Abstract
Background
Primary therapy for patients with advanced prostate cancer (PCa) consists of androgen deprivation therapy targeting the androgen receptor (AR) axis. However, most tumors progress to castration-resistant prostate cancer (CRPC) within 18–24 months. The purpose of the present study was to investigate the mechanisms through which PCa acquires drug resistance after long-term treatment with AR antagonists.
Material/Methods
Online database analysis and bioinformatics analysis were performed to identify signaling activated during anti-androgen treatment. MTT assay was used to detect cell viability. RT-qPCR was performed to examine the mRNA expression of the indicated genes. Colony formation assay was performed to observe cell proliferation. Transwell assay was conducted to demonstrate invasive ability. Protein levels were determined by Western blot analysis and immunofluorescence assays.
Results
An online database search and bioinformatics analysis indicated that bone morphogenetic protein (BMP)-6/SMAD signaling was activated in enzalutamide-resistant LNCaP cells. Furthermore, this signaling interaction was experimentally verified in bicalutamide- and enzalutamide-resistant LNCaP cells, which may be regulated by phospholipase C (PLC)ɛ and induced cell proliferation and invasion. Of note, a positive correlation was observed between PLCɛ and BMP-6 in CRPC tissue samples, which may promote bone metastasis and suggests a poor prognosis.
Conclusions
The present results suggest that targeting of PLCɛ/BMP-6/SMAD signaling may increase the sensitivity of CRPC to AR antagonists and inhibit tumor progression.
MeSH Keywords: Androgen Receptor Antagonists; Bone Morphogenetic Protein 6; Drug Resistance; Phosphoinositide Phospholipase C; Prostatic Neoplasms, Castration-Resistant
Background
Prostate cancer (PCa) is the second most common cancer type in males. It is estimated that ~1.3 million new cases and 359 000 associated deaths occurred worldwide in 2018 [1]. Radical prostatectomy and radiation therapy are effective treatment approaches for early-stage localized PCa [2]. However, ~54% of PCa patients in China present with metastasis at the time of initial diagnosis when the best treatment opportunity has already passed [3]. Bicalutamide and enzalutamide [first- and second-generation androgen receptor (AR) antagonists, respectively] initially inhibit tumor progression in the treatment of advanced PCa or castration-resistant PCa (CRPC), but their function is later limited by the development of acquired drug resistance [4]. Of note, 90% of patients who died of PCa were identified to have bone metastases on autopsy [5].
Phospholipase C (PLC)ɛ, a member of the PLC family, hydrolyzes membrane phosphoinositide 4,5-diphosphate to produce 2 second messengers, inositol-1,4,5-phosphate (IP3) and diacylglycerol (DAG) [6]. IP3 then stimulates Ca2+ signaling, while DAG activates protein kinase C (PKC), which mediates a variety of biological processes, including cancer [7]. In a previous study by our group, a higher PLCɛ expression was determined in primary PCa (PPC) tissue compared with that in benign prostatic hyperplasia tissue, and knockdown of PLCɛ reduced the protein expression levels of AR [8]. However, the specific mechanism and involvement of PLCɛ in resistance to AR antagonists remain unclear.
Bone morphogenetic proteins (BMPs) belong to the transforming growth factor (TGF)-β superfamily. In classical BMP/SMAD signaling, BMP transduces its signals through the intracellular downstream mediator SMAD1/5/9, which forms a complex with SMAD4 after phosphorylation, regulating gene expression through cooperation with other DNA binding factors or transcription factors [9,10]. BMP-6 has received a large amount of attention in PCa research [11–15]. Co-culture models of PCa/bone stromal cell lines or PCa/prostate stromal cell lines are commonly used in studies on BMP-6 [11,12]. The WNT5A/BMP-6 loop and BMP-6/interleukin-6 loop were reported to induce AR expression and bone metastasis in these models. However, whether BMP-6 is altered or participates in drug resistance when PCa cells are exposed to AR antagonist alone has remained elusive. In addition, a study indicated that BMP-9 was regulated via the PLC pathway by increasing intracellular levels of Ca2+ in human periodontal ligament cells [16]. In the present study, it was hypothesized that a link exists between PLCɛ and BMP-6 in PCa cells.
Numerous studies have focused on AR antagonist resistance, but most of them were performed shortly after the drug was applied to PCa cells, which is not in accordance with the timing of the rise of resistance in clinical practice. To investigate the mechanisms activated following long-term treatment, LNCaP cells were cultured with bicalutamide or enzalutamide for more than 6 months to obtain drug-resistant cell lines. In the present study, BMP-6 was identified as differentially expressed in acquired drug resistance through a bioinformatics analysis of a gene array dataset, and the association between PLCɛ and BMP-6 was explored at the cellular level and in clinical specimens. Overall, the present results suggest that PLCɛ promotes cell proliferation and invasion by modulating the BMP6/SMAD axis, and PLCɛ knockdown increases the sensitivity of drug-resistant cells to AR antagonist, which may therefore be a potential treatment for CRPC.
Material and Methods
Identification of differentially expressed genes (DEGs) and pathways in acquired drug resistance through online database screening
To identify genes involved in the process of acquired drug resistance, the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) was searched and the GSE78201 dataset was selected [17]. The gene expression in this dataset was then analyzed using the online tool GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). Genes that met the criteria P<0.05 and log2 |fold change| >1.5 were defined as DEGs. Gene ontology (GO) enrichment analysis and signaling pathway analysis were then performed using WebGestalt (http://www.webgestalt.org/option.php).
Cell culture, treatment, and transfection
The LNCaP cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). As previously described [18], LNCaP cells were continuously cultured with 10 μM bicalutamide or enzalutamide (Selleck Chemicals, Houston, TX, USA). Initially, the cells died in large numbers, and the surviving cells continued to undergo passaging. After 6 months of culture, cells resistant to bicalutamide or enzalutamide (referred to as LNCaP-BicaR and LNCaP-EnzaR cells, respectively) were obtained. All cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (both from Gibco-Life Technologies, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Beyotime Institute of Biotechnology) and culture of the drug-resistant cells was continued in the presence of the corresponding drugs at the above concentrations.
