Abstract
Background
The treatment of inflammatory bowel disease (IBD) is still not satisfactory and novel technologies are clinically needed. This study aimed to examine the effect of mesenchymal stromal cells (MSCs) coated with the anti-vascular cell adhesion molecule 1 (VCAM 1) antibody on experimental colitis.
Material/Methods
The antibody was coated onto the MSCs isolated from male BALB/C mice to generate anti-VCAM 1 antibody-coated MSC (V-MSC). The Transwell assay was used to detect migration rate. 2,4,6-trinitrobenzenesulfonic acid (TNBS) was used to generate experimental colitis. MSCs were injected intravenously into experimental models. Weight changes, disease activity index, and histological changes were evaluated. The SRY gene were used for cell tracking. Expression of Ki67 and claudin 1 was used to measure local repair using immunohistochemistry. T helper (Th)1, Th2, Th17, and T regulatory cells were counted.
Results
V-MSCs were successfully generated through coating MSCs with VCAM1 antibody. Analysis showed that the V-MSCs had similar surface types and differentiation as uncoated MSCs. Transwell assays showed that V-MSCs had higher migration rate than MSCs. After injection of V-MSCs, the expression of the SRY gene was enhanced in diseased colon and all indices (including weight changes, DAI score, histological changes, and the expressions of Ki67 and claudin 1) recovered rapidly. The ratio of proinflammatory Th1 and Th17 cells decreased, but the ratio of anti-inflammatory Th2 and Treg cells increased after the treatment.
Conclusions
V-MSCs enhance homing and modulating immune balance in the experimental colitis, suggesting that they are potentially useful for treating inflammatory bowel disease or other immune diseases.
MeSH Keywords: Cell Adhesion Molecules, Immunomodulation, Inflammatory Bowel Diseases, Mesenchymal Stromal Cells
Background
Inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis, have been intensively studied, and the pathogenesis is mainly related to intestinal immune reactions [1]. The treatment outcome of inflammatory bowel disease (IBD) is still not satisfactory, and novel technologies such as stem cell and gene therapy are not clinically available [2]. However, due to the immunomodulatory characteristics of stem cells, cell therapy could play a promising role in the near future [2].
2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis is an ulcerative-colitis-like disease that causes tissue destruction, inflammation, and T helper (Th)1/Th2 and Th17/T regulatory (Treg) cell imbalance [3]. Mesenchymal stromal cells (MSCs) are a type of stem cell that might be explored for therapeutic effect [2].
Intravenous transplantation of stem cells is often used in cell therapy, but the delivery of MSC to injured sites is still very low [4]. Therefore, efforts are being made to improve the delivery efficiency of MSCs. The methods attempted include cell-surface modification and use of nanoparticles and biomaterials [5]. Therefore, vascular cell adhesion molecule 1 (VCAM1), chemokine CXC receptor (CXCR) 4, E-selectin ligands, and mucosal addressin cell adhesion molecule 1 have been tested to coat the surface of MSCs to help target them to lesion sites [6]. Antibody coating technology was established several years ago in vitro [7]. VCAM1 was demonstrated to specifically target the very late antigen (VLA)-4. At injured local sites of IBD, more VLA-4 is released, leading to the attraction of more lymphocytes for immune reaction [8]. Therefore, it is possible to speculate that if MSCs were coated, they would be more efficiently targeted to diseased sites and may promote the healing of injured mucosa, improving the therapeutic outcomes of IBD.
Immune and inflammatory systems are regulated by CD4+ T cells, Treg cells, and several cytokines. CD4+ T cells include T helper (Th)1, Th2, and Th17 cells [9]. When IBD occurs, the immune balance is broken and Th cells are activated by intestinal secretions, gut bacteria, and immune-related proteins such as IL-1, IL-6, TNF-, IL-10, TGF-β, cathelicidins, lipocalins, defensins, and C-type lectins [1]. Th1 and Th17 cells cause mucosal inflammation, while Th2 and Treg cells inhibit excessive immune reactions by regulating the Th1/Th2 and Th17/Treg ratios [9,10], although Th2 cells can also be proinflammatory [11].
In this study, we applied a new technology to coat VCAM1 antibody (VACM 1-ab) onto MSCs to improve targeting efficiency in vivo. We compared the features of coated and uncoated MSCs and used them to treat acute experimental colitis mouse models induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) and showed that MSCs coated with VCAM1 antibody enhance homing and modulating immune balance in experimental colitis, suggesting that they are potentially useful for treating inflammatory bowel disease or other immune diseases.
