Abstract
Purpose
To investigate the cytotoxic effect of bleomycin, mitomycin C (MMC) and Fluorouracil (5‐FU) in combination with electroporation (EP) on human conjunctival melanoma (CM) and normal conjunctival cell lines using 2D and 3D cell culture systems in vitro.
Methods
Two CM (CRMM1, CRMM2) and one normal conjunctival epithelial cell line (HCjE‐Gi) were treated with various EP conditions and increasing concentrations of 5‐FU, MMC and bleomycin. Cell survival was assessed by MTT viability assay. All cell lines were seeded to create spheroids and were treated with bleomycin on day 3 and day 8 combined with EP. Spheroids were collected, fixed in buffered formalin and subsequently paraffin embedded for histological assessment of the effects of the treatment on cell viability.
Results
CM cell lines were resistant to electroporation alone and showed a reduction in cell number only when treated with 1000 Volts/cm and 8 pulses. HCjE‐Gi cells showed higher sensitivity to electric pulses over 750 Volts/cm. MMC and 5‐FU demonstrated a higher cytotoxicity for the HCjE‐Gi cell line. The CM cell lines were resistant to MMC and 5‐FU. Bleomycin (1 μg/ml) alone had no significant effect on the HCjE‐Gi even when combined with EP conditions ≥750 Volts/cm. In contrast, it significantly (p ‐, paired t‐test) reduced cell viability in the CM cell lines. Spheroids treated with bleomycin and EP showed a reduction in tumour mass and proliferation rates after treatment.
Conclusion
Our in vitro study using 2D and 3D models indicates that the application of EP may effectively enhance chemotherapy with bleomycin in CM. This may offer new viable perspectives for CM treatment.
Keywords: bleomycin, conjunctival melanoma, electrochemotherapy, electroporation
Introduction
Conjunctival melanoma (CM) is a rare malignancy originating from atypical melanocytes in the conjunctival or limbal epithelium and comprises 2% of all ocular melanomas (McCartney 1995). It is associated with a mortality of 25–30% (Nørregaard et al. 1996; Seregard 1998; Triay et al. 2009). The management of CM varies between ocular oncology centres and has been based on poor quality evidence of case series without a standardized therapy protocol (Damato & Coupland 2009).
Electrochemotherapy (ECT) is a tumour ablation modality that employs the effect of short electric pulses to increase the transport of non‐permeant or poorly permeant anti‐cancer drugs inside cancer cells (Sersa et al. 2008). This potentiates localized drug cytotoxicity, without affecting the surrounding tissue areas. The secondary actions of ECT include a reduction in tumour blood flow and a localized vascular disruption also enhancing drug efficacy (Gehl et al. 2002; Sersa et al. 2008; Markelc et al. 2013). A reversible effect based on high permeabilization is desirable (Sersa et al. 2008). Parameters, such as the electric pulse settings and the cell type, can have a major influence on the achievement of a transient permeabilization (Mir et al. 2006). The magnitude of the electric field depends on cell type, size, orientation and density, pulse duration and number of pulses (Gehl 2003). Currently, the standard operating procedures are guided by recommendations published by the European Standard Operating Procedures of Electrochemotherapy (ESOPE) group (Mir et al. 2006). The electrical pulses are applied via plate or needle electrodes depending on the tumour accessibility, number of cancer nodules and size. The electrical field is applied to each individual pair of adjacent needles in a sequence controlled by the electroporator. The hexagonal and the linear needle electrodes are suitable for the treatment of lesions up to 3 cm deep, whereas lesions up to 1 cm deep can be treated with finger electrodes. The use of plate electrodes is indicated for exophytic lesions where the pulses are distributed uniformly between the two plates. Individual needle electrodes can be positioned around or within bone or visceral lesions under viewer CT or ultrasound guidance (Mir et al. 2006). At present, ECT is used in the treatment of lesions in patients with different malignancies, for example metastatic breast cancer (Bourke et al. 2017), metastatic cutaneous melanoma (Kunte et al. 2017), liver metastases of carcinomas (Edhemovic et al. 2014), sarcomas (Marty et al. 2006) as well as head and neck cancer (Lenzi et al. 2017), not amenable to surgery or radiofrequency ablation. The treatment may result in complete remission of the tumour without the complications that accompany high chemotherapy doses.
