Short abstract
Chronic pain is a significant unmet medical problem. Current research regarding sodium channel function in pathological pain is advancing with the hope that it will enable the development of isoform-specific sodium channel blockers, a promising treatment for chronic pain. Before advancements in the pharmacological field, an elucidation of the roles of Nav1.7 and Nav1.8 in the pathophysiology of pain states is required. Thus, the aim of this report is to present what is currently known about the contributions of these sodium channel subtypes in the pathophysiology of neuropathic and inflammatory pain. The electrophysiological properties and localisation of sodium channel isoforms is discussed. Research concerning the genetic links of Nav1.7 and Nav1.8 in acquired neuropathic and inflammatory pain states from the scientific literature in this field is reported. The role of Nav1.7 and Nav1.8 in the generation and maintenance of abnormal neuronal electrogenesis and hyperexcitability highlights the importance of these channels in the development of pathological pain. However, further research in this area is required to fully elucidate the roles of Nav1.7 and Nav1.8 in the pathophysiology of pain for the development of subtype-specific sodium channel blockers.
Keywords: Nav1.7, Nav1.8, sodium channel, neuropathic pain, inflammatory pain, voltage-gated sodium channels, dorsal root ganglion, nociceptors, hyperexcitability
Introduction
Nociception is a physiological process involving the activation of neuronal signalling that is essential for the perception of pain. Whilst nociception is important for survival as it warns of any damaging or potentially harmful stimuli, pathological pain is not and can be extremely debilitating if it persists. Pathological pain includes nerve injury-triggered neuropathic and tissue injury-triggered inflammatory pain states, which can become chronic and unresponsive to treatment with conventional analgesics.1 The development and maintenance of these pain states involves dynamic plastic changes consisting of peripheral sensitisation (involving peripheral nociceptive neurons) and central sensitisation (involving dorsal horn and higher order central neurons), with peripheral sensitisation essential for central sensitisation, necessary for the maintenance of chronic inflammatory and neuropathic pain states. In 1974, Wall et al.2 determined that nerve injury induced a brief burst of action potentials (APs) and later it was demonstrated that following a longer interval, persistent hyperexcitability could manifest in axons of injured neurons.3 At the time, it was thought that sodium channels expressed in these axons were likely responsible for the development of abnormal neuronal electrogenesis. Decades later, molecular cloning of voltage-gated sodium channels (VGSCs) confirmed a significant role of these channels in regulating neuronal excitability in normal and pathological pain states. It is now known that the Nav1 VGSC family consists of nine members, Nav1.1–1.9 encoded by the SCN1A-SCN5A and SCN8A-SCN11A genes. The expression of these sodium channel isoforms is spatially and temporally regulated, and they possess distinct electrophysiological properties. Nav1.1, Nav1.5, Nav1.6, Nav1.7, Nav1.8 and Nav1.9 are expressed in dorsal root ganglion (DRG) neurons. Among these channel subtypes, Nav1.7 (preferentially expressed in DRG neurons), Nav1.8 and Nav1.9 (selectively expressed in DRG neurons) which are highly expressed in nociceptors and Nav1.3, which is upregulated in nociceptive neurons following injury, have been the centre of research aiming to uncover the roles of these channels in the development and maintenance of chronic pain, with the hope that these channel isoforms will make promising targets for the pharmacological treatment of pathological pain states.1 Current treatments for chronic inflammatory and neuropathic pain are not very effective and cause unwanted side effects Therefore, the development of subtype-specific sodium channel blockers may yield a more successful therapeutic outcome. Nav1.7 due to its genetic links to pathological pain and Nav1.8 as a result of its sensory neuron specificity have been focused on in particular as important in the pathophysiology of pain.4 Before the development of isoform-specific sodium channel blockers, it is important to fully elucidate the mechanisms underlying the contributions of these sodium channel isoforms in the induction and maintenance of pathological pain states. The aim of this report is to discuss current understanding of the likely roles of Nav1.7 and Nav1.8 in the pathophysiology of inherited and acquired pain, as lack of knowledge in this field is a major barrier for the development of more precise and effective analgesic treatments. The first part of this report will discuss the structure and function of VGSCs in general, followed by the biophysical properties and expression of Nav1.7 and Nav1.8, followed by how Nav1.7 and Nav1.8 may contribute in the pathophysiology of neuropathic and inflammatory pain states based on current literature.
