Abstract
Background. Value assessments and treatment decision making typically focus on clinical endpoints, especially overall survival (OS). However, OS data are not always available, and surrogate markers may also have some value to patients. This study sought to estimate preferences for progression-free survival (PFS) relative to OS in metastatic breast cancer (mBC) among a diverse set of stakeholders—patients, oncologists, and oncology nurses—and estimate the value patients and providers place on other attributes of treatment. Methods. Utilizing a combined conjoint analysis and discrete choice experiment approach, we conducted an online prospective survey of mBC patients and oncology care providers who treat mBC patients across the United States. Results. A total of 299 mBC patients, 100 oncologists, and 99 oncology nurses completed the survey. Virtually all patients preferred health state sequences with contiguous periods of PFS, compared with approximately 85% and 75% of nurses and oncologists, respectively. On average, longer OS was significantly (P < 0.01) preferred by the majority (75%) patients, but only 15% of nurses preferred longer OS, and OS did not significantly affect oncologists’ preferred health state. However, in the context of a treatment decision, whether a treatment offered continuous periods of stable disease holding OS constant significantly affected nurses’ treatment choices. Patients and providers alike valued reductions in adverse event risk and evidence from high-quality randomized controlled clinical trials. Conclusions. The strong preference for observed PFS suggests more research is warranted to better understand the reasons for PFS having positive value to patients. The results also suggest a range of endpoints in clinical trials may have importance to patients.
Keywords: surrogate endpoints, discrete choice experiment, patient preferences, provider preferences
Overall survival (OS) has long been the gold standard in establishing the efficacy of oncology therapies.1 Yet, due to improvements in the standard of care, along with constraints on study duration and design, clinical trials often provide incomplete data on the OS benefits of new therapies.2 To compensate, clinical trials often include one or more “surrogate endpoints” to quantify the potential value offered by new therapies when OS is unlikely to be shown conclusively.3–6 Progression-free survival (PFS), defined in clinical trials as the time from randomization until first evidence of tumor progression or death from any cause, is one of the most commonly used surrogate endpoints in oncology clinical trials.7 The use of PFS in clinical trials has risen rapidly since the mid-1970s, serving as a primary endpoint in 20% of clinical trials in major cancers conducted in the mid to late 2000s.8
Despite the now common use of surrogate endpoints in oncology trial designs, the value of surrogate endpoints has been questioned by some cancer researchers, and often without an evaluation of patient preferences.2,4,5 Some studies have shown a poor or modest correlation with OS in the majority of surrogate endpoints, but other studies have found that surrogate endpoints can correlate with real-world outcomes.9,10 Furthermore, data on surrogate endpoints can be collected in studies of shorter duration, which shortens access time to new therapies for patients.11,12 A great deal of controversy remains, however, as to the use of PFS as a surrogate for OS, with the association being weak in some cancers, yet strong in others.13
How data from clinical trials, including primary endpoints such as OS, and surrogate endpoints such as PFS, factor into treatment decision-making has also been questioned.14 Previous research has shown that patients often take into account a wide range of factors when making a choice of therapeutic options, and do not focus solely on OS.15,16 For example, patients consider aspects of treatment including mode of administration and dosing schedule, as well as other factors such as the impact of treatment side effects on ability to work, or a spouse or family member’s opinion. Endpoints such as PFS may factor more heavily in a patient’s decision about therapeutic options than OS benefit alone as PFS has implications in terms of quality of life not considered with OS. Physicians, on the other hand, have been shown to place heavy weight on survival when making treatment recommendation, without fully considering how treatment may affect patients’ quality of life.17,18 High levels of discordance between patients and providers have been shown to exist across the cancer treatment spectrum and tumor sites, with providers more likely to accept side effects in exchange for increased survival.19–22 These mismatched priorities may lead to suboptimal decision making when patient and provider understanding of the important attributes of therapy differ widely.
Hence, understanding the priorities patients and providers place on clinical data and treatment attributes in oncology care decision making is critical to improving the patient–provider alliance; which, when the relationship is strong, has been demonstrated to improve outcomes.23 The collection of relevant data in trials and its use in real-world decisions is of particular importance when patients are faced with potentially life-threatening diseases such as advanced cancer.
With these issues in mind, we conducted a study of metastatic breast cancer (mBC) patients and providers (oncologists and oncology nurses) across the United States to study the role information on PFS (or stable disease) plays in treatment decision making and how patients and providers weigh PFS relative to other treatment attributes. To our knowledge, the value of stable disease to patients and providers, independent from OS, has yet to be measured.
Methods
Study Design and Setting
A study of mBC patients and oncology care providers (nurses and oncologists) was conducted to evaluate the value of OS, PFS, and other treatment attributes in treatment decision making. Three individual, but complementary, surveys were developed, each tailored to the specific respondent populations. All surveys included an eligibility screener, questions about preferences for and perceptions of patient treatment decision making, a module to assess preferences for OS and PFS/stable disease, a module to assess preferences and willingness-to-pay (WTP) for specific treatment attributes, and a set of sociodemographic questions. The Chesapeake Institutional Review Board reviewed study procedures and exempted the study from full review because of low/no risk to the study participants.
A convenience sample of mBC patients, oncology nurses, and oncologists currently treating patients with mBC was invited by email or through social media postings to participate in the study. We arrived at a target sample size of n = 300 mBC patients, n = 100 oncology nurses, and n = 100 oncologists based on methodology outlined by Louviere and colleagues about minimal sample size required to conduct the main and sub-analyses for the study.24
Eligible patients were those who had been told by a doctor that they have mBC, were aged ≥18 years, and were current residents of the United States. Eligible oncology nurses were registered nurses who treated ≥5 mBC patients per month and held a nursing degree. Eligible oncologists included individuals with a medical degree who were board certified in oncology, treated ≥5 mBC patients per month, and regularly prescribed chemotherapy and/or targeted cancer treatment to cancer patients. Study participants received an email with a link to the online survey website. After providing informed consent, participants were directed to a set of instructions and then asked a series of questions to determine whether they met study inclusion criteria. Those who failed to provide informed consent or who did not meet the eligibility criteria were excluded. All respondents who successfully completed the survey, including pilot test participants, were compensated for their time and effort.