Bleomycin is clinically approved for use with electroporation (EP) (Orlowski et al. 1988; Jaroszeski et al. 2000). The exposure to electric pulses increases the drug permeation into the cells and, therefore, the cytotoxicity of the drug (Cemazar et al. 1998). Mitomycin C (MMC) has been reported to cause histopathologic degenerative changes to the conjunctival epithelium (Salomao et al. 1999) and has been used as a primary or adjuvant topical chemotherapeutic agent for the treatment of CM (Damato & Coupland 2009). Increased cytotoxicity of MMC with EP in bladder cancer has been reported in vitro and in vivo (Vásquez et al. 2000). 5‐Fluorouracil (5‐FU) is used in the treatment of a wide range of solid tumours, and its clinical efficacy in the treatment of ocular surface squamous neoplasia has been well documented (Midena et al. 2000; Yeatts et al. 2000). Previous studies showed promising results with the combination of 5‐FU and EP in ovarian and bladder carcinoma (Salomao et al. 1999; Saczko et al. 2014). Some data are available regarding the effect of EP on uveal melanoma (Fiorentzis et al. 2018).
The aim of this study was to evaluate the cytotoxic effect of bleomycin, 5‐FU and MMC on two CM cell lines and one normal epithelial conjunctival cell line grown in 2D and 3D culture systems, when used either alone or in combination with EP.
Materials and Methods
Cell lines and culture
Two CM cell lines, CRMM1 and CRMM2, were kindly provided by Prof. Dr. Martine Jager, Leiden University Medical Center, the Netherlands. A normal conjunctival epithelial cell line, HCjE‐Gi, was kindly provided by Prof. Dr. Colin Willoughby, University of Liverpool, UK. All cell lines were tested for authentication via Short Tandem Repeat (STR) before initiation of the experiments and were mycoplasma free at the time of experimentation. The CRMM1 and CRMM2 cell lines derived from a spindle cell tumour originally located in the bulbar conjunctiva (Keijser et al. 2007; Nareyeck et al. 2007). The CM cell lines were grown in Ham's F‐12K (Kaighn's) Medium (Life Technologies, Paisley, UK) containing 10% fetal bovine serum (Labtech International Ltd, East Sussex, UK) and 1% Penicillin Streptomycin (Life Technologies). The normal HCjE‐Gi cell line was grown in Keratinocyte Medium with Human Recombinant Epidermal Growth Factor and Bovine Pituitary Extract (Life Technologies) containing 10% fetal bovine serum (Labtech International Ltd). All cell lines were maintained as monolayers in 75‐cm2 tissue culture flasks (Fisher Scientific, Loughborough, UK) at 37°C in a humidified atmosphere containing 5% CO2 prior to their use as described below:
Experimental set‐up 1
In vitro electrochemotherapy (ECT)
When cells reached 70% confluence, they were harvested with 0.05% trypsin (Life technologies), counted and 1 × 106 cells were resuspended in 400 μl of culture medium, with or without the chemotherapeutic agent. Each combination was then added to a 4‐mm gap electroporation cuvette with parallel aluminium plate electrodes (Geneflow, Lichfield, UK). A range of EP conditions were applied to the cell suspensions using the voltage pulse generator (Cliniporator™) designed by IgeaS.p.A. (Capri, Modena, Italy). All cells were treated with:
0 μg/ml, 1‐, 5‐, 10‐, 50‐ and 100 μg/ml 5‐FU;
0 μg/ml, 1‐, 2.5‐, 5‐, 7.5‐, and 10 μg/ml bleomycin and
0 μg/ml, 0.5‐, 1‐, 2.5‐, 5‐ and 10 μg/ml MMC
combined with the following EP conditions:
No EP;
20 square wave electric pulses (WEP) of 500 Volts/cm strength, 100 μs pulse duration, 5 Hz repetition frequency;
40 square WEP of 500 Volts/cm strength, 100 μs pulse duration, 5 Hz repetition frequency;
8 square WEP of 750 Volts/cm strength, 100 μs pulse duration, 5 Hz repetition frequency;
20 square WEP of 750 Volts/cm strength, 100 μs pulse duration, 5 Hz repetition frequency;
8 square WEP of 1000 Volts/cm strength, 100 μs pulse duration, 5 Hz repetition frequency.
Following treatment, 2 × 104 cells were transferred into six wells of a 96‐well plate for each treatment condition and the appropriate culture medium was added up to a maximum volume of 100 μl. The plates were then incubated for 24 and 72 hr, allowing the cells to attach and grow.
The protocol was conducted for both CM cell lines and the HCjE‐Gi cells. Each experiment was performed on three different dates, giving a total of 18 technical replicates for each ECT condition.
