Abstract
Background
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a type 2‐mediated inflammatory disease associated with significant clinical, social, and economic burdens and high unmet therapeutic need. Dupilumab, a fully human monoclonal antibody targeting the interleukin‐4 receptor α (IL‐4Rα) subunit, demonstrated efficacy and acceptable safety in CRSwNP and other type 2 diseases (eg, atopic dermatitis and asthma). We now report the local effects of dupilumab on type 2 inflammatory biomarkers in nasal secretions and nasal polyp tissues of patients with CRSwNP in a randomized, placebo‐controlled, phase 2 trial (NCT01920893).
Methods
Cytokines, chemokines, and total immunoglobulin E (IgE) levels were measured using immunoassay techniques in nasal secretions and nasal polyp tissue homogenates of CRSwNP patients receiving dupilumab 300 mg or placebo weekly for 16 weeks.
Results
With dupilumab, type 2 biomarker concentrations decreased in nasal secretions (least squares mean area under the curve from 0 to 16 weeks for the change from baseline) vs placebo for eotaxin‐3 (−30.06 vs −0.86 pg/mL; P = 0.0008) and total IgE (−7.90 vs −1.86 IU/mL; P = 0.022). Dupilumab treatment also decreased type 2 biomarker levels in nasal polyp tissues at Week 16 vs baseline for eosinophilic cationic protein (P = 0.008), eotaxin‐2 (P = 0.008), eotaxin‐3 (P = 0.031), pulmonary and activation‐regulated chemokine (P = 0.016), IgE (P = 0.023), and IL‐13 (P = 0.031).
Conclusions
Dupilumab treatment reduced multiple biomarkers of type 2 inflammation in nasal secretions and polyp tissues of patients with CRSwNP, demonstrating that antagonism of IL‐4Rα signaling suppresses IL‐4‐/IL‐13‐dependent processes, such as mucosal IgE formation, as well as the expression of chemokines attracting inflammatory cells to the nasal mucosa.
Keywords: biomarkers, dupilumab, nasal polyposis, nasal secretions, type 2 inflammation
Abbreviations
- AD
atopic dermatitis
- AUC0‐16
area under the concentration‐vs‐time curve from time of first treatment to Week 16
- CCL
C‐C motif chemokine ligand
- CI
confidence interval
- CRS
chronic rhinosinusitis
- CRSwNP
chronic rhinosinusitis with nasal polyposis
- CT
computed tomography
- ECP
eosinophil cationic protein
- ELISA
enzyme‐linked immunosorbent assay
- IgE
immunoglobulin E
- IL
interleukin
- IL‐4Rα
interleukin‐4 receptor alpha
- IL‐13Rα1
interleukin‐13 receptor alpha 1
- IU
international units
- LS
least squares
- mAb
monoclonal antibody
- MFNS
mometasone furoate nasal spray
- NPS
nasal polyp score
- PARC
pulmonary and activation‐regulated chemokine
- PNIF
peak nasal inspiratory flow
- QoL
quality of life
- SD
standard deviation
- SE
standard error
- SNOT‐22
22‐item Sino‐Nasal Outcome Test
- TARC
thymus and activation‐regulated chemokine
- Th2
T helper type 2 cell
- TNF‐α
tumor necrosis factor alpha
- UPSIT
University of Pennsylvania Smell Identification Test
- VAS
visual analog scale
1. INTRODUCTION
Chronic rhinosinusitis (CRS) affects 11%‐15% of the general population and causes a considerable socioeconomic burden.1, 2 CRS is a heterogeneous inflammatory disease with two distinct clinical presentations based on pathophysiology and histology.3 CRS without nasal polyposis is a mainly neutrophilic disease with local elevations in T helper type 1‐derived cytokines, whereas CRS with nasal polyposis (CRSwNP) is characterized by type 2/T helper type 2 cell (Th2) inflammation, with marked infiltration of eosinophils and mast cells in the mucosa and polyp tissue.4 Increased concentrations of eosinophil cationic protein (ECP; a marker of activated, degranulated eosinophils), eotaxins, total immunoglobulin E (IgE), and interleukin (IL)‐4, IL‐5, and IL‐13 have been observed in the serum, plasma, and nasal polyp tissue of patients with CRSwNP.5, 6, 7 Type 2 innate lymphoid cells8, 9 and type 2/Th2 lymphocytes10 accumulate in nasal polyps and mucosa of CRSwNP patients and secrete IL‐4, IL‐5, and IL‐13,11, 12 key drivers of type 2 inflammation, which also play roles in CRSwNP‐associated diseases such as aspirin‐exacerbated respiratory disease and asthma. Another feature of CRSwNP is the presence of mucosal B cells and plasma cells that secrete IgE,13, 14 providing evidence for local IL‐4‐modulated switching of Ig class.
The current standard of care for CRSwNP is focused on controlling inflammation and, depending on disease severity, consists of intranasal corticosteroid spray or drops, nasal saline lavage, short courses of oral corticosteroids, antibiotics, and functional endoscopic sinus surgery.2 A subgroup of patients with CRSwNP does not respond well to the current standard of care and has a rapid relapse of polyp regrowth after surgery.15 Blocking the underlying immunological pathomechanisms with monoclonal antibodies (mAbs) targeting IL‐4, IL‐5, and IL‐13 has shown promising results in these patients.16, 17 In clinical trials, omalizumab, a mAb that recognizes IgE, significantly reduced nasal polyp size and showed improvement in both upper and lower airway functions in patients with CRSwNP;18 mepolizumab, a mAb targeting IL‐5, significantly reduced nasal polyp size and computed tomography (CT) scores in patients with severe nasal polyposis.19
IL‐4 and IL‐13 are key drivers of type 2‐mediated inflammation; these cytokines have structural similarities and signal via the IL‐4 Type II heterodimeric receptor complex composed of IL‐4 receptor alpha (IL‐4Rα) and IL‐13 receptor alpha 1 (IL‐13Rα1). IL‐4 also signals via the IL‐4 Type I heterodimeric receptor, composed of the IL‐4Rα subunit and the common gamma chain. IL‐4 and IL‐13 have overlapping functions, but can demonstrate varying degrees of specificity in inducing type 2 inflammation based on the relative cellular distribution of the Type I and II receptors. IL‐4 predominantly controls IgE class switching, Th2 lymphocyte differentiation, and upregulation of IgE receptors on the surfaces of mast cells, basophils, and B lymphocytes.20, 21 In contrast, IL‐13 is thought to have a greater role in stimulating mucus hypersecretion, airway hyperresponsiveness and subepithelial fibrosis, and secretion of chemokines such as eotaxins.21 Both IL‐4 and IL‐13 induce secretion of thymus and activation‐regulated chemokine (TARC) and pulmonary and activation‐regulated chemokine (PARC), which attract and activate inflammatory cells.22, 23 Based on what is known about the overlapping functions of IL‐4 and IL‐13, targeting signaling via both cytokines may be beneficial in patients with type 2‐driven disease.