Structure and function of VGSCs
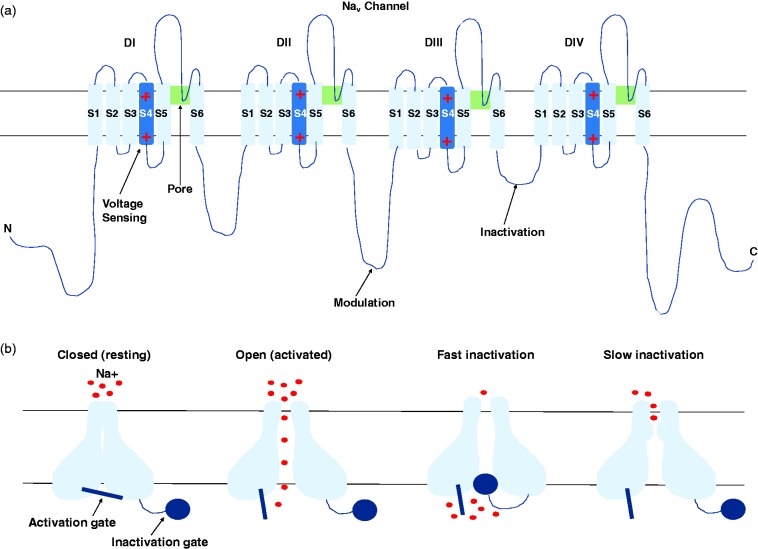
VGSCs are transmembrane proteins important in the generation and conduction of APs in response to supra-threshold stimuli in excitable cells. A large pore-forming α-subunit and one or two smaller β-subunits are the essential components of a VGSC. The α-subunit is arranged into four homologous domains (DI–DIV), each with six membrane-spanning segments (S1–S6, Figure 1(a)).5 The sodium channel ion-conducting pore is formed by the P-loop region between the helical segments S5 and S6 from each of the repeated domains, which are closely assembled at the centre of the quaternary structure.5 VGSCs have distinct states, which consist of the resting closed state, activated open state and the inactivated closed state (Figure 1(b)). The S4 segments (in each of the four domains) possess multiple positively charged amino acid residues, and these are able to ‘sense’ changes in voltage across the membrane upon opening of the channel as a result of depolarisation of the cell. Above a critical threshold, positively charged residues of the S4 segments are displaced outwards to a position nearer to the extracellular surface of the cell membrane, which triggers a series of conformational changes resulting in channel activation.5,6 The current that subsequently passes through the channel pore constitutes the upstroke (depolarising phase) of the AP. For more detailed information regarding the structure and function of VGSCs, refer to the following comprehensive reviews.5,6
Figure 1.
The structure and states of Nav channels. (a) The secondary structure of the α-subunit of VGSCs. The pore forming α-subunit is arranged into four domains (DI-DIV) each with six transmembrane alpha helices (S1–S6). The S4 segments are voltage sensors containing positively charged ions. The intracellular loop between DIII and DIV is believed to be the fast inactivation gate. (b) The three distinct states of VGSCs, with the inactivated closed state present with either fast inactivation (within milliseconds) or slow inactivation (seconds) kinetics, which differs among VGSC isoforms.
Electrophysiological properties of Nav1.7 and Nav1.8
Nav1.7 and Nav1.8 channels differ with respect to their kinetic and voltage-dependent properties and their sensitivity to the sodium channel blocker tetrodotoxin (TTX). These differences allow the Nav1.7 and Nav1.8 channels to produce distinct sodium currents and contribute to the electrogenic properties of neurons under normal and pathogenic conditions in specific ways. The Nav1.7 channel is sensitive to block by nanomolar concentrations of TTX (TTX-S), while the Nav1.8 channel is resistant to concentrations of TTX that are 100–1000 folds greater (TTX-R). Using HEK293 cells transiently transfected with only an expression plasmid containing the Nav1.7 α-subunit sequence or in conjunction with another expression plasmid containing the β1-subunit sequence, Klugbauer et al.7 were able to determine the electrophysiological properties of Nav1.7. Using whole-cell patch clamp techniques, the researchers found that the α-subunit gave rise to activating and inactivating inward currents with fast kinetics, which were rapidly blocked by TTX in a reversible manner. Nav1.7 possesses slow repriming kinetics in contrast to other channel isoforms and exhibits a slow development of closed-state inactivation.8 The slow closed-state inactivation of Nav1.7 allows the channel to respond to small, slow depolarisations by producing a ramp current. In accordance with this, Nav1.7 channels have been found to be deployed at nociceptor nerve terminals9 where generator potentials occur in response to stimulation of the sensory nerve endings. Therefore, the presence of Nav1.7 here may serve the purpose of amplifying generator potentials. Thus, Nav1.7 is thought to act as a threshold channel, setting the gain in nociceptors.10
On the other hand, Nav1.8 channels expressed in Xenopus oocytes display a slow activating and inactivating TTX-R current.11 In addition, Nav1.8 channels recover rapidly from inactivation and have a more depolarised voltage-dependency of activation and inactivation compared with other sodium channel isoforms. Using whole-cell patch clamp recordings, Dib-Hajj et al.12 found these properties of the Nav1.8 channel also exist in human DRG neurons, affirming that previous results obtained with Nav1.8-expressing oocytes are relevant to human nociceptors. The biophysical characteristics of the Nav1.8 channel highlight its important contribution to repetitive firing and neuronal excitability.
In contrast to Nav1.7 channels, which may play a role as threshold channels in peripheral sensory neurons, Nav1.8 channels have been found to carry most of the sodium current responsible for the rising phase of the AP.13 The authors13 used the AP clamp technique on individual small-diameter rat DRG neurons with similar electrophysiological characteristics as nociceptors. To isolate the TTX-R sodium current, sodium ions were substituted with the impermeable cation, N-methyl-D-glucamine, in a physiological solution containing the neurons. In addition, 300 nm of TTX was also added to the solution to block the TTX-S currents. The researchers13 determined that the TTX-R current contributes most of the sodium current throughout the duration of the AP, especially during the upstroke of the AP. Although both the TTX-R channels Nav1.8 and Nav1.9 are expressed in DRG neurons, Blair and Bean13 determined that Nav1.8 carries most of the TTX-R sodium current due to the kinetics of activation and inactivation and the more depolarised voltage-dependent properties observed, which matched those identified of heterologously expressed Nav1.8 channels.11 Thus, the modulation of Nav1.8 could have a significant impact on the excitability of neurons.
The different biophysical properties of Nav1.7 and Nav1.8 enable these sodium channels to make specific contributions to neuronal electrogenesis in normal and pathological conditions.
