Abstract
Purpose
The aim of this study was to investigate the effects of the glaucoma drugs latanoprost, brimonidine, and the combination of both on the central corneal temperature (CT) of healthy subjects by means of infrared thermography. Changes of the central CT may reflect changes of ocular blood flow.
Methods
Before application and during 2 hours after the application of latanoprost, brimonidine, or the combination of both in one eye, the CT in both eyes of 40 healthy subjects was measured repeatedly.
Results
Brimonidine reduced CT by approximately 0.5°C. This effect was statistically significant (P < 0.0001). Latanoprost, however, had a very small and insignificant influence (P = 0.47). Accordingly, the combination of brimonidine and latanoprost also reduced CT up to 0.5°C, and this effect was statistically significant (P < 0.0001).
Conclusions
Brimonidine, but not latanoprost, had a significant effect on central CT. This cooling effect of brimonidine is most probably due to a drug-induced reduction of blood circulation in the ciliary body and iris and to a certain extent also to a reduction of blood flow in the fundus of the eye.
Translational Relevance
This study shows evidence that thermography of the cornea provides indirect information on the influence of drugs on the blood flow to the anterior segment of the patient's eye.
Keywords: corneal temperature, ocular surface thermography, ocular blood flow, latanoprost, brimonidine, glaucoma, healthy subjects
Introduction
Topically applied glaucoma drugs, besides reducing intraocular pressure (IOP), might have other effects, such as changing blood flow, particularly in the ciliary body1 or in other parts of the eye.2,3 Blood flow in the ciliary body can be measured reliably in experimental animals.1,4 However, for human clinical studies, we rely on surrogates, such as corneal temperature (CT). CT can be measured easily and reliably in human.5–14 CT depends on many parameters, such as core and environmental temperatures. However, an intraindividual relative short-term change in central CT may serve as a surrogate for changes in blood flow, particularly the blood flow in the ciliary body. Therefore, central CT changes within minutes or hours after a single application of a drug yield indirect information about the influence of the corresponding drug on blood flow in the anterior part of the eye.
We tested the influence of the IOP-lowering drugs brimonidine and latanoprost on central CT. Brimonidine is a selective alpha-2-adrenergic receptor agonist that is up to 30-fold more selective for alpha-2 than for alpha-1 receptors.15 After topical installation, brimonidine reduces IOP within 1 hour, and the peak effect occurs at 2 to 3 hours.16 Brimonidine has a dual mechanism of IOP lowering: It reduces aqueous humor production and stimulates aqueous humor outflow through the uveoscleral pathway.17 It is primarily absorbed through the cornea and conjunctiva. Upon its rapid absorption, brimonidine is also distributed throughout the iris, ciliary body, vitreous body, choroid, retina, and optic nerve.18
Latanoprost is a prostaglandin F2α analogue and a highly selective agonist of endogenous prostanoid FP receptors. It reduces IOP by increasing the uveoscleral outflow of aqueous humor.19 The early effect of prostaglandins on uveoscleral outflow may be mediated by a relaxation of the ciliary muscle.20 The main effect of latanoprost is an increase in the gene expression of metalloproteinases, which then degrade the extracellular matrix. This remodeling of the ciliary muscle leads to an increased space for outflow.21,22 Latanoprost is an isopropyl ester prodrug that becomes biologically active after hydrolysis to the acid form during passage through the cornea. The release from the cornea into the anterior segment of the eye is slow.23 The maximal effect in lowering IOP is achieved within 8 to 12 hours.23
The aim of this study was to investigate the effect of topical latanoprost, brimonidine, and the combination of both latanoprost and brimonidine on central CT of healthy subjects, by means of infrared thermography. Changes in CT could potentially serve as an indicator of changes in blood flow.
Methods
Study Participants
Forty healthy subjects, 20 men and 20 women aged between 19 and 47 years, were recruited in public places and included in this study in the Department of Ophthalmology of the University of Basel, Switzerland. After explanation of the nature and possible consequences of the study, written informed consent was obtained from all subjects before admission to the study.
The inclusion criteria were as follows: normal findings in a routine ophthalmological examination, including slit-lamp examination and fundoscopy, a refractive error between +3.0 and −3.0 diopters of spherical equivalent, corrected visual acuity of 0.8 or higher, IOP not higher than 21 mm Hg, and blood pressure (BP) not higher than 140 mm Hg systolic and 90 mm Hg diastolic. The exclusion criteria were as follows: chronic or current systemic or ocular disease (including dry eyes), medication use (except contraceptives), pregnancy or lactation, recreational drug consumption, alcohol abuse, and allergy against latanoprost or brimonidine. Subjects were asked to refrain from alcohol and caffeine for at least 12 hours before the trial measurements.
Ethical approval for the study project was obtained from the local medical ethics committee, “Ethikkommission beider Basel/EKBB” (EK-Nr. 53/10), before participants were entered into the study. The prospective clinical trial was registered in Clinical Trial Register (NCT01201551). The study was designed and conducted in accordance with the tenets of the Declaration of Helsinki.
