Hormonal results may be compromised in field samples maintained without cold storage. This study determined that progestagen, testosterone and cortisol concentrations in blood with anticoagulant were stable at 37°C up to 62 h. Without anticoagulant, progestagens also were stable, but cortisol and testosterone in blood decreased over time.
Keywords: Asian elephant, cortisol, hormone degradation, progestagens, testosterone
Abstract
The value of biological samples collected in the field is compromised if storage conditions result in analyte degradation, especially in warmer climates like Thailand. We evaluated the effects of time and temperature on immunoactive steroid hormone stability in Asian elephant (Elephas maximus) blood stored with and without an anti-coagulant before centrifugation. For each elephant (5 male, 5 female), whole blood was aliquoted (n = 2 ml each) into 13 red top (without anticoagulant) or purple top (with anticoagulant) tubes. One tube from each treatment was centrifuged immediately and the serum or plasma frozen at −20°C (Time 0, T0). The remaining 12 aliquots were divided into stored temperature groups: 4°C, room temperature (RT, ~22°C), and 37°C, and centrifuged after 6, 24, 48 and 62 h of storage. Serum and plasma concentrations of progestagens in females, testosterone in males and cortisol in both sexes were quantified by validated enzyme immunoassays. Steroid concentration differences from T0 were determined by a randomized complete block ANOVA and Dunnett’s tests. The only evidence of hormone degradation was cortisol and testosterone concentrations in serum stored at 37°C. Testosterone concentrations declined by 34% at 48 h and 52% at 62 h, cortisol was decreased by 19% after 48 h and 27% after 62 h at 37°C, respectively. None of the other aliquots displayed significant changes over time at any temperature. In conclusion, steroids appear to be stable in blood for nearly 3 days at room or refrigeration temperatures before centrifugation; steroids in samples with ethylenediaminetetraacetic acid were particularly stable. However, warmer temperatures may negatively affect steroids stored without anti-coagulant, perhaps due to red blood cell metabolism. Thus, under field conditions with no access to cold or freezer temperatures, collection of plasma is a better choice for elephants up to at least 62 h before centrifugation.
Introduction
Asian elephants (Elephas maximus) are endangered throughout most of their natural ranges (EN-A2c, ver. 3.1; IUCN Red list 2009), with several populations heading toward extinction unless mitigating efforts are successful in stemming population declines. From studies on captive animals, much is known about elephant biology, particularly through analyses of serum or plasma hormones (Brown, 2014). Assessments of progestagens are key to monitoring female reproductive condition (Brown, 2014), whereas testosterone is useful in studying musth, a period characterized by temporal gland secretions, urine dribbling and more antagonistic behaviors (Rasmussen et al., 1984; Dickerman et al., 1997). Cortisol increases under acute and chronic stress conditions (Woolley et al., 2008; Ghosal et al., 2013; Boyle et al., 2015; Moltesen et al., 2016; Benítez-Dorta et al., 2017) and if prolonged, can suppress reproductive function (Wayland et al., 2002; Wingfield and Sapolsky, 2003; Breuner et al., 2008), leading to irregular cycling and acyclicity (Fanson et al., 2014). Cortisol increases during normal physiological states as well, including the follicular phase of the estrous cycle (Fanson et al., 2014) and musth (Brown et al., 2007). Non-invasive steroid monitoring methods (urine, feces, saliva, milk and hair) have been developed (Verkerk et al., 1998; Brown et al., 2010; Marcilla et al., 2012; Mack and Fokidis, 2017; Pawluski et al., 2017; Rakotoniaina et al., 2017), with feces being particularly well suited for field studies. However, under some circumstances (e.g. collections under field anesthesia), measures of circulating hormones in serum or plasma are desired.
