Abstract
Plant natural products have served as a prominent source of medicines throughout human history, and are still used today as clinically-approved pharmaceuticals. However, many medicinal plants that produce useful compounds are slow-growing or recalcitrant to cultivation, making it difficult to investigate the underlying genetic/enzymatic machinery responsible for biosynthesis. To better understand the metabolism of bioactive natural products in slow-growing medicinal plants, we used D2O labeling and LC-MS-based metabolomics to explore the biosynthesis of medically-relevant alkaloids in three plant species. Our results provide evidence for sites of active biosynthesis for these alkaloids, and demonstrate that D2O labeling can be used as a general method to determine sites of active secondary metabolism over relatively short time scales. We anticipate that these results will facilitate discovery of complete metabolic pathways for plant natural products of medicinal importance, especially for approaches that rely upon transcriptomics and knowledge of active metabolism to identify biosynthetic enzymes.
INTRODUCTION
Plants have served as a major source of medically and commercially important bioactive compounds in both ancient and modern times.1–3 Indeed, many FDA-approved medicines such as colchicine, omacetaxine mepesuccinate (homoharringtonine), and etoposide originate from plants, and extraction of these compounds or precursors from living plant tissues has been a major source of several clinically-used therapeutics.3,4 However, unlike common model plants like Nicotiana benthamiana or Arabidopsis thaliana that can grow to near maturity in weeks or months, many plants used for medicinal purposes grow very slowly, with times to maturity of years or more. Additionally, many medicinal plants can also require particular growth environments that are difficult to replicate for regular cultivation.4,5 Consequently, biological experimentation with slow growing plants can prove to be difficult, as the length of experimentation and propagation of these plants can be prohibitive to pursuing complex plant molecules as clinical candidates. As a result, the biology, genetics, and biochemistry of many slow-growing medicinal plants are not particularly well characterized. For example, very few medicinal plants have sequenced genomes, and the biosynthetic pathways underlying the production of many medicinal compounds are unknown at the genetic or biochemical levels.6 Understanding the genes that are involved in the biosynthesis of medicinal natural products will not only expand our knowledge of plant chemistry and enzymology, but will also enable metabolic engineering strategies in heterologous expression hosts.
Research towards the discovery of natural product biosynthetic pathways has included activity guided fractionation, gene expression analysis, and genome mining for biosynthetic gene clusters.7 Although there are many examples for clustering of biosynthetic enzymes in plants,8 this phenomenon does not apply to all plant natural products, and also necessitates a sufficiently sequenced and annotated genome.
In lieu of genomic information, a particularly powerful method for interrogating biosynthesis in non-model plants involves combining transcriptomics with biochemical deduction, specifically by correlating gene expression of likely candidate enzymes to the accumulation of a molecule of interest under certain conditions.9,10 This approach is broadly applicable, as genes involved in the same metabolic pathway are often co-expressed during active biosynthesis, and transcriptomes can be readily determined from any plant, regardless of the availability of genomic information.11
In many cases, plant natural product biosynthesis can be elicited by treating plant species with a biotic or abiotic stimulus. For example, wounding by herbivorous insects can stimulate nicotine biosynthesis in tobacco plants,12 and microbial infections induce the production of camalexin in Arabidopsis.13 The ability to induce biosynthesis of a plant natural product allows for facile identification of biosynthetic genes via transcriptomic analysis, as the expression of biosynthetic genes would be expected to correlate with an induction and increase of metabolite production. Indeed, this has proven to be a successful strategy for the identification of biosynthetic genes for a number of plant natural products.9,14
Many plant natural products are not induced by external stimuli, and instead accumulate within certain tissues or during specific developmental stages.15 In the absence of inducible expression, a possible approach to discover biosynthetic genes in these scenarios is to compare conditions or tissues in which the compound is present to those in which it is absent, or significantly lower. As such, this method would involve the assumption that the observed accumulation of a metabolite is indicative of active biosynthesis. However, this assumption should be made with caution, as there are examples in which accumulation of a compound does not necessarily equate to active biosynthesis in the same location. For example, after leaf wounding, nicotine biosynthesis is initiated in root tissue, after which this alkaloid is transported to the leaves.12 This phenomenon is not unique to tobacco; long distance transport of gibberellin phytohormone precursors has also been demonstrated, showing that sequentially-acting biosynthetic enzymes can be distally localized within a plant.16 Additional examples include the transport of glucosinolates into seeds following their biosynthesis in Arabidopsis siliques,17 and biosynthetic steps for the anti-cancer drugs vinblastine and vincristine that have been shown to be separated into multiple distinct cell and tissue types.18 Thus, it cannot necessarily always be assumed that accumulation of a compound in one sampled location will be correlated to de novo biosynthesis. Accordingly, without knowledge on the site of biosynthesis, the identification of biosynthetic genes with correlated expression may be confounded.
Heavy water (D2O) labeling in plants -- a simple and inexpensive experiment that can be performed on any species-- can be utilized for a variety of quantitative and qualitative examinations of metabolic activity, including natural product biosynthesis. This is because plants are “hydrotrophic”, with all of the hydrogen atoms needed for biosynthesis of new organic biomass originating from water. During photosynthesis, photosystem II “fixes” hydrogen for biosynthesis by converting water-derived hydrogens to plastoquinol-bound hydrogens.19 Eventually these plastoquinol hydrogens are fixed to a relatively stable carbon-bound form by conversion to NADPH (and NADH), a key biosynthetic intermediate from which essentially all carbon-bound hydrogen atom formed in plants are ultimately derived. Prior to complete labeling, metabolites become partially labeled to a degree that is a complex function of the metabolic fluxes involved in its biosynthesis (Figure 1). This biochemistry can be co-opted for analyzing active biosynthesis; if a molecule is being actively produced in the presence of D2O, then incorporation of deuterium, and therefore a change in its mass isotopologue distribution (MID), would be expected. MIDs can be measured quantitatively using mass spectrometry, and this type of analysis has proven to be useful for measuring active metabolism in plants. For example, supplementing plants with D2O has been used to uncover active sites of plant hormone biosynthesis,20,21 and for phototrophic organisms that grow rapidly, deuterium labeling has been used to quantitatively assess metabolite turnover and net flux.22
Figure 1. Schematic of deuterium incorporation into secondary metabolites in plants and analysis workflow.
Deuterium from heavy water (D2O) is incorporated into plant metabolism via several routes. Initial conversion of D2O to labeled plastoquinol occurs in photosystem II; subsequent steps from photosystem I and related enzymes result in the fixation of solvent-exchangeable atoms (red) to relatively stable carbon-bound hydrogen atoms (blue) during the formation of NADPH. These labeled atoms are incorporated into primary metabolites by central metabolic enzymes. Eventually, all non-exchangeable hydrogen atoms would become labeled, but on the timescales studied in this paper, several routes may contribute to the biosynthesis of secondary metabolites, including direct incorporation of hydrogen from water, reductive incorporation of hydrogen from labeled NAD(P)D, and recycling or turnover of incompletely labeled primary metabolites, resulting in partially deuterated secondary metabolites. In this study, we use LC-MS-based metabolomics approaches to quantify label incorporation into plant metabolomes by finding mass isotopologue distributions (MIDs) using both targeted and untargeted approaches, and using these distributions to estimate relative deuterium abundance in a metabolite by comparison to the MID of an unlabeled control sample.
Here, we report three case studies in which D2O labeling of slow-growing, medicinal plant species has been combined with LC-MS-based metabolomics to determine sites of active metabolism for bioactive plant alkaloids. Modern LC-MS instruments can detect very low amounts of deuterium incorporation into a huge variety of metabolites, and this sensitivity means that instead of waiting for complete labeling to occur, a situation that could take years for many plants, informative D2O labeling experiments can be done in a matter of weeks. The use of short-term D2O labeling of plants and plant tissues provides a simple experimental technique that may serve as a starting point to probe topics related to plant natural product biosynthesis such as (a) localization of biosynthetic activity to particular tissues, (b) quantification of the relative rates of metabolite synthesis across a specific metabolic pathway, and (c) estimations for metabolome-wide relative biosynthetic rates. In addition to describing our approach, we have published scripts and code that may be useful for those wishing to perform LC-MS analysis of deuterium labeling experiments, which is available at https://github.com/Stanford-ChEMH-MCAC/d2o_metabolomics.
