Abstract
The oral cavity contains a rich consortium of exopolysaccharide-producing microbes. These extracellular polysaccharides comprise a major component of the oral biofilm. Together with extracellular proteins, DNA, and lipids, they form the biofilm matrix, which contributes to bacterial colonization, biofilm formation and maintenance, and pathogenesis. While a number of oral microbes have been studied in detail with regard to biofilm formation and pathogenesis, the exopolysaccharides have been well characterized for only select organisms, namely Streptococcus mutans and Aggregatibacter actinomycetemcomitans. Studies on the exopolysaccharides of other oral organisms, however, are in their infancy. In this review, we present the current research on exopolysaccharides of oral microbes regarding their biosynthesis, regulation, contributions to biofilm formation and stability of the matrix, and immune evasion. In addition, insight into the role of exopolysaccharides in biofilms is highlighted through the evaluation of emerging techniques such as pH probing of biofilm colonies, solid-state nuclear magnetic resonance for macromolecular interactions within biofilms, and super-resolution microscopy analysis of biofilm development. Finally, exopolysaccharide as a potential nutrient source for species within a biofilm is discussed.
Keywords: bacteria, extracellular matrix, microbiology, caries, plaque/plaque biofilms, periodontal disease
Introduction
Over 700 bacterial species have been identified in the oral cavity (Dewhirst et al. 2010), which reside on the salivary-pellicle-coated tooth surface, in the anaerobic, nutrient-rich gingival sulcus, or on mucosal surfaces. To persist in the oral cavity, many oral bacteria produce self-synthesized extracellular polysaccharide (EPS) that can be specific to and shaped by a particular niche. In general, EPSs promote bacterial aggregation and surface attachment, and they protect bacteria from desiccation, predation, antimicrobial agents, antibodies, and bacteriophages (Vu et al. 2009). In this review, we present the current research on oral EPS, an understudied and critical component of the extracellular matrix of biofilm-forming oral microbes (Fig. 1).
Figure 1.
Various aspects of exopolysaccharides in oral microbes. A major component of the oral biofilm matrix is composed of exopolysaccharides. This self-synthesized polymer is essential to the structure of the biofilm and maintenance of the bacteria in the oral cavity. Presented here is the current state of extracellular polysaccharide (EPS) research: its synthesis, contribution to matrix development, regulation, and promotion of immune evasion. Future and novel directions are covered in detail to highlight emerging technologies by which to study EPS.
Extracellular Polysaccharide Synthesis in Oral Bacteria
Among the oral microbiota, the well studied belong to select Gram-positive and Gram-negative bacterial species (Table). Depending on the bacterium, the genes responsible for the synthesis and/or export of EPS exist either in an operon or at separate regions of the DNA.
Table.
Genes Responsible for Exopolysaccharide Synthesis in Oral Bacteria.
| Bacterial Species | Surface Polysaccharide | Genes |
|---|---|---|
| Streptococcus mutans | Dextran/D-glucose glucan | gtfA, gtfB, gtfC, gtfD |
| Streptococcus sanguinis | D-glucose glucan; capsular | gtfA, gtfP, brpT, brpL, ciaR, cps loci |
| Streptococcus gordonii | Dextran based | gtf |
| Porphyromonas gingivalis | Capsular | PG0106-PG0120, huβ, sinR |
| Aggregatibacter actinomycetemcomitans | Poly-N-acetylglucosamine | pgaABCD |
| Kingella kingae | Linear polymer of galactofuranose | pamABCDE |
| Prevotella intermedia | Mannose rich | wbsX |
In Gram-positive oral bacteria, the synthesis of EPS is carried out by a family of glucosyltransferases (Gtfs), which generate insoluble and soluble glucans that can be species specific and linkage specific. For example, the cariogenic bacterium Streptococcus mutans produces 3 well-characterized enzymes, GtfB, GtfC, and GtfD, which are capable of producing distinct soluble and insoluble glucans using sucrose as a substrate to build the EPS (Kuramitsu 2003; Koo et al. 2013). To date, only the structure of GtfC of S. mutans is available; therefore, our understanding of synthesis is limited (Ito et al. 2011). Although numerous microbial species are found in oral biofilms, most of them do not contribute to the synthesis of glucans until they are coated by Gtfs generated by S. mutans (Koo et al. 2013). Non-Gtfs-synthesizing oral microbes such as Actinomyces viscosus, Lactobacillus casei, and Candida albicans become extracellular glucan producers when bound by S. mutans Gtfs and contribute to the growing multispecies biofilm (Koo et al. 2013).
Streptococcus sanguinis contains 2 distal gtf genes, gtfA and gtfP, but only gtfP encodes for an enzyme that produces glucan (Yoshida et al. 2014; Liu et al. 2017). In addition to EPS, it appears that a number of oral streptococcal species, including S. sanguinis and Streptococcus oralis, contain capsular polysaccharide genes, some of which are homologous to those in Streptococcus pneumoniae (Skov-Sorensen et al. 2016). The contribution of the capsular polysaccharides to the extracellular matrix in these bacteria remains to be determined.