Dupilumab is a fully human VelocImmune ®‐derived mAb24, 25 that binds specifically to IL‐4Rα, the shared α chain subunit of the IL‐4 and IL‐13 receptors, inhibiting signaling of both IL‐4 and IL‐13. These cytokines have been implicated in numerous type 2 atopic/allergic diseases, including asthma and atopic dermatitis (AD), which are often associated as comorbidities.26 Placebo‐controlled clinical studies have demonstrated efficacy of dupilumab in both AD and asthma; asthma patients experienced significantly reduced exacerbation rates and improved lung function,27, 28 as well as reduced oral glucocorticoid use,28 and AD patients experienced significantly reduced disease severity and pruritus and improved quality of life (QoL).29, 30, 31 Dupilumab had an acceptable safety profile and was well tolerated in all of these trials. Dupilumab is approved in the European Union, USA, Japan, and other countries for the treatment of adults with inadequately controlled moderate‐to‐severe AD.
In a randomized, double‐blind, placebo‐controlled phase 2 study of dupilumab in patients with CRSwNP with and without asthma,32 the dupilumab group experienced significant improvement in endoscopic, radiographic, and QoL endpoints relative to placebo. These clinical changes were accompanied by a statistically significant reduction in circulating concentrations of the type 2 biomarkers total IgE and eotaxin‐3. Transient increases in blood eosinophil count were observed in some patients after dupilumab treatment initiation. In addition to the main study assessments of blood and serum, assessments of the effect of dupilumab on nasal secretions and nasal polyp tissues were performed in a substudy of this trial. Here, we report the local effect of dupilumab on total IgE levels and markers of eosinophilic inflammation in nasal secretions and polyp tissues from patients with CRSwNP.
2. METHODS
2.1. Study design and population
This randomized, double‐blind, placebo‐controlled, parallel‐group study was conducted in the USA and Europe between August 2013 and August 2014 (ClinicalTrials.gov Identifier: NCT01920893).32 The study was conducted following ethical principles in accordance with the Declaration of Helsinki, International Conference on Harmonisation guidelines, and Good Clinical Practice. All study documents and procedures were approved by the appropriate institutional review boards and ethics committees at each study site; each patient provided written informed consent before study participation. Eligible patients were aged 18‐65 years with bilateral nasal polyposis and chronic symptoms of sinusitis despite intranasal corticosteroid treatment lasting ≥2 months. Patients were required to have bilateral endoscopic nasal polyp score (NPS) ≥5 (maximum score = 8) with ≥2 polyps per nostril and to exhibit ≥2 of the following prior to screening: nasal obstruction or discharge, facial pain or pressure, or a reduced or lost sense of smell. Exclusion criteria included previous participation in dupilumab clinical trials and treatment with systemic corticosteroids, mAbs, immunosuppressive treatments, or anti‐IgE therapy during the 2‐month pre‐screening period.32
2.2. Study treatments
After 4 weeks of treatment with mometasone furoate nasal spray (MFNS; 100 mg/nostril twice daily), 60 patients were randomly allocated (1:1) to receive a 600 mg loading dose of dupilumab followed by 16 weeks of 300 mg dupilumab (n = 30) or matched placebo (n = 30).32 MFNS was continued at a stable dose throughout the study, and inhaled asthma control therapies were allowed.
2.3. Study outcomes
Primary and secondary endpoints in the overall population have been previously reported32 and are described in detail in Table S1. The primary efficacy endpoint was mean change in bilateral endoscopic NPS from baseline to Week 16. Secondary endpoints included change in Lund‐Mackay CT score, percentage of maxillary sinus volume occupied by disease, 22‐item Sino‐Nasal Outcome Test (SNOT‐22) score, University of Pennsylvania Smell Identification Test (UPSIT) score, and peak nasal inspiratory flow, as well as patient‐rated nasal congestion or obstruction, anterior and posterior rhinorrhea, loss in sense of smell, nocturnal awakenings, and overall symptom severity. Safety outcomes were assessed as previously described.32
2.4. Sample collection
Nasal secretion samples were collected from 60 patients (n = 30 placebo, n = 30 dupilumab) in the outpatient clinic on Day 1, and then every 4 weeks. IVALON® Post‐Op Sinus Packings (Fabco®, New London, CT, USA) were inserted bilaterally into the nasal cavities for 5 minutes. The two packings were transferred to a tube, and the adsorbed analytes were then eluted from the packings by adding 3 mL of saline (0.9% NaCl) followed by centrifugation. The supernatant aliquots were stored at −70°C. Nasal polyp biopsies were collected with forceps from 14 patients at baseline; 12 of these patients provided a second biopsy at Week 16 (n = 4 placebo, n = 8 dupilumab). Fresh tissue biopsies were weighed, snap‐frozen in liquid nitrogen, and stored at −80°C. Pharmacodynamic measurements of blood eosinophil count, total serum IgE, serum TARC, and plasma eotaxin‐3 concentrations were collected as previously described.32
2.5. Measurement of cytokines and total IgE in nasal secretions
Concentrations of eotaxin‐3, total IgE, and ECP in nasal secretions were assayed in saline eluates from nasal swabs and reported without further normalization. No additional biomarkers were measured in nasal secretions. Eotaxin‐3 was analyzed using a human C‐C motif chemokine ligand (CCL) 26 / eotaxin‐3 enzyme‐linked immunosorbent assay (ELISA) (Quantikine ELISA Kit®, R&D Systems, Minneapolis, MN, USA) according to manufacturer instructions. Total IgE and ECP concentrations were analyzed using the ImmunoCAP® fluorescent enzyme immunoassay (Phadia AB, Uppsala, Sweden) according to manufacturer instructions.
