Figure 6.
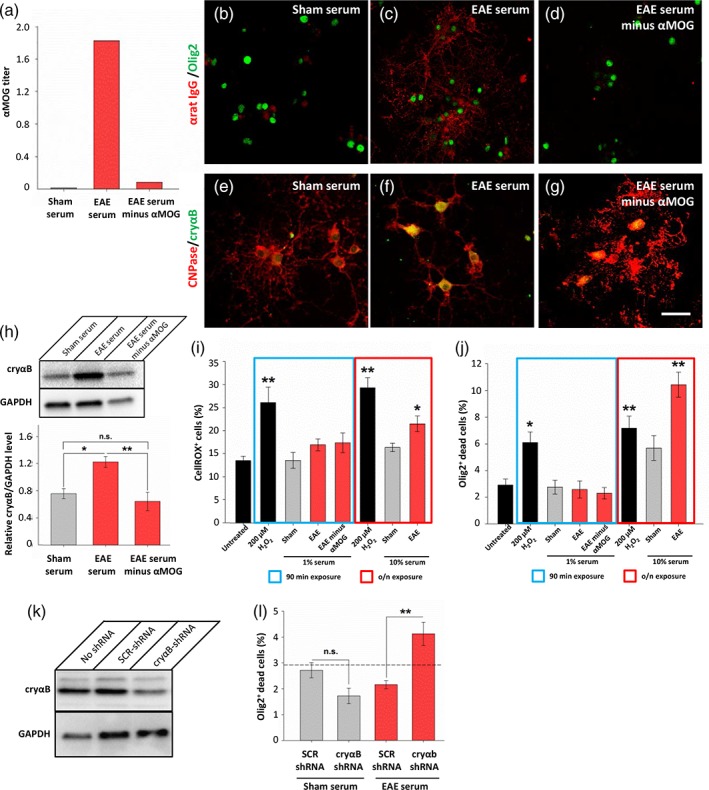
Anti‐MOG antibodies are necessary for EAE sera to induce αB‐crystallin upregulation in primary oligodendrocyte cultures. (a) ELISA of anti‐MOG antibody titers demonstrates efficacy of anti‐MOG antibody depletion. (b–d) EAE serum, but not that depleted of anti‐MOG antibodies, is able to immunolabel unfixed oligodendrocytes (red, anti‐IgG; green, olig2). (e–g) Oligodendrocyte cultures exposed to 1% sera for 90 min followed by overnight recovery were immunolabeled with antibodies against CNPase (red) and αB‐crystallin (cryαB, green). (h) Western blot with quantification showing upregulation of αB‐crystallin following 90 min exposure to 1% EAE serum, but not with EAE serum depleted of anti‐MOG antibodies. (i) Reactive oxygen species were quantified in oligodendrocyte cultures using CellROX assay. Cells exposed to H2O2 had increased ROX positivity, but not cells exposed to 1% serum. Overnight exposure of cells to 10% serum or H2O2 served as positive controls. (j) Oligodendrocyte toxicity was assessed using a fixable LIVE/DEAD assay demonstrating increased cell death upon exposure to H2O2, and also EAE serum when exposed overnight. (k) Lentiviral delivery of shRNA against αB‐crystallin successfully reduced αB‐crystallin expression in oligodendrocyte cultures compared to delivery of a scrambled control (SCR) shRNA sequence. All cultures were exposed to 10% EAE serum for 90 min followed by overnight recovery. (l) Oligodendrocytes treated with shRNA targeting αB‐crystallin had increased cell death upon 90 min exposure to 1% EAE sera compared to those treated with scrambled shRNA, or exposure to sham sera. Dashed line indicates the untreated control levels (as shown in panel j). Scale bar = 50 μm; *p < .05, **p < .01, ANOVA