CT Measurements
CT measurements were performed using the contactless infrared Ocular Surface Thermographer TG-1000 (Tomey Corporation, Nagoya, Japan). The instrument has two cameras: one for regular pictures (sensitive to visible light) and one for thermography (sensitive to infrared light). With the help of the infrared light camera, the instrument quantifies surface temperatures by measuring emitted thermal energy within an electromagnetic spectrum of 8 to 17 μm wavelengths and a topographic resolution of 240 × 320. The outcome is then presented in the form of false-color images. All CT measurements were performed under a constant room temperature of 24°C and a humidity of 50%. The examination room had no windows, and the door was kept closed to avoid air flow.24 The room was illuminated with neon light. The subjects first rested for 10 minutes in the examination room in order to adapt to the environment. At the start of each recording session, the subjects were asked to blink normally, then to keep both eyes closed for about 5 seconds. For the recordings, the subjects were asked to put their chin on the chin rest, to look at the fixation target, and to try to keep their eyes open widely for 10 seconds. The pupils were not dilated. The eye drops have been stored in a refrigerator at the temperature of approximately +4°C. One hour before they were applied to the individual subjects, we took them out of the refrigerator and stored them at room temperature.
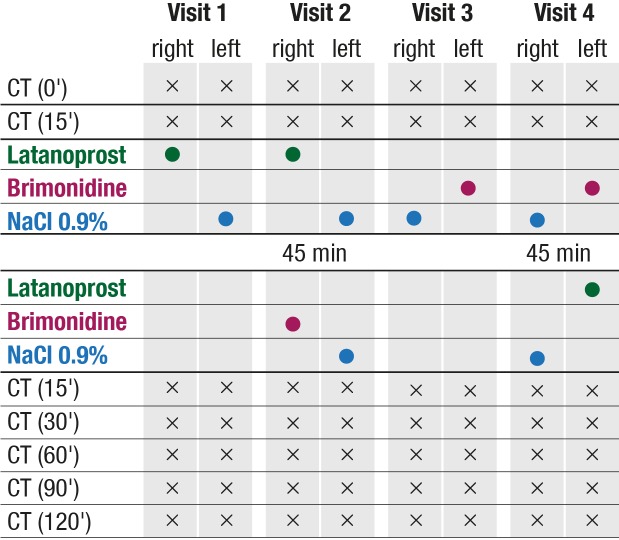
Study Plan
The CT measurements took place during four visits within two consecutive weeks: Visits 1 and 2 took place during two consecutive days of the first study week (week 1), and visits 3 and 4 during two consecutive days of the second study week (week 2). The visits and readings always took place at the same time of day because ocular surface temperature has been shown to vary throughout the day.25
Each visit consisted of seven central CT recording sessions. First, two baseline recording sessions (15 minutes apart) took place. Then eye drops were applied:
At visit 1, one drop of latanoprost was applied in the right eye and one drop of 0.9% NaCl in the left eye;
at visit 2, one drop of latanoprost and one drop of brimonidine (45 minutes apart) were applied in the right eye, and two drops of 0.9% NaCl (45 minutes apart) were applied in the left eye;
at visit 3, one drop of 0.9% NaCl was applied in the right eye, and one drop of brimonidine in the left eye;
at visit 4, two drops of 0.9% NaCl were applied in the right eye (45 minutes apart), and one drop of brimonidine and one drop of latanoprost were applied (45 minutes apart) in the left eye.
The examiner, but not the patient, knew which drop was a drug and which was just 0.9% NaCl.
The central CT measurements of both eyes took place twice before application of the eye drops, and 15, 30, 60, 90, and 120 minutes after the last drug application. In total, 28 recording sessions took place for each eye, and 80 eyes were investigated.
IOP was measured twice during each visit: before the first CT measurement and 105 minutes after application of the eye drops. BP was also measured twice during each visit: before the first CT measurement and 45 minutes after application of the eye drops. The study plan is depicted in Figure 1.
Figure 1.
Study plan. Right, the right eye; left, the left eye.
Data Recording and Data Processing
The basic methods for data recording and processing have already been described in another recent publication.26 The most important aspects are repeated here.
One recording session (lasting 10 seconds) consisted of 21 repeated measurements with time intervals of 0.5 seconds. The central CT values were then exported as a comma-separated value (CSV) file. This export tool, which stores individual temperature readings in a suitable data format, was not implemented by the device; it was provided by the manufacturer on request.
The Ocular Surface Thermographer TG-1000 quantifies the temperature of the eye and its surroundings. In this study, we focused on the central cornea. The test grid was slightly but constantly shifted toward the lower part of the cornea to avoid the unwanted influence of the upper lid. All CSV files were arranged in 21 rectangular blocks, each with 320 × 240 pixels corresponding to the visual display on the device screen. For further evaluations, data were imported into the statistical software R. We focused on 7 × 4 = 28 equidistant test locations, each representing the mean of nine neighboring pixels. The grid constant (the distance between adjacent test locations) was 20 pixels, corresponding to approximately 1.8 mm on the cornea. The chosen field of interest covered a region only marginally affected by the eyebrows and eyelids. Because the CSV files do not take the eye side into account, the coordinates of the left eyes had to be turned around the vertical axis.
