Abstract
Aim
To evaluate the efficacy and safety of fast‐acting insulin aspart (faster aspart) vs insulin aspart (IAsp) used in continuous subcutaneous insulin infusion (CSII) in participants with type 1 diabetes (T1D).
Materials and Methods
This was a double‐blind, treat‐to‐target, randomized, 16‐week trial investigating CSII treatment with faster aspart (n = 236) or IAsp (n = 236). All available information, regardless of treatment discontinuation, was used for the evaluation of effect.
Results
Faster aspart was non‐inferior to IAsp regarding the change from baseline in glycated haemoglobin (HbA1c; primary endpoint). The mean HbA1c changed from 58.4 mmol/mol (7.5%) at baseline to 57.8 mmol/mol (7.4%) with faster aspart and to 56.8 mmol/mol (7.4%) with IAsp after 16 weeks' treatment, with an estimated treatment difference (ETD) of 1.0 mmol/mol (95% confidence interval [CI] 0.14; 1.87) or 0.09% (95% CI 0.01; 0.17; P < 0.001) for non‐inferiority (0.4% margin; P < 0.02 for statistical significance in favour of IAsp). Faster aspart was superior to IAsp in change from baseline in 1‐hour postprandial glucose (PPG) increment after a meal test (ETD −0.91 mmol/L [95% CI −1.43; −0.39] or −16.4 mg/dL [95% CI −25.7; −7.0]; P = 0.001), with statistically significant reductions also at 30 minutes and 2 hours. The improvement in PPG was reflected in the change from baseline in 1‐hour interstitial glucose increment after all meals (ETD −0.21 mmol/L [95% CI −0.31; −0.11] or −3.77 mg/dL [95% CI −5.53; −2.01]). There was no statistically significant difference in the overall rate of severe or blood glucose‐confirmed hypoglycaemia (estimated rate ratio 1.00 [95% CI 0.85; 1.16]). A numerical imbalance in severe hypoglycaemic episodes between faster aspart and IAsp was seen in the treatment (21 vs 7) and 4‐week run‐in periods (4 vs 0).
Conclusions
Faster aspart provides an effective and safe option for CSII treatment in T1D.
Keywords: clinical trial, CSII, insulin therapy, type 1 diabetes
1. INTRODUCTION
Insulin pump therapy (or continuous subcutaneous insulin infusion [CSII]) in people with type 1 diabetes (T1D) presents advantages over multiple daily insulin injection (MDI) regimens. These include improved glycaemic control and a reduced rate of hypoglycaemic episodes1, 2; however, real‐world data show that, despite using CSII with or without continuous monitoring devices, only 30% of adults with T1D achieve glycated haemoglobin (HbA1c) of <53 mmol/mol (<7.0%).3 In addition to better insulin delivery and monitoring technologies, there is also a need to develop insulins with pharmacological and glucose‐lowering profiles that more closely resemble physiological insulin action. To this end, ultra‐fast‐acting insulins, such as fast‐acting insulin aspart (faster aspart),4 BioChaperone lispro5 and treprostinil lispro,6 that target postprandial glucose (PPG) excursions, are under study or in development for use with MDI and CSII regimens; inhaled insulin is also under study as an alternative approach.7 PPG is an important component of improving overall glycaemic control.
Faster aspart is conventional insulin aspart (IAsp) in a new formulation, in which two excipients, niacinamide and L‐arginine, have been added.4 In a pooled analysis in participants with T1D, faster aspart demonstrated an earlier onset of appearance, a higher early insulin exposure and a greater early glucose‐lowering effect vs IAsp, when both were administered by subcutaneous injection.4 More pronounced clinical pharmacological improvements and a greater glucose‐lowering effect were demonstrated in people with T1D using CSII.8, 9
With regard to change in HbA1c in people with T1D, faster aspart was non‐inferior (0.4% margin) to mealtime IAsp when combined with insulin detemir after 26 weeks of treatment. In addition, faster aspart was associated with a significantly greater reduction in HbA1c and superior 2‐hour PPG increment (meal test), with no difference in the incidence of overall severe or blood glucose (BG)‐confirmed hypoglycaemia.10 Faster aspart was compared with IAsp during CSII therapy in a randomized study of 37 participants with T1D. There were no detected microscopically confirmed infusion‐set occlusions in either treatment arm over a 6‐week period.11
The aim of the present onset 5 study was to confirm the effect of CSII treatment with faster aspart regarding glycaemic control by comparing it to CSII treatment with IAsp, in adults with T1D. The trial aimed to test superiority in terms of PPG regulation and time spent with low interstitial/sensor glucose levels, while also evaluating the CSII safety profile of both treatments. The trial was designed to quantify a population average effect for participants with T1D irrespective of adherence to randomized treatment and use of ancillary therapies. The primary objective was to estimate the effect based on difference in HbA1c from baseline to 16 weeks under these circumstances.