To study the pathways by which PLCɛ regulates BMP-6, 10 nM phorbol 12-myristate 13-acetate (PMA; Abcam, Cambridge, UK, USA) was added to the drug-resistant cells with PLCɛ knockdown, followed by culture for 24 h.
Lentivirus (LV) expressing small hairpin (sh)RNA targeting human PLCɛ (LV-shPLCɛ; sequence, 5′-GGTTCTCTCCTAGAAGCAACC-3′) and negative control (LV-NC; sequence, 5′-TTCTCCGAACGTGTCACGT-3′) were purchased from Shanghai Gene Pharma Corp. (Shanghai, China). In brief, for transfection, LNCaP-BicaR and LNCaP-EnzaR cells were seeded in 6-well plates, and when cells reached 60–70% confluence, they were transfected with 15 μl LV-shPLCɛ or LV-NC stock solution with 2 μl polybrene, followed by incubation at 37°C for 48 h. Stably transfected cells were selected using medium containing puromycin (1 μg/ml).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol reagent following the manufacturer’s protocol and stored at −80°C. RT was performed using the Prime Script™ RT reagent kit (Takara Bio, Inc., Otsu, Japan). The comparative 2−ΔΔCq method [19] was used for calculating the gene expression, which was normalized against β-actin.
The sequences of primers were as follows:
PLCɛ sense, 5′-GCAACTACAACGCTGTCATGGAG-3′ and
antisense, 5′-GCAACTACAACGCTGTCATGGAG-3′;
BMP-6 sense, 5′-CATGAGCTTTGTGAACCTGG-3′ and
antisense, 5′-CACCTCACCCTCAGGAATCT-3′;
inhibitor of DNA binding 2 (ID2) sense, 5′-TGAACGACTGCTACTCCAAGCTGA-3′ and
antisense, 5′-TAGTCGATGACGTGCTGCAGGATT-3′;
β-actin sense, 5′-GGGACCTGACTGACTACCTC-3′ and
antisense, 5′-ACGAGACCACCTTCAACTCCAC-3′.
Western blot analysis
In brief, cells were lysed with radioimmunoprecipitation assay buffer containing phenylmethane sulfonyl fluoride and phosphatase inhibitors (NaF and Na3VO4). The protein concentration was determined using the BCA Protein Assay kit (Beyotime Institute of Biotechnology), and 30 μg of proteins from each sample were loaded. Proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA), incubated with primary antibodies overnight at 4°C and secondary antibodies for 2 h at room temperature, and, finally, protein bands were visualized using an enhanced chemiluminescence kit (Merck Millipore, Billerica, MA, USA). The antibodies were as follows: PLCɛ (1: 300 dilution; cat. no. sc28402; Santa Cruz Biotechnology, Dallas, TX, USA), BMP-6 (1: 1,000 dilution; cat. no. ab155963), ID2 (1: 1,000 dilution; cat. no. ab166708) and β-actin (1: 3,000 dilution; cat. no. ab8226) were from Abcam (Cambridge, MA, USA), SMAD1/5/9 (1: 500 dilution; cat. no. 131100) and AR (1: 500 dilution; cat. no. GTX29474) were from GeneTex (San Antonio, TX, USA), phosphorylated (p)-SMAD1/5/9 (cat. no. 13820T), E-cadherin (cat. no. 3195), N-cadherin (cat. no. 4061) and matrix metallopeptidase (MMP9) (cat. no. 3852) were from Cell Signaling Technology (Danvers, MA, USA; 1: 1,000 dilution), goat anti-mouse IgG (cat. no. SA00001-1) and goat anti-rabbit IgG (cat. no. SA00001-2) were obtained from ProteinTech (Chicago, IL, USA; 1: 3,000 dilution).
MTT assay
For the cell viability assays, 2×103 cells were seeded into each well of a 96-well plate, followed by incubation overnight and subsequent treatment with 10 μM bicalutamide or enzalutamide, with or without 10 ng/ml human recombinant (r)BMP-6 (R&D Systems, Minneapolis, MN, USA) over 4 days, with the proliferation assessed once per day in designated wells. MTT was added, followed by incubation for 4 h at 37°C. Subsequently, the medium was discarded and 150 ml dimethyl sulfoxide was added per well. The absorbance was measured on a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm.
For determining the half-maximal inhibitory concentration (IC50), various concentrations of bicalutamide or enzalutamide were added, and for the LV-shPLCɛ+rBMP-6 group, 10 ng/ml rBMP-6 was additionally added. The absorbance was measured after 48 h of treatment.
Colony formation assay and Transwell assay
All cells were treated with AR antagonist, with or without 10 ng/ml rBMP-6, and incubated for 3 days. A total of 500 cells per well were seeded in 6-well plates and cultured for 10 days to form colonies. For the Transwell assay, 1.0×104 cells were seeded in the upper chamber of the insert coated with Matrigel for 48 h, as described previously [20].
Patients and tissue samples
A total of 48 PPC and 33 CRPC samples were collected from the Department of Urology at the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) between July 2012 and June 2018. The patients with CRPC were diagnosed according to the EAU-ESTRO-SIOG guidelines [21]. All patients with CRPC had received bicalutamide (n=25) or flutamide (n=8). Bone metastasis was identified by bone scan. The present study was approved by the Ethics Committee of Chongqing Medical University (Chongqing, China) and all patients provided written informed consent.
