Abstract
Purpose
ErbB-2 (human epidermal growth factor receptor 2) overexpression may be predictive of relative resistance and/or sensitivity to specific chemotherapeutic agents. Results from a previous study from the Cancer and Leukemia Group B (CALGB 8541) demonstrated an interaction between ErbB-2 and increasing dose of adjuvant cyclophosphamide, doxorubicin, and fluorouracil (CAF) chemotherapy. Other studies have suggested that evaluation of the phosphorylated/activated form of ErbB-2 might be more precise in defining the impact of ErbB-2 in breast cancer. We have evaluated tumor tissue sections from CALGB 8541 patients to determine whether the interaction of ErbB-2 with CAF dose is dependent on ErbB-2 activation state, and whether phosphorylated ErbB-2 is an adverse prognostic factor in patients treated with CAF.
Patients and Methods
Patients were randomly assigned to one of three dosing regimens of CAF. Paraffin samples from 992 of 1,572 patients who participated in CALGB 8541 were available. Of the 570 tumors with any staining for ErbB-2, 488 had tissue available for assay for phosphorylated ErbB-2, which was performed by immunohistochemistry.
Results
Of 910 total assessable cases, 112 of 488 ErbB-2-positive cases (23%) stained positively for phosphorylated ErbB-2. The previously described interaction of dosing regimen of CAF with ErbB-2 was not dependent on phosphorylation status of ErbB-2.
Conclusion
Monitoring phosphorylation of ErbB-2 with an antiphospho-ErbB-2 antibody did not add further precision to identifying those patients most likely to benefit from increased dose of anthracycline-based adjuvant chemotherapy. Favorable outcomes are observed in ErbB-2-overexpressing patients treated with high-dose CAF regardless of ErbB-2 phosphorylation state.
INTRODUCTION
ErbB-2 (human epidermal growth factor receptor 2 [HER-2]) overexpression is both prognostic and predictive in breast cancer.1-4 Assaying ErbB-2 activity by phospho-ErbB-2-specific immunohistochemistry (IHC) showed that the minority of ErbB-2-overexpressing tumors had phosphorylated receptor, and in a large series of patients most of whom did not receive adjuvant chemotherapy, it was specifically the tumors with phospho-ErbB-2 that had poor prognosis.5-8 In this article, we study the influence of ErbB-2 activation on outcome of patients treated with a defined modern adjuvant chemotherapy regimen.
Cancer and Leukemia Group B (CALGB) 8541 was a randomized comparison of three doses and dose intensities (referred to as high, medium, and low-dose, detailed in Patients Methods) of adjuvant cyclophosphamide, doxorubicin, and fluorouracil (CAF) for stage II, lymph node-positive breast cancer.9,10 The high-dose regimen (a standard dose in current practice) was found to be superior. In a companion basic science correlative study, a subset of cases were analyzed for ErbB-2.11-13 All three dosing regimens had equivalent outcomes for patients expressing ErbB-2-negative/low level, while patients with high ErbB-2 expression had a significant improvement in outcome with the high-dose regimen. This observation raised the hypothesis that ErbB-2-overexpressing tumors may be relatively chemotherapy resistant, requiring higher doses of chemotherapy. While high ErbB-2 expression was an adverse prognostic factor in this overall study, curiously the ErbB-2-high patients that received the high-dose chemotherapy regimen had the best disease-free survival (DFS) and overall survival (OS). This latter finding raised the hypothesis that ErbB-2 overexpression might be associated with enhanced sensitivity to this regimen, such that if high-dose CAF is used, ErbB-2 overexpression becomes a favorable prognostic factor.
In this study, we examine the impact of ErbB-2 phosphorylation on outcomes in CALGB 8541. The goal was to determine if assaying phospho-ErbB-2 further refines the impact previously noted for ErbB-2 in this population, and to determine the prognostic impact of phosphorylated ErbB-2 in patients with breast cancer treated with adjuvant anthracycline.
PATIENTS AND METHODS
Patient Selection
CALGB 8541 enrolled 1,572 women with American Joint Committee on Cancer (fifth edition) stage II breast cancer (T1-2, N1, M0) from January 1985 through April 1991.9,10 Patients were randomly assigned to one of three regimens of adjuvant CAF. The regimens differed in their dose and dose intensity, and were referred to as high, medium, and low dose. The high-dose group received four cycles of cyclophosphamide 600 mg/m2, doxorubicin 60 mg/m2, and fluorouracil 600 mg/m2; the medium dose group received six cycles of the same drugs at 400 mg/m2, 40 mg/m2, and 400 mg/m2, respectively; and the low-dose group received four cycles of the same drugs at 300 mg/m2, 30 mg/m2, and 300 mg/m2, respectively. All drugs were administered intravenously on day 1 of a 28-day cycle, with fluorouracil repeated on day 8. In the fourth year of the trial, an amendment was made to recommend tamoxifen for 5 years after chemotherapy in a nonrandomized manner for postmenopausal patients with estrogen receptor–positive tumors. All patients gave written institutional review board–approved informed consent in accordance with institutional and existing federal guidelines. The median follow-up was slightly longer than 11 years.
IHC Analysis of ErbB-2 Overexpression
ErbB-2 IHC was performed in the laboratory (of A.D.T.) as described previously.11,12 Some samples were assayed using the OA-11-854 polyclonal anti-ErbB-2 antibody (Cambridge Research Biochemicals, Wilmington, DE), while others were assayed using the monoclonal CB11 antibody (BioGenex Laboratories, San Ramon, CA) after the former became unavailable. Results were recorded as the estimated percentage of invasive tumor cells demonstrating distinct membrane staining. For this study, cases were considered ErbB-2-low if the score was 0% to 49% and ErbB-2-high if ≥ 50%. This cutoff was used in previous publications regarding ErbB-2 expression in this data set.11,12
IHC Analysis of Activated (Phosphorylated) ErbB-2 (P-ErbB-2) With Antibody PN2A
IHC was performed as described previously.8 Briefly, 4 μm formalin-fixed, paraffin-embedded sections were deparaffinized, rehydrated, and endogenous peroxidase was blocked with 2% hydrogen peroxide. Antigen retrieval was performed with 10 mmol/L sodium citrate buffer, pH 6.0, and microwaving for 15 minutes. After slides cooled to room temperature, they were washed with distilled water and phosphate-buffered saline (PBS)/0.05% Tween-20. Affinity purified6 PN2A5 was applied at 5 μg/mL overnight at 4°C. Sections were incubated at room temperature for 30 minutes with biotinylated horse-antimouse secondary antibody (Vector Labs, Burlingame, CA; diluted at 1:200) followed by streptavidin-HRP (Zymed, South San Francisco, CA; diluted 1:400) and DAB.