2. MATERIALS AND METHODS
2.1. Study design
In this double‐blind, randomized, multicentre, parallel‐group, treat‐to‐target trial with a 4‐week run‐in and 16‐week treatment period (Clinicaltrials.gov: NCT02825251), faster aspart was compared with IAsp, both administered via CSII, in adults with T1D (Figure S1, Supporting Information). The trial was conducted at 92 sites in nine countries (Belgium, Canada, France, Germany, the Netherlands, Russian Federation, Slovenia, UK, USA). A list of study sites and investigators is included in the Supplementary Appendix within Supporting Information. The trial was conducted according to the Declaration of Helsinki Amended 2013 and International Conference on Harmonization Good Clinical Practice (1996). All patients provided written informed consent.
2.2. Participants
Adults (≥18 years) with T1D (diagnosed clinically for ≥12 months) were eligible if they were using the same insulin pump (MiniMed530G, Paradigm Veo, Paradigm Revel or Paradigm; Medtronic Inc, Minneapolis, Minnesota) for CSII therapy with a rapid‐acting insulin analogue for ≥6 months prior to screening, and they were willing to stay on the same pump model throughout the trial. Further eligibility criteria were HbA1c 53 to 75 mmol/mol (7.0%‐9.0%) and body mass index ≤35 kg/m2. Full inclusion and exclusion criteria are listed in the Supplementary Appendix within Supporting Information.
2.3. Procedures
During the 4‐week run‐in period, participants remained on their pre‐trial insulin, and basal pump rates and bolus dose calculator settings were not adjusted unless for safety reasons. At randomization, participants switched from pre‐trial insulin to faster aspart or IAsp (both 100 U/mL), both double‐blind, on a unit‐for‐unit basis, keeping current pump parameters the same. During the 16‐week treatment period (considered sufficient to reach a stable HbA1c level), the fasting and preprandial BG glycaemic target was 4.0 to 6.0 mmol/L (71‐108 mg/dL). Participants performed basal rate checks based on frequent measurements of self‐measured BG (SMBG) values and according to instructions from the investigator. Basal rates were adjusted to ensure that BG was kept in a stable range (within 2 mmol/L [35 mg/dL]) while in a fasting state. Mealtime insulin (initiated 0‐2 minutes before a meal) was titrated based on carbohydrate counting using a bolus dose calculator according to usual practice. Basal rates, as well as insulin:carbohydrate ratios, insulin sensitivity factors and active insulin time, were adjusted by the investigator at each telephone call and site visit if needed. Follow‐up occurred 7 and 30 days after end of treatment.
2.4. Standardized meal test
Participants had venous PPG levels assessed before and after a bolus dose of faster aspart or IAsp (0.1 U/kg, calculated by the investigator), which was followed by a standardized liquid meal (Ensure; Abbott Nutrition, Columbus, Ohio; 78 g carbohydrate consumed within 12 minutes). Only a standard‐wave bolus, where all bolus insulin is delivered at once, was allowed. Participants were required to attend the visit in a fasting state, with an SMBG of 4.0 to 8.8 mmol/L (71‐160 mg/dL). Changes to basal rate settings 0 to 4 hours before the test were not allowed. Blood samples were taken immediately before as well as 30 minutes, and 1, 2, 3 and 4 hours after the meal. The meal test was conducted with pre‐trial insulin before randomization at week 0 and with participant's study medication at week 16.
2.5. Continuous glucose monitoring
No more than 50% of participants were allowed to use their own real‐time continuous glucose monitoring (CGM) during the trial. Participants who did not use CGM pre‐trial did not start using CGM after enrolment. Participants who were using CGM pre‐trial did not change normal practice after enrolment. They were not allowed to use low glucose suspend mode if it was a pump feature. Randomization was stratified according to use of unblinded CGM. All participants were provided with a blinded CGM device to wear during three trial periods: (a) before randomization (including during the pre‐treatment meal test); (b) before the 8th week after randomization; and (c) before the 16th week after randomization (including during the within‐treatment meal test).
2.6. Self‐measured blood glucose
At the start of the run‐in, participants were supplied with a BG meter to measure glucose values and to calibrate the blinded CGM. All SMBG values were automatically transferred to the pump. Four‐point profiles were recorded daily for insulin titration purposes, and 7‐7‐9‐point profiles were recorded on 3 consecutive days before scheduled clinic visits (and the morning of the visit) at weeks 0, 8 and 16, and were used to evaluate the glucose profile.
2.7. Outcomes
The primary endpoint was change from baseline in HbA1c 16 weeks after randomization.
Confirmatory secondary endpoints were change from baseline 16 weeks after randomization in: 1‐hour PPG increment (meal test), 1,5‐anhydroglucitol (1,5‐AG), and time spent with low interstitial glucose (IG) levels (≤3.9 mmoL/L [70 mg/dL]) during CGM. Supportive secondary endpoints are listed in Table S1, Supporting Information.
