Abstract
Purpose:
To evaluate the safety, recommended phase II dose (RP2D) and efficacy of pexidartinib, a colony stimulating factor receptor 1 (CSF-1R) inhibitor, in combination with weekly paclitaxel in patients with advanced solid tumors.
Patients and Methods:
In part 1 of this phase Ib study, 24 patients with advanced solid tumors received escalating doses of pexidartinib with weekly paclitaxel (80 mg/m2). Pexidartinib was administered at 600 mg/day in cohort 1. For subsequent cohorts, the dose was increased by ⩽50% using a standard 3+3 design. In part 2, 30 patients with metastatic solid tumors were enrolled to examine safety, tolerability and efficacy of the RP2D. Pharmacokinetics and biomarkers were also assessed.
Results:
A total of 51 patients reported ≥1 adverse event(s) (AEs) that were at least possibly related to either study drug. Grade 3–4 AEs, including anemia (26%), neutropenia (22%), lymphopenia (19%), fatigue (15%), and hypertension (11%), were recorded in 38 patients (70%). In part 1, no maximum tolerated dose was achieved and 1600 mg/day was determined to be the RP2D. Of 38 patients evaluable for efficacy, 1 (3%) had complete response, 5 (13%) partial response, 13 (34%) stable disease, and 17 (45%) progressive disease. No drug–drug interactions were found. Plasma CSF-1 levels increased 1.6- to 53-fold, and CD14dim/CD16+ monocyte levels decreased by 57–100%.
Conclusions:
The combination of pexidartinib and paclitaxel was generally well tolerated. RP2D for pexidartinib was 1600 mg/day. Pexidartinib blocked CSF-1R signaling, indicating potential for mitigating macrophage tumor infiltration.
Keywords: advanced solid tumors, colony stimulating factor receptor 1 inhibitor, paclitaxel, phase I trials, tumor associated macrophages
Translational relevance
Colony stimulating factor 1 receptor (CSF-1R) signaling has been implicated in homing of monocytes to the tumor microenvironment and their differentiation to tumor associated macrophages (TAMs). TAMs induce immune suppression and neo-angiogenesis, facilitating tumor growth and metastases. Work in a mouse model of mammary carcinoma revealed that following exposure to chemotherapy, malignant cells increase expression of colony stimulating factor (ligand of CSF-1R) leading to recruitment of TAMs and chemotherapy resistance. Pexidartinib, a small molecule inhibitor of CSF-1R (IC50 17 nM), was shown to abrogate TAM recruitment following chemotherapy, and this was associated with a less suppressed immune tumor microenvironment, slower tumor growth, and improved survival of study animals. Here, we present the results of the first-in-human phase Ib study, which established recommended phase II dose of pexidartinib in combination with paclitaxel in patients with advanced, treatment refractory solid tumors.
Introduction
CSF-1R, also known as macrophage colony-stimulating factor receptor (M-CSFR) and CD115, is a transmembrane receptor tyrosine kinase that is widely expressed by monocytes, macrophages, granulocytes, and some tumor cells.1,2 The receptor has two known ligands: colony stimulating factor 1 (CSF-1) and interleukin 34 (IL-34). Upon stimulation, CSF-1R activates intracellular signaling integral to the differentiation, maturation, migration, and survival of monocytes and macrophages.3–5 In cancer, CSF-1R signaling facilitates recruitment and survival of TAMs within the tumor microenvironment, leading to suppression of host antitumor immunity. TAMs also secrete proangiogenic factors and promote growth and invasiveness of malignant cells.6
Several types of solid tumors (including breast, renal cell carcinoma, leiomyosarcoma, and epithelial ovarian carcinomas) express high levels of CSF-1.7–11 Experiments performed in mammary carcinoma bearing transgenic MMTV–polyoma middle T (PyMT) mice demonstrated that expression of CSF-1 by carcinoma cells increases following treatment with chemotherapy. This increase in CSF-1 expression is then associated with higher proportion of TAMs and lower levels of CD8+ and CD4+ T lymphocytes.12
Treatment of tumor-bearing PyMT mice with a combination of paclitaxel and CSF-1R inhibitor led to blockade of macrophage recruitment to the tumor microenvironment and significant increases in CD8+ T cells. This was associated with CD8+ T-cell dependent reduction in tumor progression, metastases and improvement in survival of study animals. These preclinical data provided justification for therapeutic approaches in patients with solid tumors aimed at mitigating macrophage recruitment and function by inhibition of CSF-1R in combination with cytotoxic chemotherapy.12,13
Pexidartinib (PLX3397) is a novel, orally available, small molecule kinase inhibitor that blocks CSF-1R at an IC50 of 17 nM. Furthermore, pexidartinib inhibits oncogenically activated FLT3 (FLT3-ITK) and interferes with stromal cell factor-induced auto-phosphorylation of c-Kit protein (Kit) at IC50 concentrations below 1 µM. In addition, pexidartinib is known to inhibit differentiation of osteoclast precursors in a RANK-L and CSF-1 dependent manner (Investigator’s Brochure).14
Based on the results of the above preclinical studies, we conducted a phase Ib clinical trial of pexidartinib in combination with weekly paclitaxel in patients with advanced solid tumors. The primary objectives were to evaluate the safety of the drug combination and determine the recommended phase II dose (RP2D). Secondary objectives included evaluating the potential for a drug–drug interaction effect on pharmacokinetic parameters and exploring preliminary efficacy. In addition, the effect of the treatment combination on peripheral blood CSF-1 and CD14dim/CD16+ monocyte levels (potential pharmacodynamic markers of pexidartinib) was explored.
