Abstract
For more than a century, studies on tunicate muscle formation have revealed many principles of cell fate specification, gene regulation, morphogenesis, and evolution. Here, we review the key studies that have probed the development of all the various muscle cell types in a wide variety of tunicate species. We seize this occasion to explore the implications and questions raised by these findings in the broader context of muscle evolution in chordates.
Keywords: Muscle, Maternal determinants, Myoplasm, MRF, Cardiopharyngeal mesoderm, Tunicates, Ascidians, Neuromesodermal
Introduction
Muscles are formed by cells that contract through actin–myosin interactions. This common mechanism is performed with deep variation according to muscle type, within the same organism as well as across taxa [303, 307]. For instance, the human body contains several hundred distinct muscles, including several dozens in the neck and the head [47, 278]. In vertebrates, muscles can be classified into three major categories according to their structure regardless of their developmental and evolutionary origins: smooth muscles, cardiac striated muscles, and non-cardiac striated muscles, of which the large majority are skeletal muscles [303]. Muscle striations refer to repeated contractile actin–myosin units called sarcomeres [148, 149]. However, phylogenomic comparisons between bilaterians and cnidarians revealed that striated muscles emerged independently in both groups, suggesting that close structural similarities do not necessarily indicate homology of muscle cell types [307]. Likewise, striated cardiac and non-cardiac muscles might have arisen independently in distinct groups of bilaterians [45], further complicating already complex evolutionary scenarios. To help illuminate the evolutionary history of muscles, especially in chordates, we present here a detailed review of what is known about muscle development in the tunicates.
Tunicates (Fig. 1) are the extant invertebrates most closely related to us [81]. They are the sister group to the vertebrates, with whom they form a monophyletic group known as Olfactores [158]. The tunicates are a large group of marine organisms, most of them sessile, though many are pelagic. The vast majority of the Tunicata are suspension filter feeders, though there are some carnivorous deep sea tunicates [223]. What unites the tunicates and gives them their name is a thick outer covering, or tunic, made in large part of crystalline cellulose fibrils [171]. They are in fact the only animals capable of synthesizing cellulose, thanks to a single horizontal gene transfer event that introduced the Cellulose synthase (celA) gene from some ancient prokaryote into the genome of the ancestral tunicate [204, 229, 282]. It is possible that the newly acquired ability of the epidermis to synthesize a protective mantle allowed tunicates to eschew the mobility or reclusiveness of their presumed vermiform ancestors [281].
Fig. 1.
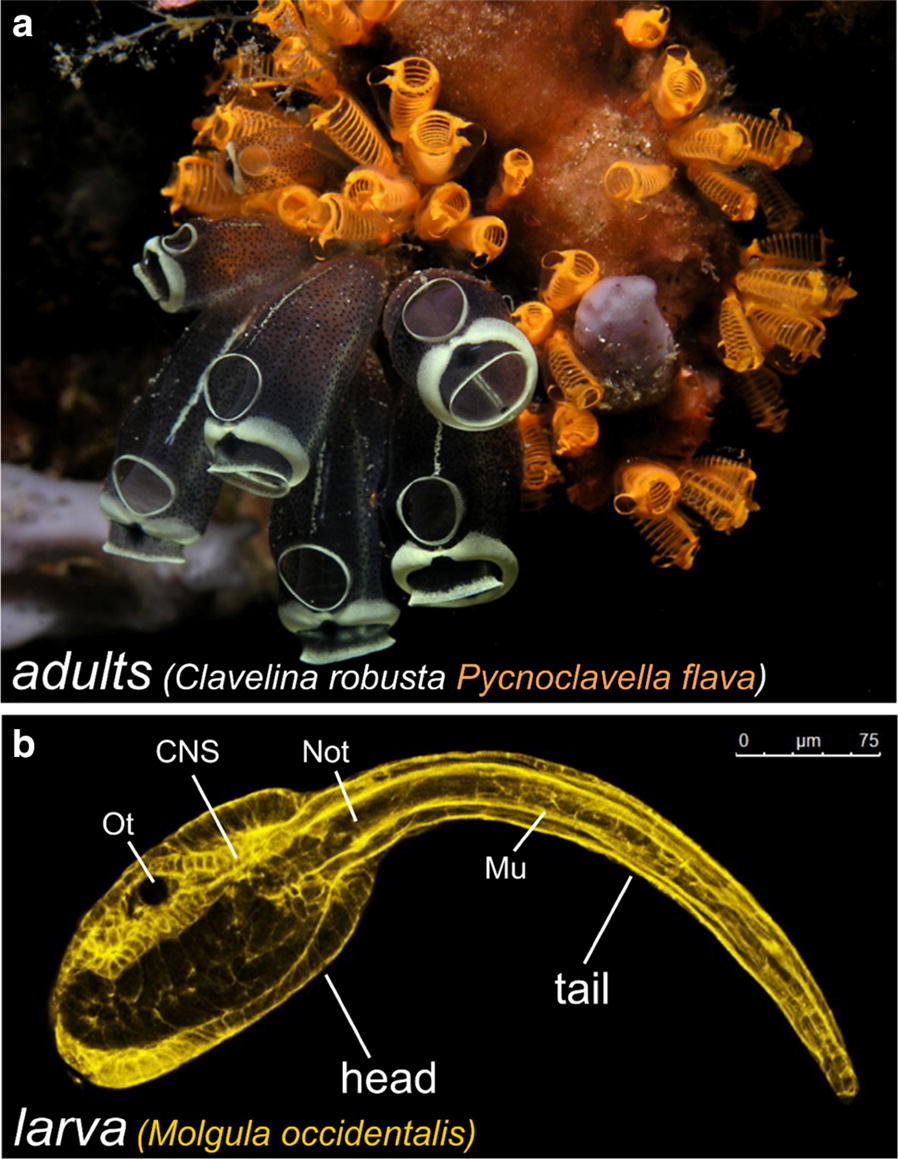
Tunicates. a A cluster of adult ascidians (benthic tunicates): Clavelina robusta (black and white) and Pycnoclavella flava (orange). Image by Nick Hobgood (https://commons.wikimedia.org/w/index.php?curid=5616579). b Tadpole larva of Molgula occidentalis, stained with Phalloidin-Alexa Fluor 546. Anterior to the left. Ot, otolith; CNS, central nervous system; Not, notochord; Mu, tail muscles
Tunicates have long been the intense subject of biological inquiry, from the seminal descriptive embryology carried out by Kovalevsky 150 years ago [177], to the emergence of experimental embryology following the micromanipulations of Chabry [53] and more lately molecular developmental genetics in the post-genomic era [284]. Their many advantages as laboratory organisms have carried the tunicates through the centuries of experimental biology, and their status as the sister group to the vertebrates has helped secure a spot for them at the biomedical research table. Studies in tunicates have helped establish basic concepts in developmental biology such as invariant lineages and mosaic development [64, 66, 191, 352], and have shed light on transcriptional and cellular mechanisms of development [26, 59, 68, 69, 84, 101, 136, 167, 226, 234, 262, 265, 323]. Furthermore, comparative studies using tunicates have refined models of chordate and vertebrate evolution [1, 2, 9, 49, 92, 112, 156, 192, 205, 275, 311, 345].
Here, we specifically review the many studies that have focused on the development of tunicate muscles. We will cover what is known about the genetic and molecular basis of muscle cell specification and differentiation in tunicates, and how this knowledge has contributed to our broader understanding of gene regulation, evolution, and development in animals. While certain inferences about chordate evolution have been drawn by comparing muscle development between vertebrates and tunicates, inter- and intra-specific comparisons between different tunicate muscles continuously hint at the fascinating, but enigmatic evolutionary history of the tunicates themselves.
Muscle anatomy in ascidians
Most of our knowledge on the regulation and evolution of muscle formation in tunicates has been coaxed from solitary ascidians, both in their “swimming tadpole” larval stage (Fig. 1b) and in their sessile adult stage (Fig. 1a). Ascidians comprise a polyphyletic group of benthic, sessile tunicates distributed in several distantly related families [297]. Here, we review the basics of muscle anatomy in this group, since they are the most numerous and well-studied of the tunicates. However, this knowledge is also indispensable to the larger discussion of muscle evolution within the tunicates, since even pelagic groups such as the thaliaceans and appendicularians are thought to have evolved from an ascidian-like ancestor.
The ascidian larva
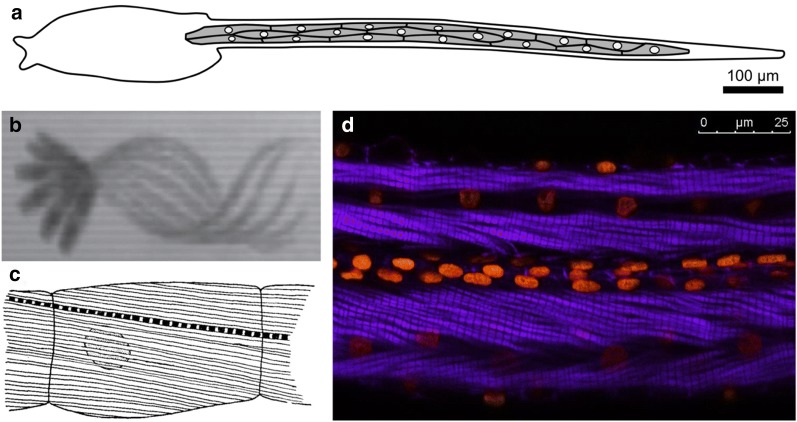
The swimming larva represents the dispersal phase of the ascidian life cycle. Breeding populations of sessile adults depend upon this mobility to settle new locations. The larval stage is when the chordate affinity of the tunicates is most obvious, as the ascidian larva has a body plan that has been described as “tadpole-like” (Fig. 1b). The larval body plan is roughly divided into a head (sometimes referred to as “trunk”) and a tail, though these terms do not accurately describe homology to similar structures in other chordate body plans. While the trunk/head contains most of the undifferentiated primordia of the juvenile and adult body [141], the “tail” is primarily composed of differentiated cells purposed for the swimming behavior of the larva. Among these are the chordate-defining notochord, which functions as an axial hydrostatic “skeleton” [123], neurons involved in swimming or touch sensing [196], and the larval tail muscles. In the larvae of solitary tunicates such as Ciona and Halocynthia, two groups, or “bands” of striated, mononucleate muscle cells flank the notochord (Fig. 2a). These two lateral muscle bands contract alternatively to bend the tail laterally, and the alternated left/right contractions result in the whiplike beating of the tail to propel the larva forward (Fig. 2b) [30, 41, 244]. Asymmetric tail flicks in one or the other direction serve to re-orient the larva in the water column and are driven by graded control of muscle contraction by specialized acetylcholine receptors expressed by the tail muscles [30, 243]. In most solitary ascidians, there are 18–21 muscle cells on either side of the tail, depending on the species. These cells are electrically coupled to one another, and their myofibrils are connected between cells via intercellular structures resembling fascia adherens junctions between striated cardiomyocytes in vertebrates (Fig. 2c, d) [21, 123, 334]. As a result, all muscle cells on the same side of the larva behave as a single syncitium, with each band comprising a discrete functional unit.
Fig. 2.
Larval tail muscles and swimming behavior. a Diagram of a Ciona robusta tadpole, showing the arrangement of 18 mononucleated muscle cells in a muscle band on the one side of the tail. b Overlaid images taken at 5-millisecond intervals, showing half of a tail beat in the repetitive swimming behavior of the Ciona tadpole. c Illustration of a tail muscle cell in the larva of Aplidium constellatum, showing the oblique position of the myofibrils relative to the anterior–posterior axis of the cell, and the continuous nature of the striated fibrils from cell to cell. d Image of Diplosoma listerianum larva tail stained with phalloidin-Alexa Fluor 488 (myofibrils, purple) and DAPI (nuclei, orange). a and b Adapted from Nishino et al. [244]. c Adapted from Grave [123]
Adult and juvenile ascidians
While only the swimming larva is truly motile, the sessile juveniles and adults are not devoid of muscles. Their musculature consists mostly of muscle fibers of the body wall, which cover the mantle as well as the siphons (Fig. 3a, b) and cardiomyocytes of the heart (Fig. 3c). There are also two rarely reported muscles, about which very little is known: a small sphincter muscle associated with thin longitudinal fibers around the anal region of the digestive tract might assist defecation, and a specific sphincter muscle around the gonoduct of the adult might control the release of the gametes [118, 273].
Fig. 3.
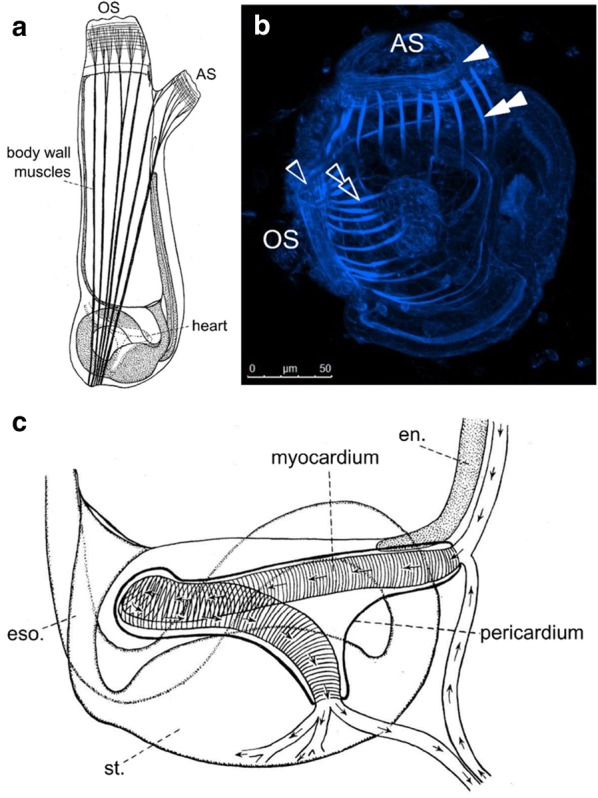
Siphon, body wall, and cardiac muscles of adult ascidians. a Diagram of an adult Ciona intestinalis, showing the muscle fibers of oral siphon (OS), atrial siphon (AS), and body wall muscles. Illustration adapted from Berrill [25]. b Juvenile Molgula occidentalis, stained with Phalloidin-Alexa Fluor 488 to visualize developing myofibril bundles. Hollow arrowhead: oral siphon muscle rings. Hollow double arrowhead, oral siphon-derived latitudinal body wall muscles; solid arrowhead, atrial siphon muscles; solid double arrowhead, atrial siphon-derived longitudinal body wall muscles; OS, oral siphon; AS, atrial siphon. c Diagram of the heart of an adult Ciona intestinalis. Arrows indicate blood flow in one direction, although ascidian hearts will periodically reverse their beat and pump blood in the opposite direction. Eso, esophagus; en, endoderm; st, stomach.
Tunicate body wall muscles control a limited number of movements of the adult, mainly opening and closing of the siphons by circular atrial and oral siphon muscles, retraction of the siphons and body by longitudinal and/or latitudinal body wall muscles, and contraction of the mantle wall by circular body wall muscles. The retraction of the body, named cowering or withdrawal, can be as much as 50% the total length in Ciona [118, 233]. The rapid contraction of the mantle wall results in the eponymous squirting of water from the branchial and atrial cavities through the siphons. Although the constant flow of water required for filtering food is assured by the movements of the ciliated gills and not by the muscles, periodic muscle contraction and squirting appears important to expel particles that cannot be digested (see video, http://videotheque.cnrs.fr/doc=44). In some species, longitudinal muscles bend the body instead of retracting it [18].
Body wall muscle bands are comprised of a small number of strands, each of which in turn is composed of many bundles of individual unstriated, multinucleated muscle fibers. In Halocynthia, up to 50 nuclei may be found in the largest fibers [233, 299]. The variation in the orientation of body wall muscle bands across different species notwithstanding [224], the overall anatomy of body wall muscles is thought to be shared in most ascidians. The two distantly related genera Ciona and Halocynthia, in which the musculature has been the most precisely described, display similarities which suggest a common ancestral form [218, 233, 299]. As mentioned, ascidian body wall muscles are smooth, not striated: their thin and thick filaments do not form sarcomeres [233, 299, 326]. However, like vertebrate skeletal muscles, they are multinucleated. In vertebrates, skeletal myofibers are multinucleated due to the fusion of multiple myoblasts [219]. Whether the multinucleation of the tunicate body wall muscles is the result of repeated nuclear divisions or the fusion of several myoblasts as in vertebrate skeletal muscle is yet to be investigated.
Although they lack sarcomeres, ascidian body wall muscles are not homologous to vertebrate smooth muscles. Smooth muscles are primarily defined by the absence of sarcomeres but do not form a homogeneous group of homologous cell types across the metazoa since several smooth-to-striated and striated-to-smooth transitions certainly occurred during bilaterian evolution [45]. The body wall muscles of ascidians share several molecular, structural, and physiological features with vertebrate skeletal muscles instead [207]. For instance, ascidian body wall muscles express tropomyosins that most closely resemble the tropomyosin expressed in the striated muscles of vertebrates [207]. The body wall muscles use a troponin–tropomyosin complex, which is similar to the striated muscles in vertebrates. Furthermore, while both tunicate body wall and vertebrate skeletal muscle fibers are multinucleated, smooth muscles are generally mononucleated [326]. Homology between tunicate body wall (and larval tail) muscles and vertebrate skeletal muscles is further supported by their shared dependence on specification by myogenic regulatory factor (MRF) transcription factors, which are not involved in vertebrate smooth muscle specification [208].
The ascidian heart is a simple tube that pumps blood through an open circulatory system, often reversing the direction of flow [73, 218]. In most solitary species, the heart develops on the right side of the juvenile, an asymmetry that is especially obvious in stolidobranch ascidians. In Ciona, and likely in all ascidians, this right-sided position is a result of the development of left/right asymmetry of the surrounding endoderm [256]. The heart tube itself consists of a single-layer epithelium of cardiomyocytes, encased in a pericardial sheath (Fig. 3c) [218, 252]. As in vertebrates, the tunicate heart has a pacemaker region that expresses HCN channels [140] and the cardiomyocytes are striated and mononucleated. The striated, loosely organized myofilaments are restricted to the basal surface of the epithelium, facing the lumen of the heart [252].
Maternal determinants of primary tail muscle lineage specification in larvae
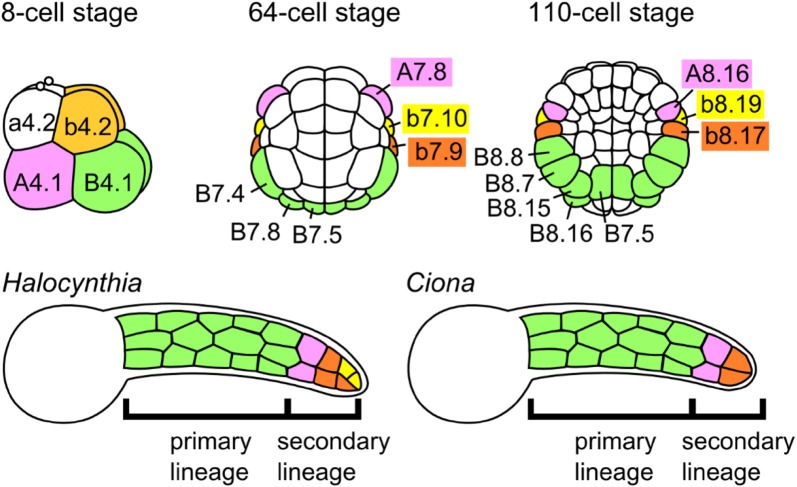
In most ascidian species, the only fully differentiated and functional muscles in the larva are those of the tail muscles. Even though juvenile/adult siphon and body wall muscle progenitors are specified and patterned during late embryogenesis and throughout the larval phase, these do not differentiate until after metamorphosis [77, 141, 268]. Accordingly, the majority of tail muscle cells are specified quite early by a gene regulatory cascade triggered by localized maternal determinants. These “primary lineage” muscle cells are derived from the vegetal-posterior pair of blastomeres (“B4.1” blastomeres, by Edwin Conklin’s cell lineage nomenclature) at the 8-cell stage (Fig. 4) [66]. In all solitary species studied, the primary lineage gives rise to 14 muscle cells on either side of the tail. This example of cell lineage specification by a maternal determinant was initially described by Conklin, who visually tracked the segregation of yellow-colored myogenic ooplasm, or “myoplasm,” into the tail muscle lineage of the Styela canopus (formerly Styela partita) embryo, triggered by fertilization [65].
Fig. 4.
Cell lineages of the tail muscles of ascidian larvae. Diagram of primary and secondary tail muscle lineage development in two species, Halocynthia roretzi and Ciona robusta. Bottom row: tailbud stages of the two species showing divergent muscle cell contributions color-coded according to conserved lineages indicated in top row (which are identical between the two species). 8-cell and tailbud stage views are lateral, 64-cell and 110-cell views are vegetal. In Halocynthia, the b4.2 blastomeres contribute to 5 muscle cells on each side of the tail, descended from b8.17 and b8.19. In Ciona, the b4.2 blastomeres gives rise to only 2 muscle cells on each side, and only b8.17 is myogenic.
Illustration adapted from Tokuoka et al. [329]
Subsequent studies showed that the myoplasm is necessary and sufficient for cell-autonomous specification and differentiation of tail muscle cells. Expression of muscle differentiation markers occurs in descendants of isolated B4.1 blastomeres [94, 237, 269, 353] and in cleavage-arrested embryos [283, 352]. Similarly, transplantation or mis-segregation of myoplasm is sufficient for muscle differentiation in non-muscle lineages [85, 238, 254, 270, 355, 356]. However, highly organized myofibrils do not form in isolated primary lineage cells, suggesting that interactions with surrounding tissues are important for the complete morphogenesis of tail muscles [71, 88, 264]. Further experiments and observations indicated that the determinant was not freely diffusable in the myoplasm per se, but rather tightly associated with the cell cortex [67, 159, 238]. The “yellow crescent” followed by Conklin is actually pigment associated with densely packed mitochondria, which are likely required for the energy expenditure of the muscles during swimming.
Some early studies proposed that autonomous specification of the primary lineage tail muscle was due to segregation of maternally expressed muscle proteins and mRNAs present in the myoplasm. However, subsequent studies revealed zygotic transcription of muscle genes starting as early as the 32-cell stage [200, 212, 213, 260, 283, 325, 333, 365]. Thus, the search for the elusive maternal determinant of muscle formation shifted instead to potential upstream regulators, more specifically maternal mRNAs [203].
That search culminated in identifying, in Halocynthia roretzi, the maternal mRNA Macho-1, which encodes an ortholog of vertebrate Zic transcription factors [242]. Macho-1 has since been renamed Zic-related.a (Zic-r.a), according to the proposed guidelines for standardized tunicate gene nomenclature [312], but in this review we retain the name Macho-1 to highlight its historical role. Orthologs of Macho-1 have been identified in several tunicate species [43], and their roles as maternal determinants seem conserved [130, 290]. In these embryos, maternally deposited, Macho-1 mRNA is tethered to the myoplasmic cytoskeletal domain and is therefore presumed to be locally translated only in the descendants of the B4.1 domain [242, 280]. Macho-1 protein triggers the regulatory cascade for primary tail muscle specification by potentiating the transcription of Tbx6-related (Tbx6-r) genes and downstream factors [292, 361, 362]. More specifically, Macho-1 physically interacts with a beta-catenin/Lef-1 transactivation complex, which in turn directly binds the promoters of its target genes to activate them. Macho-1 also relieves the repressive effect of certain cis-regulatory sequences that silence these genes outside the posterior-vegetal domain [249].
Macho-1 does not appear to directly regulate many terminal differentiation genes [292], further indicating that downstream transcription factors are required to mediate the formation of muscle. Interestingly, other Zic-related paralogs are zygotically expressed and participate in tail muscle specification in Ciona [155, 290]. Various Zic genes are expressed in vertebrate somites [227], and mesodermal expression of Zic orthologs appears to be a pan-bilaterian trait [188], hinting at a more deeply conserved role for Zic factors in muscle specification. Indeed, zygotically expressed Zic1 and Zic2 are involved in the activation of Myf5 in vertebrate somite myogenesis [257]. Altogether, this suggests that maternally expressed Macho-1 could have originated from a tunicate-specific gene duplication followed by subfunctionalization. Like vertebrate Zic genes, tunicate Macho-1 (together with other Zic-related genes) is zygotically transcribed in the developing central nervous system. This suggests that Macho-1 was co-opted as a maternal determinant specifically in the tunicate lineage, even though it may have had an ancestral, zygotic role in neural and muscle development [188, 290, 346].
Induction of secondary tail muscle lineages
The elegance of a localized, maternally deposited “organ-forming substance” held such sway that it was thought for a long time that only the primary lineage, descended from the myoplasm-rich B4.1 blastomeres, gave rise to tail muscle cells in the tunicate larva. This view was held even in the face of experimental evidence of alternative sources of tail muscle cells [269, 341]. Lineage tracing of labeled blastomeres in various species revealed that, indeed, the muscle cells flanking the tip of the tail did not descend from the B4.1 blastomeres, but rather from the A4.1 and b4.2 blastomeres (Fig. 4) [235, 240, 241]. These “secondary” lineages had been missed in part because they rarely form tail muscle cells when isolated. This is because muscle fate in these lineages, unlike in the primary lineage, is not autonomously specified by Macho-1 [209, 236]. Instead, secondary lineage muscle specification depends on a complex series of cell fate choices, instructed in part by precise cell–cell interactions.
In spite of the clear requirement for precise cell contact-based induction events in the specification of secondary lineage muscle cells, it is interesting to note that these lineages also inherit mitochondria-rich cytoplasm, which segregates to a “marginal zone” of cells that form the boundary between the animal and vegetal hemispheres and give rise to all the muscle and neural progenitors of the animal [368]. It is tempting to speculate that the secondary muscle lineage cells depend on these dense mitochondria for their activity, even though their specification is not autonomously determined by Macho-1. This would mean that maternally inherited materials like mitochondria, and possibly other unidentified molecules, may be necessary, but not sufficient, for the proper function of secondary lineage muscles.
