The global public health community has set ambitious treatment targets to end the HIV/AIDS pandemic. With the notable absence of a cure, the goal of HIV treatment is to achieve sustained suppression of an HIV viral load, which allows for immunological recovery and reduces the risk of onward HIV transmission.
KEYWORDS: AIDS, HIV, differentiated care, point-of-care, viral load
SUMMARY
The global public health community has set ambitious treatment targets to end the HIV/AIDS pandemic. With the notable absence of a cure, the goal of HIV treatment is to achieve sustained suppression of an HIV viral load, which allows for immunological recovery and reduces the risk of onward HIV transmission. Monitoring HIV viral load in people living with HIV is therefore central to maintaining effective individual antiretroviral therapy as well as monitoring progress toward achieving population targets for viral suppression. The capacity for laboratory-based HIV viral load testing has increased rapidly in low- and middle-income countries, but implementation of universal viral load monitoring is still hindered by several barriers and delays. New devices for point-of-care HIV viral load testing may be used near patients to improve HIV management by reducing the turnaround time for clinical test results. The implementation of near-patient testing using these new and emerging technologies may be an essential tool for ensuring a sustainable response that will ultimately enable an end to the HIV/AIDS pandemic. In this report, we review the current and emerging technology, the evidence for decentralized viral load monitoring by non-laboratory health care workers, and the additional considerations for expanding point-of-care HIV viral load testing.
INTRODUCTION
Currently, over 20 million people living with HIV (PLHIV), mostly in low- and middle-income countries (LMICs), are receiving antiretroviral therapy (ART) (1). The World Health Organization (WHO) recommends that all PLHIV be offered ART, regardless of CD4 count, which means that almost 20 million more people still require treatment (2). In the absence of a cure, the goal of providing ART is to achieve HIV virological suppression, which has been defined as an HIV viral load (VL) of <1,000 copies/ml. PLHIV who achieve virological suppression have more robust immune reconstitution, better clinical outcomes, and lower mortality rates (3). Furthermore, PLHIV who maintain an undetectable viral load have effectively no risk of sexual transmission to an HIV-negative partner, which has become the message of the U = U (Undetectable = Untransmittable) campaign (4).
The Joint United Nations Programme on HIV/AIDS (UNAIDS) launched the 90-90-90 project to achieve the following goals by the year 2020: (i) identify 90% of all PLHIV, (ii) initiate 90% of all PLHIV who know their status on ART, and (iii) maintain viral suppression among 90% of all PLHIV receiving ART (5). To reach these targets, coordinated efforts are required to diagnose PLHIV and initiate ART, as well as to provide ongoing care for those already on ART. Innovative models of care delivery and patient monitoring, including advanced diagnostic tools, provide opportunities to further accelerate progress toward high levels of HIV viral load suppression.
Despite scale-up of routine viral load monitoring in LMICs, viral load test results do not always lead to appropriate clinical action, including adherence counselling and follow-up viral load testing, to improve the HIV cascade of care. In Swaziland, one-third of PLHIV with viremia (>1,000 copies/ml) received appropriate adherence counseling (6, 7). In South Africa, fewer than 20% of PLHIV with viremia (>1,000 copies/ml) received a follow-up viral load test within 3 months (8, 9). A separate study in four South African provinces reported a median time from viremia (>1,000 copies/ml) to confirmation of virological failure of 30 weeks (interquartile range [IQR], 17 to 53 weeks), and the median time from viremia to ART regimen switch was 68 weeks (IQR, 35 to 124 weeks) (10, 11). While these problems may reflect broader weaknesses in the public health systems, point-of-care (POC) VL testing could expedite detection of viremia, thereby increasing viral resuppression and reducing community viral loads.
Diagnostic POC tests have rapidly emerged and are expanding into laboratories and clinics in high-income countries and in LMICs (12). In recent years, several POC HIV viral load tests have become available, and more are expected soon (13). These new technologies have the potential to decentralize and expand viral load coverage, enhance efficiency in health care services, and improve viral suppression. POC HIV viral load assays are important to measure individual treatment success as well as monitor the overall progress in achieving the goals of the HIV care and treatment. However, several important technological challenges and clinical research questions remain. In this review, we highlight the existing technologies for POC HIV viral load testing, the existing evidence for decentralized testing by health care workers within HIV programs, and evidence from modeling and cost-effectiveness studies.
TECHNOLOGIES FOR POC HIV VIRAL LOAD TESTING
Requirements of an Ideal POC HIV Viral Load Test
A variety of molecular platforms exist for accurate, high-throughput HIV viral load testing, but they require sophisticated laboratory infrastructure and highly skilled laboratory technicians. The logistical requirements to reliably transport samples to centralized laboratory facilities, which include scheduling specimen pick-up and maintaining sample integrity, can also present challenges. POC assays may allow testing outside of centralized laboratories but face a variety of constraints (12). In more remote clinical settings with fewer resources, the assays must remain functional under extreme environmental conditions (e.g., high temperature, humidity, and dust), have minimal reliance on a power supply, allow for simple sample collection, and feature automated equipment to allow for operation by users following minimal training (13–15).
In 2013, the International Diagnostics Centre presented a draft target product profile (TPP) review for a POC HIV viral load test (16). We have modified those criteria to make them more appropriate for emerging technologies (Table 1). In brief, test results should be rapid, accurate, and unambiguous, in order to be actionable during the patient visit and archivable to a centralized management system to record and monitor test volumes, results, and error rates (17–19). A POC HIV viral load assay must provide information on the level of viremia consistent with clinical standards for indicating virological failure, the threshold of which is currently defined by the WHO as 1,000 RNA copies/ml (2). Given the availability of newer medications with greater efficacy for maintaining viral suppression, newer POC HIV viral load assays should achieve a lower limit of quantification, 200 copies/ml. In addition, any POC HIV viral load assay must be easy to use by local health care workers with a minimal amount of training. With POC viral load testing, it is crucial that diagnostic accuracy is established in a wide range of HIV subtypes that represents global HIV subtype diversity. Finally, the capital equipment, consumables, and service conditions must be affordable to resource-limited HIV programs that are funded by governments in LMICs and global donors.
TABLE 1.
Ideal product attributes for POC HIV viral load test
| Domain | Optimal criteria |
|---|---|
| Goal | Quantification of HIV RNA viral load copies/ml |
| Limit of detection | 200 HIV RNA viral load copies/ml |
| Precision | Less than 0.3 log10 HIV viral load copies/ml, as compared to reference gold standard test |
| Time to results | Less than 30 min total |
| Throughput | Minimum of 15 tests per 8-h workday |
| Equipment | Small, portable, and robust, with uninterrupted power supply for use in primary health care clinics at a wide range of temperatures |
| Sample specimen | Finger capillary whole blood (maximum, 200 μl) or heel stick for young children; dried blood spot would be acceptable |
| Simple operation | The device should be capable of being used by health care workers, including technicians, nurses, and physicians, after a minimal amount of training |
| Quality control | Internal full positive and full process negative controls to monitor individual test results |
| Patient identification | Results can be easily linked to a specific patient |
| Data export for quality assurance | Full data export over mobile phone network with computer or tablet interface |
Currently Available Tests
We provide an update to a prior landscape review with details on POC HIV viral load tests, describing three products that are commercially available and one project near commercialization in India, Africa, and South America by Molbio Diagnostics (Table 2 and Fig. 1) (14). We excluded the COBAS Liat system (formerly the IQuum Liat HIV viral load assay), which demonstrated good diagnostic accuracy in two validation studies (20, 21), as the technology has been acquired by Roche and further development has been postponed. Among products from six companies that were previously described as being in the early to middle development stages (14), only one product (Molbio Diagnostics; Goa, India) is still being actively advanced (22, 23).
TABLE 2.
Point-of-care HIV viral load assays that are commercially available or in late developmenta
| Manufacturer | Assay | HIV type(s) | Amplification/detection | Analytic sensitivity (copies/ml) | Testing timeb (min) | Sample type and vol | Instrument dimensions | Test instrument | WHO PQ, CE-IVD | Marketed |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbott Inc. (formerly Alere) | m-PIMA (formerly Alere q HIV-1/2 Detect) | HIV-1 groups M/N and O, HIV-2c | RT-PCR/real time with competitive reporter hybridization | 800–1,000 | <70 | Plasma, 50 μl | 220 by 200 by 310 mm; weight, 7.8 kg | 1 unit | Yes, yes | Only in nonregulated markets |
| Cepheid | Xpert HIV-1 Viral Load | HIV-1 groups M, N, and Od | RT-PCR/real time with molecular beacons | 40 | 90 | Plasma, 1 ml | 102 by 305 by 298 mm; weight, 8.2 kg | 1 unit | Yes, yes | Yes |
| Diagnostics for the Real World | SAMBA I PSQ | HIV-1 | NASBA/ICS | 1,000 | 90 | Plasma, 200 μl | Display module: 215 by 170 by 180 mm Assay module: 190 by 330 by 330 mm Display module: 2.1 kg Assay module: 9.9 kg |
2 units | No, yes | Yes |
| Diagnostics for the Real World | SAMBA II WBSQ | HIV-1 | NASBA/ICS | 1,000 | 90 | Blood, 120 μl | Display module: 215 by 170 by 180 mm Assay module: 190 by 330 by 330 mm Display module: 2.1 kg Assay module: 9.9 kg |
2 units | No, yes | No |
| Molbio Diagnostics | TrueNat HIV Viral Load | HIV-1, group M | RT-PCR/real time with conventional TaqMan probes | 500 | 55 | Plasma, 500 μl Blood, 250 μl |
240 by 300 by 120 mm; weight, 3 kg | 2 units | No, no | No |
Abbreviations: WHO, World Health Organization; PQ, prequalification; CE-IVD, Conformité Européene in vitro diagnostic; TAT, turnaround time; PSQ, plasma semiquantitative; WBSQ, whole-blood semiquantitative; RT-PCR, reverse transcription PCR; NASBA, nucleic acid sequence-based amplification; ICS, immunochromatographic strip.
Testing time does not include time for plasma separation.
Limits of detection for m-PIMA: HIV-1 group M, 342 copies/ml (95% CI, 279 to 451) and 595 IU/ml (95% CI, 487 to 785); HIV-1 group O, 228 copies/ml (95% CI, 187 to 295); HIV-2 group A, 364 copies/ml (95% CI, 292 to 484) and 200 IU/ml (95% CI, 160 to 260).
Diagnostic sensitivity at limit of detection with WHO 3rd International Standard, 18.3 copies/ml; diagnostic specificity, 100% (95% CI, 96.7 to 100.0); limit of quantitation, 40 copies/ml.
FIG 1.
Point-of-care HIV viral load equipment and test cartridges currently marketed (A to F) or in development (G to K). Attendant key equipment for some devices, such as modems, barcode scanners, or printers, is not included. (A) The Abbott m-PIMA analyzer; (B) the Abbott m-PIMA HIV-1/2 test cartridge; (C) the Cepheid GX4 instrument; (D) the Cepheid Xpert HIV-1 viral load test cartridge; (E) the DRW SAMBA II test module with controller; (F) the DRW SAMBA II plasma semiquantitative test; (G) the Cepheid Omni instrument and controller; (H) the Molbio Diagnostics Trueprep sample preparation device; (I to K) the Molbio Diagnostics Truelab amplification instruments in Uno (I), Duo (J), and Quattro (K) module formats. (Images are reprinted with permission of the respective manufacturers.)
m-PIMA by Abbott.
The Alere q instrument and assays were recently rebranded to m-PIMA after the acquisition of Alere by Abbott (Abbott Park, IL, USA). The instrument is a rugged, battery-powered instrument designed for fully automated processing to enable testing outside of a laboratory (Table 2) (24). The technology uses real-time reverse transcriptase PCR (RT-PCR) monitored via competitive reporter hybridization to the amplified target sequence and can quantify HIV-1 RNA from groups M, N, and O, in addition to HIV-2. The m-PIMA is the only POC assay to measure HIV-2 viral load. The lower limit of quantification is 800 to 1,000 copies/ml. The test cartridge contains all required reagents and has a capillary port to introduce 50 μl of plasma that must be separated from venous or capillary whole blood. Such a step requires a refrigerated centrifuge and adds further 15 to 20 min to the test processing time, with a refrigerator required to store the samples prior to adding to the test cartridge. The cartridge is sealed and inserted into a single-module automated instrument that contains internal quality control measures, and the total time to result is approximately 70 min, excluding plasma separation. Abbott has a qualitative HIV-1/2 test, the Alere q HIV 1/2 Detect qualitative viral load assay for EID (early infant diagnosis), that had received WHO prequalification (PQ) status in 2016 for the diagnosis of HIV-positive children and adults using whole blood (25). In an early study comparing the whole-blood Alere q NAT POC viral load against the Roche Cobas Ampliprep/Cobas TaqMan v2 using plasma, the Alere q had a mean bias of 0.86 log copy/ml, a Pearson coefficient of 0.59 (confidence interval [CI] not provided), and a sensitivity and specificity to detect a viral load >1,000 copies/ml of 96.8% (95% CI, 92.1% to 99.1%) and 47.8% (95% CI, 42.2% to 53.4%), respectively (24). These results reflect the difficulty of separating cell-associated DNA from whole-blood specimens. More recent evaluations of the quantitative m-PIMA HIV-1/2 viral load assay remain unpublished; the assay received CE-IVD marking in December 2018 and is currently undergoing review by the WHO PQ program (26).
GeneXpert by Cepheid.
Cepheid produces the Xpert HIV-1 viral load assay, which has received WHO PQ and CE-IVD marking (Table 2) (27). The assay requires 1 ml of plasma, and a 15- to 20-min blood fractionation step using a refrigerated centrifuge is necessary prior to testing (28). The assay uses real time RT-PCR with molecular beacons to target RNA from HIV-1 group M (including subtypes A, B, C, D, F, G, H, J, K, CRF01_AE, CRF02_AG, and CRF03_AB), group N, and group O (http://www.cepheid.com/en/cepheid-solutions/clinical-ivd-tests/virology/xpert-hiv-1-viral-load). On-cartridge sample processing and test analysis are automated, with the total machine processing time, not including plasma separation, taking approximately 90 min. The cartridge can be used in GeneXpert machines, which also run similar cartridge tests for tuberculosis (TB), sexually transmitted infections (STIs) and other infections (30). The machines require temperatures below 30°C and dust-free environments, which restrict their use to controlled settings (31, 32). While they may be considered near POC, several modifications using batteries and even solar panels have enabled its implementation outside of traditional laboratories (33, 34). Cepheid has been developing the Omni, a battery-powered mobile instrument capable of processing a single test cartridge at the clinical point of care (30). Test cartridges for the Omni are fitted with a near-field communication chip, instead of a barcode, to activate the instrument. In July 2018, Cepheid launched the GeneXpert Edge for decentralized testing while working to fix production issues for Omni, which can be battery operated and controlled via tablet instead of computer.