Case Study 1: Timing and tissue specificity of huperzine A biosynthesis in Huperzia tetrasticha
Background
Club mosses from the Lycopodiaceae family produce a structurally-related class of molecules called the Lycopodium alkaloids.23 These alkaloids were initially isolated from plants of the Lycopodium genus, and have subsequently been found in other major genera from this family, including Huperzia and Phlegmariurus. The most prominent of the Lycopodium alkaloids is huperzine A (HupA), which was initially isolated from Huperzia serrata,24 a traditional Chinese medicinal plant that has been used for over a millennium.25 HupA exhibits reversible inhibition of human acetylcholinesterase,24 and this bioactivity has led to a great deal of interest in pursuing this alkaloid as a pharmaceutical for the treatment of neurological disorders such as Alzheimer’s dementia and schizophrenia.26 HupA has traditionally been extracted directly from wild H. serrata plants, but these species are very slow growing; it can take 10–20 years for them to go from spore germination to a mature plant that has a significant level of HupA.25 Additionally, cultivation of Huperzia species has proven to be difficult, making any large scale agricultural production of HupA untenable. Collectively, the slow growth and lack of successful cultivation of Huperzia species has resulted in non-sustainable overharvest of native populations that threatens the ecosystems in which these plants reside.25 Furthermore, although chemical syntheses of this molecule have been accomplished,27 these may not be amenable to industrial scale-up, and thus alternative means of HupA production would help to alleviate the need for harvest of wild plants.
Through previous isotope labeling studies,28,29 the Lycopodium alkaloids have been shown to be derived from L-lysine and malonyl-CoA (Figure 2a). Although biosynthetic hypotheses have been developed based upon these studies in addition to several others,23 the underlying enzymes for the majority of Lycopodium alkaloid biosynthesis have not yet been elucidated. Previous analyses of alkaloid content in multiple Huperzia and Lycopodium species have shown that these compounds accumulate to the highest levels within leaves at the new growth of the plant, suggesting this to be the active site of biosynthesis.30–32 However, these alkaloids can also be detected in other tissues of the plant, and thus it is unclear whether Lycopodium alkaloid biosynthesis is specific to one tissue, or if it is present in all tissue types.
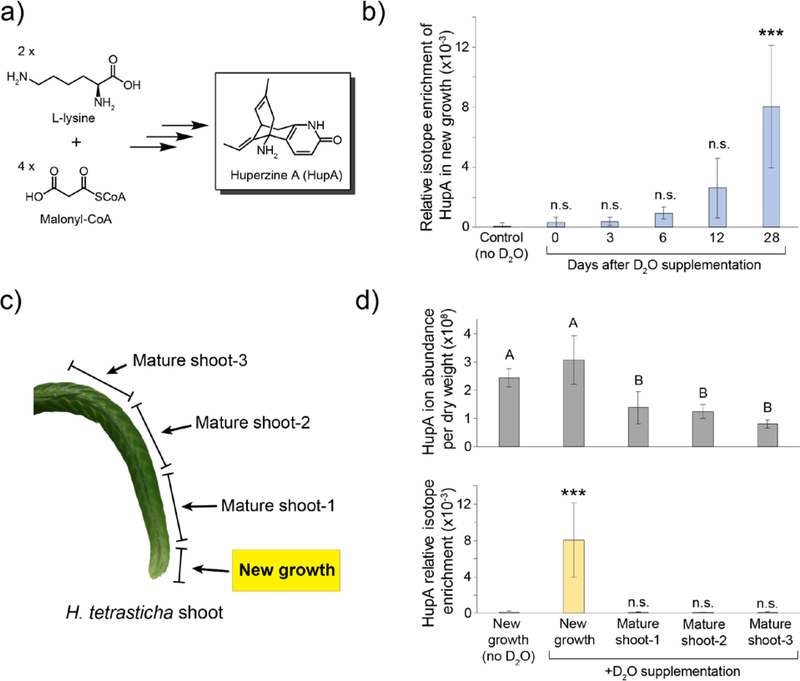
Figure 2. Analysis of HupA biosynthetic localization using deuterium labeling.
a) HupA is derived from L-lysine and malonyl-CoA, but the underlying biosynthesis remains unknown. b) Isotope enrichment of HupA over time in new growth. n=3–4 for each time point; Statistical significance determined using Dunnett’s test with comparison to the no D2O control, which was measured at the 28-day time point; *** = p<0.001, n.s. = not significant. c) HupA labeling was analyzed in different sections of the D2O-supplemented shoots to assess if biosynthesis is specific to a specific region of the shoot. d) Comparison of total HupA levels (top panel) in each tissue to the relative isotope enrichment (bottom panel) in new growth and in mature shoot tissue. n=3–7 per tissue; Statistical significance for the HupA content per tissue was performed using the Tukey-Kramer HSD test, with different letters indicating p<0.01. Statistical significance for isotope enrichment between tissues was determined using Dunnett’s test with comparison to the no D2O control; *** = p<0.001. Error bars on all graphs represent ± standard deviation.
Results
In order to determine an active site of biosynthesis, shoots from Huperzia tetrasticha, which produces HupA as a major alkaloid, were detached from the plant and placed for growth in 10% D2O. Analysis of isotope enrichment in HupA over time demonstrated that deuterium is indeed being incorporated into this molecule in new growth at the shoot tip (Figure 2b), showing that HupA biosynthesis is active in this tissue. As expected from the 10 to 15 year maturation time of Huperzia spp. and from the length of our experiment (28 days), the maximal observed deuterium incorporation was relatively low. Additionally, we did not observe maximum labeling of HupA (in which the majority of molecules would have 10% of their hydrogens replaced with deuterium), implying that complete de novo biosynthesis may not have occurred to an appreciable extent during the time frame of our experiment, or that newly synthesized HupA is only making up a small portion of the total HupA pool in this tissue.
As previously mentioned, HupA can be detected in all tissues of Huperzia plants. Although HupA abundance is significantly higher in the new growth than in mature shoot tissue, the difference between these two regions is only ~2-fold (Figure 2c and 2d). However, under D2O supplementation, HupA isotope enrichment was only observed in the new growth of the plant shoot and not in the mature shoot tissue (Figure 2c and 2d). This shows that at least in the shoot, HupA biosynthetic activity is specifically located within the new growth of shoot tips. Conversely, the lack of deuterium incorporation into HupA in the mature tissue indicates that HupA biosynthesis in this area is not active, at least to a detectable level. Collectively, the data from this experiment suggest that biosynthesis of HupA is occurring in the location of highest accumulation, specifically the new growth at the tip of shoots, and that transport (e.g. from root to shoot tips) is unlikely to be a major contributor to the observed HupA biosynthesis. Because HupA is present in the mature shoot tissue but is not being actively labeled, it seems likely that this pool of HupA was produced prior to the labeling experiments. In this scenario, production of HupA could be occurring actively at the shoot tip, and as the plant grows, the previously synthesized HupA is essentially left behind in what becomes “mature tissue”.
As an internal control, the incorporation of deuterium into the amino acid L-phenylalanine was also assessed in all of the same H. tetrasticha tissue extracts. As expected, this primary metabolite was enriched with deuterium over time in samples supplemented with D2O. Though this incorporation was highest in the new growth of the shoot, which would be expected because this tissue is actively growing, non-zero deuterium labeling could also be reliably detected in the mature shoot tissue (Figure S1). Thus, the lack of deuterium incorporation into HupA in the mature shoot tissue is not due to a lack of plant metabolic activity in this tissue, but presumably absence of HupA biosynthetic machinery.
