Abstract
Rationale
lcohol impairs the brain’s detection of performance errors as evidenced by attenuated error-related negativity (ERN), an event-related potential (ERP) thought to reflect a brain system that monitors one’s behavior. However, it remains unclear whether alcohol impairs performance-monitoring capacity across a broader range of contexts, including those entailing external feedback.
Objective
This study sought to determine whether alcohol-related monitoring deficits are specific to internal recognition of errors (reflected by the ERN) or occur also in external cuing contexts. We evaluated the impact of alcohol consumption on the feedback-related negativity (FRN), an ERP thought to engage a similar process as the ERN but elicited by negative performance feedback in the environment.
Methods
In an undergraduate sample randomly assigned to drink alcohol (n=37; average peak BAC=0.087 g/100 ml, estimated from breath alcohol sampling) or placebo beverages (n =42), ERP responses to gain and loss feedback were measured during a two-choice gambling task. Time–frequency analysis was used to parse the overlapping theta-FRN and delta-P3 and clarified the effects of alcohol on the measures.
Results
Alcohol intoxication attenuated both the theta-FRN and delta-P3 brain responses to feedback. The theta-FRN attenuation was stronger following loss than gain feedback.
Conclusions
Attenuation of both theta-FRN and delta-P3 components indicates that alcohol pervasively attenuates the brain’s response to feedback in this task. That theta-FRN attenuation was stronger following loss trials is consistent with prior ERN findings and suggests that alcohol broadly impairs the brain’s recognition of negative performance outcomes across differing contexts.
Keywords: Alcohol, Event-related potentials, Feedback-related negativity, Performance monitoring
Analysis of the neurocognitive effects of alcohol intoxication is important for understanding basic pharmacologic processes and also for the insights it can provide about a range of disinhibitory phenomena. Although various cognitive deficits have been associated with alcohol, one specific functional deficit that has garnered attention involves impairments in the ability to monitor one’s behavior, which is a critical function for carrying out goal-directed activity and avoiding negative outcomes. Compelling evidence of alcohol-related deficits in behavioral monitoring comes from findings of alcohol-related reductions in the amplitude of the error-related negativity (ERN; Easdon et al. 2005; Ridderinkhof et al. 2002), an event-related potential (ERP) elicited by erroneous responses that is thought to reflect the brain’s internal detection of errors or competing response tendencies (Carter et al. 1998; Falkenstein et al. 1991; Gehring et al. 1993). However, monitoring our behavior involves more than simply recognizing that we made an error: At times, we must also process environmental feedback indicating that we should modify our behavior. The current study extended prior work on the ERN by evaluating the impact of alcohol intoxication on an ERP response that reflects the extent to which individuals process external negative performance cues—the feedback-related negativity (FRN). In addition to clarifying the nature of performance-monitoring impairments associated with alcohol intoxication, the findings are pertinent to understanding abnormalities in responding to external behavioral cues contribute to disinhibited behavior that occurs with intoxication.
The impact of alcohol intoxication on behavioral, affective, cognitive, and neurological functioning has been widely studied. As a central nervous system depressant, alcohol reduces the amplitude of various physiological responses, including the aforementioned ERN, thought to index online error detection or conflict monitoring (for a review, see Taylor et al. 2007), and the P3/P300 (e.g., Colrain et al. 1993; Rohrbaugh et al. 1987), thought to reflect attention and updating of mental representations of the task context based on incoming stimuli (Donchin 1981). However, alcohol does not suppress neural activity indiscriminately. Rather, primary stimulus processing (e.g., as reflected by N1 or P2 components of the ERP) appears to be spared at mild to moderate levels of intoxication, as are ERP responses to stimuli within the focus (as opposed to the periphery) of attention (e.g., P3b vs. P3a; Jääsekeläinen et al. 1996; Little 1999). Furthermore, that alcohol-related reductions in the amplitude of ERN are greater following errors versus correct responses (Easdon et al. 2005; Ridderinkhof et al. 2002) supports the idea that alcohol selectively impairs error detection rather than generally disrupting processing in performance contexts.
The finding that alcohol attenuates the ERN implies that intoxication affects a specific neural system critical to monitoring ongoing behavior and guiding goal-directed activity. Specifically, the anterior cingulate cortex (ACC) is thought to play a central role in monitoring for behavioral errors and signaling the prefrontal cortex (PFC) when performance strategies need to be adjusted to effectively achieve goals (Miller and Cohen 2001). Because the ERN is known to reflect activity associated with the ACC and other anterior structures that interface with the ACC (e.g., the PFC; Dehaene et al. 1994; Gehring and Knight 2000), the documented ERN deficit that occurs with alcohol consumption suggests that intoxication fundamentally impairs this frontal brain monitoring system.
If alcohol intoxication broadly impairs monitoring processes, it should do so in performance contexts other than those that give rise to the ERN. The current study sought to evaluate this possibility by testing for effects of alcohol intoxication on the FRN, a brain response believed to reflect a process similar to the ERN. Like the ERN, the FRN appears to index the activity of a frontally based brain system that identifies negative outcomes in performance contexts (Holroyd and Coles 2002; Miltner et al. 1997).
Based in part on similar scalp topographies, it is believed that the FRN, like the ERN, involves activity of the ACC and affiliated brain structures (Gehring and Willoughby 2002; Holroyd and Coles 2002; Luu et al. 2003). Unlike the ERN, however, which reflects the brain’s internal recognition that an emitted response failed to match performance goals, the FRN reflects the brain’s response to explicit feedback from the environment indicating that a behavior resulted in a negative outcome. From this viewpoint, data indicating attenuation of the FRN response as a function of alcohol intoxication would indicate a more pervasive effect of alcohol on performance monitoring—one that extends into contexts in which feedback about behavior is externally presented rather than self-generated.