Only cases that demonstrated any staining more than 0% for ErbB-2 by total ErbB-2 IHC were subject to analysis of phospho-ErbB-2. If a case had a score of 0 for ErbB-2 by conventional IHC, analysis of the phosphorylation state of ErbB-2 was not performed. Such cases were included in all analyses and it was assumed that cases having a score of zero for ErbB-2 by conventional IHC must be negative for phospho-ErbB-2 (ie, ErbB-2-zero is equated with ErbB-2-zero/PN2A-negative). In a previous series containing more than 500 ErbB-2-negative breast cancer cases defined as ErbB-2 IHC zero, phospho-ErbB-2 was never detectable.8 Slides were scored as percentage of invasive tumor cells demonstrating distinct membranous staining. For dichotomizing results, PN2A-negativity was defined as 0% staining (or ErbB-2 score of 0). Any PN2A value greater than zero was considered positive. Cytoplasmic staining without membrane staining was considered negative.
Fluorescence In Situ Hybridization Analysis of ERBB-2 Gene Copy Number
ERBB-2 gene copy number by fluorescence in situ hybridization (FISH) was performed previously for this data set.12,13 Of ErbB-2 IHC low cases, 3.1% were FISH positive (12 of 396), and of ErbB-2 IHC high cases, 61.4% were FISH positive.12,13
Statistical Analysis
PN2A and ErbB-2 IHC were modeled as dichotomous (positive/negative) variables in Cox proportional hazards models and in Kaplan-Meier survival curves. For survival analyses (DFS, OS), five groups were considered based on ErbB-2 status: ErbB-2 negative (0% cells positive); ErbB-2 low (< 50% cells positive)/P-ErbB-2 negative (0% cells positive); ErbB-2 low (< 50% cells positive)/P-ErbB-2 positive (≥ 1% cells positive); ErbB-2 high (≥ 50% cells positive)/P-ErbB-2 negative; and ErbB-2 high/P-ErbB-2 positive. Several demographic, clinical, and laboratory variables were examined. These included treatment arm of CALGB 8541, menopausal status, tumor size, number of positive lymph nodes, estrogen receptor status, patient age, ERBB-2 gene amplification by FISH, and ErbB-2 by IHC. The interaction of PN2A and treatment arm was also examined.
OS and DFS rates were estimated according to the Kaplan-Meier product limit method. DFS was calculated as time from study entry to disease progression or death, whichever occurred first. Patients who were disease free were censored at the date of last follow-up visit. OS was calculated from time of study entry to death, and patients who were alive were censored at date of last follow-up visit.
Both univariate and multivariate Cox regression models were developed to relate various prognostic variables with DFS and OS. DFS and OS were calculated in the same manner as previously stated.14 Data quality was ensured by careful review of data by the CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
RESULTS
Tissue Collection and Incidence of ErbB-2 and Activated (Phosphorylated) ErbB-2 (P-ErbB-2)
Tissue blocks were collected by the CALGB Pathology Coordinating Office from 992 of 1,572 patients who participated in CALGB 8541 (Fig 1A).Two hundred sixty-seven of these 992 cases (27%) had high ErbB-2 (≥ 50% staining), 303 (31%) had low ErbB-2 (1% to 49% staining), and 422 (43%) had no detectable ErbB-2 (0% staining); the latter were not analyzed for P-ErbB-2. Four hundred eight-eight of 570 tissues with detectable ErbB-2 were available for PN2A staining. The 82 cases unassessable for P-ErbB-2 either did not have any slides remaining or had no tumor left on remaining sections, and were therefore excluded from the analyses. Of the 910 cases assessable for P-ErbB-2 (including the 422 cases with ErbB-2 score of zero assumed P-ErbB-2 negative), 112 were positive (12%). When analyzed with respect to low versus high ErbB-2 (< v ≥ 50% cells stained positive for ErbB-2), only 20 of 260 tissues with low ErbB-2 were positive for P-ErbB-2 (8%) compared with 92 of 228 cases with high ErbB-2 (40%). Among those with the highest ErbB-2, those with ≥ 80% of cells staining, 49% were positive for P-ErbB-2. Of the cases positive for ERBB-2 gene amplification by FISH, 41% were positive P-ErbB-2.
Fig 1.
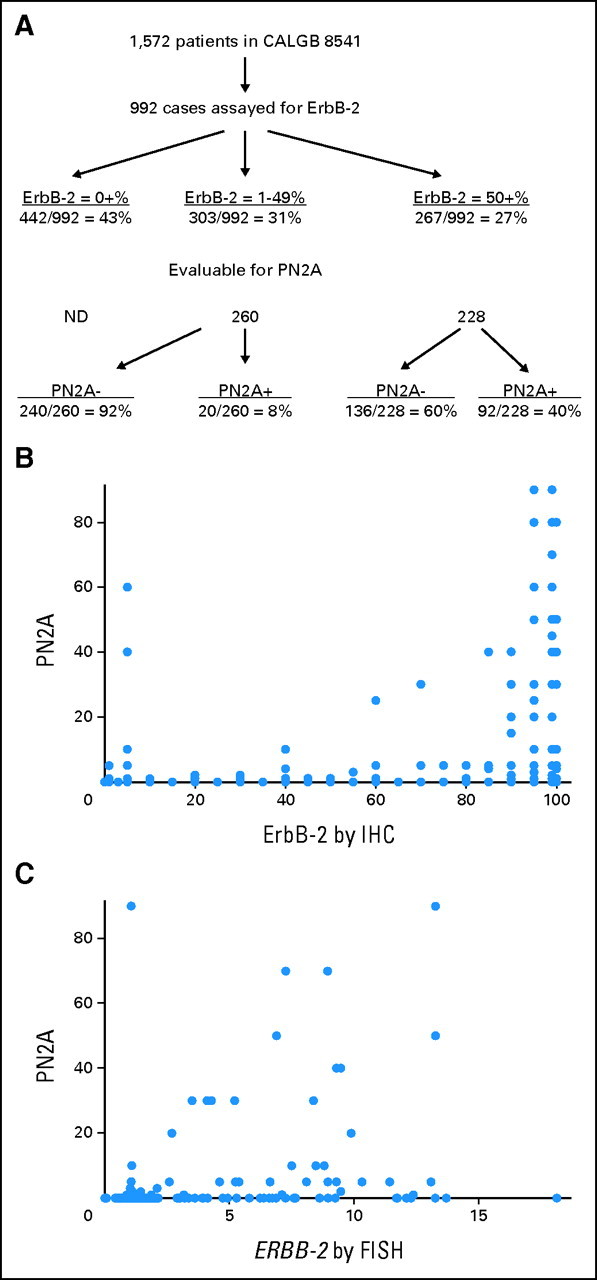
(A): Flow diagram showing distribution of patients by ErbB-2 status. Scattergrams of the relationship of phospho-ErbB-2 (PN2A) score to (B) ErbB-2 immunohistochemical score and (C) to ERBB-2 gene amplification ratio by fluorescence in situ hybridization. Of note, each dot may represent more than one case (particularly for points along the x-axis). ND, not done.
