Abstract
Background:
Osteotomies aimed at correcting adult spinal deformity are associated with higher complications and perioperative morbidity. Recently, oblique lumbar interbody fusion (OLIF) was introduced for degenerative lumbar diseases. The aim of our study is to demonstrate the effectiveness of OLIF on the management of adult degenerative lumbar deformity (ADLD).
Materials and Methods:
Patients with ADLD who underwent deformity correction and decompression using OLIF and posterior instrumentation were enrolled. For radiologic evaluation, Cobb's angle (CA), sagittal vertical axis (SVA), lumbar lordosis (LL), thoracic kyphosis (TK), pelvic tilt (PT), sacral slope (SS), and pelvic incidence (PI) were evaluated. Visual analog scale (VAS), Oswestry disability index (ODI), and perioperative parameters were recorded for clinical evaluation.
Results:
Fifteen patients with a mean age of 67 years (63–74 years) were enrolled prospectively and an average of 3 OLIFs (range 1–4) was performed. Posterior instrumentations were done at average of six levels (range 4–8). The mean operative blood loss was 863 ml (range 500–1400 ml) with a mean surgical duration of 7 h (range 3–11 h). SVA, TK, LL, CA, PT, and SS showed significant correction (P < 0.05) in immediate postoperative period and all parameters except TK were maintained at final followup. At the end of 24 months of average followup, 86% (13/15) showed fusion. VAS (leg pain), VAS (back pain), and ODI improved by 74% (range 40–100), 58% (range 20%–80%), and 69.5% (range 4%–90%), respectively. There were two major complications requiring revision (1 infection and 1 adjacent vertebral body fracture). Transient hip weakness present in two patients (13%) recovered within 6 weeks.
Conclusions:
OLIF gives favorable short term clinical and radiological outcomes in patients of ADLD. It could potentially reduce the need for morbid pelvic fixation and posterior osteotomies in patients with degenerative lumbar deformity.
Keywords: Adult deformity, degenerative lumbar deformity, degenerative lumbar kyphoscoliosis, oblique lateral interbody fusion, spine
Introduction
The treatment of patients with degenerative spinal deformity is often difficult and technically challenging. In symptomatic patients, the treatment may vary from decompression alone to long fusion and deformity correction. Various factors such as type of symptoms, magnitude of deformity, age at presentation, comorbidities, functional expectations, and bone quality influence the treatment.1,2,3,4,5 The treatment goals are different in different patients and the management is to be tailored for each individual patient on case-to-case basis. The selection of the most appropriate approach, optimal technique of fusion, and ideal method of deformity correction is of paramount importance while dealing with patients having advanced spinal deformity. Procedures such as pedicle subtraction osteotomies (PSOs) or Smith–Peterson osteotomies (SPOs) were conventionally described for these patients.3,6,7,8,9,10 These procedures have been associated with longer surgical duration, risk of injury to major vessels, excessive blood loss from segmental or epidural vessels, neurological deficits, loss of correction, and pseudoarthrosis.8,9,11 Recently, lateral lumbar interbody fusion (LLIF) and anterior lumbar interbody fusion (ALIF) have been described as an alternative to the conventional posterior only procedures with an aim to minimize the perioperative morbidities and complications.10
In addition to deformity correction, LLIFs and ALIFs also cause indirect decompression of the neural structure that helps to improve functional outcomes.12,13 However, these are certain disadvantages associated with these techniques. LLIF through a transpsoas approach has been associated with a significant risk of neural deficit.13,14,15,16 On the other hand, ALIFs have been associated with the risk of major vessel injury, retrograde ejaculation, and neural deficit.10,15,17 As a result, oblique lumbar interbody fusion (OLIF) was introduced with a view to minimize complications and achieve same surgical goals as LLIF and ALIF. OLIF utilizes the corridor between aorta and psoas muscle in the retroperitoneal space. The risk of permanent neural deficit with OLIF is minimal, and it can be performed without the need for intraoperative neuromonitoring.18 There has been a dearth of literature demonstrating the effectiveness of OLIF in patients with symptomatic adult degenerative lumbar deformity (ADLD). In the present study, we aim to demonstrate the effectiveness of OLIF in correction of ADLD. The study also highlights that OLIF can reduce the perioperative morbidity and complications of deformity correction surgery by reducing the need to perform posterior osteotomies such as PSO/SPO.
Materials and Methods
The regional ethical committee approved the study protocol and all patients provided informed consent.
Patient selection
Fifteen patients who underwent OLIF and posterior decompression fusion for symptomatic ADLD between January 2014 and December 2015 were evaluated prospectively. The inclusion criteria were patients with (1) spinal deformity in form of lumbar degenerative scoliosis or lumbar degenerative kyphoscoliosis and (2) symptomatic spinal stenosis.