Tissue and subcellular distribution of Nav1.7 and Nav1.8
Nociceptive neurons express both TTX-R and TTX-S currents, which together significantly contribute to shaping the APs of these neurons. Histochemical methods have shown that the TTX-S α-subunit Nav1.7 is preferentially expressed in the peripheral nervous system. In particular, Nav1.7 is expressed at high levels in sensory neurons of the DRG, in sympathetic ganglion neurons and in trigeminal ganglion neurons.9 Nav1.7 deposition at nerve terminals may be associated with its poised role as a threshold channel. Nav1.8 is a sensory neuron-specific channel with preferential expression in the DRG and trigeminal ganglion neurons.14 The biophysical properties and high expression of Nav1.7 and Nav1.8 channels in nociceptors, their distinct contributions to neuronal firing and their deployment at sensory nerve endings, where nociception is initiated, indicate the crucial roles that these channel isoforms play in determining the excitability of nociceptors, emphasising their importance in normal pain-signalling. Thus, the dysregulation of Nav1.7 and Nav1.8 channels can significantly influence the electrogenic properties of neurons, resulting in neuronal hyperexcitability and leading to the development of chronic pain states such as neuropathic and inflammatory pain.
Neuropathic pain
Neuropathic pain is defined as ‘pain arising as direct consequence of a lesion or disease affecting the somatosensory system’ by the International Association for the Study of Pain. It is characterised by pain in the region of sensory abnormality, which typically presents with hypersensitivity to various stimuli and in some cases allodynia. In its most usual form, neuropathic pain occurs following disease or nerve injury involving peripheral nerves, which results in demyelination and axonopathy.15 A lesion within the nervous system causes plastic changes that may occur at any point along the neuraxis. Chronic neuropathic pain is a result of a maladaptive manifestation of this plasticity.16 As a result of nerve injury, affected neurons undergo membrane remodelling and become hyperexcitable due to a shift in the pain threshold. Ectopic electrogenesis occurs when afferent excitability is deregulated as a result of nerve injury, which leads to spontaneous ectopic activity, reductions in the threshold for nociceptor activation and a heightened response to suprathreshold stimuli.15 Persistent ectopic firing in primary afferent neurons results in a persistent drive from the periphery essential for the development and maintenance of central sensitisation, which is essential for the development and maintenance of chronic neuropathic pain that is characterised by an increase in the excitability of central neurons, receptive field expansions, pain perception in response to activation of low threshold mechanoreceptive Aβ neurons (believed to give rise to allodynia) and the recruitment of non-nociceptive neurons.16 As discussed in the following sections, Nav1.7 and Nav1.8 channel expression in sensory neurons is considerably altered following peripheral nerve transection and in animal models of neuropathy. It is widely believed that the neuropathic changes that cause spontaneous abnormal activity in peripheral neurons involve subtype-specific alterations in the density, distribution and functions including changes in the kinetic properties of VGSCs. The following sections will be looking into current literature and evidence behind such changes involving Nav1.7 and Nav1.8 channels.
Nav1.7
The role of Nav1.7 in neuropathic pain is still unclear with many contradicting studies. It thus remains questionable whether Nav1.7 does contribute in the development of neuropathic pain. It is generally believed that upregulation of specific subtypes of TTX-S sodium channels contributes significantly in the development of ectopic discharges in sensory neurons. However, reduced Nav1.7 messenger RNA (mRNA) levels have been reported following axotomy in spinal ligation (SNL) and spared nerve injury (SNI) animal models of neuropathic pain, suggesting Nav1.7 is not important in the development of ectopic discharges in experimental models of neuropathic pain.17 The downregulation of Nav1.7 in injured neurons is consistent with the transition of TTX-S currents in rat DRG neurons from slow-repriming to rapid-repriming.18
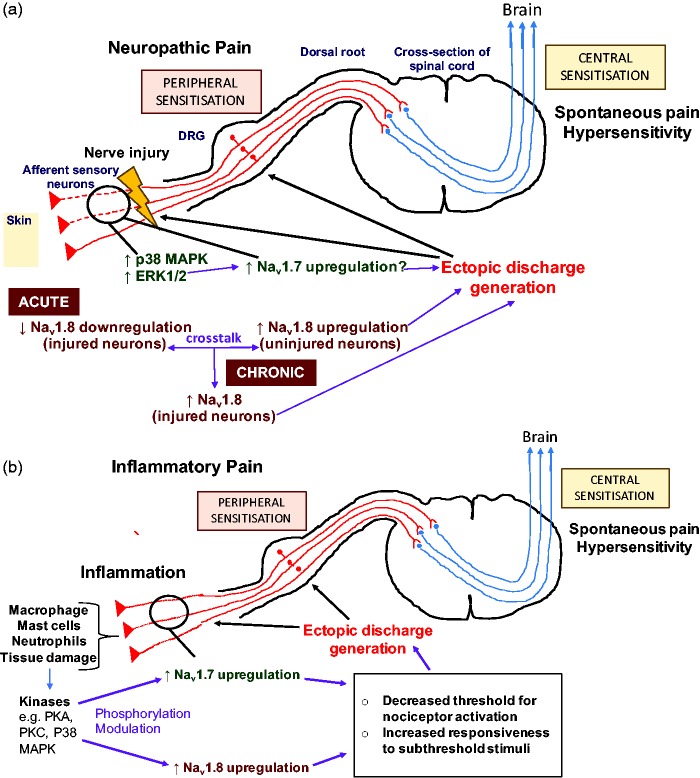
