Abstract
Facial angiofibromas, composed of fibrous tissue and blood vessels appearing on the face, are closely associated with tuberous sclerosis complex. Historically, oral rapamycin, a mammalian target of the rapamycin inhibitor of cell proliferation, has been used to treat visceral tuberous sclerosis–related tumors; however, the side effect profile of this medicine generally precludes its use in patients lacking significant internal involvement. The authors developed a novel topical formulation of rapamycin cream to treat the facial angiofibroma without exposing patients to possible systemic side effects. We followed 11 patients in a long-term, open-label, prospective study to evaluate the safety and effectiveness of rapamycin cream when used chronically. All of the patients showed an improvement in the appearance of their facial angiofibroma which was maintained in long-term follow-up without safety concerns or systemic absorption. The novel rapamycin cream was tolerated well by all patients and represents a way to address the cutaneous manifestation of tuberous sclerosis complex.
Keywords: tuberous sclerosis complex, angiofibroma, rapamycin, treatment
Tuberous sclerosis complex is an autosomal dominant disorder affecting 1 in 6000 to 1 in 10 000 individuals.1 It is characterized by inactivation of either of the 2 tumor suppressor genes, TSC1 (locus 9q34) or TSC2 (locus 16p13.3),2 which play an important role in the control of cell proliferation and differentiation through negative regulation of the mammalian target of the rapamycin pathway. This inactivation usually results in benign tumor formations in different tissues and organs.3,4 One of these benign tumors is facial angiofibromas that can appear at various ages in affected persons, usually between 2 and 5 years of age and progressively increasing in number and size before stabilizing after adolescence. These lesions are usually the cause of significant morbidity and historically have been resistant to medical and surgical treatments.5–9
Previously, 2 of the authors (J.W. and A.H.) developed a novel topical rapamycin cream for treatment of facial angiofibromas in tuberous sclerosis complex. Initially, we evaluated the safety and efficacy of our novel formulation in 2 patients.10 We then expanded our previous study and enrolled an additional 9 patients (for a total of 11 patients) in an open-label study to evaluate the safety and long-term efficacy of this rapamycin cream when used chronically. Here, we demonstrated similar results following enrollment of a greater number of patients for an extended duration of treatment.
Methods
To prepare the rapamycin cream, the authors obtained rapamycin powder (Pfizer Inc, New York, New York). The rapamycin cream was prepared in the Le Bonheur Children’s Hospital pharmacy. To formulate this cream, 150 mg of rapamycin were weighed and mixed with 150 g of the hydrophilic ointment (United States Pharmacopeia, Rockville, Maryland) using an Unguator (EGS Elektro-und, Zella-Mehlis, Germany) for one-and-half minutes. The cream was separated into several glass jars (each jar contained about 12 g of cream) and was ready for patient use. The rapamycin concentration in each jar was 0.1% by weight. Each jar of this compound will typically last with daily facial application approximately 3 months.
After written informed consent was obtained, patients with ages ranging from 9 to 27 years had baseline pictures taken of their face. These were repeated at the scheduled study visits: 2, 4, 26, and 52 weeks after initiation of treatment and at the end of the study, ∼2.5 years after initiation of treatment. A serum rapamycin level was obtained after 2 and 4 weeks of treatment. Patients applied a small amount of the topical cream twice daily for 2 weeks and then once daily. They were instructed not to apply the cream over any abrasions and to report any side effects. Patients were queried as to any side effects at each visit by study personnel. The study was approved by the institutional review board of University of Tennessee Health Science Center (UTHSC Protocol 2014-017).
Results
A total of 11 patients, ages ranging from 9 to 27 years, suffering from facial angiofibroma were instructed to apply rapamycin facial cream. Photographs were taken prior to initiating therapy and then periodically (Figure 1). At 2 and 4 weeks, the serum rapamycin level was undetectable in all patients (LabCorp, Birmingham, Alabama). The study ran for approximately 2.5 years with patients enrolled sequentially throughout this time period. Six of the 11 patients used the cream for more than 1 year: 1 patient discontinued use after a year when started on oral everolimus (Afinitor) for systemic manifestations of tuberous sclerosis complex, the other 5 continued until study termination. Of the remaining 5 patients, 2 patients used the cream for 26 weeks, 1 for 30 weeks, 1 for 21 weeks, and 1 for under 4 weeks (and was then lost to follow-up).
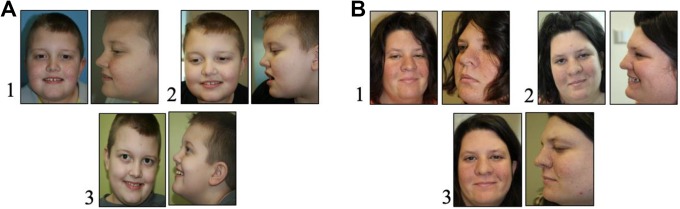
Figure 1.