An analogous phenomenon known to be associated with impairments in performance monitoring and affiliated reductions in ERN response is trait disinhibition. Deficits in behavioral regulation are strongly characteristic of impulsive personality and disorders of impulse control, and reductions in ERN amplitude have consistently been reported in relation to traits and problems of this type (Dikman and Allen 2000; Hall et al. 2007; Pailing and Segalowitz 2004). In view of this, a plausible hypothesis is that the FRN response to explicit performance feedback would be attenuated in individuals high in disinhibitory tendencies. Bernat et al. (2011) recently tested this hypothesis by examining the FRN response within a gambling task in high-disinhibited individuals who showed ERN reductions in a separate task. Contrary to the hypothesis, the FRN response to loss feedback was not attenuated in high- versus low-disinhibited individuals. The implication of this finding was that the dysregulated behavior of high-disinhibited individuals reflects deficits in internally mediated monitoring of performance and not necessarily deficits in processing of external feedback cues in performance contexts.
Is the effect of alcohol on performance monitoring likewise limited to impairment of the brain’s own internal detection of conflict between goals and emitted responses—or does intoxication result in a more pervasive impairment that extends to performance contexts in which external feedback cues guide behavior? The current study was conducted to address this question. The null hypothesis was that alcohol intoxication, in parallel with results for trait disinhibition, would not be associated with reduction in the FRN response to loss-related feedback despite its documented negative impact on the amplitude of the ERN. The alternative hypothesis was that reduced FRN response would occur due to intoxication. Lending support to this alternative hypothesis are data indicating that alcohol impairs a range of higher level cognitive functions and frontal brain areas including the ACC and PFC (Curtin and Fairchild 2003), which are theorized to be important for production of both the ERN and the FRN.
A second major hypothesis of the current study was that alcohol would reduce the amplitude of P3 brain response to performance feedback stimuli. This hypothesis was based on (a) prior published work demonstrating effects of alcohol intoxication on P3 in performance tasks (Colrain et al. 1993; Krull et al. 1993; Rohrbaugh et al. 1987; Wall and Ehlers 1995) and (b) the findings of Bernat et al. (2011), indicating that high levels of trait disinhibition were associated with reductions in the P3 response to feedback cues despite the absence of a reduction in the FRN component. Because the FRN and P3 responses to feedback stimuli overlap in time, the technique of time–frequency (TF) analysis was used to separate these two ERPs.
Method
Participants
Social drinkers (N = 92) were recruited through undergraduate psychology classes and newspaper advertisements. A pre-experimental phone screening determined eligibility for testing. Candidates were advised during the phone session that they would be randomly assigned to receive beverages with or without alcohol. Individuals were deemed eligible if they reported recently drinking three alcoholic beverages in 1 h (i.e., commensurate with the study’s alcohol manipulation) without any ill effects, and if they reported no visual or hearing impairments, neurologic or health-related problems, or problematic substance use (i.e., daily alcohol/drug use; >28 drinks per week for females or >35 for males; inability to control alcohol or drug use; or problems at home, school, work, or with the law due to alcohol or drug use).
Participants received either course credit or monetary compensation ($7.50/h) for participation in the study, and all received money for the task-related bonus. Thirteen individuals were excluded from analyses: nine due to equipment or procedural problems, two due to excessive artifacts, and two because they discontinued the session prior to completing the task. The final analysis sample consisted of 79 subjects (42 males; M age = 25 years) assigned randomly to either the alcohol (n = 37; 17 males) or the placebo group (n = 42; 25 males).
Measures
During attachment of electroencephalogram (EEG) sensors, participants completed a demographic and health history questionnaire along with three additional questionnaires: a brief Drinking Behavior Questionnaire (DBQ), which inquired about average frequency of drinking occasions over the past year, mean drinks consumed per occasion, and maximum number of drinks consumed per hour on one occasion over the last year; the Alcohol Dependence Scale (ADS; Skinner and Allen 1982), a 29-item measure of alcohol use, abuse, and dependence; and the Short Drug Abuse Screening Test (SDAST; Skinner 1982), a 20-item self-report measure of behaviors and symptoms relevant to drug abuse and dependence. As a manipulation check following completion of the study, participants completed a questionnaire that asked about (1) the number of standardsized “total alcoholic drinks” (e.g., 12-oz. beer, 5-oz. wine, or 1.5-oz. hard liquor) they believed they had consumed during the study and (2) the highest level of intoxication they experienced during the experiment (rated on a 5-point scale, with 1= “not at all intoxicated” and 5=“extremely intoxicated”).
Procedure
Participants first presented identification (to ensure a minimum age of 21 years) and completed informed written consent. The consent form stated that participants would be randomly assigned to an alcohol group (in which they would drink the equivalent of three to four servings of alcohol) or a placebo group (in which they would drink only juice). A pre-experiment breath alcohol content (BrAC) reading was obtained from each participant using an AlcoSensor IV breathalyzer instrument (Intoximeters, Inc.; St. Louis, MO, USA), and female participants completed a urinalysis test to rule out pregnancy. Two drinks were then prepared in front of the participant, placed in a refrigerator out of the participant’s sight, and were later brought back for consumption near the end of EEG sensor attachment. The alcohol group received two beverages containing fruit juice and 95% ethyl alcohol mixed at a 7:1 ratio. The target blood-alcohol level, to be assessed by BrAC testing, was 0.100 g/100 ml after a 40-min drinking period and a 10-min absorption period. Custom software (Curtin 2000) was used to calculate the amounts of ethyl alcohol and fruit juice required to yield the target BrAC for each participant based on height, weight, gender, and age. Placebo group participants received a volume of fruit juice equivalent to the liquid consumed by those in the alcohol group. To increase the believability of the placebo, 2 ml of 95% ethyl alcohol was “floated” on top of the juice (and a small amount misted on the cup rim) out of sight of the participant, just prior to presentation for consumption.
After observing the experimenter mix the drinks, participants filled out the questionnaire measures during attachment of EEG sensors. Twenty minutes prior to completion of these attachments, participants began to drink their beverages at a specified rate of 20 min for each of the two glasses. They completed additional personality questionnaires (separate from those reported on in the current study) during the remainder of the beverage administration period and gave a second BrAC reading just prior to beginning the experimental task.
