Abstract
Annual vaccination is not effective in conferring cross-protection against antigenically different influenza viruses. Therefore, it is of high priority to improve the cross protective efficacy of influenza vaccines. We investigated the adjuvant effects of monophosphoryl lipid A (MPL) and oligodeoxynucleotide CpG (CpG) on promoting homologous protection and cross-protection after vaccination of C57BL/6 and BALB/c mice with inactivated split virus. Combination adjuvant effects of MPL and CpG on improving homologous and cross protective vaccine efficacy were evident as shown by higher levels of homologous and cross-reactive binding IgG and hemagglutination inhibiting antibodies. Combination adjuvant effects on enhancing the protective efficacy against homologous and heterosubtypic virus were demonstrated by less weight loss, lower airway inflammatory disease, and better control of viral loads as well as prevention of inflammatory cytokines and cellular infiltrates. Overall, the findings in this study suggest that a combination adjuvant of different toll-like receptor ligands exhibits a unique pattern of innate and adaptive immune responses, contributing to improved homologous and heterosubtypic cross-protection by inactivated split virion influenza vaccination.
Keywords: MPL, CpG, Adjuvant, Influenza virus, Cross-protection
1. Introduction
Influenza causes severe respiratory disease, hospitalization, and substantial deaths worldwide annually. Inactivated split influenza virus vaccine is the most common platform used for human vaccination. Influenza virus continues to mutate and evolve new strains with high antigenic diversity as represented by 18 different hemagglutinin (HA) subtypes and 11 neuraminidase (NA) subtypes (Tong et al., 2013). Thus, it is highly significant to develop vaccine formulations inducing cross-reactive antibodies and providing cross-protection (Neuzil et al., 2017; Paules et al., 2018).
Aluminum hydroxide (alum) has a long history of usage as an adjuvant in human vaccines, but it is biased to promote T helper type 2 (Th2) antibody responses. Alum adjuvanted vaccines are often not effective against influenza viral pathogens (Young et al., 2015). An oil-in-water emulsion adjuvant MF59® has been licensed for human influenza vaccination (O’Hagan et al., 2013). MF59 adjuvanted vaccination with inactivated split influenza viruses derived from seasonal strains or prepandemic H5N1 strains was shown to be effective in enhancing immunogenicity in humans but the cross-reactive antibodies are limited within the same subtypes of virus (Bihari et al., 2012).
The innate immune system recognizes pathogens via interactions between pathogen-associated molecular patterns and pattern-recognition receptors, and induces inflammatory responses (Akira et al., 2001). Toll-like receptors (TLRs) are a family of pattern-recognition receptor transmembrane proteins with leucine-rich ectodomains recognizing pathogen components and intracellular toll-interleukin 1 domains for downstream signal transduction (Kawai and Akira, 2010). Various intracellular signaling pathways as a result of the interactions between TLRs and their ligands lead to the activation of transcription factors (NF-kB, AP-1, IRF 3/7), upregulating the expression of proinflammatory cytokines and recruiting innate immune cells (Kawai and Akira, 2010). Monophosphoryl lipid A (MPL) is an attenuated version of lipopolysaccharides (LPS) known to be a ligand for TLR4 and its coreceptor MD2 (Ireton and Reed, 2013). The adjuvant system 04 (AS04) is a combination of alum and MPL and licensed for its inclusion in the hepatitis B vaccine (Fendrix®) (Kundi, 2007) and human papilloma virus vaccine (Cervarix®) (Szarewski, 2012). Synthetic oligodeoxynucleotides containing unmethylated CpG motifs are well known to act as an agonist for TLR9 present in endosomal vesicles (Takeshita et al., 2001). CpG promotes the induction of T helper type 1 (Th1) and proinflammatory cytokines, and was shown to be tolerated in humans when administered as vaccine adjuvant (Klinman, 2003).
In this study, we investigated whether vaccination with inactivated split virus retaining internal proteins in the presence of MPL + CpG combination adjuvant at a low dose would enhance homologous and cross-protection in the different strains of mice. The presence of MPL + CpG combination adjuvant in the inactivated split influenza vaccination was found to promote the induction of homologous and cross-reactive antibodies, and the efficacy of homologous and heterosubtypic cross-protection. Innate and adaptive immune profiles were investigated to further provide the mechanistic insight into the actions of adjuvant effects.
2. Materials and methods
2.1. Animals, reagents and virus
C57BL/6 and BALB/c mice were purchased from Harlan and maintained in the animal facility at Georgia State University. Animal experiments were performed under the guidelines of the approved IACUC protocol (A14025). Monophosphoryl lipid A (MPL) was purchased from Sigma Aldrich and dissolved in dimethyl sulfoxide following the manufacturer’s protocol. Oligodeoxynucleotides (ODN) with CpG motifs (ODN1826, 5′-tcc atg acg ttc ctg acg tt-3′) were synthesized by Integrated DNA Technologies. The lyophilized CpG was resuspended in ultra-pure water. Both MPL and CpG adjuvants were aliquoted and saved in −80 °C until use. A/California/04/2009 (A/Cal) H1N1 virus was the strain for inactivated split virus vaccine (sCal) preparation and also used as a homologous challenge virus. For sCal vaccine preparation, A/Cal H1N1 influenza virus grown in egg substrates was inactivated with 1% neutral formalin and then concentrated by ultracentrifugation (SW32 Ti rotor, 123,760 × g, 1 h). The inactivated virus was resuspended in phosphate-based saline (PBS) and treated with 1% triton x-100 to disrupt virus particles. The disrupted virus particles were dialyzed with PBS more than 3 times overnight incubation by using 30,000 molecular weight cutoff dialysis cassettes (Thermo Fisher Scientific), retaining most internal proteins in the vaccine. The total protein concentration of sCal was determined by DC protein assay kit (Bio-Rad). The contents of HA and NA in inactivated sCal vaccines prepared were estimated using monoclonal antibody (mAb) and quantitative ELISA by mouse mAb specific for H1 HA of A/California/04/2009 (BEI Cat # FR-505) and rabbit mAb HCA-2 specific for pan NA proteins (Gravel et al., 2010) (HCA-2 mAb kindly provided by Dr. Xuguang Li). The estimates are 0.25 μg HA (25%) and 0.05 μg NA (5%) per 1 μg total proteins of split vaccines. The standard proteins purified and expressed in recombinant baculovirus were H1 HA protein (BEI Cat# NR-15749) and N1 NA protein (BEI Cat# NR-19234) derived from A/Cal H1N1 virus. Reassortant H5N1 (rgH5N1) virus containing HA and NA derived from A/Vietnam/1203/2004 and six internal genes from A/Puerto Rico/8/1934 was described previously (Song et al., 2011). A/Cal H1N1 and rgH5N1 viruses were amplified in embryonated chicken eggs and collected from allantoic fluids of the eggs. The viruses and sCal were aliquoted and saved at −80 °C until use.