Immunohistochemical analysis
All tissue samples were fixed in 10% neutral formalin, embedded in paraffin, and cut into 5-μm-thick sections. Immunohistochemical staining was performed using a standard immunoperoxidase staining procedure. Semi-quantitative analysis was performed to analyze the immunohistochemical results. Scoring for the immunoreactivity ratio was as follows: 0, 0%; 1, <5%; 2, 5–50%; and 3, >50%. Scoring for intensity was performed as follows: 0, no staining; 1, light yellow; 2, light brown; and 3, brown. The 2 scores were added together to obtain the final immunoreactivity scores, and samples with scores of ≤2 were considered as having no expression, while those with scores of ≥3 were considered to be positive [22].
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0 and GraphPad Prism software ver. 5. The significant differences among experimental groups were evaluated using the t test, one-way analysis of variance (ANOVA), and two-way ANOVA, while Bonferroni’s adjustment was made for multiple comparisons. The Kaplan-Meier method was used for survival analysis. Continuous and categorical data were analyzed by Spearman’s correlation analysis, chi-square test, Mann-Whitney test, and the Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference. All experiments were performed at least 3 times.
Results
Online database screening for DEGs
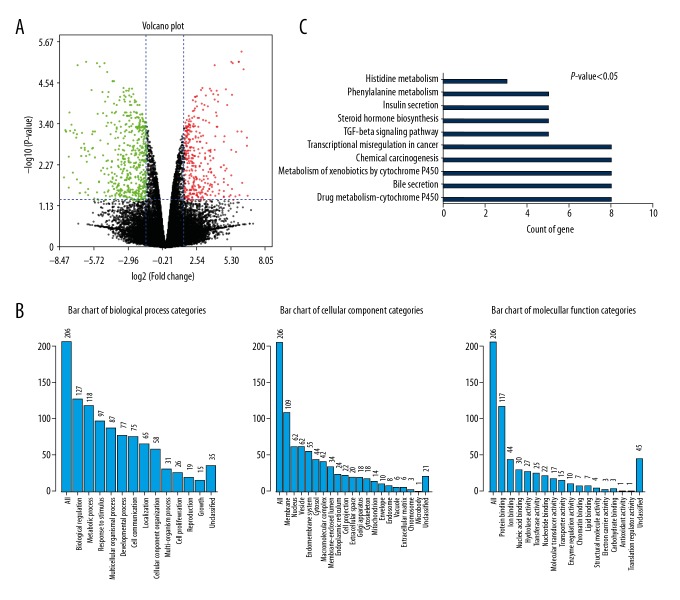
The dataset GSE78201 was retrieved from the GEO database and analyzed. Comparisons were made between LNCaP and LNCaP-EnzaR cells (Table 1). Volcano plots were drawn to visualize the distribution of the DEGs, with green or red dots representing significantly downregulated or upregulated genes, respectively (Figure 1A). A total of 206 DEGs were identified (Table 2). GO biological process analysis indicated that these genes are mainly involved in biological regulation and metabolic processes. In the GO category ‘cellular component’, terms enriched by the DEGs were associated with membranes and vesicles. Enriched terms in the GO category ‘molecular function’ mainly indicated involvement in protein binding, ion binding, and nucleic acid binding (Figure 1B). Pathway enrichment analysis suggested that these DEGs mainly participate in the regulation of drug metabolism, transcriptional dysregulation in cancer, and in the TGF-β signaling pathway (Figure 1C). As an important member of the TGF-β family, BMP-6 signaling was identified as the focus of subsequent in vitro research.
Table 1.
Comparison of gene expression between LNCaP and LNCaP-EnzaR cells in GEO database.
| Organism | Homo sapiens | |
|
| ||
| GEO ID | GSE78201 | |
|
| ||
| Sample Information | AR-positive prostate cancer cell line LNCaP, parental untreated | LNCaP cells were incubated in enzalutamide for more than 6 months, obtained enzalutamide-resistant cells |
|
| ||
| Data Contents | GSM2069526 | GSM2069518 |
| GSM2069527 | GSM2069519 | |
| GSM2069528 | GSM2069520 | |
| GSM2069529 | GSM2069521 | |
|
| ||
| Platform | Illumina HumanHT-12 V4.0 expression BeadChip | |
AR – androgen receptor.
Figure 1.
Differential gene expression analysis between LNCaP and LNCaP-EnzaR cell lines. (A) Volcano plots were used to visualize the distribution of the DEGs. The red/green color of the symbols indicates the upregulated/downregulated DEGs that met the criteria (P<0.05, log2 |fold change| >1.5). (B) GO Slim summary based upon 206 DEGs. (C) Ten signaling pathways were associated with DEGs identified by enrichment analysis. The X-axis indicates the number of genes enriched in each pathway. DEG – differentially expressed gene; GO – gene ontology.
Table 2.
Statistical distribution of differentially expressed genes between LNCaP and LNCaP-EnzaR cells.