The relationship of ErbB-2 expression (by IHC) to P-ErbB-2 IHC is shown graphically in Figure 1B. ErbB-2 was correlated with P-ErbB-2 (r = 0.49; P = .0001), confirming previous findings.8 While cases with the highest ErbB-2 were most likely to have high P-ErbB-2, 133 cases had high ErbB2 scores (≥ 80%) and low or zero P-ErbB2 score (≤ 20%), and several cases with low ErbB-2 had high P-ErbB-2. A similar correlation was noted for the relationship between ERBB-2 gene amplification (by FISH) and P-ErbB-2 (r = 0.33; P < .0001), though again many cases with a high degree of amplification had a low or zero P-ErbB-2 score, and several cases with minimal amplification had detectable P-ErbB-2 (Fig 1C). These data indicate that P-ErbB-2 is not simply a surrogate marker of ERBB-2 gene amplification, or level of ErbB-2 expression.
ErbB-2 and P-ErbB-2 As Prognostic Factors in Patients Treated With Adjuvant CAF
In CALGB 8541, for the subset of patients in whom ErbB-2 was analyzed, high ErbB-2 was previously found to be an adverse prognostic factor in multivariate analyses11,12; when ErbB-2 expression was examined as a continuous variable, there was greater benefit for high CAF with each increase in percent of cells staining.11,12 We examined the effect of ErbB-2 category on outcomes (all doses pooled; Figs 2A and 2B). In general, as noted previously, the groups with low or undetectable ErbB-2 fared slightly better than those with high ErbB-2, with the exception of the ErbB-2-low/P-ErbB-2-positive group, which had poor outcome; however this result must be interpreted cautiously because of the small number of tumors (n = 20) in this category. For the high ErbB-2 groups, phosphorylation status did not influence outcome.
Fig 2.
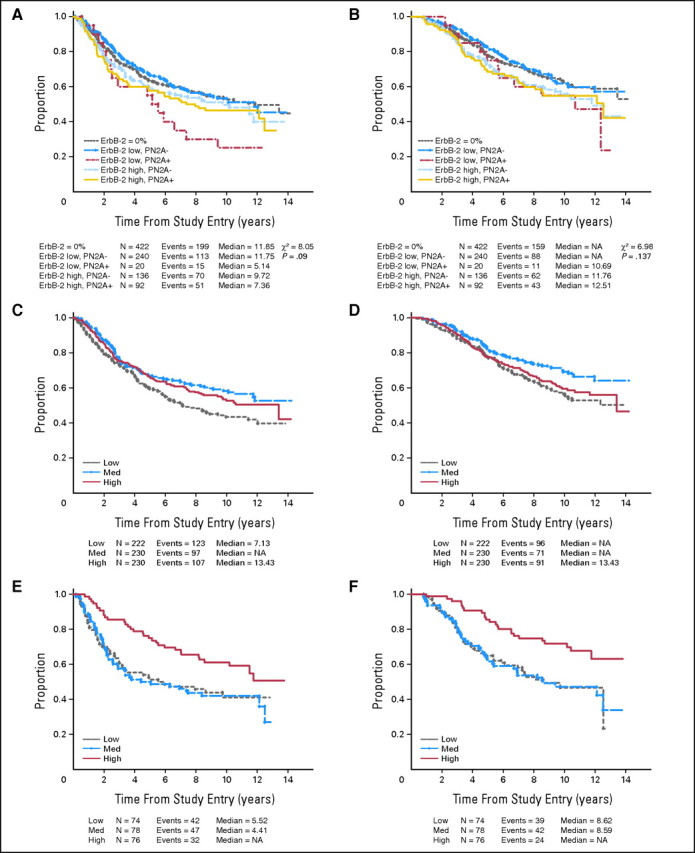
(A, C, E) Disease-free survival (DFS) and (B, D, F) overall survival (OS). (A) DFS and (B) OS of all patients, subdivided by ErbB-2 status into five groups: ErbB-2 = 0%, ErbB-2-low/P-ErbB-2-negative, ErbB-2-low/P-ErbB-2-positive, ErbB-2-high/P-ErbB-2-negative, and ErbB-2-high/P-ErbB-2-positive. (C) DFS and (D) OS of patients in the ErbB-2-low (< 50%) category subdivided by cyclophosphamide, doxorubicin, and fluorouracil (CAF) dose. (E) DFS and (F) OS of patients in the Erb-2-high (≥ 50%) category, subdivided by CAF dose.
In univariate analyses (Table 1), high ErbB-2 was associated with a slightly higher risk of death (risk ratio[RR], 1.31; P, .02), although not with an increased risk of recurrence. ERBB-2 gene amplification was also associated with an elevated risk of death (RR, 1.51; P = .019), and an elevated risk of recurrence (RR, 1.32) that did not reach statistical significance (P = .093). P-ErbB-2 was associated with an approximately 30% increased risk of both relapse and death (RRs, 1.33; P = .032 and 1.30; P = .078, respectively). When separated into the five ErbB-2 categories, most groups did not have a statistically significantly different outcome in comparison to the ErbB-2 = 0% group, although subdividing into five groups reduces statistical power.