Severe hypoglycaemia was defined according to the American Diabetes Association classification12 and BG‐confirmed hypoglycaemia was defined as a plasma glucose value <3.1 mmol/L (56 mg/dL), with or without symptoms consistent with hypoglycaemia. Meal‐related hypoglycaemic episodes were evaluated from start of meal (0–1‐hour, >1–2‐hour, >2–3‐hour and >3–4‐hour time points).
2.8. Statistical analysis
All statistical analyses were prespecified. Efficacy endpoints were summarized and analysed using the full analysis set, and results are presented based on data from all randomized participants for the entire trial period, which includes data collected after participants prematurely discontinued treatment. Safety endpoints (and insulin dose and pump parameters) were summarized using the safety analysis set (participants who received ≥1 dose of IAsp or faster aspart) and are presented based on data collected up to and including 7 days after discontinuation of treatment. Statistical analysis of the primary and secondary confirmatory endpoints followed a stepwise hierarchical procedure (Figure S2, Supporting Information). Non‐inferiority (primary endpoint) was confirmed if the upper boundary of the two‐sided 95% confidence interval (CI) was ≤0.4%. One‐sided P values are presented for non‐inferiority analyses and for the other confirmatory analyses, with two‐sided P values for treatment differences presented for all other analyses.
Change from baseline in HbA1c, PPG and PPG increment (meal test) 16 weeks after randomization was analysed using a multiple imputation model. HbA1c responder endpoints were analysed using a logistic regression model. Change from baseline in mean 7‐7‐9‐point profiles, mean PPG, mean PPG increments (7‐7‐9‐point profiles), 1,5‐AG, time spent with low IG levels, mean prandial IG increments, FPG and body weight were analysed using a multiple imputation model similar to the model used for the primary endpoint. The number of treatment‐emergent severe or BG‐confirmed hypoglycaemic episodes was analysed using a negative binomial regression model.
The sample‐size calculation and further details on statistical methods for the primary and secondary endpoints are provided in the Supplementary Appendix within Supporting Information.
3. RESULTS
A total of 472 participants were randomized to CSII treatment with either faster aspart (n = 236) or IAsp (n = 236) between July 2016 and July 2017. All randomized participants were exposed to treatment; 463 participants (98.1%) completed the trial period, while 455 (96.4%) completed the treatment period without premature discontinuation of randomized treatment (Figure S3, Supporting Information). The most frequent reason for discontinuing treatment or withdrawing from the trial was “participant decision.” Baseline characteristics were similar between treatment arms (Table 1). Approximately 25% of participants in each treatment arm were using their own CGM device.
Table 1.
Baseline characteristics
| Faster aspart (n = 236) | Insulin aspart (n = 236) | Total (n = 472) | |
|---|---|---|---|
| Age, years | 43.3 (14.8) | 43.6 (14.7) | 43.5 (14.7) |
| Male, n (%) | 103 (43.6) | 100 (42.4) | 203 (43.0) |
| Body weight | |||
| kg | 76.9 (15.2) | 78.2 (14.5) | 77.5 (14.8) |
| lb | 169.5 (33.5) | 172.4 (31.9) | 170.9 (32.7) |
| BMI, kg/m2 | 26.2 (4.1) | 26.5 (3.9) | 26.3 (4.0) |
| Duration of diabetes, years | 25.0 (12.7) | 23.3 (11.3) | 24.2 (12.0) |
| HbA1c, % | 7.5 (0.5) | 7.5 (0.5) | 7.5 (0.5) |
| mmol/mol | 58.4 (6.0) | 58.4 (5.8) | 58.4 (5.9) |
| FPG | |||
| mmol/L | 7.6 (2.6) | 7.4 (2.3) | 7.5 (2.5) |
| mg/dL | 136.9 (47.6) | 133.3 (41.7) | 135.1 (44.7) |
| Previous insulin use, n (%) | |||
| Insulin aspart | 126 (53.4) | 142 (60.2) | 268 (56.8) |
| Insulin lispro | 102 (43.2) | 86 (36.4) | 188 (39.8) |
| Insulin glulisine | 8 (3.4) | 8 (3.4) | 16 (3.4) |
| Pump model at screening, % | |||
| Paradigm Veoa | 132 (55.9) | 119 (50.4) | 251 (53.2) |
| Minimed 530Ga | 47 (19.9) | 49 (20.8) | 96 (20.3) |
| Paradigm | 35 (14.8) | 35 (14.8) | 70 (14.8) |
| Paradigm Revel | 22 (9.3) | 33 (14.0) | 55 (11.7) |
| Infusion set first dispensedb, % | |||
| Quick‐set | 154 (65.3) | 170 (72.0) | 324 (68.6) |
| Silhouette | 41 (17.4) | 35 (14.8) | 76 (16.1) |
| Mio | 24 (10.2) | 19 (8.1) | 43 (9.1) |
| Sure‐T (Easy set) | 17 (7.2) | 12 (5.1) | 29 (6.1) |
Abbreviations: BMI, body mass index; faster aspart, fast‐acting insulin aspart; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin.