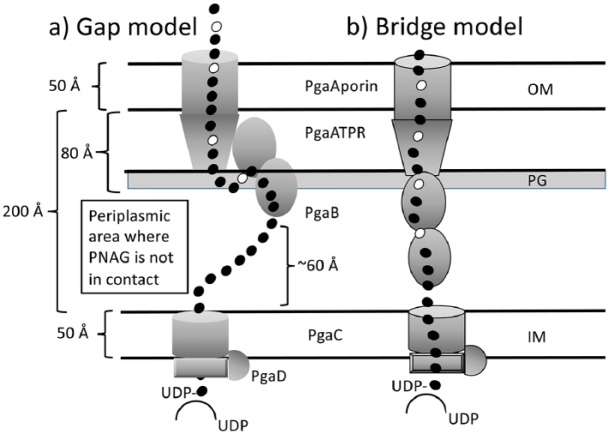
Unlike the EPSs of Gram-positive bacteria, the EPSs of Gram-negative bacteria need to be exported out of the outer membrane. In these bacteria, a set of proteins and enzymes works in concert to synthesize and export EPS (Fig. 2; an overview of the structural aspects of synthesis and export in Gram-negative bacteria is provided in the supplementary appendix). Among the Gram-negative oral microbes, the EPS of Aggregatibacter actinomycetemcomitans, a homopolymer of N-acetyl-D-glucosamine residues in a β(1,6) linkage, is the most well characterized. It is similar to the EPS produced by several other Gram-negative and Gram-positive bacteria including Escherichia coli, Yersinia pestis, Staphylococcus aureus, and Staphylococcus epidermidis.
Figure 2.
Exopolysaccharide export in Aggregatibacter actinomycetemcomitans. Schematic representation of the export mechanisms of extracellular polysaccharide (EPS) in A. actinomycetemcomitans. The PgaABCD proteins make a gap (a) or a bridge (b) for the EPS to traverse the periplasmic space. In the gap model, both N- and C-terminal domains of PgaB can interact, whereas in the bridge model, only 1 domain of PgaB can interact with the PgaATPR domain. IM, inner membrane; OM, outer membrane; PG, peptidoglycan. Open circles represent glucosamine and dark circles represent N-acetylglucosamine.
Role of Extracellular Polysaccharide in Matrix Development in Oral Bacteria
The biofilm matrix is a complex mixture of EPS, extracellular DNA (eDNA), proteins, and lipoteichoic acid (LTA; Gram positive) that are responsible for the strength of the matrix (Izano et al. 2008; Das et al. 2010). The production of the matrix has long been known to facilitate survival of cells in their respective niches. Within the matrix, EPS has a critical role in enmeshing the microbial cells and in providing a diffusion-limiting 3-dimensional (3D) scaffold that shapes spatial and microenvironment microbial heterogeneity (Stewart and Franklin 2008; Klein et al. 2015; Castillo-Pedraza et al. 2017). The roles of EPS and other matrix components have been studied in many oral bacteria as presented below.
S. mutans
The highly structured matrix produced by the cariogenic bacterium, S. mutans, is a key contributor to pathogenic dental biofilms. The EPS in cariogenic biofilms provides an abundance of primary binding sites for other species and helps form the core of the matrix-scaffold (information on the role of calcium liberation in S. mutans EPS production is provided in the supplementary appendix; Bowen and Koo 2011; Bowen et al. 2018). To understand how the biofilm matrix confers highly heterogeneous yet cohesive environments within a 3D matrix scaffold, an innovative technique was recently developed to examine the 3D spatiotemporal and structural organization during the development of the EPS matrix (Xiao et al. 2012). It was discovered that there was a compartmentalized architecture of the biofilm structure, which ultimately could easily accommodate other glucan-producing microbes. The presence of these microbes in the mixed biofilm influenced gene expression of gtf, dexA (the gene product produces an extracellular dextranase that can partially degrade the soluble dextran), and gbp genes (which produce glucan binding proteins GbpA, GbpB, and GbpC) in S. mutans. Production of glucan in situ establishes a biofilm matrix into which resident microorganisms are enmeshed and protected against antimicrobials and can become highly acidogenic or aciduric (Xiao et al. 2012). Although oral microbiota tend to produce niche-specific EPSs in the oral cavity, the cooperative EPS synthesis among them and the conducive 3D biofilm architecture may create environmental and biological niches within biofilms that can directly modulate the pathogenesis of dental caries (Koo et al. 2013).