Table 1.
Univariate Proportional Hazards Models for Prediction of DFS and OS
| Factor | DFS |
OS |
Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||||
| ErbB-2 by IHC (dichotomous) | 1.19 | 0.96 to 1.46 | .11 | 1.31 | 1.04 to 1.64 | .021* | Positive v negative | ||||
| ≥ 50% v < 50% | |||||||||||
| ERBB-2 by FISH (dichotomous) | 1.32 | 0.95 to 1.83 | .093 | 1.51 | 1.07 to 2.14 | .019* | Positive v negative | ||||
| < 2.0 v ≥ 2.0 | |||||||||||
| PN2A (dichotomous) | 1.33 | 1.03 to 1.73 | .032* | 1.30 | 0.97 to 1.73 | 0.078 | Positive v negative | ||||
| > 0% v 0% | |||||||||||
| ErbB-2/PN2A† | .09 | .14 | |||||||||
| ErbB-2 = 0% | 1.00 | 1.00 | |||||||||
| ErbB-2 low/PN2A− | 0.99 | 0.78 to 1.24 | 0.97 | 0.75 to 1.26 | |||||||
| ErbB-2 low/PN2A+ | 1.83 | 1.08 to 3.10 | 1.47 | 0.80 to 2.70 | |||||||
| ErbB-2 high/PN2A− | 1.16 | 0.89 to 1.53 | 1.31 | 0.97 to 1.75 | |||||||
| ErbB2 high/PN2A+ | 1.26 | 0.93 to 1.72 | 1.32 | 0.94 to 1.85 | |||||||
NOTE. For ErbB-2 by IHC, n = 910; for ERBB-2 by FISH, n = 500.
Abbreviations: DFS, disease-free survival; OS, overall survival; HR, hazard ratio; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization.
Statistically significant at P < .05.
Compare each following row to first row (ie, ErbB-2 = 0%).
Multivariate analysis of factors associated with outcome using the proportional hazards model is presented for both ErbB-2 and P-ErbB-2 in Table 2. Dichotomized variables were used in these tables for both P-ErbB-2 (0% v > 0%) and ErbB-2 (< 50% v ≥ 50% defining low v high). For DFS, higher doses of CAF are favorable prognostic factors (medium dose of borderline significance), while adverse prognostic factors included higher number of involved lymph nodes, larger tumor size, and premenopausal state (data not shown). P-ErbB-2 conferred an adverse relative risk comparable to these other adverse risk factors, with borderline statistical significance. The results were similar for ErbB-2-high status. For OS, higher doses of CAF were a borderline statistically significant favorable prognostic variable, while again nodal status and tumor size were adverse prognostic factors and premenopausal state was borderline (data not shown). ErbB-2-high was associated with poorer overall survival; P-ErbB-2 positivity conferred an adverse relative risk of similar magnitude (though borderline statistical significance). Multivariate analysis using ERBB-2 gene amplification by FISH, in place of ErbB-2 IHC, is presented in Table 3; trends for FISH results are similar to those for ErbB-2 IHC. In this smaller subset of patients for whom FISH assay results were available, there was not a prognostic trend for P-ErbB-2.
Table 2.
Results of Proportional Hazards Multivariate Models for DFS and OS Using ErbB-2 Score as a Dichotomous Variable (dichotomized at ≥ 50%) and Phosphorylated ErbB-2 (P-ErbB-2, PN2A) Score Modeled as a Dichotomous Variable (0 v > 0)
| Parameter | DFS |
OS |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | df | χ2 | P | RR | 95% CI | df | χ2 | P | |||||||||
| RR | ||||||||||||||||||
| ErbB-2 | 1.30 | 0.91 to 1.85 | 1.54 | 1.09 to 2.31 | ||||||||||||||
| P-ErbB-2 | 1.39 | 0.88 to 2.18 | 1.56 | 0.97 to 2.52 | ||||||||||||||
| Medium dose | ||||||||||||||||||
| ErbB-2 × CAF | 1.26 | 0.73 to 1.99 | 1.18 | 0.69 to 2.03 | ||||||||||||||
| P-ErbB-2 × CAF | 1.11 | 0.60 to 2.08 | 0.83 | 0.42 to 1.63 | ||||||||||||||
| High dose | ||||||||||||||||||
| ErbB-2 × CAF | 0.56 | 0.33 to 0.96 | 0.34 | 0.22 to 0.71 | ||||||||||||||
| P-ErbB-2 × CAF | 0.72 | 0.37 to 1.42 | 0.55 | 0.25 to 1.16 | ||||||||||||||
| Likelihood-ratio tests | ||||||||||||||||||
| ErbB-2 | 1 | 2.13 | .14 | 1 | 5.85 | .016 | ||||||||||||
| P-ErbB-2 | 1 | 2.02 | .15 | 1 | 3.32 | .069 | ||||||||||||
| ErbB-2 × CAF | 2 | 8.35 | .015 | 2 | 14.97 | .0006 | ||||||||||||
| P-ErbB-2 × CAF | 2 | 1.77 | .41 | 2 | 2.58 | .28 | ||||||||||||
NOTE. Variables included in the multivariate model included dose, square of the number of positive axillary lymph nodes, tumor size, menopausal status, and ErbB-2 or P-ErbB-2 status. For ErbB-2 immunohistochemical assay, n = 910. ErbB-2×CAF and P-ErbB-2 × CAF indicate tests of the interaction of ErbB-2 and P-ErbB-2 status, respectively (as dichotomous variables), with dose of CAF. Bold font indicates statistical significance.
Abbreviations: DFS, disease-free survival; OS, overall survival; RR, risk ratio; CAF, cyclophosphamide, doxorubicin, and fluorouracil.
Table 3.