On the other hand, Black et al.19 observed that Nav1.7 was upregulated in blind-ending axons of painful human neuromas compared with control tissue obtained more proximally from the same nerve. In addition, levels of activated p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK1/2) were increased in these blind-ending axons. Suggesting that modulation of Nav1.7 by activated MAPKs may be important in the development of ectopic discharges in nociceptors and contribute to neuroma associated pain in humans.19 Using cultured DRG neurons, Stamboulian et al.20 demonstrated that inhibition of ERK1/2 caused a depolarising shift in the voltage-dependence of Nav1.7 activation and steady-state fast inactivation. This suggests that in the presence of ERK1/2, the opposite may occur, where phosphorylation of Nav1.7 by the protein kinase may lead to a hyperpolarising shift in the voltage-dependence of Nav1.7 activation and AP generation. Thus, modulation of Nav1.7 by ERK1/2 may contribute to the channels ability to amplify subthreshold inputs by lowering the voltage-dependence of Nav1.7 channel activation, leading to increased neuronal responsiveness and ectopic discharge. This would ultimately lead to an increase in the output from peripheral nociceptive neurons and thus contribute to the development and maintenance of central sensitisation and chronic neuropathic pain. This may therefore demonstrate the role of Nav1.7 in acquired painful peripheral neuropathy (Figure 2(a)). The discrepancy between the role of Nav1.7 in animal models of neuropathic pain and in human neuropathic pain, with regard to upregulation or downregulation of Nav1.7 expression and activity17,19 is not well understood and may be due to a lack in the predictive validity of experimental models resulting in poor translation of results from rodents to humans. Moreover, different splice variants of Nav1.7 exist, which may have slight functional differences1 and species differences in the relative amounts and types of Nav1.7 isoforms expressed in nociceptors may contribute to the discrepancies observed.
Figure 2.
Proposed mechanisms of the role of Nav1.7 and Nav1.8 in the pathophysiology of neuropathic pain and inflammatory pain. (a) Nerve injury leads to an increased number of kinases such as p38 MAPK and ERK1/2. This leads to the modulation of Nav1.7 channels which are upregulated as a result of nerve injury. This contributes to increased generation of ectopic discharge. In the acute phase following nerve damage, Nav1.8 is downregulated in injured neurons and upregulated in neighbouring uninjured neurons, which contributes to increased spontaneous discharge. In the chronic phase via a form crosstalk between injured and intact neurons Nav1.8 is also upregulated in injured neurons resulting in a further increase and maintenance of the ectopic discharge. This leads to spontaneous pain and hypersensitivity. (b) Inflammatory cells and mediators are present at increased numbers at the site of tissue injury and inflammation. This results in an increased number of kinases that phosphorylate and modulate the Nav1.7 and Nav1.8 channels, which are upregulated in nociceptors innervating the damaged tissue. This leads to an increase in ectopic action potentials. Together, these mechanisms result in spontaneous pain and hypersensitivity, that is, hyperalgesia and allodynia. PKA: protein kinase A; PKC: protein kinase C; ERK: extracellular signal-regulated kinase; MAPK: mitogen-activated protein kinase; DRG: dorsal root ganglion.
Interestingly, although research suggests certain types of neuropathic and inflammatory pain states are dependent on Nav1.7, there are some Nav1.7 independent pain states that have been discovered in mice and humans.21 Pain caused by bone cancer and the chemotherapeutic agent oxaliplatin have been found to not be dependent on the presence of Nav1.7 and Nav1.8, occurring in the absence of these VGSCs in mice.22 Furthermore, a recent human case report of a female with the autosomal recessive loss-of-function mutation in the SCN9A gene causing congenital insensitivity to pain (CIP) found that despite the condition, symptoms and signs of neuropathic pain were still evident.23 This suggests that certain neuropathic pain states are not dependent on the presence of Nav1.7, highlighting the multitude and complexity of mechanisms involved in the development and maintenance of chronic pain. These findings give support to the argument for the use of polypharmacy in treating chronic pain states and may explain the lack of analgesic success from on-going studies into a range of Nav1.7-specific inhibitors.
Nav1.8
The specific contributions of Nav1.8 to neuropathic pain are not as clear as its role in inflammatory pain. Lai et al.24 developed antisense oligonucleotides (AS ODNs) complimentary to Nav1.8 mRNA, intrathecal administration of these AS ODNs to rodents caused a selective ‘knockdown’ of Nav1.8 expression, as evidenced by decreased Nav1.8 immunoreactivity in Western blot analysis of DRG extracts from AS ODN-treated rats compared to mismatch ODN- and saline-treated rats. Voltage-clamp recordings revealed that the reduction of the Nav1.8 protein correlated with a significant reduction in the TTX-R sodium current attributed to the Nav1.8 channel. Importantly, the researchers24 found that reduction in Nav1.8 expression reversed neuropathic pain behaviour, that is, hyperalgesia and tactile allodynia induced by L5/L6 SNL. This indicates that Nav1.8 is involved in neuropathic pain and that the Nav1.8 sodium current is important in the development of hyperalgesia and allodynia, the behavioural manifestations of chronic neuropathic pain.