Case Study 2: Homoharringtonine biosynthesis and whole-metabolome labeling in Cephalotaxus harringtonia
Background
Evergreen shrubs of the Cephalotaxaceae family produce a variety of alkaloids known as the Cephalotaxus alkaloids.33 This class of alkaloids is derived from the parent compound cephalotaxine and the remaining constituents are largely characterized by the addition of an aliphatic-ester side chain of varying complexity. Of the cephalotaxine esters, homoharringtonine has drawn considerable attention due to its significant inhibition of P388 lymphoid leukemia in mice34 as well as the ability to inhibit colony formation of myeloid and lymphocytic cell lines.35 Research into the mechanism of action of homoharringtonine has identified it as a potent, reversible inhibitor of the initiation of protein synthesis36, and it was approved for the treatment of chronic myeloid leukemia by the FDA in 2012.37,38 Procurement of this compound from natural sources is complicated by several factors including protection due to endangerment and relatively low levels of the desired alkaloid.39 Additionally, Cephalotaxus plants display slow rates of propagation and growth with cuttings taking at least 6 months to root and an additional 2–3 years to produce a commercially salable shrub.40 Numerous total chemical syntheses have been accomplished, however, they are not currently amenable to industrial scale production.33,41 Presently, homoharringtonine is produced by semi-synthesis from the more highly abundant parent compound, cephalotaxine, and the synthetically prepared side chain ester.42
The precise details of the biosynthesis for cephalotaxine and its esters remain undetermined; however, hypothetical pathways have been proposed based on isotope labeling studies.43,44 These endeavors have determined that the cephalotaxine core is derived from tyrosine and phenylalanine and that the side chain ester is produced by a chain elongation mechanism analogous to that of leucine biosynthesis. The location of biosynthesis within Cephalotaxus also has yet to be elucidated, but the levels of alkaloid content have been found to be highest in the needles.39 However, the observation of these compounds in other tissues suggests accumulation in needles could also be a result of metabolite transport.
Results
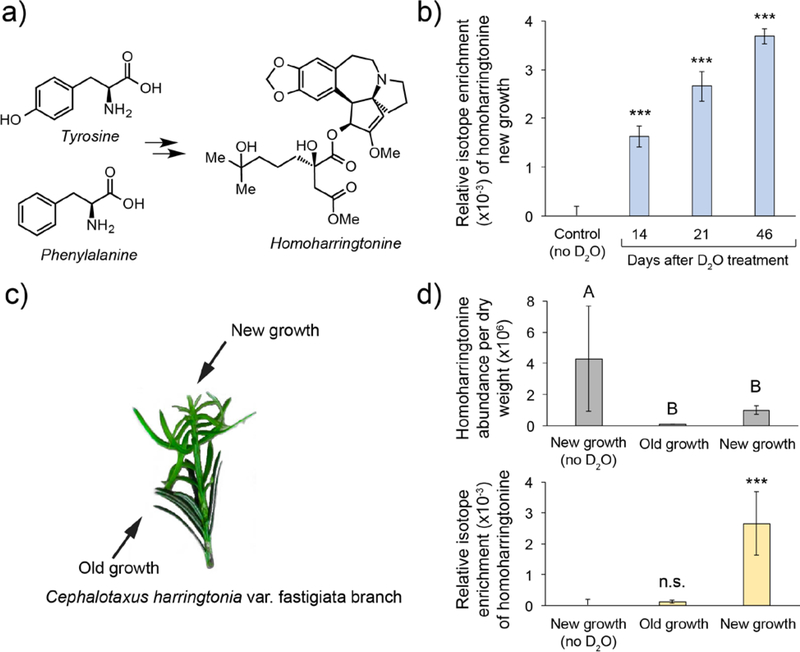
In an effort to identify a site of active biosynthesis for homoharringtonine, a small branch was removed from the base of the main plant and grown in a 10% D2O solution (Figure 3c). Analysis of deuterium incorporation over time revealed that the amount of labeled homoharringtonine continued to increase over time in areas of new growth (Figure 3b). Additionally, substantially more isotopic enrichment was observed in samples of new growth when compared to older needles (Figure 3d). While the overall levels of deuterium incorporation observed were low, which is likely attributable to the slow growth rates of Cephalotaxus plants, isotope enrichment was negligible in older needles. Thus, the important metric in this analysis is the relative amount of deuterium incorporation between new and old needles. These results are consistent with the notion that production of homoharringtonine is localized to areas of new growth despite its presence in needles that do not appear to be actively producing it. In order to confirm that the old growth needles were still metabolically active, we also analyzed deuterium incorporation of the amino acid phenylalanine (Figure S2). Significant isotope incorporation into phenylalanine was found in needles of both new and old growth relative to the control. These data suggest that both needles of new and old growth are metabolically active and that the levels of deuterium incorporation observed in homoharringtonine are indeed due to a localization of requisite biosynthetic machinery to areas of new growth. Overall, the data presented is consistent with the hypothesis that homoharringtonine biosynthesis is localized to newly grown needles and that transport of the metabolite from another location in the plant is unlikely to be a major pathway of accumulation.
Figure 3. Analysis of homoharringtonine biosynthetic location using D2O labeling.
a) Prior labeling studies have shown the core of homoharringtonine to be derived from tyrosine and phenylalanine but the details of the biosynthesis remain undetermined. b) Analysis of homoharringtonine isotope enrichment over time in new growth tissue. n=3 for each time point; Statistical significance was determined using Dunnett’s test with comparison to the no D2O control measured at a 30-day time point; *** = p<0.0001; c) Incorporation of deuterium was analyzed in needles present prior to D2O supplementation (old growth) and compared with needles that appeared post D2O supplementation (new growth). d) (top) Relative abundance of homoharringtonine in new growth vs. old growth (normalized to mg of dry weight). Statistical significance for the homoharringtonine abundance per tissue was performed using the Tukey-Kramer HSD test with different letters indicating p<0.005. (bottom) Relative isotope enrichment in new growth (with and without D2O supplementation) and old growth. n=3 for each tissue; Statistical significance for isotope enrichment between tissues was determined using Dunnett’s test with comparison to the no D2O control; *** = p<0.0001; Error bars on all graphs represent ± standard deviation.