Deformity correction and fusion were indicated in these patients due to combination of the factors below: radiographic deformity such as scoliosis angle >30°, positive sagittal balance ≥5 cm and coronal imbalance ≥4 cm, mechanical back pain, progressive deformity, radicular pain, or claudication along with symptoms associated with spinal curvature.11 We excluded patients with (1) previous lumbar surgery, (2) posttraumatic kyphosis, (3) kyphosis due to osteoporotic compression fracture, and (4) suspected infection. Whole-spine AP and lateral radiographs were obtained along with dynamic flexion extension lateral radiographs. For sagittal alignment, sagittal vertical axis (SVA), pelvic incidence (PI), pelvic tilt (PT), sacral slope (SS), lumbar lordosis (LL), and thoracic kyphosis (TK) were evaluated. For coronal alignment, coronal Cobb's angle (CA) was evaluated.
The change in foraminal area was measured to determine the indirect decompression of OLIF procedure before and after surgery. The foraminal space was measured as the area around the center of the foramen using the sagittal cut of computed tomography (CT) [Figure 1]. The measurement of foraminal area was performed using measurement tools in Picture Archiving and Communication System (LG infinity, Seoul, South Korea).
Figure 1.
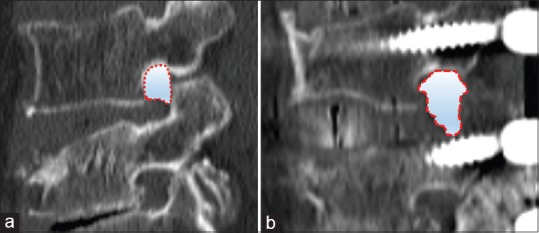
Preoperative (a) and postoperative (b) foraminal area measurements. Measurement of the foraminal area was performed at the center of the foramen using the pre and postoperative CT. The area which was surrounded with pedicle, intervertebral disk and a superior articular process were measured using a Picture Archiving and Communication System program with unit of square millimeter
In all enrolled patients, medical history that affects the surgical risk was surveyed. If comorbidities such as hypertension, diabetes mellitus, heat problem, and rheumatism disease exist, physical condition was consulted with a specialist in internal medicine. If the stage operation was planned, health condition was reevaluated during resting period by internal medicine doctor and anesthesiologist.
Surgical procedures
The surgical procedure comprised of anterior and posterior approaches. The surgeries were staged if any of these factors were contemplated: long segment posterior instrumentation, multilevel (>2) posterior decompression or need for posterior osteotomies. In all our patients, OLIF was performed first through retroperitoneal approach. The corridor between psoas muscle and the major vessels was utilized and the disc spaces were exposed after retraction of the psoas muscle. We approached disc from the left side usually unless in a case of severe ADLD with right convex scoliosis. We preferably used a 12° poly ethyl ether ketone cage to achieve lordosis and the size of the cages used varied from 10 to 16 mm height. Demineralized bone matrix was used in these long cages. To accommodate large lordotic cages at L1–L2 and L2–L3 levels, anterior longitudinal ligaments (ALLs) were released by creating a plane between the great vessels and ALL. The safety and feasibility of ALL resection have been already described.17 L5–S1 fusion using bilateral posterior lumbar interbody fusion (PLIF) cage was done in patients with a fixed tilt at L4–L5 or in patients with listhesis, stenosis, or degeneration at L5–S1.11 In all cases with fusion extending to S1, we used bilateral bicortical S1 screws.
Before operations, we measured the PI-LL mismatch using standing whole-spine X-ray in all patients and a plan for correction was made. However, for the patient who had staged operation due to comorbidities, surgical time, and other causes, we took postoperative radiography to check the PI-LL mismatch to do additional bony procedures after first operation. In case of staged surgeries, X-rays were obtained 2–3 days later and second stage surgery was usually performed 7 days after the first surgery. After that, conventional open posterior pedicle instrumentation and overcontoured rods with multiple partial facetectomies were performed for better correction.
The desired amount of correction was estimated using PI-LL mismatch.19 PI-LL mismatch was preferred because we believe that compensatory mechanisms secondary to positive sagittal balance such as retroversion of pelvis may influence all spinopelvic parameters except PI.10 PI is closely related to LL and it does not change with the change in the position of pelvis.8,19 Thus, if the desired LL was not achieved, we preferred combining posterior instrumented fusion with additional bony procedure such as PSO, SPO, and multiple crack osteotomy (n = 3). We did not require pelvic fixation in any of our patients.