A variety of studies conducted in high- and low-income settings have compared the diagnostic accuracy of the Xpert HIV-1 viral load assay to those of conventional laboratory assays, including the Abbott RealTime HIV-1 m2000rt (Abbott Molecular) (35–40), Roche COBAS AmpliPrep/COBAS TaqMan (Roche Diagnostics) (41–44), NucliSENS EasyQ HIV-1 v2.0 (bioMérieux) (45), and Versant HIV-1 RNA 1.5 assay (Siemens HealthCare Diagnostics) (46). In a recent meta-analysis of these studies, the pooled Pearson coefficient was 0.94 (95% CI, 0.89 to 0.97; n = 8 studies) and the pooled Spearman coefficient was 0.96 (95% CI, 0.86 to 0.99; n = 3), demonstrating good correlation with laboratory reference results (50). The Xpert HIV-1 had a mean bias of within 0.35 log copy/ml of the reference viral load assays. Variability in viral load thresholds meant that pooling of results for sensitivity and specificity was not performed. However, for WHO prequalification, the Xpert HIV-1 viral load assay had 94% sensitivity and 99% specificity for detecting HIV viral loads of >1,000 copies/ml (27). In one study, the Xpert HIV-1 did not detect a viral load in one patient with high titers of an HIV-1 CRF02_AG subtype variant, which had a 25-nucleotide insert that was not matched to reference wild-type sequences (42). The concordance between the Xpert HIV-1 and the reference Roche TaqMan 2.0 was high among other patients with CRF02_AG subtype, which is predominant in West Africa (42). The authors of the meta-analysis noted that the Xpert HIV-1 assay could be of significant value to decentralized HIV viral load testing due to its accuracy, ease of use, and relatively low infrastructure requirements (50). The Xpert HIV viral load assay, used in mobile community-based clinics in Botswana, showed high levels of agreement across a broad range of HIV-1 RNA values with the Abbott m2000sp/m2000rt comparator and that its use in rural communities (after home-based collection of venous blood) was feasible (38). However, to date there are no published studies demonstrating whether it is feasible to provide same-day, true POC viral load results using the Xpert HIV-1 in programmatic settings in LMICs. Instead, the need for centrifugation may prevent the Xpert HIV-1 from being used as a true POC test. Currently, there are three ongoing trials of this assay being conducted in the United States and/or sub-Saharan Africa (NCT03187964 [47], NCT03533868 [48], and NCT03066128 [49]).
SAMBA by Diagnostics for the Real World.
Diagnostics for the Real World (DRW) has developed a simple amplification-based assay (SAMBA) for semiquantitative HIV viral load measurement, called SAMBA semi-Q (https://www.drw-ltd.com/copy-of-samba-overview). The SAMBA I system has a semiautomated format with separate sample preparation, amplification, and detection units capable of processing four samples simultaneously (Table 2). The SAMBA II system is a fully automated modular system, comprised of a tablet-based controller module and up to four processing units per controller. The sample extraction, amplification and detection components are contained within a single sealed cartridge. The SAMBAs use nucleic acid based-sequence amplification (NASBA) (52), an RNA-based isothermal amplification method to amplify HIV-1 RNA, detecting the single-stranded NASBA amplicons via sequence-specific oligonucleotide capture followed by colorimetric detection on a nitrocellulose strip (53). The time to process a specimen is approximately 90 min. The test result is visually scored on a lateral flow assay (LFA) format with a viral load threshold of 1,000 copies/ml. The SAMBAs currently use plasma, adding 15 to 20 min to the test processing time and requiring a refrigerated centrifuge, although a whole-blood testing format is in development (54, 55). The SAMBA I semi-Q prototype assay had a reported 96.6% (CI not reported) concordance with the Roche COBAS AmpliPrep/COBAS TaqMan (57). In a later study, the SAMBA HIV-1 Semi-Q on the SAMBA I demonstrated 98.1% (95% CI, 96.5% to 99.1%) concordance with the Roche TaqMan 2.0 assay (56). However, in these two analyses, any result on the Roche TaqMan 2.0 that was between 500 and 2000 copies was automatically considered concordant with the SAMBA result, which may have biased the concordance results. In a separate analysis, the SAMBA HIV-1 Semi-Q on the SAMBA II was found to have 98.0% (95% CI, 94.3% to 99.6%) concordance with the Abbott RealTime assay (56).
Molbio Diagnostics.
Molbio Diagnostics intends to launch an HIV viral load device in 2019 (Table 2). According to the manufacturer, the technology uses real-time RT-PCR and can quantify HIV RNA from HIV-1 group M with a lower limit of quantification, 500 copies/ml. The testing process is fully automated using tabletop-based battery powered instruments, but sample preparation and subsequent amplification and detection occur in two separate instruments (22, 23). The total time to test results is approximately 55 min, which includes a 20-min sample preparation step to process either whole blood or plasma, though a further 15 to 20 min is required to first separate the plasma. The amplification and detection of the RNA extracts are performed on a test chip containing all necessary reagents. The current equipment is a single-, two-, or four-module instrument that uses an Android software platform for processing test chips, storing data, and communicating results and test location via mobile telephone networks (Fig. 1). Currently, there are no published evaluations of the Molbio assay.
Technologies To Advance Next-Generation Tests
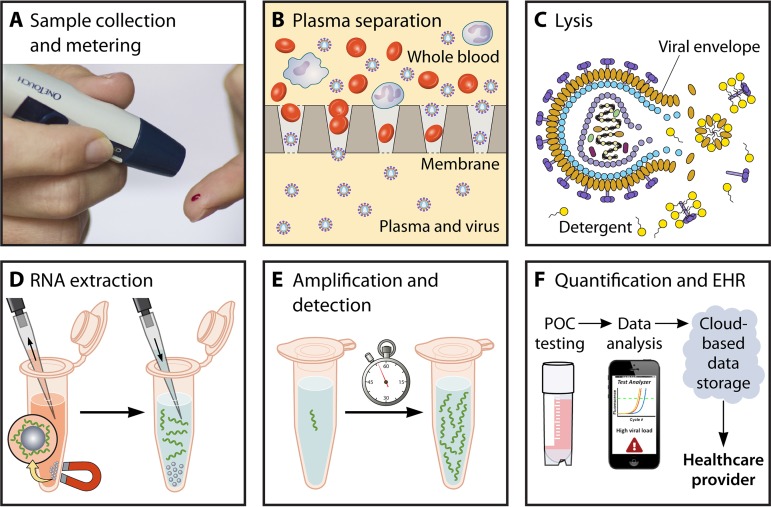
While several commercial near-POC HIV viral load tests are available or in late-stage development, further innovation is required to reduce test time, enable the use of whole blood as a starting sample, lower the costs of capital equipment and disposable test cartridges, and improve assay sensitivity and quantitative accuracy. HIV viral load testing is a complex, multistep process requiring sample collection, blood fractionation, viral lysis, RNA extraction, amplification, and detection, and communication of test results (Fig. 2). Sample preparation is a significant challenge for “sample-to-answer” viral load tests requiring automation of blood fractionation, virion lysis, and nucleic acid extraction prior to downstream amplification and detection.
FIG 2.
Examples of various steps which must be integrated into potential POC HIV viral load tests. (A) Sample collection should be simple to perform and provide precise volume metering. (B) Membrane-based plasma separation to exclude proviral HIV DNA in PBMCs. (C) Viral lysis using a detergent to denature the viral envelope and release HIV RNA. (D) Magnetic bead-based RNA extraction to purify RNA from blood-related inhibitors and lytic agents. (E) Amplification of nucleic acids to detectable concentrations. (F) Algorithm and device to provide quantitative results and communicate results to electronic health records (EHR).
POC viral load testing should be conducted on whole blood, via either venipuncture (0.2 to 2 ml), heel prick, or finger prick sampling (<200 μl) (58, 59). Plasma separation has been used to remove red blood cells and peripheral blood mononuclear cells (PBMCs) that can inhibit nucleic acid amplification tests or cause spurious results by inclusion of proviral DNA (60, 61). Several equipment-free solutions for integrated plasma separation have emerged to eliminate additional user steps (62). Sedimentation techniques often require microchip fabrication and struggle to process volumes greater than 5 to 10 μl of undiluted blood (63–65). Other techniques using separation membranes, which can be sized to process large sample volumes, are easier to integrate in paper-based assays (66–70).
A crucial step is thorough lysis of the HIV virion, which degrades the lipid and protein viral envelope that encapsulates RNA strands. Lysis can be achieved by heating, detergents, enzymes, or chaotropic salts (71–74). After virion lysis, efficient isolation of highly purified HIV RNA is required, as even trace amounts of inhibitors can significantly affect performance of quantitative amplification. Solid-phase, silica-based extraction methods are used in gold standard tests (75) and have been translated into POC microfluidic or paper-based formats (76–78). Silica-based extraction requires exchanges of wash and elution buffers, a task difficult to automate in simple POC devices. Several single-step nucleic acid purification strategies have been developed in paper-based devices, such as chitosan-coated membranes (79) and electrophoretic separation of nucleic acids from blood (69). Despite these recent advancements in sample preparation techniques, automating HIV lysis and RNA extraction from blood samples remains a formidable challenge (80–83).
Real-time PCR has long been the staple of nucleic acid amplification testing (NAAT), including most of the current gold standard viral load tests (74). PCR assays are difficult to implement in POC devices due to the significant power demands of thermocycling and the need for highly purified nucleic acids. Other options may include isothermal amplification techniques, such as loop-mediated isothermal amplification (LAMP) (84, 85), helicase dependent amplification (86), and recombinase polymerase amplification (87). Isothermal assays do not require thermal cycling and therefore have reduced power demands and require less time for amplification. While isothermal methods are convenient for POC implementation, few assays have demonstrated reliable quantitative results (88–90). For researchers developing HIV viral load isothermal amplification assays, the primary challenge remains demonstrating quantitative accuracy comparable to that of real-time PCR across wide ranging viral loads (103 to 106 copies/ml) and subtypes.
While not unique to POC testing, NAAT for HIV is also complicated by the virus’s high rate of mutation, recombinant forms, and different clades associated within regions of high prevalence (74, 91). NAATs can account for sequence diversity with degenerate primers (91), long primers capable of tolerating mismatches (87) and targeting highly conserved regions of the HIV genome (92, 93). Still, the HIV sequence diversity can impact the performance of an assay, especially when applied to a variety of subtypes (94).
When it comes to readout, quantitative NAATs most commonly use fluorescence-based methods to detect amplification (14), while LFA-based detection methods can provide semiquantitative results (95). Additional strategies have targeted reverse transcriptase activity (58) or optically sense virus particles (96). While recent studies have reported innovative devices integrating NAAT operations, these platforms used assays for various infectious diseases, with no specific demonstration of HIV detection or quantification (69, 78, 97–100).
Moving forward, the central challenge facing POC HIV viral load tests is automating all diagnostic steps, including sample preparation and amplification, ideally in a manner that circumvents the high electrical power demands and instrumentation costs for robotics, optics, or pumps. Additionally, test developers face an obstacle in the target product profile, which proposes an optimal limit of detection of 200 copies/ml and sample specimen of less than 200 μl of finger capillary blood (Table 1). A low sample volume reduces the number of input of virions, elevating the limit of detection. HIV viral load tests will need to improve efficiency of sample preparation and sensitivity of amplification assays to achieve a low detection limit via capillary blood.
GENERATING THE EVIDENCE FOR IMPLEMENTATION OF DECENTRALIZED HIV VIRAL LOAD TESTING
Approaches to improve the delivery of HIV viral load testing that are effective, scalable, and sustainable are urgently needed, but under resource constraints, the allocation of resources for decentralizing HIV viral load testing should be based on evidence (101–104). If health facility factors are not properly taken into consideration, then implementation of decentralized HIV viral load testing will be jeopardized (105, 106). Introducing new diagnostic devices into clinics with heavy workloads may cause disruptions in the clinical workflow (107), which can result in a loss of efficiency and a decline in service quality (108, 109). Alternatively, the experience of staff in primary health facilities with routine use of rapid diagnostic tests (RDTs) for HIV diagnosis may facilitate the expansion of POC HIV viral load testing (110). An assessment of the barriers and facilitators is required to address challenges at both the facility and health system levels (111–117). In a systematic review of 132 studies, the most common barriers to POC implementation in HIV programs were related to integration into clinical practice, followed by concerns regarding diagnostic accuracy (29).
Evidence and Results from Clinical Studies
The implementation of RDTs for HIV (118) and CD4 count testing (119, 120) at the point of service has resulted in improvements of the HIV care cascade (121). A case in point is the use of qualitative POC technologies for early infant diagnosis (EID). A field evaluation in South Africa comparing POC Xpert HIV-1 Qual and lab-based molecular platforms showed that decentralized EID testing was accurate and increased the rate of result return when used in a maternity hospital (122). A recent cluster randomized clinical trial in Mozambique (123), and an observational study in Malawi (124), showed that the qualitative Alere q HIV-1/2 Detect accelerated the time to ART initiation, with an associated increase in the proportion of HIV-positive infants who were initiated on ART within 180 days of sample collection (90.3% and 91.1%, respectively), compared to standard centralized laboratory testing (40.2% and 48.4%, respectively). More recent evidence comes from a large POC EID program evaluation incorporating over 20,000 tests into routine clinical services as part of a “hub and spoke” design, which reduced sample turnaround time across 339 clinical sites in 8 African countries (125). The proportion of HIV-positive infants who were initiated on ART by 60 days increased from 43.3% to 92.3%, with cost per result returned in 30 days decreasing from $131 (U.S. dollars) with conventional referral laboratory testing to $38 with POC testing. In a study for diagnosing acute HIV infection, 91% of the patients attending a community clinic in Spain were notified of their HIV-positive status the same day using the Xpert HIV-1 Qual assay (126). These studies demonstrate that POC platforms can be successfully implemented in a number of settings and have led to guidelines for the introduction of HIV POC diagnostics into national programs (127). The WHO recommended POC EID testing, and a programmatic update in 2016 confirmed that POC EID assays can be used for confirmatory testing (2).
Several randomized clinical trials in LMICs are investigating the clinical impact of POC HIV viral monitoring for PLHIV receiving ART (Table 3). Study populations vary but include children, adolescents, adults, and pregnant women, with interventions of POC viral load test alone or combined with new models of HIV care. Most studies include viral suppression as an outcome, alongside various process evaluation measures.
TABLE 3.
