Abstract
Purpose
We evaluate the antimicrobial effect of toluidine blue O (TBO)-mediated photodynamic antimicrobial chemotherapy (PACT) on Staphylococcus epidermidis and Staphylococcus aureus isolated from ocular surface infection.
Methods
We selected 24 strains of bacteria for this study. The antimicrobial effect of different TBO concentrations, light irradiation, and duration were evaluated. After determining the optimal PACT parameters, four experimental groups were included: Group 1, TBO alone (T+L−); Group 2, light-emitting diode (LED) irradiation alone (T−L+); Group 3, TBO–LED irradiation combination (T+L+); and Group 4, no treatment group (T−L−). The antibacterial effect of PACT was evaluated with the colony survival fraction (SF) methods.
Results
The antibacterial effect of PACT on S. epidermidis and S. aureus was dose-dependent to light irradiation and TBO concentration. The optimal PACT parameters were a TBO concentration of 60 μM, light irradiation of 5.27 mW/cm2, and an irradiation duration of 30 minutes. In group 1, 60 μM TBO without irradiation did not show any antibacterial effect on S. epidermidis (1.48E7 ± 1.5E6 colony-forming units [CFU]/mL) or S. aureus (1.45E7 ± 9E5 CFU/mL). In group 2, irradiation alone with 5.27 mW/cm2 did not modify bacterial growth (S. epidermidis, 1.49E7 ± 1.43E6; S. aureus, 1.5E7 ± 1.2E6). In group 3, after treatment, bacteria density dropped to 4000 ± 1000 and 3E5 ± 1E5 CFU/mL in S. epidermidis and S. aureus groups, respectively (P < 0.001, P = 0.030). In group 4, there was uniform bacterial growth (S. epidermidis, 1.51E7 ± 1.5E6; S. aureus, 1.48E7 ± 1.5E6) before and after treatment.
Conclusions
TBO-mediated PACT had an antibacterial efficacy in vitro on S. epidermidis and S. aureus isolated from ocular surface infection.
Translational Relevance
As TBO-mediated PACT has a strong antibacterial effect to S. epidermidis and S. aureus in vitro, this approach may assist in the treatment of ocular surface infectious diseases.
Keywords: red light, toluidine blue O (TBO), photodynamic therapy, bacteria, ocular infection
Introduction
Infectious keratitis is a potentially sight-threatening condition which can be caused by bacteria, virus, fungus, or parasites. In China, there are 3 million corneal blind patients with an annual increase rate of 100,000 cases.1 In India, 2 million people suffer a corneal ulcer every year.2 In the United States, the Center for Disease Control (CDC) has reported an incidence of 71,000 patients having infectious keratitis per year, and infectious keratitis has become the most widespread complication of contact lens use.3,4 In infectious keratitis, bacteria are the most common infectious agents, in particular Gram-positive cocci, such as Staphylococcus epidermidis and Staphylococcus aureus.5 Considering the rapid progression and severe complications induced by bacterial keratitis, empiric broad-spectrum antibiotic therapy should be started promptly and most cases of bacterial keratitis are treated effectively with topical antibiotic eye drops. However, recent studies have shown the increasing incidence of antibiotic-resistant bacterial infections.2,6 In the United States, 80% of ocular isolates of methicillin-resistant S. aureus were resistant to the commonly prescribed antibiotics.3,7 In addition, the localization of microorganisms within deep corneal structure makes antibacterial eye drops less effective and requires multiple instillations or increased concentrations with ocular surface toxicity issues.8 Therefore, it is important to find other effective adjuvant therapies for the management of bacterial keratitis.
Photodynamic therapy (PDT) is a process that combines photosensitizers (PS) and light irradiation to produce reactive oxygens species (ROS) that will oxidize biologic components and lead to cell death.9,10 After its initial use for tumor treatment, photodynamic antimicrobial chemotherapy (PACT) has been shown to be effective for the elimination of microorganisms.11 PACT can inactivate or kill a wide range of microbial pathogens,12 especially antibiotic-resistant microbial pathogens, such as vancomycin-resistant Enterococcus species, methicillin-resistant S. aureus and multidrug-resistant Mycobacterium tuberculosis.13–17 To date, the main PACT strategy against infectious keratitis is based on the application of riboflavin (RFV, vitamin B2) with ultraviolet light A (UVA) irradiation at 365 nm.18,19 However, its moderate antimicrobial effect limited its clinical application for the treatment of infectious keratitis.