Material and Methods
Animal care and use
BALB/c mice, aged 2–3 weeks, special pathogen-free, were obtained from Vital River Laboratory Animal Technology Co., Beijing, China. The animals were housed at controlled temperature with a 12-h light/dark cycle, and fed with standard mouse chow and tap water. They were kept for at least 1 week in our animal facilities before the experiments. Appropriate measures were taken to minimize their pain or discomfort. All animal experimental protocols in this study were approved by the Institutional Animal Care and Use Committee of the Chinese PLA General Hospital, Beijing, China.
Cell culture
Conditionally immortalized mouse MSCs (BMSC-3 cell line from male BABL/C mice) [10] were cultured in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37°C. The culture medium was refreshed every 3 days. MSCs were subcultured when they reached ~90% confluency. After 4 passages, cells were used for experiments.
VCAM-1 antibody coating
The coating was carried out essentially as described previously [8]. Briefly, recombinant protein G (Sigma, St. Louis, MO, USA) was dissolved in PBS to generate a protein G solution at 1 mg/mL. N-Hydroxysuccinimide ester of palmitic acid (Sigma) was dissolved in absolute ethanol at 10 mg/mL and slowly heated to 50°C. The solution (10 μL) was derivatized with 1 mL protein G solution at 4°C for 1.5 h. The resultant solution, palmitated protein G (PPG), was purified using Sephadex G-25 (10 mg/mL; Pharmacia, Piscataway, NJ, USA) and filter-sterilized PPG was stored at 4°C until use.
MSCs incubated with 0.25% trypsin + 0.02% EDTA for 5 min at 37°C in 5% CO2 were collected, washed with α-MEM, and adjusted to 3×106 cells/mL. After resuspension, 1×106 cells were incubated with 3 μL CM-Dil (Invitrogen, Carlsbad, CA, USA) at 37°C in 5% CO2 for 5 min and 4°C for 15 min. The cells were then washed twice and suspended in α-MEM. CM-Dil staining was observed using fluorescent microscopy. MSCs were incubated with PPG (100 μg/mL) at 37°C on a shaker operated at 100 rpm for 10 min. The cells were then washed twice and suspended in α-MEM. We added 500 μL
FITC-conjugated goat anti-mouse IgG (100μg/mL, Zhongshan Biotechnology, Beijing, China) to the suspension at 37°C for 1 h and the cells were again washed twice and suspended in α-MEM. We transferred 500 μL cells to the wells of 24-well plates (Corning International, Tokyo, Japan) for fluorescence detection and used them as the PPG-MSC group.
For VACM 1-ab coating, MSCs coated with PPG were incubated with rabbit anti-mouse VCAM 1 antibody (1: 200; Abcam, Cambridge, UK) on ice for 1–2 h. These cells were termed V-MSCs and were incubated with FITC-conjugated goat anti-rabbit IgG and measured for fluorescence as described above. For fluorescence assays, 3 fields were randomly selected from each well for quantification.
Flow cytometry
Flow cytometry was used to examine the coating efficiency VCAM 1 antibody. We stained 106 V-MSCs and MSCs with 5 μL ALEXA-labeled anti-mouse CD106 (Becton Dickinson, San Jose, CA, USA) at room temperature for 20 min. Unstained cells were used as a control. The cells were then washed twice, resuspended in 500 μL PBS, and examined using a BD FACS Calibur cytometer (Becton Dickinson).
To examine the differentiation and phenotypes of MSCs, the MSCs were seeded in the wells of 24-well plates at a concentration of 5×104 cells/well. Differentiation of MSCs into osteogenic and adipogenic lineages was assayed as reported previously [10]. Briefly, MSCs were harvested, digested with trypsin-EDTA, and stained with ALEXA-labeled anti-mouse CD105, phycoerythrin (PE)-conjugated anti-mouse CD90, fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45, and PE Cy-conjugated anti-mouse CD11b (Becton Dickinson) and analyzed on a BD FACS Calibur cytometer.