Data collection and analysis
Perioperative data were collected using the hospital's patient database system. Details of duration of surgery, intraoperative blood loss, medical or surgical complications, neurological examinations, and reoperations or readmissions were recorded. The clinical evaluation was done using visual analog scale (VAS) for back and leg and Oswestry disability index (ODI).
Standing whole-spine radiographs were obtained in all patients after about 12–14 days of the primary surgery (single-staged surgery) or second stage of staged surgery. Followup examinations were done every month for first 3 months, then on 6th, 9th, and 12th month of followup. Further followups were done at 6 monthly intervals. At the end of 2 years of followup, a CT scan was obtained to determine the status of fusion.
Statistical analysis was done using SPSS software version 22.0.0 (IBM Corporation, NY, USA). A P < 0.05 was considered statistically significant. The Wilcoxon signed-rank test was used to compare the preoperative and postoperative values of the variables. Because the number of enrolled patients was small, the power of this statistical analysis was evaluated with G power program. If the power was more than 0.8, then it is considered to be valuable.20
Results
Our study consisted of 15 patients (3 men and 12 women) with a mean age of 67 ± 3.38 years (range 63–74 years). Among them, 11 patients had comorbidities. The demographic and operative details are depicted in Table 1. Ten patients underwent single-stage surgeries and five patients underwent two-staged surgeries. In patients with two staged surgeries, OLIFs were done in the first stage and posterior fixation with or without osteotomies in the second stage. OLIF was performed on a mean of 3 ± 0.78 levels (range 1–4). Posterior instrumentation was performed on an average of 6 ± 1.5 levels (range 4–8). Additional SPOs were performed in three patients. L5–S1 level was fused in five patients (2 patients with fixed tilt of L4–5, 2 patients with disc degeneration and stenosis, and 1 patient with L5–S1 spondylolisthesis) and none of the patients required extension of fusion to iliac bone. The mean followup period was 24 ± 2.4 months (range 21–30 months). The operative parameters are depicted in Table 2.
Table 1.
Demographic and operative details
| Factors | Mean±SD (median, range) |
|---|---|
| Age (years) | 67±3.38 (65, 63-74) |
| Number of levels at which OLIF was performed | 3±0.78 (3, 1-4) |
| Number of level of posterior instrumentation | 6±1.5 (6, 4-8) |
| Followup (months) | 24±2.4 (24, 21-30) |
OLIF=Oblique lateral interbody fusion, SD=Standard deviation
Table 2.
Operative parameters
| Factors | Mean±SD (median, range) |
|---|---|
| Duration of surgery (h) | 7±2.04 (7, 3-11) |
| Blood loss during surgery (mL) | 863±296 (800, 500-1400) |
| Complications | |
| Major | 2 (infection and L2 fracture) |
| Minor | 2 (transient hip flexion weakness) |
SD=Standard deviation
Radiological parameters
Compared with the preoperative values, parameters such as LL, TK, SS, PT, SVA, and CA improved significantly in the immediate postoperative period and maintained on final followup except with TK which showed significant increment with P = 0.003 as depicted in Table 3 [Figures 2 and 3].
Table 3.
Radiological parameters assessed in the study
| Radiological parameters | Preoperative (°) | Immediate postoperative (°) | Final follow-up (°) |
|---|---|---|---|
| CA (range) | 17.1±10.3 (18, 4-31) | 7.06±9.58 (2, 2-21) (P=0.0009, power=0.97)* | 11.3±19.97 (2, 0-21) (P=0.55)** |
| SVA (range) | 70.5±31.8 mm (68, −25-129) | 15.2±18.83 mm (15, −35-39) (P<0.001, power=0.99)* | 15.13±22.07 mm (15, −37-54) (P=0.9)** |
| LL (range) | 24±7 (28, 4-42) | 44.5±8.16 (45, 23-61) (P<0.001, power=0.99)* | 45.3±9.05 (46, 23-61) (P=0.6)** |
| TK (range) | 16.3±8.5 (17, 5-31) | 24.8±8.89 (10, 28-36) (P=0.0009, power=0.96)* | 29.6±8.85 (30, 12-41) (P=0.003)* |
| SS (range) | 27±10.11 (24, 12-46) | 31.6±4.09 (31, 24-42) (P=0.04, power=0.59)* | 29.5±5.37 (28, 21-43) (P=0.15)** |
| PT (range) | 34.1±11.2 (33, 12-54) | 28.7±7.27 (31, 15-40) (P=0.04, power=0.62)* | 30.2±8.2 (30, 14-45) (P=0.15)** |
| PI (range) | 63.6±12.7 (64, 44-82) | 61.7±7.24 (61, 46-80) (P=0.5)** | 62.3±6.67 (62, 49-73) (P=0.72)** |
| Foraminal area (range) | 68±6 mm2 (69, 56-77) | 79±6 mm2 (79, 67-90) (P<0.001, power=0.99) |
P<0.05 was considered significant, *Significant, **No significant relation. Powers of all significant P values were analyzed and if the power is more than 0.8, it is considered to be valuable. The P values of final follow-up indicate changes in comparison to immediate postoperative measurements. The values are expressed as mean±SD (median, range). SD=Standard deviation, PI=Pelvic incidence, PT=Pelvic tilt, SS=Sacral slope, TK=Thoracic kyphosis, LL=Lumbar lordosis, SVA=Sagittal vertical axis, CA=Cobb’s angle
Figure 2.