Toluidine blue O (TBO), another hydrophilic cationic PS, is considered an effective membrane-destroying photosensitizing agent with a good interaction and high affinity to bacterial membranes in vitro. Compared to the antimicrobial effect of RFV-mediated UVA, the PACT effect of TBO and red light may be much stronger.20,21 The lower ROS production from RFV compared to the use of TBO could explain these results. TBO has become one of the PSs used clinically for antimicrobial treatments, especially in the field of dentistry.22,23 For ocular infection, PACT with TBO treatment has been evaluated only in vitro for fungal and Acanthamoeba keratitis.24,25 To our knowledge, no research has focused on its effectiveness against ocular bacterial infection. Therefore, we determined whether TBO-mediated PDT could affect the bioactivity of S. epidermis and S. aureus in vitro.
Materials and Methods
Bacteria Isolation and Culture
Twenty-four strains of bacteria (12 isolates of S. epidermidis and 12 of S. aureus) were selected for this study. All bacterial strains were isolated from patients with bacterial keratitis provided by the department of microbiology, Beijing Institute of Ophthalmology, Beijing Tongren Hospital. The bacterial strains stored in a glycerol tube were revived and inoculated on blood agar (Jinzhang Technique Development Co., Tianjin, China) at 36.5°C for 48 hours; then bacterial colonies were scraped with an inoculation loop and 0.5 McFarland bacterial suspensions (concentration, 1∼2 × 108 colony-forming units [CFU]/mL) were prepared.
Light Source and Photosensitizer
The experimental setup, including the light system and 96-well microtiter plates, is shown in Figure 1. The light system consisted of a high-power light-emitting diode (LED) array and a power supply. The wavelength of the LED array was centered at 625 nm and its bandwidth was 20 nm. The LED light source was fed by a small direct current (DC) power supply (MCH-K305D; MCH Instruments Co., Ltd., Shenzhen, China). The size of the LED array was able to cover part of the 96-well plate with an adjustable power intensity. The light power output was measured with an optical power meter (VLP-2000; Femto-second Technology Co., Ltd., Changchun, China).
Figure 1.
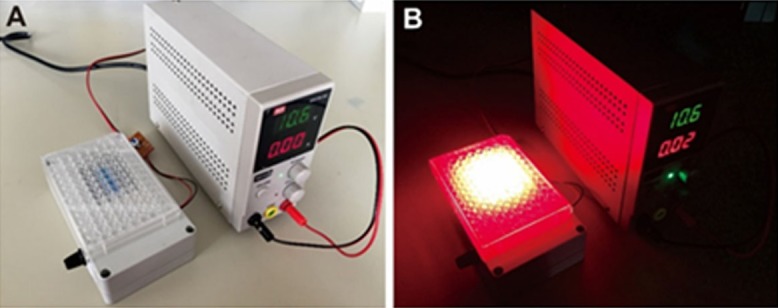
Experimental setup including the light system and the 96-well microtiter plates. (A) Overview of the experimental system, (B) Light irradiation on 96-well microtiter plates in the dark.
The photosensitizer used in this study was TBO (Sigma-Aldrich Technology Co., Ltd., St. Louis, MO). The TBO solution was prepared at a concentration of 1 mM with distilled water. After sterilization by passage through 0.22-μm pore size membrane filters, the TBO solution was stored at 4°C in the dark until experimental use.
The Effect of PS Concentration and Light Irradiation Intensity
The effect of PS concentration on S. epidermidis and S. aureus was studied. Bacterial suspensions (1 × 108 CFU/mL) were incubated with a gradient in the TBO concentration (20, 40, 60, and 80 μM) in 96-well microtiter tissue culture plates. The control wells were incubated with PBS. The population density of the experimental bacterial samples was maintained at approximately 1 × 107 CFU/mL. All culture plates were kept in the dark at room temperature for 20 minutes. After incubation, irradiation was performed with red light (irradiance, 7.30 mW/cm2) for 30 minutes and the fluency level was 13.14 J/cm2.