Randomized controlled trials assessing clinical impacts of POC HIV viral load testing
| Clinical trial | Location | Study population | Interventions | Outcomes | Estimated completion date |
|---|---|---|---|---|---|
| STREAM, NCT03066128 (49) | Single site, South Africa | 390 nonpregnant adults on ART who are due a viral load test at 6 mo after initiation | POC monitoring including Xpert HIV-1 viral load testing, combined with task shifting to enrolled/auxiliary nurses, versus standard of care | Primary: (i) Proportion of patients who are retained in care with viral suppression at <200 copies/ml at 12 mo after enrollment (18 mo on ART) Secondary: (i) Proportion of patients with viral load of <1,000 copies/ml 12 mo from enrollment (ii) Costs per patient virologically suppressed and retained in care (iii) Time to detection of virological failure, subsequent intensive adherence counseling, and initiation of second-line regimen (iv) Proportion of patients entered appropriately into differentiated ART delivery programs and time to appropriate entry into these programs |
Q4 2018 |
| NCT03288246 (189) | Single site, Haiti | 150 persons aged 10–24 yrs on ART for >6 mo | POC HIV viral load testing at enrollment, 3 and 6 mo compared to standard laboratory testing at the same time points | Primary: (i) Number of steps in the HIV viral load cascade Secondary: (i) Comprehension of the relationship between ART adherence and HIV viral load assessed 1 mo after receiving viral load result (ii) Viral suppression at <1,000 copies/ml at 6 mo from enrollment |
Q4 2018 |
| UltraHIV NCT03187964 (47) | Single site, South Africa | 1,500 adults on ART who are due annual HIV viral load testing (another component of the study assesses Xpert Ultra for TB in a separate group of 1,500 adults) | POC Xpert HIV-1 viral load testing at site versus standard laboratory viral load testing | Primary: (i) Related to TB component of the study. Secondary: (i) Proportion of patients diagnosed with viremia by 8 wks (ii) Of those with viremia, the proportion who are identified to require adherence counselling and/or HIV resistance testing by 1 wk, are referred for adherence counselling and/or second-line ART by 8 wks, and do not successfully start adherence counseling, HIV resistance testing, or second-line ART by 12 wks |
Q4 2019 |
| NCT03533868 (48) | Two sites, Nigeria | 794 adults being initiated on ART | POC Xpert HIV-1 Viral Load monitoring at 6 and 12 mo from enrollment versus standard laboratory viral load | Primary: (i) Proportion of patients with viral suppression at <1,000 copies/ml at 12 mo on ART Secondary: (i) Average adherence from pharmacy refill data (ii) Loss to follow-up at 12 mo (iii) Time to adherence counselling, confirmation of virological failure and switch to second-line ART (iv) Time from specimen collection to result availability and communication to patient (v) HIV drug resistance patterns (vi) Patient and health care worker satisfaction |
Q4 2019 |
| RAPID-Viral Load NCT03553693 (190) | 20 sites, Uganda | 2,440 children, adolescents, and adults on ART, including pregnant women and patients with unsuppressed HIV viral loads | RAPID-Viral Load study intervention testing and counselling package, which includes near-point-of-care HIV viral load testing at local testing hubs, structured viral load counseling, and forms to track viral load ordering and testing, with feedback and performance evaluations at regular intervals | Primary: (i) Proportion of patients with HIV viral load ordered when indicated by country guidelines (ii) Mean time to delivering HIV viral load result to patient Secondary: (i) HIV viral load suppression at 12 mo from enrollment (ii) HIV viral load suppression (iii) Proportion switched to second-line ART (iv) Proportion of results present in Uganda’s Central Public Health Laboratory system |
Q4 2019 |
Results from an observational study in Zimbabwe (32) suggest that implementation of Xpert HIV-1 Viral Load for ART monitoring is feasible in this setting. In addition, use of the GeneXpert platform allows multidisease POC testing for sexually transmitted infections, tuberculosis, and hepatitis C (128, 129). The same study in rural Zimbabwe showed that multidisease POC testing with GeneXpert at district and subdistrict health care facilities was achievable but relied on supervision and quality management systems to ensure the accuracy of testing services (32). In some countries, large-scale, centralized HIV viral load testing is being performed using dried blood spot cards (130–132). The diversity of approaches that have shown promise implies that a combination of centralized and decentralized models should be considered for scale-up (133, 134).
Regulatory Issues, Quality Assurance, Supply Chain, and Data Connectivity
The lessons learned from establishing centralized models for HIV viral load testing could inform building networks for decentralized testing (135–137). Some challenges would be bypassed by POC testing, such as sample transportation. However, experience and planning for supply chain, reagent forecasting, human resources, maintenance of equipment, and laboratory-clinic interfaces would remain relevant for both centralized and decentralized HIV viral load testing (138–142).
Decentralizing HIV viral load requires that resources and processes are in place for training, monitoring, and quality assurance of test results at the clinic sites (143, 144). Unclear national regulatory pathways pose a challenge (145, 146), increasing the risk of delayed marketing of POC devices across neighboring countries in East Africa (147). Ensuring quality of POC testing through an external quality assessment (EQA) program poses numerous challenges, as the costs of setting up and maintaining such programs are often underestimated (148). The use of digital technologies can support strengthening EQA activities and supply chains within a network of peripheral testing centers using POC technologies (149, 150), but their full potential has not yet been realized due to issues around data ownership and confidentiality (151).
ROLE OF TASK SHIFTING FOR DIFFERENTIATED HIV CARE
Task shifting has been effectively implemented to facilitate HIV diagnostic screening and CD4 count testing activities and may be an effective strategy for POC HIV viral load testing in decentralized settings (18, 152, 153). A key consideration for decentralized HIV viral load testing is who will perform the test. Task shifting from physicians to nurses, and nurses to community health care workers has been successfully implemented for both the diagnosis and management of HIV (154–156). Task shifting in LMICs has facilitated the creation of innovative “differentiated care” services, which have increased health care coverage, improved monitoring, and reduced costs. However, the decentralization of POC HIV viral load testing poses a new set of opportunities and challenges at both facility and health system levels (113–117).
The Role of Nurses and Community Health Care Workers for HIV Care
Early ART programs in LMICs were provided through hospital-based, physician-led services, in a resource-intense model of HIV care that was neither sustainable nor scalable. To provide ART to the large numbers of people living with HIV/AIDS in LMICs, the WHO recommended decentralization and task shifting in public sector ART programs in 2004 (157, 158). Since then, numerous observational cohorts and clinical trials have demonstrated that decentralizing ART care from hospitals to primary care clinics improved retention in care and reduced mortality (159, 160), while nurse-led ART care is equivalent to physician-led care when measured by clinical and immunological outcomes (161). In southern and eastern Africa, nurses now routinely manage ART initiation and HIV care, as well as screening for and treating TB, STIs, and noncommunicable diseases among PLHIV.
As ART programs have matured, decentralization and task shifting have been integrated into differentiated care services (156). These services aim to meet the various needs of clients by adapting the location of care and ART delivery, the person providing care, and the interval between clinical visits and ART collection (162). Differentiated care services may be based in clinical or community settings, may involve health care workers or lay community health workers (CHWs), and include services for HIV prevention, testing and treatment (142). Examples of differentiated care for ART delivery in LMICs include community-based ART clubs (163), community ART delivery programs (164), streamlined ART pick-up, and multimonth prescriptions (165). Home-based ART provided by trained CHWs has also been found to be equivalent to physician-led care (166). South Africa has been implementing fully automated kiosks for dispensing ART with support provided via telemedicine interactions between clients and off-site pharmacists (167). These strategies can make treatment less burdensome for stable, suppressed patients who can receive longer refills and care closer to home, increase availability of staff for patients in need for more intensive follow-up, and reduce health care costs through appointment spacing and task shifting (103, 166, 168).
Use of POC Assays by Nurses and Health Care Workers within HIV Programs
A key component of decentralized, differentiated care services has been the development of POC technologies that have enabled testing to be conducted by nonlaboratory staff in both clinic and community settings (12). The use of antibody-based POC HIV tests by trained lay workers has been successful and recommended by the WHO (117, 169). The ease of use and portability of RDTs has allowed them to be implemented in large community-based HIV testing campaigns to increase overall HIV testing coverage for achieving the first 90-90-90 target (170–172). Despite the simplicity of RDTs, quality assurance and supply chain management have remained challenging in programmatic settings (173, 174).
The WHO recommends CD4 count testing to stage disease at HIV diagnosis, and rapid, portable POC CD4 count assays have been developed to measure CD4 counts using venous or capillary whole blood in under 30 min (175, 176). The Pima CD4 (Abbott, Chicago, IL) can be operated by nurses in primary care clinics (177, 178) and had acceptable performance characteristics in two meta-analyses, with sensitivities to detect a CD4 count of <350 cells/mm3 of 93% and 92% and specificities of 86% and 87% (175, 176). POC CD4 count testing has been accepted by patients and health care workers in many resource-limited settings (176), although failure rates ranging from 0% to 23.3% have been reported (179). These may have been due to deficiencies in quality assurance procedures, such as inadequate sample collection or machine calibration (176, 179).
When CD4 counts were used to determine ART eligibility, POC CD4 count testing was shown to increase retention in pre-ART care and increase ART initiation (104, 180), particularly when performed by lay workers in home-based HIV testing services (181). POC CD4 count assays have been used by nurses within health care facilities to allow same-day ART initiation, which was associated with increased overall ART initiation (182) and improved retention in care and subsequent viral suppression (183). In addition, availability of POC creatinine, alanine aminotransferase, and hemoglobin assays has similarly facilitated decentralized access to clinical monitoring. With the universal test-and-treat strategy, whereby all people living with HIV are started on ART, CD4 count testing remains important for staging HIV disease and assessing eligibility for prophylactic treatment (184). Four large trials of the universal test-and-treat strategy in southern and eastern Africa (ANRS TasP, SEARCH, BCPP, and MaxART) have incorporated POC CD4 count testing by nurses or lay health care workers into community-, home-, and facility-based HIV testing strategies (185). In Lesotho, a home-based, same-day ART initiation program in which nurses performed POC CD4 count and creatinine and hemoglobin testing improved ART initiation, retention in care, and viral suppression (186).
Looking Ahead: The Role of POC HIV Viral Load Testing within Differentiated Care Services
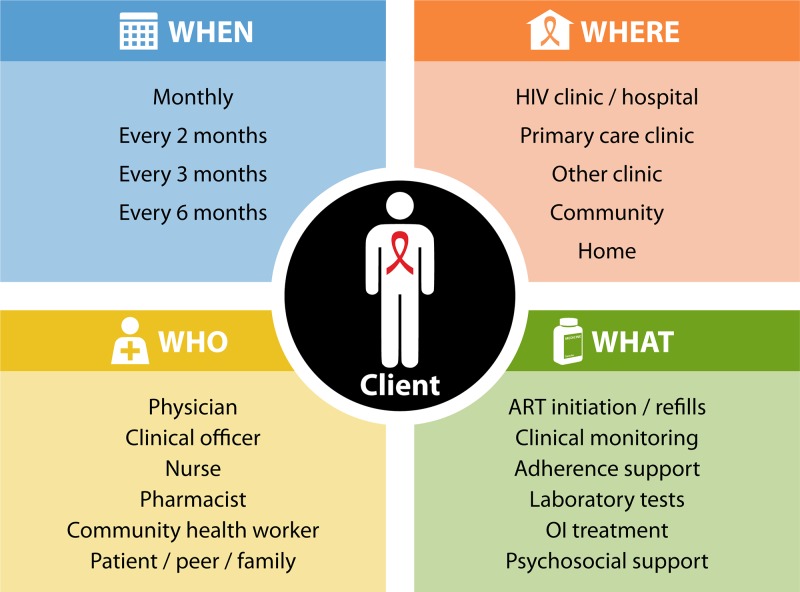
While nurses and CHWs have used POC assays to improve HIV testing and ART initiation, there is less evidence for implementing POC diagnostics to monitor PLHIV receiving ART. Evidence for the feasibility of task shifting POC viral load testing is lacking (50), although some testing has occurred within primary care or mobile clinics (24, 38, 43). The development of more portable assays and use of finger prick whole-blood testing should make POC viral load testing easier for nurses and CHWs in a range of settings (30). POC viral load assays have the potential to streamline ART monitoring and quickly triage patients into more or less intense care pathways (187), a strategy known as “viral-load informed differentiated care” (133, 162). The International AIDS Society (IAS) provides a decision framework for designing differentiated care services, which encourages policy makers to focus on the clinical characteristics, subpopulations, and contextual factors surrounding clients and to adapt the location of services, the care provider, and the services offered accordingly (Fig. 3) (162, 187).
FIG 3.
The building blocks of differentiated ART delivery with POC HIV viral load testing. OI, opportunistic infection. (Adapted from reference 187 with permission.)
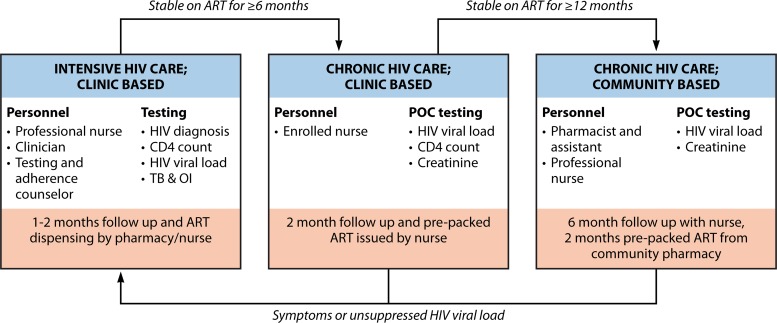
Clinical trials of POC viral load testing may facilitate task shifting to nurses and referral into differentiated care programs (Table 3) (47, 48, 188–190). In one study, integrated POC monitoring using POC CD4 count (Pima), POC creatinine, and POC HIV viral load (Xpert HIV-1) is being evaluated to rapidly identify stable patients who can be seen by an enrolled/auxiliary nurse every 2 months and then be down-referred to a community ART delivery program, in which they are seen by a nurse every 6 months (Fig. 4) (188). The primary outcome is viral suppression and retention in care after 12 months of POC monitoring, with secondary outcomes including costs of POC monitoring, time to receipt of results, time to adherence counseling, and time to switch to second-line ART. If found to be cost-effective, models of differentiated care with POC HIV viral load monitoring could be used to help achieve the 90-90-90 targets.
FIG 4.
A conceptual model of integrated point-of-care testing within differentiated care.
EVIDENCE FROM MODELING AND COST-EFFECTIVENESS
Role of Modeling in Evaluation of POC HIV Viral Load Testing
Modeling population-level impact and cost-effectiveness plays an important role in translating clinical trial findings into a broader set of population estimates (47–48, 188–191). Through modeling, trial results can be translated into standardized impact metrics such as disability-adjusted life years (DALYs) averted or quality-adjusted life years (QALYs) gained, as well as indirect benefits such as reduced HIV transmission (48, 136, 137, 192, 193). Models are typically individual based and simulate a cohort of individuals with HIV or a population with births, deaths, and dynamic disease transmission (194–198). Modeling can help to define the timing and detection threshold for POC viral load assays, based on cost-effectiveness analyses that account for the higher cost of second-line ART (194–198). The impact of POC HIV viral load testing on health outcomes can be estimated at multiple levels, including individual patients, the population, or national health systems.
Evidence from Modeling Studies for Benefit of POC HIV Viral Load Monitoring
The direct health gains from replacing clinical or laboratory-based CD4 count-based monitoring with laboratory-based viral load monitoring appear to be modest. Randomized trials in Zimbabwe and Uganda (199), Cameroon (200), and Thailand (201) found CD4 count-based monitoring to be noninferior to viral load monitoring and not substantially different from clinical monitoring over the first 3 years of ART. In contrast to centralized testing, POC HIV viral load testing provides results more rapidly, allowing for earlier adherence counseling and/or ART regimen switching to reduce the risk of HIV drug resistance, worsening immunosuppression, and onward HIV transmission. In multiple modeling studies, the benefits at the individual level of POC HIV viral load testing were estimated to be small relative to the impact of increasing coverage and retention on first-line ART (135, 194, 197, 198, 202). However, these studies were based on modeled estimates of the effectiveness of POC viral load testing, which were not informed by clinical trial results.
The estimated impact of laboratory-based (203–210) HIV viral load on transmission of HIV is beneficial but modest. In Malawi, 0.8% of HIV infections between 2017 and 2020 could have been averted through immediate scale-up of annual lab-based HIV viral load monitoring (210). A modeling analysis suggested that faster switching to second-line ART, more effective adherence counseling, and reduced transmission through viral suppression each contributed similar health gains, 0.15 QALY per PLHIV receiving ART (197).
The largest effects of POC HIV viral load testing may come from expanding access to viral load-informed differentiated care. In clinical trials (199–201, 211, 212) and modeling (139, 203–208, 213–216) of laboratory-based HIV viral load monitoring, the ability to implement differentiated care had an impact comparable to or greater than the direct health impact of viral load monitoring (217). Similar trends have emerged for models evaluating POC HIV viral load testing (135, 198). However, centralized HIV viral load testing involves sample transportation and transfer of results (218), which has been challenging to forecast and adds uncertainty to model estimates (48, 137, 192, 193, 219, 220).