Results of Proportional Hazards Multivariate Models for DFS and OS Using ERBB-2 FISH Score as a Dichotomous Variable (dichotomized at ≥ 2.0) and Phosphorylated ErbB-2 (P-ErbB-2, PN2A) Score Modeled as a Dichotomous Variable (0 v > 0)
| Parameter | DFS |
OS |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | df | χ2 | P | RR | 95% CI | df | χ2 | P | |||||||||
| RR | ||||||||||||||||||
| FISH | 1.24 | 0.66 to 2.34 | 1.33 | 0.66 to 2.70 | ||||||||||||||
| P-ErbB-2 | 0.89 | 0.37 to 2.17 | 0.94 | 0.37 to 2.35 | ||||||||||||||
| Medium dose | ||||||||||||||||||
| FISH × CAF | 1.69 | 0.66 to 4.28 | 2.03 | 0.77 to 5.39 | ||||||||||||||
| P-ErbB-2 × CAF | 1.72 | 0.51 to 5.72 | 1.28 | 0.37 to 4.44 | ||||||||||||||
| High dose | ||||||||||||||||||
| FISH × CAF | 0.68 | 0.25 to 1.83 | 0.88 | 0.30 to 2.56 | ||||||||||||||
| P-ErbB-2 × CAF | 1.50 | 0.44 to 5.08 | 0.85 | 0.21 to 3.42 | ||||||||||||||
| Likelihood-ratio tests | ||||||||||||||||||
| FISH | 1 | 1.86 | .17 | 1 | 5.81 | .016 | ||||||||||||
| P-ErbB-2 | 1 | 0.94 | .33 | 1 | 0.002 | .97 | ||||||||||||
| FISH × CAF | 2 | 6.64 | .036 | 2 | 6.26 | .044 | ||||||||||||
| P-ErbB-2 × CAF | 2 | 0.81 | .67 | 2 | 0.38 | .83 | ||||||||||||
NOTE. Variables included in the multivariate model included dose, square of the number of positive axillary lymph nodes, tumor size, menopausal status, and FISH or P-ErbB-2 status. For FISH assay, n = 497. FISH × CAF and P-ErbB-2 × CAF indicate tests of the interaction of FISH and P-ErbB-2 status, respectively (as dichotomous variables), with dose of CAF. Bold font indicates statistical significance.
Abbreviations: DFS, disease-free survival; OS, overall survival; FISH, fluorescence in situ hybridization; RR, risk ratio; CAF, cyclophosphamide, doxorubicin, and fluorouracil.
Impact of Dose of CAF According to ErbB-2 Status
The effect of dosing regimen on outcome, stratified by ErbB-2 status, which has been published previously,11,12 is updated in Figures 2C to 2F. The data continue to demonstrate that for ErbB-2-low patients, the dose of CAF did not significantly impact outcomes. For the ErbB-2-high patients, patients treated with high-dose CAF had significantly superior outcome compared with those treated with either of the less intensive regimens.
Effect of dose was examined for each of the five ErbB-2 category groupings: ErbB-2 = 0%, ErbB-2-low/P-ErbB-2-negative, ErbB-2-low/P-ErbB-2 positive, ErbB-2-high/P-ErbB-2-negative, and ErbB-2-high/P-ErbB-2-positive (Fig 3). Patients with high ErbB-2 had a better outcome if given high dose compared with medium or low dose, regardless of phosphorylation state of ErbB-2. For patients with low or undetectable ErbB-2, outcome was independent of dose regardless of phosphorylation state of ErbB-2, with the exception of the ErbB-2 = 0% group, in which DFS was inferior for the low-dose group compared with the medium- and high-dose groups. Hence, dose impacts outcome for patients whose tumors have high ErbB-2, regardless of ErbB-2 phosphorylation state.
Fig 3.
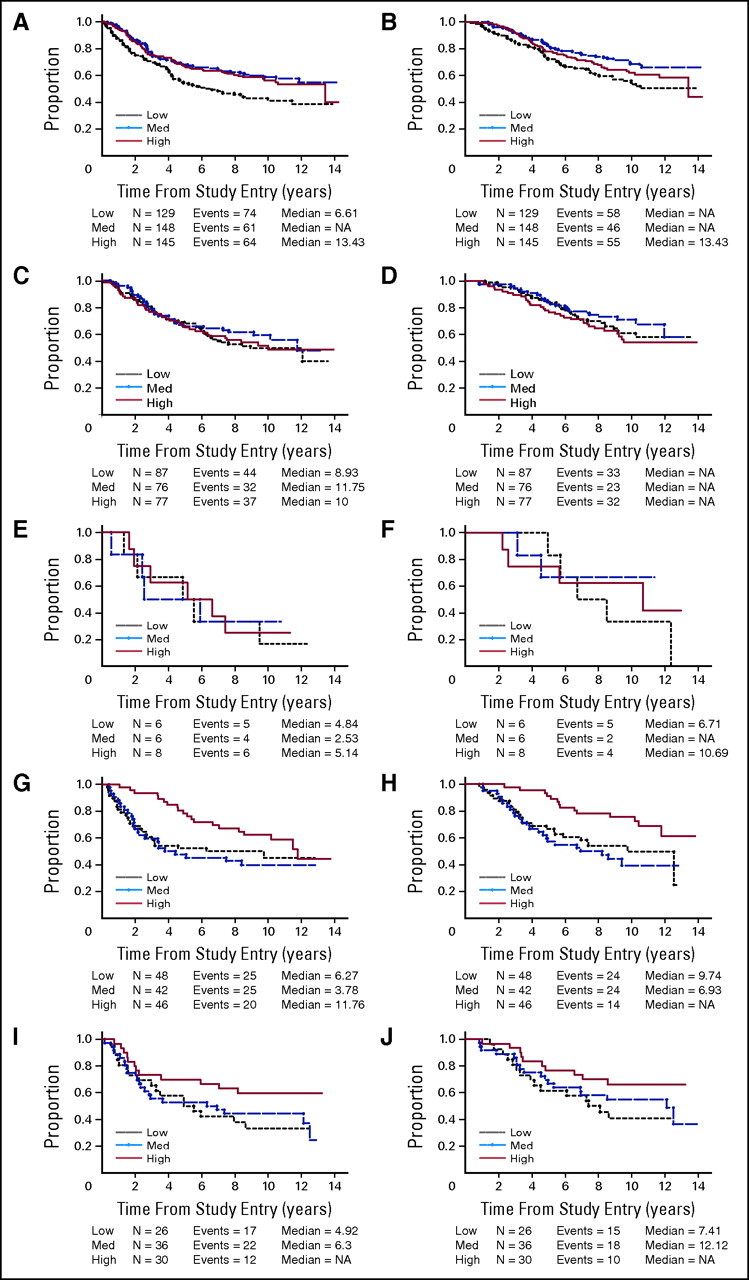
(A, C, E, G, I) Disease-free survival and (B, D, F, H, J) overall survival. For each ErbB-2 category shown, patients are subdivided by cyclophosphamide, doxorubicin, and fluorouracil dose. (A, B) ErB-2 = 0%; (C, D) ErB-2-low/P-ErB-2-negative; (E, F) ErB-2-low/P-ErB-2-positive; (G, H) Erb-2-high/P-ErB-2-negative; (I, J) ErB-2-high/P-ErB-2-positive.
