Invasive fungal diseases carry high morbidity and mortality in patients undergoing chemotherapy for hematological malignancies or allogeneic hematopoietic stem cell transplantation. In order to prevent these life-threatening infections, antifungal chemoprophylaxis plays an important role in daily clinical practice.
Keywords: antifungal prophylaxis, intermittent dosing, amphotericin B, echinocandin
SUMMARY
Invasive fungal diseases carry high morbidity and mortality in patients undergoing chemotherapy for hematological malignancies or allogeneic hematopoietic stem cell transplantation. In order to prevent these life-threatening infections, antifungal chemoprophylaxis plays an important role in daily clinical practice. Broad-spectrum antifungal triazoles are widely used but exhibit disadvantages such as relevant drug-drug interactions. Therefore, amphotericin B products or echinocandins can be an alternative in selected patient populations. As these compounds are available as intravenous formulations only, there is growing interest in extended dosing regimens. Although not approved for these agents, this strategy is a rational option, as these compounds have properties suitable for this strategy, including dose-proportional pharmacokinetics, prolonged elimination half-life, and a large therapeutic window. As the use of extended dosing regimens in antifungal prophylaxis is expanding in clinical practice, we reviewed the pharmacokinetic and pharmacodynamic rationale for this strategy, animal model data, dose escalation studies, and clinical trials supporting this concept.
INTRODUCTION
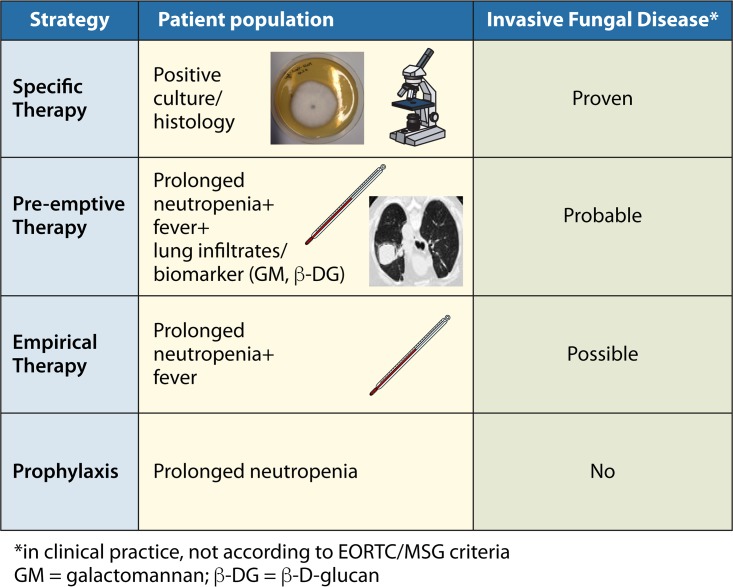
Invasive fungal diseases (IFD) have high morbidity and mortality in children and adults with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) or who are undergoing hematopoietic stem cell transplantation (HSCT), as well as in solid organ transplant recipients and patients with primary immunodeficiencies like chronic granulomatous disease. As early diagnosis of IFD is difficult and early treatment is associated with better outcome, antifungal chemoprophylaxis, empirical antifungal therapy, and preemptive treatment are widely used strategies and play an important role in daily clinical practice (Fig. 1) (1–6).
FIG 1.
Antifungal strategies in immunocompromised patients at high risk for invasive fungal infection.
Regarding prevention of IFD, the majority of clinical trials on antifungal prophylaxis investigated the use of triazoles, such as fluconazole, itraconazole, voriconazole, or posaconazole. All these agents are available as oral and intravenous formulations and are approved for the prophylactic setting. Unfortunately, antifungal triazoles exhibit numerous drug-drug interactions, and the concurrent use of several chemotherapeutic drugs, including but not limited to vincristine, a cornerstone in the treatment of patients with ALL, is contraindicated. In addition, due to considerable variability in gastrointestinal absorption and drug metabolism, plasma concentrations of itraconazole and voriconazole are unpredictable, which may impair clinical efficacy or increase the risk for toxicity. Therefore, therapeutic drug monitoring (TDM) is strongly recommended, which may also detect a low blood level in a patient with poor compliance with medication regimens (7). Unfortunately, TDM of antifungal drugs is not available in many clinical centers. Further issues associated with azole prophylaxis include long-term safety, the threat of resistance (8, 9), and limited treatment options in the case of breakthrough infections.
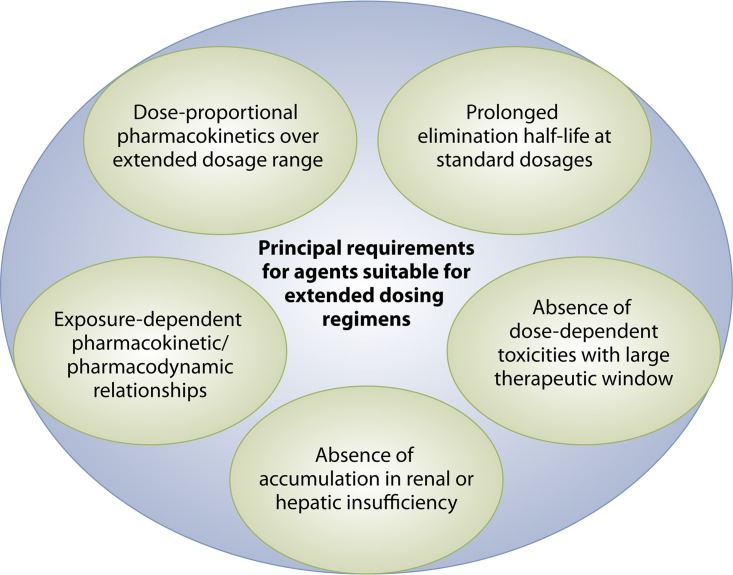
In contrast to antifungal triazoles, amphotericin B formulations and the class of echinocandins are available as intravenous formulation only and are administered on a once-daily (QD) basis. The daily intravenous administration may be of advantage for patients not tolerating or being unable to adhere to oral medication but, at the same time, is inconvenient and consumes resources. Extended dosing regimens of antifungal prophylaxis are not approved for amphotericin B formulations and echinocandins but are a rational option, as some of these compounds have properties suitable for extended dosing intervals. These properties include dose-proportional pharmacokinetics, exposure-dependent pharmacokinetic/pharmacodynamic relationships, a prolonged elimination half-life, and a large therapeutic window (Fig. 2) (10, 11). In contrast, a number of reasons speak against the use of oral or intravenous extended dosing regimens of azoles. For example, for compounds available for oral administration that have a long half-life, display linear pharmacokinetics, and are dosed once daily, such as fluconazole, there is no true advantage to using extended dosing intervals. Indeed, extending the dosing interval to 48 or 72 h may negatively impact upon patient compliance, as drug adherence is more difficult with extended dosing intervals than it is with a fixed daily dosing regimen. As it concerns the intravenous route, some of the antifungal azoles do not have stable linear pharmacokinetics, and there is a major concern of dose-dependent hepatic toxicity that is a class effect of the azoles and that is well documented, at least for itraconazole and voriconazole. In addition, TDM is recommended for most of the azoles, which further complicates administration at extended dosing intervals.
FIG 2.
Principal requirements for agents suitable for extended dosing regimens.
Since there is increasing interest in the concept of extended dosing regimens in antifungal prophylaxis and an expanding use of this strategy in daily practice, we aimed to review the pharmacokinetic properties of antifungal agents used for this approach, as well as the available preclinical and clinical data.
PHARMACOKINETIC AND PHARMACODYNAMIC CONSIDERATIONS
Amphotericin B Products
The currently licensed amphotericin B formulations (amphotericin B deoxycholate [generic; AmB-D], amphotericin B lipid complex [Abelcet], and the liposomal formulation of amphotericin B [LAMB; AmBisome]) are available as intravenous formulation only and exhibit activity against an extended spectrum of fungi. They are approved for first- or second-line treatment of IFD and, limited to AmB-D and LAMB, empirical antifungal therapy in persistently neutropenic children and adults. However, since the label has never been pursued by the respective manufacturers, they are not approved for the prophylactic setting.
Amphotericin B, as a compound, displays concentration-dependent fungicidal activity against susceptible Candida and Aspergillus spp. and prolonged postantifungal effects of up to 12 h duration in Candida albicans (12–16). In neutropenic mice with experimental disseminated candidiasis or pulmonary aspergillosis receiving amphotericin B by the intraperitoneal route, the ratio of peak plasma concentration (Cmax)/MIC of the infecting isolate was the parameter that provided the best correlation with outcome, which was assessed by the residual fungal tissue burden in target organs (17, 18). Notably, the different amphotericin B formulations display different physicochemical and pharmacokinetic characteristics and demonstrate important differences in antifungal efficacy which depend on the agent, the dose, and the type and site of infection (16, 19, 20). However, only LAMB fulfills the criteria of dose-proportional, prolonged exposure with potentially therapeutic concentrations in plasma coupled with a large therapeutic window that allow for the administration of larger doses (Table 1) (16). Apart from a prolonged residence time in plasma, LAMB distributes into and remains up to weeks in various tissues in a dose-dependent manner at drug concentrations above the MIC for many fungi. The observation that antifungal efficacy in animals seems to correlate with drug tissue concentration and the long-term retention of bioactive LAMB in plasma and different tissues provides support for the concept that extended dosing intervals may be efficacious in the prophylaxis of invasive fungal infections (21, 22).