The bacterial survival fraction (antimicrobial effect parameters) with different TBO concentrations was calculated and the optimal TBO concentration was obtained. Bacterial survival after treatment was assessed using the colony counting and colony survival fraction (SF) method.26 After experimental treatments, 100-μL aliquots were withdrawn from each well and serially diluted 10-fold with PBS. Then, a 10-μL mixture dilution from each well was inoculated onto blood agar plates in triplicate. After an incubation of 48 hours at 37°C, bacterial colonies were counted. The survival fractions of bacteria were determined with the equation N/N0 , where N0 is the number of bacterial colonies before treatment (CFUs per mL of bacteria) and N is the number of bacterial colonies after light and PS treatment.
After determining the appropriate TBO concentration, the effect of different light irradiation intensities was also studied. All bacterial strains were incubated with TBO concentration solution for 20 minutes. Then, illumination was performed with red light for 20 minutes at various light power intensities (0.68, 1.07, 2.47, 5.27, and 7.30 mW/cm2). All experiments were performed with a control group kept in the dark as described above. With these tests and the analysis of survival fractions of bacteria, the appropriate irradiance of red light was determined. Following the same method, 24 strains of bacteria were exposed to red light at the optimum power intensity (based on the above results) for 5, 10, 20, 30, or 40 minutes. Eventually, the best irradiation duration was determined similarly with the calculation of survival fractions after treatment.
PACT with TBO and Red Light
Based on the above results on optimal TBO concentration, irradiation intensity, and duration, the effect of PACT with TBO and red light was investigated. Bacterial suspensions (150 μL; isolates of S. epidermidis and S. aureus) were incubated with TBO solution in the dark at room temperature for 20 minutes and placed in 96-well microtiter plates (height of bacterial suspension was 3.6 mm). The bacterial cell density of the experimental samples was maintained at 1 × 107 CFU/mL and the TBO concentration was kept at the optimal level mentioned above. After incubation, four experimental groups were established as follows: Group 1, TBO alone (T+L−); wells containing TBO were kept in the dark without red light exposure. Group 2, LED irradiation alone (T−L+), suspensions with PBS were exposed to the optimal intensity of red light. Group 3, TBO–LED irradiation combination (T+L+), the TBO-containing wells were exposed to red light with the optimal TBO concentration, light irradiance, and irradiation time. Group 4, within the control group (T−L−) suspensions with PBS were kept in the dark. After experimental procedures, 100 μL of bacterial suspensions were inoculated on blood agar plates at 37°C for 48 hours and bacterial colonies were counted. The survival fractions (N/N0 ) of bacteria were determined.
Statistical Analysis
All results were presented as mean ± standard deviation (SD). Statistical analyses were performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL). The normal distribution of the results was tested using the Kolmogorov-Smirnov test. Descriptive statistics were used to summarize the data in multiplex analyses. The statistical significance between groups was determined using 2-way analysis of variance. P < 0.05 was considered statistically significant.