Stability of hormones in blood varies among species, sample types and preservation methods (Wiseman et al., 1983; Abal et al., 1996; Taylor and Schuett, 2004; Hegstad-Davies, 2006; Jones et al., 2007; Tahir et al., 2013). It is recommended that blood be centrifuged soon after collection to obtain serum or plasma, and frozen immediately. However, for samples collected in the field, it can take hours or even days to reach laboratory processing facilities. Little is known about how steroids degrade in elephant blood, so the goal of this study was to determine the effects of storage time and temperature on immunoactive stability of steroids in Asian elephant blood: progestagens in females, testosterone in males and cortisol in both sexes.
Materials and methods
Animals and sample collection
This study was approved by the Faculty of Veterinary Medicine, Chiang Mai University, Animal Care and Use Committee (FVM-ACUC; permit number S39/2559). Female (n = 5; age range, 9–35 yr; mean, 19.6 ± 10.6 yr) and male (n = 5; age range, 15–50 yr; mean, 26.2 ± 14.0 yr) Asian elephants were housed at the Baan Chang Elephant Camp in northern Thailand (latitude, 19°06′51.6“N; longitude 98°53’39.2”E). Elephants were fed primarily corn stalk, napier grass (Pennisetum purpureum) and bana grass (P. purpureum X, Petalophyllum americanum hybrid) with regular access to water. Elephants participated in tourist activities, including bareback riding, bathing and feeding, and were in good health at the time of the study based on physical examinations by elephant camp veterinarians. Blood samples were collected from an ear vein by elephant camp staff or Chiang Mai University veterinarians.
In Study 1, 30 ml of blood was collected from each elephant using a 22-gauge IV catheter and 50-ml syringe between 1000–1100 h. Blood in 2-ml aliquots was divided among 13 red top tubes without anticoagulant (serum) and was kept in a styrofoam box with ice (maintained ~4°C) for transportation to Chiang Mai University. Upon arrival at the laboratory (<4 h), one tube from each elephant was centrifuged at 1500× g for 10 minutes, representing Time 0 (T0). The other 12 tubes were centrifuged after 6, 24, 48 and 62 h of storage at 4°C, room temperature (~22°C), or in a 37°C controlled temperature chamber (typical ambient temperature in the warm season).
Study 2 was conducted 2 days later using the same elephants, with 30-ml of blood aliquoted into 13 purple top tubes with ethylenediaminetetraacetic acid (EDTA) anticoagulant (plasma), and the plasma harvested after 0, 6, 24, 48 and 62 h of storage at 4°C, RT or 37°C before centrifugation.
Serum and plasma samples (0.5–0.8 ml) were stored at −20°C until hormone analyses.
Hormone analysis
All chemicals were obtained from Sigma Chemical Company (St. Louis, MO), unless otherwise stated. Concentrations of testosterone and cortisol in males, progestogens and cortisol in females were quantified by enzyme immunoassays (EIAs) validated for elephants using antibodies for progesterone (monoclonal, CL425; Brown et al., 2004), testosterone (polyclonal, R156/7; Somgird et al., 2016) and cortisol (polyclonal, R4866; Somgird et al., 2016). The monoclonal progesterone antibody crossreacts with reduced pregnanes present in elephant serum (Brown, 2014), and are herein referred to as ‘progestagens’. Briefly, 96-well plates (catalog no. 07–200-39; Fisher Scientific, Pittsburgh, PA, USA) were pre-coated with secondary antibody diluted in coating buffer (catalog no. X108, 20X; Arbor Assays, Ann Arbor, MI): 150 μl (10 μg/ml) goat anti-mouse IgG for progesterone, and goat anti-rabbit IgG (Arbor Assays) for cortisol and testosterone EIAs. Coated plates were prepared by incubating at RT for 15–24 h. Wells were emptied and blotted dry, followed by adding 250 μl blocking buffer (100 mM phosphate, 150 mM sodium chloride, 1% Tween20, 0.09% sodium azide, 10% sucrose, pH 7.5) and incubating for 15–24 h at RT. After incubation, wells were emptied, blotted and dried in a Sanpla Dry Keeper (Sanplatec Corp., Auto A-3, Japan) with loose desiccant in the bottom. After drying (humidity <20%), plates were heat-sealed in a foil bag with a 1-g desiccant packet, and stored at 4°C until use.