2.2. Immunization and infection
To determine the protective efficacy of combination adjuvants against homologous virus (A/Cal H1N1), C57BL/6 mice (n = 5) were intramuscularly immunized with sCal (3 μg/mouse) alone or sCal (3 μg/mouse) plus MPL (1 μg/mouse), CpG (4 μg/mouse), or MPL + CpG (1 + 4 μg/mouse) one time. Immune sera were taken at 2 weeks after immunization. At 4 weeks after immunization, the mice were infected with a lethal dose of A/Cal H1N1 virus (3 × LD50 based on 20% body weight loss as an endpoint). For cross-protection studies, C57BL/6 mice (n = 5–6) were intramuscularly immunized with MPL + CpG (0.5 + 2 μg/mouse), sCal (3 μg/mouse) alone or sCal (3 μg/mouse) plus MPL (0.5 μg/mouse), CpG (2 μg/mouse), or MPL + CpG (0.5 + 2 μg/mouse) two times (prime and boost) with a 3-week interval. Six-week old BALB/c mice (n = 10) were immunized with MPL + CpG (0.5 + 2 μg/mouse), sCal (0.3 μg/mouse) alone or sCal (0.3 μg/mouse) plus MPL (0.5 μg/mouse), CpG (2 μg/mouse), or MPL + CpG (0.5 + 2 μg/mouse) two times (prime and boost) with a 3-week interval. Immune sera were taken at 2 weeks after each immunization. At 4 weeks after boost, the immunized mice were challenged with rgH5N1 virus (1.5 × LD50). After challenge, a set of rgH5N1-infected C57BL/6 and BALB/c mice was monitored for 14 days to record body weight changes and survival rates. To determine the cross protective efficacy and detail immunological profiles after rgH5N1 infection, another set of BALB/c mice were euthanized at day 7 post infection and bronchoalveolar lavage (BAL) and lung tissues were collected for further analysis.
2.3. Lung virus titration
The lungs of the immunized BALB/c were harvested at day 7 after rgH5N1 infection and grinded mechanically in 1.5 ml of PBS per each lung. The lung extracts and lung cells were separated after centrifugation. Embryonated chicken eggs were prepared for 9–12 days to be inoculated with diluted lung extracts. The virus titers were determined by hemagglutination assay of the allantoic fluids collected after 3-days of incubation. Virus titers as 50% egg infection dose (EID50)/ml were evaluated according to the Reed and Muench method (Reed and Muench, 1938).
2.4. Hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) assay
To access the ability of immune sera to inhibit HA and NA activity, we performed HAI and NAI assays with boost immune sera. Sera were inactivated by incubation at 56 °C for 30 min before assays. For HAI assay, the immune sera of each group were treated with receptor destroying enzymes (RDE, Sigma-Aldrich) for 16 h and then serially diluted in PBS. The serially diluted sera were incubated with 4 HA units of A/Cal H1N1 or rgH5N1 virus for 30 min in room temperature and then 0.5% chicken red blood cells were added to determine HAI titers. NAI assays were carried out as previous described (Doyle et al., 2013). Sera were serially diluted and incubated with A/Cal H1N1 or rgH5N1 virus for 30 min. The virus dose was determined by 50% of the maximal NA activity. And then the sera plus virus mixtures were added to fetuin coated plates. Horseradish peroxidase (HRP)-labeled peanut agglutinin (PNA) was used to detect desialylated fetuin. Tetramethylbenzidine (TMB) and phosphoric acid were used as a substrate and stop solution, respectively. Optical density (OD) measured at 450 nm by an ELISA reader (Bio-Rad) and percentages (%) of inhibitions were presented.
2.5. Intraperitoneal adjuvant treatment
To evaluate cellular mechanisms of MPL + CpG combination adjuvant, naïve BALB/c mice were injected intraperitoneally with 200 μl of PBS, MPL (1 μg), CPG (4 μg), or MPL + CpG (1 + 4 μg). Sera were collected from the mice at 2- and 20-h post injection to analyze systemic cytokine and chemokine levels. At 20 h after injection, peritoneal cells and exudates from the mice were collected in 2 ml of PBS and then separated by centrifugation.
2.6. Enzyme-linked immunosorbent assay (ELISA)
To measure antigen-specific antibody levels in immune sera, inactivated A/Cal H1N1 or rgH5N1 virus (200 ng/well) were coated onto the ELISA plates before adding the diluted immune sera. Anti-mouse immunoglobulin (Ig) G, IgG1 and IgG2a (or IgG2c) HRP-labeled secondary antibodies were used to detect the antigen-specific Ig in the sera. To detect cytokines and chemokines in lung extracts and peritoneal exudates, interleukin(IL)-1β, IL-6, IL-10, interferon (IFN)-γ ready-set-go kits (eBioscience) and monocyte chemoattractant protein 1 (MCP-1, CCL2), regulated on activation, normal T cell expressed and secreted (RANTES, CCL5), KC (CXCL1), Interferon gamma-induced protein 10 (IP-10, CXCL10) chemokine kits (R&D systems) were used by following the manufacturer’s manual.
2.7. Flow cytometry
For cellular phenotypic analysis in lung or peritoneal cavity, the single cells were prepared and stained with fluorophore-labeled antibodies specific for anti-mouse CD45 (clone 30-F11), CD11b (clone M1/70), CD11c (clone N418), F4/80 (clone BM8), Ly6c (clone HK1.4), MHC class II (clone M5/114.15.2), Siglec F (clone E50–2440), B220 (clone RA3–6B2), CD103 (clone 2E7), CD3 (clone 17A2), CD4 (clone GK1.5), CD8 (clone 53–6.7) and pan-natural killer (NK) cell marker CD49b (clone DX5) after anti-CD16/32 antibody (Fc blocker) treatment. To identify nucleoprotein (NP)-specific memory responses, BAL and lung cells were stained with anti- CD3 (clone 17A2), CD4 (clone GK1.5), CD8 (clone 53–6.7), CD44 (clone IM7), and influenza NP147–155 H-2Kd (TYQRTRALV)-tetramer (National Institutes of Health Tetramer Core Facility). The stained cells were acquired by BD LSRFortessa and BD FACS Diva program (BD Biosciences). The data analysis was performed by FlowJo (Tree Star Inc).