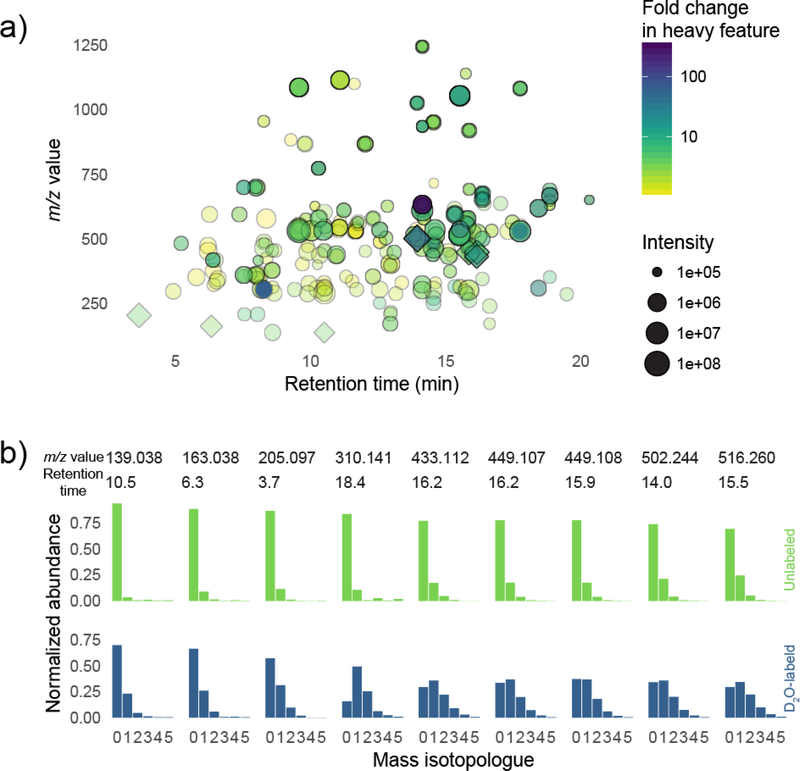
With this dataset, we also assessed the extent of labeling in detected metabolites in an untargeted manner (Figure 4). Using XCMS,45 we compared an extract of D2O-labeled new growth tissue to an unlabeled control of the same tissue type. Our analysis uncovered 10711 mass spectral features. We focused on 1769 of these which met retention time and intensity criteria (between 5 and 21.6 minutes of the LC gradient, and with ion counts greater than 3×104) and which had an m/z of less than 700 (to focus on small molecules). From among all possible 1.5 million unique pairwise combinations of these features, we identified pairs where (a) features co-eluted, and (b) the heavier feature was upregulated in the labeled sample and the lighter feature was upregulated in the unlabeled sample, and (c) where the m/z difference between the features was consistent with incorporation of deuterium. This relatively simple filtering procedure revealed that labeling was extensive throughout the Cephalotaxus metabolome (Figure 4a). For nine of the most intense or most labeled features, we show the corresponding mass isotopologue distribution (Figure 4b). Although we report neither empirical formulae nor structural identities for any of the chosen metabolites, our analysis shows many metabolites in Cephalotaxus are labeled significantly in new growth tissue after only a 46-day labeling experiment. It also shows that metabolites vary in their deuterium incorporation, and that metabolites containing relatively more or less isotope can be identified computationally, and, if desired, chosen for further study in an untargeted way. For this initial proof-of-concept experiment we used only three replicates; a more thorough untargeted metabolomics study of deuterium incorporation would be better served by a higher number of replicates.
Figure 4. Untargeted metabolomics analysis of deuterium labeling in new growth of Cephalotaxus plants.
a) XCMS analysis and pairwise feature comparisons identified coeluting features differing in mass by one or more deuterium neutrons. Each point corresponds to a coeluting feature pair where the heavier feature is more abundant in the labeled sample and where the lighter feature is more abundant in the unlabeled sample. Labeled metabolites span a range of retention times and m/z values, and the degree of label incorporation varies from metabolite to metabolite. Points plotted with diamonds correspond to metabolites whose mass isotopologue distribution is shown in (b). b) Mass isotopologue distributions of 9 highly labeled and/or spectrally intense features identified from the analysis. MIDs for metabolites extracted from labeled plants (blue bars; bottom) compared to MIDs for the same metabolites extracted from unlabeled plants (green bars; top). The extent of label corporation differs across metabolites.
We also used LC-MS2, or tandem mass spectrometry, to help assess which positions in specific deuterated molecules are most enriched for the heavy deuterium (2H) isotope. Homoharringtonine is an excellent testbed for this approach, because it ionizes efficiently, and because it’s MS2 spectrum is relatively simple: the sole intense fragment ion at a collision energy of 20 V arises from neutral loss of the ester side chain, leaving an anhydro-cephalotaxine ion at 298.1438 m/z (Figure S3). To pinpoint the locations within homoharringtonine that are more (or less) enriched for deuterium compared to the molecule as a whole, we fragmented the M2 isotopologue of homoharringtonine from D2O-labeled plant tissue. As previously observed, selective fragmentation of heavy isotopologues gives rise to fragment spectra that exhibit “splitting”.46 The relative intensities of these split peaks differ in unlabeled and labeled samples, showing that heavy isotopes (arising from the natural abundance of 13C in the unlabeled sample) are distributed differently. We hope in future work to develop a framework for quantitative analysis of these differences in order to precisely assess the degree to which different parts of secondary metabolites such as cephalotaxine are labeled.
Case Study 3: Acutumine biosynthesis and labeling of pathway metabolites in Menispermum canadense
Background
Benzylisoquinoline alkaloids (BIAs) are a family of alkaloids with more than 3,000 unique structures.47 This group includes molecules such as morphine, berberine, sanguinarine and noscapine, which all have well-characterized bioactivities in humans. Acutumine and acutumidine are BIAs produced by Menispermum species, which have been used as part of herbal remedies in east Asian countries for the treatment of virus infection, cough and amnesia.48 Both acutumine and acutumidine are known to be produced in roots of species from the Menispermum genus.49 As such, root tissues of these medicinal plants are the major source of these two alkaloids. However, these acutumine-producing plants grow slowly, with root development taking up to several years.
Acutumine and acutumidine are proposed to be synthesized from reticuline, a common intermediate of most BIAs. Most genes responsible for biosynthesis of reticuline from tyrosine have been biochemically characterized in other BIA-producing plants,47 and intermediates in the reticuline pathway are norcoclaurine, coclaurine, N-methylcoclaurine, 3’-hydroxy-N-methylcoclaurine (Figure 5a). Although many of the biosynthetic intermediates between reticuline and acutumine/acutumidine are currently unknown, the metabolic pathway for these alkaloids is proposed to require the action of three different enzymes; namely, a halogenase that converts dechloroacutumine to acutumine,50 and methylase and demethylase enzymes that interconvert acutumine and acutumidine.51 However, the genes encoding these enzymes are not known.
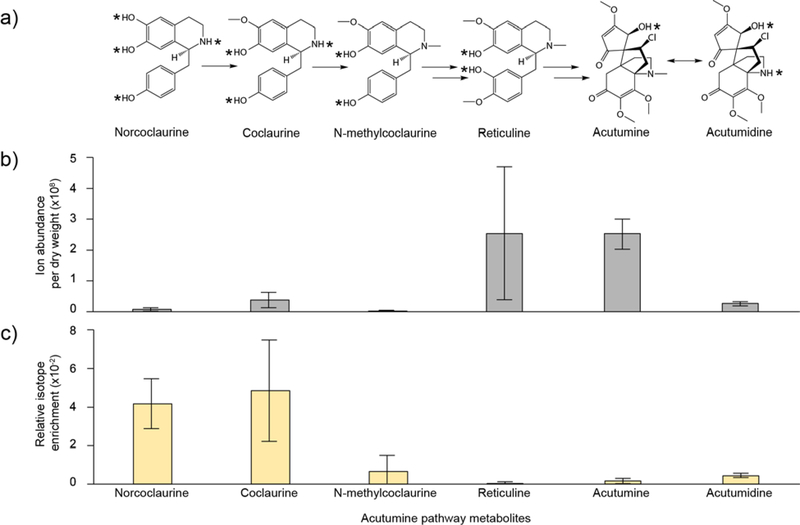
Figure 5. Acutumine pathway metabolites and their responses to D2O labeling in leaves of Menispermum plants.
a) Proposed pathway toward the biosynthesis of acutumine and acutumidine. Exchangeable protons are indicated with asterisks. b) Extracted ion current (EIC) for pathway metabolites. Results are means (6 replicates) ± standard deviation c) Relative isotope enrichment of pathway metabolites in response to D2O labeling. Results are the average of 3 replicates. Error bars represent ± standard deviation.
Results
Menispermum canadense rhizomes were reported to accumulate acutumine and its demethylated derivative acutumidine (Figure 5a).52,53 LC-MS profiling performed on this plant species indicated that mass signals putatively corresponding to acutumine, acutumidine, and most known pathway intermediates (i.e. norcoclaurine, coclaurine, N-methylcoclaurine and reticuline) are present in all organs tested (i.e. roots, rhizomes and leaves). Reticuline and acutumine accumulate to the highest level in leaves (Figure 5b, Figure S4).