We performed the study in two phases: a pilot phase, conducted from January to February 2017, and a primary data collection phase, conducted from March to June 2017. Eight respondents participated in pilot testing and debriefing interviews to further hone the survey instrument and assess respondent comprehension of the background content and survey questions. This testing included ensuring that patients understood the definitions for PFS and OS, as well as the iconography used to represent PFS and OS in the study design. Results from the pilot testing and consultation with clinical and patient experts informed revisions to the final versions of the three surveys, which were all hosted on a web-based survey platform.
Decision Scenarios
To establish survival preferences, we used a stated preference approach. For establishing preferences for a set of treatment attributes, we used a discrete choice experiment (DCE). Discrete choice experiments are based on economic (random utility) theory about how consumers make choices among goods given a limited budget.25–27 This design allows for the estimation of consumer WTP, or value, for distinct product attributes. These methods are well established in the literature and endorsed by the ISPOR Conjoint Analysis Good Research Practices Task Force for Pharmacoeconomic Research.28 Both the conjoint analysis and DCE were designed using the AlgDesign software package for R based on the D-efficiency criteria.
Survival Preference Scenarios
To assess patient and provider preferences for PFS versus OS, measured as the timing and duration of stable disease versus progressive disease and death, a set of choice alternatives were presented to survey participants. Nine choice alternatives were presented, each differing in terms of the number of periods of stable disease (PFS), number of periods of progressive disease, and OS (time to death). Choices also varied in terms of the sequencing of periods of stable and progressive disease. The nine choice alternatives presented are depicted in Figure 1. Respondents were asked to select which sequence they preferred for each of the nine combinations.
Figure 1.
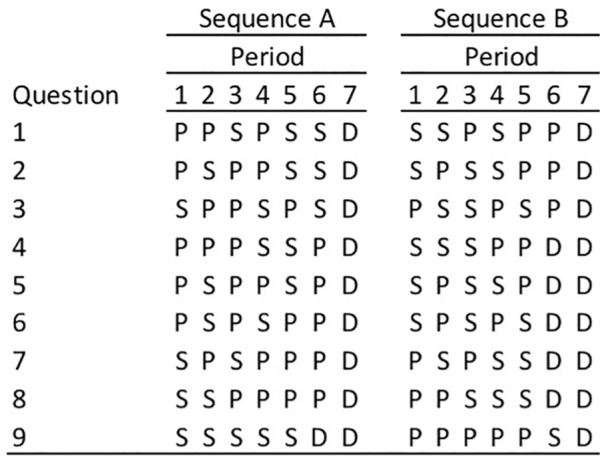
Health state choice alternatives.
P, progressive disease; S, stable disease; D, death.
A pictorial example of choice alternative four is shown in Figure 2. Instructions provided to participants are detailed in the Online Appendix.
Figure 2.
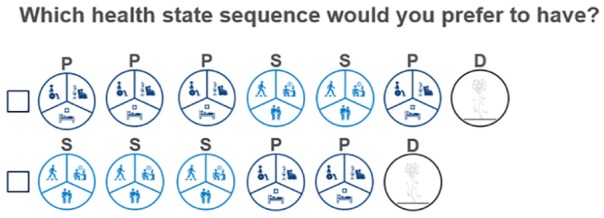
Health state choice set example.
P, progressive disease; S, stable disease; D, death.
Treatment Attribute Preference Scenarios
To gain additional insights into patient and provider treatment preferences, 12 choice sets comparing two hypothetical treatment plans varying on four key attributes were presented to survey participants. The attributes included in the survey were generated from a review of the literature and qualitative data collected through focus groups conducted with mBC patients, oncology nurses, and oncologists. Findings from the focus groups have been reported previously.16 To arrive at an appropriate and feasible number of attributes, we considered six primary factors: 1) relevance of the attribute to patient’s choice of mBC treatment, 2) relevance of the attribute to physician’s choice of mBC treatment, 3) ease of quantifying the attribute in the survey, 4) overlap or correlation with other attributes, 5) relevance to the objectives of the study, and 6) variation in the attribute across currently available mBC treatments. We aimed to have a parsimonious list of attributes to make the questions as simple as possible for the study population. Most DCE surveys in the health care literature use six or fewer attributes.25
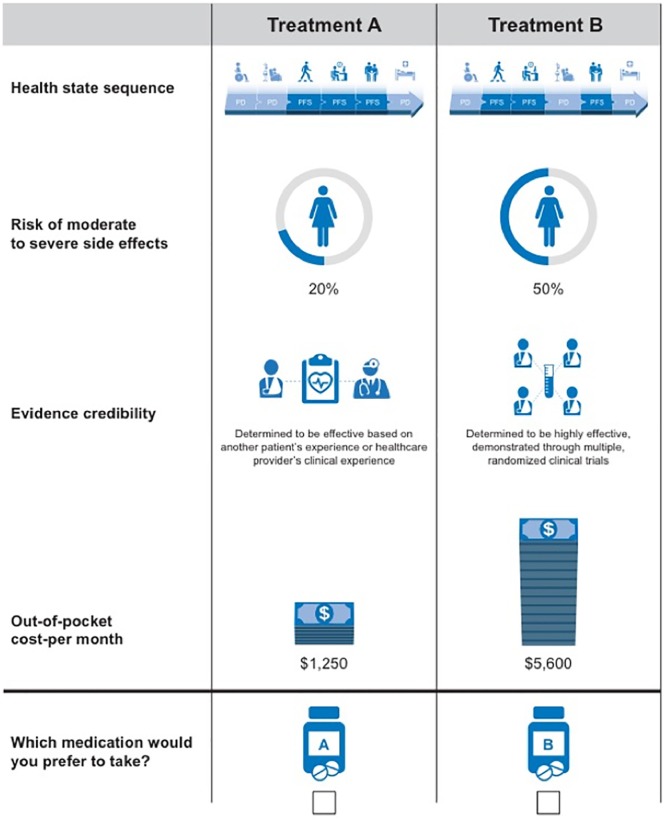
The attribute levels were selected based on the characteristics of currently available mBC treatments. Treatments considered in the development of the attribute descriptions were hormone therapy, chemotherapy, and targeted therapies such as mTOR inhibitors, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, and anti-HER2/neu targeted therapies. For each choice set, respondents were asked which of the two unlabeled treatment options they preferred for themselves or for their patients, as depicted in Figure 3.
Figure 3.
Treatment attributes choice question example.