| Probe | Gene | |
|---|---|---|
| All | 47287 | 20752 |
|
| ||
| |log2(fold change)| >1.5 & adjusted p value <0.05 | 240 | 206 |
| 90 (up) | 79 (up) | |
| 150 (down) | 127 (down) | |
BMP-6/SMAD signaling is activated in LNCaP-BicaR and LNCaP-EnzaR cells
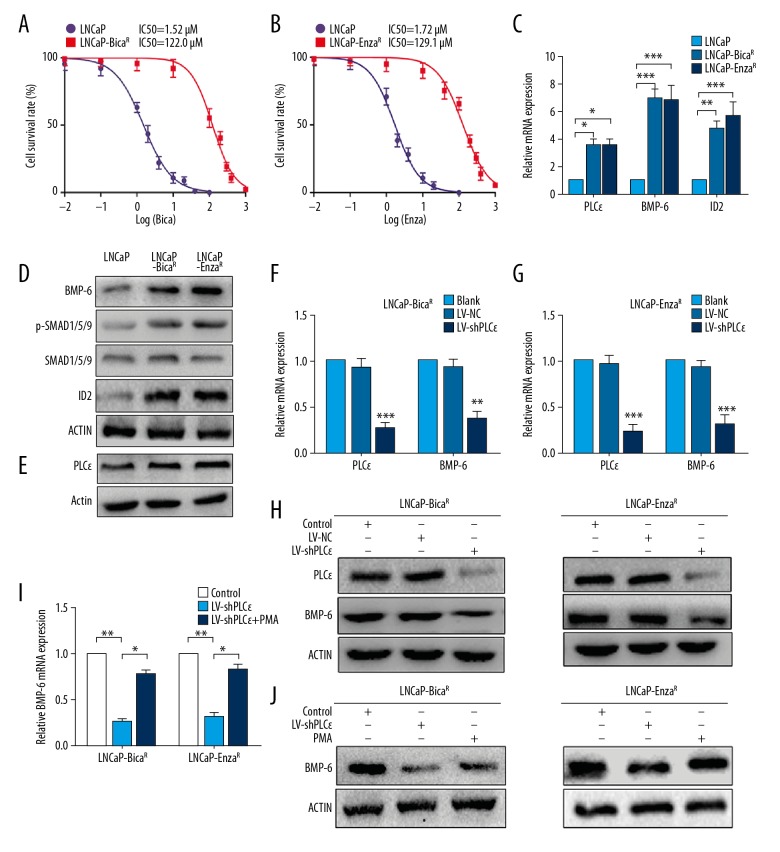
An MTT assay was performed to verify the drug-resistant cell lines. The IC50 for bicalutamide on LNCaP-BicaR cells was >80-fold higher than in LNCaP cells (IC50: 1.52 μM for LNCaP and 122.0 μM for LNCaP-BicaR; Figure 2A). Similarly, the IC50 on LNCaP-EnzaR cells was ~70-fold higher than in LNCaP cells (IC50: 1.72 μM for LNCaP and 129.1 μM for LNCaP-EnzaR; Figure 2B), suggesting that drug-resistant cells were successfully generated. RTq-PCR verified that BMP-6 and ID2 mRNA was consistently upregulated, and BMP-6, p-SMAD1/5/9 and ID2 proteins were increased in LNCaP-BicaR and LNCaP-EnzaR cells, while SMAD1/5/9 was unchanged (Figure 2C, 2D). In conclusion, AR antagonist treatment activated BMP-6/SMAD signaling in CRPC cell lines.
Figure 2.
LNCaP-BicaR and LNCaP-EnzaR cells maintained BMP-6/SMAD signaling, and PLCɛ knockdown decreased BMP-6 expression. (A, B) An MTT assay was used to determine the IC50 values of LNCaP and drug-resistant cells. (C) RT-qPCR was performed to examine the mRNA expression of the indicated genes. (D, E) Protein levels in LNCaP, LNCaP-BicaR, and LNCaP-EnzaR cells determined by Western blot analysis. (F, G) PLCɛ and BMP-6 mRNA was measured using RT-qPCR after transfection with LV-NC or LV-shPLCɛ. (H) Protein levels of PLCɛ and BMP-6 in 2 drug-resistant cell lines with or without PLCɛ knockdown. (I, J) PMA (10 nM) was added to drug-resistant cells with PLCɛ knockdown, followed by culturing for 24 h. BMP-6 mRNA and protein levels were measured using RT-qPCR and Western blot analysis, respectively, with β-actin serving as the control. * P<0.05; ** P<0.01; *** P<0.001. RT-qPCR – reverse transcription-quantitative polymerase chain reaction; PLC – phospholipase C; BMP – bone morphogenetic protein; NC – negative control; LV-shPLCɛ – lentivirus expressing small hairpin RNA targeting PLCɛ; IC50 – drug concentration causing 50% cell inhibition; PMA – phorbol 12-myristate 13-acetate.
BMP-6 is induced by PLCɛ through activation of PKC in drug-resistant cells
To verify the previous hypothesis that BMP-6 is induced by PLCɛ, the mRNA and protein levels of PLCɛ were first assessed using RT-qPCR and Western blot analysis. The results indicated that the mRNA and protein levels of PLCɛ were upregulated in drug-resistant cells (Figure 2C, 2E). Knockdown of PLCɛ in LNCaP-BicaR and LNCaP-EnzaR cells was then performed using lentivirus. Compared with those in the negative controls (Blank and LV-NC), the mRNA levels of PLCɛ and BMP-6 were downregulated (Figure 2F, 2G), and similar results were obtained at the protein level (Figure 2H). To further elucidate the mechanisms through which PLCɛ mediates BMP-6 expression, PKC activator (PMA) was added to the PLCɛ knockdown group. The result indicated that PMA treatment increased BMP-6 at the mRNA (Figure 2I) and protein levels (Figure 2J) in LNCaP-BicaR and LNCaP-EnzaR cells, suggesting that PLCɛ increases BMP-6 expression at the transcriptional level in a PKC-dependent manner.