The phenotypes of the acquired cells were gated by the phenotypic markers (GeurtsvanKessel and Lambrecht, 2008; Rose et al., 2012): alveolar macrophages; CD45+CD11b−CD11c+F4/80+, macrophages; CD45+CD11b+F4/80+, monocyte-derived macrophages; CD45+CD11b+ Ly6chighF4/80+, neutrophils; CD45+CD11b+Ly6cloF4/80−, eosinophils; CD45+CD11b+SiglecF+, total DCs; CD45+F4/80−CD11c+ MHCIIhigh, plasmacytoid DCs; CD45+F4/80−CD11c+ MHCIIhighB220+, CD103+DC; CD45+F4/80−CD11c+ MHCIIhighCD11b−CD103+, CD11b+ DC; CD45+F4/80−CD11c+MHCIIhighCD11b+, NK cells; CD45+CD3−CD49b+, NKT cells; CD45+CD3+ CD49b+.
2.8. Statistical analysis
All results were presented as means ± standard deviation (SD). The statistical significance was determined by 1-way ANOVA and followed Tukey’s multiple comparison test. p < 0.05 was considered as significance. We analyzed all data with Prism Software (GraphPad Software Inc, San Diego, CA).
3. Results
3.1. A combination of MPL + CpG adjuvants in split virus vaccination enhances homologous and heterosubtypic protection in C57BL/6 mice
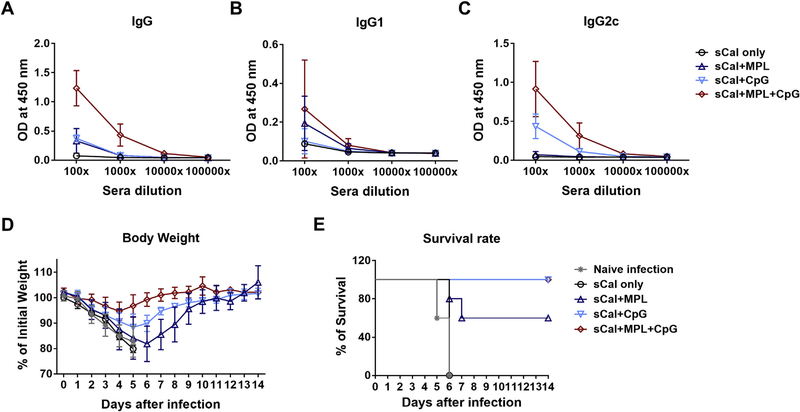
To determine whether TLR agonist combination adjuvants included in the split influenza virus vaccination would improve the efficacy of a single dose vaccination, the groups of C57BL/6 mice (n = 5 or 6) were primed with sCal vaccine (3 μg/mouse) alone or together with the adjuvants (MPL, CpG, MPL + CpG) (Fig. 1). MPL + CpG combination induced higher levels of virus-specific total IgG levels after a single dose immunization than each individual adjuvant groups (Fig. 1A). The MPL or CpG alone adjuvanted scal vaccine group also showed increased IgG antibody levels than the sCal only immunized group. MPL adjuvant induced IgG1 isotype antibody rather than IgG2c isotype antibodies. Meanwhile, the CpG and MPL + CpG adjuvant groups showed higher levels of Th1 type IgG2c than Th2 type IgG1 isotype antibodies, indicating Th1-biased immune responses (Fig. 1B and C). After challenge with a lethal dose of the homologous virus, the MPL + CpG combination group displayed significantly enhanced protection (~5% weight loss and 100% of survival) against A/Cal H1N1 virus compared to those in the MPL (~20% weight loss and 60% of survival) and CpG (~11% weight loss) after a single dose vaccination (Fig. 1D and E). These data suggest that a single immunization with split virus together with MPL + CpG combination adjuvant induces higher efficacy of protection against homologous virus compared to that of sCal only and individual MPL or CpG adjuvanted vaccination.
Fig. 1. IgG isotype antibodies and homologous protection in C57BL/6 mice after prime immunization with split vaccine or in the presence of adjuvants.
C57BL/6 mice (n = 5–6) were intramuscularly immunized one time with sCal (3 μg) split vaccine only or in the presence of adjuvants (MPL 1 μg, CpG 4 μg, or MPL 1 μg + CpG 4 μg). (A-C) A/Cal H1N1 virus specific antibody levels. The immune sera were taken 2 weeks post immunization from C57BL/6 mice. (D) Body weight changes of the immunized mice were monitored for 14 days after lethal infection with A/Cal H1N1 virus (3 × LD50). All data were shown in mean ± standard deviation (SD). (E) Survival rate of the immunized mice after lethal infection with A/Cal H1N1 virus.
To determine the effects of MPL + CpG adjuvant on the cross-protective efficacy of split vaccination, C57BL/6 mice were intramuscularly immunized with sCal vaccine (3 μg/mouse) two times (prime and boost at a 3-week interval) and challenged with rgH5N1 virus. Split vaccination with MPL + CpG adjuvant induced higher levels of rgH5N1-specific IgG, IgG1, and IgG2c antibodies compared to those in the sCal vaccine only group (Supplementary Fig. 1A–C). C57BL/6 mice with split vaccination in the presence of MPL + CpG adjuvant displayed better protection against heterosubtypic rgH5N1 challenge as shown by ~10% of body weight loss and 100% survival rates than those with split vaccine only (over 20% weight loss, 20% survival) or adjuvant (MPL + CpG) only group (0% survival) (Supplementary Fig. 1D and E). The naïve C57BL/6 mice showed a slower pattern of weight loss compared to that of the adjuvant only group after virus infection (Supplementary Fig. 1D). These results support that MPL + CpG combination adjuvants included in split influenza virus vaccination can enhance homologous and heterosubtypic protection.