TABLE 1.
Pharmacokinetic and pharmacodynamic properties of agents investigated for extended dosing regimensa
| Property | Liposomal amphotericin B | Anidulafungin | Caspofungin | Micafungin | Rezafungin |
|---|---|---|---|---|---|
| Formulation | i.v. | i.v. | i.v. | i.v. | i.v. |
| Dose linearity | Up to 10 mg/kg | Yes | Yes | Yes | Yes |
| Protein binding (%) | Not applicable | 84 | 97 | 99 | 98 |
| Vol of distribution at steady state (liters/kg) | 0.22 | 0.7–0.9 | Not available | 0.24 | 0.4 |
| Elimination half-life (h) | 6–10 | 24–26 | 9–11 | 11–17 | 80–150 |
| Substrate/inhibitor of CYP450 | No | No | Poor substrate/weak inhibitor | Poor substrate/weak inhibitor | No |
| Metabolism | Not metabolized | Via chemical degradation to inactive metabolites | Via peptide hydrolysis and N-acetylation | Via arylsulfatase and catechol-O-methyltransferase | Not metabolized |
| Elimination | Unchanged in feces and urine (<10% over 7 days) | Primarily in feces (10% unchanged); 1% in urine | 35% in feces, 41% in urine (1.4% unchanged) | 40% in feces, <15% in urine (<1% unchanged) | Primarily in feces (25% unchanged), <1% in urine |
| Dosage adjustment in renal impairment | No adjustment needed | No adjustment needed | No adjustment needed | No adjustment needed | No adjustment needed |
| Dosage adjustment in hepatic impairment | No adjustment needed | No adjustment needed | Child-Pugh: <7, no adjustment; 7–9, 35 mg maintenance; >9, no data | Child-Pugh: ≤9, no adjustment; >9, no data | Not yet examined |
| Pharmacodynamics in vitro (by time kill and PAFE) | Concn-dependent fungicidal activity; prolonged PAFEs against Candida | Concn- and time-dependent fungicidal activity against Candida; inhibitory against Aspergillus, prolonged PAFEs against Candida | As described for anidulafungin | As described for anidulafungin | Concn- and time-dependent fungicidal activity against Candida |
| Best pharmacodynamic parameter in vivob | Cmax/MIC | AUC/MIC (Candida); Cmax/MEC (Aspergillus) | As described for anidulafungin | As described for anidulafungin | AUC/MIC (Candida) |
Based in part on data from references 81 to 83. i.v., intravenous; PAFE, postantifungal effect; IFD, invasive fungal disease; Cmax, peak plasma concentration; AUC, area under the concentration-time curve; MEC, minimal effective concentration.
Parameter best associated with efficacy in animal models/patients with IFD.
Echinocandin Lipopeptides
The echinocandins are a newer class of systemic antifungal agents with broad-spectrum activity against Candida and Aspergillus. The compounds are available as intravenous formulation, display linear pharmacokinetics over a large dosage range, have a long half-life that allows for once-daily dosing, and are generally very well tolerated (Table 1). Among the currently licensed echinocandins anidulafungin, caspofungin, and micafungin, only the latter is licensed for prophylaxis against invasive infections by Candida spp. in the setting of prolonged neutropenia (23), but it is not licensed for prophylaxis against invasive mold infection. Interestingly, a retrospective observational study identified echinocandin prophylaxis as an independent risk factor for invasive fungal infection in patients receiving remission induction chemotherapy for AML (24), and the higher risk for breakthrough infection was seen for both yeast and molds. The reason for this observation is unclear, but the results have to be confirmed in future analyses before reconsidering the use of echinocandins in the prophylactic setting. It is also important to note that preclinical studies suggested that echinocandins are effective against Pneumocystis jirovecii, but the clinical relevance regarding the prophylactic setting is unclear to date (25).
In vitro studies demonstrated that the echinocandins are able to kill most Candida species. In contrast, when echinocandins are coincubated with Aspergillus fumigatus, microscopy shows concentration-dependent damage to the fungus, which is, however, able to recover in the absence of the antifungal compound (16, 20). For all three echinocandins, fungicidal activity for Candida spp. was mainly dependent on concentration and time; in addition, studies showed postantifungal effects for up to 12 h at concentrations above the MIC (26, 27).
In a model using persistently neutropenic rabbits which were inoculated with Candida spp., anidulafungin demonstrated highly predictable concentration-effect relationships which were not seen for an experimental pulmonary aspergillosis model (27). Murine kidney target models of disseminated candidiasis demonstrated that the area under the concentration-time curve (AUC)/MIC ratio is the pharmacodynamic parameter that predicts efficacy of all current echinocandins (11, 26, 28), whereas in mice with invasive pulmonary aspergillosis, the Cmax/minimal effective concentration (MEC) ratio was the parameter which was closely associated with efficacy (26, 29). Using large data sets from two phase III trials of micafungin for invasive candidiasis, a significant relationship between the AUC/MIC ratio of micafungin and mycological response was found by population pharmacokinetics and regression analysis. Monte Carlo simulations revealed a lower AUC/MIC target for C. parapsilosis than for other Candida spp., which supported the concept of species-specific echinocandin susceptibility breakpoints (11, 26, 28).
Rezafungin (CD101) is a novel intravenous echinocandin which is structurally related to anidulafungin. Compared to other echinocandins, the compound has increased chemical stability and a long elimination half-life in plasma that provides the opportunity for extended dosing regimens (30, 31). Similar to other echinocandins, the compound has broad-spectrum fungicidal activity against Candida and inhibitory activity against Aspergillus in vitro (31–33), as well as potent dose-dependent antifungal efficacy in neutropenic animal models of invasive aspergillosis and candidiasis (34). Rezafungin showed dose-proportional plasma exposures, minor accumulation (30% to 55%), low clearance (<0.28 liter/h), long half-life (>80 h), and minimal renal excretion, enabling once-weekly dosing (31, 35, 36). Rezafungin has undergone phase I/II clinical trials, and the compound might be a candidate for prophylaxis of invasive Candida and Aspergillus infections.
ANIMAL STUDIES EXPLORING EXTENDED DOSING REGIMENS
Liposomal Amphotericin B
The prophylactic administration of LAMB at dosages of up to 90 mg/kg of body weight given daily or in an extended dosing regimen was investigated in a murine model (Table 2). Mice were inoculated with Candida glabrata at 1 to 7 days or with C. albicans at 3 or 6 weeks after the last administration of LAMB. Compared to the results for untreated controls, significantly lower or no fungal burden was detected in target tissues of the animals which had received LAMB prophylaxis (37). Similar results were observed when LAMB was given at a single prophylactic dose of up to 20 mg/kg in neutropenic mice challenged with A. fumigatus (38) or in immunocompetent and immunocompromised mice challenged with Histoplasma capsulatum or C. albicans, respectively (39). Notably, in a murine model, the highest concentrations of LAMB given at cumulative doses of up to 225 mg/kg were measured in spleen and liver. The concentrations of LAMB remained above the MIC for many fungal pathogens for up to 6 weeks in kidneys and spleen and for 1 week in lungs (37).
TABLE 2.