PLCɛ knockdown inhibits cellular drug resistance, proliferation, and invasion via BMP-6/SMAD signaling
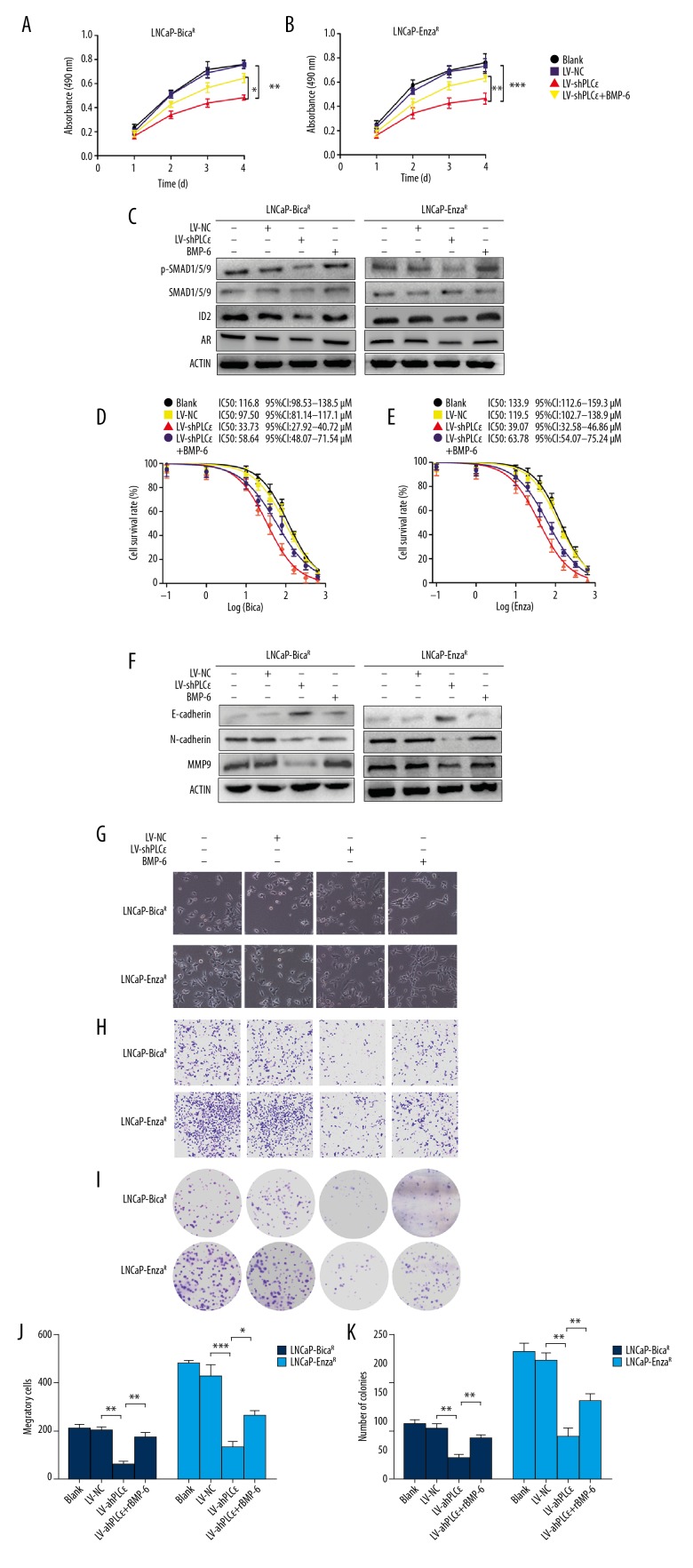
An MTT assay indicated that the viability of cells was markedly inhibited in a time-dependent manner after transfection with LV-shPLCɛ (Figure 3A, 3B). At the same time, rBMP-6 replenishment in the LV-shPLCɛ group partly recovered the suppressive effect produced by PLCɛ knockdown. As presented in Figure 3C, the protein levels of p-SMAD1/5/9, ID2 and AR were significantly downregulated following knockdown of PLCɛ, and when rBMP-6 was added to PLCɛ-knockdown cells, these proteins were partially upregulated. In addition, the IC50 of bicalutamide in the LNCaP-BicaR-shPLCɛ cells dropped to one-third of that in LNCaP-BicaR cells, which was partially reversed when cultured with rBMP6 (IC50: 116.8 μM for LNCaP-BicaR, 33.73 μM for LNCaP-BicaR-shPLCɛ, and 58.64 μM for LNCaP-BicaR-shPLCɛ+BMP-6; Figure 3D). Similar changes were observed in LNCaP-EnzaR cells with the same treatment (Figure 3E), indicating that PLCɛ promoted PCa cell resistance to AR antagonist via BMP-6 signaling.
Figure 3.
PLCɛ knockdown partially restored the sensitivity to androgen receptor antagonist through BMP-6/SMAD signaling, inhibiting cell proliferation and invasion. (A, B) LNCaP-BicaR and LNCaP-EnzaR cells were transfected with LV-PLCɛ or LV-shNC, treated with or without rBMP-6 (10 ng/ml), and an MTT assay was used to detect cell viability. (C) Protein levels were detected after 48 h of treatment by Western blot analysis. (D, E) All cells were treated with different drug concentrations for 48 h and IC50 values were determined by an MTT assay. (F) Protein expression of E-cadherin, N-cadherin, and MMP9 was detected by Western blot analysis with β-actin serving as the loading control. (G) Morphology of cells after various treatments under a microscope. (H, J) The invasive ability of the cells was evaluated by Transwell assay. (I, K) A clonogenic assay was performed to assess the ability of single cells to form colonies. * P<0.05; ** P<0.01; *** P<0.001. PLC – phospholipase C; rBMP – recombinant bone morphogenetic protein; NC – negative control; LV-shPLCɛ – lentivirus expressing small hairpin RNA targeting PLCɛ; MMP – matrix metallopeptidase; IC50 – drug concentration causing 50% cell inhibition.
Previous studies reported that BMP-6 is associated with bone metastasis [15]. To investigate whether this process is regulated by PLCɛ, epithelial-to-mesenchymal transition (EMT)-associated molecules were detected by Western blot analysis following different treatments. PLCɛ knockdown resulted in an increased protein expression of E-cadherin and had a suppressive effect on N-cadherin and MMP9 expression, while the opposite effect was observed with the addition of rBMP-6 (Figure 3F). The size of the resistant cells was different and showed a multi-polar morphology under a microscope. After knocking down PLCɛ, the cell morphology became round and the cluster growth was more obvious. After treatment with rBMP-6, the cell morphology became long and fusiform, and migratory capacity was enhanced (Figure 3G).
