Abstract
Hypertension is a global health challenge: it affects one billion people worldwide and is estimated to account for > 60% of all cases or types of cardiovascular disease. In part because sex differences in blood pressure regulation mechanisms are not sufficiently well understood, fewer hypertensive women achieve blood pressure control compared to men, even though compliance and treatment rates are generally higher in women. Thus, the objective of this study is to identify which factors contribute to the sexual dimorphism in response to anti-hypertensive therapies targeting the renin angiotensin system (RAS). To accomplish that goal, we develop sex-specific blood pressure regulation models. Sex differences in the RAS, baseline adosterone level, and the reactivity of renal sympathetic nervous activity (RSNA) are represented. A novel aspect of the model is the representation of sex-specific vasodilatory effect of the bound angiotensin II type two receptor (AT2R-bound Ang II) on renal vascular resistance. Model simulations suggest that sex differences in RSNA are the largest cause of female resistance to developing hypertension due to the direct influence of RSNA on afferent arteriole resistance. Furthermore, the model predicts that the sex-specific vasodilatory effects of AT2R-bound Ang II on renal vascular resistance may explain the higher effectiveness of angiotensin receptor blockers in treating hypertensive women (but not men), compared to angiotensin converting enzyme inhibitors.
Keywords: renin angiotensin system, ACE inhibitors, angiotensin receptor blockers, hypertension, blood pressure
Introduction
Hypertension is a global health challenge: it affects one billion people and is estimated to account for > 60% of all cases or types of cardiovascular disease. Interestingly, women have lower blood pressure than men before menopause but higher afterwards [1, 2]. Yet, hypertensive men and women are typically treated using the same approach. In part due to our insufficient knowledge regarding sex-specific blood pressure regulation mechanisms, fewer women achieve blood pressure control compared to men, even though compliance and treatment rates are higher in women [3]. These data highlight the critical need to better understand the mechanisms of blood pressure control in both sexes and cast doubt on the current “one size fits all” therapeutic approach.
Sex differences in blood pressure and the prevalence of hypertension have been reported in a number of mammalian and avian species [4]. In humans [5] and in genetic models of hypertension such as spontaneously hypertensive rats (SHR) and Dahl salt-sensitive rats [6], males develop earlier and more severe hypertension than females. To date, the mechanisms underlying male-female differences in blood pressure control remain incompletely understood.
Hypertension is undoubtedly a multifactorial disease with contributions from and affecting multiple organs [7, 8, 9, 10, 11, 12]. In particular, the kidney is a key (albeit not sole) determinant of blood pressure and of sex differences in hypertension [13]. That role is evident in transplantation studies, where blood pressure “goes with” the kidney: transplanting a kidney from a hypertensive rat into a normotensive rat induces hypertension in the recipient [14]. Essential for the kidneys long-term control of blood pressure is the pressure-natriuresis mechanism, whereby increases in renal perfusion pressure lead to increases in Na+ excretion, which in turn lowers salt and water retention and reduces effective circulating volume. Under physiological conditions, females exhibit a leftward shift in the pressure-natriuresis relation relative to males, such that females excrete the same amount of Na+ as males at a lower arterial pressure [15, 16]. Pressure-natriuresis responses encompass multiple levels of Na+ transporter regulation [17, 18], and are substantially modulated by the renin-angiotensin system (RAS) [19]. The RAS is a non-sex hormonal system critical for maintaining blood pressure and effective circulating volume. For instance, one of the peptides angiotensin (Ang) II, through the angiotensin II type 1 receptor (AT1R), induces vasoconstriction and, via its effects on kidney function, enhance Na+ and fluid retention. These vascular and tubular actions serve to maintain or raise blood pressure. However, Ang II can also bind to the angiotensin II type 2 receptor (AT2R) which induces vasodilation.
In the past few years, an explosion of data has emerged concerning sex differences in the RAS [20, 21, 22, 23], in kidney function [15, 24, 25], and in responses to anti-hypertensive therapies targeting the RAS. For instance, the benefits of chronic treatment of angiotensin converting enzyme inhibitors (ACEI) in women have been reported to lessen over time [26], while angiotensin receptor blockers (ARB) reduce blood pressure more in women than in men [27]. That observed stronger advantage of ARB over ACEI in women relative to men may be attributed to the sex differences in intrarenal RAS components. To identify the physiological mechanisms underlying those differences, we develop sex-specific computational models of blood pressure control that include the kidney and the RAS. Our model is based on major components of the seminal computational model for long-term blood pressure regulation by Guyton et al. [28]; that model has since been revised and extended by a number of researchers [29, 30, 31, 32, 33]. However, none of these models are gender specific. Thus, the present sex-specific models are the first to capture the key sex differences in blood pressure regulation mechanisms. This distinguishing feature allows our models to identify the physiological mechanisms that lead to the sexual dimorphism in the pathophysiology of hypertension and discrepancies in men’s and women’s responses to ACEI and ARB.
Materials and methods
Blood pressure regulation model
The present models are an expansion of the long-term blood pressure regulation models by Hallow et al. [30] and by Karaaslan et al. [29], which are in terms based on the model by Guyton et al. [28]. These models describe, using a large system of coupled nonlinear algebraic differential equations, the interactions among the cardiovascular system, the renal system, the renal sympathetic nervous system, the endocrine (renin-angiotensin) system, and how these systems regulate blood pressure and respond to various perturbations. For instance, renal blood flow is adjusted, via renal autoregulatory mechanisms [34, 35], according to hormonal and nervous inputs; and renal blood flow determines, in part, Na+ excretion, which impacts blood pressure. We incorporate the RAS as done in [30] while keeping the single nephron model as done in [29].
A major goal of this study is to identify the mechanisms that underlie the stronger cardiovascular benefits of ARB over ACEI in women compared to men. To that end, we incorporate into the published blood pressure regulation models [30, 29] a detailed, sex-specific representation of the RAS, the target of ACEI and ARB. The RAS model is adopted from a rat RAS model previously published by us [36], with parameters refit separately for men and women (see below). Sex-specific model parameters are identified to represent the well-known sex differences in the RAS and in renal sympathetic nervous activity (RSNA; see below). A schematic diagram of the blood pressure regulation model is shown in Fig. 1. Model equations and parameters not described in detail here can be found in Ref. [29].
Figure 1:

Schematic model of blood pressure regulation. Red nodes denote variables that describe cardiovascular function; green nodes, renal hemodynamics; orange nodes, renal Na+ handling and urine production; blue nodes, the RAS. ADH, anti-diuretic hormone; MAP, mean arterial pressure; ANP, atrial natruietic peptide; RSNA, renal sympathetic nervous activity; PRC, plasma renin concentration; PRA, plasma renin activity; AGT, angiotensinogen; Ang I, angiotensin I; Ang II, angiotensin II; AT1R-bound Ang II, angiotensin II type 1 receptor bound angiotensin II; AT2R-bound Ang II, angiotensin II type 2 receptor bound angiotensin II; ALD, aldosterone.
Renin-angiotensin system model
The present models are the first long-term blood pressure regulation models to include a sex-specific RAS component. It describes the reaction cascade from angiotensinogen (AGT) to Ang (1–7)/Ang II/Ang IV. A schematic diagram that depicts that reaction cascade is shown in Fig. 2. Below we describe the RAS model equations.