Overview of preclinical studies exploring large or extended dosing regimens for prophylaxis and treatmenta
| Authors, yr (reference) | Compound | Animal model | Most relevant observations |
|---|---|---|---|
| Garcia et al., 2000 (39) | LAMB | Prophylaxis in normal and nonneutropenic mice against C. albicans; LAMB given 2–7 days prior to inoculation, response monitoring for 7 days | Prophylactic administration of single doses of LAMB at 5–20 mg/kg reduced the fungal burden in kidney tissue relative to that in untreated controls with a trend toward dose-dependent efficacy |
| Garcia et al., 2000 (39) | LAMB | Prophylaxis in normal and nonneutropenic mice against H. capsulatum; LAMB given 2–7 days prior to inoculation, response monitoring for 10–24 days | Prophylactic administration of single doses of LAMB at 10–20 mg/kg prolonged survival with absence of Histoplasma in spleen tissue |
| Lewis et al., 2008 (38) | LAMB | Prophylaxis in neutropenic mice against A. fumigatus; LAMB given 24 h prior to inoculation, response monitoring for 7 days | Prophylaxis with single intraperitoneal doses of LAMB at ≥5 mg/kg improved animal survival and suppressed lung fungal burden |
| Smith et al., 2007 (37) | LAMB | Prophylaxis in neutropenic mice; LAMB at dosages up to 90 mg given 1–7 days prior to inoculation with C. glabrata or at a dosage of 60 mg/kg 3 or 6 weeks prior to inoculation with C. albicans; response monitoring for 21 days, assessment of drug concn for 6 weeks | LAMB levels above the MIC for many fungi for 1 wk in lungs and for as long as 6 wks in kidneys and spleen; tissue fungal burden significantly reduced or undetectable |
| Adler-Moore et al., 2004 (40) | LAMB | Treatment of neutropenic mice with invasive candidiasis; doses of 4–20 mg/kg administered intermittently in various regimens starting 24–48 h after inoculation, response monitoring for up to 1 mo | Intermittent high-dose treatments with LAMB were able to clear Candida from the kidneys; single doses of LAMB at 4 mg/kg were as efficacious as four 1-mg/kg treatments |
| Clemons and Stevens, 1998 (42) | LAMB | Treatment of nonimmunocompromised mice with disseminated cryptococcosis; doses of up to 10 mg/kg given 3 times per wk for 2 wks starting 4 days after inoculation, response monitoring for 49 days | Intermittent treatment with LAMB at 5 or 10 mg/kg prolonged survival and reduced fungal tissue burdens in a dose-dependent manner |
| Graybill and Bocanegra, 1995 (43) | LAMB | Treatment of athymic mice with disseminated histoplasmosis; doses of up to 3 mg/kg given intermittently for up to 15 days after inoculation, response monitoring for 40 days | Intermittent treatment with LAMB at 1 and 3 mg/kg improved survival |
| Lewis et al., 2008 (38) | Micafungin | Prophylaxis of neutropenic mice against A. fumigatus; single doses of up to 20 mg/kg administered 24 h prior to inoculation, response monitoring for 7 days | Prophylaxis with single doses of micafungin at ≥5 mg/kg improved survival and suppressed lung fungal burden |
| Gumbo et al., 2007 (45) | Micafungin | Treatment of neutropenic mice with C. glabrata infection; single doses of up to 100 mg/kg administered 4 h after inoculation, response monitoring for 7 days | Maximal fungal decline after single treatment doses of ≥50 mg/kg without regrowth at day 7 |
| Petraitiene et al., 2015 (46) | Micafungin | Treatment of neutropenic rabbits with disseminated candidiasis; doses of 1 mg/kg q24h, 2 mg/kg q48h, and 3 mg/kg q72h beginning 24 h after inoculation, response monitoring for 12 days | All regimens showed similar exposures to micafungin and were equally safe and effective |
| Lepak et al., 2018 (47) | Rezafungin | Treatment of neutropenic mice with C. albicans, C. glabrata, or C. parapsilosis infection; single doses of up to 64 mg/kg given 2 h after inoculation, response monitoring for 7 days | Dose-dependent therapeutic efficacy; AUC/MIC correlated with efficacy |
| Bader et al., 2018 (48) | Rezafungin | PK-PD target attainment analyses using a human population PK model; Monte Carlo simulations of various 400-mg dose regimens | At the MIC90s for C. albicans and C. glabrata, a single 400-mg dose achieved PK-PD target attainment probabilities of ≥90% through week 3 |
LAMB, liposomal amphotericin B; q24h, every 24 h; AUC, area under the concentration-time curve; PK-PD, pharmacokinetics-pharmacodynamics.
In addition to extended dosing regimens investigated for prophylaxis, extended dosing intervals of LAMB were successfully evaluated as a therapeutic option in IFD. For example, it has been demonstrated that extended dosing regimens using a maximum dose of 20 mg/kg LAMB administered three times a week were successful in treating neutropenic mice suffering from invasive C. albicans infection (40). Similar results were observed in preclinical studies assessing therapy with LAMB given in extended dosing intervals for coccidiomycosis, cryptococcosis, and histoplasmosis (21, 41–44).
Micafungin
One study in neutropenic mice demonstrated the potential utility of large doses of micafungin administered at infrequent intervals for prophylaxis against pulmonary aspergillosis. In this study, single intraperitoneal doses of micafungin at 5, 10, or 20 mg/kg administered 24 h prior to inoculation improved survival and suppressed the lung fungal burden relative to the burden in untreated controls (38).
In the therapeutic setting, the effect of micafungin dose scheduling was investigated in transiently neutropenic mice with disseminated C. glabrata infection. Dose-ranging studies demonstrated that single doses of ≥50 mg/kg resulted in maximal fungal decline without regrowth at day 7. The dose associated with 50% of maximal kill (50% effective dose [ED50]) was then administered as a single dose at day 0, two equal doses at days 0 and 3.5, or 7 equal doses given daily, which all had equivalent day 7 effects. Pharmacokinetic/pharmacodynamic modeling using human pharmacokinetic data demonstrated that a single dose of micafungin of 1,400 mg would achieve maximal fungal decline in humans, which corresponds to a single dose of 100 mg/kg in the mouse model (45). The pharmacokinetics, efficacy, and safety of alternate dosing regimens of micafungin were further investigated in a subacute neutropenic disseminated C. albicans infection model in rabbits. Micafungin was given over 12 days at 1, 2, or 3 mg/kg every 24, 48, or 72 h, respectively. The pharmacokinetics of micafungin on day 7 were linear, and all treatment groups showed significantly higher clearance of C. albicans from target tissues than was observed in untreated controls, indicating that less-fractionated regimens of micafungin were as effective as once-daily treatment (46).
Taken together, these studies in neutropenic animals demonstrated that extended dosing intervals have both preventive effects in invasive pulmonary aspergillosis and therapeutic effects in disseminated candidiasis. While these experimental data may be transferable to the other currently licensed echinocandins, no animal data on extended dosing regimens exist to date for anidulafungin and caspofungin, respectively.
Rezafungin
Whereas data on extended dosing intervals for the prophylactic administration of rezafungin are lacking to date, one preclinical study evaluated a single dose of rezafungin in mice infected with select C. albicans, C. glabrata, and Candida parapsilosis strains with a range of MICs. Assessing the residual fungal burden in kidneys after 7 days, dose-dependent activity was seen for each pathogen; the AUC/MIC ratio correlated well with efficacy and was numerically lower for all three species than were those of other echinocandins (47). In different pharmacokinetic/pharmacodynamic models of invasive C. glabrata infection, target attainment over 4 weeks of therapy was very likely after administration of a single-dose regimen at the MIC90 of 0.06 mg/liter (48). These data suggest that rezafungin has great potential for treatment and prophylaxis of invasive Candida and Aspergillus infections with extended dosing regimens and in preventing emergence of resistance through enhanced potency.
CLINICAL PHARMACOKINETICS AND SAFETY OF ESCALATED DOSAGES
Data on the clinical pharmacokinetics and safety of escalated dosages of antifungal compounds are important in order to study these drugs given prophylactically in an extended dosing regimen. Notably, many of the available data were retrieved from studies performed in patients requiring empirical, preemptive, or targeted antifungal therapy, whereas there is much less information from studies evaluating escalated doses of antifungal compounds given as prophylaxis.
Liposomal Amphotericin B
Whereas there are no published data on the safety and pharmacokinetics of escalated doses of LAMB given as prophylaxis, LAMB as empirical antifungal therapy has been investigated in an open-label, sequential-dose-escalation, multidose pharmacokinetic study in persistently febrile neutropenic adults (Table 3). Daily doses of up to 7.5 mg/kg were well tolerated and followed dose-dependent pharmacokinetics with increases of the mean AUC on the first day of treatment, consistent with reticuloendothelial uptake and redistribution (49). In a subsequent phase I/II study, the maximum tolerated dosage (MTD) of the compound was investigated at escalating dosages of up to 15 mg/kg/day, concluding the MTD of LAMB to be at least 15 mg/kg/day. There was an increase of serum creatinine of two times above baseline in one-third of the patients, but this increase was not related to the dose. The mean AUC at 24 h revealed dose-related, nonlinear, saturation-like pharmacokinetics with maximum plasma exposure following administration of 10 mg/kg/day and decline in plasma exposure at 12.5 and 15 mg/kg/day (50).
TABLE 3.
Overview of clinical trials exploring dose escalation of agents investigated for extended dosing regimensa
| Authors, yr (reference) | Compound, dose range, and patients | Most relevant observations |
|---|---|---|
| Walsh et al., 1998 (49) | LAMB at 1, 2.5, 5, or 7.5 mg/kg QD in 36 adults with fever and neutropenia | Dose-dependent pharmacokinetics with slightly more than dose-proportional increases in AUC; MTD criteria not reached |
| Walsh et al., 2001 (50) | LAMB at 7.5, 10, 12.5, or 15 mg/kg QD in 44 adults with possible, probable, or proven IFD | Dose-related nonlinear saturation-like pharmacokinetics with maximal exposure following administration of 10 mg/kg; MTD criteria not reached |
| Lestner et al., 2016 (52); Seibel et al., 2017 (51) | LAMB at 2.5, 5, 7.5, or 10 mg/kg QD in 35 pediatric patients requiring empirical or targeted therapy | Dose-dependent pharmacokinetics with slightly more than dose-proportional increases in AUC; MTD criteria not reached |
| Brüggemann et al., 2015 (55) | Anidulafungin as prophylaxis at 200 mg q48h or 300 mg q72h in 20 adult HSCT recipients | Similar exposures of both regimens relative to the 100-mg QD standard regimen as measured by the AUC0–144; no safety issues at the higher dose |
| Cornely et al., 2011 (58); Würthwein et al., 2013 (59) | Caspofungin at 70, 100, 150, or 200 mg QD in 46 adults with probable/proven invasive aspergillosis | Linear pharmacokinetics with dose-proportional increases in AUC; MTD criteria not reached |
| Betts et al., 2009 (57) | Caspofungin at 50 mg QD vs 150 mg QD in 204 adults with invasive candidiasis | Not investigated; no safety issues at the higher dose |
| Hiemenz et al., 2005 (62) | Micafungin as prophylaxis at 12.5, 25, 50, 75, 100, 150, or 200 mg QD in 74 adult HSCT recipients | Linear pharmacokinetics with dose-proportional increases in AUC; MTD criteria not reached |
| Sirohi et al., 2006 (63) | Micafungin as prophylaxis at 3, 4, 6, or 8 mg/kg QD in 36 adult HSCT recipients | Linear pharmacokinetics with dose proportional increases in AUC; MTD criteria not reached |
| Muilwijk et al., 2018 (64) | Micafungin as prophylaxis at 100 mg QD vs 300 mg twice wkly in 20 adults with hematological malignancies | Similar exposures of both regimens as measured by the AUC0–168; no safety issues at the higher dose |
| Sandison 2017 (35) | Rezafungin at 50, 100, 200, or 400 mg in single dose/multiple once-wkly doses (maximum of 3) in 56 healthy adults | Linear pharmacokinetics with dose-proportional increases in AUC; no safety issues |
LAMB liposomal amphotericin B; QD, once daily; AUC, area under the concentration-time curve; IFD, invasive fungal disease; MTD, maximum tolerated dose; q48h, every 48 h; AUC0–144, area under the concentration-time curve from 0 to 144 h; HSCT, hematopoietic stem cell transplantation.