Materials and methods
Study population
The study recruited patients from three academic institutions (University of California San Francisco, Case Western Reserve University, and The Ohio State University Comprehensive Cancer Center). The respective institutional review boards have approved the study. Western IRB was used at OSU (WIRB # 20120818). Case Western Reserve University and University of California San Francisco used their institutional IRB (IRB # 062752 and 149357, respectively) and the study was registered with ClinicalTrials.gov (NCT01525602). The study had three parts. In part 1, dose escalation of pexidartinib in combination with standard dose of weekly paclitaxel given continuously was conducted using a 3+3 design. In part 2, 30 patients with advanced solid tumors were enrolled. part 3 has enrolled 18 patients with platinum-resistant or -refractory advanced ovarian cancer. Here, we report the results from parts 1 and 2.
Patients in part 1 had advanced, incurable solid tumors. Patients in part 2 had advanced, incurable solid tumors for which a taxane would be considered a reasonable chemotherapy option. Patients were to be 18 years or older, have an Eastern Cooperative Oncology Group (ECOG) performance score (PS) of 0–2, have an anticipated life expectancy of at least 12 weeks, and adequate bone marrow reserve as well as renal, hepatic, and cardiac function. A washout period was required after any prior chemotherapy, radiation, investigational, biologic, hormonal, or targeted therapy. Additionally, bone-directed therapy was not to be started within 2 weeks prior to study day 1 or during the first 28 days on study therapy. Patients were also required to have had resolution of all prior treatment-related toxicities to grade 1 or less except for grade 2 fatigue or alopecia.
Patients were excluded from study participation if they had a secondary active malignancy, refractory nausea and vomiting, malabsorption, external biliary shunt, or significant small bowel resection that would preclude adequate absorption of oral pexidartinib, ongoing treatment with any other investigational therapy, unstable brain metastases requiring systemic steroid treatment, prior anaphylactic or severe hypersensitivity reaction to paclitaxel or cremaphor-containing agents, grade 2 or higher neuropathy, persistent grade 2 fatigue, or an active untreated infection.
Study objectives
The main objectives of part 1 were to explore the safety, tolerability and dose limiting toxicities of escalating doses of daily oral pexidartinib with weekly intravenous paclitaxel and to establish a RP2D. The main objective of part 2 was to determine the safety of pexidartinib administered at the RP2D in combination with paclitaxel in patients with advanced, nonresectable, solid tumors.
The secondary objectives of this study were to explore the efficacy of pexidartinib in combination with paclitaxel in patients with advanced solid tumors, and determine the pharmacokinetics (PK) of pexidartinib when administered in combination with paclitaxel.
Exploratory objectives included correlating the increases in plasma CSF-1 levels and the decreases in blood CD14dim/CD16+ monocyte levels during treatment with specific dose levels of pexidartinib.
Dose escalation (part 1)
The study employed a traditional 3+3 design. Cohort 1 started treatment with oral pexidartinib at a dose of 600 mg/day (divided to twice daily). Paclitaxel at a dose of 80 mg/m2 was administered intravenously over 1 h once weekly (±48 h) in all cohorts. The cycle length was 28 days. Following cycle 1, the protocol permitted skipping one of four paclitaxel doses in each cycle per discretion of the treating physician. The planned pexidartinib dose escalation schedule is provided in Table 1.
Table 1.
Summary of dosing cohorts and dose limiting toxicities.
| Dose levels | Pexidartinib mg/day PO divided BIDc | Paclitaxel mg/m2 IV | Patient number (N = 54) | Number of DLTs (N = 2) |
|---|---|---|---|---|
| Cohort -1 | 400 | 80 | 0 | 0 |
| Cohort 1a | 600 | 80 | 9 | 0 |
| Cohort 2 | 800 | 80 | 3 | 0 |
| Cohort 3 | 1000 | 80 | 3 | 0 |
| Cohort 4 | 1200 | 80 | 6 | Grade 3 hypophosphatemia |
| Cohort 5b | 1600 | 80 | 3 | Grade 3 atrial fibrillation |
| Cohort 6 | 2000 | 80 | 0 | Not applicable |
| Part 2 | 1600 | 80 | 30 | 0 |
Paclitaxel was administered intravenously over approximately 60 min on days 1, 8, 15 and 22 per 28 day cycle. Following cycle 1 of study therapy patients were permitted to skip 1 of 4 paclitaxel doses in each cycle.
Starting dose level.
Recommended phase II dose.
BID stands for twice daily.
Between three and six patients were to be enrolled at each dose level. Enrollment into the next higher dose level was to begin only if the first three patients enrolled into the cohort completed the 28-day observation period without the occurrence of a dose-limiting toxicity (DLT). If one of the three initial patients at a given dose level experienced a DLT, the cohort at this dose level was to be expanded to include an additional three patients (six patients total).
If two or more out of six patients experienced a DLT, dose escalation was to be stopped and the preceding dose level was considered to be the maximum tolerated dose (MTD) and, therefore, the RP2D.
Dose-limiting toxicities
A DLT was defined as any treatment-related adverse event (AE) that met the criteria described below and occurred within the first 28 days after the start of combination therapy. Patients must have received at least 21 days of pexidartinib and at least three of the four planned doses of paclitaxel during the first 28 days to be considered evaluable for a DLT. Patients not meeting these criteria were replaced.
DLTs included hematological AEs (grade 4 neutropenia lasting for ⩾7 days, grade 4 thrombocytopenia, and grade 3 thrombocytopenia associated with bleeding) and any other grade ⩾3 toxicity unless the event was clearly unrelated to treatment with pexidartinib or paclitaxel and with the exclusion of the following: grade ⩾3 nausea, vomiting, or diarrhea, that resolved to grade ⩽2 within 48 h with or without medical intervention or prophylaxis; allergic reaction to paclitaxel; grade 3 fatigue that resolved to grade ⩽2 within 14 days; grade ⩾3 hyperglycemia; transient (<14 days) increase in LFTs of less than or equal to one grade in severity compared with baseline levels in patients with baseline liver metastases; grade 3 peripheral neuropathy in patients with baseline grade ⩾1 peripheral neuropathy or a history of chemotherapy-associated peripheral neuropathy; grade 3 myalgia or arthralgia in patients with baseline grade ⩾1 myalgia or arthralgia; or grade 3 rash for which symptoms were easily managed with supportive care and there was no evidence of superinfection or limitation of self-care activities.