2.6. Measurement of cytokines, total IgE, and ECP concentrations in tissue homogenates
Analysis of biomarker levels in the tissue of nasal polyps was carried out as previously described.5 To create tissue homogenates, the tissue was disrupted at 50 Hz for 2 minutes with the Tissue Lyser LT (Qiagen Benelux, Antwerp, Belgium), and 1 mL 0.9% NaCl in the presence of cOmplete™, an EDTA‐free protease inhibitor (Roche Diagnostics Belgium, Vilvoorde, Belgium), per 0.1 g of tissue was added. Homogenates were centrifuged at 1800 × g for 10 minutes at 4°C. Supernatants were removed and stored at −25°C until analysis.
All supernatants were analyzed for the presence of cytokines, chemokines, ECP, and total IgE. Total IgE and ECP levels were measured using the UniCAP system (Thermo Fisher Scientific, Phadia, Groot‐Bijgaarden, Belgium) according to manufacturer instructions. Cytokines were assayed using the Luminex Performance Assay (IL‐4, IL‐5, IL‐17, tumor necrosis factor alpha [TNF‐α], IL‐10, IL‐1β, IL‐6, and vascular endothelial growth factor) and Luminex Screening Human Assay (IL‐13, IL‐33, TARC [CCL17], eotaxin‐3 [CCL26], eotaxin‐2 [CCL24], eotaxin‐1 [CCL11], and PARC [CCL18]) (R&D Systems Belgium), according to manufacturer instructions.
2.7. Statistical analyses
Descriptive statistics were used for demographics and baseline characteristics. For biomarkers in nasal secretions, the areas under the concentration‐vs‐time curves from time of first treatment to Week 16 (AUC0–16) for the change from baseline were estimated by trapezoidal analysis. Comparison of treatment effects from an analysis of covariance model was based on least squares (LS) mean differences in AUC0‐16 between patients in the dupilumab group vs the placebo group (with 95% confidence intervals [CI] and P values). The model included AUC0‐16 as the response variable, and treatment, stratification factor (comorbid asthma, biopsy performed), and baseline biomarker value as the covariates. Since the number of placebo‐treated patients who were successfully biopsied was small (n = 4), dupilumab treatment effect on biopsy biomarkers was assessed as change from baseline, analyzed using the Wilcoxon matched‐pairs signed‐rank test, in addition to a comparison of dupilumab vs placebo, which was analyzed using the Mann‐Whitney U test. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Study patients
The baseline clinical characteristics of the overall study population were similar to those of patients with biopsy data available, except for numerical differences between posterior rhinorrhea in the morning (Table 1, Table S2). Baseline demographics and/or clinical characteristics were also comparable between treatment groups in both the overall population and biopsy subgroup, except for numerical differences between total IgE in serum, and ECP and total IgE in nasal secretions (overall population), and posterior rhinorrhea in the morning (biopsy subgroup) (Table 1, Table S2). The majority of patients in both the placebo (80%) and dupilumab (83.3%) groups completed the study.32 Fourteen patients provided biopsies at baseline, 12 of whom also provided biopsies at Week 16.
Table 1.
Baseline characteristics and clinical responses in the biopsy subgroup
| Baseline and change from baseline at Week 16 | ||||||
|---|---|---|---|---|---|---|
| Placebo + MFNS (n = 4) | Dupilumab + MFNS (n = 8) | Absolute difference from baseline vs placebo, LS mean (95% CI) | P value vs placebo | |||
| Baseline, mean (SD) | Change from baseline at Week 16, LS mean (SE) | Baseline, mean (SD) | Change from baseline at Week 16, LS mean (SE) | |||
| Bilateral endoscopic NPS | 6.25 (0.50) | −0.77 (0.85) | 5.63 (1.19) | −1.83 (0.59) | −1.06 (−3.43, 1.31) | 0.3391 |
| Lund–Mackay total score | 18.25 (3.95) | 1.10 (1.49) | 18.13 (4.76) | −9.52 (1.01) | −10.62 (−14.70, −6.54) | 0.0005 |
| Percentage of maxillary sinus volume occupied by disease | 72.20 (24.50) | 6.46 (9.03) | 61.54 (25.30) | −33.43 (5.98) | −39.89 (−64.38, −15.39) | 0.0063 |
| PNIF (AM) | 108.21 (88.57) | 0.11 (21.35) | 108.38 (66.18) | 57.36 (15.28) | 57.25 (−2.05, 116.54) | 0.0568 |
| SNOT‐22 score | 42.50 (25.89) | −0.60 (7.49) | 45.50 (13.61) | −32.11 (5.68) | −31.52 (−55.12, −7.91) | 0.0177 |
| Sinusitis symptom severity assessed on VAS, cm | 6.20 (1.84) | 1.13 (1.41) | 5.46 (3.02) | −3.65 (0.77) | −4.78 (−8.99, −0.58) | 0.0332 |
| Sense of smell assessed by UPSIT | 12.00 (2.16) | −2.27 (3.58) | 14.38 (8.60) | 9.84 (2.61) | 12.11 (1.74, 22.48) | 0.0277 |
| Nasal congestion or obstruction (AM) | 1.61 (0.66) | −0.11 (0.38) | 1.82 (0.57) | −0.93 (0.29) | −0.81 (−1.90, 0.27) | 0.1220 |
| Posterior rhinorrhea (AM) | 2.32 (0.47) | −0.03 (0.31) | 1.20 (1.03) | −0.64 (0.21) | −0.61 (−1.52, 0.30) | 0.1650 |
| Serum total IgE, IU/mL | 151.75 (125.84) | 10.47 (17.04) | 142.50 (119.29) | −61.17 (13.92) | −71.63 (−124.94, −18.33) | 0.0163 |
| Serum TARC, pg/mL | 425.09 (305.46) | 36.12 (108.45) | 441.94 (256.06) | 4.94 (97.00) | −31.18 (−387.31, 324.96) | 0.8375 |
| Plasma eotaxin‐3,a pg/mL | 56.75 (14.78) | 7.86 (6.24) | 65.70 (30.70) | −20.26 (5.24) | −28.12 (−48.03, −8.21) | 0.0131 |
| Blood eosinophil count, × 109/L | 0.29 (0.18) | 0.03 (0.08) | 0.32 (0.20) | −0.04 (0.06) | −0.07 (−0.38, 0.24) | 0.5199 |
AM, in the morning; CI, confidence interval; IgE, immunoglobulin E; IU, international units; LS, least squares; MFNS, mometasone furoate nasal spray; NPS, nasal polyp score; PNIF, peak nasal inspiratory flow; SD, standard deviation; SE, standard error; SNOT‐22, 22‐item Sino‐Nasal Outcome Test; TARC, thymus and activation‐regulated chemokine; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
For plasma eotaxin‐3 data, heterogeneous compound symmetry covariance structure was used instead of unstructured covariance structure, as the model with unstructured covariance structure does not converge.