In a similar study format, the safety and pharmacokinetics of LAMB was evaluated at daily dosages of up to 10 mg/kg in neutropenic children requiring empirical or targeted antifungal therapy. Infusion-related side effects occurred in 11% of 565 infusions, and serum creatinine levels increased significantly in cohorts receiving 5 and 10 mg/kg/day. Pharmacokinetic analyses revealed dose-dependent pharmacokinetics similar to observations in adults and support pediatric dosages similar to those in adults (51, 52).
Whereas the approved dosage range of LAMB is 3 to a maximum of 6 mg/kg/day, and current guidelines recommend 3 mg/kg/day for empirical therapy and for treatment of invasive candidiasis and aspergillosis (5, 6, 53, 54), these systematic phase II dose-escalation trials demonstrate dose-dependent exposure and safety of single daily dosages of up to 10 mg/kg in both children and adults and the feasibility of administration of larger dosages within this dosage range in extended intervals. Of note, a population pharmacokinetic study of conventional (3 mg/kg/day) and intermittent (10 mg/kg on day 1 and 5 mg/kg on days 3 and 6) dosages of LAMB in adults revealed linear pharmacokinetics for both regimens, with body weight as a significant parameter of impact on clearance (10).
Anidulafungin
Whereas published formal dose-escalation or MTD studies are lacking, the principal feasibility of the administration of higher doses in an intermittent fashion has been shown in patients receiving anidulafungin prophylactically at 200 or 300 mg every 48 or 72 h, respectively, which demonstrated an AUC over a 6-day period comparable to that of the licensed regimen (55). Dosages explored in children resulted in exposure equivalent to that obtained with approved standard doses in adults (56). However, data on escalated dosages are lacking, and anidulafungin is not yet approved in the pediatric population.
Caspofungin
Several trials in adults suffering from invasive candidiasis or from invasive aspergillosis evaluated the safety and pharmacokinetics of escalated dosages of caspofungin (57–59). The data from these clinical trials demonstrate that caspofungin at dosages of up to 200 mg/day is well tolerated and displays linear pharmacokinetics without dose-limiting toxicity.
Administration of a dosage of 70 mg/m2/day to 12 children in the initial dose-finding pharmacokinetic study and comparison to an adult dose of 70 mg/day showed that the pharmacokinetic parameters (AUC0−24; Cmax and Cmin) at these higher doses were similar to the results from comparisons at the approved lower dose (pediatric dose, 50 mg/m2/day; adult dose, 50 mg/day) (60). These data and physiology-based pharmacokinetic modeling of caspofungin suggest that, in the absence of different safety target sensitivities, no fundamental differences are to be expected between children and adolescents upon dose escalation (61).
Micafungin
In an early dose-escalation study, adult HSCT recipients received fluconazole (400 mg/day) and either saline or micafungin (12.5 to 200 mg/day) prophylactically for up to 4 weeks. The MTD of micafungin was not reached, and drug-related toxicities were rare (62). In a formal dose-escalation study in HSCT recipients requiring antifungal prophylaxis, participants received up to 8 mg/kg/day micafungin for a median of 18 days. The relationship of AUC to the micafungin dose was linear. Adverse events (AEs) were not different from those expected for this setting, and there was no evidence of dose-related toxicity. No patient had a grade 3/4 AE, and criteria for MTD of micafungin were not met (63). Finally, a recent study compared the pharmacokinetics of prophylactic micafungin at 300 mg given twice weekly versus 100 mg given daily. The exposure as measured by the AUC from 0 to 168 h (AUC0–168) was similar in both treatment arms, and Monte Carlo simulations also projected similar exposures for a weekly dose of 700 mg (64).
Collectively, the results of these studies imply that micafungin has linear pharmacokinetics at dosages of up to 8 mg/kg and that the MTD is 8 mg/kg/day or higher. Micafungin has linear pharmacokinetics in children and adolescents at dosages of up to 4 mg/kg (65). While the dosage approved for prophylaxis is 1 mg/kg/day, the approved dosage range in the treatment setting is 2 to 4 mg/kg/day, providing room for intermittent administration of larger dosages for prophylaxis. Moreover, supporting the overall safety of the compound in the pediatric population, dosages of up to 15 mg/kg QD have been safely administered to neonates, and a dosage of 10 mg/kg QD is within the label specifications of the European Medicines Agency for treatment of invasive candidiasis in neonates (66, 67).
Rezafungin
The pharmacokinetics and safety of rezafungin have been investigated in two randomized, double-blind, placebo-controlled, dose-escalation studies in healthy normal volunteers, who received dosages of up to 400 mg in single or multiple once-weekly doses. There were no serious or severe AEs, or no individual was withdrawn from the study due to an AE (35). However, further pharmacokinetic and pharmacodynamic evaluations and demonstration of efficacy and safety in patients with the target infections are required to develop the potential of this promising investigational antifungal agent.
CLINICAL STUDIES OF EXTENDED DOSING REGIMENS IN FUNGAL PROPHYLAXIS
Retrospective Data
Liposomal amphotericin B.
A retrospective study analyzed the prophylactic use of LAMB (3 mg/kg once weekly) in 16 adult allogeneic HSCT recipients with graft-versus-host disease (GvHD) and receiving at least 20 mg prednisone per day (Table 4) (68). The incidence of IFD did not differ between patients with intermittent LAMB prophylaxis in which 73 and 12 patients received antifungal prophylaxis with triazoles or an echinocandin, respectively. In none of the patients was LAMB prophylaxis prematurely discontinued due to an AE.
TABLE 4.