Pharmacokinetics
A noncompartmental method of analysis was used to analyze the plasma concentrations of pexidartinib. The Cmax, Tmax, and a partial AUC (AUC0-4) at steady state were determined using samples taken on cycle 1, day 15. Plasma concentrations of paclitaxel were also determined in parallel.
Statistical analyses
Safety and tolerability were evaluated from the results of reported signs and symptoms, scheduled and symptom-directed physical examinations, vital sign measurements, 12 lead ECGs (including QTcF intervals), and clinical laboratory test results. The safety population included patients who received at least one dose of study drug. AEs were recorded from the time the patient received the first dose of study drug up to 28 days after the last dose, or prior to start of new antitumor therapy, whichever occurred first.
AEs were summarized descriptively by attribution of study therapy (unlikely, probably, likely, and definitely related) and grade using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Laboratory variables were summarized using mean change in value from baseline to scheduled time points for each dose level group and 95% confidence interval. Laboratory values were also categorized according to their CTCAE version 4 toxicity grade and tabulated by worst on-study toxicity grade and dose level group. Concomitant medications were also summarized.
Response to treatment according to RECIST version 1.1 criteria was summarized descriptively by dose level. The duration of response was calculated for each patient with a response to therapy. The duration of response was defined as number of days from the date of initial response (confirmed at least 28 days later) to the date of first documented disease progression or death, whichever occurred first.
Pexidartinib and paclitaxel pharmacokinetic parameters were analyzed based on their plasma concentrations using noncompartmental model with Phoenix, Version 6.4 (Pharsight, CA, USA). Descriptive statistics were used to analyze exploratory pharmacodynamic endpoints. SAS 9.3 software was used to conduct all statistical analysis.
Study oversight
An academic steering group, including representatives from the sponsor (Plexxikon), designed the study. All the authors vouch that the study adhered to the protocol and confirm the accuracy and completeness of the data. The first author prepared the first draft of the manuscript with assistance from the sponsor. Subsequent revisions and interpretation of the study data were completed by all authors. An institutional review board, at each site, approved the study and all the patients gave written informed consent before enrollment. The study was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. A study steering committee reviewed the study conduct.
Results
Demographics and patient characteristics
A total of 54 patients were enrolled, 24 in part 1 (dose escalation) and 30 in part 2 (RP2D expansion). Study patients ranged from 32 to 82 years of age with a median of 60 years, 61% were female and 89% white (Table 2). The majority (65%) of subjects at baseline had an ECOG PS of 1 and 28% an ECOG PS of 0. The most frequent primary tumor sites were breast and ovarian (13% each) and neuroendocrine (11%). Patients received a median of 3.5 prior lines of anticancer therapy (range 1–8 lines), with 80% of subjects having received a platinum-based therapy, and 57% having received prior taxane therapy.
Table 2.
Demographics and patient characteristics.
| Parameter | Total (N = 54) |
|---|---|
| Age | |
| Mean | 58.4 |
| SD | 11.49 |
| Median | 60.0 |
| Min, Max | 32, 82 |
| Gender n (%) | |
| Male | 21 (39) |
| Female | 33 (61) |
| Ethnicity n (%) | |
| Hispanic or Latino | 3 (6) |
| Not Hispanic or Latino | 51 (94) |
| Race n (%) | |
| American Indian or Alaska Native | 0 |
| Asian | 1 (2) |
| Black or African American | 4 (7) |
| Native Hawaiian or Other Pacific Islander | 0 |
| White | 48 (89) |
| Other | 1 (2) |
| ECOG Status n (%) | |
| 0 - Fully Active | 15 (28) |
| 1 - Restricted | 35 (65) |
| 2 - Ambulatory | 4 (7) |
| 3 - Limited Self Care | 0 |
| 4 - Completely Disabled | 0 |
| Primary Malignancy (Those in ⩾10% of patients) |
|
| Breast | 7 (13) |
| Ovarian | 7 (13) |
| Neuroendocrine | 6 (11) |
| Prior Anticancer Therapy | |
| Number of lines of therapy, median (range) | 3.5 (1, 8) |
| Prior platinum therapy n (%) | 43 (79.6) |
| Prior taxane therapy n (%) | 31 (57.4) |
Exposure and dosing compliance
Patients were on daily oral pexidartinib for a mean of 70.5 days, with a range across dose levels from 50.8 days (600 mg) to 109.2 days (1200 mg). Patient compliance with pexidartinib was high, with 89% of the patients taking over 80% assigned dose; 25 (46%) patients reported missing doses of pexidartinib due to AEs of any cause, and 23 (43%) patients missed doses due to noncompliance. Because of an AE that was at least possibly related to pexidartinib, the drug was temporarily withdrawn from 19 (35%) patients, and permanently withdrawn from 5 (9%) patients. A total of 14 (26%) patients had pexidartinib dose reductions; 6 patients had dose reductions as an immediate action following pexidartinib-related AE, while the remainder had pexidartinib held due to an AE and then restarted at a reduced dose (see Supplementary Table S1 for summary of actions).