3.2. Safety
Safety outcomes were previously reported in the overall population of this study.32 The most common treatment‐emergent adverse events were mild‐to‐moderate nasopharyngitis, injection‐site reactions, and headache, and were reported more frequently in the dupilumab treatment group vs placebo.
3.3. Nasal secretion analysis
Dupilumab treatment was associated with overall decreases in nasal secretion concentrations of eotaxin‐3, total IgE, and ECP (mean changes from baseline shown in Figure 1). The mean AUC0‐16 for changes from baseline values, when adjusted for covariates, was significantly lower vs placebo for levels of eotaxin‐3 (LS mean AUC0‐16 [±SE]: −30.06 [5.9] vs −0.86 [6.6] pg/mL; P = 0.0008) and total IgE (−7.90 [1.9] vs −1.86 [2.1] IU/mL; P = 0.0221) in nasal secretions of the overall population. The decrease in ECP in the dupilumab group compared to placebo did not reach statistical significance (−5.82 [4.3] vs −3.07 [4.6] ng/mL; P = 0.6364). In the dupilumab group, the mean absolute total IgE, ECP, and eotaxin‐3 concentrations in nasal secretions at baseline were numerically higher than in the placebo group and declined over time, reaching concentrations either similar to or lower than the placebo group at Week 16 (Table S3)
Figure 1.
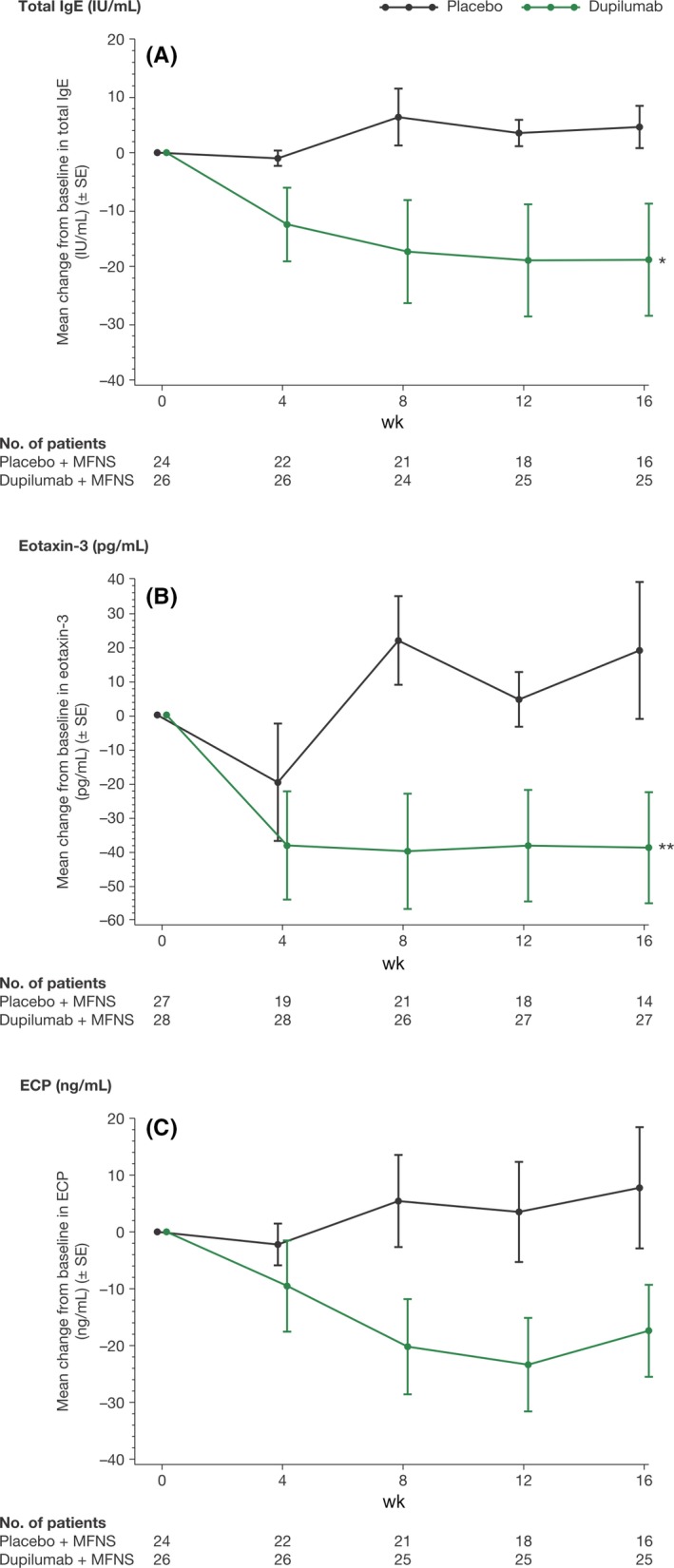
Biomarker concentrations in nasal secretions from patients with CRSwNP in the overall study population. Mean changes from baseline to Week 16 in (A) total IgE, (B) eotaxin‐3, and (C) ECP concentrations. ECP, eosinophil cationic protein; IgE, immunoglobulin E; IU, international units; MFNS, mometasone furoate nasal spray; SE, standard error. *P < 0.05; **P < 0.001 vs placebo. P values are nominal, not corrected for multiplicity, and based on the LS mean differences in AUC0‐16 between patients in the dupilumab group vs the placebo group
3.4. Clinical responses in biopsy subgroup
In the biopsy subgroup (and consistent with that previously reported for the overall study population32), dupilumab significantly improved radiographic and patient‐reported measures of disease activity after 16 weeks of treatment vs placebo, including the Lund‐Mackay total score, percentage of maxillary sinus volume occupied by disease, SNOT‐22 score, sinusitis symptom severity assessed by the visual analog scale, and sense of smell assessed by UPSIT, and significantly reduced circulating concentrations of total IgE and eotaxin‐3 (P < 0.05 for all; Table 1). In this small subset of patients, improvements in bilateral endoscopic NPS, peak nasal inspiratory flow in the morning, and nasal congestion or obstruction in the morning and posterior rhinorrhea in the morning, as well as shifts in blood eosinophil counts and serum TARC on dupilumab, were not significantly different with dupilumab vs placebo (Table 1).