Projections of Cost-Effectiveness Analysis for POC HIV Viral Load Implementation
Modeling studies have estimated the cost-effectiveness of laboratory-based viral load monitoring (139, 203–209, 213–216) and, more recently, POC viral load monitoring (135, 194, 197, 198, 202). Models incorporating health systems effects of monitoring, such as differentiated care, find increased evidence of cost-effectiveness (217). A comparison of three independently developed mathematical models estimated that implementing laboratory-based HIV viral load monitoring was not cost-effective prior to universal test-and-treat guidelines and concluded that a lack of resources to provide viral load monitoring should not preclude further expansion of ART (202, 221). In the era of the universal test-and-treat strategy, viral load monitoring is cost-effective at the individual level, assuming similar costs for laboratory-based and POC viral load testing (135, 194, 197, 198, 202). Annual viral load testing was generally found to be more cost-effective than 6-monthly viral load testing (197, 202). Patient monitoring contributes a small fraction of overall HIV care costs and thus can become cost-effective and even cost-saving when it enables other aspects of HIV care to become more efficient (197). Enabling viral load-informed differentiated care may be critical to making viral load monitoring more cost-effective and potentially cost-saving (103, 135, 166, 168, 187). As the technology becomes more widely available, new synergies between POC HIV viral load testing and optimal health care delivery may emerge. However, the rollout of dolutegravir-based first-line ART, which appears to have a lower risk of acquired drug resistance, may impact the role of HIV viral load monitoring (222, 223).
ADDITIONAL DIAGNOSTICS NEEDED FOR HIV MONITORING
In LMICs, the management of PLHIV who are identified as having unsuppressed HIV viral loads is complicated by the lack of objective measures of drug adherence and HIV drug resistance, which continues to emerge in LMICs (221, 224). The technological advances that have made POC NAAT available are also applicable to the development of POC HIV drug resistance assays that may allow for decentralized HIV resistance testing (225). Several assays are in development, while target mutations need to be fully characterized for specific drugs and drug regimens. In addition, being able to more accurately and objectively assess drug adherence may become more critical (137), and there are efforts to develop POC tests to measure drug concentrations of various antiretrovirals (ARVs) (226).
Additional tests could be considered for POC delivery to ensure more complete assessment of the health of PLHIV. Offering a package of services to identify patients with advanced HIV disease (defined as CD4 count less than 200 cells/ml) and test for common opportunistic infections is critical (158). Fortunately, POC CD4 count, POC testing for TB using GeneXpert or the lipoarabinomannan (LAM) assay, and screening for cryptococcal meningitis using a cryptococcal antigen RDT are available (184, 227, 228).
Integrated POC testing to include recommended clinical chemistry or hematology tests may be important to further improve HIV treatment services (229). Simple POC hemoglobin assays are becoming more widespread in primary care services and can be used for the monitoring PLHIV receiving zidovudine. For patients receiving tenofovir-based ART, annual creatinine measurement is recommended by the WHO to monitor for nephrotoxicity. Simple POC creatinine assays have been developed, but performance in the field requires further evaluation (230). A paper-based test or RDT for measurement of alanine transaminase could offer more reliable access for monitoring of liver function (231, 232). As PLHIV are living longer due to better access to life-saving ART, management of noncommunicable diseases, such as diabetes and cardiovascular disease, may also benefit from an existing decentralized POC testing infrastructure, such as for lipids and hemoglobin A1c testing (233).
RESEARCH NEEDS AND PRIORITIES
POC HIV viral load testing may be an essential tool for accelerating declines in HIV/AIDS burden and incidence. However, since technologies and implementation are rapidly evolving, many clinical, translational, and basic science questions remain. In Table 4, we list some of the key research needs and priorities for each of the domains presented in this review. In general, these research questions fall into the domains of technology development, clinical testing, and implementation science.
TABLE 4.
Priority research questions for point-of-care HIV viral load testing
| Category | Questions |
|---|---|
| Technology development | Can a rapid test be valid and reliable when used on finger prick capillary blood? |
| Can the test processing time be reduced to <30 min? | |
| Can the device be made portable and rugged enough for use in community settings? | |
| Can the technology assist in communicating the result to the patients more efficiently? | |
| Can proxy measures, such as virion markers, provide adequate measures of viral load? | |
| Clinical testing | Are clinic personnel capable of running and interpreting rapid viral load tests? |
| How accurate are current POC HIV viral load devices when used in clinical settings? | |
| Can POC HIV viral load assays be efficiently integrated with other required tests? | |
| Does clinic-based HIV viral load monitoring improve patient outcomes? | |
| Implementation science | How do point-of-care viral load tests fit within models of differentiated HIV care? |
| What is the optimal interval for clinic-based HIV viral load testing? | |
| Is it possible to conduct viral load testing in decentralized locations for ART refills? | |
| Is the implementation of POC viral load testing cost-effective? |
Several technology development questions pertain to the future design of devices and potential use of easily obtainable finger prick capillary blood. Additional benefits could be achieved by reducing the processing time and developing a more durable system for use in community-based settings. Clinical research questions pertain to the accuracy of individual tests when used at the clinical point of care. Perhaps one of the most pressing research priorities is having a better understanding of the efficacy of clinic-based HIV viral load testing in terms of patient outcomes, and several clinical trials will provide results soon. Implementation science research questions pertain to how frequently HIV viral load testing should be performed and whether current or future devices may allow for further decentralization of testing. Finally, the cost-effectiveness of POC HIV viral load testing will need to be explored with empirical clinical trial data and mathematical modeling.
CONCLUSIONS
While the rapid expansion of access to ART remains the priority, access to HIV viral load testing will be critical for ensuring HIV treatment success, maintaining virological suppression, and ending the HIV/AIDS pandemic. Given the complexity and delays associated with laboratory-based HIV viral load testing, rapid clinic-based POC testing holds promise for facilitating HIV management and improving patient outcomes. In this review, we have highlighted the current knowledge of the technology for POC HIV viral load testing and offered opportunities and priorities for further exploration.
Through the development of better diagnostic tests and a greater understanding of the clinical role of POC HIV viral load testing, we may be able to devise more advanced and specific solutions to HIV/AIDS. By synthesizing the expanding knowledge and addressing existing research gaps of POC HIV viral load testing, the research and public health communities may move forward together on developing sustainable solutions to end the HIV/AIDS pandemic.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (AI136648, AI124719, and AI127200) and the National Institute of Biomedical Imaging and Bioengineering (EB022630) of the National Institutes of Health. A.B. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award no. TL1TR000422 as well as a National Science Foundation Graduate Research Fellowship.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Biographies

Paul K. Drain, M.D., M.P.H., is Assistant Professor in the Departments of Global Health, Medicine (Infectious Diseases), and Epidemiology, a practicing infectious disease physician, and Associate Director of the Tuberculosis Research and Training Center at the University of Washington in Seattle. His research group focuses on development, evaluation, and implementation of diagnostic testing and clinic-based screening, including novel point-of-care technologies, to improve clinical care and patient-centered outcomes for tuberculosis and HIV in resource-limited settings. He research has been supported by several institutes of the National Institutes of Health, the Infectious Diseases Society of America, Bill and Melinda Gates Foundation, Harvard Global Health Institute, the University of Washington’s and Harvard’s Center for AIDS Research, the AIDS Healthcare Foundation, and others. He completed his medicine training at Stanford University and his infectious disease training at Massachusetts General Hospital and Brigham and Women’s Hospital at Harvard Medical School in Boston.

Jienchi Dorward, B.Sc., M.B.Ch.B., M.Sc., is a primary care physician and a Wellcome Trust Clinical Ph.D. Fellow at the Nuffield Department of Primary Care Health Sciences in the University of Oxford. In his Ph.D. studies he is investigating point-of-care viral load testing to improve management of HIV in primary care clinics in South Africa. He is an Honorary Associate Scientist at the Centre for the AIDS Programme of Research in South Africa (CAPRISA), having previously worked there as a research clinician. He completed UK General Practice specialty training through clinical placements in London and rural KwaZulu-Natal South Africa. He has an M.Sc. in Public Health in Developing Countries at the London School of Hygiene & Tropical Medicine and aims to develop a career that combines academic and clinical work in international primary care and HIV.

Andrew Bender received a B.S. in mechanical engineering from Montana State University in 2015. He is currently completing his Ph.D. at the University of Washington under the supervision of Dr. Jonathan D. Posner. He was a graduate fellow at the Institute of Translational Health Sciences in Seattle. He is currently an NSF graduate research fellow. His research interests include microfluidics, electrokinetics, and developing point-of-care molecular tests for infectious diseases.

Lorraine Lillis is a Scientific Program officer in the diagnostics group at PATH, which she joined in 2011. Her research is focused on the development and evaluation of diagnostic tools for use in low-resource settings (LRS) and is primarily concerned with the applicability and use of isothermal amplification assays in such locations. She has been the lead author on a number of publications describing the development of a recombinase polymerase assay (RPA) for the detection of HIV-1. She received her B.Sc. and Ph.D. degrees from University College Dublin, Ireland.

Francesco Marinucci is the Head of the HCV and HIV unit at the Foundation for Innovative New Diagnostics. His major focus is on catalyzing the development of innovative products and accelerate their introduction in country programs. From 2007 to 2012, he worked at the Institute of Human Virology of the University of Maryland, where he directed the Global Laboratory Program. Under his leadership, the Institute supported 229 laboratories and trained hundreds of laboratory workers in the sub-Saharan African region, as well as in other PEPFAR countries in the Caribbean region. He earned a Ph.D. in Public Health and Microbiology from the University of Rome La Sapienza with a research study on the epidemiology and transmission patterns of HIV and other sexually transmitted diseases (STDs) among rural populations in sub-Saharan Africa. He also holds a postgraduate diploma in clinical biochemistry from the School of Medicine of the University of Rome Tor Vergata.

Jilian Sacks is the Senior Scientist on the Lab Services Team at the Clinton Health Access Initiative (CHAI), where she oversees research across HIV, hepatitis, TB and human papillomavirus (HPV) to facilitate access to affordable diagnostics and monitoring technologies in resource-limited settings. Dr. Sacks received her Ph.D. in immunology and has previously held positions with the Earth Institute at Columbia University and a joint fellowship at Rutgers University and the Africa Health Research Institute in Durban.

Anna Bershteyn leads the HIV and TB research center at the Institute for Disease Modeling and is an Affiliate Assistant Professor of Global Health at the University of Washington. She received a Ph.D. in materials science and engineering from the Massachusetts Institute of Technology (MIT), where she studied lipid self-assembly at nanoparticle surfaces as a biomimetic approach to vaccine development. Her modeling research focuses on HIV transmission dynamics and impact evaluation of biomedical and programmatic improvements to HIV care and prevention

David S. Boyle is a Scientific Director in the Diagnostics Program at PATH, a global health not-for-profit organization. He leads the tuberculosis and HIV portfolios and serves as a technical expert concerning the application of molecular assays for diagnostic solutions. His research experience and interests primarily focus on the application and development of nucleic acid amplification technologies to diagnose infectious diseases. His current projects involve assessing and developing nucleic acid amplification technologies for use in low-resource settings. His interests also involve the development of population and environmental surveillance tools, including multiplexed biomarker immunoassays that indicate micronutrient deficiency, malaria, and host seroconversion after vaccination. He identifies clinical needs and gaps in critical services and identifies potential partners and tools to expand the use of existing diagnostic technologies and assays to facilitate their development and introduction, with a specific aim to promote their continued use in low-resource settings.

Jonathan D. Posner, Ph.D., is an Associate Professor in the Departments of Mechanical and Chemical Engineering and an Adjunct Associate Professor in the Department of Family Medicine at the University of Washington in Seattle. Dr. Posner’s research program is focused on developing instrument free, point-of-care diagnostics that meet the clinical requirements for sensitivity and accuracy as well as clinicians’ needs for ease to use, accuracy, and cost, including both nucleic acid amplification and immunoassay-based diagnostics. The Posner lab has made significant contributions to novel sample preparation strategies for low-resource environments that allow complex samples to be adapted to assays that typically require many human handling steps.

Nigel Garrett, M.B.B.S. M.R.C.P. M.Sc., is a Specialist Physician in HIV and Sexual Health from the UK. He is the Head of HIV Pathogenesis and Vaccine Research at the Centre for the AIDS Programme of Research in South Africa (CAPRISA). As Principal Investigator (PI) and Co-Investigator, he has contributed to more than 20 randomized clinical trials and is the CAPRISA PI for the ongoing HIV Vaccine Trials Network (HVTN) trials, including the AMP study and Mosaic vaccine trials. Nigel has published widely in the field of HIV and sexual health, including on point-of-care diagnostics, viral load assays, HIV resistance, and antiretroviral agents. As part of his position at CAPRISA he has directed the CAPRISA 002 Acute HIV infection Study, a cohort study that has significantly contributed to our understanding of broadly neutralizing antibody evolution and HIV disease progression in South African women.
REFERENCES
- 1.UNAIDS. 2018. UNAIDS data 2018. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 3.The Antiretroviral Therapy Cohort Collaboration. 2017. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2018. U=U campaign, 2017. https://www.preventionaccess.org. Accessed 6 September 2018.
- 5.UNAIDS. 2014. 90-90-90. An ambitious treatment target to help end the AIDS epidemic. World Health Organization, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [Google Scholar]
- 6.Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Jouquet G, Kerschberger B, Mekeidje C, Cyr J, Mafikudze A, Han W, Lujan J, Teck R, Antierens A, van Griensven J, Reid T. 2014. Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 67:45–51. doi: 10.1097/QAI.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etoori D, Ciglenecki I, Ndlangamandla M, Edwards CG, Jobanputra K, Pasipamire M, Maphalala G, Yang C, Zabsonre I, Kabore SM, Goiri J, Teck R, Kerschberger B. 2018. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second-line switches in Swaziland. J Int AIDS Soc 21:e25194. doi: 10.1002/jia2.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MP, Pascoe SJ, Huber A, Murphy J, Phokojoe M, Gorgens M, Rosen S, Wilson D, Pillay Y, Fraser-Hurt N. 2018. Assessing the impact of the National Department of Health’s National Adherence Guidelines for Chronic Diseases in South Africa using routinely collected data: a cluster-randomised evaluation. BMJ Open 8:e019680. doi: 10.1136/bmjopen-2017-019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MP, Pascoe SJS, Huber AN, Murphy J, Phokojoe M, Gorgens M, Rosen S, Wilson D, Pillay Y, Fraser-Hurt N. 2018. Effectiveness of interventions for unstable patients on antiretroviral therapy in South Africa: results of a cluster-randomised evaluation. Trop Med Int Health 23:1314–1325. doi: 10.1111/tmi.13152. [DOI] [PubMed] [Google Scholar]
- 10.Hermans LE, Tempelman HA, Carmona S. 2018. High rates of viral resuppression on first-line ART after initial virological failure, abstr 1118. Conf Retroviruses Opportunistic Infect.
- 11.Hermans LE, Moorhouse M, Carmona S, Grobbee DE, Hofstra LM, Richman DD, Tempelman HA, Venter WDF, Wensing AMJ. 2018. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 18:188–197. doi: 10.1016/S1473-3099(17)30681-3. [DOI] [PubMed] [Google Scholar]
- 12.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV. 2014. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNITAID. 2015. HIV/AIDS diagnostics technology landscape, 5th ed World Health Organization, Geneva, Switzerland: http://www.unitaid.org/assets/UNITAID_HIV_Nov_2015_Dx_Landscape-1.pdf. [Google Scholar]
- 14.Niemz A, Ferguson TM, Boyle DS. 2011. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol 29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigl BH, Boyle DS, de los Santos T, Peck RB, Steele MS. 2009. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices 6:461–464. doi: 10.1586/erd.09.31. [DOI] [PubMed] [Google Scholar]
- 16.The International Diagnostics Centre, London School of Hygiene and Tropical Medicine. 5 November 2013. Target product profile: point-of-care HIV viral load test. http://www.idc-dx.org/resources/target-product-profile-point-of-care-hiv-viral-load-test. Accessed 6 August 2018.