Using the DCE design, attributes included in the choice sets were the following: 1) risk of moderate to severe side effects, 2) monthly out-of-pocket cost, 3) evidence credibility, and 4) health state sequences reflecting OS, PFS, and, implicitly, health-related quality of life. The health state sequence attribute allowed us to test whether respondents were willing to pay significantly more for contiguous periods of stable disease holding OS constant. The module was not designed, however, to measure marginal willingness to substitute periods of OS for PFS.
Other Study Measurements
Demographics
For patients, we queried respondents about age, marital status, education, ethnicity, employment status, type of health insurance, and household income. For providers, we queried respondents about age, gender, ethnicity, medical training, and licensure.
Clinical History
For patients, we queried respondents about mBC disease diagnosis and duration, past and current therapies, line of treatment, hormone receptor status, presence of comorbid conditions, functional ability, and receipt of palliative or hospice care. These were included to explore whether specific patient characteristics influenced treatment preferences.
Practice Characteristics
We queried provider respondents about their practice setting (e.g., academic center, private general hospital, solo office practice) and the average number of breast cancer and mBC patients treated each month.
Analysis
Our first objective was to establish a baseline understanding of whether the timing and sequencing of stable disease/PFS intervals was meaningful to respondents, that is, whether patients/providers preferred PFS sooner and/or contiguous periods of PFS. Positive (negative) time preference implies patients prefer their stable disease in earlier (later) periods rather than later (earlier) periods. The health state/survival preference module was designed to meet the first study objective. The second objective was to estimate patient preferences for cancer treatments and WTP for changes in cancer treatment attribute levels, for example, reduced risk of side effects. The DCE module was designed to meet the second objective. All analyses were conducted using Stata statistical software package Version 14.0 (Stata Corporation, College Station, TX).
Responses to the health state/survival preference choice sets were evaluated and patient preferences were estimated using a random utility model. In a random utility model, utility has a deterministic (observable) component separable in price (if applicable) and nonprice attributes, as well as a random (unobservable) component that is distributed as an independent and identically distributed extreme value type I (Gumbel). The health state/survival attributes included in the utility model included the following: 1) an indicator for the health state sequence containing any contiguous periods of PFS, 2) length of OS, 3) cumulative periods of PFS, 4) an indicator for the contiguous periods of PFS occurring at the beginning of the health state sequence, 5) and an indicator for the contiguous periods of PFS occurring at the end of the health state sequence. A random parameters/mixed logistic model was used to estimate utility weights based on responses to the survival preference scenarios.
Responses to the treatment attribute choice scenarios were evaluated and patient preferences and WTP for specific attributes were also estimated using a random utility model. As noted previously, the attributes selected are risk of treatment side effects, evidence credibility, health state sequence over remaining life, and monthly out-of-pocket cost. A random parameters/mixed logistic model was used to estimate attribute utility weights and WTP based on responses to the treatment attribute choice scenarios. We explored specifications that were linear and quadratic in the risk of adverse events. Utility was linear in all other treatment attributes. WTP was estimated as the ratio of the nonprice attribute utility weight and the price attribute disutility weight from the random parameter logit model. Goodness of fit was evaluated based on the log-likelihood ratio statistic.
Funding
Financial support for this manuscript was provided by Novartis Oncology. The funding agreement ensured the authors independence in designing the study, interpreting the data, writing, and publishing the report.
Results
Participant Characteristics
Of 519 patient respondents screened, 189 (36.4%) did not meet study eligibility criteria, 330 (63.6%) were enrolled into the study, and 300 (57.8%) completed the survey. Of 515 oncology nurse respondents screened, 244 (47.3%) met study eligibility criteria, and 101 (19.6%) completed the survey. Of 238 oncologist respondents screened, 73 (30.7%) did not meet study eligibility criteria, and 100 (42.0%) completed the survey. We excluded providers who reported not seeing at least five mBC patients per month. Brief email reminders were sent to noncompleters on a weekly basis for up to 4 weeks. We closed each survey as soon as the accrual targets for the sample population were achieved. Ultimately, we excluded from the final data set one nurse because he/she reported not treating mBC patients, and one patient who reported having an earlier stage of breast cancer. Hence, in the completed sample data were analyzed for 299 patients, 100 oncologists, and 99 oncology nurses. We were unable to calculate the overall response rate because we employed a combination of direct invitation, social media advertising, and snowball sampling. Respondent demographic characteristics are presented in Table 1.
Table 1.
Respondent Demographic Characteristics