Cell viability and proliferation test
Viability of cells was tested using trypan blue exclusion. The numbers of live and dead cells were recorded after the staining. MTT (Sigma, USA) assay kits (Abcam, USA) were utilized to evaluate the proliferation of MSCs according to the manufacturer’s protocols. Briefly, MSCs were seeded onto 96-well plates (2×104 cells per well) and incubated in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37°C for up to 12 days. The MTT solution (10 μL, 5 mg/mL) was added to the wells. After removing the supernatant, dimethyl sulfoxide (100 μL) (Sigma, USA) was added to the wells, and the absorbance at 590 nm was measured with a plate reader.
RT-PCR
Total RNA was extracted MSC using the Total RNA Kit (SinoGene Scientific, Beijing, China). The sequences of primers for PCR were as follows: VCAM 1 F, 5′-ACC CAA ACA GAG GCA GAG, VCAM 1 R, 5′ GAG CAG GTC AGG TTC ACA. Sry F, 5′-TCG GAG GGC TAA AGT GTC, Sry R. TCT TGC CTG TAT GTG ATGG. Actin was used in internal control (F: 5′-CGT TGA CAT CCG TAA AGA CC, RL 5′-CTA GGA GCC AGA GCA GTA ATC). The PCR amplification conditions were as follows: pre-denature at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 20 s, and annealing at 60°C for 30 s, with a final extension at 60°C for 30 s.
Samples were run in triplicate and the mean value was calculated for each case. The data were managed based on a previously described protocol [12].
Transwell migration assay
Transwell chambers with 8-μm pore filters (Corning International, Tokyo, Japan) were used to determine the migration ability of MSCs. The upper chambers were plated with 2×104 cells in 200 μL α-MEM containing 0.1% bovine serum albumin (BSA), and the lower chambers with 600 μL VLA-4 (1 μ/μL, Biofine, Beijing, China) in 5% CO2 at 37°C for 10 h. The migrated MSCs on the lower side of the filter were stained by crystal violet and then counted under a microscope.
Establishment of TNBS colitis model and MSC injection
Experimental colitis was induced using 2.0 mg TNBS/50% ethanol enema (Sigma) as previously described [10]. Female BALB/c mice were randomly divided into 4 groups (n=12 in each group). Mice in the control group (Ctr) were all males and were raised normally without any treatment. Mice in the TNBS model group (T) were all females and were given 2.0 mg TNBS by enema. Mice in the MSC and V-MSC group (MT and VMT)
were infused with MSC and V-MSC (106 cells/mouse in PBS) via the tail veins 24 h after the establishment of the colitis models. PBS containing no MSC was used as negative control. Body weight, DAI, and hematoxylin and eosin (HE)-stained slices of colon tissue were used to assess the severity of inflammation as described previously [10]. On days 3, 5, 9, and 13 after MSC injection, the animals were sacrificed and serum, spleen, and colon were collected for further analysis.
Tracking of MSC
Red fluorescence and sex-determining region Y (SRY) protein were used to track the MSCs after injection. The transverse sections of colonic specimens were examined under fluorescent microscope (Olympus, Tokyo, Japan).
Immunohistochemistry
Colon sections were incubated with rabbit anti-mouse antibody against Ki67 (ab66155, 1: 500; Abcam), or claudin-1 (ab15098, 1: 500; Abcam) at 4°C overnight. The sections were then incubated with goat anti-rabbit secondary antibody (1: 1000, Beijing Zhongshan Biotechnology, Beijing, China) at 37°C for 30 min and were finally stained with HE and examined for staining density.
Immunocyte detection
The spleens were isolated, ground, and filtered through a 200-μm mesh screen to obtain cell suspensions. The suspensions were centrifuged at 1000 rpm for 5 min and the pellets were resuspended in 500 μL RPMI 1640 medium (Gibco-Invitrogen, Carlsbad, CA, USA) and 2 μL stimulant (Becton Dickinson) was added. 1×106 cells were resuspended in 100 μl PBS and stained with 5 μl CD4-FITC and CD25-APC in the dark at room temperature for 15 min. We added 1 μl FOXP3 Fixation/Permeabillzation Buffer (1×) for 30 min, followed by addition of 1 μl Permeabillzation Buffer (1×) for 10 min. We added 5 μl of IFN-γ-PE, IL-4-APC, and IL-17-PE in the dark at room temperature for 15 min. Then, the suspensions were washed in PBS and analyzed by flow cytometry.
Statistical analysis
Data are presented as means ±SD. Statistical comparisons were performed using one-way ANOVA by SPSS version 17.0 software. When P was ≤0.05, the results were considered statistically significant.