Facial angiofibroma: Patient A (left), B (right). 1. Week 0; 2. Week 4; 3. Week 52.
The use of the rapamycin cream improved the appearance of the facial angiofibroma. In addition to improvement in the general appearance, the angiofibroma was less erythematous and no longer bled with minor abrasions or light touch. This effect was noticeable as early as in the first 4 weeks of therapy, and the improvement persisted throughout the entire course of treatment (Figure 1A and B). Importantly, the improvement persisted in patients who used it for over a year (Figure 1A and B). Families and patients reported that the cream is easy to apply and did not report any local side effects.
Discussion
Products of the tumor suppression gene TSC1 and TSC2, hamartin and tuberin, inhibit cell proliferation by regulating the mammalian target of the rapamycin pathway. Rapamycin has been shown to regulate the key kinase that plays a central role in tuberous sclerosis complex. Inactivation of either gene results in loss of inhibitory effect on mammalian target of the rapamycin resulting in uncontrolled cell division and tumor promoting development. Rapamycin suppresses tumor growth by reestablishing inhibition of the mammalian target of rapamycin.
Oral use of rapamycin has been used to induce the regression of visceral tuberous sclerosis–related tumors (eg, subependymal giant cell astrocytomas, angiomyolipomas, lymphangioleiomyomatosis), including patient with facial angiofibroma.11–14 Historically, oral rapamycin has not been used to treat facial angiofibromas due to the side effects associated with systemic use of the compound. Treatment for facial angiofibroma has historically included surgical and medical options (Table 1). None of these have been entirely satisfactory. In recent years, various topical inhibitors of mammalian target of the rapamycin have emerged with more favorable outcomes but none have been commercialized yet.15–22 Different strengths of topical rapamycin cream have been formulated, with varying responses, all of which are dependent on the concentration and formulation and the penetration of the rapamycin into the dermis. The most positive results have been demonstrated when the lesions were treated with topical mammalian target of the rapamycin inhibitors, with younger aged children showing more promising outcomes than older patients.23–27 Angiofibromas start small and gradually increase in size, with their growth being augmented by puberty. The more positive results seen in younger patients may be due to the angiofibroma being smaller in size and lacking excess fibrous growths when treatment was initiated.
Table 1.
Treatments for facial angiofibromas.
We had previously reported developing a novel rapamycin cream that proved effective as a treatment of facial angiofibroma and performed a proof of concept trial in 2 patients who were treated for a 26-week period.10 Our compounded cream effectively penetrated the dermis and dramatically cleared the facial lesions. Rapamycin negatively regulates the mammalian target of rapamycin, inhibiting angiogenesis and the proliferation of collagen fibers. This occurs through a decrease in the vascular endothelial growth factor. This effect was rapid and produced the observed decrease in the redness of facial angiofibroma. The compounded cream is a novel preparation that is easy to prepare, had no systemic absorption, and was without systemic or local side effects. The current report encompasses our prior observations and extends them to a real-world therapy where the patients would likely be on therapy for many weeks or even years. We document a sustained, long-term efficacy associated with our rapamycin cream without safety concerns. These results indicate that compounded rapamycin cream is a viable option for patients with facial angiofibroma.
Footnotes
Author Contributions: MCW critically revised the manuscript. JWW contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. AAT contributed to analysis and drafted the manuscript. HA contributed to conception.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Shainberg Neuroscience Fund.
ORCID iD: James W. Wheless  https://orcid.org/0000-0002-6847-3387
https://orcid.org/0000-0002-6847-3387
Ethical Approval: This study was performed with approval of the University of Tennessee Health Science Center IRB. Patients were enrolled and treated only after signing informed consent (by patient and/or caregiver).