Comparisons between distantly related Ciona and Halocynthia revealed that the cell lineages giving rise to secondary muscle cells development are remarkably conserved (Fig. 4) [147]. The A4.1 lineage (referred to as “A-line”) gives rise to exactly 4 total muscle cells (2 on either side of the embryo) in both species, though more cells are specified from the b4.2 lineage (“b-line”) of Halocynthia (10 total cells) than in Ciona (4 total cells) [240]. Remarkably, this is the only identified difference to date among these species’ astoundingly conserved embryonic cell lineages. These extra b-line muscle cells appear to be a Halocynthia-specific novelty and may be adaptive, as their larvae are nearly twice the size of larvae of Ciona and most other solitary tunicate species [240].
In the A-line, muscle fate is restricted to the A9.31 pair of blastomeres on either side of the neural plate, each of which gives rise to two muscle cells. In fact, these muscles derive from neuromesodermal progenitors, the A8.16 blastomeres, in which the muscle determination gene Myogenic regulatory factor (Mrf) appears to be weakly expressed alongside the proneural bHLH gene Neurogenin (Neurog) [145, 154, 208]. After A8.16 divides, the A9.31 daughter cell is specified as a muscle progenitor, marked by downregulation of Neurog and upregulation of muscle determinants Tbx6-r.b and Mrf. Its sister cell A9.32 downregulates Mrf, upregulates Neurog and Ebf, and is specified instead as a neural progenitor [145], eventually giving rise to tail nerve cord cells and a motor neuron [231].
The b4.2 lineage gives rise to epidermis and endoderm in addition to nervous system and tail muscles. In Halocynthia, both b8.17 and b8.19 cells will give rise to muscle cells, while in Ciona only b8.17 will contribute to tail muscles [235]. Similar to their A-line counterparts, b-line secondary muscle progenitors also appear to have neuromesodermal potential, but little is known about the cell fate choices that result in the segregation of neural, muscle, and endodermal fates in this lineage. It will be very interesting to see how these compare to the fate choices governing tail muscle specification in B- and A-lines, as well as understanding the evolution of supernumerary muscle cell formation in the tail tip of Halocynthia.
While secondary muscle specification is highly conserved between Ciona and Halocynthia, especially in the A-line, more in-depth studies have revealed surprising differences in the molecular basis of this process (Fig. 5). In both species, A-line muscle potential is initially induced by direct contact with the more laterally placed b6.5 lineage cells [145, 146, 329]. In Ciona, this is effected through the Nodal and Delta/Notch signaling pathways. Surprisingly, these pathways are not required for A-line muscle specification in Halocynthia, in spite of the conserved role of b6.5 as the inducing cell [329]. The necessary signaling molecules emanating from b6.5 have yet to be identified in Halocynthia. Although Nodal is required for neural fate in A7.8, it does not seem to be required specifically for muscle fate [329]. This is in contrast to Ciona, where Nodal regulates both neural and muscle fate in A7.8. Another difference is that, in Ciona, Nodal signaling from b6.5 also activates the expression of a Delta-like ligand in another cell neighboring the lineage, A7.6. Delta signaling from A7.6 then cooperates with Delta signaling from b6.5 to promote neuromesodermal potential in A8.16 [146]. In Halocynthia, an unknown signal from b6.5 activates the expression of Wnt5.a ligand in A7.6 instead. Wnt5.a signaling then promotes muscle fate in A8.16 [329]. Lastly, the muscle/neural fate choice in Ciona is regulated by FGF/ERK signaling: FGF signaling activates Tbx6-r.b and Mrf expression in A9.31, while suppression of FGF signaling allows for Ebf and Neurogenin expression in A9.32 [145]. In Halocynthia, it is not known what regulates this final cell fate decision, but the data suggest that FGF/ERK signaling is not involved [329]. Thus, although an intricate feed-forward signaling relay from b6.5 to A7.6 to A8.16 exists in both species, the nature of the ligands and pathways involved has diverged.
Fig. 5.
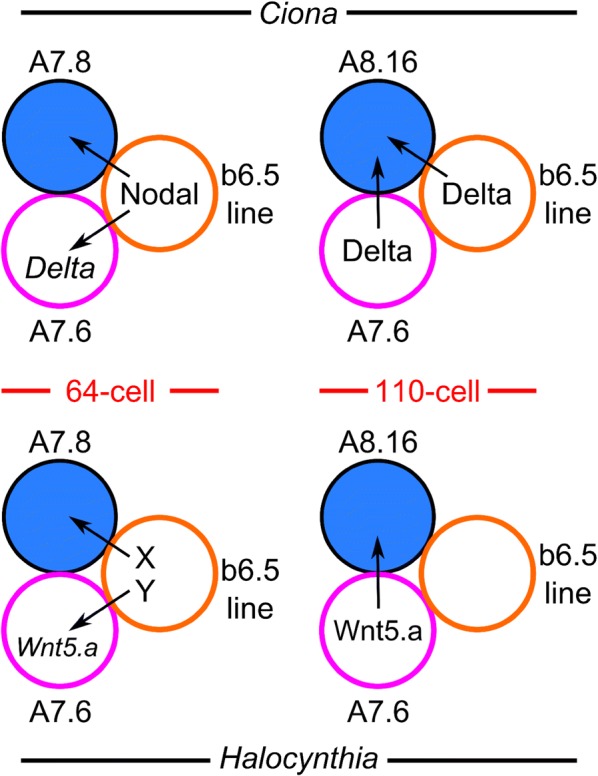
Conserved and divergent mechanisms of secondary muscle lineage induction. Schematic of the currently proposed models of secondary lineage muscle specification compared between Ciona (top row) and Halocynthia (bottom). In Ciona, Nodal from the b6.5 lineage at the 64-cell stage is required for neuromesodermal potential (represented by blue fill) in A7.8, and for expression of Delta ligand in A7.6. At the 110-cell stage, Delta signals from A7.6 and b6.5 line cells are also required for neuromesodermal potential in A8.16, a daughter cell of A7.8. In Halocynthia, unknown signal (“X”) from b6.5 line cells is required for muscle (but not neural) potential in A7.8, while unknown signal “Y” is required for Wnt5.a ligand expression in A7.6. At the 110-cell stage, Wnt5.a from A7.6 is likely required for secondary muscle specification in the A8.16 lineage. Diagram based on illustrations by Hudson and Yasuo [147] and data from Hudson and Yasuo [146], Hudson et al. [145], and Tokuoka et al. [329]
The deep conservation of the specific cell lineage relationships and cell–cell contacts that specify the secondary muscles is in stark contrast to the variable nature of the actual intercellular signaling molecules and pathways that have been selected to carry out these tasks in the different species. And yet both pathways converge on similar transcriptional programs (neural vs. muscle fate). This is an intriguing case of developmental system drift, in other words, changes to the molecular basis of a conserved developmental process (see below) [335]. The extreme conservation of the cell lineages and intercellular contacts probably reflects a strong constraint on the tunicate embryogenesis, with streamlined developmental processes that likely cannot accommodate much topological flexibility. However, why the divergence in signaling molecules deployed? Perhaps in the tunicate ancestor, several partially redundant signaling pathways were involved in secondary muscle lineage specification. Over the course of the tunicate radiation, different pathways may have become central among the others in the different tunicate families. Comparing across a larger sampling of species across the tunicate tree might help resolve whether this specialization of secondary muscle inductive signaling in fact occurred.
In vertebrates, common progenitors of paraxial mesoderm and spinal cord arise in the posterior lateral epiblast, near the tailbud [6, 120, 337]. Wnt and Fgf signaling are required in combination to imbue these cells with neuromesodermal potential and appear to further favor paraxial mesoderm over neural fate [121]. This is similar to the specification of the secondary muscle lineage in tunicates. Thus, the tunicate ancestor may have used a similar dual Wnt/Fgf signaling strategy to specify the secondary muscle lineage. While Halocynthia would have retained the role of Wnt in this process, Ciona would have kept Fgf instead.
This brings us to the outstanding evolutionary question: which muscle lineage and associated mechanism of specification, if any, represents the more ancestral, or original paraxial muscle of the tunicate tadpole? The “primary” lineage gives rise to the majority of the larval tail muscles, and its mode of autonomous specification seems especially robust compared to the intricate inductive events required for the less conspicuous, secondary muscle lineage. Thus, one might think of this as the “ancestral” tail muscle specification program, while the secondary lineages were co-opted (from neurogenic territory) to increase the number of muscle cells added to the tip of the tail. However, certain clues hint at the opposite evolutionary scenario: that the primary lineage program is a derived, tunicate-specific specialization and that the mechanisms for secondary muscle lineage induction may represent a vestige of the ancestral, pre-tunicate paraxial muscle regulatory network.
First, the arrangement of both lineages in a contiguous, mitochondria-rich crescent of cells along the posterior marginal zone of the pre-gastrula embryo [368] suggests that they might have shared a common regulatory program, but that these diverged early in tunicate evolutionary history to give rise to distinct primary (cell-autonomous) and secondary (non-cell-autonomous) inductive mechanisms.
Second, the continuity of an ancestral, cell signaling-dependent mode of muscle induction is supported by the aforementioned parallels to the neuromesodermal progenitors of vertebrate embryos, which similarly give rise to both skeletal muscles and spinal cord neurons, in response to similar Wnt/Fgf signals. This would argue specifically against a tunicate-specific co-option of neurogenic progenitors for the secondary lineage.
Third, Macho-1 is clearly a tunicate-specific Zic-related paralog, and functions somewhat redundantly with other, Macho-1-independent, zygotically expressed Zic-r paralogs, at least in Ciona [155]. The tunicate-specific role of Macho-1 is consistent with the interpretation of the primary muscle lineage specification cascade as a derived, tunicate-specific mechanism.
Finally, Pax3 and Pax7 are important regulators of myogenesis in vertebrate paraxial mesoderm [295, 319] and their tunicate ortholog, Pax3/7, is expressed in the b-line secondary muscle lineage but not in the primary muscle lineage [344]. Currently, it is not known whether the specification of b-line secondary muscles depends on Pax3/7. However, Pax3/7 expression begins in the b8.19 pair of blastomeres, when these cells are still located along the lateral borders of the neural plate [344, 345]. Pax3/7 is also a conserved marker of the neural plate borders in all chordates [142]. Therefore, this expression may not reflect a conserved role for Pax3/7 in paraxial myogenesis in tunicates.
Mrf: myogenic regulatory factor
The myogenic regulatory factors (MRFs) are basic helix–loop–helix (bHLH) transcription factors that control skeletal muscle development [47]. Despite a more discreet role in insects and nematodes, the association of MRFs with myogenesis is conserved across bilaterians [7, 57, 110, 225]. This includes the central role of a single MRF ortholog in tunicates, in whom its function is more critical for myogenesis than in the classic protostome models of genetics, Caenorhabditis elegans and Drosophila melanogaster [7, 110, 208].
In vertebrates, overexpression of MyoD, the founding member of the MRF family, was shown to be sufficient, albeit in a context-dependent manner, to induce a skeletal muscle phenotype in a non-muscle cell and remains a classic example of cell type conversion by a single master regulator gene [78, 107, 350]. In vertebrates, the other members of the MRF family (Myf5, MRF4, Myogenin) have similar and partially overlapping or redundant, yet not identical, functions in the specification and the differentiation of the non-cardiac striated muscles [47]. Myf5, MRF4, and MyoD are myogenic determination factors, while MRF4, MyoD and Myogenin can promote differentiation of myoblasts into skeletal myofibers [47, 166]. It is notable that no equivalent single master gene has been documented in the more elusive differentiation programs of cardiac and smooth muscles, where none of the MRF genes are expressed [253, 347].
A tunicate ortholog of the MRF genes was first discovered in Halocynthia roretzi and originally named AMD1 for Ascidian MyoD-related factor 1 [5]. Consistent with its role in the development of skeletal-like muscles, expression of AMD1 is restricted to embryonic tail muscle precursors and body wall muscles of the juvenile and adult. Its regulation in the tail muscles depends on Tbx6-r proteins (see “T-box 6-related factors” section), while its regulation in the body wall muscles appears to depend instead on another transcription factor, Ebf (see “The cardiopharyngeal mesoderm” and “Oral siphon muscles” sections). In Ciona spp. the ortholog of AMD1 was formerly referred to as Muscle Determination Factor (Ci-MDF) [210, 211] or MyoD [76], but was finally named Myogenic regulatory factor (Mrf) as it is equally related to the four MRF paralogs in vertebrates [208]. Based on the new nomenclature guidelines, we henceforth use the name Mrf to refer to its orthologs in all tunicates, including AMD1 in Halocynthia. Mrf is the sole member of the MRF gene family in Ciona but produces two isoforms by alternative mRNA splicing [210]. The small Mrf isoform 1 (Mrf-i1) lacks a C-terminal Helix III-coding sequence, while the larger Mrf-i2 encodes all the functional domains conserved in vertebrate MRF proteins [210]. During embryogenesis, Mrf transcripts are restricted to larval tail muscle precursors. Nonetheless, Mrf-i1 transcripts are expressed earlier than Mrf-i2, while Mrf-i2 transcripts persist for longer in the tail muscles [211]. Mrf transcripts are maintained in the tail muscles in the swimming larva in Ciona, while its ortholog is only transiently expressed in the tail muscles precursors at early embryonic stages in the stolidobranch Molgula occidentalis (A.S., unpublished data). In the larva, prior to settlement and metamorphosis, Mrf transcripts are also detected in siphon muscle precursors of Ciona [268]. Like in vertebrates, expression of Mrf has not been detected in the cardiac lineage of tunicates [5, 210, 268]. As mentioned previously, Mrf expression is a key bit of evidence to support a close relationship between striated vertebrate skeletal muscles and unstriated body wall muscles in tunicates.
Functional studies of Mrf have first been reported from early stages in Ciona [157, 208]. Knockdown experiments using antisense morpholinos targeting Mrf transcripts resulted in marked downregulation of terminal differentiation genes in the tail muscles. Even though not all differentiation markers were similarly affected, Mrf loss of function caused paralysis and loss of tail muscle myofibrils [208]. Conversely, misexpression of either isoform of Mrf is sufficient to induce expression of muscle markers in non-muscle cells, and repress notochord and endoderm development [266]. Comparable to the action of vertebrate MRFs, myogenic conversion by Mrf in Ciona is not equally successful in all cell lineages and does not induce expression of all documented muscle markers, suggesting that other lineage-specific cofactors are required for a complete myogenic fate switch [157, 208]. Despite its missing C-terminal Helix III, the shorter Mrf-i1 displays the same ability as Mrf-i2 to induce ectopic expression of terminal differentiation genes. Although eliminating either the Helix III domain or the more N-terminal histidine/cysteine-rich domain alone does not appear to affect the myogenic activity of Mrf, truncated proteins lacking both domains are not capable of activating target muscle genes, suggesting considerable overlap in the functions of these two domains [157]. In contrast, the N-terminus encodes an ascidian-specific domain that is poorly conserved between distantly related ascidians but indispensable for myogenic activity in Ciona [266]. In fact, Mrf proteins from non-ascidian species are not myogenic in Ciona unless fused to an ascidian Mrf N-terminus. Finally, the mutation of the alanine–threonine dipeptide of the bHLH domain impaired the myogenic potential of misexpressed Mrf, consistent with its conserved role as a bHLH myogenic code in vertebrates [157].
The various effects of Mrf perturbations on the expression of different muscle markers suggest that Mrf activates their transcription through various mechanisms in the embryo. Several computational studies detected an enriched sequence motif in the promoters of muscle terminal genes matching the E-box consensus sequence for Mrf binding [163, 184]. Nevertheless, Mrf activity is also proposed to function alternatively without E-box binding [184]. Finally, Mrf is also expressed in the body wall muscles [5, 210, 268, 308], where its long-anticipated role in differentiation has only recently been studied using the CRISPR/Cas9 system [332]. There, Mrf activity appears to be necessary for the expression of at least two specific structural genes of the body wall muscles, namely Mrlc4 and Mhc3 [332]. Potential differences between the tail and the body wall muscles in the transcriptional mechanisms controlled by MRF remain unexplored (see “Regulation of muscle type identity” section).
T-box 6-related factors
As for many key developmental regulators, the first tunicate gene related to Tbx6 was cloned from Halocynthia roretzi [220, 363]. This gene, first named Ascidian T-box 2, or As-T2, was initially proposed to be one of two tunicate orthologs (the other being As-T) of the T gene, also known as Brachyury [363]. It was quickly realized that, although both As-T genes encode T-box proteins, As-T2 was more closely related to Tbx6/16 genes in vertebrates. Analyses of molecular phylogenies and gene synteny revealed that the Tbx6/16 subfamily has experienced a complex history of independent duplications and losses in different chordate lineages [3, 13]. Following two round of whole genome duplications in early vertebrates, the Tbx6/16 subfamily must have contained four distinct paralogs that were later differentially lost in teleosts and tetrapods. Only one paralog, Tbx6, was conserved in placental mammals [3]. There are between two and four Tbx6-r paralogs identified in each tunicate species whose genome has been sequenced, but, surprisingly, those paralogous genes appear to be the results of multiple duplications and losses that may be specific to certain families or genera. As a result, it is impossible to assign 1:1 orthology of Tbx6-r duplicates in different tunicate species [310, 312]. Molecular phylogenies support their monophyly (they all appear to derive from a single, ancestral Tbx6-related gene in the tunicate forebear), but question their true orthology with the vertebrate Tbx6/16 subfamily. They could alternatively be descended from another group of T-box genes that have been lost in vertebrates and cephalochordates. In Ciona, there are four apparent Tbx6-r paralogs: Tbx6-r.a, Tbx6-r.b, Tbx6-r.c, and Tbx6-r.d. However, Tbx6-r.c and Tbx6-r.d are so similar in sequence that this apparent recent duplication may be instead a genome sequence assembly error. To avoid confusion, we will refer to only the three “confirmed” paralogs and ignore the possible existence of Tbx6-r.d.
Tbx6-r as a myogenic regulator
A search for myogenic regulators beyond Mrf was motivated by the detection of transcripts coding for structural muscle protein (such as muscle actins and myosins) as early as the 32-cell stage, before the onset of detected Mrf transcripts at the 64-cell stage [5, 287]. Out of this, orthologs of the Tbx6-related (Tbx6-r) family of factors came to be among the most intensely studied factors that regulate myogenesis downstream of Macho-1 (Zic-r.a) in tunicates. The early expression of As-T2 at the 32-cell stage embryo and its maintenance in the primary muscle precursors made it a plausible candidate for regulating the transcription of muscle-specific gene expression, supported by the emergence of the first reports that Tbx6 is required for specification of paraxial mesoderm in vertebrates [56]. The first functional evidence of a myogenic role for As-T2 was the induction of ectopic expression of structural muscle genes following microinjection of As-T2 mRNA in fertilized eggs [220]. Conversely, microinjection of mRNA encoding a fusion of the As-T2 DNA binding domain and the Engrailed repressor domain suppressed the expression of the same muscle genes [221]. This dominant repressor also inhibited transcription of an As-T2 reporter construct, revealing a positive autoregulatory feedback loop. Cis-regulatory analysis revealed putative T-box binding sites necessary for the activation of As-T2 and its target muscle structural genes [221]. This early tentative model, in which As-T2 directly activates terminal muscle genes and maintains its own expression through positive transcriptional feedback, primed numerous follow-up studies performed mostly in the genus Ciona.
In the Ciona embryo, all three Tbx6-r genes are first expressed at the 16-cell stage in the B5.1 and then in the B6.4 blastomeres at the 32-cell stage. However, at this stage only Tbx6-r.a is expressed in B6.2 (a daughter cell of B5.1) [322]. From the 64-cell stage onwards, the different Tbx6-r genes are expressed in various B-line muscle and mesenchyme cells, but their patterns begin to diverge slightly. At gastrulation, all three Tbx6-r genes are expressed in tail muscle precursors, including the secondary muscle lineage where their expression persists throughout the neurulation. This expression finally goes away at the tailbud stage, though Tbx6-r.a expression continues in a small subset of epidermal cells at the tail tip [322].
Tbx6-r genes were identified among the direct targets activated by the maternal muscle determinant Macho-1 in Ciona [361]. Binding sequences for Macho-1 were found in the 5’ sequence of Tbx6-r.b [179, 249, 361]. Similar results in Halocynthia point to an ancestral mechanism in tunicates [292]. Among the numerous downstream targets of Macho-1 [361], only Tbx6-r.b and Tbx6-r.c, but not Tbx6-r.a, were found to induce ectopic muscle differentiation [362]. The other 13 documented transcription factors and signaling molecules expressed in the muscle precursors downstream of Macho-1 are not myogenic but may be required for proper muscle specification and differentiation [362]. One such factor is Snail, which represses the expression of notochord genes in the muscles [109].
Overexpression of Tbx6-r.b or Tbx6-r.c restores muscle differentiation in a context of Macho-1 loss of function, confirming that they are the main direct mediators of Macho-1 in embryonic myogenesis [362]. Although expression of Tbx6-r.b and Tbx6-r.c and muscle differentiation are incompletely blocked by injection of antisense morpholinos against Macho-1, knocking down both Macho-1 and its zygotically expressed Zic-r paralogs completely blocks the expression of downstream muscle genes in Ciona [155, 362]. Indeed, expression of Tbx6-r.b and Tbx6-r.c relies on early inputs from Macho-1 and late inputs from other Zic-r factors and Mrf acting on different cis-regulatory modules [179, 362, 365]. Surprisingly, zygotic Zic-r can drive the transcription of a subset of structural muscle genes in the context of double Tbx6-r.b/Tbx6-r.c knockdown [362].
Although Tbx6-r.b and Tbx6-r.c are also expressed in mesenchyme precursors in Ciona, their myogenic activity needs to be suppressed in mesenchymal cells in order to block ectopic muscle differentiation. In Halocynthia roretzi, an FGF/ERK signal blocks the transcriptional activity and positive autoregulation of As-T2 and promotes the expression of the mesenchyme determinant Twist-related [180]. How the Tbx6-r positive feedback loop is terminated in the muscles, where transcripts of the Tbx6-related genes are not detected after the neurula stage, remains to be studied. One possible explanation is repression by Tbx15/18/22 (formerly known as VegTR), which starts being expressed in the tail muscle precursors around the same time Tbx6-r transcripts disappear [87]. This regulatory connection might differ in vertebrates, in which Tbx18 antagonizes Tbx6-mediated activation through competitive binding of target sites and recruitment of the corepressor Groucho [100, 139].
What is the molecular basis for the myogenic activity of Tbx6-r factors in tunicates? Even with an apparently simple transcriptional cascade regulating tail muscle differentiation, we have already mentioned several transcription factors proposed to interact directly with the cis-regulatory sequences of muscle structural genes. Tbx6-r factors can directly initiate the transcription of structural muscle genes, as well as activate genes coding for additional myogenic transcription factors, such as Mrf. Elements containing a functional T-box binding motif have been documented in the promoters of muscle genes in Halocynthia and Ciona [99, 163, 288, 366]. Both Tbx6-r.b and Tbx-r.c recognize a similar sequence, 5’-GWTCACACCT-3’, as determined by systematic evolution of ligands by exponential enrichment (SELEX) [362]. Large-scale mutant reporter construct assays revealed the conservation of Tbx6-r motifs with variable activity in all 19 documented cis-regulatory elements of structural genes expressed in the tail muscles [40]. The binding of Tbx6-r.b to the promoter of 10 known structural genes was further validated by a combination of chromatin immunoprecipitation followed by tiling microarrays (ChIP-chip), using overexpressed, GFP-tagged Tbx6-r.b [178].
Other evidence suggests that Tbx6-r myogenic activity is also mediated in part by downstream transcription factors. In the comprehensive studies of the genetic interactions of the transcription factors expressed during the early development of Ciona, Tbx6-r.b and Tbx6-r.c were found to directly activate Snail and Mrf [154, 178]. Recently, it was demonstrated that Snail is regulated in primary muscles by two distinct mechanisms, only one of which is Tbx6-r-dependent [328]. In the B5.1 lineage, Snail is directly activated by Tbx6-r.b, while in the B6.4 lineage Snail is activated by Macho-1 and ERK signaling. This ensures that Snail is activated simultaneously in both lineages at the 32-cell stage, even though Tbx6-r.b is expressed earlier in B5.1 (16-cell stage) than in B6.4 (32-cell stage). While the main action of Snail is to repress genes such as Brachyury and suppress notochord specification in muscle precursors [109, 154], Mrf is clearly a potent myogenic gene. Indeed, most of the promoters of the structural genes expressed in the tail muscles not only display Tbx6-r binding sites but also Mrf binding sites [40, 163, 178, 184]. Of the 155 genes whose promoters are directly bound by Mrf and Tbx6-r.b, according to ChIP-chip data, 60 were validated as being expressed in tail muscles [178]. Fibrillar collagen 1 and Creatine kinase might be two of the few muscle terminal genes for which no Mrf binding site could be identified in cis-regulatory sequences [40, 178, 179]. In contrast, other muscle structural genes appear to be directly regulated by Mrf with no detected Tbx6-r.b binding [178]. This is consistent with the ectopic expression of these markers induced by forced Mrf misexpression in cells that do not express Tbx6-r genes [208].
Overall, despite the exceptions mentioned above, these published studies have defined a regulatory network with a central coherent feed-forward loop consisting of Tbx6-r.b upstream of Mrf and Tbx6-r.b and Mrf upstream of many muscle structural genes. However, there are several ways to interpret this regulatory motif. Is the binding of Mrf and Tbx6-r.b required concomitantly for the transcription of a target gene, or do they act in temporally distinct phases? In the second model, Tbx6-r.b and to a lesser extent Tbx6-r.c would initiate the early transcription of muscle markers, before Mrf takes the relay and becomes the major activator, especially when Tbx6-r factors are downregulated. A third, intermediate model posits that Tbx6-r factors act as “pioneers” [369] and are required for the subsequent binding of Mrf to the element. A recent article answers some of these questions with a detailed investigation of the transcriptional regulation of the structural gene Mrlc3. In brief, Tbx6-r.b directly initiates Mrlc3 transcription without Mrf before the cooperation of both transcription factors is required for sustained expression of Mrlc3 [365].