| Patient (N = 299) | Provider (N = 199) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Age in years, mean (SD) | 47.5 (10.1) | 49.7 (10.1) | ||
| Race | ||||
| White/Caucasian | 250 | 83.6 | 146 | 73.4 |
| African American | 22 | 7.4 | 2 | 1.0 |
| Asian | 3 | 1.0 | 35 | 17.6 |
| Mixed/Other | 23 | 7.6 | 8 | 4.0 |
| Missing/refused | 1 | 0.3 | 8 | 4.0 |
| Hispanic ethnicity | ||||
| Hispanic | 15 | 5.0 | 10 | 5.0 |
| Not Hispanic | 282 | 94.3 | 175 | 87.9 |
| Missing/refused | 2 | 0.7 | 14 | 7.0 |
| Marital status | ||||
| Married or living as married | 207 | 69.2 | — | — |
| Not married (separated, divorced, widowed, single) | 90 | 30.1 | — | — |
| Missing/refused | 2 | 0.7 | — | — |
| Education | ||||
| Some high school | 2 | 0.7 | — | — |
| High school graduate | 22 | 7.4 | — | — |
| Some college or associate degree | 106 | 35.5 | — | — |
| College graduate | 76 | 25.4 | — | — |
| Some graduate | 21 | 7.0 | — | — |
| Graduate degree | 71 | 23.7 | — | — |
| Missing/refused | 1 | 0.3 | — | — |
| Employment status | ||||
| Full time | 81 | 27.1 | — | — |
| Part time | 19 | 6.4 | — | — |
| Unemployed: medical reasons | 154 | 51.5 | — | — |
| Unemployed: nonmedical reasons | 22 | 7.4 | — | — |
| Retired | 22 | 7.4 | — | — |
| Missing/refused | 1 | 0.2 | — | — |
| Household income | ||||
| Less than $25,000 | 67 | 22.5 | — | — |
| $25,000–$49,999 | 50 | 16.7 | — | — |
| $50,000–$99,999 | 87 | 29.0 | — | — |
| $100,000 or more | 80 | 26.8 | — | — |
| Missing/refused | 15 | 5.0 | — | — |
| Insurance | ||||
| Medicare | 32 | 10.7 | — | — |
| Medicaid | 34 | 11.4 | — | — |
| Private plan (employer or self-purchased) | 178 | 59.5 | — | — |
| Multiple (one or more types) | 48 | 16.1 | — | — |
| Other | 5 | 1.6 | — | — |
| Missing/refused/none | 2 | 0.7 | — | — |
| Stages I–III | 170 | 56.9 | — | — |
| Stage IV (de novo) | 129 | 43.1 | — | — |
| ER/PR positive | 220 | 73.6 | — | — |
| HER2/neu positive | 77 | 25.8 | — | — |
| Triple positivea | 33 | 11.0 | — | — |
| Triple negativeb | 30 | 10.1 | — | — |
| First | 95 | 31.8 | — | — |
| Second | 46 | 15.4 | — | — |
| Third or more | 110 | 36.8 | — | — |
| Currently in hospice or palliative care | 47 | 15.7 | — | — |
| Specialtyc | ||||
| Breast oncology | — | — | 116 | 58.3 |
| Medical oncology | — | — | 185 | 93.0 |
| Surgical oncology | — | — | 23 | 11.6 |
| Radiation oncology | — | — | 19 | 9.5 |
| Gynecologic oncology | — | — | 37 | 18.6 |
| Certification | ||||
| Registered nurse | — | — | 65 | 32.7 |
| Nurse practitioner | — | — | 32 | 16.1 |
| Oncology certified nurse | — | — | 5 | 2.5 |
| Certified breast care nurse | — | — | 5 | 2.5 |
| Average number of breast cancer patients treated/month | ||||
| 1–10 | — | — | 13 | 6.5 |
| 11–50 | — | — | 87 | 43.7 |
| 51–100 | — | — | 60 | 30.2 |
| 100+ | — | — | 39 | 20.0 |
| Number of stage IV breast cancer patients treated/month | ||||
| 1–4 | — | — | 10 | 5.0 |
| 5–9 | — | — | 48 | 24.1 |
| 10–19 | — | — | 62 | 31.2 |
| 20–49 | — | — | 62 | 31.2 |
| 50+ | — | — | 26 | 13.1 |
| Number of years in practice | ||||
| ≤5 | — | — | 12 | 6.0 |
| 6–10 | — | — | 31 | 15.6 |
| 11–15 | — | — | 44 | 22.1 |
| 16–20 | — | — | 43 | 21.6 |
| 20+ | — | — | 52 | 26.1 |
| Gender | ||||
| Female | 100 | 100 | 112 | 56.3 |
| Male | 0 | 0.0 | 85 | 42.7 |
| Missing/refused | 0 | 0.0 | 2 | 1.0 |
| Primary practice setting | ||||
| Solo office practice | — | — | 3 | 1.5 |
| Group office practice | — | — | 63 | 31.7 |
| Public general hospital | — | — | 7 | 3.5 |
| Private general hospital | — | — | 5 | 2.5 |
| Academic medical center/comprehensive cancer center | — | — | 22 | 11.1 |
ER/PR, estrogen receptor/progesterone receptor; HER2/neu, human epidermal growth factor receptor 2; SD, standard deviation.
Estrogen receptor/progesterone receptor and HER2/neu positive.
Estrogen receptor/progesterone receptor and HER2/neu negative.
Indicates multiple response options.
Survival Preferences: Utility parameters
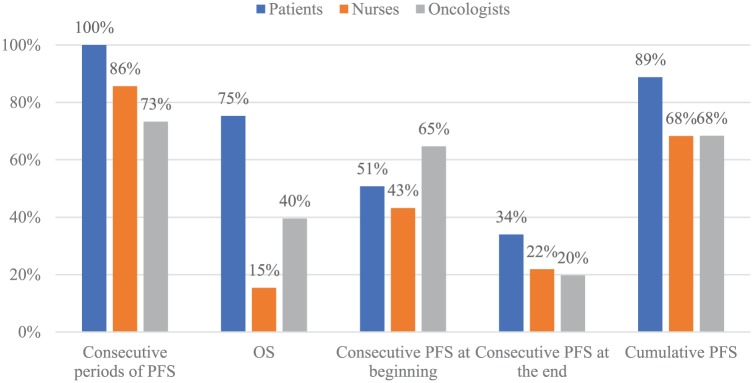
Table 2 displays the estimated coefficients from the random parameters/mixed logistic regression where all health state attribute coefficients were treated as normally distributed random variables. Figure 4 illustrates the share of respondents with positive utility weights by health state attribute and respondent group. One nurse, two oncologists, and six patients were excluded from the analysis because they chose the same option (A or B) in every choice scenario. All else equal, health states with consecutive periods of PFS were on average preferred across the three respondent groups; however, there was significant variation among nurses and oncologists, as indicated by the significant standard deviations estimated for these respondent groups. Virtually all patients preferred health state sequences with contiguous periods of PFS (i.e., had an estimated coefficient >0), compared with approximately 85% and 75% of nurses and oncologists, respectively (Figure 4). On average, longer OS was significantly preferred by the majority (75%) patients, but only 15% of nurses preferred longer OS, and OS did not significantly affect oncologists’ preferred health state. Relative to health states with no contiguous periods of stable disease/PFS, no respondent group had significant preferences for health states with the contiguous periods of PFS in the first half of the health state sequence; however, all three respondent groups preferred on average not to have the contiguous periods of PFS at the end (Table 2). Last, the cumulative periods of PFS only significantly influenced health state sequence choices among patients, for whom 89% preferred longer cumulative periods of PFS (Figure 4).
Table 2.