Overview of clinical trials exploring extended dosing intervals for antifungal prophylaxisa
| Authors, yr (reference) | Compound and dosage | Study characteristics | Most relevant observations |
|
|---|---|---|---|---|
| Infection/outcome | Adverse event | |||
| Kelsey et al., 1999 (73) | LAMB at 2 mg/kg 3×/wk | Randomized, double blind, placebo controlled; 74 patients, 87 controls | No IFD in patients receiving LAMB, 3 IFD in placebo arm (NS); colonization in 20% versus 40% (P < 0.01) | Infusion-related AEs in 5 and 1 patients receiving LAMB and placebo, respectively; these patients discontinued study |
| Penack et al., 2006 (74) | LAMB at 50 mg every other day | Randomized, open label; 75 patients, 57 controls without prophylaxis | Proven/probable IFD in patients receiving LAMB at 4.6% versus 22.2% in patients receiving placebo (P < 0.01) | Early discontinuation of LAMB in 2.8% of patients; no grade III/IV toxicities in patients receiving LAMB |
| El-Cheickh et al., 2007 (72) | LAMB at 7.5 mg/kg 1×/wk | Prospective; 21 patients | IA in 1 patient 2 mo after discontinuation of the study drug | 7 patients with premature discontinuation of LAMB (infusion related/nephrotoxicity) |
| Cordonnier et al., 2008 (71) | LAMB at 10 mg/kg 1×/wk | Prospective; 29 patients | Proven/probable IFD in 4 patients | 6 patients discontinued study due to AEs (dyspnea, renal insufficiency, anuria) |
| Arrieta et al., 2010 (78) | LAMB at 5 mg/kg 1×/wk | Randomized, open label, placebo controlled, prophylaxis against Candida infection; 20 patients, 20 controls | No Candida infection in patients receiving LAMB, 1 in placebo group; colonization in 1 and 3 patients, respectively | No difference between groups regarding hypokalemia, nephrotoxicity, NEC, hemorrhage grade III/IV |
| Bochennek et al., 2011 (76) | LAMB at 2.5 mg/kg 2×/wk | Prospective; 46 patients, 45 historical controls | No proven/probable IFD in patients receiving LAMB; significantly less than in historical controls (7 patients; P = 0.01) | 4 patients with early discontinuation of LAMB (13.5% of prophylactic episodes) due to hypokalemia of <3 mmol/liter |
| Chaftari et al., 2012 (75) | ABLC at 7.5 mg/kg 1×/wk | Randomized, open label; 19 patients, 21 controls receiving posaconazole | 1 IFD among patients receiving ABLC, no IFD in patients receiving posaconazole | Nephrotoxicity higher in ABLC arm (53% versus 5%; P = 0.001), leading to premature discontinuation |
| Ginocchio et al., 2012 (77) | LAMB at 10 mg/kg 1×/wk | Prospective; 7 patients | 2 failures (6 of the 7 patients suffered from possible IFD) | No premature discontinuation of LAMB, hypokalemia in 2 patients, premature stop of study due to low patient accrual |
| Annino et al., 2013 (70) | LAMB at 15 mg/kg once, eventually repeated after 15 days of neutropenia | Prospective; 48 patients | Proven IFD in 4 patients (2 IA, 1 Mucor, 1 Geotrichum) | Hypokalemia grade 3 in 6 patients, no other grade 3/4 AEs, 1 patient with early study discontinuation |
| Cornely et al., 2017 (1) | LAMB at 5 mg/kg 2×/wk | Randomized, double blind, placebo controlled; 288 patients, 111 controls | 8/288 proven/probable IFD in LAMB group versus 13/111 proven/probable IFD in placebo group (NS) | 8.4% severe AEs of any grade related to study drug (placebo group, 1.7%; P = 0.02) |
| Bochennek et al., 2015 (79) | Micafungin at 3 or 4 mg/kg 2×/wkb | Retrospective; 21 patients | No proven/probable IFD | No significant AEs |
| Neofytos et al., 2015 (69) | Micafungin at ≥300 mg 2 or 3×/wkb | Retrospective; 83 patients | 4 proven/probable IA cases, 1 Rhodotorula bloodstream infection | No significant AEs |
| Luu Tran et al., 2016 (68) | LAMB at 3 mg/kg 1×/wk | Retrospective; 16 patients, 85 controls receiving triazoles or an echinocandin | Proven/probable IFD in patients receiving LAMB (n = 0), triazoles (n = 1), or echinocandins (n = 2) | No premature discontinuation of LAMB due to infusion reaction, nephrotoxicity, or electrolyte disturbances; no information on creatinine |
LAMB, liposomal amphotericin B; IFD, invasive fungal disease; NS, not significant; AE, adverse event; NEC, necrotizing enterocolitis; ABLC, amphotericin B lipid complex; IA invasive aspergillosis.; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; HSCT, hematopoietic stem cell transplantation; GvHD, graft-versus-host disease.
Reason for strategy: intolerance/contraindication for other antifungal agents.
Micafungin.
Extended dosing intervals of micafungin prophylaxis in adults were analyzed in a single-center, observational, 5-year study including 83 adult allogeneic HSCT recipients who received the compound as antifungal prophylaxis and 21 adult patients in whom micafungin was given as an antifungal treatment (69). All patients received at least five doses of micafungin at a dosage of 300 mg or higher twice or three times weekly. Breakthrough IFD occurred in five patients (6.0%) receiving micafungin prophylactically. None of the patients experienced an infusion-related reaction, and renal and liver function were not impaired by the antifungal compound.
Prospective Studies without Controls
Liposomal amphotericin B.
Three clinical trials prospectively evaluated extended dosing intervals of antifungal prophylaxis with LAMB. One study included 48 adults receiving induction chemotherapy for AML (70). LAMB was given at a dosage of 15 mg/kg once and was repeated in 5 patients after 15 days of persistent neutropenia. Proven IFD was diagnosed in 4 (8.3%) patients. The drug was prematurely discontinued in one patient due to infusion-related toxicity.
Prophylactic LAMB was given once weekly at a dosage of 10 mg/kg to adults with acute leukemia (n = 21) or receiving allogeneic HSCT (n = 8) (71). Proven/probable IFD occurred in 3 leukemia patients and in 1 HSCT recipient, respectively. Whereas no AE related to LAMB led to premature discontinuation in leukemia patients, antifungal prophylaxis was discontinued in 6 of the 8 HSCT patients due to AEs which were considered to be related to the study drug, such as lung and renal problems or anaphylactic shock. Due to the high frequency of grade 3/4 AEs, the study was prematurely stopped in the HSCT group.
Another trial used once-weekly LAMB at a dosage of 7.5 mg/kg as antifungal prophylaxis in 21 adult patients receiving more than 2 mg/kg/day of prednisone for acute GvHD therapy after allogeneic HSCT (72). In one patient, invasive aspergillosis occurred 2 months after discontinuation of the study drug. Seven patients discontinued LAMB prophylaxis due to study drug-related AEs, including hypotension, chest pain, arrhythmia, and elevated serum creatinine.
Randomized Trials
Liposomal amphotericin B.
Three clinical trials investigated extended dosing intervals in LAMB prophylaxis in a prospective randomized fashion (1, 73, 74). An early double-blind and placebo-controlled study evaluated prophylactic LAMB given three times weekly at a dosage of 2 mg/kg to patients receiving chemotherapy (n = 74) or undergoing HSCT (n = 87) (73). Prophylaxis started on day 1 of chemotherapy and was given until neutrophil recovery or until an infection was suspected. As the overall incidence of proven IFD was low (no IFD in patients receiving LAMB and three IFD in controls), no statistically significant difference between the two arms was detected. However, fungal colonization of at least one body site, which was one of the endpoints, was seen in significantly more patients in the placebo arm (15 versus 35 patients). The two study arms did not differ significantly regarding clinically significant AEs and laboratory values.
In an open-label study, 132 patients with hematological malignancies and prolonged neutropenia were randomized to receive either LAMB (50 mg every other day) or placebo (74). When looking at the first neutropenic episode of each patient or at all 219 neutropenic episodes, the incidence of proven/probable IFD was significantly lower in patients receiving LAMB prophylaxis (5 versus 20, P = 0.001, and 5 versus 22, P < 0.01, respectively). The results are clearly limited by the possibility of multiple patient reentries. Due to toxicity, LAMB was prematurely stopped in three patients.
A double-blind placebo-controlled trial enrolled adult patients receiving remission induction chemotherapy for newly diagnosed ALL in order to evaluate the efficacy of prophylactic LAMB given twice weekly at a dosage of 5 mg/kg (1). Eight of 288 patients (7.9%) receiving LAMB experienced a proven/probable IFD compared to 13 of 111 patients of the placebo group (11.7%), which was statistically not significant. The most common drug-related AEs were hypokalemia in 10.5% and increased creatinine in 3.5% of the patients receiving LAMB.
ABLC.
In a prospective randomized study, prophylactic intravenous amphotericin B lipid complex (ABLC) at a dosage of 7.5 mg/kg once weekly was compared to posaconazole administered orally at a dosage of 200 mg three times a day in allogeneic HSCT recipients (75). A total of 19 and 21 adult HSCT recipients received ABLC or posaconazole, respectively, for up to 6 weeks. Definitive IFD occurred in one patient from the ABLC group and in none of the posaconazole group. Significantly more patients doubled their serum creatinine levels to abnormal ranges in the ABLC arm, and therefore, the study was prematurely stopped after an interim analysis.
Pediatric Data
Liposomal amphotericin B.
In a prospective study, LAMB was given as antifungal prophylaxis two times per week at a dosage of 2.5 mg/kg to 46 pediatric patients at high risk for IFD (76). In contrast to 7 children and adolescents of a historical control group who developed proven/probable IFD, no breakthrough IFD occurred in 46 study patients. Acute allergic reactions were seen in four patients, leading to early discontinuation of LAMB prophylaxis. Hypokalemia was observed in 25 of the 187 episodes (13.5%) in which LAMB prophylaxis was administered, whereas there were no significant changes between the baseline and end-of-treatment values of creatinine and liver enzymes.
Only one study investigated secondary antifungal prophylaxis in 7 children who received further immunosuppressive therapy after a diagnosis of deep organ IFD (six of them with possible pulmonary fungal infection) (77). LAMB was administered at a dosage of 10 mg/kg once weekly. Prophylaxis failed in 2 patients, and hypokalemia occurred in 3 patients. The study was prematurely stopped because of low patient accrual.
A randomized, placebo-controlled pediatric study on extended dosing intervals in antifungal prophylaxis outside the cancer setting investigated LAMB given once weekly at a dosage of 5 mg/kg to very low birth weight neonates (n = 40) (78). Enrolled were neonates <32 weeks of gestational age, younger than 7 days old, and with a birth weight of less than 1,500 g. Antifungal therapy was applied until 6 weeks after birth or until the discontinuation of high-risk treatments and invasive devices, whichever occurred first. Analysis was reported in a descriptive way without statistical analysis. Colonization defined as Candida spp. in any surface culture (primary endpoint) was detected in 1 (5%) and 3 (15%) patients in the LAMB and placebo group, respectively. No patient receiving LAMB developed invasive candidiasis, which occurred in 1 patient of the placebo group. No differences between groups were observed regarding the incidence of grade III/IV intraventricular hemorrhage, necrotizing enterocolitis, hypokalemia, and nephrotoxicity.
Micafungin.