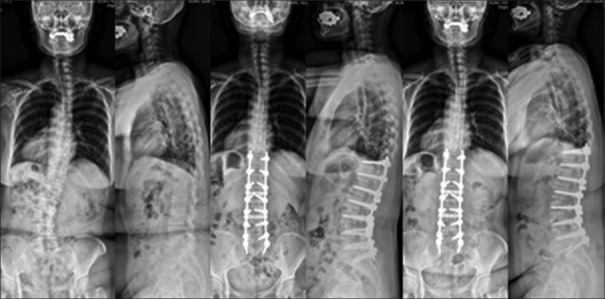
A 68-year-old female with adult lumbar degenerative deformity with 30° scoliosis T12–L5 and lumbar lordosis of 28° underwent L5–S1 posterior lumbar interbody fusion, L2–L5 OLIF, and T11-S1 posterior instrumentation and decompression L2–L5. Lumbar lordosis improved by 16° and Cobb's angle improved by 28° and maintained at final followup
Figure 3.
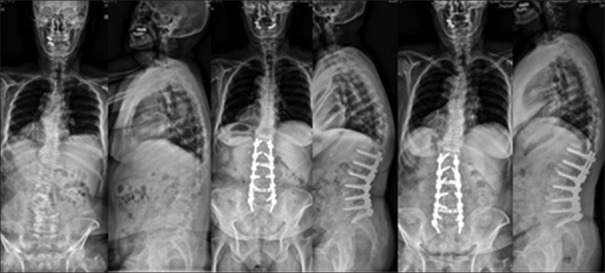
A 71-year-old female with adult lumbar degenerative deformity with T12–L4 Cobb's angle 34° and lumbar kyphosis. The patient was treated with OLIF L2–L5 with anterior longitudinal ligament release at L2–L3, T12–S1 posterior instrumentation. The correction of LL by 30°, Cobb's angle by 31° was maintained at final followup
The amount of sagittal and coronal angle correction after OLIF was evaluated by grouping the patients into three groups: “OLIF with instrumentation,” “OLIF with decompression-related/cage insertion-related bony procedures (laminectomy, facetectomy, or ALL release), and “OLIF with additional bony procedures.” The values are described in Table 4.
Table 4.
Comparison of correction of oblique lateral interbody fusion alone with different bony procedures
| Factors | Preoperative values (°) | Postoperative values (°) | Correction amount (°) |
|---|---|---|---|
| Correction amount of OLIF only with posterior instrumentation (7 cases) | 17.9±13.2 | 30.3±10.6 | 12.4±10.9 (P=0.01, power=0.74) |
| Correction amount of OLIF and facetectomy or ALL resection over OLIF site (5 cases) | 11.8±6.2 | 39.8±3.6 | 28.0±4.8 (P=0.043, power=0.99) |
| Correction amount of OLIF and bony resection procedures (3 cases) | 12.0±16.5 | 25.7±9.7 | 13.3±11.1 |
P<0.05 was considered statistically significant. Powers of all significant P values were analyzed and if the power is more than 0.8, it is considered to be valuable. Bony resection procedures include pedicle subtraction osteotomy, Smith-Peterson osteotomy, and multiple crack osteotomy. OLIF=Obloquie lumbar interbody fusion, ALL=Anterior longitudinal ligament
The mean foraminal area changed from 68 ± 6 mm2 (range 56–77 mm2) to 79 ± 6 mm2 (range 67–90 mm2) in levels that had OLIF cage (P < 0.001, power = 0.99) [Table 3].
Clinical parameters
There is an improvement of 74% ±37% (range 40%–100%) in VAS leg pain scores, 58% ±20% (range 20%–80%) in VAS back pain scores, and 69.5% ±27.6% (range 4%–90%) in ODI scores.