Consistent with this, a Transwell assay demonstrated that the invasive ability was significantly decreased by PLCɛ knockdown and was increased by rBMP-6 (Figure 3H, 3J). Furthermore, a colony formation assay confirmed that PLCɛ inhibited the proliferation of cells by regulating BMP-6 (Figure 3I, 3K).
Taken together, these results suggest that PLCɛ knockdown partially restored the sensitivity of CRPC cells to AR antagonist and simultaneously inhibited cell proliferation and invasion via BMP-6/SMAD signaling.
PLCɛ and BMP-6 are correlated with bone metastasis and shorter time to progression (TTP)
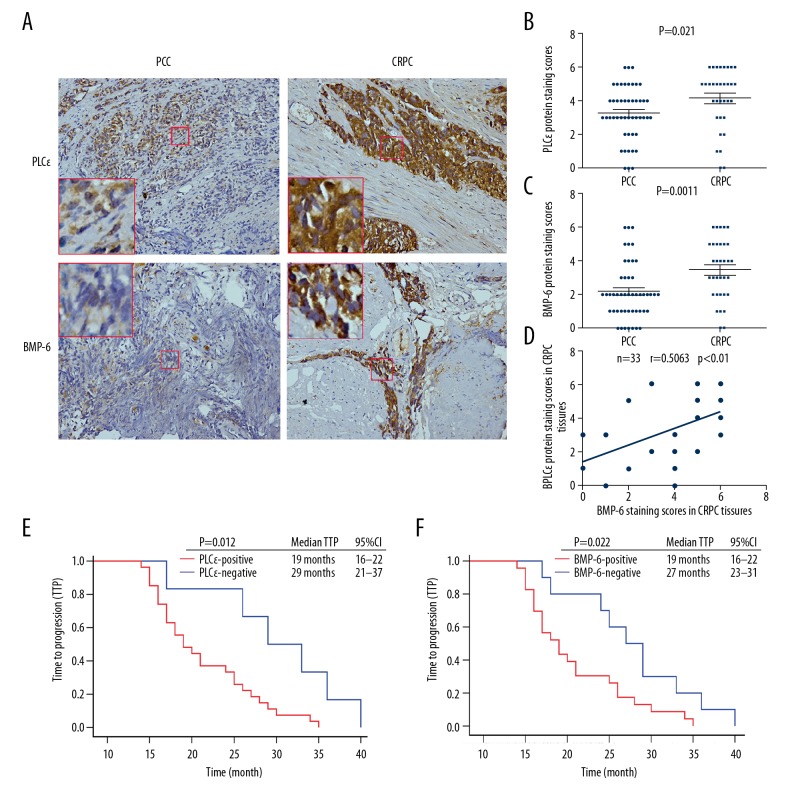
As the in vitro research suggested that PLCɛ promotes cell proliferation and invasion via the BMP-6/SMAD axis, the expression of PLCɛ and BMP-6 in PPC and CRPC tissue specimens was then verified by immunohistochemistry and their association with the demographic and clinical characteristics of the subjects was determined (Figure 4A). The results demonstrated that PLCɛ and BMP-6 were upregulated in CRPC compared with PCC tissue samples (P<0.05; Figure 4B, 4C). Furthermore, Pearson’s linear correlation analysis revealed that PLCɛ expression was positively associated with BMP-6 expression (r=0.5063, P<0.01; Figure 4D). Regarding the clinical characteristics, PLCɛ and BMP-6 expression were associated with bone metastasis (P<0.05; Table 3).
Figure 4.
PLCɛ is positively correlated with BMP-6 expression and indicates a poor outcome. (A) Immunohistochemical staining of PLCɛ and BMP-6 in PCC and CRPC tissue samples (magnification, ×100). (B, C) Staining scores for PLCɛ and BMP-6 expression in PCC and CRPC tissues. (D) Correlation dot plot for PLCɛ protein vs. corresponding BMP-6 protein in CRPC specimens and Pearson’s linear correlation analysis. (E, F) Kaplan-Meier survival analysis of the TTP of 33 patients with CRPC. P<0.05 was considered to indicate statistical significance. TTP – time to progression; PLC – phospholipase C; BMP – bone morphogenetic protein; PPC – primary prostate cancer; CRPC – castration-resistant prostate cancer.
Table 3.
Demographic and clinical characteristics of patients with primary prostate cancer or castration-resistant prostate cancer.
| Characteristics | PLCɛ | BMP-6 | ||||
|---|---|---|---|---|---|---|
| Negative % | Positive % | P-value | Negative % | Positive % | P-value | |
| PPC (n=48) | 25.0 (12/48) | 75.0 (36/48) | 70.8 (34/48) | 29.2 (14/48) | ||
| CRPC (n=33) | 18.2 (6/33) | 81.8 (27/33) | 30.3 (10/33) | 69.7 (23/33) | ||
| PSA of PPC (ng/ml) | 0.739 | 0.751 | ||||
| Median | 70.0 | 57.7 | 70.0 | 46.1 | ||
| Quartiles 25–75 | 18.9–128.6 | 25.4–241.9 | 22.7–227.6 | 26.7–165.5 | ||
| PSA of CRPC (ng/ml) | 0.132 | 0.862 | ||||
| Median | 11.2 | 17.7 | 18.6 | 15.6 | ||
| Quartiles 25–75 | 2.3–19.6 | 8.6–63.5 | 6.9–50.7 | 7.5–59.8 | ||
| Age of PPC | 0.676 | 0.237 | ||||
| Median | 67 | 69 | 69 | 66 | ||
| Quartiles 25–75 | 63–72 | 63–72 | 63–73 | 61–71 | ||
| Age of CRPC | 0.424 | 0.237 | ||||
| Median | 69 | 71 | 70 | 72 | ||
| Quartiles 25–75 | 66–73 | 69–75 | 67–72 | 70–75 | ||
| Metastases sites of PCC | 0.013 | 0.010 | ||||
| Bone (n=23) | 16.7 (2/12) | 58.3 (21/36) | 35.3 (12/34) | 78.6 (11/14) | ||
| Metastases sites of CRPC | 0.034 | 0.010 | ||||
| Bone (n=24) | 33.3 (2/6) | 81.5 (22/27) | 40.0 (4/10) | 87.0 (20/23) | ||
PPC – primary prostate cancer; CRPC – castration-resistant prostate cancer; PSA – prostate-specific antigen. Bold data indicates the statistical significance of relationships.