The mean number of paclitaxel doses was 9.8, with a range across dose levels of 7.0 doses (600 mg) to 15.5 doses (1200 mg); 39% of the patients received > 80% of paclitaxel dosing. However, following cycle 1, the study protocol permitted omitting one paclitaxel dose in each cycle (i.e., patients could receive 75% of the planned dose per cycle). Of the 45 study patients that completed the first 28-day cycle, 36 (80%) received at least three paclitaxel doses in each full 28-day cycle. Of the 27 study patients who completed two or more cycles, 20 (74%) received at least three paclitaxel doses in each full cycle following cycle 1 of therapy. The reasons for omitting paclitaxel dose were AEs (54%), physician or patient preference to omit one paclitaxel in each cycle (34%), disease progression (4%), and other (8%). Eight (15%) patients had paclitaxel dose reductions: seven as an immediate action following paclitaxel related AE, while the remaining patient had paclitaxel treatment interrupted and restarted at a reduced dose (Supplementary Table S2).
Adverse events
Treatment emergent AEs as they relate to severity grade, dose level, and attribution to the study therapy, are documented in Supplementary Tables S3 and S4. A detailed list of AEs occurring in ⩾10% of patients that were at least possibly related to study therapy are presented in Table 3. A total of 51 (94.4%) patients reported one or more AE, including anemia, fatigue, decreased appetite, diarrhea, nausea, increased AST, and alopecia, that was at least possibly related to either study drug. The most common AE was fatigue (65%), followed by anemia (59%), diarrhea, and nausea (both 39%). The most frequent type of AE by body system was gastrointestinal disorders, reported in 89% (48/54) patients, most of which were nausea, vomiting, and diarrhea. The majority of these toxicities were grade 1 and 2 in severity.
Table 3.
Summary of toxicities that occurred in >10% of patients and were at least possibly related to either study drug.
| Toxicity | Grades 1 & 2 | Grades 3 & 4 | All Grades |
|---|---|---|---|
| Number (%) | |||
| Fatigue | 27 (50) | 8 (15) | 35 (65) |
| Anemia | 18 (33) | 14 (26) | 32 (59) |
| Neutropenia/Decreased Neutrophils | 11 (20) | 12 (22) | 23 (43) |
| Diarrhea | 17 (31) | 4 (7) | 21 (39) |
| Nausea | 18 (33) | 3 (6) | 21 (39) |
| Aspartate Aminotransferase Increased | 15 (28) | 4 (7) | 19 (35) |
| Decreased Appetite | 19 (35) | 0 (0) | 19 (35) |
| Lymphocyte Count Decreased | 8 (15) | 10 (19) | 18 (33) |
| White Blood Cell Count Decreased | 16 (30) | 2 (4) | 18 (33) |
| Dysgeusia | 17 (31) | 0 (0) | 17 (31) |
| Blood Creatine Phosphokinase Increased | 17 (31) | 0 (0) | 17 (31) |
| Vomiting | 13 (24) | 2 (4) | 15 (28) |
| Alopecia | 15 (28) | 0 (0) | 15 (28) |
| Hypertension | 7 (13) | 6 (11) | 13 (24) |
| Rash | 8 (15) | 3 (6) | 11 (20) |
| Alanine Aminotransferase Increased | 9 (17) | 1 (2) | 10 (19) |
| Blood Alkaline Phosphatase Increased | 8 (15) | 1 (2) | 9 (17) |
| Hypophosphatasemia | 3 (6) | 5 (9) | 8 (15) |
| Neuropathy Peripheral | 6 (11) | 2 (4) | 8 (15) |
| Pyrexia | 8 (15) | 0 (0) | 8 (15) |
| Pruritus | 7 (13) | 0 (0) | 7 (13) |
| Periorbital Edema | 7 (13) | 0 (0) | 7 (13) |
| Edema Peripheral | 6 (11) | 0 (0) | 6 (11) |
| Constipation | 6 (11) | 0 (0) | 6 (11) |
| Hypokalemia | 6 (11) | 0 (0) | 6 (11) |
Grade 3–4 AEs that were at least possibly related to study therapy were recorded in 38 patients (70%) with most of them representing hematologic toxicities. Grade 3–4 hematological toxicities occurring with a frequency ⩾10% were anemia (26%), neutropenia (22%), and decreased lymphocytes (19%). Grade 3–4 nonhematological toxicities occurring with a frequency ⩾10% were fatigue (15%) and hypertension (11%). Grade 4 AEs occurred in five patients (9%). One patient died due to sudden cardiac death (grade 5), which was assessed by the investigator as not related to either study drug. Treatment was discontinued in six (11%) patients due to AEs. Low subject numbers in most cohorts did not allow for between-cohort statistical comparison.
Elevated AST occurred in 19 (35%) patients, of which 14 (26%) were grade 1, 1 (2%) grade 2, and 4 (7%) grade 3. Elevated ALT occurred in 10 (19%) patients, of which 6 (11%) were grade 1, 3 (6%) grade 2, and 1 (2%) grade 3. Elevated CPK occurred in 17 (31%) patients, of which 12 (22%) were grade 1 and 5 (9%) grade 2. One patient had a grade 3 elevation of bilirubin. The elevation of transaminases and CPK is a class-specific AE associated with CSF1R inhibitors thought to be related to inhibition of Kupffer cell function in the liver, which is involved in clearance of these enzymes.20 Supplementary Tables S5 and S6 list toxicities that were assessed to be at least possibly related to either pexidartinib or paclitaxel, respectively, and occurred in at least 10% of patients.
Serious adverse events
Of the 54 patients in the safety population, 17 patients (31%) experienced 29 treatment-emergent serious adverse events (SAEs). SAEs occurred in 1 of 9 patients (11%) in cohort 1, 1 of 3 patients (33%) in cohort 2, 3 of 3 patients (100%) in cohort 3, 2 of 6 patients (33%) in cohort 4, and 10 of 33 patients (30%) in cohort 5 and part 2 (RP2D). Most types of SAEs were reported in a single patient. The only treatment-emergent SAEs reported by two or more patients overall were tooth infection [two patients (4%), one patient each in cohorts 3 and 4], pyrexia [two patients (4%), one patient each in cohorts 1 and 5], and febrile neutropenia (one patient in cohort 3 and cohort 5/part 2). Of the 17 patients with 29 SAEs, 12 had 17 SAEs considered related to one or both study drugs (2 were related to pexidartinib, 5 were related to paclitaxel, and 10 were related to both study drugs). SAEs considered to be related to study treatment included pyrexia, febrile neutropenia, tooth infection, cellulitis, abscess, puncture site infection, dehydration, nausea, supraventricular tachycardia, atrial fibrillation, clostridium difficile colitis, staphylococcal bacteremia, and blood bilirubin and blood transaminase elevation.