To determine if the acutumine biosynthetic pathway is occurring in leaves of Menispermum canadense plants, excised leaves were grown in 100% D2O. This higher concentration was used in an attempt to maximize the amount of deuterium incorporation, which was indeed observed to be noticeably higher in this experiment (Figure 5c). Because of this higher deuterium incorporation, results are presented both in terms of relative isotope enrichment, as presented for HupA and homoharringtonine (Figure 5c), and also for individual MIDs in order to highlight and emphasize the shift in mass isotopologue abundance as result of this deuterium labeling (Figure S5). The isotope enrichments of two early intermediates, norcoclaurine and coclaurine, are the highest among all proposed pathway metabolites. In agreement with these results, the M0 value (which is normally the most abundant mass isotopologue of the parent ion under non-labeling conditions) of each is decreased, whereas the higher isotopologues (M1, M2, M3) values are increased. These results demonstrate that the metabolic module toward biosynthesis of N-methylcoclaurine is metabolically active in leaves of the Menispermum plants.
In contrast, however, increased isotope enrichment (Figure 5c) and an altered shift in MIDs (Figure S5) are barely detectable for reticuline in response to D2O feeding. It is possible that the enzyme responsible for converting N-methylcoclaurine to reticuline is not expressed to a significant level in leaves. However, it is also possibility that this enzyme is active, but that it is only converting a small amount of deuterium-labeled substrate to reticuline, which may be diluted by the relatively large unlabeled reticuline pool that preexisted in the plant leaf. Indeed, the latter possibility is supported by the fact that the pool size for N-methylcoclaurine is less than 1% of that of reticuline, and that the isotope enrichment of N-methylcoclaurine is also very low.
Finally, acutumine and acutumidine also exhibit an elevated relative isotope enrichment (Figure 5c), and a shift in MID (Figure S5). Although labeling is occurring at a lower level in these compounds than in the putative precursors, these data illustrate that biosynthetic processes leading to acutumine and acutumidine are active in leaves. However, the deuterium labeling observed is unlikely to be derived from reticuline, because, as previously mentioned, this proposed intermediate does not appear to be getting labeled to a significant extent. A possible explanation for this labeling is that it could be a consequence of potential NAD(P)H-dependent reactions occurring downstream of reticuline, but preceding acutumine/acutumidine, or a homeostasis among acutumine, acutumidine and other structural variants that involves reduction via NAD(P)H.
DISCUSSION
Taken collectively, our data show that informative deuterium labeling experiments can be conducted on slow-growing plants during relatively short time scales. In both the HupA and homoharringtonine examples, the rate of label incorporation was specific to new growth, with other tissues exhibiting significantly less labeling. At least for HupA, this result is consistent with what has previously been presented in the literature.30,32 Many of the previous analyses have relied solely upon relative accumulation of compound as an indication of the location of biosynthesis, and in this instance, it appears that those assumptions have proven to be accurate. In general, however, we view combined results from both relative accumulation (i.e. pool size) measurements and metabolic activity measurements (D2O labeling studies) as a much stronger evidence linking specific tissues to biosynthetic activity. This information can be used as a basis for future biosynthetic gene discovery, as the relevant enzymes that produce these compounds are likely to be differentially expressed among these different tissue types.
In the case of the Menispermum alkaloids, not only was label incorporation observed in the final alkaloid of interest, but also in many of the likely precursors. Assuming that these compounds are indeed precursors, this D2O labeling method can thus be a powerful tool for probing the activity of multiple biosynthetic transformations in a pathway. Crucially, this could be informative when searching for biosynthetic genes; if a particular intermediate is being actively labeled in a tissue, then it would be reasonable to conclude that the biosynthetic machinery is present. Conversely, this approach could also help to rule out other intermediates that are not incorporating deuterium to a significant level.
We also show that these short-term D2O labeling studies can be combined with untargeted analysis to identify particularly highly labeled (or unlabeled) metabolites, and provide some evidence that the specific regions of label incorporation within a single molecule can be assessed with LC-MS2 techniques. These techniques can be applied broadly as tools to intricately probe metabolism, and also demonstrate the wealth of information that can be obtained from a relatively simple and cheap labeling experiment. Although beyond the scope of this study, future analysis of the rates and levels of deuterium incorporation into certain metabolites may serve as a platform for detailed study of the metabolism related to production of bioactive alkaloids in plants.
While these D2O labeling methods can facilitate the study of metabolism in medicinal plants, the slow growth of the species tested here has important consequences for the design and analysis of deuterium-based isotopic labeling experiments. First, slow growth implies lower rates of biomass synthesis and of carbon fixation, which in turn implies that reaching isotopic steady state may take very long periods of time.54,55 Although the alkaloids of interest examined in this study each accumulated deuterium at a detectable and significant level, none of them were observed to have reached equilibrium with the deuterium concentration that was supplemented, and thus have not approached isotopic steady state. Additionally, many of the experimental perturbations described here -- for example, excising particular plant organs and tissues for separate incubation in labeled media -- are undoubtedly strong biological perturbations, which could have an impact on the transcriptional regulation associated with natural product biosynthesis. Slow metabolic dynamics coupled with strong perturbations make it unlikely that we attain a metabolic steady state in all of our experimental conditions, and therefore results should not be taken as an absolute measure of metabolism, but rather as an observation of relative biosynthetic capability.
An additional aspect to consider in designing and analyzing these labeling experiments is that D2O is toxic to eukaryotes, including plants, at high concentrations.56,57 In barley, D2O concentrations up to approximately 37.5% by volume are relatively well-tolerated, but above that level, growth attenuation and germination arrest increase significantly with D2O concentration.58 As shown in the first two case studies, using a D2O concentration lower than this allows for readily detectable incorporation of label over an extended experiment (greater than one month). Surprisingly, excised Menispermum leaves tolerated 100% D2O reasonably well, as metabolic activity and label incorporation could be detected for up to 15 days. Importantly, this increased D2O concentration allowed for the observation of drastically more label incorporation, although this plant tissue was noticeably stressed by the end of the experiment. Thus, depending on the question seeking to be answered by this labeling method, consideration should be taken into the concentration of D2O to be supplied; although higher concentrations may lead to faster and higher deuterium incorporation, the overall time of viable plant metabolism may be decreased.
The use of deuterium as an isotopic tracer for active biosynthesis is not without technical complications. The usual assumption of isotopic tracer studies is that the tracer is inert; specifically, that only the mass spectrometer, and not the enzymes, cells, and organisms targeted by the experiment, can tell the difference between a heavy isotope and a lighter one. This assumption is often approximately true, but in the case of deuterium (2H) vs. protium (1H), is maximally false.59 In fact, this isotopic fractionation of hydrogen isotopes can be large. For example, lipids in phototrophic microorganisms were found to be approximately 300 per mille (i.e. 30 relative percent) lighter than the growth water.60
Deuterium atoms bound to heteroatoms such as nitrogen or oxygen exchange rapidly with the hydrogen in protic solvents, but carbon-bound hydrogens, as a general rule, do not. Thus, analysis of label incorporation patterns needs to differentiate between “exchangeable” and “non-exchangeable” positions, because during metabolite extraction from plant tissues (with unlabeled methanol and water) and LC-MS analysis (with unlabeled water and acetonitrile), the exchangeable positions will rapidly lose label.61 However, isotopic exchange of even some carbon-bound hydrogen can occur during biosynthesis through enzyme-catalyzed processes.62 Thus, the labeling of a compound in plants supplemented with D2O does not necessarily indicate proof of total de novo biosynthesis, but merely that some metabolic processes leading to production of the compound are active.
It is worth noting that 13C, for example in 13CO2, does not suffer from many of these issues. Enzymatic fractionation is much reduced, solvent exchange is negligible, and 13CO2 is not toxic to plants. However, 13CO2 is a gas, thus requiring far more complex systems for reliable introduction of label.