In order to achieve stable values unaffected by blinking and brow artifacts, the median of each test location (CTm) was calculated across the 21 repetitions. Finally, the CTms of the 28 test locations of each eye were used for further statistical evaluations.
Statistical Analysis
The basic methods for evaluating and processing row data have already been described in another recent publication.26 To simplify calculations, the median of the CTm values across the 28 test locations was calculated for each subject and timepoint (CTM).
In order to analyze the influence of medication on the time course of CTM, linear mixed-effect models were performed. Mixed-effect models are suitable for modeling repeated measures data. The results are presented as differences in relation to the baseline. Accordingly, the calculation of P-values is also based on the comparison of drug effects in relation to the baseline. Additionally, the time courses of CTM are displayed as line plots with error bars.
In order to assess the association of IOP change from baseline and CTM change from baseline, a linear mixed-effect model was applied. In addition, the association was visualized with a scattergram.
A P-value of <0.05 was considered significant. All evaluations were conducted using the statistical software R version 3.2.1.27
Results
Effect of 0.9% NaCl on CT
Treatment with 0.9% NaCl had a statistically insignificant influence on central CT (P = 0.10), whether it was given once or twice (45 minutes apart) (Fig. 2).
Figure 2.
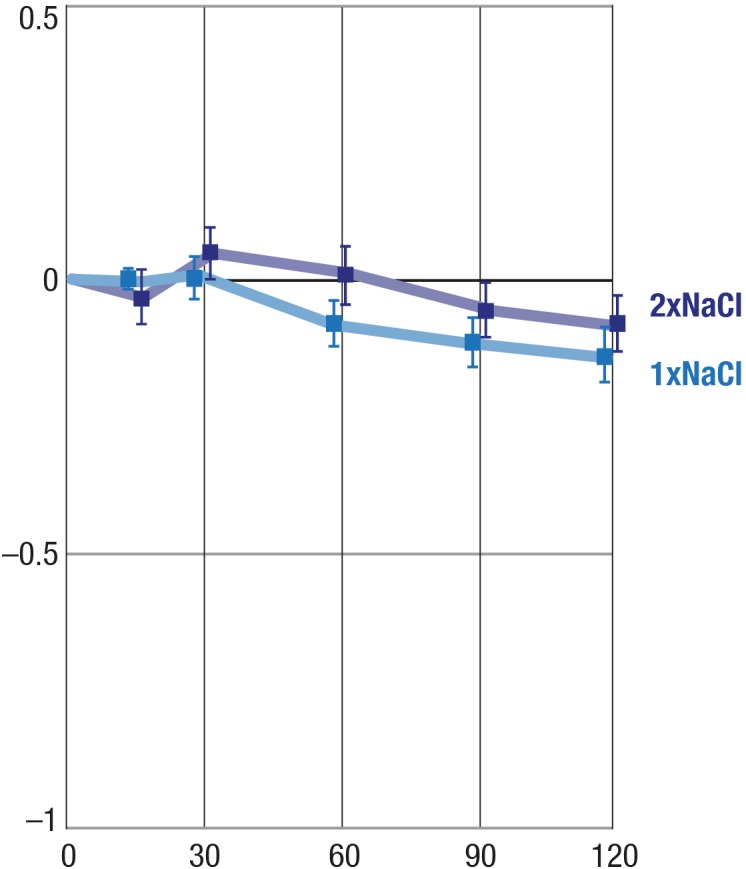
Effects of one drop and two drops of 0.9% NaCl on CT. X-axis, time in minutes; Y-axis, mean change from baseline (°C); balks, SEM.
Effect of Latanoprost on CT
Latanoprost had nearly no effect on central CT (P = 0.4718); see Figure 3.
Figure 3.
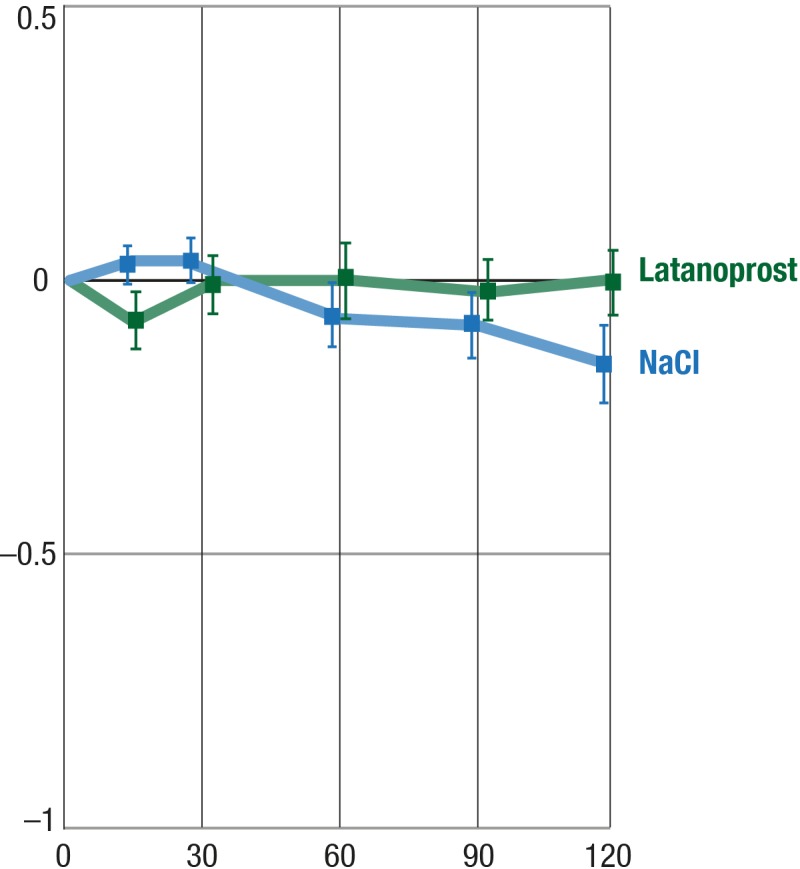
Effect of latanoprost on CT. Y-axis, mean change from baseline (°C); balks, SEM.