3.5. Tissue homogenate analysis
Dupilumab treatment was associated with significantly lower total IgE (P = 0.023), ECP (P = 0.008), eotaxin‐2 (P = 0.008), eotaxin‐3 (P = 0.031), PARC (P = 0.016), and IL‐13 (P = 0.031) concentrations in tissue homogenates of the biopsy subgroup (n = 8) at the end of treatment compared with baseline (Figure 2). No significant differences were found in the levels of IL‐6, IL‐1β, eotaxin‐1, IL‐4, IL‐5, IL‐10, IL‐17, IL‐33, TNF‐α, or TARC compared with baseline for dupilumab (Table S4). Furthermore, significant differences in median changes from baseline with dupilumab vs placebo treatment at Week 16 were found for eotaxin‐1 (P < 0.05), PARC (P < 0.01), and ECP (P < 0.01) (Figure 3). No significant differences were found for IL‐6, IL‐33, SE‐IgE, TARC, total IgE, eotaxin‐2 and 3, IL‐1b, IL‐4, IL‐5, IL‐6, IL‐10, IL‐13, IL‐17, and IL‐33 (Table S5). Overall, no consistent modulation pattern was observed in the placebo group between baseline and end of treatment for any of these biomarkers in the tissue homogenates, with a limited sample size (n = 4) (Figures 2, 3).
Figure 2.
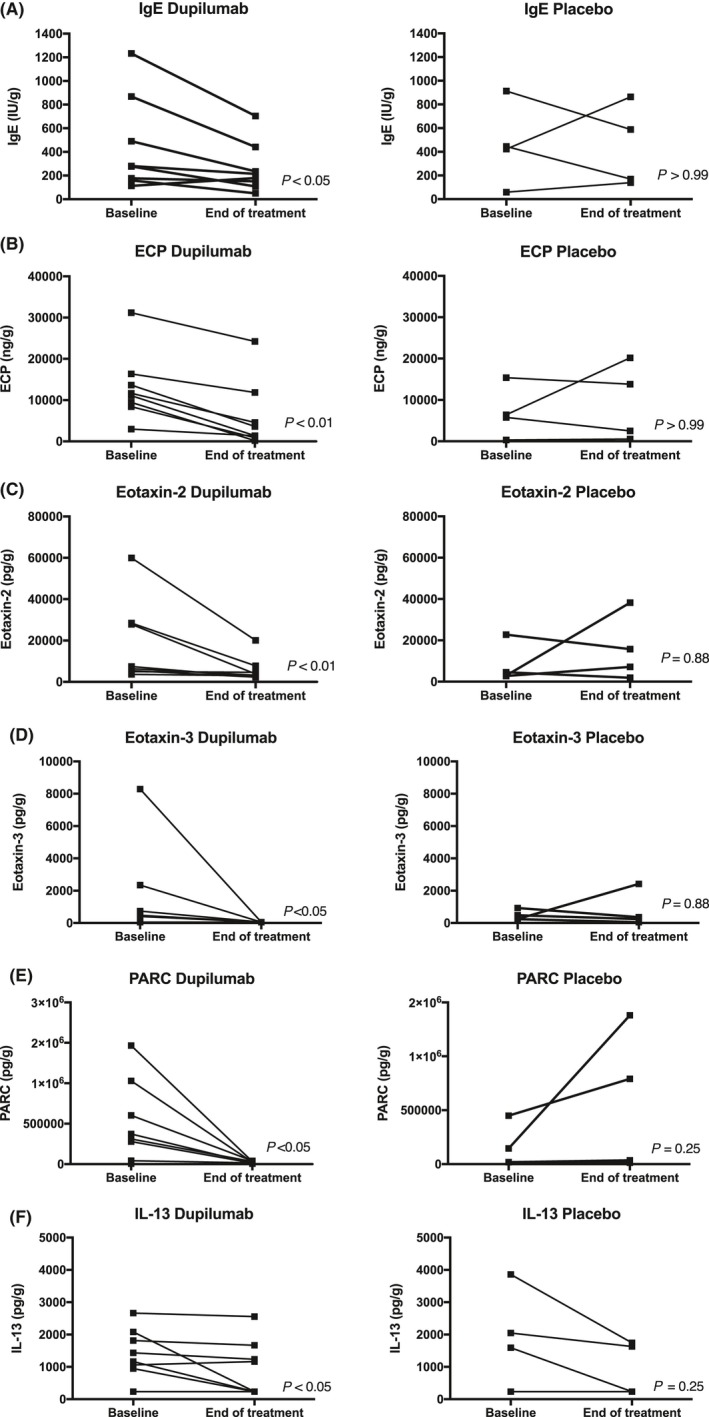
Biomarker concentrations in the nasal polyp tissue biopsies of patients with CRSwNP in the biopsy subgroup. Concentrations at baseline and Week 16 (end of treatment) in the dupilumab (n = 8) and placebo (n = 4) groups. (A) Total IgE, (B) ECP, (C) eotaxin‐2, (D) eotaxin‐3, (E) PARC, and (F) IL‐13. ECP, eosinophil cationic protein; IgE, immunoglobulin E; IL, interleukin; PARC, pulmonary and activation‐regulated chemokine. P values for end of treatment vs baseline are reported
Figure 3.