In contrast to the case in tunicates, Tbx6 factors play a relatively indirect role in vertebrate myogenesis, mostly through their role in somitogenesis. In vertebrates, skeletal muscles of the trunk and the limbs derive from the myotome, a subdivision of the somite. Somites are the serial product of the segmentation of trunk paraxial mesoderm [14]. The early specification of paraxial mesoderm depends on the expression of Tbx6 [55, 56, 338]. Loss of function of Tbx6 induces ectopic neural structures in lieu of paraxial mesoderm in vertebrates, while early overexpression of Tbx6 promotes a mesodermal fate at the expense of a neural tissue [56, 338]. This is due to altered specification of cells descended from neuromesodermal progenitors that normally give rise to posterior spinal cord and paraxial mesoderm [120]. Among the targets of Tbx6 is the Mesp/Mesogenin family of bHLH genes [324, 358]. Together, Mesp and Mesogenin participate in the formation of presomitic mesoderm and in the segmental patterning of the somites [54, 250, 357]. Nonetheless, the upregulation of Pax3 in the somite (interpreted as the earliest step toward myogenic specification) requires a termination of Tbx6-driven activation of Mesp through a negative feedback loop [357]. Expression of a Tbx6 ortholog in the paraxial mesoderm of cephalochordates suggests an ancestral role for Tbx6/16 family of genes in paraxial mesoderm specification [13].
In brief, Tbx6-r factors in tunicates play a much more direct and terminal role in myogenesis than their orthologs do in vertebrates. This might be traced to the probable secondary loss of somitogenesis and other paraxial mesoderm-derived tissues in tunicates. In vertebrates, Tbx6 genes control somitogenesis through the activation Mesp1/2 and Mesogenin, while in Ciona this process is absent and paraxial mesoderm only gives rise to tail muscles. In Ciona, the activation of Mesp/Mesogenin by Tbx6-r.b and a delay of Mrf expression occur only in the B7.5 blastomeres [60, 76, 154]. These cells give rise to the anterior tail muscles and the cardiopharyngeal precursors, the latter differentiating only after metamorphosis [268, 308]. We can speculate that the direct activation of muscle structural genes by Tbx6-r factors was a key step in the acceleration of myogenesis in the tunicate embryo, together with the co-option of a maternal myogenic determinant (Macho-1) and the loss of self-renewing intermediary precursors. The rapid differentiation and morphogenesis of tail muscles might have allowed precocious hatching and larval swimming in a hypothetical race for dispersal.
Unanswered questions in tail muscle specification
Despite the wealth of data acquired over the past two decades, our current knowledge of the regulation of ascidian tail muscle specification is far from definitive. MicroRNAs have emerged as important players of myogenesis regulation in vertebrates [143]; Kusakabe and Inoue [181]. The gene regulatory network underlying tail muscle specification in tunicates will likely never be completely understood without further understanding the roles of miR-1 and miR-133, two microRNAs that are conserved among chordates and are processed from a single primary transcript that accumulates in the tail muscle nuclei of Ciona [182].
The functions of several transcription factors expressed in the tail muscle precursors also remain to be investigated. In the tail muscle precursors of Ciona, Mrf is upstream of the homeodomain transcription factors Otp and Meox whose functions remain unknown [154]. The transcription factors Mef2, Paraxis, and Tbx15/18/22 are also expressed in the tail muscles, but neither their regulators nor their targets have been studied in those cells [153, 268, 289, 322]. However, their orthologs in vertebrates play important roles in myogenesis [222] or mesoderm patterning [48, 100].
Interestingly, several computational studies revealed that, in addition to Mrf and Tbx6 putative binding motifs, typical CRE (cAMP response element) putative binding sites are enriched in the promoters of the muscle terminal genes [163, 184]. Furthermore, their mutation in the promoters of the Mlc2 and Mrlc2 genes impairs reporter expression in the tail muscles [184]. These sites might also be crucial to mediate Mrf activity on these promoters, especially since these sequences lack regular E-box sites. Whether a CREB transcription factor is expressed in tail muscle precursors and recruits Mrf is among the pending questions concerning the tail muscle gene regulatory network. In contrast to the spatial regulation of tail muscle specification, the temporal control of myogenesis is largely unknown. In addition to the poor knowledge of mRNA and protein kinetics in the embryo, it is not understood how the myogenic transcriptional network proceeds in step with the cell cycle. The exact timing and number of mitotic divisions is precisely controlled in the ascidian embryo, including the tail muscle lineages [185]. In other lineages, developmental events are tightly coordinated with the cell cycle [93, 152, 251, 267], suggesting that such temporal control may also be a crucial component of tail muscle development [285].
Evolutionary loss of tail muscle differentiation
The swimming larva plays a crucial role in the dispersal and attachment phases of the sessile tunicate life cycle. As such, it is highly conserved in form and function across the various tunicate clades. One notable exception is the Molgulidae family, in which various species have independently lost the defining anatomical structures of the tail and as a result, the ability to swim (Fig. 6) [22, 186]. The parallel losses of the tail and swimming behavior in multiple species in this family, and in the styelid Pelonaia corrugata, appear to correlate with certain substrates such as sand flats or exposed rock [133, 144, 217, 364]. This may not simply be a case in which the swimming larval stage is lost due to relaxed selective pressure—it has been proposed that in these environments, precocious metamorphosis without a swimming phase may provide a selective advantage [144, 201]. This is most obvious for certain species that make use of highly adhesive egg coats to attach to solid rock in areas heavily battered by waves, such as Molgula bleizi and M. pacifica [22, 364].
Fig. 6.
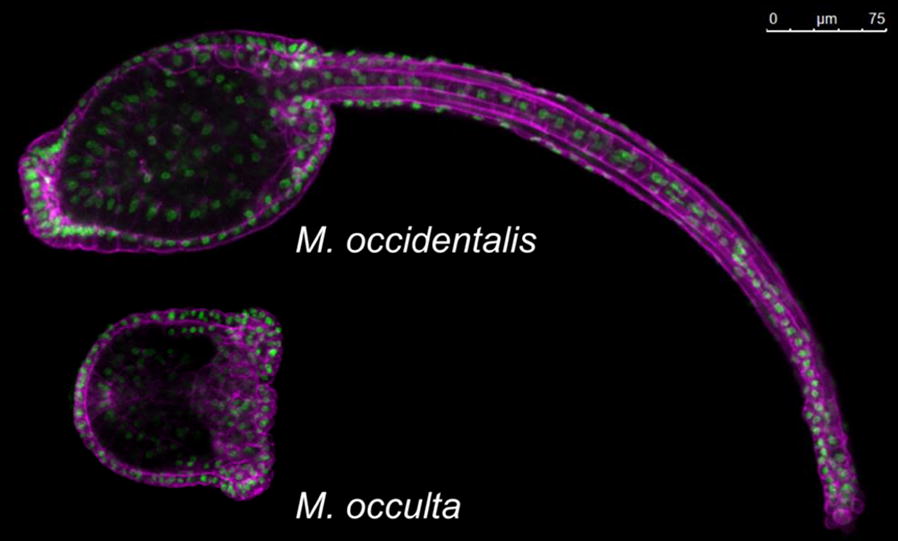
Tailed versus tailless Molgulid larvae. A tailed larva of Molgula occidentalis (top) compared to a tailless larva of Molgula occulta (bottom), stained with phalloidin Alexa Fluor conjugates (magenta) and DAPI (green). Note vestigial, amorphous tail in the posterior part of the tailless larva
Because of the independent evolutionary origins of the anural, or “tailless” condition, the developmental and genetic bases for this are homoplastic in the different species studied. Nevertheless, the numerous tailless species appear to fall somewhere along evolutionary trajectories converging on a total breakdown of the development of anatomical structures associated with swimming behavior: the notochord, tail muscles, tail epidermis, and central nervous system. The specification of these tissues does not appear to be lost, but rather the tailless condition arises from a failure of differentiation and morphogenesis [72, 354]. The embryos of these tailless Molgulids do not form a recognizable swimming tadpole like their tailed counterparts, but they develop according to the same developmental program [316, 354], with the possible exception of M. pacifica [11]. Some species may not even hatch before initiating metamorphosis and, therefore, have been distinguished as direct developers, metamorphosing into juveniles while still encased in the chorion [11, 318]. Thus, the contrast between tailed and tailless species is not the same as that between indirect and direct development; there are tailless species (e.g., M. occulta and M. arenata) that still hatch through the chorion before initiating metamorphosis and are thus classified as indirect developers, like their tailed relatives.
Although the loss of a differentiated notochord and larval neural structures in Molgula has been documented to a certain extent [195, 316–318], we will focus specifically on the loss of tail muscles. This loss of tail muscle does not appear to involve a loss of the blastomeres that give rise to tail muscles [160] nor the loss of muscle regulatory genes such as Macho-1, Tbx6-r, and Mrf, which are still present in the genomes and expressed in the presumptive tail muscle cells in tailless species [130, 131, 310, 320]. Rather, tail muscles fail to undergo proper differentiation and morphogenesis. Given the presence of some of the major muscle regulatory factors in larval muscle progenitors, we speculate that muscle differentiation is halted due to the loss of terminal differentiation gene expression instead, either through pseudogenization of larval-specific genes or loss of larval-specific cis-regulatory elements. Tail muscle differentiation has been assayed in embryos of various tailless Molgula and related species, using a series of different assays. The hallmarks of muscle differentiation are observed in their vestigial tails to varying degrees. For instance, the tailless larvae of Molgula occulta, M. arenata, and M. provisionalis express vestigial acetylcholinesterase (AChE) activity in presumptive tail muscle cells during embryonic development [11, 316, 354]. In contrast, tailless larvae of M. bleizi, M. retortiformis, M. pacifica, Bostrichobranchus digonas, and B. pilularis show no sign of vestigial AChE activity [10, 11, 317, 354]. The muscle differentiation program may be in the process of being gradually lost in each of these clades, and this process may be more or less incomplete in different species.
In some tailless species, muscle actin genes have lost their protein-coding function, rendering them “pseudogenes” [161, 183]. These losses appear to have occurred independently, in parallel, in orthologous Muscle actin 1 (MA1) genes in M. occulta and M. bleizi [161, 183]. Interestingly, two MA1 paralogs have been independently inactivated in M. occulta—a recent gene duplication resulted in two MA1 genes in the M. occulta genome (MA1.a and MA1.b), both of which have accumulated different inactivating mutations after their initial duplication [183].
The transcriptional activity of certain muscle differentiation genes has also been lost in some tailless species. For instance, transcripts of neither MA1 nor AChE were detected in Molgula tectiformis embryos [318], while MA1 and Myosin heavy chain transcripts were not detected in Bostrichobranchus digonas embryos [317], both tailless. The expression of MA1 genes was also silent or downregulated in tailless M. occulta embryos, though it partially rescued in interspecific hybrids with the tailed species M. oculata. The 5’ region upstream of M. occulta MA1.a has retained its cis-regulatory activity and can drive reporter gene expression in tailed Ciona embryos, but the corresponding region upstream of M. occulta MA1.b is not active in Ciona [183]. Conversely, the MA1 promoter from M. oculata is active in Ciona but only weakly active in M. occulta embryos, suggesting that regulatory changes in both cis and trans underlie the loss of expression [183]. It is not clear whether these regulatory changes preceded or followed the inactivating protein-coding changes. However, the restricted, exclusively larval domain of MA1 expression and function likely predisposed this gene to rapid inactivation and loss in species for whom swimming was wholly dispensable. On the other hand, it will be interesting to study the regulation and function of more pleiotropic terminal genes (notably, those required also for adult/juvenile muscles) in tailless Molgulids. Indeed, the vestigial expression of Mrf in the presumptive tail muscle cells of M. occulta could indicate that the transcriptional regulation of this gene in the different muscle types and at different stages may not be easily uncoupled.
The cardiopharyngeal mesoderm
The heart is a muscle organ acting as a rhythmic pump in animal circulatory systems. The typical tunicate heart consists of a monolayer of pericardium surrounding a monolayer of myocardium surrounded by fluid (Fig. 3c). It possesses no endocardium. Despite its tubular V-shape, which resembles a vertebrate looping embryonic heart tube, the tunicate heart had long been interpreted as homologous to the pericardium in vertebrates [301] until homologs of vertebrate cardiac regulatory genes such as Gata, Hand, and Nk4 were shown to be expressed in the heart primordium of Ciona [74, 286]. In Ciona and other ascidians, the heart derives from a single bilateral pair of embryonic blastomeres, the B7.5 cells (Fig. 7a) [141, 286]. The B7.5 blastomeres and their progeny transiently express the bHLH transcription factor gene Mesp, equally related to the multiple vertebrate paralogs Mesp and Mesogenin [286]. Like vertebrate Mesp genes, Ciona Mesp is crucial for cardiogenic specification [76, 286].
Fig. 7.
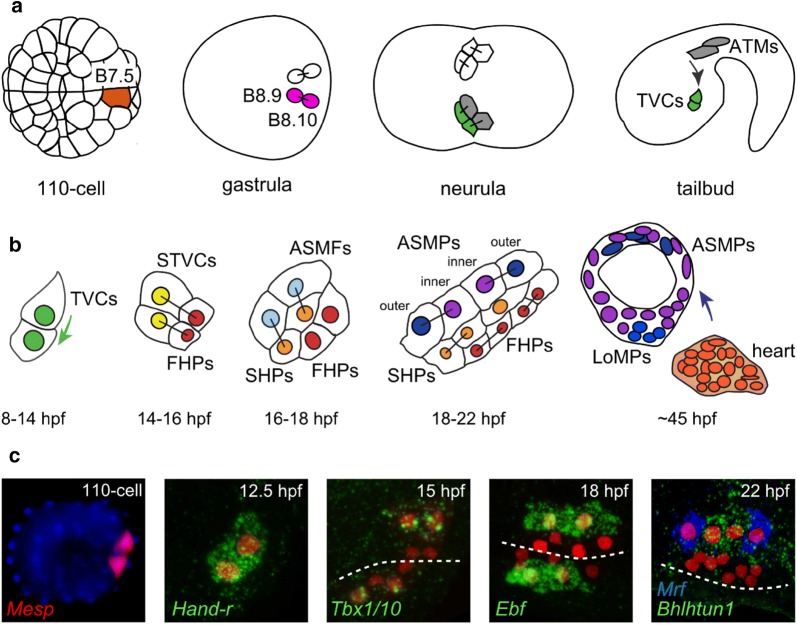
Cardiopharyngeal mesoderm development. a Schematic diagram of the cell divisions of the B7.5 lineage in Ciona robusta, up to the specification and migration of the trunk ventral cells (TVCs). ATMs, anterior tail muscles. Vegetal views in all panels except the last panel, which shows a lateral view (left side only). b Diagram indicating the divisions of the TVCs and specification of heart and atrial siphon muscle precursors. STVCs, secondary TVCs; FHPs, first heart precursors; SHPs, second heart precursors; ASMFs, atrial siphon muscle founder cells; ASMPs, atrial siphon muscle progenitors; LoMPs, longitudinal muscle progenitors. Colored arrows indicate cell migration events (TVCs, ASMPs). c Gene expression patterns in the B7.5 lineage. 1st panel: in situ hybridization for mCherry mRNA (red) expressed in the B7.5 blastomeres by a Mesp > mCherry reporter plasmid. Nuclei stained by DAPI (blue). All other panels show mRNA in situ hybridization for endogenous gene expression in embryos electroporated with Mesp > LacZ reporter plasmid. Immunostaining for beta-galactosidase, the product of the LacZ gene, reveals the descendants of the B7.5 lineage throughout development (red nuclei). Dotted lines indicate ventral midline of the embryo. See text for details. a Adapted from Stolfi et al. [310]. b and c Adapted from Kaplan et al. [165]
On each side of the embryo, the B7.5 blastomeres will give rise to two daughter cells, B8.10 and B8.9. These cells in turn divide asymmetrically, each giving rise to an anterior tail muscle cell (ATM) and a trunk ventral cell (TVC), which migrates toward the ventral side of the head (“trunk”). Specification and migration of the TVCs are sequentially controlled by the activation of the FGF-dependent ERK/Ets pathway and the transcription factor FoxF [12, 16, 68–70, 75, 246, 265, 331]. In the head, each TVC then undergoes an oriented asymmetric cell division to give rise to a medial first heart precursor (FHP) and a large, lateral secondary TVC (STVC), the latter undergoing a second oriented asymmetric division to produce a small, medial second heart precursor (SHP) and a large, lateral atrial siphon muscle founder cell (ASMF) (Fig. 7b) [268, 308, 348, 349]. FHPs and SHPs seem functionally distinct: FHPs express the myocyte marker Mhc2 early on, while SHPs form the pericardium that encases the FHPs. Mhc2 expression then expands to a subset of the SHPs in the juvenile heart [348], suggesting that the pericardium is a source of cell progenitors for continued growth of the heart in juvenile/adult development. Meanwhile, ASMFs give rise to the progenitors of the atrial siphon muscles (ASMs) and other body wall muscles surrounding the pharyngeal atrium of the juvenile and adult [141]. The FHP/STVC and SHP/ASMF fate choices are driven primarily by FGF/ERK signaling in a feed-forward circuit [267]. After each round of oriented asymmetric cell division, ERK activity is the highest always in the lateral daughter cells, and perturbations to the FGF/ERK pathway predictably converted cells in the lineage to one fate or the other. More specifically, FGF/ERK signaling activates Tbx1/10 in the STVCs, while FGF/ERK signaling and Tbx1/10 cooperate to activate Ebf in the ASMFs. Ebf in turn is sufficient to specify an ASM precursor fate [308, 349]. Later in development, some ASM precursors activate Mrf expression downstream of Ebf and begin to differentiate just before metamorphosis. Meanwhile, other ASM precursors keep dividing and repress Mrf expression through a Notch-regulated, Hes-mediated process reminiscent of vertebrate skeletal muscle stem cells (Fig. 7c) [63, 268]. As in vertebrates, the later outgrowth of body wall muscles, both circular and longitudinal, appears to depend on the reactivation of Mrf expression within this pool of undifferentiated precursors [268].
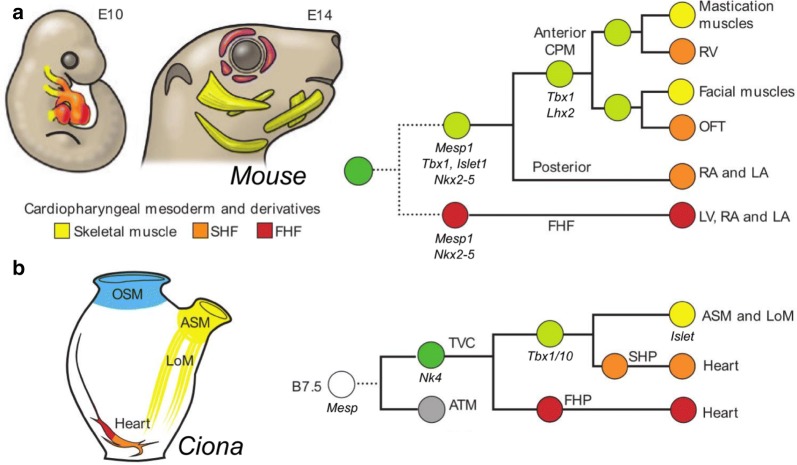
Because the TVCs give rise to both cardiac and pharyngeal muscles, these unique progenitors are known as the cardiopharyngeal mesoderm (CPhM). The “ontogenetic motif” of the CPhM of Ciona shares some remarkable parallels with heart and head muscle development in vertebrates. Despite the diversity of heart shapes in vertebrates [176, 301, 360], recent studies revealed the existence of two distinct mesodermal sources of cardiac precursors, namely the first heart field (FHF) and the second heart field (SHF), which are conserved across vertebrate groups from fish to mammals [37, 46, 169, 189, 340, 372]. The FHF gives rise to the early embryonic heart tube, while the SHF participates later by contributing to both arterial (i.e., outflow tract and right ventricle) and venous (i.e., right atrium) poles [44]. Based on early retrospective clonal analysis, the existence of common precursors specific to all cardiac cell types of both fields has been predicted in vertebrates [214]. This model has been further elaborated as evidence and also points to a common pharyngeal origin of the SHF and branchiomeric muscles, which contribute to large parts of the neck and head musculature [89, 135, 194]. Molecular commonalities between the SHF and the pharyngeal muscles have also been shown and are best illustrated by cardio-velo-facial/DiGeorge syndrome, in which the loss of function of the transcription factor TBX1 is responsible for malformations of both cardiac outflow tract and non-cardiac pharyngeal structures [170, 215].
Conserved cell lineage topologies and fate maps invite a comparison of vertebrate and tunicate CPhM development in which TVCs, STVCs, FHPs, and SHPs would be the ascidian counterparts of putative cardiopharyngeal precursors, pharyngeal precursors of the SHF and branchiomeric muscles, FHF and SHF, respectively (Fig. 8). The cell resolution reached in Ciona has allowed for a better understanding of the gene regulatory network behind the progressive specification and patterning of the CPhM. Whole genome studies from sorted cells have identified distinct waves of transcriptional activation of genes in the TVCs prior to their divisions [59, 359], and subsequently throughout the segregation of CPhM fates [268]. Recently, single-cell RNAseq analysis was used to document in great detail the developmental trajectories of FHPs, SHPs, and ASMFs [348]. This not only confirmed the conserved role of Tbx1/10 in regulating CPhM multipotency, but also revealed Dachshund homolog (Dach) as a conserved SHF-specific transcription factor that represses FHP fate in the SHPs of Ciona [348].
Fig. 8.
Comparative development of cardiopharyngeal mesoderm in vertebrates (mouse) and tunicates (Ciona). a Schematic of the mouse embryo at embryonic day (E) 10 and the mouse head at E14, and lineage tree depicting the origins of cardiac compartments and branchiomeric muscles in mice. First heart field (FHF) and its derivatives are indicated in red: left ventricle (LV), and parts of left atrium (LA) and right atrium (RA); second heart field (SHF) and derivatives are in orange: right ventricle (RV), parts of left and right atria, and outflow tract (OFT); branchiomeric skeletal muscles are in yellow; extraocular muscles are in purple. All cells derive from hypothetical common pan-cardiopharyngeal progenitors (dark green) that produce the FHF and the second Tbx1/10+ cardiopharyngeal progenitors (CPM, light green). Broken lines indicate that the common FHF/SHF progenitor remains to be identified in mice. b Schematic of the different muscle tissues of the Ciona juvenile, and lineage tree depicting clonal relationships and gene expression in the cardiopharyngeal precursors. The first heart precursors (FHP) (red) and second heart precursors (SHP) (orange) contribute to the heart (red and orange mix). The exact contributions of the FHP and SHP to the compartments and cell types in the juvenile heart remain to be elucidated. Atrial siphon muscle precursors (ASM, yellow) form atrial siphon and longitudinal muscles (LoM, yellow) of the body wall. Oral siphon muscles (OSM, blue) arise from a different, non-cardiac lineage (A7.6, see text for details). Daughter cells of the Mesp+ B7.5 blastomeres (white) produce anterior tail muscles (ATM, gray) and trunk ventral cells (TVC, dark green). The latter are pan-cardiopharyngeal progenitors that express Nk4 and divide asymmetrically to produce the FHP (red) and Tbx1/10 + STVCs (light green disk). The latter divide again asymmetrically to produce SHP (orange) and the Islet+ precursors of ASM and LoM.
Figure adapted from Diogo et al. [89]
The asymmetric divisions of the TVCs are also accompanied by progressively restricted expression of different TVC genes into either the heart precursors or the ASMFs [268]. Among the early TVC genes restricted to the STVCs and then to the ASMFs, Hand-related is necessary for the expression of Ebf. Meanwhile, Hand and Gata are early TVC genes that are eventually restricted to the heart precursors [268, 349]. This suggests that the TVCs are transcriptionally primed for both pharyngeal and cardiac fate specification [268], a feature which has not yet been documented in vertebrate cardiopharyngeal mesoderm. These primed regulatory states are resolved in part through mutual antagonism of Tbx1/10 and Nk4, which repress the transcription of each other and favor ASM and heart fate, respectively [349].
Developmental system drift in the cardiopharyngeal mesoderm
The extreme conservation of cell lineages, shapes, and positions between embryos of distantly related tunicates has allowed for detailed interspecific comparisons of developmental mechanisms, at single-cell resolution. In combination with bioinformatic comparisons of various tunicate genomes [43, 83, 86, 310, 342], this has uncovered a surprising preponderance of phenogenetic drift [351], or more precisely developmental system drift (DSD) [335]. DSD was specifically coined to describe the divergence (“drift”) of molecular mechanisms underlying otherwise identical, homologous traits between two different species. This assumes the trait was present in the common ancestor, excluding convergent or parallel evolution. It does not assume “drift” in the classical meaning of genetic drift, as selection may play a role in DSD [164]. The study of tunicate development has revealed several examples of DSD [147, 199, 248, 321]. Their rapidly evolving genomes and developmentally constrained embryos signal a conservation of phenotype and not genotype—a hallmark of phenogenetic drift.
The DSD label has been applied to diverse phenomena such as variation in the morphogenesis of an identical anatomical structure [172], divergence of transcription factor-binding DNA sequences underlying otherwise conserved cis-regulatory logic [134, 248], and deployment of different signaling pathways for the same inductive event, such as the case of secondary muscle lineage induction in Ciona versus Halocynthia [147]. Here, we further review a recent survey of cardiopharyngeal mesoderm (CPhM) development in the species Molgula occidentalis, which revealed distinct examples of divergent developmental processes that all fall under the broad DSD umbrella [310].
The solitary ascidian Molgula occidentalis is a divergent member of the genus Molgula, a Stolidobranch genus more closely related to Halocynthia than to Ciona [82, 174, 336]. Previous comparative studies and discovery of DSD in the tunicates have been limited to research contrasting Halocynthia and Ciona. However, Halocynthia eggs and embryos are nearly twice the size of those of Ciona, develop substantially more slowly, and cannot be electroporated like Ciona [370]. In contrast, M. occidentalis embryos are of similar size, develop at similar rates, and can be electroporated en masse, like Ciona [310]. These parallels allowed for a more detailed comparison of the developing B7.5-derived CPhM across these distantly related taxa [310].