The experimental task was a modified version of Gehring and Willoughby’s (2002) gambling task in which participants select one of two monetary choices and receive random feedback indicating whether they have won or lost the chosen amount. Target stimuli were two adjacent squares, each enclosing a number (5 or 25) representing cents. Each target remained on the screen until participants selected the left or right monetary amount (via a left or right button press). A blank screen appeared next for 100 ms, followed by a feedback stimulus for 1,000 ms, followed by a blank screen again for 1,500 ms prior to presentation of the next target stimulus. The feedback stimulus was identical to the target stimulus (two side-by-side numbers enclosed in boxes) except that the background of the chosen box turned red or green to indicate a win or loss (the color-outcome mapping was counterbalanced across participants), and the unchosen box turned red or green to indicate what the outcome would have been had the individual made the other choice. All four possible combinations of 5 and 25 (i.e., 5–5, 5–25, 25–5, and 25–25) and all possible outcome color combinations (green–green, green–red, etc.) were presented with equal probability across the task. Participants completed a short practice set of trials in which they were instructed how to make responses and interpret the feedback stimuli (they were not informed that the feedback was random). Participants completed 12 blocks of 32 trials and received feedback following each block regarding their cumulative bonus. Total task duration was about 20 min. Mean percentage of loss (versus gain) trials was 50% for each group.
Psychophysiological data acquisition and reduction
The EEG was recorded from 64-channel Quik-Caps (Compumedics, Inc.) with sintered Ag-AgCl electrodes (10–20 system). Electrodes placed above and below the left eye recorded ocular activity. Impedances were kept below 10 KΩ. EEG signals were digitized on-line at 1,000 Hz (referenced to CPz), epoched off-line from 1,000 ms before to 2,000 ms after feedback cue onset, and re-referenced to linked mastoids. Trial-level data were corrected for eye-blink and movement artifacts using an algorithm developed by Semlitsch et al. (1986), implemented in Neuroscan EDIT (version 4.3). Processed data were downsampled to 128 Hz using the Matlab (Mathworks, Inc.) resample function to handle anti-aliasing filtering before downsampling.
To exclude ocular artifacts remaining after ocular correction, trials on which activity at electrodes F1 or F2 exceeded 75 μV within the 0 to 1,500 ms window (relative to median activity from −750 to 0 ms) were excluded. Then, within each trial, individual electrode sites at which activity exceeded ±75 μV in either the pre- (−750 to 0) or post-stimulus (0 to 1,500) time regions (relative to one another) were omitted from analysis. Applying these criteria, 14% of trials were excluded. Finally, across all subjects and electrodes, 51 electrodes (out of 4,187) became disconnected during the task and were dropped from the dataset. Finally, gain and loss condition averages were computed for each participant, and epochs were baseline-corrected for the 150-ms pre-stimulus. The median number of trials per condition average was 177 (range, 19 to 210).
The FRN and P3 were quantified in two ways: as time-domain (TD) components and as TF components. Traditional TD methods quantify ERPs as peak or mean amplitude within designated time windows. Because the FRN and P3 overlap closely in time, alternative approaches to TD component analysis are needed to effectively parse the two responses (Bernat et al. 2011; Miltner et al. 1997). For this reason, TF analysis was used in addition to standard TD analysis. TF analysis quantifies ERP signals in terms of frequency and amplitude across time and is useful for parsing signals that overlap temporally, but are distinguishable in terms of frequency composition. Because the FRN and P3 reflect activity in distinct frequency bands, they can be disentangled using TF methods (see Bernat et al. 2011, for a demonstration using the same gambling task as the current study).
Time–domain measures: FRN and P3 peak amplitude
The ERP windows correspond to bins of 128 Hz resampled signals, and thus, specified windows entailed fractional times. Based on prior analyses of these ERPs in other datasets, the FRN was defined as the maximum negative-voltage peak in a window of 203.13 to 328.13 ms post-stimulus (feedback) onset, relative to a −101.56 to −7.71 ms pre-stimulus baseline, and the P3 was defined as the maximum positivity between 296.88 and 500 ms post-stimulus relative to the same baseline. FRN analyses were performed at electrode FCz, as the effect of interest for this measure (i.e., gain versus loss condition difference) was maximal topographically at that site. Similarly, the P3 was measured at Cz, given that maximal amplitude of response for this component occurred more centrally.
Time–frequency measures: theta-FRN and delta-P3 component amplitude
Using the technique of Bernat et al. (2011), we separated the feedback-elicited ERP into two distinct TF components: theta-FRN and delta-P3. Briefly, this entailed filtering the TD waveform separately for theta (>3 Hz) and delta (<3 Hz) frequency bands and computing TF surfaces for each. Using PCA, we then extracted a single dominant principal component for each surface, yielding one theta and one delta TF component. Pre-filtering was done in order to directly target the relevant activity known to be associated with FRN and P3 (theta and delta, respectively; e.g., Bernat et al. 2007, 2011; Demiralp et al. 2001; Gehring and Willoughby 2004; Gilmore et al. 2010; Trujillo and Allen 2007) and because it yielded the most parsimonious TF representation of FRN and P3. The “Results” section presents evidence in support of these TF variables as effective indicators of their TD counterparts. As with TD FRN and P3, analyses of the TF components focused on theta-FRN at scalp site FCz and delta-P3 at Cz.
Results
Alcohol and drug use questionnaires and manipulation checks
Table 1 presents descriptive statistics for self-report drinking and drug inventories (DBQ, ADS, and SDAST) for the alcohol and placebo groups. No group differences were found in item-level responses to the DBQ, or in mean scores on the ADS or SDAST (ts ranged from 0.72 to 1.35, all ps>0.10).
Table 1.