Figure 2:
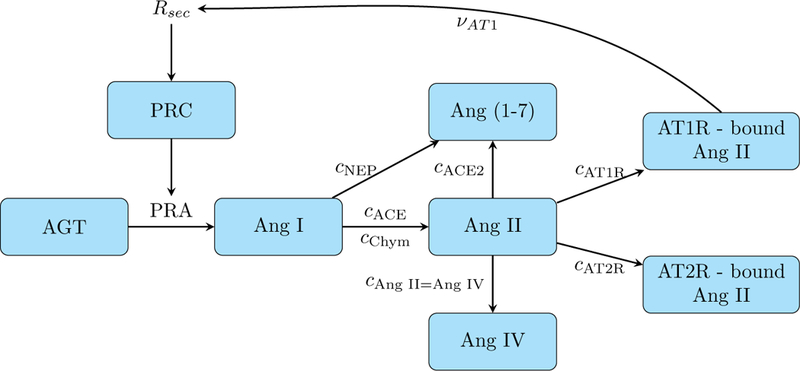
Schematic model of the renin-angiotensin system. Corresponds to the blue nodes in Fig. 1 with added details.
AGT is catalytically cleaved by plasma renin activity (PRA) to produce Ang I. The rate of change of [AGT] is given by the production rate kAGT, its conversion to Ang I, and the decay based on its half life hAGT.
| (1) |
Renin is secreted at the rate Rsec. It has a baseline rate of Nrs and is dependent on the feedback from [AT1R-bound Ang II](νAT1), where A and B are fitting constants taken from [30].
The plasma renin concentration (PRC) is dependent on Rsec and decays with a half life of hrenin. PRA is related to [PRC] by a fixed constant of XP RC−P RA which was determined by [30] from the ratio of PRA to PRC in normotensive subjects in the absence of RAS-blocking therapies.
| (2) |
| (3) |
Ang I is converted into other forms through angiotensin converting enzyme (ACE), chymase, and neutral endopeptidase (NEP) activity; the respective reaction rate constants are denoted cACE, cchym, and cNEP. The half life of Ang I is denoted hAng I.
| (4) |
Ang II is converted from Ang I through ACE and chymase and then converted into Ang (1–7) through ACE2 at the rate cACE2. Ang II also binds to the AT1R and AT2R at rates cAT1R and cAT2R, respectively. We assume that Ang II, AT1R-bound Ang II, and AT2R-bound Ang II have half lives hAng II, hAT1R, and hAT2R, respectively.
| (5) |
| (6) |
| (7) |
Ang (1–7) is converted by NEP from Ang I and by ACE2 from Ang II, and it decays with a half life of hAng (1–7).
| (8) |
Ang IV is converted from Ang II at the rate cAng II=Ang IV and decays with a half life of hAng IV.
| (9) |
Effects of RAS on blood pressure
The RAS regulates blood pressure and fluid balance primarily via its effects on the kidney. Specifically,
ALD enhances Na+ reabsorption along the distal tubule and the collecting duct;
AT1R-bound Ang II enhances Na+ reabsorption along the proximal tubule, and increases afferent and efferent arteriole resistance.
A less well-studied aspect is the blood pressure-lowering effects of AT2R-bound Ang II: it decreases afferent and efferent arteriole resistance (opposite effect of AT1R-bound Ang II) [37]. In general, males have a higher AT1R:AT2R ratio than females [37, 38]. In fact, in the kidney of the adult male rat, AT2R have been reported to be absent or only present at low levels [39]. Given these observations, it has been proposed that AT2R-bound Ang II may play a significant role in the sex differences in blood pressure regulation and in patients’ responses to ACEI and ARB.
To test the validity of this hypothesis, we incorporate the vasodilatory effect of AT2R-bound Ang II into only the female blood pressure regulation model. The equations below present the interaction between AT2R-bound Ang II and renal vascular resistance. We adopt the following notations. Afferent arteriole resistance (Raa) is the baseline resistance (Raa−ss = 31.67 mmHg min l−1) multiplied by the effects of RSNA (βRSNA), tubuloglomerular feedback (ΣTGF), the myogenic effect (Σmyo), AT1R-bound Ang II (ΨAT1R−AA) and AT2R-bound Ang II (ΨAT1R−AA). Efferent arteriole resistance (Rea) is the baseline resistance (Rea−ss = 51.66 mmHg min l−1) multiplied by the effects of AT1R-bound Ang II (ΨAT 1R−EA) [30] and AT2R-bound Ang II (ΨAT2R−EA). βRSNA and ΣTGF are given by Karaaslan et al. [29] while ΨAT1R−AA and ΨAT1R−EA are given by Hallow et al. [30].
| (10) |
| (11) |
We assume that ΨAT2R−AA and ΨAT2R−EA are linearly decreasing functions of [AT2R-bound Ang II], where [AT2R-bound Ang II]eq denotes the equilibrium concentration of the bound peptide.


Together, the effects on arteriolar resistance ΨAT2R−AA and ΨAT2R−EA will impact glomerular filtration rate (GFR), which in turn determines fluid and Na+ excretion, and eventually blood pressure and fluid balance.
Afferent and efferent arteriole resistance together control the renal blood flow (ϕrb)
| (14) |
| (15) |
The glomurlar filtration rate (ϕGFR) is the product of the net filtration pressure (Pf ) and a constant glomerular capillary filtration coefficient (Cgcf ). The net filtration pressure (Pf ) is in turn given by the difference between the glomerular hydrostatic pressure (Pgh) and the sum of the Bowman hydrostatic pressure (PB) and the glomerular osmotic pressure (Pgo). PB and Pgo are assumed known a priori. The glomerular hydrostatic pressure (Pgh) is calculated from the difference between MAP and the mean afferent arteriolar pressure, which is given by the product of ϕrb and Raa.
| (16) |
| (17) |
| (18) |
Sex-specific parameters
Besides the effects of AT2R-bound Ang II, the models also represent sex differences in the RAS, baseline aldosterone (ALD) levels, and RSNA sensitivity.
The RAS.
To identify sex-specific RAS model parameters, we apply RAS hormone peptide levels from the literature (see Table 2 and description below) and solve for sex-specific RAS reaction rate constants as done in [36]. These RAS reaction rates, shown in Table 3, are used in the hypertensive simulations.
Table 2:
Human RAS hormone levels.
Table 3:
Reaction rate constants solved for in the linear system for male and female normotensive humans.
| Reaction Rate Constant | Unit | Male | Female |
|---|---|---|---|
| [PRC] | fmol/ml/min | 17.72 | 17.72 |
| PRA | fmol/ml/min | 18.02 | 18.02 |
| kAGT | fmol/ml/min | 577.04 | 610.39 |
| cACE | 1/min | 0.88 | 1.41 |
| cchym | 1/min | 0.09 | 0.15 |
| cNEP | 1/min | 0.04 | 0.06 |
| cACE2 | 1/min | 0.0078 | 0.004 |
| cAngII=AngIV | 1/min | 0.25 | 0.04 |
| cAT1 | 1/min | 0.17 | 0.03 |
| cAT2 | 1/min | 0.07 | 0.04 |
| [AT1R − boundAngII] | fmol/ml | 13.99 | 3.78 |
| [AngIV] | fmol/ml | 0.86 | 1.36 |
Baseline ALD.
Males have higher levels of ALD than females [48]. For the male model, we keep the baseline level of 85 ng/l from [29]. For the female model, we use the ratio of male to female ALD reported in Ref. [48] to determine the female baseline ALD level to be 69.2 ng/l.