While eDNA is often a product of autolysis, it is also secreted via lysis-independent vesicles (Liao et al. 2014). eDNA appears to have multiple roles in the biofilm matrix. In 1 study, exogenously added eDNA-enhanced glucan synthesis by both the purified enzyme GtfB when adsorbed to saliva-coated hydroxyapatite and by S. mutans (Liao et al. 2014; Klein et al. 2015). Furthermore, it contributes to stress relaxation by modulating its interaction with other matrix components in biofilms of S. mutans and other bacteria (Peterson et al. 2013). It also aids in strengthening the biofilm matrix by interacting with EPS (Klein et al. 2015). The interaction with EPS might be enhanced at low pH, which is relevant for cariogenic biofilms. Strengthening of the extracellular matrix by eDNA through direct interaction with EPSs has also been demonstrated in Myxococcus xanthus (Hu et al. 2012).
Similar to eDNA, LTA also strengthens the matrix and induces insoluble glucan synthesis (Kuramitsu et al. 1980). Thus, while EPS is critical for the strong assembly and structural organization of the matrix during cariogenic biofilm formation, the other matrix molecules are also necessary for the strength of the matrix. In this regard, in S. mutans, as was observed with eDNA, LTA enhanced the structure and stability of biofilms by influencing the activity of GtfB to increase insoluble glucan synthesis (Klein et al. 2015). In the early stages of biofilm development, EPS interacts with eDNA, while in the later stages, LTA interaction with EPS dominates (Castillo-Pedraza et al. 2017), illustrating how these 2 components of the matrix may act in concert with EPS during cariogenic biofilm development.
S. sanguinis
Similar to what is observed in S. mutans, in S. sanguinis, it is hypothesized that eDNA may play a role prior to the synthesis of large amounts of glucans or in an environment that lacks the carbon sources required for glucan synthesis. However, this is not supported by the limited experimental data that are currently available (Zhu et al. 2018).
Porphyromonas gingivalis
Porphyromonas gingivalis is known to produce only capsular polysaccharide but not extracellular polysaccharide (Davey and Duncan 2006). P. gingivalis decorates its surface with at least 3 sugar macromolecules: lipopolysaccharide, capsular polysaccharide, and the anionic cell surface polysaccharide, ALPS. However, additional work is warranted in understanding the biofilm architecture and polysaccharide-based matrix development in P. gingivalis.
A. actinomycetemcomitans
It is known that the EPS in A. actinomycetemcomitans is associated with eDNA and lipopolysaccharide (Izano et al. 2008; Das et al. 2010), but the architectural role of these matrix components is undefined.
Interaction of eDNA and Extracellular DNA Binding Proteins
In many bacteria, DNABII proteins, which include IHF (integration host factor), and HU (histone-like protein), function intracellularly to bind DNA and regulate gene expression. Interestingly, in some bacteria, DNABII proteins have been associated with eDNA within the biofilm matrix. Together they stabilize and maintain the integrity of the EPS matrix (Goodman et al. 2011; Devaraj et al. 2015; Rocco et al. 2017). Coexistence of DNABII proteins and eDNA has been demonstrated in Streptococcus intermedius biofilms as well (Nur et al. 2013). In addition, in mixed Streptococcus sp. and P. gingivalis biofilms, the use of specific antibodies targeting HU proteins weakened the biofilm and prevented P. gingivalis colonization (Rocco et al. 2018).
With limited studies available investigating EPS and biofilm formation of oral bacteria, with the exception of S. mutans, experimentation in nonoral species can help direct future directions of study. In this regard, the 3D biofilm development of Vibrio cholerae has been studied using stochastic optical reconstruction spectroscopy with a resolution of 19 to 42 nm (Berk et al. 2012). The exopolysaccharide secretion and its interaction with the matrix proteins were examined using Cy3-labeled wheat germ agglutinin, which recognizes N-acetylglucosamine sugars in the exopolysaccharide. This study showed the complementary architectural roles played by the matrix components in generating ordered envelopes that encased cell clusters in the biofilm. Similar studies are warranted for studying oral bacterial biofilm architecture whose EPS is made up of specific saccharides in the mono- and mixed-species conditions (information on matrix development in mixed species biofilms is provided in the supplementary appendix).
Regulation of Extracellular Polysaccharide Production in Oral Bacteria
The synthesis of EPSs can be regulated at the DNA or at the protein level. In this section, these 2 mechanisms of regulation are presented.
P. gingivalis
An ortholog of the gene sinR, which controls EPS synthesis in Bacillus subtilis, has been identified in P. gingivalis (Yamamoto et al. 2013). The sinR gene encodes for a transcriptional activator, which has been suggested to negatively regulate 1 or more of the surface polysaccharides that are associated with the 3D structure of the biofilm (Yamamoto et al. 2013). In addition, capsule synthesis in P. gingivalis is controlled by HUβ, a DNABII histone-like protein, and a 77-base-pair inverted repeat element (Alberti-Segui et al. 2010; Bainbridge et al. 2015).