Samples or standards (50 μl) (progestagens: Sigma Diagnostics Cat. #P0130, range 0.78–200 pg/well; testosterone: Steraloids Cat. #A6950, range 2.3–600 pg/well; cortisol: Sigma Diagnostics Cat. #H4001, range 3.9–1000 pg/well) were added to appropriate wells. Next, 25 μl of steroid horseradish peroxidase conjugate (HRP; progestagens 1:40000 dilution; testosterone 1:10000 dilution; cortisol 1:16000 dilution) was immediately added to each well, followed by 25 μl of primary antibody (progestagens, 1:10000 dilution; testosterone, 1:8500 dilution; cortisol, 1:75000 dilution) added to all but non-specific binding wells and incubated at RT for 1 h. Plates were then washed four times with wash buffer (1:20 dilution, 20X Wash Buffer Part No. X007; Arbor Assays, MI) and 100 μl of TMB substrate solution was added, followed by incubation for 45–60 min at RT without shaking. The absorbance was measured at 405 nm by a microplate reader (TECAN). Assay sensitivities were 0.078, 0.047 and 0.078 ng/ml for progestagens, testosterone and cortisol, respectively. Samples were analyzed in duplicate; inter- and intra-assay coefficients of variation were <10% and <15% (n = 6 for progestagens, n = 5 for testosterone, n = 11 assays for cortisol), respectively. Progesterone, testosterone and cortisol EIAs were validated in Thailand for elephant serum by demonstrating parallelism between serial dilutions of neat serum or plasma and the respective standard curves (Pearson’s correlation coefficients, r > 0.95).
Statistical analyses
Aliquots from the same animals were assigned as a block following a randomized complete block design. Each aliquot was randomly assigned to a time and temperature treatment. Hormone concentrations were converted into percentages of T0 values by the following equation: [concentration Tx (x = 6, 24, 48, 62)]/(concentration T0). Percentage data (n = 5 for testosterone and progesterone, n = 10 for cortisol; no missing data points) are presented as mean ± standard deviation (SD). The effect of time (0, 6, 24, 48 or 62 h) on hormone concentration was assessed using a randomized complete block ANOVA, with concentration as the dependent variable and time as a fixed effect. Separate models were run for each substrate, temperature and hormone combination. Normality of residuals was evaluated by plotting QQ graphs, and the homogeneity of variance assessed by plotting residuals and fitted values. Most models did not violate normality and homogeneity of variance assumptions; however, slight deviations from a normal distribution were observed in some models as evidenced by residuals deviating from a straight line. Results were still used because ANOVA is particularly robust to normality problems (Glass et al. 1972; Harwell et al. 1992: Lix et al. 1996; Khan and Rayner, 2003; Blanca et al., 2017). If time was significant at P < 0.05, a post hoc Dunnett’s test was used to compare differences in hormone concentrations between time points. Statistical significance was set as α = 0.05. All statistical analyses were performed using R version 3.4.4 (R Development Core Team, 2015).
Results
Hormone concentrations
Descriptive data are presented in Table 1, highlighting the variability in mean and mean range progestagens, testosterone and cortisol values across individuals. Progestagen concentrations were above baseline (0.05 ng/ml) indicating females were in the luteal phase of the cycle (Brown et al., 2004). Bulls were not in musth, as reflected by testosterone values <5 ng/ml (Brown et al., 2007).
Table 1.
Mean ± SD and range values for progestagens, testosterone and cortisol concentration in samples at T0 in female (n = 5) and male (n = 5) Asian elephants in Thailand
| Hormone | Number of elephants | Serum | Plasma |
|---|---|---|---|
| Progestagens (ng/ml) | 5 | 3.16 ± 2.20 (0.26–5.99) | 2.80 ± 1.79 (0.34–5.00) |
| Testosterone (ng/ml) | 5 | 1.85 ± 1.19 (0.58–3.3) | 1.88 ± 1.48 (0.35–3.97) |
| Cortisol (ng/ml) | |||
| Male | 5 | 1.12 ± 0.50 (0.65–1.73) | 0.97 ± 0.61 (0.33–1.88) |
| Female | 5 | 0.60 ± 0.28 (0.26–1.00) | 0.56 ± 0.35 (0.22–1.12) |
Study 1
Progestagen concentrations in female serum were not significantly affected by either storage time or temperature.