MTT viability assay
Cell viability was examined using the MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) tetrazolium reduction assay (MTT; Sigma‐Aldrich, Dorset, UK). Culture medium was aspirated from each well after 24 or 72 hr, and the MTT stock solution (5 mg/ml) was added according to the manufacturer's protocol (90 μl medium and 10 μl MTT). All plates were incubated at 37° for 4 hr. The solution was then removed, and the formazan formed in the cells was dissolved using 100 μl of a 1:1 solution of dimethyl sulfoxide (DMSO; Sigma‐Aldrich) and 2‐propanol (isopropanol; Sigma‐Aldrich). The absorbance of formazan was determined at 570 nm on a plate reader (SPECTRAFLUOR, Tecan, Austria). The viability of the treated cells was expressed relative to untreated control cells. Relative EC50 values were calculated for each of the drugs tested in the cell lines examined using a freeware tool (https://www.aatbio.com/tools/ec50-calculator/). The relative EC50 value calculated represents the estimated drug concentration that caused cell death halfway between the baseline (i.e. no drug present) and the maximal response to the drug that was observed. For this relative estimation, at least one additional value beyond the maximal cell death observed was required. This was not possible for CRMM1 and CRMM2 cells treated with bleomycin and CRMM1 cells treated with MMC (Table 1), where these criteria were not met.
Table 1.
Relative EC50 values for conjunctival melanoma and normal conjunctival cell lines treated with 5FU, MMC and bleomycin for 72 hr
| EC50 values (μg/ml) | |||
|---|---|---|---|
| 5FU | MMC | Bleomycin | |
| CRMM1 | 1.07 | NC | NC |
| CRMM2 | 16.18 | 0.98 | NC |
| HCjE | 10.97 | 0.58 | 3.36 |
The relative EC50 values represent the estimated drug concentration causing cell death halfway between the baseline (i.e. no drug present) and the maximal response to the drug.
NC = noncalculable.
Experimental set‐up 2
Ultralow attachment plate
The two above‐mentioned CM cell lines (CRMM1 and CRMM2) and the normal conjunctival epithelial cells (HCjE‐Gi) were cultured using Corning® 96‐well ultralow attachment plates (Sigma‐Aldrich). Cells were seeded at 5 × 103 cells/well to form spheroids at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was exchanged every other day. To remove culture medium, the end of a pipette tip was placed at the neck region of the well to avoid spheroid disruption. Data that are not included in this paper indicated a continuous growth of the spheroids until day 3, a stable size until day 7 and a further growth on day 8. The size of the spheroids was maintained until day 30. Combining the results of the Experimental set‐up 1, the spheroids were treated with two bleomycin concentrations (1 μg/ml and 2.5 μg/ml) for 24 hr on day 3 and day 8. Three EP conditions were applied using the voltage pulse generator (CliniporatorTM) designed by IgeaS.p.A. (Capri, Modena, Italy). Details of all experimental conditions are given below.
No EP;
20 square WEP of 500 Volts/cm pulse strength, 100 μs pulse duration, 5 Hz repetition frequency;
8 square WEP of 750 Volts/cm pulse strength, 100 μs pulse duration, 5 Hz repetition frequency.
The EP was conducted in the wells using two flat parallel stainless steel electrodes on day 3. At 24 and 72 hr following treatment, the spheroids were collected, fixed in 10% neutral buffered formalin (Leica Microsystems UK Ltd, Milton Keynes, UK) and prepared for histological and immunohistochemical (IHC) analysis.
Histology and Immunohistochemistry
Molten agar was added to the formalin‐fixed spheroids, collected at the bottom of a microfuge tube and allowed to set. The agar‐embedded spheroids were then removed from the tubes and placed in tissue cassettes before being loaded onto the BAYER VIP E300 tissue processor. Following dehydration, agar‐embedded spheroids were orientated in wax blocks for sectioning. 4‐μm sections were cut onto Xtra™ adhesive slides (Leica Microsystems UK Ltd) and stained with haematoxylin and eosin (H&E) according to standard protocols. The spheroids were evaluated for their size, shape regularity, the presence/absence of central spheroid necrosis, the presence/absence of apoptotic cells and the presence/absence of adjacent dispersed cells surrounding them.
IHC was performed to detect the Ki‐67 antigen using a mouse anti‐human Ki67 antibody (Clone MM1; Leica Microsystems UK Ltd) at a dilution of 1:100 and a Dako EnVision Flex kit (Agilent Technologies LDA UK Limited, Cheadle, UK) according to the manufacturer's instructions following dewaxing and heat‐induced epitope retrieval in the Dako pretreatment module; slides were incubated in a high‐pH bath containing EnVisionTM FLEX target retrieval solution (Tris/EDTA buffer pH9.0) at 96°C for 20 min. Bound antibody was visualized with EnVision™FLEX 3,3′‐diaminobenzidine (DAB+). Sections were counterstained with Mayer's haematoxylin (VWR International Ltd, Lutterworth, UK), blued with Scott's tap water (Leica Microsystems UK Ltd) and mounted with DPX mountant (Sigma, St. Louis, Mo, USA). Human tonsil tissue was used as a positive control. Negative control sections were incubated with mouse IgG1. ImageJ2 (NIH Image, 2009) was used as a processing program for the multidimensional image analysis and for the Ki‐67 proliferation evaluation.