Data are mean (SD) unless otherwise stated.
The recommended frequency for changing each infusion set was every 3 days for the Quick‐set, Silhouette and Mio, and every 2 days for the Sure‐T, as per the manufacturers' instructions.
Low glucose suspend feature not allowed as per protocol.
Participants were free to change infusion sets during the trial.
In the faster aspart and IAsp treatment arms, HbA1c decreased from 61.61 mmol/mol (7.79%) and 61.80 mmol/mol (7.80%) to 58.38 mmol/mol (7.49%) and 58.41 mmol/mol (7.49%), respectively, during the run‐in period, followed by a further change 16 weeks after randomization to 57.77 mmol/mol (7.44%) in the faster aspart arm and 56.83 mmol/mol (7.35%) in the IAsp arm (Figure 1). Non‐inferiority of faster aspart to IAsp in terms of change from baseline in HbA1c was confirmed with an estimated treatment difference (ETD) of 1.0 mmol/mol (95% CI 0.14; 1.87) or 0.09% (95% CI 0.01; 0.17; P < 0.001) for non‐inferiority (0.4% margin). This difference was statistically significantly in favour of IAsp (P < 0.02). Superiority of faster aspart to IAsp with regard to the change from baseline in HbA1c could not be confirmed; therefore, the hierarchical testing was stopped after step 3 (Table S2, Supporting Information).
Figure 1.
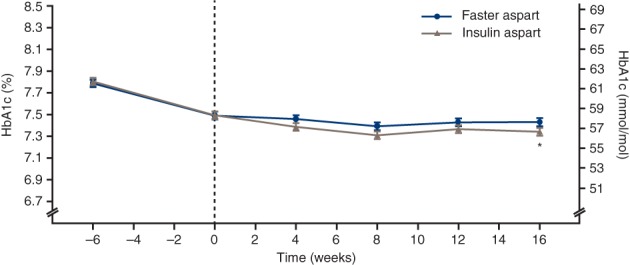
Mean glycated haemoglobin (HbA1c) over time. Error bars: ± SE (mean). *Estimated treatment difference was in favour of insulin aspart: 1.00 mmol/mol (95% confidence interval [CI] 0.14; 1.87) or 0.09% (95% CI 0.01; 0.17); P = 0.022. Non‐inferiority confirmed at 0.4% level (one‐sided test for non‐inferiority evaluated at the 2.5% level: P < 0.001). All available information regardless of treatment discontinuation was used. Faster aspart = fast‐acting insulin aspart
The proportion of participants achieving HbA1c target <53 mmol/mol (7.0%) is presented in Table S3, Supporting Information. The odds of achieving HbA1c <53 mmol/mol were not statistically significantly different between faster aspart and IAsp (ETD 0.76 [95% CI 0.46; 1.26]).
The observed change from baseline in 1‐hour PPG increment was −0.89 mmol/L (−16.0 mg/dL) and 0.05 mmol/L (0.98 mg/dL) in the faster aspart and IAsp arms, respectively (Figure 2). Superiority of faster aspart to IAsp in terms of change from baseline in 1‐hour PPG increment was confirmed (ETD −0.91 mmol/L [95% CI −1.43; −0.39] or −16.4 mg/dL [95% CI −25.7; −7.0]; P = 0.001). The estimated change from baseline in 30‐minute and 2‐hour PPG increment was also significantly in favour of faster aspart (30‐minute: −0.66 mmol/L [95% CI −1.00; −0.31] or −11.8 mg/dL [95% CI −18.1; −5.6], P < 0.001; 2‐hour: −0.90 mmol/L [95% CI −1.58; −0.22] or −16.2 mg/dL [95% CI −28.5; −4.0], P = 0.01). There were no statistically significant differences at 3 and 4 hours (Figure 2). The ETD for the change from baseline in PPG was also statistically significant in favour of faster aspart at 30 minutes, 1 hour and 2 hours (Table S3, Supporting Information). IG measurements during the meal test also support the PPG findings above (Table S3, Supporting Information).
Figure 2.