Dose limiting toxicities
As listed in Table 1, two patients had AEs that were DLTs: one in the 1200 mg Cohort (grade 3 hypophosphatemia) and one in the 1600 mg Cohort (grade 3 atrial fibrillation). However, expansion of the cohorts did not confirm DLT for these dose levels. No maximum tolerated dose was determined and dose escalation was stopped at 1600 mg/day because PK assessment indicated adequate exposure. Therefore, this dose was determined to be the RP2D for patients in part 2.
Pharmacokinetics
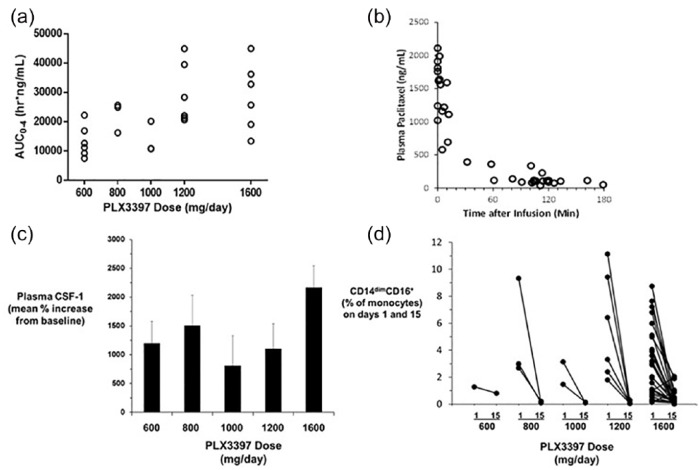
Average pexidartinib plasma concentration and exposure increased with increasing pexidartinib dose, and reached saturation at the 1200 mg dose (Figure 1a, Supplementary Table S7). Paclitaxel PK appeared to be nonlinear, and the plasma concentration decreased rapidly after infusion (Figure 1b).
Figure 1.
Summary of pharmacokinetic and pharmacodynamics analysis: (a) pexidartinib steady-state plasma exposure on day 15; (b) pacilitaxel plasma concentration after IV infusion; (c) plasma CSF-1 concentration; (d) CD14dim/16+ monocyte levels on days 1 and 15 of study treatment.
Plasma paclitaxel PK was consistent with reports for single agent paclitaxel, indicating no drug–drug interaction at steady-state pexidartinib levels (Figure 1b).15
Pharmacodynamic biomarkers
CSF-1 levels in plasma increased after pexidartinib treatment, possibly due to the slowing of the internalization of ligand with its receptor, which is thought to be the primary mechanism of plasma CSF-1 clearance or the compensatory increase in CSF-1 expression. All pexidartinib doses tested showed a robust average CSF-1 increase of from 800 to 2100% (Figure 1c).16
The percentage of CD14dim/CD16+ monocytes of total blood monocytes at baseline decreased by 57% to 100% after 2 weeks of pexidartinib treatment in nearly every patient, indicating blockade of CSF-1R signaling. This decrease was observed even at the 600 mg dose level (Figure 1d). The CD14dim/CD16+ monocyte subset is known to be sensitive to CSF-1 treatment, and thus serves as a pharmacodynamic marker of CSF-1R inhibition. Levels of CD14dim/CD16+ monocytes have also been correlated with increased tumor burden, and preliminary research has implicated these cells in tumor-induced suppression of host immune response and promotion of tumor invasiveness and angiogenesis.6
Antitumor activity
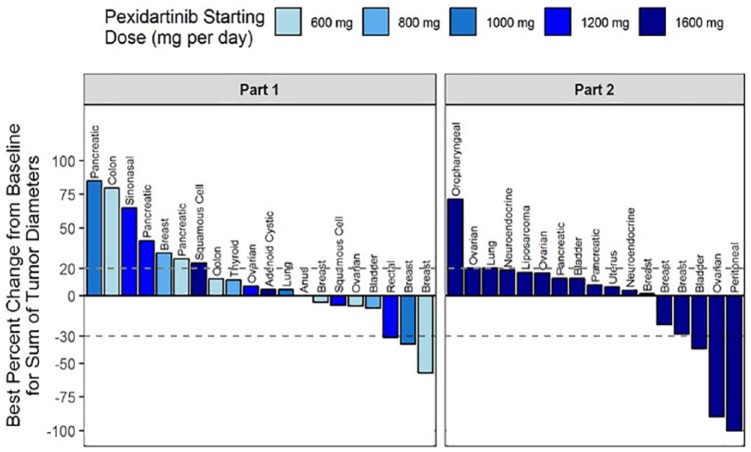
Table 4 provides summary of tumor response in 38 patients evaluable for efficacy. Overall, 3% of patients had complete response (CR), 13% had partial response (PR), 34% had stable disease, and 45% progressive disease as the best response to study therapy. The remaining 5% of patients could not be assessed or did not have confirmatory scans. Of the five patients who experienced partial response to study therapy, two had breast cancer, and the remaining three had rectal, bladder and ovarian cancers, respectively. One patient with peritoneal carcinoma had CR (see below). In addition, one patient with neuroblastoma harboring a missense mutation V32G in the csf-1r gene was on study therapy for approximately 48 weeks until disease progression developed. Figure 2 is a waterfall diagram that summarizes the maximum percentage change in the size of the target lesions for patients in parts 1 and 2. The swimmers plot depicting the duration of all 54 patients on study is shown in Supplementary Figure S1.