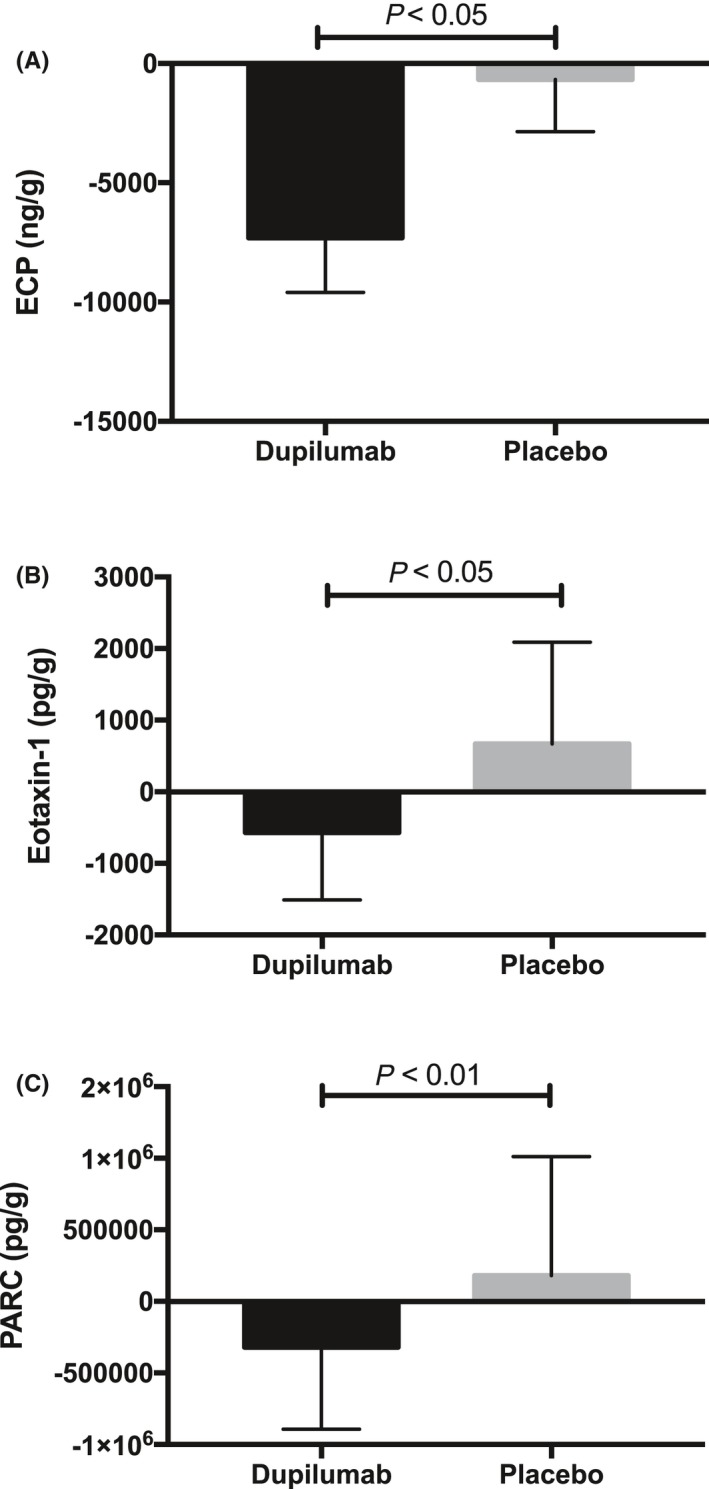
Biomarker concentrations in the nasal polyp tissue biopsies of patients with CRSwNP in the biopsy subgroup. Median changes from baseline at Week 16 (end of treatment) in the dupilumab (n = 8) and placebo (n = 4) groups in (A) ECP, (B) eotaxin‐1, and (C) PARC concentrations. CRSwNP, chronic rhinosinusitis with nasal polyposis; ECP, eosinophil cationic protein; PARC, pulmonary and activation‐regulated chemokine. P values are nominal, not corrected for multiplicity. Error bars represent the interquartile range
4. DISCUSSION
CRSwNP is characterized by a type 2‐predominant eosinophilic endotype in most patients. The presence of high levels of total IgE and IL‐5 in the nasal tissues and secretions of patients with CRSwNP, along with increased recognition of their respective roles in inflammation, has led to the testing of mAbs specifically targeting IgE (omalizumab) or IL‐5 (mepolizumab) in trials. These therapeutic agents reduced the signs and symptoms of CRSwNP.18, 19, 33, 34 However (with the exception of periostin), local reductions in IL‐5, ECP, or total IgE levels in nasal secretions and homogenates were not demonstrated with these therapeutic agents.34, 35 We recently reported that dupilumab, an IL‐4Rα inhibitor that blocks IL‐4 and IL‐13 signaling, was well tolerated, reduced polyp size, and rapidly improved smell in patients with CRSwNP.32 The post hoc analyses reported here were conducted to further investigate the local effects of dupilumab on eosinophilic inflammation and obtain more information on the relationship between local and serum levels of type 2 chemokines and total IgE in patients with CRSwNP.
Dupilumab treatment was associated with a significant decrease in biomarkers of type 2 inflammation, including total IgE and eotaxin‐3, in nasal secretions, as well as a decrease in ECP that did not reach statistical significance, vs placebo. In nasal polyp homogenates, dupilumab significantly reduced total IgE, the chemokines eotaxin‐2, eotaxin‐3, and PARC, and ECP concentrations at Week 16 compared with baseline, and eotaxin‐1, ECP, and PARC only when compared as mean changes from baseline at Week 16 vs placebo; similar trends were not observed with placebo. These data suggest that dupilumab not only reduces mucosal IgE production via antagonism of IL‐4Rα signaling, but most likely also affects the eosinophil pathway locally via suppression of eosinophil chemokine release; this is consistent with prior observations of increased levels of total IgE, ECP, and eosinophils in nasal polyps that may respond to therapy.5 We have previously reported that dupilumab markedly lowers total IgE and eotaxin‐3 levels in the blood of patients with CRSwNP.32 Thus, dupilumab demonstrates both systemic and local effects on these markers of type 2 inflammation in CRSwNP.
Eosinophil cationic protein is a cytotoxic ribonuclease produced and released by degranulating eosinophils. ECP concentrations are associated with the number of activated eosinophils in the blood.36 Elevation in eosinophil activity has not been completely elucidated, though it is elevated in CRSwNP and asthma, as well as other eosinophilic inflammatory diseases.6, 36 An increase in ECP concentrations in nasal mucosa may cause epithelial damage. Supporting this hypothesis, high concentrations of ECP have been reported in recurrent CRSwNP.6, 36 Dupilumab treatment resulted in a significant reduction in ECP concentrations in nasal polyp tissue at Week 16 compared with baseline, with a decline in ECP levels that did not reach statistical significance in nasal secretions, suggesting a decrease in local eosinophil activation. However, because of insufficient tissue, the effect of dupilumab on local eosinophil counts could not be determined.