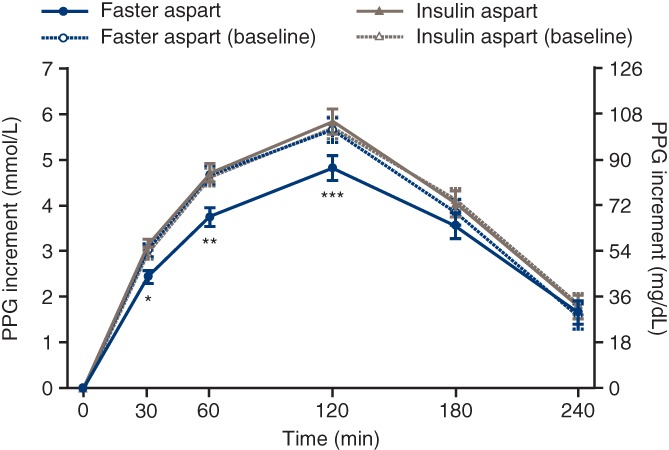
Postprandial glucose (PPG) increment after a standardized meal test at baseline and week 16. Error bars: ± SE (mean). *Estimated treatment difference (ETD) at week 16: −0.66 mmol/L (95% confidence interval [CI] −1.00; −0.31] or −11.8 mg/dL (95% CI −18.1; −5.6; P < 0.001). **ETD at week 16: −0.91 mmol/L (95% CI −1.43; −0.39) or −16.4 mg/dL (95% CI −25.7; −7.1; P = 0.001). ***ETD at week 16: −0.90 mmol/L (95% CI −1.58; −0.22) or −16.2 mg/dL (95% CI −28.5; −4.0; P = 0.01). All available information regardless of treatment discontinuation was used. Faster aspart = fast‐acting insulin aspart
Prandial IG and IG increment profiles at baseline and week 16 are presented in Figure 3. For breakfast, lunch, main evening meal, and across all meals, the incremental rise in mean IG after 30 minutes, 1 hour and 2 hours was lower with faster aspart vs IAsp, with statistically significant ETDs after 1 hour and 2 hours for each individual meal and the mean across all meals (Figure S4, Supporting Information). There was no statistically significant difference between treatments in the change from baseline to IG peak or time to IG peak (Table S3, Supporting Information). The mean time spent with low IG (≤3.9 mmol/L [70 mg/dL]) changed from 85.4 min/d and 79.9 min/d at baseline to 78.6 min/d and 83.0 min/d at week 16 with faster aspart and IAsp, respectively, with no statistical difference between treatments (ETD −6.74 min/d [95% CI −15.56; 2.09]). At week 16, the percentage of time spent with low IG (≤3.9 mmol/L [70 mg/dL]) was 5.5% and 5.8%, and for IG in target range (4.0‐10.0 mmol/L [71‐180 mg/dL]) it was 52.4% and 54.5% with faster aspart and IAsp, respectively. The means of the IG profile and the coefficient of variation in the IG profile were similar from baseline to 16 weeks for both treatments (Table S3, Supporting Information). The 24‐hour IG profiles at week 16 between faster aspart and IAsp were broadly similar, although the median and interquartile range IG values during the night trended higher compared with baseline in the faster aspart group (Figure S5, Supporting Information).
Figure 3.
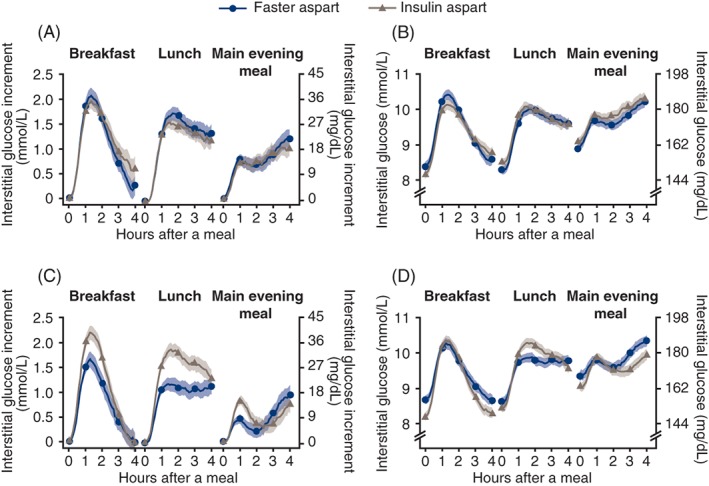
Prandial interstitial glucose (IG) profiles. A and B, IG increment and IG at baseline. C and D, IG increment and IG at week 16. Error bars: ± SE (mean). Prandial IG increment is derived as the IG values subtracted by the mean of IG values within 15 minutes before the start of the meal. Meal times during the continuous glucose monitoring period were captured in participants' diaries. All available information regardless of treatment discontinuation was used. Faster aspart = fast‐acting insulin aspart
The observed change from baseline in 1,5‐AG 16 weeks after randomization was 0.14 μg/mL and 0.25 μg/mL in the faster aspart and IAsp arms, respectively, with no statistically significant difference between treatments (ETD −0.10 μg/mL [95% CI −0.36; 0.17]).
