0.206, P = 0.020), USP6 (R2 = 0.207, P = 0.020), and FLYWCH1P1 (R2 = 0.228, P = 0.014); The following genes were decreased in the High Susceptibility Group: IGFN1 (R2 =0.182, P = 0.030), APOB (R2 = 0.153, P = 0.048), and PLXNA4 (R2 = 0.181, P = 0.030).
Fig. 5.
The six genes correlated with change in insulin sensitivity that also increased more than twofold in the High Susceptibility Group. BBOX1-AS1(A) USP6 (B), FLYWCH1P1 (C), IGFN1 (D), APOB (E), and PLXNA4 (F). Statistical design included CLIX-IR category + CLIX-IR:nested + CLIX-IR:time. Fold changes were determined using maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. Low Susceptibility Group: n = 14; High Susceptibility Group: n = 12.
DISCUSSION
Our primary finding was that High Susceptibility (individuals who had the largest increase in insulin resistance with short-term bed rest) participants are characterized with muscle gene responses described as a decreased preference for lipid oxidation, decreased capacity for triglyceride export (APOB), increased lipogenesis (i.e., PFKFB3, FASN), and increased amino acid export (SLC43A1). Together, these data provide a unique glimpse into the transcriptome of inactivity-induced insulin resistance that could open up avenues for targeted therapies to minimize insulin resistance during protracted periods of muscle disuse.
The top 10 GO:biological process families yielded only three families in common between Low and High Susceptibility in response to bed rest (muscle contraction, muscle filament sliding, mitochondrial ATP synthesis-coupled proton transport), suggesting divergent pathway activity between the two distinct insulin sensitivity cohorts. Pathways uniquely altered with High Susceptibility were related to lipid metabolism and storage, as well as protein assembly. In agreement with others, changes to various processes of lipid metabolism, including decreased uptake (27, 56), increased synthesis, and decreased oxidation, have been observed following diet (10) and inactivity-induced (1, 7, 27) insulin resistance. Indeed, genes within these pathways that were altered in the High Susceptibility Group were related to decreased fatty acid uptake (APOC1↑, APOL2↑, CD36↓, and LPL↓) (56) and increased lipid biosynthesis (MID1IP1↑, OSBPL10↓, and PNPLA7↑), three of which were altered >1.5-fold (APOC1, PAFAH1B3, and ACVR1C). ACVR1C codes for the activin type I receptor, Alk7, and in adipose cells, this protein has been shown to mediate growth differentiation factor 3 (or GDF3) activity, the increase of which was related to diet-induced fat accumulation (2, 8). The lipid metabolism-associated gene, PAFAH1B3, was correlated with the change in insulin sensitivity (R2 = 0.278, P = 0.0057), although not much information exists as to its function, therefore, warranting further investigation.
The most notable gene change identified from the volcano plot (Fig. 1) was the nearly 400% increase in inducible 6-phosphofructo-2-kinase (PFKFB3) with high susceptibility. The PFKFB3 gene codes for a regulatory enzyme, which can be activated by cell stress (mitogens (37), Toll-like receptor 4-mediated inflammation (41), hypoxia (49)), and whose product activates glycolysis through enhancement of 6-phosphofructo-1-kinase (PFK-1) activity. Of the four PFKFB enzymes, PFKFB3 has the highest kinase activity for synthesis of fructose-2,6-bisphosphate (F2,6BP), and while not a glycolytic intermediate itself, F2,6BP is the most potent activator of PFK-1 such that it can release PFK-1 from inhibition by ATP (54). The literature yields little information on PFKFB3 within skeletal muscle in the context of insulin resistance. However, its protein levels have been shown to be decreased in adipose tissue of insulin-resistant (vs. insulin sensitive) obese women (4), and its overexpression in adipose of high-fat-fed mice was shown to promote lipogenesis within the adipocyte, while decreasing inflammation and improving insulin sensitivity (24). Increased lipogenesis within adipocytes may be advantageous for maintaining glucose homeostasis, but the opposite seems to be true within muscle. In a rodent model of improved insulin sensitivity (through pharmacologic activation of SIRT1), investigators note that PFKFB3 protein levels decreased in skeletal muscle compared with less insulin-sensitive controls (16). Indeed, an increased level of PFKFB3 within inactivity-induced insulin-resistant skeletal muscle (in the current study) agrees with the general observations that glycolytic enzymes increase within unloaded skeletal muscle (45).