Dupilumab significantly reduced the concentrations of eotaxin chemokines in both plasma32 and nasal secretions in patients with CRSwNP. Eotaxin levels are elevated in CRSwNP and other allergic and eosinophilic diseases such as asthma and AD.37 The eotaxin family consists of eotaxin‐1 (CCL11), eotaxin‐2 (CCL24), and eotaxin‐3 (CCL26), all of which are potent chemotactic factors for eosinophils and other inflammatory cells that express their receptors, and are released from epithelial cells following exposure to IL‐4 and IL‐13.38, 39 Eotaxins act on eosinophils and T lymphocytes to prolong the survival of eosinophils via the production and secretion of type 2‐associated cytokines37 and mediate transepithelial eosinophil migration and activation.40, 41 Eotaxin‐3, in particular, has been suggested to be an important recruiter of eosinophils in asthma,42 eosinophilic esophagitis,43 and nasal polyposis.44 Inhibiting IL‐4/IL‐13 signaling with dupilumab blockade of IL‐4Rα resulted in a decrease of eotaxin‐2 in nasal polyp tissue and eotaxin‐3 in both nasal polyp tissue and secretions.
PARC (also known as CCL18, MIP‐4, AMAC‐1, and DC‐CK1) is elevated in patients with CRSwNP.45 Secreted by mast cells, monocytes, dendritic cells, and macrophages, its expression is regulated by IL‐4 and IL‐13, as well as IL‐10. PARC is chemotactic for multiple cell types, including Th2‐cells, naive T cells, and dendritic cells. PARC is highly expressed in inflammatory diseases such as asthma and AD.23, 46, 47 In this substudy, dupilumab significantly reduced PARC concentrations in nasal polyp tissue compared with baseline, suggesting IL‐4 and IL‐13 are key drivers of tissue infiltration of not only eosinophils, but also other inflammatory cell types implicated in CRSwNP pathobiology.
Overall, these effects of dupilumab on biomarkers are consistent with blockade of IL‐4Rα and indicate a local anti‐inflammatory effect in the nose and sinuses. A possible mechanism for reducing eosinophil infiltration and activation in tissues, suggested by reduced levels of eotaxins in nasal secretions and polyps, may be downregulation of the chemotactic gradient, signaling eosinophil migration from the bloodstream into the tissue. Such a mechanism is further supported by the transient increase in eosinophils observed in blood shortly after the start of dupilumab treatment.32
Clinical improvements in the subgroup with polyp biopsies (n = 8) were comparable to those observed in the overall treatment group (n = 30) before and after 16 weeks of dupilumab treatment (LS mean differences vs placebo at Week 16 [95% CI] in NPS and SNOT‐22 score in biopsy subgroup vs total population: −1.06 [−3.43, 1.31] vs −1.55 [−2.43, −0.67] and −31.52 [−55.12, −7.91] vs −18.11 [−25.62, −10.60], respectively), suggesting that reductions in local inflammation biomarkers were attended by parallel improvements in symptoms and reduction in polypoid mass, as assessed by endoscopic exam and CT imaging. However, in contrast to the overall population, not all improvements in the biopsy subgroup were significant due to the small number of patients in the cohort, and no analyses were performed assessing associations between individual‐level improvements in biomarkers with individual‐level reductions in symptoms and polypoid mass.
Indeed, a limitation of this study was the small cohort of patients available for nasal secretions and tissue biopsy analyses. In a larger, ongoing phase 3 study (SINUS‐52; ClinicalTrials.gov Identifier: NCT02898454), additional biomarkers in nasal secretions and mRNA expression analyses and cytology of nasal mucosa brushings will be assessed. Another limitation of this study is that it enrolled almost exclusively Caucasian patients; as racial and regional differences in underlying inflammation associated with nasal polyps have been reported,10 the findings for biomarkers in this study may not be universally applicable.
Further studies are warranted to investigate dupilumab's mechanisms of action and identify markers of response in greater detail.
5. CONCLUSIONS
The reduction of multiple inflammatory biomarkers, including total IgE and markers of eosinophilic inflammation, in both nasal secretions and polyp tissues of patients with CRSwNP treated with dupilumab, relative to placebo and baseline, respectively, demonstrated a local effect of dupilumab on type 2 disease mechanisms, which were consistent with overall, parallel improvements in nasosinal symptoms and reductions in polypoid tissue. This suggests that the antagonism of IL‐4Rα signaling with dupilumab leads to clinical benefits that are mediated by reductions in underlying type 2 mucosal inflammation, although further analyses in larger patient populations are required to confirm these findings. Overall, we demonstrate that dupilumab has a broad mechanism of action, with its ability to target two central driver cytokines of type 2 inflammation, which might be more advantageous than targeting a single type 2 cytokine (eg, IL5 or IL13 alone) or solely targeting total IgE for the treatment of atopic diseases.
CONFLICTS OF INTEREST
K. Jonstam is the sub‐investigator of the study. B.N. Swanson, L. Mannent L, N. Tian, Y. Wang, D. Zhang, C. Fan, A. Grabher, and G. Pirozzi are employees of and may hold stock and/or stock options in Sanofi. L‐O. Cardell is an associate investigator of the study. G. Holtappels has nothing to disclose. J.D. Hamilton and N.M.H. Graham are employees and shareholders of Regeneron Pharmaceuticals, Inc. C. Bachert is the principal investigator of the study.
AUTHOR CONTRIBUTIONS
K.J., G.H., B.N.S., L.P.M., J.D.H., A.G., N.M.H.G., G.P., and C.B. involved in study concept and design. K.J., G.H., B.N.S., L.P.M., L‐O.C., N.T., Y.W., D.Z., C.F., J.D.H., A.G., N.M.H.G., G.P., and C.B. contributed to the acquisition, analysis, or interpretation of data. K.J. drafted the manuscript. K.J., G.H., B.N.S., L.P.M., L‐O.C., N.T., Y.W., D.Z., C.F., J.D.H., A.G., N.M.H.G., G.P., and C.B. performed critical revision of the manuscript for important intellectual content. All authors approved the manuscript for submission.
Supporting information
Table S1. Study outcomes
ACKNOWLEDGMENTS
We thank Megan Rice, ScD, for providing support with statistical analyses for this manuscript. This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Editorial assistance with drafting the manuscript following author guidance, incorporating comments according to author feedback, and submission support was provided by Jamie Lim, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro‐inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74:743–752. 10.1111/all.13685
Funding Information
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT01920893.