Breast Cancer Survival Attribute Mixed Logit Model Coefficients by Respondent Groupa
| Patients | Nurses | Oncologists | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Contiguous periods of PFS | 0.918** (0.139) | −0.0024 (0.169) | 1.274** (0.336) | 1.196** (0.373) | 0.569* (0.257) | 0.915** (0.340) |
| OS | 1.003** (0.293) | 1.467** (0.225) | −1.732** (0.415) | 1.699** (0.299) | −0.221 (0.336) | −0.832* (0.403) |
| Consecutive periods of PFS at beginning | 0.0129 (0.115) | −0.676** (0.138) | −0.103 (0.201) | −0.604 (0.319) | 0.332 (0.184) | 0.880** (0.192) |
| Consecutive periods of PFS at the end | −1.154** (0.238) | 2.792** (0.263) | −1.377** (0.342) | 1.775** (0.423) | −1.434** (0.362) | 1.683** (0.450) |
| Cumulative PFS | 2.440** (0.324) | 2.006** (0.220) | 0.141 (0.238) | 0.296 (0.227) | 0.530 (0.338) | 1.108** (0.262) |
| Observations | 5274 | 1764 | 1764 | |||
| Log likelihood | −1304 | −395.1 | −467.0 | |||
OS, overall survival; PFS, progression-free survival; SD, standard deviation.
Robust standard errors in parentheses. The sign of the estimated standard deviations is irrelevant. Although in practice the estimates may be negative, interpret them as being positive.
P < 0.01. *P < 0.05.
Figure 4.
Proportion of respondents with positive utility weights for timing, sequencing, overall survival, and stable disease.
OS, overall survival; PFS, progression-free survival/stable disease.
Treatment Attribute Preferences: Utility parameters
Table 3 displays the estimated coefficients from the random parameters/mixed logistic regression where all treatment attribute coefficients were treated as normally distributed random variables. This model had better goodness of fit than the simple logistic model or the random parameters logit where only the non-cost attributes were included as random parameters based on the log likelihood statistic. The significance of the estimated standard deviations suggests significant preference heterogeneity in the three populations and justifies the use of the random parameter logistic specification (Table 2). In the baseline scenario, two patients and two providers were excluded from the DCE analysis because they chose the same option, A or B, in every DCE choice set.
Table 3.
Breast Cancer Treatment Attribute Mixed Logit Model Coefficients by Respondent Group
| Patients | Nurses | Oncologists | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Non-contiguous PFS health state sequence | −0.0142 (0.0650) | −0.479** (0.101) | 0.237* (0.115) | 0.563** (0.203) | −0.0195 (0.0922) | −0.360* (0.181) |
| RCT evidence | 1.208** (0.108) | −1.636** (0.132) | 1.815** (0.213) | −1.304** (0.198) | 0.867** (0.157) | −1.139** (0.147) |
| Risk of adverse events, % | −0.0395** (0.0029) | 0.0367** (0.0031) | −0.0432** (0.0048) | 0.0218** (0.0049) | −0.0291** (0.0036) | −0.0241** (0.0038) |
| Out-of-pocket cost per month, ($100s) | −0.0626** (4.73e−03) | 0.0610** (5.26e−03) | −0.0598** (7.54e−03) | 0.0629** (0.0119) | −0.0438** (5.12e−03) | 0.0308** (4.46e−03) |
| Observations | 7,128 | 2,376 | 2,376 | |||
| Log likelihood | −1533 | −497.9 | −593.8 | |||
PFS, progression-free survival; RCT, randomized controlled trial.
Robust standard errors in parentheses. The sign of the estimated standard deviations is irrelevant. Although in practice the estimates may be negative, interpret them as being positive.
P < 0.01. *P < 0.05.
In the random parameter logit model, we found that the contiguity of periods of PFS only significantly influenced nurses’ treatment preferences. Approximately half (51.2%) of patients were less likely to choose a treatment if the health state sequence had non-contiguous periods of PFS, all else equal.* A similar share of oncologists (52.2%) were less likely to choose a treatment if the health state sequence had non-contiguous periods of PFS; however, only about one third (33.7%) of nurses were less likely to choose a treatment if the health state sequence had non-contiguous periods of PFS. This is consistent with the finding that a majority of nurses preferred health state sequences with contiguous periods of PFS and the contiguity of periods of PFS being a key determinant of nurses’ survival preferences. All respondents were significantly more likely to choose a therapy with efficacy demonstrated in a randomized controlled trial (RCT) versus patient/provider experience. Evidence from RCTs was especially influential for nurses, with nearly 92% of nurses preferring treatments with efficacy demonstrated by RCT evidence, as compared with approximately 77% of both patients and oncologists. A higher risk of adverse events decreased the probability of choosing a treatment, for all respondent groups, as did the patient out-of-pocket cost of treatment. Nearly all (97.6%) nurses preferred lower risk of adverse events, as did the majority of patients (85.9%) and oncologists (88.6%).
Treatment Attribute Preferences: Willingness-to-Pay
In the random parameter model, only oncology nurses had a significant (negative) WTP for contiguous periods of PFS holding OS constant, as depicted in Table 3. All respondents significantly valued efficacy evidence demonstrated in clinical trials over patient/provider experience, and were willing to pay approximately $67 per one-percentage point reduction in the risk of side effects (Table 4). In particular, willingness to pay for reducing the risk of side effects was very similar across the three respondent groups.
Table 4.
Willingness-to-Pay for Breast Cancer Treatment Attributes by Respondent Group
| Attribute | WTP for | Patients | Nurses | Oncologists |
|---|---|---|---|---|
| Health state sequence | Contiguous PFS (v. non-contiguous PFS) | $22.7 [−$181.1, $226.6] | −$396.0* [−$775.1, −$16.8] | $44.4 [−$368.7, $457.5] |
| Evidence credibility | Efficacy demonstrated in RCTs (v. patient/provider experience) | $1930.1** [$1529.2, $231.1] | $3037.2** [$2170.4, $3904.9] | $1977.3** [$1293.6, $2661.1] |
| AE/side event risk | One percentage point reduction in risk of side effects | $63.1** [$52.1, $74.0] | $72.3** [$52.9, $91.7] | $66.4** [$47.5, $85.1] |
AE, adverse event; PFS, progression-free survival; RCT, randomized controlled trial; WTP, willingness to pay.
95% confidence intervals in brackets.
P < 0.01. *P < 0.05.