What this revealed was a surprising prevalence of DSD in the mechanisms underlying gene regulation and morphogenesis in the CPhM. The B7.5 lineage itself is perfectly conserved between M. occidentalis and Ciona robusta (formerly Ciona intestinalis Type A), when considering cell divisions and cell fates (Fig. 9a). The B7.5 cells give rise to two migratory trunk ventral cells (TVCs) and two anterior tail muscle cells (ATMs) on either side of the embryo. As in Ciona, the TVC/ATM fate choice in Molgula is governed by FGF/ERK signaling, which promotes TVC fate at the expense of ATM fate [75]. Molgula TVCs undergo two rounds of asymmetric cell divisions to give rise to distinct heart and body wall (pharyngeal) muscle progenitors. The orientation and asymmetry of these divisions is identical in Ciona and results in the exact same segregation of cardiopharyngeal fates [310].
Fig. 9.
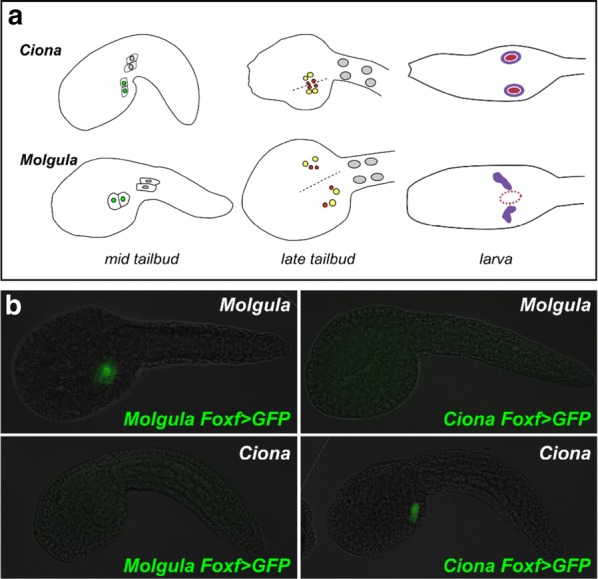
Developmental system drift in the cardiopharyngeal mesoderm between Ciona and Molgula. a Diagram comparing differences in morphogenesis of cardiopharyngeal progenitors between Ciona robusta, formerly Ciona intestinalis Type A (top) and Molgula occidentalis (bottom). Mid tailbud stage: lateral view, only one side illustrated. TVCs (green nuclei) separate from their sister anterior tail muscle cells (gray nuclei) and migrate anteriorly and ventrally on each side of the embryo. In M. occidentalis, this migration is more lateral than in C. robusta. Late tailbud stage: ventral view. TVCs divide to give rise to secondary TVCs (yellow) and first heart precursors (red). In C. robusta, these cells form a single cluster at the ventral midline (dotted line), while in M. occidentalis the cells on either side do not meet at the midline. Larva: dorsal view. In C. robusta, atrial siphon muscle precursors (ASMPs, purple) from either side surround an atrial siphon placode (burgundy circle) in the dorsal head epidermis. In M. occidentalis, the future atrial siphon of the juvenile arises from a single primordium that has not yet formed in the larva (dotted burgundy outline). At this stage, the ASMPs of M. occidentalis form two dorsal clusters of cells on either side of the dorsal midline. b Cross-species reporter plasmid assays reveal mutual unintelligibility of orthologous cis-regulatory elements between C. robusta and M. occidentalis. Foxf > GFP reporter plasmids drive identical expression patterns in homologous TVCs when electroporated into embryos of the corresponding species of origin, but are completely non-functional when electroporated into the other species. a Adapted from Kaplan et al. [165]. b Adapted from Stolfi et al. [310]
Gene expression patterns in the B7.5 lineage and its CPhM derivatives are also highly conserved between M. occidentalis and C. robusta [310]. Transcription factors known to be crucial to the development of the B7.5 lineage are all expressed in the same cells at the same stages. These include Mesp in the B7.5 cells; Ets.b in the B8.10/B8.9 founder cells; Foxf, Hand-related, and Gata4/5/6 in the TVCs; Tbx1/10 in the secondary TVCs, and Ebf in the atrial siphon muscle precursors. Identical gene expression patterns in the CPhM are not limited to transcription factors, as expression of the retinoic acid synthesis enzyme Aldh1a a [228] and cytoskeletal regulator Rhod/f [59] is also conserved between Ciona and Molgula [310].
One difference is the apparent lack of Nk4 expression in the TVCs of M. occidentalis. Nk4 is the sole tunicate ortholog of tinman in Drosophila and Nkx2-5 in humans. In C. robusta, Nk4 was shown to be expressed in migrating TVCs and to promote heart fate by antagonizing Tbx1/10 in the specification of body wall muscle from common CPhM progenitors [349]. In M. occidentalis, Nk4 expression in the TVCs was not detected [310]. This could reflect a greater reliance by M. occidentalis on the FGF/ERK pathway that acts as the major determinant of heart/body wall muscle fate in Ciona [267]. In C. robusta, Nk4 expression is reactivated in the heart primordium during metamorphosis [74], but this later expression was not assayed in M. occidentalis.
There is variation in certain morphogenetic processes between the two species’ B7.5 lineages, but none that fundamentally alter the final outcome of CPhM development. For instance, in C. robusta, the TVCs meet at the midline as they divide to give rise to distinct heart and pharyngeal muscle primordia [308], while in M. occidentalis, these cells do not meet at the midline and remain more dorsolateral. Since the juvenile heart, in both species, forms from a single primordium containing precursors from both sides of the embryo, the separate cells in M. occidentalis ultimately converge later on in development [310]. Another notable difference is related to the formation of the atrial siphon muscle primordia. In C. robusta, muscle progenitor “rings” form around the paired atrial siphon placodes, which are well-formed in the larva [308]. In M. occidentalis, the atrial siphon arises from a single placode that forms much later in development [310]. Accordingly, the atrial siphon muscle progenitors of M. occidentalis larvae appear to form unorganized clusters of cells in the vicinity of where the paired placodes would be in C. robusta; muscle rings eventually form around the atrial siphon after metamorphosis. Thus, for the most part, the differences in CPhM morphogenesis observed between Ciona and Molgula indicate heterochrony of otherwise conserved cell behaviors. They are representative of DSD in that they are cryptic changes in the developmental processes underlying conserved traits.
With identical cell lineage, cell fate decisions, and gene expression patterns largely conserved between Ciona and Molgula, the expectation was that the regulatory logic controlling the expression of CPhM genes would also be highly conserved, despite the noncoding genome sequences being completely divergent (i.e., not bioinformatically alignable) between these two distant species. Indeed, precedents for the conservation of regulatory logic in spite of enhancer sequence divergence had been observed in various instances, including between Halocynthia and Ciona [248]. However, Ciona robusta versus Molgula occidentalis cross-species reporter plasmid assays revealed a surprising, profound divergence in regulatory logic underlying otherwise identical expression patterns in the unambiguously homologous cells of the B7.5 lineage in tunicates (Fig. 9b) [310].
For instance, cis–regulatory sequences for activation of Mesp in B7.5 were found to be functionally divergent between C. robusta and M. occidentalis: The C. robusta Mesp reporter plasmid is weakly activated in B7.5 cells of M. occidentalis, with substantial “leaky” or imprecise expression in other cell lineages, while the M. occidentalis Mesp reporter plasmid is completely non-functional in C. robusta. This is in spite of the fact that both reporter plasmids drive identical expression patterns in their respective “native” species. The underlying cause of this discrepancy seems to be a re-wiring of the trans-regulatory logic for activation of Mesp in B7.5. In C. robusta, Tbx6-r.b and Lhx3/4 synergistically activate Mesp transcription [60]. In M. occidentalis, while a related Tbx6-r factor also appears to regulate Mesp¸ Lhx3/4 appears to play no role in Mesp regulation [310]. This altered logic may explain the partial loss of cross-species enhancer compatibility, or “intelligibility” between these two species. Other examples of cis-regulatory unintelligibility in the CPhM were identified between Ciona and Molgula, suggesting that such acute phenogenetic drift may be the norm, rather than the exception, between these distantly related tunicate taxa.
Why is there an apparent prevalence of cis-regulatory DSD in tunicates? It is possible that DSD has been underreported in other groups of organisms for technical reasons. A failed cross-species transgenic assay is a negative experimental result that may be hard to interpret without all the proper controls in place. Furthermore, embryos of different animal species may not be directly comparable to each other and homologous territories difficult to pinpoint (e.g., insects vs. mammals). When comparisons are limited to species within certain slow-evolving groups, like vertebrates, there may not have been enough evolutionary divergence for DSD to manifest in such an acute manner. In tunicates, we have the ability to easily electroporate various reporter constructs into distantly related species and directly compare large numbers of their nearly identical embryos. These conditions may favor the detection and testing of cryptic processes like DSD.
Alternatively, DSD might indeed be disproportionately common in the evolution of tunicates relative to other metazoan groups. It has been proposed that DSD occurs due to the interplay between directional and stabilizing selection [132, 164, 259, 335]. In simple terms, directional selection favors a new phenotype, while stabilizing selection favors the ancestral phenotype (“phenotypic stasis”). Genes with pleiotropic functions may be involved in several traits under both types of selection. Directional selection for a certain trait may modify such pleiotropic genes to the point where compensatory changes are needed to maintain another trait. In the case of tunicates, we know their genomes are rapidly evolving. At the same time, their embryos are nearly identical across vast evolutionary distances and are developmentally constrained, developing according to stereotyped, invariant cell lineages. There must be strong stabilizing selection to maintain precise and robust gene expression, since tunicate embryos cannot compensate for even the slightest of errors in cell fate specification. In tunicates, early fate restriction and invariant cell fate decisions leave little room for other cells to replace a missing cell, as in regulative embryos. Thus, an inordinate number of compensatory changes may be required to reconcile elevated molecular evolution rates and highly constrained developmental processes. Furthermore, it has also been proposed that the geometric constraints of the tunicate embryo ensure robustness to developmental mechanisms that in regulative embryos emerge instead from genomic constraints, i.e., highly conserved cis-regulatory sequences [128]. In this way, the lack of regulative abilities of the early tunicate embryo may have paradoxically relaxed such genomic constraints, allowing for further genomic sequence divergence—like an evolutionary ratchet that has resulted in acute DSD between distantly related tunicates.
Oral siphon muscles
The typical adult ascidian has two siphons—an incurrent, or oral siphon, and an excurrent, or atrial siphon. Both arise from the anterior and the posterior placodal domains, respectively [202, 205], and are muscularized by rings of myofiber bundles derived from siphon muscle progenitors that also give rise to body wall muscle fibers arrayed perpendicularly to the muscle rings around each siphon and extending to cover the rest of the body (Fig. 3a, b). In Ciona, atrial siphon- and oral siphon-derived body wall muscles end up parallel to each other (Fig. 3a), while in stolidobranchs-like Molgula (Fig. 3b), Halocynthia, and Boltenia, they end up perpendicular to each other and are thus distinguished as “longitudinal” versus “latitudinal” mantle muscles, respectively [77, 141]. Differentiated muscles originating around either the atrial or oral siphon are ultrastructurally [299] and molecularly indistinguishable [268]. However, developmentally they arise from very distinct lineages. While the muscles surrounding the atrial siphon (and other atrial siphon-derived body wall muscles) are derived from cardiopharyngeal progenitors of the B7.5 lineage, oral siphon muscles (OSMs), and other oral siphon-derived body wall muscles arise from the A7.6 blastomeres instead [141].
The A7.6 blastomeres are anterior, vegetal mesodermal cells derived from A6.3, an endomesodermal progenitor in the 32-cell stage embryo. A6.3 divides to give rise to two daughter cells of different fate: A7.6 (mesoderm) and A7.5 (endoderm). Ephrin signals from adjacent a- and b-line cells downregulate FGF/ERK signaling in the A7.6 cell, allowing for TLC fate, while FGF/ERK signaling is activated in its sister cell and specifies endoderm fate [298]. This is likely due to the local recruitment of Ras-inactivating RasGAP protein to the side of the A6.3 cell in contact with cells expressing ephrin ligands, by activated Eph receptors [138]. Additionally, Nodal signaling is also required for the specification of TLCs [146, 154, 298].
The A7.6 blastomeres give rise to a mesenchymal cell population called the trunk lateral cells (TLCs), which give rise to three broad tissue types in the juvenile/adult: OSMs, hemocytes, and epithelial cells lining the first gill slit [141, 330]. How OSMs are specified and organized within this lineage remained a mystery until recently (Fig. 10) [332]. The OSMs are derived from the anterior most cells of the TLC lineage, while more posterior cells appear to contribute to the other two fates. OSM fate restriction correlates with sustained expression of the bHLH transcription factor Hand-related, which is also a marker of atrial siphon muscle (ASM) fate restriction in the B7.5 lineage. In contrast, expression of Myt1 is progressively excluded from fate-restricted OSM lineage cells, indicating perhaps a repressive role for this particular transcription factor in muscle specification. After becoming specified and fate-restricted, OSM progenitors migrate to surround the oral siphon primordium, or stomodaeum, and further segregate into differentiating cells marked by expression of Mrf, and stem cell-like progenitors marked by Bhlhtun1 [268, 332].
Fig. 10.
Oral siphon muscle development in Ciona. a Diagram depicting the development of oral siphon muscles (OSMs) in Ciona robusta, adapted from [332]. OSM precursors (OSMPs) are derived from an OSM founder cell (OSMF), which in turn derives from the anterior trunk lateral cell (aTLC). Trunk lateral cells (TLCs) are in turn derived the A7.6 blastomere of the 110-cell-stage embryo. Timing is given in hours post-fertilization (hpf) at 18 °C. Atrial siphon muscles (ASMs) derive from the B7.5 blastomeres. b Diagram of the A7.6 lineage, with precursors of non-OSM contributions (blood, tunic cells, part of stomach, part of gill slit epithelium, etc.) depicted as white circles. At bottom is a diagram depicting the timing of expression of the transcription factors that are central to the OSM gene regulatory network, relative to each cell fate decision. Refer a for developmental timing of cell fate decisions in hours post-fertilization. c Common siphon muscle gene regulatory network, showing OSM-specific, ASM-specific, or shared hierarchical regulatory relationships. Putative, untested regulatory connections show as dotted lines. Re-wiring of the network must have occurred in either the OSM or ASM lineage, changing the hierarchy between Tbx1/10 and Ebf, and the mechanism of Mrf regulation
Despite the profound differences between the B7.5 and A7.6 lineages, the molecular profile of the OSM precursors converges on a regulatory state that is shared with ASM precursors, as suggested by the expression of structural genes such as Myosin heavy chain 3 and Myosin regulatory light chain [268, 308], as well as transcription factor-encoding genes Tbx1/10, Ebf, Islet, Bhlhtun1, and Mrf [268, 332]. Surprisingly, the temporal order of the onset of expression of important regulators Tbx1/10 and Ebf was different between OSM and ASM lineages. In the ASM lineage, Tbx1/10 is expressed in STVCs that give rise to both heart and ASM progenitors [268, 349]. In the latter, Tbx1/10 is required for expression of Ebf, which is sufficient for ASM fate [268, 308, 349]. In the OSM lineage, Ebf expression is not limited to fate-restricted OSM progenitors, but rather starts two mitotic divisions earlier. Ebf expression actually precedes the expression of Tbx1/10, which only comes on in fate-restricted OSM progenitors. Indeed, Ebf is not sufficient for muscle fate specification in the OSM lineage, but a combination of Tbx1/10 and Ebf overexpressed together is sufficient for precocious and ectopic OSM specification at the expense of other TLC derivatives [332]. Interestingly, these ectopic OSM progenitors can associate with the primordia of the atrial siphons instead, perhaps due to their closer proximity to these at earlier stages.
In sum, the two key transcription factors regulating OSM/ASM fate are the same in either lineages, yet their places in the regulatory hierarchy have been reversed. This re-wiring of the same regulatory inputs into a siphon/body wall muscle progenitor program within the same organism is unusual. One possible explanation for this is that the adult muscle program initially evolved in one lineage and was later co-opted in the other. In fact, the latter lineage may have been predisposed to this co-option; if this lineage already expressed one of the two key factors, all that would have been needed was the co-option of the second factor. To which lineage the “original” siphon/body wall muscle program belonged and which is the latecomer is difficult to know. To answer this, a comparably detailed understanding of muscle development in cephalochordates, the sister group to the olfactorians, would be needed. Regardless of the exact evolutionary trajectory, the two different configurations of the regulatory network increase the developmental repertoire of embryo. In the B7.5 lineage, Tbx1/10+/Ebf− cells are specified as heart precursors. In the A7.6 lineage, it is not known what becomes of the Ebf+/Tbx1/10− cells, but they do not contribute to OSMs.
Regulation of muscle type identity
In mammals, the classic division between slow and the fast striated fibers refers to distinct contractile properties. To a large extent, these properties depend on a finely tuned expression of particular isoforms of myosin heavy chains and illustrate the importance of muscle molecular identity. Sarcomeric myosin heavy chains are encoded by no less than 11 genes in mammalian genomes [15, 272]. While some are widely expressed across different striated muscles types, including the heart, others are exclusively restricted to certain head and neck muscles [294]. Beyond this spatial diversity, the repertoire of the myosin heavy chains also changes temporally, as embryonic and neonatal myosin heavy chains are successively expressed during development [294]. More generally, the molecular diversity of the structural components of the myofibrils across muscle subtypes relies on the differential expression of various paralogous genes, as well as alternative splicing of the same gene [293, 294].
Similarly in tunicates, specific paralogous or alternatively spliced isoforms are differentially expressed in larval tail muscles, juvenile and adult body wall muscles, and the heart, often in a mutually exclusive manner [58]. Out of 6 Myosin heavy chain genes in Ciona, 5 code for sarcomeric-like myosin heavy chains (Mhc2, 3, 4, 5 and 6). Mhc3 is exclusively expressed in the body wall muscles [308], while the expression of Mhc4, Mhc5, and Mhc6 appears to be restricted to the embryonic and larval stages in the tail muscle cells [58]. Mhc2 is primarily expressed in the heart and is absent from body wall muscles [58, 308], though its expression is also detected in a few posterior tail muscle cells in the embryo (F.R., unpublished observations).
Phylogenetic analyses of the genes involved in this contractile machinery indicate that the diversification of muscle type-specific proteins occurred after the vertebrate–tunicate split [58]. In the documented multigenic families, namely those encoding muscle actins, myosin heavy chains, myosin light chains, myosin regulatory light chains, tropomyosins, troponin C, troponin I, and troponin T, most paralogs in Ciona are equally related to the paralogs expressed differentially in the fast, slow skeletal, and cardiac muscles of humans. This supports a common origin for tunicate non-cardiac muscles and vertebrate skeletal muscles (but not smooth muscles) [58, 207]. Although the last common ancestor of tunicates and vertebrates likely possessed a single muscle actin that was expressed in both heart and paraxial muscles [4], the same ancestor also appears to have possessed two myosin heavy chain-encoding genes, the descendants of which have been conserved in both tunicate and vertebrate lineages [206]. Specifically, Ciona Mhc3 groups with a unique vertebrate gene, MYH16, in a sister clade to all other chordate Myosin heavy chain genes [206]. Intriguingly, expression of both Mhc3 and MYH16 might be restricted to muscles derived from the cardiopharyngeal mesoderm, reflecting a potentially conserved ancestral pharyngeal muscle identity [151, 272].
One example of the parallel diversification of vertebrate and tunicate muscle genes is the evolution within the tunicate troponin I (TnI), a regulatory subunit of the troponin complex. In vertebrates, distinct TnI paralogs are expressed in slow skeletal muscles, fast skeletal muscles, and cardiomyocytes, with the slow skeletal and cardiac forms being more closely related to each other [8, 137]. In Ciona, a single TnI gene is transcribed in all larval and adult muscle types, but distinct isoforms are alternatively spliced in cardiac and non-cardiac muscles [61, 198]. However, in the distantly related Halocynthia, tail muscle TnI and body wall TnI are the products of two paralogous genes instead [366, 367]. Interestingly, these subfunctionalized TnI paralogs may have coevolved with Troponin T, which is also duplicated in Halocynthia but not in Ciona [61, 95, 96]. These findings suggest that the diversification of muscle structure and function occurred in parallel in tunicates and vertebrates, even though some broad muscle anatomical classes (e.g., cardiac, pharyngeal, paraxial, etc.) were already molecularly distinct in stem olfactorians. The growing number of tunicate genome sequences that are available should allow us to trace more precisely these gene duplication events in relation to the tunicate radiation. Finally, how the diversification of such structural genes has shaped the diversity of muscle contractile properties (e.g., speed of contraction, force, resistance to fatigue) in the tunicates remains to be tested.
Until now, how the expression of distinct sets of genes is regulated in the different tunicate muscle types has been mostly left to speculation. Nonetheless, preliminary observations in Ciona combined with a closer look at how muscle diversity is achieved not only in vertebrates but also in protostomes, notably in Drosophila [91], may give us precious indication for future studies. In mammals, most of the properties of muscle fibers are established during development and regeneration, but are also modulated by neural and hormonal activity. Indeed, the contractile properties of muscle fibers can be changed through exercise [129]. This muscle plasticity can be induced by nerve-evoked electrical stimulation at determined frequencies, mediated by calcium-dependent signal transduction. This signaling impinges on the phosphorylation, nuclear localization, and activity of numerous transcription factors such as Six, MEF2, NFAT, Sox6, and myogenic regulatory factors (MRFs). These are all established actors in myogenesis in the embryo and in the adult, even though muscle differentiation is independent of neural activity in the initial phases of development [129, 294].
Among such transcription factors, the homeobox proteins of the sine oculis (Six) family have a prominent place. Six1 and Six4 are required for the expression of Pax3 and MRF genes in the mouse embryo [126], but can also reprogram adult fibers from slow-twitch to fast-twitch phenotypes [127]. Six1 is crucial for the development of fast muscle fibers in zebrafish [27], while in mouse, Six1 directly activates the enhancer of the fast myosin heavy chain gene cluster [276]. Therefore, a regulatory feed-forward loop, involving six factors upstream of and in combination with MyoD, is at work from the very first steps of myogenesis all the way to terminal muscle identity [279]. Similar regulatory motifs, but with different trans-acting factors, are found in insects. In Drosophila, the transcription factor Collier (the sole ortholog of chordate EBF proteins) is required for the activation of Nautilus (the sole ortholog of MRF genes), in a subset of embryonic muscles (DA3). The combination of these two transcription factors is then required to maintain expression of Collier and adopt a DA3 instead of a DA2 muscle identity [90, 97, 98].
In tunicates, the relative homogeneity of body wall muscles and the relative monotony of muscle activity in most tunicates do not suggest much modulation of muscle identity by varied patterns of neural excitation. However, there is ample evidence that the combinatorial logic of transcriptional regulation might explain the diversity of structural genes that expressed the different muscle types in a tunicate. As mentioned previously, the Tbx6-related factors acting upstream of and in combination with Mrf are proposed to shape tail muscle identity in the tunicate embryo. Ebf is likely to play a similar role with Mrf in the body wall muscles, which derive from oral and the atrial siphon muscle precursors [268, 308, 332]. Ebf first activates Mrf and is upstream of terminal differentiation genes such as Mhc3, Mrlc4 and Tpm1. Ebf alone might be sufficient to activate certain muscle markers on its own, but it likely requires cooperation with Mrf for the activation of others. Similar roles for EBF proteins might be deeply conserved in vertebrates. In Xenopus frogs, EBF proteins have a myogenic role upstream of MRFs [125]. In mouse, Ebf protein synergizes with MyoD to induce specific terminal genes of the diaphragm [162]. Moreover, EBF/MRF heterodimer formation has been revealed in cell cultures [300]. Whether Mrf/Ebf heterodimers bind the cis-regulatory regions of tunicate body wall muscle terminal genes remains to be explored. Finally, how cardiomyocyte differentiation and identity are regulated has yet to be investigated at all.
Muscles in other tunicates
Tunicates have traditionally been grouped into three major classes—Ascidiacea, Thaliacea, and Appendicularia (Fig. 11). Up until now, we have considered only solitary ascidians, which make up a little less than half of the more than 3000 described species of tunicates. The ascidians are a polyphyletic, catch-all grouping devoid of any cladistic validity [82, 174, 336]. In essence, they are defined as benthic tunicates, as opposed to the pelagic thaliaceans and appendicularians, which are monophyletic clades and account for ~ 150 total species (~ 5%) [297, 312]. A slight majority of tunicates are actually colonial ascidians. However, mounting embryological and phylogenomic data suggest that coloniality evolved more than once and that extant pelagic tunicates evolved from ascidian-like ancestors [42, 122, 261, 336, 371]. Although the original tunicate was probably a solitary, free-swimming, amphioxus-like animal, it appears that coloniality and the pelagic lifestyle are both secondarily derived in tunicates. In contrast to solitary ascidians, not much is known about development in colonial or pelagic tunicates. Here, we briefly review what is known about muscles in other tunicate groups: colonial ascidians, thaliaceans, and appendicularians.
Fig. 11.
Muscle diversity among the Tunicata. Diagram showing different myofilament array types among the major tunicate groups. Thick filaments lengths drawn at the same scale relative to scale bar, except for pyrosome and ascidian body wall muscles in which lengths were not known. The width of each fiber shown is the maximum distance between the sarcolemma and the most interior myofilament. For doliolids, only the body wall muscles are shown, not the larval tail muscles, which are presumed to be similar to ascidian larval tail muscles.
Figure adapted from Bone and Ryan [34]
Colonial ascidians
Although not as intensely studied as solitary species, colonial species comprise 60% of all described ascidian species [297]. Coloniality evolved in the tunicates more than once [371] though perhaps repeatedly built upon an ancestral ability to regenerate body parts [42]. Colonial ascidians are thus a polyphyletic group of benthic tunicates united by their ability to reproduce through two complementary routes: sexually via gametes and embryogenesis, and asexually through blastogenesis [23]. Both processes converge on a similar end product, the zooid, or individual within a colony. In most colonial species, zooids are encased in a common tunic and are alternatively referred to as compound ascidians (Fig. 12a).
Fig. 12.