Effect of Brimonidine on CT
Brimonidine induced a significant (P < 0.0001) and rather consistent reduction of central CT. During the 2 hours of observation, the effect was maximal at around 1 hour after drug application (shown in Fig. 4).
Figure 4.
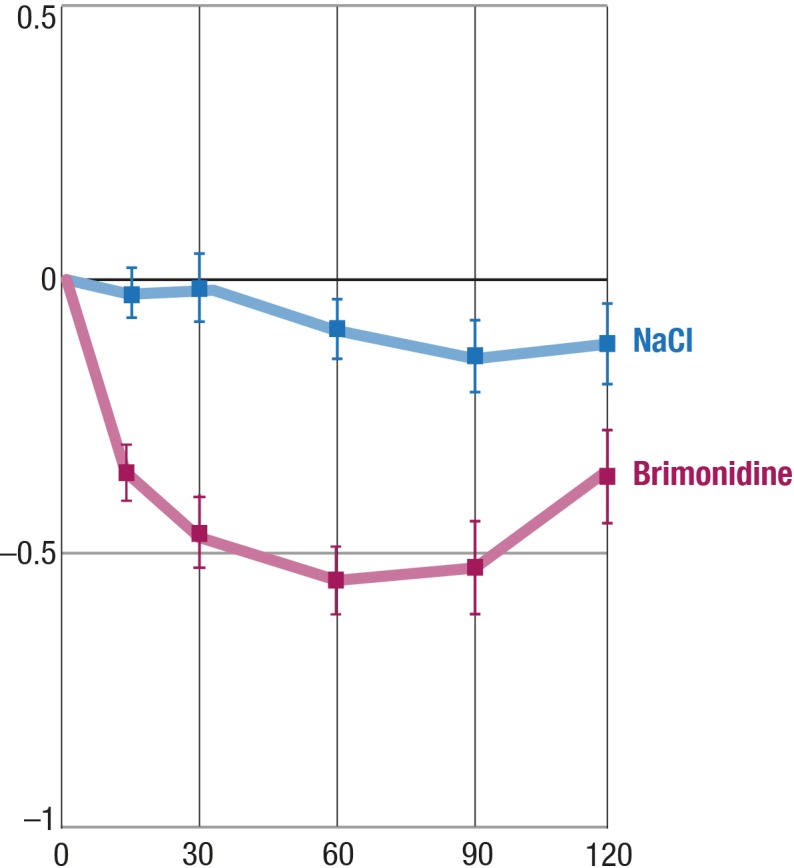
Effect of brimonidine on CT. Y-axis, mean change from baseline (°C); balks, SEM.
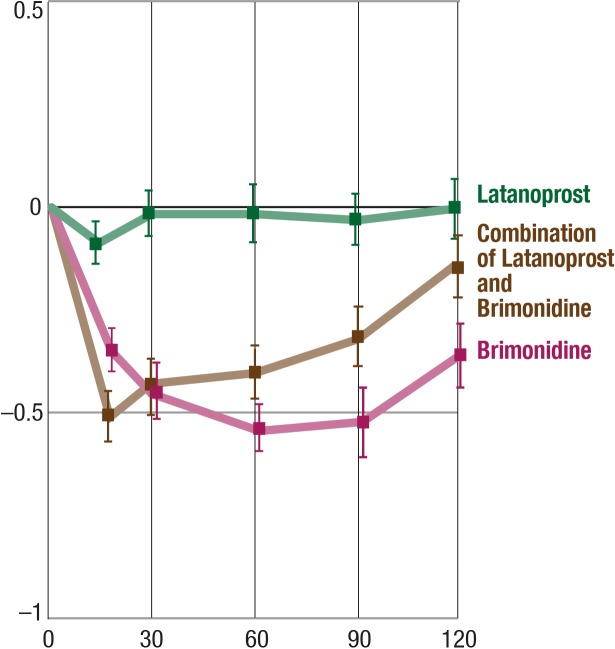
Comparison of the Effect of Latanoprost, Brimonidine, and the Combination of Both on CT
We compared the pooled data of latanoprost, of brimonidine, and of the combination of both. Treatment with latanoprost had no significant influence on central CT, whereas both brimonidine alone and the combination of latanoprost and brimonidine cooled the central cornea by approximately 0.5°C (P < 0.0001) (Fig. 5).