It seems unlikely that the role of Nav1.8 in neuropathic pain is mediated via injured nerves as Dib-Hajj et al.25 found that Nav1.8 expression was significantly reduced along with the slowly inactivating current that belongs to this channel in injured DRG neurons of a transected peripheral nerve. Instead, Nav1.8 appears to contribute to neuropathic pain development via its upregulation in adjacent uninjured sensitised neurons.25 The SNL model of neuropathic pain allows both injured and uninjured neurons from an axotomised nerve to be investigated. Gold et al.26 carried out an L5 SNL in rats, causing injury to all the neurons of the L5 DRG, while all the neurons of the L4 DRG remained intact. Immunohistochemical analysis using antibodies against Nav1.8 following an intrathecal injection of AS ODN showed that there was a significant redistribution of Nav1.8 channels to uninjured axons neighbouring injured axons in the sciatic nerve, which was accompanied by an increase in the Nav1.8 TTX-R sodium current. These effects were not observed in the sciatic nerve of control rats receiving mismatch ODN treatment. The researchers observed around 8% of the C-wave (reflecting AP conduction in unmyelinated C fibres) of the compound action potential in the sciatic nerve of sham-operated rats following a 5-min exposure to TTX, whereas around 40% of the C-wave was present in the sciatic nerve of ligated rats following exposure to TTX for the same amount of time. Highlighting the increased TTX-R current in uninjured C-fibres following SNL. The authors also observed a small rise in the TTX-R component of the A-wave that was slowly conducting, indicating that part of the increased immunostaining for Nav1.8 in the sciatic nerve is due to an increase in functional Nav1.8 channels in uninjured thinly myelinated Aδ neurons.26 The rapid-repriming kinetics of Nav1.8 enables this sodium channel isoform to support repetitive AP firing, and this is consistent with observations of spontaneous ectopic activity in uninjured C-fibres following SNL.27 Thus Nav1.8 seems to be involved in the generation of neuropathic pain via its upregulation in uninjured sensitised nociceptive fibres neighbouring injured neurons within a damaged nerve, the associated increase in the TTX-R sodium current in these neurons could lead to increased AP firing as Nav1.8 has been determined to carry most of the sodium current responsible for the AP,13 leading to enhanced electrogenesis and hyperexcitability of uninjured nociceptors. The increased excitability of uninjured nociceptors would lead to an increase in the peripheral input contributing to the development of central sensitisation and thus chronic neuropathic pain. This may therefore be the mechanism underlying the role of Nav1.8 in the generation of hyperalgesia and allodynia following nerve injury.24
Interestingly, it has been proposed that the way in which Nav1.8 channels contribute to neuropathic pain may change overtime after nerve injury.28 Coward et al.28 found that in the first couple of weeks after SNI, Nav1.8 seemed to be involved in neuropathic pain via its upregulation in intact nociceptors leading to an enhancement in the excitability of these uninjured afferent fibres. However, overtime, the role of Nav1.8 channels in neuropathic pain appeared to be mediated via its upregulation in injured nociceptors. Therefore, there may be a time-dependent shift in the mechanism underlying Nav1.8 action in the pathophysiology of neuropathic pain. The time-dependent changes in Nav1.8 function may involve some form of cross-talk between injured and non-injured neurons. The exact mechanism of this is unknown but may be mediated by products of Wallerian degeneration.27 Thus, Nav1.8 may contribute to the pathophysiology of acute and chronic neuropathic pain through somewhat different mechanisms, where the importance of its role shifts from uninjured to injured neurons overtime (Figure 2(a)).
Studies into the potential analgesic effects of targeting Nav1.8 channels have predominantly been translated from mice models of pain. This is due to the absence of loss-of-function mutations in the SCN10A gene, which has not yet been described in humans.21 In contrast, many gain-of function mutations affecting the SCN10A gene have been reported in literature, reinforcing the role of Nav1.8 in nociceptive mechanisms in humans.21 Several gain-of-function mutations of SCN10A have been identified.29 These contribute to painful peripheral neuropathy through increasing the Nav1.8 channel response to depolarisations resulting in the maintenance of neuronal hyperexcitability at the level of the DRG.29 This supports the role of Nav1.8 in the pathophysiology of specific neuropathic pain states in humans, highlighting their potential as a therapeutic target for pain relief. Although the therapeutic effects of Nav1.8 inhibitors have been demonstrated in animal models of inflammatory and neuropathic pain,30,31 there have been a lack of studies into the efficacy of Nav1.8-specific inhibitors in humans. There have been efforts to develop such agents for use in humans;32 however, the only reported clinical trial of a Nav1.8 inhibitor, Pfizer compound PF-04531083,33 was terminated, and there have been no further clinical tests into Nav1.8-specific compounds reported in the literature.34 Given the importance of Nav1.8 in the pathophysiology of chronic pain, there is currently a need for the development of Nav1.8-selective compounds for study in clinical trials.
Inflammatory pain
Inflammatory pain occurs as a result of tissue injury linked to inflammation, resulting in the production and release of inflammatory mediators such as cytokines, kinins, growth factors and prostanoids that sensitise Aδ- and C-nociceptors innervating the inflamed tissue.35 Plastic changes consisting of peripheral and central sensitisation lead to the generation and maintenance of inflammatory pain. Inflammatory mediators are important for both types of sensitisation (particularly peripheral sensitisation) and act on their corresponding receptors to activate intracellular signalling pathways, resulting in the activation of intracellular kinases that can phosphorylate and modulate the electrophysiological properties of VGSCs, leading to membrane excitability and an increase in the response of nociceptors to various stimuli.35 Nociceptor sensitisation is characterised by the presence of spontaneous ectopic discharges, decreased activation thresholds and an increase in responsiveness to stimuli. These alterations lead to the development of hyperalgesia, allodynia and spontaneous ongoing pain, which are also the behavioural symptoms present in neuropathic pain states. Therefore, it is important to determine the extent to which inflammatory and neuropathic pain states share physiological mechanisms including those in which sodium channels play key roles.