Overall, there are many caveats that can complicate analysis of D2O tracer experiments in slow-growing plants, especially for quantitative flux analyses. However, we believe that they do not preclude the use of this technique to yield insight into the metabolic processes occurring in slow-growing plants. In particular, when a tissue enjoys both (a) the highest abundance of a target metabolite relative to other tissues, and (b) the highest degree of deuterium labeling of the metabolite relative to other tissues, the most parsimonious explanation is that this is likely to be a major site of at least some key pathway steps in biosynthesis. If putative upstream intermediates have been identified (e.g. via other techniques such as labeling studies with specific precursor molecules), then deuterium incorporation can be analyzed on a “per molecule” basis to determine which biosynthetic steps may be active in a particular tissue or condition. Future experimentation to define intermediates, coupled to D2O labeling experiments, can facilitate the subsequent discovery of biosynthetic genes for natural products that are found in medicinal plants.
METHODS
Plant growth
Huperzia tetrasticha (also known as Phlegmariurus tetrastichus) plants were acquired from Charles Alford Plants (rareferns.com). Prior to labeling studies, plants were grown with ambient laboratory temperature and lighting, misted with water daily, and were given a nutrient solution (Ionic® Grow for Soil 3–1-5, Hydrodynamics International) once per week.
Cephalotaxus harringtonia var. Fastigiata was purchased from Forest Farms (forestfarm.com). Upon receiving and prior to labeling experiments, the plant was given a nutrient solution (Ionic® Grow for Soil 3–1-5, Hydrodynamics International) and was subsequently watered approximately once every two weeks. Growth conditions consisted of ambient laboratory temperature and lighting.
Menispermum canadense plants were acquired from Reeseville Ridge Nursery (reesevilleridgenursery.com). Plant growth was maintained at 22°C under a diurnal light environment of 16 hour illumination (30 μmol photons m−2 sec−1) and 8 hour dark. Nutrients (Ionic® Grow for Soil 3–1-5, Hydrodynamics International) were applied to plants once per week.
D2O labeling
Shoots from H. tetrasticha were excised from the living plant using ethanol-sterilized scissors and placed cut end down in either 100% deionized water (control) or 10% D2O in deionized water (prepared from 100% D2O, Sigma-Aldrich). Shoot tips (new growth), which were determined based upon having a lighter green hue compared to the rest of the mature shoot (see Figure 2c), were excised from shoots prior to D2O supplementation (day 0) and from shoots in 10% D2O at 3, 6, 12, and 28 days after being placed in the deuterated solution. Three shoot tips were collected at each time point. Tips (new growth) from the control shoots was harvested at the 28 day time point. In addition to shoot tips, mature shoot tissue was also collected at the 28 day time point for both control and D2O-supplemented shoots. This mature shoot tissue was divided into ~2–3 cm sections annotated as “mature shoot-1”, “mature shoot-2”, or “mature shoot-3” based upon location relative to the shoot tips, with a lower number indicating closer proximity. All tissue samples were flash-frozen in liquid N2 for subsequent metabolite extraction.
For Cephalotaxus harringtonia, two branches, approximately 6 cm in length, were removed from the base of the living plant using ethanol-sterilized scissors and placed cut end down in a 50 mL Falcon tube containing 10 mL of either 100% H2O (tap water) as a control or 10 mL of a mixture of 10% D2O in tap water. The caps were placed on the tubes such that airflow could occur and the two samples were left undisturbed for two weeks under ambient lighting and temperature. During this time, new needles appeared at the top end of both branches and were classified as new growth by the smaller size and lighter green color when compared to preexisting needles (old growth). New growth needles (1–2 needles per sample) were removed from the branch in 10% D2O at time points of 14, 21, and 46 days after being placed in the D2O solution and old growth needles (1 needle per sample) were collected at time points of 21 and 46 days. Three samples were collected at each time point. New growth needles from the control branch in 100% H2O were collected after 30 days. All samples were immediately placed in 2 mL tubes (Eppendorf) and flash-frozen in liquid N2 for subsequent metabolite extraction.
For Menispermum canadense, leaves (5 cm in diameter) with petioles (3 cm in length) were removed from stems of the Menispermum plants. Three pairs of leaves were harvested from 3 individual plants. Petioles of each pair of leaves were inserted in liquid Murashige and Skoog (MS) medium prepared with 100% H2O or 100% D2O, respectively. The 3 pairs of leaves were grown for 5, 10 and 15 days, respectively. All samples were flash-frozen in liquid N2 for subsequent metabolite extraction.
Metabolite extraction
Flash-frozen Huperzia, Cephalotaxus, and Menispermum samples were lyophilized to dryness, weighed, and homogenized to a fine powder in 2 mL safe-lock tubes (Eppendorf) with a 5 mm diameter steel ball by using a ball mill homogenizer (Retsch MM400) set to 25 s−1 for 2 minutes. Samples were extracted with 80% MeOH in water (100 μL of solvent per milligram of dry tissue) for 10 minutes at 65 ºC. Samples were centrifuged at 10,000 × g for 5 minutes to pellet plant tissue, and the supernatant was filtered into sample vials using Mini-UniPrep™ syringeless PTFE filters with 0.45 μm pore size (Whatman) for analysis via liquid chromatography-mass spectrometry (LC-MS).
LC-MS analysis
Huperzia extracts were analyzed using an Agilent 1260 HPLC paired with an Agilent 6520 Accurate-Mass Q-TOF electrospray ionization (ESI) mass spectrometer. Reversed-phase chromatography was performed with a 5 μm, 2 × 100 mm Gemini NX-C18 column (Phenomenex) by using water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B) as solvents. For each sample, 5 μL was injected and run at a flow rate of 0.4 mL min−1 using the following gradient: 0–1 min, 3% B; 3–21 min, 3–50% B, 21–22 min, 50–97% B, 22–27 min, 97% B, 27–28 min, 97–3% B, 28–32 min, 3% B. The MS data were collected in positive mode from 2–32 min using the following parameters: mass range from 50–1700 m/z, drying gas at 300 ºC with a flow rate of 11 L min−1, nebulizer at 35 psig, Vcap at 3500 V, fragmentor at 150 V, skimmer at 65 V, octupole 1 RF Vpp at 750 V, acquisition rate of 709.2 ms per spectrum. The presence of HupA in H. tetrasticha extracts was confirmed via comparison of retention and time and mass spectrum to an authentic HupA standard (Fisher Scientific). The HupA standard was prepared by dissolving HupA in MeOH to a final concentration of 10 ng μL-1. Under the aforementioned chromatographic parameters, HupA eluted at approximately 4–6 minutes, and the mass spectrum was compared to published spectra for verification (Ma and Gang 2008).
Cephalotaxus extracts were analyzed as described above for Huperzia except for the solvent gradient and MS parameters. The following parameters were used for LC-MS analysis of Cephalotaxus extracts: 5 μL was injected and run at a flow rate of 0.4 mL min−1 with the following gradient: 0–1 min, 3% B; 1–21 min, 3–35% B; 21–22 min, 35–97% B; 22–27 min, 97% B; 27–28 min, 95–3% B; 28–33 min, 3% B. The MS data were collected in positive mode from 1–33 min with source parameters as above except the fragmentor voltage was 100 V and the nebulizer pressure was 25 psig. The presence of homoharringtonine was confirmed by comparison of the MS and MS2 spectra with published spectra of authentic compounds.63
Menispermum extracts were analyzed as described above for Huperzia except for the injection volume and solvent gradient. For each sample, 2 μL was injected and run using the following gradient: 0–3 min, 3% B; 3–25 min, 3–25% B; 25–35 min, 25–65% B; 35–40 min, 65–95% B; 40–43 min, 95% B;43–44 min, 95–3% B; 44–50 min, 3% B. The presence of acutumine pathway metabolites were confirmed by comparison of the MS2 spectra with the those in the Metlin database (metlin.scripps.edu).