The change from baseline in the estimated mean of the 7‐7‐9 SMBG profiles was not significantly different between treatments (Figure S6 and Table S3, Supporting Information). The observed change from baseline in the 1‐hour PPG increment, based on the 7‐7‐9 SMBG profiles, combined across all three main meals was −0.53 mmol/L (−9.60 mg/dL) with faster aspart and 0.12 mmol/L (2.12 mg/dL) with IAsp, with a statistically significant difference in favour of faster aspart (ETD −0.46 mmol/L [95% CI −0.90; −0.02] or −8.3 mg/dL [95% CI −16.3; −0.3]; P = 0.042). There were no significant differences in 1‐hour PPG increment (and 1‐hour PPG) at any individual meal (i.e. breakfast, lunch or at the main evening meal).
In both treatment groups, mean FPG showed an increase from baseline until week 8, and thereafter a decrease until week 16, with no statistically significant difference between treatments (ETD −0.07 mmol/L [95% CI −0.50; 0.36] or −1.29 mg/dL [95% CI −9.05; 6.48]). The fasting SMBG levels on the days when the meal tests were performed had to be 4.0 to 8.8 mmol/L (71‐160 mg/dL). At week 8 there was no requirement for any particular fasting SMBG value.
Mean and median daily basal and bolus insulin doses remained stable over the treatment period in both groups (Table S4, Supporting Information), with basal:bolus ratios of 48/52 at baseline, and 48/52 and 49/51 with faster aspart and IAsp, respectively, at week 16. The median insulin:carbohydrate ratio decreased with both treatments from 9.18 to 9.00 g/U with faster aspart and from 9.05 to 8.83 g/U with IAsp. The mean insulin sensitivity factor was 2.64 and 2.50 mmol/L/U at baseline, and 2.65 and 2.60 mmol/L/U 16 weeks after randomization with faster aspart and IAsp, respectively. The mean active insulin time was 3.6 hours at baseline and week 16 for both treatment groups.
The observed rates of treatment‐emergent severe or BG‐confirmed hypoglycaemia were 45.07 and 45.29 episodes per patient‐year of exposure (PYE) with faster aspart and IAsp, respectively. The overall rate of treatment‐emergent severe or BG‐confirmed hypoglycaemic episodes was not statistically significantly different between treatments, with an estimated rate ratio of 1.00 (95% CI 0.85; 1.16). A higher rate of severe or BG‐confirmed hypoglycaemic episodes was observed for faster aspart vs IAsp within the first hour after the start of a meal (1.25 vs 0.71 events/PYE; estimated rate ratio 1.78 [95% CI 1.15; 2.75]), with no statistically significant differences at other intervals during the first 4 hours after the start of the meal (Table S5 and Figure S7, Supporting Information). A numerically higher rate of severe hypoglycaemia was observed with faster aspart (0.29 events/PYE) vs IAsp (0.10 events/PYE) during the 16 weeks after randomization. During the run‐in period, three participants who were later randomized to faster aspart reported a total of four severe hypoglycaemic episodes; these three participants also reported 10 of the 21 severe hypoglycaemic episodes that occurred with faster aspart during the treatment period. No severe hypoglycaemic episodes were reported during the run‐in period by those participants later randomized to IAsp.
The observed rate of unexplained hyperglycaemic episodes was 16.3 and 14.8 episodes/PYE with faster aspart and IAsp, respectively. The percentage of participants reporting treatment‐emergent adverse events (TEAEs) and the overall rate of TEAEs were similar in the two treatment arms (Table S6, Supporting Information). The event rate for infusion‐site reactions considered possibly or probably related to trial product was numerically higher with faster aspart (5.5% of participants [0.29 events/PYE]) vs IAsp (3.8% of participants [0.18 events/PYE]); (Table S6, Supporting Information). Allergic reactions were reported in 4.2% and 3.0% of participants with faster aspart and IAsp, respectively (0.14 vs 0.09 events/PYE). Overall, most adverse events (AEs) were non‐serious, of mild or moderate severity, and judged to be unlikely related to trial product. The mean body weight increase from baseline at week 16 was <1 kg in both treatment arms, with a statistically significant difference in favour of IAsp (ETD −0.43 kg [95% CI −0.81; −0.06]; P = 0.024). No clinically significant differences were seen from baseline to week 16 with regard to vital signs, physical examination, fundus photography/fundoscopy, laboratory assessments (biochemistry, haematology, lipids and urine analysis), and no relevant changes in ECG variables were noted.
The mean number of infusion‐set changes per week was similar in the faster aspart and IAsp groups (2.55 vs 2.49, respectively). A similar rate of non‐routine infusion‐set changes was reported with faster aspart and IAsp (6.97 vs 6.68 events), although a higher proportion of participants reported non‐routine changes with faster aspart vs IAsp (71.2% vs 57.2%). Non‐routine changes reported by participants to be caused by a perceived occlusion or unexplained hyperglycaemia were similar in the two treatment groups, while changes attributable to infusion‐site reactions were numerically higher with faster aspart vs IAsp (Table S7, Supporting Information).