A. actinomycetemcomitans
Recently, the role of EPS in biofilm formation in A. actinomycetemcomitans was analyzed using an EPS mutant strain, which lacked the pgaABCD operon (Shanmugam et al. 2015). The absence of the pga operon appears to affect genes involved in peptidoglycan recycling as well as glycogen storage. EPS modulates not only the expression levels of colonization genes (aae, apiA, flp-1, and tadA) but also other genes for cell viability. The transcriptome analysis of the wild type in comparison to the EPS mutant strain showed that the gene csrA (a gene encoding for carbon storage regulator CsrA) is highly downregulated in the EPS mutant. Similar studies performed with E. coli pga mutants demonstrated the existence of a possible relationship between csrA, the pga operon, and EPS (Wang et al. 2005). In E. coli, CsrA affects pgaABCD genes posttranscriptionally and EPS synthesis is necessary for CsrA and other Csr-mediated signaling components to affect biofilm formation.
S. sanguinis
Two transcriptional regulators, BrpT (which belongs to the tetracycline resistance family) and BrpL, a LuxR family protein, have been identified in S. sanguinis to regulate biofilm formation and EPS production (Liu et al. 2017; Zhu et al. 2018). BrpT alters the biofilm structure with an increase in the quantity of water-insoluble and soluble glucans (Liu et al. 2017). Further analysis showed that the glucosyltransferase gene gtfP is significantly upregulated in a brpT mutant strain, suggesting that the regulator, brpT, may influence the glucan synthesis and biofilm formation through the activity of GtfP (Liu et al. 2017). The absence of BrpL affected a number of phenotypic characteristics of the biofilm: 1) decreasing its susceptibility to ampicillin, 2) increasing surface attachment, and 3) altering carbohydrate metabolism (Zhu et al. 2018).
In summary, regulation of EPS biosynthesis is being explored in a number of oral bacteria. In this regard, gene network analysis in these bacteria is expected to further our understanding of the regulation of EPS synthesis. In addition, the regulatory role of second-messenger signaling in contributing to EPS production is now being explored (an expanded current state of research is provided in the supplementary appendix).
Extracellular Polysaccharide and Immune Evasion
A bacterium’s pathogenicity is attributed to its arsenal of virulence factors, such as fimbriae, proteases, toxins, and EPSs. However, the capacity of any organism to persist in a particular niche requires more than simply possessing virulence factors for functions like tissue adherence or nutrient procurement. Successful colonizing microbes have also evolved strategies to escape protective immunity of the host, often by manipulating key components of innate immune system. These strategies are thought to facilitate the bacterium’s initial colonization, retention, and growth within its niche.
Kingella kingae
The Gram-negative bacterium Kingella kingae is a member of the clinically important HACEK group of bacteria and is a common etiologic agent of pediatric infections (Yagupsky et al. 2011). K. kingae produces an EPS, which is a linear polymer of galactofuranose residues in alternating β(1→3)–β(1→6) linkages (Bendaoud et al. 2011). A recent study has shown the galactan produced by K. kingae prevents opsonin deposition and complement activation facilitating intravascular survival, resulting in enhanced virulence in an in vivo rat model (Munoz et al. 2018).
Prevotella intermedia
Clinical isolates of the oral pathogen Prevotella intermedia are reported to constitutively produce an EPS that forms a meshwork structure around their cells (Yamanaka et al. 2011). Chemical analyses of the viscous materials isolated from their culture supernatants revealed that they consist of a mostly mannose-rich polysaccharide. EPS-producing P. intermedia strains were rarely internalized by leukocytes both in vitro and in vivo, while strains of P. intermedia that lack EPS were readily engulfed and digested by phagosomes. EPS-producing P. intermedia were more pathogenic in inducing abscess formation in mice and evading phagocytosis when compared to non-EPS-producing P. intermedia or P. gingivalis (Yamanaka et al. 2011).
P. gingivalis
Survival of P. gingivalis in the presence of host immune cells and in abscess models of infection has been attributed in part to the presence of the capsular polysaccharide (Singh et al. 2011). Recently, P. gingivalis strains that were deficient in either the SinR regulator or genes associated with capsule production were less fit in an abscess model of infection or in the presence of mammalian cells, highlighting the importance of capsule in virulence (Miller et al. 2017).
A. actinomycetemcomitans
While the protection of A. actinomycetemcomitans cells rendered by leukotoxin has been studied, the role of EPS in the protection of A. actinomycetemcomitans was demonstrated recently (Venketaraman et al. 2008). The EPS mutant strain of A. actinomycetemcomitans or wild-type cells pretreated with the Dispersin B enzyme was susceptible to killing by the human macrophage cell line THP-1 when compared to untreated wild-type cells, suggesting that EPS is necessary for evasion of macrophage killing.
In summary, immune evasion by oral bacteria is understudied and more mechanistic evaluations of immune evasion by these bacteria are warranted. In this context, the mechanisms of immune evasion by nonoral bacteria are provided in the supplementary appendix, which can help drive the direction of research in oral species.
New Directions in the Study of Exopolysaccharides: Control of Biofilms through EPS Studies
Understanding the interactions among the various matrix components could lead to the development of inhibitors to disrupt the stability of the 3D matrix resulting in disease management. As an example of what is possible, recent in silico docking analysis targeting the S. mutans water-insoluble glucan-producing GtfC generated a selective inhibitor of biofilm production with no impact on growth of either S. mutans or commensal streptococci (Nijampatnam et al. 2018). Such studies could lead to virulence-selective inhibition of oral biofilms in the future.