There were two patients who developed transient hip flexion weakness that resolved over 6 weeks duration. One patient had fracture of the adjacent superior vertebrae (L2) while insertion of the cage that was managed conservatively with bed rest and bracing for 3 months. One patient encountered infection that required repeated debridements and prolonged antibiotic therapy. The infection resolved after three debridements and an antibiotic course of 2.5 months.
CT scan at final followup showed fusion in 13/15 patients (86%). The remaining two patients without fusion however did not show any sign of pseudoarthrosis.
Discussion
Conventionally, PSO or multiple SPOs have been used for the deformity correction in patients of ADLD. PSO can lead to correction of 30°–40° per segment.21 However, the risk of neural deficit after PSO is significant and ranges from 2.1%–38%.7,21 A combination of subluxation, residual dorsal impingement, and dural buckling was a proposed cause for neural injury and the deficits are usually not detected by intraoperative neuromonitoring.22 Moreover, loss of correction leading to loss of LL can occur in up to 18% and loss of sagittal alignment can occur in up to 33% of patients on followup.8 Pseudoarthrosis and implant failure rate after PSO are high and are reported in the range of 12%–22%.7,23 Anterior column support has been recommended at the osteotomy or nonosteotomy site to prevent the loss of correction and pseudoarthrosis and thereby increases the complexity and complications of the procedure.6,8 On the other hand, multiple SPOs have also been advocated used for the correction of sagittal plane deformity. The amount of correction with single SPOs is moderate in the range of about 9.3°–10.7°.7,24 SPOs are safe compared to that of PSO with lesser blood loss and lesser risk of neurological injury, but SPOs have a greater tendency toward coronal decompensation and they require a mobile anterior disc to allow for the correction.7 Hence, there was a dire need for a better correction technique in patients of ADLD.
Anterior approaches have evolved dramatically over the past decade. Previously, TLIF and PLIF were used as a component of all posterior surgery for ADLD with stenosis. However, the amount of lordosis correction or correction of sagittal imbalance was considered inadequate.24,25 As a result, LLIF and ALIF techniques were introduced to achieve better correction in sagittal and coronal plane. ALIF has an advantage of allowing insertion of a large size cage in the disc space and better sagittal correction due to release of the ALL.10,26,27,28 However, risk of injury to the great vessels, retrograde ejaculation, neural injury, and cage displacement are frequently observed with ALIF.26,27,28 On the other hand, LLIF allows adequate coronal CA correction, but the correction in the sagittal plane deformity was modest in the range of 1°–9°.15,16,28,29,30,31 Most of the early described lateral interbody fusion techniques used transpsoas approach to reach the disc space. This increases the risk of damage to the lumbosacral plexus and may result in neurological deficit, retrograde ejaculation and postoperative psoas weakness (hip flexion).15,16,28,32 Hence, the use of neuromonitoring was a must for these techniques to prevent neurological injury. OLIF that was subsequently introduced has the same advantages of LLIF, but the approach-related complications are less than that of LLIF. The trajectory of OLIF is anterior to psoas muscle, and thereby, injury to psoas and spinal nerves can be avoided.17 Moreover, patients with ADLD often present with foraminal, lateral recess or central stenosis and sagittal or lateral spondylolisthesis.14 OLIF similar to LLIF offers an attractive solution in these patients as it results in indirect decompression of the foraminal and central neural canal. This reduces the number of levels required to be decompressed and addressed posteriorly.
We used large sizes cages (12–14 mm) with a lordotic angle of 12° and observed a 20° mean change in LL and 10° mean change in CA with a mean of three fusion levels per patient. This correlated well with an average LL change of 8° per level of LLIF as reported by Anand et al. with use of 12° lordotic cages.33 Improvement in LL by 3°–37° has been reported by several authors using various lateral interbody fusion techniques. Anand et al. evaluated 90 cases undergoing minimally invasive deformity correction using direct lateral interbody fusion and observed a mean CA change of 26° and mean LL change of 3° with a mean four fusion levels.34 Caputo et al. performed extreme lateral interbody fusion (XLIF) in 30 patients and reported a mean change of LL by 5° and mean change in CA by 14.6° with a mean fusion of four levels per patient.35 Tormenti et al. reported a mean change of LL by 7° and CA change of 25° with a mean 2.8 XLIF per patient.36 Ohtori et al. employed MI-OLIF in a series of 12 patients and observed mean improvement of coronal CA by 37° and LL by 31° with a mean fusion of 2.9 levels per patients.18 ALL release has been recommended by some authors. Deukmedjian et al. in their initial study of seven patients employing anterior release observed that LL improved by a mean of 24° with anterior release and fusion in 1.6 levels per patients.17 Manwaring et al. in their series of nine patients with anterior release obtained 16.5° correction in LL and 16° correction in coronal CA with 1.6 anterior release and fusions per patient.15
To validate the correction power of OLIF with or without additional procedures such as bony and soft tissue resection, the authors compared the results in three groups to identify the effects of OLIF only and the effects of additional procedures conducted with OLIF. Although sufficient corrections were available for the OLIF alone, the OLIF and facetectomy or ALL release groups showed most effective correction amount among them [Table 4]. The effective correction of OLIF could be enhanced by interdiscal widening using a big OLIF cage with/without removal of soft tissue tethering effect though resection of ALL and additional correction acquired by removal of the posterior bone construct via facetectomy and/or laminectomy. We also observed that lower lumbar levels can accommodate large size cages without the need for ALL release, and hence, in our study, ALL resections were required only to accommodate large lordotic cases at L1/2 and L2/3 levels.