Results
The Antibacterial Effect of Different PS Concentrations
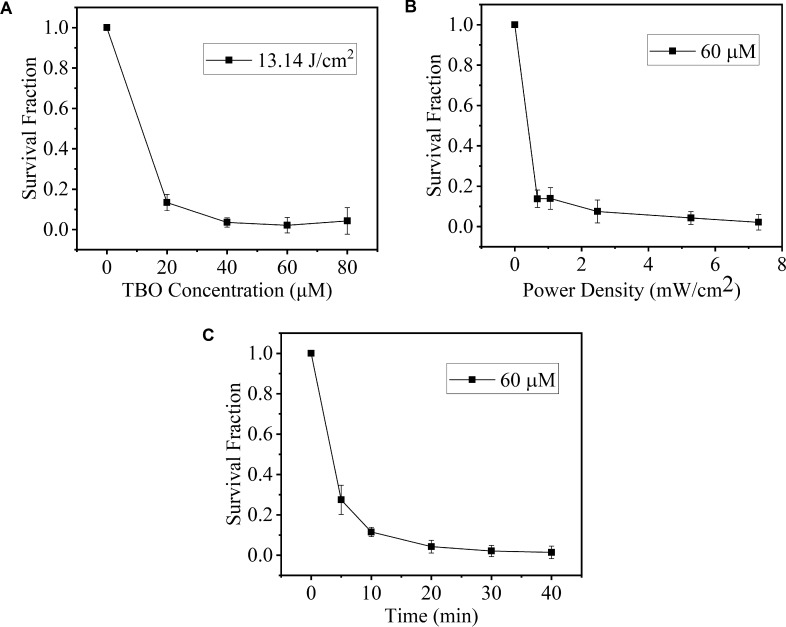
As the TBO concentration increased, the survival fraction of S. epidermidis showed a declining trend (Fig. 2A). The difference in the survival fraction among different TBO concentrations was significant (F = 3.534, P < 0.001, Table 1). The antibacterial efficacy of PACT increased as the TBO concentration increased from 20 to 60 μM and then was stable from 60 to 80 μM (t = 1.524, P = 0.139, Table 1). With regard to S. aureus (Fig. 3A), a similar significant difference in survival fraction also was noted between the control and PACT groups at all tested concentrations of TBO (F = 5.392, P < 0.001, Table 1). The survival fraction decreased as the concentration of TBO increased, at a constant red light irradiation (13.14 J/cm2). In the S. epidermidis and S. aureus groups, the antibacterial efficacy also was stable with the TBO concentrations higher than 60 μM (P = 0.139, 0.318, Table 1). Consequently, the antibacterial efficacy of PACT was TBO concentration–dependent up to 60 μM. Therefore, this concentration was used in the further experiments.
Figure 2.
Survival fraction of S. epidermidis incubated with TBO followed by irradiation. A value of 1 was assigned to the survival fraction of the control untreated cells. (A) Effect of different TBO concentrations on the survival of bacterial cells irradiated by the red light for 20 minutes (TBO concentration: 20, 40, 60, and 80 μM). (B) survival fraction as a function of irradiance (irradiance: 0.68, 1.07, 2.47, 5.27, and 7.30 mW/cm2; irradiation duration was 20 minutes). (C) effect of irradiation duration on phototoxicity of TBO against bacterial cells. Cells were irradiated with light for 5, 10, 20, 30, and 40 minutes.
Table 1.
Effect of Different TBO Concentrations on the Survival of Bacterial Cells Irradiated by the Red Light during 20 minutes.
|
TBO Concentration, μM |
S. epidermidis, N/N0 |
S. aureus, N/N0 |
||||
|
Survival Fraction |
t |
P |
Survival Fraction |
t |
P |
|
| 0 | 1 | – | – | 1 | – | – |
| 20 | 0.134 ± 0.040 | 7.337 | <0.001 | 0.340 ± 0.029 | 5.392 | <0.001 |
| 40 | 0.035 ± 0.023 | 3.121 | 0.004 | 0.162 ± 0.021 | 4.824 | <0.001 |
| 60 | 0.021 ± 0.018 | 2.182 | 0.037 | 0.132 ± 0.022 | 2.177 | 0.041 |
| 80 | 0.043 ± 0.016 | 1.524 | 0.139 | 0.111 ± 0.030 | 1.004 | 0.318 |
| F | 3.534 | 4.332 | ||||
| P | <0.001 | <0.001 | ||||
Figure 3.
Survival fraction of S. aureus incubated with TBO followed by irradiation. A value of 1 was assigned to the survival fraction of the control untreated cells. (A) Effect of different TBO concentrations on the survival of bacterial cells irradiated by the red light for 20 minutes (TBO concentration: 20, 40, 60, and 80 μM). (B) survival fraction as a function of irradiance (irradiance: 0.68, 1.07, 2.47, 5.27, and 7.30 mW/cm2; irradiation duration was 20 minutes). (C) effect of irradiation duration on phototoxicity of TBO against bacterial cells. Cells were irradiated with light for 5, 10, 20, 30, and 40 minutes.