3.2. MPL + CpG combination adjuvant enhances heterosubtypic cross-reactive antibodies in BALB/c mice
Considering broader application in genetically heterologous populations, we determined the adjuvant effects in a different mouse strain. BALB/c mice are known to be a higher responder to vaccination than C57BL/6 mice (Chen et al., 1999; Misplon et al., 2010). BALB/c mice were immunized with a low dose (0.3 μg/mouse) of inactivated-split vaccine sCal (H1N1) only, or together with adjuvants (sCal + MPL, sCal + CpG, or sCal + MPL + CpG) two times with a 3-week interval. Adjuvant only (MPL + CpG) immunization group was added as a control. After prime immunization, the vaccine virus (A/Cal)-specific IgG levels of the adjuvanted groups (MPL, CpG and MPL + CpG) were higher than the sCal only group (Supplementary Fig. 2A). After boost immunization, A/Cal virus-specific IgG levels were similar in all groups (Supplementary Fig. 2B). The sCal only and MPL adjuvanted groups showed higher IgG1 and lower IgG2a, but the CpG or MPL + CpG adjuvanted groups showed higher levels of IgG2a, Th1 type IgG isotype antibody in BALB/c mice, compared to other groups after prime or prime - boost immunizations.
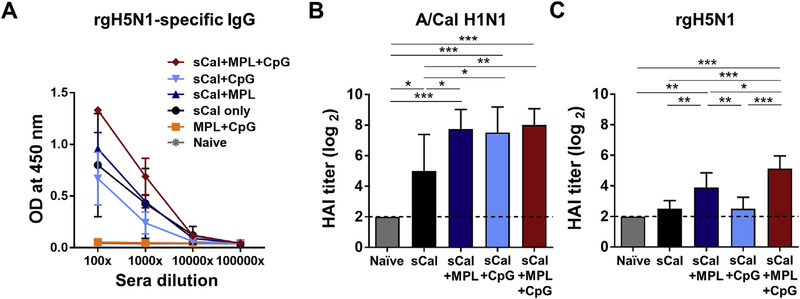
There were no significant differences between groups in the levels of IgG specific for A/Cal after boost immunization (Supplementary Fig. 2A). Nonetheless, when optical density values of ELISA were compared at different serum dilutions, the MPL + CpG combination adjuvanted group showed approximately 8 times higher levels of heterosubtypic rgH5N1 virus specific IgG antibodies compared to those in the sCal only immunized group and 5 to 10 times higher than those in the individual MPL or CpG adjuvanted group (Fig. 2A). The adjuvanted groups showed significantly higher levels of A/Cal H1N1-specific hemagglutination inhibition (HAI) titers than the sCal only group (Fig. 2B). When we used rgH5N1 virus for HAI assay, however, sCal only and sCal + CpG immune sera showed low HAI titers, but sCal + MPL and sCal + MPL + CpG immunizations significantly increased heterosubtypic HAI titers against rgH5N1 virus (Fig. 2C). All immunized groups showed high neuraminidase inhibition (NAI) titers against homologous A/Cal H1N1 virus, but MPL and MPL + CpG adjuvanted split vaccine groups only showed low to moderate levels of NA inhibiting activity against heterosubtypic rgH5N1 virus (Supplementary Fig. 3). It is likely that NAI titers detected using H1N1 and rgH5N1 virus substrates in the sCal + MPL + CpG group might be due to the combination effects of anti-HA and anti-NA antibodies.
Fig. 2. Cross reactive IgG antibody production in BALB/c mice immunized with sCal plus MPL + CpG combination adjuvant.
BALB/c mice (n = 5) were intramuscularly immunized with sCal (0.3 μg) only or in the presence of adjuvants (MPL 0.5 μg, CpG 2 μg, or MPL 0.5 μg + CpG 2 μg) or MPL + CpG adjuvant only. (A) Boost serum IgG antibodies cross reactive for rgH5N1 virus. (B) Hemagglutination inhibition (HAI) titers against homologous A/Cal H1N1 virus. (C) HAI titers against heterosubtypic rgH5N1 virus. All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison test were performed. *; 0.033, **; p < 0.002, and ***; p < 0.001 between the indicated groups.
3.3. MPL + CpG adjuvanted split virus vaccination improves the efficacy of heterosubtypic cross protection in BALB/c mice
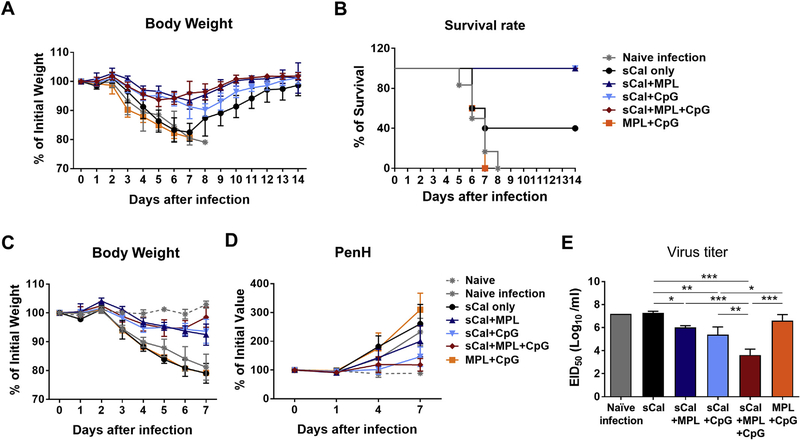
To determine the adjuvant effects on improving heterosubtypic cross protection, the immunized BALB/c mice were challenged with rgH5N1 virus at a low lethal dose (1.5 × LD50) at 4 weeks post boost immunization. Body weight changes were monitored for 14 days (Fig. 3A). From the day 3 post challenge, naïve or adjuvant only treated and infected mice and sCal only immunized mice started to lose weight (~5%), but all adjuvanted vaccine groups did not show body weight loss. By day 6–7 post challenge, the sCal vaccine only group displayed a similar pattern of ~20% weight loss similar to that observed in naïve or adjuvant only treated and infected mice, but survived better (40%) than naïve or adjuvant only mice with 0% survival rate. Notably, the MPL + CpG adjuvanted vaccine group displayed only moderate disease (~6% weight loss) with 100% survival rate, and quickly recovered body weight at day 7 post infection compared to either adjuvant vaccine alone groups (Fig. 3A and C).
Fig. 3. MPL + CpG combination adjuvant promotes the efficacy of heterosubtypic cross protection in BALB/c mice.