Table 4.
Summary of best overall tumor response in evaluable patients.
| Total (N = 38) |
|
|---|---|
| Complete Response | 1 (3%) |
| Partial Response | 5 (13%) |
| Stable Disease | 13 (34%) |
| Clinical Benefita | 19 (50%) |
| Progressive Disease | 17 (45%) |
| Unable to Assess | 2 (5%) |
| Not Evaluable | 0 |
Clinical Benefit = Complete Response + Partial Response + Stable Disease.
Figure 2.
Waterfall diagram of maximum percentage change in tumor size for patients in parts 1 and 2. Patients with at least one posttreatment radiographic assessment were included. Positive values indicate tumor growth, and negative values indicate tumor reduction. The upper and lower dashed lines depict thresholds defined in RECIST v1.1 for progressive disease and partial response, respectively.
Of the six patients with platinum-resistant or -refractory gynecologic malignancies (five with ovarian cancer and one with primary peritoneal carcinoma), one had a CR and one a PR with response durations of 189 and 94 days and progression free survival of 239 and 148 days, respectively. All these six patients had received a taxane prior to study enrollment.
Other clinical parameters of interest
No significant deterioration of ECOG PS was observed for the majority of patients between the start and end of study therapy. The percentage of patients with ECOG PS <2 at baseline was 83% (49/54), and the percentage of patients with ECOG PS <2 at the end of treatment was 79% (29/37 subjects who had ECOG assessment reported at the end or treatment visit). Three patients had a decline in ECOG PS to >1. At the end of treatment, 14% (5/37) had an ECOG PS of 0 (fully active), 65% (24/37) PS 1 (restricted), 16% (6/37) PS 2 (ambulatory), and 1 patient each (3%) PS of 3 and 4.
Discussion
Reprograming the tumor microenvironment as a potential strategy for reversing tumor-induced immune suppression has been gaining increasing interest and is supported by translational data.17 The CSF-1/CSF-1R axis is implicated in the homing of monocytes to the tumor microenvironments and their differentiation to become the M2 type of TAMs.3,6 M2 TAMs mediate immune suppression and neo-angiogenesis facilitating tumor growth and metastases.17,18 TAMs are associated with poorer patient survival, expression of immunosuppressive cytokines, and the switch to Th2 based immunity.14,19 In vivo studies in transgenic mouse model of mammary carcinoma demonstrated that TAMs increase within the tumor stroma after exposure to cytotoxic chemotherapy, and this increase mediates resistance to subsequent treatment. Inhibition of CSF-1R function not only leads to an increase in cytotoxic T lymphocytes, but is also associated with slower tumor growth, reduction in metastases, and improved survival of tumor-bearing animals treated with chemotherapy.12 This provides support for clinical development of CSF-1R inhibition in combination with cytotoxic chemotherapy. Here, we report a phase Ib clinical trial of CSF-1R inhibitor pexidartinib (PLX3397) in combination with weekly paclitaxel in patients with treatment refractory solid tumors. The study demonstrated an acceptable toxicity profile, with 89% of patients being able to maintain >80% relative dose intensity of pexidartinib and 74% of patients being able to receive at least 3 doses of paclitaxel therapy in each 28 day cycle beyond cycle 2. Fatigue (65% overall, 15% grade ⩾3), nausea (39%, 6% grade ⩾3), and diarrhea (39% overall, 7% grade ⩾3) represented the most common nonhematologic toxicities. Grade 3 anemia (59% overall, 26% grade ⩾3), lymphopenia (33% overall, 19% grade ⩾3), and neutropenia (43% overall, 22% grade ⩾3) were the most common hematologic toxicities. Despite high frequency of these events, most of these were easily manageable with supportive care and dose modifications of pexidartinib (which occurred in 26% of patients), or temporarily withholding paclitaxel (occurring in 39% of patients). The most frequent reason for discontinuation of study therapy was disease progression with only six (11%) patients discontinuing study therapy due to adverse events.
Grade 1 or 2 increases in serum transaminases have been common with pexidartinib and other CSF-1R inhibitors with grade 3 or higher increases occurring less frequently. It has been hypothesized that reversible elevations in transaminases as well as lactate dehydrogenase (LDH) and creatinine kinase in patients receiving CSF1R inhibitors may be the result of inhibition of Kupffer cells, which are resident macrophages present in the sinusoids within the liver and which express CSF1R.20 Kupffer cells function as part of the mononuclear phagocytic system.21 They also use pinocytosis and receptor-mediated phagocytosis to filter blood of particulate matter including several enzymes such as aspartate aminotransferase and creatinine kinase.22 Animal studies demonstrated that neutralizing CSF1 or treatment with clodronate (known to be toxic to macrophages) results in decreased level of Kupffer cells and elevation in ALT, AST, CK, and LDH enzymes in the circulation.20 Furthermore, knockout mice for CSF1 (CSF1op/op/CSF1op/op), also known as osteopetrotic mice, have fewer Kupffer cells and higher levels of the above enzymes compared with wild-type littermates.