Along with the increase in PFKFB3, we also noticed a corresponding increase in the fatty acid synthase gene (FASN) in the High Susceptibility Group (Table 4), a major lipogenic enzyme within muscle that converts acetyl-CoA to fatty acids, primarily palmitate (34). Fatty acid synthase protein (FAS) expression is elevated within the skeletal muscle of insulin-resistant mice induced by high-fat feeding, while muscle-specific inactivation of FAS rescues insulin sensitivity (19). Assuming increased PFKFB3 expression supports increased glycolysis within the muscle of High Susceptibility subjects during bed rest, a resultant accumulation of acetyl-CoA would presumably increase the flux of lipogenesis through FAS. In conjunction with increased fatty acid synthesis, the apolipoprotein B (APOB) gene expression decreased in the High Susceptibility Group (and correlated with insulin resistance), representing a reduced capacity for export of synthesized lipids from skeletal muscle. While apolipoproteins increase in circulation following diet-induced insulin resistance, this accumulation primarily arises from increased APOB expression in the liver, where a majority of apolipoproteins are synthesized, promoting increased delivery of triglycerides to other tissues (43). However, in a model where mice were high-fat fed for a year, transgenic overexpression of APOB within skeletal muscle reduced the accumulation of triglyceride within muscle, as well as reduced fasting serum insulin levels (5). In summary, the increase of PFKFB3 and FASN, coupled with a decrease of APOB, as well as alterations in pathways associated with lipid metabolism and storage (Fig. 2), describes a metabolic fuel shift, whereby high susceptibility to insulin resistance is characterized by increased lipogenesis and enhanced glycolysis, a phenotype consistently observed with disuse (45, 52).
Another interesting finding was that SLC43A1 [LAT3, an L-type amino acid (AA) transporter for branched-chain amino acids (BCAA) and phenylalanine] increased only in the High Susceptibility Group. LAT3 has been shown to modulate export of amino acids in the liver (26) and placenta (9) and is upregulated during starvation (when intracellular amino acid content is high, and protein synthesis is low) (18) providing evidence that this transporter likely exports amino acids in skeletal muscle. Although it is tempting to attribute increased SLC43A1/LAT3 to increased amino acid efflux as a result of increased muscle breakdown/muscle atrophy, SLC43A1 was not altered in the Low Susceptibility Group despite identical magnitude of muscle loss between the two groups. Although we did not measure intracellular amino acids levels in these subjects, BCAA and phenylalanine accumulation occurred in skeletal muscle after hindlimb unloading in rats (42). BCAAs are also found to be increased in skeletal muscle (but decreased in the liver) of high-fat-fed rats, while intervention restricting dietary BCAA completely normalized accumulation of fatty acyl CoAs (53). In contrast to our findings, SLC43A1 was downregulated in T2DM skeletal muscle (35), which we speculate may be due to long-term exposure to insulin resistance, and the chronically elevated circulating levels of BCAA’s observed in this population (36). Nonetheless, SLC43A1 was correlated to disuse-induced insulin resistance in our study and, therefore, may describe early events of dysregulated BCAA metabolism; a condition that is readily apparent in those with metabolic disease (21, 29) and in a cross-sectional cohort of individuals with low physical activity levels (17, 25).
Finally, it is worth noting that mitochondrial pathways were altered within the top 10 GO:biological process pathways in both Low and High Susceptibility Groups, including a decrease in PPARGC1A (PGC1A), which is in agreement with a microarray study examining younger men after 9 days of bed rest (1), and another after 3 wk of unilateral limb suspension (27). As a follow-up, we examined the protein expression of mitochondrial Complexes I–V before and after bed rest. Surprisingly, there was no impact of bed rest on the expression of any of the mitochondrial complexes, aside from a trend (P = 0.059) in Complex V to be lower. Studies that have examined the impact of inactivity on mitochondrial complex expression are inconsistent, with 10 days of bed rest in older adults showing an increase in Complex I (44), while 7 days of bed rest in young males was shown to decrease expression of all complexes (13) and 2 wk of limb immobilization in middle-aged men (~50 yr), identifying a decrease in Complex II (38). It should be noted that the OxPhos Western blots were not performed on all subjects (n = 10 Low, n = 6 High) due to limited paired (pre, post) muscle samples remaining; thus, we may have been underpowered to detect subtle differences as a result of bed rest. However, direct assessment of mitochondrial respiration, such as high-resolution respirometry/fluorometry, may provide improved sensitivity on the impact of short-term inactivity in humans on mitochondrial function (28), since intrinsic function has been known to be altered independent of mitochondrial content (20).
Limitations.