Mean estimated blood loss as high as 2900 mL has been reported with the PSO technique.10,37 The mean pooled blood loss (anterior + posterior approach) of 863 ml indicates that OLIF significantly reduces the intraoperative blood loss compared to that of posterior osteotomies.18 Deukmedjian et al. and Leveque et al. reported a mean blood loss of 655 and 800 ml, respectively, with lateral interbody fusion techniques.17,37
Recently, degenerative spinal deformities are treated using minimal invasive spine (MIS) techniques, and these have been reported to minimize bleeding, soft tissue injury, and infection rates.2,38 In our series, we could have considered MIS technique; however, because of need for multilevel decompression (relatively long surgical time) and additional bone procedures such as facetectomy, lamina resection, and SPO, we prefer to do an open posterior approach in our series. Hence, in selective patients, we have to resort to staged surgeries to reduce the intensity of surgical insult inflicted upon the patients. Some of the complications related to longer operative duration and excessive intraoperative blood loss can be reduced. Furthermore, the spinopelvic parameters can be reassessed between the two stages of surgery to assess the need for additional procedures such as SPO, PSO, open laminectomy, or extent of pedicle screw fixations.17,39
Four patients developed complications (two transient hip weakness, one infection, and one end plate fracture). However, only one patient (6%) required revision. Leveque et al. observed a revision rate of 15% with LLIF, whereas 28% revision was observed after PSO.37 VAS scores improved by 74% for leg pain and 58% for back pain and ODI scores improved by 69.5% in our study. Theologis et al. observed 30%–37% improvement in ODI scores and 57%–60% improvement in VAS back pain scores and 32%–65% in VAS leg scores.40 Similarly, Lee et al. obtained a 48%–60% improvement in ODI scores, 50%–75% improvement in VAS back scores, and 38%–54% improvement in VAS leg scores.10 Better ODI scores obtained in our study can be considered secondary to the fact that pelvic fixation was not performed in any of our patients and enlargement of neural foramen achieved by relatively large size OLIF cage occupying approximately 2/3 surface of vertebral body.
Our study had few limitations. We had a small series of patients and did not establish a control group. Hence, to draw valid conclusion is seemed to be difficult. To overcome these limitations, the authors once again confirmed the p value, which showed statistical significance by SPSS software, through statistical power verification by G Power program. Through such a process, we tried to reduce the statistical errors, and were careful to draw out the results. The decision pertaining to the extent of posterior fusion levels was subjective, and based on surgeon's experience, however, authors considered the clinical (age, muscular status, and bone quality) and radiological (global balance of spine, progression of degeneration and instability of spine) factors of enrolled patients. The increase in TK during followup might point toward the development of proximal junctional kyphosis. However, none of our patients showed significant clinical or radiological features of proximal junctional kyphosis. Larger series with longer followup may be required for better understanding of pseudoarthrosis, proximal junctional kyphosis, and loss of correction after OLIF in ADLD. However, given the encouraging results obtained in our study, it can be concluded that OLIF gives equal or better early outcomes compared to the conventional posterior techniques.