Demographic and questionnaire variables by beverage group
| Alcohol (n=37) | Placebo (n=42) | |
|---|---|---|
| M (SD) | M (SD) | |
| Age (years) | 24.00 (5.89) | 26.48 (9.15) |
| Percent male | 46.0% | 59.2% |
| Drinking Behavior Questionnaire | ||
| Drinking frequencya | 2.69 (1.21) | 3.00 (1.67) |
| Drinking quantity | 3.72(1.17) | 4.19 (1.90) |
| Max. drinks/h, one occasion | 3.27 (3.34) | 2.81 (2.17) |
| ADS total score | 5.69 (3.37) | 6.31 (4.18) |
| SDAST total score | 2.92 (2.40) | 3.63 (3.63) |
| Beverage Manipulation Questionnaire | ||
| Number of drinks consumedb | 3.64 (1.05) | 1.94 (1.17) |
| Subjective intoxicationb | 3.17 (0.61) | 1.80 (0.69) |
ADS Alcohol Dependence Scale, SDAST Short Drug Abuse Screening Test
Drinking frequency refers to the number of occasions per week. Drinking quantity refers to the number of standard-sized drinks (one beer, one glass of wine, or one shot of liquor) per occasion. Max. drinks/h, one occasion refers to the maximum number of standardsized drinks consumed per hour in one occasion in the past year. Number of drinks consumed refers to the estimated number of standard-sized alcoholic drinks consumed during the experiment, and subjective intoxication was rated on a 1 to 5 scale where 1 = “not at all intoxicated” and 5=“extremely intoxicated”
Alcohol vs. placebo, p<0.05
Mean BrAC just prior to starting the gambling task was 0.087 g/100 ml (SD = 0.012) for the alcohol group and 0.000 (SD = 0.000) for the placebo group. Just after the task was completed, means were 0.086 g/100 ml (SD=0.013) and 0.000, respectively, suggesting that we successfully captured the plateau surrounding the peak BrAC rather than a point on the ascending or descending limb of the blood-alcohol curve. Results for the manipulation check questionnaire (see Table 1) indicated that both groups perceived themselves to have consumed alcohol, with the alcohol group reporting higher levels of subjective intoxication. Mean estimated consumption for the alcohol group was 3.64 (SD=1.05) standard alcoholic drinks, versus M=1.94 (SD=1.17) for the placebo group, t(73)=6.52, p<0.001. Mean subjective intoxication was 3.17 (SD=0.61) for the alcohol group and 1.80 (SD=0.69) for the placebo group, t(73)=8.98, p<0.001. Self-reported drinking behaviors and manipulation effectiveness as indexed by these variables did not moderate the primary ERP effects (i.e., the addition of any one of the questionnaire measures from Table 1 to the GLM analyses reported below yielded no interactions between the questionnaire, group status, and ERP amplitude).
Behavioral data
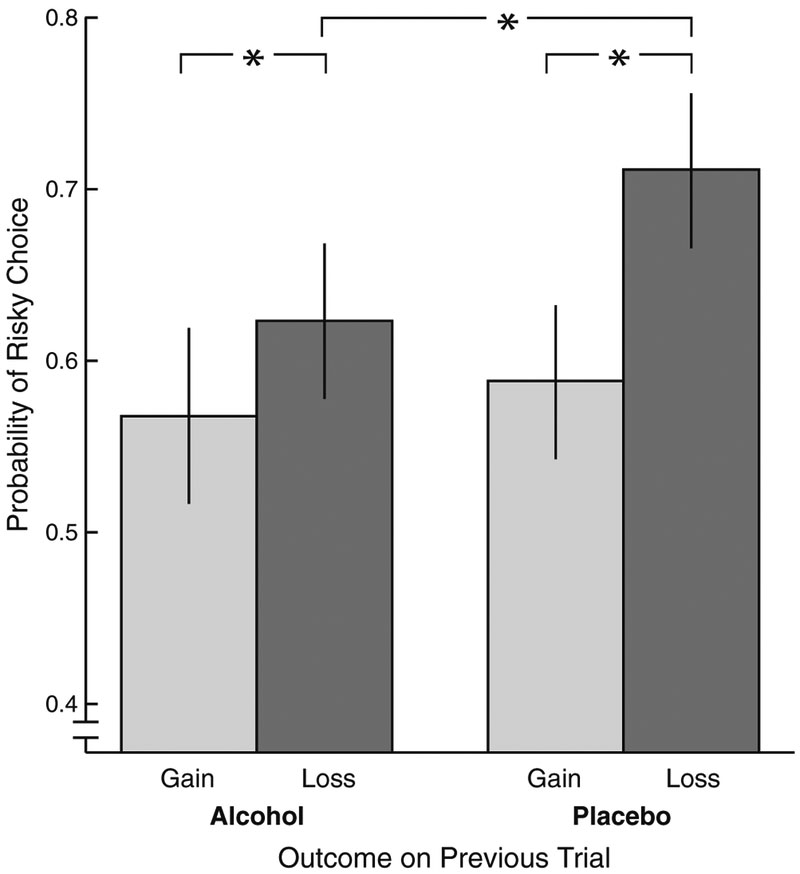
In order to explore behavioral effects of the beverage manipulation, we measured risk-taking behavior, with risky choices defined as 25–5 or 5–25 trials on which participants selected the “25” option. Prior work suggests that participants are more likely to make risky choices following negative (e.g., loss) outcomes (Gehring and Willoughby 2002). To evaluate this, we performed a previous outcome (gain, loss) × group (alcohol, placebo) GLM with the proportion of risky choices as the DV. Overall, we replicated the expected finding of increased riskiness following loss vs. gain trials, previous outcome, F(1,77)=44.27, p<0.001, and in addition found a significant group × previous outcome interaction, F(1,77)=6.34, p=0.01 (with the main effect of group nonsignificant, F(1,77)=2.76, p= 0.10). Both groups exhibited significantly greater riskiness following loss vs. gain outcomes, alcohol t(34)=2.83, p=0.008, versus placebo t(43)=6.78, p<0.001. However, as illustrated in Fig. 1, the group difference in behavior was specific to loss trials, t(77)=−2.55, p=0.01 (alcohol vs. placebo Ms=0.64 and 0.72, SDs=0.13 and 0.15, respectively); the corresponding comparison for gain trials was not significant, t(77)=−0.57, p=0.57 (alcohol vs. placebo Ms=0.58 and 0.60, SDs=0.15 and 0.15, respectively). Thus, although both groups showed increased riskiness following loss (vs. gain) outcomes, intoxicated participants demonstrated this increase to a lesser extent than non-intoxicated individuals due to a diminished carryover effect for loss trials specifically.