Results
Morphology of MSCs and VCAM1-MSCs
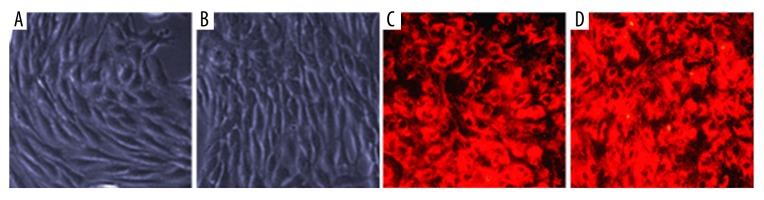
MSCs were long, spindle-shaped, and arranged as bundles or radially in culture (Figure 1A). The coating of VCAM-1 antibody did not change the morphology of MSCs (Figure 1B). CM-Dil is a cytochemical stain for tracing in vivo. When observed under green fluorescence, the cell membranes of MSCs generated red fluorescence (Figure 1C, 1D).
Figure 1.
Morphology of mesenchymal stem cells before and after coating with VCAM 1 antibody. (A) Arranged in bundles; (B) Coating with VCAM 1 antibody; (C) Stained with CM-Dil; (D) Stained with CM-Dil after coating with VCAM 1 antibody (400×).
VCAM 1 antibody coating upregulated the expression of VCAM-1 in MSCs
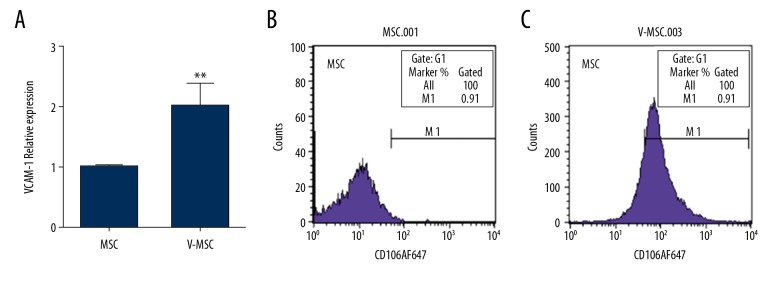
Real-time PCR and flow cytometry were performed to measure the expression of VCAM-1 in MSCs. The mRNA and protein levels were low in uncoated MSCs (Figure 2A, 2B), but were significantly increased after VCAM-1 antibody coating (Figure 2A, 2C).
Figure 2.
(A–C) Expression of VCAM1 at mRNA and protein levels before and after coating with VCAM 1 antibody. (A) Relative mRNA level; (B) Relative protein levels. * denotes P<0.05 vs. uncoated MSC.
VCAM1 antibody coating did not change the characteristics of MSCs
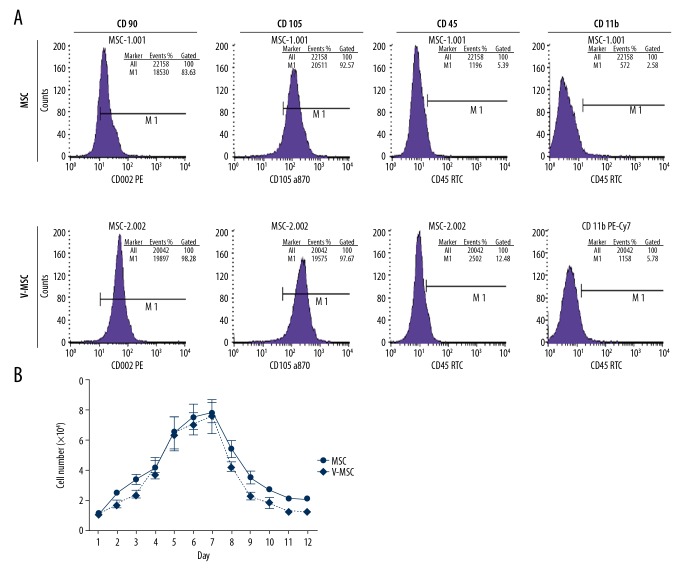
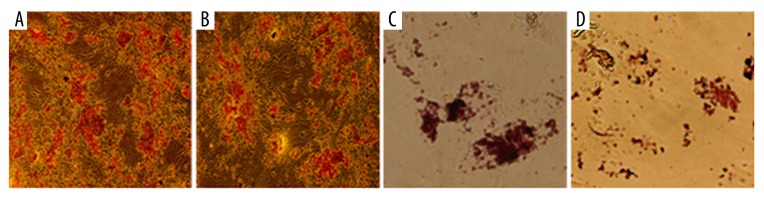
Flow cytometry revealed that after VCAM1 antibody coating, the expression profiles of MSCs were similar to these before coating. The positive rates for CD90, CD105, CD45, and CD11b were 99.28%, 97.67%, 12.48, and 5.78% after the coating and 83.63%, 92.57%, 5.39%, and 2.58% before the coating (Figure 3A). The proliferation ability of VCAM-1 antibody-coated MSCs were similar to that of uncoated MSCs over a 12-day culture-period. (Figure 3B). MSCs differentiate into osteoblasts and adipocytes under different conditions. After culturing in osteogenic medium for 3 weeks, mineralized nodules could be observed in both groups of cells (Figure 4A, 4B). After induction with adipogenic medium for 8 days, large and red particles were also observed in both groups (Figure 4C, 4D).