Results
Experimental set‐up 1
In 2D culture, the most significant effects of 5‐FU, bleomycin and MMC to reduce cell number were seen 72 hr following incubation with each of the agents alone (Fig. 1); EC50 values are shown in Table 1.
Figure 1.
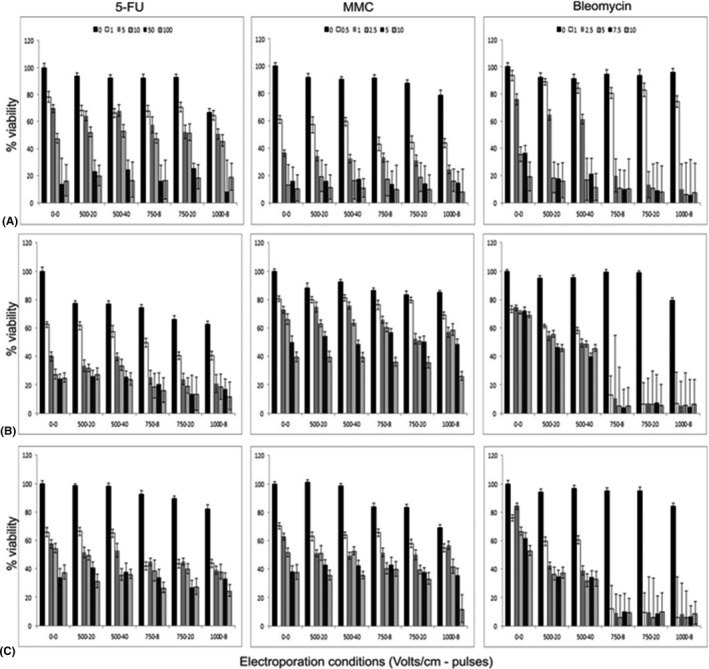
Cytotoxicity of 5‐FU, MMC and bleomycin with and without electroporation 72 hr following treatment in (A) HCjE normal conjunctival cells (B) CRMM1 and (C) CRMM2 conjunctival melanoma cells. Bars within each electroporation condition represent increasing concentrations of the drug from baseline, that is cells alone. The black bars show the effect of increasing electroporation alone on cell viability. Enhanced effects of bleomycin to reduce CRMM1 and CRMM2 viability can clearly be seen with increasing electroporation; however, this is less evident for 5FU and MMC. HCjE cells are sensitive to increasing concentrations of 5FU, MMC and bleomycin irrespective of the electroporation conditions employed. Values are the mean ± SEM.
HCjE‐Gi cells were most sensitive to MMC with an EC50 of 0.58 μg/ml. EP had no significant effect to enhance the efficacy of 5‐FU or MMC in HCjE‐Gi cells after 72 hr (Fig. 1). However, EP of 750 Volts/cm for 8 pulses increased the sensitivity of HCjE‐Gi cells to bleomycin (Fig. 1), such that the EC50 for this drug was reduced from 3.36 μg/ml to 1.42 μg/ml. This was not further enhanced by either increasing the number of pulses or increasing the voltage to 1000 Volts/cm.
CRMM1 cells were most sensitive to 5‐FU with an EC50 of 1.07 μg/ml, and the cytotoxic effect of this drug was not further enhanced by EP (Fig. 1). Similarly, EP did not further increase the sensitivity of CRMM1 cells to MMC.
CRMM2 cells were most sensitive to MMC with an EC50 of 0.98 μg/ml, effects that were not further enhanced by EP (Fig. 1). Interestingly, EP ≥750 Volts/cm further increased the sensitivity of CRMM2 cells to only the lowest dose of 5‐FU tested, 1 μg/ml (Fig. 1).
CRMM1 and CRMM2 cells were largely resistant to the cytotoxic effects of bleomycin alone; however, this was enhanced in both cell lines by EP (Fig. 1). EP conditions of ≥750 Volts/cm significantly enhanced the cytotoxic effects of bleomycin such that even at the lowest dose tested, 1 μg/ml, cell numbers were maximally reduced compared with cells treated with this dose of bleomycin alone; 6.5% cell viability compared with 73.1% (p ‐, paired t‐test) in CRMM1 cells, and 5.9% cell viability compared with 76.1% (p ‐, paired t‐test) in CRMM2 cells. This enhanced cytotoxic effect of 1 μg/ml bleomycin in the presence of 750 Volts/cm was not observed in the HCjE cells.