Effect of Light Irradiation Intensity and Duration
The bacterial survival fractions treated with 60 μm TBO at different light irradiation intensities are presented in Figures 2B and 3B. The survival of S. epidermidis and S. aureus decreased gradually as light irradiation intensities increased (F = 2.514, P = 0.006; F = 2.963, P = 0.002, respectively; Table 2). The survival fraction at light irradiation of 5.27 and 7.30 mW/cm2 was not significantly different in the S. epidermidis and S. aureus groups (P = 0.056, 0.062, respectively). Consequently, the light irradiation intensity used in the further experiments was 5.27 mW/cm2. Meanwhile, at a given light irradiation intensity, a greater decrease was observed for S. epidermidis. For example, at a lower light irradiation (0.68 mW/cm2), the cell survival of S. epidermidis (0.138 ± 0.043) was almost four times lower than that of S. aureus (0.510 ± 0.048). This difference was significant (t = 2.421, P = 0.025).
Table 2.
Survival Fraction as a Function of Irradiance (Irradiation Time was 20 Minutes).
|
Irradiance, mW/cm2 |
S. epidermidis |
S. aureus |
||||
|
Survival Fraction, N/N0 |
t |
P |
Survival Fraction, N/N0 |
t |
P |
|
| 0 | 1 | – | – | 1 | – | – |
| 0.68 | 0.138 ± 0.043 | 5.453 | <0.001 | 0.510 ± 0.048 | 9.079 | <0.001 |
| 1.07 | 0.139 ± 0.054 | 0.327 | 0.571 | 0.462 ± 0.043 | 2.907 | 0.003 |
| 2.47 | 0.075 ± 0.057 | 2.363 | 0.006 | 0.401 ± 0.025 | 0.772 | 0.052 |
| 5.27 | 0.042 ± 0.032 | 2.116 | 0.012 | 0.245 ± 0.021 | 2.213 | 0.011 |
| 7.30 | 0.021 ± 0.018 | 1.672 | 0.056 | 0.132 ± 0.021 | 1.625 | 0.062 |
| F | 2.514 | 2.963 | ||||
| P | 0.006 | 0.002 | ||||
When the light irradiance was kept at 5.27 mW/cm2 and the TBO concentration was 60 μM, the survival fractions of S. epidermidis and S. aureus exposed to red light for 5, 10, 20, 30, and 40 minutes were measured (Table 3) and plotted in Figures 2C and 3C, respectively. With 5 minutes of light irradiation, the survival fraction of S. epidermidis and S. aureus decreased to nearly 70% (0.274 ± 0.073) and 50% (0.466 ± 0.052), respectively. These results indicated that the antibacterial efficacy mainly occurred in the initial phase of illumination. Over 30 minutes, the differences in the survival fraction between every two time points were significant (P = 0.001∼0.004 in the S. epidermidis group and P = 0.001∼0.005 in the S. aureus group). Comparing the survival fraction at 40 and 30 minutes, the differences were not significant in these two groups (P = 0.083 and P = 0.261, respectively). Consequently, the light irradiation time selected for further experiments was 30 minutes.
Table 3.
Effect of Irradiation Time on Phototoxicity of TBO against Bacterial Cells
|
Irradiation Time, Minutes |
S. epidermidis |
S. aureus |
||||
|
Survival Fraction, N/N0 |
t |
P |
Survival Fraction, N/N0 |
t |
P |
|
| 0 | 1 | – | – | 1 | – | – |
| 5 | 0.274 ± 0.073 | 6.858 | <0.001 | 0.466 ± 0.052 | 5.242 | <0.001 |
| 10 | 0.115 ± 0.023 | 7.170 | <0.001 | 0.365 ± 0.030 | 2.421 | 0.005 |
| 20 | 0.042 ± 0.032 | 2.885 | 0.004 | 0.245 ± 0.021 | 3.138 | 0.003 |
| 30 | 0.021 ± 0.028 | 3.242 | 0.003 | 0.149 ± 0.022 | 8.912 | <0.001 |
| 40 | 0.014 ± 0.031 | 1.694 | 0.083 | 0.108 ± 0.031 | 1.232 | 0.261 |
| F | 4.691 | 8.852 | ||||
| P | <0.001 | <0.001 | ||||
PACT with TBO and Red Light
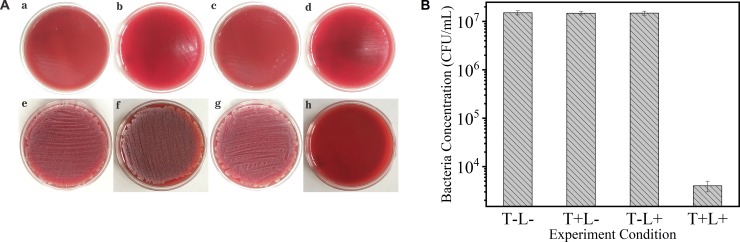