Nav1.7
Nassar et al.36 found that nociceptor-specific Nav1.7 knockout (KO) mice exhibited enhanced thermal and mechanical pain thresholds and decreased inflammatory responses to injections of various inflammatory mediators into the hindpaw of mice, including complete Freund’s adjuvant (induces longer term inflammation), Carrageenan (triggers acute inflammation through enhancement of prostanoids that act on Prostanoid (EP) receptors and lead to the activation of protein kinase A (PKA) and phosphorylation of VGSCs) and nerve growth factor (NGF causes hyperalgesia via activation of TrkA receptors). This data indicates that Nav1.7 is important in the development of inflammatory pain.
Toledo-Aral et al.9 demonstrated that administration of NGF to cultured PC12 cells expressing only Nav1.7 caused a significant upregulation of Nav1.7 expression and an increase in Nav1.7 channel density at neurite terminals. Therefore, Nav1.7 may play a role in the regulation of inflammatory pain thresholds via its increased expression and trafficking into the membrane of peripheral nociceptor terminals where generator potentials occur. The poised role of Nav1.7 as a threshold channel provides a plausible explanation of how the channel may amplify generator potentials bringing neurons closer to the threshold for Nav1.8 which contributes most of the sodium current underlying the AP. In this way, the presence of increased functional Nav1.7 channels with slow-closed state inactivation kinetics at sensory nerve terminals may cause nociceptors to fire in response to subthreshold stimuli, that is, causing a decreased threshold for nociceptor activation and increased responsiveness. This would contribute to the increased peripheral drive leading to the induction of central sensitisation and thus chronic inflammatory pain. This may thus be the mechanism via which Nav1.7 channels contribute in the pathophysiology of inflammatory pain, hyperalgesia and tactile allodynia (Figure 2(b)).
Genetic evidence highlighting the importance of Nav1.7 in the pathophysiology of pain was first reported in patients with a gain-of-function mutation in the SCN9A gene linked to inherited erythromelalgia (IEM), a rare autosomal dominant inflammatory disease characterised by repeated episodes of intense pain, erythema and warmth in the peripheries.37 Many Nav1.7 mutations located throughout DI–DIV have been identified and linked to IEM.38 Most of these mutations result in enhanced activation of Nav1.7 through a hyperpolarising shift in channel activation, increased response to small slow depolarisations and slower channel deactivation, leading to hyperexcitability of nociceptors.38 IEM has been recognised as an important model condition for studies into the efficacy of Nav1.7-selective blockers and pharmacogenomically led methods of analgesia.39 A clinical trial involving five participants from whom induced pluripotent stem cell lines were created, mirroring the clinical effects of hyperexcitability and abnormal responses to heat stimuli, demonstrated the success of a Nav1.7 inhibitor PF-05089771 in alleviating heat-triggered pain in most of these subjects.40 Another study using pharmacotherapy guided by genomic analysis, structural modelling and functional analysis found a significant attenuation of pain using carbamazepine.41 Pain in IEM patients carrying S241T mutations in Nav1.7 was found to be sensitive to carbamazepine through molecular modelling and functional profiling.41 The study showed that pain was successfully attenuated in these patients through the use of the drug.41 This demonstrates the importance of pharmacogenomically guided approaches to studying pain treatments, a method future studies should also adopt to aid the identification of potentially beneficial therapeutic agents.
Nav1.8
A significant body of evidence points to an important role of Nav1.8 modulation in inflammatory pain. Kerr et al.42 found that thermal hyperalgesia induced by NGF treatment was abolished in Nav1.8 KO mice, which had similar withdrawal latencies to noxious thermal stimuli as control saline-treated mice, supporting a role for Nav1.8 in inflammatory pain. Furthermore, Black et al.43 observed that Nav1.8 mRNA and protein levels increased in small-diameter DRG neurons extracted from rats injected with carrageenan. The researchers determined that this increase in functional Nav1.8 expression correlated with an increase in the slowly inactivating TTX-R Nav1.8 current. A similar observation was also made in another study44 using cultured small-diameter rat DRG neurons treated with a range of inflammatory mediators. Electrophysiological recordings revealed that the inflammatory mediators caused an increase in the TTX-R sodium current, an enhancement in the TTX-R current activation and inactivation rates, and a shift in the voltage-dependency of activation in the hyperpolarising direction and therefore a decrease in the threshold for activation.44 These modulations of the Nav1.8 TTX-R current induced by hyperalgesic agents would be expected to result in neuronal hyperexcitability by reducing the threshold for activation and increasing AP electrogenesis due to greater and faster depolarisation of the nociceptor membrane, leading to an increase in the response of neurons to stimuli. The increased Nav1.8 activation and inactivation rates would cause more rapid membrane repolarisation and therefore cause a reduction in the interval between each AP spike, thus promoting repetitive AP firing and an increase in the AP firing frequency in response to a prolonged stimulus.44 It is therefore thought that the modulation of Nav1.8 by inflammatory mediators may significantly contribute to peripheral sensitisation43, causing an increase in nociceptor excitability and the peripheral drive required to induce central sensitisation and thus chronic inflammatory pain (Figure 2(b)). These changes in the biophysiological properties of Nav1.8 are thought to be mediated by the activation of intracellular signalling pathways that result in the activation of specific kinases responsible for Nav1.8 phosphorylation and modulation. Inflammatory mediators such as prostaglandin E2 (PGE2) are known to lead to the activation of the protein kinases PKA and PKC. Fitzgerald et al.45 found that PKA-mediated phosphorylation of specific serine residues of the intracellular loop between DI and DII of the Nav1.8 channel transiently expressed in COS-7 cells, caused an increase in the TTX-R current density and a hyperpolarising shift in the voltage-dependence of Nav1.8 channel activation. On the other hand, administration of inflammatory cytokines such as TNFα leads to an enhancement of Nav1.8 TTX-R current density without affecting the gating properties of Nav1.8 via activation of the p38 MAPK, which has been determined to be via phosphorylation of serine residues within the intracellular loop of DI and DII of the Nav1.8 sodium channel that are distinct from the consensus sites targeted by PKA.46 Therefore, the various inflammatory mediators may modulate Nav1.8 by different methods to increase the excitability of nociceptors.