RSNA.
Females have less excitable and more easily repressed RSNA [49]. RSNA is affected by mean arterial pressure (MAP) and right atrial pressure (Pra). Mathematically, RSNA is the baseline level (Nrsna = 1) multiplied by the effects of MAP (αmap) and the effects of Pra (αrap) as shown in Eqs. 19 and 20 and defined in Ref. [29]. To qualitatively model the female’s RSNA sensitivity, we introduce a new step in the female model, Eq. 22.
| (19) |
| (20) |
| (21) |
| (22) |
Note that the baseline value of rsna0 is 1. Thus, the female rsna formula implies lower RSNA levels at higher levels of stimulation (i.e., slower increase in rsna for rsna0 > 1), and higher RSNA levels with low stimulation.
Results
Responses to angiotensin infusion
Angiotensin infusion experiments are commonly done in both animals and humans to characterize the body’s reaction. We simulate the Ang I and II infusion experiments in [50] by adding to Eqs. 4 and 5 infusion constants kI = 0.44833 fmol/ml/min and kII = 0.3633 fmol/ml/min, respectively to simulate the constant infusion rate of Ang I and II used in the study.
The key difference between the model’s responses to Ang I and Ang II infusion is the direction of change in [Ang I] (Fig. 3a). Ang I infusion, naturally, increases [Ang I], but Ang II infusion decreases [Ang I]. The opposite responses can be explained as follows. Both Ang I and Ang II infusion raises [AT1R-bound Ang II]. By means of a feedback mechanism (depicted in Fig. 2), the higher [AT1R-bound Ang II] suppresses PRA. Taken in isolation, the lower PRA would decrease [Ang I] and [Ang II]. Indeed, this is what was observed with the Ang II infusion simulation. For the Ang I infusion simulation, however, the added Ang I overwhelms competing factors, resulting in an increase in [Ang I].
Figure 3:
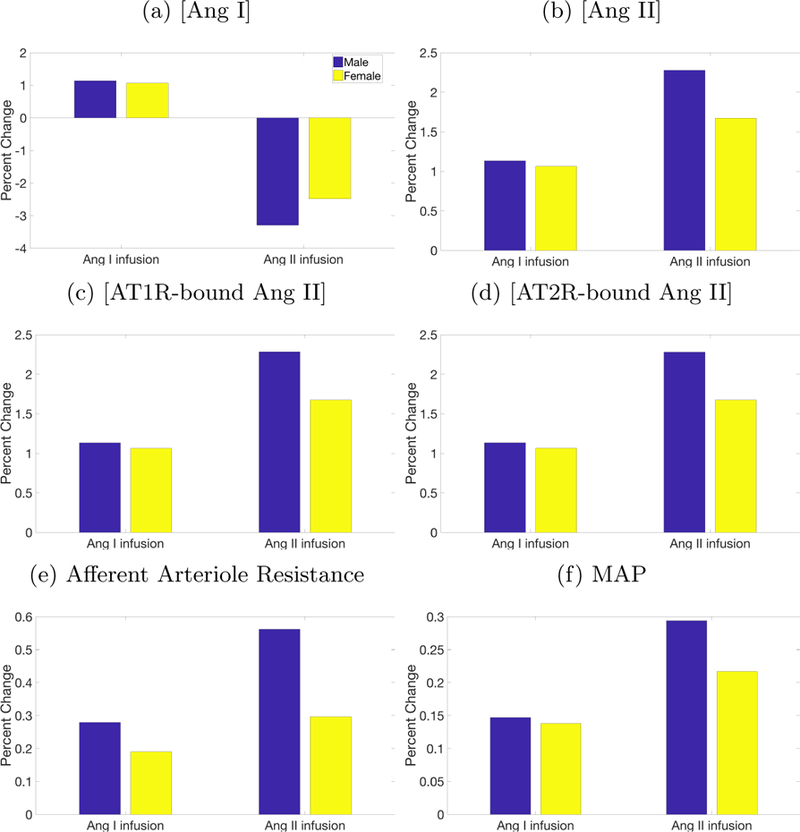
Predicted percent change from control due to Ang I and Ang II infusion.
The predicted response to Ang II infusion shows a greater sexual dimorphism than Ang I infusion. This can be attributed to the significantly larger male-to-female difference between reaction rate constants in the Ang II reaction (Eq. 5) compared to Ang I (Eq. 4); see Table 3. As a result, a given stimulus generally yields smaller changes in [AT1R-bound Ang II] and [AT2R-bound Ang II] in females than in males (Figs. 3c and 3d). With both Ang I and Ang II infusion, the lower [AT1R-bound Ang II] in females cause smaller increases in MAP by elevating afferent arteriole resistance, Na+ reabsorption in the proximal tubule, and Na+ reabsorption in the distal tubule (through effects on ALD levels) by less than in the male model.
Responses to various hypertensive stimuli
Hypertension is a multifactorial disease, with causes including arterial stiffening, impaired renal Na+ handling, and stimulation of the renal sympathetic nervous system. To assess the sensitivity of blood pressure to key contributors of hypertension, we induce hypertension, in both the male and female models, by individually increasing the following model parameters: systemic vascular resistance, afferent arteriole resistance, proximal tubule Na+ reabsorption, distal tubule Na+ reabsorption, collecting duct Na+ reabsorption, renin secretion, ALD secretion, and RSNA (Fig. 4). The amount of increase for each parameter is selected to represent a large increase within physiological bounds.
Figure 4:
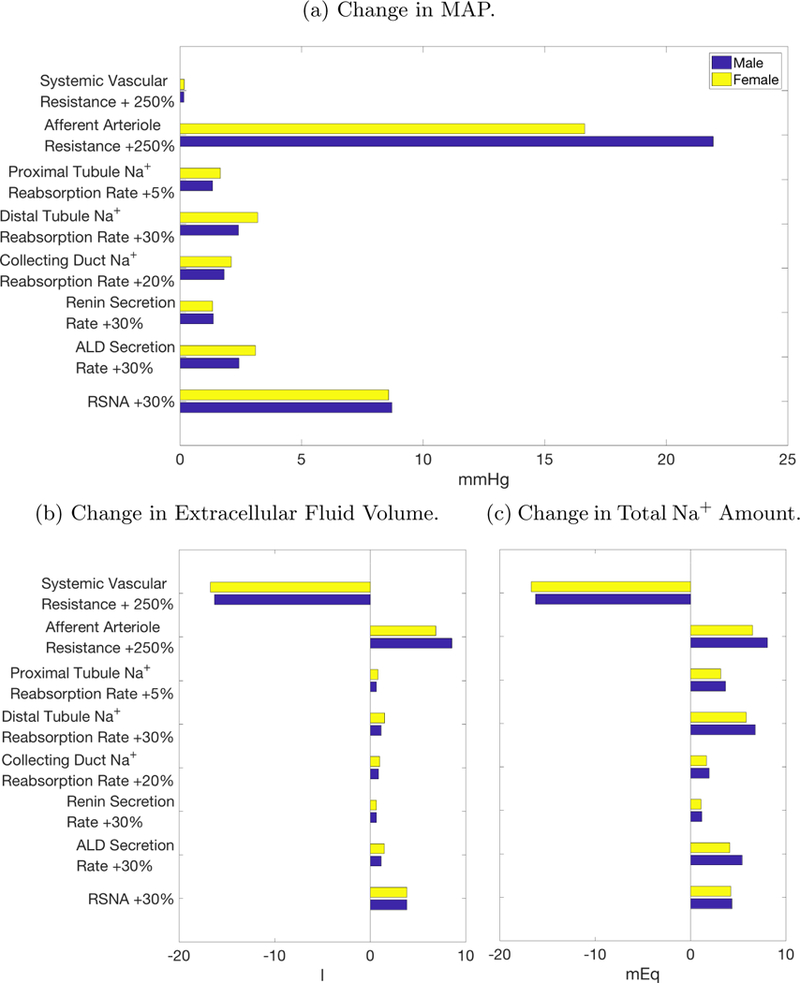
Change in selected variables from various causes of hypertension. The chosen increase in afferent arteriole resistance yields the largest rise in mean arterial pressure (MAP).