Figure 3.
Flow cytometry assays of MSC phenotypes and proliferation of MSCs before and after coating with VCAM 1 antibody. (A) Flow cytometry results; (B) Growth curves.
Figure 4.
Osteoblastic and adipocyte differentiations of MSCs before and after coating with VCAM 1 antibody. (A) Osteoblast before coating; (B) Osteoblast after coating; (C) Adipocyte before coating; (D) Adipocyte after coating (400×).
VCAM1 antibody coating did not influence cell viability
Trypan blue assay showed that the viability of uncoated and coated MSCs was >90%, indicting the VCAM1 antibody coating did not reduce cell viability.
VCAM-1 antibody coating increased the migration ability of MSCs to VLA-4
Transwell assay showed MSCs in both groups migrated to the other side of the membrane (Figure 5A, 5B). The number of migrated MSCs was higher after VCAM-1 antibody coating than before the coating (Figure 5C).
Figure 5.
The migration of MSCs towards VLA-4 in Transwell assay before and after coating with VCAM 1 antibody. (A, B) Migrated MSCs towards VLA-4 without and with VCAM 1 antibody coating (400×); (C) The numbers of migrated MSCs. * Denotes P<0.05 vs. uncoated MSC.
VCAM-1 antibody coating promoted homing of MSCs to injured colon
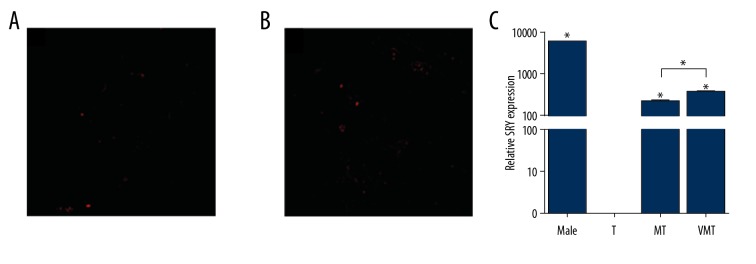
Cell tracking results showed that the injected MSCs were localized in the injured colon. As a consequence, red fluorescence was observed in the lamina propria at 48 h after the injections (Figure 6A, 6B). More red-colored MSCs were observed after injection with V-MSCs (Figure 6A, 6B), but no red fluorescence was seen in the T group. The SRY gene was detected in the Ctr, MT, and VMT groups (Figure 6C), but not in the T group. Expression of the SRY gene in the VMT group was significantly higher than in the MT group (Figure 6C).
Figure 6.
Red fluorescence and the SRY gene expression in colon lamina propria after injection with uncoated and VCAM 1 antibody-coated MSCs. (A, B) Red fluorescence after injection with uncoated and VCAM 1 antibody-coated MSCs (100×); (C) Relative mRNA level. T, TNBS model group. MT and VMT, MSC and V-MSC treated groups, respectively. * P<0.05 vs. uncoated MSC.
VCAM-1 antibody coating attenuated TNBS-induced severe colitis
Mice in the T group began to lose weight and have diarrhea, bloody stools, and abdominal pain after exposure to TNBS. After injection with MSCs and V-MSCs, the symptoms were relieved, especially in the VMT group (Figure 7A). DAI scores increased after TNBS exposure. In comparison, after injection with MSCs and
Figure 7.