Deuterium enrichment analysis
Mass isotopologue distributions (MIDs) for known compounds were determined by spectral summation over retention time windows of interest using the get_MID.py script available at https://github.com/Stanford-ChEMH-MCAC/d2o_metabolomics. Briefly, this program takes as input (a) an ion structure, from which a charge and exact monoisotopic mass is calculated; (b) a m/z tolerance t in parts per million; (c) a retention time window of interest in minutes; and (d) the number of integer masses to include in the MID. For each integer mass higher than the monoisotopic mass, all signals between (1 - t/1e6) times the mass of the completely 13C substituted isotopologue (1.0034 Da heavier for each integer) and (1 + t/1e6) times mass of completely 2H-substituted isotopologue (1.0063 Da heavier) were summed. This approach minimizes error and bias associated with peak-picking on individual isotopologue extracted ion chromatograms, which can distort the relative intensities of weak isotopologue peaks. MIDs are obtained by normalizing all of the obtained intensities to sum to 1. From MIDs, isotopic enrichment (IE) was quantified by the simple formula:
where IE is total heavy isotopic enrichment in the compound, n is the number of (integer) mass isotopologues that can be detected, and Mi the relative abundance of the ith isotopologue. Unlike other methods for estimating isotope abundance, such as fitting a single unknown isotope abundance to observed MIDs,61 this technique makes no additional assumptions about the distribution of heavy label (e.g. single-degree-of-freedom approximation). For quantitation of deuterium enrichment, the IE value of a deuterium-labeled sample was simply subtracted from the IE value of an unlabeled control sample. This subtraction corrects for the natural abundance of heavy isotopologues, mainly from 13C. A Jupyter notebook fit_isotopic_abundance_from_MID.ipynb describing the method in detail and for performing this calculation in R is available at https://github.com/Stanford-ChEMH-MCAC/d2o_metabolomics.
The relative abundance of isolated compounds was determined by adding together the intensities of all of the relevant mass isotopologues for the compound’s parent ion [M+H]+ that were calculated by the get_MID.py script. This value was then normalized via dividing by the dry mass of the tissue from which the compound was isolated. Statistical tests pertaining to deuterium enrichment and compound abundance were performed in JMP Pro 13.0.
Untargeted analysis of label incorporation
Three replicates of new Cephalotaxus harringtonia tissue grown in labeled media and extracted as described above were compared to three corresponding replicates grown in unlabeled media. The labeled samples were grown for 46 days, and the corresponding labeled controls were grown for 30 days. Features were identified and grouped in XCMS.45 We then focused on features with intensities of more than 30,000 ion counts in at least one of the six samples, and with retention times between 5 minutes and 21.6 minutes (the core of the LC gradient). Initial analysis revealed that many detected labeled features had high m/z values and high charges, indicating they may possibly derive from peptides rather than primary or secondary metabolites. We thus focused only on features with m/z values of less than 700. Meeting all these criteria were 1,769 features. We compared all pairs of these features, retaining only pairs where the retention time difference was less than 3 seconds, and where the heavy feature was increased in labeled samples and the lighter feature was increased in unlabeled samples, and where the mass difference between features was within 35 parts per million of the mass of an integer multiple of deuterium neutrons. A Jupyter notebook untargeted_d2o_labeling_analysis.ipynb with code for performing the analysis described is available at https://github.com/Stanford-ChEMH-MCAC/d2o_metabolomics.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an NIH U01 Grant GM110699, an NIH R01 Grant GM12152701 (to E.S.S.), and a collaboration with Novartis Institutes for BioMedical Research in Cambridge, MA. We would like to acknowledge Curtis Neveu for use of the photosystem II image, obtained from https://commons.wikimedia.org/w/index.php?curid=30380168.
Literature Cited
- 1.Kinghorn AD, Pan L, Fletcher JN, Chai H. The relevance of higher plants in lead compound discovery programs. J Nat Prod. 2011;74:1539–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. [DOI] [PubMed] [Google Scholar]
- 3.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015;33:1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. [DOI] [PubMed] [Google Scholar]
- 5.Miralpeix B, Rischer H, Hakkinen ST, Ritala A, Seppanen-Laakso T, Oksman-Caldentey KM, Capell T, Christou P. Metabolic engineering of plant secondary products: which way forward? Curr Pharm Des. 2013;19:5622–5639. [DOI] [PubMed] [Google Scholar]
- 6.Staniek A, Bouwmeester H, Fraser PD, Kayser O, Martens S, Tissier A, van der Krol S, Wessjohann L, Warzecha H. Natural products - learning chemistry from plants. Biotechnol J. 2014;9:326–336. [DOI] [PubMed] [Google Scholar]
- 7.Wurtzel ET, Kutchan TM. Plant metabolism, the diverse chemistry set of the future. Science. 2016;353:1232–1236. [DOI] [PubMed] [Google Scholar]
- 8.Nutzmann HW, Huang A, Osbourn A. Plant metabolic clusters – from genetics to genomics. New Phytol. 2016;211:771–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau W, Sattely ES. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science. 2015;349:1224–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputi L, Franke J, Farrow SC, et al. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science. 2018;360:1235–1239. [DOI] [PubMed] [Google Scholar]
- 11.Góngora-Castillo E, Buell CR. Bioinformatics challenges in de novo transcriptome assembly using short read sequences in the absence of a reference genome sequence. Nat Prod Rep. 2013;30:490–500. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Bennetzen JL. Current status and prospects for the study of Nicotiana genomics, genetics, and nicotine biosynthesis genes. Mol Genet Genomics. 2015;290:11–21. [DOI] [PubMed] [Google Scholar]
- 13.Moldrup ME, Geu-Flores F, Halkier BA. Assigning gene function in biosynthetic pathways: camalexin and beyond. Plant Cell. 2013;25:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein AP, Sattely ES. Two cytochromes P450 catalyze S-heterocyclizations in cabbage phytoalexin biosynthesis. Nat Chem Biol. 2015;11:837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca V, St-Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. [DOI] [PubMed] [Google Scholar]
- 16.Regnault T, Davière J-M, Wild M, et al. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat Plants. 2015;1:1–6. [DOI] [PubMed] [Google Scholar]
- 17.Nour-Eldin HH, Halkier BA. Piecing together the transport pathway of aliphatic glucosinolates. Phytochem Rev. 2009;8:53–67. [Google Scholar]
- 18.Courdavault V, Papon N, Clastre M, Giglioli-Guivarc’h N, St-Pierre B, Burlat V. A look inside an alkaloid multisite plant: the Catharanthus logistics. Curr Opin Plant Biol. 2014;19:43–50. [DOI] [PubMed] [Google Scholar]
- 19.Vinyard DJ, Ananyev GM, Dismukes GC. Photosystem II: the reaction center of oxygenic photosynthesis. Annu Rev Biochem. 2013;82:577–606. [DOI] [PubMed] [Google Scholar]
- 20.Astot C, Dolezal K, Nordström A, Wang Q, Kunkel T, Moritz T, Chua NH, Sandberg G. An alternative cytokinin biosynthesis pathway. Proc Natl Acad Sci U S A. 2000;97:14778–14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol. 2002;50:309–332. [DOI] [PubMed] [Google Scholar]