Figure 5.
Effects of latanoprost, brimonidine, and the combination of both on CT. Y-axis, mean change from baseline (°C); balks, SEM.
Effect of the Sequence of the Application of the Combination of Latanoprost and Brimonidine on CT
The effect of the sequence of the drug application is presented in Figure 6. The difference in the time course can be explained by the 45-minute interval between application of the two drugs.
Figure 6.
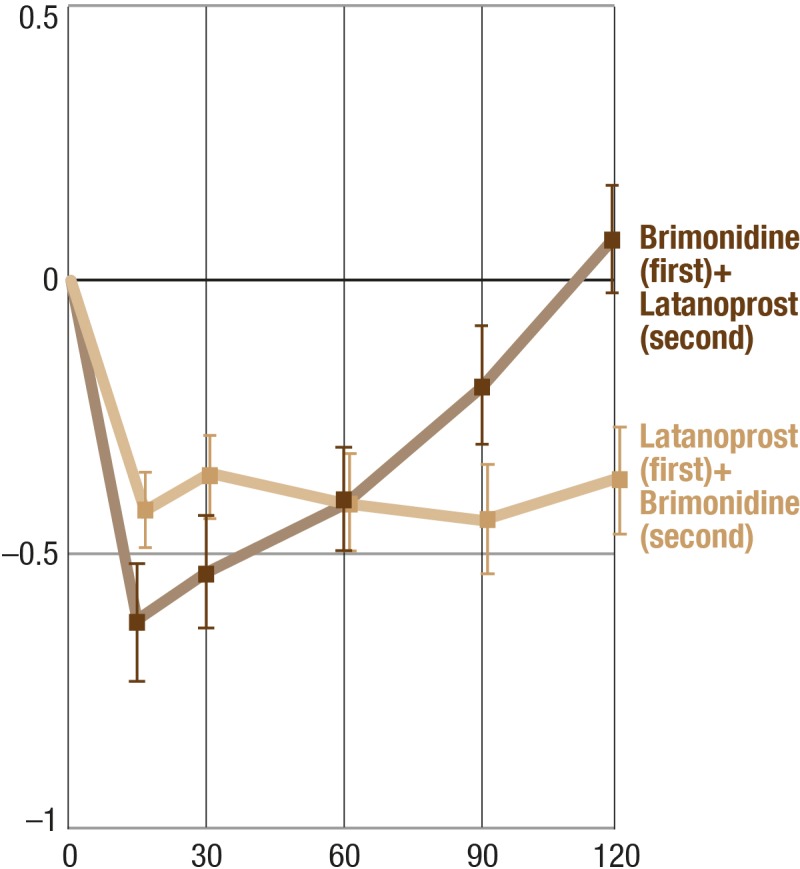
Effect of the sequence of the application of the combination of latanoprost and brimonidine on CT. Y-axis, mean change from baseline (°C); balks, SEM.
Effect of Latanoprost and Brimonidine on IOP
One hundred five minutes after the application of a single drop of latanoprost or brimonidine, the IOP decreased by 1.00 and 1.98 mm Hg, respectively. These effects were significantly different (P = 0.0334).
Relationship Between CT Changes and IOP Changes
After pooling all treatment effects together, changes in central CT correlated positively and significantly (P = 0.0053) with changes in IOP (Fig. 7), but the slope of the regression line is very flat, indicating a very weak influence.
Figure 7.
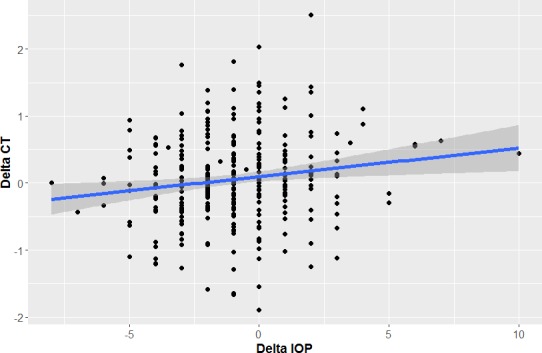
Relationship between CT and IOP changes. The decrease of CT correlates positively with the decrease of IOP. X-axis, Delta IOP (mm Hg); Y-axis, Delta CT (°C).
Effect of Latanoprost and Brimonidine on BP
Forty-five minutes after the application of a single drop of latanoprost or brimonidine, the systolic BP decreased by 4.49 and 4.51 mm Hg, respectively, and the diastolic BP by 0.97 and 2.10 mm Hg, respectively. Neither the effects on systolic BP nor on diastolic BP were significantly different (P = 0.9866 and P = 0.3206, respectively).
Discussion
The present study demonstrates that brimonidine, but not latanoprost, reduced central CT significantly. Accordingly, the combination of brimonidine and latanoprost also reduced central CT. As the cornea has no blood vessels, its central temperature depends mainly on heat conduction and convection by the aqueous humor. Short-term changes of the central CT, under otherwise constant conditions, are therefore mainly influenced by changes of blood flow in the ciliary body and the iris, and to some extent in the back of the eye. The temperature of the peripheral cornea is additionally influenced by the blood flow in the perilimbal vessels. This study does not allow any conclusion on to what extent blood flow in particular parts of the eye is influenced by brimonidine.