4. DISCUSSION
In this randomized trial, faster aspart was shown to be effective in glycaemic control because non‐inferiority to IAsp in CSII for the change from baseline in HbA1c after 16 weeks was achieved. Superiority of faster aspart over IAsp in the change in HbA1c, however, was not confirmed, with a small but statistically significant difference in favour of IAsp. Faster aspart significantly improved PPG increment vs IAsp at 30 minutes, 1 hour (superiority confirmed) and 2 hours after a standardized meal test, and this difference was supported by CGM IG postprandial increments and SMBG postprandial increments. These results align well with previous studies10, 13, 14; however, in light of the positive PPG findings in the present trial, it is surprising that faster aspart did not improve HbA1c to a greater extent than IAsp, particularly because a statistically significant difference in favour of faster aspart was demonstrated in a previous study in people with T1D using MDI (onset 1).10 Contrasting the IG profiles for faster aspart and IAsp, the higher nocturnal and pre‐meal levels of IG for participants receiving faster aspart may have countered the expected overall glycaemic benefit of improved PPG control. The reasons underlying this rise in IG are unclear; however, it is likely that both the basal rate and bolus pump settings used for this double‐blind trial required further optimization to adjust delivery according to the distinct pharmacological profile of faster aspart. Specific recommendations for the use of faster aspart in CSII may include a different distribution of insulin doses between basal and bolus.
The risk of overall severe or BG‐confirmed hypoglycaemia was similar in the two treatment groups; however, the rate of severe or BG‐confirmed hypoglycaemia for the small proportion of episodes reported 1 hour after the meal was significantly higher with faster aspart vs IAsp (with no significant differences at other time intervals). This finding was also reported in the onset 1 study after 26 and 52 weeks.10, 13 Collectively, these findings reflect the left‐shifted time–action profile of faster aspart4; that is, the increased early absorption, faster onset of action and greater early glucose‐lowering effect (vs IAsp) that can lead to earlier onset of hypoglycaemia after a meal. During the 16 weeks after randomization, a numerical imbalance in severe hypoglycaemia was observed, whereby a 3‐fold increase in the number of events was reported with faster aspart vs IAsp (0.29 vs 0.10 events/PYE). This finding may be partly attributable to an imbalance when randomizing participants who had previously experienced severe hypoglycaemia during the run‐in period. All three of these participants were randomly assigned to the faster aspart group and experienced 10 of the 21 episodes of severe hypoglycaemia during the treatment period.
The overall safety profiles for faster aspart and IAsp in terms of AEs were broadly similar and as expected for insulin aspart formulations. The rate of infusion‐set changes (routine and non‐routine) was similar in the two groups. A numerically higher number of infusion‐site reactions (a cited reason for non‐routine changes) was reported with faster aspart vs IAsp (0.29 vs 0.18 events/PYE, respectively). Further supporting the results of the onset 4 study,11 these findings indicate that faster aspart is safe to use and compatible with CSII.
The present trial is the first to evaluate the efficacy and safety of an ultra‐fast‐acting insulin in CSII therapy in a large number of participants (with a high participant retention rate) over a clinically meaningful treatment period. With the development of increasingly sophisticated insulin delivery systems comes the need for faster‐acting, more physiological insulins that are better able to control PPG fluctuations. The prospective use of ultra‐fast‐acting insulin in closed‐loop therapy is of great clinical interest and studies are underway.15, 16, 17, 18 In summary, this trial showed that faster aspart provides an effective and safe option for CSII treatment in people with T1D, with improvements in PPG control reflected in meal‐test and CGM results.
CONFLICTS OF INTEREST
D.C.K. has served as a consultant to Ascensia, Astra Zeneca, EoFlow, Lifecare, Novo Nordisk and Voluntis. M.E. has served on advisory boards for Novo Nordisk, Eli Lilly, Medtronic, Cellnovo and Roche, received speakers'/writers' fees from Novo Nordisk, Eli Lilly, Abbott Diabetes Care and Roche, and received travel support from Novo Nordisk and Roche. W.L. has served on advisory boards for Novo Nordisk, Sanofi and Insulet, has received honoraria for serving on speakers' bureaus for Novo Nordisk, Insulet and Dexcom, and has received research grant support from Novo Nordisk. H.‐P.K. has served on advisory boards for Novo Nordisk and Eli Lilly and received speakers' fees from Novo Nordisk, Eli Lilly, Dexcom, MSD, Astra Zeneca, Roche, Sanofi Aventis and Berlin Chemie. E.R. has served as a consultant for A. Menarini Diagnostics, Abbott, Becton‐Dickinson, Cellnovo, Dexcom Inc., Eli Lilly, Insulet Inc., Johnson & Johnson (Animas, LifeScan), Medtronic, Novo Nordisk, Roche and Sanofi‐Aventis, and received research support from Abbott, Dexcom Inc., Insulet Inc. and Roche. J.H.D.V. has served on advisory boards for Novo Nordisk, Roche Diabetes Care and Zealand, received research support from Dexcom, Medtronic, Novo Nordisk and Senseonics, and received speakers' fees from Novo Nordisk, Roche Diabetes Care and Senseonics. T.Gr. is an employee of, and holds shares in, Novo Nordisk A/S. A.H. and T.Go. are employees of Novo Nordisk A/S. T.B. has served on advisory boards for Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, Medtronic, DreaMed Diabetes and Bayer Health Care, received honoraria for participating on the speakers' bureaus of Eli Lilly, Bayer, Novo Nordisk, Medtronic, Sanofi and Roche, and owns stocks in DreaMed Diabetes. His institution received research grant support, with receipt of travel and accommodation expenses in some cases from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Sandoz and Diamyd.