Testosterone concentrations in male serum did not change significantly when stored at 4°C or RT. However, time did have a significant effect on testosterone concentrations when stored at 37°C (F4,16 = 11.99, P = 0.0001). Post-hoc comparisons indicated that testosterone concentrations were 34% lower than T0 after 48 h (t16 = 4.16, P = 0.0027), and 52% lower after 62 h (t16 = 6.33, P = 0.0001; Fig. 1).
Figure 1.
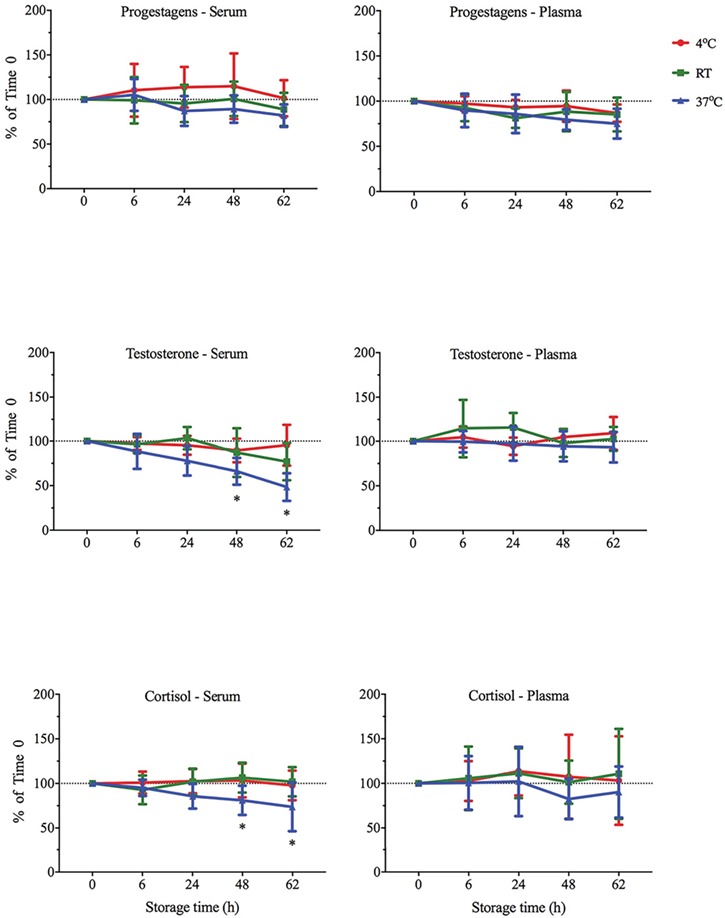
Mean ± SD concentrations of progestagen (n = 5), testosterone (n = 5) and cortisol (n = 10) in elephant serum and plasma samples stored at 4°C, RT (~22°C) and 37°C for up to 62 h before centrifugation Data are expressed as a percentage of T0 values. For each treatment, asterisks denote values that differ from the initial T0 concentration (P < 0.05).
In addition, cortisol concentrations in male and female serum did not change significantly when stored at 4°C or RT; however, time did have a significant effect on cortisol concentrations when stored at 37°C (F4,36 = 4.43, P = 0.0051). Post-hoc comparisons indicated that cortisol concentrations were 19% lower than T0 after 48 h (t36 = 2.996, P = 0.017), and 27% lower after 62 h (t36 = 3.624, P = 0.0003; Fig. 1).
Study 2
There were no significant time and temperature of storage effects on concentrations of plasma steroids relative to T0 (Fig. 1).