Both the male and female models predict that increases in afferent arteriole resistance induce the largest increase in extracellular fluid volume and thus MAP (Fig. 4a). Other parameter changes (e.g., RSNA, ALD, etc.) yield smaller effects, in part, because of the activation of the autoregulatory response, which elevates renal blood flow, GFR, and eventually urine and Na+ excretion, via the dilation of the afferent arteriole. That compensatory response is nullified in the case where afferent arteriole resistance is assumed a priori to be elevated. Indeed, increased afferent arteriole resistance lowers renal blood flow and GFR, whereas all other cases predict an opposite change in these variables. It is noteworthy that increased afferent arteriole resistance leads to similar increases in Na+ amount as other causes of hypertension. Instead, the increase in MAP stems from water retention as extracellular fluid volume increases by double that of the next largest cause of hypertension, RSNA (Fig. 4b).
Explaining sex differences in response to hypertensive stimulus
In the next set of simulations, we seek to explain men’s higher susceptibility to hypertension, compared to women, in response to increased afferent arteriole resistance. In our models, afferent arteriole resistance is controlled through multiple feedback systems, including RSNA, AT1R-bound Ang II, and AT2R-bound Ang II — all of which exhibit sex differences. When baseline afferent arteriole resistance is elevated, each feedback system reacts to keep the effective afferent arteriole resistance near normotensive levels. Consequently, male afferent arteriole resistance increases from 32.2 mmHg to 52.4 mmHg (a 62.6% increase) while female afferent arteriole resistance only increases from 32.1 mmHg to 48.2 mmHg (a 50.2% increase). Thus, despite the same increase in the baseline afferent arteriole resistance parameter, the female model exhibits a significantly smaller increase in afferent arteriole resistance. This sex difference can be attributed primarily to the stronger RSNA-mediated feedback mechanisms in females: RSNA stimulation of afferent arteriole resistance drops by 51.2% percent in the female model, but only by 47.8% in the male model. Compared to RSNA, the two other (RAS-mediated) feedback systems show a much smaller male-female difference in response to changes in baseline afferent arteriole resistance. Thus we hypothesize that RSNA is the main cause of female resistance to developing hypertension.
To test this hypothesis, we conduct model simulations using the male model with one trait set to the corresponding female value at a time (Fig. 5). Specifically, we induce hypertension in three variants of the male model by increasing afferent arteriole resistance by 250%. The three variants are: one with baseline ALD level replaced by the female value (e.g., 69.2 ng/l instead of 85 ng/l), one with female RAS parameters, and another with the female effects of RSNA (Eq. 22). Each of these traits were described previously. The variant of the male model that yields blood pressure most similar to the female model will have the female trait that may best explain the sex difference in response to increased afferent arteriole resistance. Results are shown in Fig. 5.
Figure 5:
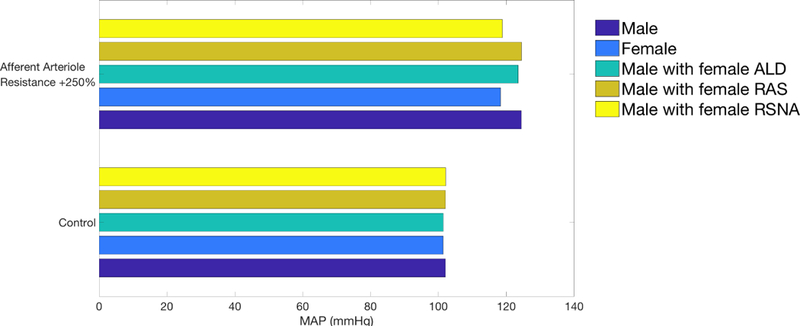
Predicted blood pressure in baseline male and female (two blues) and three instances of male model with one trait set to female value (green and two yellows). Blood pressure was obtained for control conditions (lower) and hypertensive conditions induced by elevation in afferent arteriole resistance (upper).
The male models with female ALD level or RAS parameters behave similarly to the baseline male model, in the sense that the higher afferent arteriole resistance yields similar increases in MAP. In contrast, the male model with female RSNA acts similarly to the female model. Thus, we conclude that in our models, female protection against increased afferent arteriole resistance is due, in large part, to sex differences in RSNA sensitivity.
Hypertensive drug treatments
In the next set of simulations, we investigate the sex-specific responses to two classes of hypertensive therapies that target the RAS. We focus on renal hypertension, which is caused by the narrowing of the afferent arterioles, modeled by a higher baseline afferent arteriole resistance. We consider two types of anti-hypertensive drugs. The first, ACEI, block ACE from converting Ang I into Ang II. To simulate their effects, we reduce cACE by a target level γACE:
| (23) |
where 0 ≤ γACE ≤ 1. To determine the value of γACE, we compare our model to experimental data. Nussberger et al. found that with ACEI administration, Ang II levels in normotensive men dropped by 63% [42]. To achieve this reduction in [Ang II] in our normotensive male model requires a γACE = 0.78. This γACE is used in all simulations.
The second class of hypertensive drugs considered are ARB, which stop Ang II from attaching to the AT1R. To simulate their effects, we reduce cAT1R by a target level γARB:
| (24) |
where 0 ≤ γARB ≤ 1. To determine the level of ARB, we again compare with experimental results. Tsutamoto et al. found that with ARB, Ang II rose by 90% in humans with mild to moderate congestive heart failure [51]. As their patients had normal blood pressure, we compare this to the normotensive male model, which requires γARB = 0.67 to reproduce the experimental rise in Ang II. This γARB is used in all simulations.
The effects of ACEI and ARB on MAP in men and women are summarized in Fig. 6. These results indicate that ACEI and ARB induce a similar reduction in MAP in men, whereas ARB yield a significantly larger MAP decrease in women than ACEI (Fig 6).
Figure 6:
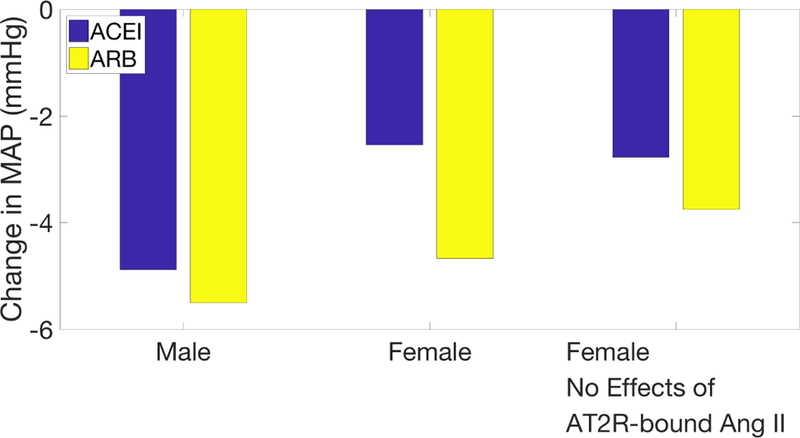
Change in MAP following ACEI (blue) and ARB (yellow) administration on hypertensive male model (first group) and on hypertensive female models with and without the vasodilatory effect of [AT2R-bound Ang II] (second and third groups, respectively).