Author contributions
D.C.K. was the principal investigator of this clinical trial and is the guarantor of this work, and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.H. was the medical specialist for the trial and had the medical responsibility on the clinical trial level. T.Gr. was the responsible statistician. All authors had access to the study data, take responsibility for the accuracy of the analysis, reviewed and contributed to the content of the manuscript, and had authority in the decision to submit the manuscript for publication, in collaboration with Novo Nordisk. All authors approved the manuscript for publication.
Data accessibility
The subject level analysis data sets for the research presented in the publication are available from the corresponding author on reasonable request.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
We are grateful to the patients for their participation in the study. Medical writing and editorial assistance were provided by Steven Barberini PhD and Erin Slobodian of Watermeadow Medical, an Ashfield company, part of UDG Healthcare PLC, funded by Novo Nordisk A/S.
Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast‐acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab. 2019;21:961–967. 10.1111/dom.13610
Funding information Novo Nordisk A/S.
REFERENCES
- 1. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;Cd005103. [DOI] [PubMed] [Google Scholar]
- 2. Pickup JC. Management of diabetes mellitus: is the pump mightier than the pen? Nat Rev Endocrinol. 2012;8:425‐433. [DOI] [PubMed] [Google Scholar]
- 3. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971‐978. [DOI] [PubMed] [Google Scholar]
- 4. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast‐acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen G, Alluis B, Meiffren G, et al. The ultra‐rapid BioChaperone insulin lispro shows a faster onset of action and stronger early metabolic effect than Humalog. Diabetes. 2014;63:LB18. [Google Scholar]
- 6. Kazda C, Leohr J, Liu R, et al. A novel formulation of insulin lispro containing citrate and treprostinil shows faster absorption and improved postprandial glucose excursions com vs Humalog in patients with T1DM. Diabetes. 2017;67:A247. [Google Scholar]
- 7. Muchmore DB. The need for faster insulin. J Diabetes Sci Technol. 2017;11:157‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster‐acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double‐blind, crossover trial. Diabetes Obes Metab. 2017;19:208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bode BW, Johnson JA, Hyveled L, Tamer SC, Demissie M. Improved postprandial glycemic control with faster‐acting insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2017;19:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell‐Jones D, Bode BW, De Block C, et al. Fast‐acting insulin aspart improves glycemic control in basal‐bolus treatment for type 1 diabetes: results of a 26‐week multicenter, active‐controlled, treat‐to‐target, randomized, parallel‐group trial (onset 1). Diabetes Care. 2017;40:943‐950. [DOI] [PubMed] [Google Scholar]
- 11. Zijlstra E, Demissie M, Graungaard T, Heise T, Nosek L, Bode B. Investigation of pump compatibility of fast‐acting insulin aspart in subjects with type 1 diabetes. J Diabetes Sci Technol. 2018;12:145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast‐acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52‐week, randomized, treat‐to‐target, phase III trial. Diabetes Obes Metab. 2018;20:1148‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal‐bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care. 2017;40:951‐957. [DOI] [PubMed] [Google Scholar]
- 15. ClinicalTrials.gov . NCT03212950. https://clinicaltrials.gov/ct2/show/NCT03212950. Accessed November 30, 2018.
- 16. ClinicalTrials.gov . NCT03579615. https://clinicaltrials.gov/ct2/show/NCT03579615. Accessed November 30, 2018.
- 17. ClinicalTrials.gov . NCT03554486. https://clinicaltrials.gov/ct2/show/NCT03554486. Accessed November 30, 2018.
- 18. ClinicalTrials.gov . NCT03262116. https://clinicaltrials.gov/ct2/show/NCT03262116. Accessed November 30, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The subject level analysis data sets for the research presented in the publication are available from the corresponding author on reasonable request.