Body weight (A), DAI score (B), histopathological change (C–F) in experimental colitis models following injection of uncoated and VCAM 1 antibody-coated MSCs. Ctr – normal mucosa; T – diffuse inflammatory response with a number of infiltrated inflammatory cells infiltration; MT – some inflammatory response; VMT – few inflammatory response. a P<0.05 versus control; b P<0.05 vs. T group; c P<0.05 vs. MT group (200×).
V-MSCs, DAI scores were significantly decreased (p<0.05), especially in the VMT group 3 days after the treatment (Figure 7B). Histological damage was observed in the HE-stained colon tissues in the T group (p<0.05) (Figure 7D) compared with the Ctr group (p<0.05) (Figure 7C). In the MT group, there was severe crypt damage and many infiltrated inflammatory cells, composed mainly of neutrophils and macrophages. After MSC and V-MSC injections, the injured colon mucosa was largely restored, resulting in fewer infiltrated inflammatory cells and mild crypt damage (p<0.05), especially in the VMT group (p<0.05) (Figure 7E, 7F).
VCAM-1 antibody coating promoted mucosal healing
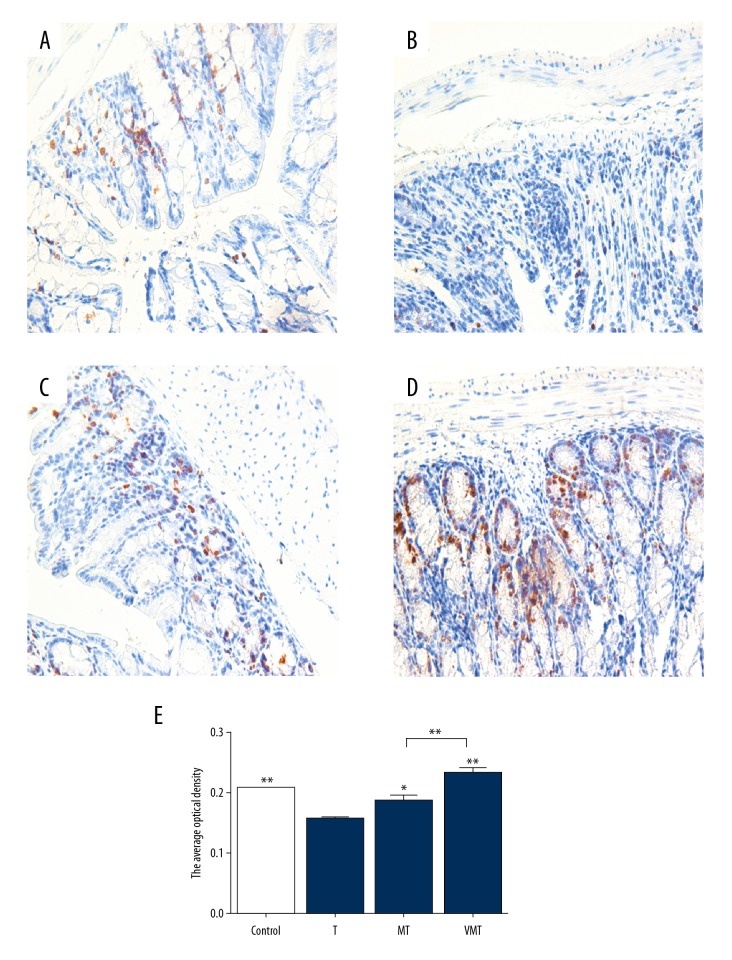
Expression of Ki67 is a marker of cell proliferation. In the Control group, there was low level of Ki67 expression (Figure 8A). In the T group, Ki67 expression decreased in the injured colon (Figure 8B). After MSC and V-MSC injection, Ki67 expression was significantly increased, especially in the VMT group (Figure 8C–8E).
Figure 8.
Immunohistochemistry results of Ki67 in colon tissue showing the expression of Ki67 (200×). Left panel, immunohistochemistry results. (A) Control group; (B) T group; (C) MT group; (D) VMT group; (E) Optical density. *, ** P<0.05 or <0.01 vs. uncoated T group.
In normal mice, the intestinal epithelial barrier was intact and updated regularly to maintain its balance. In the Control group, there was a low level of claudin 1 expression (Figure 9A). In the T group, the expression of claudin 1 decreased in the injured mucosal epithelium (Figure 9B). After MSC and V-MSC injection, the expression of claudin-1 was significantly upregulated, especially in the VMT group (Figure 9C–9E).
Figure 9.