Another interesting approach to the targeted control of cariogenic biofilms is using arginine to disrupt the biofilm 3D architecture and induce in situ pH changes within the EPS matrix. In this regard, arginine catabolism has been shown to modulate cariogenic biofilm production by S. mutans, shifting the microbiome to one that is less caries promoting (Nascimento et al. 2014). A recent study demonstrated the mechanism by which L-arginine, present in human saliva, carries out this modulation (He et al. 2016). Brief incubation with L-arginine suppressed the genes responsible for the production of insoluble EPS (gtfB of S. mutans) and bacteriocin (SMU_150). In another recent study, it was shown that the average thickness of S. mutans biofilm mass was lower when grown in the presence of arginine, compared to the growth without arginine (Huang et al. 2017). Interestingly, the EPS/bacteria ratio was also reduced in the presence of arginine (P = 0.004), showing that arginine can profoundly inhibit EPS formation, especially water-insoluble glucans in S. mutans (Huang et al. 2017). In a mixed species biofilm study using S. mutans and Streptococcus gordonii, arginine enhanced the expression of S. gordonii spxB, which is responsible for peroxide production (He et al. 2016). Together, further understanding of the changes occurring in these 2 bacteria in the presence of arginine may aid in the development of novel methods to control cariogenic biofilms.
A third approach is to use the inherent ability of S. mutans to bind to glucan, which mediates their attachment to tooth surfaces. In this regard, recently, a novel method of preferential depletion of S. mutans, even from co-cultures, was demonstrated using Sephadex beads (dextranomer microspheres) (Mashburn-Warren et al. 2017). This method could be applicable in the removal of pathogenic streptococci from the oral cavity and in the treatment of dental caries with limited perturbation of commensal microbiota.
High-resolution Microscopy
EPS has been well characterized only for a limited number of oral microbes, with an emphasis on S. mutans, which has provided important information about cross-kingdom interactions, pH microenvironments, and overall biofilm development. In this regard, well-defined 3D microcolonies formed by S. mutans where bacterial clusters are thoroughly enmeshed and sheltered in an EPS-rich matrix were characterized using multiphoton confocal microscopy (Hwang et al. 2016). When the spatial pH distribution throughout the 3D microcolony was analyzed, it was revealed that the acidic pH values persisted from the bottom to the lower-middle layers of the microcolony despite exposure to a pH 7 buffer.
In P. aeruginosa, the analysis of biofilm colonies by microscopy has revealed a central void that was hypothesized to be related to the movement of viable cells or dead cells within the structure (Webb et al. 2003). The 3D biofilm of A. actinomycetemcomitans, when visualized by confocal microscopy and viability staining, showed that cell death occurred within the microcolony (Shanmugam et al. 2015). Super-resolution microscopy coupled with selective fluorescent tagging will provide a better understanding of the fate of cells within the biofilm as they mature and whether this phenomenon occurs in oral bacteria.
Chemical mapping of EPS in biofilms has provided spatial imaging of biofilm matrix components such as proteins, polysaccharides, and lipids (Ding et al. 2016). Of particular note, biologically relevant water clusters were observed in the biofilms, suggesting the potential for intercellular small-molecule communication within biofilms. This technique could easily be adapted to study polymicrobial biofilms and differentiate between persister cells or viable but not-cultivatable cells within the interior biofilm. Thus, although only a sample of possibilities is provided, potential interactions among matrix components can be studied using microscopy methods as described for V. cholerae (see below).
Biophysical Methods to Study EPS: Solid-State Nuclear Magnetic Resonance
Studies have demonstrated the power of nuclear magnetic resonance (NMR) to determine the structures of EPSs produced by A. actinomycetemcomitans (Izano et al. 2008), K. kingae (Bendaoud et al. 2011), Lactobacillus plantarum (Fontana et al. 2015), and Streptococcus thermophilus (Sawen et al. 2010). In the future, NMR can be used to identify the structures of novel polysaccharides that are within the biofilm, which can lead to the identification and/or characterization of uncultivable bacteria.
Note that the function of the matrix within a biofilm is diverse and bacteria achieve this diversity by varying their matrix composition (Nadell et al. 2016). Some oral bacteria impart structural rigidity by using protein fibers within the matrix, which form a scaffold for the EPSs to attach. Illustrating this concept is the presence of amyloid in human dental plaque, which has been found to be produced by both laboratory strains and clinical isolates of S. mutans (Oli et al. 2012). The presence of such functional amyloids may play a role in biofilm development. In addition, an interaction between S. mutans cell-surface-localized adhesin P1 protein (antigen I/II, PAc) and its C123 fragment has been demonstrated using solid-state NMR, and the groundwork has been laid for future high-resolution characterization of specific interactions occurring between matrix components (Tang et al. 2016). A recent study highlights the strength of using solid-state NMR to analyze the various carbon pools present (sugar, lipid, and protein) within the biofilm matrix in V. cholerae (Hobley et al. 2015). The analysis of macromolecules in the extracellular matrix could determine the molecular composition of the matrix formed by genetically intractable species. Since the oral microbiome consists of over 700 species, many of which are not cultivatable, the use of NMR for EPS identification and a compilation of associated bacteria should be possible in the near future.