The H1N1 split vaccine immunized BALB/c mice (n = 5) in the presence or absence of adjuvants as described in Fig. 2 legend were challenged with rgH5N1 virus (1.5 × LD50) at 4 weeks after boost immunization. (A) Body weight changes were monitored for 14 days after infection. (B) Survival rate of the immunized mice after rgH5N1 infection. (C) Body weight changes were monitored for 7 days after infection. (D) Enhanced pause (PenH) of respiration was measured by plethysmography. Percentages of changes were based on the value in day 0. (E) Lung viral titers at day 7 post infection. Virus titration was determined by using embryonated chicken eggs and presented in 50% egg infectious titers (EID50). All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison test were performed. *; 0.033, **; p < 0.002, and ***; p < 0.001 between the indicated groups.
To better assess the cross protective efficacy, a separate independent set of BALB/c mice was used to monitor body weight changes for 7 days (Fig. 3C) and to determine PenH values (enhanced pause of air inhalation) as a measure of respiratory function. The MPL + CpG adjuvant vaccine group maintained the lowest PenH levels, but the other groups did not show a sign of recovery and kept increasing PenH (Fig. 3D). Lung samples were collected at day 7 post infection to determine the virus titers in embryonated chicken eggs (Fig. 3E). The MPL + CpG combination vaccine group showed a significantly lower level of virus titers by 1000 folds compared to the naïve infection and sCal groups, and lower than the MPL or CpG adjuvant vaccine groups. These data suggest that MPL + CpG adjuvanted split virus vaccination confers higher efficacy of protection against heterosubtypic virus than split virus vaccination with MPL or CpG adjuvant alone.
3.4. MPL + CpG adjuvant split virus vaccination prevents the induction of inflammatory cytokines after challenge
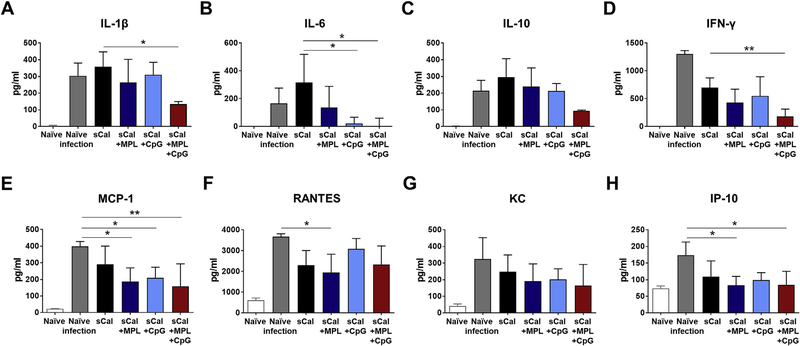
To evaluate the inflammatory responses of the immunized BALB/c mice after heterosubtypic rgH5N1 virus infection in the different adjuvant groups of sCal vaccination at 7 days after challenge, we measured cytokines and chemokines in lung samples (Fig. 4). The naïve mice with virus infection showed the highest levels of IFN-γ and chemokines like MCP-1 (CCL2), RANTES (CCL5), KC (CXCL1) and IP-10 (CXCL10) (Fig. 4D–H). The sCal vaccine only group showed the highest levels of IL-1β, IL-6 and IL-10 induction after challenge. MPL or CpG adjuvanted immunization decreased the induction of IL-6 and MCP-1 but still could not prevent the induction of IL-1β, IL-10, and IFN-γ after virus challenge. The group of sCal plus MPL + CPG combination adjuvant most effectively prevented the induction of inflammatory cytokines and chemokines (IL-1β, IL-6, IFN-γ, MCP-1, IP-10). These results suggest that the sCal vaccination with MPL + CpG adjuvants effectively prevent the induction of inflammatory cytokines after heterosubtypic virus challenge.
Fig. 4. Cytokines and chemokines in the lungs the BALB/c mice after heterosubtypic virus challenge.
Lung samples of the immunized BALB/c mice (n = 5) were harvested at day 7 post rgH5N1 virus infection. Cytokine and chemokine levels of each lung samples were measured by ELISA. All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison test were performed. *; 0.033 and **; p < 0.002 between the indicated groups.
3.5. Adjuvanted split virus vaccination inhibits inflammatory cell infiltrates due to viral infection
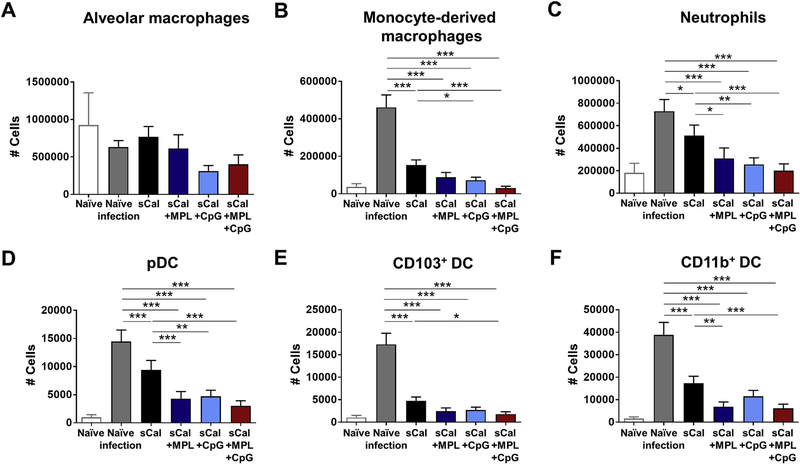
In addition to cytokine/chemokine production, we evaluated inflammatory cell infiltration into the lungs at 7 days after challenge of the immunized BALB/c mice (Fig. 5). The phenotypes of cellular infiltrates were obtained by flow cytometry. Cell numbers of each cell population were calculated by cell percentages and total cell counts. There were no significant differences in the number of alveolar macrophages (Fig. 5A). The naïve mice with viral infection showed highest cell numbers of monocyte-derived macrophages (CD45+CD11b+ Ly6chighF4/80+), neutrophils (CD45+CD11b+Ly6cloF4/80−), pDCs (CD45+F4/80−CD11c+MHCIIhighB220+), CD103+ DCs (CD45+F4/80−CD11c+MHCIIhighCD11b−CD103+), and CD11b+ DCs (CD45+F4/80−CD11c+ MHCIIhighCD11b+CD103−) (Fig. 5B–F). Vaccination with sCal only induced moderate but significant infiltration of inflammatory cells (monocyte-derived macrophages, neutrophils, pDC, CD11b+ DC) after heterosubtypic virus challenge. The MPL + CpG adjuvanted vaccine group was the most effective in suppressing the infiltration of inflammatory cells (monocyte-derived macrophages, neutrophils, DC subsets) after heterosubtypic virus challenge.