- 17.Gous N, Boeras DI, Cheng B, Takle J, Cunningham B, Peeling RW. 2018. The impact of digital technologies on point-of-care diagnostics in resource-limited settings. Expert Rev Mol Diagn 18:385–397. doi: 10.1080/14737159.2018.1460205. [DOI] [PubMed] [Google Scholar]
- 18.Pai M, Ghiasi M, Pai NP. 2015. Point-of-care diagnostic testing in global health: what is the point? Microbe 10:103–107. doi: 10.1128/microbe.10.103.1. [DOI] [Google Scholar]
- 19.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G, Hay Burgess DC. 2006. Requirements for high impact diagnostics in the developing world. Nature 444:73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 20.Scott L, Gous N, Carmona S, Stevens W. 2015. Laboratory evaluation of the Liat HIV Quant (IQuum) whole-blood and plasma HIV-1 viral load assays for point-of-care testing in South Africa. J Clin Microbiol 53:1616–1621. doi: 10.1128/JCM.03325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanriverdi S, Chen L, Chen S. 2010. A rapid and automated sample-to-result HIV load test for near-patient application. J Infect Dis 201:S52–S58. doi: 10.1086/650387. [DOI] [PubMed] [Google Scholar]
- 22.Nair CB, Manjula J, Subramani PA, Nagendrappa PB, Manoj MN, Malpani S, Pullela PK, Subbarao PV, Ramamoorthy S, Ghosh SK. 2016. Differential diagnosis of malaria on Truelab Uno(R), a portable, real-time, microPCR device for point-of-care applications. PLoS One 11:e0146961. doi: 10.1371/journal.pone.0146961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikam C, Kazi M, Nair C, Jaggannath M, M M, R V, Shetty A, Rodrigues C. 2014. Evaluation of the Indian TrueNAT micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. Int J Mycobacteriol 3:205–210. doi: 10.1016/j.ijmyco.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Jani IV, Meggi B, Vubil A, Sitoe NE, Bhatt N, Tobaiwa O, Quevedo JI, Loquiha O, Lehe JD, Vojnov L, Peter TF. 2016. Evaluation of the whole-blood Alere Q NAT point-of-care RNA assay for HIV-1 viral load monitoring in a primary health care setting in Mozambique. J Clin Microbiol 54:2104–2108. doi: 10.1128/JCM.00362-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. 2016. WHO prequalification of in vitro diagnostics. Public report. Product: Alere™ q HIV-1/2 Detect. World Health Organization, Geneva, Switzerland: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicReport_0226-032-00AlereHIVDetect_v2.pdf. [Google Scholar]
- 26.Tunari A. 2018. m PIMA, a real PoC viral load platform suitable to monitoring HIV patients at any level of the health system. 22nd Int AIDS Conf, Amsterdam, The Netherlands.
- 27.World Health Organization. 2017. WHO prequalification of in vitro diagnostics. Public report. Product: Xpert HIV-1 Viral Load with GeneXpert Dx, GeneXpert Infinity48, GeneXpert Infinity-48s and GeneXpert Infinity-80. World Health Organization, Geneva, Switzerland: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/170720_final_pq_report_pqdx_0192_0193_0194_0195_070-00.pdf?ua=1. [Google Scholar]
- 28.Fidler S, Lewis H, Meyerowitz J, Kuldanek K, Thornhill J, Muir D, Bonnissent A, Timson G, Frater J. 2017. A pilot evaluation of whole blood finger-prick sampling for point-of-care HIV viral load measurement: the UNICORN study. Sci Rep 7:13658. doi: 10.1038/s41598-017-13287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai NP, Wilkinson S, Deli-Houssein R, Vijh R, Vadnais C, Behlim T, Steben M, Engel N, Wong T. 2015. Barriers to implementation of rapid and point-of-care tests for human immunodeficiency virus infection. Point Care 14:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drain PK, Garrett NJ. 2015. The arrival of a true point-of-care molecular assay—ready for global implementation? Lancet Glob Health 3:e663–e664. doi: 10.1016/S2214-109X(15)00186-2. [DOI] [PubMed] [Google Scholar]
- 31.Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. 2016. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 48:516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndlovu Z, Fajardo E, Mbofana E, Maparo T, Garone D, Metcalf C, Bygrave H, Kao K, Zinyowera S. 2018. Multidisease testing for HIV and TB using the GeneXpert platform: a feasibility study in rural Zimbabwe. PLoS One 13:e0193577. doi: 10.1371/journal.pone.0193577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FIND. 2011. Solar energy powers GeneXpert IV Dx system for detection of tuberculosis and rifampicin resistance in district/sub-district public health care settings in Uganda. https://www.ghdonline.org/uploads/Uganda-GX-solar_for-website_190420111-SOLAR_3.pdf.
- 34.Raizada N, Sachdeva KS, Sreenivas A, Vadera B, Gupta RS, Parmar M, Kulsange S, Babre A, Thakur R, Gray C, Ramachandran R, Alavadi U, Ghedia M, Vollepore B, Dewan P, Boehme C, Paramsivan CN. 2014. Feasibility of decentralized deployment of Xpert MTB/RIF test at lower level of health system in India. PloS One 9:e89301. doi: 10.1371/journal.pone.0089301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceffa S, Luhanga R, Andreotti M, Brambilla D, Erba F, Jere H, Mancinelli S, Giuliano M, Palombi L, Marazzi MC. 2016. Comparison of the Cepheid GeneXpert and Abbott M2000 HIV-1 real time molecular assays for monitoring HIV-1 viral load and detecting HIV-1 infection. J Virol Methods 229:35–39. doi: 10.1016/j.jviromet.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Gueudin M, Baron A, Alessandri-Gradt E, Lemee V, Mourez T, Etienne M, Plantier JC. 2016. Performance evaluation of the new HIV-1 quantification assay, Xpert HIV-1 viral load, on a wide panel of HIV-1 variants. J Acquir Immune Defic Syndr 72:521–526. doi: 10.1097/QAI.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 37.Kulkarni S, Jadhav S, Khopkar P, Sane S, Londhe R, Chimanpure V, Dhilpe V, Ghate M, Yelagate R, Panchal N, Rahane G, Kadam D, Gaikwad N, Rewari B, Gangakhedkar R. 2017. GeneXpert HIV-1 Quant assay, a new tool for scale up of viral load monitoring in the success of ART programme in India. BMC Infect Dis 17:506. doi: 10.1186/s12879-017-2604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moyo S, Mohammed T, Wirth KE, Prague M, Bennett K, Holme MP, Mupfumi L, Sebogodi P, Moraka NO, Boleo C, Maphorisa CN, Seraise B, Gaseitsiwe S, Musonda RM, van Widenfelt E, Powis KM, Gaolathe T, Tchetgen Tchetgen EJ, Makhema JM, Essex M, Lockman S, Novitsky V. 2016. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol 54:3050–3055. doi: 10.1128/JCM.01594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan JA, Plantier JC, Templeton K, Wu AHB. 2016. Multi-site clinical evaluation of the Xpert HIV-1 viral load assay. J Clin Virol 80:27–32. doi: 10.1016/j.jcv.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Swathirajan CR, Vignesh R, Boobalan J, Solomon SS, Saravanan S, Balakrishnan P. 2017. Performance of point-of-care xpert HIV-1 plasma viral load assay at a tertiary HIV care centre in Southern India. J Med Microbiol 66:1379–1382. doi: 10.1099/jmm.0.000514. [DOI] [PubMed] [Google Scholar]
- 41.Gous N, Scott L, Berrie L, Stevens W. 2016. Options to expand HIV viral load testing in South Africa: evaluation of the GeneXpert HIV-1 viral load assay. PLoS One 11:e0168244. doi: 10.1371/journal.pone.0168244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avidor B, Matus N, Girshengorn S, Achsanov S, Gielman S, Zeldis I, Schweitzer I, Adler A, Turner D. 2017. Comparison between Roche and Xpert in HIV-1 RNA quantitation: a high concordance between the two techniques except for a CRF02_AG subtype variant with high viral load titters detected by Roche but undetected by Xpert. J Clin Virol 93:15–19. doi: 10.1016/j.jcv.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Garrett NJ, Drain PK, Werner L, Samsunder N, Abdool Karim SS. 2016. Diagnostic accuracy of the point-of-care Xpert HIV-1 viral load assay in a South African HIV clinic. J Acquir Immune Defic Syndr 72:e45–e48. doi: 10.1097/QAI.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nash M, Ramapuram J, Kaiya R, Huddart S, Pai M, Baliga S. 2017. Use of the GeneXpert tuberculosis system for HIV viral load testing in India. Lancet Glob Health 5:e754–e755. doi: 10.1016/S2214-109X(17)30247-4. [DOI] [PubMed] [Google Scholar]
- 45.Mor O, Gozlan Y, Wax M, Mileguir F, Rakovsky A, Noy B, Mendelson E, Levy I. 2015. Evaluation of the RealTime HIV-1, Xpert HIV-1, and Aptima HIV-1 Quant Dx assays in comparison to the NucliSens EasyQ HIV-1 v2.0 assay for quantification of HIV-1 viral load. J Clin Microbiol 53:3458–3465. doi: 10.1128/JCM.01806-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruzzone B, Caligiuri P, Nigro N, Arcuri C, Delucis S, Di Biagio A, Icardi G. 2017. Xpert HIV-1 viral load assay and VERSANT HIV-1 RNA 1.5 assay. J Acquir Immune Defic Syndr 74:e86–e88. doi: 10.1097/QAI.0000000000001227. [DOI] [PubMed] [Google Scholar]
- 47.Theron G. 2017. Xpert Ultra and Xpert HIV-VIRAL LOAD in people living with HIV (UltraHIV). ClincalTrials.gov NCT03187964. https://clinicaltrials.gov/ct2/show/NCT03187964.
- 48.Kanki P. 2018. Reaching 90% HIV suppression: the role of POC viral load monitoring in Nigeria (POC). ClinicalTrials.gov NCT03533868. https://clinicaltrials.gov/ct2/show/NCT03533868.
- 49.Drain P. 2017. Point-of-care viral load testing to enable streamlined care and task shifting for chronic HIV care (STREAM). ClinicalTrials.gov NCT03066128. https://clinicaltrials.gov/ct2/show/NCT03066128.
- 50.Nash M, Huddart S, Badar S, Baliga S, Saravu K, M P. 2018. Performance of the Xpert HIV-1 viral load assay: a systematic review and meta-analysis. J Clin Microbiol 56:e01673-17. doi: 10.1128/JCM.01673-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reference deleted.
- 52.Compton J. 1991. Nucleic acid sequence-based amplification. Nature 350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 53.Dineva MA, Candotti D, Fletcher-Brown F, Allain JP, Lee H. 2005. Simultaneous visual detection of multiple viral amplicons by dipstick assay. J Clin Microbiol 43:4015–4021. doi: 10.1128/JCM.43.8.4015-4021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titchmarsh L, Zeh C, Verpoort T, Allain JP, Lee H. 2015. Leukodepletion as a point-of-care method for monitoring HIV-1 viral load in whole blood. J Clin Microbiol 53:1080–1086. doi: 10.1128/JCM.02853-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brook G. 28 June 2018. HIV viral load point-of-care testing: the what, the whys and the wherefores. Sex Transm Infect doi: 10.1136/sextrans-2018-053688. [DOI] [PubMed] [Google Scholar]
- 56.Goel N, Ritchie AV, Mtapuri-Zinyowera S, Zeh C, Stepchenkova T, Lehga J, De Ruiter A, Farleigh LE, Edemaga D, So R, Sembongi H, Wisniewski C, Nadala L, Schito M, Lee H. 2017. Performance of the SAMBA I and II HIV-1 Semi-Q tests for viral load monitoring at the point-of-care. J Virol Methods 244:39–45. doi: 10.1016/j.jviromet.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Ritchie AV, Ushiro-Lumb I, Edemaga D, Joshi HA, De Ruiter A, Szumilin E, Jendrulek I, McGuire M, Goel N, Sharma PI, Allain JP, Lee HH. 2014. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J Clin Microbiol 52:3377–3383. doi: 10.1128/JCM.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. 2011. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med 17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 59.Pavie J, Rachline A, Loze B, Niedbalski L, Delaugerre C, Laforgerie E, Plantier J-C, Rozenbaum W, Chevret S, Molina J-M, Simon F. 2010. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS One 5:e11581. doi: 10.1371/journal.pone.0011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kran A-MB, Jonassen TO, Sannes M, Jakobsen K, Lind A, Maeland A, Holberg-Petersen M. 2009. Overestimation of human immunodeficiency virus type 1 load caused by the presence of cells in plasma from plasma preparation tubes. J Clin Microbiol 47:2170–2174. doi: 10.1128/JCM.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monleau M, Montavon C, Laurent C, Segondy M, Montes B, Delaporte E, Boillot F, Peeters M. 2009. Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing. J Clin Microbiol 47:1107–1118. doi: 10.1128/JCM.02255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiscus SA, Brambilla D, Grosso L, Schock J, Cronin M. 1998. Quantitation of human immunodeficiency virus type 1 RNA in plasma by using blood dried on filter paper. J Clin Microbiol 36:258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X-B, Wu Z-Q, Wang K, Zhu J, Xu J-J, Xia X-H, Chen H-Y. 2012. Gravitational sedimentation induced blood delamination for continuous plasma separation on a microfluidics chip. Anal Chem 84:3780–3786. doi: 10.1021/ac3003616. [DOI] [PubMed] [Google Scholar]
- 64.Dimov IK, Basabe-Desmonts L, Garcia-Cordero JL, Ross BM, Park Y, Ricco AJ, Lee LP. 2011. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 11:845–850. doi: 10.1039/c0lc00403k. [DOI] [PubMed] [Google Scholar]
- 65.Yeh E-C, Fu C-C, Hu L, Thakur R, Feng J, Lee LP. 2017. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci Adv 3:e1501645. doi: 10.1126/sciadv.1501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nabatiyan A, Parpia ZA, Elghanian R, Kelso DM. 2011. Membrane-based plasma collection device for point-of-care diagnosis of HIV. J Virol Methods 173:37–42. doi: 10.1016/j.jviromet.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Homsy A, van der Wal PD, Doll W, Schaller R, Korsatko S, Ratzer M, Ellmerer M, Pieber TR, Nicol A, de Rooij NF. 2012. Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood. Biomicrofluidics 6:012804. doi: 10.1063/1.3672188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH. 2013. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal Chem 85:10463–10470. doi: 10.1021/ac402459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bender AT, Borysiak MD, Levenson AM, Lillis L, Boyle DS, Posner JD. 2018. Semiquantitative nucleic acid test with simultaneous isotachophoretic extraction and amplification. Anal Chem 90:7221–7229. doi: 10.1021/acs.analchem.8b00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hin S, Loskyll M, Klein V, Keller M, Strohmeier O, von Stetten F, Zengerle R, Mitsakakis K. 2018. Membrane-based sample inlet for centrifugal microfluidic cartridges. Microelectron Eng 187:78–83. doi: 10.1016/j.mee.2017.12.006. [DOI] [Google Scholar]
- 71.Singh RP. 1999. A solvent-free, rapid and simple virus RNA-release method for potato leafroll virus detection in aphids and plants by reverse transcription polymerase chain reaction. J Virol Methods 83:27–33. doi: 10.1016/S0166-0934(99)00102-0. [DOI] [PubMed] [Google Scholar]
- 72.Singh RP, Dilworth AD, Singh M, McLaren DL. 2004. Evaluation of a simple membrane-based nucleic acid preparation protocol for RT-PCR detection of potato viruses from aphid and plant tissues. J Virol Methods 121:163–170. doi: 10.1016/j.jviromet.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Puren A, Gerlach JL, Weigl BH, Kelso DM, Domingo GJ. 2010. Laboratory operations, specimen processing, and handling for viral load testing and surveillance. J Infect Dis 201:S27–S36. doi: 10.1086/650390. [DOI] [PubMed] [Google Scholar]