Two pediatric centers administered micafungin to 21 children and adolescents at a dosage between 3 and 4 mg/kg twice per week, because these patients did not tolerate or had contraindications to polyenes and triazoles (79). The analysis demonstrated that none of the children and adolescents suffered from proven or probable breakthrough IFD, and in none of the patients were significant clinical AEs observed. The authors assessed plasma trough concentrations in 11 randomly selected patients, 9 of whom had values above 150 ng/ml, a concentration which is thought to be effective for the prophylactic setting.
SUMMARY AND FUTURE DIRECTIONS
There is increasing interest in the strategy of extended dosing regimens in antifungal prophylaxis, in particular in patients undergoing therapy for a hematological malignancy or undergoing allogeneic HSCT. In contrast, intermittent antifungal prophylaxis seems to be less attractive for solid organ transplant recipients or patients with primary immunodeficiencies, as these patients are able to tolerate oral antifungal compounds and usually do not receive medication which is contraindicated with the concurrent use of azoles. Data from both pharmacokinetic/pharmacodynamic analyses and animal studies clearly support this approach, and extended dosing intervals in antifungal prophylaxis using LAMB or micafungin have already been investigated in patients at risk to develop IFD. However, the results of the 15 clinical studies published to date are difficult to interpret, as the compounds were evaluated in various patient populations and at different dosages and schedules, and the few randomized studies had major limitations, such as few IFD in the control arm or the possibility of multiple patient reentries. Therefore, based on data derived by pharmacokinetic/pharmacodynamic modeling, experts should agree on specific doses and dosing schedules, which may then be investigated in sufficiently powered and financed studies. Future research in this area should also evaluate novel and promising compounds (80). Solid clinical data derived by rationally designed pharmacokinetic/pharmacodynamic analyses will help to optimize extended dosing intervals in antifungal prophylaxis, as this strategy might have important implications, in particular in the outpatient setting. For example, this strategy could potentially decrease costs and resource utilization and improve the quality of life of the patient.
ACKNOWLEDGMENTS
Each author was involved in the concept and writing of the manuscript, and the final version of the manuscript was read and approved by each author.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
T.L. has received research grants from Gilead Sciences, is a consultant to Astellas, Basilea, Gilead Sciences, and Merck/MSD, and served at the speaker’s bureaus of Astellas, Gilead Sciences, Merck/MSD, and Pfizer. G.H. received grants from Bayer AG and Medac and served at the speaker’s bureau of Grünenthal. A.H.G. has received grants from Gilead, Merck, Sharp & Dohme, Pfizer, and Schering-Plough, is a consultant to Amplyx, Astellas, Basilea, Gilead, Merck, Sharp & Dohme, and Schering-Plough, and served at the speaker’s bureaus of Astellas, Basilea, Gilead, Merck, Sharp & Dohme, Pfizer, Schering-Plough, and Zeneus/Cephalon. All the other authors do not have a conflict of interest to declare.
Biographies

Thomas Lehrnbecher is Professor of Pediatrics and has worked since 2001 as a pediatric hematologist and oncologist in the Johann Wolfgang Goethe-University, Frankfurt, Germany. He trained in the Children’s Hospital in Würzburg, Germany and from 1996 to 1999 at the Pediatric Oncology Branch of the National Cancer Institute, Bethesda, MD, USA. His clinical and laboratory research focuses on infectious complications in children with cancer, the development of immunotherapeutic strategies against invasive fungal infections in hematopoietic stem cell transplant recipients, and the interaction of host immune cells and fungi. Dr. Lehrnbecher currently chairs the Infection in the Immunocompromised Child sections of both the German Society of Pediatric Infectious Diseases and the German Society of Pediatric Oncology and Hematology and, until 2016, the Supportive Care section of the International Society of Pediatric Oncology. Since 2018, he has been a member of the International Council of the Immunocompromised Host Society.

Konrad Bochennek is a consultant at the department of Pediatric Hematology and Oncology at the J.W. Goethe University Hospital in Frankfurt am Main. He trained in the University of Frankfurt, including laboratory research in the laboratory of immunology and stem cell transplantation in the children’s hospital and the Georg-Speyer-Institute in Frankfurt am Main. He is board examined for pediatrics, for pediatric hematology and oncology, and for palliative care in childhood. His research focus includes immunotherapy against different malignancies in childhood and infectious diseases in immunocompromised children, as well as clinical didactics and medical education.

Thomas Klingebiel studied medicine from 1974 to 1980 at the Philipps University in Marburg and the Medical School Lübeck, Germany. He was board certified as Pediatrician in 1988 and as Pediatric Hematologist/Oncologist in 2006. Since 2000, he has been a full professor of pediatrics, and he is the Head of the Department for Children and Adolescents at Frankfurt University Hospital and Vice Dean of the Medical Faculty. Dr. Klingebiel’s main research areas are hematopoietic stem cell transplantation and the treatment of soft tissue sarcoma, where he was the study chair of the German Soft Tissue Sarcoma Group until recently.

Silke Gastine is a research fellow at the University College London—Great Ormond Street Institute of Child Health in the pediatric infectious diseases research group. Her postgraduate training includes a Ph.D. in clinical pharmacy and pharmacometrics at the department of pharmaceutical chemistry at the Westfälische Wilhelms-Universität Münster, as well as a research stay with the Center for Antimicrobial Pharmacodynamics at the University of Liverpool. After completion of her pharmaceutical training at the Johann Wolfgang Goethe-Universität Frankfurt am Main, she worked as a ward-based pharmacist at the University Children’s Hospital Münster with focus on neonatology and pediatric intensive care. Dr. Gastine’s main research goals are individualized dosing using pharmacometric tools, as well as pharmacokinetic and pharmacodynamic modeling in special populations with a strong focus on pediatric infectious diseases.

Georg Hempel is a research group leader at the Department of Medical and Pharmaceutical Chemistry and responsible for teaching in Clinical Pharmacy. He received his Ph.D. in Pharmaceutical Chemistry in 1995 at the University of Münster. After a postdoctoral position with Eli Lilly in London, United Kingdom, he joined the research group of Professor Joachim Boos in the Department of Pediatric Oncology. In 2002, he received his venia legendi in Pharmaceutical Chemistry and Clinical Pharmacy. In 2008, he was nominated as außerplanmäßiger Professor at the Faculty of Chemistry and Pharmacy of the University of Münster. The goal of his research is the optimization of drug therapy in patients with cancer. Dose and schedule optimization using pharmacometric models is the main focus. He was involved in several international clinical studies, is an active member in the EORTC, the Central European Association of Cancer Research, and the German Pharmaceutical Society, and has published more than 100 peer-reviewed manuscripts.

Andreas H. Groll is Professor of Pediatrics, Head of the Infectious Disease Research Programme, and Deputy Director of the Department of Haematology/Oncology at the University Children’s Hospital in Münster, Germany. Dr. Groll’s postgraduate education included fellowships in Infectious Diseases at the Children's Hospital in Boston and in Pediatric Hematology/Oncology at the National Cancer Institute in Bethesda, Maryland. Dr. Groll is board certified in pediatrics, pediatric hematology/oncology, and infectious diseases. Dr. Groll’s research interests include infectious complications in the immunocompromised host, particularly invasive fungal infections, the pharmacokinetics and pharmacodynamics of antimicrobial agents, and the design and conduct of clinical research studies. He is a member of several international medical societies, is on the editorial board of several international journals, and has published more than 200 scientific articles thus far. He is regularly engaged in patient care as Attending Physician of the inpatient and outpatient service and the hematopoietic stem cell transplant program.