We hypothesize that the above difference may be attributable to the dilatory effects of AT2R-bound Ang II on renal vascular resistance, which is present only in the female model (Eqs. 12,13). When this effect is eliminated, then the sex difference in MAP response is much attenuated. (Fig. 6). These results suggest that the vasodilatory effects of AT2R-bound Ang II are essential for explaining the significantly stronger MAP-lowering effect of ARB over ACEI seen in women but not in men.
Discussion
Despite known sex differences in responses to some anti-hypertensive therapies, male and female hypertensive patients are typically treated with the same therapeutic approach. The objective of this study is to apply sex-specific computational models of blood pressure regulation to reveal the contribution of different pathways and systems to the sexual dimorphism in the pathophysiology of hypertension and in the efficacy of commonly prescribed anti-hypertensive therapies, ACEI and ARB. Our models represent physiological systems responsible for long term blood pressure regulation, including the cardiovascular system and the kidney [28], as well as the RAS [30] and RSNA [29]. Major novel aspects of these models include sex specific descriptions of RSNA sensitivity (Eq. 22), the RAS (Table 3), ALD concentration, and the effects of AT2R-bound Ang II on renal vascular resistance (Eqs. 12 and 13).
Model simulations indicate a stronger response, measured by increases in MAP, in men compared to women to a number of hypertensive stimuli, including Ang II infusion (Fig. 3f) and increased afferent arteriole resistance (Figure 4a). This difference in responses can be attributed to the sex difference in the reaction rate constants of the RAS, to female-specific vasodilatory benefits of AT2R-bound Ang II, and to the RSNA, which is more excitable in the male (Figure 5).
Men’s stronger responses to ACEI and ABR relative to women.
The same factors that heighten men’s sensitivity to hypertensive stimuli also enhance their response to anti-hypertensive treatments that target the RAS. For instance, ACEI are predicted to yield a significantly larger decrease in MAP in men (4.88 mmHg) compared to women (2.54 mmHg, Fig. 6). This result is consistent with a study by Falconnet et al., who found that chronic ACEI decreased both systolic and diastolic blood pressure in hypertensive men more than women (18.9/13.3 mmHg decrease for men, vs 11.5/7.8 mmHg decrease for women) [26]. One discrepancy between the simulation and clinical studies is that the model predicts in both sexes significantly smaller absolute MAP reductions than those reported in Ref. [26]. That discrepancy could be due to the effects of ACEI beyond the RAS, such as its inhibition of the degradation of bradykinin, an inflammatory mediator [52]. The build up of bradykinin causes blood vessels to dilate, thereby reducing blood pressure. Bradykinin may also contribute to the enhanced effectiveness of ACEI in men; bradykinin signaling causes nitric oxide release, which reduces oxidative stress [52, 53, 54, 55]. While it is ambiguous if men or women have greater levels of oxidative stress, it has been shown that increasing oxidative stress in male SHR increases blood pressure and vice versa, whereas the same is not true in female SHR [56]. While important, these effects are beyond the scope of the model.
Furthermore, model simulations yield a slightly smaller decrease in MAP due to ARB in the female model (4.67 mmHg) as compared to the male model (5.50 mmHg, Fig. 6). This small difference is consistent with the findings that hydrochlorothiazide and ARB treatment together resulted in only a 3 mmHg greater average decrease in the female population than in the male population [57]. In contrast, in a study of ARB treatment in patients with essential hypertension, after 6 weeks of treatment ARB was found to be more effective in women (23 mmHg decrease in systolic pressure) compared to men (15 mmHg) [27]. The above differing, and sometimes contrasting, observations and model predictions are a consequence of the multiple origins of hypertension.
ARB are more effective than ACEI in women, but that difference vanishes in men.
Our models predict that ARB reduce blood pressure more than ACEI in females (4.67 versus 2.54 mmHg drop, respectively, Figure 6). This result is consistent with the findings of a study of chronic heart failure patients treated with either ACEI or ARB, which indicate that ARB reduced patient morbidity more than ACEI in females [58]. No difference in patient morbidity was found in males. It is noteworthy that our model only produces this result when the vasodilatory effects of AT2R-bound Ang II are included in the female model; more below.
In contrast, our model predicts no significant difference in MAP reductions in the male model following ACEI or ARB treatment (Figure 6). Direct comparison of our model’s prediction with clinical or experimental data is difficult, inasmuch as most studies failed to divide blood pressure measurements by sex. A number of studies have shown ARBs and ACEIs to induce similar reductions in blood pressure [59, 60, 61, 62]. In contrast, Mogensen et al. found ARB reduced systolic blood pressure more than ACEI [63] whereas Azizi et al. and Morgan et al. reported the opposite findings [64, 65]. However, as previously noted, none of these studies divided the results by sex. Most studies had a much larger participation from men than women (approximately two thirds male) [59, 61, 62, 63, 64] or were almost completely male [65].
A key contribution of the present study is the explanation of why ARB leads to a larger blood pressure reduction than ACEI in females, but that benefit is not seen in men. Both ACEI and ARB lower MAP by reducing [AT1R-bound Ang II]. In the present model, the only difference between ACEI and ARB is the direction of change of [AT2R-bound Ang II]: ACEI cause a decrease in [AT2R-bound Ang II], while ARB cause an increase. This opposite effect is the reason why the introduction of the vasodilatory effects of AT2R-bound Ang II into the model is necessary to replicating the differences in reaction to ACEI and ARB in women. Given the observation that AT2R have been reported to be absent or nearly so in the kidney of the adult rat [39], we incorporate the model assumption that AT2R-bound Ang II causes the afferent and efferent arterioles to dilate in the female kidney (Eqs. 12 and 13). The novel representation of AT2R-bound Ang II effects leads to a cardiovascular benefit that is specific to ARB and females: while both ACEI and ARB lowers MAP by reducing [AT1R-bound Ang II], in females ARB, but not ACEI, also lowers MAP by reducing arteriolar resistance (via elevating [AT2R-bound Ang II]). The MAP-lowering effect from the reduction in afferent arteriole resistance is then compounded by the RSNA response in female which is more easily repressed compared to men.
The potential importance of sexual dimorphism in the regulation of afferent arteriole resistance is consistent with other model predictions. In their model of pressure-diuresis and naturesis, Beard and Mescam found that the differential regulation of afferent and efferent arteriole resistance was sufficient to explain the observed differences between normotensive and hypertensive Dahl salt-sensitive rats [66].
In summary, the cardiovascular advantages of ARB over ACEI are stronger in women than in men because of (1) the higher AT2R expression in women especially in the afferent and efferent arterioles and (2) a less excitable and more easily repressed RSNA in women.
Model limitations and future extensions.