Of note, Kaplan-Meier survival analysis demonstrated that PLCɛ-positive patients had a shorter TTP than PLCɛ-negative patients {19 months [95% confidence interval (CI): 16–22 months] vs. 29 months (95% CI: 21–37 months); P<0.05; Figure 4E]. A similar result was obtained for the comparison between BMP-6-positive and -negative patients [19 months (95% CI: 16–22 months) vs. 27 months (95% CI: 23–31 months, P<0.05); Figure 4F]. In summary, the expression of PLCɛ and BMP-6 predicted a poor prognosis for patients with PCa.
Discussion
CRPC is an important challenge in the treatment of PCa, and bone metastasis also seriously affects the quality of life of patients. Although comprehensive research has been performed to explore the mechanisms of drug resistance arising during AR antagonist treatment, the molecular mechanisms are complex and remain to be fully elucidated. In the present study, the GEO database was screened for genes and pathways that may potentially be involved in resistance to AR antagonists. Subsequently, bicalutamide- and enzalutamide-resistant cells were generated and cultured to accurately simulate the clinical occurrence of drug resistance in patients. These drug-resistant cells may be valuable models for studying CRPC. Finally, the molecular mechanisms were explored in the cells in vitro and in clinical specimens, and a potential therapeutic target for CRPC was thereby identified.
According to previous studies, PLCɛ is associated with numerous cancer types. It is an oncogene in cancer types including head and neck cancer, bladder cancer, and PCa, but functions as a tumor suppressor gene in colorectal cancer [23]. This difference may be partly caused by the complex structure of PLCɛ and the multiple signaling pathways involved. A previous study by our group demonstrated that PLCɛ promoted the proliferation of PCa cells [24]. The present study indicated that PLCɛ knockdown suppressed the proliferation and invasion of LNCaP-BicaR and LNCaP-EnzaR cells, suggesting the promoting effect of PLCɛ in CRPC.
Due to the low expression of BMP-6 in PCa cell lines [25], most studies of BMP-6 use PCa/bone stromal or PCa/prostate stromal cell line co-culture models. It was also reported that PLCɛ promotes the expression of BMP-9 by increasing the intracellular levels of Ca2+ in periodontal ligament cells [16], while another study revealed that BMP-2 and BMP-4 were regulated by PKC in human osteosarcoma cell lines [26]. While PLCɛ regulates various different downstream molecules and signaling pathways, the present study indicated that PLCɛ may regulate BMP-6 expression by activating PKC. To the best of our knowledge, the present study is the first to report that BMP-6 is regulated by the PLCɛ/PKC pathway in drug-resistant PCa cell lines, providing a novel therapeutic approach via modification of BMP-6 expression.
There are certain controversies regarding the effect of BMP-6 on the proliferation of PCa cell lines. A study by Haudenschild et al. [27] demonstrated that BMP-6 inhibited the proliferation of DU145 and LNCaP cells, but Lee et al. [11] reported that cell proliferation was enhanced when LNCaP cells were cultured with BMP-6 in androgen-depleted media. Another study observed no marked effect of BMP-6 on the proliferation of LuCaP 23.1 or C4-2B cells [15]. Thus, in different cell lines, different BMP-6 concentrations and culture conditions may lead to different results. Studies by our and other groups [11,28] support that BMP-6 promotes cell proliferation with a parallel increase of p-SMAD, confirming the importance of the BMP-6/SMAD axis in tumor progression.
A previous study indicated that p-SMAD5 interacts with β-catenin to induce AR expression in PCa cells [11]. A previous study by our group also suggested that β-catenin is activated in LNCaP-BicaR and LNCaP-EnzaR cells. In the present study, PLCɛ knockdown suppressed BMP-6/p-SMAD1/5/9 and AR expression. Combined with the results of the above study, it may be inferred that PLCɛ-induced drug resistance is due to overexpression of AR caused by the BMP6/p-SMAD signaling pathway. The present study also indicated that downregulation of PLCɛ inhibited BMP-6/SMAD/ID2 signaling and expression of the EMT-associated molecules E-cadherin, N-cadherin, and MMP9, reducing the invasive behavior of drug-resistant cells. EMT is a recognized key process for tumor invasion and metastasis [29]. In line with the present results, previous studies have indicated that, as a molecule downstream of BMP/SMAD, ID2 induces EMT-associated factors and enhances the aggressiveness of cancers [30–32]. Immunohistochemical analysis suggested that PLCɛ and BMP-6 proteins were upregulated in CRPC, which were associated with bone metastasis and a shorter TTP. However, further studies with larger numbers of patients and matched PPC and CRPC tissues from the same patient are required.
Other pathways have been identified to be associated with drug resistance through bioinformatics analyses, including steroid hormone biosynthesis and cytochrome P450-linked drug metabolism, which we will further examine in a future study. Overall, the present results indicate that the BMP-6/SMAD pathway was activated by PLCɛ in drug-resistant cells; PLCɛ knockdown partially restored the sensitivity of CRPC cells to AR antagonists and inhibited the malignant behavior of cells. Therefore, PLCɛ/BMP-6 may have a critical role in monitoring of CRPC progression and targeted therapy.