Future avenues for therapeutic research
Drug development programmes into Nav1.7, in particular, have been on-going for many years. Despite this promising research, there has been a failure to produce effective novel analgesic treatments for chronic inflammatory and neuropathic pain states. The explanation for this lack of success may come from the idea that the greater the selectivity of an inhibitor for Nav1.7, the less effective the overall analgesic effect.21 On the other hand, less selective Nav1.7 blockers combined with agents targeting other sites may produce a stronger and more comprehensive range of therapeutic effects. Further support for this theory comes from the previously stated observation that not all pain states are Nav1.7 dependent.21–23 This highlights the complexity and magnitude of mechanisms involved in the pathophysiology of pain, supporting the use of polypharmacy in the treatment of chronic pain. Future clinical trials should explore the potential of combined treatment regimens, targeting VGSCs as well as other clinically relevant sites in alleviating chronic pain. A recent example of such additionally identified clinically relevant agents and targets includes the opioid system and enkephalinase inhibitors.21 Interestingly, there is a significant role of opioid signalling pathways in the pain-free state associated with Nav1.7 null mutant CIP mice and humans.47 Researchers found the absence of Nav1.7 resulted in the upregulation of Penk mRNA which produces enkephalin proteins, thus demonstrating an increase in opioid signalling in CIP.47 When the opioid antagonist naloxone was given, this enhanced the noxious stimuli detected, suggesting the upregulated opioid system in Nav1.7 null mutants is responsible for the analgesic effect in this state.47 Interestingly, lower sodium levels were found to be related to the upregulation of Penk mRNA expression.47 This highlights a potential mechanism of interest for future studies to explore the role of the enhanced opioid system in CIP and its contribution to the analgesic state. These findings therefore suggest Nav1.7 inhibitors combined with low doses of exogenous opioids or enkephalinase inhibitors should result in a significant analgesic effect. This effect has been demonstrated in animal models of inflammatory and neuropathic pain.22,47,48 Thus polypharmacy poses a promising avenue for future research into pain relief. Further studies are needed to elucidate other molecular pathways and targets involved in the pathophysiology of inflammatory and neuropathic pain and their relation to VGSCs. This should pave the way for the development of additional drug targets that can be used in combination with sodium channel isoform-specific blockers, leading to a greater widespread analgesic effect. The development of agents for use in such clinical studies alongside Nav1.7 and Nav1.8 inhibitors depends on further research into the key mechanisms involved in the pathophysiology of inflammatory and neuropathic pain.
Conclusions
Overall, it is clear that the dysregulation of Nav1.7 and Nav1.8 expression and alterations in the biophysical properties of these channels contribute in the development of pathological pain. Nav1.7 in particular is important in inherited human pain syndromes with genetic mutations altering the channels functional properties. It is yet to be determined whether Nav1.7 is as important in neuropathic pain with contradictory research in this field. However, modulation of the Nav1.7 channel by MAPK may be one of the mechanisms contributing to the development of ectopic discharges in acquired peripheral neuropathy in humans. Literature concerning the role of Nav1.8 in the pathophysiology of neuropathic pain has yielded clearer results, and it is thought that the channel may be important for the development of abnormal electrogenesis and hyperexcitability in uninjured sensitised primary afferents neighbouring injured neurons. Furthermore, there may be time-dependent changes in the contribution of Nav1.8 in the pathophysiology of neuropathic pain. KO studies have confirmed a role for both Nav1.7 and Nav1.8 in the pathophysiology of inflammatory pain with these animals displaying reduced/attenuated inflammatory pain behaviours. A better understanding regarding the contributions of these channels in pathological pain is beginning to develop. More studies in this area should help to elucidate the exact mechanisms by which these channels contribute to chronic pain states. Further research in this field is important for the development of subtype-specific sodium channel blockers, a promising potential therapeutic strategy for chronic pathological pain. Current analgesics cause a range of adverse effects and are not very successful in alleviating pain. Advancements in understanding of the function of specific subtypes of sodium channels in the pathophysiology of chronic neuropathic and inflammatory pain will help to satisfy the need for more precise and effective treatments for patients with chronic pain conditions.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci 2010; 33: 325–347. [DOI] [PubMed] [Google Scholar]
- 2.Wall PD, Waxman SG, Basbaum AI. Ongoing activity in peripheral nerve, III. Injury discharge. Exp Neurol 1974; 45: 578–589. [DOI] [PubMed] [Google Scholar]
- 3.Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve 1999; 22: 1177–1187. [DOI] [PubMed] [Google Scholar]
- 4.Dib-Hajj SD, Binshtok AM, Cummins TR, Jarvis MF, Samad T, Zimmermann K. Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res Rev 2009; 60: 65–83. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and function of voltage-gated sodium channels at atomic resolution. Exp Physiol 2014; 99: 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood N, Iseppon F. Sodium channels. Brain Neurosci Adv 2018; 2: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. Embo J 1995; 14: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol 2003; 551: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo-Aral JJ, Moss BL, He Z-J, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I, Halegoua S, Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA 1997; 94: 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 2007; 579: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996; 379: 257–262. [DOI] [PubMed] [Google Scholar]