Fig. 5. Cellular infiltrates in the lungs after heterosubtypic virus challenge.
The immunized BALB/c mice (n = 5) were infected with rgH5N1 virus (1.5 × LD50) after 4 weeks of boost immunization. Lung samples were harvested at day 7 post infection and then cell phenotypes were determined by flow cytometry. Cell numbers of each cell population were calculated by multiplying cell percentages with total cell numbers. (A) Alveolar macrophages (AM); CD11b−CD11c+F4/80+. (B) Monocyte-derived macrophages; CD11b+F4/80+Ly6Chigh. (C) Neutrophils; CD11b+F4/80−Ly6clo. (D) Plasmacytoid dendritic cells (pDC); CD45+F4/80−CD11c+MHCIIhighB220+. (E) CD103+DC; CD45+F4/80−CD11c+ MHCIIhighCD11b−CD103+. (F) CD11b+ DC; CD45+F4/80−CD11c+MHCIIhighCD11b+. All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison test were performed. *; 0.033, **; p < 0.002, and ***; p < 0.001 between the indicated groups.
3.6. Acute immune responses are differentially modulated by individual and combination MPL + CpG
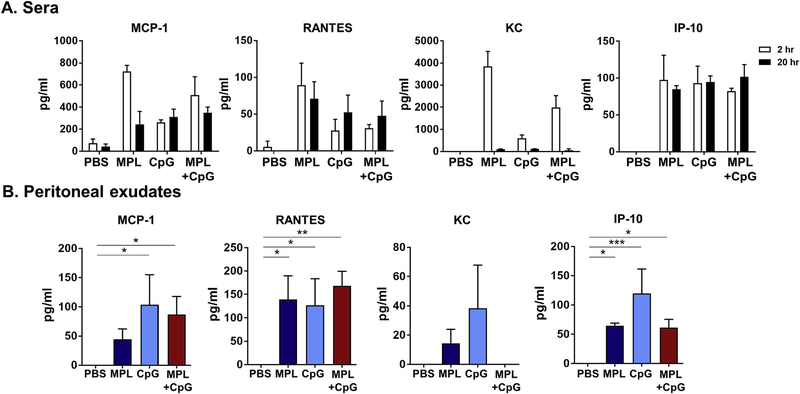
TLR agonists are known as strong immune stimulators and cell recruiters. To evaluate the acute innate immune effects of MPL + CpG on inducing chemokines and cell recruitments at the site of injection, we injected naïve BALB/c mice with PBS, MPL (1 μg), CpG (4 μg), and MPL + CpG intraperitoneally and determined chemokines and cell phenotypes in serum and peritoneal cavity. MPL was more potent in acutely inducing chemokines (MCP-1, RANTES, KC) in sera than CpG whereas MPL + CpG combination induced moderate levels of these chemokines within 2 h after injection (Fig. 6A). After 20 h, moderate to low levels of chemokines (MCP-1, RANTES, KC) were detected in sera in adjuvant-treated mice (Fig. 6A). The CpG treated mice showed high levels of chemokines (MCP-1, RANTES, KC, IP-10) whereas MPL + CpG treatment resulted in similarly high levels of MCP-1, RANTES but low levels of KC and IP-10 in peritoneal cavities at 20-h after injection (Fig. 6B).
Fig. 6. Acute induction of cytokines and chemokines after intraperitoneal injection with adjuvants.
Naïve BALB/c mice (n = 3) were intraperitoneally injected with PBS, MPL (1 μg), CpG (4 μg), or MPL (1 μg) +CpG (4 μg). Sera (A) were collected at 2 h and 20 h after injection. Peritoneal exudates (B) were harvested at 20 h after injection. Cytokine and chemokine levels of each samples were measured by ELISA. All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison tests were performed. *; 0.033, **; p < 0.002, and ***; p < 0.001 between the indicated groups.
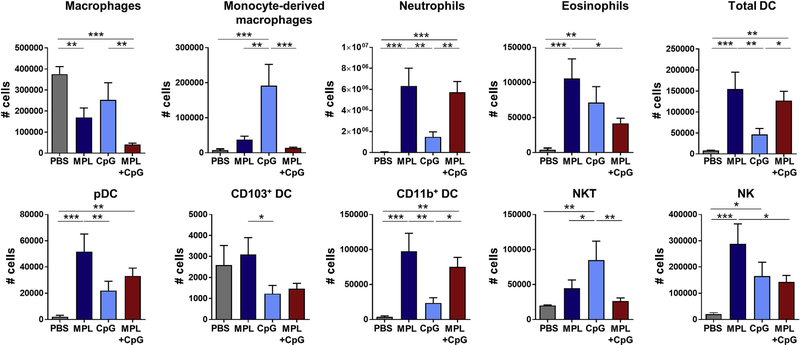
The phenotypes of innate cells infiltrated into the peritoneal cavity at 20 h after injection with adjuvants were determined by flow cytometry (Fig. 7). Most of peritoneal cells in PBS-treated naïve mice were macrophages and CD103+ DCs. CpG injection recruited monocyte-derived macrophages, eosinophils, and NKT cells in the peritoneal cavity at high levels whereas MPL induced infiltrates of neutrophils, eosinophils, DC subsets (pDC, CD11b+ DC), and NK cells at the highest levels. Injection of MPL + CpG combination adjuvant recruited neutrophils and total DCs at high levels similar to those of MPL, but other cells (eosinophils, CD103+ DCs, NK cells) at low levels similar to those acutely recruited in response to CpG injection (Fig. 7). MPL + CpG treatment resulted in further lowering macrophages while recruiting DCs at the site of injection. Overall, MPL + CpG combination modulates acute immune responses of chemokines and innate immune cells at the site of injection in a unique pattern different from either adjuvant alone.
Fig. 7. Cellular infiltrates in peritoneal cavity after intraperitoneal adjuvant injection.
Naïve BALB/c mice (n = 3) were intraperitoneally injected with PBS, MPL (1 μg), CpG (4 μg), or MPL (1 μg) +CpG (4 μg). Cells in peritoneal cavity were collected at 20 h after injection by flushing with PBS and then centrifugation. Cell phenotypes were determined by flow cytometry. Cell numbers of each cell population per mouse were calculated by multiplying cell percentages with total cell numbers. All data were shown in mean ± standard deviation (SD). For statistical analysis, One-way ANOVA and Tukey’s post-multiple comparison test were performed. *; 0.033, **; p < 0.002, and ***; p < 0.001 between the indicated groups.