The effects of glaucoma drugs such as brimonidine and latanoprost on ocular blood flow (OBF) are only partly known and may depend not only on the species but also on the tissues in the eye, such as the ciliary body, retina, or choroid.
After application of brimonidine, reduced blood flow in the choroid was observed by Weigert et al.3 and in the ciliary body by Reitsamer et al.1 These findings are in accordance with our findings. However, other authors found no significant effect on OBF, particularly at the back of the eye in glaucoma patients.28–32
The published effects of latanoprost on OBF are also heterogeneous. Whereas some authors have found a neutral effect on OBF in healthy subjects33 and in patients with primary open-angle glaucoma,28,34–37 others have described an increase of OBF in heathy volunteers38 and in patients with primary open-angle glaucoma.33,39 In ex vivo studies, latanoprost reduced the endothelin-induced vasoconstriction of isolated rabbit ciliary arteries40 and in in vivo studies with rabbits, latanoprost mitigated the endothelin-induced reduction of optic nerve head blood flow.40 Latanoprost has also been observed to improve the regulation of blood flow in the choroid of healthy subjects.41
As we applied IOP-lowering drugs, the question arises as to whether the induced changes in blood flow may be a secondary to the IOP drop, rather than a direct pharmacological effect. The effect on CT is unlikely due to IOP changes for two reasons: First, the IOP reduction (measured approximately 2 hours after drug application) was stronger after brimonidine than after latanoprost treatment; therefore, we would rather expect a higher CT after brimonidine treatment than after latanoprost treatment. Second, when we pooled all treatment effects together, delta central CT correlated positively with delta IOP. In other words, a reduction of IOP was associated with a very slight cooling of the cornea, whereas we would expect a warming due to an increase in blood flow at a lower IOP.
Theoretically, the effect of brimonidine on CT could also partly be due to BP changes. However, the BP changes were very small and similar in all treatment events, and therefore a relevant effect of BP on CT can be excluded.
Taken together, we can assume that brimonidine has an IOP- and BP-independent direct effect on blood flow.
We measured the temperature of the nonvascularized center of the cornea. It can be assumed that this temperature is mainly influenced by the blood flow in the ciliary body and iris, and to a lesser extent by blood flow in the conjunctiva and at the back of the eye.42
In our trial, brimonidine had a stronger IOP-lowering effect than latanoprost. While this appears to be inconsistent with previous studies,43 it can be explained by the fact that we measured the IOP approximately 2 hours after the drug application. At this time point brimonidine has almost its peak effect,16 while the peak effect of latanoprost is only about 8 to 12 hours after drug application.23
Our study has some limitations: (1) We have compared eye drops, as they are on the market, with 0.9% NaCl. It cannot be completely ruled out that the galenics of the eye drops may also play a role. (2) The ambient temperature can influence the CT. For this reason, the volunteers had to adapt in the examination room for 10 minutes before starting the experiment. However, we cannot rule out the possibility that this adaptation time was too short.
Overall, we provided evidence that brimonidine, but not latanoprost, reduced central CT. This cooling effect is most probably due to a decrease in blood flow, particularly in the ciliary body.
Acknowledgments
We would like to acknowledge the help of Clinical Trial Unit at Basel, Switzerland.
Disclosure: K. Konieczka, None; S. Koch, None; D. Hauenstein, None; T.N. Chackathayil, None; T. Binggeli, None; A. Schoetzau, None; and J. Flammer, None
References
- 1.Reitsamer HA, Posey M, Kiel JW. Effects of a topical alpha2 adrenergic agonist on ciliary blood flow and aqueous production in rabbits. Exp Eye Res. 2006;82:405–415. doi: 10.1016/j.exer.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Fuchsjager-Mayrl G, Wally B, Rainer G, et al. Effect of dorzolamide and timolol on ocular blood flow in patients with primary open angle glaucoma and ocular hypertension. Br J Ophthalmol. 2005;89:1293–1297. doi: 10.1136/bjo.2005.067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigert G, Resch H, Garhofer G, Fuchsjager-Mayrl G, Schmetterer L. Effects of topical clonidine versus brimonidine on choroidal blood flow and intraocular pressure during squatting. Invest Ophthalmol Vis Sci. 2007;48:4220–4225. doi: 10.1167/iovs.07-0178. [DOI] [PubMed] [Google Scholar]