With the above-determined PACT parameters (irradiance, 5.27 mW/cm2; irradiation time, 30 minutes; TBO concentration, 60 μM), the effect of TBO-mediated PDT on the ocular pathogenic bacteria was evaluated. In group 1, the treatment of 60-μM TBO in the dark environment did not provide any antibacterial efficacy to S. epidermidis (1.48E7 ± 1.5E6 CFU/mL) and S. aureus (1.45E7 ± 9E5 CFU/mL). Compared to the bacterial cell density (1 × 107 CFU/mL) of experimental samples before treatment, the differences were not significant (both P > 0.05). Similarly, in group 2, light with 5.27 mW/cm2 alone did not cause any change in bacteria growth density (S. epidermidis, 1.49E7 ± 1.43E6; S. aureus, 1.5E7 ± 1.2E6; both P > 0.05). However, in group 3, when the bacterial suspensions (S. epidermidis and S. aureus) were irradiated in the presence of TBO, greater reductions of bacteria density (4000 ± 1000, 3E5 ± 1E5) were detected (P < 0.001, P = 0.03). In group 4, there was uniform bacterial growth (S. epidermidis, 1.51E7 ± 1.5E6; S. aureus, 1.48E7 ± 1.5E6) on the surface of the agar plates, like groups 1 and 2. The results of antibacterial efficacy in different groups before (Figs. 4a–d, 5a–d) and after (Figs. 4e–h, 5e–h) irradiation are presented in Figures 4A and 5A. The number of colonies of S. epidermidis and S. aureus in each group is shown in Figures 4B and 5B. In groups 1, 2 and 4, the number of colonies of S. epidermidis and S. aureus remained nearly constant, whereas the number colonies in group 3 was significantly decreased compared to the control groups. Moreover, compared to S. epidermidis, more S. aureus colonies survived after PACT.
Figure 4.
(A) The growth situation of S. epidermidis in different treatment groups before (a, b, c, d) and after (e, f, g, h) irradiation for 48 hours. The TBO-mediated PDT group (h) showed obvious growth inhibition after irradiation; (a, e) control group, (b, f) TBO-only group, (c, g) red light only, (d, h) TBO-mediated PDT group. (B) The number of colonies of survival bacteria in the different experimental groups (T, TBO; L, red light; irradiance, 5.27 mW/cm2; irradiation duration, 30 minutes; TBO concentration, 60 μM).
Figure 5.
(A) The growth situation of S. aureus in different treatment groups before (a, b, c, d) and after (e, f, g, h) irradiation for 48 hours. The TBO-mediated PDT group (h) showed obvious growth inhibition after irradiation; (a, e) control group, (b, f) TBO-only group, (c, g) red light only, (d, h) TBO-mediated PDT group. (B) The number of colonies of survival bacteria in different treatment groups (T, TBO; L, red light; irradiance, 5.27 mW/cm2; irradiation duration, 30 minutes; TBO concentration, 60 μM).
Discussion
PACT is a photochemical reaction process in which microorganisms are treated with cytotoxic ROS produced by a photosensitizing agent irradiated with low-intensity visible light.27 We investigated the effect of the photodynamic action of TBO on the viability of S. epidermidis and S. aureus isolated from ocular surface infections.
Our results showed that a large number of S. epidermidis and S. aureus cells could be inactivated when they were irradiated using a LED light with the presence of TBO. These results were similar to those observed by Halili et al.14 showing the effect of PDT on bacterial isolates growth. These investigators used rose bengal and riboflavin mediated photodynamic therapy to inhibit methicillin-resistant S. aureus isolates. Meanwhile, a larger plate (15 × 100 mm) application and bacterial count of central zone in this study gave us good tips to get the accurate antibacterial effect. TBO is another photosensitizer considered to have higher antimicrobial effect than riboflavin.20–21 However, it has been previously evaluated in vitro only for fungal and Acanthamoeba isolates.24–25 Interestingly, the survival fraction of bacteria suspensions decreased as the concentration of TBO increased, when the dose of light irradiation was constant. Meanwhile, as the irradiance intensity or irradiation time increased, the survival of S. epidermidis and S. aureus decreased gradually. We also observed that the antibacterial effect mainly occurred in the initial phase of irradiation. As free oxygen in the solution is depleted by the photodynamic process with singlet oxygen and ROS generation, photodynamic effect became less effective with time and reaching a plateau.