Immunohistochemistry results of claudin 1 in colon tissue showing the expression of claudin 1 (200×). (A) Control group; (B) T group; (C) MT group; (D) VMT group. Left panel, immunohistochemistry results. (A) Control group; (B) T group; (C) MT group; (D) VMT group; (E) Optical density. *, ** P<0.05 or <0.01 vs. uncoated T group.
VCAM-1 antibody coating affected immune responses of Th1/Th2/Th17/Treg cells in experimental colitis
The differentiation of Th1, Th2, Th17, and Treg lymphocytes in the spleen was analyzed using flow cytometry. The proportion of Th1 cells was 10.77% in the Control group, 13.41% in the T group, 12.58% in the MT group, and 4.35% in the VMT group. The proportion of Th2 cells was 2.37% in the Control group, 0.8% in the T group, 3.63% in the MT group, and 8.96% in the VMT group. The proportion of Th17 cells was 0.05% in the Control group, 0.16% in the T group, 0.09% in the MT group and 0.07% in the VMT group. The proportion of Treg cells was 2.83% in the Control group, 1.64% in the T group, 3.54% in the MT group, and 6.87% in the VMT group. TNBS exposure caused Th1- and Th17-related inflammatory reactions. After MSC injections, the proportions of Th1 and Th17 cells decreased, and Th2- and Treg-related anti-inflammatory reactions become dominant. In the VMT group, V-MSCs showed stronger anti-inflammatory activity than MSCs did in the MT group (Figure 10).
Figure 10.
Flow cytometry determination of Th1/Th2/Th17/Treg cell ratios in the Ctr, T, MT, and VMT groups, showing the changes of proinflammatory and anti-inflammatory cells after MSC treatments.
Discussion
Recently, stem cells are delivered intravenously into the lungs or heart as a therapeutic approach [6,13]. For this purpose, it is important to deliver the cells to the diseased sites for better treatment, which is still challenging. In our earlier study, we demonstrated that few MSCs are able to home to the injured colon, and as a consequence, therapeutic effect is limited [10]. MSCs have the potential for immunosuppression, which can regulate immune imbalance during the curative process [13]. Earlier study showed that the VCAM-1/VLA-4 interaction facilitates the migration of MSCs to the target sites by promoting the adhesion of hematopoietic stem cells to MSCs [14]. This also enhances the migration of stem cells to injured cardiac tissue [15]. Therefore, we hypothesized that coating MSCs with VCAM1 antibody would enhance the delivery of cells to inflamed colon and improve IBD treatment.
In our study, we use a two-step cell-surface coating technology. Firstly, PPG, a protein with affinity for MSCs [8], was coated on MSCs. Secondly, VCAM 1 antibody was attached to PPG-MSCs, resulting in VCAM1-ab coated MSCs. We found that V-MSCs upregulated the expression of VCAM-1 expression. However, the underlying mechanism of this upregulation is not clear.
We then examined if the antibody coating changes the features of MSCs, and found that the biological features of MSCs remained unchanged after the coating. Transwell assays demonstrated that more MSCs migrated to the other side of the Transwell membrane in the V-MSC than MSC group, indicating that VCAM1 antibody promotes the migration of MSCs. Flow cytometry showed that the coating did not influence MSC characteristics of regeneration, differentiation, and immunomodulation, and enhanced targeting efficiency to specific local sites.
To investigate their therapeutic effect, we injected V-MSCs into a mouse model of experimental colitis. Compared with uncoated MSCs, V-MSCs promoted the homing of MSCs to the injured colon, as demonstrated by more red fluorescence and higher expression of SRY gene in the diseased colon. V-MSCs also generated better therapeutic effect, as shown by body weight recovery, DAI score, and histology.