Discussion
This study investigated the impact of temperature and time on steroid hormone (progestagens, testosterone and cortisol) degradation in blood of male and female Asian elephants stored with or without anticoagulant before centrifugation, and found that storage at 4°C or RT (~22°C) for at least 62 h had little impact on serum or plasma concentrations. All steroids in blood with anticoagulant were not significantly different from T0 when stored at 37°C for up to 62 h before plasma harvesting. By contrast, both testosterone and cortisol in serum stored at 37°C declined significantly within 48 h, ~34% for testosterone and 20% for cortisol, and by 62 h levels were only half to a quarter of original levels, respectively. These findings agree with reports in some species, but not others, and highlight species and steroid differences in hormone stability between sample types.
Studies on the stability of progesterone in blood have yielded mixed results. While some found progesterone to be quite stable, human serum unaltered after 48 h at 22°C (Wiseman et al., 1983; Diver et al., 1994; Jones et al., 2007) and dog serum and plasma (EDTA) stable for 2 weeks at 20–22°C (Tahir et al., 2013), others found that progesterone degrades rapidly. For example, progesterone in dog serum (2 h) declined more quickly than in dog plasma (heparin; 5 h) at 4°C (Volkmann, 2006). In cows, serum or plasma (heparin) progesterone decreased 50% within 24 h at 22°C (Wiseman et al., 1983) and > 70% after 72 h at 22–26°C (Reimers et al., 1983). In another study, serum progesterone was only 40% of initial concentrations after 8 h and < 10% after 24 h at varying temperatures (De Castro et al., 2004). Declining progesterone concentrations in blood before centrifugation may be due to the presence of blood cells and effects on steroid metabolism (Ohtsuka and Koide, 1969; Vahdat et al., 1981, 1984). Cytochrome P-450 in lymphocytes and platelets also can metabolize steroids (Lemberg and Barrett 1973; Hodgson and Guthrie, 1980). However, in elephants, degradation of progestagens in serum or plasma was not observed at any storage temperature, at least up to 62 h, suggesting blood cell steroid metabolism of this steroid did not occur during that time. Perhaps this is related to 5α-reduced pregnanes (e.g. 5α-pregnane-3,20-dione, 5α-pregnane-3-ol-20 one, 17α-hydroxyprogesterone) being the predominant luteal steroids, rather than progesterone (Hodges, 1998).
Testosterone concentrations in elephant bull samples stored at 4°C and RT were stable in serum and plasma for at least 62 h, but decreased within 48 h in samples stored at 37°C without anticoagulant. In goats, testosterone was stable in samples with fluoride-potassium oxalate for at least 24 h at 22°C (Fahmi et al., 1985). Similarly, in diamondback rattlesnakes, testosterone concentrations in plasma were unchanged during storage at 0°C for up to 24 h (Taylor and Schuett, 2004). However, concentrations in that study were equally stable at 40°C, and did not show the decline observed in elephants at an elevated temperature (37°C in our study). Testosterone in human blood exhibited no clinically relevant changes during storage at RT for 168 h (Diver et al., 1994). However, a more recent study found testosterone concentrations in human samples without anticoagulant actually increased within the first 48 h of storage at 22°C (Jones et al., 2007). Similarly, in dogs, testosterone in plasma stored at RT were unchanged for up to 144 h, but in serum, concentrations were increased at 48 h (Frankland, 1985). Thus, there can be differences in steroid immunoactive stability between samples stored with and without anticoagulant, with serum values being influenced more.
Cortisol concentrations in the blood of cows with and without anticoagulant were stable at 25°C for at least 62 h (Reimers et al., 1983), and at 4°C for up to 40 h in the blood of dogs with (EDTA, heparin) or without anticoagulant (Olson et al., 1981), and at RT in gray seals (Bennett et al., 2012), consistent with the results of this study. By contrast, cortisol in human blood stored in heparin was increased by ~15% in plasma samples stored at RT by 48 h, and at 4°C by 148 h (Diver et al., 1994).