Photo-damage to the DNA and the cytoplasmic membrane has been proposed as possible mechanisms of PDT in bacteria.22 An in vitro study using hematoporphyrin-PACT against S. aureus demonstrated that PACT resulted in photo damage to cytoplasmic membrane proteins as well as chromosomal and plasmid DNA.28 Furthermore, PACT also may induce oxidative stress-derived DNA damage and lead to the production of 7, 8-dihydro-8-oxo-20-deoxyguanosine (8-oxodG).29 This modification of guanidine could cause DNA mis-replication and fragmentation.30 These results also revealed that the photosensitivity of S. epidermidis was higher than that of S. aureus when they were irradiated under similar conditions, and this difference was more significant at lower light doses. Gad et al.31 reported a higher photo-killing effect on S. epidermidis than on S. aureus using methylene blue as photosensitizer, which is similar to TBO. Abundant production of polysaccharide from S. epidermidis has been suggested to obstruct the diffusion of the photosensitizer through the extracellular polymer matrix;32 thus, reducing the susceptibility to photosensitization. Another reason could be the intrinsic variation of S. aureus, especially the thickness of its cell wall, which may result in the S. aureus cells appearing more resistant to photo-killing, such as intrinsic variation in the cell wall thickness influencing the dye uptake.32 Hence, to achieve the same antimicrobial efficacy, a higher light dosage or higher TBO concentration could be required.
Research has shown promising capabilities of PACT. There are several advantages of PACT over conventional antimicrobial agents. First, rapid killing of the target organism mainly depends on the light irradiation dose delivered and appropriate PS concentration. Second, resistance development would be unlikely, since killing is mediated by singlet oxygen and free radicals, and high concentrations of photosensitizer do not need to be maintained at the disease site for more than a few minutes, in contrast to the hours or even days necessary in the case of conventional antimicrobial agents. Finally, antimicrobial effects can be confined to the site of the lesion by careful topical application of photosensitizer and the area of irradiation can be restricted further by using an optical fiber.33 However, the safety of PACT for ocular surface infection should be evaluated further before clinical application. Meanwhile, its clinical applicability and feasibility also should be considered, although the antimicrobial effect of PACT in vitro was obvious.
In conclusion, this study demonstrated that common pathogens of the ocular surface could be effectively inactivated by TBO-mediated PACT. The antimicrobial effect of PACT was related to PS concentration and light dosage. Further insight into PACT with in vivo experiments is required to ensure the safety and effectiveness of PACT.
Acknowledgments
Supported by National Nature Science Foundation of China (Grant No.81470607).