REFERENCES
- 1. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe – an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216‐1223. [DOI] [PubMed] [Google Scholar]
- 2. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:743‐12. [DOI] [PubMed] [Google Scholar]
- 3. Tomassen P, Vandeplas G, van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449‐1456. [DOI] [PubMed] [Google Scholar]
- 4. Gröger M, Bernt A, Wolf M, et al. Eosinophils and mast cells: a comparison of nasal mucosa histology and cytology to markers in nasal discharge in patients with chronic sino‐nasal diseases. Eur Arch Otorhinolaryngol. 2013;270:2667‐2676. [DOI] [PubMed] [Google Scholar]
- 5. Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607‐614. [DOI] [PubMed] [Google Scholar]
- 6. Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280‐1289. [DOI] [PubMed] [Google Scholar]
- 7. Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728‐732. [DOI] [PubMed] [Google Scholar]
- 8. Shaw JL, Fakhri S, Citardi MJ, et al. IL‐33‐responsive innate lymphoid cells are an important source of IL‐13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394‐403. [DOI] [PubMed] [Google Scholar]
- 10. Zhang N, Van Zele T, Perez‐Novo C, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961‐968. [DOI] [PubMed] [Google Scholar]
- 11. Koyasu S, Moro K. Innate Th2‐type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11:109‐114. [DOI] [PubMed] [Google Scholar]
- 12. Kong SK, Soo Kim B, Gi Uhm T, et al. Aspirin induces IL‐4 production: augmented IL‐4 production in aspirin‐exacerbated respiratory disease. Exp Mol Med. 2016;48:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840‐1847. [DOI] [PubMed] [Google Scholar]
- 14. Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B‐cell inflammation and EBV‐induced protein 2 expression. J Allergy Clin Immunol. 2013;131(1075–1083):1083.e1‐1083.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28:192‐198. [DOI] [PubMed] [Google Scholar]
- 16. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431‐1440. [DOI] [PubMed] [Google Scholar]
- 17. Pauwels B, Jonsta K, Bachert C. Emerging biologics for the treatment of chronic rhinosinusitis. Expert Rev Clin Immunol. 2015;11:349‐361. [DOI] [PubMed] [Google Scholar]
- 18. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(110–116):e1. [DOI] [PubMed] [Google Scholar]
- 19. Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: a randomised trial. J Allergy Clin Immunol. 2017;140(1024–1031):e14. [DOI] [PubMed] [Google Scholar]
- 20. Steinke JW. Anti‐interleukin‐4 therapy. Immunol Allergy Clin North Am. 2004;24(599–614):vi. [DOI] [PubMed] [Google Scholar]
- 21. Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546‐3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129‐135. [DOI] [PubMed] [Google Scholar]
- 23. de Nadaï P, Charbonnier AS, Chenivesse C, et al. Involvement of CCL18 in allergic asthma. J Immunol. 2006;176:6286‐6293. [DOI] [PubMed] [Google Scholar]
- 24. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111:5157‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111:5153‐5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nature Rev Drug Discov. 2016;15:35‐50. [DOI] [PubMed] [Google Scholar]
- 27. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378:2486‐2496. [DOI] [PubMed] [Google Scholar]
- 28. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378:2475‐2485. [DOI] [PubMed] [Google Scholar]
- 29. Simpson EL, Bieber T, Guttman‐Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335‐2348. [DOI] [PubMed] [Google Scholar]
- 30. Blauvelt A, de Bruin‐Weller M, Gooderham M, et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomized, double‐blinded, placebo‐controlled, phase 3 trial. Lancet. 2017;389:2287‐2303. [DOI] [PubMed] [Google Scholar]
- 31. de Bruin‐Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trials (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083‐1101. [DOI] [PubMed] [Google Scholar]
- 32. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469‐479. [DOI] [PubMed] [Google Scholar]
- 33. Vennera Mdel C, Picado C, Mullol J, Alobid I, Bernal‐Sprekelsen M. Efficacy of omalizumab in the treatment of nasal polyps. Thorax. 2011;66:824‐825. [DOI] [PubMed] [Google Scholar]
- 34. Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(989–995):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 35. De Schryver E, Derycke L, Calus L, et al. The effect of systemic treatments on periostin expression reflects their interference with the eosinophilic inflammation in chronic rhinosinusitis with nasal polyps. Rhinology. 2017;55:152‐160. [DOI] [PubMed] [Google Scholar]
- 36. Bystrom J, Amin K, Bishop‐Bailey D. Analysing the eosinophil cationic protein–a clue to the function of the eosinophil granulocyte. Respir Res. 2011;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmadi Z, Hassanshahi G, Khorramdelazad H, Zainodini N, Koochakzadeh L. An overlook to the characteristics and roles played by eotaxin network in the pathophysiology of food allergies: allergic asthma and atopic dermatitis. Inflammation. 2016;39:1253‐1267. [DOI] [PubMed] [Google Scholar]
- 38. Li L, Xia Y, Nguyen A, et al. Effects of Th2 cytokines on chemokine expression in the lung: IL‐13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999;162:2477‐2487. [PubMed] [Google Scholar]
- 39. Ramanathan M Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down‐regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olze H, Förster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin‐2 and eotaxin‐3. Rhinology. 2006;44:145‐150. [PubMed] [Google Scholar]
- 41. Yuan Q, Campanella GS, Colvin RA, et al. Membrane‐bound eotaxin‐3 mediates eosinophil transepithelial migration in IL‐4‐stimulated epithelial cells. Eur J Immunol. 2006;36:2700‐2714. [DOI] [PubMed] [Google Scholar]
- 42. Berkman N, Ohnona S, Chung FK, Breuer R. Eotaxin‐3 but not eotaxin gene expression is upregulated in asthmatics 24 hours after allergen challenge. Am J Respir Cell Mol Biol. 2001;24:682‐687. [DOI] [PubMed] [Google Scholar]
- 43. Blanchard C, Wang N, Stringer KF, et al. Eotaxin‐3 and a uniquely conserved gene‐expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poposki JA, Uzzaman A, Nagarkar DR, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peterson S, Poposki JA, Nagarkar DR, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129:119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamilton JD, Suárez‐Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate‐to‐severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293‐1300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study outcomes