Conclusions
Our study has shown that PLCɛ knockdown partially restored the sensitivity of CRPC cells to AR antagonists through the BMP-6/SMAD pathway as well as inhibiting the malignant behavior of cells. Thus, these findings may provide a new approach for treating CRPC.
Acknowledgements
The authors would like to thank the Department of Urology of the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) for providing technical assistance and collecting the specimens.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang XM, Deng YL, Ye DW, et al. [Chinese experts consensus on the treatment of metastatic prostate cancer 2018 edition]. Zhonghua Wai Ke Za Zhi. 2018;56:646–52. doi: 10.3760/cma.j.issn.0529-5815.2018.09.002. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 4.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J Clin Oncol. 2016;34:2098–106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 6.Smrcka AV, Brown JH, Holz GG. Role of phospholipase Cepsilon in physiological phosphoinositide signaling networks. Cell Signal. 2012;24:1333–43. doi: 10.1016/j.cellsig.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang RY, Du WQ, Zhang YC, et al. PLCepsilon signaling in cancer. J Cancer Res Clin Oncol. 2016;142:715–22. doi: 10.1007/s00432-015-1999-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wu X, Ou L, et al. PLCepsilon knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015;362:61–69. doi: 10.1016/j.canlet.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Corradini E, Rozier M, Meynard D, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011;141:1907–14. doi: 10.1053/j.gastro.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Bi D, Cheng C, et al. Bone morphogenetic protein 7 enhances the osteogenic differentiation of human dermal-derived CD105+ fibroblast cells through the Smad and MAPK pathways. Int J Mol Med. 2019;43:37–46. doi: 10.3892/ijmm.2018.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GT, Kang DI, Ha YS, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. Br J Cancer. 2014;110:1634–44. doi: 10.1038/bjc.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F, Chen Y, Shen T, et al. Stromal TGF-beta signaling induces AR activation in prostate cancer. Oncotarget. 2014;5:10854–69. doi: 10.18632/oncotarget.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee GT, Kwon SJ, Kim J, et al. WNT5A induces castration-resistant prostate cancer via CCL2 and tumour-infiltrating macrophages. Br J Cancer. 2018;118:670–78. doi: 10.1038/bjc.2017.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GT, Jung YS, Ha YS, et al. Bone morphogenetic protein-6 induces castration resistance in prostate cancer cells through tumor infiltrating macrophages. Cancer Sci. 2013;104:1027–32. doi: 10.1111/cas.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai J, Keller J, Zhang J, et al. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65:8274–85. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 16.Tantilertanant Y, Niyompanich J, Everts V, et al. Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J Cell Physiol. 2019;234(4):4528–39. doi: 10.1002/jcp.27257. [DOI] [PubMed] [Google Scholar]
- 17.Kregel S, Chen JL, Tom W, et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget. 2016;7:26259–74. doi: 10.18632/oncotarget.8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Li L, Sun W, et al. HepaCAM inhibits the malignant behavior of castration-resistant prostate cancer cells by downregulating Notch signaling and PF-3084014 (a gamma-secretase inhibitor) partly reverses the resistance of refractory prostate cancer to docetaxel and enzalutamide in vitro. Int J Oncol. 2018;53:99–112. doi: 10.3892/ijo.2018.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wang H, Zhang R, et al. Antitumor activity of asiaticoside against multiple myeloma drug-resistant cancer cells is mediated by autophagy induction, activation of effector caspases, and inhibition of cell migration, invasion, and STAT-3 signaling pathway. Med Sci Monit. 2019;25:1355–61. doi: 10.12659/MSM.913397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Quan Z, He Y, Luo C, et al. Interleukin 6 induces cell proliferation of clear cell renal cell carcinoma by suppressing hepaCAM via the STAT3-dependent up-regulation of DNMT1 or DNMT3b. Cell Signal. 2017;32:48–58. doi: 10.1016/j.cellsig.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Tyutyunnykova A, Telegeev G, Dubrovska A. The controversial role of phospholipase C epsilon (PLCepsilon) in cancer development and progression. J Cancer. 2017;8:716–29. doi: 10.7150/jca.17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Fan Y, Du Z, et al. Knockdown of phospholipase Cɛ (PLCɛ) inhibits cell proliferation via phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/AKT signaling pathway in human prostate cancer. Med Sci Monit. 2018;24:254–63. doi: 10.12659/MSM.908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamada H, Kitazawa R, Gohji K, Kitazawa S. Epigenetic regulation of human bone morphogenetic protein 6 gene expression in prostate cancer. J Bone Miner Res. 2001;16:487–96. doi: 10.1359/jbmr.2001.16.3.487. [DOI] [PubMed] [Google Scholar]
- 26.Helvering LM, Sharp RL, Ou X, Geiser AG. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000;256:123–38. doi: 10.1016/s0378-1119(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 27.Haudenschild DR, Palmer SM, Moseley TA, et al. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004;64:8276–84. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Jin EJ, Cheng X, et al. Opposing roles of TGFbeta and BMP signaling in prostate cancer development. Genes Dev. 2017;31:2337–42. doi: 10.1101/gad.307116.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–54. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 30.Asirvatham AJ, Carey JP, Chaudhary J. ID1-, ID2-, and ID3-regulated gene expression in E2A positive or negative prostate cancer cells. Prostate. 2007;67:1411–20. doi: 10.1002/pros.20633. [DOI] [PubMed] [Google Scholar]
- 31.Kowanetz M, Valcourt U, Bergstrom R, et al. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24:4241–54. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu DY, Wojciechowski MC, Lovicu FJ. Bone morphogenetic protein-7 suppresses TGFbeta2-induced epithelial-mesenchymal transition in the lens: Implications for cataract prevention. Invest Ophthalmol Vis Sci. 2017;58:781–96. doi: 10.1167/iovs.16-20611. [DOI] [PMC free article] [PubMed] [Google Scholar]