Proteomics
Matrix proteins play an important role in initial surface attachment, aggregate formation, and participation in 3D biofilm architectures. These contain organized motifs that are useful in cell-cell adhesion and binding to EPSs. Recent studies have begun to identify the secretome and matrix proteome, to decipher their roles in biofilm formation and in the regulation of matrix-related genes and proteins that lyse the cells for generation of eDNA (Fong and Yildiz 2015).
EPS as a Nutrient Source
In maturing biofilms, cells at the periphery are exposed to nutrients from the environment and deplete the diffusing nutrients, reducing the amount of nutrients available for interior cells. Most cells remain in the colony until conditions become nutritionally unfavorable, and without an alternative mechanism, the interior cells in a biofilm passively wait for nutrients to reach them (Zhang et al. 2014). In this regard, breakdown of EPS within the biofilm to liberate nutrients for the interior bacteria needs to be evaluated. As a start, capillary electrophoresis and a time-of-flight mass spectrometer have been used to identify metabolites in oral plaque and biofilms of S. mutans and Actinomyces naeslundii (Washio and Takahashi 2016).
Although EPS is appreciated for its role in providing structure and facilitating the adherence of the growing biofilm, the ability for EPS to serve as a nutrient source in the local environment is underappreciated and understudied. The ability to use self-synthesized EPS as a nutrient source will give a critical advantage to select oral biofilm members to survive in competitive and potentially nutrient-deficient regions. In this context, oral streptococci secrete an extracellular dextranase (DexA), which is capable of degrading soluble dextrans into isomaltooligosaccharides. S. mutans has the ability to uptake these into the cell where they are degraded by DexB into glucose. Thus, the potential exists for oral streptococci to use EPSs as sole carbon sources (Colby and Russell 1997). However, a recent study suggested that during night starvation, when a nutritional role for EPS would be expected, EPS catabolism resulting in acid production by S. mutans does not occur since enamel demineralization was not observed (Castillo-Pedraza et al. 2017). Additional detailed studies are needed to determine whether or not EPS could be a nutrition source. Some non-EPS-producing bacteria generate nutrients from EPS-producing bacteria in the soil. For example, the EPS produced by Beijerinckia indica is degraded by Planctomycetes and the products are used as nutrition for growth (Wang et al. 2015). Degradation of EPS of competing bacteria for nutrition might provide a selective advantage for survival in oral biofilms. To fully understand the availability of nutrients in the biofilm interior, combinatorial experimental designs incorporating mutagenesis, metagenomic, or transcriptomic analyses and temporal analysis of the metabolome will need to be undertaken.
Conclusion
We hope that we have presented a concise overview of the EPS research, a critically understudied area in oral biofilms. Novel methods are currently available and/or emerging that could explore this area to newer heights.
Author Contributions
C. Cugini, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; M. Shanmugam, contributed to conception and design, drafted the manuscript; N. Landge, contributed to conception and design, critically revised the manuscript; N. Ramasubbu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519845001 for The Role of Exopolysaccharides in Oral Biofilms by C. Cugini, M. Shanmugam, N. Landge and N. Ramasubbu in Journal of Dental Research
Acknowledgments
The authors thank Mr. Luke Fritzky, manager of the Confocal Imaging Facility at NJMS, Rutgers for assistance with confocal imaging.
Footnotes
A supplemental appendix to this article is available online.