This study presents the first sex-specific computational models of whole-body long-term blood pressure regulation. While comprehensive, the representation of some regulatory processes, especially those concerning renal tubular transport and autoregulation, is relatively simplistic. For example, the renal autoregulatory components, such as the tubuloglomerular feedback and the myogenic response, can be replaced by more comprehensive models (e.g., Refs. [67, 68, 34, 35]) with sex-specific responses incorporated [15]. Also, if one wishes to simulate and compare the actions of different drugs (e.g., diuretics) that target the nephron directly, one may replace the relatively simple renal transport components (orange nodes in Fig. 1) by a more detailed epithelial transport model (e.g., Refs. [69, 70, 71, 72, 73, 74, 75]), with known sexual dimorphism in renal transporter expression [76, 77] incorporated as done in Ref. [78]. With the inclusion of a detailed epithelial transport model, the drug’s effect on specific transporters can be simulated, with the influence on, e.g., Na+ transport predicted (rather than specified a priori).
Table 1:
List of Acronyms
| Acronym | Definition |
|---|---|
| ACE | angiotensin converting enzyme |
| ACEI | angiotensin converting enzyme inhibitor |
| ADH | anti-diuretic hormone |
| AGT | angiotensinogen |
| ALD | aldosterone |
| Ang I | angiotensin I |
| Ang II | angiotensin II |
| Ang (1–7) | angiotensin (1–7) |
| Ang IV | angiotensin IV |
| ANP | atrial naturetic peptide |
| ARB | angiotensin receptor blocker |
| AT1R - bound Ang II | angiotensin type 1 receptor bound angiotensin II |
| AT2R - bound Ang II | angiotensin type 2 receptor bound angiotensin II |
| GFR | glomerular filtration rate |
| MAP | mean arterial pressure |
| NEP | neutral endopeptidase |
| PRA | plasma renin activity |
| PRC | plasma renin concentration |
| RAS | renin angiotensin system |
| RSNA | renal sympathetic nervous activity |
| SHR | spontaneously hypertensive rats |
Sex specific computational models of long-term blood pressure regulation introduced
Different enzymatic activity is key to male sensitivity to angiotensin II infusion
Renal sympathetic nervous activity is key to female resistance to renal hypertension
Angiotensin type 2 receptor key to female strength of ARB over ACEI treatment
Acknowledgments
This research was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, grant R01DK106102, by the National Science Foundation, grant DMS1263995, and by the Canada 150 Research Chair program.
Appendix: Determining sex-speciftc RAS parameters
To identify sex-specific RAS model parameters, we follow the general approach in Ref. [36]. The goal is to determine the reaction rates in Eqs. 1–9. To that end, we first set the RAS hormone peptide levels to values gleaned from literature. The values and references are found in Table 2 and further discussed below.
Human male concentrations are taken from Ref. [47, 40, 42, 45, 79]. Female hormone concentrations are estimated from these male values using the male-to-female ratios reported in the references given in Table 2, column labelled “Female”.
With the hormone concentrations specified, the 12 unknowns are (i) [PRC], (ii) PRA, (iii) AGT production rate (kAGT) (iv) seven reaction rate constants (ci), (v) [Ang IV], and (vi) [AT1R-bound Ang II].
The unknowns are computed by applying the steady-state formulation of the model equations (Eqs. 1–8), and the steady-state conservation equation.
With 12 unknowns and 9 equations, we need 3 additional constraints. To that end, we impose the (i) ratio of AT1R-to-AT2R receptors (i.e., the associated reaction rate constants), (ii) ratio of NEP-to-ACE2, and (iii) ratio of ACE-to-Chymase. Sex-specified ratios are available for AT1R-to-AT2R receptors [38], but not the other ratios. See Table 4.
Table 4:
Sex-specific RAS reaction rate constant ratios.
References
- [1].Hay M, Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors, Clinical Science 130 (1) (2016) 9–18. [DOI] [PubMed] [Google Scholar]
- [2].Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, De Simone G, Ford ES, et al. , Heart disease and stroke statistics2011 update, Circulation 123 (4) (2011) e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gu Q, Burt VL, Paulose-Ram R, Dillon CF, Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among us adults with hypertension: data from the national health and nutrition examination survey 1999–2004, American journal of hypertension 21 (7) (2008) 789–798. [DOI] [PubMed] [Google Scholar]
- [4].Sandberg K, Ji H, Sex differences in primary hypertension, Biology of sex differences 3 (1) (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW, 24-h ambulatory blood pressure in 352 normal danish subjects, related to age and gender, American journal of hypertension 8 (10) (1995) 978–986. [DOI] [PubMed] [Google Scholar]
- [6].Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP, Sex difference in the development of deoxycorticosterone-salt hypertension in the rat., Hypertension 9 (2) (1987) 172–177. [DOI] [PubMed] [Google Scholar]
- [7].Dahl LK, Love R, Evidence for relationship between sodium (chloride) intake and human essential hypertension, AMA archives of internal medicine 94 (4) (1954) 525–531. [DOI] [PubMed] [Google Scholar]
- [8].Guyton AC, Renal function curve–a key to understanding the pathogenesis of hypertension., Hypertension 10 (1) (1987) 1–6. [DOI] [PubMed] [Google Scholar]
- [9].Grassi G, Mancia G, Neurogenic hypertension: is the enigma of its origin near the solution?, Hypertension 43 (2) (2004) 154–155. [DOI] [PubMed] [Google Scholar]
- [10].Grassi G, Seravalle G, Quarti-Trevano F, The neuroadrenergic hypothesis in hypertension: current evidence, Experimental physiology 95 (5) (2010) 581–586. [DOI] [PubMed] [Google Scholar]
- [11].Bugenhagen SM, Cowley AW, Beard DA, Identifying physiological origins of baroreflex dysfunction in salt-sensitive hypertension in the dahl ss rat, Physiological genomics 42 (1) (2010) 23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, Omholt SW, Arterial stiffening provides sufficient explanation for primary hypertension, PLoS computational biology 10 (5) (2014) e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang L, Wang X, Qu HY, Jiang S, Zhang J, Fu L, Buggs J, Pang B, Wei J, Liu R, Role of kidneys in sex differences in angiotensin ii–induced hypertensionnovelty and significance, Hypertension 70 (6) (2017) 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bianchi G, Fox U, Di Francesco G, Giovanetti A, Pagetti D, Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats, Clinical Science 47 (5) (1974) 435–448. [DOI] [PubMed] [Google Scholar]
- [15].Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM, Gender differences in pressure-natriuresis and renal autoregulation, Hypertension 57 (2) (2011) 275–282. [DOI] [PubMed] [Google Scholar]
- [16].Khraibi AA, Liang M, Berndt TJ, Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats, American journal of hypertension 14 (9) (2001) 893–896. [DOI] [PubMed] [Google Scholar]
- [17].McDonough AA, Isn forefronts symposium 2015: Maintaining balance under pressurehypertension and the proximal tubule, Kidney International Reports 1 (3) (2016) 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McDonough AA, Nguyen MT, Maintaining balance under pressure, Hypertension 66 (3) (2015) 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mirabito KM, Hilliard LM, Head GA, Widdop RE, Denton KM, Pressor responsiveness to angiotensin ii in female mice is enhanced with age: role of the angiotensin type 2 receptor, Biology of sex differences 5 (1) (2014) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maric-Bilkan C, Manigrasso MB, Sex differences in hypertension: contribution of the renin–angiotensin system, Gender medicine 9 (4) (2012) 287–291. [DOI] [PubMed] [Google Scholar]