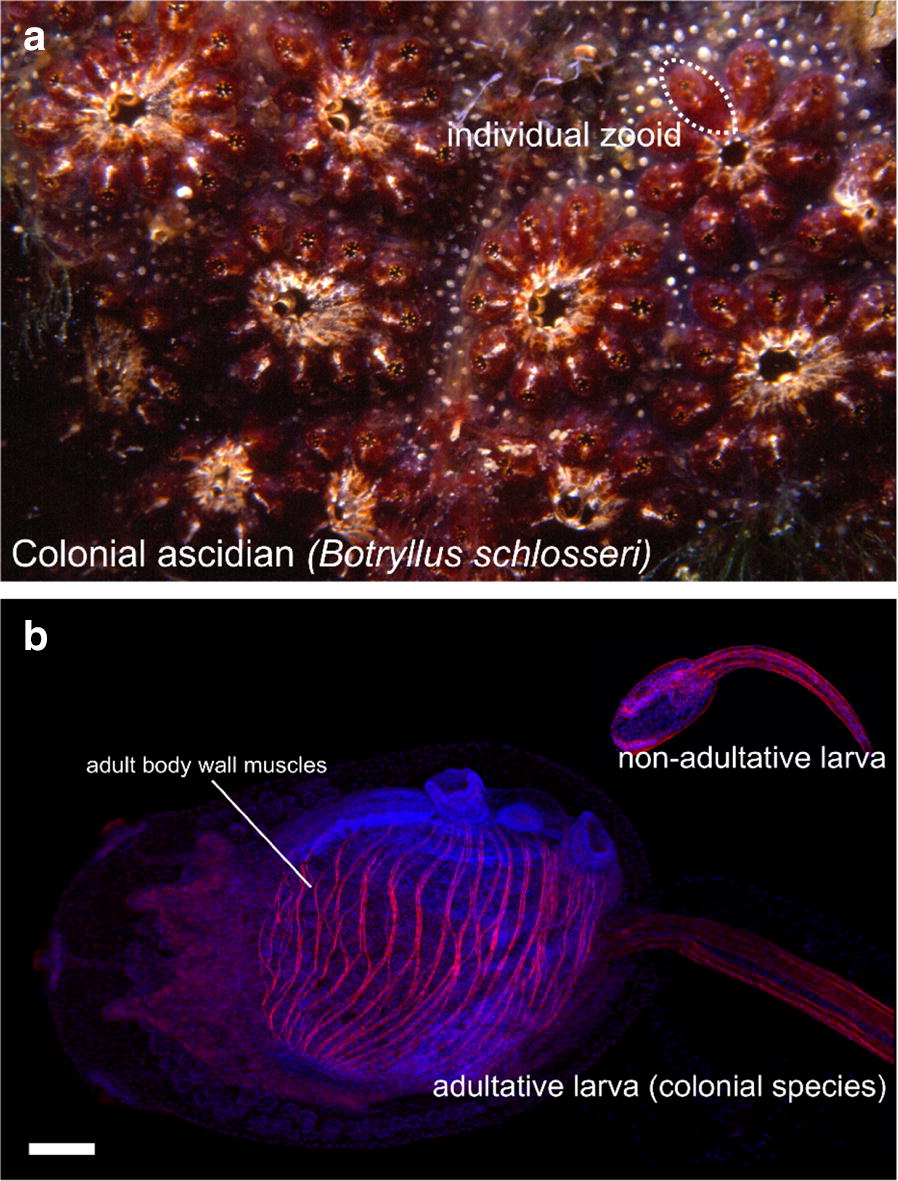
Colonial ascidians. a Colonies of Botryllus schlosseri, showing typical rosette organization of individual blastozooids (dotted outline). Adapted from image by Géry Parent (https://commons.wikimedia.org/wiki/File:Botryllus_schlosseri_(Pallas,_1766).jpg). b Precocious development of juvenile structures in the adultative larva of some colonial species. Top: Non-adultative larva of the solitary species Molgula occidentalis. Bottom: Adultative larva of an unidentified colonial aplousobranch species, showing elaborated tail as well as fully formed siphons, branchial basket, endostyle, and differentiated body wall muscles. Both larvae stained with phalloidin conjugates (red) and DAPI (blue), and shown to the same scale (bar = 100 μm)
Blastogenesis is the process of whole body regeneration to produce genetically identical individuals, or blastozooids, from the somatic tissue of a preexisting individual. The blastozooid stands in contrast to the oozooid, the original zooid and founder of each colony, derived through typical sexual reproduction similar to that seen in solitary species: fertilization of an egg by sperm, subsequent embryogenesis, a swimming larval phase, and metamorphosis.
The fine details of blastogenesis vary wildly among the many different colonial species [42]. In all species, the epidermis of new zooids always is derived from preexisting epidermis. However, the source of all other somatic tissues is different in the different clades. In the order Aplousobranchia, which is comprised almost entirely of colonial species, non-epidermal tissues derive from the epicardium, a specialized epithelium of endodermal origin. In colonial stolidobranchs, it appears that, in addition to the endoderm-derived, peribranchial epithelium, loose mesenchymal cells and circulating hemoblasts can also contribute to non-epidermal tissues [258, 313]. Blastogenesis may be irregular or precisely timed and synchronized among the zooids of each colony [17]. During such blastogenic cycles, zooids undergo programmed senescence, and a new generation of blastozooids “takes over” the colony [271].
In the colonial stolidobranch Botryllus schlosseri, there is not much to distinguish the organization of body wall muscles between oozooids and blastozooids, though blastozooids have a higher number of muscle fibers [327]. Therefore, there are two alternate developmental pathways for a nearly identical set of juvenile/adult body wall muscles in this and other species. While there is no reason to believe that the development of body wall muscles in the oozooids of colonial ascidians deviates much from that seen in individuals of solitary species, this has never been documented. Later development of body wall muscles during blastogenesis has been documented in the stolidobranchs B. schlosseri [79] and Symplegma reptans [314]. During takeover events, body wall muscles and heart degenerate along with the rest of the zooid [79]. How this programmed senescence is regulated is not known.
In Botryllus and Symplegma, body wall muscles appear to derive from mesenchyme or undifferentiated hemoblasts [79, 314]. In contrast, the heart appears to derive from an invagination of the peribranchial epithelium (endoderm) [79, 247]. Recent molecular analysis of body wall muscle and heart development during Botryllus blastogenesis further confirmed this uncoupling of cardiopharyngeal fates and lineages, suggesting that different parts of the embryonic CPhM regulatory network have been partially co-opted in different blastozooid lineages [263].
At first glance, this suggests that the atrial siphon muscles and heart do not share a common lineage in blastogenesis as they do during embryonic development. It will be interesting to identify the precise origin of the hemoblasts/mesenchyme that generate the body wall muscles, and whether they descend, together with heart progenitors, from a similar cardiopharyngeal mesoderm as seen in the embryo of solitary species.
Another feature of colonial species is that most of them have large swimming larvae that develop from yolk eggs and differentiate adult structures far in advance of hatching, settlement, or metamorphosis. This “adultation” of the larva appears to have evolved in parallel with the different colonial groups. Some species even commence their blastogenic cycles during embryogenesis, resulting in larvae that carry sometimes one or more blastozooids at different developmental stages [24], or even an entire “swimming colony” as in Hypsistozoa fasmeriana [38]. To propel these enormous, adultative larvae, many species have elaborated more powerful tail muscles, often composed of many more cells than seen in larvae of solitary species (Fig. 12b). For instance, in Distaplia occidentalis, there are about 750 muscle cells on either side of the tail [51]; in Diplosoma macdonaldi, there are 800 on either side [52]. These are mononucleated and striated, much like the tail muscle cells of solitary larvae, though the entire tail is rotated 90° to the left. All evidence suggests that these elaborated tail muscles form much like in solitary species, except for a proliferative phase after neurulation in which several rounds of mitosis generate the copious number of cells in the hatching larva [50]. Perhaps as a result, development is much slower than in solitary species and occurs in the protective environment of the colony, which broods its young tadpoles.
Thaliaceans
Thaliaceans are a monophyletic group of pelagic tunicates that is firmly nested within Ascidiacea as the sister group to the clade formed by the ascidian orders Phlebobranchia and Aplousobranchia [82, 174], and therefore clearly derived from an ascidian-like ancestor [39, 111, 122, 175, 193, 216, 232, 261, 291]. They are suspension filter feeders and an important component of zooplankton biomass and possibly in global carbon cycles [80, 119, 190]. Within the thaliaceans, there are three monophyletic orders: Salpida, Pyrosomatida, and Doliolida (Fig. 13) [114]. The phylogenetic relationships between these groups are still unresolved [122], due to the paucity of thaliacean genome sequences. Thaliaceans have evolved complex life cycles and, like colonial ascidians, can reproduce through alternate sexual and asexual modes [19, 20]. The most complex life cycles are found in the doliolids, which not only alternate sexual and asexual generations, but also form colonies of zooids specialized for different purposes—locomotion, feeding, and gamete production [255]. Despite their fascinating biology, not much is known about thaliaceans at the genetic or molecular levels.
Fig. 13.
Thaliaceans. a A chain of mature blastozooids of an unidentified salp species. Image by Ed Bierman (https://www.flickr.com/photos/edbierman/5005294079). b A young pyrosome colony (species unknown). Image by Nick Hobgood (https://commons.wikimedia.org/wiki/File:Combjelly.jpg). c Left: illustration of tailed, free-swimming larva (oozoid) of Doliolum denticulatum, showing larval tail and 9 body wall muscle bands. Adapted from Godeaux [114]. Right: illustration of an individual of the sexual generation (gonozooid) of Doliolum denticulatum. Anterior to the left. m1–m8, muscle bands 1 through 8; at, atrial aperture; at l, atrial aperture lobes; br, branchial (oral) aperture; br l, branchial aperture lobes; br s, branchial sac; dt, dorsal tubercule; end, endostyle; h, heart; i, intestine; n, nerves; ng, nerve ganglion; ov, ovary; pp, peripharyngeal band; p br, peribranchial atrium; sg, stigmata (gill slits), s gl, subneural gland, so, sense organs; st, stomach, tes, testes. Adapted from: Tunicata. In: A Guide to the Shell and Starfish Galleries (Mollusca, Polyzoa, Brachiopoda, Tunicata, Echinoderma, and Worms). London: Department of Zoology, British Museum (Natural History), 1901. Larva and gonozooid not drawn on same scale
Being derived from a sessile ancestor, thaliaceans have become secondarily adapted to a pelagic lifestyle. Salps and doliolids navigate the water column by jet propulsion [35], driven by rings of muscle fibers that encircle their barrel-like bodies (Fig. 13c) [150]. Muscular contractions of the body take water in through the anterior “siphon,” or valve, and eject it through the posterior valve. These valves are likely modified siphons homologous to the oral and atrial siphons, respectively, of sessile tunicates. Pyrosomes, on the other hand, do not move about by motor control, but rather form large colonies that float passively through the oceans [150]. However, each individual zooid in a pyrosome colony has a network of body wall muscles that act as retractors/sphincters (like in sessile tunicates) instead of generating jet propulsion as in salps and doliolids.
Because thaliaceans likely descend from an ascidian-like ancestor, their muscles are most likely homologous to the siphon and body wall muscles of sessile tunicates. Indeed, in pyrosomes, body wall muscles are very similar to those of Ciona, consisting of bands of unstriated, multinucleate “smooth” muscle fibers [150]. In doliolids, muscle bands are thick and composed of many, obliquely striated, multinucleate muscle fibers [29]. Doliolids swim using very rapid muscle contractions that can propel them through the water at speeds of up to 50 body lengths per second [35]. It has been proposed that the uniquely oblique striation of doliolid muscles allows them to undergo the large length changes that would be required for such powerful propulsion, given their lack of a rigid skeleton [34]. Some doliolid species have retained a tailed larva very similar to the larvae of ascidians, with three rows of striated muscle cells on either side of a notochord [113, 115, 339]. Furthermore, the number of muscle bands is used to distinguish two subgroups within the Doliolids: The Doliolidina has 8 muscle bands, whereas the Doliopsidina has only 5 [116, 117]. Whether these groups represent distinct phylogenetic clades remains to be studied.
In salps, muscle bands are also formed by many multinucleate fibers, though these have more conventional cross-striations [33]. They also contract more slowly than doliolid muscles and do not undergo such large length changes [197]. There is considerable dimorphism between the oozoid and blastozooid of the same species, including the number and arrangement of muscle bands [216, 261]. Piette and Lemaire proposed that differences in swimming behavior between oozoids and blastozooids have resulted in selection for generationally dimorphic muscles [261], with greater interspecies variation seen at the blastozooid stage [216].
Given the unstriated body wall muscles of sessile tunicates and pyrosomes, it is remarkable that the (presumed) homologous muscles of doliolids and salps are instead striated. If we assume that unstriated “smooth” muscles are a tunicate-specific innovation, we must conclude that the muscles of doliolids and salps are secondarily striated. This plasticity suggests that striation pattern is not a particularly useful trait to homologize muscle types among the chordates.
Appendicularians
Appendicularians (also known as larvaceans, Fig. 14) are pelagic tunicates like the thaliaceans. However, unlike all other tunicates, they retain the chordate form throughout their short life cycle, which only lasts a few days [102]. The cell lineages and fate maps of the Oikopleura dioica embryo are well documented and show a greater reduction in developmental timescale and cell numbers than even solitary ascidian embryos [108, 239, 306]. Phylogenetic classification of the appendicularians has been extremely difficult, due to their very small size and unusual morphology, as well as a paucity of appendicularian DNA sequences (only one species, Oikopleura dioica, has had its genome sequenced) which are also evolving very rapidly [86, 296]. Some phylogenetic analyses have placed them basal to all other tunicates [82, 174, 315, 336, 343]. Others have posited them as a sister group to stolidobranch [336] or aplousobranch ascidians [305]. Further complicating the evolutionary scenarios, Garstang believed they were descended from ascidians by way of doliolids [111].
Fig. 14.
Appendicularians. a An individual of the appendicularian species Oikopleura dioica. Anterior is to the top right. b Illustration of the animal and its house as depicted in (a). Black arrows indicate the direction of water currents that flow into the house by the beating of the tail. Red arrow indicates the movement of food particles toward the mouth. c Lateral view of the anatomy of Fritillaria pellucida. The tail has been rotated 90° to showcase the single band of 10 interdigitated muscle cells (m) on the one side of the tail (other side not shown). a, sensory axons; c, caudal ganglion; cr, gill slit ciliary ring (spiracle); g, gonad; gl, gland cells; h, heart; m, muscle cells; n, notochord; o, esophagus; p, pharyngeal gland (endostyle); r, receptor cells of the lower lip; s, stomach; u, upper lip. Scale bar: 100 μm. a and b Adapted from adapted from Bouquet et al. [36] and Stolfi and Brown [309]. c Adapted from Bone et al. [32]
The appendicularian tail, with its notochord and paraxial muscles, is retained throughout the animal’s brief life span. However, instead of using this tail strictly for swimming, the adult uses it to generate a flow of food-laden sea water through filters intricately sculpted with chambers and ducts (Fig. 14a, b) [104], a specialization of the cellulosic tunic that all tunicates possess. The appendicularian filter is actually an inflatable “house” which the animal inhabits, and is easily discarded and rebuilt several times a day [32, 103, 106]. Appendicularians also have a small, rapidly beating heart in the ventral part of the head. In some species, the heart is a closed pouch and may not function as a proper pump but more like a “churn” to move the acellular hemolymph around the body sinuses [187, 277]. Appendicularians do not have body wall muscles like other tunicates, probably because they use external food filters and because they do not move by jet propulsion like salps or doliolids.
Appendicularians are suspension feeders and use the tail to inflate the house and draw water and food particles through it. This tail is very similar to the tail of ascidian larvae, consisting of two bands of paraxial muscles on either side of the notochord, and is rotated 90° to the left relative to the head. The muscles are formed of by a single band of 10 cells on either side of the notochord (Fig. 14c). These cells appear to be striated and mononucleated (Fig. 11), though each nucleus has an elaborate branched morphology that could suggest polyploidization [105, 245, 302]. Motor neurons synapse onto most muscle cells, down the length of the tail, at a ratio of nearly 1 motor neuron for every muscle cell [31, 245]. This is in contrast to the ascidian tail, in which a few motor neurons innervate only a limited subset of muscle cells [274]. However, both ascidian and appendicularian tail muscle cells are electrically coupled [28]. Development of the tail muscles of Oikopleura longicauda has been observed in detail, and a single muscle actin gene cDNA was cloned from this species and found to be expressed in the tail muscle cells and the developing heart [245]. The amino acid sequence of O. longicauda muscle actin shares features with both body wall- and tail-specific muscle actins of ascidians, which could support the basal position of appendicularians relative to all other tunicates.
Although it is tempting to imagine appendicularians as the original form of the tunicate ancestor, embryological evidence suggests they are secondarily derived, descended from sessile ascidian-like ancestors. For example, the rotation of the tail is also seen in aplousobranch and perophorid ascidian larvae [51, 124]. Furthermore, the epidermis of the head in O. dioica rotates ventrally during metamorphosis [306]. In ascidians, the epidermis also rotates 90° during metamorphosis [62]. This rotation inverts the orientation of the animal relative to the substrate, since it needs to point away from the site of attachment in order to filter feed. While this inversion is crucial for the sessile ascidian to function as an adult, there is seemingly no reason for such an epidermal rotation in the pelagic appendicularians. Similarly, the intestine in O. dioica develops from a straight endodermal “strand” that extends down the length of the developing tail but later forms a U-shaped tube back toward the head [173, 304]. This is highly evocative of intestinal development in ascidians, in which endodermal strand cells must migrate into the head as the larval tail is reabsorbed, and form a U-shaped tube that is uniquely adapted to the sessile ascidian body plan [168, 230]. Finally, an adult appendicularian is only a few millimeters in length, approximately the size of an ascidian larva or juvenile but more than an order of magnitude smaller than an adult ascidian. In sum, it is hard to interpret these features as anything other than the vestiges of the larval/adult transition of a sessile ascidian-like ancestor, and that the adult appendicularian is a result of neoteny, via retention of the larval swimming structures and accelerated sexual development. The greater implication of this interpretation is that, although tunicates evolved from a free-swimming olfactorian ancestor, all extant tunicates are likely descended from an animal with motile larval and sessile adult stages. If true, this would urge caution in formulating scenarios for the evolution of tunicate muscle types based on the muscles found in extant appendicularians.
Conclusion
As we have attempted to convey in our review, research on the various muscle lineages of tunicates has produced not only a firm base of biological insights into developmental biology and evolution, but also a vast web of unanswered questions that tunicologists will be able to pursue for years to come. The near future promises to be an exciting time for further probing the mechanisms of gene regulation, cellular morphogenesis, and regeneration in a variety of tunicate species.
Acknowledgements
We thank Lionel Christiaen for his prolonged support. We thank Anna Di Gregorio for sharing her knowledge of T-box genes in Ciona. Finally, we thank the tunicate research community for reuse of their published figures throughout this review.
Authors’ contributions
FR and AS wrote this review. Both authors read and approved the final manuscript.
Funding
AS is funded by NIH award R00 HD084814.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Both authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Florian Razy-Krajka, Email: florian.razy@biosci.gatech.edu.
Alberto Stolfi, Email: alberto.stolfi@biosci.gatech.edu.