Conclusions
OLIF gives favorable early clinical and radiological outcomes in patients of symptomatic ADLD and reduces the risk of serious complications. OLIF reduces the need for supplemental posterior osteotomies and potentially obviates the need for extension of fusion to pelvis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142–7. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–48. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 3.Bradford DS, Tay BK, Hu SS. Adult scoliosis: Surgical indications, operative management, complications, and outcomes. Spine (Phila Pa 1976) 1999;24:2617–29. doi: 10.1097/00007632-199912150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop (Belle Mead NJ) 2003;32:77–82. [PubMed] [Google Scholar]
- 5.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: Perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322–30. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 6.Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS, et al. Management of fixed sagittal plane deformity: Results of the transpedicular wedge resection osteotomy. Spine (Phila Pa 1976) 2001;26:2036–43. doi: 10.1097/00007632-200109150-00020. [DOI] [PubMed] [Google Scholar]
- 7.Cho KJ, Bridwell KH, Lenke LG, Berra A, Baldus C. Comparison of smith-petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine (Phila Pa 1976) 2005;30:2030–7. doi: 10.1097/01.brs.0000179085.92998.ee. [DOI] [PubMed] [Google Scholar]
- 8.Cho KJ, Kim KT, Kim WJ, Lee SH, Jung JH, Kim YT, et al. Pedicle subtraction osteotomy in elderly patients with degenerative sagittal imbalance. Spine (Phila Pa 1976) 2013;38:E1561–6. doi: 10.1097/BRS.0b013e3182a63c29. [DOI] [PubMed] [Google Scholar]
- 9.Kim KT, Lee SH, Suk KS, Lee JH, Jeong BO. Outcome of pedicle subtraction osteotomies for fixed sagittal imbalance of multiple etiologies: A retrospective review of 140 patients. Spine (Phila Pa 1976) 2012;37:1667–75. doi: 10.1097/BRS.0b013e3182552fd0. [DOI] [PubMed] [Google Scholar]
- 10.Lee CS, Park SJ, Chung SS, Lee JY, Yum TH, Shin SK, et al. Mini-open anterior lumbar interbody fusion combined with lateral lumbar interbody fusion in corrective surgery for adult spinal deformity. Asian Spine J. 2016;10:1023–32. doi: 10.4184/asj.2016.10.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: Prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006;31:2329–36. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 12.Kepler CK, Sharma AK, Huang RC, Meredith DS, Girardi FP, Cammisa FP, Jr, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16:329–33. doi: 10.3171/2012.1.SPINE11528. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331–7. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 14.Dahdaleh NS, Smith ZA, Snyder LA, Graham RB, Fessler RG, Koski TR, et al. Lateral transpsoas lumbar interbody fusion: Outcomes and deformity correction. Neurosurg Clin N Am. 2014;25:353–60. doi: 10.1016/j.nec.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Manwaring JC, Bach K, Ahmadian AA, Deukmedjian AR, Smith DA, Uribe JS, et al. Management of sagittal balance in adult spinal deformity with minimally invasive anterolateral lumbar interbody fusion: A preliminary radiographic study. J Neurosurg Spine. 2014;20:515–22. doi: 10.3171/2014.2.SPINE1347. [DOI] [PubMed] [Google Scholar]
- 16.Tempel ZJ, Gandhoke GS, Bonfield CM, Okonkwo DO, Kanter AS. Radiographic and clinical outcomes following combined lateral lumbar interbody fusion and posterior segmental stabilization in patients with adult degenerative scoliosis. Neurosurg Focus. 2014;36:E11. doi: 10.3171/2014.3.FOCUS13368. [DOI] [PubMed] [Google Scholar]
- 17.Deukmedjian AR, Dakwar E, Ahmadian A, Smith DA, Uribe JS. Early outcomes of minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. ScientificWorldJournal. 2012;2012:789698. doi: 10.1100/2012/789698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtori S, Mannoji C, Orita S, Yamauchi K, Eguchi Y, Ochiai N, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: Oblique lateral interbody fusion for degenerated lumbar spinal kyphoscoliosis. Asian Spine J. 2015;9:565–72. doi: 10.4184/asj.2015.9.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: A fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7:99–103. doi: 10.1007/s005860050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, N.J: L. Erlbaum Associates; 1988. pp. 21–567. [Google Scholar]
- 21.Auerbach JD, Lenke LG, Bridwell KH, Sehn JK, Milby AH, Bumpass D, et al. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine (Phila Pa 1976) 2012;37:1198–210. doi: 10.1097/BRS.0b013e31824fffde. [DOI] [PubMed] [Google Scholar]