- [21].Moritz K, Cuffe J, Wilson L, Dickinson H, Wlodek M, Simmons D, Denton K, sex specific programming: a critical role for the renal renin–angiotensin system, Placenta 31 (2010) S40–S46. [DOI] [PubMed] [Google Scholar]
- [22].Hilliard LM, Sampson AK, Brown RD, Denton KM, The his and hers of the renin-angiotensin system, Current hypertension reports 15 (1) (2013) 71–79. [DOI] [PubMed] [Google Scholar]
- [23].Komukai K, Mochizuki S, Yoshimura M, Gender and the renin–angiotensin–aldosterone system, Fundamental & clinical pharmacology 24 (6) (2010) 687–698. [DOI] [PubMed] [Google Scholar]
- [24].Chen Y, Sullivan JC, Edwards A, Layton AT, Sex-specific computational models of the spontaneously hypertensive rat kidneys: factors affecting nitric oxide bioavailability, American Journal of Physiology-Renal Physiology 313 (2) (2017) F174–F183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hatano R, Onoe K, Obara M, Matsubara M, Kanai Y, Muto S, Asano S, Sex hormones induce a gender-related difference in renal expression of a novel prostaglandin transporter, oat-pg, influencing basal pge 2 concentration, American Journal of Physiology-Renal Physiology 302 (3) (2012) F342–F349. [DOI] [PubMed] [Google Scholar]
- [26].Falconnet C, Bochud M, Bovet P, Maillard M, Burnier M, Gender difference in the response to an angiotensin-converting enzyme inhibitor and a diuretic in hypertensive patients of african descent, Journal of Hypertension 22 (6). [DOI] [PubMed] [Google Scholar]
- [27].Canzanello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB, Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension, American Journal of Hypertension 21 (1) (2008) 61–66. doi: 10.1038/ajh.2007.24. [DOI] [PubMed] [Google Scholar]
- [28].Guyton AC, Coleman TG, Granger HJ, Circulation: overall regulation, Annu. Rev. Physiol 34 (1972) 13–46. [DOI] [PubMed] [Google Scholar]
- [29].Karaaslan F, Denizhan Y, Kayserilioglu A, Gulcur HO, Long-term mathematical model involving renal sympathetic nerve activity, arterial pressure, and sodium excretion, Annals of biomedical engineering 33 (11) (2005) 1607–1630. [DOI] [PubMed] [Google Scholar]
- [30].Hallow KM, Lo A, Beh J, Rodrigo M, Ermakov S, Friedman S, de Leon H, Sarkar A, Xiong Y, Sarangapani R, Schmidt H, Webb R, Kondic AG, A model-based approach to investigating the pathophysiological mechanisms of hypertension and response to antihypertensive therapies: extending the guyton model, American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 306 (9) (2014) R647–R662. doi: 10.1152/ajpregu.00039.2013. [DOI] [PubMed] [Google Scholar]
- [31].Abram SR, Hodnett BL, Summers RL, Coleman TG, Hester RL, Quantitative circulatory physiology: an integrative mathematical model of human physiology for medical education, Advances in Physiology Education 31 (2), pMID: 17562912. doi: 10.1152/advan.00114.2006. [DOI] [PubMed] [Google Scholar]
- [32].Ikeda N, Marumo F, Shirataka M, Sato T, A model of overall regulation of body fluids, Annals of Biomedical Engineering 7 (2) (1979) 135–166. doi: 10.1007/BF02363132. [DOI] [PubMed] [Google Scholar]
- [33].Thomas SR, Baconnier P, Fontecave J, Francoise J-P, Guillaud F, Hannaert P, Hernandez A, Le Rolle V, Maziere P, Tahi F, White RJ, Saphir: a physiome core model of body fluid homeostasis and blood pressure regulation, Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 366 (1878) (2008) 3175–3197. doi: 10.1098/rsta.2008.0079. [DOI] [PubMed] [Google Scholar]
- [34].Sgouralis I, Layton AT, Autoregulation and conduction of vasomotor responses in a mathematical model of the rat afferent arteriole, American Journal of Physiology-Renal Physiology 303 (2) (2012) F229–F239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sgouralis I, Layton AT, Theoretical assessment of renal autoregulatory mechanisms, American Journal of Physiology-Renal Physiology 306 (11) (2014) F1357–F1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Leete J, Gurley S, Layton AT, Modeling sex differences in the renin angiotensin system and the efficacy of antihypertensive therapies, Computers & Chemical Engineering 112 (2018) 253–264. doi: 10.1016/j.compchemeng.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zimmerman MA, Sullivan JC, Hypertension: What’s sex got to do with it?, Physiology 28 (4) (2013) 234–244. doi: 10.1152/physiol.00013.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MHC, Fortes ZB, Nigro D, A lower ratio of at1/at2 receptors of angiotensin ii is found in female than in male spontaneously hypertensive rats, Cardiovascular Research 62 (3) (2004) 587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- [39].Sullivan JC, Sex and the renin-angiotensin system: inequality between the sexes in response to ras stimulation and inhibition, American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 294 (4) (2008) R1220–R1226. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- [40].Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H, Novel sandwich elisa for human angiotensinogen, American Journal of Physiology-Renal Physiology 293 (3) (2007) F956–F960, pMID: 17553939. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schunkert H, Danser AJ, Hense H-W, Derkx FH, Kurzinger S, Riegger GA, Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women, Circulation 95 (1) (1997) 39–45. doi: 10.1161/01.CIR.95.1.39. [DOI] [PubMed] [Google Scholar]
- [42].Nussberger J, Wuerzner G, Jensen C, Brunner HR, Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril, Hypertension 39 (1) (2002) 1–8. [DOI] [PubMed] [Google Scholar]
- [43].Cohall DH, Scantlebury-Manning T, James S, Hall K, Ferrario CM, Reninangiotensinaldosterone system gender differences in an afro-caribbean population, Journal of the Renin-Angiotensin-Aldosterone System 16 (3) (2015) 539–546, pMID: 24532825. doi: 10.1177/1470320314523659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reyes-Engel A, Morcillo L, Aranda FJ, Ruiz M, Gaitan MJ, Mayor-Olea A, Aranda P, Ferrario CM, Influence of gender and genetic variability on plasma angiotensin peptides, J Renin Angiotensin Aldosterone Syst 7 (2) (2006) 92–97. [DOI] [PubMed] [Google Scholar]
- [45].Chappell MC, Pirro NT, Sykes A, Ferrario CM, Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme, Hypertension 31 (1) (1998) 362–367. doi: 10.1161/01.HYP.31.1.362. [DOI] [PubMed] [Google Scholar]
- [46].Sumino H, Ichikawa S, Miya Y, Sakamaki T, Kurabayashi M, Angiotensin ii plays an important role in maintaining blood pressure in postmenopausal women receiving hormone replacement therapy*, American Journal of Hypertension 18 (10) (2005) 1340. doi: 10.1016/j.amjhyper.2004.07.022. [DOI] [PubMed] [Google Scholar]
- [47].Lo A, Beh J, De Leon H, Hallow MK, Ramakrishna R, Rodrigo M, Sarkar A, Sarangapani R, Georgieva A, Using a Systems Biology Approach to Explore Hypotheses Underlying Clinical Diversity of the Renin Angiotensin System and the Response to Antihypertensive Therapies, Springer New York, New York, NY, 2011, pp. 457–482. [Google Scholar]
- [48].Miller JA, Anacta LA, Cattran DC, Impact of gender on the renal response to angiotensin ii, Kidney International 55 (1) (1999) 278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- [49].Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR, Gender differences in sympathetic nervous system regulation, Clin. Exp. Pharmacol. Physiol 26 (2) (1999) 122–126. [DOI] [PubMed] [Google Scholar]