Conclusion
Immunoactive concentrations of progestagens, testosterone and cortisol in blood stored with anticoagulant were not significantly different from T0 over time, and exhibited no significant changes when stored at 4°C, RT or 37°C for up to 62 h. For blood without anticoagulant, serum progestagens also were not significantly different from T0 across all temperatures and times of storage. However, serum cortisol and testosterone showed significant decreases in concentrations at 48 and 62 h of storage at 37°C.
Acknowledgements
Special thanks to the Baan Chang Elephant Camp owners, and elephant mahouts for assistance and allowing us to work with the elephants. We also thank our colleagues, Ms Patcharapa Towiboon, Mr Pallop Tankaew, Dr Patiparn Toin and Dr Tithipong Plangsangmas for help with sample processing and laboratory analyses.
Funding
This work was supported by the Faculty of Veterinary Medicine, Chiang Mai University (CMUMIS: R000017939) and Center of Elephant and Wildlife Research, Chiang Mai University, with training support to Mu-Yao Li from the Center of Elephant and Wildlife Research, Chiang Mai University.
References
- Abal MA, Ghezzi M, Quiroga M, SolanaH AN (1996) Concentrations of progesterone during storage of whole blood from llama (Lama glama): effects of anticoagulants, storage time and temperature. Acta Vet Scand 37: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-Dorta V, Caballero MJ, Betancor MB, Manchado M, Tort L, Zamorana MJ, Izquiero M, Montero D (2017) Effects of thermal stress on the expression of glucocorticoid receptor complex linked genes in Senegalese sole (Solea senegalensis): acute and adaptive stress responses. Gen Comp Endocrinol 252:173–185. doi: 10.1016/j.ygcen.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Moss SEW, PomeroyP Speakman JR, Fedas MA (2012) Effects of handling regime and sex on changes in cortisol, thyroid hormones and body mass in fasting grey seal pups. Comp Biochem Physiol A Mol Integr Physiol 161: 69–76. doi: 10.1016/j.cbpa.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Blanca MJ, Alarcón R, Arnau J (2017) Non-normal data: is ANOVA still a valid option. Psicothema 29: 552–557. [DOI] [PubMed] [Google Scholar]
- Boyle SA, RobertsB PBM, BlakeMR LSE, Marshall JJ, Smith A, Hadcke A, Falcone JF, Kouba AJ (2015) Assessment of flooring renovations on African elephant (Loxodonta africana) behavior and glucocorticoid response. PLoS ONE 10: e0141009 10.1371/journal.pone.0141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP (2008) In search of relationships between the acute adrenocortical response and fitness. Gen Com Endocrinol 157:288–295. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Brown JL, Olson D, Keele M, Freeman EW (2004) Survey of the reproductive cyclicity status of Asian and African elephants in North America. Zoo Biol 23: 309–321. [Google Scholar]
- Brown JL, Somerville M, Riddle HS, Keele M, Duer CK, Freeman EW (2007) Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen Comp Endocrinol 151: 153–162. [DOI] [PubMed] [Google Scholar]
- Brown JL, Kersey DC, Walker SL (2010) Assessment of luteinizing hormone and prolactin immunoactivity in Asian and African elephant urine using assays validated for serum. Gen Comp Endocrinol 69:138–143. doi: 10.1016/j.ygcen.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Brown JL. (2014) Comparative reproductive biology of elephants In WV Holt, JL Brown, P Comizzoli, eds, Reproductive Sciences in Animal Conservation - Progress and Prospects. Advances in Experimental Medicine and Biology. Springer Science and Business Media, New York, NY, pp. 135–169 [DOI] [PubMed] [Google Scholar]
- De Castro T, Valdez L, Rodriguez M, Benquet N, Rubianes E (2004) Decline in assayable progesterone in bovine serum under different storage conditions. Trop Anim Health Prod 36: 381–384. 10.1023/B:TROP.0000026669.06351.26. [DOI] [PubMed] [Google Scholar]