Pexidartinib may also have a more direct hepatotoxic effect. Elevations of liver transaminases and bilirubin have been observed in most studies with single agent or combination pexidartinib regimens. Based on these studies, there is a small subset of patients who have drug-induced liver injury. Possible drug-induced liver injury occurred in three patients receiving combination therapy of pexidartinib with vemurafenib (confirmed by liver biopsy in one patient). In each of these patients, the onset of liver abnormalities was noted within the first cycle of treatment. Pexidartinib was withheld for each patient, and over the following weeks, further increases were seen, predominantly in bilirubin and alkaline phosphatase. In one of these patients, liver biopsy showed changes in the biliary system with a paucity of bile ducts. Despite discontinuation of pexidartinib, bilirubin continued to increase and was associated with pruritus in two patients. In two patients, bilirubin and aminotransferases decreased significantly to nearly normal over the following 2–3 months. More data is needed to clarify the rate and contribution of the study drug to liver injury and investigate possible mechanism by which this occurs. Given this experience, future studies with pexidartinib will require careful patient selection, close monitoring of liver function with aggressive discontinuation parameters for patients who develop transaminase and bilirubin elevations and avoidance of other potentially hepatotoxic agents.
It should be noted that pexidartinib treatment leads to rapid decline in CD14dim/CD16+ monocytes in the peripheral blood, with near complete reduction of their levels even with doses as low as 800 mg daily. There was also concurrent increase in CSF1 levels in all patients. These results support the use of circulating CD14dim/CD16+ monocytes and CSF1 levels as suitable pharmacodynamic markers that may be useful in determining target occupancy and biologic effects of pexidartinib.
This study has some limitations. Firstly, tumor biopsies were not collected, and therefore we were unable to measure the effect of study therapy on tissue levels of TAMs, tumor infiltrating lymphocytes (favorable prognostic factor in some cancers), and other immune cell subsets in the tumor microenvironment. Future studies should incorporate paired tumor biopsies in order to learn whether combination of pexidartinib and paclitaxel results in favorable changes in the tumor microenvironment and whether that correlates with response. Secondly, the study had insufficient patient number to compare safety and efficacy of study therapy with historical control patients that had specific tumor types and received paclitaxel. This is a limitation of most solid tumor phase I trials, which aim at establishing the safety profile and a RP2D. Thirdly, the study enrolled patients who had history of multiple prior lines of therapy (range 1–8). Therefore, an objective response rate of 16% and clinical benefit rate of 50% is not disappointing. Future studies may limit enrollment to patient populations with restricted number of prior therapies and focus on specific tumor types.
Other inhibitors of CSF-1R signaling are also in clinical development, such as small molecule inhibitors ARRY-382, PLX7486, BLZ945, and JNJ-40346527, as well as monoclonal antibodies such as LY3022855, emactuzumab, AMG820, cabiralizumab, MCS110, and PD-0360324 (the latter two are inhibitors of CSF1). However, pexidartinib is among the CSF-1R inhibitors with the most extensive clinical development programs. Pexidartinib is studied in glioblastoma multiforme, c-kit mutated melanoma, prostate cancer, neurofibroma, sarcoma, lymphoma, and leukemias. With the exception of patients with pigmented villonodular synovitis (PVNS) or tenosynovial giant cell tumors (TGCT, see below), single agent activity of pexidartinib is very modest. Therefore, CSF-1R inhibitors will likely provide the most promise in rational combinations with other agents. Preclinical data supports concurrent use of pexidartinib with chemotherapy, radiation therapy, and immune therapy among other combinations.14,23–26 The current study found that pexidartinib given concurrently with paclitaxel resulted in a CR rate of 3% and a PR of 13%. Responses were seen in patients with breast, rectal, bladder, primary peritoneal, and ovarian cancers. The most promising signal of clinical activity was noted in six patients with platinum-resistant or -refractory epithelial gynecologic malignancies, with one patient experiencing a CR and one having a PR with responses lasting for 189 and 94 days, respectively. This led to incorporating part 3 of the study that tested pexidartinib and paclitaxel combination in patients with platinum resistant and refractory ovarian cancer, and which will be reported separately.
One exception where therapy with CSF-1R inhibitor alone could result in meaningful clinical benefit is in the case of PVNS or TGCT; a rare benign proliferative disease involving large joints. The majority of cases harbor chromosomal translocations involving the gene encoding for CSF-1 (located on chromosome 1p13) and resulting in high expression of this cytokine by cells within the synovium. This leads to substantial recruitment of the mononuclear and multi-nucleated cells that form the bulk of the mass.27,28 Cassier and colleagues reported promising clinical activity of anti CSF1R antibody emactuzumab, with CR and PR rates of 7% and 79%, respectively, in 28 patients with PVNS; 11% of patients had prolonged stable disease, and no patient had disease progression.29 In a phase II dose expansion cohort enrolling patients with PVNS/TGCT, pexidartinib at a dose of 1000 mg daily produced PRs in 7 of 12 patients that occurred within the first 4 months of treatment and lasted more than 8 months. Median PFS was not reached at the time the results were published.30 Given such promising results, a phase III study testing treatment with pexidartinib or placebo in patients in PVNS was launched (ClinicalTrials.gov identifier: NCT02371369).
In conclusion, the combination of pexidartinib and weekly paclitaxel was generally well-tolerated. No maximum tolerated dose was reached and RP2D for pexidartinib in combination with weekly paclitaxel was 1600 mg/day. Biomarker levels suggested that pexidartinib blocked CSF-1R signaling, indicating a potential for significantly mitigating macrophage tumor infiltration.
Supplemental Material
Supplemental material, Supplementary_Figure_1 for Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors by Robert Wesolowski, Neelesh Sharma, Laura Reebel, Mary Beth Rodal, Alexandra Peck, Brian L. West, Adhirai Marimuthu, Paul Severson, David A. Karlin, Afshin Dowlati, Mai H. Le, Lisa M. Coussens and Hope S. Rugo in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplementary_Tables for Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors by Robert Wesolowski, Neelesh Sharma, Laura Reebel, Mary Beth Rodal, Alexandra Peck, Brian L. West, Adhirai Marimuthu, Paul Severson, David A. Karlin, Afshin Dowlati, Mai H. Le, Lisa M. Coussens and Hope S. Rugo in Therapeutic Advances in Medical Oncology
Footnotes
Author Note: Neelesh Sharma is now a Senior Medical Director of Immuno-Oncology at Bayer in East Hanover, New Jersey.