Discussion
Our study of mBC patients and oncology providers in the United States demonstrates the value information on PFS plays in treatment decision making and how patients and providers weigh PFS in the context of other treatment attributes. Our analysis of survival and treatment preferences demonstrates that there is significant variation in preferences among and across patients and providers. Contiguous periods of stable disease/PFS, the timing of contiguous periods of stable disease/PFS, OS, and cumulative periods of stable disease/PFS were all significant drivers of average patient preferences for survival. Nurses’ preferences were also driven by contiguous periods of stable disease/PFS, the timing of the contiguous PFS, and OS, but not by cumulative periods of PFS. Oncologists’ preferences were driven primarily by contiguous periods of stable disease/PFS and their timing. All respondents were willing to pay significant amounts to reduce the risk of side effects and for efficacy demonstrated in clinical trials rather than provider experience. Willingness to pay for reduced risk of side effects was notably aligned across the three respondent groups. Our analysis of treatment attribute preferences demonstrates that the sequencing of periods of stable disease (PFS) across remaining life (OS) significantly influences the treatment decisions of nurses only when considered jointly with other treatment characteristics. The results also suggest that patients and providers alike value high-quality clinical evidence in their decision making, and prefer treatments with fewer side effects. This study points not only to the challenge of surrogate endpoints in clinical trials, but also to their potential value. Rarely do patients consider their therapy choices as merely a decision between survival and progression.
As demonstrated in previous research, patients value a host of factors when choosing a therapy.15,29 It is possible that patients start with a baseline assumption about PFS and OS—assuming any therapy they are offered will at a minimum provide survival benefits. When respondents are asked to choose between survival scenarios alone, it is possible to parse out preferences for PFS versus OS, but in the context of a treatment decision when a variety of treatment characteristics are considered—specifically side effects, strength of evidence, and cost—the PFS/OS benefit may be assumed and other factors take precedence. WTP for a lower risk of side effects is not inconsistent with a preference for PFS. Progression-free survival is in part a question of health-related quality of life, where stable disease allows patients to proceed with their daily lives in a predictable way. Similarly, a lower risk of side effects also suggests a more predictable health-related quality of life and ability to carry on with typical life events. Thus, stable disease and fewer side effects may give patients a sense of control over their disease and improve their quality of life. These findings were consistent with our previously published qualitative work in that patients and providers recognized the benefits associated with having fewer side effects—or even having to monitor for side effects, thus allowing patients to live as “normal” a life as possible, and maintain independence and overall functioning.16
In the assessment of disease progression and survival preferences, patients placed more emphasis on the cumulative periods of PFS than OS. This is an important finding as this indicates the continued value of surrogate endpoints in clinical trials and the critical nature of communicating PFS benefits to patients making choices about treatment. Again, this is bolstered by the WTP analysis whereby high-quality clinical trial data are highly valued by patients and providers alike.
However, our finding that the length, timing, and contiguity of PFS, as well as OS, all strongly influence patient survival preferences highlights the importance of patient–provider communication to ensure alignment around treatment. One approach found to be effective in addressing gaps in patient–provider communication is shared decision making, a collaborative process that allows patients and their providers to make health care decisions together, while taking into account the best clinical evidence available and the patient’s individual values and preferences.30–33 Shared decision making has been proven to demonstrate positive outcomes among patients diagnosed with preference sensitive conditions and is increasingly deployed in the oncology setting.17,34,35 The present study highlights the critical need for providers to broaden their lens beyond primary endpoints such as OS, and to elicit patient preferences associated with surrogate endpoints such as PFS.
This study has several limitations and future research would benefit from a deeper exploration of the tradeoffs between PFS and OS, as well as the individual determinants of survival and treatment preferences. Importantly, we did not explicitly ask patients if they would be willing to trade a shorter period of survival for a longer period of stable disease, but instead used a stated preferences approach. While a stated preferences survey design provides insights into what patients value, the level of detail achieved through this approach can leave questions unanswered. For example, beyond pilot testing, we did not evaluate patient understanding of the meaning and interpretation of PFS (stable disease) or progressive disease. Some patients may have interpreted stable disease to mean a period of high function and good health, whereas others may have interpreted stable disease to mean a period of continued poor health, but with higher functional ability than periods of progressive disease. Patients may have not made a connection between stable disease and functioning, but instead interpreted stable disease as their condition simply not getting worse. Such interpretation may substantially influence preference choices made by the patient.
Furthermore, the omission of an opt-out choice in the discrete choice experiment may also have an influence on the treatment choices patient respondents made, particularly in the context of treatment for mBC. Prior research shows that approximately 1% to 3.5% of all breast cancer patients opt out of treatment.36–38 Late-stage breast cancer patients have higher treatment refusal rates, with 7% of stage III or IV breast cancer patients opting out of treatment in one study.39 Comparatively, late-stage lung cancer patients opt out of treatment at rates of 19% to 24%.39–41 In reviewing the utilization of an opt-out option in discrete choice experiments, we found that several studies examining treatment preferences in breast cancer patients included an opt-out choice in their surveys. Notably, Ngorsuraches and Thongkeaw found that participants selected the opt-out choice in 42% of all observations.42 The high usage of the opt-out choice is consistent with past research finding that people are more likely to select the opt-out choice when the tradeoff is complex or emotionally difficult.43 Furthermore, Veldwijk et al. found that inclusion of opt-out option in discrete choice experiments may skew results and lead to lower data quality.44 Thus, numerous studies examining treatment preferences in breast cancer patients do not include an opt-out choice.29,45,46 For example, daCosta DiBonaventura et al. found that 35% of their sample of mBC patients either had discontinued or were nonadherent to treatment at the time of their study.29 Similarly, 29% of participants in the study by Lalla et al. were not receiving any treatment for mBC at the time of survey completion.46 Despite these rates, neither study included a hypothetical opportunity to forego treatment options presented in their DCE, thereby forcing participants to select between hypothetical treatment scenarios, as we did in the present study.
Caution should be exercised when extrapolating our results to other patient and provider populations. While our study did not draw from a nationally representative sample of oncology providers, provider respondent characteristics are generally consistent with the oncology workforce.47,48 Race, ethnicity, and gender characteristics of our sample are similar to those of the oncology workforce.48 Furthermore, as our study design was an online convenience sample, our patient population was more highly educated, more likely to be Caucasian, and more likely to be fluent in English than the general mBC patient population. Thus, our results may not be generalizable to the perspectives of mBC patients from other socioeconomic, cultural, or non-English-speaking backgrounds and results may not be generalizable to the larger population of patients and oncology care providers.