- 4.Kiel JW, Reitsamer HA. Relationship between ciliary blood flow and aqueous production: does it play a role in glaucoma therapy? J Glaucoma. 2006;15:172–181. doi: 10.1097/00061198-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Matteoli S, Favuzza E, Mazzantini L, et al. Ocular surface temperature in patients with evaporative and aqueous-deficient dry eyes: a thermographic approach. Physiol Meas. 2017;38:1503–1512. doi: 10.1088/1361-6579/aa78bd. [DOI] [PubMed] [Google Scholar]
- 6.Tan LL, Sanjay S, Morgan PB. Screening for dry eye disease using infrared ocular thermography. Cont Lens Anterior Eye. 2016;39:442–449. doi: 10.1016/j.clae.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Su TY, Ho WT, Chiang SC, Lu CY, Chiang HK, Chang SW. Infrared thermography in the evaluation of meibomian gland dysfunction. J Formos Med Assoc. 2017;116:554–559. doi: 10.1016/j.jfma.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Luong M, Jomir L, Labauge P, Dandurand M, Meunier L, Stoebner PE. Ross syndrome with sweating anomaly associated with Sjogren syndrome: an infrared thermo-graphic case study. Acta Derm Venereol. 2011;91:80–81. doi: 10.2340/00015555-0948. [DOI] [PubMed] [Google Scholar]
- 9.Vannetti F, Matteoli S, Finocchio L, et al. Relationship between ocular surface temperature and peripheral vasoconstriction in healthy subjects: a thermographic study. Proc Inst Mech Eng H. 2014;228:297–302. doi: 10.1177/0954411914523755. [DOI] [PubMed] [Google Scholar]
- 10.Belkin A, Abulafia A, Michaeli A, Ofir S, Assia EI. Wound temperature profiles of coaxial mini-incision versus sleeveless microincision phacoemulsification. Clin Exp Ophthalmol. 2017;45:247–253. doi: 10.1111/ceo.12851. [DOI] [PubMed] [Google Scholar]
- 11.Giannaccare G, Fresina M, Agnifili L, Versura P. Ocular-surface temperature modification by cataract surgery. J Cataract Refract Surg. 2016;42:983–989. doi: 10.1016/j.jcrs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Mencucci R, Mazzotta C, Corvi A, Terracciano L, Rechichi M, Matteoli S. In vivo thermographic analysis of the corneal surface in keratoconic patients undergoing riboflavin-UV-A accelerated cross-linking. Cornea. 2015;34:323–327. doi: 10.1097/ICO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 13.Sniegowski MC, Erlanger M, Olson J. Thermal imaging of corneal transplant rejection. Int Ophthalmol. 2018;38:2335–2339. doi: 10.1007/s10792-017-0731-z. [DOI] [PubMed] [Google Scholar]
- 14.Ooi EH, Ng EY, Purslow C, Acharya R. Variations in the corneal surface temperature with contact lens wear. Proc Inst Mech Eng H. 2007;221:337–349. doi: 10.1243/09544119JEIM185. [DOI] [PubMed] [Google Scholar]
- 15.Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996;41(Suppl 1):S9–S18. doi: 10.1016/s0039-6257(96)82027-3. [DOI] [PubMed] [Google Scholar]
- 16.Walters TR. Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: a review of safety, efficacy, dose response, and dosing studies. Surv Ophthalmol. 1996;41(Suppl 1):S19–S26. doi: 10.1016/s0039-6257(96)82028-5. [DOI] [PubMed] [Google Scholar]
- 17.Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006;2:337–346. doi: 10.2147/tcrm.2006.2.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur S, Cantor LB. Update on the role of alpha-agonists in glaucoma management. Exp Eye Res. 2011;93:271–283. doi: 10.1016/j.exer.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993;100:1297–1304. doi: 10.1016/s0161-6420(93)31484-3. [DOI] [PubMed] [Google Scholar]
- 20.Poyer JF, Millar C, Kaufman PL. Prostaglandin F2 alpha effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci. 1995;36:2461–2465. [PubMed] [Google Scholar]
- 21.Lutjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey JD, Kashiwagi K, Boyle D, Kashiwagi F, Firestein GS, Weinreb RN. Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr Eye Res. 1996;15:869–875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- 23.Sanford M. Preservative-free latanoprost eye drops in patients with primary open-angle glaucoma/ocular hypertension. Clin Drug Invest. 2014;34:521–528. doi: 10.1007/s40261-014-0203-4. [DOI] [PubMed] [Google Scholar]
- 24.Freeman RD, Fatt I. Environmental influences on ocular temperature. Invest Ophthalmol. 1973;12:596–602. [PubMed] [Google Scholar]
- 25.Kocak I, Orgul S, Flammer J. Variability in the measurement of corneal temperature using a noncontact infrared thermometer. Ophthalmologica. 1999;213:345–349. doi: 10.1159/000027452. [DOI] [PubMed] [Google Scholar]
- 26.Konieczka K, Schoetzau A, Koch S, Hauenstein D, Flammer J. Cornea thermography: optimal evaluation of the outcome and the resulting reproducibility. Transl Vis Sci Technol. 2018;7:14. doi: 10.1167/tvst.7.3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2015. R: A language and environment for statistical computing. Available at: http://wwwR-projectorg/ [Google Scholar]