First, as with all studies that rely on end points from skeletal muscle biopsy tissue, samples encompass the entirety of the intramuscular niche, which includes other supporting cells (e.g., satellite cells, macrophages, fibroblasts), in addition to the muscle fiber itself; thus, changes observed in this study cannot be attributed solely to the muscle fiber or specific cell type. Second, we acknowledge that the High Susceptibility subjects had higher insulin sensitivity at baseline (lower fasting insulin and higher Matsuda Index), suggesting they were more insulin-sensitive to begin with. However, there were no baseline differences between the groups in CLIX-IR, which is better representative of insulin sensitivity and more akin to a hyperinsulinemic euglycemic clamp. The associations made from this study cannot determine causation, and thus, it is not possible to determine which gene changes contribute to, or occur, as a result of the extent of insulin resistance. Finally, insulin resistance can occur rapidly, with changes in insulin sensitivity reported within 1 day of sitting (46); therefore, early transcriptome changes describing the onset of insulin resistance may not have been captured.
In conclusion, the skeletal muscle transcriptome of inactivity-induced insulin resistance, as demonstrated through comparison of a high-response group to a low-response group, is one of increased glycolysis, decreased preference for lipid oxidation, decreased capacity for lipid export and increased lipogenesis, and increased amino acid export. Understanding these transcriptional changes may help to open up avenues for therapies targeted toward lipid metabolism and storage within muscle in patients susceptible to insulin resistance during periods of disuse.
GRANTS
This project was supported by NIH Grants R01AG050781 and F32AR072481 and the National Center for Advancing Translational Sciences Grant 1ULTR001067. The study was also supported by NIH Grant T32-HL-139451.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.D. conceived and designed research; Z.S.M., M.T.H., and M.J.D. performed experiments; Z.S.M. and C.S. analyzed data; Z.S.M., P.T.R., A.I.M., C.S., M.T.H., and M.J.D. interpreted results of experiments; Z.S.M. and M.J.D. prepared figures; Z.S.M. and M.J.D. drafted manuscript; Z.S.M., P.T.R., A.I.M., M.T.H., and M.J.D. edited and revised manuscript; Z.S.M., P.T.R., A.I.M., C.S., M.T.H., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the CCTS nursing, dietary, and medical staff for their assistance with the muscle biopsies, blood sampling, and patient care during the inpatient and outpatient visits.
REFERENCES
- 1.Alibegovic AC, Sonne MP, Højbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab 299: E752–E763, 2010. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- 2.Andersson O, Korach-Andre M, Reissmann E, Ibáñez CF, Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci USA 105: 7252–7256, 2008. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderwald C, Anderwald-Stadler M, Promintzer M, Prager G, Mandl M, Nowotny P, Bischof MG, Wolzt M, Ludvik B, Kästenbauer T, Pacini G, Luger A, Krebs M. The Clamp-Like Index: a novel and highly sensitive insulin sensitivity index to calculate hyperinsulinemic clamp glucose infusion rates from oral glucose tolerance tests in nondiabetic subjects. Diabetes Care 30: 2374–2380, 2007. doi: 10.2337/dc07-0422. [DOI] [PubMed] [Google Scholar]
- 4.Arner P, Sahlqvist AS, Sinha I, Xu H, Yao X, Waterworth D, Rajpal D, Loomis AK, Freudenberg JM, Johnson T, Thorell A, Näslund E, Ryden M, Dahlman I. The epigenetic signature of systemic insulin resistance in obese women. Diabetologia 59: 2393–2405, 2016. [Erratum in Diabetologia 59: 2728, 2016]. doi: 10.1007/s00125-016-4074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartels ED, Ploug T, Størling J, Mandrup-Poulsen T, Nielsen LB. Skeletal muscle apolipoprotein B expression reduces muscular triglyceride accumulation. Scand J Clin Lab Invest 74: 351–357, 2014. doi: 10.3109/00365513.2014.893446. [DOI] [PubMed] [Google Scholar]
- 6.Bell KE, von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging 5: 33–41, 2016. doi: 10.14283/jfa.2016.78. [DOI] [PubMed] [Google Scholar]