4. Discussion
Because of continuing mutations and generation of antigenically different influenza viruses, it is of high priority to develop vaccination conferring improved cross-protection. It is interesting to find that MPL + CpG combination adjuvant promoted the induction of homologous (in C57BL/6 mice) and cross-reactive antibodies including cross-reactive HAI antibodies (in BALB/c mice). Effective vaccine adjuvants such as MPL + CpG might be playing a role in boosting cross reactive antibodies targeting to the conserved epitopes present in the vaccines. As a result, the efficacy of cross-protection in the sCal split vaccination with MPL + CpG combination adjuvant was improved as evidenced by less weight loss and quicker recovery, maintaining lung functions, and effective lung viral control by 2–3 magnitudes of log10. In addition, MPL + CpG adjuvant in the inactivated split virus vaccination resulted in the lowest levels of inflammatory cytokines and cellular infiltrates such as monocyte-derived macrophages and dendritic cells at the sites of viral replication after heterosubtypic challenge. Innate inflammatory responses were induced due to virus infection and contributing to control of early viral replication. The cytokine levels in lung after infection were inversely correlated with the virus titers in lungs. However, early innate immune responses alone would not be sufficient for conferring cross protection. This provides evidence that the cross-reactive neutralizing antibodies might have blocked the virus entry to a certain degree and contributed to lowering inflammatory cytokines in the MPL + CpG adjuvant group.
Adjuvant doses can be a parameter for determining the efficacy and safety of non-replicating subunit vaccination. MPL is a licensed adjuvant component for human vaccination and was reported in a high dose range (5–100 μg) in murine models as a vaccine adjuvant to enhance the efficacy of vaccines (Blanco et al., 2014; Didierlaurent et al., 2009; Hancock et al., 2003; Prince et al., 2001). CpG as a vaccine adjuvant has been reported to be used in a wide range of high doses (10–100 μg) in mice (Hancock et al., 2003; Ioannou et al., 2002; Klinman et al., 1999; Prince et al., 2003). In this study, we found that a combination of MPL (1 μg) and CpG (4 μg) adjuvants was more effective than either MPL or CpG adjuvant alone in conferring homologous protection after prime vaccination of C57BL/6 mice with inactivated split influenza virus. Consistently, we reported that a single dose vaccination of BALB/c mice with inactivated split influenza virus (A/PR/8/34) in the presence of MPL, CpG, or MPL + CpG adjuvant provided homologous protection by providing complete clearance of lung viral loads below the detection limit (Ko et al., 2017). Our comparative studies suggest that a combination of MPL TLR4 + CpG TLR9 agonists could be superior over aluminum hydroxide and more effective in enhancing vaccine efficacy after a single dose intramuscular vaccination than R848 TLR 7/8 agonist and natural killer T cell activator alphagalactosylceramide (data not shown). In this study, a further lower dose of adjuvants (0.5 μg of MPL and 2 μg of CpG) was applied to the primeboost strategy of inactivated split influenza virus (sCal) vaccination and then heterosubtypic cross-protection was investigated. IgG levels specific for vaccine virus were higher in the adjuvant groups (MPL, CpG, MPL + CpG) after prime vaccination. Although similar levels of homologous binding IgG antibodies were induced in all vaccine groups after boost immunization, HAI titers against the homologous virus were significantly higher in the adjuvanted groups. In addition, cross-reactive HAI titers were significantly higher in the MPL or MPL + CpG group but not CpG and sCal only groups. Despite no detectable levels of cross-reactive HAI antibodies, the CpG adjuvant group showed better control of heterosubtypic virus infection. It is possible that Th1 type T cell immunity in the CpG adjuvant group might have contributed to cross-protection. However, we did not observe significant antigen-specific T cell responses in mice with adjuvanted vaccination and after challenge (Supplementary Fig. 4). A combination of low dose MPL + CpG adjuvant might represent a unique vaccine adjuvant formulation, improving the quality of cross reactive HAI antibodies in the context of inactivated split virus containing internal proteins.
We found differential patterns of in vivo immune responses in MPL, CpG and their combination adjuvants. MPL adjuvant effects include higher levels of IgG1 (Th2) isotype and cross-reactive IgG antibodies whereas CpG adjuvant effects are more biased to induce Th1 type isotype IgG2a (or IgG2c in C57BL/6 mice) antibodies. MPL as a TLR4 ligand is known to trigger innate immune responses via MyD88-dependent (TIRAP/MyD88) and TRIF-dependent pathways, stimulating early and late nuclear factor (NF)-κB and IFN-response factor 3 (IRF3) activation leading to the induction of inflammatory cytokines and type 1 IFN (Kawai and Akira, 2010). CpG interacts with intracellular TLR9, recruiting MyD88 adaptor signaling molecules and leading to the activation of NF-κB and IRF7, eventually inducing inflammatory cytokines and type 1 IFN (Kawai and Akira, 2010). We have shown in a previous study that MPL stimulates bone marrow-derived DCs (BMDC) to secrete IL-6 and TNF-α but not IL-12p70 whereas CpG induces all these cytokines in BMDC cultures in vitro at low to moderate levels (Ko et al., 2017). Interestingly, MPL + CpG combination was found to be effective in inducing IL-12p70 and TNF-α in BMDC cultures in vitro (Ko et al., 2017). MF59 squalene oil-in-water emulsion adjuvant was shown to promote the induction of chemokines and inflammatory cytokines and recruit various innate immune cells such as neutrophils and monocytes at the injection site (Calabro et al., 2011). Similarly, in vivo acute innate immune cell recruitment at the site of injection showed that CpG was highly effective in recruiting monocyte-derived macrophages and NKT cells, and high chemokine levels whereas MPL recruited neutrophils, eosinophils, DC subsets (pDCs, CD11b+ DCs), and NK cells. MPL + CpG combination appeared to modulate acute innate immune responses in a differential pattern compared to those of either MPL or CpG alone. MPL + CpG treatment induced a similar profile of innate immune cells as in MPL treatment but lower levels of eosinophils, NKT, NK cells, and DC subsets. In contrast to CpG, macrophage populations were observed at lower levels differentially after injection of MPL or MPL + CpG, which is consistent with aluminum and MF59 licensed adjuvants (Ko et al., 2016). It might be that macrophages would be trafficked to the surrounding lymph nodes or depleted via activation-induced apoptosis (Hsu et al., 2004; Kawai and Akira, 2010).