References
- 1.Abitua PB, Gainous TB, Kaczmarczyk AN, Winchell CJ, Hudson C, Kamata K, Nakagawa M, Tsuda M, Kusakabe TG, Levine M. The pre-vertebrate origins of neurogenic placodes. Nature. 2015;524(7566):462. doi: 10.1038/nature14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abitua PB, Wagner E, Navarrete IA, Levine M. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature. 2012;492(7427):104. doi: 10.1038/nature11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn D, You K-H, Kim C-H. Evolution of the tbx6/16 subfamily genes in vertebrates: insights from zebrafish. Mol Biol Evol. 2012;29(12):3959–3983. doi: 10.1093/molbev/mss199. [DOI] [PubMed] [Google Scholar]
- 4.Almazán A, Ferrández-Roldán A, Albalat R, Cañestro C. Developmental atlas of appendicularian Oikopleura dioica actins provides new insights into the evolution of the notochord and the cardio-paraxial muscle in chordates. Dev Biol. 2018 doi: 10.1016/j.ydbio.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Araki I, Saiga H, Makabe KW, Satoh N. Expression of AMD 1, a gene for a MyoD 1-related factor in the ascidian Halocynthia roretzi. Roux’s Arch Dev Biol. 1994;203(6):320–327. doi: 10.1007/BF00457803. [DOI] [PubMed] [Google Scholar]
- 6.Attardi A, Fulton T, Florescu M, Shah G, Muresan L, Lenz MO, Lancaster C, Huisken J, van Oudenaarden A, Steventon B. Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics. Development. 2018 doi: 10.1242/dev.166728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balagopalan L, Keller CA, Abmayr SM. Loss-of-function mutations reveal that the Drosophila nautilus gene is not essential for embryonic myogenesis or viability. Dev Biol. 2001;231(2):374–382. doi: 10.1006/dbio.2001.0162. [DOI] [PubMed] [Google Scholar]
- 8.Barnes DE, Hwang H, Ono K, Lu H, Ono S. Molecular evolution of troponin I and a role of its N-terminal extension in nematode locomotion. Cytoskeleton. 2016;73(3):117–130. doi: 10.1002/cm.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassham S, Postlethwait JH. The evolutionary history of placodes: a molecular genetic investigation of the larvacean urochordate Oikopleura dioica. Development. 2005;132(19):4259–4272. doi: 10.1242/dev.01973. [DOI] [PubMed] [Google Scholar]
- 10.Bates WR. Direct development in the ascidian Molgula retortiformis (Verrill, 1871) Biol Bull. 1995;188(1):16–22. doi: 10.2307/1542063. [DOI] [PubMed] [Google Scholar]
- 11.Bates WR, Mallett JE. Ultrastructural and histochemical study of anural development in the ascidian Molgula pacifica (Huntsman) Roux’s Arch Dev Biol. 1991;200(4):193–201. doi: 10.1007/BF00361337. [DOI] [PubMed] [Google Scholar]
- 12.Beh J, Shi W, Levine M, Davidson B, Christiaen L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development. 2007;134(18):3297–3305. doi: 10.1242/dev.010140. [DOI] [PubMed] [Google Scholar]
- 13.Belgacem MR, Escande M-l, Escriva H, Bertrand S. Amphioxus Tbx6/16 and Tbx20 embryonic expression patterns reveal ancestral functions in chordates. Gene Expr Patterns. 2011;11(3):239–243. doi: 10.1016/j.gep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Bénazéraf B, Pourquié O. Formation and segmentation of the vertebrate body axis. Annu Rev Cell Dev Biol. 2013;29:1–26. doi: 10.1146/annurev-cellbio-101011-155703. [DOI] [PubMed] [Google Scholar]
- 15.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12(4):780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernadskaya YY, Brahmbhatt S, Gline SE, Wang W, Christiaen L. Discoidin-domain receptor coordinates cell-matrix adhesion and collective polarity in migratory cardiopharyngeal progenitors. Nat Commun. 2019;10(1):57. doi: 10.1038/s41467-018-07976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrill N. The development of the bud in Botryllus. Biol Bull. 1941;80(2):169–184. doi: 10.2307/1537595. [DOI] [Google Scholar]
- 18.Berrill N. The development, morphology and budding of the ascidian Diazona. J Mar Biol Assoc U K. 1948;27(02):389–399. doi: 10.1017/S0025315400025443. [DOI] [Google Scholar]
- 19.Berrill N. Budding and development in Salpa. J Morphol. 1950;87(3):553–606. doi: 10.1002/jmor.1050870308. [DOI] [PubMed] [Google Scholar]
- 20.Berrill N. Budding in pyrosoma. J Morphol. 1950;87(3):537–552. doi: 10.1002/jmor.1050870307. [DOI] [PubMed] [Google Scholar]
- 21.Berrill N, Sheldon H. The fine structure of the connections between muscle cells in ascidian tadpole larva. J Cell Biol. 1964;23(3):664–669. doi: 10.1083/jcb.23.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrill NJ. Studies in tunicate development. Part II. Abbreviation of development in the Molgulidae. Philos Trans R Soc Lond, Ser B. 1931;219:281–346. doi: 10.1098/rstb.1931.0006. [DOI] [Google Scholar]
- 23.Berrill NJ. Cell division and differentiation in asexual and sexual development. J Morphol. 1935;57(2):353–427. doi: 10.1002/jmor.1050570204. [DOI] [Google Scholar]
- 24.Berrill NJ. Studies in tunicate development. Part III. Differential retardation and acceleration. Philos Trans R Soc Lond B Biol Sci. 1935;225(525):255–326. doi: 10.1098/rstb.1935.0013. [DOI] [Google Scholar]
- 25.Berrill NJ. Studies in tunicate development. Part V. The evolution and classification of ascidians. Philos Trans R Soc B. 1936;226(530):43–70. doi: 10.1098/rstb.1936.0002. [DOI] [Google Scholar]
- 26.Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115(5):615–627. doi: 10.1016/S0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- 27.Bessarab DA, Chong S-W, Srinivas BP, Korzh V. Six1a is required for the onset of fast muscle differentiation in zebrafish. Dev Biol. 2008;323(2):216–228. doi: 10.1016/j.ydbio.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Bone Q. Evolutionary patterns of axial muscle systems in some invertebrates and fish. Am Zool. 1989;29(1):5–18. doi: 10.1093/icb/29.1.5. [DOI] [Google Scholar]
- 29.Bone Q. On the muscle fibres and locomotor activity of doliolids (Tunicata: Thaliacea) Q. Bone. J Mar Biol Assoc U K. 1989;69(03):597–607. doi: 10.1017/S0025315400031003. [DOI] [Google Scholar]
- 30.Bone Q. On the locomotion of ascidian tadpole larvae. J Mar Biol Assoc U K. 1992;72(1):161–186. doi: 10.1017/S0025315400048864. [DOI] [Google Scholar]
- 31.Bone Q. The biology of pelagic tunicates. Oxford: Oxford University Press; 1998. [Google Scholar]
- 32.Bone Q, Gorski G, Pulsford A. On the structure and behaviour of Fritillaria (Tunicata: Larvacea) J Mar Biol Assoc U K. 1979;59(02):399–411. doi: 10.1017/S0025315400042715. [DOI] [Google Scholar]
- 33.Bone Q, Ryan K. The structure and innervation of the locomotor muscles of salps (Tunicata: Thaliacea) J Mar Biol Assoc U K. 1973;53(04):873–883. doi: 10.1017/S0025315400022530. [DOI] [Google Scholar]
- 34.Bone Q, Ryan K. On the structure and innervation of the muscle bands of Doliolum (Tunicata: Cyclomyaria) Proc R Soc Lond B Biol Sci. 1974;187(1088):315–327. doi: 10.1098/rspb.1974.0077. [DOI] [PubMed] [Google Scholar]
- 35.Bone Q, Trueman E. Jet propulsion in Doliolum (Tunicata: Thaliacea) J Exp Mar Biol Ecol. 1984;76(2):105–118. doi: 10.1016/0022-0981(84)90059-5. [DOI] [Google Scholar]
- 36.Bouquet JM, Spriet E, Troedsson C, Otterå H, Chourrout D, Thompson EM. Culture optimization for the emergent zooplanktonic model organism Oikopleura dioica. J Plankton Res. 2009;31(4):359–370. doi: 10.1093/plankt/fbn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brade T, Gessert S, Kühl M, Pandur P. The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev Biol. 2007;311(2):297–310. doi: 10.1016/j.ydbio.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Brewin BI. An account of larval budding in the compound ascidian, Hypsistozoa fasmeriana. Quart J Microsc Sci. 1959;3(52):575–589. [Google Scholar]
- 39.Brien P. Contribution a l’étude de l’embryogenèse et de la blastogenèse des salpes. Brussels: l’Université des Bruxelles; 1928. [Google Scholar]
- 40.Brown CD, Johnson DS, Sidow A. Functional architecture and evolution of transcriptional elements that drive gene coexpression. Science. 2007;317(5844):1557–1560. doi: 10.1126/science.1145893. [DOI] [PubMed] [Google Scholar]
- 41.Brown E, Nishino A, Bone Q, Meinertzhagen I, Okamura Y. GABAergic synaptic transmission modulates swimming in the ascidian larva. Eur J Neurosci. 2005;22(10):2541–2548. doi: 10.1111/j.1460-9568.2005.04420.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown FD, Swalla BJ. Evolution and development of budding by stem cells: ascidian coloniality as a case study. Dev Biol. 2012;369(2):151–162. doi: 10.1016/j.ydbio.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 43.Brozovic M, Dantec C, Dardaillon J, Dauga D, Faure E, Gineste M, Louis A, Naville M, Nitta KR, Piette JJ. ANISEED 2017: extending the integrated ascidian database to the exploration and evolutionary comparison of genome-scale datasets. Nucleic Acids Res. 2017;46(1):718–725. doi: 10.1093/nar/gkx1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5(3):a008292. doi: 10.1101/cshperspect.a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet T, Fischer AH, Steinmetz PR, Lauri A, Bertucci P, Arendt D. The evolutionary origin of bilaterian smooth and striated myocytes. Elife. 2016;5:e19607. doi: 10.7554/eLife.19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6(11):826–837. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 47.Buckingham M, Rigby PW. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28(3):225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Burgess R, Rawls A, Brown D, Bradley A, Olson EN. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature. 1996;384(6609):570. doi: 10.1038/384570a0. [DOI] [PubMed] [Google Scholar]
- 49.Cañestro C, Bassham S, Postlethwait JH. Evolution of the thyroid: anterior–posterior regionalization of the Oikopleura endostyle revealed by Otx, Pax2/5/8, and Hox1 expression. Dev Dyn. 2008;237(5):1490–1499. doi: 10.1002/dvdy.21525. [DOI] [PubMed] [Google Scholar]
- 50.Cavey MJ. Myogenic events in compound ascidian larvae. Am Zool. 1982;22(4):807–815. doi: 10.1093/icb/22.4.807. [DOI] [Google Scholar]
- 51.Cavey MJ, Cloney RA. Fine structure and differentiation of ascidian muscle. I. Differentiated caudal musculature of Distaplia occidentalis tadpoles. J Morphol. 1972;138(3):349–373. doi: 10.1002/jmor.1051380304. [DOI] [PubMed] [Google Scholar]
- 52.Cavey MJ, Cloney RA. Ultrastructure and differentiation of ascidian muscle. Cell Tissue Res. 1976;174(3):289–313. doi: 10.1007/BF00220677. [DOI] [PubMed] [Google Scholar]
- 53.Chabry L. Contribution a l’embryologie normale et teratologique des Ascidies simples. J Anat Physiol. 1887;23:167–319. [Google Scholar]
- 54.Chalamalasetty RB, Dunty WC, Jr, Biris KK, Ajima R, Iacovino M, Beisaw A, Feigenbaum L, Chapman DL, Yoon JK, Kyba M. The Wnt3a/[beta]-catenin target gene Mesogenin1 controls the segmentation clock by activating a Notch signalling program. Nat Commun. 2011;2:390. doi: 10.1038/ncomms1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180(2):534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- 56.Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391(6668):695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Krause M, Sepanski M, Fire A. The Caenorhabditis elegans MYOD homologue HLH-1 is essential for proper muscle function and complete morphogenesis. Development. 1994;120(6):1631–1641. doi: 10.1242/dev.120.6.1631. [DOI] [PubMed] [Google Scholar]
- 58.Chiba S, Awazu S, Itoh M, Chin-Bow ST, Satoh N, Satou Y, Hastings KE. A genomewide survey of developmentally relevant genes in Ciona intestinalis. Dev Genes Evol. 2003;213(5–6):291–302. doi: 10.1007/s00427-003-0324-x. [DOI] [PubMed] [Google Scholar]
- 59.Christiaen L, Davidson B, Kawashima T, Powell W, Nolla H, Vranizan K, Levine M. The transcription/migration interface in heart precursors of Ciona intestinalis. Science. 2008;320(5881):1349. doi: 10.1126/science.1158170. [DOI] [PubMed] [Google Scholar]
- 60.Christiaen L, Stolfi A, Davidson B, Levine M. Spatio-temporal intersection of Lhx3 and Tbx6 defines the cardiac field through synergistic activation of Mesp. Dev Biol. 2009;328(2):552–560. doi: 10.1016/j.ydbio.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Cleto CL, Vandenberghe AE, MacLean DW, Pannunzio P, Tortorelli C, Meedel TH, Satou Y, Satoh N, Hastings KE. Ascidian larva reveals ancient origin of vertebrate-skeletal-muscle troponin I characteristics in chordate locomotory muscle. Mol Biol Evol. 2003;20(12):2113–2122. doi: 10.1093/molbev/msg227. [DOI] [PubMed] [Google Scholar]
- 62.Cloney RA. Ascidian larvae and the events of metamorphosis. Am Zool. 1982;22(4):817–826. doi: 10.1093/icb/22.4.817. [DOI] [Google Scholar]
- 63.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. doi: 10.1016/S1534-5807(02)00254-X. [DOI] [PubMed] [Google Scholar]
- 64.Conklin EG. Mosaic development in ascidian eggs. J Exp Zool. 1905;2(2):145–223. doi: 10.1002/jez.1400020202. [DOI] [Google Scholar]
- 65.Conklin EG. Organ-forming substances in the eggs of ascidians. Biol Bull. 1905;8(4):205. doi: 10.2307/1535879. [DOI] [Google Scholar]
- 66.Conklin EG. The organization and cell-lineage of the ascidian egg, vol 13; 1905.
- 67.Conklin EG. The development of centrifuged eggs of ascidians. J Exp Zool. 1931;60(1):1–119. doi: 10.1002/jez.1400600102. [DOI] [Google Scholar]
- 68.Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat Cell Biol. 2011;13(8):952–957. doi: 10.1038/ncb2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cota CD, Davidson B. Mitotic membrane turnover coordinates differential induction of the heart progenitor lineage. Dev Cell. 2015;34(5):505–519. doi: 10.1016/j.devcel.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Cota CD, Segade F, Davidson B. Heart genetics in a small package, exploiting the condensed genome of Ciona intestinalis. Brief Funct Genom. 2013;13(1):3–14. doi: 10.1093/bfgp/elt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crowther RJ, Whittaker J. Developmental autonomy of muscle fine structure in muscle lineage cells of ascidian embryos. Dev Biol. 1983;96(1):1–10. doi: 10.1016/0012-1606(83)90305-6. [DOI] [PubMed] [Google Scholar]
- 72.Damas D. Recherches sur le développement des Molgules. Arch Biol. 1902;18:599–664. [Google Scholar]
- 73.Davidson B. Ciona intestinalis as a model for cardiac development. Semin Cell Dev Biol. 2007;18(1):16–26. doi: 10.1016/j.semcdb.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davidson B, Levine M. Evolutionary origins of the vertebrate heart: specification of the cardiac lineage in Ciona intestinalis. Proc Natl Acad Sci. 2003;100(20):11469–11473. doi: 10.1073/pnas.1634991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson B, Shi W, Beh J, Christiaen L, Levine M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes Dev. 2006;20(19):2728. doi: 10.1101/gad.1467706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidson B, Shi W, Levine M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development. 2005;132(21):4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- 77.Davidson B, Wallace SES, Howsmon RA, Swalla BJ. A morphological and genetic characterization of metamorphosis in the ascidian Boltenia villosa. Dev Genes Evol. 2003;213(12):601–611. doi: 10.1007/s00427-003-0363-3. [DOI] [PubMed] [Google Scholar]
- 78.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 79.Degasperi V, Gasparini F, Shimeld SM, Sinigaglia C, Burighel P, Manni L. Muscle differentiation in a colonial ascidian: organisation, gene expression and evolutionary considerations. BMC Dev Biol. 2009;9(1):1. doi: 10.1186/1471-213X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deibel D. The abundance, distribution, and ecological impact of doliolids. Biol Pelagic Tunicates. 1998;171–186.
- 81.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439(7079):965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 82.Delsuc F, Philippe H, Tsagkogeorga G, Simion P, Tilak M-K, Turon X, López-Legentil S, Piette J, Lemaire P, Douzery EJP. A phylogenomic framework and timescale for comparative studies of tunicates. BMC Biol. 2018;16(1):39. doi: 10.1186/s12915-018-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delsuc F, Tsagkogeorga G, Lartillot N, Philippe H. Additional molecular support for the new chordate phylogeny. Genesis. 2008;46(11):592–604. doi: 10.1002/dvg.20450. [DOI] [PubMed] [Google Scholar]
- 84.Denker E, Bočina I, Jiang D. Tubulogenesis in a simple cell cord requires the formation of bi-apical cells through two discrete Par domains. Development. 2013;140(14):2985–2996. doi: 10.1242/dev.092387. [DOI] [PubMed] [Google Scholar]
- 85.Deno T, Satoh N. Studies on the cytoplasmic determinant for muscle cell differentiation in ascidian embryos: an attempt at transplantation of the myoplasm. Dev Growth Differ. 1984;26(1):38–43. doi: 10.1111/j.1440-169X.1984.00043.x. [DOI] [PubMed] [Google Scholar]
- 86.Denoeud F, Henriet S, Mungpakdee S, Aury J-M, Da Silva C, Brinkmann H, Mikhaleva J, Olsen LC, Jubin C, Cañestro C. Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science. 2010;330(6009):1381–1385. doi: 10.1126/science.1194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Gregorio A. T-box genes and developmental gene regulatory networks in ascidians. In: Frasch M, editor. Current topics in developmental biology. Amsterdam: Elsevier; 2017. pp. 55–91. [DOI] [PubMed] [Google Scholar]
- 88.Di Gregorio A, Harland RM, Levine M, Casey ES. Tail morphogenesis in the ascidian, Ciona intestinalis, requires cooperation between notochord and muscle. Dev Biol. 2002;244(2):385–395. doi: 10.1006/dbio.2002.0582. [DOI] [PubMed] [Google Scholar]
- 89.Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor EJN. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520(7548):466. doi: 10.1038/nature14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dubois L, Enriquez J, Daburon V, Crozet F, Lebreton G, Crozatier M, Vincent A. Collier transcription in a single Drosophila muscle lineage: the combinatorial control of muscle identity. Development. 2007;134(24):4347–4355. doi: 10.1242/dev.008409. [DOI] [PubMed] [Google Scholar]
- 91.Dubois L, Frendo J-L, Chanut-Delalande H, Crozatier M, Vincent A. Genetic dissection of the Transcription Factor code controlling serial specification of muscle identities in Drosophila. Elife. 2016;5:e14979. doi: 10.7554/eLife.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dufour HD, Chettouh Z, Deyts C, de Rosa R, Goridis C, Joly J-S, Brunet J-F. Precraniate origin of cranial motoneurons. Proc Natl Acad Sci. 2006;103(23):8727–8732. doi: 10.1073/pnas.0600805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dumollard R, Hebras C, Besnardeau L, McDougall A. Beta-catenin patterns the cell cycle during maternal-to-zygotic transition in urochordate embryos. Dev Biol. 2013;384(2):331–342. doi: 10.1016/j.ydbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Durante M. Cholinesterase in the anterior and posterior hemiembryos of Ciona intestinalis. Acta Embryol Morph Exp. 1957;1(2):131–133. [Google Scholar]
- 95.Endo T, Matsumoto K, Hama T, Ohtsuka Y, Katsura G, Obinata T. Distinct troponin T genes are expressed in embryonic/larval tail striated muscle and adult body wall smooth muscle of ascidian. J Biol Chem. 1996;271(44):27855–27862. doi: 10.1074/jbc.271.44.27855. [DOI] [PubMed] [Google Scholar]
- 96.Endo T, Matsumoto K, Hama T, Ohtsuka Y, Katsura G, Obinata T. Temporal and spatial expression of distinct troponin T genes in embryonic/larval tail striated muscle and adult body wall smooth muscle of ascidian. Cell Struct Funct. 1997;22(1):197–203. doi: 10.1247/csf.22.197. [DOI] [PubMed] [Google Scholar]
- 97.Enriquez J, Boukhatmi H, Dubois L, Philippakis AA, Bulyk ML, Michelson AM, Crozatier M, Vincent A. Multi-step control of muscle diversity by Hox proteins in the Drosophila embryo. Development. 2010;137(3):457–466. doi: 10.1242/dev.045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enriquez J, de Taffin M, Crozatier M, Vincent A, Dubois L. Combinatorial coding of Drosophila muscle shape by Collier and Nautilus. Dev Biol. 2012;363(1):27–39. doi: 10.1016/j.ydbio.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 99.Erives A, Levine M. Characterization of a maternal T-Box gene in Ciona intestinalis. Dev Biol. 2000;225(1):169–178. doi: 10.1006/dbio.2000.9815. [DOI] [PubMed] [Google Scholar]
- 100.Farin HF, Bussen M, Schmidt MK, Singh MK, Schuster-Gossler K, Kispert A. Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on Groucho corepressors. J Biol Chem. 2007;282(35):25748–25759. doi: 10.1074/jbc.M703724200. [DOI] [PubMed] [Google Scholar]
- 101.Farley EK, Olson KM, Zhang W, Brandt AJ, Rokhsar DS, Levine MS. Suboptimization of developmental enhancers. Science. 2015;350(6258):325–328. doi: 10.1126/science.aac6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fenaux R. Cycle vital d’un appendiculaire Oikopleura dioica Fol, 1872 description et chronologie. Ann Inst Oceanogr Paris. 1976;52:89–101. [Google Scholar]
- 103.Fenaux R. Rhythm of secretion of oikopleurid’s houses. Bull Mar Sci. 1985;37(2):498–503. [Google Scholar]
- 104.Fenaux R. The house of Oikopleura dioica (Tunicata, Appendicularia): structure and functions. Zoomorphology. 1986;106(4):224–231. doi: 10.1007/BF00312043. [DOI] [Google Scholar]
- 105.Fenaux R. Anatomy and functional morphology of the Appendicularia. Biol Pelagic Tunicates. 1998;25–34.
- 106.Flood P, Deibel D. The appendicularian house. Biol Pelagic Tunicates. 1998;105–124.
- 107.Fong AP, Tapscott SJ. Skeletal muscle programming and re-programming. Curr Opin Genet Dev. 2013;23(5):568–573. doi: 10.1016/j.gde.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fujii S, Nishio T, Nishida H. Cleavage pattern, gastrulation, and neurulation in the appendicularian, Oikopleura dioica. Dev Genes Evolut. 2008;218(2):69–79. doi: 10.1007/s00427-008-0205-4. [DOI] [PubMed] [Google Scholar]
- 109.Fujiwara S, Corbo JC, Levine M. The snail repressor establishes a muscle/notochord boundary in the Ciona embryo. Development. 1998;125(13):2511–2520. doi: 10.1242/dev.125.13.2511. [DOI] [PubMed] [Google Scholar]
- 110.Fukushige T, Krause M. The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. Elegans embryos. Development. 2005;132(8):1795–1805. doi: 10.1242/dev.01774. [DOI] [PubMed] [Google Scholar]
- 111.Garstang W. Memoirs: the morphology of the tunicata, and its bearings on the phylogeny of the chordata. Quart J Microsc Sci. 1928;2(285):51–187. [Google Scholar]
- 112.Gasparini F, Degasperi V, Shimeld SM, Burighel P, Manni L. Evolutionary conservation of the placodal transcriptional network during sexual and asexual development in chordates. Dev Dyn. 2013;242(6):752–766. doi: 10.1002/dvdy.23957. [DOI] [PubMed] [Google Scholar]
- 113.Godeaux J. Stades larvaires du Doliolum. Bulletin de la Classe des Sciences Académie Royale de Belgique. 1955;91(7):769–787. [Google Scholar]
- 114.Godeaux J. The relationships and systematics of the Thaliacea, with keys for identification. Biol Pelagic Tunicates. 1998;273–294.
- 115.Godeaux J, Bone Q, Braconnot J. Anatomy of Thaliacea. Biol Pelagic Tunicates. 1998;1–24.
- 116.Godeaux J, Harbison GJ. On some pelagic doliolid tunicates (Thaliacea, Doliolida) collected by a submersible off the eastern North American coast. Bull Mar Sci. 2003;72(3):589–612. [Google Scholar]
- 117.Godeaux JE. History and revised classification of the order Cyclomyaria (Tunicata, Thaliacea, Doliolida) Biologie. 2003;73:191–222. [Google Scholar]
- 118.Goodbody I. The physiology of ascidians. Adv Mar Biol. 1975;12:1–149. doi: 10.1016/S0065-2881(08)60457-5. [DOI] [Google Scholar]
- 119.Gorsky G, Fenaux R. The role of Appendicularia in marine food webs. Biol Pelagic Tunicates. 1998;161–169.