Fig. 1.
The effect of previous trial outcome (gain vs. loss) on risk-taking behavior for the alcohol (n=37) and placebo (n=42) groups. Risky choices were defined as 5–25 or 25–5 trials on which participants selected “25.” Error bars represent ±2 standard errors of the mean. Asterisks indicate significant (p<0.05) pairwise differences. Although both groups showed increased riskiness following loss versus gain outcomes (i.e., the loss vs. gain t test was significant for each group), intoxicated participants demonstrated this increase to a lesser extent than non-intoxicated participants due to a diminished trial-to-trial carryover effect for loss trials (i.e., the alcohol vs. placebo difference in risk taking was significant for previous-loss trials only)
Brain response data
Time–domain FRN and P3
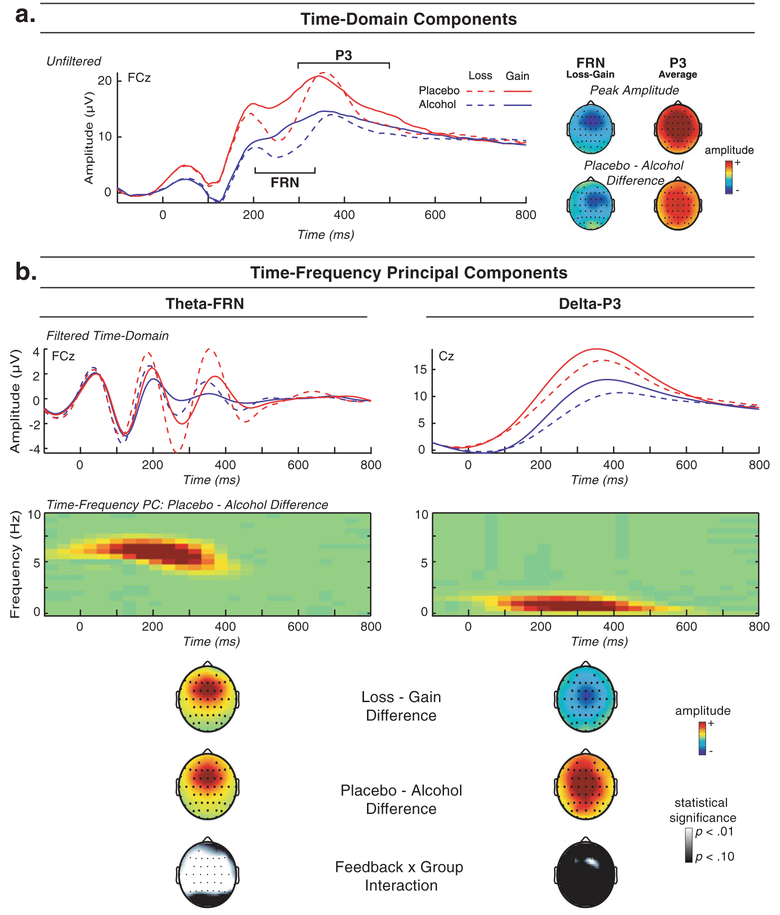
Figure 2a depicts the average feedback-locked ERP waveform at electrode site FCz as a function of feedback type (gain vs. loss) and group (alcohol vs. placebo). The FRN is evident as a negative deflection in the ERP maximal around 250 ms post-feedback onset, whereas the P3 is evident as the positive peak that follows the FRN, maximal around 350 ms post-feedback onset. Because the FRN is typically measured as a gain–loss difference, the topographical maps of the FRN in Fig. 2a reflect gain–loss difference scores, whereas the topographical maps of the P3 reflect amplitude across gain and loss trials combined. As expected based on prior work with this task (Bernat et al. 2011), the overall scalp distribution of the FRN was frontal, whereas the topography of the P3 was more central.
Fig. 2.