REFERENCES
- 1.Cornely OA, Leguay T, Maertens J, Vehreschild M, Anagnostopoulos A, Castagnola C, Verga L, Rieger C, Kondakci M, Harter G, Duarte RF, Allione B, Cordonnier C, Heussel CP, Morrissey CO, Agrawal SG, Donnelly JP, Bresnik M, Hawkins MJ, Garner W, Gokbuget N, AmBiGuard Study Group. 2017. Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia. J Antimicrob Chemother 72:2359–2367. doi: 10.1093/jac/dkx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. 2007. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood 110:3532–3539. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 3.Hol JA, Wolfs TF, Bierings MB, Lindemans CA, Versluys AB, de Wildt A, Gerhardt CE, Boelens JJ. 2014. Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 49:95–101. doi: 10.1038/bmt.2013.136. [DOI] [PubMed] [Google Scholar]
- 4.Ambasta A, Carson J, Church DL. 2015. The use of biomarkers and molecular methods for the earlier diagnosis of invasive aspergillosis in immunocompromised patients. Med Mycol 53:531–557. doi: 10.1093/mmy/myv026. [DOI] [PubMed] [Google Scholar]
- 5.Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T. Fourth European Conference on Infections in Leukemia, Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP), Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG), International Immunocompromised Host Society (ICHS), European Leukaemia Net (ELN). 2014. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 15:e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 6.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rausch CR, Kontoyiannis DP. 2019. Prolonged voriconazole treatment in a patient with chronic lymphocytic leukemia resulting in a litany of chronic overlapping toxicities. J Oncol Pharm Pract 25:747–753. doi: 10.1177/1078155218762624. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 10.Hope WW, Goodwin J, Felton TW, Ellis M, Stevens DA. 2012. Population pharmacokinetics of conventional and intermittent dosing of liposomal amphotericin B in adults: a first critical step for rational design of innovative regimens. Antimicrob Agents Chemother 56:5303–5308. doi: 10.1128/AAC.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbo T. 2015. Single or 2-dose micafungin regimen for treatment of invasive candidiasis: therapia sterilisans magna! Clin Infect Dis 61:S635–S642. doi: 10.1093/cid/civ715. [DOI] [PubMed] [Google Scholar]
- 12.Ralph ED, Khazindar AM, Barber KR, Grant CW. 1991. Comparative in vitro effects of liposomal amphotericin B, amphotericin B-deoxycholate, and free amphotericin B against fungal strains determined by using MIC and minimal lethal concentration susceptibility studies and time-kill curves. Antimicrob Agents Chemother 35:188–191. doi: 10.1128/AAC.35.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnidge JD, Gudmundsson S, Vogelman B, Craig WA. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J Antimicrob Chemother 34:83–92. doi: 10.1093/jac/34.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Klepser ME, Wolfe EJ, Jones RN, Nightingale CH, Pfaller MA. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother 41:1392–1395. doi: 10.1128/AAC.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst EJ, Klepser ME, Pfaller MA. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother 44:1108–1111. doi: 10.1128/AAC.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll AH, Piscitelli SC, Walsh TJ. 2001. Antifungal pharmacodynamics: concentration-effect relationships in vitro and in vivo. Pharmacotherapy 21:133S–148S. doi: 10.1592/phco.21.12.133S.34507. [DOI] [PubMed] [Google Scholar]
- 17.Andes D, Stamsted T, Conklin R. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother 45:922–926. doi: 10.1128/AAC.45.3.922-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 50:469–473. doi: 10.1128/AAC.50.2.469-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Nakeeb Z, Petraitis V, Goodwin J, Petraitiene R, Walsh TJ, Hope WW. 2015. Pharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 59:2735–2745. doi: 10.1128/AAC.04723-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groll AH, Piscitelly TJ, Walsh TJ. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 44:343–500. doi: 10.1016/S1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 21.Stone NR, Bicanic T, Salim R, Hope W. 2016. Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 76:485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler-Moore JP, Proffitt RT, Olson JA, Jensen GM. 2017. Tissue pharmacokinetics and pharmacodynamics of AmBisome(R) (L-AmBis) in uninfected and infected animals and their effects on dosing regimens. J Liposome Res 27:195–209. doi: 10.1080/08982104.2017.1327543. [DOI] [PubMed] [Google Scholar]
- 23.van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 24.Gomes MZ, Jiang Y, Mulanovich VE, Lewis RE, Kontoyiannis DP. 2014. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob Agents Chemother 58:2775–2780. doi: 10.1128/AAC.01527-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T, Hong HL, Lee YM, Sung H, Kim SH, Choi SH, Kim YS, Woo JH, Lee SO. 2013. Is caspofungin really an effective treatment for Pneumocystis jirovecii pneumonia in immunocompromised patients without human immunodeficiency virus infection? Experiences at a single center and a literature review. Scand J Infect Dis 45:484–488. doi: 10.3109/00365548.2012.760842. [DOI] [PubMed] [Google Scholar]
- 26.Groll AH, Schrey D, Walsh TJ. 2011. Echinocandins, p 95–112. In Kauffman CA, Sobel JD, Pappas PG, Dismukes WE (ed) Essentials of clinical mycology, 2nd ed Springer, New York, NY. [Google Scholar]
- 27.Groll AH, Mickiene D, Petraitiene R, Petraitis V, Lyman CA, Bacher JS, Piscitelli SC, Walsh TJ. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother 45:2845–2855. doi: 10.1128/AAC.45.10.2845-2855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hope W, Drusano GL, Rex JH. 2016. Pharmacodynamics for antifungal drug development: an approach for acceleration, risk minimization and demonstration of causality. J Antimicrob Chemother 71:3008–3019. doi: 10.1093/jac/dkw298. [DOI] [PubMed] [Google Scholar]
- 29.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis 190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 30.James KD, Laudeman CP, Malkar NB, Krishnan R, Polowy K. 2017. Structure-activity relationships of a series of echinocandins and the discovery of CD101, a highly stable and soluble echinocandin with distinctive pharmacokinetic properties. Antimicrob Agents Chemother 61:e01541-16. doi: 10.1128/AAC.01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong V, Hough G, Schlosser M, Bartizal K, Balkovec JM, James KD, Krishnan BR. 2016. Preclinical evaluation of the stability, safety, and efficacy of CD101, a novel echinocandin. Antimicrob Agents Chemother 60:6872–6879. doi: 10.1128/AAC.00701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2016. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. 2017. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents 50:352–358. doi: 10.1016/j.ijantimicag.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Perez WB, Jimenez-Ortigosa C, Hough G, Locke JB, Ong V, Bartizal K, Perlin DS. 2016. CD101: a novel long-acting echinocandin. Cell Microbiol 18:1308–1316. doi: 10.1111/cmi.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandison T, Ong V, Lee J, Thye D. 2017. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 61:e01627-16. doi: 10.1128/AAC.01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong V, James KD, Smith S, Krishnan BR. 2017. Pharmacokinetics of the novel echinocandin CD101 in multiple animal species. Antimicrob Agents Chemother 61:e01626-16. doi: 10.1128/AAC.01626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP. 2007. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother 59:941–951. doi: 10.1093/jac/dkm077. [DOI] [PubMed] [Google Scholar]
- 38.Lewis RE, Albert ND, Kontoyiannis DP. 2008. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 52:4178–4180. doi: 10.1128/AAC.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia A, Adler-Moore JP, Proffitt RT. 2000. Single-dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob Agents Chemother 44:2327–2332. doi: 10.1128/AAC.44.9.2327-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adler-Moore JP, Olson JA, Proffitt RT. 2004. Alternative dosing regimens of liposomal amphotericin B (AmBisome) effective in treating murine systemic candidiasis. J Antimicrob Chemother 54:1096–1102. doi: 10.1093/jac/dkh460. [DOI] [PubMed] [Google Scholar]
- 41.Albert MM, Adams K, Luther MJ, Sun SH, Graybill JR. 1994. Efficacy of AmBisome in murine coccidioidomycosis. J Med Vet Mycol 32:467–471. doi: 10.1080/02681219480000621. [DOI] [PubMed] [Google Scholar]
- 42.Clemons KV, Stevens DA. 1998. Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother 42:899–902. doi: 10.1128/AAC.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graybill JR, Bocanegra R. 1995. Liposomal amphotericin B therapy of murine histoplasmosis. Antimicrob Agents Chemother 39:1885–1887. doi: 10.1128/AAC.39.8.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gangneux JP, Sulahian A, Garin YJ, Farinotti R, Derouin F. 1996. Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisome. Antimicrob Agents Chemother 40:1214–1218. doi: 10.1128/AAC.40.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumbo T, Drusano GL, Liu W, Kulawy RW, Fregeau C, Hsu V, Louie A. 2007. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother 51:968–974. doi: 10.1128/AAC.01337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petraitiene R, Petraitis V, Hope WW, Walsh TJ. 2015. Intermittent dosing of micafungin is effective for treatment of experimental disseminated candidiasis in persistently neutropenic rabbits. Clin Infect Dis 61:S643–S651. doi: 10.1093/cid/civ817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. 2018. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother 62:e02154-17. doi: 10.1128/AAC.02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bader JC, Bhavnani SM, Andes DR, Ambrose PG. 2018. We can do better: a fresh look at echinocandin dosing. J Antimicrob Chemother 73:i44–i50. doi: 10.1093/jac/dkx448. [DOI] [PubMed] [Google Scholar]