The interaction of P-ErbB-2 and ErbB-2 with arm of CAF was further examined by proportional hazards multivariate modeling in Table 2. In a manner consistent with previous observations with shorter follow-up, the interaction between ErbB-2 positivity and CAF dose remained statistically significant through a follow-up of 11 years (Table 2). There is not a statistically significant interaction between P-ErbB-2 and CAF dose (Table 2), though this conclusion is compromised by low statistical power. The test of interaction of ERBB-2 gene amplification by FISH with arm of CAF is also statistically significant (Table 3).
High Dose CAF Overcomes the Adverse Prognostic Impact of P-ErbB-2
Because high-dose CAF is standard in current practice, it is instructive to focus specifically on this group. As noted previously, for patients in the high-dose group, the ErbB-2-high group has a better outcome than the ErbB-2-low group (Table 4). This continues to suggest that ErbB-2 overexpression may result in enhanced sensitivity to high-dose CAF, although this could be a spurious finding, particularly since the FISH results do not show the same trend. When the ErbB-2-high patients are further subdivided by phosphorylation state of ErbB-2, there is no impact of phosphorylation. Because phospho-ErbB-2 is an adverse prognostic factor in patients who do not receive adjuvant chemotherapy or receive mainly nonanthracycline based regimens, this result suggests that adequately-dosed adjuvant CAF can overcome the adverse prognostic impact of not only ErbB-2 overexpression, but also of P-ErbB-2.
Table 4.
DFS and OS of High-Dose CAF Patients Subdivided by ErbB-2 Status at Median Follow-Up of 11 Years
| ErbB-2 Status | DFS |
OS |
||||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |||
| All ErbB-2 high | 62 | 52 to 73 | 71 | 61 to 81 | ||
| All ErbB-2 low | 49 | 42 to 56 | 56 | 49 to 63 | ||
| ErbB-2 high/P-ErbB-2− | 59 | 46 to 76 | 69 | 56 to 85 | ||
| ErbB-2 high/P-ErbB-2+ | 60 | 44 to 80 | 66 | 51 to 86 | ||
| ErbB-2 low/P-ErbB-2− | 51 | 45 to 59 | 58 | 52 to 66 | ||
| ErbB-2 low/P-ErbB-2+ | 25 | 6 to 83 | 42 | 16 to 100 | ||
| All P-ErbB-2 negative | 53 | 47 to 60 | 60 | 54 to 67 | ||
| All P-ErbB-2 positive | 52 | 39 to 71 | 60 | 46 to 80 | ||
| FISH positive | 48 | 38 to 60 | 51 | 41 to 63 | ||
| FISH negative | 53 | 48 to 59 | 61 | 56 to 66 | ||
Abbreviations: DFS, disease-free survival; OS, overall survival; CAF, cyclophosphamide, doxorubicin, and fluorouracil.
DISCUSSION
The previously noted interaction of CAF with ErbB-2,11,12 in which higher dose improved outcome for high ErbB-2 expressors but did not impact the (more favorable) outcome for low ErbB-2 expressors, continues to be observed with 11-year follow-up. In this article, we add three additional findings. First, the relationship between CAF dose and outcome was independent of ErbB-2 phosphorylation status. Second, the apparent improved outcome of ErbB-2-positive patients treated with high-dose CAF is also independent of ErbB-2 phosphorylation state. Third, although P-ErbB-2 expression was an adverse prognostic factor, high-dose CAF overcomes the adverse prognostic impact of P-ErbB-2, a finding of particular clinical relevance.
There are several possible reasons for the observation that phosphorylation state did not appear to play a role in the relationship between CAF dose and outcome. It is unlikely that artifact due to dephosphorylation is a confounding factor given the robustness of this phospho-HER2 assay at predicting prognosis in our previous large studies performed retrospectively. P-ErbB-2 IHC is a semi-quantitative assay, and it could be that there is some biologically relevant signaling activity that is below the level of detection by this assay. Signaling activity that affects sensitivity to CAF (as opposed to tumor aggressiveness or ability to metastasize) could also potentially be related to the phosphorylation of an autophosphorylation site other than that recognized by PN2A (Tyr1248). Alternatively, ErbB-2 overexpression could serve as a surrogate marker of another alteration, independent of ErbB-2 signaling, that in turn impacts sensitivity to CAF.