Funding: Plexxikon, Inc.
Conflict of interest statement: All authors declare that Plexxikon, Inc. provided financial support for the conduct of this clinical trial. No other conflicts of interest were reported. Abby Klein, BS Pharm, Consultant Medical Writer for Plexxikon Inc. (a professional medical writer paid by the sponsor) provided editorial assistance.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Robert Wesolowski, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, 1800 Cannon Dr 1250 Lincoln Tower Columbus, OH, 43210, USA.
Neelesh Sharma, Case Western Reserve University, Cleveland, OH, USA.
Laura Reebel, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Mary Beth Rodal, Case Western Reserve University, Cleveland, OH, USA.
Alexandra Peck, University of California San Francisco, CA, USA.
Brian L. West, Plexxikon Inc. Berkeley, CA, USA
Adhirai Marimuthu, Plexxikon Inc. Berkeley, CA, USA.
Paul Severson, Plexxikon Inc. Berkeley, CA, USA.
David A. Karlin, Plexxikon Inc. Berkeley, CA, USA
Afshin Dowlati, Case Western Reserve University, Cleveland, OH, USA.
Mai H. Le, Plexxikon Inc. Berkeley, CA, USA
Lisa M. Coussens, Oregon Health and Sciences University, Portland, OR, USA
Hope S. Rugo, University of California San Francisco, CA, USA
References
- 1. Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med 1995; 27: 79–85. [DOI] [PubMed] [Google Scholar]
- 2. Sasmono RT, Oceandy D, Pollard JW, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 2003; 101: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 3. Bourette RP, Rohrschneider LR. Early events in M-CSF receptor signaling. Growth Factors 2000; 17: 155–166. [DOI] [PubMed] [Google Scholar]
- 4. Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 2004; 14: 628–638. [DOI] [PubMed] [Google Scholar]
- 5. Lin H, Lee E, Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008; 320: 807–811. [DOI] [PubMed] [Google Scholar]
- 6. Mathsyaraja H, Thies K, Taffany DA, et al. CSF1-ETS2-induced microRNA in myeloid cells promote metastatic tumor growth. Oncogene 2015; 34: 3651–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamimi RM, Brugge JS, Freedman ML, et al. Circulating colony stimulating factor-1 and breast cancer risk. Cancer Res 2008; 68: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scholl SM, Pallud C, Beuvon F, et al. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J Natl Cancer Inst 1994; 86: 120–126. [DOI] [PubMed] [Google Scholar]
- 9. Menke J, Kriegsmann J, Schimanski CC, et al. Autocrine CSF-1 and CSF-1 receptor coexpression promotes renal cell carcinoma growth. Cancer Res 2012; 72: 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espinosa I, Beck AH, Lee CH, et al. Coordinate expression of colony-stimulating factor-1 and colony-stimulating factor-1-related proteins is associated with poor prognosis in gynecological and nongynecological leiomyosarcoma. Am J Pathol 2009; 174: 2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chambers SK, Kacinski BM, Ivins CM, et al. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res 1997; 3: 999–1007. [PubMed] [Google Scholar]
- 12. DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Gamal MI, Al-Ameen SK, Al-Koumi DM, et al. Recent advances of colony-stimulating factor-1 receptor (CSF-1R) kinase and its inhibitors. J Med Chem 2018; 61: 5450–5466. [DOI] [PubMed] [Google Scholar]
- 14. Cannarile MA, Weisser M, Jacob W, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawate S, Takeyoshi I, Morishita Y. Pharmacokinetics of paclitaxel in a hemodialysis patient with advanced gastric cancer: a case report. World J Gastroenterol 2006; 12: 5237–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartocci A, Mastrogiannis DS, Migliorati G, et al. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci USA 1987; 84: 6179–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy 2017; 9: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedersen MB, Danielsen AV, Hamilton-Dutoit SJ, et al. High intratumoral macrophage content is an adverse prognostic feature in anaplastic large cell lymphoma. Histopathology 2014; 65: 490–500. [DOI] [PubMed] [Google Scholar]
- 20. Radi ZA, Koza-Taylor PH, Bell RR, et al. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol 2011; 179: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toth CA, Thomas P. Liver endocytosis and Kupffer cells. Hepatology 1992; 16: 255–266. [DOI] [PubMed] [Google Scholar]
- 22. Naito M, Hasegawa G, Ebe Y, et al. Differentiation and function of Kupffer cells. Med Electron Microsc 2004; 37: 16–28. [DOI] [PubMed] [Google Scholar]
- 23. Butowski, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 2016; 18: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 2013; 73: 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014; 74: 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruffell B, Chang-Strachan D, Chan V, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014; 26: 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cupp JS, Miller MA, Montgomery KD, et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol 2007; 31: 970–976. [DOI] [PubMed] [Google Scholar]
- 28. West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci USA 2006; 103: 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cassier PA, Italiano A, Gomez-Roca CA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol 2015; 16: 949–956. [DOI] [PubMed] [Google Scholar]
- 30. Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 2015; 373: 428–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_1 for Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors by Robert Wesolowski, Neelesh Sharma, Laura Reebel, Mary Beth Rodal, Alexandra Peck, Brian L. West, Adhirai Marimuthu, Paul Severson, David A. Karlin, Afshin Dowlati, Mai H. Le, Lisa M. Coussens and Hope S. Rugo in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Tables for Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors by Robert Wesolowski, Neelesh Sharma, Laura Reebel, Mary Beth Rodal, Alexandra Peck, Brian L. West, Adhirai Marimuthu, Paul Severson, David A. Karlin, Afshin Dowlati, Mai H. Le, Lisa M. Coussens and Hope S. Rugo in Therapeutic Advances in Medical Oncology