The authors acknowledge the financial support from the National Institute of Dental and Craniofacial Research of the National Institutes of Health grants DE027125 and DE022544.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: N. Ramasubbu  https://orcid.org/0000-0003-3198-8296
https://orcid.org/0000-0003-3198-8296
References
- Alberti-Segui C, Arndt A, Cugini C, Priyadarshini R, Davey ME. 2010. HU protein affects transcription of surface polysaccharide synthesis genes in Porphyromonas gingivalis. J Bacteriol. 192(23):6217–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge BW, Hirano T, Grieshaber N, Davey ME. 2015. Deletion of a 77-base-pair inverted repeat element alters the synthesis of surface polysaccharides in Porphyromonas gingivalis. J Bacteriol. 197(7):1208–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol. 193(15):3879–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk V, Fong JC, Dempsey GT, Develioglu ON, Zhuang X, Liphardt J, Yildiz FH, Chu S. 2012. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 337(6091):236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26(3):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Koo H. 2011. Biology of Streptococcus mutans–derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45(1):69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Pedraza MC, Novais TF, Faustoferri RC, Quivey RG, Jr, Terekhov A, Hamaker BR, Klein MI. 2017. Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms. Biofouling. 33(9):722–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Russell RR. 1997. Sugar metabolism by mutans streptococci. Soc Appl Bacteriol Symp Ser. 26:80S–88S. [PubMed] [Google Scholar]
- Das T, Sharma PK, Busscher HJ, van der Mei HC, Krom BP. 2010. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl Environ Microbiol. 76(10):3405–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey ME, Duncan MJ. 2006. Enhanced biofilm formation and loss of capsule synthesis: deletion of a putative glycosyltransferase in Porphyromonas gingivalis. J Bacteriol. 188(15):5510–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj A, Justice SS, Bakaletz LO, Goodman SD. 2015. DNABII proteins play a central role in UPEC biofilm structure. Mol Microbiol. 96(6):1119–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhou Y, Yao J, Szymanski C, Fredrickson J, Shi L, Cao B, Zhu Z, Yu XY. 2016. In situ molecular imaging of the biofilm and its matrix. Anal Chem. 88(22):11244–11252. [DOI] [PubMed] [Google Scholar]
- Fong JNC, Yildiz FH. 2015. Biofilm matrix proteins. Microbiol Spectr. 3(2). doi: 10.1128/microbiolspec.MB-0004-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana C, Li S, Yang Z, Widmalm G. 2015. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr Res. 402:87–94. [DOI] [PubMed] [Google Scholar]
- Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. 2011. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 4(6):625–637. [DOI] [PubMed] [Google Scholar]
- He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman L, Santarpia P, Zhou X, Koo H. 2016. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 198(19):2651–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. 2015. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 39(5):649–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li L, Sharma S, Wang J, McHardy I, Lux R, Yang Z, He X, Gimzewski JK, Li Y, et al. 2012. DNA builds and strengthens the extracellular matrix in Myxococcus xanthus biofilms by interacting with exopolysaccharides. PLoS One. 7(12):e51905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang K, Deng M, Exterkate RAM, Liu C, Zhou X, Cheng L, Ten Cate JM. 2017. Effect of arginine on the growth and biofilm formation of oral bacteria. Arch Oral Biol. 82:256–262. [DOI] [PubMed] [Google Scholar]
- Hwang G, Liu Y, Kim D, Sun V, Aviles-Reyes A, Kajfasz JK, Lemos JA, Koo H. 2016. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci Rep. 6:32841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ito S, Shimamura T, Weyand S, Kawarasaki Y, Misaka T, Abe K, Kobayashi T, Cameron AD, Iwata S. 2011. Crystal structure of glucansucrase from the dental caries pathogen Streptococcus mutans. J Mol Biol. 408(2):177–186. [DOI] [PubMed] [Google Scholar]
- Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB. 2008. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathog. 44(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. 2015. Streptococcus mutans–derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. 2013. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 92(12):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu HK. 2003. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit Rev Oral Biol Med. 14(5):331–344. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK, Wondrack L, McGuinness M. 1980. Interaction of Streptococcus mutans glucosyltransferases with teichoic acids. Infect Immun. 29(2):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn SJ, Burne RA, Koo H, Brady LJ, Wen ZT. 2014. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol. 196(13):2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Stone VN, Ge X, Tang M, Elrami F, Xu P. 2017. TetR family regulator brpT modulates biofilm formation in Streptococcus sanguinis. PLoS One. 12(1):e0169301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Downey JS, Goodman SD. 2017. Novel method for the depletion of cariogenic bacteria using dextranomer microspheres. Mol Oral Microbiol. 32(6):475–489. [DOI] [PubMed] [Google Scholar]
- Miller DP, Hutcherson JA, Wang Y, Nowakowska ZM, Potempa J, Yoder-Himes DR, Scott DA, Whiteley M, Lamont RJ. 2017. Genes contributing to Porphyromonas gingivalis fitness in abscess and epithelial cell colonization environments. Front Cell Infect Microbiol. 7:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz VL, Porsch EA, St Geme JW., 3rd 2018. Kingella kingae surface polysaccharides promote resistance to human serum and virulence in a juvenile rat model. Infect Immun. 86(6). pii: e00100-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadell CD, Drescher K, Foster KR. 2016. Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol. 14(9):589–600. [DOI] [PubMed] [Google Scholar]
- Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. 2014. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 29(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijampatnam B, Zhang H, Cai X, Michalek SM, Wu H, Velu SE. 2018. Inhibition of Streptococcus mutans biofilms by the natural stilbene piceatannol through the inhibition of glucosyltransferases. ACS Omega. 3(7):8378–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur A, Hirota K, Yumoto H, Hirao K, Liu D, Takahashi K, Murakami K, Matsuo T, Shu R, Miyake Y. 2013. Effects of extracellular DNA and DNA-binding protein on the development of a Streptococcus intermedius biofilm. J Appl Microbiol. 115(1):260–270. [DOI] [PubMed] [Google Scholar]
- Oli MW, Otoo HN, Crowley PJ, Heim KP, Nascimento MM, Ramsook CB, Lipke PN, Brady LJ. 2012. Functional amyloid formation by Streptococcus mutans. Microbiology. 158(Pt 12):2903–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, van der Mei HC, Sjollema J, Busscher HJ, Sharma PK. 2013. A distinguishable role of eDNA in the viscoelastic relaxation of biofilms. MBio. 4(5):e00497-00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco CJ, Bakaletz LO, Goodman SD. 2018. Targeting the HUbeta protein prevents Porphyromonas gingivalis from entering into preexisting biofilms. J Bacteriol. 200(11). pii: e00790-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco CJ, Davey ME, Bakaletz LO, Goodman SD. 2017. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Mol Oral Microbiol. 32(2):118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawen E, Huttunen E, Zhang X, Yang Z, Widmalm G. 2010. Structural analysis of the exopolysaccharide produced by Streptococcus thermophilus ST1 solely by NMR spectroscopy. J Biomol NMR. 47(2):125–134. [DOI] [PubMed] [Google Scholar]
- Shanmugam M, El Abbar F, Ramasubbu N. 2015. Transcriptome profiling of wild-type and pga-knockout mutant strains reveal the role of exopolysaccharide in Aggregatibacter actinomycetemcomitans. PLoS One. 10(7):e0134285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Wyant T, Anaya-Bergman C, Aduse-Opoku J, Brunner J, Laine ML, Curtis MA, Lewis JP. 2011. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun. 79(11):4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov-Sorensen UB, Yao K, Yang Y, Tettelin H, Kilian M. 2016. Capsular polysaccharide expression in commensal Streptococcus species: genetic and antigenic similarities to Streptococcus pneumoniae. MBio. 7(6). pii: e01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 6(3):199–210. [DOI] [PubMed] [Google Scholar]
- Tang W, Bhatt A, Smith AN, Crowley PJ, Brady LJ, Long JR. 2016. Specific binding of a naturally occurring amyloidogenic fragment of Streptococcus mutans adhesin P1 to intact P1 on the cell surface characterized by solid state NMR spectroscopy. J Biomol NMR. 64(2):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venketaraman V, Lin AK, Le A, Kachlany SC, Connell ND, Kaplan JB. 2008. Both leukotoxin and poly-N-acetylglucosamine surface polysaccharide protect Aggregatibacter actinomycetemcomitans cells from macrophage killing. Microb Pathog. 45(3):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu B, Chen M, Crawford RJ, Ivanova EP. 2009. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 14(7):2535–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dubey AK, Suzuki K, Baker CS, Babitzke P, Romeo T. 2005. CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli. Mol Microbiol. 56(6):1648–1663. [DOI] [PubMed] [Google Scholar]
- Wang X, Sharp CE, Jones GM, Grasby SE, Brady AL, Dunfield PF. 2015. Stable-isotope probing identifies uncultured Planctomycetes as primary degraders of a complex heteropolysaccharide in soil. Appl Environ Microbiol. 81(14):4607–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio J, Takahashi N. 2016. Metabolomic studies of oral biofilm, oral cancer, and beyond. Int J Mol Sci. 17(6). pii: E870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, Kjelleberg S. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 185(15):4585–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, III, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8(4):e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagupsky P, Porsch E, St Geme JW., 3rd 2011. Kingella kingae: an emerging pathogen in young children. Pediatrics. 127(3):557–565. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Noiri Y, Yamaguchi M, Asahi Y, Maezono H, Kuboniwa M, Hayashi M, Ebisu S. 2013. The sinR ortholog PGN_0088 encodes a transcriptional regulator that inhibits polysaccharide synthesis in Porphyromonas gingivalis ATCC 33277 biofilms. PLoS One. 8(2):e56017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Yamane K, Furukawa T, Matsumoto-Mashimo C, Sugimori C, Nambu T, Obata N, Walker CB, Leung KP, Fukushima H. 2011. Comparison of the virulence of exopolysaccharide-producing Prevotella intermedia to exopolysaccharide non-producing periodontopathic organisms. BMC Infect Dis. 11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Konno H, Nagano K, Abiko Y, Nakamura Y, Tanaka Y, Yoshimura F. 2014. The influence of a glucosyltransferase, encoded by gtfP, on biofilm formation by Streptococcus sanguinis in a dual-species model. APMIS. 122(10):951–960. [DOI] [PubMed] [Google Scholar]
- Zhang W, Seminara A, Suaris M, Brenner MP, David A, Weitz DA, Angelini TA. 2014. Nutrient depletion in Bacillus subtilis biofilms triggers matrix production. New J Physi. 16. doi: 10.1088/1367-2630/16/1/015028 [DOI] [Google Scholar]
- Zhu B, Macleod LC, Kitten T, Xu P. 2018. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 13:915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519845001 for The Role of Exopolysaccharides in Oral Biofilms by C. Cugini, M. Shanmugam, N. Landge and N. Ramasubbu in Journal of Dental Research