- [50].Admiraal PJ, Danser AH, Jong MS, Pieterman H, Derkx FH, Schalekamp MA, Regional angiotensin ii production in essential hypertension and renal artery stenosis., Hypertension 21 (2) (1993) 173–184. doi: 10.1161/01.HYP.21.2.173. [DOI] [PubMed] [Google Scholar]
- [51].Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, et al. , Angiotensin ii type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure, Journal of the American College of Cardiology 35 (3) (2000) 714–721. [DOI] [PubMed] [Google Scholar]
- [52].Taddei S, Bortolotto L, Unraveling the pivotal role of bradykinin in ace inhibitor activity, American Journal of Cardiovascular Drugs 16 (5) (2016) 309–321. [DOI] [PubMed] [Google Scholar]
- [53].Sangsree S, Brovkovych V, Minshall RD, Skidgel RA, Kininase i-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin b1 receptor agonists, American Journal of Physiology-Heart and Circulatory Physiology 284 (6) (2003) H1959–H1968. [DOI] [PubMed] [Google Scholar]
- [54].Gryglewski RJ, Uracz W, Chlopicki S, Marcinkiewicz E, Bradykinin as a major endogenous regulator of endothelial function, Pediatric pathology & molecular medicine 21 (3) (2002) 279–290. [DOI] [PubMed] [Google Scholar]
- [55].Oeseburg H, Iusuf D, van der Harst P, van Gilst WH, Henning RH, Roks AJ, Bradykinin protects against oxidative stress–induced endothelial cell senescence, Hypertension 53 (2) (2009) 417–422. [DOI] [PubMed] [Google Scholar]
- [56].Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF, Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease, Clinical and experimental pharmacology and physiology 34 (9) (2007) 938–945. [DOI] [PubMed] [Google Scholar]
- [57].Saunders E, Cable G, Neutel J, Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (inclusive) study, The Journal of Clinical Hypertension 10 (1) (2008) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L, Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failurea population study, European journal of heart failure 9 (6–7) (2007) 602–609. [DOI] [PubMed] [Google Scholar]
- [59].Gradman AH, Arcuri KE, Goldberg AI, Ikeda LS, Nelson EB, Snavely DB, Sweet CS, A randomized, placebo-controlled, double-blind, parallel study of various doses of losartan potassium compared with enalapril maleate in patients with essential hypertension, Hypertension 25 (6) (1995) 1345–1350. [DOI] [PubMed] [Google Scholar]
- [60].De Rosa M, Cardace P, Rossi M, Baiano A, De Cristofaro A, Comparative effects of chronic ace inhibition and at1 receptor blocked losartan on cardiac hypertrophy and renal function in hypertensive patients, Journal of human hypertension 16 (2) (2002) 133. [DOI] [PubMed] [Google Scholar]
- [61].Jacobsen P, Andersen S, Jensen BR, Parving H-H, Additive effect of ace inhibition and angiotensin ii receptor blockade in type i diabetic patients with diabetic nephropathy, Journal of the American Society of Nephrology 14 (4) (2003) 992–999. [DOI] [PubMed] [Google Scholar]
- [62].Ferrari P, Marti H-P, Pfister M, Frey FJ, Additive antiproteinuric effect of combined ace inhibition and angiotensin ii receptor blockade, Journal of hypertension 20 (1) (2002) 125–130. [DOI] [PubMed] [Google Scholar]
- [63].Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, Cooper ME, Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (calm) study, Bmj 321 (7274) (2000) 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Azizi M, Linhart A, Alexander J, Goldberg A, Menten J, Sweet C, Menard J, Pilot study of combined blockade of the renin–angiotensin system in essential hypertensive patients, Journal of hypertension 18 (8) (2000) 1139–1147. [DOI] [PubMed] [Google Scholar]
- [65].Morgan T, Anderson A, Bertram D, MacInnis RJ, Effect of candesartan and lisinopril alone and in combination on blood pressure and microalbuminuria, Journal of the Renin-Angiotensin-Aldosterone System 5 (2) (2004) 64–71. [DOI] [PubMed] [Google Scholar]
- [66].Beard DA, Mescam M, Mechanisms of pressure-diuresis and pressure-natriuresis in dahl salt-resistant and dahl salt-sensitive rats, BMC physiology 12 (1) (2012) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Layton AT, Feedback-mediated dynamics in a model of a compliant thick ascending limb, Mathematical biosciences 228 (2) (2010) 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Layton AT, Moore LC, Layton HE, Multistable dynamics mediated by tubuloglomerular feedback in a model of coupled nephrons, Bulletin of mathematical biology 71 (3) (2009) 515. [DOI] [PubMed] [Google Scholar]
- [69].Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT, Effects of nkcc2 isoform regulation on nacl transport in thick ascending limb and macula densa: a modeling study, American Journal of Physiology-Renal Physiology 307 (2) (2014) F137–F146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Layton AT, Vallon V, Edwards A, Predicted consequences of diabetes and sglt inhibition on transport and oxygen consumption along a rat nephron, American Journal of Physiology-Renal Physiology 310 (11) (2016) F1269–F1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Layton AT, Vallon V, Edwards A, A computational model for simulating solute transport and oxygen consumption along the nephrons, American Journal of Physiology-Renal Physiology 311 (6) (2016) F1378–F1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Layton AT, Laghmani K, Vallon V, Edwards A, Solute transport and oxygen consumption along the nephrons: effects of na+ transport inhibitors, American Journal of Physiology-Renal Physiology 311 (6) (2016) F1217–F1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Layton AT, Vallon V, Edwards A, Modeling oxygen consumption in the proximal tubule: effects of nhe and sglt2 inhibition, American Journal of Physiology-Renal Physiology 308 (12) (2015) F1343–F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Layton AT, Edwards A, Vallon V, Adaptive changes in gfr, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis, American Journal of Physiology-Renal Physiology 313 (2) (2017) F199–F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Layton AT, Vallon V, Sglt2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism, American Journal of Physiology-Renal Physiology 314 (5) (2018) F969–F984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, et al. , Expression of na+-d-glucose cotransporter sglt2 in rodents is kidney-specific and exhibits sex and species differences, American Journal of Physiology-Cell Physiology 302 (8) (2012) C1174–C1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Veiras LC, Girardi AC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu A, et al. , Sexual dimorphic pattern of renal transporters and electrolyte homeostasis, J Am Soc Nephrol 28 (12) (2017) 3504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li Q, McDonough AA, Layton HE, Layton AT, Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: Modeling and analysis, American Journal of Physiology-Renal Physiology [DOI] [PMC free article] [PubMed]
- [79].Nussberger J, Gradman AH, Schmieder RE, Lins RL, Chiang Y, Prescott MF, Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension, International Journal of Clinical Practice 61 (9) 1461–1468. doi: 10.1111/j.1742-1241.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- [80].Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM, Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study, Hypertension 48 (5) (2006) 914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]