- 74.Luft LM, Gill MJ, Church DL. 2011. HIV-1 viral diversity and its implications for viral load testing: review of current platforms. Int J Infect Dis 15:e661–e670. doi: 10.1016/j.ijid.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. 1990. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X, Cui D-F, Liu C-C, Li H. 2007. Microfabrication and characterization of porous channels for DNA purification. J Micromech Microeng 17:68–75. doi: 10.1088/0960-1317/17/1/009. [DOI] [Google Scholar]
- 77.Linnes JC, Fan A, Rodriguez NM, Lemieux B, Kong H, Klapperich CM. 2014. Paper-based molecular diagnostic for Chlamydia trachomatis. RSC Adv 4:42245–42251. doi: 10.1039/C4RA07911F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez NM, Wong WS, Liu L, Dewar R, Klapperich CM. 2016. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 16:753–763. doi: 10.1039/c5lc01392e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byrnes SA, Bishop JD, Lafleur L, Buser JR, Lutz B, Yager P. 2015. One-step purification and concentration of DNA in porous membranes for point-of-care applications. Lab Chip 15:2647–2659. doi: 10.1039/c5lc00317b. [DOI] [PubMed] [Google Scholar]
- 80.Persat A, Marshall LA, Santiago JG. 2009. Purification of nucleic acids from whole blood using isotachophoresis. Anal Chem 81:9507–9511. doi: 10.1021/ac901965v. [DOI] [PubMed] [Google Scholar]
- 81.Marshall LA, Rogacs A, Meinhart C, Santiago JG. 2014. An injection-molded device for purification of nucleic acids from whole blood using isotachophoresis. J Chromatogr A 1331:139–142. doi: 10.1016/j.chroma.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 82.Sur K, McFall SM, Yeh ET, Jangam SR, Hayden MA, Stroupe SD, Kelso DM. 2010. Immiscible phase nucleic acid purification eliminates PCR inhibitors with a single pass of paramagnetic particles through a hydrophobic liquid. J Mol Diagn 12:620–628. doi: 10.2353/jmoldx.2010.090190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casavant BP, Guckenberger DJ, Beebe DJ, Berry SM. 2014. Efficient sample preparation from complex biological samples using a sliding lid for immobilized droplet extractions. Anal Chem 86:6355–6362. doi: 10.1021/ac500574t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curtis KA, Rudolph DL, Nejad I, Singleton J, Beddoe A, Weigl B, LaBarre P, Owen SM. 2012. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS One 7:e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ocwieja KE, Sherrill-Mix S, Liu C, Song J, Bau H, Bushman FD. 2015. A reverse transcription loop-mediated isothermal amplification assay optimized to detect multiple HIV subtypes. PLoS One 10:e0117852. doi: 10.1371/journal.pone.0117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang W, Chow WHA, Li Y, Kong H, Tang Y, Lemieux B. 2010. Nucleic acid assay system for tier II laboratories and moderately complex clinics to detect HIV in low‐resource settings. J Infect Dis 201:S46–S51. doi: 10.1086/650388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lillis L, Lehman DA, Siverson JB, Weis J, Cantera J, Parker M, Piepenburg O, Overbaugh J, Boyle DS. 2016. Cross-subtype detection of HIV-1 using reverse transcription and recombinase polymerase amplification. J Virol Methods 230:28–35. doi: 10.1016/j.jviromet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crannell ZA, Rohrman B, Richards-Kortum R. 2014. Quantification of HIV-1 DNA using real-time recombinase polymerase amplification. Anal Chem 86:5615–5619. doi: 10.1021/ac5011298. [DOI] [PubMed] [Google Scholar]
- 89.van Gemen B, van Beuningen R, Nabbe A, van Strijp D, Jurriaans S, Lens P, Kievits T. 1994. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labelled probes. J Virol Methods 49:157–167. doi: 10.1016/0166-0934(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 90.Gurrala R, Lang Z, Shepherd L, Davidson D, Harrison E, McClure M, Kaye S, Toumazou C, Cooke GS. 2016. Novel pH sensing semiconductor for point-of-care detection of HIV-1 viremia. Sci Rep 6:36000. doi: 10.1038/srep36000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lecossier D, Bouchonnet F, Clavel F, Hance AJ. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. doi: 10.1126/science.1083338. [DOI] [PubMed] [Google Scholar]
- 92.Wang S, Xu F, Demirci U. 2010. Advances in developing HIV-1 viral load assays for resource-limited settings. Biotechnol Adv 28:770–781. doi: 10.1016/j.biotechadv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pas S, Rossen JW, Schoener D, Thamke D, Pettersson A, Babiel R, Schutten M. 2010. Performance evaluation of the new Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test version 2.0 for quantification of human immunodeficiency virus type 1 RNA. J Clin Microbiol 48:1195–1200. doi: 10.1128/JCM.01832-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swanson P, de Mendoza C, Joshi Y, Golden A, Hodinka RL, Soriano V, Devare SG, Hackett J Jr. 2005. Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J Clin Microbiol 43:3860–3868. doi: 10.1128/JCM.43.8.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malmsten A, Shao X-W, Aperia K, Corrigan GE, Sandström E, Källander CFR, Leitner T, Gronowitz JS. 2003. HIV‐1 viral load determination based on reverse transcriptase activity recovered from human plasma. J Med Virol 71:347–359. doi: 10.1002/jmv.10492. [DOI] [PubMed] [Google Scholar]
- 96.Shafiee H, Lidstone EA, Jahangir M, Inci F, Hanhauser E, Henrich TJ, Kuritzkes DR, Cunningham BT, Demirci U. 2014. Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Sci Rep 4:4116. doi: 10.1038/srep04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kloke A, Fiebach AR, Zhang S, Drechsel L, Niekrawietz S, Hoehl MM, Kneusel R, Panthel K, Steigert J, von Stetten F, Zengerle R, Paust N. 2014. The LabTube—a novel microfluidic platform for assay automation in laboratory centrifuges. Lab Chip 14:1527–1537. doi: 10.1039/c3lc51261d. [DOI] [PubMed] [Google Scholar]
- 98.Connelly JT, Rolland JP, Whitesides GM. 2015. “Paper machine” for molecular diagnostics. Anal Chem 87:7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 99.Lafleur LK, Bishop JD, Heiniger EK, Gallagher RP, Wheeler MD, Kauffman P, Zhang X, Kline EC, Buser JR, Kumar S, Byrnes SA, Vermeulen NMJ, Scarr NK, Belousov Y, Mahoney W, Toley BJ, Ladd PD, Lutz BR, Yager P. 2016. A rapid, instrument-free, sample-to-result nucleic acid amplification test. Lab Chip 16:3777–3787. doi: 10.1039/c6lc00677a. [DOI] [PubMed] [Google Scholar]
- 100.Liao S-C, Peng J, Mauk MG, Awasthi S, Song J, Friedman H, Bau HH, Liu C. 2016. Smart cup: a minimally-instrumented, smartphone-based point-of-care molecular diagnostic device. Sens Actuators B Chem 229:232–238. doi: 10.1016/j.snb.2016.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rustagi AS, Gimbel S, Nduati R, Cuembelo MF, Wasserheit JN, Farquhar C, Gloyd S, Sherr K. 2017. Health facility factors and quality of services to prevent mother-to-child HIV transmission in Côte d’Ivoire, Kenya, and Mozambique. Int J STD AIDS 28:788–799. doi: 10.1177/0956462416668766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barker C, Dutta A, Klein K. 2017. Can differentiated care models solve the crisis in HIV treatment financing? Analysis of prospects for 38 countries in sub-Saharan Africa. J Int AIDS Soc 20:21648. doi: 10.7448/IAS.20.5.21648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pham MD, Romero L, Parnell B, Anderson DA, Crowe SM, Luchters S. 2017. Feasibility of antiretroviral treatment monitoring in the era of decentralized HIV care: a systematic review. AIDS Res Ther 14:3. doi: 10.1186/s12981-017-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lecher S, Ellenberger D, Kim AA, Fonjungo PN, Agolory S, Borget MY, Broyles L, Carmona S, Chipungu G, De Cock KM, Deyde V, Downer M, Gupta S, Kaplan JE, Kiyaga C, Knight N, MacLeod W, Makumbi B, Muttai H, Mwangi C, Mwangi JW, Mwasekaga M, Ng'Ang'A LW, Pillay Y, Sarr A, Sawadogo S, Singer D, Stevens W, Toure CA, Nkengasong JN. 2015. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR Morb Mortal Wkly Rep 64:1287–1290. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 105.Engel N, Davids M, Blankvoort N, Dheda K, Pant Pai N, Pai M. 2017. Making HIV testing work at the point of care in South Africa: a qualitative study of diagnostic practices. BMC Health Serv Res 17:408. doi: 10.1186/s12913-017-2353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engel N, Yellappa V, Pai NP, Pai M. 2017. Diagnosing at point of care in South India: coordination work and frictions. Sci Technol Stud 30:54–72. doi: 10.23987/sts.63085. [DOI] [Google Scholar]
- 107.Stime KJ, Garrett N, Sookrajh Y, Dorward J, Dlamini N, Olowolagba A, Sharma M, Barnabas RV, Drain PK. 2018. Clinic flow for STI, HIV, and TB patients in an urban infectious disease clinic offering point-of-care testing services in Durban, South Africa. BMC Health Serv Res 18:363. doi: 10.1186/s12913-018-3154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mashamba-Thompson TP, Sartorius B, Drain PK. 2018. Operational assessment of point-of-care diagnostics in rural primary healthcare clinics of KwaZulu-Natal, South Africa: a cross-sectional survey. BMC Health Serv Res 18:380. doi: 10.1186/s12913-018-3207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murray CJL. 2015. Maximizing antiretroviral therapy in developing countries: the dual challenge of efficiency and quality. JAMA 313:359–360. doi: 10.1001/jama.2014.16376. [DOI] [PubMed] [Google Scholar]
- 110.Rutstein SE, Golin C, Wheeler SB, Kamwendo D, Hosseinipour MC, Weinberger M, Miller WC, Biddle AK, Soko A, Mkandawire M, Mwenda R, Sarr A, Gupta S, Mataya R. 2016. On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care 28:1–10. doi: 10.1080/09540121.2015.1058896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiao S, Zhang Y, Li X, Menon JA. 2018. Facilitators and barriers for HIV-testing in Zambia: a systematic review of multi-level factors. PLoS One 13:e0192327. doi: 10.1371/journal.pone.0192327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Callaghan M, Ford N, Schneider H. 2010. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health 8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chan AK, Ford D, Namata H, Muzambi M, Nkhata MJ, Abongomera G, Mambule I, South A, Revill P, Grundy C, Mabugu T, Chiwaula L, Cataldo F, Hakim J, Seeley J, Kityo C, Reid A, Katabira E, Sodhi S, Gilks CF, Gibb DM. 2014. The Lablite project: a cross-sectional mapping survey of decentralized HIV service provision in Malawi, Uganda and Zimbabwe. BMC Health Serv Res 14:352. doi: 10.1186/1472-6963-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mwangala S, Moland KM, Nkamba HC, Musonda KG, Monze M, Musukwa KK, Fylkesnes K. 2015. Task-shifting and quality of HIV testing services: experiences from a national reference hospital in Zambia. PLoS One 10:e0143075. doi: 10.1371/journal.pone.0143075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wongkanya R, Pankam T, Wolf S, Pattanachaiwit S, Jantarapakde J, Pengnongyang S, Thapwong P, Udomjirasirichot A, Churattanakraisri Y, Prawepray N, Paksornsit A, Sitthipau T, Petchaithong S, Jitsakulchaidejt R, Nookhai S, Lertpiriyasuwat C, Ongwandee S, Phanuphak P, Phanuphak N. 2018. HIV rapid diagnostic testing by lay providers in a key population-led health service programme in Thailand. J Virus Erad 4:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy CE, Yeh PT, Johnson C, Baggaley R. 2017. Should trained lay providers perform HIV testing? A systematic review to inform World Health Organization guidelines. AIDS Care 29:1473–1479. doi: 10.1080/09540121.2017.1317710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peter T, Zeh C, Katz Z, Elbireer A, Alemayehu B, Vojnov L, Costa A, Doi N, Jani I. 2017. Scaling up HIV viral load—lessons from the large-scale implementation of HIV early infant diagnosis and CD4 testing. J Int AIDS Soc 20:e25008. doi: 10.1002/jia2.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malonza IM, Richardson BA, Kreiss JK, Bwayo JJ, Stewart GCJ. 2003. The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial. AIDS 17:113–118. doi: 10.1097/01.aids.0000042934.55529.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. 2011. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 120.Gous NM, Scott LE, Potgieter J, Ntabeni L, Sanne I, Stevens W. 2016. Implementation and operational research: implementation of multiple point-of-care testing in 2 HIV antiretroviral treatment clinics in South Africa. J Acquir Immune Defic Syndr 71:e34–e43. doi: 10.1097/QAI.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 121.Holmes CB, Sanne I. 2015. Changing models of care to improve progression through the HIV treatment cascade in different populations. Curr Opin HIV AIDS 10:447–450. doi: 10.1097/COH.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 122.Technau K-G, Kuhn L, Coovadia A, Murnane PM, Sherman G. 2017. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. Lancet HIV 4:e442–e448. doi: 10.1016/S2352-3018(17)30097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jani IV, Meggi B, Loquiha O, Tobaiwa O, Mudenyanga C, Zitha A, Mutsaka D, Mabunda N, Vubil A, Bollinger T, Vojnov L, Peter TF. 2018. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients: a cluster-randomised trial. AIDS 32:1453–1463. doi: 10.1097/QAD.0000000000001846. [DOI] [PubMed] [Google Scholar]
- 124.Mwenda R, Fong Y, Magombo T, Saka E, Midiani D, Mwase C, Kandulu J, Wang M, Thomas R, Sherman J, Vojnov L. 2018. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis 67:701–707. doi: 10.1093/cid/ciy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bianchi F, Machekano R, Lemaire J.2018. Diagnosing and treating more infants, faster: Findings from the first multi-country evaluation of routine point-of-care early infant diagnosis in eight sub-Saharan countries. 22nd Int AIDS Conf, Amsterdam, The Netherlands.
- 126.Meulbroek M, Pujol F, Pérez F, Dalmau-Bueno A, Taboada H, Marazzi G, Carrillo A, Cabas A, Gata A, Aldabó E, Roldán B, Coll P, Añez F, Pantaleón J, Mochales M, Gómez V, Marín O, Mir JF, Decoca J, Saz J. 2018. BCN Checkpoint: same‐day confirmation of reactive HIV rapid test with point of care HIV‐RNA accelerates linkage to care and reduces anxiety. HIV Med 19(Suppl 1):63–65. doi: 10.1111/hiv.12595. [DOI] [PubMed] [Google Scholar]
- 127.UNICEF. 2018. Key considerations for introducing new HIV point-of-care diagnostic technologies in national health systems. UNICEF, New York NY: http://childrenandaids.org/sites/default/files/poc-toolkit/KCD_draft_English_High-Res-.pdf. [Google Scholar]
- 128.World Health Organization. 2017. Considerations for adoption and use of multidisease testing devices in integrated laboratory networks. http://www.who.int/tb/publications/2017/considerations_multidisease_testing_devices_2017/en/.