Results from time (a) and time–frequency (b) decompositions of ERP activity following loss and gain feedback for alcohol and placebo groups. a Waveform plot: average unfiltered ERP activity by feedback type (loss, gain) and group (alcohol, placebo) at FCz depicting the expected enhancement (negativity) following loss versus gain feedback for FRN, as well as the positive-going P3. Alcohol-related reductions in amplitude are apparent both in the FRN (loss–gain difference) and P3 (all trials). a Topographical maps: the upper topographical maps depict the overall frontocentral distribution of the FRN (loss–gain difference) measure as well as the more central distribution of the P3 (averaged across gain and loss trials). The lower topographical maps illustrate the topographical distribution of the group difference for each component (with the FRN group difference again being represented for the loss–gain difference). b Waveform plots, first level: average time–domain ERP activity by feedback and group, frequency-filtered (third order Butterworth) to illustrate activity in the theta (3–9 Hz bandpass) range corresponding to FRN response (FCz, left plot) and activity in the delta (3 Hz lowpass) range corresponding to P3 response (Cz, right plot). b Color surface plots, second level: time–frequency representation of the theta-FRN and delta-P3 principal component scores following feedback onset on loss and gain trials combined (depicted for the placebo vs. alcohol difference). Topographical maps, third level: scalp topography distributions for the mean of the TF-PCA energy for the theta-FRN (left map) and delta-P3 (right map). From the topographical maps, it can be seen that the theta-FRN activity is maximal frontocentrally (at FCz) and enhanced for loss versus gain trials (upper left topomap of Fig. 1b), consistent with the interpretation that this component is a measure of “FRN.” Similarly, the more central topographical distribution of delta-P3 matches that of the time–domain P3. Note that the loss–gain difference for theta-FRN is opposite to that of the negative-going time–domain FRN, since TF measures are scaled such that more amplitude is most positive. Further, delta-P3 is enhanced for gain versus loss trials (upper right topomap of b). The middle row of topographical maps illustrates the scalp distribution of the group difference for both theta-FRN and delta-P3. Further, the bottom row of topographical maps highlights the feedback × group interaction for theta-FRN, due to a larger alcohol-related amplitude reduction following loss versus gain trials. PC principal component
To test for effects of alcohol intoxication on these two ERP components, a feedback (gain, loss) × group (alcohol, placebo) GLM analysis was performed for each. For the FRN, a main effect of feedback, F(1,77) = 141.09, p<0.001, signified that the amplitude of the waveform during the FRN window was more negative following loss than gain outcomes, as is expected for this measure (Bernat et al. 2011; Gehring and Willoughby 2002). Further, a main effect of group, F(1,77)=20.84, p<0.001, indicated that the overall amplitude of the waveform was reduced for the alcohol group. Because the FRN is typically quantified in the time domain as a relative enhancement (more negative deflection) for loss relative to gain outcomes, the observed feedback × group interaction, F(1,77)=9.69, p=0.003, indicates that the FRN, as it is typically measured (i.e., as a loss–gain difference), was reduced in amplitude for intoxicated individuals (alcohol and placebo loss–gain, Ms=−3.19 and −5.46, SDs=2.11 and 3.96, respectively, both gain–loss differences significant with p <0.001). The nature of this effect is further clarified by the TF decomposition described below. The topographical map of the group difference depicted in Fig. 2a (lower left head plot) illustrates the frontocentral distribution of the alcohol-related effect on FRN for loss versus gain trials.
In contrast with the FRN, the P3 was generally enhanced (i.e., more positive) for gain outcomes as compared to loss outcomes, feedback main effect, F(1,77) = 11.35, p=0.001. Similar to the FRN, however, P3 response amplitude was generally reduced for individuals in the alcohol group, F(1,77)=29.34, p<0.001. As illustrated in Fig. 2a (lower right topographical map), the group difference for the P3 was distributed centrally on the scalp and paralleled the scalp distribution of P3 amplitude for the sample as a whole. Although the degree of the alcohol-related reduction in P3 appeared greater for loss than for gain outcomes, F(1,77)=5.08, p=0.027, TF analyses (see below) revealed this two-way interaction to be attributable to alcohol’s impact on the overlapping theta-FRN.
Time–frequency component scores
Figure 2b depicts the TF decomposition of the feedback-ERP. Consistent with prior findings using this task (Bernat et al. 2011), theta-FRN in the current sample (left side) paralleled the TD FRN in terms of its latency, frontocentral topographical distribution, and augmentation for loss versus gain feedback. Like the TD P3, delta-P3 (right side) had a somewhat later latency and more central scalp distribution than theta-FRN and, as depicted in the upper topographical maps of Fig. 2b, was enhanced for gain versus loss outcomes. Further, regression analyses indicated that each TD measure could be conceptualized as a mixture of theta-FRN and delta-P3 activity (i.e., regression analyses predicting either FRN or P3 from theta and delta measures yielded significant independent contributions of theta-FRN and delta-P3 toward prediction of each TD measure).1
As is illustrated by the delta-filtered TD waveform (Fig. 2b, top right plot), delta-P3 served to increase (i.e., make more positive) the amplitude of the feedback-ERP at the time of both the FRN and the P3 (Fig. 2a). Theta-FRN (Fig. 2b, top left plot), however, influenced the TD waveform differently. At the time of the FRN (between 200 and 300 ms post-stimulus), the phase of theta-FRN was such that increased theta energy translated into more negative TD amplitude (yielding the typical enhancement in negative-going FRN with increased theta-FRN). However, at the time of the P3 (between 300 and 400 ms), the phase of theta ERN exhibited a shift such that increased theta energy resulted in more positive amplitude in the TD. Thus, care must be taken in interpreting associations between TF and TD measures, because enhancements in TF energy can result in either more positive or more negative TD amplitude, depending on the phase of the TF waveform at the time point of interest.
Following the approach employed with the TD measures, feedback × group GLM analyses were conducted for the theta-FRN and delta-P3 variables. Similar to the results for the TD FRN, theta-FRN was enhanced for loss versus gain feedback, F(1,77)=41.52, p<0.001, and reduced in amplitude for the alcohol versus the placebo group, F(1,77)=16.05, p<0.001, with the two-way (feedback × group) interaction also emerging as significant, F(1,77)=11.04, p=0.001. Both groups show a significant loss vs. gain difference (p<0.001 in each case). However, the group difference in theta-FRN response was larger for loss trials (placebo versus alcohol difference= 0.26) than for gain trials (difference=0.08). Follow-up analyses of the interaction revealed that although the loss vs. gain difference was significant in both groups, the magnitude of the difference was smaller for the alcohol group (M=0.08, SD=0.06) than for the placebo group (M=0.26, SD=0.32). Thus, although both groups showed relatively enhanced FRN response for loss versus gain trials, the magnitude of this enhancement was diminished for intoxicated participants as compared to non-intoxicated participants.