Disclosure: J. Shen, None; Q. Liang, None; G. Su, None; Y. Zhang, None; Z. Wang, None; C. Baudouin, None; A. Labbé, None
References
- 1.Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9:e113843. doi: 10.1371/journal.pone.0113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Tandon R, Gupta SK, Sreenivas V, Vashist P. Burden of corneal blindness in India. Indian J Community Med. 2013;38:198–206. doi: 10.4103/0970-0218.120153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–1028. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 4.Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:1027–1030. [PMC free article] [PubMed] [Google Scholar]
- 5.Peng MY, Cevallos V, McLeod SD, et al. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37:84–87. doi: 10.1097/ICO.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:322–345. [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Ferreiro A, González-Barcia M, Gil-Martínez M, et al. Evaluation of the in vitro ocular toxicity of the fortified antibiotic eye drops prepared at the Hospital Pharmacy Departments. Farm Hosp. 2016;40:352–370. doi: 10.7399/fh.2016.40.5.10416. [DOI] [PubMed] [Google Scholar]
- 9.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–958. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Ray KJ, Prajna L, Srinivasan M, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131:310–313. doi: 10.1001/jamaophthalmol.2013.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Jori G, Fabris C, Soncin M, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright M, Phoenix DA, Gaskell M, Marshall B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother. 1999;44:823–825. doi: 10.1093/jac/44.6.823. [DOI] [PubMed] [Google Scholar]
- 14.Halili F, Arboleda A, Durkee H, et al. Rose bengal- and riboflavin- mediated photodynamic therapy to inhibit methicillin resistant Staphylococcus aureus keratitis isolates. Am J Ophthalmol. 2016;166:194–202. doi: 10.1016/j.ajo.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Taslı H, Akbıyık A, Topaloğlu N, et al. Photodynamic antimicrobial activity of new porphyrin derivatives against methicillin resistant Staphylococcus aureus. J Microbiology. 2018;56:828–837. doi: 10.1007/s12275-018-8244-7. [DOI] [PubMed] [Google Scholar]
- 16.Amaral L, Martins M, Viveiros M. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. J Antimicrob Chemother. 2007;59:1237–1246. doi: 10.1093/jac/dkl500. [DOI] [PubMed] [Google Scholar]
- 17.Alves F, Carmello JC, Mima EGO, Costa CAS, Bagnato VS, Pavarina AC. Photodithazine-mediated antimicrobial photodynamic therapy against fluconazole-resistant Candida albicans in vivo. Med Mycol. 2018. published online October 18. [DOI] [PubMed]
- 18.Martins SAR, Combs JC, Noguera G, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–3408. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Liang Q, Su G, et al. Effect of ultraviolet light irradiation combined with riboflavin on different bacterial pathogens from ocular surface infection. J Biophys. 2017;2017:3057329. doi: 10.1155/2017/3057329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen HK, Garcia J, Væth M, et al. Comparison of riboflavin and toluidine blue O as photosensitizers for photoactivated disinfection on endodontic and periodontal pathogens in vitro. PLoS One. 2015;10:e0140720. doi: 10.1371/journal.pone.0140720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouillaguet S, Wataha JC, Zapata O, et al. Species from photosensitizers activated with visible light sources available in dental offices. Photomed Laser Surg. 2010;28:519–525. doi: 10.1089/pho.2009.2505. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Luan X, Bi L, et al. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med Sci. 2008;23:49–54. doi: 10.1007/s10103-007-0454-x. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca MB, Junior PO, Pallota RC, et al. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed Laser Surg. 2008;26:209–213. doi: 10.1089/pho.2007.2124. [DOI] [PubMed] [Google Scholar]
- 24.Arboleda A, Miller D, Cabot F, et al. Assessment of rose bengal vs. riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158:64–70. doi: 10.1016/j.ajo.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuyoshi M, Takashi S, Takeshi K, et al. Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro. Invest Ophthalmol Vis Sci. 2012;53:6305–6313. doi: 10.1167/iovs.12-9828. [DOI] [PubMed] [Google Scholar]
- 26. Jett BD, Hatter KL, Huycke MM. Simplified agar plate method for quantifying viable bacteria, Biotechniques 1997. 23 648–650. [DOI] [PubMed] [Google Scholar]
- 27.Jori G. Photodynamic therapy of microbial infections: state of the art and perspectives. J Environ Pathol Toxicol Oncol. 2006;25:505–519. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.320. [DOI] [PubMed] [Google Scholar]
- 28.Bertoloni G, Lauro FM, Cortella G, Merchat M. Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochim Biophys Acta. 2000;1475:169e74. doi: 10.1016/s0304-4165(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 29.Sayed Z, Harris F, Phoenix D. A. A study on the bacterial phototoxicity of phenothiazinium based photosensitisers. FEMS Immunol Med Microbiol. 2005;43:367e72. doi: 10.1016/j.femsim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guaninespecific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488:65e76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 31.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 2004;48:2173–2178. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle-Vavra S, Labischinski H, Ebert CC, Ehlert K, Daum RS. A spectrum of changes occurs in peptidoglycan composition of glycopeptide- intermediate clinical Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2001;45:280–287. doi: 10.1128/AAC.45.1.280-287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson M. Lethal photosensitization of oral bacteria and its potential application in the photodynamic therapy or oral infections. J Photochem Photobiol. 2004;3:412–418. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]