Experimental set‐up 2
Based on the results of the experiments performed in 2D culture, a concentration of 1 μg/ml bleomycin and the electric field condition of 750 Volts/cm with 8 pulses, 100 μs pulse duration, 5 Hz repetition frequency were applied to a 3D spheroid model.
Bleomycin alone had only a limited effect on cell viability when examining the H&E‐stained sections for degenerative changes. For example, no cytotoxic effect of bleomycin could be seen in CRMM1 and HCjE‐Gi cell lines on day 3 (Figs 2, 4). However, a cytotoxic effect of 1 μg/mg bleomycin without EP was noted on day 3 for the CRMM2 (Fig. 3).
Figure 2.
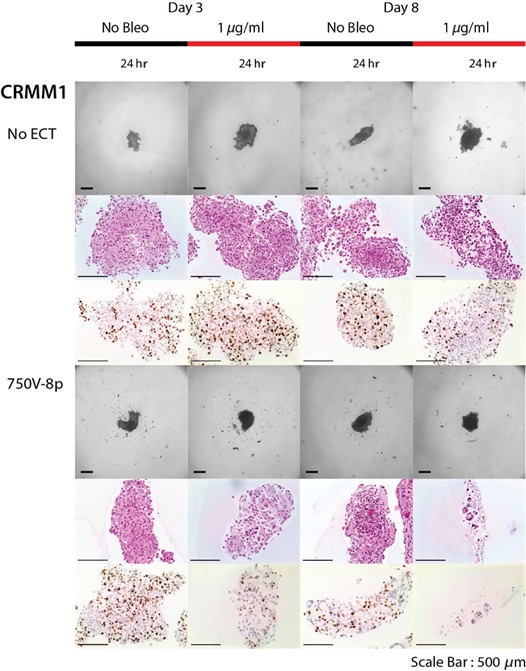
Decreased growth fraction on day 3 as well as on day 8, after treatment with 1 μg/ml bleomycin and ECT with 750 Volts/cm for 8 pulses but not for bleomycin alone for CRMM1 cell line. Reduction in the spheroid mass with necrosis and loss of cell definition with the formation of confluent cells with multiple nuclei.
Figure 4.
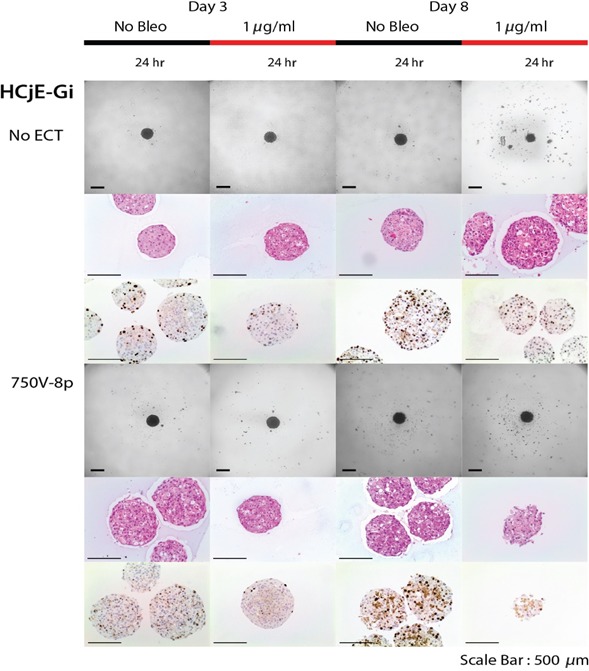
Treatment results of bleomycin (1 μg/ml) with ECT (750 Volts/cm and 8 pulses) on spheroids for HCjE‐Gi cell line. Decreased cell death 24 hr after treatment on day 3 of normal epithelial conjunctival cells.
Figure 3.