- 28.Hommer A, Sperl P, Resch H, et al. A double-masked randomized crossover study comparing the effect of latanoprost/timolol and brimonidine/timolol fixed combination on intraocular pressure and ocular blood flow in patients with primary open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2012;28:569–575. doi: 10.1089/jop.2011.0165. [DOI] [PubMed] [Google Scholar]
- 29.Siesky B, Harris A, Ehrlich R, et al. Short-term effects of brimonidine/timolol and dorzolamide/timolol on ocular perfusion pressure and blood flow in glaucoma. Adv Ther. 2012;29:53–63. doi: 10.1007/s12325-011-0092-3. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt KG, Klingmuller V, Gouveia SM, Osborne NN, Pillunat LE. Short posterior ciliary artery, central retinal artery, and choroidal hemodynamics in brimonidine-treated primary open-angle glaucoma patients. Am J Ophthalmol. 2003;136:1038–1048. doi: 10.1016/s0002-9394(03)00631-7. [DOI] [PubMed] [Google Scholar]
- 31.Jonescu-Cuypers CP, Harris A, Ishii Y, et al. Effect of brimonidine tartrate on ocular hemodynamics in healthy volunteers. J Ocul Pharmacol Ther. 2001;17:199–205. doi: 10.1089/108076801750295236. [DOI] [PubMed] [Google Scholar]
- 32.Costagliola C, Parmeggiani F, Ciancaglini M, D'Oronzo E, Mastropasqua L, Sebastiani A. Ocular perfusion pressure and visual field indice modifications induced by alpha-agonist compound (clonidine 0.125%, apraclonidine 1.0% and brimonidine 0.2%) topical administration. An acute study on primary open-angle glaucoma patients. Ophthalmologica. 2003;217:39–44. doi: 10.1159/000068249. [DOI] [PubMed] [Google Scholar]
- 33.Harris A, Garzozi HJ, McCranor L, Rechtman E, Yung CW, Siesky B. The effect of latanoprost on ocular blood flow. Int Ophthalmol. 2009;29:19–26. doi: 10.1007/s10792-008-9190-x. [DOI] [PubMed] [Google Scholar]
- 34.Liu CJ, Ko YC, Cheng CY, Chou JC, Hsu WM, Liu JH. Effect of latanoprost 0.005% and brimonidine tartrate 0.2% on pulsatile ocular blood flow in normal tension glaucoma. Br J Ophthalmol. 2002;86:1236–1239. doi: 10.1136/bjo.86.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Januleviciene I, Harris A, Kagemann L, Siesky B, McCranor L. A comparison of the effects of dorzolamide/timolol fixed combination versus latanoprost on intraocular pressure and pulsatile ocular blood flow in primary open-angle glaucoma patients. Acta Ophthalmol Scand. 2004;82:730–737. doi: 10.1111/j.1600-0420.2004.00358.x. [DOI] [PubMed] [Google Scholar]
- 36.Zeitz O, Matthiessen ET, Reuss J, et al. Effects of glaucoma drugs on ocular hemodynamics in normal tension glaucoma: a randomized trial comparing bimatoprost and latanoprost with dorzolamide [ISRCTN18873428] BMC Ophthalmol. 2005;5:6. doi: 10.1186/1471-2415-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arend O, Harris A, Wolter P, Remky A. Evaluation of retinal haemodynamics and retinal function after application of dorzolamide, timolol and latanoprost in newly diagnosed open-angle glaucoma patients. Acta Ophthalmol Scand. 2003;81:474–479. doi: 10.1034/j.1600-0420.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 38.Geyer O, Man O, Weintraub M, Silver DM. Acute effect of latanoprost on pulsatile ocular blood flow in normal eyes. Am J Ophthalmol. 2001;131:198–202. doi: 10.1016/s0002-9394(00)00797-2. [DOI] [PubMed] [Google Scholar]
- 39.Gherghel D, Hosking SL, Cunliffe IA, Armstrong RA. First-line therapy with latanoprost 0.005% results in improved ocular circulation in newly diagnosed primary open-angle glaucoma patients: a prospective, 6-month, open-label study. Eye. 2008;22:363–369. doi: 10.1038/sj.eye.6702639. [DOI] [PubMed] [Google Scholar]
- 40.Kurashima H, Watabe H, Sato N, Abe S, Ishida N, Yoshitomi T. Effects of prostaglandin F(2alpha) analogues on endothelin-1-induced impairment of rabbit ocular blood flow: comparison among tafluprost, travoprost, and latanoprost. Exp Eye Res. 2010;91:853–859. doi: 10.1016/j.exer.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Boltz A, Schmidl D, Weigert G, et al. Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Invest Ophthalmol Vis Sci. 2011;52:4410–4415. doi: 10.1167/iovs.11-7263. [DOI] [PubMed] [Google Scholar]
- 42.Gugleta K, Orgul S, Flammer J. Is corneal temperature correlated with blood-flow velocity in the ophthalmic artery? Curr Eye Res. 1999;19:496–501. doi: 10.1076/ceyr.19.6.496.5286. [DOI] [PubMed] [Google Scholar]
- 43.Einarson TR, Kulin NA, Tingey D, Iskedjian M. Meta-analysis of the effect of latanoprost and brimonidine on intraocular pressure in the treatment of glaucoma. Clin Ther. 2000;22:1502–1515. doi: 10.1016/s0149-2918(00)83048-9. [DOI] [PubMed] [Google Scholar]