A candidate surrogate might be topoisomerase II (topo II) activity, a target for doxorubicin. The gene for topo II may be coamplified (or deleted) when ERBB-2 is amplified.15-20 A better outcome after anthracycline-containing chemotherapy than after similar nonanthracycline-containing regimens for ErbB-2-positive tumors21-23 may be related to coamplification of topo II.24,25 However, thus far, we have found no interaction between TOPO II gene amplification and benefit from higher dose of CAF in this data set,26 although topo II protein level or activity have not been examined. It is possible that other elements of the ErbB-2 amplicon may contribute to treatment response. Phase III trials have now demonstrated a dramatic improvement in DFS when trastuzumab is added to standard adjuvant therapy for patients with ErbB-2-overexpressing tumors.27,28 In one adjuvant trastuzumab trial that included a nonanthracycline-containing arm (carboplatin/docetaxel/trastuzumab), preliminary results showed no significant difference between the anthracyline-containing arm and the nonanthracycline arm in the total population, but possible superiority of the anthracycline-containing arm among the subgroup of patients with coamplified topo II.29
A potential enhanced sensitivity of ErbB-2-overexpressing tumors to anthracycline has been suggested by the prior results of this CALGB study. In another CALGB study (9344), increasing the dose of doxorubicin above 60 mg/m2 did not further improve the outcome of ErbB-2-positive patients30; this raises the possibility that the association found in this study occurred by chance, but there also could be a threshold effect for response to doxorubicin. A similar interaction between ErbB-2 and responsiveness to two different anthracycline regimens (FEC50 v FEC100) has been reported in the neoadjuvant setting.31 Thus, when high-dose CAF is used, ErbB-2 overexpression may paradoxically be a favorable prognostic factor. This favorable impact of ErbB-2 may be even greater in the era of adjuvant trastuzumab. Preliminary evidence from the metastatic setting suggests that ErbB-2 phosphorylation may predict for sensitivity to trastuzumab.32,33 Hence in a chemotherapy-trastuzumab combination, activity-dependent and -independent interactions may be operative. Independently of trastuzumab, our current results show that high-dose CAF appears to overcome the adverse impact of P-ErbB-2 among ErbB-2-overexpressing tumors.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Michael P. DiGiovanna, Genentech (C) Stock Ownership: David F. Stern, Phosphoproteomics LLC Honoraria: None Research Funding: Lynn G. Dressler, Vysis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael P. DiGiovanna, David F. Stern, Lynn G. Dressler, I. Craig Henderson, Donald A. Berry, Daniel F. Hayes, Ann D. Thor
Financial support: Michael P. DiGiovanna, Ann D. Thor
Administrative support: I. Craig Henderson, Larry Norton, Ann D. Thor
Provision of study materials or patients: Michael P. DiGiovanna, Daniel R. Budman, I. Craig Henderson, Edison T. Liu, Hyman B. Muss, Daniel F. Hayes, Ann D. Thor
Collection and assembly of data: Michael P. DiGiovanna, Susan Edgerton, Lynn G. Dressler, Daniel R. Budman, I. Craig Henderson, Edison T. Liu, Daniel F. Hayes, Ann D. Thor
Data analysis and interpretation: Michael P. DiGiovanna, David F. Stern, Gloria Broadwater, Lynn G. Dressler, Larry Norton, Hyman B. Muss, Donald A. Berry, Daniel F. Hayes, Ann D. Thor
Manuscript writing: Michael P. DiGiovanna, Gloria Broadwater, Lynn G. Dressler, Larry Norton, Edison T. Liu, Hyman B. Muss, Donald A. Berry, Daniel F. Hayes, Ann D. Thor
Final approval of manuscript: Michael P. DiGiovanna, David F. Stern, Susan Edgerton, Gloria Broadwater, Lynn G. Dressler, Daniel R. Budman, I. Craig Henderson, Larry Norton, Edison T. Liu, Hyman B. Muss, Donald A. Berry, Daniel F. Hayes, Ann D. Thor
Appendix
The following institutions participated in this study:
Christiana Care Health Services Inc CCOP, Wilmington, DE, Stephen Grubbs, MD, supported by CA45418; Columbia University, New York, NY; Dana-Farber Cancer Institute, Boston, MA, Eric P. Winer, MD, supported by CA32291; Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH, Marc S. Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD, supported by CA47577; Long Island Jewish Medical Center, Lake Success, NY, Marc Citron, MD, supported by CA11028; Massachusetts General Hospital, Boston, MA, Michael L. Grossbard, MD, supported by CA12449; McGill University, Montreal, Quebec, Canada, Gerald Batist, MD; Mount Sinai Medical Center, Miami, FL, Rogerio Lilenbaum, MD, supported by CA45564; Rhode Island Hospital, Providence, RI, William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD, supported by CA02599; Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV, John Ellerton, MD, supported by CA35421; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD, supported by CA21060; Syracuse Hematology-Oncology Associates CCOP, Syracuse, NY, Jeffrey Kirshner, MD, supported by CA45389; University of Alabama Birmingham, Birmingham, AL, Robert Diasio, MD, supported by CA47545; University of California at San Diego, San Diego, CA, Joanne Mortimer, MD, supported by CA11789; University of Chicago, Chicago, IL, Gini Fleming, MD, supported by CA41287; University of Cincinnati Medical Center, Cincinnati, OH; University of Iowa, Iowa City, IA, Gerald Clamon, MD, supported by CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical School, Worcester, MA, William V. Walsh, MD, supported by CA37135; University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Michael C. Perry, MD, supported by CA12046; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by CA47559; University of Tennessee Memphis, Memphis, TN, Harvey B. Niell, MD, supported by CA47555; Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC, Thomas Reid, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO, Nancy Bartlett, MD, supported by CA77440; and Weill Medical College of Cornell University, New York, NY, Scott Wadler, MD, supported by CA07968.
Footnotes
published online ahead of print at www.jco.org on April 7, 2008
Cancer and Leukemia Group B (CALGB) 8541 was supported, in part, by grants from the National Cancer Institute (CA31946) to the CALGB (Richard L. Schilsky) the CALGB Statistical Center (Stephen George, CA33601), CA77651 (G.B.), CA47559 (L.G.D.), CA35279 (D.R.B.), CA60138 (L.C.H.), CA77651 (L.N.), and CA77406 (H.B.M.); by UO1CA64507 (D.F.H.) and UO1CA64061 (E.T.L., L.G.D.); RO1CA45708 (D.F.S.); DAMD17-97-1-7065 from the Department of Defense (M.P.D.); and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (D.F.H.). A complete list of participating institutions appears in the online-only Appendix.