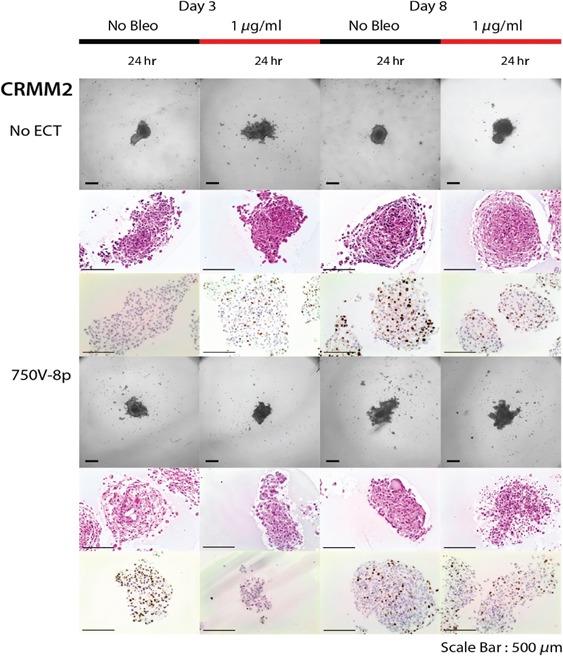
Effect of bleomycin alone (1 μg/ml) and after combination with 750 Volts/cm and 8 pulses on spheroid viability on day 3 and day 8 for CRMM2 cell line. Increased cell death 24 hr after combined treatment on day 3 as well as on day 8.
When combining 1 μg/ml bleomycin with EP at 750 Volts/cm for 8 pulses, a reduction in the spheroid mass together with necrosis, a loss of cell definition and the formation of confluent cells with multiple nuclei was observed. This was noted 24 hr following treatment on day 8 in all cell lines (Figs 2, 3, 4). In terms of a decreased spheroid size and histological changes, this appeared to be most apparent in the CRMM2 cells following ECT. Furthermore, there was a decreased Ki‐67 growth fraction in all three cell lines on day 3 as well as on day 8 compared to the findings without treatment (Figs 2, 3, 4). The combined treatment led to 89.5% reduction (p ‐, paired t‐test) in the proliferation of the CRMM1 and to 88.1% reduction (p ‐, paired t‐test) of the CRMM2 on day 3. On the other hand, the bleomycin treatment alone showed an increase in the Ki‐67 staining by 61.6% of the CRMM1 cells and 59.5% of the CRMM2 cells.
Discussion
Presented here are novel results investigating the application of ECT in CM using 2D and 3D cell culture models. ECT constitutes a new therapeutic strategy, effective even with highly reduced doses and time exposure of standard chemotherapeutic drug protocols. Indeed, the application of ECT may significantly reduce adverse drug side‐effects, including toxic effects to surrounding normal tissues. The mechanisms of increasing drug efficacy of ECT are dependent on various factors, such as the pulse duration, amplitude and the number of delivered pulses (Mir et al. 1991).
Current treatments of CM include surgical excision, cryotherapy adjunctive plaque therapy and topical chemotherapy for residual conjunctival melanoma in situ (Kenawy et al. 2013). The topical agents used include MMC and 5‑FU (Yeatts et al. 1995; Salomao et al. 1999; Midena et al. 2000; Kim & Abramson 2008), and both are associated with significant side‐effects, mainly due to their toxicity on corneal stem cells. The latter can cause limbal stem cell failure leading to in some cases in corneal erosion or ulceration, scleral ulceration, and in advanced cases pathological changes of the anterior uvea (Yeatts et al. 1995; Salomao et al. 1999). Consequently, alternative therapies are being investigated for CM.
Bleomycin is widely used to inhibit cancer cell proliferation (Domenge et al. 1996). It is a water‐soluble antibiotic, and its cytotoxicity is associated with the induction of single‐ and double‐strand DNA breaks (Byrnes et al. 1990). Due to its high molecular weight (MW 1487.49), bleomycin cannot easily penetrate the cell membrane and enter the cell, limiting bleomycin's cytotoxic effect and clinical applicability (Roy & Horwitz 1984; Takita 1984). ECT allows the penetration of molecules into the cytoplasm through the formation of reversible pores in the cell membrane (Gehl 2003; Mir et al. 2006).
In 2D culture of CM cell lines, we demonstrated that electroporation did not significantly enhance the cytotoxicity of MMC and 5‐FU when compared with HCjE‐Gi normal conjunctival cells. However, ECT combined with 1 μg/ml bleomycin at 750 Volts/cm with 8 pulses reduced cell viability of the CM cell lines, without influencing cell viability of the normal conjunctival cells.