- 7.Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985) 111: 1201–1210, 2011. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 8.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17: 446–452, 2010. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleal JK, Glazier JD, Ntani G, Crozier SR, Day PE, Harvey NC, Robinson SM, Cooper C, Godfrey KM, Hanson MA, Lewis RM. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J Physiol 589: 987–997, 2011. doi: 10.1113/jphysiol.2010.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covington JD, Johannsen DL, Coen PM, Burk DH, Obanda DN, Ebenezer PJ, Tam CS, Goodpaster BH, Ravussin E, Bajpeyi S. Intramyocellular lipid droplet size rather than total lipid content is related to insulin sensitivity after 8 weeks of overfeeding. Obesity (Silver Spring) 25: 2079–2087, 2017. doi: 10.1002/oby.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crossland H, Skirrow S, Puthucheary ZA, Constantin-Teodosiu D, Greenhaff PL. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end-points. J Physiol 597: 1259–1270, 2019. doi: 10.1113/JP275444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 13.Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJC. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65: 2862–2875, 2016. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 14.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048, 2016. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347–358, 2008. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Fukai K, Harada S, Iida M, Kurihara A, Takeuchi A, Kuwabara K, Sugiyama D, Okamura T, Akiyama M, Nishiwaki Y, Oguma Y, Suzuki A, Suzuki C, Hirayama A, Sugimoto M, Soga T, Tomita M, Takebayashi T. Metabolic profiling of total physical activity and sedentary behavior in community-dwelling men. PLoS One 11: e0164877, 2016. doi: 10.1371/journal.pone.0164877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H, Yan K. Protein characterization of NA+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. Am J Pathol 170: 888–898, 2007. doi: 10.2353/ajpath.2007.060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funai K, Song H, Yin L, Lodhi IJ, Wei X, Yoshino J, Coleman T, Semenkovich CF. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest 123: 1229–1240, 2013. doi: 10.1172/JCI65726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granata C, Jamnick NA, Bishop DJ. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med 48: 1809–1828, 2018. doi: 10.1007/s40279-018-0936-y. [DOI] [PubMed] [Google Scholar]
- 21.Haufe S, Engeli S, Kaminski J, Witt H, Rein D, Kamlage B, Utz W, Fuhrmann JC, Haas V, Mähler A, Schulz-Menger J, Luft FC, Boschmann M, Jordan J. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr Metab Cardiovasc Dis 27: 858–864, 2017. doi: 10.1016/j.numecd.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 24.Huo Y, Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Fan YY, Ong KT, Woo SL, Chapkin RS, Mashek DG, Chen Y, Dong H, Lu F, Wei L, Wu C. Targeted overexpression of inducible 6-phosphofructo-2-kinase in adipose tissue increases fat deposition but protects against diet-induced insulin resistance and inflammatory responses. J Biol Chem 287: 21492–21500, 2012. doi: 10.1074/jbc.M112.370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kujala UM, Mäkinen VP, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, Rahkila P, Würtz P, Kovanen V, Cheng S, Sipilä S, Hirvensalo M, Telama R, Tammelin T, Savolainen MJ, Pouta A, O’Reilly PF, Mäntyselkä P, Viikari J, Kähönen M, Lehtimäki T, Elliott P, Vanhala MJ, Raitakari OT, Järvelin MR, Kaprio J, Kainulainen H, Ala-Korpela M. Long-term leisure-time physical activity and serum metabolome. Circulation 127: 340–348, 2013. doi: 10.1161/CIRCULATIONAHA.112.105551. [DOI] [PubMed] [Google Scholar]
- 26.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD, Lehman-McKeeman LD, Vaillancourt RR, Cherrington NJ. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 47: 603–615, 2015. doi: 10.1007/s00726-014-1894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammers G, Poelkens F, van Duijnhoven NT, Pardoel EM, Hoenderop JG, Thijssen DH, Hopman MT. Expression of genes involved in fatty acid transport and insulin signaling is altered by physical inactivity and exercise training in human skeletal muscle. Am J Physiol Endocrinol Metab 303: E1245–E1251, 2012. doi: 10.1152/ajpendo.00356.2012. [DOI] [PubMed] [Google Scholar]
- 28.Larsen S, Lundby AM, Dandanell S, Oberholzer L, Keiser S, Andersen AB, Haider T, Lundby C. Four days of bed rest increases intrinsic mitochondrial respiratory capacity in young healthy males. Physiol Rep 6: e13793, 2018. doi: 10.14814/phy2.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerin C, Goldfine AB, Boes T, Liu M, Kasif S, Dreyfuss JM, De Sousa-Coelho AL, Daher G, Manoli I, Sysol JR, Isganaitis E, Jessen N, Goodyear LJ, Beebe K, Gall W, Venditti CP, Patti ME. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab 5: 926–936, 2016. doi: 10.1016/j.molmet.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 31.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM. Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem 55: 425–438, 2009. doi: 10.1373/clinchem.2008.115352. [DOI] [PubMed] [Google Scholar]