- 22.Baran R, Lau R, Bowen BP, Diamond S, Jose N, Garcia-Pichel F, Northen TR. Extensive turnover of compatible solutes in cyanobacteria revealed by deuterium oxide (D2O) stable isotope probing. ACS Chem Biol. 2017;12:674–681. [DOI] [PubMed] [Google Scholar]
- 23.Ma X, Gang DR. The Lycopodium alkaloids. Nat Prod Rep. 2004;21:752–772. [DOI] [PubMed] [Google Scholar]
- 24.Liu J-S, Zhu Y-L, Yu C-M, Zhou Y-Z, Han Y-Y, Wu F-W, Qi B-F. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem. 1986;64:837–839. [Google Scholar]
- 25.Ma X, Tan C, Zhu D, Gang DR, Xiao P. Huperzine A from Huperzia species - An ethnopharmacological review. J Ethnopharmacol. 2007;113:15–34. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira A, Rodrigues M, Fortuna A, Falcao A, Alves G. Huperzine A from Huperzia serrata: a review of its sources, chemistry, pharmacology and toxicology. Phytochem Rev. 2016;15:51–85. [Google Scholar]
- 27.Siengalewicz P, Mulzer J, Rinner U. Lycopodium alkaloids - synthetic highlights and recent developments In: Knolker H-J, ed. Alkaloids: Chemistry and Biology. Vol 72 Oxford, UK: Elsevier Inc.; 2013:1–151. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RN, Castillo M, MacLean DB, Spenser ID, Wrobel JT. Biosynthesis of lycopodine. J Am Chem Soc. 1968;90:1360–1361. [DOI] [PubMed] [Google Scholar]
- 29.Marshall WD, Spenser ID, Nguyen TT, MacLean DB. Biosynthesis of lycopodine. The question of the intermediacy of piperidine-2-acetic acid. Can J Chem. 1975;53:41–50. [Google Scholar]
- 30.Hemscheidt T Tropane and related alkaloids. Top Curr Chem. 2000;209:176–206. [Google Scholar]
- 31.Ma X, Tan C, Zhu D, Gang DR. Is there a better source of huperzine A than Huperzia serrata? Huperzine A content of Huperziaceae species in China. J Agric Food Chem. 2005;53:1393–1398. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Shi Y, Huang J, Gong Y, Liu Z, Hu W. Modification and validation of a high-performance liquid chromatography method for quantification of huperzine A in Huperzia crispata. J AOAC Int. 2010;93:1428–1435. [PubMed] [Google Scholar]
- 33.Jalil Miah MA, Hudlicky T, Reed JW. Cephalotaxus alkaloids In: Cordel GA, ed. Alkaloids: Chemistry and Biology. Vol 51 San Diego, CA: Academic Press, Inc.; 1998:199–269. [Google Scholar]
- 34.Powell RG, Weisleder D, Smith CR Jr.. Antitumor alkaloids from Cephalotaxus harringtonia: structure and activity. J Pharm Sci. 1972;61:1227–1230. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Chen D, Shen Z, Koeffler HP. Effect of homoharringtonine on proliferation and differentiation of human leukemic cells in vitro. Cancer Res. 1990;50:2031–2035. [PubMed] [Google Scholar]
- 36.Huang MT. Harringtonine, an inhibitor of initiation of protein biosynthesis. Mol Pharmacol. 1975;11:511–519. [PubMed] [Google Scholar]
- 37.Alvandi F, Kwitkowski VE, Ko C-W, et al. U.S. Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist. 2014;19:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lü S, Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol. 2014;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delfel NE. Alkaloid distribution and catabolism in Cephalotaxus harringtonia. Phytochemistry. 1980;19:403–408. [Google Scholar]
- 40.Southworth AL, Dirr MA. Timing and K-IBA treatments affect rooting of stem cuttings of Cephalotaxus harringtonia. HortScience. 1996;31:222–223. [Google Scholar]
- 41.Pérard-Viret J, Quteishat L, Alsalim R, Royer J, Dumas F. Cephalotaxus alkaloids In: Knölker H-J, ed. Alkaloids: Chemistry and Biology. Vol 78 Oxford, UK: Elsevier Inc.; 2017:205–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robin JP, Dhal R, Dujardin G, Girodier L, Mevellec L, Poutot S. The first semi-synthesis of enantiopure homoharringtonine via anhydrohomoharringtonine from a preformed chiral acyl moiety. Tetrahedron Lett. 1999;40:2931–2934. [Google Scholar]
- 43.Parry RJ, Chang MNT, Schwab JM, Foxmanle BM. Biosynthesis of the Cephalotaxus alkaloids. Investigations of the early and late stages of cephalotaxine biosynthesis. J Am Chem Soc. 1980;102:1099–1111. [Google Scholar]
- 44.Gitterman A, Dufresne RF, Sternbach DD, Cabelli MD, Parry RJ. Biosynthesis of the Cephalotaxus alkaloids. Investigations of the biosynthesis of deoxyharringtonine, isoharringtonine, and harringtonine. J Am Chem Soc. 1980;102:2074–2081. [Google Scholar]
- 45.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann A, Walker S, Mol G. Product ion isotopologue pattern: A tool to improve the reliability of elemental composition elucidations of unknown compounds in complex matrices. Rapid Commun Mass Spectrom. 2016;30:791–799. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol. 2008;59:735–769. [DOI] [PubMed] [Google Scholar]
- 48.King SM, Herzon SB. The hasubanan and acutumine alkaloids In: Knolker H-J, ed. Alkaloids: Chemistry and Biology. Vol 73 Oxford, UK: Elsevier Inc.; 2014:161–222. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto Y, Uchida S, Inanaga S, Kimura Y, Hashimoto M, Isogai A. Early steps of dauricine biosynthesis in cultured roots of Menispermum dauricum. Biosci Biotechnol Biochem. 1996;60:503–505. [DOI] [PubMed] [Google Scholar]
- 50.Babiker HAA, Sugimoto Y, Saisho T, Inanaga S, Hashimoto M, Isogai A. Biosynthetic relationship between acutumine and dechloroacutumine in Menispermum dauricum root cultures. Biosci Biotech Bioch. 1999;63:515–518. [DOI] [PubMed] [Google Scholar]
- 51.Sugimoto Y, Babiker HAA, Saisho T, Furumoto T, Inanaga S, Kato M. Chlorinated alkaloids in Menispermum dauricum DC. root culture. J Org Chem. 2001;66:3299–3302. [DOI] [PubMed] [Google Scholar]
- 52.Hagel JM, Morris JS, Lee EJ, et al. Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol. 2015;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagel JM, Morris JS, Lee EJ, et al. Metabolome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol. 2015;15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelleher JK. Flux estimation using isotopic tracers: common ground for metabolic physiology and metabolic engineering. Metab Eng. 2001;3:100–110. [DOI] [PubMed] [Google Scholar]
- 55.Antoniewicz MR, Kraynie DF, Laffend LA, González-Lergier J, Kelleher JK, Stephanopoulos G. Metabolic flux analysis in a nonstationary system: Fed-batch fermentation of a high yielding strain of E. coli producing 1,3-propanediol. Metab Eng. 2007;9:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis GN. Biology of heavy water. Science. 1934;79:151–153. [DOI] [PubMed] [Google Scholar]
- 57.Kushner DJ, Baker A, Dunstall TG. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol. 1999;77:79–88. [PubMed] [Google Scholar]
- 58.Bhandarkar MK, Bhattacharya S, Gaur BK. Physiological studies with heavy water. II. Germination and growth inhihition of barley by D2O. Physiol Plant. 1971;24:517–521. [Google Scholar]
- 59.Hayes JM. Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Rev Mineral Geochemistry. 2001;43:225–277. [Google Scholar]
- 60.Zhang X, Gillespie AL, Sessions AL. Large D/H variations in bacterial lipids reflect central metabolic pathways. Proc Natl Acad Sci U S A. 2009;106:12580–12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer CR, Bowen BP, Pan C, Northen TR, Banfield JF. Stable-isotope probing reveals that hydrogen isotope fractionation in proteins and lipids in a microbial community are different and species-specific. ACS Chem Biol. 2013;8:1755–1763. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt H-L, Werner RA, Eisenreich W. Systematics of 2H patterns in natural compounds and its importance for the elucidation of biosynthetic pathways. Phytochem Rev. 2003;2:61–85. [Google Scholar]
- 63.Choi YH, Yoo KP, Kim J. HPLC-electrospray ionization-MS-MS analysis of Cephalotaxus harringtonia leaves and enhancement of the extraction efficiency of alkaloids therein by SFE. J Chromatogr Sci. 2003;41:67–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.