Patients face complex decisions and tradeoffs about treatment, and their goals for treatment may not always align with clinicians’ goals for treatment. Previous research has demonstrated that when attitudes are aligned and the patient–physician alliance is strong, patients are more satisfied with their care, adherent to treatment, and optimistic about the usefulness of treatment.23 For breast cancer patients specifically, providers’ ability to effectively communicate information about treatment options has been shown to reduce treatment delays while increasing patients’ knowledge about breast cancer and receipt of breast conserving surgery.49 Despite weak evidence of the correlation between PFS and OS, these data suggest that OS is not the only priority for patients or providers. Clinical trials that focus only on prolonged longevity will likely fail to capture attributes of treatment that are important to these populations.50
Conclusion
Researchers and clinicians often presume that the value of surrogate endpoints like stable disease/PFS only have value if they increase OS. In contrast, our results suggest that mBC patients demonstrated a strong preference for longer periods of stable disease, even when OS was held fixed, highlighting the value of PFS to mBC patients even when it does not contribute to OS. Providers did not share this preference and were not WTP for stable disease. This suggests the critical need for improved patient-provider communication and shared decision-making in the oncology setting.
Supplemental Material
Supplemental material, MDM-PP-18-034.R2_appendix_online_supp for The Value of Progression-Free Survival in Metastatic Breast Cancer: Results From a Survey of Patients and Providers by Joanna P. MacEwan, Jason Doctor, Karen Mulligan, Suepattra G. May, Katharine Batt, Christopher Zacker, Darius Lakdawalla and Dana Goldman in MDM Policy & Practice
Acknowledgments
The authors would like to thank Carolyn Harley, Thanh G. N. Ton, Diana Vania, Michelle Brauer, and Meaghan Roach for research support, and Noe Marquez for assistance with designing the visual survey aids.
Footnotes
Financial support for this study was provided entirely by a contract with Novartis Oncology. Joanna MacEwan and Suepattra May are employees and Karen Mulligan is a past employee of Precision Health Economics, a research consultancy to the health and life science industries. Katharine Batt and Jason Doctor are Scientific Advisors to Precision Health Economics. Darius Lakdawalla and Dana Goldman serve as Scientific Advisors to Precision Health Economics and hold equity in its parent company, Precision Medicine Group. Christopher Zacker is an employee of Novartis Oncology, which provided funding to Precision Health Economics to conduct this study. Financial support for this study was provided entirely by a contract with Novartis Oncology. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
The share of respondents with an estimated coefficient/utility parameter falling below zero was calculated using the estimated mean and standard deviation for the cumulative normal distribution.
ORCID iDs: Suepattra G. May  https://orcid.org/0000-0002-7228-8440
https://orcid.org/0000-0002-7228-8440
Dana Goldman  https://orcid.org/0000-0001-8498-6396
https://orcid.org/0000-0001-8498-6396
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Joanna P. MacEwan, Precision Health Economics, Los Angeles, California
Jason Doctor, Precision Health Economics, Los Angeles, California.
Karen Mulligan, Precision Health Economics, Los Angeles, California.
Suepattra G. May, Precision Health Economics, Los Angeles, California.
Katharine Batt, Precision Health Economics, Los Angeles, California.
Christopher Zacker, Novartis Oncology, One Health Plaza, East Hanover, New Jersey.
Darius Lakdawalla, Precision Health Economics, Los Angeles, California.
Dana Goldman, Precision Health Economics, Los Angeles, California.
References
- 1. Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009;15(5):401–5. [DOI] [PubMed] [Google Scholar]
- 2. Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–13. BMJ. 2017;359:j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michiels S, Pugliano L, Marguet S, et al. Progression-free survival as surrogate end point for overall survival in clinical trials of HER2-targeted agents in HER2-positive metastatic breast cancer. Ann Oncol. 2016;27(6):1029–34. doi: 10.1093/annonc/mdw132 [DOI] [PubMed] [Google Scholar]
- 4. Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–98. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 5. Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of us food and drug administration approvals. JAMA Intern Med. 2015;175(12):1992–4. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 6. Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. 2014;32(12):1277–80. doi: 10.1200/jco.2013.53.8009 [DOI] [PubMed] [Google Scholar]
- 7. Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030–3. doi: 10.1200/jco.2011.38.7571 [DOI] [PubMed] [Google Scholar]
- 8. Kay A, Higgins J, Day AG, Meyer RM, Booth CM. Randomized controlled trials in the era of molecular oncology: methodology, biomarkers, and end points. Ann Oncol. 2011;23(6):1646–51. [DOI] [PubMed] [Google Scholar]
- 9. Shafrin J, Brookmeyer R, Peneva D, et al. The value of surrogate endpoints for predicting real-world survival across five cancer types. Curr Med Res Opin. 2016;32(4):731–9. doi: 10.1185/03007995.2016.1140027 [DOI] [PubMed] [Google Scholar]
- 10. Lakdawalla DN, Shafrin J, Hou N, et al. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health. 2017;20(7):866–75. doi: 10.1016/j.jval.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 11. Lakdawalla DN, Chou JW, Linthicum MT, MacEwan JP, Zhang J, Goldman DP. Evaluating expected costs and benefits of granting access to new treatments on the basis of progression-free survival in non–small-cell lung cancer. JAMA Oncol. 2015;1(2):196–202. doi: 10.1001/jamaoncol.2015.0203 [DOI] [PubMed] [Google Scholar]
- 12. Bognar K, Romley JA, Bae JP, Murray J, Chou JW, Lakdawalla DN. The role of imperfect surrogate endpoint information in drug approval and reimbursement decisions. J Health Econ. 2017;51:1–12. doi: 10.1016/j.jhealeco.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 13. Adunlin G, Cyrus JW, Dranitsaris G. Correlation between progression-free survival and overall survival in metastatic breast cancer patients receiving anthracyclines, taxanes, or targeted therapies: a trial-level meta-analysis. Breast Cancer Res Treat. 2015;154(3):591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu FX, Witt EA, Ebbinghaus S, Beyer GD, Basurto E, Joseph RW. Patient and oncology nurse preferences for the treatment options in advanced melanoma: a discrete choice experiment. Cancer Nurs. 2019;42(1):E52–E59. doi: 10.1097/ncc.0000000000000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. May SG, Chung AH, Vania DK, et al. Abstract P4-20-02: Value of cancer care for metastatic breast cancer patients and providers. Cancer Res. 2017;77(4 Suppl.):P4-20-02. doi: 10.1158/1538-7445.sabcs16-p4-20-02 [DOI] [Google Scholar]