- 35.Møller AB, Kampmann U, Hedegaard J, Thorsen K, Nordentoft I, Vendelbo MH, Møller N, Jessen N. Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with Type 2 diabetes. Sci Rep 7: 43775, 2017. doi: 10.1038/srep43775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novellasdemunt L, Bultot L, Manzano A, Ventura F, Rosa JL, Vertommen D, Rider MH, Navarro-Sabate À, Bartrons R. PFKFB3 activation in cancer cells by the p38/MK2 pathway in response to stress stimuli. Biochem J 452: 531–543, 2013. doi: 10.1042/BJ20121886. [DOI] [PubMed] [Google Scholar]
- 38.Pileggi CA, Hedges CP, D’Souza RF, Durainayagam BR, Markworth JF, Hickey AJR, Mitchell CJ, Cameron-Smith D. Exercise recovery increases skeletal muscle H2O2 emission and mitochondrial respiratory capacity following two-weeks of limb immobilization. Free Radic Biol Med 124: 241–248, 2018. doi: 10.1016/j.freeradbiomed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 107: 37–49, 2018. doi: 10.1016/j.exger.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, LaStayo PC, Drummond MJ. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5 days of bed rest. Rejuvenation Res 20: 449–461, 2017. doi: 10.1089/rej.2017.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-García A, Monsalve E, Novellasdemunt L, Navarro-Sabaté A, Manzano A, Rivero S, Castrillo A, Casado M, Laborda J, Bartrons R, Díaz-Guerra MJ. Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem 286: 19,247–19,258, 2011. doi: 10.1074/jbc.M110.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimkus KL, Shirazi-Fard Y, Wiggs MP, Ullah ST, Pohlenz C, Gatlin DM 3rd, Carroll CC, Hogan HA, and Fluckey JD. Responses of skeletal muscle size and anabolism are reproducible with multiple periods of unloading/reloading. J Appl Physiol (1985) 125: 1456–1467, 2018. doi: 10.1152/japplphysiol.00736.2017. [DOI] [PubMed] [Google Scholar]
- 43.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, Hellénius ML, Fisher RM. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J 28: 2637–2643, 2007. doi: 10.1093/eurheartj/ehm360. [DOI] [PubMed] [Google Scholar]
- 44.Standley RA, Distefano G, Pereira SL, Tian M, Kelly OJ, Coen PM, Deutz NEP, Wolfe RR, Goodpaster BH. Effects of β-hydroxy-β-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J Appl Physiol (1985) 123: 1092–1100, 2017. doi: 10.1152/japplphysiol.00192.2017. [DOI] [PubMed] [Google Scholar]
- 45.Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr 135: 1824S–1828S, 2005. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- 46.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 60: 941–949, 2011. doi: 10.1016/j.metabol.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 37: 802–806, 1988. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 48.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593: 4259–4273, 2015. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, MacNabb M, Rudd JH, Narula J, Enriquez JA, Través PG, Fernández-Velasco M, Bartrons R, Martín-Sanz P, Fayad ZA, Tejedor A, Boscá L. HIF-1α and PFKFB3 mediate a tght relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol 35: 1463–1471, 2015. doi: 10.1161/ATVBAHA.115.305551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thyfault JP, Booth FW. Lack of regular physical exercise or too much inactivity. Curr Opin Clin Nutr Metab Care 14: 374–378, 2011. doi: 10.1097/MCO.0b013e3283468e69. [DOI] [PubMed] [Google Scholar]
- 51.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) 111: 1218–1224, 2011. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 52.Wagenmakers AJ. A malonyl-CoA fuel sensing mechanism in muscle: effects of insulin, glucose and denervation. Clin Nutr 15: 144–145, 1996. doi: 10.1016/S0261-5614(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 53.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, Trimmer JK, Brosnan MJ, Rolph TP, Newgard CB. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 5: 538–551, 2016. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol 86: 174–179, 2009. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Pratley RE, Tokraks S, Bogardus C, Permana PA. Microarray profiling of skeletal muscle tissues from equally obese, non-diabetic insulin-sensitive and insulin-resistant Pima Indians. Diabetologia 45: 1584–1593, 2002. doi: 10.1007/s00125-002-0905-7. [DOI] [PubMed] [Google Scholar]
- 56.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol (1985) 100: 249–257, 2006. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]
- 57.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res 46: D754–D761, 2018. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14: 88–98, 2018. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]