- 120.Gouti M, Metzis V, Briscoe J. The route to spinal cord cell types: a tale of signals and switches. Trends Genet. 2015;31(6):282–289. doi: 10.1016/j.tig.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 121.Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 2014;12(8):e1001937. doi: 10.1371/journal.pbio.1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Govindarajan AF, Bucklin A, Madin LP. A molecular phylogeny of the Thaliacea. J Plankton Res. 2011;33(6):843–853. doi: 10.1093/plankt/fbq157. [DOI] [Google Scholar]
- 123.Grave C. Amaroucium pellucidum (Leidy) form constellatum (Verrill). I. The activities and reactions of the tadpole larva. J Exp Zool. 1920;30(2):239–257. doi: 10.1002/jez.1400300206. [DOI] [Google Scholar]
- 124.Grave C, McCosh G. Perophora viridis (Verrill) The activities and structure of the free-swimming larva. Wash Univ Stud Scient Ser. 1923;11(89):116. [Google Scholar]
- 125.Green YS, Vetter ML. EBF proteins participate in transcriptional regulation of Xenopus muscle development. Dev Biol. 2011;358(1):240–250. doi: 10.1016/j.ydbio.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132(9):2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- 127.Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti J-E, Kawakami K, Xu P-X, Kelly R, Petrof BJ. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol. 2004;24(14):6253–6267. doi: 10.1128/MCB.24.14.6253-6267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guignard L, Fiuza UM, Leggio B, Faure E, Laussu J, Hufnagel L, Malandain G, Godin C, Lemaire P. Contact-dependent cell communications drive morphological invariance during ascidian embryogenesis. bioRxiv. 2017 doi: 10.1101/238741. [DOI] [PubMed] [Google Scholar]
- 129.Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev. 2011;86(3):564–600. doi: 10.1111/j.1469-185X.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gyoja F. Expression of a muscle determinant gene, macho-1, in the anural ascidian Molgula tectiformis. Dev Genes Evol. 2006;216(5):285–289. doi: 10.1007/s00427-005-0056-1. [DOI] [PubMed] [Google Scholar]
- 131.Gyoja F, Satou Y, Shin-i T, Kohara Y, Swalla BJ, Satoh N. Analysis of large scale expression sequenced tags (ESTs) from the anural ascidian, Molgula tectiformis. Dev Biol. 2007;307(2):460–482. doi: 10.1016/j.ydbio.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 132.Haag ES. Compensatory vs. pseudocompensatory evolution in molecular and developmental interactions. Genetica. 2007;129(1):45–55. doi: 10.1007/s10709-006-0032-3. [DOI] [PubMed] [Google Scholar]
- 133.Hadfield KA, Swalla BJ, Jeffery WR. Multiple origins of anural development in ascidians inferred from rDNA sequences. J Mol Evol. 1995;40(4):413–427. doi: 10.1007/BF00164028. [DOI] [PubMed] [Google Scholar]
- 134.Hare EE, Peterson BK, Iyer VN, Meier R, Eisen MB. Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet. 2008;4(6):e1000106. doi: 10.1371/journal.pgen.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harel I, Maezawa Y, Avraham R, Rinon A, Ma H-Y, Cross JW, Leviatan N, Hegesh J, Roy A, Jacob-Hirsch J. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc Natl Acad Sci. 2012;109(46):18839–18844. doi: 10.1073/pnas.1208690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hashimoto H, Robin FB, Sherrard KM, Munro EM. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev Cell. 2015;32(2):241–255. doi: 10.1016/j.devcel.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 137.Hastings KE. Molecular evolution of the vertebrate troponin I gene family. Cell Struct Funct. 1997;22(1):205–211. doi: 10.1247/csf.22.205. [DOI] [PubMed] [Google Scholar]
- 138.Haupaix N, Stolfi A, Sirour C, Picco V, Levine M, Christiaen L, Yasuo H. p120RasGAP mediates ephrin/Eph-dependent attenuation of FGF/ERK signals during cell fate specification in ascidian embryos. Development. 2013;140(21):4347–4352. doi: 10.1242/dev.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hayashi S, Nakahata Y, Kohno K, Matsui T, Bessho Y. Presomitic mesoderm-specific expression of the transcriptional repressor Hes7 is controlled by E-box, T-box, and Notch signaling pathway. J Biol Chem. 2018;293:12167–12176. doi: 10.1074/jbc.RA118.003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hellbach A, Tiozzo S, Ohn J, Liebling M, De Tomaso AW. Characterization of HCN and cardiac function in a colonial ascidian. J Exp Zool Part A. 2011;315(8):476–486. doi: 10.1002/jez.695. [DOI] [PubMed] [Google Scholar]
- 141.Hirano T, Nishida H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. Dev Biol. 1997;192(2):199–210. doi: 10.1006/dbio.1997.8772. [DOI] [PubMed] [Google Scholar]
- 142.Holland LZ, Schubert M, Kozmik Z, Holland ND. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evolut Dev. 1999;1(3):153–165. doi: 10.1046/j.1525-142x.1999.99019.x. [DOI] [PubMed] [Google Scholar]
- 143.Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1–13. doi: 10.1016/j.ydbio.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 144.Huber JL, da Silva KB, Bates WR, Swalla BJ. The evolution of anural larvae in molgulid ascidians. Semin Cell Dev Biol. 2000;11(6):419–426. doi: 10.1006/scdb.2000.0195. [DOI] [PubMed] [Google Scholar]
- 145.Hudson C, Lotito S, Yasuo H. Sequential and combinatorial inputs from Nodal, Delta2/Notch and FGF/MEK/ERK signalling pathways establish a grid-like organisation of distinct cell identities in the ascidian neural plate. Development. 2007;134(19):3527–3537. doi: 10.1242/dev.002352. [DOI] [PubMed] [Google Scholar]
- 146.Hudson C, Yasuo H. A signalling relay involving Nodal and Delta ligands acts during secondary notochord induction in Ciona embryos. Development. 2006;133(15):2855–2864. doi: 10.1242/dev.02466. [DOI] [PubMed] [Google Scholar]
- 147.Hudson C, Yasuo H. Similarity and diversity in mechanisms of muscle fate induction between ascidian species. Biol Cell. 2008;100(5):265–277. doi: 10.1042/BC20070144. [DOI] [PubMed] [Google Scholar]
- 148.Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 149.Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- 150.Huxley TH. Observations upon the anatomy and physiology of Salpa and Pyrosoma. Philos Trans R Soc Lond. 1851;141:567–593. doi: 10.1098/rstl.1851.0027. [DOI] [Google Scholar]
- 151.Ikeda D, Ono Y, Snell P, Edwards YJK, Elgar G, Watabe S. Divergent evolution of the myosin heavy chain gene family in fish and tetrapods: evidence from comparative genomic analysis. Physiol Genomics. 2007;32(1):1–15. doi: 10.1152/physiolgenomics.00278.2006. [DOI] [PubMed] [Google Scholar]
- 152.Ikeda T, Matsuoka T, Satou Y. A time delay gene circuit is required for palp formation in the ascidian embryo. Development. 2013;140(23):4703–4708. doi: 10.1242/dev.100339. [DOI] [PubMed] [Google Scholar]
- 153.Imai KS, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131(16):4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- 154.Imai KS, Levine M, Satoh N, Satou Y. Regulatory blueprint for a chordate embryo. Science. 2006;312(5777):1183. doi: 10.1126/science.1123404. [DOI] [PubMed] [Google Scholar]
- 155.Imai KS, Satou Y, Satoh N. Multiple functions of a Zic-like gene in the differentiation of notochord, central nervous system and muscle in Ciona savignyi embryos. Development. 2002;129(11):2723–2732. doi: 10.1242/dev.129.11.2723. [DOI] [PubMed] [Google Scholar]
- 156.Imai KS, Stolfi A, Levine M, Satou Y. Gene regulatory networks underlying the compartmentalization of the Ciona central nervous system. Development. 2009;136(2):285. doi: 10.1242/dev.026419. [DOI] [PubMed] [Google Scholar]
- 157.Izzi SA, Colantuono BJ, Sullivan K, Khare P, Meedel TH. Functional studies of the Ciona intestinalis myogenic regulatory factor reveal conserved features of chordate myogenesis. Dev Biol. 2013;376(2):213–223. doi: 10.1016/j.ydbio.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jefferies R. Two types of bilateral symmetry in the Metazoa: chordate and bilaterian. In: Ciba Foundation Symposium 162-Biological Asymmetry and Handedness. Wiley Online Library; 1991. p. 94–127. [DOI] [PubMed]
- 159.Jeffery WR, Meier S. A yellow crescent cytoskeletal domain in ascidian eggs and its role in early development. Dev Biol. 1983;96(1):125–143. doi: 10.1016/0012-1606(83)90317-2. [DOI] [PubMed] [Google Scholar]
- 160.Jeffery WR, Swalla BJ. An evolutionary change in the muscle lineage of an anural ascidian embryo is restored by interspecific hybridization with a urodele ascidian. Dev Biol. 1991;145(2):328–337. doi: 10.1016/0012-1606(91)90131-L. [DOI] [PubMed] [Google Scholar]
- 161.Jeffery WR, Swalla BJ, Ewing N, Kusakabe T. Evolution of the ascidian anural larva: evidence from embryos and molecules. Mol Biol Evol. 1999;16(5):646–654. doi: 10.1093/oxfordjournals.molbev.a026147. [DOI] [PubMed] [Google Scholar]
- 162.Jin S, Kim J, Willert T, Klein-Rodewald T, Garcia-Dominguez M, Mosqueira M, Fink R, Esposito I, Hofbauer LC, Charnay P. Ebf factors and MyoD cooperate to regulate muscle relaxation via Atp2a1. Nat Commun. 2014;5:3793. doi: 10.1038/ncomms4793. [DOI] [PubMed] [Google Scholar]
- 163.Johnson DS, Zhou Q, Yagi K, Satoh N, Wong W, Sidow A. De novo discovery of a tissue-specific gene regulatory module in a chordate. Genome Res. 2005;15(10):1315–1324. doi: 10.1101/gr.4062605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johnson NA, Porter AH. Evolution of branched regulatory genetic pathways: directional selection on pleiotropic loci accelerates developmental system drift. Genetica. 2007;129(1):57–70. doi: 10.1007/s10709-006-0033-2. [DOI] [PubMed] [Google Scholar]
- 165.Kaplan N, Razy-Krajka F, Christiaen L. Regulation and evolution of cardiopharyngeal cell identity and behavior: insights from simple chordates. Curr Opin Genet Dev. 2015;32:119–128. doi: 10.1016/j.gde.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kassar-Duchossoy L, Gayraud-Morel B, Gomès D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5: myod double-mutant mice. Nature. 2004;431(7007):466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- 167.Katikala L, Aihara H, Passamaneck YJ, Gazdoiu S, José-Edwards DS, Kugler JE, Oda-Ishii I, Imai JH, Nibu Y, Di Gregorio A. Functional Brachyury binding sites establish a temporal read-out of gene expression in the Ciona notochord. PLoS Biol. 2013;11(10):e1001697. doi: 10.1371/journal.pbio.1001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kawai N, Ogura Y, Ikuta T, Saiga H, Hamada M, Sakuma T, Yamamoto T, Satoh N, Sasakura Y. Hox10-regulated endodermal cell migration is essential for development of the ascidian intestine. Dev Biol. 2015;403(1):43–56. doi: 10.1016/j.ydbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 169.Kelly RG. The second heart field. Curr Top Dev Biol. 2012;100:33–65. doi: 10.1016/B978-0-12-387786-4.00002-6. [DOI] [PubMed] [Google Scholar]
- 170.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–440. doi: 10.1016/S1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 171.Kimura S, Itoh T. Biogenesis and function of cellulose in the tunicates. In: Malcolm Brown R Jr, Saxena IM, editors. Cellulose: molecular and structural biology. Dordrecht: Springer; 2007. pp. 217–236. [Google Scholar]
- 172.Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DH, Félix M-A. Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol. 2007;17(22):1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 173.Kishi K, Onuma TA, Nishida H. Long-distance cell migration during larval development in the appendicularian, Oikopleura dioica. Dev Biol. 2014;395(2):299–306. doi: 10.1016/j.ydbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 174.Kocot KM, Tassia MG, Halanych KM, Swalla BJ. Phylogenomics offers resolution of major tunicate relationships. Mol Phylogenet Evol. 2018;121:166–173. doi: 10.1016/j.ympev.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 175.Korschelt E, Heider K. Lehrbuch der vergleichenden Entwicklungsgeschichte der wirbellosen Thiere. Schaffhausen: Fischer; 1890. [Google Scholar]
- 176.Koshiba-Takeuchi K, Mori AD, Kaynak BL, Cebra-Thomas J, Sukonnik T, Georges RO, Latham S, Beck L, Henkelman RM, Black BL. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature. 2009;461(7260):95–98. doi: 10.1038/nature08324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kovalevskij AO. Entwickelungsgeschichte der einfachen Ascidien. Commissionäre der Kaiserlichen akademie der wissenschaften. St. Petersburg: Eggers et cie und H. Schmitzdorff; 1866. [Google Scholar]
- 178.Kubo A, Suzuki N, Yuan X, Nakai K, Satoh N, Imai KS, Satou Y. Genomic cis-regulatory networks in the early Ciona intestinalis embryo. Development. 2010;137(10):1613–1623. doi: 10.1242/dev.046789. [DOI] [PubMed] [Google Scholar]
- 179.Kugler JE, Gazdoiu S, Oda-Ishii I, Passamaneck YJ, Erives AJ, Di Gregorio A. Temporal regulation of the muscle gene cascade by Macho1 and Tbx6 transcription factors in Ciona intestinalis. J Cell Sci. 2010;123(14):2453–2463. doi: 10.1242/jcs.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kumano G, Negoro N, Nishida H. Transcription factor Tbx6 plays a central role in fate determination between mesenchyme and muscle in embryos of the ascidian, Halocynthia roretzi. Dev Growth Differ. 2014;56(4):310–322. doi: 10.1111/dgd.12133. [DOI] [PubMed] [Google Scholar]
- 181.Kusakabe R, Inoue K. Developmental regulation and evolution of muscle-specific microRNAs. In: Sampath K, Hiiragi T, Hadjantonakis K, editors. Seminars in cell and developmental biology. Amsterdam: Elsevier; 2015. pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 182.Kusakabe R, Tani S, Nishitsuji K, Shindo M, Okamura K, Miyamoto Y, Nakai K, Suzuki Y, Kusakabe TG, Inoue K. Characterization of the compact bicistronic microRNA precursor, miR-1/miR-133, expressed specifically in Ciona muscle tissues. Gene Expr Patterns. 2013;13(1–2):43–50. doi: 10.1016/j.gep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 183.Kusakabe T, Swalla BJ, Satoh N, Jeffery WR. Mechanism of an evolutionary change in muscle cell differentiation in ascidians with different modes of development. Dev Biol. 1996;174(2):379–392. doi: 10.1006/dbio.1996.0082. [DOI] [PubMed] [Google Scholar]
- 184.Kusakabe T, Yoshida R, Ikeda Y, Tsuda M. Computational discovery of DNA motifs associated with cell type-specific gene expression in Ciona. Dev Biol. 2004;276(2):563–580. doi: 10.1016/j.ydbio.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 185.Kuwajima M, Kumano G, Nishida H. Regulation of the number of cell division rounds by tissue-specific transcription factors and Cdk inhibitor during ascidian embryogenesis. PLoS ONE. 2014;9(3):e90188. doi: 10.1371/journal.pone.0090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lacaze-Duthiers H. Histoire des ascidies simples des cotes de France. II. Étudies des especes. Arch Zool Exp Gen. 1877;6:457–676. [Google Scholar]
- 187.Lankester ER. Memoirs: on the heart of appendicularia furcata and the development of its muscular fibres. J Cell Sci. 1874;2(55):274–277. [Google Scholar]
- 188.Layden MJ, Meyer NP, Pang K, Seaver EC, Martindale MQ. Expression and phylogenetic analysis of the zic gene family in the evolution and development of metazoans. EvoDevo. 2010;1(1):1. doi: 10.1186/2041-9139-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lazic S, Scott IC. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev Biol. 2011;354(1):123–133. doi: 10.1016/j.ydbio.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 190.Lebrato M, Jones D. Mass deposition event of Pyrosoma atlanticum carcasses off Ivory Coast (West Africa) Limnol Oceanogr. 2009;54(4):1197–1209. doi: 10.4319/lo.2009.54.4.1197. [DOI] [Google Scholar]
- 191.Lemaire P. Unfolding a chordate developmental program, one cell at a time: invariant cell lineages, short-range inductions and evolutionary plasticity in ascidians. Dev Biol. 2009;332(1):48–60. doi: 10.1016/j.ydbio.2009.05.540. [DOI] [PubMed] [Google Scholar]
- 192.Lemaire P. Evolutionary crossroads in developmental biology: the tunicates. Development. 2011;138(11):2143–2152. doi: 10.1242/dev.048975. [DOI] [PubMed] [Google Scholar]
- 193.Lemaire P, Piette J. Tunicates: exploring the sea shores and roaming the open ocean A tribute to Thomas Huxley. Open Biol. 2015;5(6):150053. doi: 10.1098/rsob.150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lescroart F, Kelly RG, Le Garrec J-F, Nicolas J-F, Meilhac SM, Buckingham M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137(19):3269–3279. doi: 10.1242/dev.050674. [DOI] [PubMed] [Google Scholar]
- 195.Lowe EK, Racioppi C, Peyriéras N, Ristoratore F, Christiaen L, Swalla BJ, Stolfi A. A cis-regulatory change underlying the motor neuron-specific loss of terminal selector gene expression in immotile tunicate larvae. bioRxiv. 2019 doi: 10.1101/567719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mackie G, Bone Q. Skin impulses and locomotion in an ascidian tadpole. J Mar Biol Assoc U K. 1976;56(3):751–768. doi: 10.1017/S0025315400020774. [DOI] [Google Scholar]
- 197.Mackie G, Bone Q. Locomotion and propagated skin impulses in salps (Tunicata: Thaliacea) Biol Bull. 1977;153(1):180–197. doi: 10.2307/1540700. [DOI] [PubMed] [Google Scholar]
- 198.MacLean DW, Meedel TH, Hastings KE. Tissue-specific Alternative Splicing of Ascidian Troponin I Isoforms REDESIGN OF A PROTEIN ISOFORM-GENERATING MECHANISM DURING CHORDATE EVOLUTION. J Biol Chem. 1997;272(51):32115–32120. doi: 10.1074/jbc.272.51.32115. [DOI] [PubMed] [Google Scholar]
- 199.Madgwick A, Magri MS, Dantec C, Gailly D, Fiuza U-M, Guignard L, Hettinger S, Gomez-Skarmeta JL, Lemaire P. Evolution of embryonic cis-regulatory landscapes between divergent Phallusia and Ciona ascidians. Dev Biol. 2019. [DOI] [PubMed]
- 200.Makabe KW, Satoh N. Temporal expression of myosin heavy chain gene during ascidian embryogenesis. Dev Growth Differ. 1989;31(1):71–77. doi: 10.1111/j.1440-169X.1989.00071.x. [DOI] [PubMed] [Google Scholar]
- 201.Maliska ME, Pennell MW, Swalla BJ. Developmental mode influences diversification in ascidians. Biol Let. 2013;9(3):20130068. doi: 10.1098/rsbl.2013.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Manni L, Gasparini F, Hotta K, Ishizuka KJ, Ricci L, Tiozzo S, Voskoboynik A, Dauga D. Ontology for the asexual development and anatomy of the colonial chordate Botryllus schlosseri. PLoS ONE. 2014;9(5):e96434. doi: 10.1371/journal.pone.0096434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marikawa Y, Yoshida S, Satoh N. Muscle determinants in the ascidian egg are inactivated by UV irradiation and the inactivation is partially rescued by injection of maternal mRNAs. Roux’s Arch Dev Biol. 1995;204(3):180–186. doi: 10.1007/BF00241270. [DOI] [PubMed] [Google Scholar]
- 204.Matthysse AG, Deschet K, Williams M, Marry M, White AR, Smith WC. A functional cellulose synthase from ascidian epidermis. Proc Natl Acad Sci USA. 2004;101(4):986–991. doi: 10.1073/pnas.0303623101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mazet F, Hutt JA, Milloz J, Millard J, Graham A, Shimeld SM. Molecular evidence from Ciona intestinalis for the evolutionary origin of vertebrate sensory placodes. Dev Biol. 2005;282(2):494–508. doi: 10.1016/j.ydbio.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 206.McGuigan K, Phillips PC, Postlethwait JH. Evolution of sarcomeric myosin heavy chain genes: evidence from fish. Mol Biol Evol. 2004;21(6):1042–1056. doi: 10.1093/molbev/msh103. [DOI] [PubMed] [Google Scholar]
- 207.Meedel T, Hastings K. Striated muscle-type tropomyosin in a chordate smooth muscle, ascidian body-wall muscle. J Biol Chem. 1993;268(9):6755–6764. [PubMed] [Google Scholar]
- 208.Meedel TH, Chang P, Yasuo H. Muscle development in Ciona intestinalis requires the b-HLH myogenic regulatory factor gene Ci-MRF. Dev Biol. 2007;302(1):333–344. doi: 10.1016/j.ydbio.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Meedel TH, Crowther RJ, Whittaker J. Determinative properties of muscle lineages in ascidian embryos. Development. 1987;100(2):245–260. doi: 10.1242/dev.100.2.245. [DOI] [PubMed] [Google Scholar]
- 210.Meedel TH, Farmer SC, Lee JJ. The single MyoD family gene of Ciona intestinalis encodes two differentially expressed proteins: implications for the evolution of chordate muscle gene regulation. Development. 1997;124(9):1711–1721. doi: 10.1242/dev.124.9.1711. [DOI] [PubMed] [Google Scholar]
- 211.Meedel TH, Lee JJ, Whittaker J. Muscle development and lineage-specific expression of CiMDF, the MyoD-family gene of Ciona intestinalis. Dev Biol. 2002;241(2):238–246. doi: 10.1006/dbio.2001.0511. [DOI] [PubMed] [Google Scholar]
- 212.Meedel TH, Whittaker J. Development of acetylcholinesterase during embryogenesis of the ascidian Ciona intestinalis. J Exp Zool. 1979;210(1):1–10. doi: 10.1002/jez.1402100102. [DOI] [PubMed] [Google Scholar]
- 213.Meedel TH, Whittaker J. Lineage segregation and developmental autonomy in expression of functional muscle acetylcholinesterase mRNA in the ascidian embryo. Dev Biol. 1984;105(2):479–487. doi: 10.1016/0012-1606(84)90305-1. [DOI] [PubMed] [Google Scholar]
- 214.Meilhac SM, Esner M, Kelly RG, Nicolas J-F, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6(5):685–698. doi: 10.1016/S1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 215.Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104(4):619–629. doi: 10.1016/S0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 216.Metcalf MM, Bell MM. The Salpidae: a taxonomic study. In: Papers on collections gathered by the “Albatross” philippine expedition, 1907–1910. Washington: U.S. Government Printing Office; 1932.
- 217.Millar R. The breeding and development of the ascidian Pelonaia corrugata Forbes and Goodsir. J Mar Biol Assoc U K. 1954;33(03):681–687. doi: 10.1017/S0025315400026953. [DOI] [Google Scholar]
- 218.Millar RH. Ciona. Liverpool: University Press of Liverpool; 1953. [Google Scholar]
- 219.Mintz B, Baker WW. Normal mammalian muscle differentiation and gene control of isocitrate dehydrogenase synthesis. Proc Natl Acad Sci. 1967;58(2):592–598. doi: 10.1073/pnas.58.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mitani Y, Takahashi H, Satoh N. An ascidian T-box gene As-T2 is related to the Tbx6 subfamily and is associated with embryonic muscle cell differentiation. Dev Dyn. 1999;215(1):62–68. doi: 10.1002/(SICI)1097-0177(199905)215:1<62::AID-DVDY7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 221.Mitani Y, Takahashi H, Satoh N. Regulation of the muscle-specific expression and function of an ascidian T-box gene, As-T2. Development. 2001;128(19):3717–3728. doi: 10.1242/dev.128.19.3717. [DOI] [PubMed] [Google Scholar]
- 222.Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83(7):1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 223.Monniot C, Monniot F. Feeding behaviour of abyssal tunicates. In: Proceedings of the 9th European marine biology symposium. 1975. p. 357–362.
- 224.Moreno TR, Rocha RM. Phylogeny of the Aplousobranchia (Tunicata: Ascidiacea) Revista Brasileira de Zoologia. 2008;25(2):269–298. doi: 10.1590/S0101-81752008000200016. [DOI] [Google Scholar]
- 225.Müller P, Seipel K, Yanze N, Reber-Müller S, Streitwolf-Engel R, Stierwald M, ürg Spring J, Schmid V. Evolutionary aspects of developmentally regulated helix-loop-helix transcription factors in striated muscle of jellyfish. Dev Biol. 2003;255(2):216–229. doi: 10.1016/S0012-1606(02)00091-X. [DOI] [PubMed] [Google Scholar]
- 226.Munro E, Robin F, Lemaire P. Cellular morphogenesis in ascidians: how to shape a simple tadpole. Curr Opin Genet Dev. 2006;16(4):399–405. doi: 10.1016/j.gde.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 227.Nagai T, Aruga J, Takada S, Günther T, Spörle R, Schughart K, Mikoshiba K. The expression of the MouseZic1, Zic2, andZic3Gene suggests an essential role forZicGenes in body pattern formation. Dev Biol. 1997;182(2):299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- 228.Nagatomo K-i, Fujiwara SJGEP. Expression of Raldh2, Cyp26 and Hox-1 in normal and retinoic acid-treated Ciona intestinalis embryos. Gene Expr Patterns. 2003;3(3):273–277. doi: 10.1016/S1567-133X(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 229.Nakashima K, Yamada L, Satou Y, Azuma J-i, Satoh N. The evolutionary origin of animal cellulose synthase. Dev Genes Evol. 2004;214(2):81–88. doi: 10.1007/s00427-003-0379-8. [DOI] [PubMed] [Google Scholar]
- 230.Nakazawa K, Yamazawa T, Moriyama Y, Ogura Y, Kawai N, Sasakura Y, Saiga H. Formation of the digestive tract in Ciona intestinalis includes two distinct morphogenic processes between its anterior and posterior parts. Dev Dyn. 2013;242(10):1172–1183. doi: 10.1002/dvdy.24009. [DOI] [PubMed] [Google Scholar]
- 231.Navarrete IA, Levine M. Nodal and FGF coordinate ascidian neural tube morphogenesis. Development. 2016;143(24):4665–4675. doi: 10.1242/dev.144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Neumann G. Die Pyrosomen der deutschen Tiefsee-Expedition. Leipzig: Fischer; 1913. [Google Scholar]
- 233.Nevitt G, Gilly WF. Morphological and physiological properties of non-striated muscle from the tunicate, Ciona intestinalis: parallels with vertebrate skeletal muscle. Tissue Cell. 1986;18(3):341–360. doi: 10.1016/0040-8166(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 234.Newman-Smith E, Kourakis MJ, Reeves W, Veeman M, Smith WC. Reciprocal and dynamic polarization of planar cell polarity core components and myosin. Elife. 2015;4:e05361. doi: 10.7554/eLife.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nishida H. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme: III. Up to the tissue restricted stage. Dev Biol. 1987;121(2):526–541. doi: 10.1016/0012-1606(87)90188-6. [DOI] [PubMed] [Google Scholar]
- 236.Nishida H. Determinative mechanisms in secondary muscle lineages of ascidian embryos: development of muscle-specific features in isolated muscle progenitor cells. Development. 1990;108(4):559–568. doi: 10.1242/dev.108.4.559. [DOI] [PubMed] [Google Scholar]
- 237.Nishida H. Developmental potential for tissue differentiation of fully dissociated cells of the ascidian embryo. Roux’s Arch Dev Biol. 1992;201(2):81–87. doi: 10.1007/BF00420418. [DOI] [PubMed] [Google Scholar]
- 238.Nishida H. Regionality of egg cytoplasm that promotes muscle differentiation in embryo of the ascidian, Halocynthia roretzi. Development. 1992;116(3):521–529. [Google Scholar]
- 239.Nishida H. Development of the appendicularian Oikopleura dioica: culture, genome, and cell lineages. Dev Growth Differ. 2008;50(s1):S239–S256. doi: 10.1111/j.1440-169X.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 240.Nishida H, Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme: I. Up to the eight-cell stage. Dev Biol. 1983;99(2):382–394. doi: 10.1016/0012-1606(83)90288-9. [DOI] [PubMed] [Google Scholar]
- 241.Nishida H, Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme: II. The 16-and 32-cell stages. Dev Biol. 1985;110(2):440–454. doi: 10.1016/0012-1606(85)90102-2. [DOI] [PubMed] [Google Scholar]
- 242.Nishida H, Sawada K. macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature. 2001;409(6821):724–729. doi: 10.1038/35055568. [DOI] [PubMed] [Google Scholar]
- 243.Nishino A, Baba SA, Okamura Y. A mechanism for graded motor control encoded in the channel properties of the muscle ACh receptor. Proc Natl Acad Sci. 2011;108(6):2599–2604. doi: 10.1073/pnas.1013547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nishino A, Okamura Y, Piscopo S, Brown ER. A glycine receptor is involved in the organization of swimming movements in an invertebrate chordate. BMC Neurosci. 2010;11(1):6. doi: 10.1186/1471-2202-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nishino A, Satou Y, Morisawa M, Satoh N. Muscle actin genes and muscle cells in the appendicularian, Oikopleura longicauda: phylogenetic relationships among muscle tissues in the urochordates. J Exp Zool. 2000;288(2):135–150. doi: 10.1002/1097-010X(20000815)288:2<135::AID-JEZ5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 246.Norton J, Cooley J, Islam AT, Cota CD, Davidson B. Matrix adhesion polarizes heart progenitor induction in the invertebrate chordate Ciona intestinalis. Development. 2013;140(6):1301–1311. doi: 10.1242/dev.085548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nunzi M, Burighel P, Schiaffino S. Muscle cell differentiation in the ascidian heart. Dev Biol. 1979;68(2):371–380. doi: 10.1016/0012-1606(79)90211-2. [DOI] [PubMed] [Google Scholar]