- Dickerman RD, Zachariah NY, Fouraker M, Mcconathy WJ (1997) Neuroendocrine-associated behavioral patterns in the male Asian elephant (Elephas maximus). Physiol Behav 61: 771–773. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Hughes JG, Hutton JL, West CR, Hipkin LJ (1994) The long-term stability in whole blood of 14 commonly-requested hormone analytes. Ann Clin Biochem 31: 561–565. [DOI] [PubMed] [Google Scholar]
- Fahmi HA, Williamson NB, Tibary A, Hegstad RL (1985) The influence of some sample handling factors on progesterone and testosterone analysis in goats. Theriogenology 24: 227–233. [DOI] [PubMed] [Google Scholar]
- Fanson KV, Keeley T, Fanson BG (2014) Cyclic changes in cortisol across the estrous cycle in parous and nulliparous Asian elephants. Endocr Connect 3:57–66. doi: 10.1530/EC-14-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland AL. (1985) Canine testosterone concentrations in samples stored at room temperature. Br Vet J 141: 308–311. 10.1016/0007-1935(85)90068-5. [DOI] [PubMed] [Google Scholar]
- Ghosal R, Ganswindt A, Seshagiri PB, Sukumar R (2013) Endocrine correlates of musth in free-ranging Asian elephants (Elephas maximus) determined by non-invasive faecal steroid hormone metabolite measurements. PLoS ONE 8: e84787 10.1371/journal.pone.0084787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GV, Peckham PD, Sanders JR (1972) Consequences of failure to meet assumptions underlying fixed effects analyses of variance and covariance. Rev Educ Res 42: 237–288. [Google Scholar]
- Harwell MR, Rubinstein EN, Hayes WS, Olds CC (1992) Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. J Educ Stat 17: 315–339. [Google Scholar]
- Lix LM, Keselman JC, Keselman HJ (1996) Consequences of assumption violations revisited: a quantitative review of alternatives to the one-way analysis of variance F test. Rev Educ Res 66: 579–619. [Google Scholar]
- Hegstad-Davies RL. (2006) A review of sample handling considerations for reproductive and thyroid hormone measurement in serum or plasma. Theriogenology 66: 592–598. [DOI] [PubMed] [Google Scholar]
- Hodges JK. (1998) Endocrinology of the ovarian cycle and pregnancy in the Asian (Elephas maximus) and African (Loxodonta africana) elephant. Anim Reprod Sci 53: 3–18. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Guthrie FE (1980) Introduction to Biochemical Toxicology. Elsevier, North Holland, New York, p. 690 [Google Scholar]
- Jones ME, Folkerd EJ, Doody DA, Iqbal J, Dowsett M, Ashworth A, Swerdlow AJ (2007) Effect of delays in processing blood samples on measured endogenous plasma sex hormone levels in women. Cancer Epidemiol Biomarkers Prev 16: 1136–1139. [DOI] [PubMed] [Google Scholar]
- Khan A, Rayner GD (2003) Robustness to non-normality of common tests for the many-sample location problem. J Appl Math Decis Sci 7: 187–206. [Google Scholar]
- Lemberg R, Barrett J (1973) Cytochromes. Academic Press, New York: pp 4. [Google Scholar]
- Mack Z, Fokidis HB (2017) A novel method for assessing chronic cortisol concentrations in dogs using the nail as a source. Domest Anim Endocrinol 59: 53–57. doi: 10.1016/j.domaniend.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Marcilla AM, Urios V, Limiñana R (2012) Seasonal rhythms of salivary cortisol secretion in captive Asian elephants (Elephas maximus). Gen Comp Endocrinol 176: 259–264. doi: 10.1016/j.ygcen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Moltesen M, Laursen DC, Thörnqvist P, Andersson MÅ, Winberg S, Höglund E (2016) Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss). J Exp Biol 219: 3907–3914. doi: 10.1242/jeb.139857. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E, Koide SS (1969) Incorporation of steroids into human, dog, and duck erythrocytes. Gen Comp Endocriol 12: 598–603. [DOI] [PubMed] [Google Scholar]