BALB/c mice were found to be highly responsive to a lower dose of influenza split virus vaccine than C57BL/6 mice, consistent with previous studies (Chen et al., 1999; Misplon et al., 2010). MPL + CpG combination and CpG alone adjuvant effects on improving protection appeared to be more prominent in C57BL/6 mice whereas MPL + CpG combination and MPL alone adjuvant effects in BALB/c mice. Combination adjuvants might have broader application in heterogeneous population. Further studies are needed for better understanding the possible correlation of adjuvant actions between acute innate and long-term adaptive immune responses as well as the underlying mechanisms for how MPL + CpG combination adjuvant could be effective in inducing cross reactive antibodies and protection. Also, more antigenically different strains and subtypes of influenza viruses should be tested to determine the breadth of cross-protection.
Supplementary Material
Acknowledgement
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI119366 (S.M.K.), and AI093772 (S.M.K.). The following reagents were obtained through the NIH Biodefense and Emerging Infections (BEI) Research Resources Repository, NIAID: NR-15749 (HA protein), NR-19234 (NA protein), FR-505 (HA monoclonal antibody). Rabbit mAb HCA-2 specific for pan NA proteins was kindly provided by Dr. Xuguang Li.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2018.06.004.
References
- Akira S, Takeda K, Kaisho T, 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol 2, 675–680. [DOI] [PubMed] [Google Scholar]
- Bihari I, Panczel G, Kovacs J, Beygo J, Fragapane E, 2012. Assessment of antigen-specific and cross-reactive antibody responses to an MF59-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adult and elderly subjects. Clin. Vaccine Immunol 19, 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN, 2014. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/mono-phosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine 32, 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A, 2011. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yoshikawa T, Kadowaki S, Hagiwara Y, Matsuo K, Asanuma H, Aizawa C, Kurata T, Tamura S, 1999. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J. Gen. Virol 80 (Pt 10), 2559–2564. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N, 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol 183, 6186–6197. [DOI] [PubMed] [Google Scholar]
- Doyle TM, Hashem AM, Li C, Van Domselaar G, Larocque L, Wang J, Smith D, Cyr T, Farnsworth A, He R, Hurt AC, Brown EG, Li X, 2013. Universal antineuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res 100, 567–574. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Lambrecht BN, 2008. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 1, 442–450. [DOI] [PubMed] [Google Scholar]
- Gravel C, Li C, Wang J, Hashem AM, Jaentschke B, Xu KW, Lorbetskie B, Gingras G, Aubin Y, Van Domselaar G, Girard M, He R, Li X, 2010. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 28, 5774–5784. [DOI] [PubMed] [Google Scholar]
- Hancock GE, Heers KM, Pryharski KS, Smith JD, Tiberio L, 2003. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine 21, 4348–4358. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M, 2004. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428, 341–345. [DOI] [PubMed] [Google Scholar]
- Ioannou XP, Gomis SM, Karvonen B, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S, 2002. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine 21, 127–137. [DOI] [PubMed] [Google Scholar]
- Ireton GC, Reed SG, 2013. Adjuvants containing natural and synthetic Toll-like receptor 4 ligands. Expert Rev. Vaccines 12, 793–807. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373–384. [DOI] [PubMed] [Google Scholar]
- Klinman DM, 2003. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2, 305–315. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Barnhart KM, Conover J, 1999. CpG motifs as immune adjuvants. Vaccine 17, 19–25. [DOI] [PubMed] [Google Scholar]
- Ko EJ, Lee YT, Kim KH, Jung YJ, Lee Y, Denning TL, Kang SM, 2016. Effects of MF59 adjuvant on induction of isotype-switched IgG antibodies and protection after immunization with T-dependent influenza virus vaccine in the absence of CD4+ T cells. J. Virol 90, 6976–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee YT, Lee Y, Kim KH, Kang SM, 2017. Distinct effects of monophosphoryl lipid a, oligodeoxynucleotide CpG, and combination adjuvants on modulating innate and adaptive immune responses to influenza vaccination. Immune Netw. 17, 326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundi M, 2007. New hepatitis B vaccine formulated with an improved adjuvant system. Expet Rev. Vaccine 6, 133–140. [DOI] [PubMed] [Google Scholar]
- Misplon JA, Lo CY, Gabbard JD, Tompkins SM, Epstein SL, 2010. Genetic control of immune responses to influenza A matrix 2 protein (M2). Vaccine 28, 5817–5827. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Bresee JS, de la Hoz F, Johansen K, Karron RA, Krishnan A, Madhi SA, Mangtani P, Spiro DJ, Ortiz JR, Group, W.H.O.P.P.C.f.N.-G.I.V.A., 2017. Data and product needs for influenza immunization programs in low- and middle-income countries: rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine 35, 5734–5737. [DOI] [PubMed] [Google Scholar]
- O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD, 2013. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expet Rev. Vaccine 12, 13–30. [DOI] [PubMed] [Google Scholar]
- Paules CI, Sullivan SG, Subbarao K, Fauci AS, 2018. Chasing seasonal influenza - the need for a universal influenza vaccine. N. Engl. J. Med 378, 7–9. [DOI] [PubMed] [Google Scholar]
- Prince GA, Denamur F, Deschamps M, Garcon N, Prieels JP, Slaoui M, Thiriart C, Porter DD, 2001. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine 19, 2048–2054. [DOI] [PubMed] [Google Scholar]
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM, 2003. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J. Virol 77, 13156–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg 27, 493–497. [Google Scholar]
- Rose S, Misharin A, Perlman H, 2012. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM, 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl. Acad. Sci. U.S.A 108, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarewski A, 2012. Cervarix(R): a bivalent vaccine against HPV types 16 and 18, with cross-protection against other high-risk HPV types. Expert Rev. Vaccines 11, 645–657. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M, Klinman DM, 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol 167, 3555–3558. [DOI] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO, 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog. 9, e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BE, Sadarangani SP, Leo YS, 2015. The avian influenza vaccine Emerflu. Why did it fail? Expet Rev. Vaccine 14, 1125–1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.