As was found for the TD P3, delta-P3 amplitude was enhanced following gain versus loss outcomes, F(1,77)= 51.19, p<0.001, but reduced overall for the alcohol versus the placebo group, F(1,77)=12.27, p=0.001. However, in contrast with the TD P3, the feedback × group interaction for the delta-P3 TF variable was nonsignificant, F(1,77)=1.05, p=0.31, indicating similar alcohol-related amplitude reduction across gain and loss trials despite generally enhanced delta-P3 reactivity for gain outcomes. Topographical maps depicting scalp distributions of statistical effects for these TF component variables are included at the bottom of Fig. 2a (maps on the left side are for theta, those on the right are for delta).2
Discussion
Prior work reporting alcohol-related attenuation of the ERN suggests that alcohol impairs a frontal brain network critical for monitoring one’s behavior. The current study evaluated the impact of alcohol on the FRN, an ERP response similar to the ERN, but one guided by performance feedback from the environment. Using gain and loss feedback in a gambling task, we quantified FRN along with feedback stimulus P3 using both traditional TD and newer TF analysis methods. TF decomposition allowed us to parse these two overlapping components of the feedback-ERP and clarify the findings. That the theta-FRN component was attenuated more strongly by alcohol for loss than gain trials supports the idea that alcohol broadly impairs the brain’s recognition of negative behavioral outcomes, both internally and externally based.
However, an alternative possibility is that the FRN reduction resulted from a lower-level sensory or attentional deficit produced by alcohol. A more basic impairment of this sort would likely result in attenuation of the N1 ERP, an earlier brain negativity theorized to reflect the allocation of attention to task-relevant stimuli (Heinze et al. 1990; Luck et al. 1990). However, the TD waveform (Fig. 2a) has somewhat larger N1 response in the alcohol (vs. placebo) group, and a baseline-to-peak measure of N1 in fact yields a significant group difference. Nonetheless, consideration of the TF breakdown of the waveform (Fig. 2b) points to an early emerging difference in delta-frequency activity as the source of this apparent difference (Fig. 2b, right side). Examined separately from the delta component of the signal, the peak and trough of the initial theta oscillation—corresponding to the P1 and N1, respectively—overlap for the two groups (Fig. 2b, left side). The implication is that early oscillatory activity presumed to reflect processes related to selection, and allocation of attention is unaffected by intoxication, whereas subsequent oscillatory activity reflecting registration of the feedback direction (loss vs. gain; i.e., the FRN) is attenuated. Thus, while we cannot rule out the possibility that the FRN reduction might be attributable to another processing deficit, our data appear consistent with the idea proposed by others that alcohol more strongly effects higher-level evaluative, versus lower-level sensory or orienting, processes (for a review, see Jääsekeläinen et al. 1996).
Unlike the theta-FRN, which showed stronger alcohol-related attenuation for loss trials, the delta-P3 response was reduced similarly across trial types. This effect was clarified by TF analysis, which enabled us to separate the delta-P3 signal from the overlapping theta-FRN and thereby better understand the apparent gain/loss interaction in the TD P3. It showed that theta and delta energy both contributed positively to the TD P3. Given this, in conjunction with the findings for the TF measures, it is clear that the observed feedback × group interaction for the TD P3 was attributable to the contribution of later positive-going theta to P3. Specifically, the relatively greater impact of alcohol in attenuating theta-FRN reactivity following loss (compared with gain) outcomes, coupled with the absence of a feedback × group interaction for delta-P3, resulted in the larger group difference in TD P3 amplitude for loss versus gain feedback.
Our finding of delta-P3 amplitude reduction coincides with prior work on the neurocognitive effects of intoxication and mirrors findings of P3 reduction among individuals high in trait disinhibition (Bernat et al. 2011). In contrast, our finding that alcohol attenuated the FRN differs from prior work reporting intact FRN response in high-disinhibited individuals (Bernat et al. 2011), despite attenuated ERN (Hall et al. 2007). Thus, the current results, in conjunction with prior work, suggest that the deleterious effect of alcohol on the brain’s monitoring system is more pronounced and pervasive than that associated with disinhibitory personality.3 The lack of relationship between FRN and trait disinhibition also suggests that the FRN-gambling procedure is a more rigorous test of self-monitoring deficits than the ERN.
Nonetheless, follow-up research will further clarify the precise nature and basis of the ERP effects reported here. One avenue for further research will be to examine effects of alcohol on FRN response elicited by feedback that is contingent on responding (e.g., reinforcement-learning tasks, or gambling tasks in which participants can learn strategies to avoid loss or errors). Tasks of this type yield a greater array of behavioral measures and may thus allow for finer-grained analysis of the interplay between intoxication, FRN, and performance. Further, given that the current study used a relatively high dose of alcohol, it will also be worthwhile in future research to evaluate whether lower doses produce similar or lesser FRN attenuation.
In summary, intoxicated participants showed impaired processing of performance feedback, both in terms of the relatively immediate recognition of the outcome’s valence (good vs. bad, as reflected in the FRN) and in terms of later evaluative processing reflected in P3. In combination with the behavioral finding that intoxication produced weaker effects of previous trial outcome on risk-taking, these findings indicate that intoxication produces a state in which individuals neither fully interpret the significance of performance feedback nor do they carry the feedback information forward into subsequent trials to alter their behavior. In comparison to recent work finding intact FRN in high-disinhibited individuals, these data also suggest that alcohol (which produces a state of heightened disinhibition; Patrick and Lang 1999) is associated with more profound impairment of self-monitoring processes than trait disinhibition.
Acknowledgements
This work was supported by grant AA12164 from the National Institute on Alcohol Abuse and Alcoholism and grants MH088143 and MH072850 from the National Institute of Mental Health.
Footnotes
Conflicts of interest We have no conflicts of interest to report.
A regression model that included the TD FRN gain–loss difference score as the DV, and theta-gain, theta-loss, delta-gain, and delta-loss as the IVs (all measured at FCz), yielded a multiple R of 0.85, F(4,74)=49.57, p<0.001. Theta-gain, theta-loss, delta-gain, and delta-loss each contributed uniquely to the amplitude of the TD FRN (ts[74] = −2.31, 9.47, 5.80, and −5.97, respectively, all ps<0.03). A second regression model predicting TD P3 amplitude from these differing theta and delta component measures (measured at electrode Cz) yielded a similar outcome, F(4,74)= 181.63, p<0.001, R=0.95, with theta-loss, delta-gain, and delta-loss each contributing uniquely to the TD P3, ts(74)=3.00, 4.87, and 5.06, respectively, all ps<0.01. The predictive contribution of the theta-gain component in this case was non-significant, t(74)=0.96, p=0.340.