- Olson PN, Bowen RA, Husted PW, Nett TM (1981) Effects of storage on concentration of hydrocortisone (cortisol) in canine serum and plasma. Am J Vet Res 42: 1618–1620. [PubMed] [Google Scholar]
- Pawluski J, Jego P, Henry S, Bruchet A, Palme R, Coste C, Hausberger M (2017) Low plasma cortisol and fecal cortisol metabolite measures as indicators of compromised welfare in domestic horses (Equus caballus). PLoS ONE 12: e0182257 10.1371/journal.pone.0182257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotoniaina JH, Kappeler PM, Kaesler E, Hämäläinen AM, Kirschbaum C, Kraus C (2017) Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecol 17:30 doi: 10.1186/s12898-017-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LE, Buss IO, Hess DL, Schmidt MJ (1984) Testosterone and dihydrotestosterone concentrations in elephant serum and temporal gland secretions. Biol Reprod 30: 352–362. [DOI] [PubMed] [Google Scholar]
- Reimers TJ, McCann JP, Cowan RG (1983) Effects of storage times and temperatures on T3, T4, LH, prolactin, insulin, cortisol and progesterone concentrations in blood samples from cows. J Anim Sci 57: 683–691. [DOI] [PubMed] [Google Scholar]
- Development Core R. (2015) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. ISBN 3–900051–07-0. http://www.r-project.org/.
- Somgird C, Homkong P, Sripiboon S, Brown JL, Stout TA, Colenbrander B, Mahasawangkul S, Thitaram C (2016) Potential of a gonadotropin-releasing hormone vaccine to suppress musth in captive male Asian elephants (Elephas maximus). Anim. Reprod. Sci 164: 111–120. doi: 10.1016/j.anireprosci.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Tahir MZ, Thoumire S, Raffaelli M, Grimard B, Reynaud K, Chastnt-Maillard S (2013) Effect of blood handling conditions on progesterone assay results obtained by chemiluminescence in the bitch. Domest Anim Endocrinol 45:141–144. doi: 10.1016/j.domaniend.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Schuett GW (2004) Effect of temperature and storage duration on the stability of steroid hormones in blood samples from western diamond-backed rattlesnakes (Crotalus atrox). Herpetol Rev 35: 14–17. [Google Scholar]
- Vahdat F, Hurtgen JP, Whitmore HL, Seguin BE, Johnston SD (1981) Decline in assayable progesterone in bovine plasma: effect of time, temperature, anticoagulant, and presence of blood cells. Am J Vet Res 42: 521–522. [PubMed] [Google Scholar]
- Vahdat F, Seguin BE, Whitmore HL, Johnston SD (1984) Role of blood cells in degradation of progesterone in bovine blood. Am J Vet Res 45: 240–243. [PubMed] [Google Scholar]
- Verkerk GA, Phipps AM, Carragher JF, Matthews LR, Stelwagen K (1998) Characterization of milk cortisol concentrations as a measure of short-term stress responses in lactating dairy cows. Anim Welf 7: 77–86(10). [Google Scholar]
- Volkmann DH. (2006) The effects of storage time and temperature and anticoagulant on laboratory measurements of canine blood progesterone concentrations. Theriogenology 66: 1583–1586. [DOI] [PubMed] [Google Scholar]
- Wayland M, Gilchrist HG, Marchant T, Keating J, Smits JE (2002) Immune function, stress response, and body condition in arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ Res 90: 47–60. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM (2003) Reproduction and resistance to stress: when and how. J Neuroendocrinol 15: 711–724. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Vincent DL, Thomford PL, Scheffrahn NS, Sargent GF, Kesler DJ (1983) Changes in porcine, ovine, bovine and equine blood progesterone concentrations between collection and centrifugation. Anim Reprod Sci 5: 157–165. [Google Scholar]
- Woolley L, Millspaugh JJ, Woods RJ, Rensburg SJ, Mackey RL, Page B, Slotow R (2008) Population and individual elephant response to a catastrophic fire in Pilanesberg National Park. PLoS ONE 3: e3233 10.1371/journal.pone.0003233. [DOI] [PMC free article] [PubMed] [Google Scholar]