- 248.Oda-Ishii I, Bertrand V, Matsuo I, Lemaire P, Saiga H. Making very similar embryos with divergent genomes: conservation of regulatory mechanisms of Otx between the ascidians Halocynthia roretzi and Ciona intestinalis. Development. 2005;132(7):1663–1674. doi: 10.1242/dev.01707. [DOI] [PubMed] [Google Scholar]
- 249.Oda-Ishii I, Kubo A, Kari W, Suzuki N, Rothbächer U, Satou Y. A maternal system initiating the zygotic developmental program through combinatorial repression in the ascidian embryo. PLoS Genet. 2016;12(5):e1006045. doi: 10.1371/journal.pgen.1006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oginuma M, Niwa Y, Chapman DL, Saga Y. Mesp2 and Tbx6 cooperatively create periodic patterns coupled with the clock machinery during mouse somitogenesis. Development. 2008;135(15):2555–2562. doi: 10.1242/dev.019877. [DOI] [PubMed] [Google Scholar]
- 251.Ogura Y, Sasakura Y. Developmental control of cell-cycle compensation provides a switch for patterned mitosis at the onset of chordate neurulation. Dev Cell. 2016;37(2):148–161. doi: 10.1016/j.devcel.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 252.Oliphant LW, Cloney RA. The ascidian myocardium: sarcoplasmic reticulum and excitation-contraction coupling. Zeitschrift für Zellforschung und mikroskopische Anatomie. 1972;129(3):395–412. doi: 10.1007/BF00307296. [DOI] [PubMed] [Google Scholar]
- 253.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313(5795):1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ortolani G. Cleavage and development of egg fragments in ascidians. Acta Embryol Morphol Exp. 1958;1:247–272. [Google Scholar]
- 255.Paffenhöfer G-A, Köster M. From one to many: on the life cycle of Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea) J Plankton Res. 2011;33(7):1139–1145. doi: 10.1093/plankt/fbr001. [DOI] [Google Scholar]
- 256.Palmquist K, Davidson B. Establishment of lateral organ asymmetries in the invertebrate chordate, Ciona intestinalis. EvoDevo. 2017;8(1):12. doi: 10.1186/s13227-017-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pan H, Gustafsson MK, Aruga J, Tiedken JJ, Chen JC, Emerson CP. A role for Zic1 and Zic2 in Myf5 regulation and somite myogenesis. Dev Biol. 2011;351(1):120–127. doi: 10.1016/j.ydbio.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pancer Z, Gershon H, Rinkevich B. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biol Bull. 1995;189(2):106–112. doi: 10.2307/1542460. [DOI] [PubMed] [Google Scholar]
- 259.Pavlicev M, Wagner GP. A model of developmental evolution: selection, pleiotropy and compensation. Trends Ecol Evol. 2012;27(6):316–322. doi: 10.1016/j.tree.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 260.Perry H, Melton D. A rapid increase in acetylcholinesterase mRNA during ascidian embryogenesis as demonstrated by microinjection into Xenopus laevis oocytes. Cell Differ. 1983;13(3):233–238. doi: 10.1016/0045-6039(83)90094-5. [DOI] [PubMed] [Google Scholar]
- 261.Piette J, Lemaire P. Thaliaceans, the neglected pelagic relatives of Ascidians: a developmental and evolutionary enigma. Q Rev Biol. 2015;90(2):117–145. doi: 10.1086/681440. [DOI] [PubMed] [Google Scholar]
- 262.Prodon F, Chenevert J, Hébras C, Dumollard R, Faure E, Gonzalez-Garcia J, Nishida H, Sardet C, McDougall A. Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development. 2010;137(12):2011–2021. doi: 10.1242/dev.047845. [DOI] [PubMed] [Google Scholar]
- 263.Pruenster MM, Ricci L, Brown FD, Tiozzo S. Modular co-option of cardiopharyngeal genes during non-embryonic myogenesis. EvoDevo. 2019;10(1):3. doi: 10.1186/s13227-019-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pucci-Minafra I, Ortolani G. Differentiation and tissue interaction during muscle development of ascidian tadpoles. An electron microscope study. Dev Biol. 1968;17(6):692–712. doi: 10.1016/0012-1606(68)90014-6. [DOI] [PubMed] [Google Scholar]
- 265.Racioppi C, Wiechecki KA, Christiaen L. Combinatorial chromatin dynamics foster accurate cardiopharyngeal fate choices. bioRxiv. 2019;546945. [DOI] [PMC free article] [PubMed]
- 266.Ratcliffe LE, Asiedu EK, Pickett CJ, Warburton MA, Izzi SA, Meedel TH. The Ciona myogenic regulatory factor functions as a typical MRF but possesses a novel N-terminus that is essential for activity. Dev Biol. 2018 doi: 10.1016/j.ydbio.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Razy-Krajka F, Gravez B, Kaplan N, Racioppi C, Wang W, Christiaen L. An FGF-driven feed-forward circuit patterns the cardiopharyngeal mesoderm in space and time. eLife. 2018;7:e29656. doi: 10.7554/elife.29656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Razy-Krajka F, Lam K, Wang W, Stolfi A, Joly M, Bonneau R, Christiaen L. Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle specification from primed cardiopharyngeal progenitors. Dev Cell. 2014;29(3):263–276. doi: 10.1016/j.devcel.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reverberi G, Minganti A. La distribuzione delle potenze nel germe di Ascidie allo stadio di otto blastomeri, analizzata mediante le combinazioni ei trapianti di blastomeri. Pubbl Stn Zool Napoli. 1947;21(1):1–35. [Google Scholar]
- 270.Reverberi G, Ortolani G. Twin larvae from halves of the same egg in ascidians. Dev Biol. 1962;5(1):84–100. doi: 10.1016/0012-1606(62)90005-2. [DOI] [PubMed] [Google Scholar]
- 271.Rinkevich B, Lauzon RJ, Brown B, Weissman IL. Evidence for a programmed life span in a colonial protochordate. Proc Natl Acad Sci. 1992;89(8):3546–3550. doi: 10.1073/pnas.89.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol. 2010;588(2):353–364. doi: 10.1113/jphysiol.2009.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Roule L. Recherches sur les Ascidies simples des côtes de Provence (Phallusiadées), Marseille: Typ. J. Cayer; 1884. [Google Scholar]
- 274.Ryan K, Lu Z, Meinertzhagen IA. The CNS connectome of a tadpole larva of Ciona intestinalis (L.) highlights sidedness in the brain of a chordate sibling. Elife. 2016;5:e16962. doi: 10.7554/eLife.16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ryan K, Lu Z, Meinertzhagen IA. Circuit homology between decussating pathways in the Ciona larval CNS and the vertebrate startle-response pathway. Curr Biol. 2017;27(5):721–728. doi: 10.1016/j.cub.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 276.Sakakibara I, Santolini M, Ferry A, Hakim V, Maire P. Six homeoproteins and a linc-RNA at the fast MYH locus lock fast myofiber terminal phenotype. PLoS Genet. 2014;10(5):e1004386. doi: 10.1371/journal.pgen.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Salensky W. Etudes anatomiques sur les Appendicularies: 1., Oikopleura vanhoeffeni Lohmann. St. Pétersbourg: Académie Impériale des sciences de St.-Pétersbourg; 1903. [Google Scholar]
- 278.Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138(12):2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- 279.Santolini M, Sakakibara I, Gauthier M, Ribas-Aulinas F, Takahashi H, Sawasaki T, Mouly V, Concordet J-P, Defossez P-A, Hakim V. MyoD reprogramming requires Six1 and Six4 homeoproteins: genome-wide cis-regulatory module analysis. Nucleic Acids Res. 2016;44(18):8621–8640. doi: 10.1093/nar/gkw512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sardet C, Nishida H, Prodon F, Sawada K. Maternal mRNAs of PEM and macho 1, the ascidian muscle determinant, associate and move with a rough endoplasmic reticulum network in the egg cortex. Development. 2003;130(23):5839–5849. doi: 10.1242/dev.00805. [DOI] [PubMed] [Google Scholar]
- 281.Sasakura Y. Cellulose production and the evolution of the sessile lifestyle in ascidians. Sessile Organ. 2018;35(2):21–29. doi: 10.4282/sosj.35.21. [DOI] [Google Scholar]
- 282.Sasakura Y, Nakashima K, Awazu S, Matsuoka T, Nakayama A, Azuma J, Satoh N. Transposon-mediated insertional mutagenesis revealed the functions of animal cellulose synthase in the ascidian Ciona intestinalis. Proc Natl Acad Sci USA. 2005;102(42):15134. doi: 10.1073/pnas.0503640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Satoh N. On the ‘clock’mechanism determining the time of tissue-specific enzyme development during ascidian embryogenesis. Development. 1979;54(1):131–139. [PubMed] [Google Scholar]
- 284.Satoh N. Developmental genomics of ascidians. Hoboken: Wiley; 2013. [Google Scholar]
- 285.Satou Y. Gene regulatory systems that control gene expression in the Ciona embryo. Proc Jpn Acad Ser B. 2015;91(2):33. doi: 10.2183/pjab.91.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Satou Y, Imai KS, Satoh N. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131(11):2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- 287.Satou Y, Kusakabe T, Araki L, Satoh N. Timing of initiation of muscle-specific gene expression in the ascidian embryo precedes that of developmental fate restriction in lineage cells. Dev Growth Differ. 1995;37(3):319–327. doi: 10.1046/j.1440-169X.1995.t01-2-00010.x. [DOI] [PubMed] [Google Scholar]
- 288.Satou Y, Satoh N. Two cis-regulatory elements are essential for the muscle-specific expression of an actin gene in the ascidian embryo. Dev Growth Differ. 1996;38(5):565–573. doi: 10.1046/j.1440-169X.1996.t01-1-00013.x. [DOI] [PubMed] [Google Scholar]
- 289.Satou Y, Takatori N, Yamada L, Mochizuki Y, Hamaguchi M, Ishikawa H, Chiba S, Imai K, Kano S, Murakami SD. Gene expression profiles in Ciona intestinalis tailbud embryos. Development. 2001;128(15):2893–2904. doi: 10.1242/dev.128.15.2893. [DOI] [PubMed] [Google Scholar]
- 290.Satou Y, Yagi K, Imai KS, Yamada L, Nishida H, Satoh N. macho-1-related genes in Ciona embryos. Dev Genes Evol. 2002;212(2):87–92. doi: 10.1007/s00427-002-0218-3. [DOI] [PubMed] [Google Scholar]
- 291.Savigny J. Mémoires sur les animaux sans vertèbres. La Rochelle: G. Dufour; 1816. [Google Scholar]
- 292.Sawada K, Fukushima Y, Nishida H. Macho-1 functions as transcriptional activator for muscle formation in embryos of the ascidian Halocynthia roretzi. Gene Expr Patterns. 2005;5(3):429–437. doi: 10.1016/j.modgep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 293.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76(2):371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 294.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 295.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/S0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 296.Seo H-C, Kube M, Edvardsen RB, Jensen MF, Beck A, Spriet E, Gorsky G, Thompson EM, Lehrach H, Reinhardt R. Miniature genome in the marine chordate Oikopleura dioica. Science. 2001;294(5551):2506. doi: 10.1126/science.294.5551.2506. [DOI] [PubMed] [Google Scholar]
- 297.Shenkar N, Swalla BJ. Global diversity of Ascidiacea. PLoS ONE. 2011;6(6):e20657. doi: 10.1371/journal.pone.0020657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shi W, Levine M. Ephrin signaling establishes asymmetric cell fates in an endomesoderm lineage of the Ciona embryo. Development. 2008;135(5):931–940. doi: 10.1242/dev.011940. [DOI] [PubMed] [Google Scholar]
- 299.Shinohara Y, Konishi K. Ultrastructure of the body-wall muscle of the ascidian Halocynthia roretzi: smooth muscle cell with multiple nuclei. J Exp Zool. 1982;221(2):137–142. doi: 10.1002/jez.1402210203. [DOI] [Google Scholar]
- 300.Sigvardsson M. Overlapping expression of early B-cell factor and basic helix-loop-helix proteins as a mechanism to dictate B-lineage-specific activity of the λ5 promoter. Mol Cell Biol. 2000;20(10):3640–3654. doi: 10.1128/MCB.20.10.3640-3654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Simões-Costa MS, Vasconcelos M, Sampaio AC, Cravo RM, Linhares VL, Hochgreb T, Yan CY, Davidson B, Xavier-Neto J. The evolutionary origin of cardiac chambers. Dev Biol. 2005;277(1):1–15. doi: 10.1016/j.ydbio.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 302.Søviknes AM, Glover JC. Spatiotemporal patterns of neurogenesis in the appendicularian Oikopleura dioica. Dev Biol. 2007;311(1):264–275. doi: 10.1016/j.ydbio.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 303.Squire JM, Al-khayat HA, Knupp C, Luther PK. Molecular architecture in muscle contractile assemblies. Adv Protein Chem. 2005;71:17–87. doi: 10.1016/S0065-3233(04)71002-5. [DOI] [PubMed] [Google Scholar]
- 304.Stach T. Ontogeny of the appendicularian Oikopleura dioica (Tunicata, Chordata) reveals characters similar to ascidian larvae with sessile adults. Zoomorphology. 2007;126(3):203–214. doi: 10.1007/s00435-007-0041-5. [DOI] [Google Scholar]
- 305.Stach T, Turbeville JM. Phylogeny of Tunicata inferred from molecular and morphological characters. Mol Phylogenet Evol. 2002;25(3):408–428. doi: 10.1016/S1055-7903(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 306.Stach T, Winter J, Bouquet J-M, Chourrout D, Schnabel R. Embryology of a planktonic tunicate reveals traces of sessility. Proc Natl Acad Sci. 2008;105(20):7229–7234. doi: 10.1073/pnas.0710196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Steinmetz PR, Kraus JE, Larroux C, Hammel JU, Amon-Hassenzahl A, Houliston E, Wörheide G, Nickel M, Degnan BM, Technau U. Independent evolution of striated muscles in cnidarians and bilaterians. Nature. 2012;487(7406):231–234. doi: 10.1038/nature11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Stolfi A, Gainous TB, Young JJ, Mori A, Levine M, Christiaen L. Early chordate origins of the vertebrate second heart field. Science. 2010;329(5991):565. doi: 10.1126/science.1190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Stolfi A, Brown FD. Tunicata. In: Wanninger A, editor. Evolutionary developmental biology of invertebrates 6. Springer: Vienna; 2015. pp. 135–204. [Google Scholar]
- 310.Stolfi A, Lowe EK, Racioppi C, Ristoratore F, Brown CT, Swalla BJ, Christiaen L. Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. Life. 2014 doi: 10.7554/elife.03728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Stolfi A, Ryan K, Meinertzhagen IA, Christiaen L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature. 2015;527(7578):371. doi: 10.1038/nature15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stolfi A, Sasakura Y, Chalopin D, Satou Y, Christiaen L, Dantec C, Endo T, Naville M, Nishida H, Swalla BJ. Guidelines for the nomenclature of genetic elements in tunicate genomes. Genesis. 2015;53(1):1–14. doi: 10.1002/dvg.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Stoner DS, Weissman IL. Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proc Natl Acad Sci. 1996;93(26):15254–15259. doi: 10.1073/pnas.93.26.15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sugino YM, Matsumura M, Kawamura K. Body muscle-cell differentiation from coelomic stem cells in colonial tunicates. Zool Sci. 2007;24(6):542–546. doi: 10.2108/zsj.24.542. [DOI] [PubMed] [Google Scholar]
- 315.Swalla BJ, Cameron CB, Corley LS, Garey JR. Urochordates are monophyletic within the deuterostomes. Syst Biol. 2000;49(1):52–64. doi: 10.1080/10635150050207384. [DOI] [PubMed] [Google Scholar]
- 316.Swalla BJ, Jeffery WR. Interspecific hybridization between an anural and urodele ascidian: differential expression of urodele features suggests multiple mechanisms control anural development. Dev Biol. 1990;142(2):319–334. doi: 10.1016/0012-1606(90)90353-K. [DOI] [PubMed] [Google Scholar]
- 317.Swalla BJ, Jeffery WR. Vestigial brain melanocyte development during embryogenesis of an anural ascidian. Dev Growth Differ. 1992;34(1):17–25. doi: 10.1111/j.1440-169X.1992.00017.x. [DOI] [PubMed] [Google Scholar]
- 318.Tagawa K, Jeffery WR, Satoh N. The recently-described ascidian species Molgula tectiformis is a direct developer. Zool Sci. 1997;14(2):297–303. doi: 10.2108/zsj.14.297. [DOI] [PubMed] [Google Scholar]
- 319.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89(1):127–138. doi: 10.1016/S0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 320.Takada N, York J, Davis J, Schumpert B, Yasuo H, Satoh N, Swalla BJ. Brachyury expression in tailless Molgulid ascidian embryos. Evolut Dev. 2002;4(3):205–211. doi: 10.1046/j.1525-142X.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- 321.Takahashi H, Mitani Y, Satoh G, Satoh N. Evolutionary alterations of the minimal promoter for notochord-specific Brachyury expression in ascidian embryos. Development. 1999;126(17):3725–3734. doi: 10.1242/dev.126.17.3725. [DOI] [PubMed] [Google Scholar]
- 322.Takatori N, Hotta K, Mochizuki Y, Satoh G, Mitani Y, Satoh N, Satou Y, Takahashi H. T-box genes in the ascidian Ciona intestinalis: characterization of cDNAs and spatial expression. Dev Dyn. 2004;230(4):743–753. doi: 10.1002/dvdy.20082. [DOI] [PubMed] [Google Scholar]
- 323.Takatori N, Oonuma K, Nishida H, Saiga H. Polarization of PI3 K activity initiated by ooplasmic segregation guides nuclear migration in the mesendoderm. Dev Cell. 2015;35(3):333–343. doi: 10.1016/j.devcel.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 324.Tazumi S, Yabe S, Yokoyama J, Aihara Y, Uchiyama H. PMesogenin1 and 2 function directly downstream of Xtbx6 in Xenopus somitogenesis and myogenesis. Dev Dyn. 2008;237(12):3749–3761. doi: 10.1002/dvdy.21791. [DOI] [PubMed] [Google Scholar]
- 325.Terakado K. The effects of actinomycin D on muscle cells of ascidian embryo. Dev Growth Differ. 1973;15(3):179–192. doi: 10.1111/j.1440-169X.1973.00179.x. [DOI] [PubMed] [Google Scholar]
- 326.Terakado K, Obinata T. Structure of multinucleated smooth muscle cells of the ascidian Halocynthia roretzi. Cell Tissue Res. 1987;247(1):85–94. doi: 10.1007/BF00216550. [DOI] [Google Scholar]
- 327.Tiozzo S, Murray M, Degnan BM, De Tomaso AW, Croll RP. Development of the neuromuscular system during asexual propagation in an invertebrate chordate. Dev Dyn. 2009;238(8):2081–2094. doi: 10.1002/dvdy.22023. [DOI] [PubMed] [Google Scholar]
- 328.Tokuoka M, Kobayashi K, Satou Y. Distinct regulation of Snail in two muscle lineages of the ascidian embryo achieves temporal coordination of muscle development. Development. 2018;145(11):dev163915. doi: 10.1242/dev.163915. [DOI] [PubMed] [Google Scholar]
- 329.Tokuoka M, Kumano G, Nishida H. FGF9/16/20 and Wnt-5α signals are involved in specification of secondary muscle fate in embryos of the ascidian, Halocynthia roretzi. Dev Genes Evolut. 2007;217(7):515–527. doi: 10.1007/s00427-007-0160-5. [DOI] [PubMed] [Google Scholar]
- 330.Tokuoka M, Satoh N, Satou Y. A bHLH transcription factor gene, Twist-like1, is essential for the formation of mesodermal tissues of Ciona juveniles. Dev Biol. 2005;288(2):387–396. doi: 10.1016/j.ydbio.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 331.Tolkin T, Christiaen L. 4 Development and evolution of the ascidian cardiogenic mesoderm. Curr Top Dev Biol. 2012;100:107. doi: 10.1016/B978-0-12-387786-4.00011-7. [DOI] [PubMed] [Google Scholar]
- 332.Tolkin T, Christiaen L. Rewiring of an ancestral Tbx1/10-Ebf-Mrf network for pharyngeal muscle specification in distinct embryonic lineages. Development. 2016;143:3852–3862. doi: 10.1242/dev.136267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Tomlinson CR, Beach RL, Jeffery WR. Differential expression of a muscle actin gene in muscle cell lineages of ascidian embryos. Development. 1987;101(4):751–765. doi: 10.1242/dev.101.4.751. [DOI] [PubMed] [Google Scholar]
- 334.Trotter J. Functional morphology of force transmission in skeletal muscle. Cells Tissues Organs. 1993;146(4):205–222. doi: 10.1159/000147459. [DOI] [PubMed] [Google Scholar]
- 335.True JR, Haag ES. Developmental system drift and flexibility in evolutionary trajectories. Evolut Dev. 2001;3(2):109–119. doi: 10.1046/j.1525-142x.2001.003002109.x. [DOI] [PubMed] [Google Scholar]
- 336.Tsagkogeorga G, Turon X, Hopcroft RR, Tilak M-K, Feldstein T, Shenkar N, Loya Y, Huchon D, Douzery EJ, Delsuc F. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC Evol Biol. 2009;9(1):187. doi: 10.1186/1471-2148-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas J-F. Redefining the progression of lineage segregations during mammalian embryogenesis by Clonal analysis. Dev Cell. 2009;17(3):365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 338.Uchiyama H, Kobayashi T, Yamashita A, Ohno S, Yabe S. Cloning and characterization of the T-box gene Tbx6 in Xenopus laevis. Dev Growth Differ. 2001;43(6):657–669. doi: 10.1046/j.1440-169X.2001.00606.x. [DOI] [PubMed] [Google Scholar]
- 339.Uljanin VN. Die arten der gattung Doliolum im golfe von Neapel und den angrenzenden meeresabschnitten. Leipzig: W. Engelmann; 1884. [Google Scholar]
- 340.Vincent SD, Buckingham ME. Chapter one-how to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 341.Von Ubisch L. über die Entwicklung von Ascidienlarven nach frühzeitiger Entfernung der einzelnen organbildenden Keimbezirke. Dev Genes Evol. 1939;139(3):438–492. doi: 10.1007/BF00578751. [DOI] [PubMed] [Google Scholar]
- 342.Voskoboynik A, Neff NF, Sahoo D, Newman AM, Pushkarev D, Koh W, Passarelli B, Fan HC, Mantalas GL, Palmeri KJ. The genome sequence of the colonial chordate, Botryllus schlosseri. Elife. 2013;2:e00569. doi: 10.7554/eLife.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wada H. Evolutionary history of free-swimming and sessile lifestyles in urochordates as deduced from 18S rDNA molecular phylogeny. Mol Biol Evol. 1998;15(9):1189–1194. doi: 10.1093/oxfordjournals.molbev.a026026. [DOI] [PubMed] [Google Scholar]
- 344.Wada H, Holland PW, Sato S, Yamamoto H, Satoh N. Neural tube is partially dorsalized by overexpression ofHrPax-37: the Ascidian Homologue ofPax-3andPax-7. Dev Biol. 1997;187(2):240–252. doi: 10.1006/dbio.1997.8626. [DOI] [PubMed] [Google Scholar]
- 345.Wada H, Holland PWH, Satoh N. Origin of patterning in neural tubes. Nature. 1996;384(6605):123. doi: 10.1038/384123a0. [DOI] [PubMed] [Google Scholar]
- 346.Wada S, Saiga H. HrzicN, a new Zic family gene of ascidians, plays essential roles in the neural tube and notochord development. Development. 2002;129(24):5597–5608. doi: 10.1242/dev.00156. [DOI] [PubMed] [Google Scholar]
- 347.Wang D-Z, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14(5):558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 348.Wang W, Niu X, Jullian E, Mauck WM, Kelly RG, Satija R, Christiaen L. A single cell transcriptional roadmap for cardiopharyngeal fate diversification. Nat Cell Biol. 2019;21(6):674–686. doi: 10.1038/s41556-019-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang W, Razy-Krajka F, Siu E, Ketcham A, Christiaen L. NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol. 2013;11(12):e1001725. doi: 10.1371/journal.pbio.1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci. 1989;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Weiss KM, Fullerton SM. Phenogenetic drift and the evolution of genotype–phenotype relationships. Theor Popul Biol. 2000;57(3):187–195. doi: 10.1006/tpbi.2000.1460. [DOI] [PubMed] [Google Scholar]
- 352.Whittaker J. Segregation during ascidian embryogenesis of egg cytoplasmic information for tissue-specific enzyme development. Proc Natl Acad Sci. 1973;70(7):2096–2100. doi: 10.1073/pnas.70.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Whittaker J. Segregation during cleavage of a factor determining endodermal alkaline phosphatase development in ascidian embryos. J Exp Zool. 1977;202(2):139–153. doi: 10.1002/jez.1402020202. [DOI] [PubMed] [Google Scholar]
- 354.Whittaker J. Development of vestigial tail muscle acetylcholinesterase in embryos of an anural ascidian species. Biol Bull. 1979;156(3):393–407. doi: 10.2307/1540926. [DOI] [PubMed] [Google Scholar]
- 355.Whittaker J. Acetylcholinesterase development in extra cells caused by changing the distribution of myoplasm in ascidian embryos. Development. 1980;55(1):343–354. [PubMed] [Google Scholar]
- 356.Whittaker J. Muscle lineage cytoplasm can change the developmental expression in epidermal lineage cells of ascidian embryos. Dev Biol. 1982;93(2):463–470. doi: 10.1016/0012-1606(82)90134-8. [DOI] [PubMed] [Google Scholar]
- 357.Windner SE, Doris RA, Ferguson CM, Nelson AC, Valentin G, Tan H, Oates AC, Wardle FC, Devoto SH. Tbx6, Mesp-b and Ripply1 regulate the onset of skeletal myogenesis in zebrafish. Development. 2015;142(6):1159–1168. doi: 10.1242/dev.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Wittler L, Shin Eh, Grote P, Kispert A, Beckers A, Gossler A, Werber M, Herrmann BG. Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. EMBO Rep. 2007;8(8):784–789. doi: 10.1038/sj.embor.7401030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Woznica A, Haeussler M, Starobinska E, Jemmett J, Li Y, Mount D, Davidson B. Initial deployment of the cardiogenic gene regulatory network in the basal chordate, Ciona intestinalis. Dev Biol. 2012;368(1):127–139. doi: 10.1016/j.ydbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Xavier-Neto J, Castro R, Sampaio A, Azambuja A, Castillo H, Cravo R, Simoes-Costa M. Parallel avenues in the evolution of hearts and pumping organs. Cell Mol Life Sci. 2007;64(6):719–734. doi: 10.1007/s00018-007-6524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yagi K, Satoh N, Satou Y. Identification of downstream genes of the ascidian muscle determinant gene Ci-macho1. Dev Biol. 2004;274(2):478–489. doi: 10.1016/j.ydbio.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 362.Yagi K, Takatori N, Satou Y, Satoh N. Ci-Tbx6b and Ci-Tbx6c are key mediators of the maternal effect gene Ci-macho1 in muscle cell differentiation in Ciona intestinalis embryos. Dev Biol. 2005;282(2):535–549. doi: 10.1016/j.ydbio.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 363.Yasuo H, Kobayashi M, Shimauchi Y, Satoh N. The ascidian genome contains another T-domain gene that is expressed in differentiating muscle and the tip of the tail of the embryo. Dev Biol. 1996;180(2):773–779. doi: 10.1006/dbio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 364.Young CM, Gowan RF, Dalby J, Pennachetti CA, Gagliardi D. Distributional consequences of adhesive eggs and anural development in the ascidian Molgula pacifica (Huntsman, 1912) Biol Bull. 1988;174(1):39–46. doi: 10.2307/1541757. [DOI] [PubMed] [Google Scholar]
- 365.Yu D, Oda-Ishii I, Kubo A, Satou Y. The regulatory pathway from genes directly activated by maternal factors to muscle structural genes in ascidian embryos. Development. 2019;146(3):dev173104. doi: 10.1242/dev.173104. [DOI] [PubMed] [Google Scholar]
- 366.Yuasa HJ, Kawamura K, Yamamoto H. The structural organization of ascidian Halocynthia roretzi troponin I genes. J Biochem. 2002;132(1):135–141. doi: 10.1093/oxfordjournals.jbchem.a003191. [DOI] [PubMed] [Google Scholar]
- 367.Yuasa HJ, Sato S, Yamamoto H, Takagi T. Structure of the ascidian, Halocynthia roretzi, troponin C gene. J Biochem. 1997;121(4):671–676. doi: 10.1093/oxfordjournals.jbchem.a021638. [DOI] [PubMed] [Google Scholar]
- 368.Zalokar M, Sardet C. Tracing of cell lineage in embryonic development of Phallusia mammillata (Ascidia) by vital staining of mitochondria. Dev Biol. 1984;102(1):195–205. doi: 10.1016/0012-1606(84)90184-2. [DOI] [PubMed] [Google Scholar]
- 369.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zeller RW. Electroporation in ascidians: history, theory and protocols. In: Sasakura Y, editor. Transgenic ascidians. Singapore: Springer; 2018. pp. 37–48. [DOI] [PubMed] [Google Scholar]
- 371.Zeng L, Jacobs MW, Swalla BJ. Coloniality has evolved once in stolidobranch ascidians. Integr Comp Biol. 2006;46(3):255–268. doi: 10.1093/icb/icj035. [DOI] [PubMed] [Google Scholar]
- 372.Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE. Latent TGF-[bgr] binding protein 3 identifies a second heart field in zebrafish. Nature. 2011;474(7353):645–648. doi: 10.1038/nature10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.