References
- 1. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. [DOI] [PubMed] [Google Scholar]
- 2. Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9(1):73–79. [DOI] [PubMed] [Google Scholar]
- 3. Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz RA, Fernandez G, Kotulska K, Jozwiak S. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57(2):189–202. [DOI] [PubMed] [Google Scholar]
- 5. Boixeda P, Sanchez-Miralles E, Azana JM, Arrazola JM, Moreno R, Ledo A. CO2, argon, and pulsed dye laser treatment of angiofibromas. J Dermatol Surg Oncol. 1994;20(12):808–812. [DOI] [PubMed] [Google Scholar]
- 6. Gomes AA, Gomes YV, Lima FB, Pessoa SG. Multiple facial angiofibromas treated with high-frequency equipment. An Bras Dermatol. 2011;86(4 suppl 1):S186–S189. [DOI] [PubMed] [Google Scholar]
- 7. Hori K, Soejima K, Nozaki M, et al. Treatment of facial angiofibroma of tuberous sclerosis using cultured epithelial autografts. Ann Plast Surg. 2006;57(4):415–417. [DOI] [PubMed] [Google Scholar]
- 8. Turkmen M, Ertam I, Unal I, Dereli T. Facial angiofibromas of tuberous sclerosis: successful treatment with podophyllin. J Eur Acad Dermatol Venereol. 2009;23(6):713–714. [DOI] [PubMed] [Google Scholar]
- 9. Weiss ET, Geronemus RG. New technique using combined pulsed dye laser and fractional resurfacing for treating facial angiofibromas in tuberous sclerosis. Lasers Surg Med. 2010;42(5):357–360. [DOI] [PubMed] [Google Scholar]
- 10. Wheless JW, Almoazen H. A novel topical rapamycin cream for the treatment of facial angiofibromas in tuberous sclerosis complex. J Child Neurol. 2013;28(7):933–936. [DOI] [PubMed] [Google Scholar]
- 11. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. [DOI] [PubMed] [Google Scholar]
- 12. Hofbauer GF, Marcollo-Pini A, Corsenca A, et al. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. Br J Dermatol. 2008;159(2):473–475. [DOI] [PubMed] [Google Scholar]
- 13. Kaufman McNamara E, Curtis AR, Fleischer AB., Jr Successful treatment of angiofibromata of tuberous sclerosis complex with rapamycin. J Dermatolog Treat. 2012;23(1):46–48. [DOI] [PubMed] [Google Scholar]
- 14. Micozkadioglu H, Koc Z, Ozelsancak R, Yildiz I. Rapamycin therapy for renal, brain, and skin lesions in a tuberous sclerosis patient. Ren Fail. 2010;32(10):1233–1236. [DOI] [PubMed] [Google Scholar]
- 15. DeKlotz CM, Ogram AE, Singh S, Dronavalli S, MacGregor JL. Dramatic improvement of facial angiofibromas in tuberous sclerosis with topical rapamycin: optimizing a treatment protocol. Arch Dermatol. 2011;147(9):1116–1117. [DOI] [PubMed] [Google Scholar]
- 16. Dill PE, De Bernardis G, Weber P, Losch U. Topical everolimus for facial angiofibromas in the tuberous sclerosis complex. A first case report. Pediatr Neurol. 2014;51(1):109–113. [DOI] [PubMed] [Google Scholar]
- 17. Foster RS, Bint LJ, Halbert AR. Topical 0.1% rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: a pilot study of four patients. Australas J Dermatol. 2012;53(1):52–56. [DOI] [PubMed] [Google Scholar]
- 18. Haemel AK, O’Brian AL, Teng JM. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146(7):715–718. [DOI] [PubMed] [Google Scholar]
- 19. Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D. 2012;12(3):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mutizwa MM, Berk DR, Anadkat MJ. Treatment of facial angiofibromas with topical application of oral rapamycin solution (1 mgmL(-1)) in two patients with tuberous sclerosis. Br J Dermatol. 2011;165(4):922–923. [DOI] [PubMed] [Google Scholar]
- 21. Truchuelo T, Diaz-Ley B, Rios L, Alcantara J, Jaen P. Facial angiofibromas treated with topical rapamycin: an excellent choice with fast response. Dermatol Online J. 2012;18(1):15. [PubMed] [Google Scholar]
- 22. Wataya-Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): a pilot study of nine Japanese patients with TSC of different disease severity. Br J Dermatol. 2011;165(4):912–916. [DOI] [PubMed] [Google Scholar]
- 23. Bae-Harboe YS, Geronemus RG. Targeted topical and combination laser surgery for the treatment of angiofibromas. Lasers Surg Med. 2013;45(9):555–557. [DOI] [PubMed] [Google Scholar]
- 24. Knopfel N, Martin-Santiago A, Bauza A, Hervas JA. Topical 0.2% rapamycin to treat facial angiofibromas and hypomelanotic macules in tuberous sclerosis. Actas Dermosifiliog. 2014;105(8):802–803. [DOI] [PubMed] [Google Scholar]
- 25. Park J, Yun SK, Cho YS, Song KH, Kim HU. Treatment of angiofibromas in tuberous sclerosis complex: the effect of topical rapamycin and concomitant laser therapy. Dermatology. 2014;228(1):37–41. [DOI] [PubMed] [Google Scholar]
- 26. Wataya-Kaneda M, Tanaka M, Yang L, et al. Clinical and histologic analysis of the efficacy of topical rapamycin therapy against hypomelanotic macules in tuberous sclerosis complex. JAMA Dermatol. 2015;151(7):722–730. [DOI] [PubMed] [Google Scholar]
- 27. Jozwiak S, Sadowski K, Kotulska K, Schwartz RA. Topical use of mammalian target of rapamycin (mTOR) inhibitors in tuberous sclerosis complex—a comprehensive review of the literature. Pediatr Neurol. 2016;61:21–27. [DOI] [PubMed] [Google Scholar]