The aim of the second part of our study was to develop a 3D in vitro model of CM cells, which would more accurately mimic in vivo conditions and enable successful EP and ECT. Cancer cell lines traditionally grown as monolayers for investigating the mechanisms and treatment of cancer fail to reproduce the physiological phenomena observed in vivo (Desoize 2000). They do not reproduce the morphology and biochemical features that cells possess in the original tissue. As an alternative, 3D in vitro models of cells offer the possibility to study different parameters under conditions that more closely resemble the in vivo situation. In the 2D in vitro model that we applied in Experiment 1, only the combination of bleomycin together with ECT significantly reduced cell viability in CRMM1, CRMM2 and HCjE‐Gi cell lines. However, the effect of the combined treatment on the spheroid viability was lower in the HCjE‐Gi cell line. Because MMC and 5‐FU molecules are smaller than bleomycin molecules (MMC, MW 334.33; 5‐FU, MW 130.8), they pass easily through the cellular membrane and the influence of electroporation on their cytotoxic effect was negligible (Yeatts et al. 1995; Salomao et al. 1999). Therefore, bleomycin in combination with the optimal ECT conditions from the first part of our study was used to evaluate the cytotoxic effect in the 3D model of CRMM1, CRMM2 and HCjE‐Gi cell lines. We show for the first time that EP sensitizes CM cells to doses that have a minimal effect on the normal conjunctival cells on 2D and 3D models.
Further development of this platform using more advanced in vitro 3D experiments as well as in preclinical animal models (e.g. chick embryo (Kalirai et al. 2015), or murine CM models (de Waard et al. 2015)) will facilitate the acceptance of irreversible electroporation (IRE) as a viable cancer therapy.
This study was supported by Gertrud Kusen‐Stiftung and by Dr. Rolf M. Schwiete‐Stiftung.
The authors are thankful to Prof. Dr. Martine J. Jager from the Laboratory of Ophthalmology at LUMC, Leiden, The Netherlands, for providing the CM cell lines; to Prof. Dr. Colin Willoughby, University of Liverpool, UK, for providing the normal conjunctival cell line; and to Mr Simon Biddolph for his advice regarding spheroid embedding and for help in morphological staining. Mr Periklis Katopodis was partially funded from the LOORG Group, Liverpool, UK (www.loorg. org).
References
- Bourke MG, Salwa SP, Sadadcharam M et al. (2017): Effective treatment of intractable cutaneous metastases of breast cancer with electrochemotherapy: ten‐year audit of single centre experience. Breast Cancer Res Treat 161: 289–297. [DOI] [PubMed] [Google Scholar]
- Byrnes RW, Templin J, Sem D, Lyman S & Petering DH (1990): Intracellular DNA strand scission and growth inhibition of Ehrlich ascites tumor cells by bleomycin. Cancer Res 50: 5275–5286. [PubMed] [Google Scholar]
- Cemazar M, Milacic R, Miklavcic D, Dolzan V & Sersa G (1998): Intratumoral cisplatin administration in electrochemotherapy: antitumor effectiveness, sequence dependence and platinum content. Anticancer Drugs 9: 525–530. [DOI] [PubMed] [Google Scholar]
- Damato B & Coupland SE (2009): Management of conjunctival melanoma. Expert Rev Anticancer Ther 9: 1227–1239. [DOI] [PubMed] [Google Scholar]
- Desoize B (2000): Contribution of three‐dimensional culture to cancer research. Crit Rev Oncol Hematol 36: 59–60. [DOI] [PubMed] [Google Scholar]
- Domenge C, Orlowski S, Luboinski B, De Baere T, Schwaab G, Belehradek J Jr & Mir LM (1996): Antitumor electrochemotherapy: new advances in the clinical protocol. Cancer 77: 956–963. [DOI] [PubMed] [Google Scholar]
- Edhemovic I, Brecelj E, Gasljevic G et al. (2014): Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol 110: 320–327. [DOI] [PubMed] [Google Scholar]
- Fiorentzis M, Kalirai H, Katopodis P, Seitz B, Viestenz A & Coupland SE (2018): Electrochemotherapy with bleomycin and cisplatin enhances cytotoxicity in primary and metastatic uveal melanoma cell lines in vitro. Neoplasma 65: 210–215. [DOI] [PubMed] [Google Scholar]
- Gehl J (2003): Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand 177: 437–447. [DOI] [PubMed] [Google Scholar]
- Gehl J, Skovsgaard T & Mir LM (2002): Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta 1569: 51–58. [DOI] [PubMed] [Google Scholar]
- Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R & Heller R (2000): Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs 11: 201–208. [DOI] [PubMed] [Google Scholar]