V-MSC accelerated the healing of intestinal cells and restored the intestinal epithelial barrier, as indicated by the increased expression of Ki67 and claudin 1. Although V-MSCs and MSCs could be induced to differentiate into osteoblasts and adipocytes, the cell types that play biological role in vivo are still undifferentiated MSCs. Furthermore, injection of V-MSCs inhibited the production of proinflammatory Th1 and Th17 cells, and activated the production of anti-inflammatory Th2 and Treg cells. Possible mechanisms underlying the improved therapeutic effects could result from increased homing of MSCs to the injured tissue. MSCs, when cultured in vitro, had low or no expression of VCAM1 on their surface. However, more VCAM1 ligands are released in inflamed tissue, resulting in more migration of V-MSCs to diseased sites through antibody/ligand binding. It is expected that MSCs at the diseased tissue would generate better therapeutic response. MSCs could regenerate and differentiate in the injured site to facilitate the restoration of functions of the intestinal cells and barrier. The intestinal epithelial barrier has intestinal epithelial cells, endocrine cells, and tight junctions between cells. Occludin, claudins, and junctional adhesion molecules are transmembrane tight junction proteins [16, 17]. Increased expression claudin may therefore enhance healing of wounds. MSCs possess strong immune-regulatory activity in vitro and in vivo. TNBS-induced experimental colitis causes imbalance of Th1/Th2 and Th17/Treg cell ratios [10]. When MSCs are localized to the inflamed tissue, they release many chemotactic factors and upregulate the expression of cell adhesion molecules, such as VCAM1. This results in more CD4+ T cells and Treg cells accumulating in the diseased sites, which strengthens the ability of MSCs to regulate the imbalance in immune cells [17,18]. Therefore, we speculate that V-MSC plays a greater role in reducing the number of proinflammatory Th1 and Th17 cells and increasing the number of anti-inflammatory Th2 and Treg cells in vivo.
Conclusions
We generated targeting MSCs by coating their surface with VCAM1 antibody. This approach could be used to coat cells with other antibodies. We showed that VCAM1 antibody-coated MSCs enhance homing of MSCs and suppress inflammation in acute experimental colitis. This offers a possible future therapy for IBD and other immune or inflammatory diseases.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (grant no. 81370503) and the Natural Science Foundation of Beijing Municipality, (grant no. 7142148)
Declarations
Ethics approval and consent to participate: All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the Chinese PLA General Hospital, Beijing, China. All animals received humane care in compliance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996).
Conflict of interests
None.
References
- 1.Ohkusa T, Koido S. Intestinal microbiota and ulcerative colitis. J Infect Chemother. 2015;21:761–68. doi: 10.1016/j.jiac.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Dave M, Mehta K, Luther J, et al. Mesenchymal stem cell therapy for inflammatory bowel disease: A systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2696–707. doi: 10.1097/MIB.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321–29. doi: 10.1097/MIB.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 4.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013 doi: 10.1155/2013/732742. 732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xinaris C, Morigi M, Benedetti V, et al. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22:423–36. doi: 10.3727/096368912X653246. [DOI] [PubMed] [Google Scholar]
- 6.Ansboro S, Greiser U, Barry F, Murphy M. Strategies for improved targeting of therapeutic cells: implications for tissue repair. Eur Cell Mater. 2012;23:310–18. doi: 10.22203/ecm.v023a24. discussion 318–19. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Peacock J. The use of palmitate-conjugated protein A for coating cells with artificial receptors which facilitate intercellular interactions. J Immunol Methods. 1993;158:57–65. doi: 10.1016/0022-1759(93)90258-9. [DOI] [PubMed] [Google Scholar]
- 8.Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–10. doi: 10.1016/j.biomaterials.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong S, Guo R, Yang Z, et al. Treg depletion attenuates irradiation-induced pulmonary fibrosis by reducing fibrocyte accumulation, inducing Th17 response, and shifting IFN-gamma, IL-12/IL-4, IL-5 balance. Immunobiology. 2015;220:1284–91. doi: 10.1016/j.imbio.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen QQ, Yan L, Wang CZ, et al. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702–17. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol. 2018;18:121–33. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Cao F, Liu T, Xu Y, et al. Culture and properties of adipose-derived mesenchymal stem cells: characteristics in vitro and immunosuppression in vivo. Int J Clin Exp Pathol. 2015;8:7694–709. [PMC free article] [PubMed] [Google Scholar]
- 14.Ghobadi A, Rettig MP, Cooper ML, et al. Bortezomib is a rapid mobilizer of hematopoietic stem cells in mice via modulation of the VCAM-1/VLA-4 axis. Blood. 2014;124:2752–54. doi: 10.1182/blood-2014-08-595967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner S, Theiss HD, Leiss M, et al. Enhanced stem cell migration mediated by VCAM-1/VLA-4 interaction improves cardiac function in virus-induced dilated cardiomyopathy. Basic Res Cardiol. 2013;108:388. doi: 10.1007/s00395-013-0388-3. [DOI] [PubMed] [Google Scholar]
- 16.Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. 2013;91:204–14. doi: 10.1038/icb.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 18.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]