- 22.Enercan M, Ozturk C, Kahraman S, Sarıer M, Hamzaoglu A, Alanay A, et al. Osteotomies/spinal column resections in adult deformity. Eur Spine J. 2013;22(Suppl 2):S254–64. doi: 10.1007/s00586-012-2313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boachie-Adjei O, Ferguson JA, Pigeon RG, Peskin MR. Transpedicular lumbar wedge resection osteotomy for fixed sagittal imbalance: Surgical technique and early results. Spine (Phila Pa 1976) 2006;31:485–92. doi: 10.1097/01.brs.0000199893.71141.59. [DOI] [PubMed] [Google Scholar]
- 24.Cho KJ, Suk SI, Park SR, Kim JH, Kim SS, Lee TJ, et al. Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J. 2008;17:650–6. doi: 10.1007/s00586-008-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Homma T. One-stage three-dimensional correction and fusion: A multilevel posterior lumbar interbody fusion procedure for degenerative lumbar kyphoscoliosis. Technical note. J Neurosurg. 2003;99:125–31. doi: 10.3171/spi.2003.99.1.0125. [DOI] [PubMed] [Google Scholar]
- 26.Dorward IG, Lenke LG, Bridwell KH, OʼLeary PT, Stoker GE, Pahys JM, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: A matched cohort analysis. Spine (Phila Pa 1976) 2013;38:E755–62. doi: 10.1097/BRS.0b013e31828d6ca3. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh PC, Koski TR, O'Shaughnessy BA, Sugrue P, Salehi S, Ondra S, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: Implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379–86. doi: 10.3171/SPI-07/10/379. [DOI] [PubMed] [Google Scholar]
- 28.Watkins RG, 4th, Hanna R, Chang D, Watkins RG., 3rd Sagittal alignment after lumbar interbody fusion: Comparing anterior, lateral, and transforaminal approaches. J Spinal Disord Tech. 2014;27:253–6. doi: 10.1097/BSD.0b013e31828a8447. [DOI] [PubMed] [Google Scholar]
- 29.Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: A radiographic study. J Neurosurg Spine. 2011;15:92–6. doi: 10.3171/2011.3.SPINE10425. [DOI] [PubMed] [Google Scholar]
- 30.Baghdadi YM, Larson AN, Dekutoski MB, Cui Q, Sebastian AS, Armitage BM, et al. Sagittal balance and spinopelvic parameters after lateral lumbar interbody fusion for degenerative scoliosis: A case-control study. Spine (Phila Pa 1976) 2014;39:E166–73. doi: 10.1097/BRS.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro C, Oliveira L, Amaral R, Marchi L, Pimenta L. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res. 2014;472:1776–83. doi: 10.1007/s11999-013-3263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RD, Valore A, Villaminar A, Comisso M, Balsano M. Pelvic parameters of sagittal balance in extreme lateral interbody fusion for degenerative lumbar disc disease. J Clin Neurosci. 2013;20:576–81. doi: 10.1016/j.jocn.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Anand N, Cohen RB, Cohen J, Kahndehroo B, Kahwaty S, Baron E, et al. The influence of lordotic cages on creating sagittal balance in the CMIS treatment of adult spinal deformity. Int J Spine Surg. 2017;11:23. doi: 10.14444/4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand N, Baron EM, Khandehroo B. Limitations and ceiling effects with circumferential minimally invasive correction techniques for adult scoliosis: Analysis of radiological outcomes over a 7-year experience. Neurosurg Focus. 2014;36:E14. doi: 10.3171/2014.3.FOCUS13585. [DOI] [PubMed] [Google Scholar]
- 35.Caputo AM, Michael KW, Chapman TM, Jennings JM, Hubbard EW, Isaacs RE, et al. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci. 2013;20:1558–63. doi: 10.1016/j.jocn.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 37.Leveque JC, Yanamadala V, Buchlak QD, Sethi RK. Correction of severe spinopelvic mismatch: Decreased blood loss with lateral hyperlordotic interbody grafts as compared with pedicle subtraction osteotomy. Neurosurg Focus. 2017;43:E15. doi: 10.3171/2017.5.FOCUS17195. [DOI] [PubMed] [Google Scholar]
- 38.Bach K, Ahmadian A, Deukmedjian A, Uribe JS. Minimally invasive surgical techniques in adult degenerative spinal deformity: A systematic review. Clin Orthop Relat Res. 2014;472:1749–61. doi: 10.1007/s11999-013-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand N. How to create sagittal balance in MIS correction of adult spinal deformity. Spine (Phila Pa 1976) 2017;42(Suppl 7):S17–8. doi: 10.1097/BRS.0000000000002029. [DOI] [PubMed] [Google Scholar]
- 40.Theologis AA, Mundis GM, Jr, Nguyen S, Okonkwo DO, Mummaneni PV, Smith JS, et al. Utility of multilevel lateral interbody fusion of the thoracolumbar coronal curve apex in adult deformity surgery in combination with open posterior instrumentation and L5-S1 interbody fusion: A case-matched evaluation of 32 patients. J Neurosurg Spine. 2017;26:208–19. doi: 10.3171/2016.8.SPINE151543. [DOI] [PubMed] [Google Scholar]