- 12.Dib-Hajj SD, Tyrrell L, Cummins TR, Black JA, Wood PM, Waxman SG. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett 1999; 462: 117–120. [DOI] [PubMed] [Google Scholar]
- 13.Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 2002; 22: 10277–10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel NaV1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 2003; 550: 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain 2006; 7: S3–S12. [DOI] [PubMed] [Google Scholar]
- 16.Finnerup NB, Sindrup SH, Jensen TS. Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharmacol 2007; 21: 129–136. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Oh Y, Chung JM, Chung K. Changes in three subtypes of tetrodotoxin sensitive sodium channel expression in the axotomized dorsal root ganglion in the rat. Neurosci Lett 2002; 323: 125–128. [DOI] [PubMed] [Google Scholar]
- 18.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 1997; 17: 3503–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol 2008; 64: 644–653. [DOI] [PubMed] [Google Scholar]
- 20.Stamboulian S, Choi J-S, Ahn H-S, Chang Y-W, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 2010; 30: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emery EC, Luiz AP, Wood JN. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets 2016; 20: 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard A-M, Wood JN. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep 2014; 6: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler DW, Lee MCH, Harrison EK, Menon DK, Woods CG. Case report: neuropathic pain in a patient with congenital insensitivity to pain [version 2; referees: 2 approved]. F1000Res 2014; 3: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 2002; 95: 143–152. [DOI] [PubMed] [Google Scholar]
- 25.Dib-Hajj S, Black JA, Felts P, Waxman S G. Down-regulation of transcripts for Na channel alpha-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci USA 1996; 93: 14950–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MS, Weinreich D, Kim C-S, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003; 23: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 2001; 21: RC140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, Bountra C, Anand P. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain 2000; 85: 41–50. [DOI] [PubMed] [Google Scholar]
- 29.Faber CG, Lauria G, Merkies ISJ, Cheng X, Han C, Ahn H-S, Persson A-K, Hoeijmakers JGJ, Gerrits MM, Pierro T, Lombardi R, Kapetis D, Dib-Hajj SD, Waxman SG. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci USA 2012; 109: 19444–19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kort ME, Atkinson RN, Thomas JB, Drizin I, Johnson MS, Secrest MA, Gregg RJ, Scanio MJC, Shi L, Hakeem AH, Matulenko MA, Chapman ML, Krambis MJ, Liu D, Shieh C-C, Zhang X, Simler G, Mikusa JP, Zhong C, Joshi S, Honore P, Roeloffs R, Werness S, Antonio B, Marsh KC, Faltynek CR, Krafte DS, Jarvis MF, Marron BE. Subtype-selective NaV1.8 sodium channel blockers: identification of potent, orally active nicotinamide derivatives. Bioorg Med Chem Lett 2010; 20: 6812–6815. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis MF, Honore P, Shieh C-C, Chapman M, Joshi S, Zhang X-F, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 2007; 104: 8520–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA. Voltage gated sodium channels as drug discovery targets. Channels (Austin) 2015; 9: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagal SK, Chapman ML, Marron BE, Prime R, Storer RI, Swain NA. Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett 2014; 24: 3690–3699. [DOI] [PubMed] [Google Scholar]
- 34.Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 2017; 16: 545–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain 2006; 7: S1–S29. [DOI] [PubMed] [Google Scholar]
- 36.Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA 2004; 101: 12706–12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet 2004; 41: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetter I, Deuis JR, Mueller A, Israel MR, Starobova H, Zhang A, Rash LD, Mobli M. NaV1.7 as a pain target – from gene to pharmacology. Pharmacol Ther 2017; 172: 73–100. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Mis MA, Estacion M, Dib-Hajj SD, Waxman SG. NaV1.7 as a pharmacogenomic target for pain: moving toward precision medicine. Trends Pharmacol Sci 2018; 39: 258–275. [DOI] [PubMed] [Google Scholar]
- 40.Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot P-P, Loucif A JC, Brown AR, Young G, Mis M, Randall A, Waxman SG, Stanley P, Kirby S, Tarabar S, Gutteridge A, Butt R, McKernan RM, Whiting P, Ali Z, Bilsland J, Stevens EB. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 2016; 8: 335ra56. [DOI] [PubMed] [Google Scholar]
- 41.Geha P, Yang Y, Estacion M, Schulman BR, Tokuno H, Apkarian AV, Dib-Hajj SD, Waxman SG. Pharmacotherapy for pain in a family with inherited erythromelalgia guided by genomic analysis and functional profiling. JAMA Neurol 2016; 73: 659–667. [DOI] [PubMed] [Google Scholar]
- 42.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport 2001; 12: 3077–3080. [DOI] [PubMed] [Google Scholar]
- 43.Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004; 108: 237–247. [DOI] [PubMed] [Google Scholar]
- 44.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA 1996; 93: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol 1999; 516: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 2008; 28: 3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minett MS, Pereira V, Sikandar S, et al. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nat Commun 2015; 6: 8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minett MS, Eijkelkamp N, Wood JN. Significant determinants of mouse pain behaviour. PLoS One 2014; 9: e104458. [DOI] [PMC free article] [PubMed] [Google Scholar]