To test for possible moderating effects of gender on these primary ERP variables of interest, we re-ran the feedback × group GLMs for theta-FRN and delta-P3 with gender included as an additional between-subjects factor. For delta-P3, no interactions involving gender were found, feedback × gender, group × gender, and feedback × group × gender, Fs(1,75)=2.41, 1.00, and 2.79, allps≥0.10. For theta-FRN, a significant three-way feedback × group × gender interaction was evident, F(1,75)=4.72, p=0.03. However, in two-way GLMs conducted separately for men and women, the feedback × group interaction emerged as significant in each analysis, F(1,35)=9.58, p=0.004 for women and F(1,40)=4.63, p=0.038 for men—with the three-way interaction attributable not to a difference in the nature of the two-way interaction but only to a difference in the relative magnitude of alcohol’s effect on loss versus gain differentiation, with women showing greater attenuation of loss/gain differentiation as a function of alcohol (relative to placebo) than men.
Although possible differences in sample and study characteristics necessitate tentative conclusions regarding comparisons between these studies, it is worth noting that direct comparison of the theta-FRN amplitude across this and the Bernat et al. (2011) samples supports the claim that theta-FRN is more strongly affected by alcohol than trait disinhibition. Specifically, substituting the high-disinhibited subgroup (n=94) from the Bernat et al. (2011) sample for the placebo control group indicated that, like the placebo group in the current study (both of whom completed the same experimental task), intoxicated individuals had attenuated theta-FRN in comparison to high-disinhibited participants, group F(1,92)=21.09, p<0.001, with relatively greater amplitude reduction for loss vs. gain trials, gain/loss × group, F(1,92) = 15.35, p<0.001.
References
- Bernat EM, Malone SM, Williams, WJ, Patrick CJ, Iacono WG(2007) Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International J Psycho-physiology 64:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ (2011) Externalizing psychopathology and brain responses to gain/loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. J Abnorm Psychol. doi: 10.1037/a0022124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Sci 280:747–749 [DOI] [PubMed] [Google Scholar]
- Colrain IM, Taylor J, McLean S, Butter R, Wise G, Montgomery I (1993) Dose dependent effects of alcohol on visual evoked potentials. Psychopharmocol 112:383–388 [DOI] [PubMed] [Google Scholar]
- Curtin JJ (2000) Unpublished computer program
- Curtin JJ, Fairchild BA (2003) Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnormal Psychol 3:424–436 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM (1994) Localization of a neural system for error detection and compensation. Psychol Sci 5:303–305 [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroglu C, & Başar E (2001) Wavelet analysis of oddball P300. International J Psychophysiology 39:221–227 [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJB (2000) Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology 37:43–54 [PubMed] [Google Scholar]
- Donchin E (1981) Surprise!…Surprise? Psychophysiology 18:493–513 [DOI] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C (2005) Alcohol consumption impairs stimulus- and error-related processing during a go/no-go task. Cognit Brain Res 25:873–883 [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J (1991) Effects of cross-modal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78:447–455 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT (2000) Prefrontal–cingulate interactions in action monitoring. Nat Neurosci 3:516–520 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2002) The medial frontal cortex and the rapid processing of monetary gains and losses. Sci 295:2279–2282 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2004) Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring In Ullsperger M & Falkenstein M (eds) Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig, Germany, Max Planck Institute of Cognitive Neuroscience, pp 14–20 [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390 [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG (2010) Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology 47:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ (2007) Externalizing psychopathology and the error-related negativity. Psychol Sci 18:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze HJ, Luck SJ, Mangun GR, Hillyard SA (1990) Visual event-related potentials index focused attention within bilateral stimulus arrays. I. Evidence for early selection. Electroencephalagr Clin Neurophysiol 75:511–527 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH (2002) The neural basis of human error-processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709 [DOI] [PubMed] [Google Scholar]
- Jääsekeläinen IP, Näätänen R, Sillanaukee P (1996) Effect of acute ethanol on auditory and visual event-related potentials: a review and reinterpretation. Biol Psychiatry 40:284–291 [DOI] [PubMed] [Google Scholar]
- Krull KR, Smith LT, Sinha R, Parsons O (1993) Simple reaction time event-related potentials: effects of alcohol and sleep deprivation. Alcohol Clin Exp Res 17:771–777 [DOI] [PubMed] [Google Scholar]
- Little HJ (1999) The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Ther 84:333–353 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA (1990) Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalagr Clin Neurophysiol 75:528–542 [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C (2003) Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci 14:47–53 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH (1997) Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. J Cognit Neurosci 9:788–798 [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ (2004) The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology 41:84–95 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Lang AR (1999) Psychopathic traits and intoxicated states: affective concomitants and conceptual links In: Dawson ME, Schell AM, Böhmelt AH (eds) Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge University Press, New York, pp 209–230 [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GPH (2002) Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Sci 298:2209–2211 [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff R et al. (1987) Dose-related effects of ethanol on visual-sustained attention and event-related potentials. Alcohol 4:293–300 [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O (1986) A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 23:695–703 [DOI] [PubMed] [Google Scholar]
- Skinner A (1982) The drug abuse screening test. Addict Behav 7:363–371 [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA (1982) Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91:199–209 [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ (2007) Neural systems for error monitoring: recent findings and theoretical perspectives. Neuro-scientist 13:160–172 [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ (2007) Theta EEG dynamics of the error-related negativity. Clin Neurophysiology 118:645–668 [DOI] [PubMed] [Google Scholar]
- Wall TL, Ehlers CL (1995) Acute effects of alcohol and P300 in Asians with different ALDH2 genotypes. Alcohol Clin Exp Res 19:617–622 [DOI] [PubMed] [Google Scholar]