- 17. Brom L, Snoo-Trimp D, Janine C, et al. Challenges in shared decision making in advanced cancer care: a qualitative longitudinal observational and interview study. Health Expect. 2017;20(1):69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mühlbacher AC, Nübling M. Analysis of physicians’ perspectives versus patients’ preferences: direct assessment and discrete choice experiments in the therapy of multiple myeloma. Eur J Health Econ. 2011;12(3):193–203. doi: 10.1007/s10198-010-0218-6 [DOI] [PubMed] [Google Scholar]
- 19. Bruera E, Willey JS, Palmer JL, Rosales M. Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–80. [DOI] [PubMed] [Google Scholar]
- 20. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489–95. doi: 10.1200/JCO.2008.20.3232 [DOI] [PubMed] [Google Scholar]
- 21. de Bekker-Grob EW, Bliemer MC, Donkers B, et al. Patients’ and urologists’ preferences for prostate cancer treatment: a discrete choice experiment. Br J Cancer. 2013;109(3):633–40. doi: 10.1038/bjc.2013.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krammer R, Heinzerling L. Therapy preferences in melanoma treatment—willingness to pay and preference of quality versus length of life of patients, physicians and healthy controls. PLoS One. 2014;9(11):e111237. doi: 10.1371/journal.pone.0111237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuertes JN, Mislowack A, Bennett J, et al. The physician-patient working alliance. Patient Educ Couns. 2007;66(1):29–36. doi: 10.1016/j.pec.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 24. Louviere J, Hensher D, Swait J. Conjoint preference elicitation methods in the broader context of random utility theory preference elicitation methods. In: Gustafsson A, Herrmann A, Huber F, eds. Conjoint Measurement. Berlin: Springer; 2000. p 167–98. [Google Scholar]
- 25. de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–72. [DOI] [PubMed] [Google Scholar]
- 26. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77. [DOI] [PubMed] [Google Scholar]
- 27. Hauber AB, Fairchild AO, Johnson FR. Quantifying benefit-risk preferences for medical interventions: an overview of a growing empirical literature. Appl Health Econ Health Policy. 2013;11(4):319–29. doi: 10.1007/s40258-013-0028-y [DOI] [PubMed] [Google Scholar]
- 28. Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15. [DOI] [PubMed] [Google Scholar]
- 29. daCosta DiBonaventura M, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–96. [PMC free article] [PubMed] [Google Scholar]
- 30. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. doi: 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–92. [DOI] [PubMed] [Google Scholar]
- 32. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–61. [DOI] [PubMed] [Google Scholar]
- 33. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32(2):276–84. [DOI] [PubMed] [Google Scholar]
- 34. Politi MC, Studts JL, Hayslip JW. Shared decision making in oncology practice: what do oncologists need to know? Oncologist. 2012;17(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Stam MA, Pieterse AH, van der Poel HG, et al. Shared decision-making in prostate cancer care—encouraging every patient to be actively involved in decision-making, or ensuring patients’ preferred level of involvement? J Urol. 2018;200(3):582–9. [DOI] [PubMed] [Google Scholar]
- 36. Joseph K, Vrouwe S, Kamruzzaman A, et al. Outcome analysis of breast cancer patients who declined evidence-based treatment. World J Surg Oncol. 2012;10:118. doi: 10.1186/1477-7819-10-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roder DM, Silva PD, Zorbas HN, et al. Adherence to recommended treatments for early invasive breast cancer: decisions of women attending surgeons in the breast cancer audit of Australia and New Zealand. Asian Pac J Cancer Prev. 2012;13(4):1675–82. doi: 10.7314/apjcp.2012.13.4.1675 [DOI] [PubMed] [Google Scholar]
- 38. Chen SJ, Kung PT, Huang KH, Wang YH, Tsai WC. Characteristics of the delayed or refusal therapy in breast cancer patients: a longitudinal population-based study in Taiwan. PLoS One. 2015;10(6):e0131305. doi: 10.1371/journal.pone.0131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149 [DOI] [PubMed] [Google Scholar]
- 40. Tabchi S, Kassouf E, Florescu M, Tehfe M, Blais N. Factors influencing treatment selection and survival in advanced lung cancer. Curr Oncol. 2017;24(2):e115–e122. doi: 10.3747/co.24.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suh WN, Kong KA, Han Y, et al. Risk factors associated with treatment refusal in lung cancer. Thorac Cancer. 2017;8(5):443–50. doi: 10.1111/1759-7714.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ngorsuraches S, Thongkeaw K. Patients’ preferences and willingness-to-pay for postmenopausal hormone receptor-positive, HER2-negative advanced breast cancer treatments after failure of standard treatments. Springerplus. 2015;4:674. doi: 10.1186/s40064-015-1482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luce MF. Choosing to avoid: coping with negatively emotion-laden consumer decisions. J Consum Res. 1998;24(4):409–33. doi: 10.1086/209518 [DOI] [Google Scholar]
- 44. Veldwijk J, Lambooij MS, de Bekker-Grob EW, Smit HA, de Wit GA. The effect of including an opt-out option in discrete choice experiments. PLoS One. 2014;9(11):e111805. doi: 10.1371/journal.pone.0111805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279–87. doi: 10.2147/IJWH.S31331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lalla D, Carlton R, Santos E, Bramley T, D’Souza A. Willingness to pay to avoid metastatic breast cancer treatment side effects: results from a conjoint analysis. Springerplus. 2014;3:350. doi: 10.1186/2193-1801-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. American Society of Clinical Oncology. The State of Cancer Care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract. 2017;13(4):e353–e394. doi: 10.1200/JOP.2016.020743 [DOI] [PubMed] [Google Scholar]
- 48. Towle E. Demographics of the US oncology workforce. J Oncol Pract. 2016;12(2):99. doi: 10.1200/JOP.2015.010124 [DOI] [PubMed] [Google Scholar]
- 49. Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52(7):1138–45. doi: 10.1111/j.1532-5415.2004.52312.x [DOI] [PubMed] [Google Scholar]
- 50. Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18(1):122. doi: 10.1186/s13063-017-1870-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MDM-PP-18-034.R2_appendix_online_supp for The Value of Progression-Free Survival in Metastatic Breast Cancer: Results From a Survey of Patients and Providers by Joanna P. MacEwan, Jason Doctor, Karen Mulligan, Suepattra G. May, Katharine Batt, Christopher Zacker, Darius Lakdawalla and Dana Goldman in MDM Policy & Practice