- 129.Garrett NJ, Osman F, Maharaj B, Naicker N, Gibbs A, Norman E, Samsunder N, Ngobese H, Mitchev N, Singh R, Abdool Karim SS, Kharsany ABM, Mlisana K, Rompalo A, Mindel A. 2018. Beyond syndromic management: opportunities for diagnosis-based treatment of sexually transmitted infections in low- and middle-income countries. PLoS One 13:e0196209. doi: 10.1371/journal.pone.0196209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rutstein SE, Hosseinipour MC, Kamwendo D, Soko A, Mkandawire M, Biddle AK, Miller WC, Weinberger M, Wheeler SB, Sarr A, Gupta S, Chimbwandira F, Mwenda R, Kamiza S, Hoffman I, Mataya R. 2015. Dried blood spots for viral load monitoring in Malawi: feasible and effective. PLoS One 10:e0124748. doi: 10.1371/journal.pone.0124748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smit PW, Sollis KA, Fiscus S, Ford N, Vitoria M, Essajee S, Barnett D, Cheng B, Crowe SM, Denny T, Landay A, Stevens W, Habiyambere V, Perriens JH, Peeling RW. 2014. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS One 9:e86461. doi: 10.1371/journal.pone.0086461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kebede Y, Fonjungo PN, Tibesso G, Shrivastava R, Nkengasong JN, Kenyon T, Kebede A, Gadde R, Ayana G. 2016. Improved specimen-referral system and increased access to quality laboratory services in Ethiopia: the role of the public-private partnership. J Infect Dis 213:S59–S64. doi: 10.1093/infdis/jiv576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, Bonner K, Rousseau C, Garnett G, Cambiano V, Nakagawa F, Ford D, Bansi-Matharu L, Miners A, Lundgren JD, Eaton JW, Parkes-Ratanshi R, Katz Z, Maman D, Ford N, Vitoria M, Doherty M, Dowdy D, Nichols B, Murtagh M, Wareham M, Palamountain KM, Chakanyuka Musanhu C, Stevens W, Katzenstein D, Ciaranello A, Barnabas R, Braithwaite RS, Bendavid E, Nathoo KJ, van de Vijver D, Wilson DP, Holmes C, Bershteyn A, Walker S, Raizes E, Jani I, Nelson LJ, Peeling R, Terris-Prestholt F, Murungu J, Mutasa-Apollo T, Hallett TB, Revill P. 2015. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 528:S68–S76. doi: 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dorward J, Drain PK, Garrett N. 2018. Point-of-care viral load testing and differentiated HIV care. Lancet HIV 5:e8–e9. doi: 10.1016/S2352-3018(17)30211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mwau M, Syeunda CA, Adhiambo M, Bwana P, Kithinji L, Mwende J, Oyiengo L, Sirengo M, Boeke CE. 2018. Scale-up of Kenya’s national HIV viral load program: findings and lessons learned. PLoS One 13:e0190659. doi: 10.1371/journal.pone.0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, Toure CA, Agolory S, Appiah-Pippim G, Beard S, Borget MY, Carmona S, Chipungu G, Diallo K, Downer M, Edgil D, Haberman H, Hurlston M, Jadzak S, Kiyaga C, MacLeod W, Makumb B, Muttai H, Mwangi C, Mwangi JW, Mwasekaga M, Naluguza M, Ng'Ang'A LW, Nguyen S, Sawadogo S, Sleeman K, Stevens W, Kuritsky J, Hader S, Nkengasong J. 2016. Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep 65:1332–1335. doi: 10.15585/mmwr.mm6547a2. [DOI] [PubMed] [Google Scholar]
- 137.Boillot F, Serrano L, Muwonga J, Kabuayi JP, Kambale A, Mutaka F, Fujiwara PI, Decosas J, Peeters M, Delaporte E. 2016. Implementation and operational research: programmatic feasibility of dried blood spots for the virological follow-up of patients on antiretroviral treatment in Nord Kivu, Democratic Republic of the Congo. J Acquir Immune Defic Syndr 71:e9–e15. doi: 10.1097/QAI.0000000000000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouattara EN, Robine M, Eholié SP, MacLean RL, Moh R, Losina E, Gabillard D, Paltiel AD, Danel C, Walensky RP, Anglaret X, Freedberg KA. 2016. Laboratory monitoring of antiretroviral therapy for HIV infection: cost-effectiveness and budget impact of current and novel strategies. Clin Infect Dis 62:1454–1462. doi: 10.1093/cid/ciw117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Craik A, Patel P, Patel P, Mallewa J, Malisita K, Bitilinyu-Bangoh J, van Oosterhout JJ, Kelly C. 2017. Challenges with targeted viral load testing for medical inpatients at Queen Elizabeth Central Hospital in Blantyre, Malawi. Mal Med J 28:179–181. doi: 10.4314/mmj.v28i4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Habiyambere V, Ford N, Low-Beer D, Nkengasong JN, Sands A, Pérez González M, Fernandes P, Milgotina E. 2016. Availability and use of HIV monitoring and early infant diagnosis technologies in WHO member states in 2011–2013: analysis of annual surveys at the facility level. PLoS Med 13:e1002088. doi: 10.1371/journal.pmed.1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kilmarx PH, Simbi R. 2016. Progress and challenges in scaling up laboratory monitoring of HIV treatment. PLoS Med 13:e1002089. doi: 10.1371/journal.pmed.1002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grimsrud A, Barnabas RV, Ehrenkranz P, Ford N. 2017. Evidence for scale up: the differentiated care research agenda. J Int AIDS Soc 20:22024. doi: 10.7448/IAS.20.5.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fonjungo PN, Osmanov S, Kuritsky J, Ndihokubwayo JB, Bachanas P, Peeling RW, Timperi R, Fine G, Stevens W, Habiyambere V, Nkengasong JN. 2016. Ensuring quality: a key consideration in scaling-up HIV-related point-of-care testing programs. AIDS 30:1317–1323. doi: 10.1097/QAD.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fonjungo PN, Boeras DI, Zeh C, Alexander H, Parekh BS, Nkengasong JN. 2016. Access and quality of HIV-related point-of-care diagnostic testing in global health programs. Clin Infect Dis 62:369–374. doi: 10.1093/cid/civ866. [DOI] [PubMed] [Google Scholar]
- 145.Rugera SP, McNerney R, Poon AK, Akimana G, Mariki RF, Kajumbula H, Kamau E, Mpawenimana S, Said SY, Toroitich A, Ronoh W, Sollis KA, Sonoiya S, Peeling RW. 2014. Regulation of medical diagnostics and medical devices in the East African community partner states. BMC Health Serv Res 14:524. doi: 10.1186/s12913-014-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McNerney R, Peeling RW. 2015. Regulatory in vitro diagnostics landscape in Africa: update on regional activities. Clin Infect Dis 61:S135–S140. doi: 10.1093/cid/civ553. [DOI] [PubMed] [Google Scholar]
- 147.Boeras DI, Peeling RW. 2016. External quality assurance for HIV point-of-care testing in Africa: a collaborative country-partner approach to strengthen diagnostic services. Afr J Lab Med 5:556. doi: 10.4102/ajlm.v5i2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyers AFA, Sandstrom P, Denny TN, Hurlston M, Ball TB, Peeling RW, Boeras DI. 2016. Quality assurance for HIV point-of-care testing and treatment monitoring assays. Afr J Lab Med 5:557. doi: 10.4102/ajlm.v5i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng B, Cunningham B, Boeras DI, Mafaune P, Simbi R, Peeling RW. 2016. Data connectivity: a critical tool for external quality assessment. Afr J Lab Med 5:535. doi: 10.4102/ajlm.v5i2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Minior T, Douglas M, Edgil D, Srivastava M, Crowley J, Firth J, Lapidos-Salaiz I, Williams J, Lee L. 2017. The critical role of supply chains in preventing human immunodeficiency virus drug resistance in low- and middle-income settings. J Infect Dis 216:S812–S815. doi: 10.1093/infdis/jix403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stevens W, Marshall T. 2010. Challenges in implementing viral load testing in South Africa. J Infect Dis 201:S78–S84. doi: 10.1086/650383. [DOI] [PubMed] [Google Scholar]
- 152.Roberts T, Cohn J, Bonner K, Hargreaves S. 2016. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 62:1043–1048. doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Minchella PA, Chipungu G, Kim AA, Sarr A, Ali H, Mwenda R, Nkengasong JN, Singer D. 2017. Specimen origin, type and testing laboratory are linked to longer turnaround times for HIV viral load testing in Malawi. PLoS One 12:e0173009. doi: 10.1371/journal.pone.0173009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, Cornell M, Heiberg C, Ingram C, Panchia R, Rassool M, Gonin R, Stevens W, Truter H, Dehlinger M, van der Horst C, McIntyre J, Wood R. 2010. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet 376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sherr K, Pfeiffer J, Mussa A, Vio F, Gimbel S, Micek M, Gloyd S. 2009. The role of nonphysician clinicians in the rapid expansion of HIV care in Mozambique. J Acquir Immune Defic Syndr 52:S20–S23. doi: 10.1097/QAI.0b013e3181bbc9c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.World Health Organization. 2008. Task shifting: rational redistribution of tasks among health workforce teams: global recommendations and guidelines. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 157.Farmer P, Léandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. 2001. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy). Bull World Health Organ 79:1145–1151. [PMC free article] [PubMed] [Google Scholar]
- 158.World Health Organization. 2004. Integrated management of adolescent and adult illness. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 159.Kredo T, Ford N, Adeniyi FB, Garner P. 2013. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev 2013:CD009987. doi: 10.1002/14651858.CD009987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suthar AB, Rutherford GW, Horvath T, Doherty MC, Negussie EK. 2014. Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS 28:S175–S185. doi: 10.1097/QAD.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 161.Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. 2014. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. Cochrane Database Syst Rev 2014:CD007331. doi: 10.1002/14651858.CD007331.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, Ford N, Killingo B, Mabote L, Mansell T, Reinisch A, Zulu I, Bekker L. 2016. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 19:21484. doi: 10.7448/IAS.19.1.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wilkinson L, Harley B, Sharp J, Solomon S, Jacobs S, Cragg C, Kriel E, Peton N, Jennings K, Grimsrud A. 2016. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Trop Med Int Health 21:743–749. doi: 10.1111/tmi.12699. [DOI] [PubMed] [Google Scholar]
- 164.Vogt F, Kalenga L, Lukela J, Salumu F, Diallo I, Nico E, Lampart E, Van den Bergh R, Shah S, Ogundahunsi O, Zachariah R, Van Griensven J. 2017. Dentralizing ART supply for stable HIV patients to community based distribution centers: program outcomes from an urban context in Kinshasa, DRC. J Acquir Immune Defic Syndr 74:326–331. doi: 10.1097/QAI.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prust ML, Banda CK, Nyirenda R, Chimbwandira F, Kalua T, Jahn A, Eliya M, Callahan K, Ehrenkranz P, Prescott MR, McCarthy EA, Tagar E, Gunda A. 2017. Multi-month prescriptions, fast-track refills, and community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. J Int AIDS Soc 20:21650. doi: 10.7448/IAS.20.5.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. 2013. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health 5:169–179. doi: 10.1093/inthealth/iht016. [DOI] [PubMed] [Google Scholar]
- 167.Mkhinze D. 15 March 2018. ATM pharmacy to cut queues for South Africa's AIDS patients. Reuters Health News. Accessed 12 April 2019 https://www.reuters.com/article/us-safrica-aids-arv-atm/atm-pharmacy-to-cut-queues-for-south-africas-aids-patients-idUSKCN1GR2CO.
- 168.Revill P, Walker S, Cambiano V, Phillips A, Sculpher MJ. 2018. Reflecting the real value of health care resources in modelling and cost-effectiveness studies—the example of viral load informed differentiated care. PLoS One 13:e0190283. doi: 10.1371/journal.pone.0190283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.World Health Organization. 2015. Consolidated guidelines on HIV testing services. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 170.Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, Ajose O, Fakoya AO, Granich RM, Negussie EK, Baggaley RC. 2013. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med 10:e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharma M, Ying R, Tarr G, Barnabas R. 2015. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 528:S77–S85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, Lavoy G, Kwarisiima D, Sang N, Jain V, Thirumurthy H, Liegler T, Balzer LB, Petersen ML, Cohen CR, Bukusi EA, Kamya MR, Havlir DV, Charlebois ED. 2016. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV 3:e111–e119. doi: 10.1016/S2352-3018(15)00251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jaya Z, Drain PK, Mashamba-Thompson TP. 2017. Evaluating quality management systems for HIV rapid testing services in primary healthcare clinics in rural KwaZulu-Natal, South Africa. PLoS One 12:e0183044. doi: 10.1371/journal.pone.0183044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kufa T, Kharsany AB, Cawood C, Khanyile D, Lewis L, Grobler A, Chipeta Z, Bere A, Glenshaw M, Puren A. 2017. Misdiagnosis of HIV infection during a South African community-based survey: implications for rapid HIV testing. J Int AIDS Soc 20:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scott LE, Campbell J, Westerman L, Kestens L, Vojnov L, Kohastsu L, Nkengasong J, Peter T, Stevens W. 2015. A meta-analysis of the performance of the PimaTM CD4 for point of care testing. BMC Med 13:168. doi: 10.1186/s12916-015-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pham MD, Agius PA, Romero L, McGlynn P, Anderson D, Crowe SM, Luchters S. 2016. Performance of point-of-care CD4 testing technologies in resource-constrained settings: a systematic review and meta-analysis. BMC Infect Dis 16:592. doi: 10.1186/s12879-016-1931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, Lehe JD, Peter TF. 2011. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS 25:807–812. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 178.Gous N, Scott L, Potgieter J, Ntabeni L, Enslin S, Newman R, Stevens W. 2013. Feasibility of performing multiple point of care testing for HIV anti-retroviral treatment initiation and monitoring from multiple or single fingersticks. PLoS One 8:e85265. doi: 10.1371/journal.pone.0085265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. 2014. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc 17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vojnov L, Markby J, Boeke C, Harris L, Ford N, Peter T. 2016. POC CD4 testing improves linkage to HIV care and timeliness of ART initiation in a public health approach: a systematic review and meta-analysis. PLoS One 11:e0155256. doi: 10.1371/journal.pone.0155256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Desai MA, Okal DO, Rose CE, Ndivo R, Oyaro B, Otieno FO, Williams T, Chen RT, Zeh C, Samandari T. 2017. Effect of point-of-care CD4 cell count results on linkage to care and antiretroviral initiation during a home-based HIV testing campaign: a non-blinded, cluster-randomised trial. Lancet HIV 4:e393–e401. doi: 10.1016/S2352-3018(17)30091-7. [DOI] [PubMed] [Google Scholar]
- 182.Stevens WS, Gous NM, MacLeod WB, Long LC, Variava E, Martinson NA, Sanne I, Osih R, Scott LE. 2017. Multidisciplinary point-of-care testing in South African primary health care clinics accelerates HIV ART initiation but does not alter retention in care. J Acquir Immune Defic Syndr 76:65–73. doi: 10.1097/QAI.0000000000001456. [DOI] [PubMed] [Google Scholar]
- 183.Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, Sanne I, Bokaba D, Sauls C, Rohr J, Long L. 2016. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med 13:e1002015. doi: 10.1371/journal.pmed.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peeling RW, Ford N. 2017. Reprising the role of CD4 cell count in HIV programmes. Lancet HIV 4:e377–e378. doi: 10.1016/S2352-3018(17)30096-6. [DOI] [PubMed] [Google Scholar]
- 185.Perriat D, Balzer L, Hayes R, Lockman S, Walsh F, Ayles H, Floyd S, Haviral Loadir D, Kamya M, Lebelonyane R, Mills LA, Okello V, Petersen M, Pillay D, Sabapathy K, Wirth K, Orne-Gliemann J, Dabis F. 2018. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc 21:e25048. doi: 10.1002/jia2.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Labhardt ND, Ringera I, Lejone TI, Klimkait T, Muhairwe J, Amstutz A, Glass TR. 2018. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho. JAMA 319:1103. doi: 10.1001/jama.2018.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.International AIDS Society. 2016. Differentiated care for HIV: a decision framework for antiretroviral therapy delivery. International AIDS Society, Durban, South Africa. [Google Scholar]
- 188.Dorward J, Garrett N, Quame-Amaglo J, Samsunder N, Ngobese H, Ngomane N, Moodley P, Mlisana K, Schaafsma T, Donnell D, Barnabas R, Naidoo K, Abdool Karim S, Celum C, Drain PK. 2017. Protocol for a randomised controlled implementation trial of point-of-care viral load testing and task shifting: the Simplifying HIV TREAtment and Monitoring (STREAM) study. BMJ Open 7:e017507. doi: 10.1136/bmjopen-2017-017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fitzgerald D. 2017. Point-of-care viral load testing among HIV-infected adolescents in Haiti. ClinicalTrials.gov NCT03288246. https://clinicaltrials.gov/ct2/show/NCT03288246.