- 49.Walsh TJ, Yeldandi V, McEvoy M, Gonzalez C, Chanock S, Freifeld A, Seibel NI, Whitcomb PO, Jarosinski P, Boswell G, Bekersky I, Alak A, Buell D, Barret J, Wilson W. 1998. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother 42:2391–2398. doi: 10.1128/AAC.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M, Barrett J, Anaissie EJ. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother 45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seibel NL, Shad AT, Bekersky I, Groll AH, Gonzalez C, Wood LV, Jarosinski P, Buell D, Hope WW, Walsh TJ. 2017. Safety, tolerability, and pharmacokinetics of liposomal amphotericin B in immunocompromised pediatric patients. Antimicrob Agents Chemother 61:e01477-16. doi: 10.1128/AAC.01477-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lestner JM, Groll AH, Aljayyoussi G, Seibel NL, Shad A, Gonzalez C, Wood LV, Jarosinski PF, Walsh TJ, Hope WW. 2016. Population pharmacokinetics of liposomal amphotericin B in immunocompromised children. Antimicrob Agents Chemother 60:7340–7346. doi: 10.1128/AAC.01427-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehrnbecher T, Robinson P, Fisher B, Alexander S, Ammann RA, Beauchemin M, Carlesse F, Groll AH, Haeusler GM, Santolaya M, Steinbach WJ, Castagnola E, Davis BL, Dupuis LL, Gaur AH, Tissing WJE, Zaoutis T, Phillips R, Sung L. 2017. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol 35:2082–2094. doi: 10.1200/JCO.2016.71.7017. [DOI] [PubMed] [Google Scholar]
- 54.Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, Frere P, Gachot B, Heinz WJ, Lass-Florl C, Ribaud P, Thiebaut A, Cordonnier C, Third European Conference on Infections in Leukemia. 2011. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 update. Bone Marrow Transplant 46:709–718. doi: 10.1038/bmt.2010.175. [DOI] [PubMed] [Google Scholar]
- 55.Bruggemann RJ, Van Der Velden WJ, Knibbe CA, Colbers A, Hol S, Burger DM, Donnelly JP, Blijlevens NM. 2015. A rationale for reduced-frequency dosing of anidulafungin for antifungal prophylaxis in immunocompromised patients. J Antimicrob Chemother 70:1166–1174. doi: 10.1093/jac/dku477. [DOI] [PubMed] [Google Scholar]
- 56.Benjamin DK Jr, Driscoll T, Seibel NL, Gonzalez CE, Roden MM, Kilaru R, Clark K, Dowell JA, Schranz J, Walsh TJ. 2006. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother 50:632–638. doi: 10.1128/AAC.50.2.632-638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, Herbrecht R, Ruiz-Palacios G, Young JA, Baddley JW, Strohmaier KM, Tucker KA, Taylor AF, Kartsonis NA, Caspofungin High-Dose Study Group. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 48:1676–1684. doi: 10.1086/598933. [DOI] [PubMed] [Google Scholar]
- 58.Cornely OA, Vehreschild JJ, Vehreschild MJ, Wurthwein G, Arenz D, Schwartz S, Heussel CP, Silling G, Mahne M, Franklin J, Harnischmacher U, Wilkens A, Farowski F, Karthaus M, Lehrnbecher T, Ullmann AJ, Hallek M, Groll AH. 2011. Phase II dose escalation study of caspofungin for invasive Aspergillosis. Antimicrob Agents Chemother 55:5798–5803. doi: 10.1128/AAC.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wurthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJ, Farowski F, Muller C, Boos J, Hempel G, Hallek M, Groll AH. 2013. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 57:1664–1671. doi: 10.1128/AAC.01912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh TJ, Adamson PC, Seibel NL, Flynn PM, Neely MN, Schwartz C, Shad A, Kaplan SL, Roden MM, Stone JA, Miller A, Bradshaw SK, Li SX, Sable CA, Kartsonis NA. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother 49:4536–4545. doi: 10.1128/AAC.49.11.4536-4545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stader F, Wuerthwein G, Groll AH, Vehreschild JJ, Cornely OA, Hempel G. 2015. Physiology-based pharmacokinetics of caspofungin for adults and paediatrics. Pharm Res 32:2029–2037. doi: 10.1007/s11095-014-1595-9. [DOI] [PubMed] [Google Scholar]
- 62.Hiemenz J, Cagnoni P, Simpson D, Devine S, Chao N, Keirns J, Lau W, Facklam D, Buell D. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother 49:1331–1336. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sirohi B, Powles RL, Chopra R, Russell N, Byrne JL, Prentice HG, Potter M, Koblinger S. 2006. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 64.Muilwijk E, Maertens J, van der Velde W, Ter Heine R, Colbers A, Burger D, Andes D, Theunissen K, Blijlevens N, Brüggemann R. 2018. Pharmacokinetics of extended dose intervals of micafungin in haematology patients: optimizing antifungal prophylaxis, abstr O0794. Abstr 28th Eur Congr Clin Microbiol Infect Dis (ECCMID), Madrid, Spain, 21 to 24 April 2018. [DOI] [PubMed]
- 65.Seibel NL, Schwartz C, Arrieta A, Flynn P, Shad A, Albano E, Keirns J, Lau WM, Facklam DP, Buell DN, Walsh TJ. 2005. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother 49:3317–3324. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith PB, Walsh TJ, Hope W, Arrieta A, Takada A, Kovanda LL, Kearns GL, Kaufman D, Sawamoto T, Buell DN, Benjamin DK Jr. 2009. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 28:412–415. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.European Medicines Agency (EMA). 2009. Mycamine. Annex I. Summary of product characteristics, 1 March 2018 update https://www.ema.europa.eu/en/documents/product-information/mycamine-epar-product-information_en.pdf. Accessed 18 April 2019.
- 68.Luu Tran H, Mahmoudjafari Z, Rockey M, Henry D, Grauer D, Aljitawi O, Abhyankar S, Ganguly S, Lin T, McGuirk J. 2016. Tolerability and outcome of once weekly liposomal amphotericin B for the prevention of invasive fungal infections in hematopoietic stem cell transplant patients with graft-versus-host disease. J Oncol Pharm Pract 22:228–234. doi: 10.1177/1078155214560920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neofytos D, Huang YT, Cheng K, Cohen N, Perales MA, Barker J, Giralt S, Jakubowski A, Papanicolaou G. 2015. Safety and efficacy of intermittent intravenous administration of high-dose micafungin. Clin Infect Dis 61:S652–S661. doi: 10.1093/cid/civ818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Annino L, Chierichini A, Anaclerico B, Finolezzi E, Norata M, Cortese S, Cassetta MI, Fallani S, Novelli A, Girmenia C. 2013. Prospective phase II single-center study of the safety of a single very high dose of liposomal amphotericin B for antifungal prophylaxis in patients with acute myeloid leukemia. Antimicrob Agents Chemother 57:2596–2602. doi: 10.1128/AAC.00155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cordonnier C, Mohty M, Faucher C, Pautas C, Robin M, Vey N, Monchecourt F, Mahi L, Ribaud P. 2008. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME Study. Int J Antimicrob Agents 31:135–141. doi: 10.1016/j.ijantimicag.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 72.El-Cheikh J, Faucher C, Furst S, Duran S, Berger P, Vey N, Stoppa AM, Bouabdallah R, Gastaut JA, Viens P, Blaise D, Mohty M. 2007. High-dose weekly liposomal amphotericin B antifungal prophylaxis following reduced-intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant 39:301–306. doi: 10.1038/sj.bmt.1705592. [DOI] [PubMed] [Google Scholar]
- 73.Kelsey SM, Goldman JM, McCann S, Newland AC, Scarffe JH, Oppenheim BA, Mufti GJ. 1999. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant 23:163–168. doi: 10.1038/sj.bmt.1701543. [DOI] [PubMed] [Google Scholar]
- 74.Penack O, Schwartz S, Martus P, Reinwald M, Schmidt-Hieber M, Thiel E, Blau IW. 2006. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single-center trial. Ann Oncol 18:1306–1312. doi: 10.1093/annonc/mdl128. [DOI] [PubMed] [Google Scholar]
- 75.Chaftari AM, Hachem RY, Ramos E, Kassis C, Campo M, Jiang Y, Prince RA, Wang W, Raad II. 2012. Comparison of posaconazole versus weekly amphotericin B lipid complex for the prevention of invasive fungal infections in hematopoietic stem-cell transplantation. Transplantation 94:302–308. doi: 10.1097/TP.0b013e3182577485. [DOI] [PubMed] [Google Scholar]
- 76.Bochennek K, Tramsen L, Schedler N, Becker M, Klingebiel T, Groll AH, Lehrnbecher T. 2011. Liposomal amphotericin B twice weekly as antifungal prophylaxis in paediatric haematological malignancy patients. Clin Microbiol Infect 17:1868–1874. doi: 10.1111/j.1469-0691.2011.03483.x. [DOI] [PubMed] [Google Scholar]
- 77.Ginocchio F, Faraci M, Fioredda F, Moroni C, Caviglia I, Barabino P, Haupt R, Castagnola E. 2012. Weekly high-dose liposomal amphotericin B for secondary prophylaxis of invasive fungal disease in immunocompromised children: experience from a pediatric case series. J Chemother 24:243–244. doi: 10.1179/1973947812Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 78.Arrieta AC, Shea K, Dhar V, Cleary JP, Kukreja S, Morris M, Vargas-Shiraishi OM, Ashouri N, Singh J. 2010. Once-weekly liposomal amphotericin B as Candida prophylaxis in very low birth weight premature infants: a prospective, randomized, open-label, placebo-controlled pilot study. Clin Ther 32:265–271. doi: 10.1016/j.clinthera.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Bochennek K, Balan A, Muller-Scholden L, Becker M, Farowski F, Muller C, Groll AH, Lehrnbecher T. 2015. Micafungin twice weekly as antifungal prophylaxis in paediatric patients at high risk for invasive fungal disease. J Antimicrob Chemother 70:1527–1530. doi: 10.1093/jac/dku544. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Lara MF, Sifuentes-Osornio J, Ostrosky-Zeichner L. 2017. Drugs in clinical development for fungal infections. Drugs 77:1505–1518. doi: 10.1007/s40265-017-0805-2. [DOI] [PubMed] [Google Scholar]
- 81.European Medicines Agency (EMA). 2009. Ecalta. Annex I. Summary of product characteristics, 22 January 2019 update https://www.ema.europa.eu/en/documents/product-information/ecalta-epar-product-information_en.pdf. Accessed 18 April 2019.
- 82.European Medicines Agency (EMA). 2009. Cancidas (previously Caspofungin MSD). Annex I. Summary of product characteristics, 10 April 2019 update https://www.ema.europa.eu/en/documents/product-information/cancidas-epar-product-information_en.pdf. Accessed 18 April 2019.
- 83.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother 46:828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]