M.P.D. and D.F.S. contributed equally to this article.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.DiGiovanna MP: Clinical significance of HER-2/ overexpression: Part I, in DeVita VT Jr, Hellman S, Rosenberg SA (eds): Principles and Practice of Oncology: Principles and Practice of Oncology Updates (ed 9). Cedar Knolls, NJ, Lippincott Williams & Wilkins, 1999. neu
- 2.DiGiovanna MP: Clinical significance of HER-2/ overexpression: Part II, in DeVita VT Jr, Hellman S, Rosenberg SA (eds): Principles and Practice of Oncology: Principles and Practice of Oncology Updates (ed 10). Cedar Knolls, Lippincott Williams & Wilkins, 1999. neu
- 3.Yamauchi H, Stearns V, Hayes DF: When is a tumor marker ready for prime time? A case study of c-B-2 as a predictive factor in breast cancer. J Clin Oncol 19::2334,2001-2356, erb [DOI] [PubMed] [Google Scholar]
- 4.Isaacs C, Stearns V, Hayes DF: New prognostic factors for breast cancer recurrence. Semin Oncol 28::53,2001-67, [DOI] [PubMed] [Google Scholar]
- 5.DiGiovanna MP, Stern DF: Activation state-specific monoclonal antibody detects tyrosine phosphorylated p185 in a subset of human breast tumors overexpressing this receptor. Cancer Res 55::1946,1995-1955, neu/erbB-2 [PubMed] [Google Scholar]
- 6.DiGiovanna MP, Roussel RR, Stern DF: Production of antibodies that recognize specific tyrosine-phosphorylated peptides, in Ausubel FM, Brent R, Kingston RE, et al (eds): Current Protocols in Molecular Biology. New York, John Wiley & Sons, Inc, , pp 18.6.1,1996-18.6.19 [DOI] [PubMed]
- 7.DiGiovanna MP, Carter D, Flynn SD, et al: Functional assay for HER-2/ demonstrates active signalling in a minority of HER-2/-overexpressing invasive human breast tumours. Br J Cancer 74::802,1996-806, neu neu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thor AD, Liu S, Edgerton S, et al: Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/): A study of incidence and corrrelation with outcome in breast cancer. J Clin Oncol 18::3230,2000-3239, neu [DOI] [PubMed] [Google Scholar]
- 9.Wood WC, Budman DR, Korzun AH, et al: Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med 330::1253,1994-1259, [DOI] [PubMed] [Google Scholar]
- 10.Budman DR, Berry DA, Cirrincione CT, et al: Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J Natl Cancer Inst 90::1205,1998-1211, [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Thor AD, Berry DA, et al: C-B-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 330::1260,1994-1266, erb [DOI] [PubMed] [Google Scholar]
- 12.Thor AD, Berry DA, Budman DR, et al: ErbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst 90::1346,1998-1360, [DOI] [PubMed] [Google Scholar]
- 13.Dressler LG, Berry DA, Broadwater G, et al: Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol 23::4287,2005-4297, [DOI] [PubMed] [Google Scholar]
- 14.Cox DR: Regression models and life-tables (with discussion). Journal Royal Statistical Society, Series B 34::187,1972-220, [Google Scholar]
- 15.Keith WN, Douglas F, Wishart GC, et al: Co-amplification of B2, topoisomerase II a and retinoic acid receptor a genes in breast cancer and allelic loss at topoisomerase I on chromosome 20. Eur J Cancer 29A::1469,1993-1475, erb [DOI] [PubMed] [Google Scholar]
- 16.Jarvinen TAH, Tanner M, Barlund M, et al: Characterization of topoisomerase II alpha gene amplification and deletion in breast cancer. Genes Chromosomes Cancer 26::142,1999-150, [PubMed] [Google Scholar]
- 17.Jarvinen TAH, Tanner M, Rantanen V, et al: Amplification and deletion of topoisomerase II alpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. American J Pathol 156::839,2000-847, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Järvinen TAH, Liu ET: Her-2/neu and topoisomerase IIa in breast cancer. Breast Cancer Res Trt 78::299,2003-311, [DOI] [PubMed] [Google Scholar]
- 19.Harris LN, Yang L, Tang C, et al: Induction of sensitivity to doxorubicin and etoposide by transfection of MCF-7 breast cancer cells with heregulin β-2. Clinical Cancer Res 4::1005,1998-1012, [PubMed] [Google Scholar]
- 20.Harris LN, Yang L, Liotcheva V, et al: Induction of topoisomerase II activity after ErbB2 activation is associated with a differential response to breast cancer chemotherapy. Clinical Cancer Res 7::1497,2001-1504, [PubMed] [Google Scholar]
- 21.Paik S, Bryant J, Park C, et al: ErbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst 90::1361,1998-1370, [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Bryant J, Tan-Chiu E, et al: HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-15. J Natl Cancer Inst 92::1991,2000-1998, [DOI] [PubMed] [Google Scholar]
- 23.Pritchard KI, Shepherd LE, O'Malley FP, et al: HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354::2103,2006-2111, [DOI] [PubMed] [Google Scholar]
- 24.Di Leo A, Gancberg D, Larsimont D, et al: HER-2 amplification and topoisomerase II alpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clinical Cancer Res 8::1107,2002-1116, [PubMed] [Google Scholar]
- 25.Tanner M, Isola J, Wiklund T, et al: Topoisomerase IIa gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Cancer Group Trial 9401. J Clin Oncol 24::2428,2006-2436, [DOI] [PubMed] [Google Scholar]
- 26.Harris L, Dressler L, Cowan D, et al: Comparison of HER-2 and topoisomerase IIa amplification with outcome after cyclophosphoamide, doxorubicin and 5 FU-based chemotherapy for Stage II breast cancer (CALGB 8541/150013). Proc Am Soc Clin Oncol 23::832,2004, (abst) [Google Scholar]
- 27.Piccart-Gebhart MJ, Proctor M, Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353::1659,2005-1672, [DOI] [PubMed] [Google Scholar]
- 28.Romond EH, Perez EA, Bryant J, et al: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353::1673,2005-1684, [DOI] [PubMed] [Google Scholar]
- 29.Slamon D, Eiermann W, Robert N, et al: BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC/T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC/TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. Presented at San Antonio Breast Cancer Symposium, San Antonio, TX, December 14-17, 2006. (abstr 52)
- 30.Hayes DF, Thor A, Dressler L, et al: HER2 predicts benefit from adjuvant paclitaxel after AC in node-positive breast cancer: CALGB 9344. J Clin Oncol 24::5s,2006, (abstr 510) [Google Scholar]
- 31.Petit T, Borel C, Ghnassia J-P, et al: Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant setting. Clinical Cancer Res 7::1577,2001-1581, [PubMed] [Google Scholar]
- 32.Hudelist G, Kostler WJ, Attems J, et al: Her-2/-triggered intracellular tyrosine kinase activation: relevance of ligand-independent activation mechanisms and impact upon the efficacy of trastuzumab-based treatment. Br J Cancer 89::983,2003-991, neu In vivo [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudelist G, Köstler WJ, Czerwenka K, et al: Her-2/neu and EGFR tyrosine kinase activation predict the efficacy of trastuzumab-based therapy in patients with metastatic breast cancer. Int J Cancer 118::1126,2006-1134, [DOI] [PubMed] [Google Scholar]