- 190.Jain V. 2018. Rapid HIV viral load monitoring in high risk patients in Uganda (RAPID-VL). ClinicalTrials.gov NCT03553693. https://clinicaltrials.gov/ct2/show/NCT03553693.
- 191.Peter T, Ellenberger D, Kim AA, Boeras D, Messele T, Roberts T, Stevens W, Jani I, Abimiku A, Ford D, Katz Z, Nkengasong JN. 2017. Early antiretroviral therapy initiation: access and equity of viral load testing for HIV treatment monitoring. Lancet Infect Dis 17:e26–e29. doi: 10.1016/S1473-3099(16)30212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Killingo BM, Taro TB, Mosime WN. 2017. Community-driven demand creation for the use of routine viral load testing: a model to scale up routine viral load testing. J Int AIDS Soc 20:e25009. doi: 10.1002/jia2.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Drain PK, Rousseau C. 2017. Point-of-care diagnostics: extending the laboratory network to reach the last mile. Curr Opin HIV AIDS 12:175–181. doi: 10.1097/COH.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Estill J, Salazar-Vizcaya L, Blaser N, Egger M, Keiser O. 2015. The cost-effectiveness of monitoring strategies for antiretroviral therapy of HIV infected patients in resource-limited settings: software tool. PLoS One 10:e0119299. doi: 10.1371/journal.pone.0119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lesosky M, Glass T, Mukonda E, Hsiao N-Y, Abrams EJ, Myer L. 2017. Optimal timing of viral load monitoring during pregnancy to predict viraemia at delivery in HIV-infected women initiating ART in South Africa: a simulation study. J Int AIDS Soc 20:e25000. doi: 10.1002/jia2.25000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ellman TM, Alemayehu B, Abrams EJ, Arpadi S, Howard AA, El‐Sadr WM. 2017. Selecting a viral load threshold for routine monitoring in resource-limited settings: optimizing individual health and population impact. J Int AIDS Soc 20:e25007. doi: 10.1002/jia2.25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Estill J, Egger M, Blaser N, Vizcaya LS, Garone D, Wood R, Campbell J, Hallett TB, Keiser O, IeDEA Southern Africa. 2013. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS 27:1483–1492. doi: 10.1097/QAD.0b013e328360a4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Phillips AN, Cambiano V, Nakagawa F, Ford D, Apollo T, Murungu J, Rousseau C, Garnett G, Ehrenkranz P, Bansi-Matharu L, Vojnov L, Katz Z, Peeling R, Revill P. 2016. Point-of-care viral load testing for sub-Saharan Africa: informing a target product profile. Open Forum Infect Dis 3:ofw161. doi: 10.1093/ofid/ofw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.DART Trial Team. 2010. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet 375:123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Mbougua JBT, Boyer S, Carrieri MP, Mben J-M, Dontsop M, Kazé S, Molinari N, Bourgeois A, Mpoudi-Ngolé E, Spire B, Koulla-Shiro S, Delaporte E. 2011. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis 11:825–833. doi: 10.1016/S1473-3099(11)70168-2. [DOI] [PubMed] [Google Scholar]
- 201.Jourdain G, Le Cœur S, Ngo-Giang-Huong N, Traisathit P, Cressey TR, Fregonese F. Leurent B, Collins IJ, Techapornroong M, Banchongkit S, Buranabanjasatean S, Halue G, Nilmanat A, Luekamlung N, Klinbuayaem V, Chutanunta A, Kantipong P, Bowonwatanuwong C, Lertkoonalak R, Leenasirimakul P, Tansuphasawasdikul S, Sang-A-Gad P, Pathipvanich P, Thongbuaban S, Wittayapraparat P, Eiamsirikit N, Buranawanitchakorn Y, Yutthakasemsunt N, Winiyakul N, Decker L, Barbier S, Koetsawang S, Sirirungsi W, McIntosh K, Thanprasertsuk S, Lallemant M, PHPT-3 study team. 2013. Switching HIV treatment in adults based on CD4 count versus viral load monitoring: a randomized, non-inferiority trial in Thailand. PLoS Med 10:e1001494. doi: 10.1371/journal.pmed.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Keebler D, Revill P, Braithwaite S, Phillips A, Blaser N, Borquez A, Cambiano V, Ciaranello A, Estill J, Gray R, Hill A, Keiser O, Kessler J, Menzies NA, Nucifora KA, Vizcaya LS, Walker S, Welte A, Easterbrook P, Doherty M, Hirnschall G, Hallett TB. 2014. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Health 2:e35–e43. doi: 10.1016/S2214-109X(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Phillips A, Cambiano V, Nakagawa F, Mabugu T, Magubu T, Miners A, Ford D, Pillay D, De Luca A, Lundgren J, Revill P. 2014. Cost-effectiveness of HIV drug resistance testing to inform switching to second line antiretroviral therapy in low income settings. PLoS One 9:e109148. doi: 10.1371/journal.pone.0109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Negoescu DM, Zhang Z, Bucher HC, Bendavid E. 2017. Differentiated human immunodeficiency virus RNA monitoring in resource-limited settings: an economic analysis. Clin Infect Dis 64:1724–1730. doi: 10.1093/cid/cix177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Braithwaite RS, Nucifora KA, Toohey C, Kessler J, Uhler LM, Mentor SM, Keebler D, Hallett T. 2014. How do different eligibility guidelines for antiretroviral therapy affect the cost-effectiveness of routine viral load testing in sub-Saharan Africa? AIDS 28:S73–S83. doi: 10.1097/QAD.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. 2008. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet 371:1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 207.Pham QD, Wilson DP, Nguyen TV, Do NT, Truong LX, Nguyen LT, Zhang L. 2016. Projecting the epidemiological effect, cost-effectiveness and transmission of HIV drug resistance in Vietnam associated with viral load monitoring strategies. J Antimicrob Chemother 71:1367–1379. doi: 10.1093/jac/dkv473. [DOI] [PubMed] [Google Scholar]
- 208.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. 2008. Cost-effectiveness of HIV monitoring strategies in resource-limited settings—a Southern African analysis. Arch Intern Med 168:1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vijayaraghavan A, Efrusy MB, Mazonson PD, Ebrahim O, Sanne IM, Santas CC. 2007. Cost-effectiveness of alternative strategies for initiating and monitoring highly active antiretroviral therapy in the developing world. J Acquir Immune Defic Syndr 46:91. [DOI] [PubMed] [Google Scholar]
- 210.Estill J, Kerr CC, Blaser N, Salazar-Vizcaya L, Tenthani L, Wilson DP, Keiser O. 2018. The effect of monitoring viral load and tracing patients lost to follow-up on the course of the HIV epidemic in Malawi: a mathematical model. Open Forum Infect Dis 5:ofy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kahn JG, Marseille E, Moore D, Bunnell R, Were W, Degerman R, Tappero JW, Ekwaru P, Kaharuza F, Mermin J. 2011. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ 343:d6884. doi: 10.1136/bmj.d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Boyer S, March L, Kouanfack C, Laborde-Balen G, Marino P, Aghokeng AF, Mpoudi-Ngole E, Koulla-Shiro S, Delaporte E, Carrieri MP, Spire B, Laurent C, Moatti JP. 2013. Monitoring of HIV viral load, CD4 cell count, and clinical assessment versus clinical monitoring alone for antiretroviral therapy in low-resource settings (Stratall ANRS 12110/ESTHER): a cost-effectiveness analysis. Lancet Infect Dis 13:577–586. doi: 10.1016/S1473-3099(13)70073-2. [DOI] [PubMed] [Google Scholar]
- 213.Hamers RL, Sawyer AW, Tuohy M, Stevens WS, Rinke de Wit TF, Hill AM. ART-A Consortium. 2012. Cost-effectiveness of laboratory monitoring for management of HIV treatment in sub-Saharan Africa: a model-based analysis. AIDS 26:1663–1672. doi: 10.1097/QAD.0b013e3283560678. [DOI] [PubMed] [Google Scholar]
- 214.Kimmel AD, Weinstein MC, Anglaret X, Goldie SJ, Losina E, Yazdanpanah Y, Messou E, Cotich KL, Walensky RP, Freedberg KA. 2010. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr 54:258–268. doi: 10.1097/QAI.0b013e3181d0db97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bishai D, Colchero A, Durack DT. 2007. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS 21:1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 216.Schneider K, Puthanakit T, Kerr S, Law MG, Cooper DA, Donovan B, Phanuphak N, Sirisanthana V, Ananworanich J, Ohata J, Wilson DP. 2011. Economic evaluation of monitoring virologic responses to antiretroviral therapy in HIV-infected children in resource-limited settings. AIDS 25:1143–1151. doi: 10.1097/QAD.0b013e3283466fab. [DOI] [PubMed] [Google Scholar]
- 217.Barnabas RV, Revill P, Tan N, Phillips A. 2017. Cost-effectiveness of routine viral load monitoring in low- and middle-income countries: a systematic review. J Int AIDS Soc 20:e25006. doi: 10.1002/jia2.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.El‐Sadr WM, Rabkin M, Nkengasong J, Birx DL. 2017. Realizing the potential of routine viral load testing in sub-Saharan Africa. J Int AIDS Soc 20:e25010. doi: 10.1002/jia2.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alemnji G, Onyebujoh P, Nkengasong JN. 2017. Improving laboratory efficiencies to scale-up HIV viral load testing. Curr Opin HIV AIDS 12:165. doi: 10.1097/COH.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 220.Carmona S, Peter T, Berrie L. 2017. HIV viral load scale-up: multiple interventions to meet the HIV treatment cascade. Curr Opin HIV AIDS 12:157. doi: 10.1097/COH.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 221.Kessler J, Braithwaite RS. 2013. Modeling the cost-effectiveness of HIV treatment: how to buy the most ‘health’ when resources are limited. Curr Opin HIV AIDS 8:544–549. doi: 10.1097/COH.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dorward J, Lessells R, Drain PK, Naidoo K, de Oliveira T, Pillay Y, Abdool Karim SS, Garrett N. 2018. Dolutegravir for first-line antiretroviral therapy in low-income and middle-income countries: uncertainties and opportunities for implementation and research. Lancet HIV 5:e400–e404. doi: 10.1016/S2352-3018(18)30093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Phillips AN, Cambiano V, Nakagawa F, Revill P, Jordan MR, Hallett TB, Doherty M, De Luca A, Lundgren JD, Mhangara M, Apollo T, Mellors J, Nichols B, Parikh U, Pillay D, Rinke de Wit T, Sigaloff K, Havlir D, Kuritzkes DR, Pozniak A, van de Vijver D, Vitoria M, Wainberg MA, Raizes E, Bertagnolio S, Phillips AN, Cambiano V, Nakagawa F, Revill P, Jordan MR, Hallett TB, Doherty M, De Luca A, Lundgren JD, Mhangara M, Apollo T, Mellors J, Nichols B, Parikh U, Pillay D, Rinke de Wit T, Sigaloff K, Havlir D, Kuritzkes DR, Pozniak A, van de Vijver D, Vitoria M, Wainberg MA, Raizes E, Bertagnolio S. 2018. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV 5:e146–e154. doi: 10.1016/S2352-3018(17)30190-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McCluskey SM, Boum Y II, Muginguzi N, Jaberer JE, Martin JN, Hunt PW, Marconi VC, Bangsberg DR, Siedner MJ. 2017. Brief report: appraising viral load thresholds and adherence support recommendations in the World Health Organization guidelines for detection and management of virologic failure. J Acquir Immune Defic Syndr 76:183–187. doi: 10.1097/QAI.0000000000001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duarte HA, Panpradist N, Beck IA, Lutz B, Lai J, Kanthula RM, Kantor R, Tripathi A, Saravanan S, MacLeod IJ, Chung MH, Zhang G, Yang C, Frenkel LM. 2017. Current status of point-of-care testing for human immunodeficiency virus drug resistance. J Infect Dis 216:S824–S828. doi: 10.1093/infdis/jix413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Castillo-Mancilla JR, Haberer JE. 2018. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Curr HIV/AIDS Rep 15:49–59. doi: 10.1007/s11904-018-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Greene G, Sriruttan C, Le T, Chiller T, Govender NP. 2017. Looking for fungi in all the right places: screening for cryptococcal disease and other AIDS-related mycoses among patients with advanced HIV disease. Curr Opin HIV AIDS 12:139–147. doi: 10.1097/COH.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 228.Sabur NF, Esmail A, Brar MS, Dheda K. 2017. Diagnosing tuberculosis in hospitalized HIV-infected individuals who cannot produce sputum: is urine lipoarabinomannan testing the answer? BMC Infect Dis 17:803. doi: 10.1186/s12879-017-2914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ondoa P, van der Broek A, Jansen C, de Bruijn H, Schultsz C. 2017. National laboratory policies and plans in sub-Saharan African countries: gaps and opportunities. Afr J Lab Med 6:578. doi: 10.4102/ajlm.v6i1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dorward J, Yende-Zuma N, Samsunder N, Karim QA, Drain PK, Garrett N. 2017. Clinic-based evaluation of a point-of-care creatinine assay to screen for renal impairment amongst HIV-positive patients receiving tenofovir disoproxil fumarate. J Acquir Immune Defic Syndr 77:e36–e39. doi: 10.1097/QAI.0000000000001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pollock NR, McGray S, Colby DJ, Noubary F, Nguyen H, Nguyen TA, Khormaee S, Jain S, Hawkins K, Kumar S, Rolland JP, Beattie PD, Chau NV, Quang VM, Barfield C, Tietje K, Steele M, Weigl BH. 2013. Field evalution of a prototype paper-based point-of-care fingerstick transaminase test. PLoS One 8:e75616. doi: 10.1371/journal.pone.0075616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xu H, Cheng S, Liu X, Li J, Zheng W, Lu G, Zhang J, Zheng J, Zhang J. 2016. Development of monoclonal antibodies and immunochromatographic lateral flow device for rapid test of alanine aminotransferase isoenzyme 1. Protein Expr Purif 119:94–101. doi: 10.1016/j.pep.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 233.Harris TG, Rabkin M, El-Sadr WM. 2018. Achieving the fourth 90: healthy aging for people living with HIV. AIDS 32:1563–1569. doi: 10.1097/QAD.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]