- Kalirai H, Shahidipour H, Coupland SE & Luyten GPM (2015): Use of the chick embryo model in uveal melanoma. Ocul Oncol Pathol 1: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser S, Maat W, Missotten GS & de Keizer RJ (2007): A new cell line from a recurrent conjunctival melanoma. Br J Ophthalmol 91: 1566–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenawy N, Lake SL, Coupland SE & Damato BE (2013): Conjunctival melanoma and melanocytic intra‐epithelial neoplasia. Eye (Lond) 27: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW & Abramson DH (2008): Topical treatment options for conjunctival neoplasms. Clin Ophthalmol 2: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte C, Letulé V, Gehl J et al. (2017): Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol 176: 1475–1485. [DOI] [PubMed] [Google Scholar]
- Lenzi R, Muscatello L, Saibene AM, Felisati G & Pipolo C (2017): The controversial role of electrochemotherapy in head and neck cancer: a systematic review of the literature. Eur Arch Otorhinolaryngol 274: 2389–2394. [DOI] [PubMed] [Google Scholar]
- Markelc B, Sersa G & Cemazar M (2013): Differential mechanisms associated with vascular disrupting action of electrochemotherapy: intravital microscopy on the level of single normal and tumor blood vessels. PLoS ONE 8: e59557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M, Sersa G, Garbay JR et al. (2006): Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl 4: 3–13. [Google Scholar]
- McCartney AC (1995): Pathology of ocular melanomas. Br Med Bull 51(3): 678e93. [DOI] [PubMed] [Google Scholar]
- Midena E, Angeli CD, Valenti M, de Belvis V & Boccato P (2000): Treatment of conjunctival squamous cell carcinoma with topical 5‐fluorouracil. Br J Ophthalmol 84: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir LM, Belehradek M, Domenge C et al. (1991): Electrochemotherapy, a new antitumor treatment: first clinical trial. C R Acad Sci III 313: 613–618. [PubMed] [Google Scholar]
- Mir LM, Gehl J, Sersa G et al. (2006): Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non‐invasive electrodes. EJC Suppl 4: 14–25. [Google Scholar]
- Nareyeck G, Wuestemeyer H, von der Haar D & Anastassiou G (2007): Establishment of two cell lines derived from conjunctival melanomas. Exp Eye Res 81: 361–362. [DOI] [PubMed] [Google Scholar]
- Nørregaard JC, Gerner N, Jensen OA & Prause JU (1996): Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol 234: 569–572. [DOI] [PubMed] [Google Scholar]
- Orlowski S, Belehradek J Jr, Paoletti C & Mir LM (1988): Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol 37: 4727–4733. [DOI] [PubMed] [Google Scholar]
- Roy SN & Horwitz SB (1984): Characterization of the association of radiolabeled bleomycin A2 with HeLa cells. Cancer Res 44: 1541–1546. [PubMed] [Google Scholar]
- Saczko J, Kamińska I, Kotulska M et al. (2014): Combination of therapy with 5‐fluorouracil and cisplatin with electroporation in human ovarian carcinoma model in vitro. Biomed Pharmacother 68: 573–580. [DOI] [PubMed] [Google Scholar]
- Salomao DR, Mathers WD, Sutphin JE, Cuevas K & Folberg R (1999): Cytologic changes in the conjunctiva mimicking malignancy after topical mitomycin C chemotherapy. Ophthalmology 106: 1756–1760. [DOI] [PubMed] [Google Scholar]
- Seregard S (1998): Conjunctival melanoma. Surv Ophthalmol 42: 321–350. [DOI] [PubMed] [Google Scholar]
- Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G & Snoj M (2008): Electrochemotherapy in treatment of tumours. Eur J SurgOncol 34: 232–240. [DOI] [PubMed] [Google Scholar]
- Takita T (1984): Mechanism of action of bleomycin at the molecular level. GanTo Kagaku Ryoho 11: 2659–2665. [PubMed] [Google Scholar]
- Triay E, Bergman L, Nilsson B, All‐Ericsson C & Seregard S (2009): Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol 93: 1524–1528. [DOI] [PubMed] [Google Scholar]
- Vásquez JL, Ibsen P, Lindberg H & Gehl J (2000): In vitro and in vivo experiments on electrochemotherapy for bladder cancer. J Urol 193: 1009–1015. [DOI] [PubMed] [Google Scholar]
- de Waard NE, Cao J, McGuire SP, Kolovou PE, Jordanova ES, Ksander BR & Jager MJ (2015): A murine model for metastatic conjunctival melanoma. Invest Ophthalmol Vis Sci 56: 2325–2333. [DOI] [PubMed] [Google Scholar]
- Yeatts RP, Ford JG, Stanton CA et al. (1995): Topical 5‐fluorouracil in treating epithelial neoplasia of the conjunctiva and cornea. Ophthalmology 102(9): 1338–1344. [DOI] [PubMed] [Google Scholar]
- Yeatts RP, Engelbrecht NE, Curry CD, Ford JG & Walter KA (2000): 5‐Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology 107: 2190–2195. [DOI] [PubMed] [Google Scholar]


